Dear Sir:
Pseudoxanthoma elasticum (PXE) is an inherited metabolic disease characterized by elastic fiber fragmentation and ectopic calcification in various tissues [1]. Cerebrovascular abnormalities have been sporadically reported in PXE [2]. The aims of this study are: (1) to investigate the prevalence of internal carotid artery hypoplasia (ICAH) in a PXE patient cohort by comparison with a non-PXE patient cohort; (2) to evaluate the prevalence of intracranial aneurysms (IAs) or arterial malformations (AMs) in ICAH PXE patients; and (3) to investigate the prevalence of stroke in ICAH PXE versus non-ICAH PXE patients.
We retrospectively compared 151 PXE patients from the French PXE cohort (National Reference Center for PXE at Angers University Hospital) to 402 non-PXE patients. ICAH was defined as diameter <3 mm as assessed by ultrasound imaging and confirmed by computed tomography angiography imaging. Stroke was defined as an acute neurological event with focal symptoms and signs lasting ≥24 hours consistent with focal cerebral ischemia and confirmed by cerebral imaging. Patients were deemed to have experienced a stroke once this information was retrieved from the medical record database.
Continuous variables were described as mean±standard deviation, and categorical variables were expressed as numbers and percentages. Chi-square test was used for categorical variables. In order to take into account possible heterogeneity between PXE- and non-PXE patients at baseline, P-value adjustments (Padjusted) were made using multivariate logistic regression analysis (MLRA) with body mass index, Framingham-Laurier scores, common carotid artery diameter and ankle-brachial-index as explanatory variables of ICAH, and the same variables in addition to internal carotid artery (ICA) diameter and mean resistive index as explanatory variables of stroke. Statistical analysis was performed using SAS version 9.4 software (SAS Institute, Cary, NC, USA). A significance threshold of 0.05 was applied to all of the statistical tests.
In this study, rules of ethics were followed for all patients in accordance with the Declaration of Helsinki of 2008. All patients provided informed written consent and the study was approved by our local ethics committee (CPP Ouest II, Angers, France).
Thirteen PXE patients and one non-PXE patient (8.6% vs. 0.2%, P<0.0001) presented with ICAH (Figures 1 and 2). MLRA indicated that ICAH was associated with PXE (odds ratio [OR], 7.44; 95% confidence interval [CI], 1.20 to 46.25; Padjusted=0.0314). IA and AM were found in 38.5% (5/13) of ICAH PXE patients (Figure 2B). No statistically significant difference in stroke prevalence between ICAH and non-ICAH PXE patients was observed (23.1% vs. 7.2%, P=0.0861) confirmed by MLRA where stroke prevalence was not statistically significant between PXE and non-PXE patients (OR, 1.73; 95% CI, 0.50 to 6.01; Padjusted=0.3853).
Figure 1.
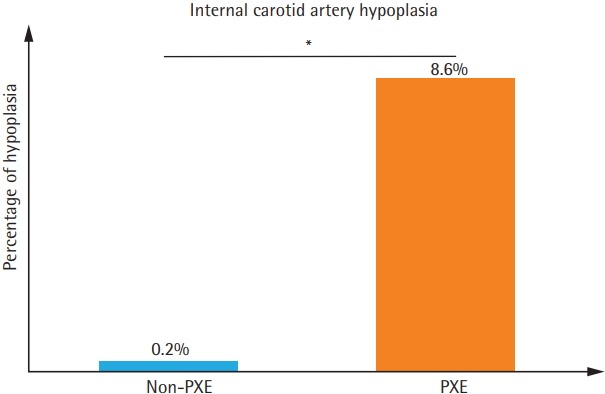
Prevalence of internal carotid artery hypoplasia (ICAH) in one cohort of pseudoxanthoma elasticum (PXE) patients and another cohort of non-PXE patients. *P<0.0001.
Figure 2.
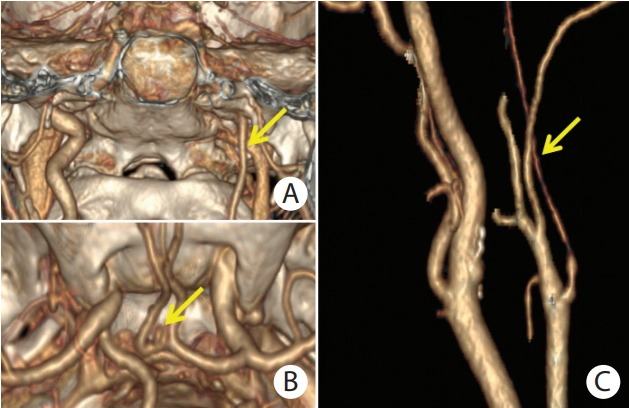
(A) Computed tomography angiography (CTA) with 3D volume rendering reconstruction of unilateral left internal carotid artery hypoplasia (ICAH) in a female pseudoxanthoma elasticum (PXE) patient in coronal plane (yellow arrow). (B) Upper view of small aneurysm of anterior communicating artery (yellow arrow) with visualization of circle of Willis and base of skull. (C) Reconstructed CTA image of internal carotid artery in a female PXE patient with left ICAH (yellow arrow).
The present study shows that ICAH is common in patients with PXE and is frequently associated with IA but does not increase the risk of stroke. The typical appearance of ICAH encountered in our PXE patients differed from that of atherosclerotic lesions or medial calcification and was devoid of echogenic densification or any lesions or irregularities suggestive of fibrodysplasia. Prevalence of ICAH (8.6%) was strikingly higher than that found in non-PXE patients (0.2%). The results we obtained from non-PXE patients are consistent with those documented in the general population where prevalence of unilateral or bilateral ICAH is reportedly below 0.1% [3]. In the general population ICAH is bilateral in 40%, unilateral right-sided in 40% and unilateral left-sided in 20% of cases [3]. This distribution echoes our findings (31% of bilateral, 38% of left-sided, and 31% of right-sided ICAH in 13 PXE patients with ICAH), suggesting similar mechanisms despite higher incidence in PXE patients.
In our study, we found that the carotid canal match to the ICA diameter suggesting an impaired developmental process. Indeed, given that the carotid canals in the skull base form secondary to the presence of the embryonic ICA, asymmetric diameters of the two carotid canals imply a developmental defect of the narrow internal carotid artery [4]. Computed tomography imaging of the skull base is the best means of studying the carotid canal and has become the main tool for diagnosing ICAH [4]. From a pathophysiological point of view, ICAH has been observed in association with various conditions, such as NF2 [5,6], polycystic kidney disease [7] (PKD) and Klippel-Trenaunay syndrome [8], but its etiology is largely unknown. Merlin and PKD protein mutations cause NF2 and PKD, respectively [9,10]. These proteins are implicated in endothelial cell mechanostransduction [9,10]. They contribute to vascular morphogenesis by bloodflow sensing [9,10].
ICAH in PXE displays unique morphological characteristics that differ from the clinical features usually observed in other vascular diseases involving stenosis of the ICA. The latter is encountered in several acquired diseases such as arteriosclerosis [11], large vessel vasculitis [12], fibromuscular dysplasia [13], intimal dissection [14], and moyamoya disease [15]. ICAH may be caused by abnormal signaling between the extracellular matrix and vascular cells. Indeed elastic fibers, which are fragmented in PXE, are a key regulator of vascular wall development [16]. Patients with inherited elastin defects develop segmental stenosis of the internal lumen in various vascular beds which differs from ICAH [17]. Furthermore, elastin matrix deposition in the arterial wall during late fetal development is essential for normal arterial morphogenesis [18]. We cannot, however, rule out the possibility that abnormalities in the fibroblasts of PXE patients might be instrumental in the pathogenesis of ICAH [18]. Nevertheless, specific ATP-binding cassette sub-family C member 6 (ABCC6) mutations could not be linked to ICAH-patients because no phenotype-genotype correlation was observed in ICAH PXE patients in this study (data not shown). Furthermore, ICAH should be treated as an additional phenotypic expression of PXE. In our study we observed 38.5% of IA and AM in ICAH PXE patients. Hence patients with ICAH should be screened for potential IA in the circle of Willis or AM, since the incidence of aneurysm is 24% to 34% in these patients compared to 2% to 4% in the general population [3].
ICAH was associated with hemorrhagic stroke in 23.1% of cases. In non-ICAH PXE patients, 7.2% had a history of ischemic stroke. No significant association was found between ICAH and stroke. In the general population, narrowing of the carotid lumen diameter results mainly from atherosclerosis and contributes to a higher risk of recurrent ischemic stroke [19].
Furthermore, stroke prevalence of 8.6% in our cohort of PXE patients (including ICAH and non-ICAH) correlates with a recent study [20] which reports 8% of strokes in 178 PXE patients from a Dutch cohort. Prevalence of stroke in the general population of a similar age approximates 3% [21], reflecting an increased risk of stroke in PXE patients.
ICAH is common in PXE patients and vascular physicians might test for this new feature during vascular assessment. Furthermore, this study suggests that PXE patients might be screened for ICAH. In all cases where ICAH is present, patients might undergo cerebral imaging to identify any aneurysms.
References
- 1.Lefthériotis G, Omarjee L, Le Saux O, Henrion D, Abraham P, Prunier F, et al. The vascular phenotype in Pseudoxanthoma elasticum and related disorders: contribution of a genetic disease to the understanding of vascular calcification. Front Genet. 2013;4:4. doi: 10.3389/fgene.2013.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van den Berg JS, Hennekam RC, Cruysberg JR, Steijlen PM, Swart J, Tijmes N, et al. Prevalence of symptomatic intracranial aneurysm and ischaemic stroke in pseudoxanthoma elasticum. Cerebrovasc Dis. 2000;10:315–319. doi: 10.1159/000016076. [DOI] [PubMed] [Google Scholar]
- 3.Taşar M, Yetişer S, Taşar A, Uğurel S, Gönül E, Sağlam M. Congenital absence or hypoplasia of the carotid artery: radioclinical issues. Am J Otolaryngol. 2004;25:339–349. doi: 10.1016/j.amjoto.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 4.Osborn RE, Mojtahedi S, Hay TC, DeWitt JD. Internal carotid artery hypoplasia. Comput Radiol. 1986;10:283–287. doi: 10.1016/0730-4862(86)90032-6. [DOI] [PubMed] [Google Scholar]
- 5.Chen MC, Liu HM, Huang KM. Agenesis of the internal carotid artery associated with neurofibromatosis type II. AJNR Am J Neuroradiol. 1994;15:1184–1186. [PMC free article] [PubMed] [Google Scholar]
- 6.Wali AR, Santiago-Dieppa DR, Steinberg JA, Alattar A, Cheung VJ, Modir R, et al. Hypoplastic internal carotid artery co-presenting with neurofibromatosis and intracranial masses. Cureus. 2016;8:e750. doi: 10.7759/cureus.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Afifi AK, Godersky JC, Menezes A, Smoker WR, Bell WE, Jacoby CG. Cerebral hemiatrophy, hypoplasia of internal carotid artery, and intracranial aneurysm. A rare association occurring in an infant. Arch Neurol. 1987;44:232–235. doi: 10.1001/archneur.1987.00520140090024. [DOI] [PubMed] [Google Scholar]
- 8.Goldstein SJ, Lee C, Young AB, Guidry GJ. Aplasia of the cervical internal carotid artery and malformation of the circle of Willis associated with Klippel-Trenaunay syndrome. Case report. J Neurosurg. 1984;61:786–789. doi: 10.3171/jns.1984.61.4.0786. [DOI] [PubMed] [Google Scholar]
- 9.Wong HK, Shimizu A, Kirkpatrick ND, Garkavtsev I, Chan AW, di Tomaso E, et al. Merlin/NF2 regulates angiogenesis in schwannomas through a Rac1/semaphorin 3F-dependent mechanism. Neoplasia. 2012;14:84–94. doi: 10.1593/neo.111600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Forman JR, Qamar S, Paci E, Sandford RN, Clarke J. The remarkable mechanical strength of polycystin-1 supports a direct role in mechanotransduction. J Mol Biol. 2005;349:861–871. doi: 10.1016/j.jmb.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 11.Casadei A, Floreani M, Catalini R, Serra C, Assanti AP, Conci P. Sonographic characteristics of carotid artery plaques: implications for follow-up planning? J Ultrasound. 2012;15:151–157. doi: 10.1016/j.jus.2012.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Giordana P, Baqué-Juston MC, Jeandel PY, Mondot L, Hirlemann J, Padovani B, et al. Contrast-enhanced ultrasound of carotid artery wall in Takayasu disease: first evidence of application in diagnosis and monitoring of response to treatment. Circulation. 2011;124:245–247. doi: 10.1161/CIRCULATIONAHA.110.006668. [DOI] [PubMed] [Google Scholar]
- 13.Arning C, Grzyska U. Color Doppler imaging of cervicocephalic fibromuscular dysplasia. Cardiovasc Ultrasound. 2004;2:7. doi: 10.1186/1476-7120-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chandra A, Suliman A, Angle N. Spontaneous dissection of the carotid and vertebral arteries: the 10-year UCSD experience. Ann Vasc Surg. 2007;21:178–185. doi: 10.1016/j.avsg.2006.10.020. [DOI] [PubMed] [Google Scholar]
- 15.Cali RL, Berg R, Rama K. Bilateral internal carotid artery agenesis: a case study and review of the literature. Surgery. 1993;113:227–233. [PubMed] [Google Scholar]
- 16.Karnik SK, Brooke BS, Bayes-Genis A, Sorensen L, Wythe JD, Schwartz RS, et al. A critical role for elastin signaling in vascular morphogenesis and disease. Development. 2003;130:411–423. doi: 10.1242/dev.00223. [DOI] [PubMed] [Google Scholar]
- 17.Pober BR, Johnson M, Urban Z. Mechanisms and treatment of cardiovascular disease in Williams-Beuren syndrome. J Clin Invest. 2008;118:1606–1615. doi: 10.1172/JCI35309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Quaglino D, Boraldi F, Annovi G, Ronchetti I. The multifaceted complexity of genetic diseases: a lesson from pseudoxanthoma elasticum. In: Ikehara K. Advances in the Study of Genetic Disorders. Rijeka, HR: Intech; 2011. pp. 289–318. [Google Scholar]
- 19.Johansson E, Cuadrado-Godia E, Hayden D, Bjellerup J, Ois A, Roquer J, et al. Recurrent stroke in symptomatic carotid stenosis awaiting revascularization: a pooled analysis. Neurology. 2016;86:498–504. doi: 10.1212/WNL.0000000000002354. [DOI] [PubMed] [Google Scholar]
- 20.Kauw F, Kranenburg G, Kappelle LJ, Hendrikse J, Koek HL, Visseren FLJ, et al. Cerebral disease in a nationwide Dutch pseudoxanthoma elasticum cohort with a systematic review of the literature. J Neurol Sci. 2017;373:167–172. doi: 10.1016/j.jns.2016.12.053. [DOI] [PubMed] [Google Scholar]
- 21.Benjamin EJ, Virani SS, Callaway CW, Chamberlain AM, Chang AR, Cheng S, et al. Heart disease and stroke statistics-2018 update: a report from the American Heart Association. Circulation. 2018;137:e67–e492. doi: 10.1161/CIR.0000000000000558. [DOI] [PubMed] [Google Scholar]


