Abstract
Objectives
The aim of study was to evaluate the influence of pharmacist intervention based on “CMO model”, to improve activation in HIV-patients.
Material and methods
Longitudinal, prospective, single-center study. Eligible patients were HIV-infected, taking antiretroviral treatment. The collected data included demographic characteristics, clinical and HIV-related and pharmacotherapeutic variables. The primary outcome was the variation of patient activation measured by Spanish adapted patient activation measure questionnaire. This questionnaire assesses people’s knowledge, skills and confidence in managing their own health care. The assessment was performed at the beginning and 6 months after the program start, which consisted of individualized interventions planned in the stratification model, a motivational interview and a specific pharmacotherapeutic follow-up.
Results
A total of 140 patients were included. The most common regimens prescribed were based on non-nucleoside plus nucleoside reverse transcriptase inhibitor (44.0%) and more than half of the patients had chronic concomitant medication. The patients who achieved the highest activation level increased from 28.1% to 68.3% (p<0.0005). The relationship between this increase in patient activation and the stratification level that occurs in largest increases in patients with a low need level, where it was observed an improvement in the percentage of patients with high activation from 28.3% to 74.3% (p<0.001) after intervention. The percentage of patients with adequate adherence to concomitant treatment increased by 18.4% (p = 0.035). Baseline PAM values showed high activation for 28.6% (40 patients), intermediate for 43.6% (61) and low for 27.9% (39).
Conclusion
CMO model has an important role for patient activation, improving adherence and health outcomes for HIV+ patients.
Key-words: patient activation, HIV, pharmaceutical care, adherence
Abstract
Objetivo
Evaluar la influencia de un programa de intervenciones basadas en el nuevo modelo CMO para pacientes VIH+ en la mejora de su activación con su tratamiento.
Material y métodos
Estudio longitudinal, prospectivo, unicéntrico. Se incluyeron pacientes VIH adultos en tratamiento antirretroviral activo. Se recogieron variables demográficas, clínicas y relacionadas con el VIH y variables farmacoterapéuticas. La variable principal fue la variación del nivel de Activación de los pacientes medidos por el cuestionario “patient activation measure” (PAM). Este cuestionario valora el conocimiento, las habilidades y la confianza de los pacientes para ser responsable de su propio cuidado. El cuestionario se facilitó a los pacientes en la visita de inicio y a los 6 meses del inicio del programa que consistió en aplicar las intervenciones diseñadas en el modelo de estratificación junto con una entrevista motivacional y un seguimiento farmacoterapéutico específico fuera de las consultas habituales.
Resultados
Se incluyeron 140 pacientes. El 44% de los pacientes estaba en tratamiento con un régimen compuesto por 2ITIAN+ITINN y más del 50% presentaban medicación concomitante crónica. En relación a la variable principal, la evolución del número de pacientes que alcanzaron el nivel más alto de activación pasó de un 28,1% a un 68,3% (p<0,0005). El análisis de esta relación determinó que los mayores incrementos se producen en los pacientes con un nivel de necesidad de Atención Farmacéutica bajo, donde se observó un incremento del porcentaje de pacientes con activación alta de un 28,3% a un 74,3% (p<0,001). El porcentaje de pacientes con buena adherencia al tratamiento antirretroviral se incrementó un 18,4% al tratamiento concomitante (p=0,035). Los valores de PAM basales recogidos incluyeron una alta activación para el 28,6% (40 pacientes), medio para el 43,6% (61) y bajo para el 27,9% (39).
Conclusión
El programa de intervenciones basado en el modelo CMO influye en la activación de los pacientes y puede mejorar la adherencia y otros resultados en salud en los pacientes VIH+.
Palabras clave: activación paciente, VIH, Atención farmacéutica, adherencia
INTRODUCTION
The field of viral pathologies has undergone a real revolution in recent years, especially in HIV. Since the appearance of highly active antiretroviral treatment (HAART) in 1996, the morbidity and mortality associated with the disease has been drastically reduced, and patients’ life expectancy has approached to general population’s one [1–3].
From Pharmaceutical Care perspective, we find ourselves dealing with a new reality in the outpatient pharmacy clinics with a sharp rise in the number of patients, a greater complexity in most of them and the incorporation of new expensive treatments in a crisis time. All this involves changes and restructuring in the working model of Hospital Pharmacy. A stratification system was necessary to optimise the use of resources and time. In this way, targeted interventions for each kind of patients were designed to have a beneficial impact on achieve the individual aims proposed by each patient [4].
Priority interventions include enhance patients’ empowerment. In chronic diseases, such as HIV, self-management education has been shown to improve the treatment adherence, increase CD4 count, decrease viral loan and reduce risk-taking behaviors [5–11].
The patient’s central role in decision making and management of care is becoming increasingly recognized [12]. In this section, it is particularly important patient’s activation.
A health active patient is those who are activated believe patients have important role to play in self-managing collaborating with providers, and maintaining their health. In addition, they know how to manage their condition and maintain functioning and prevent health declines. This involves having the skills and behavioral repertoire to manage these competently and achieve a high-quality care [13].
The term “patient activation” should not be confused with the term “patient engagement”, which refers to interventions designed to increase activation and the resulting patient behaviors [14].
Currently, the Patient Activation Measure (PAM) in the only validated instrument that comprehensively measures the degree to which patients are activated to manage their own health care [15]. The PAM, which was developed by Hibbard et al [13], contains Likert-response questions each soliciting some information on the patient’s knowledge, skills, and beliefs to self-manage their own care, collaborate with their health care providers, and maintain health behaviors while preventing decline [16].
Patient activation has been studied in patients with other chronic illnesses, such as inflammatory bowel disease [14], diabetes [17], multiple sclerosis [18] or heart failure [19].
In general, evidence exists which shows a close relationship between the patient’s activation and improvements in health results, improvements in patients experience with the health care system and cost reduction [12,15,20].
Research literature available in HIV-infected patients suggests that higher activation was associated with viral suppression, mediated by greater antiretroviral adherence [10].
In order to address a new approach to viral diseases care within an innovative care framework, a new model of Pharmaceutical Care called “CMO model” has been developed [21].
This system includes a risk-stratified model for pharmaceutical care in HIV-patients of Spanish Society of Hospital Pharmacy, motivational interviews and the use of new technologies. This new model facilitates the optimization of resources and the development of the most appropriate intervention strategies for each of the established levels, identifying those patients who can benefit more from certain interventions of Pharmaceutical Care [22,23]. In this way, pharmacotherapeutic follow-up can be carried out based on the patients’ needs and their established pharmacotherapeutic objectives.
One of the main objectives to be achieved with this new approach is to increase the co-responsibility of patients with their own treatment through information and education about self-care. To do this, patients compromised and activated in relation to their health and their treatment are essential. Until now, there are not published studies that show how structured interventions can help increase the level of activation in the specific population of HIV + patients.
Therefore, the aim of this study was to evaluate the influence of pharmacist intervention, based on “CMO model”, to improve activation in HIV patients.
MATERIAL AND METHODS
The present work is a longitudinal, prospective, single-center study. Patients were eligible for inclusion if they were HIV-infected, were over 18 years of age, had been taking antiretroviral treatment (ART) for more than six months and had signed the informed consent. Patients who participated in clinical trials, not signed consent form or missed pharmaceutical follow-up program for any reason were excluded.
Patient recruitment was conducted in November 2015, in a monographic consultation of Pharmaceutical Care to viral diseases patients.
The collected data included demographic characteristics (age, gender, HIV risk factor and economic status), clinical and HIV related variables such as plasmatic viral load and CD4 absolute count (Baseline and at 6 months), type and number of comorbidities, HCV and/or HBV coinfection, and pharmacotherapeutic variables as prescribed ART, kind and number of concomitant drugs, polypharmacy (defined as taking more than 5 drugs a day) [24]. In addition, we determined ART and co-medication adherence and the complexity index of the treatment baseline and 6 months after the intervention.
Co-medication was considered if it was prescribed with a minimum duration of 60 days. Calculation of complexity index was performed through “Medication regimen complexity index” (MRCI) tool of Colorado University [25] available in http://www.ucdenver.edu/academics/colleges/pharmacy/Research/researchareas/Pages/researchareas.aspx).
Patients were stratified by level of risk according to the model of selection and Pharmaceutical Care for HIV patients with or without HCV [22].
The primary outcome of interest is the variation of patient activation measured by PAM questionnaire, developed and validated for this purpose. This assessment was performed at the beginning of the study and later at 6 months.
The PAM is a 10-item survey tool adapted to Spanish and designed to assess a person’s knowledge, skills and confidence in managing their own health care. This questionnaire contains 10 items and use a Likert scale with four response options: The options are strongly disagree (1), disagree (2), agree (3), strongly agree (4) and N/A (5).
A chart provided by the creators of the questionnaire convert this punctuation to the activation score with a score range from 0 to 100. Based on activation score cut points provides by the survey developers, patients are stratified into 1 of 3 stages of progressive activation. Level I (PAM score of <52.9) where patients are not prepared to play an active role in their own health or believe they play an important role but lack confidence and/or knowledge to take action; Level II (PAM score of 53.0 to 75.4) with patients who are beginning to take action, but may still lack confidence or support to achieve the desired changes; Level III ( PAM score of ≥75.5) with individuals who have adopted many self-management behaviors, but may not be able to maintain actions over time or during times of stress. The full is included as supplementary material. Patient activation was measured at the start visit of the study to determine the degree of basal activation and 6 months after the start of the intervention. In patients with HCV coinfection in active treatment for both conditions, an intermediate measure was performed at week 4 of treatment for hepatitis.
Other study endpoints are the analysis of activation in HIV+ patients in a real clinical practice cohort and the implications of the intervention program on patients’ viro-immunological status, their influence on the variation of pharmacotherapeutic complexity, and ART/ concomitant medication adherence.
Medication adherence was assessed using two different methods, electronic pharmacy refill records calculated based on the formula: [(pills dispensed/pills prescribed per day)/days between refills] x 100 and specifics adherence scales SMAQ (antiretroviral treatment) and morisky-green (concomitant medication).
The ART medication was obtained from a pharmacy-dispensing program to outpatients (Dominion-Farmatools). The rest of treatment was collected from electronic health prescriptions program of Andalusian Public Health System. The remaining variables were obtained by consulting analytics, microbiology reports, and from review of the medical history of each patient.
To analyze the relationship between the stratification group and the activation of the patients, the classification of the patients was performed using the model designed and validated for HIV + patients [22].
The individualized interventions planned in the stratification model, detailed in supplemental material 2, were applied to all patients. Additionally, a motivational interview and a specific pharmacotherapeutic follow-up were carried out outside the usual consultations, with a web tool [26] and an own app.
The face-to-face interventions were conducted at the initial screening visit and at the intermediate follow-up visit, approximately 3 months after the study began. Non-presence interventions were carried out continuously throughout the study period.
The sample size was estimated based on previous studies in which the detected differences are estimated at 2 points on the PAM scale, with a 95% confidence level and a statistical power of 90%. Considering these facts, at least 65 patients were necessary. These calculations were made using the Bonett equations [27,28].
With regard to the statistical analysis, quantitative variables are expressed as mean and standard deviation or as median and percentile P25 and P75 in the case of a skewed distribution. Qualitative variables are expressed as percentages (%).
To assess the differences in the variables collected before and after the intervention, we ran the following statistical analysis: when data were consistent with a normal distribution, a t-test for related samples was used to compare two means of quantitative variables. Otherwise, a nonparametric Wilcoxon test was performed. The confidence interval established to determine the differences between mean or median was 95%. The McNemar’s test was applied to analyze the changes in dichotomous variables. In order to establish the relationship between the different quantitative variables, we calculated the Pearson’s correlation coefficient and the non-parametric Mann-Whitney U test for unrelated samples. Data analysis was carried out using the statistical package SPSS 22.0 for Windows (IBM Corp., Armonk, NY, USA).
The study received approval from Research Ethics Committee Sevilla-Sur
RESULTS
One hundred forty patients were included in the study. The 85.3% were men with a median age of 47.8 years (IQR: 43.0-49.0). Of all patients included in the study, 92.1% had at least one comorbidity, being the chronic liver disease the main condition with 56.4%. The remaining demographic and clinical characteristics of the patients and their respective variation at 6 months are shown in table 1.
Table 1.
Demographic and clinical characteristics of the initial study population and their variation at 6 months.
| Variable (n=140 patients) | Frequency | |
|---|---|---|
| Basal | After 6 Months | |
| Gender (male), n (%) | 120 (85.4) | - |
| Age (years) (median + IQR) | 47.8 (43.0-49.0) | - |
| HIV risk factor, n (%) | ||
| IDU | 60 (42.9) | - |
| Sexual | 71 (50.7) | - |
| Unknown | 9 (6.4) | - |
| Economic Status, n (%) | ||
| No-contribution | 34 (24.5) | - |
| Pensioner | 36 (25.9) | - |
| Active population and unemployed with benefits | 59 (42.4) | - |
| Population with wages greater than 18,000 | 8 (5.7) | - |
| Population with wages greater than 100.000 | 2 (1.4) | - |
| Undetectable Plasmatic Viral Load (<50 cop/mL), n(%) | 108 (94.7) | 117 (92.1) |
| CD4 (cel/ µL) (median + IQR) | 636.0 (434.0-842.0) | 681.0 (476.5-841.0) |
| CD4 Levels, n (%) | ||
| <250 cel/µL | 9 (6.8) | 10 (8.8) |
| 250-500 cel/µL | 34 (25.6) | 22 (19.5) |
| >500 cel/µL | 90 (67.7) | 81 (71.7) |
| Coinfection HVC, n (%) | 53 (37.8) | - |
| Comorbidities, n (%) | ||
| Liver Diseases | 79 (56.4) | - |
| Lipid disorders | 33 (23.6) | - |
| STD/HPV | 30 (21.4) | - |
| Metabolic disorders | 18 (12.8) | - |
| Anxiety / Depressive Syndrome | 14 (10.0) | - |
| Gastrointestinal disorders | 12 (8.6) | - |
| High blood pressure | 12 (8.6) | - |
| Others | 43 (30.7) | - |
IQR: Interquartile range; IDU: intravenous drug user; HCV: Hepatitis C virus; STD: Sexual transmission diseases; HPV: human papillomavirus.
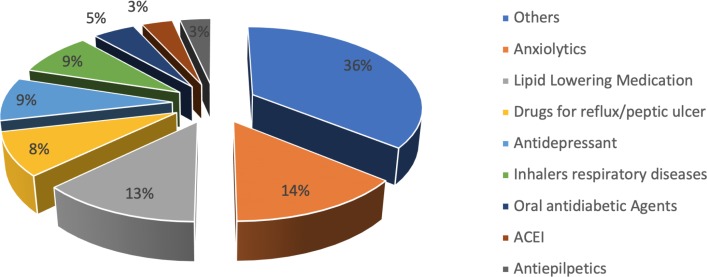
At baseline, the most common regimens were those including a combination of a non-nucleoside and a nucleoside reverse transcriptase inhibitor (44.0%) and more than half of the patients had chronic concomitant medication, with an average of 1.64 ± 2.17 drugs per person. The most prescribed pharmacotherapeutic group was anxiolytics with 14.1%, followed by lipid-lowering agents (12.8%). The remaining pharmacological groups are detailed in figure 1.
Figure 1.
Description of the drugs prescribed as part of the concomitant treatment of study patients at baseline.
ACEI: Inhibitors of the angiotensin-converting enzyme.
Regarding adherence, most patients were adherent to antiretroviral treatment (77.9%). However, of the patients with prescribed concomitant medication, only 35.29% had adequate adherence to these drugs.
Applying the selection and stratification model, seven patients (5.0%) were at priority level 1 (upper), 14 patients (10.0%) in the intermediate and the rest 119 (85.0%) in level 3 (basal).
The percentage of patients with concomitant medication grew by 11% (p= 0.008), increasing the average number of drugs prescribed per patient. Full pharmacotherapeutic characteristics and their evolution at 6 months are shown in table 2.
Table 2.
Pharmacotherapy variables of study patients.
| Variables (n=140 patients) | Frequency | p | |
|---|---|---|---|
| Basal | 6 Months | ||
| Combination of antiretroviral drugs, n (%) | |||
| 2 NRTI+NNRTI | 62 (44.3) | 57 (40.7) | p>0.05 |
| 2 NRTI + PI/r | 15 (10.7) | 10 (7.1) | p>0.05 |
| 2 NRTI + INSTI | 33 (23.6) | 38 (27.1) | p>0.05 |
| Other combinations | 30 (21.4) | 35 (25.0) | p>0.05 |
| STR, n (%) | 64 (45.7) | 81 (57.9) | p<0.000 |
| Concomitant treatment for Hepatitis C | 6 (4.3) | 11 (7.8) | p=0.630 |
| Polypharmacy | 43 (30.7) | 49 (35.0) | p=0.109 |
| Patients with concomitant medication | 72 (51.4) | 83 (59.3) | p=0.008 |
| Number of concomitant drugs prescribed (Mean ± sd) | 1.64 ± 2.17 | 2.02 ± 2.34 | p<0.05 |
| Complexity index global treatment (Median + IQR) | 7 (3-12) | 7 (4-12) | 0.02 |
| Complexity index global treatment categorized | |||
| High (≥14 points) | 26 (18.6) | 27 (19.3) | P=0.5 |
| Low (<14 points) | 114 (81.4) | 113 (80.7) | |
2 NRTI+NNRTI: 2 Nucleoside reverse transcriptase inhibitors + 1 Non-nucleoside reverse transcriptase inhibitors; 2 NRTI + PI/r: 2 Nucleoside reverse transcriptase inhibitors + 1 boosted protease inhibitors; 2 NRTI + INSTI: 2 Nucleoside reverse transcriptase inhibitors + 1 integrase inhibitors; STR: Single-tablet regimen; sd: standard deviation. IQR: Interquartile range.
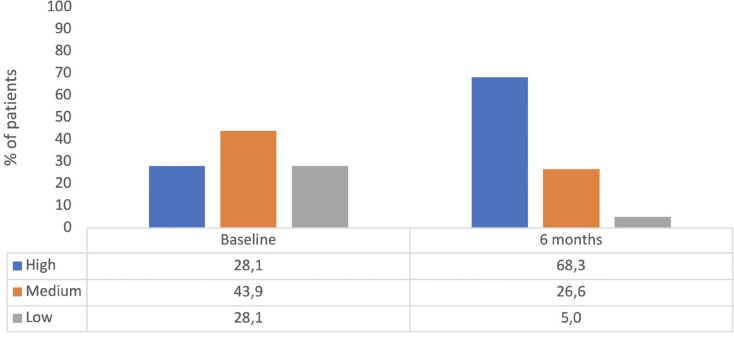
With regard to the primary endpoint, the number of patients who achieved the highest activation level increased from 28.1% to 68.3% yield statistical significance (p <0.001).
The analysis of the relationship between this increase in patient activation and the stratification level of need for pharmaceutical care of Spanish Society of Hospital Pharmacy determined that the largest increases occur in patients with a low need level (level 3), where it was observed an improve in the percentage of patients with high activation from 28.3% to 74.3% (p <0.001) after the intervention. Figure 2 shows the complete outcomes.
Figure 2.
Patient activation evolution during the study.
Finally, analyzing the secondary objectives, baseline PAM values showed high activation for 28.6% (40 patients), intermediate for 43.6% (61) and low for 27.9% (39). Table 3 describes the scores of the different items of the adapted PAM scale.
Table 3.
Scores of the different items of the adapted PAM scale.
| Score | ||||||
|---|---|---|---|---|---|---|
| Strongly disagree | Disagree | Agree | Strongly agree | NA | n | |
| When all is said and done, I am the person who is responsible for managing my health condition(s). | 0 (0%) | 1 (0.7%) | 76 (54.3%) | 63(45.0%) | 0 (0%) | 140 (100%) |
| Taking an active role in my own health care is the most important factor in determining my health and ability to function. | 0 (0%) | 0 (0%) | 83 (59.3%) | 57 (40.7%) | 0 (0%) | 140 (100%) |
| I know what each of my prescribed medications does. | 3 (2.1%) | 22 (15.7%) | 75 (53.6%) | 38 (27.1%) | 2 (1.4%) | 140 (100%) |
| I am confident that I can tell when I need to go to get medical care and when I can handle a health problem myself. | 0 (0%) | 8 (5.7%) | 78 (55.7%) | 54 (38.6%) | 0 (0%) | 140 (100%) |
| I am confident I can tell doctor concerns I have even when he or she does not ask. | 0 (0%) | 1 (0.7%) | 84 (60.0%) | 55 (39.3%) | 0 (0%) | 140 (100%) |
| I am confident that I can follow through on medical treatments I need to do at home. | 0 (0%) | 6 (4.3%) | 76 (54.3%) | 57 (40.7%) | 1 (0.7%) | 140 (100%) |
| I have been able to maintain the lifestyle changes for my health condition(s) that I have made. | 0 (0%) | 23 (16.4%) | 71 (50.7%) | 46 (32.9%) | 0 (0%) | 140 (100%) |
| I know how prevent further problems with my health condition(s) | 0 (0%) | 7 (5.0%) | 94 (67.1%) | 36 (25.7%) | 5 (2.1%) | 140 (100%) |
| I am confident that I can figure out solutions when new situations or problems arise with my health condition(s). | 1 (0.7%) | 14 (10.0%) | 84 (60.0%) | 38 (27.1%) | 3 (2.1%) | 140 (100%) |
| I am confident that I can maintain lifestyle changes, like diet and exercise, even during time of stress. | 0 (0%) | 23 (16.4%) | 74 (52.9%) | 42 (30.0%) | 1 (0.7%) | 140 (100%) |
PAM = Patient Activation Measure; NA = Not applicable
All patients recognized the importance of their active involvement in their health and most trusted health workers fully in solving their health problems. In contrast, more than 17.2% of patients had serious doubts in identifying the effect of their prescribed drugs and 16.4% on the ability to maintain lifestyle changes in both normal and stressful situations.
The implementation of the interventions improved the number of CD4 lymphocytes, without reaching the statistical significance.
The percentage of patients with an adequate adherence to antiretroviral therapy increased by 5.9% (78.4 vs. 84.2) and 18.4% (34.9 vs. 52.4) to concomitant treatment, the latter difference being statistically significant (p = 0.035).
DISCUSSION
This study found that a personalized and tailored pharmaceutical care model interventions based on patient stratification, motivational interview and used of new technologies can improve patient activation. In addition, this pharmaceutical care model was independently associated with better results in antiretroviral and non-antiretroviral medication adherence for HIV+ patients.
There are evidences from other cross-sectional studies that pharmaceutical care can support the improvement of patient health outcomes in HIV+, including adherence, but this is the first time that one specific model of pharmaceutical care enhance patient activation [29–31].
In our study, individualized and tailored interventions for every-single stratified population, including motivational interviews and the use of new technologies, improve patient activation, as measured through the PAM questionnaire.
This score can provide insight into possible strategies to improve patient activation at different stages on the pharmaceutical care process.
The use of the PAM tool as the basis for designing action plans and evaluating the progress of patients at the individual and collective level seems to be a viable and easy to incorporate approach. It is necessary to ensure that patients with follow-up plans based on the activation level obtain better health outcomes and require less resource than those that do not take this parameter into account.
However, we consider it essential to accompany this measure with the implementation of patient care stratification, since in this way it will be possible to identify the main care needs that the patient demands and design a pharmacotherapeutic follow-up and an individualized care plan.
The lack of experience in supporting patient motivation and engagement has been mentioned as a potential barrier for health organizations in their mission to improve care quality and decrease health care costs [32]. Carrying out motivational interviews during the patient’s follow-up will allow patients to achieve their pharmacotherapeutic objectives In this way, patients will be able to identify in a simpler way what are health behaviours that best suit them or, in case of difficulties or stress, to agree on an appropriate strategy over time to achieve it.
In general, it is believed that the use of new technologies in the health system offers patients better access to knowledge and a greater opportunity to become involved in their own care than in traditional face-to-face consultations.
To our knowledge, this is the first study assessing activation and empowerment of a pharmaceutical care model integrated for HIV patients. Although different approaches are being carried out in this area [11, 33], it is yet to define which is the ideal tool to be incorporated in a generalized way.
Hibbard et al developed the PAM questionnaire to efficiently assess patient knowledge, skills and confidence in managing their health [13, 16]. Our results indicate that the main points of improvement are in providing information about what the prescribed drugs do and the importance of maintaining healthy living habits. Usually, traditional models of pharmaceutical care have provided oral and written information on this aspect. Currently, we consider that, adapted to each educational level, it is necessary to incorporate visual (through new technologies) and emotional information (sharing experiences with patients in similar situations) to approach a strategy of success in this kind of patients.
The levels of activation of the patients have varied in the different studies depending on the kind of population (33, 34) and state of health (35). In this work, there were too few participants, only 5%, who maintained a low level of activation.
The success of efforts to implement CMO model in these populations may require employing additional individual characteristics study and strategies to aid them to become more activated.
Marshall R et al [10] study suggested that interventions which enhance patient activation may improve HIV clinical outcomes, and provide some insight regarding who would most benefit from their interventions. Higher levels of patient activation were associated with CD4 count>200 cells/mL, HIV-1 RNA viral suppression, and optimal antiretroviral adherence. However, they only included antiretroviral but no concomitant prescriptions. Our model improves not only adherence to antiretroviral treatment but in general to all prescription drugs. In this way, we were able to provide a key self-management skill for patients, that is essential not only to viral suppression but also to achieve other pharmacotherapeutic objectives, since HIV population is increasingly aging and has a greater number of comorbidities [34, 35]. Patients with high levels of activation have improved self-management skills, including better knowledge, confidence, and the ability to take medications as prescribed. Patient activation, as measured through the PAM, may represents a summary indicator that can potentially be used to predict adherence to all drug prescribed.
The present study had some limitations. The study population may not be representative of the general target population because patients from a single hospital were included in a limited period of time.
In addition, antiretroviral treatments have been changing annually, according to the guidelines and the new co-formulations available. In the last few years, strategies based on STR have increased, which may influence adherence. In our study, 45.7% of patients had STR regimen, a percentage lower than the current values.
On the other hand, the distribution of the percentage of patients included in each level of stratification was in line with what was expected and published for other patient cohorts analysed [22].
In addition, interventions were only drawn for a little period of time (6 months). However, this population is very similar to other cohorts in our country and although interventions supporting patient activation is not yet well developed, different studies have shown improvements in activation over similar periods of time [36].
Additionally, due to study design no other health care outcomes, such as hospital admission or mortality have been studied. Other studies have demonstrated the relationship between patient activation with health outcome measures for adult population with chronic conditions. More studies are needed to address the limitations of the current investigation and provide further insight into how to support patients best to be more effective participants in their care.
Specifically, for HIV-infected population further investigation research is needed to examine the association of PAM with prospective long-term follow-up studies Moreover, it is necessary to examine the impact of incremental changes in PAM scores on subsequent changes in key outcomes, in a multicentric and randomized study. And finally, it would be very interesting to know how people are capable of continuing to maintain this significant change overtime.
In conclusion, the findings highlight the important role that CMO model has for patient activation and which may involve improving adherence and health outcomes for HIV+ patients.
ACKNOWLEDEGMENTS
We wish to thank Ana Isabel Rosales Sanchez and Maria Gracia Hinojosa Hidalgo for their participation and support in this project.
FUNDING
PAM questionnaire has been used with the permission of the University of Oregon and with the support of Abbvie®. AbbVie® funded this research without interference in its contents.
CONFLICTS OF INTEREST
None to declare
REFERENCES
- 1.Palella FJ, Delaney KM, Moorman AC, Loveless MO, Fuhrer J, Satten GA, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med. 1998;338:853–60. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- 2.Mocroft A, Vella S, Benfield TL, Chiesi A, Miller V, Gargalianos P, et al. Changing patterns of mortality across Europe in patients infected with HIV-1. EuroSIDA Study Group. Lancet. 1998;352:1725–30. PMID: . [DOI] [PubMed] [Google Scholar]
- 3.Lohse N, Obel N. Update of Survival for Persons With HIV Infection in Denmark. Ann Intern Med. 2016;165:749. doi: 10.7326/L16-0091. [DOI] [PubMed] [Google Scholar]
- 4.XXX Sociedad Española de Farmacia Hospitalaria Modelo de Selección y Atención Farmacéutica de Pacientes Crónicos de la SEFH. Depósito. :25474–2013. [Google Scholar]
- 5.Marks G, Crepaz N, Senterfitt JW, Janssen RS. Meta-analysis of high-risk sexual behavior in persons aware and unaware they are infected with HIV in the United States: implications for HIV prevention programs. J Acquir Immune Defic Syndr. 2005;39:446–53. PMID: . [DOI] [PubMed] [Google Scholar]
- 6.Berg MB, Safren SA, Mimiaga MJ, Grasso C, Boswell S, Mayer KH. Nonadherence to medical appointments is associated with increased plasma HIV RNA and decreased CD4 cell counts in a community-based HIV primary care clinic. AIDS Care. 2005;17:902–7. doi: 10.1080/09540120500101658. [DOI] [PubMed] [Google Scholar]
- 7.Park WB, Choe PG, Kim S-H, Jo JH, Bang JH, Kim HB, et al. One-year adherence to clinic visits after highly active antiretroviral therapy: a predictor of clinical progress in HIV patients. J Intern Med. 2007;261:268–75. doi: 10.1111/j.1365-2796.2006.01762.x. [DOI] [PubMed] [Google Scholar]
- 8.Metsch LR, Pereyra M, Messinger S, Rio C del, Strathdee SA, Anderson-Mahoney P, et al. HIV Transmission Risk Behaviors among HIV-Infected Persons Who Are Successfully Linked to Care. Clin Infect Dis. 2008;47:577–84. doi: 10.1086/590153. [DOI] [PubMed] [Google Scholar]
- 9.Mugavero MJ, Davila JA, Nevin CR, Giordano TP. From access to engagement: measuring retention in outpatient HIV clinical care. AIDS Patient Care STDS. 2010;24:607–13. doi: 10.1089/apc.2010.0086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marshall R, Beach MC, Saha S, Mori T, Loveless MO, Hibbard JH, et al. Patient Activation and Improved Outcomes in HIV-Infected Patients. J Gen Intern Med. 2013;28:668–74. doi: 10.1007/s11606-012-2307-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morillo-Verdugo R, Robustillo-Cortes MA. Desarrollo de un programa de paciente experto 2.0 para pacientes VIH+. Revista multidisciplinar del Sida, 2015 (monográfico 2015); 1(6): 40-52. [Google Scholar]
- 12.Hibbard JH, Greene J. What the evidence shows about patient activation: better health outcomes and care experiences; fewer data on costs. Health Aff (Millwood). 2013;32:207–14. doi: 10.1377/hlthaff.2012.1061. [DOI] [PubMed] [Google Scholar]
- 13.Hibbard JH, Mahoney ER, Stockard J, Tusler M. Development and testing of a short form of the patient activation measure. Health Serv Res. 2005;40:1918–30. doi: 10.1111/j.1475-6773.2005.00438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shah SL, Siegel CA. Increasing Patient Activation Could Improve Outcomes for Patients with Inflammatory Bowel Disease. Inflamm Bowel Dis. 2015;21:2975–8. doi: 10.1097/MIB.0000000000000575. [DOI] [PubMed] [Google Scholar]
- 15.Kinney RL, Lemon SC, Person SD, Pagoto SL, Saczynski JS. The association between patient activation and medication adherence, hospitalization, and emergency room utilization in patients with chronic illnesses: a systematic review. Patient Educ Couns. 2015;98:545–52. doi: 10.1016/j.pec.2015.02.005. [DOI] [PubMed] [Google Scholar]
- 16.Hibbard JH, Stockard J, Mahoney ER, Tusler M. Development of the Patient Activation Measure (PAM): conceptualizing and measuring activation in patients and consumers. Health Serv Res. 2004;39:1005–26. doi: 10.1111/j.1475-6773.2004.00269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lorig K, Ritter PL, Villa FJ, Armas J. Community-Based Peer-Led Diabetes Self-management. Diabetes Educ. 2009;35:641–51. doi: 10.1177/0145721709335006. [DOI] [PubMed] [Google Scholar]
- 18.Stepleman L, Rutter M-C, Hibbard J, Johns L, Wright D, Hughes M. Validation of the patient activation measure in a multiple sclerosis clinic sample and implications for care. Disabil Rehabil. 2010;32:1558–67. doi: 10.3109/09638280903567885. [DOI] [PubMed] [Google Scholar]
- 19.Shively MJ, Gardetto NJ, Kodiath MF, Kelly A, Smith TL, Stepnowsky C, et al. Effect of Patient Activation on Self-Management in Patients With Heart Failure. J Cardiovasc Nurs. 2013;28:20–34. doi: 10.1097/JCN.0b013e318239f9f9. [DOI] [PubMed] [Google Scholar]
- 20.Hendriks M, Rademakers J. Relationships between patient activation, disease-specific knowledge and health outcomes among people with diabetes; a survey study. BMC Health Serv Res. 2014;14:393. doi: 10.1186/1472-6963-14-393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Calleja-Hernández MÁ, Morillo-Verdugo R. El modelo CMO en consultas externas de Farmacia Hospitalaria. Barcelona; 2016. Menarini: ISBN: 978-84-608-6548-3 [Google Scholar]
- 22.Morillo-Verdugo R, Martínez-Sesmero JM, Lázaro-López A, Sánchez-Rubio J, Navarro-Aznárez H, DeMiguel-Cascón M. Development of a risk stratification model for pharmaceutical care in HIV patients. Farm Hosp. 2017. 1;41(3):346-356.doi: 10.7399/fh.2017.41.3.10655. [DOI] [PubMed] [Google Scholar]
- 23.Morillo Verdugo R, Villarreal Arevalo AL, Alvarez De Sotomayor M, Robustillo Cortes MA. Development of a taxonomy for pharmaceutical interventions in HIV+ patients based on the CMO model. Farm Hosp. 2016;40:544–68. doi: 10.7399/fh.2016.40.6.10567. [DOI] [PubMed] [Google Scholar]
- 24.Grupo de expertos de la Secretaría del Plan Nacional sobre el SIDA (SPNS) , Sociedad Española de Geriatría y Gerontología (SEGG). Documento de consenso sobre edad avanzada e infección por el virus de la inmunodeficiencia humana. 2015; Noviembre Avaible at: https://www.segg.es/media/descargas/Documento-de-edad-avanzada-y-VIH.pdf ( Accesed 20 Feb 2017) [Google Scholar]
- 25.Libby AM, Fish DN, Hosokawa PW, Linnebur SA, Metz KR, Nair K V, et al. Patient-level medication regimen complexity across populations with chronic disease. Clin Ther. 2013;35:385–398.e1. doi: 10.1016/j.clinthera.2013.02.019. [DOI] [PubMed] [Google Scholar]
- 26.Inicio - Consulta de Atención Farmacéutica al Paciente con Patologías Víricas del Hospital de Valme. https://www.farmaciavalmecpv.com/. Accessed 26 Feb 2017. [Google Scholar]
- 27.Bonett DG, Wright TA. Sample size requirements for estimating pearson, kendall and spearman correlations. Psychometrika. 2000;65:23–8. doi: 10.1007/BF02294183. [DOI] [Google Scholar]
- 28.Bonett DG. Sample size requirements for estimating intraclass correlations with desired precision. Stat Med. 2002;21:1331–5. doi: 10.1002/sim.1108. [DOI] [PubMed] [Google Scholar]
- 29.Glasgow RE, Wagner EH, Schaefer J, Mahoney LD, Reid RJ, Glasgow RE Greene SM., Wagner EH, Schaefer J, Mahoney LD, Reid RJ, Greene SM. Development and validation of the Patient Assessment of Chronic Illness Care (PACIC). Med Care. 2005;43(5):436-44. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 30.Parchman ML, Zeber JE, Palmer RF. Participatory decision making, patient activation, medication adherence, and intermediate clinical outcomes in type 2 diabetes: a STARNet study. Ann Fam Med. 2010;8:410–7. doi: 10.1370/afm.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Greene J, Hibbard JH. Why does patient activation matter? An examination of the relationships between patient activation and health-related outcomes. J Gen Intern Med. 2012;27:520–6. doi: 10.1007/s11606-011-1931-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Deen D, Lu WH, Weintraub MR, Maranda MJ, Elshafey S, Gold MR. The impact of different modalities for activating patients in a community health center setting. Patient Educ Couns. 2012;89:178–83. doi: 10.1016/j.pec.2012.04.012. [DOI] [PubMed] [Google Scholar]
- 33.Crouch P-CB, Rose CD, Johnson M, Janson SL. A pilot study to evaluate the magnitude of association of the use of electronic personal health records with patient activation and empowerment in HIV-infected veterans. PeerJ. 2015;3:e852. doi: 10.7717/peerj.852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gleason LJ, Luque AE, Shah K. Polypharmacy in the HIV-infected older adult population. Clin Interv Aging. 2013;8:749–63. doi: 10.2147/CIA.S37738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marzolini C, Elzi L, Gibbons S, Weber R, Fux C, Furrer H, et al. Prevalence of comedications and effect of potential drug-drug interactions in the Swiss HIV Cohort Study. Antivir Ther. 2010;15:413–23. doi: 10.3851/IMP1540. [DOI] [PubMed] [Google Scholar]
- 36.Harvey L, Fowles JB, Xi M, Terry P. When activation changes, what else changes? the relationship between change in patient activation measure (PAM) and employees’ health status and health behaviors. Patient Educ Couns. 2012;88:338–43. doi: 10.1016/j.pec.2012.02.005. [DOI] [PubMed] [Google Scholar]