In recent years, the clinical incidence of thyroid cancer has been increasing year by year, and its risk assessment and clinical management methods have also been accordingly modified and constantly improved. There are great differences between the clinical diagnostic and therapeutic modes and disease management of thyroid cancer employed by various medical institutions in China, particularly with regard to the clinical application of serum marker of thyroid cancer. To this end, the China Anti-Cancer Association Thyroid Cancer Specialized Committee Chinese Association of Thyroid Oncology organized this compilation of Expert Consensus on Clinical Application of Serum Marker of Thyroid Cancer to help and impel relevant clinical institutions and professionals to standardize clinical diagnosis, treatment, and long-term management of thyroid cancer, and to properly utilize the serum marker for scientific auxiliary clinical diagnosis and assessment of thyroid cancer before and after operation. A total of 14 recommendations have been put forward in this consensus for measuring the levels of serum marker of thyroid cancer. In view of the limited references, especially regarding the perspective data currently available at home and abroad, omissions are inevitable. Moreover, we also hope that the professionals can give more valuable suggestions for regular revision in the future (see categories of recommendations in Table 1 and detailed recommendations in Table 2).
1.
Categories of recommendations
| Strength of recommendation | Categories and implications |
| A | Highly recommend. Quality of evidence is high, and benefits of clinical application outweigh harms. |
| B | Recommend. Quality of evidence is moderate, and benefits of clinical application outweigh harms. |
| C | Recommend. It is based on expert opinions. |
| D | Not recommend. It is based on expert opinions. |
| E | Not recommend. Quality of evidence is moderate, and harms of clinical application outweigh benefits. |
| F | Strongly not recommend. Quality of evidence is high, and harms of clinical application outweigh benefits. |
| I | Not routinely recommend. Evidence is insufficient or absent, or controversial, and balance of benefits and harms cannot be judged. |
2.
Recommendations
| No. | Recommendations | Grades of recommendation |
| 1 | For all patients with thyroid cancer considered for operative treatment, thyroid function including serum TSH level should be tested before operation | A |
| 2 | It is not recommended to use Tg for diagnosis and differentiation of benign and malignant thyroid tumor | E |
| 3 | Tg and TgAb can be taken as routine tests before operation of thyroid cancer, and it is recommended to simultaneously test both for assessment of initial clinical state and serological index baseline | A |
| 4 | If suspected metastatic lymph nodes are observed in preoperative neck examination of DTC, measurement of Tg value in FNA washout fluid can be optionally used as an auxiliary method to evaluate metastatic lymph nodes | B |
| 5 | Tg and TgAb should be routinely tested after total thyroidectomy of DTC, using Tg and TgAb detection reagents from the same manufacturer, and it is recommended that continuous test is used for sustainable assessment of postoperative recurrence risk and treatment response | A |
| 6 | Unstimulated Tg and TgAb tests’ results can be taken as the baseline values for treatment response assessment after thyroid lobectomy of DTC, and further examination shall be performed to confirm whether there is recurrence and metastasis if Tg level continuously elevates | B |
| 7 | It is recommended to continuously monitor postoperative serum Tg and TgAb and assess the dynamic risk stratification to guide the adjustment of DTC follow-up scheme and treatment decisions | A |
| 8 | For DTC patients with positive TgAb, postoperative disease states shall be continuously assessed by measuring the variation tendency of serum Tg and TgAb levels | C |
| 9 | For patients suspected of malignant thyroid tumor, serum Ctn shall be routinely tested before operation to identify and screen for MTC, and for patients with elevated Ctn or considered for MTC, CEA shall also be tested | B |
| 10 | Elevated serum Ctn value can reflect tumor burden in patients with MTC, and can be taken as a strong evidence to guide clinical assessment of MTC | A |
| 11 | Patients diagnosed with MTC shall be mainly treated with total thyroidectomy, and cervical lymph node metastasis and extent of dissection shall be preliminarily evaluated with reference to imageology and serum Ctn value | B |
| 12 | For carriers with HMTC family mutation gene, serum Ctn can be monitored regularly from infancy, which is helpful to discover change of disease early and consider whether operative treatment shall be performed discretionarily according to the patient’s condition | B |
| 13 | For patients with MTC, it is not recommended to test serum Ctn and CEA during operation to assess the thoroughness of excision | F |
| 14 | Ctn and CEA can be taken as important monitoring indexes of postoperative management and prognostic prediction of MTC | A |
Criteria for setting of recommendation grade
The recommendations on the clinical application of serum marker of thyroid cancer in Expert Consensus are based on evidence-based medicine and expert opinions, and the categories of recommendations are as follows:
For the clinical laboratory test of serum marker of thyroid cancer, immunological test methods are more commonly used at present, including methods used for estimating levels of thyroglobulin (Tg) and calcitonin (Ctn). Ctn is the serum marker of medullary thyroid carcinoma (MTC), and Tg can be used as the serum marker of differentiated thyroid carcinoma (DTC). Anti-thyroglobulin antibody (TgAb) is the autoimmune antibody generated against Tg, and the existence of serum TgAb and the quantitative changes associated with it have a direct impact on the measurement of the serum Tg value. The carcinoembryonic antigen (CEA) is associated with diagnosis and clinical progress in some patients with MTC, and it can be used as a serum marker for MTC along with Ctn.
DTC
Preoperative serological test and assessment of DTC
Recommendation 1: For all patients with thyroid cancer considered for operative treatment, thyroid function including serum thyroid-stimulating hormone level shall be tested before the operation (grade of recommendation: A).
For all patients with thyroid tumor considered for operative treatment, thyroid function, including the level of thyroid-stimulating hormone (TSH), shall be tested. Assessing thyroid function can help surgeons and anesthetists to judge the safety in operative treatment of patients, and preoperative thyroid function shall be routinely tested. For patients with abnormal thyroid function, medical treatment shall be appropriately performed to ensure that the thyroid function is within the acceptable range for surgery. A previous study1 showed that the incidence of malignant nodules in patients with thyroid tumor and TSH level below the normal range is lower than that in patients with normal or elevated TSH level. Therefore, to some extent, TSH level serves as a meaningful reference for malignant tumor assessment, and it is routinely recommended to measure it.
Recommendation 2: It is not recommended to use Tg for diagnosis and differentiation of benign and malignant thyroid tumor (grade of recommendation: E).
Tg is the specific protein generated by thyroid and secreted by the thyroid follicular epithelial cells. Many thyroid diseases can cause the elevation of serum Tg level, including DTC, goiter, thyroiditis, thyroid injury, hyperthyroidism, etc. At present, the detection reagents are unable to distinguish “tumor-induced” Tg from the “normal tissue-derived.” Therefore, serum Tg cannot be used to distinguish the difference between benign and malignant thyroid tumor2-4.
Recommendation 3: Tg and TgAb levels can be tested routinely before operation of thyroid cancer, and it is recommended to simultaneously test both for assessment of the initial clinical state and serological index baseline (grade of recommendation: A).
High level of preoperative serum Tg suggests its better sensitivity in postoperative monitoring, and preoperative measurements of Tg and TgAb baseline values can theoretically assess the reliability of Tg and TgAb tests in postoperative assessment. Trumboli et al.5 conducted a large sample analysis on serum Tg levels in patients with thyroid nodules and indicated that the preoperative serum Tg level can be taken as an index for assessment of the initial clinical state of thyroid cancer and should be routinely tested before operation. The expert group recommends that a simultaneous test of Tg and TgAb levels should be conducted for an assessment of the initial clinical state and serological index baseline.
Marker measurements in washout fluid from preoperative fine needle aspiration (FNA) of lymph nodes of DTC
Recommendation 4: If suspected metastatic lymph nodes are observed in preoperative neck examination of DTC, measurement of Tg level in FNA washout fluid can be optionally used as an auxiliary method to evaluate metastatic lymph nodes (grade of recommendation: B).
Cervical lymph node metastasis is commonly observed in DTC. Ultrasonography and CT are commonly used to assess the cervical lymph node metastasis, but they have limitations. For a few suspicious metastatic lymph nodes undetermined in imaging examination, ultrasound-guided FNA pathology diagnosis and/or Tg measurement in washout fluid can be used as auxiliary diagnostic methods6-7. If the evidence for assessment of lymph node cytology is insufficient or if the cytological findings are not in consensus with the imaging findings, measurement of Tg values in washout fluid must be performed. A study showed that the sensitivity, specificity, and accuracy of FNA combined with Tg measurement in washout fluid were 87.0%, 100%, and 92.2%, respectively8-10. Therefore, the measurement of Tg value in FNA washout fluid can be used as an auxiliary method to determine the lymphatic metastasis. However, a minority of false-positive results may occur, particularly during evaluation of central compartment lymph nodes in the presence of thyroid11-12.
As normal saline does not contain Tg and is the most commonly used solution in clinical practice, puncture needles must be washed with normal saline and dilute into washout fluid. A study showed that the FNA-Tg levels in serum separation tubes (pro-coagulation tubes) and heparin anti-coagulation tubes significantly decreased compared with those in common serum tubes. Therefore, a common serum tube is recommended to be used in the collection of FNA washout fluid. The requirements for lymph node FNA-Tg detection reagents are in accordance with those for serum Tg detection reagent. Meanwhile, reagents with high sensitivity shall be used to ensure that the little amount of Tg in washout fluid can be detected.
Serological test in DTC postoperative assessment and follow-up
Recommendation 5: Tg and TgAb should be routinely tested after total thyroidectomy of DTC, using the same manufacturer’s reagents, and should be evaluated serially over time at various time points during follow-up for continuous assessment of postoperative recurrence risk and treatment response (grade of recommendation: A).
Serum Tg can be a tumor maker of DTC after total thyroidectomy (particularly after the treatment with radioactive iodine ablation), and its level has a positive correlation with tumor burden in DTC patients, which can be used as a clinical marker for assessment of tumor recurrence and metastasis. However, as the serological Tg test is affected by TgAb level in the body, clinical studies in different populations and with test assays showed that positive TgAb can be found in 25%–30% of DTC patients in the first diagnosis13. To accurately evaluate, TgAb shall be routinely measured along with serum Tg. The fist measurement shall be generally conducted within 3–4 weeks after operation or ablation, and the expert group recommends to continuously measure Tg and TgAb for assessment of postoperative recurrence risk and treatment response based on the trend in changing values.
DTC treatment responses are as follows: 1) excellent response: no disease-related evidence, including no clinical and biochemical indexes or structural-related evidence; 2) biochemical incomplete response: continuous elevation of serum Tg or TgAb, without evidence of local lesion; 3) structural incomplete response: continuous or new local/distant metastasis; 4) indeterminate response: no specific biochemical or structural abnormality or unable to confirm whether the lesion is benign or malignant. Patients may have stable or decreased TgAb value, without relevant evidence to prove the existence of the lesion.
Recurrence risk of DTC and disease-related mortality can change at any time under the impact of clinical disease course and treatment response. Therefore, the initial recurrence risk stratification cannot remain stubbornly unchanged and shall be continuously amended during follow-up14. See Tables 3 and 4 for the summary for dynamic risk stratification with total thyroidectomy15.
3.
Dynamic risk stratification in DTC patients with total thyroidectomy and radioiodine remnant ablation15
| Item | Excellent response | Biochemical incomplete response | Structural incomplete response | Indeterminate response |
| * Without TgAb | ||||
| Suppressed Tg level | < 0.2 ng/mL* | > 1 ng/mL* | Any | 0.2-1 ng/mL* |
| TSH-stimulated Tg level | < 1 ng/mL* | > 10 ng/mL* | Any | 1-10 ng/mL* |
| TgAb level | Not detected | Higher than normal | Any | Stable or gradually declining |
| Imaging examination | Negative result | Negative result | Structural or functional lesion indicated | Non-specific findings, or minor uptake in thyroid bed on radioactive iodine scanning |
4.
Dynamic risk stratification in DTC patients with total thyroidectomy only15
| Item | Excellent response | Biochemical incomplete response | Structural incomplete response | Indeterminate response |
| * Without TgAb | ||||
| Suppressed Tg level | < 0.2 ng/mL* | < 5 ng/mL*; or gradually increase under the similar level of TSH* | Any | 0.2-5 ng/mL* |
| TSH-stimulated Tg level | < 2 ng/mL* | > 10 ng/mL*; or gradually increase under the similar level of TSH* | Any | 2-10 ng/mL* |
| TgAb level | Not detected | Elevating | Any | Stable or gradually declining |
| Imaging examination | Negative result | Negative result | Structural or functional lesion indicated | Non-specific findings, or minor uptake in thyroid bed on radioactive iodine scanning |
Recommendation 6: Unstimulated Tg and TgAb test results can be taken as the baseline values for treatment response assessment after thyroid lobectomy of DTC, and further examination shall be performed to confirm whether there is recurrence and metastasis if Tg level continuously elevates (grade of recommendation: B).
After thyroid lobectomy of DTC, accurately assessing the cutoff value of serum Tg level for treatment response has no clear definition. However, the results of unstimulated serum Tg test performed 1 month after operation can be considered as the baseline Tg value for long-term follow-up and dynamic risk assessment of patients according to the present available data16,17. For patients with continuous elevation in unstimulated serum Tg level, clinically visible lesions mostly exist, and further imaging examination is recommended to confirm lesions18. A stable or declined unstimulated serum Tg value is a good predictive index for “disease-free status” with a predicating accuracy up to above 80%19. See Table 5 for the dynamic risk stratification with thyroid lobectomy of DTC15.
5.
Dynamic risk stratification in DTC patients with thyroid lobectomy only15
| Item | Excellent response | Biochemical incomplete response | Structural incomplete response | Indeterminate response |
| * Without TgAb | ||||
| Suppressed Tg level | Stable, < 30 ng/mL* | > 30 ng/mL*; or gradually increasing with similar TSH* level | Any | - |
| TSH-stimulated Tg level | Not applicable | Not applicable | Not applicable | Not applicable |
| TgAb level | Not detected | Elevating | Any | Stable or gradually declining without structural or functional lesion |
| Imaging examination | Negative result | Negative result | Structural or functional lesion indicated | Non-specific findings |
Along with the update on Tg detection reagents20, the detection sensitivity, and sensitivity and precision in low concentration range of highly sensitive Tg (functional sensitivity less than 0.1 ng/mL) have greatly improved. With the emergence of highly sensitive Tg test, the “TSH-stimulated Tg level” might no longer be used for routine assessment of “excellent response” in DTC patients who underwent total thyroidectomy. Suppressed Tg level test is effective in monitoring the postoperative remission of diseases16.
Recommendation 7: It is recommended to frequently measure serum Tg and TgAb after surgery and assess the dynamic risk stratification to guide the adjustment of DTC follow-up scheme and treatment decisions (grade of recommendation: A).
After total thyroidectomy, Tg level in most patients with DTC reaches the lowest concentration 1 month after operation20-21. As the postoperative early assessment indexes and important predictive factors, serum Tg and TgAb values can be used to guide the selection of clinical treatment regimen. The major guidance is listed below:
1) Excellent response: The follow-up intensity and frequency shall be decreased, and the goal of TSH suppressive treatment shall be broadened (lower limit of normal: 2.0 mU/L). Definition of low-risk patients with treatment of total thyroidectomy is to have a postoperative stimulated or suppressed Tg of less than 1 ng/mL, whose prognosis is reliable. Intermediate-risk patients are to have postoperative Tg of less than 1 ng/mL, whose prognosis is reliable with a possibility of micrometastasis. For low-risk and intermediate-risk patients without radioactive iodine treatment, postoperative unstimulated Tg of less than 1 ng/mL indicates favorable treatment response, and the recurrence risk is less than 1%22.
2) Biochemical incomplete response: If Tg value is stable or gradually declining, most patients can be continuously observed on the premise of constant TSH suppressive treatment (lower limit of normal: 0.1 mU/L), and it is not recommended to immediately perform exploratory/preventive operation or radioactive iodine treatment. An elevated Tg or TgAb level is associated with risk for recurrence; because of this, the follow-up frequency might increase, other examinations might be conducted, or other possible treatments might be given. After total thyroidectomy, suppressed or stimulated Tg of 5–10 ng/mL23-26 indicates a higher probability of positive imaging on radioactive iodine scan and confirmation of local or distant metastasis. If postoperative Tg is more than 10 ng/mL, other assessments and treatments are probably required. However, Tg may also be very low or undetectable once the tumor becomes undifferentiated or dedifferentiated.
3) Structural incomplete response: Imaging examination shows that the diseases persistently exist or recur with Tg of more than 10–30 ng/mL after total thyroidectomy, which mostly occurs in patients with failed initial ablation who have local or distant metastasis. Incomplete structure response can cause an increase in mortality27-28. Therefore, multidisciplinary diagnosis and treatment shall be recommended.
4) Indeterminate response: The goal of TSH suppressive treatment is slightly broadened (lower limit of normal: 1.0 mU/L), and treatment response classification can be re-assessed based on the results of imaging examinations and serum Tg/TgAb tests. The initial examination frequency of imaging and serum Tg/TgAb is 1–2 times per year, and the interval can be appropriately prolonged if the condition is stable.
Recommendation 8: For DTC patients with positive TgAb, postoperative disease states shall be continuously assessed by measuring the variation tendency of serum TgAb levels (grade of recommendation: C).
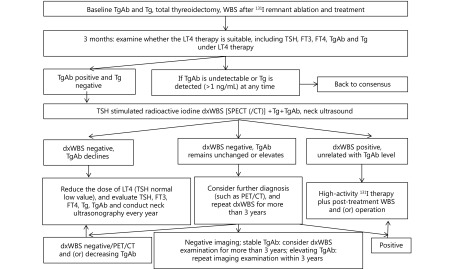
Positive TgAb on immunological detection usually means that serum TgAb value is greater than the upper limit of normal reference range of population. However, for postoperative patients with DTC, some scholars think that it is more appropriate to select the lower limit of detection of TgAb as the positive cutoff value of interfering TgAb29-30. For DTC patients who are TgAb positive preoperatively, TgAb value must be monitored during follow-up. As a surrogate indicator, the trend of TgAb is more important than the numerical value. Decreasing serum TgAb level indicates that the disease remits. Conversely, disease recurrence shall be suspected with a continuous increase in serum TgAb levels. Patients with stable serum TgAb shall be diagnosed as indetermination. For follow-up recommendations on DTC patients with positive TgAb after total thyroidectomy, see Figure 130.
1.
Treatment and follow-up flow chart of patients with differentiated thyroid cancer who have positive TgAb WBS: whole body scintiscanning; LT4: levothyroxine; TSH: thyroid stimulating hormone; FT3: free triiodothyronine; FT4: free thyroxine; TgAb: antithyroglobulin antibody; Tg: thyroglobulin; dxWBS: whole body imaging of diagnostic radioactive iodine; PET/CT: positron emission tomography/computed X-ray tomography.
MTC
Postoperative serological test and assessment of MTC
Recommendation 9: For patients suspected of malignant thyroid tumor, serum Ctn shall be routinely tested before operation to identify and screen for MTC. For patients with elevated Ctn or considered for MTC, CEA shall also be tested (grade of recommendation: B).
A series of prospective non-randomized studies have assessed the utility of Ctn and proved that routine serum Ctn screening can discover early C-cell hyperplasia and MTC, so as to improve the detectable rate and overall survival rate of MTC31-35. The American Thyroid Association maintains a neutral attitude to Ctn screening28 but still accepts that Ctn screening is of significant value in some patient subgroups. The consensus recommends that preoperative Ctn test should be routinely performed on patients suspected of having malignant thyroid tumor.
Meanwhile, the expert group suggests that basal values of serum Ctn and CEA should be simultaneously tested if MTC is considered clinically. The serum Ctn value in a few MTC patients may be in normal range, and significant elevation in serum CEA but relative reduction in Ctn may occur in some patients with advanced MTC; some scholars think that patients with poorly differentiated MTC may either have normal serum Ctn and CEA levels or experience a simultaneous decline in serum Ctn and CEA levels36. Therefore, for judgment and assessment of MTC, clinicians should fully analyze the clinical and pathological results in addition to serum Ctn and CEA as reference.
Recommendation 10: Elevated serum Ctn value can reflect tumor burden in patients with MTC and can be taken as a strong evidence to guide clinical assessment of MTC (grade of recommendation: A).
MTC is characterized by high malignancy, commonly with lymph node metastasis and distant metastasis. Primary and metastatic tumor burden of MTC co-determine and is positively correlated with serum Ctn level. Clinicians can perform a clinical assessment of MTC based on the level of serum Ctn. A study showed that the risk of lymph node metastasis increased when serum Ctn value is more than 20 pg/mL, and the possibility of distant metastasis increased when serum Ctn value is more than 500 pg/mL37. For patients with preoperative serum Ctn value less than 10 pg/mL, “biochemical cure” can be achieved after treatment with complete lymphadenectomy, and postoperative 10-year survival rate is 97.7%38.
Recommendation 11: Patients diagnosed with MTC shall be mainly treated with total thyroidectomy, and cervical lymph node metastasis and extent of dissection shall be preliminarily evaluated using imaging studies and serum Ctn value (grade of recommendation: B).
The incidence of lymph node metastasis in MTC patients is high, about 70%–90%, and the lymph node metastasis’ behavior is associated with the size and location of primary tumor39,40. Necessary lymph node dissection is required during initial thyroidectomy, and comprehensive assessment shall be conducted on the probability of cervical lymph node metastasis based on the location and size of primary MTC lesion and serum Ctn values. Imaging studies are routine methods employed to assess whether the lymph nodes have metastasis, and preoperative serum Ctn values can also effectively assist in determining the extent of lymph node metastasis. Previous studies showed41,42 that it is generally indicated that suspicious lymph nodes had metastasized to the ipsilateral central zone and ipsilateral lateral neck zone, contralateral central zone, and contralateral lateral neck zone and superior mediastinum zone when the serum Ctn values are more than 50, 200, and 500 pg/mL, respectively.
Recommendation 12: For carriers with hereditary MTC (HMTC) family mutation gene, serum Ctn can be monitored regularly from infancy, which helps discover the changes in disease status early and determine whether operative treatment shall be performed discretionarily according to the patient’s condition (grade of recommendation: B).
At present, it is recommended in all guidelines at home and abroad that the carriers with HMTC family mutation genes should undergo total thyroidectomy. Due to limited knowledge, most of patients’ family members in China refuse to undergo preventive surgery, while the clinicians shall fully inform them of the serious condition, closely monitor with imaging, and follow up the changes in Ctn.
In principle, for patients with asymptomatic multiple endocrine neoplasia 2A and familial MTC above 5 years old, and patients with asymptomatic multiple endocrine neoplasia 2B above 1 year old, thorough operative treatment shall be carried out if basal serum Ctn value is more than 40 pg/mL. For adolescent HMTC patients with RET mutation genes and serum Ctn less than 30 pg/mL, preventive thyroidectomy shall be carried out. For patients with serum Ctn value higher than threshold value (10 pg/mL), close follow-up will be the best choice, and preventive thyroidectomy can also be considered43-44. It must be carefully decided whether young infants should undergo surgery or not because they can have elevated serum calcitonin levels and yet still normal for their age group45.
Intraoperative serological test in MTC
Recommendation 13: For patients with MTC, it is not recommended to test serum Ctn and CEA during operation to assess the thoroughness of excision (grade of recommendation: F).
Ctn and parathyroid hormone (PTH) co-participate in the regulation of calcium in the body and maintain stability of calcium metabolism. The half-life of Ctn is more than 1 h, mainly undergoing degradation and excretion from the kidney. However, the half-life of its precursor serum procalcitonin in human body is about 20–24 hours with good stability, continuously forming calcitonin. For patients with high level of preoperative Ctn, the intraoperative Ctn value after tumor resection cannot immediately reflect the thoroughness of operative excision. Similarly, serum CEA is mainly eliminated by Kupffer cells and liver cells with a half-life of 1–7 days. However, depending on liver function, the half-life of serum CEA is prolonged in cholestasis and hepatocellular diseases. Therefore, it is not recommended to routinely test serum Ctn and CEA levels after tumor resection during operation.
Serology-assisted postoperative management of MTC
Recommendation 14: Ctn and CEA levels can be considered as important monitoring indexes of postoperative management and prognostic prediction of MTC (grade of recommendation: A).
Postoperative serum Ctn test can be used to assess the effect of operative treatment in MTC patients, and the normalization of postoperative serum Ctn usually indicates favorable outcome. A previous study showed that serum Ctn value would be even lower than the lower limit of detection after total resection of thyroid tissues46. In view of half-life of Ctn, regarding its metabolism and other factors, it is generally suggested that the optimal time for test of postoperative Ctn minimum value be 3 months after the operation47. However, considering the different tumor burden in various patients, the test time of postoperative serum Ctn and CEA can be 1 week, 1 month, 3 months, and half a year. Regular postoperative re-examination shall be carried out if test values are less than the lower limit of detection or the normal reference range. Initial re-examination period is half a year and can be gradually prolonged to once per year if the condition is stable.
A study of postoperative long-term observation and follow-up of MTC demonstrated that48 the 3-year and 5-year survival rates were 94% and 90%, respectively, if postoperative serum Ctn value was less than 10 pg/mL. Moreover, the 3-year and 5-year survival rates were reduced to 78% and 61%, respectively, if postoperative serum Ctn value was more than 10 pg/mL. If patients exhibit abnormal postoperative basal serum Ctn values after total thyroidectomy, this might indicate the presence of residual lymph nodes or lesions, or that recurrence risk may exist, even if serum Ctn value is less than 150 pg/mL. Therefore, the expert group recommends that neck ultrasound examination shall be performed if postoperative serum Ctn level increases but is less than 150 pg/mL, and Ctn, CEA, and neck ultrasound shall be repeated semiannually for monitoring if test results are negative. If postoperative serum Ctn value is more than 150 pg/mL, neck ultrasound, chest and abdomen CT/MRI, and whole body bone examination must be carried out, and PET/CT examination should be performed when necessary, in order to discover the lesions early.
Prospect
Continuous exploration of new methods and new markers for laboratory diagnosis of thyroid cancer: We hope that more molecular makers are used for diagnosis, prognosis assessment, and confirmation of therapeutic targets.
How to use Tg to assess the treatment response in patients without total thyroidectomy: For patients without total thyroidectomy, serum Tg level is greatly affected and assessment of treatment response cannot entirely rely on it, and new markers or new methods might be used for follow-up in patients who did not undergo total thyroidectomy.
Exploration of serum Ctn value in evaluating efficacy of targeted drug treatment of MTC: Serum Ctn levels in MTC patients can greatly reduce after receiving some drug therapies, while those declines have no significant correlation with the changes in tumor size and regression; hence, further studies are needed to verify the feasibility of using serum Ctn as a reliable index for drug effect assessment.
Exploration of optimum clinical cutoff value of serum markers of thyroid cancer to achieve the optimal clinical specificity and sensitivity: Along with the continuous development of test methodology, the accuracy of reagents constantly improves, and more studies are needed to find the optimal cutoff value and apply that to clinical practice in future, so as to enhance the predictive value in the assessment of tumor stage and tumor recurrence.
Acknowledgements
This article was published originally in Chinese Journal of Clinical Oncology 2017; 48: 7-13 (in Chinese).
Conflict of interest statement
No potential conflicts of interest are disclosed.
References
- 1.Li JZ, Jin YJ, Liu X, Zhang LY Association between the serum TSH concentration and thyroid cancer incidence. Chin J Oncol. 2011;33:921–4. [PubMed] [Google Scholar]
- 2.Sohn YM, Kim MJ, Kim EK, Kwak JY Diagnostic performance of thyroglobulin value in indeterminate range in fine needle aspiration washout fluid from lymph nodes of thyroid cancer. Yonsei Med J. 2012;53:126–31. doi: 10.3349/ymj.2012.53.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Teng WP, Liu YF, Gao M, Huang G Guidelines for diagnosis and treatment of thyroid nodules and differentiated thyroid carcinoma. Chin J Clin Oncol. 2012;39:1249–70. [Google Scholar]
- 4.Bournaud C, Charrié A, Nozières C, Chikh K, Lapras V, Denier ML, et al Thyroglobulin measurement in fine-needle aspirates of lymph nodes in patients with differentiated thyroid cancer: a simple definition of the threshold value, with emphasis on potential pitfalls of the method. Clin Chem Lab Med. 2010;48:1171–7. doi: 10.1515/CCLM.2010.220. [DOI] [PubMed] [Google Scholar]
- 5.Trimboli P, Treglia G, Giovanella L Preoperative measurement of serum thyroglobulin to predict malignancy in thyroid nodules: a systematic review. Horm Metab Res. 2015;47:247–52. doi: 10.1055/s-0034-1395517. [DOI] [PubMed] [Google Scholar]
- 6.Lee EK, Chung KW, Min HS, Kim TS, Kim TH, Ryu JS, et al Preoperative serum thyroglobulin as a useful predictive marker to differentiate follicular thyroid cancer from benign nodules in indeterminate nodules. J Korean Med Sci. 2012;27:1014–8. doi: 10.3346/jkms.2012.27.9.1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu LL, Li SY, Xu HS, Zhao BW, Gao L, Zhang MR, et al Clinical value of high frequency ultrasound guided fine needle aspirates(FNA) and FNA-thyroglobulin detection for diagnosing cervical metastatic lymph nodes in patients underwent thyroidectomy for papillary thyroid carcinoma. Chin J Ultrason. 2014;23:679–82. [Google Scholar]
- 8.Zhao XW, Guan HX, Sun H Thyroglobulin measurement in lymph node fine-needle aspiration washout: clinical application advances and some puzzles caner. Chin J Pract Int Med. 2016;36:37–40. [Google Scholar]
- 9.Salmaslıoğlu A, Erbil Y, Çıtlak G, Ersöz F, Sarı S, Olmez A, et al Diagnostic value of thyroglobulin measurement in fine- needle aspiration biopsy for detecting metastatic lymph nodes in patients with papillary thyroid carcinoma. Langenbecks Arch Surg. 2011;396:77–81. doi: 10.1007/s00423-010-0723-1. [DOI] [PubMed] [Google Scholar]
- 10.Chung J, Kim E K, Lim H, et al Optimal indication of thyroglobulin measurement in fine-needle aspiration for detecting lateral metastatic lymph nodes in patients with papillary thyroid carcinoma. Head Neck. 2014;36(6):795–801. doi: 10.1002/hed.v36.6. [DOI] [PubMed] [Google Scholar]
- 11.Li Q, Zhao BW, Lyu JH, Shou JD, Xu HS, Xu LL, et al The value of thyroglobulin measurement in fine-needle aspiration for diagnosis of suspicious lymph nodes in patients with thyroid carcinoma after thyroidectomy. Chin J Otorhinolaryngol Head Neck Surg. 2016;51:378–82. doi: 10.3760/cma.j.issn.1673-0860.2016.05.012. [DOI] [PubMed] [Google Scholar]
- 12.Spencer CA, Bergoglio LM, Kazarosyan M, Fatemi S, LoPresti JS Clinical impact of thyroglobulin (Tg) and Tg autoantibody method differences on the management of patients with differentiated thyroid carcinomas. J Clin Endocrinol Metab. 2005;90:5566–75. doi: 10.1210/jc.2005-0671. [DOI] [PubMed] [Google Scholar]
- 13.Giovanella L, Clark PM, Chiovato L, Duntas L, Elisei R, Feldt-Rasmussen U, et al Thyroglobulin measurement using highly sensitive assays in patients with differentiated thyroid cancer: a clinical position paper. Eur J Endocrinol. 2014;171:R33–46. doi: 10.1530/EJE-14-0148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tuttle RM, Tala H, Shah J, Leboeuf R, Ghossein R, Gonen M, et al Estimating risk of recurrence in differentiated thyroid cancer after total thyroidectomy and radioactive iodine remnant ablation: using response to therapy variables to modify the initial risk estimates predicted by the new American Thyroid Association staging system. Thyroid. 2010;20:1341–9. doi: 10.1089/thy.2010.0178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guan HX, Liang N Dynamic risk stratification of differentiated thyroid cancer: a new concept that arose from the view of disease characteristics. Chin J Gen Surg. 2016;25:1536–43. [Google Scholar]
- 16.Reiners C, Hänscheid H, Luster M, Lassmann M, Verburg FA Radioiodine for remnant ablation and therapy of metastatic disease. Nat Rev Endocrinol. 2011;7:589–95. doi: 10.1038/nrendo.2011.134. [DOI] [PubMed] [Google Scholar]
- 17.Momesso DP, Tuttle RM Update on differentiated thyroid cancer staging. Endocrinol Metab Clin North Am. 2014;43:401–21. doi: 10.1016/j.ecl.2014.02.010. [DOI] [PubMed] [Google Scholar]
- 18.Vaisman F, Momesso D, Bulzico DA, Pessoa CHCN, da Cruz MDG, Dias F, et al Thyroid lobectomy is associated with excellent clinical outcomes in properly selected differentiated thyroid cancer patients with primary tumors greater than 1 cm. J Thyroid Res. 2013;2013:398194. doi: 10.1155/2013/398194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baloch Z, Carayon P, Conte-Devolx B, et al Laboratory European Journal of Endocrinology Review L Giovanella and others Implications of highly sensitive Tg 171: 2 R44 www.ejeonline.org support for the diagnosis and monitoring of thyroid diseases. Thyroid. 2013;13:3–126. [Google Scholar]
- 20.Wunderlich G, Zöphel K, Crook L, Smith S, Smith BR, Franke WG A high-sensitivity enzyme-linked immunosorbent assay for serum thyroglobulin. Thyroid. 2001;11:819–24. doi: 10.1089/105072501316973064. [DOI] [PubMed] [Google Scholar]
- 21.Frasoldati A, Pesenti M, Gallo M, Caroggio A, Salvo D, Valcavi R Diagnosis of neck recurrences in patients with differentiated thyroid carcinoma. Cancer. 2003;97:90–6. doi: 10.1002/(ISSN)1097-0142. [DOI] [PubMed] [Google Scholar]
- 22.Ibrahimpasic T, Nixon IJ, Palmer FL, Whitcher MM, Tuttle RM, Shaha A, et al Undetectable thyroglobulin after total thyroidectomy in patients with low- and intermediate-risk papillary thyroid cancer-is there a need for radioactive iodine therapy? Surgery. 2012;152:1096–105. doi: 10.1016/j.surg.2012.08.034. [DOI] [PubMed] [Google Scholar]
- 23.Giovanella L, Ceriani L, Ghelfo A, Keller F Thyroglobulin assay 4 weeks after thyroidectomy predicts outcome in low-risk papillary thyroid carcinoma. Clin Chem Lab Med. 2005;43:843–7. doi: 10.1515/CCLM.2005.142. [DOI] [PubMed] [Google Scholar]
- 24.Robenshtok E, Grewal RK, Fish S, Sabra M, Tuttle RM A low postoperative nonstimulated serum thyroglobulin level does not exclude the presence of radioactive iodine avid metastatic foci in intermediate-risk differentiated thyroid cancer patients. Thyroid. 2013;23:436–42. doi: 10.1089/thy.2012.0352. [DOI] [PubMed] [Google Scholar]
- 25.Rosário PWS, Guimaraes VC, Maia FFR, Fagundes TA, Purisch S, Padrao EL, et al Thyroglobulin before ablation and correlation with posttreatment scanning. Laryngoscope. 2005;115:264–7. doi: 10.1097/01.mlg.0000154730.31281.0c. [DOI] [PubMed] [Google Scholar]
- 26.Oyen WJG, Verhagen C, Saris E, van den Broek WJM, Pieters GFFM, Corsten FHM Follow-up regimen of differentiated thyroid carcinoma in thyroidectomized patients after thyroid hormone withdrawal. J Nucl Med. 2013;41:643–6. [PubMed] [Google Scholar]
- 27.Zhao D, Liang J, Lin YS Progress in diagnosis and treatment of radioactive iodine-refractory differentiated thyroid carcinomas. Chin J Nucl Med Mol Imaging. 2013;33:505–9. [Google Scholar]
- 28.American Thyroid Association (ATA) Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer, Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL, et al Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19:1167–214. doi: 10.1089/thy.2009.0110. [DOI] [PubMed] [Google Scholar]
- 29.Spencer C, Petrovic I, Fatemi S Current thyroglobulin autoantibody (TgAb) assays often fail to detect interfering TgAb that can result in the reporting of falsely low/undetectable serum Tg IMA values for patients with differentiated thyroid cancer. J Clin Endocrinol Metab. 2011;96:1283–91. doi: 10.1210/jc.2010-2762. [DOI] [PubMed] [Google Scholar]
- 30.Verburg FA, Luster M, Cupini C, Chiovato L, Duntas L, Elisei R, et al Implications of thyroglobulin antibody positivity in patients with differentiated thyroid cancer: a clinical position statement. Thyroid. 2013;23:1211–25. doi: 10.1089/thy.2012.0606. [DOI] [PubMed] [Google Scholar]
- 31.Elisei R, Bottici V, Luchetti F, Di Coscio G, Romei C, Grasso L, et al Impact of routine measurement of serum calcitonin on the diagnosis and outcome of medullary thyroid cancer: experience in 10, 864 patients with nodular thyroid disorders. J Clin Endocrinol Metab. 2004;89:163–8. doi: 10.1210/jc.2003-030550. [DOI] [PubMed] [Google Scholar]
- 32.Hahm JR, Lee MS, Min YK, Lee MK, Kim KW, Nam SJ, et al Routine measurement of serum calcitonin is useful for early detection of medullary thyroid carcinoma in patients with nodular thyroid diseases. Thyroid. 2001;11:73–80. doi: 10.1089/10507250150500694. [DOI] [PubMed] [Google Scholar]
- 33.Niccoli P, Wion-Barbot N, Caron P, Henry JF, de Micco C, Saint Andre JP, et al Interest of routine measurement of serum calcitonin: study in a large series of thyroidectomized patients. J Clin Endocrinol Metab. 1997;82:338–41. doi: 10.1210/jcem.82.2.3737. [DOI] [PubMed] [Google Scholar]
- 34.Costante G, Meringolo D, Durante C, Bianchi D, Nocera M, Tumino S, et al Predictive value of serum calcitonin levels for preoperative diagnosis of medullary thyroid carcinoma in a cohort of 5817 consecutive patients with thyroid nodules. J Clin Endocrinol Metab. 2007;92:450–5. doi: 10.1210/jc.2006-1590. [DOI] [PubMed] [Google Scholar]
- 35.Chambon G, Alovisetti C, Idoux-Louche C, Reynaud C, Rodier M, Guedj AM, et al The use of preoperative routine measurement of basal serum thyrocalcitonin in candidates for thyroidectomy due to nodular thyroid disorders: results from 2733 consecutive patients. J Clin Endocrinol Metab. 2011;96:75–81. doi: 10.1210/jc.2010-0162. [DOI] [PubMed] [Google Scholar]
- 36.Malandrino P, Latina A, Marescalco S, Spadaro A, Regalbuto C, Fulco RA, et al Risk-adapted management of differentiated thyroid cancer assessed by a sensitive measurement of basal serum thyroglobulin. J Clin Endocrinol Metab. 2011;96:1703–9. doi: 10.1210/jc.2010-2695. [DOI] [PubMed] [Google Scholar]
- 37.Wells SA Jr, Asa SL, Dralle H, Elisei R, Evans DB, Gagel RF, et al Revised American Thyroid Association guidelines for the management of medullary thyroid carcinoma. Thyroid. 2015;25:567–610. doi: 10.1089/thy.2014.0335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Machens A, Dralle H Biomarker-based risk stratification for previously untreated medullary thyroid cancer. J Clin Endocrinol Metab. 2010;95:2655–63. doi: 10.1210/jc.2009-2368. [DOI] [PubMed] [Google Scholar]
- 39.Cheung K, Roman SA, Wang TS, Walker HD, Sosa JA Calcitonin measurement in the evaluation of thyroid nodules in the United States: a cost-effectiveness and decision analysis. J Clin Endocrinol Metab. 2008;93:2173–80. doi: 10.1210/jc.2007-2496. [DOI] [PubMed] [Google Scholar]
- 40.Gagel RF, Hoff AO, Cote GE. Medullary thyroid carcinoma. In: Braverman LE, Utiger RD. Werner & Ingbar's the Thyroid. 9th ed. Philadelphia: Lippincott Williams & Wilkins; 2005; 967-88.
- 41.Park JH, Lee YS, Kim BW, Chang HS, Park CS Skip lateral neck node metastases in papillary thyroid carcinoma. World J Surg. 2012;36:743–7. doi: 10.1007/s00268-012-1476-5. [DOI] [PubMed] [Google Scholar]
- 42.Machens A, Hauptmann S, Dralle H Prediction of lateral lymph node metastases in medullary thyroid cancer. Br J Surg. 2008;95:586–91. doi: 10.1002/(ISSN)1365-2168. [DOI] [PubMed] [Google Scholar]
- 43.Modigliani E, Cohen R, Campos JM, Conte-Devolx B, Maes B, Boneu A, et al Prognostic factors for survival and for biochemical cure in medullary thyroid carcinoma: results in 899 patients. Clin Endocrinol (Oxf) 1998;48:265–73. doi: 10.1046/j.1365-2265.1998.00392.x. [DOI] [PubMed] [Google Scholar]
- 44.Schilling T, Bürck J, Sinn HP, Clemens A, Otto HF, Höppner W, et al Prognostic value of codon 918(ATG→ACG) RET proto-oncogene mutations in sporadic medullary thyroid carcinoma . Int J Cancer. 2001;95:62–6. doi: 10.1002/(ISSN)1097-0215. [DOI] [PubMed] [Google Scholar]
- 45.Basuyau JP, Mallet E, Leroy M, Brunelle P Reference intervals for serum calcitonin in men, women, and children. Clin Chem. 2004;50:1828–30. doi: 10.1373/clinchem.2003.026963. [DOI] [PubMed] [Google Scholar]
- 46.Engelbach M, Görges R, Forst T, Pfützner A, Dawood R, Heerdt S, et al Improved diagnostic methods in the follow-up of medullary thyroid carcinoma by highly specific calcitonin measurements. J Clin Endocrinol Metab. 2000;85:1890–4. doi: 10.1210/jcem.85.5.6601. [DOI] [PubMed] [Google Scholar]
- 47.Elisei R, Pinchera A Advances in the follow-up of differentiated or medullary thyroid cancer. Nat Rev Endocrinol. 2012;8:466–75. doi: 10.1038/nrendo.2012.38. [DOI] [PubMed] [Google Scholar]
- 48.Pellegriti G, Leboulleux S, Baudin E, Bellon N, Scollo C, Travagli JP, et al Long-term outcome of medullary thyroid carcinoma in patients with normal postoperative medical imaging. Br J Cancer. 2003;88:1537–42. doi: 10.1038/sj.bjc.6600930. [DOI] [PMC free article] [PubMed] [Google Scholar]