Abstract
Glioblastoma (GBM) is one of the deadliest tumors and has a median survival of 3 months if left untreated. Despite advances in rationally targeted pharmacological approaches, the clinical care of GBM remains palliative in intent. Since the majority of altered signaling cascades involved in cancer establishment and progression eventually affect cell cycle progression, an alternative approach for cancer therapy is to develop innovative compounds that block the activity of crucial molecules needed by tumor cells to complete cell division. In this context, we review promising ongoing and future strategies for GBM therapeutics aimed towards G2/M inhibition such as anti-microtubule agents and targeted therapy against G2/M regulators like cyclin-dependent kinases, Aurora inhibitors, PLK1, BUB, 1, and BUBR1, and survivin. Moreover, we also include investigational agents in the preclinical and early clinical settings. Although several drugs were shown to be gliotoxic, most of them have not yet entered therapeutic trials. The use of either single exposure or a combination with novel compounds may lead to treatment alternatives for GBM patients in the near future.
Keywords: Chemotherapy, PLK1, AURK, survivin, BUB, BUR1
Introduction
Glioblastoma
For many years, tumors of the central nervous system (CNS) were primarily categorized according to histopathological criteria determined under microscopic observation, where similarities and phenotypic differences depended on cellular origin and their presumed levels of differentiation. In particular, gliomas are diffusely infiltrating glial cell tumors that are responsible for 80% of malignant tumors initiated in the brain and have been classified by the World Health Organization (WHO) depending on the type of the affected glial cell, thus integrating the nomenclature with grading system1. Histological diagnosis is correlated with tumor grade on a scale of I to IV based on how normal or abnormal the cells appear2. Accordingly, a grade IV astrocytoma (also called "glioblastoma", "glioblastoma multiforme", "grade IV glioblastoma", and "GBM") represents the most common and most aggressive primary malignant brain tumor, with 3 per 100,000 people diagnosed every year3. GBM is histopathologically characterized by brisk mitotic activity, cellular and nuclear atypia, vascular thrombosis, and microvascular hyperproliferation and necrosis, with 80% being primary or de novo occurring though malignant transformation from lower-grade gliomas (sometimes referred to as secondary GBMs)4.
GBM is one of the most deadly types of tumors5. If left untreated, this dismal tumor has a median survival of 3 months6. In addition to maximal safe surgical resection and radiotherapy (RT), the standard chemotherapeutic agent for its treatment since 2005 is the alkylant prodrug temozolomide (TMZ), which was first approved by the Food and Drug Administration (FDA) for use in recurrent GBM based on the phase II trial by Yung and colleagues7. Posteriorly, in the pivotal phase III study, Stupp and colleagues randomized ~600 patients diagnosed with GBM from various treatment centers. Their investigation consisted of radiation alone or radiotherapy with continuous daily TMZ, which demonstrated an improved 14.6-month median survival in the second group, versus 12.1 months in control patients. Two-year survival was also increased by 26.5% compared to 10.4% for those treated with radiotherapy alone. Nowadays, RT combined with concomitant and adjuvant TMZ after surgical resection, namely STUPP treatment, is widely used for newly diagnosed GBM patients8,9. Nonetheless, only 15%–20% of patients survive 5 years after diagnosis, and no other therapies have demonstrated a robust survival benefit in recurrent disease6,10.
TMZ is an imidazotetrazine derivative of the alkylating agent dacarbazine that delivers a methyl group to the purine bases of DNA (O6-guanine, N7-guanine, and N3-adenine). Although O6-methylguanine (O6-MeG) is the primary cytotoxic lesion, it can be reversed by the action of the repair enzyme methylguanine methyltransferase (MGMT), thereby neutralizing the cytotoxic effects of TMZ11. Accordingly, high expression of MGMT in glioma cells is the predominant mechanism underlying tumor resistance to alkylating agents12. Moreover, patients with methylated-MGMT treated with TMZ showed a 21.7-month median overall survival (OS) compared with 12.7 months in those with unmethylated promoters13, proving a direct association between MGMT expression and tumor response to TMZ therapy14.
Moreover, results from the European Organization for Research and Treatment of Cancer and National Cancer Institute of Canada trial recognized methylated-MGMT as the strongest predictor of outcome and benefit from TMZ treatment8,15. Similarly, the recent meta-analysis by Zhao and colleagues16 involving 7,886 patients, highlighted the universal predictive value of MGMT methylation in newly diagnosed GBM patients, elderly GBM patients, and recurrent GBM patients16.
Over the last two decades, many researchers have highlighted the importance of GBM molecular subtyping, but only recently was the WHO Classification for CNS Tumors able to integrate phenotypic and genotypic parameters, and subdivided GBM in three categories based on the status of the isocitrate dehydrogenase (IDH) gene17. Consequently, GBM are currently classified as IDH-wildtype (approximately 90% of cases that correspond most frequently to the clinically defined primary GBM), GBM IDH-mutant (approximately 10% of cases that closely correspond to the so-called secondary GBM), and GBM not otherwise specified (NOS), a diagnosis that is reserved for tumors without full IDH evaluation17. Importantly, some studies already have showed that OS of IDH-mutants are greater than IDH-wildtype gliomas18,19.
This current classification represents a conceptual and practical advance over its 2007 predecessor, reinforcing the need for molecular/genomic diagnosis, new molecular approaches, as well as further studies to gain a better understanding of the role of these mutational profiles in the survival of patients and their prognostic values.
Accordingly, many analyses of the genomic landscape of GBM were published by the Cancer Genome Atlas Research Network in 200820-22 and revealed specific genomic, epigenomic, transcriptomic, and proteomic alterations in core pathways that define novel specific tumor subgroups. Thus, some studies have predicted that genomic diagnoses will overrule and dictate the diagnosis in the future23. However, these subdivisions still play no role in current diagnostics and treatment decisions, but do help to overcome some of the molecular heterogeneity in GBM and improve treatment.
The concept of exploiting cell division as a therapeutic target has been in practice since the advent of chemotherapy. Briefly, antitumor treatments can affect the cell cycle through three modes of action: blocking DNA synthesis, causing DNA damage, or perturbing mitotic processes. As with many solid tumors, GBM is defined as a highly heterogeneous cancer with different cell populations coexisting within the tumor mass, each one with a distinct proliferative status directly connected to key molecules regulating cell cycle progression and mitosis. In this regard, the diagnostic and prognostic relevance of cell cycle biomarkers strongly reinforce the need to characterize signaling pathways and highlight their potential for novel targeted therapies for GBM. Hence, in the present review, we feature classic compounds used in anti-GBM therapy, and review recent advances on new therapeutic approaches that are based on the inhibition of cell cycle molecules.
Anti-microtubule agents
Mitosis disruption is widely used in clinics, and even with the rapid expansion in the number of classes of compounds with antineoplastic activity, anti-microtubule agents are still among the most strategic and have played a pivotal role in the curative and palliative treatment of cancer over the last 50 years.
In general, anti-microtubule compounds can be divided into tubulin destabilizing and stabilizing agents. In the first group, we can include vinblastine and vincristine, alkaloids isolated from the periwinkle plant Cantharanthus rosea, and colchicine, isolated from the meadow saffron Colchicum autumnale, all of which have been shown to inhibit microtubule assembly and to depolymerize steady state microtubules at substoichiometric concentrations24. In marked contrast to these classical agents, paclitaxel (the prototype of the taxane family), is another natural occurring anticancer agent originally isolated from the stem bark Taxus brevifolia that induces tubulin polymerization and forms extremely stable and nonfunctional microtubules, ultimately resulting in apoptosis25.
Microtubule-targeting drugs were long believed to induce cellular death by disrupting the spindle and delaying mitosis, however, it is now recognized that aside from their role in proper chromosome segregation, microtubules also play a significant role in many interphase functions, such as intracellular trafficking of proteins and organelles, migration, and maintenance of cellular shape. Consequently, microtubule interphase function impairment also brings an overall effect that ensures the efficacy of taxanes and vinca alkaloids26. Accordingly, agents targeting microtubule dynamics are widely used in the clinic, both alone and in combination with other chemotherapeutic agents, against multiple types of cancer. Nonetheless, these agents possess substantial liabilities and their use has been restricted by dose-limiting peripheral neurotoxicity27, severe myelosuppression28, and acquired resistance29-31.
In recent years, many new anti-microtubule drugs have emerged, most of which are still under clinical phase I/II trials26. Of note, a third-generation taxane cabazitaxel was approved in 2010 by FDA as the first therapy to show a survival benefit for the treatment of patients with docetaxel-refractory castration-resistant prostate cancer32. Moreover, the low solubility and susceptibility to elimination by multi drug resistance (MDR) pumps have triggered the development of alternative formulations. Nab-paclitaxel (Abraxane®), for instance, results from the covalent binding of albumin to the classical taxane to improve its cellular uptake and is currently being implemented in the treatment of pancreatic ductal adenocarcinoma33. Other innovative approaches that include the encapsulation of paclitaxel in nanocarrier systems, such as nanoparticles, liposomes, micelles, bioconjugates, or dendrimers34, hold promise to possess stronger antitumor activity and/or to minimize undesired side effects.
In line with this, new possibilities have emerged for GBM treatment (Table 1). As classical taxanes do not cross the blood-brain barrier (BBB)29, additional microtubule-stabilizing compounds such as epothilone D35 and dictyostatin36 are now showing therapeutic potential against GBM, with BBB permeability and slow brain clearance. Other strategies, including conjugates of paclitaxel and poly-L-glutamic acid37,38, paclitaxel delivered in nanoparticles39-43, or in biodegradable polyethylene-glycol filomicelles44,45, have also shown auspicious pre-clinical results.
1.
Microtubule inhibitors and selective G2/M targeted compound tested in GBM in pre-clinical and therapeutic studies
| G2/M inhibitor | Development status | Reference |
| Anti-microtubule | ||
| 7-Deazahypoxanthine | Pre-clinical | 46 |
| AD-1 | Pre-clinical | 47 |
| Cabazitaxel | Phase III (did not include GBM) | 48 |
| Chalcone | Pre-clinical | 49 |
| CI-980 | Phase I/II (study was stopped) | 50 |
| Colcemid | Pre-clinical | 51 |
| Colchicine | Phase III (did not include GBM) | 51 |
| Combretastatin A4 | Pre-clinical | 52 |
| CP248 | Pre-clinical | 53 |
| Cucurbitacin B | Pre-clinical | 54 |
| D-24851 | Phase I (did not include GBM) | 55,56 |
| Docetaxel | Phase II | 57,58 |
| DTA0100 | Pre-clinical | 59 |
| Epothilone B | Phase I/II | 60,61 |
| Epothilone D | Phase I (did not include GBM) | 62 |
| Ixabepilone | Phase I/II | 61 |
| JAI-51 | Pre-clinical | 63 |
| Mebendazole | Pre-clinical | 64,65 |
| Sagopilone | Phase II | 66 |
| ST-11 | Pre-clinical | 67 |
| TTI-237 | Phase I (did not include GBM) | 68 |
| Vitilevuamide | Pre-clinical | 69 |
| PLK1 inhibitors | ||
| BI 2536 | Phase II (did not include GBM) | 70-73 |
| BI 6727 | Phase I/II (did not include GBM) | 71,74 |
| GSK461364 | Phase I/II (did not include GBM) | 71,75 |
| GW843682X | Pre-clinical | 71 |
| JNJ-10198409 | Pre-clinical | 70 |
| CDK inhibitors | ||
| Abemaciclib | Phase I | 76,77 |
| CVT-313 | Pre-clinical | 78 |
| Dinaciclib | Pre-clinical | 79 |
| Flavopiridol | Phase I (did not include GBM) | 80-83 |
| JNJ-7706621 | Pre-clinical | 84 |
| MK-8776 | Phase I/II (did not include GBM) | 85 |
| ON123300 | Pre-clinical | 86 |
| Palbociclib | Pre-clinical | 77,87-90 |
| Continued | ||
Targeted therapy against G2/M regulators
The success of spindle poisons and the vulnerability of cancer cells to mitotic arrest have directed the search for new compounds to attain alternative mitotic targets (Figure 1), raising the necessity to overcome the limitations of tubulin-based antimitotic drugs and to expand the clinical effectiveness previously shown by these drugs. Currently, many of these selective drugs are being tested (Table 1).
1.
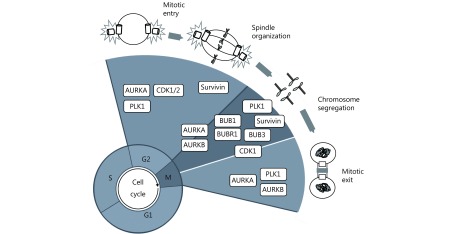
Schematic representation of plausible G2/M cell cycle regulators as therapeutic targets in GBM. CDK1 is essential for the G2–M transition and mitosis. BUBR1, BUB1, and BUB3 are fundamental to guarantee spindle assembly and correct chromosome separation by allowing their appropriate attachment to kinetochores. PLK1 is considered a master cell cycle regulator and is essential for the initiation of mitosis, centrosome maturation, bipolar spindle formation, cytokinesis, and the DNA damage response. AURKA and AURKB are crucial for spindle formation and guarantee the organization and alignment of the chromosomes during prometaphase, centrosome separation, and participate during cytokinesis. Survivin participates in cell division through its functions in the chromosomal passenger complex (CPC), spindle formation, checkpoint control, and assembling on polymerized microtubules.
Cyclin-dependent kinase (CDK) inhibitors
Cell cycle begins with a single cell that divides into two daughter cells with identical genomes107. This complex process is a well-ordered series of irreversible transitions from one state to the next. CDKs orchestrate progression through the consecutive phases, including entry into the cell cycle from quiescence, the G1/S phase transition, DNA replication in S phase, nuclear breakdown, chromosome condensation and segregation, and cytokinesis108.
Some CDKs (CDK1, CDK2, CDK3, CDK4, CDK6, CDK10, and CDK11) coordinate cell cycle regulation at different stages. CDK1 and CDK2, for instance, direct S and G2 phase transit, while CDK1 alone governs the G2/M transition and mitotic progression109. CDKs are generally activated when complexed with the adaptor molecules cyclins, but are also regulated by phosphorylation, interactions with inhibitory proteins, transient intracellular translocations, or by periodic proteolytic degradation of their activating cyclin partner. Other CDKs regulate cell cycle indirectly by activating other members of the family (CDK7, CDK20) or via transcription (CDK7, CDK8, CDK9, CDK19)110.
Amplification or mutation of cyclins, CDKs genes, or genes encoding their endogenous inhibitors, ultimately leads to deregulated CDK activity and loss of cell cycle control, a universal characteristic of cancer cells111-113. Changes in CDKs expression and/or regulation have been described in many human tumors, and occur frequently in the development of GBM114.
The search for CDK inhibitors began approximately 20 years ago and has led to the development of a plethora of compounds with variable selectivity that efficiently block cell-cycle progression and display potentially promising antitumor activities115,116. So far, the most thoroughly studied CDK inhibitors are flavopiridol and dinaciclib.
Flavopiridol is a semi-synthetic flavone that inhibits the activity of CDK1, CDK2, CDK4, and CDK6 by competitive binding at the adenosine triphosphate (ATP)-binding pocket117,118. In preclinical studies, its antitumor activity was rapidly recognized and confirmed in a variety of human tumor cell lines and xenograft models119,120. Specifically, in vitro testing of flavopiridol in GBM cell lines (including T98G, U87MG, U118MG, U251MG, and U373MG) induced apoptosis in a caspase-independent manner and independent of retinoblastoma and p53 tumor suppressor pathway alterations81. This drug also inhibited growth and cell migration in murine GBM, induced apoptosis, and reduced the expression of cyclin D1, CDK4, p21, and BCL-2. However, in contrast to reports on human cell lines, apoptosis was mediated by mitochondria121.
Nonetheless, despite its initial promise, flavopiridol has not displayed effectiveness in clinical trials as a single agent, and only marginal responses have been reported in the treatment of hematological malignancies122-124. This narrow therapeutic window and the identification of several off-target effects encouraged the development of the second-generation CDK inhibitor dinaciclib (SCH727665).
Dinaciclib inhibits CDK 1, 2, 5, and 9 with a better therapeutic index in preclinical studies125,126, and showed potent growth inhibiting activity in leukemia, osteosarcoma, and pancreatic cancer cells126-128. The drug also showed antitumor effects in a variety of human cell lines and xenograft models from the pediatric preclinical testing program129. Remarkably, treatment induced significant delays in event-free survival distribution compared to that of control treatment in more than 60% of evaluable solid tumors and in 3 of 7 leukemia xenografts, although poor results were obtained in the 3 xenograft GBM models129. Nevertheless, an initial clinical experience in patients with advanced leukemia showed transient cytoreductions and did not correlate with clinical outcome126. Many other compounds such as TG02130, P276-00131, SLM6132, and VMY-1-103133, among others, have been described to inhibit CDKs and may eventually enter clinical trials. Those previously tested in GBM are listed in Table 1.
Nonetheless, the inability of CDK inhibitors to produce prolonged remissions as single agents has increased the tendency towards their use in combination with other drugs. For instance, in vitro models have provided evidence of cooperative activity between flavopiridol and carboplatin in human ovarian cancer cells134, and between the CDK inhibitor and bortezomib in lymphoid and myeloid cell lines135,136. Moreover, flavopiridol has been shown to enhance TMZ-induced toxicity in human GBM cells and xenographic U87MG tumors in a p53-independent manner80, and to increase the pro-apoptotic and cytotoxic effects of ara-C in leukemia137. Early results in humans demonstrated 67% complete remission in patients with newly diagnosed acute myeloid leukemia (AML) after treatment with flavopiridol/cytarabine/mitoxantrone138. Auspicious results were also observed in a phase I trial of bortezomib and flavopiridol in patients with recurrent or refractory B-cell neoplasms139, providing additional prospects for pharmacological intervention.
Aurora kinases inhibitors
The accurate order of cell cycle progression events is ensured by tightly orchestrated feedback control mechanisms called "checkpoints", which prevent progression from phase to phase until particular critical conditions have been satisfied140,141. The metaphase checkpoint for example, also known as the spindle checkpoint, prevents the separation of chromosome or chromatid until they are properly attached to the spindle apparatus, guaranteeing correct chromosome placement on the metaphase plate and equitable chromosome segregation, resulting in the preservation of a stable diploid karyotype142. Conversely, abrogation of this mitotic checkpoint impairs the fidelity of chromosome segregation and induces chromosomal instability (CIN), a driving force of oncogenic transformation and tumor progression143,144.
The Aurora family of serine/threonine kinases includes Aurora A (AURKA), Aurora B (AURKB) and Aurora C (AURKC), all of which are essential for mitosis (AURKA and AURKB) and meiosis (AURKC) control145. Although the three AURK are involved in cell division, each one possesses specific functions. AURKA regulates the progression of mitosis by phosphorylating multiple substrates and promotes mitotic entry by governing the activation of Cyclin-B/CDK1145. Additionally, it controls centrosome maturation146, chromosome segregation, and bipolar spindle assembly147. AURKA expression, localization, and activity are consistent with its function as a centrosomal kinase. Specifically, there is an increase in its levels during the G2/M transition, and it is early localized at the centrosome and progressively associates with the mitotic poles and the adjacent spindle microtubules148.
On the other hand, AURKB is one of the most intensively studied kinases because it provides catalytic activity to the chromosome passenger complex (CPC). The CPC coordinates highly diversified processes, such as chromosome alignment, histone modification, and cytokinesis. For these reasons, AURKB is considered the "enzymatic heart" of this complex149,150. CPC can be considered as similar to the cyclin/CDK kinase complex, although instead of one nonenzymatic/regulatory subunit, the CPC contains three regulatory nonenzymatic subunits: survivin, borealin, and inner centromere protein (INCENP), all of which are subject to phosphorylation by AURKB151. AURKB kinase activity is critical for proper chromosome segregation150, initially through the phosphorylation of histone H3 on serine 10 (H3S10ph) to aid in mitotic chromosome condensation152,153. This kinase also contributes to the spindle checkpoint through phosphorylation of microtubule depolymerase mitotic centromere-associated kinesin (MCAK), which targets the protein to kinetochores, where it acts to mend any inadequate kinetochore attachment to the spindle154. Finally, AURKB is indispensable for accurate cytokinesis through phosphorylation of MgcRacGAP, a GTPase-activating protein, which is converted into RhoGAP and thus promotes cytokinesis155.
AURKC, on the other hand, appears to be the major enzymatic component of the CPC during meiosis, playing a specific role during female meiotic division and coordinating meiotic spindles in spermatogenesis156. Human AURKC is first expressed at the pericentric heterochromatin in pachytene spermatocytes157. In preimplantation embryos, it appears to be the major AURK expressed during the first three embryonic cell cycles, where it can be visualized on prometaphase chromosomes in zygotes and two- and four-cell-stage human embryos. Conversely, the endogenous AURKB protein is expressed at low-to-untraceable levels during these embryonic stages, but increases substantially after the eight-cell stage. It is interesting to highlight that the expression of AURKC occurs earlier, and is entirely substituted by AURKB at the blastocyst stage. Thus, it is tempting to hypothesize that AURKC could be the main enzymatic component of the CPC and thus, plays a specific role during human female meiosis and preimplantation embryo development157.
In terms of their role as mitotic regulators, deletion of AURKs could lead to cell division failure and embryonic development impairment. Increased expression or gene amplification of AURKs has been described in numerous cancers158, including breast159,160, ovarian161,162, gastric/gastrointestinal163,164, colorectal165,166, lung167,168, cervical169,170, prostate160,171,172, oral173,174, AML175,176, and glioma177,178. Importantly, in glioma tumors, AURKA and AURKB expression increases with tumor grade,179-182, and is significantly associated with GBM poorer patient survival183-185.
Many studies have confirmed the determinant role of AURKA in tumorigenesis through multiple mechanisms such as control of proliferation186, epithelial-mesenchymal transition (EMT)187, and metastasis188, as well as in the self-renewal capacity of cancer stem cells (CSCs)189. On the other hand, AURKB endorses cell cycle progression190 and the survival of cancer cells191, and AURKC kinase may encourage tumor progression160,192.
Thus, over the last decades, a series of AURK inhibitors have shown to effectively repress the progression and growth of many cancers (both in vivo and in vitro), suggesting that these kinases could represent novel therapeutic targets.
ZM447439, a selective ATP-competitor, was reported as the first Aurora kinase inhibitor in 2003193. This compound inhibits the phosphorylation of histone H3 on serine 10, a physiological target of AURKB194, causing AURKB to be more selectively inhibited both in vitro and in vivo compared to AURKA195. Borges et al.106 found that this inhibitor decreased proliferation and acted synergistically with TMZ in primary cultures and cell lines of GBM, while inducing apoptotic cell death.
Moreover, MLN8054, a small-molecule inhibitor of AURKA, induces accumulation of cells in G2/M phase and spindle defects in vitro196, leading to aneuploidy through chromosome congressional defects197. This compound was administered orally to breast, colon, pancreatic, and bladder cancer patients198 in a phase I clinical trial via capsules (5 or 25 mg) once daily for 7 uninterrupted days every 21 days and was later extended to evaluate increasing durations of oral dosing in patients with advanced malignancies199. The main side effect was grade 2/3 somnolence, which was attributed to the binding of the agent to the gamma-aminobutyric acid α-1 benzodiazepine (GABAAα1 BZD) receptor. Posteriorly, MLN8237 (alisertib), a more potent inhibitor was introduced and the structural modification of a methoxy group to either end of the MLN8054 molecule resulted in less benzodiazepine-like side effects200. This second generation inhibitor readily crosses the BBB and acts as a specific AURKA inhibitor at concentrations lower than and equal to the maximally tolerated dose in animal models and is currently being tested in a variety of phase I-II clinical trials201. Of note, two independent groups showed that MLN8237 exhibited potent effectiveness against glioblastoma neurosphere tumor stem-like cells in vitro and protracted the median survival of mice bearing intracranial human GBM neurosphere tumor xenografts202, while potentiating the effects of TMZ100.
Another interesting compound VX 680, also known as MK-0457, tozasertib, or VE465, is a synthetic, pyrimidine derivative with affinity for AURKA, AURKAB, and AURKAC. Nanomolar concentrations of MK-0457 show powerful antitumor activity by inhibiting phosphorylation of histone H3, causing accumulation of cells with 4N DNA, thus preventing cytokinesis and inducing massive apoptosis in various cancer cell lines. In preclinical models, MK-0457 has been shown to impede tumor xenograft growth and prompt tumor regression203. Moreover, in its first phase I clinical trial, intravenous continuous infusion of MK-0457 was given over several days to patients that had been previously treated for solid tumors204, and was found to show activity in a phase II study, administrated to patients with Philadelphia chromosome-positive acute leukemia205.
In GBM cells, MK-0457 decreased colony formation, increased polyploidy, and p53 expression, resulting in cell growth inhibition in a caspase-independent manner105. In parallel, AZD1152, a quinazoline prodrug, showed high affinity for AURKB and AURKC, with comparable effects to MK-0457 in cancer cells206. Specifically in GBM, AZD1152 induced polyploidy and non-apoptotic cell death regardless of p53 status and was accompanied by poly-merotelic kinetochore-microtubule attachments and DNA damage in all the cell lines tested102. Moreover, AZD1152 treatment enhanced the expression levels of the death receptor TRAIL-R2, thus increasing the natural killer (NK) cell ligand MIC A/B in p53-deficient cells, along with an induction of FAS/CD95 in p53-proficient cells leading to NK-cell-mediated lysis, thus highlight a p53-independent mode of action102. Additional AURK inhibitors that are less well studied but have been tested in GBM are listed in Table 1.
PLK1 inhibitors
Polo-like kinase (PLK) 1 is the most well studied member of the PLK family. This serine threonine kinase has a highly conserved N-terminal Ser/Thr kinase catalytic domain and plays a critical role in multiple steps of cell cycle progression and the DNA damage response207. Recently, PLK1 has emerged as a potential target for cancer therapy, as its overexpression is prevalent in various malignant tumor types and acts as a prognostic factor208,209.
This master cell cycle checkpoint protein shows its expression peak at G2/M phase and is involved in mitosis initiation, centrosome maturation, bipolar spindle formation, cytokinesis, and participates during the DNA damage response207. Preclinical studies in vitro and in vivo have shown that its blockage has a significant impact and can be explored for cancer treatment of both solid and hematologic malignancies.
Several anticancer drug candidates targeting PLK1 have been developed, and some agents have shown auspicious outcomes in early-phase clinical trials, though none of them have achieved clinical applications. PLK1 inhibitors can be classified according to their mode of action, even though most of them competitively bind to the ATP-binding site such as BI 2536, BI 6727, and GSK461364210. However, there are other inhibitors that target regions outside the ATP pocket, such as ONO1910, and those that can even bind to PLK1 through the PDB domain210,211.
In general, PLK1 inhibitors have shown favorable pharmacokinetic profiles, safety, and efficacy in patients with solid tumors. BI 2536, the first PLK1 inhibitor to be tested as monotherapy in humans, is currently being explored in a combinational study conducted in patients with diverse solid tumors212. Nonetheless, most data from clinical studies reported several adverse effects on patients including neutropaenia, thrombocytopaenia, anaemia, and pain213. Contrarily, the second-generation BI 6727 (also known as volasertib) showed improved pharmacokinetic profile, safety, and efficacy in phase II studies. Of note, the benefits obtained by treating AML with volasertib awarded the Breakthrough Therapy status for this drug by the FDA214, though its adverse effects are still an important issue to be considered . Comparatively, a recent phase I dose escalation study with NMS-1286937 successfully identified the maximum tolerated dose and toxicity, but even with disease stabilization in several patients, hematological toxicity was also limiting215.
Other multi-tyrosine kinase inhibitors, such as pazopanib and dasatinib, that are known to inhibit PLK1 at high concentrations, have been approved for therapeutic use by the FDA216,217 despite the fact that they may not inhibit PLK1 activity in a direct manner217.
On the other hand, PLK1 inhibitors have shown to be more effective when other genetic alterations are present in cancer cells. For instance, the thiophene benzimidazole GSK461364 has shown a superior antitumor effect in p53-mutated tumors in a preclinical study, although it must be co-administered with anticoagulants because of the high occurrence of venous thrombotic emboli212. Others, like RO3280, poloxin, and ON 01910, that are also being tested in vitro218, have shown to cause mitotic impairment and apoptosis in cancer cells219,220.
In GBM, BI 2536 has shown to cause G2 arrest, restrain cell proliferation and survival, and even synergize when combined with TMZ treatment71,221. Furthermore, when analyzing the CD133(+) tumor-propagating cells from primary GBM, a high level of PLK1 has been shown and its inhibition has an antitumor effect when combined with anti-BRAF drugs222. Other compounds like J10198409 have also shown to disrupt GBM stem cell proliferation70, and are sensitive to TMZ (Table 1).
BUB1 and BUBR1 inhibition
The mitotic spindle checkpoint (also known as spindle assembly checkpoint or SAC) is one of most important cell controls to guarantee the correct segregation of chromosomes141. Any dysregulation of SAC genes expression leads to DNA aneuploidy223. Several genes have been identified as part of this process, among which the budding uninhibited by benzimidazole (BUB) and the mitotic arrest deficient (MAD, also known in humans as BUBR1) family members are the most explored224,225.
The BUB family includes BUB1 and BUB3, while the BUBR1 family is composed by MAD1, MAD2, and MAD3. These kinases ensure correct chromosome segregation by playing key roles in averting the premature separation of sister chromatids until all chromosomes are appropriately attached to kinetochores226. BUBR1 is not only required for spindle checkpoint, but is also needed for chromosome alignment227. BUB1/BUBR1 and MAD2 operate as elements of distinct pathways sensing tension and attachment.
Variation on SAC gene expression was reported in human CNS tumors228. Alterations of different mitotic checkpoint proteins are important for GBM development and maintenance, and their levels are frequently inversely correlated to prognosis. Recently, it was shown that senescent GBM cells have aberrant centrosome morphology, and depletion of protein kinase C, which is fundamental to induce mitotic slippage-induced senescence229. Moreover, other kinases such as the monopolar spindle 1 (MSP1) are also important for spindle integrity and have shown to be regulated by the miR-21 in GMB cells230.
Mutations on BUB1, BUB3, and BUBR1 do not play substantial roles in the causation of chromosomal instability in GBM231. Nonetheless, a correlation between glioma grade and expression level of SAC genes was already reported, and BUB1 was associated with survival rates and proposed as a survival predictor232. Upregulation of BUB1 and BUBR1 expression and the downregulation of BUB3 were described in GBM samples and cell lines. Moreover, inhibition of BUB1 and BUBR1 via siRNA has shown to be efficient in decreasing cell proliferation and colony formation and, when combined with other drugs such as TMZ, increasing cell cycle arrest and apoptosis233.
In this way, SAC proteins are considered viable options for GBM therapy, though only few inhibitors are currently available, and none of them are FDA approved. Inhibitors of MSP-1 have already been tested in vitro showing promising results by sensitizing GBM cells to anticancer drugs234. The BUB1 inhibitors cycloalkenepyrazoles235, BAY-320 and BAY-524 have also shown to be efficient to sensitize cells to treatment with paclitaxel by compromising chromosome segregation and cell proliferation236.
Survivin
Survivin (also known as BIRC5) is a member of the inhibitor of apoptosis (IAP) family with key roles in the control of cell division and inhibition of apoptosis237-239. Of all the IAPs, survivin is the smallest with a single N-terminus BIR domain and C-terminus coiled coil (CC) domain and six alternative splicing variants or isoforms described until today240: wildtype (WT), 2B, ΔEx3, 3B, 2α, and 3α. Of those, survivin WT, 2B, and ΔEx3 variants have been extensively investigated for clinical and prognostic associations in cancer241. Notably, the role of novel isoforms in the regulation of apoptosis shows conservation of anti-apoptotic properties for survivin-WT and –ΔEx3 variants and a markedly reduced anti-apoptotic potential for survivin-2B242.
Functionally, survivin is considered a nodal protein, meaning that it is a protein involved in multiple signaling mechanisms in tumor maintenance and interacts with a large number of molecules, regulators, transcriptional networks, and modifiers that are involved in its functions, either directly or indirectly243. Consequently, it is possible to include survivin in multiple cellular networks. It participates in cell division through its functions in the chromosomal passenger complex (CPC), spindle formation, checkpoint control, and assembling on polymerized microtubules. Furthermore, in the anti-apoptotic network, survivin provides a heightened cell survival threshold and cooperates intermolecularly with adaptor or cofactor molecules of the cytosol and mitochondria243. Interestingly, increasing evidence indicates that survivin restricts autophagy, and its downregulation may induce apoptosis through autophagy-dependent mechanisms, including interactions with the autophagy regulator beclin 1244. In addition, recent reports show that in cancer cells, the translocation of survivin into the nucleus may increase DNA repair by upregulating Ku70245. Furthermore, survivin forms a complex with Ku70 and γH2AX in cells following irradiation246, and it has been widely demonstrated that its overexpression causes resistance to various chemotherapeutic (vincristine, cisplatin, bortezomib, tamoxifen) and pro-apoptotic agents (TNF-α, TRAIL)247-253. Of note, survivin is almost untraceable in normal adult tissues, but presents very high and unique expression patterns in most human tumors, making it an ideal selective target for cancer treatment243. Moreover, its plethora of functions allows for the disruption of numerous tumor-promoting networks with global anti-proliferative effects. For these reasons, many compounds have been developed to block its transcription, translation, and function in tumor cells243.
In gliomas, particularly GBM254, the high levels of survivin have been strongly related with a poor prognosis and resistance to chemotherapy and radiotherapy255,256. Among the more well studied survivin antagonists, the small molecules 1-(2-methoxyethyl)-2-methyl-4,9-dioxo-3-(pyrazin-2-ylmethyl)-4,9-dihydro-1H-naphtho (2,3-d) imidazolium bromide (YM155) and tetra-O-methyl nordihydroguaiaretic acid (M4N) have already been tested in GBM cells. YM155, specifically inhibits survivin gene promoter activity257 and its anti-neoplastic effect have now been reported in GBM cell lines with normal or deficient DNA-dependent protein kinase activity258. In addition, this drug caused a drastic decrease in the invasive and metastatic capacities of the GBM cells259, However, phase I and II trials have shown modest efficacy260,261.
On the other hand, M4N can inhibit the transcription of survivin by interfering with the binding of the Sp1 transcription factor to its promoter262. Recently, Castro-Gamero et al.263 showed that M4N treatment downregulated the expression of survivin and the survivin-ΔEx3 variant, and augmented the levels of survivin-2B variant, while decreasing cell proliferation, inducing apoptosis and acting synergistically with chemotherapy in GBM primary cultures and cell lines. Comparable to YM155, M4N has also been included in clinical trials. Safe profiles and partial responses have been demonstrated in a few patients with chronic myeloid leukemia (CML) or AML264. Interestingly, in a phase I study that included high-grade glioma patients, the safe daily dose was established as 1,700 mg, although the long-term stability and the lack of associated myelosuppression suggested that M4N could be safely combined with radiation and TMZ in newly diagnosed high-grade gliomas265.
On the other hand, while targeting the transcription of survivin is already being tested in clinical trials, YM155 and M4N are not selective and may also act on one or more upstream transcription factors that regulate the expression of many other downstream genes. Consequently, designating these compounds as specific survivin inhibitors is difficult266.
G2/M inhibition and GBM standard treatment
As previously described, TMZ is the first line drug for GBM treatment. The drug was initially approved in the 1980s for grade III gliomas. Due to its oral administration and ability to cross the BBB, TMZ was rapidly incorporated into GBM treatment. Nonetheless, its efficacy is hampered by the presence of active MGMT and has only brought mild improvements to treatment efficacy, even in patients that do not express the DNA repair protein. Thus, there is a constant search for drugs to potentiate its cytotoxic effects. Within this review, we have highlighted G2/M inhibitors that have already shown synergistic effects with TMZ in GBM pre-clinical studies and summarized these findings in Table 2.
2.
G2/M inhibitors that have proven synergistic effects with GBM standard treatments (TMZ and radioitherapy) and other drugs in vitro
| Inhibitor | Target | Synergistic effect | Radiosensitizer | Reference |
| Docetaxel | Microtubules | TMZ | Not tested | 56 |
| BI 2536 | PLK1 | TMZ | Yes | 70,71,221 |
| BI 6727 | PLK1 | TMZ | Not tested | 71,74 |
| GSK461364 | PLK1 | TMZ | Yes | 71,75 |
| GW843682X | PLK1 | TMZ | Not tested | 71 |
| JNJ-10198409 | PLK1 | Not tested | 70 | |
| Abemaciclib | CDK4/6 | TMZ | Not tested | 76,77 |
| Dinaciclib | CDK2/CDK5/ CDK1/CDK9 | Not tested | 79 | |
| Flavopiridol | CDK1/CDK2/CDK4/CDK6 | TMZ | Not tested | 80-83 |
| MK-8776 | CDK2 | Gemcitabine | Not tested | 85 |
| ON123300 | CDK4 | Gefitinib | Not tested | 86 |
| Palbociclib | CDK4/6 | TMZ ; mTOR inhibitor | Yes | 77,87-90 |
| Roscovitine | CDK2/CDK5 | PI3K inhibitor PIK-90 | Not tested | 92,93 |
| Alisertib | AURKA | TMZ | Yes | 97-100 |
| AZD1152 | AURKA/B | TMZ | Yes | 101,102 |
| VX-680 | pan-AURK | Yes | 104 | |
| ZM 447439 | AURKA/B | TMZ | Yes | 106 |
| YM155 | Survivin | TMZ | Yes | 267 |
| M4N | Survivin | TMZ | Yes | 263 |
Radiotherapy, on the other hand, is considered one of most effective cancer treatments after surgery. However, despite the combination of treatment strategies, GBM prognosis remains low. Several drug combinations have been proposed to increase radiotherapy response. Many of the cell cycle inhibitors cited above have proven encouraging results in vitro (Table 2).
PLK1 blockage by BI 2536, has a radiosensitizing effect on GBM by causing a G2/M arrest and leading to an increase in cell death71. Similar results were seen when another PLK1 inhibitor (GSK461364) was used75.
Similarly, Lehman et al.182 found that MLN8237 was potently cytotoxic and sensitized GBM cells to ionizing radiation in vitro through AURKA inhibition. Moreover, this drug inhibited the proliferation of GBM neurospheres and potentiated the effects of TMZ and ionizing radiation via inhibition of phosphor-Thr(288) AURKA100. ZM447439 (associated with TMZ) also enhanced the effects of radiation in GBM cells106. Furthermore, YM155 has shown to increase the percentage of giant multinucleated cells and centrosomal overduplication of U87 cells after irradiation, causing mitotic cell death267. Similar results were obtained after treatment of GBM cells with M4N263.
Collectively, these findings highlight the potential of using cell cycle proteins for improved outcome of GBM by enhancing the response to radiation treatment.
Conclusions
Targeting the cell cycle machinery, especially mitotic proteins, continues to hold great promise as a potential strategy to combat cancer progression, showing very encouraging results in the preclinical setting. At present, a plethora of drugs have proven to be gliotoxic, but only a small number have entered therapeutic trials and from those, even fewer have proven to have effective responses (Figure 2). Nonetheless, many compounds are being tested for GBM and in the near future, single exposure or combinations may lead to treatment alternatives for patients with this devastating tumor.
2.
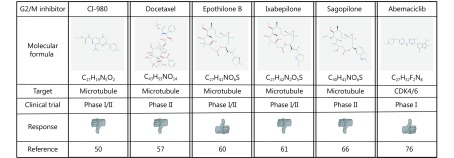
G2/M inhibitors that have been tested in patients with GBM. Formulas were obtained at the NIH Pubchem Open Chemistry Database (https://pubchem.ncbi.nlm.nih.gov).
Acknowledgements
This work was supported by The São Paulo Research Foundation – FAPESP (Grant No. 12/16888-8 and 2009/50118-2).
Conflict of interest statement
No potential conflicts of interest are disclosed.
References
- 1.Goodenberger ML, Jenkins RB Genetics of adult glioma. Cancer Genet. 2012;205:613–21. doi: 10.1016/j.cancergen.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 2.Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, et al The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114:97–109. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ostrom QT, Gittleman H, Fulop J, Liu M, Blanda R, Kromer C, et al CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2008-2012. Neuro Oncol. 2015;17:iv1–62. doi: 10.1093/neuonc/nov189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ohgaki H, Kleihues P Genetic pathways to primary and secondary glioblastoma. Am J Pathol. 2007;170:1445–53. doi: 10.2353/ajpath.2007.070011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khosla D Concurrent therapy to enhance radiotherapeutic outcomes in glioblastoma. Ann Transl Med. 2016;4:54. doi: 10.21037/atm. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Malmström A, Grønberg BH, Marosi C, Stupp R, Frappaz D, Schultz H, et al Temozolomide versus standard 6-week radiotherapy versus hypofractionated radiotherapy in patients older than 60 years with glioblastoma: the nordic randomised, phase 3 trial. Lancet Oncol. 2012;13:916–26. doi: 10.1016/S1470-2045(12)70265-6. [DOI] [PubMed] [Google Scholar]
- 7.Yung WKA, Albright RE, Olson J, Fredericks R, Fink K, Prados MD, et al A phase II study of temozolomide vs. procarbazine in patients with glioblastoma multiforme at first relapse. Br J Cancer. 2000;83:588–93. doi: 10.1054/bjoc.2000.1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJB, Janzer RC, et al Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC Trial. Lancet Oncol. 2009;10:459–66. doi: 10.1016/S1470-2045(09)70025-7. [DOI] [PubMed] [Google Scholar]
- 9.Nagasawa DT, Chow F, Yew A, Kim W, Cremer N, Yang I Temozolomide and other potential agents for the treatment of glioblastoma multiforme. Neurosurg Clin North Am. 2012;23:307–22. doi: 10.1016/j.nec.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 10.Chen R, Cohen AL, Colman H Targeted therapeutics in patients with high-grade gliomas: past, present, and future. Curr Treat Options Oncol. 2016;17:42. doi: 10.1007/s11864-016-0418-0. [DOI] [PubMed] [Google Scholar]
- 11.Zhang JH, Stevens MFG, Bradshaw TD Temozolomide: mechanisms of action, repair and resistance. Curr Mol Pharmacol. 2012;5:102–14. doi: 10.2174/1874467211205010102. [DOI] [PubMed] [Google Scholar]
- 12.Jaeckle KA, Eyre HJ, Townsend JJ, Schulman S, Knudson HM, Belanich M, et al Correlation of tumor O6 methylguanine-DNA methyltransferase levels with survival of malignant astrocytoma patients treated with bis-chloroethylnitrosourea: a southwest oncology group study. J Clin Oncol. 1998;16:3310–15. doi: 10.1200/JCO.1998.16.10.3310. [DOI] [PubMed] [Google Scholar]
- 13.Hegi ME, Diserens AC, Gorlia T, Hamou MF, de Tribolet N, Weller M, et al MGMT gene silencing and benefit from temozolomide in glioblastoma . N Engl J Med. 2005;352:997–1003. doi: 10.1056/NEJMoa043331. [DOI] [PubMed] [Google Scholar]
- 14.Newlands ES, Stevens MFG, Wedge SR, Wheelhouse RT, Brock C Temozolomide: a review of its discovery, chemical properties, pre-clinical development and clinical trials. Cancer Treat Rev. 1997;23:35–61. doi: 10.1016/S0305-7372(97)90019-0. [DOI] [PubMed] [Google Scholar]
- 15.Gorlia T, van den Bent MJ, Hegi ME, Mirimanoff RO, Weller M, Cairncross JG, et al Nomograms for predicting survival of patients with newly diagnosed glioblastoma: prognostic factor analysis of EORTC and NCIC trial 26981-22981/CE.3. Lancet Oncol. 2008;9:29–38. doi: 10.1016/S1470-2045(07)70384-4. [DOI] [PubMed] [Google Scholar]
- 16.Zhao YH, Wang ZF, Cao CJ, Weng H, Xu CS, Li K, et al The clinical significance of O6-methylguanine-DNA methyltransferase promoter methylation status in adult patients with glioblastoma: a meta-analysis . Front Neurol. 2018;9:127. doi: 10.3389/fneur.2018.00127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, et al The 2016 world health organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016;131:803–20. doi: 10.1007/s00401-016-1545-1. [DOI] [PubMed] [Google Scholar]
- 18.Ohno M, Narita Y, Miyakita Y, Matsushita Y, Arita H, Yonezawa M, et al Glioblastomas with IDH1/2 mutations have a short clinical history and have a favorable clinical outcome . Jpn J Clin Oncol. 2016;46:31–9. doi: 10.1093/jjco/hyv170. [DOI] [PubMed] [Google Scholar]
- 19.Hartmann C, Hentschel B, Simon M, Westphal M, Schackert G, Tonn JC, et al Long-term survival in primary glioblastoma with versus without isocitrate dehydrogenase mutations. Clin Cancer Res. 2013;19:5146–57. doi: 10.1158/1078-0432.CCR-13-0017. [DOI] [PubMed] [Google Scholar]
- 20.The Cancer Genome Atlas Research Network Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature. 2008;455:1061–8. doi: 10.1038/nature07385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Verhaak RGW, Hoadley KA, Purdom E, Wang V, Qi Y, Wilkerson MD, et al Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1 . Cancer Cell. 2010;17:98–110. doi: 10.1016/j.ccr.2009.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brennan CW, Verhaak RGW, McKenna A, Campos B, Noushmehr H, Salama SR, et al The somatic genomic landscape of glioblastoma. Cell. 2013;155:462–77. doi: 10.1016/j.cell.2013.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nørøxe DS, Poulsen HS, Lassen U Hallmarks of glioblastoma: a systematic review. ESMO Open. 2016;1:e000144. doi: 10.1136/esmoopen-2016-000144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Manfredi JJ, Horwitz SB Taxol: an antimitotic agent with a new mechanism of action. Pharmacol Ther. 1984;25:83–125. doi: 10.1016/0163-7258(84)90025-1. [DOI] [PubMed] [Google Scholar]
- 25.Wani MC, Taylor HL, Wall ME, Coggon P, McPhail AT Plant antitumor agents. VI. isolation and structure of taxol, a novel antileukemic and antitumor agent from taxus brevifolia. J Am Chem Soc. 1971;93:2325–7. doi: 10.1021/ja00738a045. [DOI] [PubMed] [Google Scholar]
- 26.Field JJ, Kanakkanthara A, Miller JH Microtubule-targeting agents are clinically successful due to both mitotic and interphase impairment of microtubule function. Bioorg Med Chem. 2014;22:5050–9. doi: 10.1016/j.bmc.2014.02.035. [DOI] [PubMed] [Google Scholar]
- 27.Gornstein E, Schwarz TL The paradox of paclitaxel neurotoxicity: mechanisms and unanswered questions. Neuropharmacology. 2014;76:175–83. doi: 10.1016/j.neuropharm.2013.08.016. [DOI] [PubMed] [Google Scholar]
- 28.Gidding CEM, Kellie SJ, Kamps WA, de Graaf SSN Vincristine revisited. Crit Rev Oncol Hematol. 1999;29:267–87. doi: 10.1016/S1040-8428(98)00023-7. [DOI] [PubMed] [Google Scholar]
- 29.Fellner S, Bauer B, Miller DS, Schaffrik M, Fankhänel M, Spruß T, et al Transport of paclitaxel (taxol) across the blood-brain barrier in vitro and in vivo. J Clin Invest. 2002;110:1309–18. doi: 10.1172/JCI0215451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vergara D, Tinelli A, Iannone A, Maffia M The impact of proteomics in the understanding of the molecular basis of paclitaxel-resistance in ovarian tumors. Curr Cancer Drug Targets. 2012;12:987–97. doi: 10.2174/156800912803251171. [DOI] [PubMed] [Google Scholar]
- 31.Murray S, Briasoulis E, Linardou H, Bafaloukos D, Papadimitriou C Taxane resistance in breast cancer: mechanisms, predictive biomarkers and circumvention strategies. Cancer Treat Rev. 2012;38:890–903. doi: 10.1016/j.ctrv.2012.02.011. [DOI] [PubMed] [Google Scholar]
- 32.Bahl A, Oudard S, Tombal B, Ozgüroglu M, Hansen S, Kocak I, et al Impact of cabazitaxel on 2-year survival and palliation of tumour-related pain in men with metastatic castration-resistant prostate cancer treated in the TROPIC trial. Ann Oncol. 2013;24:2402–8. doi: 10.1093/annonc/mdt194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Neesse A, Michl P, Tuveson DA, Ellenrieder V Nab-paclitaxel: novel clinical and experimental evidence in pancreatic cancer . Z Gastroenterol. 2014;52:360–6. doi: 10.1055/s-00000094. [DOI] [PubMed] [Google Scholar]
- 34.Nehate C, Jain S, Saneja A, Khare V, Alam N, Dubey RD, et al Paclitaxel formulations: challenges and novel delivery options. Curr Drug Deliv. 2014;11:666–86. doi: 10.2174/1567201811666140609154949. [DOI] [PubMed] [Google Scholar]
- 35.Sang F, Feng P, Chen J, Ding YH, Duan XY, Zhai JD, et al Epothilone D and its 9-methyl analogues: combinatorial syntheses, conformation, and biological activities. Eur J Med Chem. 2013;68:321–32. doi: 10.1016/j.ejmech.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 36.Brunden KR, Gardner NM, James MJ, Yao YM, Trojanowski JQ, Lee VMY, et al MT-stabilizer, dictyostatin, exhibits prolonged brain retention and activity: potential therapeutic implications. ACS Med Chem Lett. 2013;4:886–9. doi: 10.1021/ml400233e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jeyapalan S, Boxerman J, Donahue J, Goldman M, Kinsella T, Dipetrillo T, et al Paclitaxel poliglumex, temozolomide, and radiation for newly diagnosed high-grade glioma: a brown university oncology group study. Am J Clin Oncol. 2014;37:444–9. doi: 10.1097/COC.0b013e31827de92b. [DOI] [PubMed] [Google Scholar]
- 38.Luo ZM, Yan ZQ, Jin K, Pang Q, Jiang T, Lu H, et al Precise glioblastoma targeting by AS1411 aptamer-functionalized poly (L-γ-glutamylglutamine)-paclitaxel nanoconjugates . J Colloid Interface Sci. 2017;490:783–96. doi: 10.1016/j.jcis.2016.12.004. [DOI] [PubMed] [Google Scholar]
- 39.di Mauro V, Catalucci D The importance of being NcRNAs: from bit players as " junk DNA” to rising stars on the stage of the pharmaceutical industry. Ann Transl Med. 2017;5:147. doi: 10.21037/atm. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Balzeau J, Pinier M, Berges R, Saulnier P, Benoit JP, Eyer J The effect of functionalizing lipid nanocapsules with NFL-TBS.40-63 peptide on their uptake by glioblastoma cells. Biomaterials. 2013;34:3381–9. doi: 10.1016/j.biomaterials.2013.01.068. [DOI] [PubMed] [Google Scholar]
- 41.Xu YY, Shen M, Sun Y, Gao P, Duan YR Polymer nanocomposites based thermo-sensitive gel for paclitaxel and temozolomide co-delivery to glioblastoma cell. J Nanosci Nanotechnol. 2015;15:9777–87. doi: 10.1166/jnn.2015.12338. [DOI] [PubMed] [Google Scholar]
- 42.Gu GZ, Xia HM, Hu QY, Liu ZY, Jiang MY, Kang T, et al PEG-Co-PCL nanoparticles modified with mmp-2/9 activatable low molecular weight protamine for enhanced targeted glioblastoma therapy. Biomaterials. 2013;34:196–208. doi: 10.1016/j.biomaterials.2012.09.044. [DOI] [PubMed] [Google Scholar]
- 43.Gu GZ, Gao XL, Hu QY, Kang T, Liu ZY, Jiang MY, et al The influence of the penetrating peptide IRGD on the effect of paclitaxel-loaded MT1-AF7p-conjugated nanoparticles on glioma cells. Biomaterials. 2013;34:5138–48. doi: 10.1016/j.biomaterials.2013.03.036. [DOI] [PubMed] [Google Scholar]
- 44.Baumann BC, Kao GD, Mahmud A, Harada T, Swift J, Chapman C, et al Enhancing the efficacy of drug-loaded nanocarriers against brain tumors by targeted radiation therapy. Oncotarget. 2013;4:64–79. doi: 10.18632/oncotarget.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tsao CT, Kievit FM, Ravanpay A, Erickson AE, Jensen MC, Ellenbogen RG, er al Thermoreversible poly(ethylene glycol)-g-chitosan hydrogel as a therapeutic t lymphocyte depot for localized glioblastoma immunotherapy. Biomacromolecules. 2014;15:2656–62. doi: 10.1021/bm500502n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Scott R, Karki M, Reisenauer MR, Rodrigues R, Dasari R, Smith WR, et al Synthetic and biological studies of tubulin targeting c2-substituted 7-deazahypoxanthines derived from marine alkaloid rigidins. ChemMedChem. 2014;9:1428–35. doi: 10.1002/cmdc.v9.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fang KM, Liu JJ, Li CC, Cheng CC, Hsieh YT, Chai KM, et al Colchicine derivative as a potential anti-glioma compound. J Neurooncol. 2015;124:403–12. doi: 10.1007/s11060-015-1874-2. [DOI] [PubMed] [Google Scholar]
- 48.Ghoochani A, Hatipoglu-Majernik G, Sehm T, Wach S, Buchfelder M, Taubert H, et al Cabazitaxel operates anti-metastatic and cytotoxic via apoptosis induction and stalls brain tumor angiogenesis . Oncotarget. 2016;7:38306–18. doi: 10.18632/oncotarget.9439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shankaraiah N, Nekkanti S, Brahma UR, Praveen Kumar N, Deshpande N, Prasanna D, et al Synthesis of different heterocycles-linked chalcone conjugates as cytotoxic agents and tubulin polymerization inhibitors. Bioorg Med Chem. 2017;25:4805–16. doi: 10.1016/j.bmc.2017.07.031. [DOI] [PubMed] [Google Scholar]
- 50.Kunschner LJ, Fine H, Hess K, Jaeckle K, Kyritsis AP, Yung WKA CI-980 for the treatment of recurrent or progressive malignant gliomas: national central nervous system consortium phase I-II evaluation of CI-980. Cancer Invest. 2002;20:948–54. doi: 10.1081/CNV-120005910. [DOI] [PubMed] [Google Scholar]
- 51.Kato K, Ito H, Inaguma Y, Okamoto K, Saga S Synthesis and accumulation of αb crystallin in c6 glioma cells is induced by agents that promote the disassembly of microtubules. J Biol Chem. 1996;271:26989–94. doi: 10.1074/jbc.271.43.26989. [DOI] [PubMed] [Google Scholar]
- 52.Friesen DE, Barakat KH, Semenchenko V, Perez-Pineiro R, Fenske BW, Mane J, et al Discovery of small molecule inhibitors that interact with γ-tubulin. Chem Biol Drug Des. 2012;79:639–52. doi: 10.1111/jpp.2012.79.issue-5. [DOI] [PubMed] [Google Scholar]
- 53.Yoon JT, Palazzo AF, Xiao DH, Delohery TM, Warburton PE, Bruce JN, et al CP248, a derivative of exisulind, causes growth inhibition, mitotic arrest, and abnormalities in microtubule polymerization in glioma cells. Mol Cancer Ther. 2002;1:393–404. [PubMed] [Google Scholar]
- 54.Yin D, Wakimoto N, Xing HT, Lu DN, Huynh T, Wang X, et al Cucurbitacin B markedly inhibits growth and rapidly affects the cytoskeleton in glioblastoma multiforme. Int J Cancer. 2008;123:1364–75. doi: 10.1002/ijc.v123:6. [DOI] [PubMed] [Google Scholar]
- 55.Bacher G, Nickel B, Emig P, Vanhoefer U, Seeber S, Shandra A, et al D-24851, a novel synthetic microtubule inhibitor, exerts curative antitumoral activity in vivo, shows efficacy toward multidrug-resistant tumor cells, and lacks neurotoxicity . Cancer Res. 2001;61:392–9. [PubMed] [Google Scholar]
- 56.Tamaskar I, Mekhail T, Dreicer R, Olencki T, Roman S, Elson P, et al Phase I trial of weekly docetaxel and daily temozolomide in patients with metastatic disease. Invest New Drugs. 2008;26:553–9. doi: 10.1007/s10637-008-9153-0. [DOI] [PubMed] [Google Scholar]
- 57.Sanson M, Napolitano M, Yaya R, Keime-Guibert F, Broët P, Hoang-Xuan K, et al Second line chemotherapy with docetaxel in patients with recurrent malignant glioma: a phase II study. J Neurooncol. 2000;50:245–9. doi: 10.1023/A:1006494032052. [DOI] [PubMed] [Google Scholar]
- 58.Forsyth P, Cairncross G, Stewart D, Goodyear M, Wainman N, Eisenhauer E Phase II trial of docetaxel in patients with recurrent malignant glioma: a study of the national cancer institute of canada clinical trials group. Invest New Drugs. 1996;14:203–6. doi: 10.1007/BF00210791. [DOI] [PubMed] [Google Scholar]
- 59.Podolski-Renić A, Banković J, Dinić J, Ríos-Luci C, Fernandes MX, Ortega N, et al DTA0100, dual topoisomerase II and microtubule inhibitor, evades paclitaxel resistance in P-glycoprotein overexpressing cancer cells. Eur J Pharm Sci. 2017;105:159–68. doi: 10.1016/j.ejps.2017.05.011. [DOI] [PubMed] [Google Scholar]
- 60.Fogh S, Machtay M, Werner-Wasik M, Curran WJ Jr, Bonanni R, Axelrod R, et al Phase I trial using patupilone (Epothilone B) and concurrent radiotherapy for central nervous system malignancies. Int J Radiat Oncol Biol Phys. 2010;77:1009–16. doi: 10.1016/j.ijrobp.2009.06.050. [DOI] [PubMed] [Google Scholar]
- 61.Peereboom DM, Supko JG, Carson KA, Batchelor T, Phuphanich S, Lesser G, et al A phase I/II trial and pharmacokinetic study of ixabepilone in adult patients with recurrent high-grade gliomas. J Neurooncol. 2010;100:261–8. doi: 10.1007/s11060-010-0190-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dietzmann A, Kanakis D, Kirches E, Kropf S, Mawrin C, Dietzmann K Nanomolar concentrations of epothilone D inhibit the proliferation of glioma cells and severely affect their tubulin cytoskeleton. J Neurooncol. 2003;65:99–106. doi: 10.1023/B:NEON.0000003679.40609.63. [DOI] [PubMed] [Google Scholar]
- 63.Boumendjel A, McLeer-Florin A, Champelovier P, Allegro D, Muhammad D, Souard F, et al A novel chalcone derivative which acts as a microtubule depolymerising agent and an inhibitor of P-Gp and BCRP in in-vitro and in-vivo glioblastoma models . BMC Cancer. 2009;9:242. doi: 10.1186/1471-2407-9-242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.de Witt M, Gamble A, Hanson D, Markowitz D, Powell C, Al Dimassi S, et al Repurposing mebendazole as a replacement for vincristine for the treatment of brain tumors. Mol Med. 2017;23:50–6. doi: 10.1007/s00894-017-3212-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bai RY, Staedtke V, Wanjiku T, Rudek MA, Joshi A, Gallia GL, et al Brain penetration and efficacy of different mebendazole polymorphs in a mouse brain tumor model. Clin Cancer Res. 2015;21:3462–70. doi: 10.1158/1078-0432.CCR-14-2681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stupp R, Tosoni A, Bromberg JEC, Hau P, Campone M, Gijtenbeek J, et al Sagopilone (ZK-EPO, ZK 219477) for recurrent glioblastoma. a phase II multicenter trial by the european organisation for research and treatment of cancer (EORTC) brain tumor group. Ann Oncol. 2011;22:2144–9. doi: 10.1093/annonc/mdq729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fung S, Xu C, Hamel E, Wager-Miller JB, Woodruff G, Miller A, et al Novel indole-based compounds that differentiate alkylindole-sensitive receptors from cannabinoid receptors and microtubules: characterization of their activity on glioma cell migration. Pharmacol Res. 2017;115:233–41. doi: 10.1016/j.phrs.2016.10.025. [DOI] [PubMed] [Google Scholar]
- 68.Beyer CF, Zhang N, Hernandez R, Vitale D, Lucas J, Nguyen T, et al TTI-237: a novel microtubule-active compound with in vivo antitumor activity . Cancer Res. 2008;68:2292–300. doi: 10.1158/0008-5472.CAN-07-1420. [DOI] [PubMed] [Google Scholar]
- 69.Edler MC, Fernandez AM, Lassota P, Ireland CM, Barrows LR Inhibition of tubulin polymerization by vitilevuamide, a bicyclic marine peptide, at a site distinct from colchicine, the vinca alkaloids, and dolastatin 10. Biochem Pharmacol. 2002;63:707–15. doi: 10.1016/S0006-2952(01)00898-X. [DOI] [PubMed] [Google Scholar]
- 70.Danovi D, Folarin A, Gogolok S, Ender C, Elbatsh AMO, Engström PG, et al A high-content small molecule screen identifies sensitivity of glioblastoma stem cells to inhibition of polo-like kinase 1. PLoS One. 2013;8:e77053. doi: 10.1371/journal.pone.0077053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pezuk JA, Brassesco MS, Morales AG, de Oliveira JC, de Paula Queiroz RG, Machado HR, et al Polo-like kinase 1 inhibition causes decreased proliferation by cell cycle arrest, leading to cell death in glioblastoma. Cancer Gene Ther. 2013;20:499–506. doi: 10.1038/cgt.2013.46. [DOI] [PubMed] [Google Scholar]
- 72.Lee BL, Liedke PE, Barrios CH, Simon SD, Finkelstein DM, Goss PE Breast cancer in brazil: present status and future goals. Lancet Oncol. 2012;13:e95–102. doi: 10.1016/S1470-2045(11)70323-0. [DOI] [PubMed] [Google Scholar]
- 73.Pezuk JA, Brassesco MS, Morales AG, de Oliveira JC, de Oliveira HF, Scrideli CA, et al Inhibition of polo-like kinase 1 induces cell cycle arrest and sensitizes glioblastoma cells to ionizing radiation. Cancer Biother Radiopharm. 2013;28:516–22. doi: 10.1089/cbr.2012.1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gorlick R, Kolb EA, Keir ST, Maris JM, Reynolds CP, Kang MH, et al Initial testing (stage 1) of the polo-like kinase inhibitor volasertib (BI 6727), by the pediatric preclinical testing program. Pediatr Blood Cancer. 2014;61:158–64. doi: 10.1002/pbc.v61.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tandle AT, Kramp T, Kil WJ, Halthore A, Gehlhaus K, Shankavaram U, et al Inhibition of polo-like kinase 1 in glioblastoma multiforme induces mitotic catastrophe and enhances radiosensitisation. Eur J Cancer. 2013;49:3020–8. doi: 10.1016/j.ejca.2013.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Patnaik A, Rosen LS, Tolaney SM, Tolcher AW, Goldman JW, Gandhi L, et al Efficacy and safety of abemaciclib, an inhibitor of CDK4 and CDK6, for patients with breast cancer, non-small cell lung cancer, and other solid tumors. Cancer Discov. 2016;6:740–53. doi: 10.1158/2159-8290.CD-16-0095. [DOI] [PubMed] [Google Scholar]
- 77.Raub TJ, Wishart GN, Kulanthaivel P, Staton BA, Ajamie RT, Sawada GA, et al Brain exposure of two selective dual cdk4 and cdk6 inhibitors and the antitumor activity of CDK4 and CDK6 inhibition in combination with temozolomide in an intracranial glioblastoma xenograft. Drug Metab Dispos. 2015;43:1360–71. doi: 10.1124/dmd.114.062745. [DOI] [PubMed] [Google Scholar]
- 78.Liu LJ, Michowski W, Inuzuka H, Shimizu K, Nihira NT, Chick JM, et al G1 cyclins link proliferation, pluripotency and differentiation of embryonic stem cells. Nat Cell Biol. 2017;19:177–88. doi: 10.1038/ncb3474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jane EP, Premkumar DR, Cavaleri JM, Sutera PA, Rajasekar T, Pollack IF Dinaciclib, a cyclin-dependent kinase inhibitor promotes proteasomal degradation of mcl-1 and enhances ABT-737-mediated cell death in malignant human glioma cell lines. J Pharmacol Exp Ther. 2016;356:354–65. doi: 10.1124/jpet.115.230052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hayashi T, Adachi K, Ohba S, Hirose Y The cdk inhibitor flavopiridol enhances temozolomide-induced cytotoxicity in human glioma cells. J Neurooncol. 2013;115:169–78. doi: 10.1007/s11060-013-1220-5. [DOI] [PubMed] [Google Scholar]
- 81.Alonso M, Tamasdan C, Miller DC, Newcomb EW Flavopiridol induces apoptosis in glioma cell lines independent of retinoblastoma and p53 tumor suppressor pathway alterations by a caspase-independent pathway. Mol Cancer Ther. 2003;2:139–50. doi: 10.4161/cbt.214. [DOI] [PubMed] [Google Scholar]
- 82.Cimini A, d’Angelo M, Benedetti E, D’Angelo B, Laurenti G, Antonosante A, et al Flavopiridol: an old drug with new perspectives? Implication for development of new drugs. J Cell Physiol. 2017;232:312–22. doi: 10.1002/jcp.v232.2. [DOI] [PubMed] [Google Scholar]
- 83.Cobanoglu G, Turacli ID, Ozkan AC, Ekmekci A Flavopiridol’s antiproliferative effects in glioblastoma multiforme. J Cancer Res Ther. 2016;12:811–7. doi: 10.4103/0973-1482.172132. [DOI] [PubMed] [Google Scholar]
- 84.Zhong S, Wu B, Dong XC, Han YJ, Jiang SS, Zhang Y, et al Identification of driver genes and key pathways of glioblastoma shows JNJ-7706621 as a novel antiglioblastoma drug. World Neurosurg. 2018;109:e329–42. doi: 10.1016/j.wneu.2017.09.176. [DOI] [PubMed] [Google Scholar]
- 85.Hou HG, Krishnamurthy Nemani V, Du GX, Montano R, Song R, Gimi B, et al Monitoring oxygen levels in orthotopic human glioma xenograft following carbogen inhalation and chemotherapy by implantable resonator-based oximetry. Int J Cancer. 2015;136:1688–96. doi: 10.1002/ijc.v136.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhang XP, Lv H, Zhou QY, Elkholi R, Chipuk JE, Reddy MVR, et al Preclinical pharmacological evaluation of a novel multiple kinase inhibitor, ON123300, in brain tumor models. Mol Cancer Ther. 2014;13:1105–16. doi: 10.1158/1535-7163.MCT-13-0847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hashizume R, Zhang AL, Mueller S, Prados MD, Lulla RR, Goldman S, et al Inhibition of DNA damage repair by the CDK4/6 inhibitor palbociclib delays irradiated intracranial atypical teratoid rhabdoid tumor and glioblastoma xenograft regrowth. Neuro Oncol. 2016;18:1519–28. doi: 10.1093/neuonc/now106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Olmez I, Brenneman B, Xiao AZ, Serbulea V, Benamar M, Zhang Y, et al Combined CDK4/6 and MTOR inhibition is synergistic against glioblastoma via multiple mechanisms . Clin Cancer Res. 2017;23:6958–68. doi: 10.1158/1078-0432.CCR-17-0803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Michaud K, Solomon DA, Oermann E, Kim JS, Zhong WZ, Prados MD, et al Pharmacologic inhibition of cyclin-dependent kinases 4 and 6 arrests the growth of glioblastoma multiforme intracranial xenografts. Cancer Res. 2010;70:3228–38. doi: 10.1158/0008-5472.CAN-09-4559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Whittaker S, Madani D, Joshi S, Chung SA, Johns T, Day B, et al Combination of palbociclib and radiotherapy for glioblastoma. Cell Death Discov. 2017;3:17033. doi: 10.1038/cddiscovery.2017.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Erbayraktar Z, Alural B, Erbayraktar RS, Erkan EP Cell division cycle 7-kinase inhibitor PHA-767491 hydrochloride suppresses glioblastoma growth and invasiveness. Cancer Cell Int. 2016;16:88. doi: 10.1186/s12935-016-0364-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kolodziej M, Goetz C, di Fazio P, Montalbano R, Ocker M, Strik H, et al Roscovitine has anti-proliferative and pro-apoptotic effects on glioblastoma cell lines: a pilot study. Oncol Rep. 2015;34:1549–56. doi: 10.3892/or.2015.4105. [DOI] [PubMed] [Google Scholar]
- 93.Cheng CK, Gustafson WC, Charron E, Houseman BT, Zunder E, Goga A, et al Dual blockade of lipid and cyclin-dependent kinases induces synthetic lethality in malignant glioma. Proc Natl Acad Sci USA. 2012;109:12722–7. doi: 10.1073/pnas.1202492109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ali MA, Reis A, Ding LH, Story MD, Habib AA, Chattopadhyay A, et al SNS-032 prevents hypoxia-mediated glioblastoma cell invasion by inhibiting hypoxia inducible factor-1alpha expression. Int J Oncol. 2009;34:1051–60. doi: 10.3892/ijo_00000231. [DOI] [PubMed] [Google Scholar]
- 95.Ali MA, Choy H, Habib AA, Saha D SNS-032 prevents tumor cell-induced angiogenesis by inhibiting vascular endothelial growth factor. Neoplasia. 2007;9:370–81. doi: 10.1593/neo.07136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Greenall SA, Lim YC, Mitchell CB, Ensbey KS, Stringer BW, Wilding AL, et al Cyclin-dependent kinase 7 is a therapeutic target in high-grade glioma. Oncogenesis. 2017;6:e336. doi: 10.1038/oncsis.2017.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Liu Z, Wang F, Zhou ZW, Xia HC, Wang XY, Yang YX, et al Alisertib induces G2/M arrest, apoptosis, and autophagy via PI3K/Akt/MTOR- and P38 MAPK-mediated pathways in human glioblastoma cells . Am J Transl Res. 2017;9:845–73. [PMC free article] [PubMed] [Google Scholar]
- 98.Kurokawa C, Geekiyanage H, Allen C, Iankov I, Schroeder M, Carlson B, et al Alisertib demonstrates significant antitumor activity in bevacizumab resistant, patient derived orthotopic models of glioblastoma. J Neurooncol. 2017;131:41–8. doi: 10.1007/s11060-016-2285-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Daniele S, Sestito S, Pietrobono D, Giacomelli C, Chiellini G, di Maio D, et al Dual inhibition of PDK1 and aurora kinase a: an effective strategy to induce differentiation and apoptosis of human glioblastoma multiforme stem cells. ACS Chem Neurosci. 2017;8:100–14. doi: 10.1021/acschemneuro.6b00251. [DOI] [PubMed] [Google Scholar]
- 100.Hong X, O’Donnell JP, Salazar CR, van Brocklyn JR, Barnett KD, Pearl DK, et al The selective aurora-a kinase inhibitor MLN8237 (alisertib) potently inhibits proliferation of glioblastoma neurosphere tumor stem-like cells and potentiates the effects of temozolomide and ionizing radiation. Cancer Chemother Pharmacol. 2014;73:983–90. doi: 10.1007/s00280-014-2430-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Pacaud R, Cheray M, Nadaradjane A, Vallette FM, Cartron PF Histone H3 phosphorylation in GBM: a new rational to guide the use of kinase inhibitors in anti-GBM therapy. Theranostics. 2015;5:12–22. doi: 10.7150/thno.8799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wiedemuth R, Klink B, Fujiwara M, Schröck E, Tatsuka M, Schackert G, et al Janus face-like effects of aurora b inhibition: antitumoral mode of action versus induction of aneuploid progeny. Carcinogenesis. 2016;37:993–1003. doi: 10.1093/carcin/bgw083. [DOI] [PubMed] [Google Scholar]
- 103.Matsuhashi A, Ohno T, Kimura M, Hara A, Saio M, Nagano A, et al Growth suppression and mitotic defect induced by JNJ-7706621, an inhibitor of cyclin-dependent kinases and aurora kinases. Curr Cancer Drug Targets. 2012;12:625–39. doi: 10.2174/156800912801784839. [DOI] [PubMed] [Google Scholar]
- 104.Li N, Maly DJ, Chanthery YH, Sirkis DW, Nakamura JL, Berger MS, et al Radiotherapy followed by aurora kinase inhibition targets tumor-propagating cells in human glioblastoma. Mol Cancer Ther. 2015;14:419–28. doi: 10.1158/1535-7163.MCT-14-0526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lee PY, Chen CL, Lin ZZ, Cheng AL, Chen EIT, Whang-Peng J, et al The aurora kinases inhibitor VE-465 is a novel treatment for glioblastoma multiforme. Oncology. 2013;84:326–35. doi: 10.1159/000347021. [DOI] [PubMed] [Google Scholar]
- 106.Borges KS, Castro-Gamero AM, Moreno DA, da Silva Silveira V, Brassesco MS, de Paula Queiroz RG, et al Inhibition of Aurora kinases enhances chemosensitivity to temozolomide and causes radiosensitization in glioblastoma cells. J Cancer Res Clin Oncol. 2012;138:405–14. doi: 10.1007/s00432-011-1111-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Saka Y, Giuraniuc CV, Ohkura H Accurate chromosome segregation by probabilistic self-organisation. BMC Biol. 2015;13:65. doi: 10.1186/s12915-015-0172-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Morgan DO. The cell cycle: principles of control. London: New Science Press, 2007.
- 109.Pines J The cell cycle kinases. Semin Cancer Biol. 1994;5:305–13. [PubMed] [Google Scholar]
- 110.Bruyère C, Meijer L Targeting cyclin-dependent kinases in anti-neoplastic therapy. Curr Opin Cell Biol. 2013;25:772–9. doi: 10.1016/j.ceb.2013.08.004. [DOI] [PubMed] [Google Scholar]
- 111.Malumbres M Cyclins and related kinases in cancer cells. J BUON. 2007;12(Suppl 1):S45–52. [PubMed] [Google Scholar]
- 112.Sherr CJ Cancer cell cycles. Science. 1996;274:1672–7. doi: 10.1126/science.274.5293.1672. [DOI] [PubMed] [Google Scholar]
- 113.Hall M, Peters G Genetic alterations of cyclins, cyclin-dependent kinases, and cdk inhibitors in human cancer. Adv Cancer Res. 1996;68:67–108. doi: 10.1016/S0065-230X(08)60352-8. [DOI] [PubMed] [Google Scholar]
- 114.Lam PYP, di Tomaso E, Ng HK, Pang JCS, Roussel MF, Hjelm NM Expression of p19 INK4d, CDK4, CDK6 in glioblastoma multiforme. Br J Neurosurg. 2000;14:28–32. doi: 10.1080/02688690042870. [DOI] [PubMed] [Google Scholar]
- 115.Knockaert M, Greengard P, Meijer L Pharmacological inhibitors of cyclin-dependent kinases. Trends Pharmacol Sci. 2002;23:417–25. doi: 10.1016/S0165-6147(02)02071-0. [DOI] [PubMed] [Google Scholar]
- 116.Meijer L Chemical inhibitors of cyclin-dependent kinases. Trends Cell Biol. 1996;6:393–7. doi: 10.1016/0962-8924(96)10034-9. [DOI] [PubMed] [Google Scholar]
- 117.de Azevedo WF Jr, Canduri F, da Silveira NJF Structural basis for inhibition of cyclin-dependent kinase 9 by flavopiridol. Biochem Biophys Res Commun. 2002;293:566–71. doi: 10.1016/S0006-291X(02)00266-8. [DOI] [PubMed] [Google Scholar]
- 118.Brüsselbach S, Nettelbeck DM, Sedlacek HH, Müller R Cell cycle-independent induction of apoptosis by the anti-tumor drug flavopiridol in endothelial cells. Int J Cancer. 1998;77:146–52. doi: 10.1002/(ISSN)1097-0215. [DOI] [PubMed] [Google Scholar]
- 119.Bible KC, Kaufmann SH Flavopiridol: a cytotoxic flavone that induces cell death in noncycling A549 human lung carcinoma cells. Cancer Res. 1996;56:4856–61. [PubMed] [Google Scholar]
- 120.Lee HG, Baek JW, Shin SJ, Kwon SH, Cha SD, Park WJ, et al Antitumor effects of flavopiridol on human uterine leiomyoma in vitro and in a xenograft model. Reprod Sci. 2014;21:1153–60. doi: 10.1177/1933719114525266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Newcomb EW, Tamasdan C, Entzminger Y, Alonso J, Friedlander D, Crisan D, et al Flavopiridol induces mitochondrial-mediated apoptosis in murine glioma GL261 cells via release of cytochrome c and apoptosis inducing factor . Cell Cycle. 2003;2:242–9. doi: 10.4161/cc.2.3.357. [DOI] [PubMed] [Google Scholar]
- 122.Hofmeister CC, Poi M, Bowers MA, Zhao WQ, Phelps MA, Benson DM, et al A phase I trial of flavopiridol in relapsed multiple myeloma. cancer chemother. Cancer Chemother Pharmacol. 2014;73:249–57. doi: 10.1007/s00280-013-2347-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Jones JA, Rupert AS, Poi M, Phelps MA, Andritsos L, Baiocchi R, et al Flavopiridol can be safely administered using a pharmacologically derived schedule and demonstrates activity in relapsed and refractory non-hodgkin’s lymphoma. Am J Hematol. 2014;89:19–24. doi: 10.1002/ajh.23568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Blum W, Phelps MA, Klisovic RB, Rozewski DM, Ni WJ, Albanese KA, et al Phase I clinical and pharmacokinetic study of a novel schedule of flavopiridol in relapsed or refractory acute leukemias. Haematologica. 2010;95:1098–105. doi: 10.3324/haematol.2009.017103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Parry D, Guzi T, Shanahan F, Davis N, Prabhavalkar D, Wiswell D, et al Dinaciclib (SCH 727965), a novel and potent cyclin-dependent kinase inhibitor. Mol Cancer Ther. 2010;9:2344–53. doi: 10.1158/1535-7163.MCT-10-0324. [DOI] [PubMed] [Google Scholar]
- 126.Gojo I, Sadowska M, Walker A, Feldman EJ, Iyer SP, Baer MR, et al Clinical and laboratory studies of the novel cyclin-dependent kinase inhibitor dinaciclib (SCH 727965) in acute leukemias. Cancer Chemother Pharmacol. 2013;72:897–908. doi: 10.1007/s00280-013-2249-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Feldmann G, Mishra A, Bisht S, Karikari C, Garrido-Laguna I, Rasheed Z, et al Cyclin-dependent kinase inhibitor dinaciclib (SCH727965) inhibits pancreatic cancer growth and progression in murine xenograft models. Cancer Biol Ther. 2011;12:598–609. doi: 10.4161/cbt.12.7.16475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Fu W, Ma L, Chu B, Wang X, Bui MM, Gemmer J, et al The cyclin-dependent kinase inhibitor SCH 727965 (Dinacliclib) induces the apoptosis of osteosarcoma cells. Mol Cancer Ther. 2011;10:1018–27. doi: 10.1158/1535-7163.MCT-11-0167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Gorlick R, Kolb EA, Houghton PJ, Morton CL, Neale G, Keir ST, et al Initial testing (stage 1) of the cyclin dependent kinase inhibitor SCH 727965 (dinaciclib) by the pediatric preclinical testing program. Pediatr Blood Cancer. 2012;59:1266–74. doi: 10.1002/pbc.v59.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Goh KC, Novotny-Diermayr V, Hart S, Ong LC, Loh YK, Cheong A, et al TG02, a novel oral multi-kinase inhibitor of CDKs, JAK2 and FLT3 with potent anti-leukemic properties. Leukemia. 2012;26:236–43. doi: 10.1038/leu.2011.218. [DOI] [PubMed] [Google Scholar]
- 131.Rathos MJ, Khanwalkar H, Joshi K, Manohar SM, Joshi KS Potentiation of in vitro and in vivo antitumor efficacy of doxorubicin by cyclin-dependent kinase inhibitor p276-00 in human non-small cell lung cancer cells . BMC Cancer. 2013;13:29. doi: 10.1186/1471-2407-13-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Dolloff NG, Allen JE, Dicker DT, Aqui N, Vogl D, Malysz J, et al Sangivamycin-like molecule 6 exhibits potent anti-multiple myeloma activity through inhibition of cyclin-dependent kinase-9. Mol Cancer Ther. 2012;11:2321–30. doi: 10.1158/1535-7163.MCT-12-0578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ringer L, Sirajuddin P, Heckler M, Ghosh A, Suprynowicz F, Yenugonda VM, et al VMY-1-103 is a novel CDK inhibitor that disrupts chromosome organization and delays metaphase progression in medulloblastoma cells. Cancer Biol Ther. 2011;12:818–26. doi: 10.4161/cbt.12.9.17682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Baumann KH, Kim H, Rinke J, Plaum T, Wagner U, Reinartz S Effects of alvocidib and carboplatin on ovarian cancer cells in vitro . Exp Oncol. 2013;35:168–73. [PubMed] [Google Scholar]
- 135.Dai Y, Rahmani M, Pei XY, Dent P, Grant S Bortezomib and flavopiridol interact synergistically to induce apoptosis in chronic myeloid leukemia cells resistant to imatinib mesylate through both Bcr/Abl-dependent and -independent mechanisms. Blood. 2004;104:509–18. doi: 10.1182/blood-2003-12-4121. [DOI] [PubMed] [Google Scholar]
- 136.Dai Y, Rahmani M, Grant S Proteasome inhibitors potentiate leukemic cell apoptosis induced by the cyclin-dependent kinase inhibitor flavopiridol through a SAPK/JNK- and NF-κB-dependent process . Oncogene. 2003;22:7108–22. doi: 10.1038/sj.onc.1206863. [DOI] [PubMed] [Google Scholar]
- 137.Karp JE, Ross DD, Yang WD, Tidwell ML, Wei YT, Greer J, et al Timed sequential therapy of acute leukemia with flavopiridol: in vitro model for a phase i clinical trial . Clin Cancer Res. 2003;9:307–15. [PubMed] [Google Scholar]
- 138.Karp JE, Blackford A, Smith BD, Alino K, Seung AH, Bolaños-Meade J, et al Clinical activity of sequential flavopiridol, cytosine arabinoside, and mitoxantrone for adults with newly diagnosed, poor-risk acute myelogenous leukemia. Leuk Res. 2010;34:877–82. doi: 10.1016/j.leukres.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Holkova B, Perkins EB, Ramakrishnan V, Tombes MB, Shrader E, Talreja N, et al Phase I trial of bortezomib (PS-341; NSC 681239) and alvocidib (Flavopiridol; NSC 649890) in patients with recurrent or refractory b-cell neoplasms. Clin Cancer Res. 2011;17:3388–97. doi: 10.1158/1078-0432.CCR-10-2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Lu XP, Koch KS, Lew DJ, Dulic V, Pines J, Reed SI, et al Induction of cyclin mRNA and cyclin-associated histone H1 kinase during liver regeneration. J Biol Chem. 1992;267:2841–4. [PubMed] [Google Scholar]
- 141.Hartwell LH, Weinert TA Checkpoints: controls that ensure the order of cell cycle events. Science. 1989;246:629–34. doi: 10.1126/science.2683079. [DOI] [PubMed] [Google Scholar]
- 142.D’Assoro AB, Lingle WL, Salisbury JL Centrosome amplification and the development of cancer. Oncogene. 2002;21:6146–53. doi: 10.1038/sj.onc.1205772. [DOI] [PubMed] [Google Scholar]
- 143.Knauf JA, Ouyang B, Knudsen ES, Fukasawa K, Babcock G, Fagin JA Oncogenic RAS induces accelerated transition through G2/M and promotes defects in the G2 DNA damage and mitotic spindle checkpoints . J Biol Chem. 2006;281:3800–9. doi: 10.1074/jbc.M511690200. [DOI] [PubMed] [Google Scholar]
- 144.D’Assoro AB, Busby R, Suino K, Delva E, Almodovar-Mercado GJ, Johnson H, et al Genotoxic stress leads to centrosome amplification in breast cancer cell lines that have an inactive G1/S cell cycle Checkpoint. Oncogene. 2004;23:4068–75. doi: 10.1038/sj.onc.1207568. [DOI] [PubMed] [Google Scholar]
- 145.Goldenson B, Crispino JD The aurora kinases in cell cycle and leukemia. Oncogene. 2015;34:537–45. doi: 10.1038/onc.2014.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Toji S, Yabuta N, Hosomi T, Nishihara S, Kobayashi T, Suzuki S, et al The centrosomal protein lats2 is a phosphorylation target of aurora-a kinase. Genes Cells. 2004;9:383–97. doi: 10.1111/gtc.2004.9.issue-5. [DOI] [PubMed] [Google Scholar]
- 147.Musacchio A, Hardwick KG The spindle checkpoint: structural insights into dynamic signalling. Nat Rev Mol Cell Biol. 2002;3:731–41. doi: 10.1038/nrm929. [DOI] [PubMed] [Google Scholar]
- 148.Crane R, Gadea B, Littlepage L, Wu H, Ruderman JV Aurora a, meiosis and mitosis. Biol Cell. 2004;96:215–29. doi: 10.1016/j.biolcel.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 149.Vagnarelli P, Earnshaw WC Chromosomal passengers: the four-dimensional regulation of mitotic events. Chromosoma. 2004;113:211–22. doi: 10.1007/s00412-004-0307-3. [DOI] [PubMed] [Google Scholar]
- 150.Vader G, Medema RH, Lens SMA The chromosomal passenger complex: guiding Aurora-B through mitosis. J Cell Biol. 2006;173:833–7. doi: 10.1083/jcb.200604032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Adams RR, Carmena M, Earnshaw WC Chromosomal passengers and the (aurora) ABCs of mitosis. Trends Cell Biol. 2001;11:49–54. doi: 10.1016/S0962-8924(00)01880-8. [DOI] [PubMed] [Google Scholar]
- 152.Murnion ME, Adams RR, Callister DM, Allis CD, Earnshaw WC, Swedlow JR Chromatin-associated protein phosphatase 1 regulates aurora-b and histone h3 phosphorylation. J Biol Chem. 2001;276:26656–65. doi: 10.1074/jbc.M102288200. [DOI] [PubMed] [Google Scholar]
- 153.Hsu JY, Sun ZW, Li XM, Reuben M, Tatchell K, Bishop DK, et al Mitotic phosphorylation of histone H3 is governed by ipl1/aurora kinase and Glc7/PP1 phosphatase in budding yeast and nematodes. Cell. 2000;102:279–91. doi: 10.1016/S0092-8674(00)00034-9. [DOI] [PubMed] [Google Scholar]
- 154.Lan WJ, Zhang X, Kline-Smith SL, Rosasco SE, Barrett-Wilt GA, Shabanowitz J, et al Aurora B phosphorylates centromeric MCAK and regulates its localization and microtubule depolymerization activity. Curr Biol. 2004;14:273–86. doi: 10.1016/j.cub.2004.01.055. [DOI] [PubMed] [Google Scholar]
- 155.Minoshima Y, Kawashima T, Hirose K, Tonozuka Y, Kawajiri A, Bao YC, et al Phosphorylation by aurora B converts MgcRacGAP to a RhoGAP during cytokinesis. Dev Cell. 2003;4:549–60. doi: 10.1016/S1534-5807(03)00089-3. [DOI] [PubMed] [Google Scholar]
- 156.Yang KT, Li SK, Chang CC, Tang CJC, Lin YN, Lee SC, et al Aurora-c kinase deficiency causes cytokinesis failure in meiosis i and production of large polyploid oocytes in mice. Mol Biol Cell. 2010;21:2371–83. doi: 10.1091/mbc.e10-02-0170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Avo Santos M, van de Werken C, de Vries M, Jahr H, Vromans MJM, Laven JSE, et al A role for aurora c in the chromosomal passenger complex during human preimplantation embryo development. Hum Reprod. 2011;26:1868–81. doi: 10.1093/humrep/der111. [DOI] [PubMed] [Google Scholar]
- 158.Tang AQ, Gao KY, Chu LL, Zhang R, Yang J, Zheng JN Aurora kinases: novel therapy targets in cancers. Oncotarget. 2017;8:23937–54. doi: 10.18632/oncotarget.14893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Cirak Y, Furuncuoglu Y, Yapicier O, Aksu A, Cubukcu E Aurora a overexpression in breast cancer patients induces taxane resistance and results in worse prognosis. J BUON. 2015;20:1414–9. [PubMed] [Google Scholar]
- 160.Zekri A, Lesan V, Ghaffari SH, Tabrizi MH, Modarressi MH Gene amplification and overexpression of aurora-c in breast and prostate cancer cell lines. Oncol Res. 2012;20:241–50. doi: 10.3727/096504013X13589503482978. [DOI] [PubMed] [Google Scholar]
- 161.Do TV, Xiao F, Bickel LE, Klein-Szanto AJ, Pathak HB, Hua X, et al Aurora kinase a mediates epithelial ovarian cancer cell migration and adhesion. Oncogene. 2014;33:539–49. doi: 10.1038/onc.2012.632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Davidson B, Nymoen DA, Elgaaen BV, Staff AC, Tropé CG, Kærn J, et al BUB1 mRNA is significantly co-expressed with AURKA and AURKB mRNA in advanced-stage ovarian serous carcinoma . Virchows Arch. 2014;464:701–7. doi: 10.1007/s00428-014-1577-7. [DOI] [PubMed] [Google Scholar]
- 163.Honma K, Nakanishi R, Nakanoko T, Ando K, Saeki H, Oki E, et al Contribution of Aurora-A and -B expression to DNA aneuploidy in gastric cancers. Surg Today. 2014;44:454–61. doi: 10.1007/s00595-013-0581-x. [DOI] [PubMed] [Google Scholar]
- 164.Katsha A, Belkhiri A, Goff L, El-Rifai W Aurora kinase a in gastrointestinal cancers: time to target. Mol Cancer. 2015;14:106. doi: 10.1186/s12943-015-0375-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Hosseini S, Hashemzadeh S, Estiar MA, Ebrahimzadeh R, Fakhree MBA, Yousefi B, et al Expression analysis of aurora-c and survivin, two testis-specific genes, in patients with colorectal cancer. Clin Lab. 2015;61:475–80. doi: 10.7754/clin.lab.2014.141017. [DOI] [PubMed] [Google Scholar]
- 166.Liu JM, Long XH, Zhang GM, Zhou Y, Chen XY, Huang SH, et al Let-7g reverses malignant phenotype of osteosarcoma cells by targeting Aurora-B. Int J Clin Exp Pathol. 2014;7:4596–606. [PMC free article] [PubMed] [Google Scholar]
- 167.Lo Iacono M, Monica V, Saviozzi S, Ceppi P, Bracco E, Papotti M, et al Aurora Kinase a expression is associated with lung cancer histological-subtypes and with tumor de-differentiation. J Transl Med. 2011;9:100. doi: 10.1186/1479-5876-9-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Takeshita M, Koga T, Takayama K, Ijichi K, Yano T, Maehara Y, et al Aurora-B overexpression is correlated with aneuploidy and poor prognosis in non-small cell lung cancer. Lung Cancer. 2013;80:85–90. doi: 10.1016/j.lungcan.2012.12.018. [DOI] [PubMed] [Google Scholar]
- 169.Fujii S, Srivastava V, Hegde A, Kondo Y, Shen LL, Hoshino K, et al Regulation of AURKC expression by CpG island methylation in human cancer cells . Tumour Biol. 2015;36:8147–58. doi: 10.1007/s13277-015-3553-5. [DOI] [PubMed] [Google Scholar]
- 170.Twu NF, Yuan CC, Yen MS, Lai CR, Chao KC, Wang PH, et al Expression of Aurora Kinase A and B in normal and malignant cervical tissue: high aurora a kinase expression in squamous cervical cancer. Eur J Obstet Gynecol Reprod Biol. 2009;142:57–63. doi: 10.1016/j.ejogrb.2008.09.012. [DOI] [PubMed] [Google Scholar]
- 171.Toughiri R, Li X, Du QS, Bieberich CJ Phosphorylation of NuMA by Aurora-A kinase in PC-3 prostate cancer cells affects proliferation, survival, and interphase NuMA localization. J Cell Biochem. 2013;114:823–30. doi: 10.1002/jcb.v114.4. [DOI] [PubMed] [Google Scholar]
- 172.Fadri-Moskwik M, Weiderhold KN, Deeraksa A, Chuang C, Pan J, Lin SH, et al Aurora B is regulated by acetylation/deacetylation during mitosis in prostate cancer cells. FASEB J. 2012;26:4057–67. doi: 10.1096/fj.12-206656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Tatsuka M, Sato S, Kitajima S, Suto S, Kawai H, Miyauchi M, et al Overexpression of Aurora-A potentiates HRAS-mediated oncogenic transformation and is implicated in oral carcinogenesis. Oncogene. 2005;24:1122–7. doi: 10.1038/sj.onc.1208293. [DOI] [PubMed] [Google Scholar]
- 174.Pannone G, Hindi SAH, Santoro A, Sanguedolce F, Rubini C, Cincione RI, et al Aurora B expression as a prognostic indicator and possibile therapeutic target in oral squamous cell carcinoma. Int J Immunopathol Pharmacol. 2011;24:79–88. doi: 10.1177/039463201102400110. [DOI] [PubMed] [Google Scholar]
- 175.Kim SJ, Jang JE, Cheong JW, Eom JI, Jeung HK, Kim Y, et al Aurora A kinase expression is increased in leukemia stem cells, and a selective Aurora A kinase inhibitor enhances Ara-C-induced apoptosis in acute myeloid leukemia stem cells. Korean J Hematol. 2012;47:178–85. doi: 10.5045/kjh.2012.47.3.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Hartsink-Segers SA, Zwaan CM, Exalto C, Luijendijk MWJ, Calvert VS, Petricoin EF, et al Aurora kinases in childhood acute leukemia: the promise of aurora b as therapeutic target. Leukemia. 2013;27:560–8. doi: 10.1038/leu.2012.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Premkumar DR, Jane EP, Pollack IF Cucurbitacin-I inhibits aurora kinase a, aurora kinase b and survivin, induces defects in cell cycle progression and promotes ABT-737-induced cell death in a caspase-independent manner in malignant human glioma cells. Cancer Biol Ther. 2015;16:233–43. doi: 10.4161/15384047.2014.987548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Diaz RJ, Golbourn B, Shekarforoush M, Smith CA, Rutka JT Aurora kinase B/C inhibition impairs malignant glioma growth in vivo. J Neurooncol. 2012;108:349–60. doi: 10.1007/s11060-012-0835-2. [DOI] [PubMed] [Google Scholar]
- 179.Klein A, Reichardt W, Jung V, Zang KD, Meese E, Urbschat S Overexpression and amplification of STK15 in human gliomas. Int J Oncol. 2004;25:1789–94. [PubMed] [Google Scholar]
- 180.Araki K, Nozaki K, Ueba T, Tatsuka M, Hashimoto N High expression of aurora-b/aurora and ipl1-like midbody-associated protein (AIM-1) in astrocytomas. J Neurooncol. 2004;67:53–64. doi: 10.1023/B:NEON.0000021784.33421.05. [DOI] [PubMed] [Google Scholar]
- 181.Madhavan S, Zenklusen JC, Kotliarov Y, Sahni H, Fine HA, Buetow K Rembrandt: helping personalized medicine become a reality through integrative translational research. Mol Cancer Res. 2009;7:157–67. doi: 10.1158/1541-7786.MCR-08-0435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Lehman NL, O’Donnell JP, Whiteley LJ, Stapp RT, Lehman TD, Roszka KM, et al Aurora a is differentially expressed in gliomas, is associated with patient survival in glioblastoma and is a potential chemotherapeutic target in gliomas. Cell Cycle. 2012;11:489–502. doi: 10.4161/cc.11.3.18996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Samaras V, Stamatelli A, Samaras E, Arnaoutoglou C, Arnaoutoglou M, Stergiou I, et al Comparative immunohistochemical analysis of aurora-a and aurora-b expression in human glioblastomas. Associations with proliferative activity and clinicopathological features. Pathol Res Pract. 2009;205:765–73. doi: 10.1016/j.prp.2009.06.011. [DOI] [PubMed] [Google Scholar]
- 184.Zeng WF, Navaratne K, Prayson RA, Weil RJ Aurora b expression correlates with aggressive behaviour in glioblastoma multiforme. J Clin Pathol. 2007;60:218–21. doi: 10.1136/jcp.2006.036806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Barton VN, Foreman NK, Donson AM, Birks DK, Handler MH, Vibhakar R Aurora kinase A as a rational target for therapy in glioblastoma. J Neurosurg Pediatr. 2010;6:98–105. doi: 10.3171/2010.3.PEDS10120. [DOI] [PubMed] [Google Scholar]
- 186.Rong R, Jiang LY, Sheikh MS, Huang Y Mitotic kinase Aurora-A phosphorylates RASSF1A and modulates RASSF1A-mediated microtubule interaction and M-phase cell cycle regulation. Oncogene. 2007;26:7700–8. doi: 10.1038/sj.onc.1210575. [DOI] [PubMed] [Google Scholar]
- 187.Wan XB, Long ZJ, Yan M, Xu J, Xia LP, Liu L, et al Inhibition of Aurora-A suppresses epithelial-mesenchymal transition and invasion by downregulating MAPK in nasopharyngeal carcinoma cells. Carcinogenesis. 2008;29:1930–7. doi: 10.1093/carcin/bgn176. [DOI] [PubMed] [Google Scholar]
- 188.Noh EM, Lee YR, Hong OY, Jung SH, Youn HJ, Kim JS Aurora kinases are essential for PKC-induced invasion and matrix metalloproteinase-9 expression in MCF-7 breast cancer cells. Oncol Rep. 2015;34:803–10. doi: 10.3892/or.2015.4027. [DOI] [PubMed] [Google Scholar]
- 189.Xia ZB, Wei P, Zhang H, Ding ZM, Yang LX, Huang ZS, et al AURKA governs self-renewal capacity in glioma-initiating cells via stabilization/activation of β-catenin/wnt signaling . Mol Cancer Res. 2013;11:1101–11. doi: 10.1158/1541-7786.MCR-13-0044. [DOI] [PubMed] [Google Scholar]
- 190.González-Loyola A, Fernández-Miranda G, Trakala M, Partida D, Samejima K, Ogawa H, et al Aurora b overexpression causes aneuploidy and p21cip1 repression during tumor development . Mol Cell Biol. 2015;35:3566–78. doi: 10.1128/MCB.01286-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Porcelli L, Guida G, Quatrale AE, Cocco T, Sidella L, Maida I, et al Aurora kinase B inhibition reduces the proliferation of metastatic melanoma cells and enhances the response to chemotherapy. J Transl Med. 2015;13:26. doi: 10.1186/s12967-015-0385-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Pacchierotti F, Adler ID, Eichenlaub-Ritter U, Mailhes JB Gender effects on the incidence of aneuploidy in mammalian germ cells. Environ Res. 2007;104:46–69. doi: 10.1016/j.envres.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 193.Ditchfield C, Johnson VL, Tighe A, Ellston R, Haworth C, Johnson T, et al Aurora B couples chromosome alignment with anaphase by targeting BubR1, mad2, and Cenp-E to kinetochores. J Cell Biol. 2003;161:267–80. doi: 10.1083/jcb.200208091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Gadea BB, Ruderman JV Aurora kinase inhibitor ZM447439 blocks chromosome-induced spindle assembly, the completion of chromosome condensation, and the establishment of the spindle integrity checkpoint in xenopus egg extracts . Mol Biol Cell. 2005;16:1305–18. doi: 10.1091/mbc.e04-10-0891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Kitzen JJEM, de Jonge MJA, Verweij J Aurora kinase inhibitors. Crit Rev Oncol Hematol. 2010;73:99–110. doi: 10.1016/j.critrevonc.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 196.Manfredi MG, Ecsedy JA, Meetze KA, Balani SK, Burenkova O, Chen W, et al Antitumor activity of MLN8054, an orally active small-molecule inhibitor of Aurora A kinase. Proc Natl Acad Sci USA. 2007;104:4106–11. doi: 10.1073/pnas.0608798104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Hoar K, Chakravarty A, Rabino C, Wysong D, Bowman D, Roy N, et al MLN8054, a Small-molecule inhibitor of aurora a, causes spindle pole and chromosome congression defects leading to aneuploidy. Mol Cell Biol. 2007;27:4513–25. doi: 10.1128/MCB.02364-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Clinical Study Medical Monitor, Millennium Pharmaceuticals, Inc. A study of MLN8054 in patients with advanced solid tumors. [Accessed June 20, 2018], Available from: https://www.clinicaltrials.gov/ct2/show/NCT00249301?cond=NCT00249301&rank=1.
- 199.Millennium Pharmaceuticals, Inc. A phase 1 trial of extended MLN8054 dosing in patients with advanced malignancies. [Accessed June 20, 2018], Available from: https://www.clinicaltrials.gov/ct2/show/NCT00652158?cond=NCT00652158&rank=1.
- 200.Dees EC, Cohen RB, von Mehren M, Stinchcombe TE, Liu H, Venkatakrishnan K, et al Phase I study of aurora a kinase inhibitor MLN8237 in advanced solid tumors: safety, pharmacokinetics, pharmacodynamics, and bioavailability of two oral formulations. Clin Cancer Res. 2012;18:4775–84. doi: 10.1158/1078-0432.CCR-12-0589. [DOI] [PubMed] [Google Scholar]
- 201.Liewer S, Huddleston A Alisertib: a review of pharmacokinetics, efficacy and toxicity in patients with hematologic malignancies and solid tumors. Expert Opin Investig Drugs. 2018;27:105–12. doi: 10.1080/13543784.2018.1417382. [DOI] [PubMed] [Google Scholar]
- 202.van Brocklyn JR, Wojton J, Meisen WH, Kellough DA, Ecsedy JA, Kaur B, et al Aurora-a inhibition offers a novel therapy effective against intracranial glioblastoma. Cancer Res. 2014;74:5364–70. doi: 10.1158/0008-5472.CAN-14-0386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Harrington EA, Bebbington D, Moore J, Rasmussen RK, Ajose-Adeogun AO, Nakayama T, et al VX-680, a potent and selective small-molecule inhibitor of the Aurora kinases, suppresses Tumor growth in vivo . Nat Med. 2004;10:262–7. doi: 10.1038/nm1003. [DOI] [PubMed] [Google Scholar]
- 204.Traynor AM, Hewitt M, Liu G, Flaherty KT, Clark J, Freedman SJ, et al Phase I dose escalation study of MK-0457, a novel aurora kinase inhibitor, in adult patients with advanced solid tumors. cancer chemother. Cancer Chemother Pharmacol. 2011;67:305–14. doi: 10.1007/s00280-010-1318-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Giles FJ, Swords RT, Nagler A, Hochhaus A, Ottmann OG, Rizzieri DA, et al MK-0457, an Aurora kinase and BCR-ABL inhibitor, is active in patients with BCR-ABL T315I leukemia. Leukemia. 2013;27:113–7. doi: 10.1038/leu.2012.186. [DOI] [PubMed] [Google Scholar]
- 206.Wilkinson RW, Odedra R, Heaton SP, Wedge SR, Keen NJ, Crafter C, et al AZD1152, a selective inhibitor of aurora b kinase, inhibits human tumor xenograft growth by inducing apoptosis. Clin Cancer Res. 2007;13:3682–8. doi: 10.1158/1078-0432.CCR-06-2979. [DOI] [PubMed] [Google Scholar]
- 207.Hyun SY, Hwan HI, Jang YJ Polo-like kinase-1 in DNA damage response. BMB Rep. 2014;47:249–55. doi: 10.5483/BMBRep.2014.47.5.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Li HF, Wang HB, Sun ZQ, Guo Q, Shi HY, Jia YC The clinical and prognostic value of polo-like kinase 1 in lung squamous cell carcinoma patients: immunohistochemical analysis. Biosci Rep. 2017;37:BSR20170852. doi: 10.1042/BSR20170852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Ramani P, Nash R, Sowa-Avugrah E, Rogers C High levels of polo-like kinase 1 and phosphorylated translationally controlled tumor protein indicate poor prognosis in neuroblastomas. J Neurooncol. 2015;125:103–11. doi: 10.1007/s11060-015-1900-4. [DOI] [PubMed] [Google Scholar]
- 210.Garuti L, Roberti M, Bottegoni G Polo-like kinases inhibitors. Curr Med Chem. 2012;19:3937–48. doi: 10.2174/092986712802002455. [DOI] [PubMed] [Google Scholar]
- 211.Li SS, Zhang YJ, Xu WF Developments of polo-like kinase 1 (plk1) inhibitors as anti-cancer agents. Mini Rev Med Chem. 2013;13:2014–25. doi: 10.2174/13895575113136660103. [DOI] [PubMed] [Google Scholar]
- 212.Yim H Current clinical trials with polo-like kinase 1 inhibitors in solid tumors. Anticancer Drugs. 2013;24:999–1006. doi: 10.1097/CAD.0000000000000007. [DOI] [PubMed] [Google Scholar]
- 213.Schöffski P, Blay JY, de Greve J, Brain E, Machiels JP, Soria JC, et al Multicentric parallel phase II trial of the polo-like kinase 1 inhibitor BI 2536 in patients with advanced head and neck cancer, breast cancer, ovarian cancer, soft tissue sarcoma and melanoma. the first protocol of the European Organization for Research and Treatment of Cancer (EORTC) Network of Core Institutes (NOCI) Eur J Cancer. 2010;46:2206–15. doi: 10.1016/j.ejca.2010.03.039. [DOI] [PubMed] [Google Scholar]
- 214.van den Bossche J, Lardon F, Deschoolmeester V, de Pauw I, Vermorken JB, Specenier P, et al Spotlight on volasertib: preclinical and clinical evaluation of a promising plk1 inhibitor. Med Res Rev. 2016;36:749–86. doi: 10.1002/med.2016.36.issue-4. [DOI] [PubMed] [Google Scholar]
- 215.Weiss GJ, Jameson G, von Hoff DD, Valsasina B, Davite C, di Giulio C, et al Phase I dose escalation study of NMS-1286937, an orally available Polo-Like kinase 1 inhibitor, in patients with advanced or metastatic solid tumors. Invest New Drugs. 2018;36:85–95. doi: 10.1007/s10637-017-0491-7. [DOI] [PubMed] [Google Scholar]
- 216.Uitdehaag JCM, Verkaar F, Alwan H, de Man J, Buijsman RC, Zaman GJR A guide to picking the most selective kinase inhibitor tool compounds for pharmacological validation of drug targets. Br J Pharmacol. 2012;166:858–76. doi: 10.1111/j.1476-5381.2012.01859.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Xu L, Zhu YR, Shao JJ, Chen M, Yan H, Li GQ, et al Dasatinib synergises with irinotecan to suppress hepatocellular carcinoma via inhibiting the protein synthesis of PLK1 . Br J Cancer. 2017;116:1027–36. doi: 10.1038/bjc.2017.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Shin SB, Woo SU, Yim H Differential cellular effects of plk1 inhibitors targeting the ATP-binding domain or polo-box domain. J Cell Physiol. 2015;230:3057–67. doi: 10.1002/jcp.25042. [DOI] [PubMed] [Google Scholar]
- 219.Zhang Z, Zhang GJ, Kong CZ Targeted inhibition of polo-like kinase 1 by a novel small-molecule inhibitor induces mitotic catastrophe and apoptosis in human bladder cancer cells. J Cell Mol Med. 2017;21:758–67. doi: 10.1111/jcmm.2017.21.issue-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Wang NN, Li ZH, Zhao H, Tao YF, Xu LX, Lu J, et al Molecular targeting of the oncoprotein PLK1 in pediatric acute myeloid leukemia: RO3280, a novel plk1 inhibitor, induces apoptosis in leukemia cells. Int J Mol Sci. 2015;16:1266–92. doi: 10.3390/ijms16011266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Lee C, Fotovati A, Triscott J, Chen J, Venugopal C, Singhal A, et al Polo-like kinase 1 inhibition kills glioblastoma multiforme brain tumor cells in part through loss of SOX2 and delays tumor progression in mice. Stem Cells. 2012;30:1064–75. doi: 10.1002/stem.1081. [DOI] [PubMed] [Google Scholar]
- 222.Lerner RG, Grossauer S, Kadkhodaei B, Meyers I, Sidorov M, Koeck K, et al Targeting a plk1-controlled polarity checkpoint in therapy-resistant glioblastoma-propagating cells. Cancer Res. 2015;75:5355–66. doi: 10.1158/0008-5472.CAN-14-3689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Ando K, Kakeji Y, Kitao H, Iimori M, Zhao Y, Yoshida R, et al High expression of BUBR1 is one of the factors for inducing DNA aneuploidy and progression in gastric cancer. Cancer Sci. 2010;101:639–45. doi: 10.1111/cas.2010.101.issue-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Li R, Murray AW Feedback control of mitosis in budding yeast. Cell. 1991;66:519–31. doi: 10.1016/0092-8674(81)90015-5. [DOI] [PubMed] [Google Scholar]
- 225.Rieder CL, Schultz A, Cole R, Sluder G Anaphase onset in vertebrate somatic cells is controlled by a checkpoint that monitors sister kinetochore attachment to the spindle. J Cell Biol. 1994;127:1301–10. doi: 10.1083/jcb.127.5.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Bolanos-Garcia VM, Blundell TL BUB1 and BUBR1: multifaceted kinases of the cell cycle. Trends Biochem Sci. 2011;36:141–50. doi: 10.1016/j.tibs.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Skoufias DA, Andreassen PR, Lacroix FB, Wilson L, Margolis RL Mammalian mad2 and bub1/bubR1 recognize distinct spindle-attachment and kinetochore-tension checkpoints. Proc Natl Acad Sci USA. 2001;98:4492–7. doi: 10.1073/pnas.081076898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Kokkinakis DM, Liu XY, Chada S, Ahmed MM, Shareef MM, Singha UK, et al Modulation of gene expression in human central nervous system tumors under methionine deprivation-induced stress. Cancer Res. 2004;64:7513–25. doi: 10.1158/0008-5472.CAN-04-0592. [DOI] [PubMed] [Google Scholar]
- 229.Restall IJ, Parolin DAE, Daneshmand M, Hanson JEL, Simard MA, Fitzpatrick ME, et al PKCι depletion initiates mitotic slippage-induced senescence in glioblastoma. Cell Cycle. 2015;14:2938–48. doi: 10.1080/15384101.2015.1071744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Maachani UB, Tandle A, Shankavaram U, Kramp T, Camphausen K Modulation of MiR-21 signaling by MPS1 in human glioblastoma. Oncotarget. 2016;7:52912–27. doi: 10.18632/oncotarget.4143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Reis RM, Nakamura M, Masuoka J, Watanabe T, Colella S, Yonekawa Y, et al Mutation analysis of hBUB1, hBUBR1 and hBUB3 genes in glioblastomas . Acta Neuropathol. 2001;101:297–304. doi: 10.1007/s004010100366. [DOI] [PubMed] [Google Scholar]
- 232.Bie L, Zhao G, Cheng P, Rondeau G, Porwollik S, Ju Y, et al The accuracy of survival time prediction for patients with glioma is improved by measuring mitotic spindle checkpoint gene expression. PLoS One. 2011;6:e25631. doi: 10.1371/journal.pone.0025631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Morales AG, Pezuk JA, Brassesco MS, de Oliveira JC, de Paula Queiroz RG, Machado HR, et al BUB1 and BUBR1 inhibition decreases proliferation and colony formation, and enhances radiation sensitivity in pediatric glioblastoma cells . Childs Nerv Syst. 2013;29:2241–8. doi: 10.1007/s00381-013-2175-8. [DOI] [PubMed] [Google Scholar]
- 234.Tannous BA, Kerami M, van der Stoop PM, Kwiatkowski N, Wang JH, Zhou WJ, et al Effects of the selective MPS1 inhibitor MPS1-IN-3 on glioblastoma sensitivity to antimitotic drugs. J Natl Cancer Inst. 2013;105:1322–31. doi: 10.1093/jnci/djt168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Brazeau JF, Rosse G Novel cycloalkenepyrazoles as inhibitors of bub1 kinase. ACS Med Chem Lett. 2014;5:280–1. doi: 10.1021/ml5000492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Baron AP, von Schubert C, Cubizolles F, Siemeister G, Hitchcock M, Mengel A, et al Probing the catalytic functions of bub1 kinase using the small molecule inhibitors BAY-320 and BAY-524. eLife. 2016;5:e12187. doi: 10.7554/eLife.12187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Altieri DC Survivin, versatile modulation of cell division and apoptosis in cancer. Oncogene. 2003;22:8581–9. doi: 10.1038/sj.onc.1207113. [DOI] [PubMed] [Google Scholar]
- 238.Chen X, Duan N, Zhang CG, Zhang WT Survivin and tumorigenesis: molecular mechanisms and therapeutic strategies. J Cancer. 2016;7:314–23. doi: 10.7150/jca.13332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Kelly RJ, Lopez-Chavez A, Citrin D, Janik JE, Morris JC Impacting tumor cell-fate by targeting the inhibitor of apoptosis protein survivin. Mol Cancer. 2011;10:35. doi: 10.1186/1476-4598-10-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Garg H, Suri P, Gupta JC, Talwar GP, Dubey S Survivin: a unique target for tumor therapy. Cancer Cell Int. 2016;16:49. doi: 10.1186/s12935-016-0326-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.de Necochea-Campion R, Chen CS, Mirshahidi S, Howard FD, Wall NR Clinico-pathologic relevance of Survivin splice variant expression in cancer. Cancer Lett. 2013;339:167–74. doi: 10.1016/j.canlet.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Mahotka C, Wenzel M, Springer E, Gabbert HE, Gerharz CD Survivin-ΔEx3 and survivin-2b: two novel splice variants of the apoptosis inhibitor survivin with different antiapoptotic properties . Cancer Res. 1999;59:6097–102. [PubMed] [Google Scholar]
- 243.Altieri DC Survivin, cancer networks and pathway-directed drug discovery. Nat Rev Cancer. 2008;8:61–70. doi: 10.1038/nrc2293. [DOI] [PubMed] [Google Scholar]
- 244.Niu TK, Cheng Y, Ren XC, Yang JM Interaction of beclin 1 with survivin regulates sensitivity of human glioma cells to trail-induced apoptosis. FEBS Lett. 2010;584:3519–24. doi: 10.1016/j.febslet.2010.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Jiang GF, Ren B, Xu L, Song SZ, Zhu CC, Ye FL Survivin may enhance dna double-strand break repair capability by up-regulating ku70 in human kb cells. Anticancer Res. 2009;29:223–8. [PubMed] [Google Scholar]
- 246.Capalbo G, Dittmann K, Weiss C, Reichert S, Hausmann E, Rödel C, et al Radiation-induced survivin nuclear accumulation is linked to DNA damage repair. Int J Radiat Oncol Biol Phys. 2010;77:226–34. doi: 10.1016/j.ijrobp.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 247.Cheung CHA, Chen HH, Kuo CC, Chang CY, Coumar MS, Hsieh HP, et al Survivin counteracts the therapeutic effect of microtubule de-stabilizers by stabilizing tubulin polymers. Mol Cancer. 2009;8:43. doi: 10.1186/1476-4598-8-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Zhang MC, Hu CP, Chen Q Effect of down-regulation of survivin gene on apoptosis and cisplatin resistance in cisplatin resistant human lung adenocarcinoma A549/CDDP cells. Chin J Oncol. 2006;28:408–12. [PubMed] [Google Scholar]
- 249.Cheung CHA, Sun XY, Kanwar JR, Bai JZ, Cheng LT, Krissansen GW A cell-permeable dominant-negative survivin protein induces apoptosis and sensitizes prostate cancer cells to tnf-α therapy. Cancer Cell Int. 2010;10:43. doi: 10.1186/1475-2867-10-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Liu JL, Wang Y, Jiang J, Kong R, Yang YM, Ji HF, et al Inhibition of survivin expression and mechanisms of reversing drug-resistance of human lung adenocarcinoma cells by SiRNA. Chin Med J (Engl) 2010;123:2901–7. [PubMed] [Google Scholar]
- 251.Ling X, Calinski D, Chanan-Khan AA, Zhou MX, Li FZ Cancer cell sensitivity to bortezomib is associated with survivin expression and p53 status but not cancer cell types. J Exp Clin Cancer Res. 2010;29:8. doi: 10.1186/1756-9966-29-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.He SQ, Rehman H, Gong MG, Zhao YZ, Huang ZY, Li CH, et al Inhibiting survivin expression enhances TRAIL-induced tumoricidal activity in human hepatocellular carcinoma via cell cycle arrest . Cancer Biol Ther. 2007;6:1258–68. doi: 10.4161/cbt.6.8.4444. [DOI] [PubMed] [Google Scholar]
- 253.Moriai R, Tsuji N, Moriai M, Kobayashi D, Watanabe N Survivin plays as a resistant factor against tamoxifen-induced apoptosis in human breast cancer cells. Breast Cancer Res Treat. 2009;117:261–71. doi: 10.1007/s10549-008-0164-5. [DOI] [PubMed] [Google Scholar]
- 254.Chakravarti A, Noll E, Black PM, Finkelstein DF, Finkelstein DM, Dyson NJ, et al Quantitatively determined survivin expression levels are of prognostic value in human gliomas. J Clin Oncol. 2002;20:1063–8. doi: 10.1200/JCO.2002.20.4.1063. [DOI] [PubMed] [Google Scholar]
- 255.Chakravarti A, Zhai GG, Zhang M, Malhotra R, Latham DE, Delaney MA, et al Survivin enhances radiation resistance in primary human glioblastoma cells via caspase-independent mechanisms . Oncogene. 2004;23:7494–506. doi: 10.1038/sj.onc.1208049. [DOI] [PubMed] [Google Scholar]
- 256.Reichert S, Rödel C, Mirsch J, Harter PN, Tomicic MT, Mittelbronn M, et al Survivin inhibition and DNA double-strand break repair: a molecular mechanism to overcome radioresistance in glioblastoma. Radiother Oncol. 2011;101:51–8. doi: 10.1016/j.radonc.2011.06.037. [DOI] [PubMed] [Google Scholar]
- 257.Nakahara T, Takeuchi M, Kinoyama I, Minematsu T, Shirasuna K, Matsuhisa A, et al YM155, a novel small-molecule survivin suppressant, induces regression of established human hormone-refractory prostate tumor xenografts. Cancer Res. 2007;67:8014–21. doi: 10.1158/0008-5472.CAN-07-1343. [DOI] [PubMed] [Google Scholar]
- 258.Lai PC, Chen SH, Yang SH, Cheng CC, Chiu TH, Huang YT Novel survivin inhibitor YM155 elicits cytotoxicity in glioblastoma cell lines with normal or deficiency DNA-dependent protein kinase activity. Pediatr Neonatol. 2012;53:199–204. doi: 10.1016/j.pedneo.2012.04.008. [DOI] [PubMed] [Google Scholar]
- 259.Guo H, Wang YX, Song T, Xin T, Zheng ZM, Zhong P, et al Silencing of survivin using YM155 inhibits invasion and suppresses proliferation in glioma cells. Cell Biochem Biophys. 2015;71:587–93. doi: 10.1007/s12013-014-0238-4. [DOI] [PubMed] [Google Scholar]
- 260.Tolcher AW, Mita A, Lewis LD, Garrett CR, Till E, Daud AI, et al Phase I and pharmacokinetic study of YM155, a small-molecule inhibitor of survivin. J Clin Oncol. 2008;26:5198–203. doi: 10.1200/JCO.2008.17.2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Giaccone G, Zatloukal P, Roubec J, Floor K, Musil J, Kuta M, et al Multicenter phase II trial of YM155, a small-molecule suppressor of survivin, in patients with advanced, refractory, non-small-cell lung cancer. J Clin Oncol. 2009;27:4481–6. doi: 10.1200/JCO.2008.21.1862. [DOI] [PubMed] [Google Scholar]
- 262.Hwu JR, Tseng WN, Gnabre J, Giza P, Huang RCC Antiviral activities of methylated nordihydroguaiaretic acids. 1. synthesis, structure identification, and inhibition of tat-regulated HIV transactivation. J Med Chem. 1998;41:2994–3000. doi: 10.1021/jm970819w. [DOI] [PubMed] [Google Scholar]
- 263.Castro-Gamero AM, Borges KS, Moreno DA, Suazo VK, Fujinami MM, de Paula Gomes Queiroz R, de Oliveira HF, et al Tetra-o-methyl nordihydroguaiaretic acid, an inhibitor of Sp1-mediated survivin transcription, induces apoptosis and acts synergistically with chemo-radiotherapy in glioblastoma cells. Invest New Drugs. 2013;31:858–70. doi: 10.1007/s10637-012-9917-4. [DOI] [PubMed] [Google Scholar]
- 264.Tibes R, McDonagh KT, Lekakis L, Bogenberger JM, Kim S, Frazer N, et al Phase i study of the novel Cdc2/CDK1 and akt inhibitor terameprocol in patients with advanced leukemias. Invest New Drugs. 2015;33:389–96. doi: 10.1007/s10637-014-0198-y. [DOI] [PubMed] [Google Scholar]
- 265.Grossman SA, Ye XB, Peereboom D, Rosenfeld MR, Mikkelsen T, Supko JG, et al Phase I study of terameprocol in patients with recurrent high-grade glioma. Neuro Oncol. 2012;14:511–7. doi: 10.1093/neuonc/nor230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Peery RC, Liu JY, Zhang JT Targeting survivin for therapeutic discovery: past, present, and future promises. Drug Discov Today. 2017;22:1466–77. doi: 10.1016/j.drudis.2017.05.009. [DOI] [PubMed] [Google Scholar]
- 267.Lanvin O, Monferran S, Delmas C, Couderc B, Toulas C, Cohen-Jonathan-Moyal E Radiation-induced mitotic cell death and glioblastoma radioresistance: a new regulating pathway controlled by integrin-linked kinase, hypoxia-inducible factor 1alpha and survivin in U87 cells. Eur J Cancer. 2013;49:2884–91. doi: 10.1016/j.ejca.2013.05.003. [DOI] [PubMed] [Google Scholar]