Abstract
Objective:
Spontaneous hepatocellular carcinoma (HCC) rupture can be fatal, and hepatic resection could achieve a favorable long-term survival among all strategies of tumor rupture. However, there is no available prognostic scoring system for patients with ruptured HCC who underwent partial hepatectomy.
Methods:
From January 2005 to May 2015, 129 patients with spontaneous HCC rupture underwent partial hepatectomy. Preoperative clinical data were collected and analyzed. Independent risk factors affecting overall survival (OS) were used to develop the new scoring system. Harrell’s C statistics, Akaike information criterion (AIC), the relative likelihood, and the log likelihood ratio were calculated to measure the homogeneity and discriminatory ability of a prognostic system.
Results:
In the multivariable Cox regression analysis, three factors, including tumor size, preoperative α-fetoprotein level, and alkaline phosphatase level, were chosen for the new tumor-associated antigen (TAA) prognostic scoring system. The 1-year OS rates were 88.1%, 43.2%, and 30.2% for TAA scores of 0–5 points (low-risk group), 6–9 points (moderate-risk group), and 10–13 points (high-risk group), respectively. The TAA scoring system had superior homogeneity and discriminatory ability (Harrell’s C statistics, 0.693 vs. 0.627 and 0.634; AIC, 794.79 vs. 817.23 and 820.16; relative likelihood, both < 0.001; and log likelihood ratio, 45.21 vs. 22.77 and 21.84) than the Barcelona Clinic Liver Cancer staging system and the Cancer of the Liver Italian Program in predicting OS. Similar results were found while predicting disease-free survival (DFS).
Conclusions:
The new prognostic scoring system is simple and effective in predicting both OS and DFS of patients with spontaneous ruptured HCC.
Keywords: Spontaneous rupture, prognostic scoring system, homogeneity, discriminatory ability, overall survival, disease-free survival, hepatocellular carcinoma
Introduction
Spontaneous rupture of a tumor is considered fatal in patients with hepatocellular carcinoma (HCC), with a reported incidence rate ranging from 3% to 15%1-4. HCC ruptures may lead to dismal clinical outcomes, and mortality rates in the acute phase remain high5-8. The treatment and prognosis of ruptured HCC remain controversial as yet. Some treatment strategies have been reported, including supportive care, transcatheter arterial embolization (TAE), emergency liver resection, and staged hepatectomy1,4,5. Many studies have confirmed that surgical resection (whether emergency or staged) could achieve a better long-term survival than other treatments in selected patients and is a safe and effective therapy in hospital5,6,9-13.
However, given this condition’s rare occurrence, only a limited number of studies have been conducted to determine the prognostic factors for postoperative survival; to our knowledge, no postoperative prognostic model has been proposed. Therefore, a new prognostic scoring system for patients with ruptured HCC who underwent partial hepatectomy is needed.
The present study aimed to create and validate a new postoperative scoring system for patients with spontaneous HCC rupture based on the 10-year clinical data of a large single center. We also aimed to compare the discriminatory ability and homogeneity of the new model with the existing classification systems [Barcelona Clinic Liver Cancer (BCLC), the Cancer of the Liver Italian Program (CLIP), and the Okuda system].
Patients and methods
Patient selection
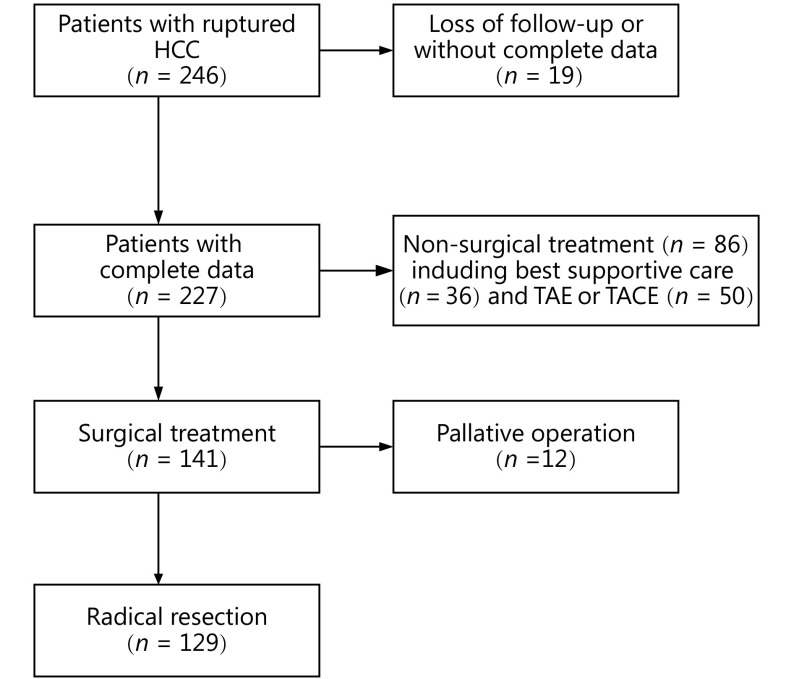
Between January 2005 and May 2015, 246 patients with complete data were diagnosed with spontaneous rupture of HCC at Tongji Hospital of Tongji Medical College of Huazhong University of Science and Technology, Wuhan, China. Among them, 86 patients underwent non-surgical treatment (50 for transcatheter hepatic arterial embolization or transcatheter hepatic arterial chemoembolization and 36 for best supportive care), and 141 patients underwent surgical treatment ultimately. About 12 of the 141 patients underwent palliative surgery such as microwave coagulation therapy, ligation of the hepatic artery, or suturing ligation surgery instead of partial hepatectomy. A total of 129 patients were included in the analysis (Figure 1).
1.
Study flowchart. TACE, transcatheter arterial chemoembolization; TAE, transcatheter arterial embolization.
Diagnosis, treatment, and follow-up
Normally, the diagnosis of HCC rupture was based on signs and symptoms upon admission, such as acute abdominal pain and shock, and confirmed by at least two of the following kinds of imaging examinations: abdominal ultrasonography (US), magnetic resonance imaging (MRI), and/or spiral computed tomography (CT) scanning. Diagnostic abdominal paracentesis was performed in patients without contraindications. Ultimately, hemorrhage was confirmed by surgical exploration. Routine pre-operation testing included a peripheral blood test; liver and kidney function checks; serum electrolyte, serum markers of hepatitis B and C viruses; and serologic α-fetoprotein (AFP) measurements; electrocardiography; coagulation function; and chest X-ray.
The primary goal of acute management was maintaining stable hemodynamics (to avoid hemorrhagic shock) by means of active vein fluid resuscitation, and blood products would be used if indicated. All hospitalized patients who had unstable hemodynamics were closely monitored in the intensive care unit or ward. Following the initial immediate treatment in most cases, bleeding could be controlled spontaneously. TAE was deemed an effective therapy in achieving hemostasis in patients without severely impaired liver function or other contraindications. Child-Pugh C grade was recognized as an absolute contraindication for both liver surgery and TAE. After TAE, partial hepatectomy was taken into account. Definitive treatment was based on tumor extent, the status of liver function, the general condition of the patient, and the treatment wishes of the patient and his or her family members.
Partial hepatectomy comprised single or multiple hepatic resections aiming at excising all visible malignant tissue. All operative procedures were performed by experienced surgeons, and the abdominal cavity was extensively explored during the operation. During the resection, Pringle’s maneuver was applied when necessary to occlude the hepatic blood inflow. The desirable tumor margin during resection was more than 1 cm. Transection of liver parenchyma was traditionally implemented using the clamp-crushing technique or sometimes using a BiClamp or ultrasonic scalpel. Sutures and an argon beam coagulator were used for hemostasis. The degree and type of differentiation and cirrhosis were confirmed by postoperative pathological examination.
During the study period, various types of outpatient examinations, telephone interview, or re-admission were performed. The follow-up period ended on December 31, 2017, or when the patient died or was lost to follow-up. All patients were followed up once a month for 6 months after the operation and then every 3 months if there was no recurrence. Tests for complete blood count, liver and kidney function, coagulation function, and serum AFP level; abdominal ultrasonography; and chest X-ray were performed routinely after the operation. Digital subtraction angiography, abdominal CT or MRI, bone scan, chest CT, head CT, or positron emission tomography (PET) might be performed in patients with suspected relapse. The treatment recommendations in cases of tumor recurrence have been decided at a multidisciplinary liver tumor conference and include reoperation, percutaneous ablative therapy, percutaneous ethanol injection, transarterial chemoembolization (TACE), radiotherapy, intravenous chemotherapy, and sorafenib (since 2008) oral tablets. The treatment decision was usually made after a consultation with the patient and the family members. DFS was defined as the time interval from the date of surgery to recurrence or the latest follow-up if no evidence of recurrence was found. OS also was recorded. This study adhered to the ethical principles set by the Declaration of Helsinki.
Statistical analysis
The optimum cut-off value of alkaline phosphatase (ALP) was 100.5 U/L, based on the maximum sum of sensitivity and specificity of the ALP value for predicting OS in receiver operating characteristic curve analysis. Tumor size was divided into three levels using cut-off values of 5 and 10 cm. Likewise, AFP was divided based on the cut-off values of 20 and 400 ng/mL. All statistical analyses were conducted using SPSS 24.0 (IBM Corp., Armonk, NY, USA) and R software 3.4.3 for Windows (http://www.r-project.org), combined with other packages (Harrell Miscellaneous, survival, survminer, ggsurvplot, and ggpar). A two-sided P-value of < 0.05 was considered significant. Continuous data were summarized as mean ± standard deviation (SD) or median (range or quartile) for ordinal and non-normally distributed variables, respectively. Categorical data were expressed as number ( n) or proportion (%). Continuous data between groups were compared using Student’s t-test when it met the application conditions; otherwise, the Mann-Whitney U test was used. Categorical data were compared using χ2 test with (or without) the Yates correction or Fisher’s exact test, depending on the situation. Univariate survival analysis of OS and DFS was performed, and the results were compared using a log-rank test and presented with Kaplan-Meier curves. After initial univariate survival analysis, multivariate Cox survival analysis was carried out, and the hazard ratios (HR) and 95% confidence intervals (95% CI) were estimated.
The results of regression coefficient ratios in the final Cox model for OS were obtained and rounded to the nearest whole number for further analysis. We received the final weights of each factor using this approach. After adding up the weights of all risk factors, the evaluation risk scores of tumor-associated antigen (TAA) were obtained. All cases were ultimately divided into three risk groups according to their TAA scores. The survival rate among the three risk groups was compared by log-rank test.
The patients were divided into risk groups based on the TAA scoring system (stages I, II, and III), BCLC staging system (stages A, B, and C), and CLIP classification. Homogeneity and discriminatory ability were observed to measure the performance of a classification system14,15. Harrell’s C statistics (C-index) was used to determine the discriminatory ability of the TAA scoring system, BCLC staging system, and CLIP classification system. The C-index ranges from 0.5 (no discrimination) to 1.0 (perfect discrimination). A higher C-index implies superior discriminative ability in each prognostic system. Homogeneity was evaluated by the log likelihood ratio generated from the Cox proportional hazard model. A higher log likelihood ratio in the model suggests better homogeneity of postoperative survival of those patients in the same stage. In addition, the Akaike Information Criteria (AIC) was used to determine the discriminatory ability of a given prognostic model. A lower AIC indicates lower information loss of the model and better goodness of fit. The relative likelihood of models represents the probability that TAA minimizes information loss as effectively as BCLC (or CLIP) and can be interpreted succinctly as a P-value for the comparison of AIC differences. The relative likelihood of TAA vs. BCLC or CLIP model was calculated using the following formula: exp [(AICTAA/AICBCLC or CLIP)/2].
Results
Patient and tumor characteristics
All patients (113 men and 16 women) during the study period who underwent partial liver resection had a mean age of 46.3 ± 11.5 years. Table 1 summarizes patients’ demographic, clinical, and laboratory characteristics. Among them, 116 patients (83.5%) were HBsAg positive. The median size (maximum diameter) of the liver tumor was 70 mm (interquartile range, 50–100 mm), among which most were single tumors (n = 98, 76.0%). On admission, shock was present in 23 (17.7%) patients in our cohort. Further details could be found in Table 1.
1.
Demographic, clinical, and laboratory characteristics of patients with ruptured HCC who underwent partial liver resection
| Characteristics | All patients (n = 139) |
| Age (year)* | 46.3 ± 11.5 |
| Gender | |
| Male | 113 (87.6%) |
| Female | 16 (12.4%) |
| HBs-Ag (+) | 116 (83.5%) |
| HCV-Ab (+) | 8 (6.2%) |
| Tumor size (cm)** | 7.0 (5.0–10.0) |
| Tumor number | |
| Solitary | 98 (76.0%) |
| Multiple | 31 (24.0%) |
| Tumor differentiation | |
| Well or moderately | 67 (51.9%) |
| Poorly | 62 (48.1%) |
| Preoperative TAE | |
| Yes | 18 (14.0%) |
| No | 111 (86.0%) |
| Hepatic inflow occlusion | |
| Yes | 68 (52.7%) |
| No | 61 (47.3%) |
| Cirrhosis | |
| Yes | 89 (69.0%) |
| No | 40 (31.0%) |
| Portal hypertension | |
| Yes | 11 (8.5%) |
| No | 118 (91.5%) |
| MVI | |
| Yes | 24 (18.6%) |
| No | 105 (81.4%) |
| Shock | |
| Yes | 23 (17.8%) |
| No | 106 (82.2%) |
| Alcohol consumption | |
| Yes | 32 (24.8%) |
| No | 97 (75.2%) |
| Preoperative blood loss (mL) | |
| ≤ 500 | 69 (49.6%) |
| Continued | |
At the end of follow-up, 99 (70.7%) patients died, including 98 (69.7%) who died due to tumor-related or liver-related causes. Only 1 (1.0%) patient died due to myocardial infarction, and that was the only case of postoperative hospital death reported in this study. OS rates for 1, 2, 3, and 5 years were 53.5%, 37.2%, 30.8%, and 19.6%, respectively, and 1-, 2-, 3-, and 5-year DFS rates were 33.3%, 21.7%, 18.3%, and 10.7%, respectively.
Prognostic factors
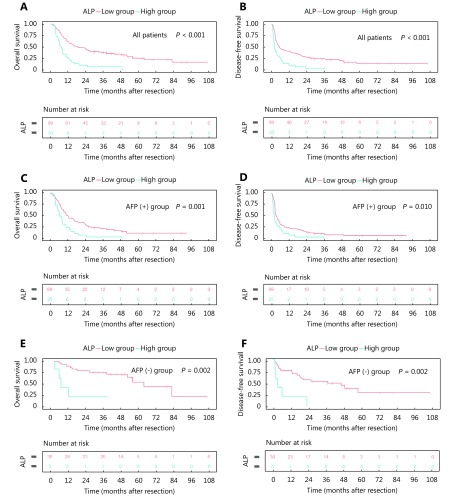
Univariate survival analysis identified the following indexes associated with OS: tumor size, macroscopic vascular invasion, total bilirubin level, AFP level, ALP level, and blood transfusion in the hospital. In addition, aspartate aminotransferase factor level >40 U/L was a risk factor for poor DFS not OS ( Supplementary Table S1). Each variable with a P-value <0.05 in the univariate analysis was used in the multivariable model. Multivariable Cox regression showed that tumor size (T), AFP (A) and ALP (A) ( Supplementary Figure S1) were independent risk factors associated with OS. Similar results were also found for DFS (Table 2).
S1.
Univariate analysis of predictive factors associated with overall survival (OS) and disease-free survival (DFS)
| Variables | OS | DFS | |||
| HR(95%CI) | P | HR(95%CI) | P | ||
| Gender | |||||
| Male/Famale | 1.010 (0.551–1.851) | 0.974 | 0.814 (0.456–1.454) | 0.487 | |
| Age (year) | |||||
| > 50/≤ 50 | 0.878 (0.586–1.316) | 0.529 | 0.821 (0.561–1.200) | 0.309 | |
| Tumor size (cm) | |||||
| > 5/≤ 5 | 3.284 (1.884–5.723) | < 0.001 | 2.989 (1.827–4.892) | < 0.001 | |
| Tumor number | |||||
| Multiple/Solitary | 1.460 (0.925–2.305) | 0.104 | 1.892 (1.238–2.894) | 0.003 | |
| Tumor differentiation | |||||
| Poorly/Well or moderately | 1.411 (0.950–2.095) | 0.088 | 1.234 (0.849–1.794) | 0.271 | |
| Cirrhosis | |||||
| Yes/No | 0.993 (0.648–1.523) | 0.976 | 1.109 (0.740–1.662) | 0.617 | |
| Portal hypertension | |||||
| Yes/No | 1.221 (0.614–2.427) | 0.570 | 1.252(0.651–2.409) | 0.501 | |
| MVI | |||||
| Yes/no | 3.035 (1.870–4.924) | <0.001 | 2.775 (1.736–4.437) | <0.001 | |
| Alcohol | |||||
| Yes/no | 0.878 (0.550–1.402) | 0.586 | 0.765 (0.492–1.188) | 0.233 | |
| Shock | |||||
| Yes/no | 1.606 (0.990–2.606) | 0.055 | 1.417 (0.887–2.265) | 0.145 | |
| Preoperative TAE | |||||
| Yes/no | 0.767 (0.427–1.377) | 0.375 | 1.129 (0.673–1.896) | 0.646 | |
| Preoperative blood loss (mL) | |||||
| > 600/≤ 600 | 0.935 (0.628–1.393) | 0.743 | 0.933 (0.642–1.356) | 0.716 | |
| BT in hospital | |||||
| Yes/no | 1.594 (1.053–2.412) | 0.027 | 1.570 (1.062–2.323) | 0.024 | |
| Cholesterin (mmol/L) | |||||
| > 3.7/≤ 3.7 | 1.040 (0.697–1.551) | 0.848 | 0.982 (0.672–1.435) | 0.924 | |
| Creatinine (μmol/L) | |||||
| > 104/≤ 104 | 1.465 (0.459–4.670) | 0.519 | 1.236 (0.388–3.935) | 0.720 | |
| ALT (U/L) | |||||
| > 40/≤ 40 | 0.755 (0.489–1.165) | 0.204 | 0.913 (0.611–1.364) | 0.657 | |
| AST (U/L) | |||||
| > 40/≤ 40 | 1.395 (0.940–2.071) | 0.099 | 1.554 (1.068–2.259) | 0.021 | |
| Continued | |||||
S1.
Overall survival (A) (C) (E) and disease-free survival (B) (D) (F) in all patients (A) (B). Patients were classified into an AFP-positive group (n = 94) (C) (D) and AFP-negative group (n = 35) (E) (F). Differences between different ALP groups were analyzed using the log-rank test for the significance of differences.
2.
Multivariate analysis of predictive factors associated with overall survival (OS) and disease-free survival (DFS)
| Variables | OS | DFS | |||||
| β | HR (95%CI) | P | β | HR (95%CI) | P | ||
| β, the coefficients in the COX regression; HR, hazard ratio; CI, confidence interval; MVI, macroscopic vascular invasion; TBIL, total bilirubin; AFP, α-fetoprotein; ALP, alkaline phosphatase; BT, blood transfusion; AST, aspartate aminotransferase. | |||||||
| Tumor size (cm) | 0.015 | 0.005 | |||||
| 5–10/<5 | 0.789 | 2.200 (1.185–4.085) | 0.013 | 0.576 | 1.779 (1.019–3.104) | 0.043 | |
| ≥ 10/<5 | 0.950 | 2.586 (1.346–4.968) | 0.004 | 1.002 | 2.724 (1.490–4.981) | 0.001 | |
| MVI | |||||||
| Yes/no | 0.509 | 1.664 (0.962–2.879) | 0.069 | 0.508 | 1.129 (0.729–1.750) | 0.587 | |
| TBIL (μmol/L) | |||||||
| > 17.1/≤ 17.1 | 0.238 | 1.269 (0.808–1.991) | 0.301 | 0.191 | 1.211 (0.789–1.859) | 0.382 | |
| AFP (ng/mL) | 0.003 | 0.008 | |||||
| 20–400/≤ 20 | 0.576 | 1.779 (0.782–4.048) | 0.170 | 0.491 | 1.633 (0.758–3.518) | 0.210 | |
| > 400/≤ 20 | 0.967 | 2.629 (1.495–4.624) | 0.001 | 0.794 | 2.212 (1.337–3.659) | 0.002 | |
| ALP (U/L) | |||||||
| > 100.5/≤ 100.5 | 0.560 | 1.751 (1.070–2.865) | 0.026 | 0.559 | 1.749 (1.074–2.848) | 0.025 | |
| BT in hospital | |||||||
| Yes/no | 0.157 | 1.170 (0.748–1.831) | 0.491 | 0.121 | 1.129 (0.729–1.750) | 0.587 | |
| AST (U/L) | |||||||
| > 40/≤ 40 | 0.097 | 0.907 (0.579–1.422) | 0.672 | ||||
Prognostic index
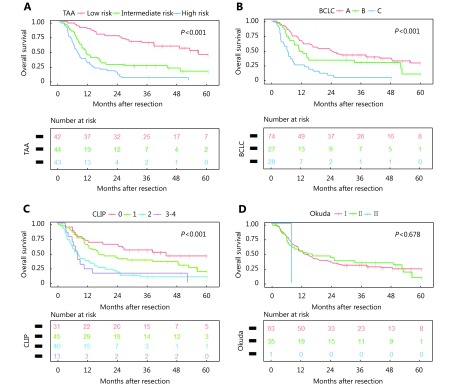
In Cox regression, the coefficients (β) and hazard ratio (HR) for each risk factor were reported in Table 2. In the final model, three factors for OS were adopted. The three prognostic factors were tumor size < 5 cm (weight, 0), 5–10 cm (weight, 4), or ≥ 10 cm (weight, 5); AFP ≤ 20 ng/mL (weight, 0), 20–400 ng/mL (weight, 3), or >400 ng/mL (weight, 5); and ALP >100.5 U/L (weight, 3) ( Table 3). Risk scores ranged from 0 to 13 points. All patients were grouped based on their risk scores; approximately 88.1% of patients with low risk (0–5 points, n = 42) survived more than 1 year, compared with 43.2% of those with moderate risk (6–9 points, n = 44) and 30.2% of those with high risk (10–13 points, n = 43). OS rates among different risk score groups classified based on the TAA scoring system were significantly different, as shown in Figure 2 (P < 0.001). OS rates for 2, 3, and 5 years were 73.8%, 64.1%, and 44.2%, respectively, in the low-risk group and 27.3%, 24.8%, and 15.5%, respectively, in the moderate-risk group. However, the OS rates were only 9.3%, 4.7%, and 0, respectively, in the high-risk group.
3.
Components of the TAA risk scores
| Risk factor | Score |
| Low-risk group, scores 0–5; moderate-risk group, scores 6–9; high-risk group, scores 10–13. AFP, α-fetoprotein; ALP, alkaline phosphatase. | |
| Tumor size (cm) | |
| < 5 | 0 |
| 5–10 | 4 |
| ≥ 10 | 5 |
| AFP (ng/mL) | |
| ≤ 20 | 0 |
| 20–400 | 3 |
| > 400 | 5 |
| ALP (U/L) | |
| ≤ 100.5 | 0 |
| > 100.5 | 3 |
2.
Overall survival predicted by TAA scoring systems (A), the Barcelona Clinic Liver Cancer (BCLC) classification systems (B), the Cancer of the Liver Italian Program (C), and the Okuda system (D). Differences were analyzed using the log-rank test.
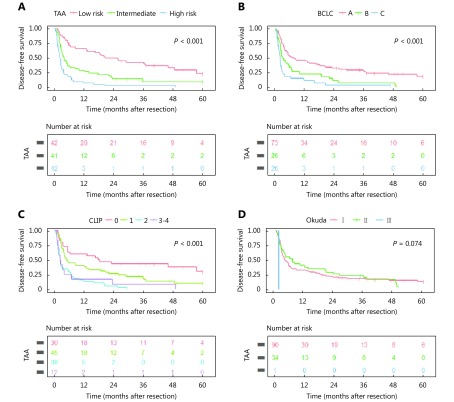
In addition, the difference in DFS was also highly significant in the new staging system (Supplementary Figure S2).
S2.
Disease-free survival in different TAA scoring systems (A) and BCLC classification systems (B), CLIP system (C), and Okuda system (D). Differences were analyzed by the log-rank test for the significance of differences.
The CLIP and BCLC showed significant difference in terms of predicting both OS (Figure 2) and DFS (Supplementary Figure S2) (both P < 0.001). Unfortunately, no significant difference was found for Okuda system neither in OS ( Figure 2) (P = 0.678) nor in DFS (Supplementary Figure S2) (P = 0.074); thus, it was not included in the further analysis.
Model assessment
Homogeneity and discriminatory ability were evaluated by Harrell’s C statistics, AIC, and log likelihood ratio. The TAA scoring system showed a higher Harrell’s C statistic (0.693) compared with the traditional BCLC (0.627) and the CLIP (0.634) classification system. This result demonstrates that the discriminatory ability of the new TAA staging is better than the old ones. Similarly, the TAA scoring system had a lower AIC (794.79) compared with the BCLC (817.23) and the CLIP (820.16) classification system. Likewise, the TAA scoring system correlated with less information loss than both the BCLC and CLIP in predicting OS (both the relative likelihood < 0.001). Similar results were found for the log likelihood ratio (TAA to BCLC to CLIP, 45.21 : 22.77 : 21.81) ( Table 4). Thus, the TAA score system has better discriminatory ability and homogeneity for predicting OS than the BCLC classification system.
4.
Comparison of TAA, BCLC and CLIP prognostic scoring system in predicting overall survival (OS)
| Predictor | TAA
model |
BCLC
model |
CLIP
model |
| AIC, Akaike information criterion. | |||
| Harrell’s C statistic | 0.693 | 0.627 | 0.634 |
| AIC | 794.79 | 817.23 | 820.16 |
| Log likelihood ratio | 45.21 | 22.77 | 21.84 |
| Relative likelihood of AIC
(BCLC or CLIP vs. TAA) |
< 0.001 | < 0.001 | |
Furthermore, the new staging system showed higher homogeneity and discriminatory ability in predicting DFS (Supplementary Table S2).
S2.
Comparison of TAA, BCLC and CLIP prognostic scoring systems in predicting disease-free survival (DFS)
| Predictor | TAA model | BCLC model | CLIP model |
| AIC, Akaike information criterion. | |||
| Harrell’s C statistic | 0.686 | 0.633 | 0.640 |
| AIC | 884.15 | 902.32 | 900.57 |
| Log likelihood ratio | 42.30 | 24.13 | 27.88 |
| Relative likelihood of AIC
(BCLC or CLIP vs. TAA) |
< 0.001 | < 0.001 | |
Hence, the TAA scoring system could be more informative than the Okuda, BCLC, and CLIP classification system for predicting survival.
Discussion
Spontaneous tumor rupture is uncommon but fatal in patients with HCC5,6. The mechanism of tumor rupture is still uncertain. Risk of tumor rupture was reported to associate with expanding growth tumor size, tumor necrosis, and hepatic vein obstruction by a tumor thrombus4,16-19. The leading cause of death after tumor rupture was either bleeding complications or hepatic failure in the short term20. Compared with TAE or conservative management alone, hepatic resection was reported to achieve a better in-hospital or 1-month survival. In addition, the cause of death was related to tumor-related events in the long-term survival, consisting of tumor recurrence or metastasis20. By contrast, surgical resection could achieve a more favorable long-term outcome20. Hence, partial hepatectomy would be the best treatment due to its effect on both short-term and long-term survival5,9-12,20.
In the current HCC staging systems, tumor rupture is arbitrarily classified as T4 disease regardless of other tumor or clinical index21-23, although this classification of ruptured HCC remains controversial because of its inability in reflecting a true prognosis accurately24. Thus, tumor node metastasis (TNM) staging systems are unavailable to reclassify HCC rupture. The Japan Integrated Staging score was based on the TNM staging and consequently, was not included in the analysis. The BCLC classification system25-28 could be used in patients with this type of disease, but the Eastern Cooperative Oncology Group performance scores may not be applicable to bed-bound patients. In our hepatic surgery center, about half of the patients with spontaneous ruptured HCC were treated with partial hepatectomy, and we consequently analyzed the postoperative risk factors of these 129 patients. Results showed that there is a significant relationship between the following factors: tumor size, AFP, ALP, and postoperative outcomes in patients with spontaneous ruptured HCC. Furthermore, we developed and validated a simple TAA scoring system that can effectively predict the OS and DFS of these patients.
In the present study, tumor size can be an independent risk factor for survival of patients with spontaneous ruptured HCC, and this finding is consistent with that reported in a previous large patient cohort study in China12. Tumor size was classified by the cut-off value of 5 and 10 cm in our study (median diameter of the tumor was 7.0 cm). To date, the largest proportion of patients with HCC rupture was the subject of a nationwide survey in Japan reported by Aoki et al.24 In this study, tumor size was considered as the main independent risk factor of survival, with the largest odds ratio. In addition, relatively high tension in large tumors suggested an association with the occurrence of spontaneous tumor rupture1. A study in China also suggested a link between tumor size ≥ 5 cm and high propensity for rupture29. In summary, large tumor size suggests not only a rupture tendency but also worse chance of survival. Furthermore, tumor size has been included in many prognostic evaluation systems for HCC, such as TNM, BCLC, Okuda, Chinese University Prognostic Index (CUPI), and CLIP21,25,30-32.
AFP, as a serum cancer marker, is used frequently for HCC diagnosis and prognosis prediction including CLIP and CUPI31-37. Levels of AFP were also reported as a survival risk factor in patients with spontaneous ruptured HCC12. This present study showed that AFP could predict overall survival and tumor recurrence. Occasionally, the sensitivity of AFP may be unsatisfactory for the detection of HCC37-39; however, in our study, ALP may have a prognostic role, even though serum AFP was negative (Supplementary Figure S1).
ALP, not a tissue-specific marker, can exist in all tissues and organs of the body. As the cause of liver damage and cholestasis, an elevated ALP level can be found in some hepatobiliary disease, such as primary biliary cirrhosis, cholangitis, choledocholithiasis, hepatitis40-43. The common ground of all these diseases is inflammation, which could play a role in tumor formation. Meanwhile, ALP was observed to have prognostic value for tumors such as HCC44 and cholangiocarcinoma45. Moreover, it has been used in prognostic scoring systems for HCC (CUPI)31 and intrahepatic cholangiocarcinoma46. Furthermore, ALP can affect tumor proliferation by undergoing nucleolar localization, as observed under an electron microscope47. In a cancer cell line such as Hep-G2, ALP was found to participate in cell cycles47. In general, ALP participates in tumor formation and represents inflammation reactions in various ways. In patients with ruptured HCC, the state of stress, blood loss, complex tumor status, poor liver function, and the worse general situation may suggest a more obvious inflammatory condition that may contribute to tumor formation. However, further research is needed to explore the mechanism underlying this.
This study showed that ALP has a predictive role for the survival of patients with spontaneous ruptured HCC who underwent partial hepatectomy. ALP is an easily accessible and relatively inexpensive laboratory index after admission. As a liver function parameter, ALP level can be a predictor of both OS and DFS independently after adjusting for other factors in the multivariate analysis.
Based on these three factors, we developed a new prognostic scoring system (TAA). The optimal prognostic classification system should have the maximal discriminatory ability to predict survival outcomes of different stages of ruptured HCC while keeping the variability of survival outcomes for each stage of the classification system to a minimum. The BCLC staging system, as a commonly accepted clinical classification, has provided recommended treatment strategies for different stages of HCC accordingly. Unfortunately, tumor rupture, a complication of HCC that requires urgent care, has not been fully considered in the BCLC staging system. This might be one of the possible reasons that the BCLC classification system did not display superior homogeneity and discriminatory ability in our study.
This research was implemented in one of the largest liver surgery centers in China; moreover, all surgeons had several years of practical experience in liver resection as treatment for hepatic tumors. When patients were diagnosed with HCC rupture, partial hepatectomy was first considered after admission. Partial hepatectomy would be performed when the patient met the application conditions. In our study, the proportion of patients with HCC rupture who underwent hepatic resection was 52.4% (129 of 246), which was higher than those reported in previous studies4,5,12,48.
This study had a few limitations. First, because of the retrospective nature of this study, its inherent shortcomings are unavoidable. Second, as a relatively rare disease, only a limited number of patients with HCC rupture underwent partial hepatectomy. Thus, only fewer patients were available for further analysis. Finally, owing to a larger proportion of hepatitis B virus-related HCC patients in this study, the patients’ clinical features may differ from those in Western countries.
Acknowledgements
We thank Professor Xiaoping Chen for patient management, Ms. Hongping Luo for patient care, and Dr. Hua Xiao, Ms. Jingjing Yu, and Miss Huiyuan Yang for patient register and follow-up.
Conflict of interest statement
No potential conflicts of interest are disclosed.
Contributor Information
Peng Zhu, Email: zhupeng@tjh.tjmu.edu.cn.
Bixiang Zhang, Email: bixiangzhang@163.com.
References
- 1.Zhu LX, Wang GS, Fan ST Spontaneous rupture of hepatocellular carcinoma. Br J Surg. 1996;83:602–7. doi: 10.1002/(ISSN)1365-2168. [DOI] [PubMed] [Google Scholar]
- 2.Leung KL, Lau WY, Lai PBS, Yiu RYC, Meng WCS, Leow CK Spontaneous rupture of hepatocellular carcinoma: conservative management and selective intervention. Arch Surg. 1999;134:1103–7. doi: 10.1001/archsurg.134.10.1103. [DOI] [PubMed] [Google Scholar]
- 3.Nagasue N, Inokuchi K Spontaneous and traumatic rupture of hepatoma. Br J Surg. 1979;66:248–50. doi: 10.1002/(ISSN)1365-2168. [DOI] [PubMed] [Google Scholar]
- 4.Lai ECH, Lau WY Spontaneous rupture of hepatocellular carcinoma: a systematic review. Arch Surg. 2006;141:191–8. doi: 10.1001/archsurg.141.2.191. [DOI] [PubMed] [Google Scholar]
- 5.Liu CL, Fan ST, Lo CM, Tso WK, Poon RTP, Lam CM, et al Management of spontaneous rupture of hepatocellular carcinoma: single-center experience. J Clin Oncol. 2001;19:3725–32. doi: 10.1200/JCO.2001.19.17.3725. [DOI] [PubMed] [Google Scholar]
- 6.Cherqui D, Panis Y, Rotman N, Fagniez PL Emergency liver resection for spontaneous rupture of hepatocellular carcinoma complicating cirrhosis. Br J Surg. 1993;80:747–9. doi: 10.1002/(ISSN)1365-2168. [DOI] [PubMed] [Google Scholar]
- 7.Xu HS, Yan JB Conservative management of spontaneous ruptured hepatocellular carcinoma. Am Surg. 1994;60:629–33. [PubMed] [Google Scholar]
- 8.Chen CY, Lin XZ, Shin JS, Lin CY, Leow TC, Chen CY, et al Spontaneous rupture of hepatocellular carcinoma. A review of 141 taiwanese cases and comparison with nonrupture cases. J Clin Gastroenterol. 1995;21:238–42. doi: 10.1097/00004836-199510000-00015. [DOI] [PubMed] [Google Scholar]
- 9.Shimada R, Imamura H, Makuuchi M, Soeda J, Kobayashi A, Noike T, et al Staged hepatectomy after emergency transcatheter arterial embolization for ruptured hepatocellular carcinoma. Surgery. 1998;124:526–35. doi: 10.1016/S0039-6060(98)70099-6. [DOI] [PubMed] [Google Scholar]
- 10.Liu H, Peng YH, Fu Y, Li RF One-stage liver resection for spontaneous rupture of hepatocellular carcinoma. World J Surg. 2005;29:1316–8. doi: 10.1007/s00268-005-7626-2. [DOI] [PubMed] [Google Scholar]
- 11.Vergara V, Muratore A, Bouzari H, Polastri R, Ferrero A, Galatola G, et al Spontaneous rupture of hepatocelluar carcinoma: surgical resection and long-term survival. Eur J Surg Oncol. 2000;26:770–2. doi: 10.1053/ejso.2000.1001. [DOI] [PubMed] [Google Scholar]
- 12.Yang T, Sun YF, Zhang J, Lau WY, Lai ECH, Lu JH, et al Partial hepatectomy for ruptured hepatocellular carcinoma. Br J Surg. 2013;100:1071–9. doi: 10.1002/bjs.9167. [DOI] [PubMed] [Google Scholar]
- 13.Yeh CN, Lee WC, Jeng LB, Chen MF, Yu MC Spontaneous tumour rupture and prognosis in patients with hepatocellular carcinoma. Br J Surg. 2002;89:1125–9. doi: 10.1046/j.1365-2168.2002.02188.x. [DOI] [PubMed] [Google Scholar]
- 14.Ueno S, Tanabe G, Sako K, Hiwaki T, Hokotate H, Fukukura Y, et al Discrimination value of the new western prognostic system (clip score) for hepatocellular carcinoma in 662 Japanese patients. Hepatology. 2001;34:529–34. doi: 10.1053/jhep.2001.27219. [DOI] [PubMed] [Google Scholar]
- 15.Marrero JA, Fontana RJ, Barrat A, Askari F, Conjeevaram HS, Su GL, et al Prognosis of hepatocellular carcinoma: comparison of 7 staging systems in an American cohort. Hepatology. 2005;41:707–15. doi: 10.1002/(ISSN)1527-3350. [DOI] [PubMed] [Google Scholar]
- 16.Ong GB, Taw JL Spontaneous rupture of hepatocellular carcinoma. Br Med J. 1972;4:146–9. doi: 10.1136/bmj.4.5833.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kew MC, Dos Santos HA, Sherlock S Diagnosis of primary cancer of the liver. Br Med J. 1971;4:408–11. doi: 10.1136/bmj.4.5784.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alpert ME, Hutt MSR, Davidson CS Primary hepatoma in uganda: a prospective clinical and epidemiologic study of forty-six patients. Am J Med. 1969;46:794–802. doi: 10.1016/0002-9343(69)90030-8. [DOI] [PubMed] [Google Scholar]
- 19.Hermann RE, David TE Spontaneous rupture of the liver caused by hepatomas. Surgery. 1973;74:715–9. [PubMed] [Google Scholar]
- 20.Moris D, Chakedis J, Sun SH, Spolverato G, Tsilimigras DI, Ntanasis-Stathopoulos I, et al Management, outcomes, and prognostic factors of ruptured hepatocellular carcinoma: a systematic review. J Surg Oncol. 2018;117:341–53. doi: 10.1002/jso.v117.3. [DOI] [PubMed] [Google Scholar]
- 21.Amin MB, Edge SB, Greene FL, Byrd DR, Brookland RK, Washington MK, et al. AJCC cancer staging manual. 8th ed. New York: Springer; 2017.
- 22.Chun YS, Pawlik TM, Vauthey JN 8th edition of the ajcc cancer staging manual: pancreas and hepatobiliary cancers. Ann Surg Oncol. 2018;25:845–7. doi: 10.1245/s10434-017-6025-x. [DOI] [PubMed] [Google Scholar]
- 23.Subramaniam S, Kelley RK, Venook AP A review of hepatocellular carcinoma (HCC) staging systems. Chin Clin Oncol. 2013;2:33. doi: 10.3978/j.issn.2304-3865.2013.07.05. [DOI] [PubMed] [Google Scholar]
- 24.Aoki T, Kokudo N, Matsuyama Y, Izumi N, Ichida T, Kudo M, et al Prognostic impact of spontaneous tumor rupture in patients with hepatocellular carcinoma: an analysis of 1160 cases from a nationwide survey. Ann Surg. 2014;259:532–42. doi: 10.1097/SLA.0b013e31828846de. [DOI] [PubMed] [Google Scholar]
- 25.Llovet JM, Brú C, Bruix J Prognosis of hepatocellular carcinoma: the bclc staging classification. Semin Liver Dis. 1999;19:329–38. doi: 10.1055/s-2007-1007122. [DOI] [PubMed] [Google Scholar]
- 26.Llovet JM, Burroughs A, Bruix J Hepatocellular carcinoma. Lancet. 2003;362:1907–17. doi: 10.1016/S0140-6736(03)14964-1. [DOI] [PubMed] [Google Scholar]
- 27.Forner A, Llovet JM, Bruix J Hepatocellular carcinoma. Lancet. 2012;379:1245–55. doi: 10.1016/S0140-6736(11)61347-0. [DOI] [PubMed] [Google Scholar]
- 28.Forner A, Reig M, Bruix J Hepatocellular carcinoma. Lancet. 2018;391:1301–14. doi: 10.1016/S0140-6736(18)30010-2. [DOI] [PubMed] [Google Scholar]
- 29.Zhu Q, Li J, Yan JJ, Huang L, Wu MC, Yan YQ Predictors and clinical outcomes for spontaneous rupture of hepatocellular carcinoma. World J Gastroenterol. 2012;18:7302–7. doi: 10.3748/wjg.v18.i48.7302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Okuda K, Ohtsuki T, Obata H, Tomimatsu M, Okazaki N, Hasegawa H, et al Natural history of hepatocellular carcinoma and prognosis in relation to treatment study of 850 patients. Cancer. 1985;56:918–28. doi: 10.1002/(ISSN)1097-0142. [DOI] [PubMed] [Google Scholar]
- 31.Leung TWT, Tang AMY, Zee B, Lau WY, Lai PBS, Leung KL, et al Construction of the chinese university prognostic index for hepatocellular carcinoma and comparison with the tnm staging system, the okuda staging system, and the cancer of the liver italian program staging system. Cancer. 2002;94:1760–9. doi: 10.1002/(ISSN)1097-0142. [DOI] [PubMed] [Google Scholar]
- 32.The Cancer of the Liver Italian Program (CLIP) Investigators Prospective validation of the clip score: a new prognostic system for patients with cirrhosis and hepatocellular carcinoma. Hepatology. 2000;31:840–5. doi: 10.1053/he.2000.5628. [DOI] [PubMed] [Google Scholar]
- 33.Collier J, Sherman M Screening for hepatocellular carcinoma. Hepatology. 1998;27:273–8. doi: 10.1002/(ISSN)1527-3350. [DOI] [PubMed] [Google Scholar]
- 34.Daniele B, Bencivenga A, Megna AS, Tinessa V α-fetoprotein and ultrasonography screening for hepatocellular carcinoma. Gastroenterology. 2004;127:S108–12. doi: 10.1053/j.gastro.2004.09.023. [DOI] [PubMed] [Google Scholar]
- 35.Blank S, Wang Q, Fiel MI, Luan W, Kim KW, Kadri H, et al Assessing prognostic significance of preoperative alpha-fetoprotein in hepatitis b-associated hepatocellular carcinoma: normal is not the new normal. Ann Surg Oncol. 2014;21:986–94. doi: 10.1245/s10434-013-3357-z. [DOI] [PubMed] [Google Scholar]
- 36.Llovet JM, Peña CEA, Lathia CD, Shan M, Meinhardt G, Bruix J Plasma biomarkers as predictors of outcome in patients with advanced hepatocellular carcinoma. Clin Cancer Res. 2012;18:2290–300. doi: 10.1158/1078-0432.CCR-11-2175. [DOI] [PubMed] [Google Scholar]
- 37.Silva JP, Gorman RA, Berger NG, Tsai S, Christians KK, Clarke CN, et al The prognostic utility of baseline alpha-fetoprotein for hepatocellular carcinoma patients. J Surg Oncol. 2017;116:831–40. doi: 10.1002/jso.v116.7. [DOI] [PubMed] [Google Scholar]
- 38.Spangenberg HC, Thimme R, Blum HE Serum markers of hepatocellular carcinoma. Semin Liver Dis. 2006;26:385–90. doi: 10.1055/s-2006-951606. [DOI] [PubMed] [Google Scholar]
- 39.Bertino G, Ardiri A, Malaguarnera M, Malaguarnera G, Bertino N, Calvagno GS Hepatocellualar carcinoma serum markers. Semin Oncol. 2012;39:410–33. doi: 10.1053/j.seminoncol.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 40.Lu LG, Zeng MD, Wan MB, Li CZ, Mao YM, Li JQ, et al Grading and staging of hepatic fibrosis, and its relationship with noninvasive diagnostic parameters. World J Gastroenterol. 2003;9:2574–8. doi: 10.3748/wjg.v9.i11.2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lammers WJ, van Buuren HR, Hirschfield GM, Janssen HLA, Invernizzi P, Mason AL, et al Levels of alkaline phosphatase and bilirubin are surrogate end points of outcomes of patients with primary biliary cirrhosis: an international follow-up study. Gastroenterology. 2014:1338–1349.e5. doi: 10.1053/j.gastro.2014.08.029. [DOI] [PubMed] [Google Scholar]
- 42.LaRusso NF, Shneider BL, Black D, Gores GJ, James SP, Doo E, et al Primary sclerosing cholangitis: summary of a workshop. Hepatology. 2006;44:746–64. doi: 10.1002/(ISSN)1527-3350. [DOI] [PubMed] [Google Scholar]
- 43.Padda MS, Singh S, Tang SJ, Rockey DC Liver test patterns in patients with acute calculous cholecystitis and/or choledocholithiasis. Aliment Pharmacol Ther. 2009;29:1011–8. doi: 10.1111/apt.2009.29.issue-9. [DOI] [PubMed] [Google Scholar]
- 44.Yeh CN, Chen MF, Lee WC, Jeng LB Prognostic factors of hepatic resection for hepatocellular carcinoma with cirrhosis: univariate and multivariate analysis. J Surg Oncol. 2002;81:195–202. doi: 10.1002/(ISSN)1096-9098. [DOI] [PubMed] [Google Scholar]
- 45.Bhudhisawasdi V, Muisuk K, Areejitranusorn P, Kularbkaew C, Khampitak T, Saeseow OT, et al Clinical value of biliary alkaline phosphatase in non-jaundiced cholangiocarcinoma. J Cancer Res Clin Oncol. 2004;130:87–92. doi: 10.1007/s00432-003-0515-x. [DOI] [PubMed] [Google Scholar]
- 46.Jiang W, Zeng ZC, Tang ZY, Fan J, Sun HC, Zhou J, et al A prognostic scoring system based on clinical features of intrahepatic cholangiocarcinoma: the fudan score. Ann Oncol. 2011;22:1644–52. doi: 10.1093/annonc/mdq650. [DOI] [PubMed] [Google Scholar]
- 47.Yamamoto K, Awogi T, Okuyama K, Takahashi N Nuclear localization of alkaline phosphatase in cultured human cancer cells. Med Electron Microsc. 2003;36:47–51. doi: 10.1007/s007950300006. [DOI] [PubMed] [Google Scholar]
- 48.Miyamoto M, Sudo T, Kuyama T Spontaneous rupture of hepatocellular carcinoma: a review of 172 japanese cases. Am J Gastroenterol. 1991;86:67–71. [PubMed] [Google Scholar]