Abstract
Background
With the development of new techniques to easily obtain lower respiratory tract specimens, bronchoalveolar lavage fluid and other lung fluids are gaining importance in pulmonary disease diagnosis. We aimed to review and summarize lung fluid biomarkers associated with acute respiratory distress syndrome diagnosis and mortality.
Methods
After searching PubMed, Embase, Web of Science, and the Cochrane Library for articles published prior to January 11, 2018, we performed a meta-analysis on biomarkers for acute respiratory distress syndrome diagnosis in at-risk patients and those related to disease mortality. From the included studies, we then extracted the mean and standard deviation of the biomarker concentrations measured in the lung fluid, acute respiratory distress syndrome etiologies, sample size, demographic variables, diagnostic criteria, mortality, and protocol for obtaining the lung fluid. The effect size was measured by the ratio of means, which was then synthesized by the inverse-variance method using its natural logarithm form and transformed to obtain a pooled ratio and 95% confidence interval.
Results
In total, 1156 articles were identified, and 49 studies were included. Increases in total phospholipases A2 activity, total protein, albumin, plasminogen activator inhibitor-1, soluble receptor for advanced glycation end products, and platelet activating factor-acetyl choline were most strongly associated with acute respiratory distress syndrome diagnosis. As for biomarkers associated with acute respiratory distress syndrome mortality, interleukin-1β, interleukin-6, interleukin-8, Kerbs von Lungren-6, and plasminogen activator inhibitor-1 were significantly increased in the lung fluid of patients who died. Decreased levels of Club cell protein and matrix metalloproteinases-9 were associated with increased odds for acute respiratory distress syndrome diagnosis, whereas decreased levels of Club cell protein and interleukin-2 were associated with increased odds for acute respiratory distress syndrome mortality.
Conclusions
This meta-analysis provides a ranking system for lung fluid biomarkers, according to their association with diagnosis or mortality of acute respiratory distress syndrome. The performance of biomarkers among studies shown in this article may help to improve acute respiratory distress syndrome diagnosis and outcome prediction.
Electronic supplementary material
The online version of this article (10.1186/s13054-019-2336-6) contains supplementary material, which is available to authorized users.
Keywords: Respiratory distress syndrome, Adult, Acute lung injury, Bronchoalveolar lavage fluid, Biomarkers, Diagnosis, Mortality
Background
Acute respiratory distress syndrome (ARDS) is a clinical syndrome comprising a rapid onset of respiratory failure in patients with risk factors, such as refractory arterial hypoxemia with low reaction to supplemental oxygen and the presence of bilateral infiltrates on radiographic imaging [1]. To date, the diagnosis of ARDS and acute lung injury (ALI) is mostly based on clinical characterization. Frequently-used criteria are the American European Consensus Conference (AECC) criteria [2] and the Berlin definition [3].
As the accuracy of a diagnosis of ARDS based only on the clinical syndrome has been questioned, countless studies have focused on the identification of biomarkers for ARDS. Terpstra et al. conducted a meta-analysis in 2014 focused on plasma biomarkers for ARDS in humans and provided a ranking system for distinguishing the disease from at-risk patients and determining the prognosis [4]. They reviewed multiple plasma biomarkers for ARDS, ranked by pooled odds ratio (OR). However, they only summarized biomarkers for ARDS in plasma; biomarkers in other fluids, such as bronchoalveolar lavage fluid (BALF), were not evaluated.
BALF and other lung fluids, such as pulmonary edema fluid (PEF), epithelial lining fluid (ELF), and lung aspirational fluid (LAF), are definitive in respiratory disease diagnosis. Since BALF provides a sample closest to the site of the disease process, it reflects the local lung environment directly. In 2017, García-Laorden et al. reported that biomarkers representing epithelial apoptosis, such as Fas and FasL, as well as biomarkers reflecting extracellular matrix injury, such as procollagen peptide III (PCP III) and procollagen peptide I (PCP I), were elevated in ARDS BALF samples [5]. The aim of the present study was to compare biomarker levels in lung fluid samples among patients with ARDS and the at-risk controls, as well as those of non-survivors versus survivors of ARDS.
Methods
Data source and study selection
We manually searched PubMed, Embase, Wed of Science, and the Cochrane Library for studies on biomarkers for ARDS in lung fluid samples published prior to January 11, 2018. Details of the search strategy are listed in Additional file 1. We also searched the references of included studies. Two researchers screened and evaluated the eligibility of all studies independently, and a third reviewer intervened whenever there was a disagreement. The inclusion criteria were (1) original research report of adult with or at-risk of ARDS, (2) report of exact values of biomarker concentration in lung fluid related to a clinical outcome (diagnosis of ARDS in at-risk patients and/or mortality of ARDS), (3) description of demographic variables, and (4) written in English. The exclusion criteria were (1) written in languages other than English, (2) not related to ARDS/ALI, (3) not an original research, (4) in vivo/in vitro studies, (5) pediatric studies, (6) biomarker not measured in lung fluid, (7) biomarker used for treatment monitoring, and (8) only one article available for a specific biomarker for no mergeable effect size and low reliability.
Data extraction and quality assessment
We built Excel spreadsheets (Microsoft Corp., Redmond, WA) to extract data from the included studies, and the two researchers finished data extraction independently. The ARDS etiology and the mean or median level and standard deviation (SD) of the biomarker in the lung fluid were obtained. When a biomarker was measured sequentially, only the day 1 measurement was extracted. We extracted lung fluid biomarker levels from different subgroups as follows: patients with ARDS versus critically ill non-ARDS controls and survivors versus non-survivors in patients with ARDS. The mean value of a biomarker’s concentration was equal to the median level in this study, and standard error (SE) was converted to SD using an Excel formula. In addition, demographic variables (age, sex, and number of participants for each subgroup), diagnostic criteria for ARDS, ARDS mortality, the moment the lung fluid sample was retrieved, the sample type (BALF/other than BALF), sample retrieval location, and volume of BALF irrigation solution used were recorded. The recovery rate of BALF was also recorded, if provided.
All studies were assessed for quality according to the Quality Assessment of Diagnostic Accuracy Studies Score-2 (QUADAS-2), and the content was tailored according to the guideline of QUADAS-2 [6]. Details of the tailored QUADAS-2 are listed in Additional file 2. Risk of bias and an applicability concerns graph/summary was conducted using Review Manager version 5.3 (Cochrane Collaboration, Oxford, UK).
Data synthesis and data analysis
Meta-analysis was performed with Stata 13.1 (StataCorp LLC, College Station, TX). The ratio of means (RoM) was employed to assess the effect size [7–9]. RoM is the mean value of a biomarker in the ARDS group divided by the mean value in the at-risk group (meanARDS/meanat-risk) or the mean value of a biomarker in the non-survivors group divided by the mean value in the survivors group (meannon-survivor/meansurvivor). RoM of each study was log transformed and pooled using the inverse-variance method to gain a pooled, transformed RoM, which was then back-transformed to determine the pooled RoM and 95% confidence interval, using the fixed effect model of the Stata software. The significance level for this meta-analysis model was set at p < .05. Forest plots were provided for biomarkers of which four or more studies were included in this meta-analysis. Biomarkers were ranked according to pooled RoM and statistical significance. We used the Q statistic to test the existence of heterogeneity; a p value of less than 0.10 was considered significant for heterogeneity. I2 was employed to assess the proportion of total variability due to heterogeneity. An I2 value of approximately 25% was regarded as low heterogeneity, 50% as medium, and 75% as high heterogeneity. Publication bias was assessed with Egger’s regression test [10], where a p value of less than 0.10 was considered significant for publication bias. Duval and Tweedie’s trim and fill was then conducted [11].
For the biomarkers with a significant RoM and existence of heterogeneity, we performed a subgroup meta-analysis on study type (case-control study versus another study type) or sample type (BALF versus other lung fluid), when three or more studies were included.
Results
Literature search
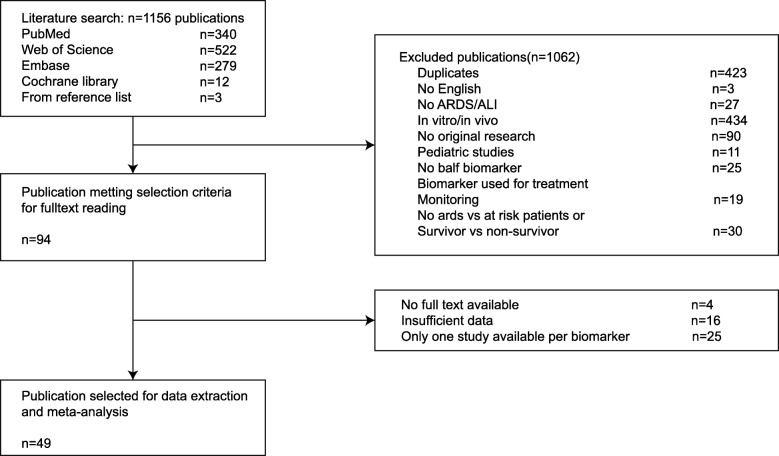
The total literature search yielded 1156 articles from the databases as follows: PubMed, 340 articles; Web of Science, 522 articles; Embase, 279 articles; Cochrane Library, 12 articles; and 3 articles from the reference lists of included studies. By reviewing the titles and abstracts, studies were mainly excluded due to the following: in vitro/animal studies (n = 434), duplication (n = 423), not an original research (reviews, editorials, or case reports, n = 90), and biomarkers not related with occurrence or mortality of ARDS (n = 30). After the initial screening, 95 articles remained for full-text review. Of these, 25 articles only reported on a specific biomarker, 16 articles contained insufficient data, and 4 articles had no full-text copy available, despite attempts to contact the authors. The remaining 49 articles were used for the meta-analysis [12–60] (Fig. 1).
Fig. 1.
Flowchart of study selection. ARDS, acute respiratory distress syndrome; ALI, acute lung injury; vs, versus
Study characteristics and quality assessment
Demographic variables of the included studies are summarized in (Table 1). A total of 49 articles involving 2189 patients were included in this meta-analysis. ARDS/ALI was diagnosed according to the AECC criteria in 71% of the studies. Other criteria, such as edema fluid/plasma protein ratio [61], lung injury score [62], Fowler criteria [63], and clinical criteria, were used along with the AECC criteria. The mean age ranged from 37 to 70 years, mortality rate ranged from 15 to 77%, and lung fluid was collected between 30 min of intubation and 72 h of ARDS diagnosis. As for sample retrieval location, the right middle lobe and lingular lobe were the most common. Other locations were based on abnormal areas identified on chest radiographs, and a blind sampling of BALF was performed in two studies. In regard to sample type, 69% of the studies measured biomarkers in BALF with a certain volume of irrigation solution. Ten articles used pulmonary edema fluid, and four articles measured a biomarker in epithelial lining fluid. Only three articles provided the recovery rate of the irrigation solution; therefore, we could only assume a stable recovery rate between subgroups for this study.
Table 1.
Demographic variables
| Reference | Biomarkers | Diagnostic criteria for ARDS | Study size | Male (%) | Age | Mortality (%) | Sample retrieved time | Sample retrieved location | Sample type (vol. of irrigation solution) |
|---|---|---|---|---|---|---|---|---|---|
| Studies related to diagnosis | |||||||||
| Armstrong [14] | Procollagen peptide I | AECC | 66 | 66.7 | 64.65 | 48.5 (nsp.) | Within 48 h of ICU admission | Right middle lobe | BALF (20 mL*6) |
| Bersten [15] | Total phospholipids | Clinical criteria | 21 | np. | np. | np. | Within 7 h of study entry | np. | LAF |
| Calfee [16] | Soluble intercellular adhesion molecule-1 | Clinical criteria | 67 | 59.84 | 50.63 | 44.79 (hosp mort) | Within 4 h of intubation | np. | PEF |
| Chollet-Martin [18] | Interleukin-8 | LIS | 29 | np. | 62.12 | np. | Within 72 h of ARDS diagosis | np. | ELF |
| Tumor necrosis factor-α | |||||||||
| Interleukin-6 | |||||||||
| Conner [20] | Soluble intercellular adhesion molecule-1 | Clinical criteria+EF/plasma protein | 27 | np. | np. | np. | Within 1 h of intubation | np. | PEF |
| Delclaux [21] | Albumin | Clinical criteria | 29 | 71.43 | 54 | 64.29 (nsp.) | np. | np. | BALF (3*50mL) |
| El Solh [22] | Plasminogen activator inhibitor-1 | AECC | 51 | 43.14 | 36.57 | 5.88 (hosp mort) | Within 8 h of intubation | Blind BAL | PEF |
| Prabhakaran [52] | Plasminogen activator inhibitor-1 | AECC | 51 | 58 | 50 | 47 (hosp mort) | Within 12h of intubation | np. | PEF |
| Song [56] | Plasminogen activator inhibitor-1 | Clinical criteria | 33 | 39 | 70 | 15 (28-day mort) | When patients were suspected to have VAP | np. | BALF (20mL*3) |
| Farjanel [23] | Procollagen peptide III | LIS | 61 | 60.7 | 49.23 | 47.5 (hosp mort) | 3 days after intubation | A subsegmental of middle lobe | BALF (3*50mL) |
| Procollagen peptide I | |||||||||
| Albumin | |||||||||
| Geerts [25] | Club cell protein | AECC | 26 | 73.1 | 51 | np. | Within 12 h of ARDS diagnosis | Right middle lobe | BALF (50mL*3) |
| Total protein | |||||||||
| González-López [26] | Interleukin-8 | AECC | 22 | np. | 50.1 | 31.8 (ICU mort) | np. | np. | BALF (20mL*3) |
| Matrix metalloproteinases-9 | |||||||||
| Procollagen peptide III | |||||||||
| Interleukin-6 | |||||||||
| Vascular endothelial growth factor | |||||||||
| Hallgren [27] | Albumin | Clinical criteria | 40 | 66.67 | 46.87 | 25 (nsp.) | np. | Right middle lobe | BALF (3*20mL) |
| Hamacher [28] | Tumor necrosis factor-α | AECC | 56 | 75 | 45.41 | 25.07 (hosp mort) | np. | np. | BALF (nsp.) |
| Soluble TNF-α receptors II | |||||||||
| Transforming growth factor-β1 | |||||||||
| Idell [29] | Total protein | Clinical criteria | 16 | np. | np. | np. | Within 96 h of ARDS diagnosis | Right middle lobe/right lower lobe/lingula | BALF (30mL*5) |
| Jabaudon [60] | Soluble receptor for advanced glycation end products | AECC | 60 | 55 | 61 | 66.67 | Within 24 h of disease onset. | np. | LAF |
| Karagiorga [32] | Total protein | Clinical criteria | 31 | 64.52 | 41.43 | 22.58 (nsp.) | Within 72 h of ARDS diagnosis | np. | BALF (20mL*6) |
| Platelet activating factor-acetyl choline | |||||||||
| Total phospholipids | |||||||||
| Total phospholipases A2 activity | |||||||||
| Keane [33] | Interleukin-8 | AECC | 9 | np. | np. | np. | np. | np. | BALF (nsp.) |
| Procollagen peptide III | |||||||||
| Procollagen peptide I | |||||||||
| Kurdowska [36] | Interleukin-8 | LIS | 26 | np. | np. | 41.2 (nsp.) | Upon ICU admission | Right middle lobe | BALF (60mL*3) |
| Lanchou [37] | Matrix metalloproteinases-9 | AECC | 21 | 47.6 | 54 | 33.3 (nsp.) | Within 24 h of ARDS diagnosis | Right middle lobe | BALF (20mL*3) |
| Matrix metalloproteinases-2 | |||||||||
| Martin [44] | Total protein | AECC | 69 | 68 | 45 | 56 (nsp.) | Within 72 h of ARDS diagnosis | Right middle lobe/lingula | BALF (30mL*5) |
| Martin [43] | Soluble receptor for advanced glycation end products | AECC | 69 | 59 | 53.9 | 46 (nsp.) | np. | np. | BALF (nsp.) |
| Medford [45] | Vascular endothelial growth factor | AECC | 70 | 40 | 59.37 | 38.57 (28-day mort) | With 72 h of intubation | np. | ELF |
| Miller [46] | Interleukin-8 | AECC | 35 | np. | 48.54 | np. | Within 30 min of intubation | np. | PEF |
| Nakos [49] | Total protein | AECC | 21 | 76.2 | 61 | np. | Within 24 h of intubation | Right middle lobe/lingula. | BALF (20mL*6) |
| Albumin | |||||||||
| Platelet activating factor-acetyl choline | |||||||||
| Total phospholipids | |||||||||
| Nakos [48] | Total protein | AECC | 19 | 66.67 | 45 | 33.33 (nsp.) | Within 12 h of intubation | np. | BALF (20mL*6) |
| Albumin | |||||||||
| Platelet activating factor-acetyl choline | |||||||||
| Total phospholipids | |||||||||
| Nakos [47] | Platelet activating factor-acetyl choline | AECC | 31 | 29 | 55.54 | 32.2 (ICU mort) | Upon ARDS diagnosis | np. | BALF (20mL*6) |
| Total phospholipases A2 activity | |||||||||
| Park [51] | Tumor necrosis factor-α | AECC | 54 | 59.1 | 44.67 | 20.1 (nsp.) | Within 24 h of ARDS diagnosis | Right middle lobe/lingula | BALF (30mL*5) |
| Soluble TNF-α receptors II | |||||||||
| Interleukin-1β | |||||||||
| Interleukin-6 | |||||||||
| Pugin [53] | Interleukin-8 | AECC | 31 | 54.84 | 48.87 | 57.65 (nsp.) | np. | blind BAL | PEF |
| Matrix metalloproteinases-2 | |||||||||
| Procollagen peptide III | |||||||||
| Ricou [55] | Interleukin-6 | Clinical criteria+LIS | 24 | 79.2 | 50.5 | 33.3 (nsp.) | Within 24 h of ARDS diagnosis | np. | BALF (nsp.) |
| Stern [57] | Procollagen peptide III | AECC | 25 | 64 | 67.08 | 60 (30-day mort) | Within 72 h of ARDS diagnosis | The abnormal area on the chest radiography | BALF (20mL*6) |
| Albumin | |||||||||
| Hepatic growth factor | |||||||||
| Uchida [58] | Soluble receptor for advanced glycation end products | AECC | 33 | 57.67 | 43 | 51.33 (hosp mort) | np. | np. | PEF |
| Studies related to mortality | |||||||||
| Adamzik [12] | Tumor necrosis actor-α | AECC | 47 | 68.09 | 44.53 | 36.17 (30-day mort) | Within 24 h of ICU admission | np. | BALF (40mL*4) |
| Total protein | |||||||||
| Interleukin-6 | |||||||||
| Interleukin-2 | |||||||||
| Interleukin-1β | |||||||||
| Interleukin-10 | |||||||||
| Clark [19] | Procollagen peptide III | AECC | 117 | 64.1 | 42.78 | 41 (hosp mort) | Within 72h of ARDS diagosis | Right middle lobe/lingula | BALF (30mL*5) |
| Frenzel [24] | Interleukin-6 | AECC | 46 | 60.9 | 62 | 45.7 (28-day mort) | Within 96h of intubation | Right middle lobe/lingula | BALF (20mL*5) |
| Interleukin-8 | |||||||||
| Tumor necrosis factor-α | |||||||||
| Interleukin-1β | |||||||||
| Interleukin-10 | |||||||||
| Kondo [34] | Kerbs von Lungren-6 | AECC | 32 | 84.38 | 70.1 | 31.3 (hosp mort) | Within 24 h of ARDS diagnosis | Right middle lobe | ELF |
| Lee [38] | Interleukin-8 | AECC | 31 | 51.6 | 54.5 | 51.6 (28-day mortality) | Within 48 h of ARDS diagnosis | Right middle lobe/lingular | BALF (30mL*5) |
| Lin [41] | Interleukin-6 | AECC | 39 | 69.2 | 68 | 43.6 (hosp mort) | Within 24h of ARDS diagnosis | Right middle lobe/lingula | BALF (20mL*6) |
| Interleukin-8 | |||||||||
| Nathani [50] | Kerbs von Lungren-6 | AECC | 42 | 57.1 | 60.13 | np. | Upon study entry | Middle lobe | BALF (50mL*3) |
| Ishizaka [30] | Kerbs von Lungren-6 | AECC | 38 | 77% | 68 | 32 (hosp mort) | Upon onset of ARDS | Right middle lobe | ELF |
| Studies related to diagnosis and mortality | |||||||||
| Agouridakis [13] | Interleukin-2 | AECC | 34 | 74.42 | 47.79 | 27.9 (nsp.) | Within 2h of ICU admission | np. | BALF (nsp.) |
| Chesnutt [17] | Procollagen peptide III | AECC+EF/plasma protein | 44 | 47.73 | 56.25 | 63.64 (hosp mort) | Within 1h of intubation | np. | PEF |
| Jorens [31] | Club cell protein | Fowler+LIS | 35 | 88.6 | 55 | 42.9 (nsp.) | Within 12 h of ARDS diagnosis | Right middle lobe | BALF (50mL*3) |
| Kropski [35] | Club cell protein | AECC+EF/plasma protein | 32 | 46.9 | 48 | 56.5 (nsp.) | Within 24 h of intubation | np. | PEF |
| Lee [39] | IL-8 | Clinical criteria | 112 | 78.6 | 66.5 | 77.3 (nsp) | With 24h of ICU admission | The most abnormal area on the chest radiography/right middle lobe/lingula | BALF (20mL*6) |
| Tumor necrosis factor-α | |||||||||
| Interleukin-1β | |||||||||
| Interleukin-6 | |||||||||
| Interleukin-10 | |||||||||
| Lesur [40] | Interleukin-2 | AECC | 33 | 36.4 | 51.52 | 24.2 (hosp mort) | Within 72h of intubation | Right middle lobe/lingula | BALF (20mL*5) |
| Marshall [42] | Procollagen peptide III | AECC | 60 | 53.33 | 51.35 | 31.67 (nsp.) | Within 24h of ARDS diagnosis | np. | BALF (nsp.) |
| Quesnel [54] | Interleukin-8 | AECC | 122 | 64.8 | 67 | 33.6 (28-day mort) | np. | np. | BALF (20mL*6) |
| Procollagen peptide I | |||||||||
| Transforming growth factor-β1 | |||||||||
| Hepatic growth factor | |||||||||
| Ware [59] | Vascular endothelial growth factor | AECC | 102 | 62.8 | 49 | 60 (nsp.) | np. | np. | PEF |
ARDS acute respiratory distress syndrome, AECC American-European Consensus Conference, EF edema fluid, LIS lung injury score, np not provided, nsp not specific, mort mortality, hosp mort hospital mortality, ICU intensive care unit, BALF bronchoalveolar lavage fluid, LAF lung aspirational fluid, ELF epithelial lining fluid, PEF pulmonary edema fluid
The ARDS etiologies are summarized in Additional file 3. The most common cause of ARDS was sepsis (30.87%), followed by pneumonia (23.70%), trauma (10.94%), aspiration (8.53%), transfusion (4.23%), and major surgery (3.47%). Other etiologies included vasculitis, retroperitoneal hematoma-DIC, drug overdose, reperfusion injury, and diabetic ketoacidosis.
The quality assessment is displayed in Additional file 4, including the risk of bias and applicability of studies to the review question.
Biomarkers associated with ARDS diagnosis
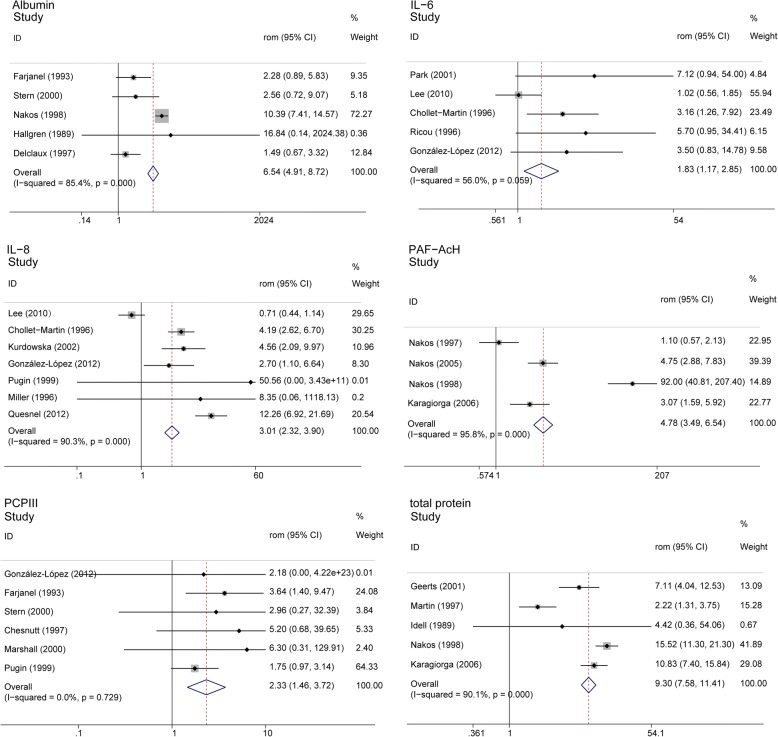
We performed a meta-analysis on 22 biomarkers in lung fluid associated with the diagnosis of ARDS in the at-risk population (Table 2); Fig. 2 shows the forest plots for biomarkers available in at least 3 studies. Pooled RoM values for total phospholipases A2 activity (total PLA2 activity) (17.995 [11.381, 28.454]), total protein (9.299 [7.575, 11.414]), albumin (6.544 [4.908, 8.725]), plasminogen activator inhibitor-1 (PAI-1) (5.525 [3.876, 7.877]), soluble receptor for advanced glycation end products (sRAGE) (4.901 [3.603, 7.673]), platelet activating factor-acetyl choline (PAF-AcH) (4.783 [3.495, 6.545]), soluble tumor necrosis factor-α receptors II (STNF-RII) (3.253 [1.765, 5.993]), hepatic growth factor (HGF) (3.199 [1.668, 6.135]), and interleukin-8 (IL-8) (3.008 [2.322, 3.896]) were the highest. The overall effect size ranged from 0.548 to 17.995, among biomarkers with significant RoM between subgroups, and decreased Club cell protein (CC16) (0.553 [0.369, 0.827]) and matrix metalloproteinases-9 (MMP-9) (0.548 [0.336, 0.893]) levels in lung fluid indicated a higher possibility of ARDS diagnosis in the at-risk population. However, a pervasive heterogeneity was displayed.
Table 2.
Biomarkers associated with ARDS diagnosis
| Biomarker | No. of study | No. of patients | RoM (95% CI) | p | Heterogeneity | |
|---|---|---|---|---|---|---|
| Q (p value) | I2 (%) | |||||
| Total phospholipases A2 activity | 2[32, 47] | 62 | 17.995 (11.381, 28.454) | < 0.05 | 10.54 (0.001) | 90.50 |
| Total protein | 5[25, 29, 32, 44, 48] | 179 | 9.299 (7.575, 11.414) | < 0.05 | 40.48 (< 0.1) | 90.10 |
| Albumin | 5[21, 23, 27, 48, 57] | 191 | 6.544 (4.908, 8.725) | < 0.05 | 27.35 (< 0.1) | 85.40 |
| Plasminogen activator inhibitor-1 | 3[22, 52, 56] | 135 | 5.525 (3.876, 7.877) | < 0.05 | 3.69 (0.158) | 45.8 |
| Soluble receptor for advanced glycation end products | 3[43, 58, 60] | 162 | 4.901 (3.603, 7.673) | < 0.05 | 31.19 (0.000) | 93.6 |
| Platelet activating factor-acetyl choline | 4[32, 47–49] | 120 | 4.783 (3.495, 6.545) | < 0.05 | 71.83 (< 0.1) | 95.80 |
| Soluble TNF-α receptors II | 2[28, 51] | 110 | 3.253 (1.765, 5.993) | < 0.05 | 4.95 (0.026) | 79.80 |
| Hepatic growth factor | 2[54, 57] | 144 | 3.199 (1.668, 6.135) | < 0.05 | 0.02 (0.892) | 0 |
| Interleukin-8 | 7[18, 26, 36, 39, 46, 53, 54] | 377 | 3.008 (2.322, 3.896) | < 0.05 | 62.08 (< 0.1) | 90.30 |
| Soluble intercellular adhesion molecule-1 | 2[16, 20] | 96 | 2.952 (1.902, 4.581) | < 0.05 | 0.28 (0.6) | 0 |
| Procollagen peptide I | 2[14, 23] | 133 | 2.949 (1.867, 4.659) | < 0.05 | 0.11 (0.743) | 0.00 |
| Interleukin-2 | 2[13, 40] | 67 | 2.761 (1.508, 5.057) | 0.001 | 17.34 (< 0.1) | 94.20 |
| Procollagen peptide III | 6[17, 23, 26, 42, 53, 57] | 195 | 2.328 (1.456, 3.723) | < 0.05 | 2.81 (0.729) | 0 |
| Interleukin-6 | 5[18, 26, 39, 51, 55] | 250 | 1.826 (1.170, 2.852) | 0.008 | 9.1 (0.0059) | 56 |
| Club cell protein | 3[25, 31, 35] | 93 | 0.553 (0.369, 0.827) | 0.004 | 3.6 (0.166) | 44.40 |
| Matrix metalloproteinases-9 | 2[26, 37] | 43 | 0.548 (0.336, 0.893) | 0.016 | 15.45 (< 0.1) | 93.50 |
| Transforming growth factor-β1 | 2[28, 54] | 116 | 1.32 (0.575, 3.034) | 0.513 | 0.93 (0.334) | 0 |
| Tumor necrosis factor-α | 4[18, 28, 39, 51] | 247 | 1.3 (0.917, 1.843) | 0.14 | 2.97 (0.397) | 0 |
| Matrix metalloproteinases −2 | 2[37, 53] | 52 | 1.066 (0.889, 1.278) | 0.493 | 0.06 (0.814) | 0 |
| Total phospholipids | 4[15, 32, 48, 49] | 110 | 1.003 (0.862, 1.166) | 0.973 | 34.25 (< 0.1) | 91.20 |
| Interleukin-1β | 2[39, 51] | 166 | 0.952 (0.628, 1.444) | 0.817 | 4.23 (0.04) | 76.30 |
| Vascular endothelial growth factor | 3[26, 45, 59] | 194 | 0.812 (0.544, 1.212) | 0.309 | 3.4 (0.183) | 41.20 |
Numbers within the square brackets were reference numbers
RoM ratio of means, CI confident interval
Fig. 2.
Forest plot for acute respiratory distress syndrome (ARDS) diagnosis. RoM, ratio of means; CI, confident interval; IL-6, interleukin-6; IL-8, interleukin-8; PAF-ACH, platelet activating factor-acetyl choline; PCPIII, procollagen peptide III
We performed an influence analysis to examine the sensitivity of the results. Influence analysis showed that the heterogeneity was possibly caused by the limited number of studies. By removing the studies with extreme RoM, we observed a robust effect on the biomarkers. The outcome of the influence analysis is displayed in Additional file 5.
Subgroup analysis was performed for biomarkers IL-8, total protein, albumin, sRAGE, PAF-AcH, and IL-6. Since most of the included studies were case-control studies, we excluded studies with other design types. Heterogeneity for total protein, albumin, and IL-6 was partly explained. However, heterogeneity for IL-8 was not clarified. Only one article was case-control study; therefore, the source of heterogeneity was not determined for PAF-AcH because of a limited number of study. We also excluded studies with ARDS that was not diagnosed using AECC criteria, which significantly reduced the heterogeneity for IL-6, but not for albumin. In addition, we assumed sample type may be a variable between studies because biomarker measurement in BALF was influenced by recovery rate and dilution. IL-8 remained significantly increased when BALF studies were excluded. As only one article measured biomarkers in lung fluid other than BALF, it was not evaluated. Results of the subgroup analysis are presented in Additional file 6.
Biomarkers associated with ARDS mortality
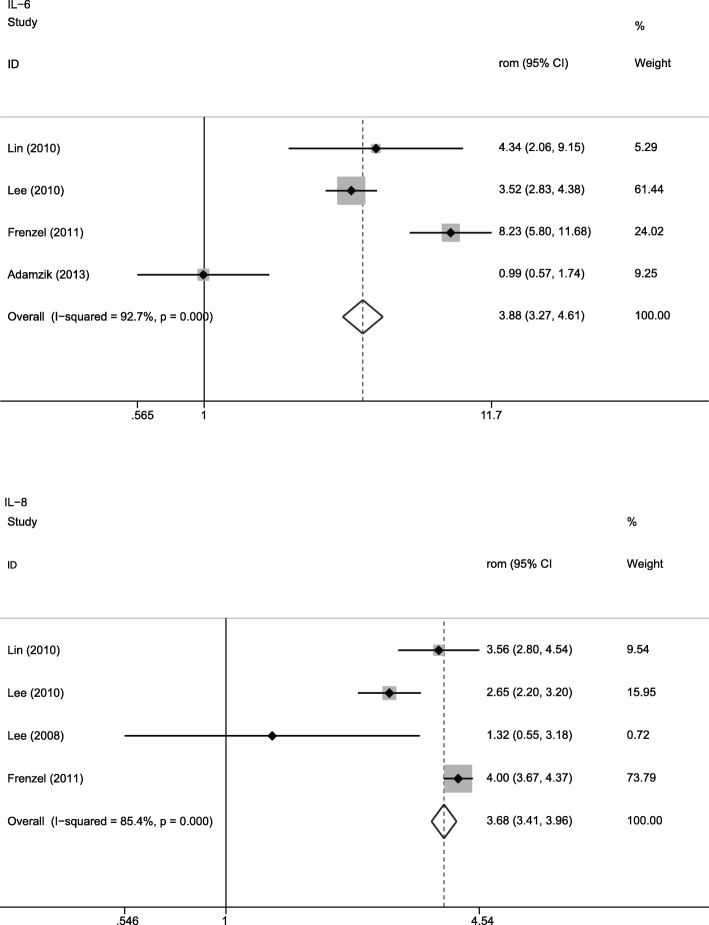
We performed a meta-analysis on 11 biomarkers in lung fluid associated with ARDS mortality (Table 3); Fig. 3 shows the forest plots for biomarkers associated with ARDS mortality. Interleukin-1β (IL-1β) (4.617 [4.331, 4.921]), IL-6 (3.882 [3.270, 4.608]), IL-8 (3.679 [3.414, 3.964]), and Kerbs von Lungren-6 (KL-6) (3.178 [2.931, 3.446]) ranked the highest in biomarkers associated with ARDS mortality. The overall effect size ranged from 0.406 to 4.617. Among the biomarkers with a significant difference between survivors and non-survivors, decreased levels of interleukin-2 (IL-2) (0.828 [0.715, 0.959]) and CC16 (0.406 [0.362, 0.405]) were associated with a high mortality rate. Heterogeneity was displayed for many of the biomarkers, and influence analysis indicated that the heterogeneities were not likely caused by extreme RoM values. Due to the small number of studies, subgroup analysis based on design type or sample type could not be performed. Subgroup analysis for tumor necrosis factor-α (TNF-α) was performed when excluding patients with ARDS not diagnosed using AECC criteria. Results of the subgroup analysis are presented in Additional file 7.
Table 3.
Biomarkers associated with ARDS mortality
| Biomarker | No. of study | No. of patients | RoM (95% CI) | p | Heterogeneity | |
|---|---|---|---|---|---|---|
| Q (p value) | I2 (%) | |||||
| Interleukin-1β | 3[12, 24, 39] | 137 | 4.617 (4.331, 4.921) | < 0.05 | 11.24 (0.004) | 82.20 |
| Interleukin-6 | 4[12, 24, 39, 41] | 176 | 3.882 (3.270, 4.608) | < 0.05 | 41.09 (< 0.1) | 92.70 |
| Interleukin-8 | 4[24, 38, 39, 41] | 160 | 3.679 (3.414, 3.964) | < 0.05 | 20.59 (< 0.1) | 85.40 |
| Kerbs von Lungren-6 | 2[30, 34] | 65 | 3.178 (2.931, 3.446) | < 0.05 | 0.83 (0.363) | 0.00 |
| Plasminogen activator inhibitor-1 | 2[52, 56] | 32 | 2.085 (2.039, 2.133) | < 0.05 | 1.31 (0.252) | 23.70 |
| Tumor necrosis factor-α | 3[12, 24, 39] | 137 | 1.923 (1.656, 2.233) | < 0.05 | 11.47 (0.003) | 82.60 |
| Procollagen peptide III | 3[17, 19, 42] | 194 | 1.714 (1.613, 1.822) | < 0.05 | 34.08 (< 0.1) | 94.10 |
| Total protein | 3[12, 19, 42] | 208 | 1.667 (1.595, 1.742) | < 0.05 | 82.47 (< 0.1) | 97.60 |
| Interleukin-2 | 2[13, 40] | 27 | 0.828 (0.715, 0.959) | 0.012 | 1.49 (0.223) | 32.70 |
| Club cell protein | 2[31, 35] | 37 | 0.406 (0.362, 0.405) | < 0.05 | 21.08 (< 0.1) | 95.30 |
| Interleukin-10 | 3[12, 24, 39] | 137 | 1.019 (0.922, 1.127) | 0.709 | 54.54 (< 0.1) | 96.30 |
Numbers within the square brackets were reference numbers
RoM ratio of means, CI confident interval
Fig. 3.
Forest plot for acute respiratory distress syndrome (ARDS) mortality. RoM, ratio of means; CI, confident interval; IL-6, interleukin-6; IL-8, interleukin-8
Publication bias
Among the biomarkers associated with ARDS, Egger’s regression test demonstrated a p value of less than 0.10 for IL-6; furthermore, when we adjusted for possible publication bias by Duval and Tweedie’s trim and fill, the RoM remained significant for IL-6. Among the biomarkers associated with mortality, no publication bias was noted. The results of the publication bias analysis are presented in Additional file 8.
Discussion
In this systematic review and meta-analysis, we summarized the biomarkers related to ARDS diagnosis in the at-risk population and those related to ARDS mortality.
By searching several databases and screening for related articles, 49 studies involving 2189 patients were identified.
We discovered that total protein, albumin, PAI-1, PAF-ACH, sTNFα-RII, HGF, IL-8, PCP I, PCP III, soluble receptor for advanced glycation end products (SRAGE), and IL-6 were significantly increased in the lung fluid of patients with ARDS. Although total PLA2 activity, soluble intercellular adhesion molecule-1 (SICAM-1), IL-2, CC16, and MMP-9 were also significantly different between patients with ARDS and at-risk patients, few studies were included on each of these biomarkers, so the results are unreliable.
IL-1β, IL-6, IL-8, TNF-α, PCPIII, and total protein were significantly increased in lung fluid of patients who died in the ARDS cohort. Furthermore, few studies for KL-6, plasminogen activator inhibitor-1 (PAI-1), IL-2, and CC16 were included, although these biomarkers were significantly different between survivors and non-survivors.
Pioneering work by Terpstra et al. reported on plasma biomarkers for ARDS diagnosis and prognosis. They reported that KL-6, lactate dehydrogenase (LDH), SRAGE, von Willebrand factor (vWF), and IL-8 displayed the highest effect size for ARDS diagnosis, and interleukin-4 (IL-4), IL-2, Angiopoietin-2 (Ang-2), and KL-6 had the highest effect size for ARDS prognosis (assessed by pooled odds ratio). These biomarkers represent pathophysiological processes, which led to the hypothesis that ARDS diagnosis is correlated with tissue damage, whereas ARDS mortality is correlated with systemic inflammation (4). In our meta-analysis, biomarkers for ARDS diagnosis were related to inflammation (IL-8 and IL-6), endothelial injury (SICAM), epithelial injury (SRAGE and HGF), lung fibroproliferation (PCPI and PCPIII), and coagulopathy (PAF-ACH). With regard to ARDS mortality, biomarkers related to inflammation (IL-8, IL-6, and IL-1β), epithelial injury (KL-6), and lung fibroproliferation (PCPIII) were elevated in the lung fluid of patients with ARDS presenting the worst outcomes. Therefore, we assume that, aside from tissue damage and systemic inflammation, lung fibroproliferation is vital in both ARDS diagnosis and prognosis. Several studies on lung biopsy of patients with ARDS showed a strong relationship between fibrosis activity and ARDS mortality [64, 65].
To our knowledge, this is the first meta-analysis of biomarkers in lung fluid for ARDS. Since lower respiratory tract specimens are now easily obtained, BALF and other lung fluids have become frequently used clinical samples for pulmonary disease diagnosis, secondary to plasma/serum. Since the most intensive physiological processes in ARDS occur in the lung, theoretically, lung fluid can reflect the pathophysiological process differently from other body fluids.
In this analysis, we applied RoM to assess the effect size and to attempt to eliminate the bias caused by dilution of different kinds of lung fluid. For example, edema fluid was completely undiluted, whereas BALF might be quite diluted. This methodology has been proved to be robust and is widely used [7–9, 66, 67].
This systematic review and meta-analysis may prompt further research on ARDS diagnosis and prognosis in many different ways. First, it demonstrates the research priorities and indicates that research on ARDS biomarkers in lung fluid and other compartments is needed. Second, it establishes ARDS biomarkers and their performance in lung fluid as an innovative research field. Third, it identifies numerous novel translational approaches for biomarker measurement in different compartments, such as chromatography for metabolomics separation and mass spectrometry or nuclear magnetic resonance spectroscopy for biomarker detection [68]. Although some non-quantitative methods were not included in this meta-analysis, they are still worth exploring.
There were limitations in this meta-analysis as well. First, although we performed a subgroup analysis of the biomarkers related to ARDS diagnosis and mortality, heterogeneity was not explainable for every biomarker. We assume that this could be related to the different etiologies of ARDS, variation in BALF procedures between studies, multiple control types used in the studies, wide range of intervals between study inclusion and biomarker measurement, and different treatments for ARDS. We were not able to conduct further analysis due to the limited information.
Second, a limited number of studies were included for each biomarker, which impedes the reproducibility of the results. The number of studies for each biomarker should be taken into consideration while assessing the performance in the ranking system.
Third, only the biomarkers addressed by two or more studies were included. Because of this, promising biomarkers evaluated in a single study were not considered, which may limit the view on lung fluid biomarker research as a whole.
Finally, the use of lung fluid as a study object itself had some limitations. Due to the lack of information on specific BALF procedures, we could only assume the recovery rates between subgroups in one study were within an acceptable range, which may have caused some of the heterogeneity between studies.
Conclusions
This systematic review and meta-analysis included 49 studies with 2189 participants, providing an overview of research on lung fluid biomarkers for ARDS. The ranking system provided by evaluating the effect size for ARDS diagnosis and prognosis may serve as a reference for further research on biomarkers for ARDS.
Additional files
Search strategy. (DOCX 17 kb)
Tailored QUADAS-2. (DOCX 17 kb)
ARDS etiologies. (DOCX 13 kb)
Result of quality assessment. Low = low possibility of risk of bias. High = means high possibility of high risk of bias. Index test(s) = measurement used in an article for biomarker concentration detection. Reference standard = criteria used for acute respiratory distress syndrome diagnosis, including American European Consensus Conference criteria, lung injury score, Fowler criteria and so on. Flow and timing is a part evaluating possible bias of the process including patients recruiting and biomarker measurement. (EPS 2305 kb)
Outcome of influence analysis. (DOCX 14 kb)
Result of subgroup analysis for diagnosis. (DOCX 14 kb)
Result of subgroup analysis for mortality. (DOCX 12 kb)
Result of publication bias. (DOCX 13 kb)
Acknowledgements
We thank Dr. Ting Li, Dr. Wen Wang, and Dr. Kan Zhai for their support in conducting this systematic review and meta-analysis.
Funding
This study was funded by the National Natural Science Foundation of China (NO. 81500003), Beijing Natural Science Foundation of China (NO. 7172084), the Beijing Municipal Administration of Hospitals Clinical Medicine Development of Special Funding Support (ID: ZYLX201312), and the Beijing Municipal Administration of Hospitals’ Ascent Plan (ID: DFL20150302).
Availability of data and materials
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
Abbreviations
- AECC
American European Consensus Conference
- ALI
Acute lung injury
- Ang-2
Angiopoietin-2
- ARDS
Acute respiratory distress syndrome
- BALF
Bronchoalveolar lavage fluid
- CC16
Club cell protein
- ELF
Epithelial lining fluid
- IL-1β
Interleukin-1β
- IL-2
Interleukin-2
- IL-4
Interleukin-4
- IL-8
Interleukin-8
- KL-6
Kerbs von Lungren-6
- LAF
Lung aspirational fluid
- LDH
Lactate dehydrogenase
- MMP-9
Matrix metalloproteinases-9
- OR
Odds ratio
- PAF-ACH
Platelet activating factor-acetyl choline
- PAI-1
Plasminogen activator inhibitor-1
- PCP I
Procollagen peptide I
- PCP III
Procollagen peptide III
- PEF
Pulmonary edema fluid
- PLA2
Phospholipases A2
- QUADAS-2
Quality Assessment of Diagnostic Accuracy Studies Score-2
- RoM
Ratio of means
- SD
Standard deviation
- SE
Standard error
- SICAM
Soluble intercellular adhesion molecule-1
- SRAGE
Soluble receptor for advanced glycation end products
- STNF-RII
Soluble TNF-α receptors II
- TNF-α
Tumor necrosis factor-α
- vWF
von Willebrand factor
Authors’ contributions
YW was in charge of study design and data analysis and was a major contributor in writing the manuscript. HW helped with the quality assessment and revised the manuscript. CoZ participated in the design of the study and the literature search. RG performed the literature search and data extraction and revised the manuscript. CfZ participated in the quality assessment of the study, data extraction, and statistical analysis. HY helped to revise the manuscript. ZT helped with the study design and revised the manuscript. All authors have read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med. 2000;342(18):1334–1349. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- 2.Bernard GR, Artigas A, Brigham KL, Carlet J, Falke K, Hudson L, Lamy M, LeGall JR, Morris A, Spragg R: Report of the American-European Consensus Conference on acute respiratory distress syndrome: definitions, mechanisms, relevant outcomes, and clinical trial coordination. Consensus Committee. J Crit Care 1994, 9(1):72–81. [DOI] [PubMed]
- 3.Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, Camporota L, Slutsky AS. Acute respiratory distress syndrome: the Berlin definition. Jama. 2012;307(23):2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 4.Terpstra ML, Aman J, GPvN A, ABJ G. Plasma biomarkers for acute respiratory distress syndrome: a systematic review and meta-analysis. Crit Care Med. 2014;42(3):691–700. doi: 10.1097/01.ccm.0000435669.60811.24. [DOI] [PubMed] [Google Scholar]
- 5.Garcia-Laorden MI, Lorente JA, Flores C, Slutsky AS, Villar J. Biomarkers for the acute respiratory distress syndrome: how to make the diagnosis more precise. Ann Transl Med. 2017;5(14):283. doi: 10.21037/atm.2017.06.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, Leeflang MM, Sterne JA, Bossuyt PM. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155(8):529–536. doi: 10.7326/0003-4819-155-8-201110180-00009. [DOI] [PubMed] [Google Scholar]
- 7.Friedrich JO, Adhikari N, Herridge MS, Beyene J. Meta-analysis: low-dose dopamine increases urine output but does not prevent renal dysfunction or death. Ann Intern Med. 2005;142(7):510–524. doi: 10.7326/0003-4819-142-7-200504050-00010. [DOI] [PubMed] [Google Scholar]
- 8.Friedrich JO, Adhikari NK, Beyene J. The ratio of means method as an alternative to mean differences for analyzing continuous outcome variables in meta-analysis: a simulation study. BMC Med Res Methodol. 2008;8:32. doi: 10.1186/1471-2288-8-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Friedrich JO, Adhikari NKJ, Beyene J. Ratio of means for analyzing continuous outcomes in meta-analysis performed as well as mean difference methods. J Clin Epidemiol. 2011;64(5):556–564. doi: 10.1016/j.jclinepi.2010.09.016. [DOI] [PubMed] [Google Scholar]
- 10.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56(2):455–463. doi: 10.1111/j.0006-341x.2000.00455.x. [DOI] [PubMed] [Google Scholar]
- 12.Adamzik M, Broll J, Steinmann J, Westendorf AM, Rehfeld I, Kreissig C, Peters J. An increased alveolar CD4 + CD25 + Foxp3 + T-regulatory cell ratio in acute respiratory distress syndrome is associated with increased 30-day mortality. Intensive Care Med. 2013;39(10):1743–1751. doi: 10.1007/s00134-013-3036-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Agouridakis P, Kyriakou D, Alexandrakis MG, Perisinakis K, Karkavitsas N, Bouros D. Association between increased levels of IL-2 and IL-15 and outcome in patients with early acute respiratory distress syndrome. Eur J Clin Investig. 2002;32(11):862–867. doi: 10.1046/j.1365-2362.2002.01081.x. [DOI] [PubMed] [Google Scholar]
- 14.Armstrong L, Thickett DR, Mansell JP, Ionescu M, Hoyle E, Billinghurst RC, Poole AR, Millar AB. Changes in collagen turnover in early acute respiratory distress syndrome. Am J Respir Crit Care Med. 1999;160(6):1910–1915. doi: 10.1164/ajrccm.160.6.9811084. [DOI] [PubMed] [Google Scholar]
- 15.Bersten AD, Doyle IR, Davidson KG, Barr HA, Nicholas TE, Kermeen F. Surfactant composition reflects lung overinflation and arterial oxygenation in patients with acute lung injury. Eur Respir J. 1998;12(2):301–308. doi: 10.1183/09031936.98.12020301. [DOI] [PubMed] [Google Scholar]
- 16.Calfee CS, Eisner MD, Parsons PE, Thompson BT, Conner ER, Jr, Matthay MA, Ware LB, Network NARDSCT. Soluble intercellular adhesion molecule-1 and clinical outcomes in patients with acute lung injury. Intensive Care Med. 2009;35(2):248–257. doi: 10.1007/s00134-008-1235-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chesnutt AN, Matthay MA, Tibayan FA, Clark JG. Early detection of type III procollagen peptide in acute lung injury. Pathogenetic and prognostic significance. Am J Respir Crit Care Med. 1997;156(3 Pt 1):840–845. doi: 10.1164/ajrccm.156.3.9701124. [DOI] [PubMed] [Google Scholar]
- 18.Chollet-Martin S, Jourdain B, Gibert C, Elbim C, Chastre J, Gougerot-Pocidalo MA. American journal of respiratory and critical care medicine. Vol. 154. 1996. Interactions between neutrophils and cytokines in blood and alveolar spaces during ARDS; pp. 594–601. [DOI] [PubMed] [Google Scholar]
- 19.Clark JG, Milberg JA, Steinberg KP, Hudson LD. Type III procollagen peptide in the adult respiratory distress syndrome. Association of increased peptide levels in bronchoalveolar lavage fluid with increased risk for death. Ann Intern Med. 1995;122(1):17–23. doi: 10.7326/0003-4819-122-1-199501010-00003. [DOI] [PubMed] [Google Scholar]
- 20.Conner ER, Ware LB, Modin G, Matthay MA. Elevated pulmonary edema fluid concentrations of soluble intercellular adhesion molecule-1 in patients with acute lung injury: biological and clinical significance. Chest. 1999;116(1 Suppl):83s–84s. doi: 10.1378/chest.116.suppl_1.83s. [DOI] [PubMed] [Google Scholar]
- 21.Delclaux C, d’Ortho MP, Delacourt C, Lebargy F, Brun-Buisson C, Brochard L, Lemaire F, Lafuma C, Harf A. Gelatinases in epithelial lining fluid of patients with adult respiratory distress syndrome. Am J Phys. 1997;272(3 Pt 1):L442–L451. doi: 10.1152/ajplung.1997.272.3.L442. [DOI] [PubMed] [Google Scholar]
- 22.El Solh AA, Bhora M, Pineda L, Aquilina A, Abbetessa L, Berbary E. Alveolar plasminogen activator inhibitor-1 predicts ARDS in aspiration pneumonitis. Intensive Care Med. 2006;32(1):110–115. doi: 10.1007/s00134-005-2847-2. [DOI] [PubMed] [Google Scholar]
- 23.Farjanel J, Hartmann DJ, Guidet B, Luquel L, Offenstadt G. Four markers of collagen metabolism as possible indicators of disease in the adult respiratory distress syndrome. Am Rev Respir Dis. 1993;147(5):1091–1099. doi: 10.1164/ajrccm/147.5.1091. [DOI] [PubMed] [Google Scholar]
- 24.Frenzel J, Gessner C, Sandvoss T, Hammerschmidt S, Schellenberger W, Sack U, Eschrich K, Wirtz H. Outcome prediction in pneumonia induced ALI/ARDS by clinical features and peptide patterns of BALF determined by mass spectrometry. PLoS One. 2011;6(10):e25544. doi: 10.1371/journal.pone.0025544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Geerts L, Jorens PG, Willems J, De Ley M, Slegers H. Natural inhibitors of neutrophil function in acute respiratory distress syndrome. Crit Care Med. 2001;29(10):1920–1924. doi: 10.1097/00003246-200110000-00012. [DOI] [PubMed] [Google Scholar]
- 26.González-López A, García-Prieto E, Batalla-Solís E, Amado-Rodríguez L, Avello N, Blanch L, Albaiceta GM. Lung strain and biological response in mechanically ventilated patients. Intensive Care Med. 2012;38(2):240–247. doi: 10.1007/s00134-011-2403-1. [DOI] [PubMed] [Google Scholar]
- 27.Hallgren R, Samuelsson T, Laurent TC, Modig J. Accumulation of hyaluronan (hyaluronic acid) in the lung in adult respiratory distress syndrome. Am Rev Respir Dis. 1989;139(3):682–687. doi: 10.1164/ajrccm/139.3.682. [DOI] [PubMed] [Google Scholar]
- 28.Hamacher J, Lucas R, Lijnen HR, Buschke S, Dunant Y, Wendel A, Grau GE, Suter PM, Ricou B. Tumor necrosis factor-a and angiostatin are mediators of endothelial cytotoxicity in bronchoalveolar lavages of patients with acute respiratory distress syndrome. Am J Respir Crit Care Med. 2002;166(5):651–656. doi: 10.1164/rccm.2109004. [DOI] [PubMed] [Google Scholar]
- 29.Idell S, Thrall RS, Maunder R, Martin TR, McLarty J, Scott M, Starcher BC. Bronchoalveolar lavage desmosine in bleomycin-induced lung injury in marmosets and patients with adult respiratory distress syndrome. Exp Lung Res. 1989;15(5):739–753. doi: 10.3109/01902148909062858. [DOI] [PubMed] [Google Scholar]
- 30.Ishizaka A, Matsuda T, Albertine KH, Koh H, Tasaka S, Hasegawa N, Kohno N, Kotani T, Morisaki H, Takeda J, et al. Elevation of KL-6, a lung epithelial cell marker, in plasma and epithelial lining fluid in acute respiratory distress syndrome. Am J Phys Lung Cell Mol Phys. 2004;286(6):L1088–L1094. doi: 10.1152/ajplung.00420.2002. [DOI] [PubMed] [Google Scholar]
- 31.Jorens PG, Sibille Y, Goulding NJ, van Overveld FJ, Herman AG, Bossaert L, De Backer WA, Lauwerys R, Flower RJ, Bernard A. Potential role of Clara cell protein, an endogenous phospholipase A2 inhibitor, in acute lung injury. Eur Respir J. 1995;8(10):1647–1653. doi: 10.1183/09031936.95.08101647. [DOI] [PubMed] [Google Scholar]
- 32.Karagiorga G, Nakos G, Galiatsou E, Lekka ME. Biochemical parameters of bronchoalveolar lavage fluid in fat embolism. Intensive Care Med. 2006;32(1):116–123. doi: 10.1007/s00134-005-2868-x. [DOI] [PubMed] [Google Scholar]
- 33.Keane MP, Donnelly SC, Belperio JA, Goodman RB, Dy M, Burdick MD, Fishbein MC, Strieter RM. Imbalance in the expression of CXC chemokines correlates with bronchoalveolar lavage fluid angiogenic activity and procollagen levels in acute respiratory distress syndrome. J Immunol. 2002;169(11):6515–6521. doi: 10.4049/jimmunol.169.11.6515. [DOI] [PubMed] [Google Scholar]
- 34.Kondo T, Hattori N, Ishikawa N, Murai H, Haruta Y, Hirohashi N, Tanigawa K, Kohno N. KL-6 concentration in pulmonary epithelial lining fluid is a useful prognostic indicator in patients with acute respiratory distress syndrome. Respir Res. 2011;12:32. doi: 10.1186/1465-9921-12-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kropski JA, Fremont RD, Calfee CS, Ware LB. Clara cell protein (CC16), a marker of lung epithelial injury, is decreased in plasma and pulmonary edema fluid from patients with acute lung injury. Chest. 2009;135(6):1440–1447. doi: 10.1378/chest.08-2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kurdowska A, Noble JM, Grant IS, Robertson CR, Haslett C, Donnelly SC. Anti-interleukin-8 autoantibodies in patients at risk for acute respiratory distress syndrome. Crit Care Med. 2002;30(10):2335–2337. doi: 10.1097/00003246-200210000-00024. [DOI] [PubMed] [Google Scholar]
- 37.Lanchou J, Corbel M, Tanguy M, Germain N, Boichot E, Theret N, Clement B, Lagente V, Malledant Y. Imbalance between matrix metalloproteinases (MMP-9 and MMP-2) and tissue inhibitors of metalloproteinases (TIMP-1 and TIMP-2) in acute respiratory distress syndrome patients. Crit Care Med. 2003;31(2):536–542. doi: 10.1097/01.CCM.0000048626.02184.F8. [DOI] [PubMed] [Google Scholar]
- 38.Lee KS, Choi YH, Kim YS, Baik SH, Oh YJ, Sheen SS, Park JH, Hwang SC, Park KJ. Evaluation of bronchoalveolar lavage fluid from ARDS patients with regard to apoptosis. Respir Med. 2008;102(3):464–469. doi: 10.1016/j.rmed.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 39.Lee YL, Chen W, Chen LY, Chen CH, Lin YC, Liang SJ, Shih CM. Systemic and bronchoalveolar cytokines as predictors of in-hospital mortality in severe community-acquired pneumonia. J Crit Care. 2010;25(1):176. doi: 10.1016/j.jcrc.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 40.Lesur O, Kokis A, Hermans C, Fulop T, Bernard A, Lane D. Interleukin-2 involvement in early acute respiratory distress syndrome: relationship with polymorphonuclear neutrophil apoptosis and patient survival. Crit Care Med. 2000;28(12):3814–3822. doi: 10.1097/00003246-200012000-00010. [DOI] [PubMed] [Google Scholar]
- 41.Lin W-C, Lin C-F, Chen C-L, Chen C-W, Lin Y-S. Prediction of outcome in patients with acute respiratory distress syndrome by bronchoalveolar lavage inflammatory mediators. Exp Biol Med. 2010;235(1):57–65. doi: 10.1258/ebm.2009.009256. [DOI] [PubMed] [Google Scholar]
- 42.Marshall RP, Bellingan G, Webb S, Puddicombe A, Goldsack N, McAnulty RJ, Laurent GJ. Fibroproliferation occurs early in the acute respiratory distress syndrome and impacts on outcome. Am J Respir Crit Care Med. 2000;162(5):1783–1788. doi: 10.1164/ajrccm.162.5.2001061. [DOI] [PubMed] [Google Scholar]
- 43.Martin R, Martin GS, Harris F, Brown L, Esper AM. The rage signaling pathway in sepsis induced ARDS: role of HMGB1 and MMPS. Am J Respir Crit Care Med. 2015;191:A2372.
- 44.Martin TR, Rubenfeld GD, Ruzinski JT, Goodman RB, Steinberg KP, Leturcq DJ, Moriarty AM, Raghu G, Baughman RP, Hudson LD. Relationship between soluble CD14, lipopolysaccharide binding protein, and the alveolar inflammatory response in patients with acute respiratory distress syndrome. Am J Respir Crit Care Med. 1997;155(3):937–944. doi: 10.1164/ajrccm.155.3.9117029. [DOI] [PubMed] [Google Scholar]
- 45.Medford ARL, Godinho SIH, Keen LJ, Bidwell JL, Millar AB. Relationship between vascular endothelial growth factor + 936 genotype and plasma/epithelial lining fluid vascular endothelial growth factor protein levels in patients with and at risk for ARDS. Chest. 2009;136(2):457–464. doi: 10.1378/chest.09-0383. [DOI] [PubMed] [Google Scholar]
- 46.Miller EJ, Cohen AB, Matthay MA. Increased interleukin-8 concentrations in the pulmonary edema fluid of patients with acute respiratory distress syndrome from sepsis. Crit Care Med. 1996;24(9):1448–1454. doi: 10.1097/00003246-199609000-00004. [DOI] [PubMed] [Google Scholar]
- 47.Nakos G, Kitsiouli E, Hatzidaki E, Koulouras V, Touqui L, Lekka ME. Phospholipases A2 and platelet-activating-factor acetylhydrolase in patients with acute respiratory distress syndrome. Crit Care Med. 2005;33(4):772–779. doi: 10.1097/01.ccm.0000158519.80090.74. [DOI] [PubMed] [Google Scholar]
- 48.Nakos G, Kitsiouli EI, Tsangaris I, Lekka ME. Bronchoalveolar lavage fluid characteristics of early intermediate and late phases of ARDS - alterations in leukocytes, proteins, PAF and surfactant components. Intensive Care Med. 1998;24(4):296–303. doi: 10.1007/s001340050571. [DOI] [PubMed] [Google Scholar]
- 49.Nakos G, Pneumatikos J, Tsangaris I, Tellis C, Lekka M. Proteins and phospholipids in BAL from patients with hydrostatic pulmonary edema. Am J Respir Crit Care Med. 1997;155(3):945–951. doi: 10.1164/ajrccm.155.3.9117030. [DOI] [PubMed] [Google Scholar]
- 50.Nathani N, Perkins GD, Tunnicliffe W, Murphy N, Manji M, Thickett DR. Kerbs von Lungren 6 antigen is a marker of alveolar inflammation but not of infection in patients with acute respiratory distress syndrome. Crit Care. 2008;12(1):R12. doi: 10.1186/cc6785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Park WY, Goodman RB, Steinberg KP, Ruzinski JT, Radella F, 2nd, Park DR, Pugin J, Skerrett SJ, Hudson LD, Martin TR. Cytokine balance in the lungs of patients with acute respiratory distress syndrome. Am J Respir Crit Care Med. 2001;164(10 Pt 1):1896–1903. doi: 10.1164/ajrccm.164.10.2104013. [DOI] [PubMed] [Google Scholar]
- 52.Prabhakaran P, Ware LB, White KE, Cross MT, Matthay MA, Olman MA. Elevated levels of plasminogen activator inhibitor-1 in pulmonary edema fluid are associated with mortality in acute lung injury. Am J Phys Lung Cell Mol Phys. 2003;285(1):L20–L28. doi: 10.1152/ajplung.00312.2002. [DOI] [PubMed] [Google Scholar]
- 53.Pugin J, Verghese G, Widmer MC, Matthay MA. The alveolar space is the site of intense inflammatory and profibrotic reactions in the early phase of acute respiratory distress syndrome. Crit Care Med. 1999;27(2):304–312. doi: 10.1097/00003246-199902000-00036. [DOI] [PubMed] [Google Scholar]
- 54.Quesnel C, Piednoir P, Gelly J, Nardelli L, Garnier M, Lecon V, Lasocki S, Bouadma L, Philip I, Elbim C, et al. Alveolar fibrocyte percentage is an independent predictor of poor outcome in patients with acute lung injury. Crit Care Med. 2012;40(1):21–28. doi: 10.1097/CCM.0b013e31822d718b. [DOI] [PubMed] [Google Scholar]
- 55.Ricou B, Nicod L, Lacraz S, Welgus HG, Suter PM, Dayer JM. Matrix metalloproteinases and TIMP in acute respiratory distress syndrome. Am J Respir Crit Care Med. 1996;154(2 Pt 1):346–352. doi: 10.1164/ajrccm.154.2.8756805. [DOI] [PubMed] [Google Scholar]
- 56.Song Y, Lynch SV, Flanagan J, Zhuo H, Tom W, Glidden D, Brown R, Garcia O, Wiener-Kronish JP, Dotson RH, et al. Increased plasminogen activator inhibitor-1 concentrations in bronchoalveolar lavage fluids are associated with increased mortality in a cohort of patients with Pseudomonas aeruginosa. Anesthesiology. 2007;106(2):252–261. doi: 10.1097/00000542-200702000-00012. [DOI] [PubMed] [Google Scholar]
- 57.Stern JB, Fierobe L, Paugam C, Rolland C, Dehoux M, Petiet A, Dombret MC, Mantz J, Aubier M, Crestani B. Keratinocyte growth factor and hepatocyte growth factor in bronchoalveolar lavage fluid in acute respiratory distress syndrome patients. Crit Care Med. 2000;28(7):2326–2333. doi: 10.1097/00003246-200007000-00024. [DOI] [PubMed] [Google Scholar]
- 58.Uchida T, Shirasawa M, Ware LB, Kojima K, Hata Y, Makita K, Mednick G, Matthay ZA, Matthay MA. Receptor for advanced glycation end-products is a marker of type I cell injury in acute lung injury. Am J Respir Crit Care Med. 2006;173(9):1008–1015. doi: 10.1164/rccm.200509-1477OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ware LB, Kaner RJ, Crystal RG, Schane R, Trivedi NN, McAuley D, Matthay MA. VEGF levels in the alveolar compartment do not distinguish between ARDS and hydrostatic pulmonary oedema. Eur Respir J. 2005;26(1):101–105. doi: 10.1183/09031936.05.00106604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jabaudon M, Blondonnet R, Roszyk L, Pereira B, Guerin R, Perbet S, Cayot S, Bouvier D, Blanchon L, Sapin V, et al. Soluble forms and ligands of the receptor for advanced glycation end-products in patients with acute respiratory distress syndrome: an observational prospective study. PLoS One. 2015;10(8):e0135857. doi: 10.1371/journal.pone.0135857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ware LB, Fremont RD, Bastarache JA, Calfee CS, Matthay MA. Determining the aetiology of pulmonary oedema by the oedema fluid-to-plasma protein ratio. Eur Respir J. 2010;35(2):331–337. doi: 10.1183/09031936.00098709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Murray JF, Matthay MA, Luce JM, Flick MR. An expanded definition of the adult respiratory distress syndrome. Am Rev Respir Dis. 1988;138(3):720–723. doi: 10.1164/ajrccm/138.3.720. [DOI] [PubMed] [Google Scholar]
- 63.Fowler AA, Hamman RF, Zerbe GO, Benson KN, Hyers TM. Adult respiratory distress syndrome. Prognosis after onset. Am Rev Respir Dis. 1985;132(3):472–478. doi: 10.1164/arrd.1985.132.3.472. [DOI] [PubMed] [Google Scholar]
- 64.Martin C, Papazian L, Payan MJ, Saux P, Gouin F. Pulmonary fibrosis correlates with outcome in adult respiratory distress syndrome. A study in mechanically ventilated patients. Chest. 1995;107(1):196–200. doi: 10.1378/chest.107.1.196. [DOI] [PubMed] [Google Scholar]
- 65.Hill JD, Ratliff JL, Parrott JC, Lamy M, Fallat RJ, Koeniger E, Yaeger EM, Whitmer G. Pulmonary pathology in acute respiratory insufficiency: lung biopsy as a diagnostic tool. J Thorac Cardiovasc Surg. 1976;71(1):64–71. [PubMed] [Google Scholar]
- 66.Friedrich JO, Adhikari NKJ, Beyene J. Ratio of geometric means to analyze continuous outcomes in meta-analysis: comparison to mean differences and ratio of arithmetic means using empiric data and simulation. Stat Med. 2012;31(17):1857–1886. doi: 10.1002/sim.4501. [DOI] [PubMed] [Google Scholar]
- 67.Sun WJ, Grosser S, Tsong Y. Ratio of means vs. difference of means as measures of superiority, noninferiority, and average bioequivalence. J Biopharm Stat. 2017;27(2):338–355. doi: 10.1080/10543406.2016.1265536. [DOI] [PubMed] [Google Scholar]
- 68.Meyer NJ, Calfee CS. Novel translational approaches to the search for precision therapies for acute respiratory distress syndrome. Lancet Respir Med. 2017;5(6):512–523. doi: 10.1016/S2213-2600(17)30187-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Search strategy. (DOCX 17 kb)
Tailored QUADAS-2. (DOCX 17 kb)
ARDS etiologies. (DOCX 13 kb)
Result of quality assessment. Low = low possibility of risk of bias. High = means high possibility of high risk of bias. Index test(s) = measurement used in an article for biomarker concentration detection. Reference standard = criteria used for acute respiratory distress syndrome diagnosis, including American European Consensus Conference criteria, lung injury score, Fowler criteria and so on. Flow and timing is a part evaluating possible bias of the process including patients recruiting and biomarker measurement. (EPS 2305 kb)
Outcome of influence analysis. (DOCX 14 kb)
Result of subgroup analysis for diagnosis. (DOCX 14 kb)
Result of subgroup analysis for mortality. (DOCX 12 kb)
Result of publication bias. (DOCX 13 kb)
Data Availability Statement
All data generated or analyzed during this study are included in this published article [and its supplementary information files].