Abstract
Objective:
Epidermal growth factor receptor (EGFR) activation was reported to upregulate programmed death-ligand 1 (PD-L1) expression in lung cancer cells and subsequently contribute to immune escape, indicating its critical role in EGFR-driven lung tumors. This study characterized PD-L1 expression in patients with surgically resected EGFR-mutant non-small cell lung cancer (NSCLC). The effect of PD-L1 expression on clinical outcomes was also investigated in advanced EGFR-mutant NSCLC treated with EGFR-tyrosine kinase inhibitors (TKIs).
Methods:
In total, 73 patients with surgically resected NSCLC and EGFR mutations were identified. PD-L1 expression and CD8+ tumor-infiltrating lymphocyte (TIL) density were assessed by immunohistochemistry. A literature review of publications that assessed the predictive and prognostic value of PD-L1 expression in advanced EGFR-mutant NSCLC patients treated with EGFR-TKIs was performed.
Results:
Nineteen (26.0%) patients were positive for PD-L1 expression, which was significantly associated with concomitant KRAS mutation (P = 0.020) and marginally associated with higher CD8+ TILs density (P = 0.056). Positive PD-L1 expression was associated with markedly inferior overall survival (OS) in multivariate analysis (P = 0.032). The combination of PD-L1 and CD8+ TILs expression could be used to stratify the population into three groups with distinct prognoses. A meta-analysis of six publications showed that positive PD-L1 expression was not associated with OS [hazard ratio (HR) = 0.90; 95% confidence interval (CI), 0.42–1.38] or progression-free survival (HR = 1.03; 95 CI, 0.73–1.33) in advanced EGFR-mutant NSCLC patients receiving EGFR-TKIs.
Conclusions:
PD-L1 expression tended to correlate with CD8+ TIL expression, concomitant KRAS mutation, and poor survival in surgically resected EGFR-mutant NSCLC. PD-L1 expression was neither the predictive nor the prognostic factor in advanced EGFR-mutant NSCLC patients treated with EGFR-TKIs.
Keywords: Non-small cell lung cancer, EGFR mutation, PD-L1, CD8, survival
Introduction
Targeted therapy on epidermal growth factor receptor (EGFR)-activating mutations has revolutionized the treatment landscape of patients with advanced EGFR-mutant non-small cell lung cancer (NSCLC)1. Several large-scale phase 3 trials have demonstrated the superior efficacy of first- and second-generation EGFR tyrosine kinase inhibitors (TKIs) to standard platinum-based chemotherapy in the first-line setting2-4. However, almost all responders acquired drug resistance after 9–12 months (acquired resistance), and 30% of the initial cohorts showed no response to EGFR-TKIs (primary resistance)1,5-7. A series of resistance mechanisms have been associated with primary and/or acquired resistance, including Bcl-2 family member (BIM) deletion polymorphism, second site mutations, downstream or bypass signaling pathway activation, and histological transformation; however, 25%–30% of acquired resistance and half of primary resistance mechanisms remain undetermined1,7. Therefore, drug resistance is the major challenge for the application of EGFR-TKI in EGFR-mutant NSCLC. There is an urgent need to identify more effective biomarkers to better predict the efficacy of EGFR-TKIs.
Immune checkpoints are orchestrated by a set of co-stimulatory and co-inhibitory molecules to regulate the activation and effector functions of tumor-infiltrating lymphocytes (TILs)8. Blockade of inhibitory immune checkpoints by antibodies may release the immune inhibition of effector T cells, especially CD8+ T cells9. To date, therapeutic antibodies targeting programmed cell death 1 (PD-1) and its ligand (PD-L1) have been associated with remarkable response rates in various cancers and are another significant breakthrough in the treatment of patients with advanced NSCLC. Three PD-1/PD-L1 antibodies, including nivolumab, pembrolizumab, and atezolizumab, have been approved for second-line or subsequent treatment of advanced NSCLC for their promising anti-tumor effect compared to that of standard chemotherapy10-13. Furthermore, pembrolizumab was approved in the first-line setting for patients with PD-L1 expression ≥ 50%14,15. However, in clinical practice, Gainor et al.16 reported a limited effect of PD-1/PD-L1 inhibitors in patients with EGFR-mutant NSCLC. A recent phase 2 clinical study reported a 12.2% objective response rate (ORR) to an anti-PD-L1 antibody among EGFR-mutant NSCLC patients, with at least 25% of tumor cells expressing PD-L1, compared to an ORR of 3.6% in those with PD-L1 expression < 25% 17, suggesting the clinical value of PD-L1 expression in EGFR-mutant NSCLC patients treated with anti-PD-L1 antibody. However, there are limited data to characterize the PD-L1 expression in EGFR-mutant NSCLC patients. A recent preclinical study demonstrated that the expression of mutant EGFR in bronchial epithelial cells could significantly induce PD-L1 expression and that EGFR-TKIs could reduce PD-L1 expression in NSCLC cell lines with activated EGFR18, indicating a potential relationship between PD-L1 expression and EGFR mutations in NSCLC. Several groups, therefore, investigated the predictive and prognostic value of PD-L1 expression on EGFR-mutant NSCLC patients treated with EGFR-TKIs. However, their findings were paradoxical. Lin et al.19 reported that PD-L1 expression was associated with a better response to EGFR-TKI and longer survival, while Tang et al.20 suggested that PD-L1 expression was neither the predictive nor the prognostic factor in EGFR-mutant NSCLC patients treated with EGFR-TKIs.
Therefore, the current study aimed to characterize PD-L1 expression in patients with surgically resected EGFR-mutant NSCLC and to investigate the effect of PD-L1 expression on clinical outcomes in patients with advanced EGFR-mutant NSCLC treated with EGFR-TKIs. Because the antitumor effect of anti- PD-1/PD-L1 antibodies is mainly dependent on the proliferation and activation of CD8+ TILs8,9, we further evaluated the prognostic value of the combination of PD-L1 expression and CD8+ TIL density in surgically resected NSCLC with EGFR mutations.
Patients and methods
Patient cohort
We retrospectively identified patients who underwent surgical resection of the lung (lobectomy or pulmonectomy) due to the histologically-confirmed NSCLC, between 2011 and 2015, at Shanghai Pulmonary Hospital and our cooperative medical centers. The major patient baseline characteristics including age, sex, smoking history, histology [World Health Organization (WHO) classification]21, and stage of NSCLC at initial diagnosis were collected. Never smoker was defined as a person who has smoked fewer than 100 cigarettes during their lifetime. This study was conducted in accordance with the provisions of the Declaration of Helsinki and was approved by the ethics committee of Shanghai Pulmonary Hospital. The exclusion criteria included histologically-confirmed SCLC, stage IIIB-IV before surgery, and chemotherapy and/or radiotherapy administered before surgery. Patients who could not provide adequate samples for EGFR detection and PD-L1/CD8 staining or who disagreed with the study protocols were ineligible.
PD-L1 expression analysis
PD-L1 expression was evaluated by immunohistochemistry (IHC) as described previously22. In brief, tumor sections of formalin-fixed and paraffin-embedded specimens were treated with 3% H2O2 for 10 min, blocked with 5% goat serum, and incubated with a primary anti-human PD-L1 antibody (#13684, clone E1L3N, Cell Signaling Technology, Danvers, MA, diluted 1:200). Next, a peroxidase-labeled secondary antibody (REAL EnVision Detection Reagent Peroxidase Rabbit/Mouse, DAKO, Denmark) was applied to the sections for 30 min at room temperature. PD-L1 positive/negative expression was defined using a cut-off value of 5%23-26.
CD8+ TIL analysis
A mouse anti-CD8 monoclonal antibody (M7103, clone C8144B, DAKO, Denmark) was utilized for CD8+ TIL density assessment as described previously27,28. TILs were enumerated on immunostained CD8 preparations and scored using a four-tier scale (details in supplemental file). The cut-off point for high/low CD8+ TIL expression was 5%.
EGFR and KRAS mutation detection
EGFR and KRAS mutation analyses were conducted at Shanghai Pulmonary Hospital & Thoracic Cancer Institute, Tongji University School of Medicine (Shanghai, China). The samples were tested using an Amplification Refractory Mutation System (ARMS) as previously described (Amoy Diagnostics Co., Ltd., Xiamen, China) 22,29,30.
Meta-analysis
We performed a publication search of the PubMed/Medline, EMBASE, Google Scholar, Cochrane Library, and Web of Science databases through December 31, 2017, using “lung cancer” and “PD-L1” and “EGFR-TKI” and their related words. Data on the relationship between PD-L1 expression and overall survival (OS), progression-free survival (PFS), and clinicopathological characteristics in EGFR-mutant NSCLC patients treated with EGFR-TKIs were collected from publications meeting the eligibility criteria (Supplementary Table S1). The details of our methodology are described in the supplemental file.
S1.
Search strategies included: PubMed and EMBASE (date was from the inception through June 2017)
| 1) PubMed search strategy |
| 1. "Lung neoplasms" [Mesh] |
| 2. Lung cancer [Title/Abstract] |
| 3. Lung tumor [Title/Abstract] |
| 4. Lung tumour [Title/Abstract] |
| 5. Lung carcinoma* [Title/Abstract] |
| 6. Lung neoplas* [Title/Abstract] |
| 7. Lung malignan* [Title/Abstract] |
| 8. "B7-H1 antigen" [Mesh] |
| 9. "CD274 protein" [Mesh] |
| 10. PD-L1 [Title/Abstract] |
| 11. PDL1 [Title/Abstract] |
| 12. B7-H1 [Title/Abstract] |
| 13. CD274 [Title/Abstract] |
| 14. "EGFR gene" [Mesh] |
| 15. "erbB1 gene" [Mesh] |
| 16. EGFR [Title/Abstract] |
| 17. TKI [Title/Abstract] |
| 18. EGFR Tyrosine kinase inhibitor [title/Abstract] |
| 19. (1 OR 2 OR 3 OR 4 OR 5 OR 6 OR 7) AND (8 OR 9 OR 10 OR 11 OR 12 OR 13) AND (14 OR 15 OR 16 OR 17 OR 18) |
| 2) EMBASE search strategy |
| 1. 'lung neoplasm'/exp |
| 2. lung cancer:ab,ti |
| 3. lung tumor:ab,ti |
| 4. lung tumour:ab,ti |
| 5. lung carcinoma:ab,ti |
| 6. lung neoplas*:ab,ti |
| 7. lung malignan*:ab,ti |
| 8. lung adenoma*:ab,ti |
| 9. 'B7-H1 antigen'/exp |
| 10. B7-H1 |
| 11. 'CD274 protein'/exp |
| 12. CD274 |
| 13. 'PD-L1 expression'/exp |
| 14. PDL1 expression:ab,ti |
| 15. 'EGFR'/exp |
| 16. 'TKI'/exp |
| 17. 'EGFR-TKI'/exp |
| 18. EGFR:ab,ti |
| 19. TKI:ab,ti |
| 20. EGFR tyrosine kinase inhibitor:ab,ti |
| 21. (1 OR 2 OR 3 OR 4 OR 5 OR 6 OR 7 OR 8) AND (((9 OR 10) OR (11 OR 12)) OR 13 OR 14) AND (15 OR 16 17 OR 18 OR 19 OR 20) |
Statistical analysis
Categorical variables were analyzed using the Chi-square or Fisher’s exact tests. Continuous variables were analyzed by analysis of variance and Tukey’s multiple comparison tests. Kaplan-Meier curves were used to assess patient survival, and log-rank tests were used to evaluate the significance of differences between two or four groups. Cox proportional hazards models were used for uni- and multivariate survival analyses to calculate the hazard ratios (HRs) and corresponding 95% confidence intervals (CIs). The OS was calculated from the date of lung cancer diagnosis to death from any cause or was censored at the last follow-up date. P-values less than 0.05 (two-sided) were considered to be significant. Meta-analysis was conducted using STATA version 12.0 (Stata Corporation, TX). All statistical analyses were performed using IBM SPSS Statistics for Windows, version 22.0 (IBM Corp., Armonk, NY, USA).
Results
Characterization of PD-L1 expression in EGFR-mutant NSCLC
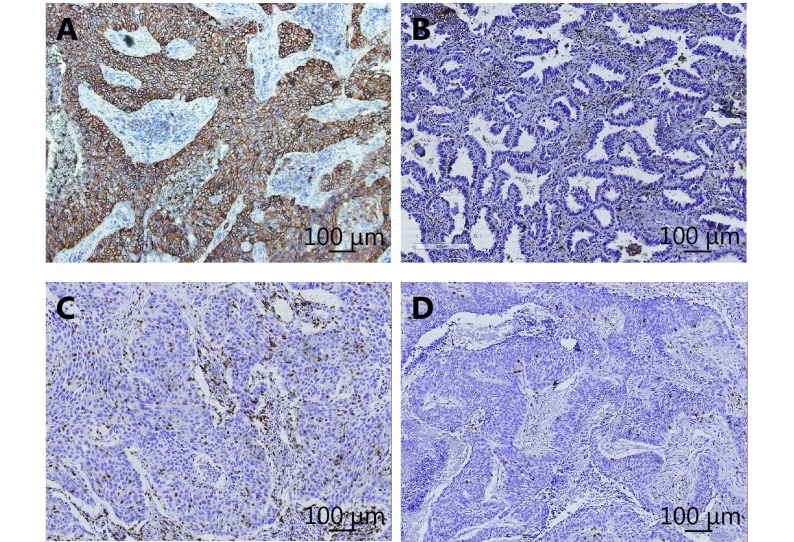
In total, 201 patients with surgically resected NSCLC were initially enrolled. After EGFR detection, 73 patients with surgically resected EGFR-mutant NSCLC were finally identified. Most (63.0%) were < 65 years old at the time of diagnosis. Forty-two (57.5%) of the patients were female and 44 (60.3%) were never-smokers. The most predominant histological type was adenocarcinoma (71 patients). Three patients (4.1%) had concomitant KRAS mutations. Nineteen patients (26.0%) had positive PD-L1 expression and 22 (30.1%) had high CD8+ TILs density. Representative images of IHC for PD-L1 expression and CD8+ TILs are shown in Supplementary Figure S1. The clinical features of the 73 patients are summarized in Table 1.
S1.
Positive (A) and negative staining (B) pattern for programmed cell death ligand-1 (PD-L1); positive (C) and negative staining (D) pattern for CD8+ tumor infiltrating lymphocytes (TILs).
1.
Baseline characteristics of the study population
| Variables | All patients | PD-L1 positive | PD-L1 negative | P |
| pTNM, pathological TNM; TILs, tumor infiltrating lymphocytes. | ||||
| Total | 73 | 19 | 54 | |
| Age at diagnosis, years | 0.570 | |||
| < 65 | 46 | 13 | 33 | |
| ≥ 65 | 27 | 6 | 21 | |
| Gender | 0.297 | |||
| Male | 31 | 10 | 21 | |
| Female | 42 | 9 | 33 | |
| Smoking history | 0.429 | |||
| Never-smoker | 44 | 10 | 34 | |
| Former/current smoker | 29 | 9 | 20 | |
| Pathological classification | 0.973 | |||
| Adenocarcinoma | 71 | 18 | 53 | |
| Non-adenocarcinoma | 2 | 1 | 1 | |
| pTNM stage | 0.591 | |||
| pI-IIIA | 69 | 17 | 52 | |
| pIIIB-IV | 4 | 2 | 2 | |
| Lymph node metastasis | 0.661 | |||
| Yes | 30 | 7 | 23 | |
| No | 43 | 12 | 31 | |
| KRAS mutation | 0.020 | |||
| KRAS mutation | 3 | 3 | 0 | |
| KRAS wild type | 70 | 16 | 54 | |
| CD8+ TILs expression | 0.056 | |||
| Yes | 22 | 9 | 13 | |
| No | 51 | 10 | 41 | |
There was a significant correlation between PD-L1 expression and concomitant KRAS mutations (P = 0.020). Patients with high CD8+ TIL density were more likely to have positive PD-L1 expression, but the relationship did not reach statistical significance (P = 0.056). There were no significant differences in PD-L1 expression in terms of age (P = 0.570), gender (P = 0.297), smoking status (P = 0.429), pathologic types (adenocarcinoma vs. non-adenocarcinoma) (P = 0.973), pathologic stages (I/IIIA vs. IIIB/IV) (P = 0.591), and lymph node metastasis (P = 0.661).
Prognostic value of PD-L1 expression in surgically resected NSCLC with EGFR mutation
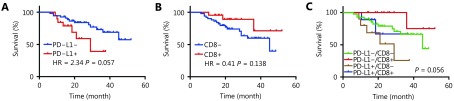
The median follow-up time was 25.7 months (range, 5.6–54.9 months). Univariate analysis revealed that patients with positive PD-L1 expression showed a marginally shorter OS than that in patients with negative expression (HR = 2.339, 95% CI: 0.992–9.835; P = 0.057) (Table 2 and Figure 1A). High CD8+ TIL density tended to be associated with longer OS but it did not reach the statistical significance (HR = 0.409, 95% CI: 0.185–1.246; P = 0.138) (Table 2 and Figure 1B). Other factors including gender, age, smoking status, lymph node metastasis, and EGFR mutant types were not associated with OS (Table 2). Multivariate analyses suggested that positive PD-L1 expression was significantly associated with a shorter OS (HR = 2.995, 95% CI: 1.097–8.178; P = 0.032) (Table 2). While patients with high CD8+ TIL expression showed a longer OS than that in those with low CD8+ TIL expression, the difference was not statistically significant (HR = 0.296, 95% CI: 0.080–1.103; P = 0.070) (Table 2).
2.
Univariate and multivariate analyses of clinical parameters on OS in patients with EGFR muttaions.
| Factor | Univariate analysis | Multivariate analysis | |||||
| HR (log rank) | 95% CI | P | HR (log rank) | 95% CI | P | ||
| HR: hazard ratio; CI: confidence interval; *: Sensitizing mutations include EGFR exon 19 deletion and L858R; rare mutations include EGFR mutations except exon 19 deletion and L858R. | |||||||
| Gender (male/female) | 0.990 | 0.404–2.413 | 0.982 | 1.904 | 0.541–6.695 | 0.316 | |
| Age, years (≥ 65/<65) | 1.221 | 0.494–3.096 | 0.656 | 1.098 | 0.431–2.795 | 0.845 | |
| Smoking (smoking/never) | 1.339 | 0.523–3.425 | 0.544 | 1.988 | 0.557–7.095 | 0.290 | |
| Lymph node metastases (yes/no) | 1.591 | 0.679–4.113 | 0.279 | 1.544 | 0.587–4.060 | 0.579 | |
| PD-L1 expression (yes/no) | 2.339 | 0.992–9.385 | 0.057 | 2.995 | 1.097–8.178 | 0.032 | |
| CD8 expression (yes/no) | 0.409 | 0.185–1.246 | 0.138 | 0.296 | 0.080–1.103 | 0.070 | |
| EGFR (rare/sensitizing)* | 1.279 | 0.343–5.025 | 0.693 | 1.451 | 0.389–5.408 | 0.579 | |
| KRAS (mutation/wild type) | 1.089 | 0.135–8.839 | 0.934 | 1.357 | 0.132–13.978 | 0.798 | |
1.
Prognostic value of PD-L1 expression (A) CD8 TILs expression (B) and combination of PD-L1 and CD8 TILS expression (C) in surgically resected NSCLC with EGFR mutation.
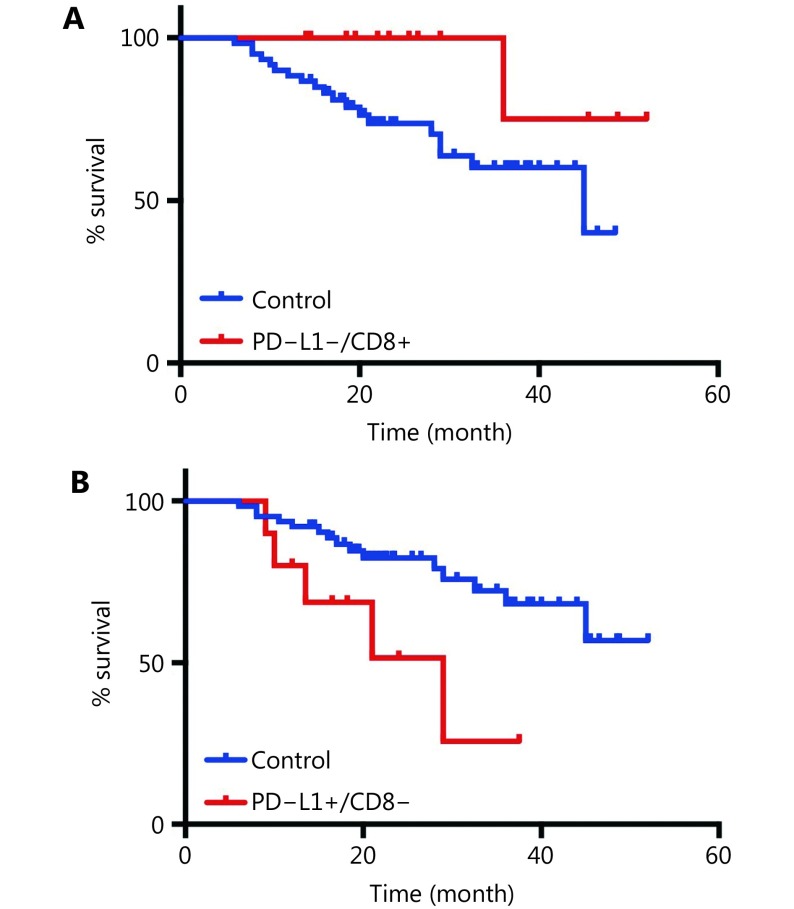
We further assigned the population into four groups according to PD-L1 and CD8+ TIL expression. The median OS was not reached in the PD-L1+/CD8-high and PD-L1-/CD8-high expression groups but was 29.0 months in the PD-L1+/CD8-low group and 45.0 months in the PD-L1-/CD8-low group (P = 0.056, Figure 1C). Patients with PD-L1-/high CD8+ TIL density expression had the longest OS (HR = 0.196, P = 0.072), while the PD-L1+/CD8-low group had the shortest OS (HR = 3.087, P = 0.020) (Supplementary Figure S2).
S2.
Patients with PD-L1-/CD8+ TILs expression had the longest OS (A) and PD-L1+/CD8- low group had the shortest OS (B).
Features of the studies included in the meta-analysis
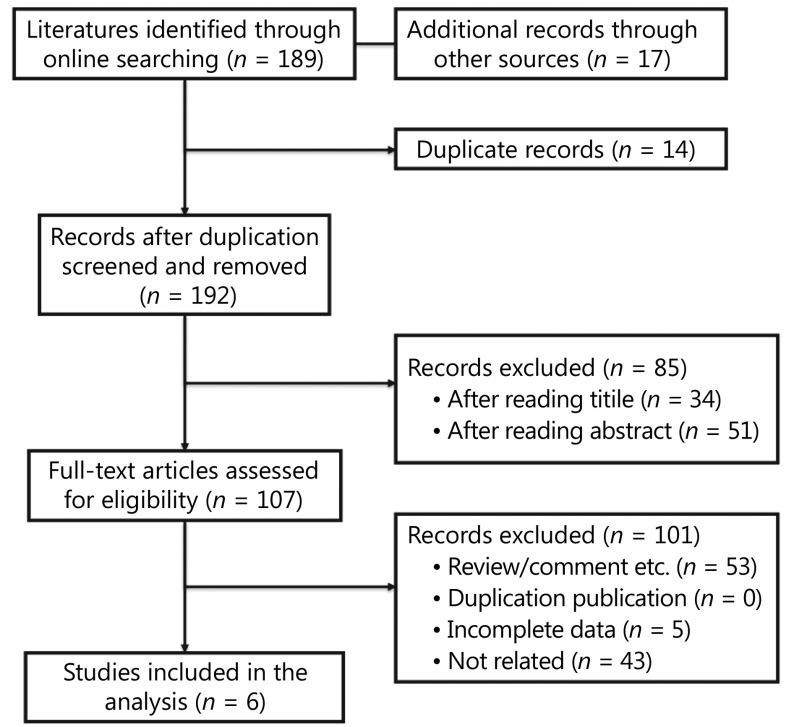
A total of 192 potentially relevant studies were screened. Most of the excluded publications were reviews, comments, duplications, or studies with incomplete data. The current study assessed 648 cases from six publications19,20,31-34 to investigate both the predictive and prognostic value of PD-L1 expression in advanced EGFR-mutant NSCLC patients treated with EGFR-TKIs. The main features of the eligible studies are shown in Table 3 and Supplementary Table S2. In addition, prognostic and predictive data on OS and PFS were obtained from the included studies. Figure 2 depicts a flowchart of publication inclusion.
3.
Baseline characteristics of included studies
| Author | Year | Treatment record | No. of
cases |
No. < 65 years | No. of
female |
No. of
ECOG = 0–1 |
No. of
never smoker |
No. of
adenocarcinoma |
Outcome assessment |
| NA: not available; OS: overall survival; PFS: progression-free survival; ORR, overall response rate; DCR, disease control rate. | |||||||||
| Lin et al. | 2015 | Yes | 56 | 40 | 35 | 39 | 38 | 56 | OS, PFS |
| Tang et al. | 2015 | Yes | 170 | 85 | 77 | NA | 113 | 145 | OS, PFS |
| Meniawy et al. | 2016 | Yes | 33 | 12 | 17 | 32 | NA | 23 | OS, PFS |
| Sorensen et al. | 2016 | Yes | 38 | 13 | 23 | 31 | 9 | NA | OS, PFS |
| Cho et al. | 2017 | Yes | 319 | NA | 194 | 318 | 114 | 319 | OS, PFS |
| Kobayashi et al. | 2018 | Yes | 32 | 13 | 20 | NA | 27 | NA | OS, PFS |
S2.
Methodological characteristics of included studies and quality score
| No. | Authors | Year | Representativ-eness of population | Non exposed cohort | Ascertainment of exposure | Outcome not present at start of study | Appropriate confounding measurement and account | Sufficient measurement of outcomes | Complete-ness of follow-up |
| 1 | Lin et al. | 2015 | 0 | 1 | 1 | 1 | 2 | 2 | 2 |
| 2 | Tang et al. | 2015 | 0 | 1 | 1 | 1 | 2 | 1 | 2 |
| 3 | Meniawy et al. | 2016 | 0 | 1 | 1 | 1 | 1 | 1 | 2 |
| 4 | Sorensen et al. | 2016 | 0 | 1 | 1 | 1 | 1 | 1 | 2 |
| 5 | Cho et al. | 2017 | 0 | 1 | 1 | 1 | 2 | 2 | 2 |
| 6 | Kobayashi et al. | 2018 | 0 | 1 | 1 | 1 | 2 | 1 | 2 |
2.
The flowchart of publication selection.
Predictive and prognostic value of high PD-L1 expression in EGFR-mutant NSCLC treated with EGFR-TKIs
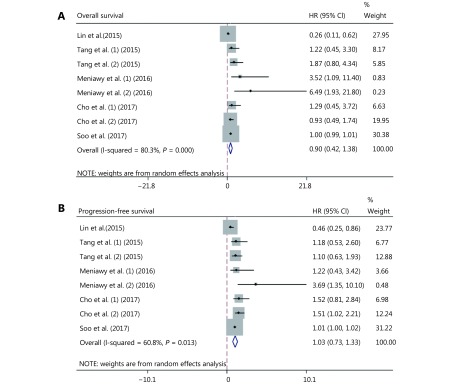
A meta-analysis was performed to evaluate the overall effect of positive PD-L1 expression in the studies containing predictive and prognostic data. The pooled results showed that positive PD-L1 expression was not correlated with OS (HR = 0.90, 95% CI: 0.42–1.38; P > 0.05; Figure 3A). Similarly, the pooled results indicated that positive PD-L1 expression was not associated with PFS (HR = 1.03, 95% CI: 0.73–1.33; P > 0.05; Figure 3B). Both results showed high heterogeneity (I2 = 80.3%, P < 0.001; I2 = 60.8%; P = 0.013; respectively).
3.
Meta-analysis to evaluate the predictive (A) and prognostic (B) value of high PD-L1 expression in EGFR-mutant NSCLC treated with EGFR-TKIs.
Publication bias
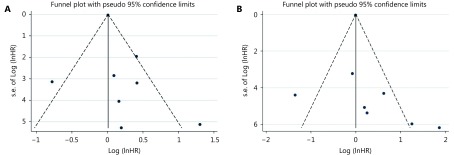
Sensitivity analysis was conducted by deleting one study at one time to assess its effect on the pooled HRs. Deletion of the study by Meniawy et al.31 slightly decreased the heterogeneity in the analysis of pooled HRs of PFS and OS. No other studies influenced the pooled results. Begg’s funnel plots and Egger’s tests were used to evaluate the publication bias. The Begg’s funnel plot was symmetric, and Egger’s tests suggested no evidence of publication bias (Supplementary Figure S3).
S3.
Funnel plots for progression-free survival (A) and overall survival (B) from included studies.
Discussion
Emerging evidence suggested that blocking PD-1/PD-L1 showed a limited efficacy in patients with EGFR-mutant NSCLC. A preclinical study reported that EGFR activation could regulate PD-L1 expression in lung cancer, indicating a sophisticated interaction between EGFR mutations and PD-L1 expression. Improved understanding of PD-L1 expression in EGFR-mutant NSCLC would contribute to the development of more effective immunotherapy. Additionally, clarifying the predictive and prognostic value of PD-L1 expression in EGFR-mutant NSCLC patients treated with EGFR-TKIs would be helpful to precisely determine the populations who could most benefit from EGFR-TKIs therapy. To achieve these goals, the current study systematically investigated the clinicopathological features of PD-L1 expression in surgically resected EGFR-mutant NSCLC and the effect of this expression on clinical outcome patients with advanced EGFR-mutant NSCLC treated with EGFR-TKIs. Our results showed that positive PD-L1 expression was significantly correlated with concomitant KRAS mutations and seemed to be associated with high CD8+ TILs density without statistical significance, mainly due to the small sample size. Positive PD-L1 expression was associated with a significantly shorter OS, and the combination of PD-L1 and CD8+ expression could further stratify the population into three groups with distinct prognoses. Furthermore, a meta-analysis of six publications indicated that positive PD-L1 expression was neither a predictive nor a prognostic factor in advanced EGFR-mutant NSCLC patients treated with EGFR-TKIs.
Several studies have reported a positive association between EGFR mutations and positive PD-L1 expression in completely resected NSCLC20,35-37. However, few studies have characterized PD-L1 expression in patients with EGFR-mutant NSCLC. In our study, we observed that positive PD-L1 expression was significantly correlated with concomitant KRAS mutations in patients with EGFR-mutant NSCLC. PD-L1 is frequently highly expressed in NSCLC with driver mutations, which subsequently activates key oncogenic pathways such as PI3K-AKT and RAS-MAPK. Hence, it is anticipated that more cases with high PD-L1 expression had concomitant EGFR and KRAS mutations. Notably, only three patients in the present study had concomitant EGFR and KRAS mutations. We should interpret this result with caution. In addition, positive PD-L1 expression was marginally significantly associated with high CD8+ TIL density in this population, suggesting high PD-L1 expression of CD8+ TILs in EGFR-mutant NSCLC. Further investigation of the potential role of PD-1/PD-L1 pathway in inhibiting CD8+ TIL function in EGFR-mutant NSCLC is required.
We further evaluated the prognostic value of PD-L1 expression in surgically resected NSCLC with EGFR mutations. As expected, positive PD-L1 expression was significantly associated with inferior OS and CD8+ TIL density was marginally correlated with a longer OS. When we combined PD-L1 expression and CD8+ TIL density, the total populations could be divided into three groups with distinct prognoses. Similarly, a large number of studies consistently reported positive PD-L1 expression to be an independent negative prognostic factor in NSCLC. In 2014, Azuma et al.35 assessed the prognostic value of PD-L1 expression in 164 surgically resected NSCLC, reporting high PD-L1 expression to be significantly associated with a shorter OS independently of other factors. Two recent meta-analyses also demonstrated the prognostic value of PD-L1 expression in NSCLC. However, another meta-analysis reported no statistically significant difference between PD-L1 expression and prognosis in patients with NSCLC38,39. Of note, the driver gene mutations of the populations in these studies were unknown. Thus, high-quality studies with larger sample sizes are needed to determine the prognostic value of PD-L1 expression in NSCLC with specific driver mutations. Our finding of the prognostic value of PD-L1 expression combined with CD8+ TIL density was consistent with those of other studies28. For example, Tokito et al.28 observed that a combination of a lack of PD-L1 expression and CD8+ TIL density was significantly associated with favorable survival in patients with locally advanced NSCLC receiving concurrent chemoradiotherapy, indicating that PD-L1 expression in combination with CD8+ TIL density was a useful predictive biomarker in patients with stage III NSCLC. These results suggested that the combination of PD-L1 expression and CD8+ TIL density may be useful to predict the prognosis and efficacy of immunotherapy in NSCLC.
To clarify the predictive and prognostic value of PD-L1 expression in EGFR-mutant NSCLC patients treated with EGFR-TKIs, we conducted a meta-analysis of six eligible publications. Although preclinical studies reported that EGFR activation could induce PD-L1 expression to facilitate immune escape and that EGFR-TKI could significantly down-regulate PD-L1 expression in EGFR-mutant NSCLC cells18,35, the pooled results indicated that positive PD-L1 expression was not correlated with OS or PFS in this population. Whether PD-L1 expression is a reliable biomarker for EGFR-TKI treatment in advanced EGFR-mutant NSCLC patients requires further investigation.
The current study had several limitations. First, the number of publications included in the meta-analysis was relatively small and all were retrospective studies. Second, publication bias is inevitable in this area of research. We identified several abstracts published at meetings without further detailed publications and excluded these abstracts from the meta-analysis. Third, the quality of data was heterogeneous due to a multitude of confounding factors (laboratory conditions, PD-L1 antibody, cutoff values of PD-L1 expression, etc.) that made direct comparisons difficult.
In conclusion, the present study showed that PD-L1 expression had a particular clinicopathological feature and was correlated with a shorter OS in patients with EGFR-mutant NSCLC. The combination of PD-L1 expression and CD8+ TIL density more precisely differentiated sub-populations with distinct prognoses after surgery. Positive PD-L1 expression was neither the predictive nor the prognostic factor in EGFR-mutant NSCLC patients treated with EGFR-TKIs. These results suggested that a meaningful graded prognostic assessment for patients with surgical EGFR-mutant NSCLC should incorporate PD-L1 and CD8+ TILs and that PD-L1 expression should not be evaluated as a biomarker of EGFR-TKIs.
Acknowledgements
This study was supported in part by grants from the National Natural Science Foundation of China (Grant No. 81672286, 81772467 and 81874036), “Shuguang Program” supported by Shanghai Education Development Foundation and Shanghai Municipal Education Commission (Grant No. 16SG18), the Chronic Diseases Program of Shanghai Shen Kang Pharmaceutical Development Co. Ltd (Grant No. SHDC 12015314).
Conflict of interest statement
No potential conflicts of interest are disclosed.
Contributor Information
Jun Qian, Email: fishman1977@163.com.
Tao Jiang, Email: tonyjiangdr@163.com.
References
- 1.Rotow J, Bivona TG Understanding and targeting resistance mechanisms in NSCLC. Nat Rev Cancer. 2017;17:637–58. doi: 10.1038/nrc.2017.84. [DOI] [PubMed] [Google Scholar]
- 2.Zhou CC, Wu YL, Chen GY, Feng JF, Liu XQ, Wang CL, et al Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study . Lancet Oncol. 2011;12:735–42. doi: 10.1016/S1470-2045(11)70184-X. [DOI] [PubMed] [Google Scholar]
- 3.Rosell R, Carcereny E, Gervais R, Vergnenegre A, Massuti B, Felip E, et al Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13:239–46. doi: 10.1016/S1470-2045(11)70393-X. [DOI] [PubMed] [Google Scholar]
- 4.Wu YL, Zhou CC, Hu CP, Feng JF, Lu S, Huang YC, et al Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations (LUX-Lung 6): an open-label, randomised phase 3 trial. Lancet Oncol. 2014;15:213–22. doi: 10.1016/S1470-2045(13)70604-1. [DOI] [PubMed] [Google Scholar]
- 5.Tan CS, Gilligan D, Pacey S Treatment approaches for EGFR-inhibitor-resistant patients with non-small-cell lung cancer. Lancet Oncol. 2015;16:e447–59. doi: 10.1016/S1470-2045(15)00246-6. [DOI] [PubMed] [Google Scholar]
- 6.Hirsch FR, Suda K, Wiens J, Bunn Jr PA New and emerging targeted treatments in advanced non-small-cell lung cancer. Lancet. 2016;388:1012–24. doi: 10.1016/S0140-6736(16)31473-8. [DOI] [PubMed] [Google Scholar]
- 7.Westover D, Zugazagoitia J, Cho BC, Lovly CM, Paz-Ares L Mechanisms of acquired resistance to first- and second-generation EGFR tyrosine kinase inhibitors. Ann Oncol. 2018;29:i10–9. doi: 10.1093/annonc/mdx703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boussiotis VA Molecular and biochemical aspects of the PD-1 checkpoint pathway. N Engl J Med. 2016;375:1767–78. doi: 10.1056/NEJMra1514296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Teng MWL, Ngiow SF, Ribas A, Smyth MJ Classifying cancers based on T-cell infiltration and PD-L1. Cancer Res. 2015;75:2139–45. doi: 10.1158/0008-5472.CAN-15-0255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Herbst RS, Baas P, Kim DW, Felip E, Pérez-Gracia JL, Han JY, et al Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387:1540–50. doi: 10.1016/S0140-6736(15)01281-7. [DOI] [PubMed] [Google Scholar]
- 11.Rittmeyer A, Barlesi F, Waterkamp D, Park K, Ciardiello F, von Pawel J, et al Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet. 2017;389:255–65. doi: 10.1016/S0140-6736(16)32517-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brahmer J, Reckamp KL, Baas P, Crinò L, Eberhardt WEE, Poddubskaya MDE, et al Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373:123–35. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready N E, et al Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373:1627–39. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reck M, Rodríguez-Abreu D, Robinson AG, Hui RN, Csőszi T, Fülöp A, et al Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016;375:1823–33. doi: 10.1056/NEJMoa1606774. [DOI] [PubMed] [Google Scholar]
- 15.Attili I, Passaro A, Pavan A, Conte P, De Marinis F, Bonanno L Combination immunotherapy strategies in advanced non-small cell lung cancer (NSCLC): does biological rationale meet clinical needs? Crit Rev Oncol Hematol. 2017;119:30–9. doi: 10.1016/j.critrevonc.2017.09.007. [DOI] [PubMed] [Google Scholar]
- 16.Gainor JF, Shaw AT, Sequist LV, Fu XJ, Azzoli CG, Piotrowska Z, et al EGFR mutations and ALK rearrangements are associated with low response rates to PD-1 pathway blockade in non-small cell lung cancer: a retrospective analysis . Clin Cancer Res. 2016;22:4585–93. doi: 10.1158/1078-0432.CCR-15-3101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garassino MC, Cho BC, Kim JH, Mazières J, Vansteenkiste J, Lena H, et al Durvalumab as third-line or later treatment for advanced non-small-cell lung cancer (ATLANTIC): an open-label, single-arm, phase 2 study. Lancet Oncol. 2018;19:521–36. doi: 10.1016/S1470-2045(18)30144-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Akbay EA, Koyama S, Carretero J, Altabef A, Tchaicha JH, Christensen CL, et al Activation of the PD-1 pathway contributes to immune escape in EGFR-driven lung tumors. Cancer Discovery. 2013;3:1355–63. doi: 10.1158/2159-8290.CD-13-0310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin C, Chen X, Li MF, Liu JN, Qi XF, Yang WT, et al Programmed death-ligand 1 expression predicts tyrosine kinase inhibitor response and better prognosis in a cohort of patients with epidermal growth factor receptor mutation-positive lung adenocarcinoma. Clin Lung Cancer. 2015;16:e25–35. doi: 10.1016/j.cllc.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 20.Tang YN, Fang WF, Zhang YX, Hong SD, Kang SY, Yan Y, et al The association between PD-L1 and EGFR status and the prognostic value of PD-L1 in advanced non-small cell lung cancer patients treated with EGFR-TKIs. Oncotarget. 2015;6:14209–19. doi: 10.18632/oncotarget.3694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Travis WD, Brambilla E, Noguchi M, Nicholson AG, Geisinger KR, Yatabe Y, et al International association for the study of lung cancer/american thoracic society/european respiratory society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol. 2011;6:244–85. doi: 10.1097/JTO.0b013e318206a221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang H, Shi JP, Lin DM, Li XF, Zhao C, Wang Q, et al Prognostic value of PD-L1 expression in combination with CD8+ TILs density in patients with surgically resected non-small cell lung cancer . Cancer Med. 2018;7:32–45. doi: 10.1002/cam4.2018.7.issue-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Herbst RS, Soria JC, Kowanetz M, Fine GD, Hamid O, Gordon MS, et al Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515:563–7. doi: 10.1038/nature14011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Taube JM, Anders RA, Young GD, Xu HY, Sharma R, McMiller TL, et al Colocalization of inflammatory response with B7-h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci Transl Med. 2012;4:127ra37. doi: 10.1126/scitranslmed.3003689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–54. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brahmer JR, Drake CG, Wollner I, Powderly JD, Picus J, Sharfman WH, et al Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol. 2010;28:3167–75. doi: 10.1200/JCO.2009.26.7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang CY, Lin MW, Chang YL, Wu CT, Yang PC Programmed cell death-ligand 1 expression in surgically resected stage I pulmonary adenocarcinoma and its correlation with driver mutations and clinical outcomes. Eur J Cancer. 2014;50:1361–9. doi: 10.1016/j.ejca.2014.01.018. [DOI] [PubMed] [Google Scholar]
- 28.Tokito T, Azuma K, Kawahara A, Ishii H, Yamada K, Matsuo N, et al Predictive relevance of PD-L1 expression combined with CD8+ TIL density in stage III non-small cell lung cancer patients receiving concurrent chemoradiotherapy. Eur J Cancer. 2016;55:7–14. doi: 10.1016/j.ejca.2015.11.020. [DOI] [PubMed] [Google Scholar]
- 29.Shi JP, Yang H, Jiang T, Li XF, Zhao C, Zhang LM, et al Uncommon EGFR mutations in a cohort of Chinese NSCLC patients and outcomes of first-line EGFR-TKIs and platinum-based chemotherapy . Chin J Cancer Res. 2017;29:543–52. doi: 10.21147/j.issn.1000-9604.2017.06.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jiang T, Su CX, Li XF, Zhao C, Zhou F, Ren SX, et al EGFR TKIs plus WBRT demonstrated no survival benefit other than that of TKIs alone in patients with NSCLC and EGFR mutation and brain metastases . J Thorac Oncol. 2016;11:1718–28. doi: 10.1016/j.jtho.2016.05.013. [DOI] [PubMed] [Google Scholar]
- 31.Meniawy TM, Lake RA, McDonnell AM, Millward MJ, Nowak AK PD-L1 on peripheral blood T lymphocytes is prognostic in patients with non-small cell lung cancer (NSCLC) treated with EGFR inhibitors. Lung Cancer. 2016;93:9–16. doi: 10.1016/j.lungcan.2015.12.006. [DOI] [PubMed] [Google Scholar]
- 32.Cho JH, Zhou W, Choi YL, Sun JM, Choi H, Kim TE, et al Retrospective molecular epidemiology study of PD-L1 expression in patients with EGFR-mutant non-small cell lung cancer . Cancer Res Treat. 2018;50:95–102. doi: 10.4143/crt.2016.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kobayashi K, Seike M, Zou FF, Noro R, Chiba M, Ishikawa A, et al Prognostic significance of NSCLC and response to EGFR-TKIs of EGFR-mutated NSCLC based on PD-L1 expression. Anticancer Res. 2018;38:753–62. doi: 10.21873/anticanres.12281. [DOI] [PubMed] [Google Scholar]
- 34.Sorensen SF, Demuth C, Weber B, Sorensen BS, Meldgaard P Increase in soluble PD-1 is associated with prolonged survival in patients with advanced EGFR-mutated non-small cell lung cancer treated with erlotinib . Lung Cancer. 2016;100:77–84. doi: 10.1016/j.lungcan.2016.08.001. [DOI] [PubMed] [Google Scholar]
- 35.Azuma K, Ota K, Kawahara A, Hattori S, Iwama E, Harada T, et al Association of PD-L1 overexpression with activating EGFR mutations in surgically resected nonsmall-cell lung cancer. Ann Oncol. 2014;25:1935–40. doi: 10.1093/annonc/mdu242. [DOI] [PubMed] [Google Scholar]
- 36.D'Incecco A, Andreozzi M, Ludovini V, Rossi E, Capodanno A, Landi L, et al PD-1 and PD-L1 expression in molecularly selected non-small-cell lung cancer patients. Br J Cancer. 2015;112:95–102. doi: 10.1038/bjc.2014.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li J, Chen YQ, Shi XS, Le XB, Feng FL, Chen JY, et al A systematic and genome-wide correlation meta-analysis of PD-L1 expression and targetable NSCLC driver genes. J Thorac Dis. 2017;9:2560–71. doi: 10.21037/jtd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang A, Wang HY, Liu Y, Zhao MC, Zhang HJ, Lu ZY, et al The prognostic value of PD-L1 expression for non-small cell lung cancer patients: a meta-analysis. Eur J Surg Oncol. 2015;41:450–6. doi: 10.1016/j.ejso.2015.01.020. [DOI] [PubMed] [Google Scholar]
- 39.Pan ZK, Ye F, Wu X, An HX, Wu JX Clinicopathological and prognostic significance of programmed cell death ligand1 (PD-L1) expression in patients with non-small cell lung cancer: a meta-analysis. J Thorac Dis. 2015;7:462–70. doi: 10.3978/j.issn.2072-1439.2015.02.13. [DOI] [PMC free article] [PubMed] [Google Scholar]