Abstract
Degenerative retinal disease leads to significant visual morbidity worldwide. Diabetic retinopathy and macular degeneration are leading causes of blindness in the developed world. While current therapies for these diseases slow disease progression, stem cell and gene therapy may also reverse the effects of these, and other, degenerative retinal conditions. Novel therapies being investigated include the use of various types of stem cells in the regeneration of atrophic or damaged retinal tissue, the prolonged administration of neurotrophic factors and/or drug delivery, immunomodulation, as well as the replacement of mutant genes, and immunomodulation through viral vector delivery. This review will update the reader on aspects of stem cell and gene therapy in diabetic retinopathy, age-related macular degeneration, retinitis pigmentosa and other less common inherited retinal dystrophies. These therapies include the use of adeno-associated viral vector-based therapies for treatment of various types of retinitis pigmentosa and dry age-related macular degeneration. Other potential therapies reviewed include the use of mesenchymal stem cells in local immunomodulation, and the use of stem cells in generating structures like three-dimensional retinal sheets for transplantation into degenerative retinas. Finally, aspects of stem cell and gene therapy in diabetic retinopathy, age-related macular degeneration, retinitis pigmentosa, and other less common inherited retinal dystrophies will be reviewed.
Background
Degenerative retinal disease afflicts many around the world and can lead to blindness. Age related macular degeneration is the leading cause of blindness in Caucasians greater than 40 years of age in the USA [1]. Diabetic retinopathy is the leading cause of vision loss in those between the ages of 20 and 74 [2]. Retinitis pigmentosa affects 1 in 3000–7000 people, making it one of the most common causes of inherited retinal disease leading to blindness [3, 4].
Current FDA (Food and Drug Administration)-approved treatment for neovascular age-related macular degeneration (AMD) and complications associated with diabetic retinopathy involve frequent anti-vascular endothelial growth factor (VEGF) intravitreal injections. Similarly, diabetic retinopathy is treated with anti-VEGFs and laser photocoagulation. Though effective in treating the complications associated with these diseases, they do little to reverse the course. Until recently, treatment for retinitis pigmentosa (RP) has consisted of measures to reduce further damage or slow the disease. However, FDA approval has been received of the gene therapy Luxturna (voretigene neparvovec-rzyl), which targets RPE65 [5–7].
Stem cell and gene therapy may also reverse the effects of these degenerative retinal conditions. Efforts have been made to develop novel therapies involving the regeneration of atrophic or damaged retinal tissue, prolonged administration of neurotrophic factors and/or drug delivery, immunomodulation, replacement of mutant genes, and immunomodulation through viral vector delivery. The purpose of this review is to introduce the retinal conditions and diseases most prevalent in patient populations, and to explore some of the novel treatment approaches currently under investigation; these include the use of stem cells and gene therapy techniques.
Stem cells
While there is ambiguity in the definitions suggested, stem cells are generally identified as populations of cells that are both self-renewing, and capable of differentiating into multiple cell types, thus receiving the description of multipotent or pluripotent, depending on the situation [8]. It had been thought that the mature retina of mammals is incapable of regeneration; however, reports have shown that there are a population of retinal stem cells localized to the pigmented ciliary margin that are capable of differentiating into several types of retinal cells such as rod photoreceptors, bipolar cells, and Müller cells [9–11]. This population of cells has since been described as late-stage neuronal progenitors or pigmented ciliary epithelial cells [12, 13]. Neural progenitor/stem cells are important to retinal development, as the retina is a specialized appendage of the nervous system.
Among the types of stem or progenitor cells, identified by source, are human embryonic stem cells (hESCs), bone marrow stromal cells (BMSCs), human mesenchymal stem cells (hMSCs), human pluripotent stem cells (hPSCs), and human retinal progenitor cells (hRPCs). hESCs are derived from the transfer of preimplantation embryo cells into culture, and are classified as a type of hPSC along with human induced pluripotent stem cells; these cell lines maintain pluripotency until being differentiated, and were among the first progenitor cells used in regenerative research [14, 15]. hMSCs can differentiate into the various mesenchymal tissues such as osteoblasts, chondrocytes, and adipocytes. There is disagreement over the appropriateness of terms such as mesenchymal stem cell, and the related terms bone marrow stromal cell, mesenchymal progenitor cell, and bone marrow progenitor cell; hMSCs are generally understood to refer to the fibroblast-like cells shown, more recently, to also be capable of differentiating into non-mesenchymal lineages such as cardiac, renal, hepatic, and neural cells [16]. They are important to the normal function of hematopoietic stem cells, and have been investigated for use in cancer therapy due to their tendency to localize to solid tumors [17]. Sources for deriving hRPCs include fetal retinas, ESCs, and induced pluripotent stem cells (iPSCs); there is suggestion in the literature that the fetal-derived RPCs may be more suitable for therapy due to lower immunogenicity and increased stability [18].
The multipotent characteristic of the progenitor cells is important to the proper development of the structures in the eye and retina, and for this reason many have thought that there could be regenerative potential in the damaged or degenerate retina through the transplantation or activation of stem cells. Initial attempts at transplanting neural and retinal stem cells into the degenerating retina proved unsuccessful, and it was seen that these cells neither integrated into the retina, nor restored vision [19–22]. However, MacLaren et al. [23] noted that the mammalian retina can incorporate rod photoreceptor precursors derived from the post-natal day 1 (P1) retina of mice into the outer nuclear layer (ONL). They observed that these cells differentiated, formed functional synapses, and improved vision in mouse models of degenerative retinal disease. The conclusion drawn was that the ontogenetic stage of the precursor cells, defined in this case by the expression of neural retina leucine zipper (Nrl), is vital to the integration of the stem cells into the retina [23].
Others have observed that the transplantation of embryonic stem cell-derived neural progenitors leads to the enhanced survival of host retinal cells, such as photoreceptors, and improved preservation of visual function in the mnd mouse model of neuronal ceroid lipofuscinoses [24]. It has been suggested that this enhanced survival of host cells could be mediated by the secretion of growth factors, as IGF-1 administration has been seen to produce similar results [24–27].
Aharony et al. [28] investigated stem cells and their effects on ocular conditions. They organized the roles of stem cells in treatment of the eyes into three categories: vehicles for drug delivery, immunomodulatory agents, and mediators of tissue regeneration. Progress is being made in the understanding of each of these roles and the ways to effectively utilize them. In terms of tissue regeneration, researchers are often capable of inducing growth and development of transplanted cells, and in some cases, notable integration into the framework of the host cells is observed. In a study with primates treated with the hypoxia-inducing agent cobalt chloride and irradiation to mimic retinal disease, the transplantation of human embryonic stem cells (hESCs) was noted to result in survival and maturation of the transplanted cells, as well as some integration of the cells with host bipolar cells [29]. Singhal et al. [30] demonstrated that Müller glia which exhibit multipotentiality can be induced to differentiate into retinal ganglionic cells (RGCs) upon treatment with fibroblast growth factor 2 (FGF2) and γ-secretase inhibitor N-[N-(3,5-difluorophenacetyl)-l-alanyl]-S-phenylglycine t-butyl ester (DAPT) to inhibit Notch signaling. This differentiation into RGCs correlated to a significant improvement in retinal function as measured by electroretinogram.
Some studies have attempted to prime stem cells with environmental factors such as epidermal growth factor (EGF) prior to their transplantation in an effort to improve their incorporation into the host [31]. However, stem cells have been noted to migrate to the retina and differentiate into glia and ganglion cells in the absence of priming before transplantation when injected intravitreally or subretinally [28, 31]. Canola et al. [31] observed that this cell incorporation occurred to a greater extent in more advanced stages of disease. Importantly, few transplanted cells expressed photoreceptor markers, which may indicate that priming prior to or along with transplantation is necessary for the differentiation of photoreceptor lineages [28].
Bone marrow-derived stem cells (BMSCs) can differentiate into ganglion cells when administered intravitreally and intravenously following optic nerve injury in mice [28, 32, 33]. This differentiation effect was increased by administration of neuronal growth factors along with the cells [33].
Zhou and Xia [34] observed that transplantation of retinal stem cells in a state of glaucoma reduced levels of IFN-γ in both the serum and the aqueous humor which led to a decrease in inflammation. On the other hand, it has also been noted that in vitro study of mesenchymal stem cells (MSCs) in a rat retina-explant model show differentiation into microglia, which would induce inflammation rather than reducing it [28, 35].
Ischemia-associated retinal degeneration is a major cause of vision loss; Mathew et al. [36] found that intravitreal administration of BMSCs aided the survival of the retina in rats suffering ischemic damage. BMSCs had an anti-apoptotic effect through decreased TUNEL and caspase-3 expression, attenuated inflammation by reducing levels of TNF-α, IL-1β, and IL-6, and preserved autophagy. The transmembrane glycoprotein Prominin-1 (Prom1) has been shown to be an important regulator of autophagy in the RPE [37]. Understanding this role will aid in anticipating and controlling tumorigenesis in stem cell therapy.
One of the barriers to stem cell transplantation therapy is the difficulty of inducing incorporation of transplanted cells into the host cell structure; this could be complicated by immune reaction. Chao et al. [38] observed that human ESC-derived retinal neurons injected into the submacular space of a squirrel monkey continued to survive 3 months following the injection, and that some donor cells integrated into the host retina while some axons from donor cells extended into the optic nerve within the same time period.
Wahlin et al. [39] recently discussed a method for differentiating human pluripotent stem cells (hPSCs) into three dimensional (3D) retina models that bear many similarities to mature retinas. These similarities include structural outgrowth of resembling the outer segment of photoreceptors, neurotransmitter expression, and synaptic vesicle fusion. It is suggested that this model could aid researchers in studying the retina and the effects of various therapies on it in vitro. 3D retinal sheets generated using stem cells are being investigated for their ability to be transplanted into retinal degenerative mice [40, 41].
While inflammation can cause significant tissue damage, indications show that there is a link between inflammation and retina regeneration. The Xenopus genus of frog and other amphibians have the interesting ability to regenerate the whole retina upon its removal through activation of RPE cells to adopt multipotent characteristics [42]. Naitoh et al. [43] observed that the upregulation of matrix metalloproteinases through inflammatory cytokine upregulation was vital to retinal regeneration as dexamethasone or Withaferin A administration significantly suppressed RPE cell migration and transdifferentiation in the African clawed frog (Xenopus laevis).
Qu et al. [44] investigated the effects of combined human mesenchymal stem cell (hMSC) and human retinal progenitor cell (hRPC) subretinal transplant in rats. It was observed that the combined therapy resulted in improved electroretinogram performance, improved outer nuclear layer thickness, increased migration of grafted cells, and reduced activation of microglia and Müller cell gliosis as compared to single transplantation of hRPCs or hMSCs. Both MSCs and RPCs have been transplanted clinically for treatment of retinal disease, but this study suggests that a more optimal result may be achieved through combination therapy with both types of cells.
While stem cell transplantation has not yet developed into the unhindered, regenerative solution to all degenerative conditions as was initially hoped, much research is currently ongoing. Our understanding of stem cell capabilities and the varying modes that may be used therapeutically continues to progress (Table 1). Other applications will likely materialize into beneficial therapies following continued investigation.
Table 1.
Stem cell therapy
| Condition | Technique | Result |
|---|---|---|
| Diabetic retinopathy | Mesenchymal stem cells | Absorption of ROS through expression of sulfoxide reductase A |
| Endothelial progenitor cells | Incorporation into host retinal tissue and prevention of neovascularization | |
| Macular degeneration | Induction of pluripotent stem cells | Differentiation into photoreceptors and RPE cells and integration into the host cell structure |
| Subretinal ESC transplantation | Regenerative therapy | |
| Retinitis pigmentosa | Treatment with brain-derived neurotrophic factor | Improved survival of neurons and retinal ganglionic cells and preserves structure of the optic nerve |
| Induction of neural stem cell secretion of ciliary neurotrophic factor | Protection of photoreceptor cells |
Gene therapy
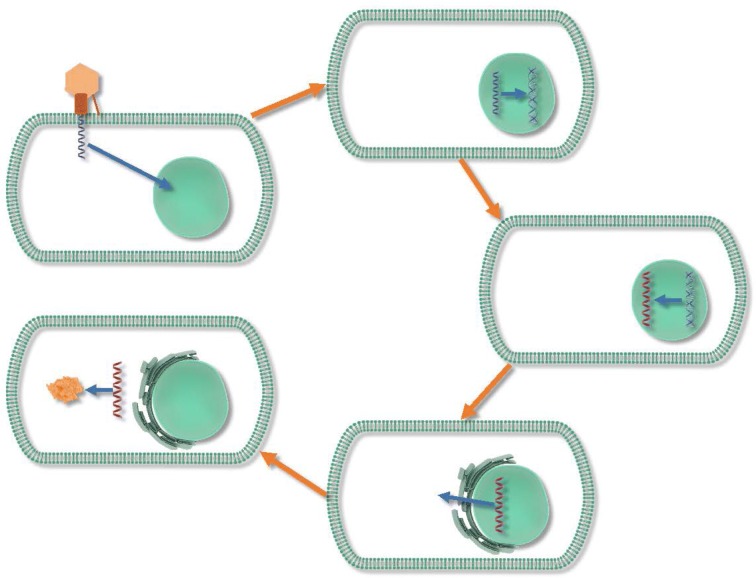
Interest has grown in the potential of gene therapy, the delivery of nucleic acid polymers into host cells to treat underlying conditions, for retinal diseases in recent decades (Fig. 1). The first demonstration of retroviruses acquiring cellular genes occurred in the mid-1970s, and this was followed by experimentation with retroviruses, simian virus 40 (SV40), bovine papilloma virus (BPV), vaccinia, and herpes simplex virus (HSV) [45]. In the time since the first use of viral vectors, much experience has been gained with vectors such as measles, vaccinia, polio, reovirus, adenovirus, vesicular stomatitis virus (VSV), lentivirus, γ-retrovirus, HSV, and adeno-associated virus (AAV). AAV has emerged as a favored vector for direct gene delivery in vivo due to its lack of pathogenicity and ability to incorporate into a variety of tissues in a directed manner (Table 2); one of its primary drawbacks is a limitation of packaged genetic material to 4.7 kb [45].
Fig. 1.
The general process of gene transduction with a viral vector: the AAV or other viral vector inserts its single-stranded DNA into the targeted cell, and the DNA is taken up into the nucleus where it is converted into double-stranded DNA by host cell machinery. This gene, along with its accompanying promoter is inserted between inverted terminal repeats to form an episomal concatemer in the host cell nucleus. Normal transcription and translation processes take place to produce the protein product of interest. DNA is depicted by the blue coils, and RNA by red coils
Table 2.
Gene therapy
| Condition | Goal | Method |
|---|---|---|
| Diabetic retinopathy | Reduction of angiogenesis | Downregulation of VEGF via gene targeting of sFlt-1, Flt23k, and PEDF |
| Reduction of oxidative stress | Vector-mediated delivery of superoxide dismutase | |
| Regulation of renin–angiotensin system | Targeting genes of ACE 2, Ang, or Mas receptor | |
| Macular degeneration | Inhibition of angiogenesis and neovascularization | Binding of VEGF by proteins delivered by AAV2 vectors |
| AAV2 vectors carrying sFLT-1 | ||
| Viral vectors carrying endostatin and angiostatin | ||
| Retinitis pigmentosa | Decreased loss of photoreceptors and preserved retinal function due to improved phagocytic function | Vector delivery of MERTK gene |
| Increased cell survival | Increased expression of GDNF | |
| Restore proper expression of the RPGR gene | Production of the retinitis pigmentosa GTPase regulator gene (commonly mutated in X-linked retinitis pigmentosa) |
Boye et al. [46] reviewed the literature, and noted that gene therapy was particularly promising for the treatment of ocular disease due to the accessibility, immune-privileged nature, and compartmentalization of the eye. Some of the primary obstacles to long-term gene delivery include DNA degradation and promoter inactivation; as a result, many treatment strategies focus on the implementation of controlled release systems, optimization of promoters to aid DNA stability, and the reduction of regions heavy in cytosine and guanine (CpG sequences) [47].
Ezati et al. [48] investigated the efficacy of reprogramming RPE cells through AAV vector transduction of retinal progenitor cell genes. Larger increases were observed in neonatal RPE cell cultures than in adult cell cultures in general. Analysis indicated that there was an 80-fold increase in expression of the stem cell marker SOX2 (sex determining region Y-box 2) in neonatal culture as compared to control, and a 12-fold increase in adult culture. The increases in gene expression of other genes was less dramatic with there being 3.8-fold and 2.5-fold overexpression of nestin, and 3-fold and 2.5-fold increases in PAX6 expression, in neonatal and adult cells respectively. The ability to induce de-differentiation or reprogramming of RPE cells could have important implications for the regeneration of the retina in a diseased state.
2017 was a year of advances in gene therapy. It saw the first Food and Drug Administration (FDA) approval of gene therapies for certain forms of acute lymphoblastic leukemia, large B cell lymphoma, and biallelic RPE65-associated retinal dystrophy [49]. The excitement surrounding gene therapy and its potential is evident from the abundance of start-up companies devoted solely to the research and development of specific therapies, and the many millions of dollars being invested into such ventures [45].
Retinal diseases and novel therapies
Diabetic retinopathy
Approximately 422 million people around the world have been diagnosed with diabetes [50]. It is estimated that approximately 35% of those with diabetes have DR. Proliferative diabetic retinopathy (PDR) is the most common vision-threatening condition in individuals with type 1 diabetes, and DME is the leading cause of vision loss in people with type 2 diabetes [2, 51, 52].
Diabetes leads to damage retinal vasculature. This damage results largely from the effects of hyperglycemia on the basement membrane, endothelium, and pericytes of the retinal blood vessels [53]. Some of these changes have been noted to be induced by activation of protein kinase C, increased formation of glycation products, activation of polyol pathway, oxidation, and inflammation [54–57]. These processes lead to microaneurysm formation, vascular leakage, capillary non-perfusion, and neovascularization [53]. The presence of neovascularization defines PDR. Ultimately, diabetic retinopathy and secondary retinal ischemia lead to neuroretinal damage through neurodegeneration, gliosis, and neuroinflammation [53].
Current treatments for diabetic retinopathy, beyond management of the diabetes and hyperglycemia, most often focus on the vascular aspects of the condition. Anti-VEGF treatments (bevacizumab, ranibizumab, and aflibercept) have proven effective in reducing the vision loss in patients with DR. Anti-VEGFs decrease retinal edema by mediating VEGF’s action on endothelial cells and their adjoining junctions [53]. Anti-VEGFs also cause regression of neovascularization in PDR [58]. Panretinal photocoagulation and focal retinal photocoagulation are also used to effectively treat PDR and DME respectively [59–62].
Mesenchymal stem cells (MSCs) demonstrate potential as immunomodulatory agents in DR [63]. Through expression of sulfoxide reductase A, MSCs have been shown to absorb reactive oxygen species [64]. MSCs have demonstrated neuroprotective effects in animal models of retinal degeneration, and light- and ischemia-damaged retinas [65–67]. Endothelial progenitor cells (EPCs) have displayed some ability to repair damage in ischemic and diabetic retinopathy, though increased inflammation has also been reported; it appears that the EPC subtype, endothelial colony forming cells (ECFCs), are the cells capable of incorporating into the host retinal tissue and preventing neovascularization [63, 68, 69]. EPC deficiencies in diabetic animal models exhibit some improvement with the administration of modulating agents of granulocyte colony-stimulating factor (G-CSF), stromal cell-derived factor 1 (SDF-1), and some peroxisome proliferator-activated receptors (PPAR), as well as the drugs rosiglitazone, and atorvastatin [63, 70–75].
Wang et al. [76] recently suggested that gene therapy investigations into DR either focus on targeting existing neovascularization and vascular hyperpermeability, or protecting nerves and vessels from damage. Several studies have demonstrated an ability to downregulate VEGF including therapies targeting sFlt-1, Flt23k, and PEDF (which also decreased expression of matrix metalloproteinase and connective tissue growth factor) [77–82]. Other transgenes that have been targeted to reduce angiogenesis include endostatin, angiostatin, and tissue inhibitor metalloproteinase-3 [82–85].
Adhi et al. [86] observed that soluble cluster of differentiation 59 (sCD59) protected retinal neurons and the blood-retinal barrier from membrane attack complex-mediated damage. Other studies have aimed to increase neurotrophic factors like brain-derived neurotrophic factor (BDNF), decrease oxidative stress through manganese-dependent superoxide dismutase (MnSOD) delivery, or regulate the renin–angiotensin system with angiotensin-converting enzyme 2, Ang- [1–7], and the Mas receptor [87–92]. While therapies targeting each of the transgenes mentioned have shown efficacy in reducing or preventing damage in animal models of DR-related disease, there remains further investigation necessary into long-term efficacy and safety in humans.
Macular degeneration
Age-related macular degeneration (AMD) is a disease of neurosensory retina and retinal pigment epithelium (RPE). AMD accounts for nearly 9% of worldwide blindness, and has become the leading cause of legal blindness in individuals over age 65 in the United States, Australia, Japan, and western Europe [93, 94]. In 2010, approximately 2.07 million people in the United States had AMD, which was up from 1.75 million in 2000 [95, 96]. AMD has a markedly increased prevalence in whites when compared to individuals of other ethnicities, with 1.85 million of the 2.07 million reported cases in 2010 being white [96]. It is estimated that 196 million people will have AMD globally in 2020, and 288 million in 2040 [93].
Presently, for dry AMD, the only treatment is oral administration of high-dose antioxidants (vitamins C and E, and beta carotene) and zinc which have been shown to slow the progression of AMD in a minority of patients [97]. Anti-VEGFs are the current standard of care in the treatment of wet AMD [98]. They lead to regression of choroidal neovascularization, prevention subretinal fibrosis, and decrease rates of severe vision loss in patients with wet AMD. Other options that are employed include laser therapy and photodynamic therapy targeting the choroidal neovascularization [99].
Induced pluripotent stem cells (iPSCs) have demonstrated similar effects to those previously discussed. The ability has been demonstrated to differentiate into photoreceptors and RPE cells and integrate into the host cell structure to significantly improve retinal function in retinal dystrophic and degenerative rat and mouse models [100–103]. It was also observed that these therapies were not accompanied by significant tumorigenesis which is important since rapid tumor formation is one of the primary concerns associated with iPSCs and ESCs [28]. Mandai et al. [104] transplanted an autologous iPSC-derived RPE cell sheet subretinally in a patient with advanced, wet AMD, and found that 1 year post-transplant, there was neither improvement nor worsening of visual acuity, and the graft remained intact. Cystoid macular edema was noted to be present. Further investigation is needed, but this study suggests that it could be feasible to transplant cell sheets to preserve remaining vision in degenerative diseases, but significantly improved results have not yet been demonstrated.
The initial studies of subretinal ESC transplantation in human patients with AMD and Stargardt disease indicated improved vision in the majority of patients on the order of 9–19 letters on the visual acuity test within a few months, and did not raise concern of serious safety issues in the small patient population thus treated [105, 106]. These studies suggest that stem cell transplantation has great promise as a potential regenerative therapy for individuals with AMD and Stargardt disease.
As mentioned previously, VEGF is a major mediator of angiogenesis in exudative AMD, and its inhibition can result in improved prognosis. VEGF antibodies and inhibitory agents have been used to good effect in the management of exudative AMD [46, 107, 108]. One of the inconveniences associated with VEGF antibody administration is that it requires regular intravitreal injections. AAV2 vectors carrying proteins that bind VEGF components have been effective in limiting new vessel formation in wet AMD. Extracellular VEGF is bound and prevented from interacting with the endothelial receptors FLT-1 and FLK by soluble FLT-1 (sFLT-1) [46]. Treatments with AAV2 vectors carrying sFLT-1 have inhibited neovascularization, and the use of rAAV.sFLT-1 and AAV2-sFLT01 have been shown to be safe in humans [109–112]. In phase 1 and phase 2a clinical trials, rAAV2-mediated gene delivery of sFLT-1 resulted in improved best corrected visual acuity (BCVA) in 62% of patients [111, 113, 114]. AAV2-sFLT01 is a novel chimeric protein that can be used to inhibit choroidal neovascularization (CNV). Pechan et al. [77] described the generation of two novel chimeric VEGF-binding proteins: sFLT01 and sFLT02. These proteins are comprised of an IgG-like chain of Flt-1 fused to either a human IgG1 Fc region or to its methyl domain. Several studies have confirmed the safety and efficacy of the AAV2 vector-mediated treatment of neovascularization in wet AMD models and patients [111, 112, 115–118]. Viral vectors carrying endostatin and angiostatin, the collagen and fibrinogen cleavage products, have efficacy in the inhibition of angiogenesis in exudative AMD [85, 119–121]. The safety of lentiviral Equine Infectious Anemia Virus (EIAV) as a method for ocular gene therapy in humans has been demonstrated in clinical trials [122]. Campochiaro et al. [122] noted that long-term expression of angiostatin and endostatin was observed through the latest measurement of eight neovascular AMD subjects after more than 2.5 years, and two subjects after more than 4 years. It was recently demonstrated that insulin-like growth factor binding protein-related protein 1 (IGFBP-rP1) inhibits retinal angiogenesis in mice with oxygen-induced retinopathy by blocking the extracellular signal-related kinase (ERK) signaling pathway and inhibiting VEGF expression [123].
The investigational drug HMR59 developed by Hemera Biosciences (Boston, Massachusetts, USA), uses an AAV2 vector to increase sCD59 expression; it aims to provide a therapy for dry AMD, and was granted ‘safe to proceed’ status by the FDA in January 2017 [124, 125].
Retinitis pigmentosa and other inherited retinal dystrophies
Retinits pigmentosa (RP) is a group of inherited degenerative conditions that affect the photoreceptor cells of the retina. In the classic presentation of RP, the rods are preferentially targeted first, which leads to a loss of night vision and limits peripheral vision. As the disease progresses, central vision also becomes compromised resulting in legal blindness. The prevalence of RP globally is estimated at approximately 1 in 4000 [126, 127]. Depending on its form, RP can be inherited in an autosomal dominant, autosomal recessive, or x-linked recessive manner [126, 128–130]. Spontaneous mutations undoubtedly account for some cases of RP, as approximately 40% of RP cases are isolated instances that present without any other family members being affected [131]. Many mutations associated with RP have been identified [132–138].
Stem cells have been investigated as agents for prolonged administration of neuroprotective or neurotrophic factors. Aharony et al. [28] note that stem cells can be induced to secrete neurotrophic factors (NTFs) such as brain-derived neurotrophic factor (BDNF), ciliary neurotrophic factor (CNTF), glial cell-line-derived neurotrophic factor (GDNF), and vascular endothelial growth factor (VEGF) to treat degenerative ophthalmic conditions; however, it is mentioned that the effect of these agents administered through stem cell engraftment is currently suboptimal. Promising treatments do exist in this area: BDNF has been shown to improve survival of neurons and RGCs and to preserve the structure of the optic nerve [139–143]. CNTF-secreting NSCs have been noted to provide protection to photoreceptors cells in models of retinitis pigmentosa [144]. GDNF-secreting ESCs and BMSCs secreting a combination of GDNF, BDNF, and VEGF have also been shown to significantly improve RGC survival [28, 145, 146].
The mer receptor tyrosine kinase (MERTK) has received attention due to the involvement of its mutation in a very rare form of autosomal recessive retinitis pigmentosa [46, 147–149]. Retinal degeneration results from a subretinal accumulation of debris from the outer segment of photoreceptors due to inhibited phagocytic activity, and this leads to apoptotic photoreceptor loss and progressively worsening performance on eletroretinography [150–153]. Gene replacement studies targeting the MERTK gene have involved the vectors adeno-associated virus (AAV), adenovirus, and lentivirus [154–156]. Tschernutter et al. [156] used a lentivirus-mediated process, and observed improvement of phagocytic function, decreased loss of photoreceptors, and preserved retinal function for the 7 month examination period included in the published report. It has been shown that AAV-mediated CTNF expression suppresses electrophysiological retinal responses; however, AAV-mediated GDNF expression was not associated with the same adverse effects, but improved cell survival in combination therapy with lentivirus-mediated gene replacement [157]. The tyrosine-mutant AAV8 Y733F vector expressing a human MERTK cDNA driven by an RPE-selective promoter administered subretinally has been observed to improve retinal function in RP models for an 8 month study period, with improvement in phagocytic function, decreased retinal vascular degeneration, and inhibition of Müller cell activation being noted [158]. Interestingly, AAV8 vectors appear to exhibit a greater spread in a dog model than other AAV vectors such as AAV2; in a study conducted in a primate model, both vector types transduced RPE efficiently, but the AAV8 vectors were significantly better at targeting photoreceptors than the AAV2 vectors [159, 160]. Petit et al. [161] observed that the developmental stage of rods has an effect on the gene transfer efficiency of AAV vectors, suggesting that the ability of AAV vectors to infect dying rod cells could be limited, and the gene transfer efficiency markedly reduced. Following subretinal injection, subjects with altered development of rod outer segments exhibited significantly reduced AAV transduction of rods, and increased preference for cones. A preference for rods was observed when cells had matured. This type of increase in cone transduction was also observed in adult mice with retinal degeneration as compared to wild-type mice. An understanding of vector preferences to photoreceptors will aid researchers in developing effective delivery systems and treatments.
One of the major causes of X-linked retinitis pigmentosa (XLRP) is mutation of the retinitis pigmentosa GTPase regulator (RPGR) gene [162–165]. There has been difficulty in producing AAV vectors with RPGR due to the relative instability of its sequence [166–170]. Fischer et al. [166] optimized the coding sequence of RPGRORF15 in an effort to increase sequence stability, increase expression levels of the RPGR transgene, and to remove cryptic splice sites. They demonstrated production of an AAV8 vector that consistently produced the full-length, correct RPGR protein, which rescued the disease phenotype in animal models. The glutamylation pattern of the vector-derived RPGR and that of the wild-type protein were indistinguishable which indicates a lack of significant alteration to post-translational modification. Appropriate safety was demonstrated in mice [166].
There have also been efforts to treat autosomal dominant RP (ADRP) using gene therapy. ADRP can be caused by mutations in more than 20 genes. Boye et al. [46] note that mutations may take the form of haploinsufficiency, dominant negative gene product, or toxic gain-of-function. In haploinsufficiency, the product produced by a single wild-type allele is not sufficient to maintain normal function. In dominant negative gene product mutation, there is usually interference with the movement or assembly of the product. Toxic gain-of-function mutations result in products that exhibit direct toxicity to the cell. More than 100 dominant mutations in the RHO gene alone have been identified [46]. Gene therapy differs depending on the type of mutation. In haploinsufficiency, therapy has involved delivery of wild-type cDNA to increase the level of normal protein to adequate levels such as in the treatment of retinal degeneration due to mutation in PRPH2 by Cai et al. [171]. Due to the complexity and diversity of dominant mutations, even those of the RHO gene alone, there have been efforts to treat the condition by using molecules such as ribozymes, synthetic miRNAs, or siRNAs to target and degrade both mutant and wild-type mRNA; due to the degenerative effect this has on the retina, these therapeutic agents must be accompanied by delivery and expression of cDNA resistant to degradation because of silent mutations [46, 172–179]. Some other approaches that exhibit promise in the treatment of ADRP include AAV vector delivery of wild-type cDNA to slow retinal degeneration, and the gene transfer of molecular chaperones to assist in proper protein folding or suppress the unfolding response [46, 180, 181].
There are many institutions, both private and academic, pursuing research into gene therapeutic agents to treat RP, XLRP, ADRP, and MERTK-related autosomal recessive RP [182]. Nightstar Therapeutics (London, England, UK) has an AAV vector encoding RPGR in phase I/II clinical trial [183]. The AAV2/5-hPDE6B vector from Horama (Paris, France) targets rod cGMP phosphodiesterase 6 β (PDE6B), and is in phase I/II clinical trial in patients with RP due to mutation of this gene [184]. MeiraGTx’s (New York, New York, USA) AAV2/5-hRKp.RPGR has also entered phase I/II clinical trial for the treatment of XLRP [185]. The RestroSense (acquired by Allergan in 2016) AAV vector RST-001 (Chop2) or ChR2 is intended primarily to treat retinitis pigmentosa, but advanced dry AMD is cited as a follow-on indication. It increases expression of the photosensitivity gene, channelrhodopsin-2 [186]. RST-001 is currently in phase I/II clinical trial for its application in advanced retinitis pigmentosa [187]. As an area of great interest currently, there are many investigations ongoing into gene therapies targeting various genes causing multiple types of retinal dystrophies.
In December 2017, the FDA approved Luxturna (voretigene neparvovec-rzyl, Spark Therapeutics. Philadelphia, Pennsylvania, USA) for the treatment of Leber’s congenital amaurosis type 2 and RP due to a mutation in RPE65 [7]. The therapy employs an AAV2 vector carrying complementary DNA (cDNA) encoding human RPE65. Voretigene neparvovec-rzyl is injected subretinally. The FDA indications for the drug require that the patient have a confirmed biallelic RPE65 mutation and viable retinal cells [188]. This drug is notable for being the first in vivo gene therapy approved by the FDA. In phase III clinical trial, it was demonstrated that patients receiving the drug experienced statistically significant improvements in multi-luminance mobility testing (MLMT), and full-field stimulus testing (FST) as compared to control, indicating restoration of RPE65 enzymatic activity resulting in improved navigation in low-to-moderate light and increased light perception. There were also slight improvements in best-corrected visual acuity (BCVA) noted. Ocular adverse events observed in the study participants were generally mild, with the most common being elevated intraocular pressure, cataract, retinal tear, and ocular inflammation [5]. Voretigene neparvovec appears to be a relatively safe therapy that could dramatically improve the vision of those with biallelic RPE65 mutation-associated retinal dystrophy.
Future directions
Considering research into stem cells and their use in treating ocular conditions in recent years, it appears that their greatest utility is likely in environment modification through such practices as drug delivery and immunomodulation. The role of curing retinal diseases will be more directly addressed in the immediate future through gene modulatory therapies. Gene transduction through viral vector delivery is an area of aggressive research. While some of these novel treatments are already being used in clinical medicine, their continued potential for attenuating degenerative processes and improving vision is significant and deserves continued investigation. While there have been many mutations reported, there undoubtedly remain others yet undiscovered, and identification of genes and mutations amenable to targeted therapy should continue. AAV and lentiviral vectors are the staple of therapeutic gene delivery in retinal research, and while these techniques have yielded good results, it could be of benefit to further investigate the feasibility of retroviral transduction in conjunction with retinal stem cell proliferation induction or non-viral transfection techniques in retinal gene therapy. Non-viral gene therapy is another promising area of retinal research [189, 190]. Recent investigations have made encouraging progress and showcased remarkable potential therapies to improve the visual acuity and quality of life of millions of individuals around the world. The future of treatment in retinal disease likely lies in the utilization of some form of genetic modification to combat these blinding conditions.
Authors’ contributions
PEL and ACJ conceived of the design of the work and performed the later stages of editing. PEL performed data collection and interpretation, and major drafting. SCF assisted in initial editing of the manuscript, and creation of tables. ACJ outlined the structure of the paper. All authors read and approved the final manuscript.
Acknowledgements
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
All articles and resources referenced herein were accessed between 1 May 2017 and 5 April 2018 and located through PubMed/MEDLINE database and Google searches using the relevant keywords.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Not applicable.
Funding
Not applicable.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Parker E. Ludwig, Email: parkerludwig@creighton.edu
S. Caleb Freeman, Email: stevenfreeman@creighton.edu.
Adam C. Janot, Email: acjanot1@gmail.com
References
- 1.Congdon N, O’Colmain B, Klaver CC, Klein R, Muñoz B, Friedman DS, et al. Causes and prevalence of visual impairment among adults in the United States. Arch Ophthalmol. 2004;122(4):477–485. doi: 10.1001/archopht.122.4.477. [DOI] [PubMed] [Google Scholar]
- 2.Lee R, Wong TY, Sabanayagam C. Epidemiology of diabetic retinopathy, diabetic macular edema and related vision loss. Eye Vis (Lond). 2015;2. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4657234/. Cited 16 May 2017. [DOI] [PMC free article] [PubMed]
- 3.Tsujikawa M, Wada Y, Sukegawa M, Sawa M, Gomi F, Nishida K, et al. Age at onset curves of retinitis pigmentosa. Arch Ophthalmol. 2008;126(3):337–340. doi: 10.1001/archopht.126.3.337. [DOI] [PubMed] [Google Scholar]
- 4.Ferrari S, Di Iorio E, Barbaro V, Ponzin D, Sorrentino FS, Parmeggiani F. Retinitis pigmentosa: genes and disease mechanisms. Curr Genom. 2011;12(4):238–249. doi: 10.2174/138920211795860107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Russell S, Bennett J, Wellman JA, Chung DC, Yu Z-F, Tillman A, et al. Efficacy and safety of voretigene neparvovec (AAV2-hRPE65v2) in patients with RPE65-mediated inherited retinal dystrophy: a randomised, controlled, open-label, phase 3 trial. Lancet. 2017;390(10097):849–860. doi: 10.1016/S0140-6736(17)31868-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dias MF, Joo K, Kemp JA, Fialho SL, da Silva CA, Woo SJ, et al. Molecular genetics and emerging therapies for retinitis pigmentosa: basic research and clinical perspectives. Prog Retin Eye Res. 2017;63:107–131. doi: 10.1016/j.preteyeres.2017.10.004. [DOI] [PubMed] [Google Scholar]
- 7.FDA approves novel gene therapy to treat patients with a rare form of inherited vision loss. Available at: https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm589467.htm. Cited 4 Jan 2018.
- 8.Morrison SJ, Shah NM, Anderson DJ. Regulatory mechanisms in stem cell biology. Cell. 1997;88(3):287–298. doi: 10.1016/S0092-8674(00)81867-X. [DOI] [PubMed] [Google Scholar]
- 9.Tropepe V, Coles BLK, Chiasson BJ, Horsford DJ, Elia AJ, McInnes RR, et al. Retinal stem cells in the adult mammalian eye. Science. 2000;287(5460):2032–2036. doi: 10.1126/science.287.5460.2032. [DOI] [PubMed] [Google Scholar]
- 10.Coles BLK, Angénieux B, Inoue T, Rio-Tsonis KD, Spence JR, McInnes RR, et al. Facile isolation and the characterization of human retinal stem cells. PNAS. 2004;101(44):15772–15777. doi: 10.1073/pnas.0401596101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Perron M, Harris WA. Retinal stem cells in vertebrates. BioEssays. 2000;22(8):685–688. doi: 10.1002/1521-1878(200008)22:8<685::AID-BIES1>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 12.Bernardos RL, Barthel LK, Meyers JR, Raymond PA. Late-stage neuronal progenitors in the retina are radial Müller glia that function as retinal stem cells. J Neurosci. 2007;27(26):7028–7040. doi: 10.1523/JNEUROSCI.1624-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cicero SA, Johnson D, Reyntjens S, Frase S, Connell S, Chow LML, et al. Cells previously identified as retinal stem cells are pigmented ciliary epithelial cells. PNAS. 2009;106(16):6685–6690. doi: 10.1073/pnas.0901596106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stem Cell Basics III. stemcells.nih.gov. https://stemcells.nih.gov/info/basics/3.htm. Cited 28 Dec 2018.
- 15.Zhu Z, Huangfu D. Human pluripotent stem cells: an emerging model in developmental biology. Development. 2013;140(4):705–717. doi: 10.1242/dev.086165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marion NW, Mao JJ. Mesenchymal stem cells and tissue engineering. Methods Enzymol. 2006;420:339–361. doi: 10.1016/S0076-6879(06)20016-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cruet-Hennequart S, Prendergast AM, Barry FP, Carty MP. Human mesenchymal stem cells (hMSCs) as targets of DNA damaging agents in cancer therapy. Curr Cancer Drug Targets. 2010;10(4):411–421. doi: 10.2174/156800910791208553. [DOI] [PubMed] [Google Scholar]
- 18.Liu Y, Chen SJ, Li SY, Qu LH, Meng XH, Wang Y, et al. Long-term safety of human retinal progenitor cell transplantation in retinitis pigmentosa patients. Stem Cell Res Ther. 2017;8. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5622579/. Cited 28 Dec 2018. [DOI] [PMC free article] [PubMed]
- 19.Chacko DM, Rogers JA, Turner JE, Ahmad I. Survival and differentiation of cultured retinal progenitors transplanted in the subretinal space of the rat. Biochem Biophys Res Commun. 2000;268(3):842–846. doi: 10.1006/bbrc.2000.2153. [DOI] [PubMed] [Google Scholar]
- 20.Sakaguchi DS, Van Hoffelen SJ, Theusch E, Parker E, Orasky J, Harper MM, et al. Transplantation of neural progenitor cells into the developing retina of the Brazilian opossum: an in vivo system for studying stem/progenitor cell plasticity. Dev Neurosci. 2004;26(5–6):336–345. doi: 10.1159/000082275. [DOI] [PubMed] [Google Scholar]
- 21.Van Hoffelen SJ, Young MJ, Shatos MA, Sakaguchi DS. Incorporation of murine brain progenitor cells into the developing mammalian retina. Invest Ophthalmol Vis Sci. 2003;44(1):426–434. doi: 10.1167/iovs.02-0269. [DOI] [PubMed] [Google Scholar]
- 22.Young MJ, Ray J, Whiteley SJ, Klassen H, Gage FH. Neuronal differentiation and morphological integration of hippocampal progenitor cells transplanted to the retina of immature and mature dystrophic rats. Mol Cell Neurosci. 2000;16(3):197–205. doi: 10.1006/mcne.2000.0869. [DOI] [PubMed] [Google Scholar]
- 23.MacLaren RE, Pearson RA, MacNeil A, Douglas RH, Salt TE, Akimoto M, et al. Retinal repair by transplantation of photoreceptor precursors. Nature. 2006;444(7116):203–207. doi: 10.1038/nature05161. [DOI] [PubMed] [Google Scholar]
- 24.Meyer JS, Katz ML, Maruniak JA, Kirk MD. Embryonic stem cell-derived neural progenitors incorporate into degenerating retina and enhance survival of host photoreceptors. Stem Cells. 2006;24(2):274–283. doi: 10.1634/stemcells.2005-0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lu P, Jones LL, Snyder EY, Tuszynski MH. Neural stem cells constitutively secrete neurotrophic factors and promote extensive host axonal growth after spinal cord injury. Exp Neurol. 2003;181(2):115–129. doi: 10.1016/S0014-4886(03)00037-2. [DOI] [PubMed] [Google Scholar]
- 26.Klassen HJ, Imfeld KL, Kirov II, Tai L, Gage FH, Young MJ, et al. Expression of cytokines by multipotent neural progenitor cells. Cytokine. 2003;22(3–4):101–106. doi: 10.1016/S1043-4666(03)00120-0. [DOI] [PubMed] [Google Scholar]
- 27.Cooper JD, Messer A, Feng AK, Chua-Couzens J, Mobley WC. Apparent loss and hypertrophy of interneurons in a mouse model of neuronal ceroid lipofuscinosis: evidence for partial response to insulin-like growth factor-1 treatment. J Neurosci. 1999;19(7):2556–2567. doi: 10.1523/JNEUROSCI.19-07-02556.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aharony I, Michowiz S, Goldenberg-Cohen N. The promise of stem cell-based therapeutics in ophthalmology. Neural Regen Res. 2017;12(2):173–180. doi: 10.4103/1673-5374.200793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shirai H, Mandai M, Matsushita K, Kuwahara A, Yonemura S, Nakano T, et al. Transplantation of human embryonic stem cell-derived retinal tissue in two primate models of retinal degeneration. Proc Natl Acad Sci USA. 2016;113(1):E81–E90. doi: 10.1073/pnas.1512590113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Singhal S, Bhatia B, Jayaram H, Becker S, Jones MF, Cottrill PB, et al. Human Müller glia with stem cell characteristics differentiate into retinal ganglion cell (RGC) precursors in vitro and partially restore RGC function in vivo following transplantation. Stem Cells Transl Med. 2012;1(3):188–199. doi: 10.5966/sctm.2011-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Canola K, Angénieux B, Tekaya M, Quiambao A, Naash MI, Munier FL, et al. Retinal stem cells transplanted into models of late stages of retinitis pigmentosa preferentially adopt a glial or a retinal ganglion cell fate. Invest Ophthalmol Vis Sci. 2007;48(1):446–454. doi: 10.1167/iovs.06-0190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goldenberg Cohen N, Avraham Lubin B-CR, Sadikov T, Goldstein RS, Askenasy N. Primitive stem cells derived from bone marrow express glial and neuronal markers and support revascularization in injured retina exposed to ischemic and mechanical damage. Stem Cells Dev. 2012;21(9):1488–1500. doi: 10.1089/scd.2011.0366. [DOI] [PubMed] [Google Scholar]
- 33.Goldenberg Cohen N, Avraham Lubin B-CR, Sadikov T, Askenasy N. Effect of coadministration of neuronal growth factors on neuroglial differentiation of bone marrow-derived stem cells in the ischemic retina. Invest Ophthalmol Vis Sci. 2014;55(1):502–512. doi: 10.1167/iovs.13-12223. [DOI] [PubMed] [Google Scholar]
- 34.Zhou X, Xia X-B. Retinal stem cells transplantation combined with copolymer-1 immunization reduces interferon-gamma levels in an experimental model of glaucoma. Int J Ophthalmol. 2011;4(6):594–598. doi: 10.3980/j.issn.2222-3959.2011.06.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Azizi SA, Stokes D, Augelli BJ, DiGirolamo C, Prockop DJ. Engraftment and migration of human bone marrow stromal cells implanted in the brains of albino rats—similarities to astrocyte grafts. Proc Natl Acad Sci USA. 1998;95(7):3908–3913. doi: 10.1073/pnas.95.7.3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mathew B, Poston JN, Dreixler JC, Torres L, Lopez J, Zelkha R, et al. Bone-marrow mesenchymal stem-cell administration significantly improves outcome after retinal ischemia in rats. Graefes Arch Clin Exp Ophthalmol. 2017;255:1581–1592. doi: 10.1007/s00417-017-3690-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bhattacharya S, Yin J, Winborn CS, Zhang Q, Yue J, Chaum E. Prominin-1 is a novel regulator of autophagy in the human retinal pigment epithelium. Invest Ophthalmol Vis Sci. 2017;58(4):2366–2387. doi: 10.1167/iovs.16-21162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chao JR, Lamba DA, Klesert TR, Torre AL, Hoshino A, Taylor RJ, et al. Transplantation of human embryonic stem cell-derived retinal cells into the subretinal space of a non-human primate. Transl Vis Sci Technol. 2017;6(3):4. doi: 10.1167/tvst.6.3.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wahlin KJ, Maruotti JA, Sripathi SR, Ball J, Angueyra JM, Kim C, et al. Photoreceptor outer segment-like structures in long-term 3D retinas from human pluripotent stem cells. Sci Rep. 2017;7. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC5429674/. Cited 23 May 2017. [DOI] [PMC free article] [PubMed]
- 40.Sasai Y. Next-generation regenerative medicine: organogenesis from stem cells in 3D culture. Cell Stem Cell. 2013;12(5):520–530. doi: 10.1016/j.stem.2013.04.009. [DOI] [PubMed] [Google Scholar]
- 41.Assawachananont J, Mandai M, Okamoto S, Yamada C, Eiraku M, Yonemura S, et al. Transplantation of embryonic and induced pluripotent stem cell-derived 3D retinal sheets into retinal degenerative mice. Stem Cell Rep. 2014;2(5):662–674. doi: 10.1016/j.stemcr.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yoshii C, Ueda Y, Okamoto M, Araki M. Neural retinal regeneration in the anuran amphibian Xenopus laevis post-metamorphosis: transdifferentiation of retinal pigmented epithelium regenerates the neural retina. Dev Biol. 2007;303(1):45–56. doi: 10.1016/j.ydbio.2006.11.024. [DOI] [PubMed] [Google Scholar]
- 43.Naitoh H, Suganuma Y, Ueda Y, Sato T, Hiramuki Y, Fujisawa-Sehara A, et al. Upregulation of matrix metalloproteinase triggers transdifferentiation of retinal pigmented epithelial cells in Xenopus laevis: a link between inflammatory response and regeneration. Dev Neurobiol. 2017;77:1086–1100. doi: 10.1002/dneu.22497. [DOI] [PubMed] [Google Scholar]
- 44.Qu L, Gao L, Xu H, Duan P, Zeng Y, Liu Y, et al. Combined transplantation of human mesenchymal stem cells and human retinal progenitor cells into the subretinal space of RCS rats. Sci Rep. 2017;7(1):199. doi: 10.1038/s41598-017-00241-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Finer M, Glorioso J. A brief account of viral vectors and their promise for gene therapy. Gene Ther. 2017;24(1):1–2. doi: 10.1038/gt.2016.71. [DOI] [PubMed] [Google Scholar]
- 46.Boye SE, Boye SL, Lewin AS, Hauswirth WW. A comprehensive review of retinal gene therapy. Mol Ther. 2013;21(3):509–519. doi: 10.1038/mt.2012.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Oliveira AV, Rosa da Costa AM, Silva GA. Non-viral strategies for ocular gene delivery. Mater Sci Eng C. 2017;77:1275–1289. doi: 10.1016/j.msec.2017.04.068. [DOI] [PubMed] [Google Scholar]
- 48.Ezati R, Etemadzadeh A, Soheili Z-S, Samiei S, Pirmardan ER, Davari M, et al. The influence of rAAV2-mediated SOX2 delivery into neonatal and adult human RPE cells; a comparative study. J Cell Physiol. 2017;233:1222–1235. doi: 10.1002/jcp.25991. [DOI] [PubMed] [Google Scholar]
- 49.Approved Cellular and Gene Therapy Products - FDA. Available at: https://www.fda.gov/BiologicsBloodVaccines/CellularGeneTherapyProducts/ApprovedProducts/default.htm. Cited 8 Mar 2018.
- 50.World Health Organization . Global report on diabetes. Geneva: World Health Organization; 2016. [Google Scholar]
- 51.Tong L, Vernon SA, Kiel W, Sung V, Orr GM. Association of macular involvement with proliferative retinopathy in Type 2 diabetes. Diabet Med. 2001;18(5):388–394. doi: 10.1046/j.1464-5491.2001.00483.x. [DOI] [PubMed] [Google Scholar]
- 52.Lightman S, Towler HM. Diabetic retinopathy. Clin Cornerstone. 2003;5(2):12–21. doi: 10.1016/S1098-3597(03)90015-9. [DOI] [PubMed] [Google Scholar]
- 53.Abcouwer SF, Gardner TW. Diabetic retinopathy: loss of neuroretinal adaptation to the diabetic metabolic environment. Ann NY Acad Sci. 2014;1311(1):174–190. doi: 10.1111/nyas.12412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Marques-Neves C. Diabetic retinopathy—pathophysiology. Acta Ophthalmol. 2015 doi: 10.1111/j.1755-3768.2015.0198. [DOI] [Google Scholar]
- 55.Joussen AM, Poulaki V, Le ML, Koizumi K, Esser C, Janicki H, et al. A central role for inflammation in the pathogenesis of diabetic retinopathy. FASEB J. 2004;18(12):1450–1452. doi: 10.1096/fj.03-1476fje. [DOI] [PubMed] [Google Scholar]
- 56.Stitt AW. The role of advanced glycation in the pathogenesis of diabetic retinopathy. Exp Mol Pathol. 2003;75(1):95–108. doi: 10.1016/S0014-4800(03)00035-2. [DOI] [PubMed] [Google Scholar]
- 57.Asnaghi V, Gerhardinger C, Hoehn T, Adeboje A, Lorenzi M. A role for the polyol pathway in the early neuroretinal apoptosis and glial changes induced by diabetes in the rat. Diabetes. 2003;52(2):506–511. doi: 10.2337/diabetes.52.2.506. [DOI] [PubMed] [Google Scholar]
- 58.Prompt panretinal photocoagulation versus ranibizumab + deferred panretinal photocoagulation for proliferative diabetic retinopathy—study results—ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/results/NCT01489189. Cited 6 Jan 2018.
- 59.Diabetic Retinopathy Study (DRS). Department of Ophthalmology Academic Resources, Boston University. http://www.bu.edu/eye/evidence-based-medicine/vitreo-retinal-studies/diabetic-retinopathy-study-drs/. Cited 6 Jan 2018.
- 60.Royle P, Mistry H, Auguste P, Shyangdan D, Freeman K, Lois N, et al. The landmark trials: Diabetic Retinopathy Study and Early Treatment Diabetic Retinopathy Study. NIHR J Libr; 2015. https://www.ncbi.nlm.nih.gov/books/NBK305100/. Cited 6 Jan 2018.
- 61.Dowler JGF. Laser management of diabetic retinopathy. J R Soc Med. 2003;96(6):277–279. doi: 10.1177/014107680309600605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kozak I, Luttrull JK. Modern retinal laser therapy. Saudi J Ophthalmol. 2015;29(2):137–146. doi: 10.1016/j.sjopt.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kramerov AA, Ljubimov AV. Stem cell therapies in the treatment of diabetic retinopathy and keratopathy. Exp Biol Med (Maywood) 2016;241(6):559–568. doi: 10.1177/1535370215609692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Salmon AB, Pérez VI, Bokov A, Jernigan A, Kim G, Zhao H, et al. Lack of methionine sulfoxide reductase A in mice increases sensitivity to oxidative stress but does not diminish life span. FASEB J. 2009;23(10):3601–3608. doi: 10.1096/fj.08-127415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Inoue Y, Iriyama A, Ueno S, Takahashi H, Kondo M, Tamaki Y, et al. Subretinal transplantation of bone marrow mesenchymal stem cells delays retinal degeneration in the RCS rat model of retinal degeneration. Exp Eye Res. 2007;85(2):234–241. doi: 10.1016/j.exer.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 66.Zhang Y, Wang W. Effects of bone marrow mesenchymal stem cell transplantation on light-damaged retina. Invest Ophthalmol Vis Sci. 2010;51(7):3742–3748. doi: 10.1167/iovs.08-3314. [DOI] [PubMed] [Google Scholar]
- 67.Li N, Li X, Yuan J. Effects of bone-marrow mesenchymal stem cells transplanted into vitreous cavity of rat injured by ischemia/reperfusion. Graefes Arch Clin Exp Ophthalmol. 2009;247(4):503–514. doi: 10.1007/s00417-008-1009-y. [DOI] [PubMed] [Google Scholar]
- 68.Medina RJ, O’Neill CL, Sweeney M, Guduric-Fuchs J, Gardiner TA, Simpson DA, et al. Molecular analysis of endothelial progenitor cell (EPC) subtypes reveals two distinct cell populations with different identities. BMC Med Genom. 2010;3:18. doi: 10.1186/1755-8794-3-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yoon C-H, Hur J, Park K-W, Kim J-H, Lee C-S, Oh I-Y, et al. Synergistic neovascularization by mixed transplantation of early endothelial progenitor cells and late outgrowth endothelial cells: the role of angiogenic cytokines and matrix metalloproteinases. Circulation. 2005;112(11):1618–1627. doi: 10.1161/CIRCULATIONAHA.104.503433. [DOI] [PubMed] [Google Scholar]
- 70.Cho H-J, Kim H-S, Lee M-M, Kim D-H, Yang H-J, Hur J, et al. Mobilized endothelial progenitor cells by granulocyte-macrophage colony-stimulating factor accelerate reendothelialization and reduce vascular inflammation after intravascular radiation. Circulation. 2003;108(23):2918–2925. doi: 10.1161/01.CIR.0000097001.79750.78. [DOI] [PubMed] [Google Scholar]
- 71.Butler JM, Guthrie SM, Koc M, Afzal A, Caballero S, Brooks HL, et al. SDF-1 is both necessary and sufficient to promote proliferative retinopathy. J Clin Invest. 2005;115(1):86–93. doi: 10.1172/JCI22869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hwang J-H, Kim S-W, Park S-E, Yun H-J, Lee Y, Kim S, et al. Overexpression of stromal cell-derived factor-1 enhances endothelium-supported transmigration, maintenance, and proliferation of hematopoietic progenitor cells. Stem Cells Dev. 2006;15(2):260–268. doi: 10.1089/scd.2006.15.260. [DOI] [PubMed] [Google Scholar]
- 73.Cheang WS, Fang X, Tian XY. Pleiotropic effects of peroxisome proliferator-activated receptor γ and δ in vascular diseases. Circ J. 2013;77(11):2664–2671. doi: 10.1253/circj.CJ-13-0647. [DOI] [PubMed] [Google Scholar]
- 74.Sorrentino SA, Bahlmann FH, Besler C, Müller M, Schulz S, Kirchhoff N, et al. Oxidant stress impairs in vivo reendothelialization capacity of endothelial progenitor cells from patients with type 2 diabetes mellitus: restoration by the peroxisome proliferator-activated receptor-gamma agonist rosiglitazone. Circulation. 2007;116(2):163–173. doi: 10.1161/CIRCULATIONAHA.106.684381. [DOI] [PubMed] [Google Scholar]
- 75.Mohler ER, Shi Y, Moore J, Bantly A, Hamamdzic D, Yoder M, et al. Diabetes reduces bone marrow and circulating porcine endothelial progenitor cells, an effect ameliorated by atorvastatin and independent of cholesterol. Cytometry A. 2009;75(1):75–82. doi: 10.1002/cyto.a.20691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wang J-H, Ling D, Tu L, van Wijngaarden P, Dusting GJ, Liu G-S. Gene therapy for diabetic retinopathy: are we ready to make the leap from bench to bedside? Pharmacol Ther. 2017;173(Supplement C):1–18. doi: 10.1016/j.pharmthera.2017.01.003. [DOI] [PubMed] [Google Scholar]
- 77.Pechan P, Rubin H, Lukason M, Ardinger J, DuFresne E, Hauswirth WW, et al. Novel anti-VEGF chimeric molecules delivered by AAV vectors for inhibition of retinal neovascularization. Gene Ther. 2009;16(1):10–16. doi: 10.1038/gt.2008.115. [DOI] [PubMed] [Google Scholar]
- 78.Haurigot V, Villacampa P, Ribera A, Bosch A, Ramos D, Ruberte J, et al. Long-term retinal PEDF overexpression prevents neovascularization in a murine adult model of retinopathy. PLoS ONE. 2012;7(7):e41511. doi: 10.1371/journal.pone.0041511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lamartina S, Cimino M, Roscilli G, Dammassa E, Lazzaro D, Rota R, et al. Helper-dependent adenovirus for the gene therapy of proliferative retinopathies: stable gene transfer, regulated gene expression and therapeutic efficacy. J Gene Med. 2007;9(10):862–874. doi: 10.1002/jgm.1083. [DOI] [PubMed] [Google Scholar]
- 80.Ideno J, Mizukami H, Kakehashi A, Saito Y, Okada T, Urabe M, et al. Prevention of diabetic retinopathy by intraocular soluble flt-1 gene transfer in a spontaneously diabetic rat model. Int J Mol Med. 2007;19(1):75–79. [PubMed] [Google Scholar]
- 81.Gehlbach P, Demetriades AM, Yamamoto S, Deering T, Xiao WH, Duh EJ, et al. Periocular gene transfer of sFlt-1 suppresses ocular neovascularization and vascular endothelial growth factor-induced breakdown of the blood-retinal barrier. Hum Gene Ther. 2003;14(2):129–141. doi: 10.1089/104303403321070829. [DOI] [PubMed] [Google Scholar]
- 82.Auricchio A, Behling KC, Maguire AM, O’Connor EM, Bennett J, Wilson JM, et al. Inhibition of retinal neovascularization by intraocular viral-mediated delivery of anti-angiogenic agents. Mol Ther. 2002;6(4):490–494. doi: 10.1006/mthe.2002.0702. [DOI] [PubMed] [Google Scholar]
- 83.Biswal MR, Prentice HM, Dorey CK, Blanks JC. A hypoxia-responsive glial cell-specific gene therapy vector for targeting retinal neovascularization. Invest Ophthalmol Vis Sci. 2014;55(12):8044–8053. doi: 10.1167/iovs.14-13932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Le Gat L, Gogat K, Bouquet C, Saint-Geniez M, Darland D, Van Den Berghe L, et al. In vivo adenovirus-mediated delivery of a uPA/uPAR antagonist reduces retinal neovascularization in a mouse model of retinopathy. Gene Ther. 2003;10(25):2098–2103. doi: 10.1038/sj.gt.3302122. [DOI] [PubMed] [Google Scholar]
- 85.Igarashi T, Miyake K, Kato K, Watanabe A, Ishizaki M, Ohara K, et al. Lentivirus-mediated expression of angiostatin efficiently inhibits neovascularization in a murine proliferative retinopathy model. Gene Ther. 2003;10(3):219–226. doi: 10.1038/sj.gt.3301878. [DOI] [PubMed] [Google Scholar]
- 86.Adhi M, Cashman SM, Kumar-Singh R. Adeno-associated virus mediated delivery of a non-membrane targeted human soluble CD59 attenuates some aspects of diabetic retinopathy in mice. PLoS ONE. 2013;8(10):e79661. doi: 10.1371/journal.pone.0079661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhang L, Xia H, Han Q, Chen B. Effects of antioxidant gene therapy on the development of diabetic retinopathy and the metabolic memory phenomenon. Graefes Arch Clin Exp Ophthalmol. 2015;253(2):249–259. doi: 10.1007/s00417-014-2827-8. [DOI] [PubMed] [Google Scholar]
- 88.Verma A, Shan Z, Lei B, Yuan L, Liu X, Nakagawa T, et al. ACE2 and Ang-(1-7) confer protection against development of diabetic retinopathy. Mol Ther. 2012;20(1):28–36. doi: 10.1038/mt.2011.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gong Y, Chang Z-P, Ren R-T, Wei S-H, Zhou H-F, Chen X-F, et al. Protective effects of adeno-associated virus mediated brain-derived neurotrophic factor expression on retinal ganglion cells in diabetic rats. Cell Mol Neurobiol. 2012;32(3):467–475. doi: 10.1007/s10571-011-9779-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Fletcher EL, Phipps JA, Ward MM, Vessey KA, Wilkinson-Berka JL. The renin–angiotensin system in retinal health and disease: its influence on neurons, glia and the vasculature. Prog Retin Eye Res. 2010;29(4):284–311. doi: 10.1016/j.preteyeres.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 91.Marchesi C, Paradis P, Schiffrin EL. Role of the renin–angiotensin system in vascular inflammation. Trends Pharmacol Sci. 2008;29(7):367–374. doi: 10.1016/j.tips.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 92.Ferreira AJ, Santos RAS, Bradford CN, Mecca AP, Sumners C, Katovich MJ, et al. Therapeutic implications of the vasoprotective axis of the renin–angiotensin system in cardiovascular diseases. Hypertension. 2010;55(2):207–213. doi: 10.1161/HYPERTENSIONAHA.109.140145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wong WL, Su X, Li X, Cheung CMG, Klein R, Cheng C-Y, et al. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob Health. 2014;2(2):e106–e116. doi: 10.1016/S2214-109X(13)70145-1. [DOI] [PubMed] [Google Scholar]
- 94.Ambati J, Ambati BK, Yoo SH, Ianchulev S, Adamis AP. Age-related macular degeneration: etiology, pathogenesis, and therapeutic strategies. Surv Ophthalmol. 2003;48(3):257–293. doi: 10.1016/S0039-6257(03)00030-4. [DOI] [PubMed] [Google Scholar]
- 95.Prevalence of age-related macular degeneration in the United States. National Eye Institute. https://nei.nih.gov/eyedata/pbd4. Cited 17 May 2017.
- 96.Age-related macular degeneration (AMD) tables. National Eye Institute. https://nei.nih.gov/eyedata/amd/tables. Cited 17 May 2017.
- 97.Randomized A. Placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss. Arch Ophthalmol. 2001;119(10):1417–1436. doi: 10.1001/archopht.119.10.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Martin DF, Maguire MG, Fine SL, Ying G, Jaffe GJ, Grunwald JE, et al. Ranibizumab and bevacizumab for treatment of neovascular age-related macular degeneration: two-year results. Ophthalmology. 2012;119(7):1388–1398. doi: 10.1016/j.ophtha.2012.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Nm B. Photodynamic therapy of subfoveal choroidal neovascularization in age-related macular degeneration with verteporfin: two-year results of 2 randomized clinical trials-tap report 2. Arch Ophthalmol. 2001;119(2):198–207. [PubMed] [Google Scholar]
- 100.Carr A-J, Vugler AA, Hikita ST, Lawrence JM, Gias C, Chen LL, et al. Protective effects of human iPS-derived retinal pigment epithelium cell transplantation in the retinal dystrophic rat. PLoS ONE. 2009;4(12):e8152. doi: 10.1371/journal.pone.0008152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kanemura H, Go MJ, Shikamura M, Nishishita N, Sakai N, Kamao H, et al. Tumorigenicity studies of induced pluripotent stem cell (iPSC)-derived retinal pigment epithelium (RPE) for the treatment of age-related macular degeneration. PLoS ONE. 2014;9(1):e85336. doi: 10.1371/journal.pone.0085336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Li Y, Tsai Y-T, Hsu C-W, Erol D, Yang J, Wu W-H, et al. Long-term safety and efficacy of human-induced pluripotent stem cell (iPS) grafts in a preclinical model of retinitis pigmentosa. Mol Med. 2012;18:1312–1319. doi: 10.2119/molmed.2012.00242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Tucker BA, Park I-H, Qi SD, Klassen HJ, Jiang C, Yao J, et al. Transplantation of adult mouse iPS cell-derived photoreceptor precursors restores retinal structure and function in degenerative mice. PLoS ONE. 2011;6(4):e18992. doi: 10.1371/journal.pone.0018992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mandai M, Watanabe A, Kurimoto Y, Hirami Y, Morinaga C, Daimon T, et al. Autologous induced stem-cell-derived retinal cells for macular degeneration. N Engl J Med. 2017;376(11):1038–1046. doi: 10.1056/NEJMoa1608368. [DOI] [PubMed] [Google Scholar]
- 105.Schwartz SD, Regillo CD, Lam BL, Eliott D, Rosenfeld PJ, Gregori NZ, et al. Human embryonic stem cell-derived retinal pigment epithelium in patients with age-related macular degeneration and Stargardt’s macular dystrophy: follow-up of two open-label phase 1/2 studies. Lancet. 2015;385(9967):509–516. doi: 10.1016/S0140-6736(14)61376-3. [DOI] [PubMed] [Google Scholar]
- 106.Song WK, Park K-M, Kim H-J, Lee JH, Choi J, Chong SY, et al. Treatment of macular degeneration using embryonic stem cell-derived retinal pigment epithelium: preliminary results in Asian patients. Stem Cell Rep. 2015;4(5):860–872. doi: 10.1016/j.stemcr.2015.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Aiello LP, Pierce EA, Foley ED, Takagi H, Chen H, Riddle L, et al. Suppression of retinal neovascularization in vivo by inhibition of vascular endothelial growth factor (VEGF) using soluble VEGF-receptor chimeric proteins. Proc Natl Acad Sci USA. 1995;92(23):10457–10461. doi: 10.1073/pnas.92.23.10457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Brown DM, Regillo CD. Anti-VEGF agents in the treatment of neovascular age-related macular degeneration: applying clinical trial results to the treatment of everyday patients. Am J Ophthalmol. 2007;144(4):627–637. doi: 10.1016/j.ajo.2007.06.039. [DOI] [PubMed] [Google Scholar]
- 109.Lai YKY, Shen WY, Brankov M, Lai CM, Constable IJ, Rakoczy PE. Potential long-term inhibition of ocular neovascularisation by recombinant adeno-associated virus-mediated secretion gene therapy. Gene Ther. 2002;9(12):804–813. doi: 10.1038/sj.gt.3301695. [DOI] [PubMed] [Google Scholar]
- 110.Lai C-M, Shen W-Y, Brankov M, Lai YKY, Barnett NL, Lee S-Y, et al. Long-term evaluation of AAV-mediated sFlt-1 gene therapy for ocular neovascularization in mice and monkeys. Mol Ther. 2005;12(4):659–668. doi: 10.1016/j.ymthe.2005.04.022. [DOI] [PubMed] [Google Scholar]
- 111.Constable IJ, Pierce CM, Lai C-M, Magno AL, Degli-Esposti MA, French MA, et al. Phase 2a randomized clinical trial: safety and post hoc analysis of subretinal rAAV.sFLT-1 for wet age-related macular degeneration. EBioMedicine. 2016;14:168–175. doi: 10.1016/j.ebiom.2016.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Heier JS, Kherani S, Desai S, Dugel P, Kaushal S, Cheng SH, et al. Intravitreous injection of AAV2-sFLT01 in patients with advanced neovascular age-related macular degeneration: a phase 1, open-label trial. Lancet. 2017;390:50–61. doi: 10.1016/S0140-6736(17)30979-0. [DOI] [PubMed] [Google Scholar]
- 113.Rakoczy EP. Gene therapy for the long term treatment of wet AMD. Lancet. 2017;390:6–7. doi: 10.1016/S0140-6736(17)31262-X. [DOI] [PubMed] [Google Scholar]
- 114.Rakoczy EP, Lai C-M, Magno AL, Wikstrom ME, French MA, Pierce CM, et al. Gene therapy with recombinant adeno-associated vectors for neovascular age-related macular degeneration: 1 year follow-up of a phase 1 randomised clinical trial. Lancet. 2015;386(10011):2395–2403. doi: 10.1016/S0140-6736(15)00345-1. [DOI] [PubMed] [Google Scholar]
- 115.Lai C-M, Estcourt MJ, Wikstrom M, Himbeck RP, Barnett NL, Brankov M, et al. rAAV.sFlt-1 gene therapy achieves lasting reversal of retinal neovascularization in the absence of a strong immune response to the viral vector. Invest Ophthalmol Vis Sci. 2009;50(9):4279–4287. doi: 10.1167/iovs.08-3253. [DOI] [PubMed] [Google Scholar]
- 116.Lukason M, DuFresne E, Rubin H, Pechan P, Li Q, Kim I, et al. Inhibition of choroidal neovascularization in a nonhuman primate model by intravitreal administration of an AAV2 vector expressing a novel anti-VEGF molecule. Mol Ther. 2011;19(2):260–265. doi: 10.1038/mt.2010.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Igarashi T, Miyake K, Masuda I, Takahashi H, Shimada T. Adeno-associated vector (type 8)-mediated expression of soluble Flt-1 efficiently inhibits neovascularization in a murine choroidal neovascularization model. Hum Gene Ther. 2010;21(5):631–637. doi: 10.1089/hum.2009.153. [DOI] [PubMed] [Google Scholar]
- 118.Maclachlan TK, Lukason M, Collins M, Munger R, Isenberger E, Rogers C, et al. Preclinical safety evaluation of AAV2-sFLT01—a gene therapy for age-related macular degeneration. Mol Ther. 2011;19(2):326–334. doi: 10.1038/mt.2010.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Mori K, Ando A, Gehlbach P, Nesbitt D, Takahashi K, Goldsteen D, et al. Inhibition of choroidal neovascularization by intravenous injection of adenoviral vectors expressing secretable endostatin. Am J Pathol. 2001;159(1):313–320. doi: 10.1016/S0002-9440(10)61697-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lai L-J, Xiao X, Wu JH. Inhibition of corneal neovascularization with endostatin delivered by adeno-associated viral (AAV) vector in a mouse corneal injury model. J Biomed Sci. 2007;14(3):313–322. doi: 10.1007/s11373-007-9153-7. [DOI] [PubMed] [Google Scholar]
- 121.Raisler BJ, Berns KI, Grant MB, Beliaev D, Hauswirth WW. Adeno-associated virus type-2 expression of pigmented epithelium-derived factor or Kringles 1-3 of angiostatin reduce retinal neovascularization. Proc Natl Acad Sci USA. 2002;99(13):8909–8914. doi: 10.1073/pnas.122247299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Campochiaro PA, Lauer AK, Sohn EH, Mir TA, Naylor S, Anderton MC, et al. Lentiviral vector gene transfer of endostatin/angiostatin for macular degeneration (GEM) study. Hum Gene Ther. 2016;28(1):99–111. doi: 10.1089/hum.2016.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zhang P, Wang H, Cao H, Xu X, Sun T. Insulin-like growth factor binding protein-related protein 1 inhibit retinal neovascularization in the mouse model of oxygen-induced retinopathy. J Ocul Pharmacol Ther. 2017;33:459–465. doi: 10.1089/jop.2016.0171. [DOI] [PubMed] [Google Scholar]
- 124.Gene therapy for dry AMD allowed to proceed. American Academy of Ophthalmology. https://www.aao.org/headline/gene-therapy-dry-amd-allowed-to-proceed (2017). Cited 6 Jan 2018.
- 125.Treatment of advanced dry age related macular degeneration with AAVCAGsCD59—full text view—ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT03144999. Cited 6 Jan 2018.
- 126.Hartong DT, Berson EL, Dryja TP. Retinitis pigmentosa. Lancet. 2006;368(9549):1795–1809. doi: 10.1016/S0140-6736(06)69740-7. [DOI] [PubMed] [Google Scholar]
- 127.Facts about retinitis pigmentosa. National Eye Institute. https://nei.nih.gov/health/pigmentosa/pigmentosa_facts. Cited 18 May 2017.
- 128.Bunker CH, Berson EL, Bromley WC, Hayes RP, Roderick TH. Prevalence of retinitis pigmentosa in Maine. Am J Ophthalmol. 1984;97(3):357–365. doi: 10.1016/0002-9394(84)90636-6. [DOI] [PubMed] [Google Scholar]
- 129.Grøndahl J. Estimation of prognosis and prevalence of retinitis pigmentosa and Usher syndrome in Norway. Clin Genet. 1987;31(4):255–264. doi: 10.1111/j.1399-0004.1987.tb02804.x. [DOI] [PubMed] [Google Scholar]
- 130.Novak-Lauš K, Kukulj S, Zorić-Geber M, Bastaić O. Primary tapetoretinal dystrophies as the cause of blindness and impaired vision in the Republic of Croatia. Acta Clin Croat. 2002;41(1):23–25. [Google Scholar]
- 131.Baumgartner WA. Etiology, pathogenesis, and experimental treatment of retinitis pigmentosa. Med Hypotheses. 2000;54(5):814–824. doi: 10.1054/mehy.1999.0957. [DOI] [PubMed] [Google Scholar]
- 132.Dryja TP, McGee TL, Reichel E, Hahn LB, Cowley GS, Yandell DW, et al. A point mutation of the rhodopsin gene in one form of retinitis pigmentosa. Nature. 1990;343(6256):364–366. doi: 10.1038/343364a0. [DOI] [PubMed] [Google Scholar]
- 133.Kajiwara K, Berson EL, Dryja TP. Digenic retinitis pigmentosa due to mutations at the unlinked peripherin/RDS and ROM1 loci. Science. 1994;264(5165):1604–1609. doi: 10.1126/science.8202715. [DOI] [PubMed] [Google Scholar]
- 134.McLaughlin ME, Sandberg MA, Berson EL, Dryja TP. Recessive mutations in the gene encoding the β-subunit of rod phosphodiesterase in patients with retinitis pigmentosa. Nat Genet. 1993;4(2):130–134. doi: 10.1038/ng0693-130. [DOI] [PubMed] [Google Scholar]
- 135.Olsson JE, Gordon JW, Pawlyk BS, Roof D, Hayes A, Molday RS, et al. Transgenic mice with a rhodopsin mutation (Pro23His): a mouse model of autosomal dominant retinitis pigmentosa. Neuron. 1992;9(5):815–830. doi: 10.1016/0896-6273(92)90236-7. [DOI] [PubMed] [Google Scholar]
- 136.Gal A, Li Y, Thompson DA, Weir J, Orth U, Jacobson SG, et al. Mutations in MERTK, the human orthologue of the RCS rat retinal dystrophy gene, cause retinitis pigmentosa. Nat Genet. 2000;26(3):270–271. doi: 10.1038/81555. [DOI] [PubMed] [Google Scholar]
- 137.Meindl A, Dry K, Herrmann K, Manson E, Ciccodicola A, Edgar A, et al. A gene (RPGR) with homology to the RCC1 guanine nucleotide exchange factor is mutated in X-linked retinitis pigmentosa (RP3) Nat Genet. 1996;13(1):35–42. doi: 10.1038/ng0596-35. [DOI] [PubMed] [Google Scholar]
- 138.Wells J, Wroblewski J, Keen J, Inglehearn C, Jubb C, Eckstein A, et al. Mutations in the human retinal degeneration slow (RDS) gene can cause either retinitis pigmentosa or macular dystrophy. Nat Genet. 1993;3(3):213–218. doi: 10.1038/ng0393-213. [DOI] [PubMed] [Google Scholar]
- 139.Johnson JE, Barde YA, Schwab M, Thoenen H. Brain-derived neurotrophic factor supports the survival of cultured rat retinal ganglion cells. J Neurosci. 1986;6(10):3031–3038. doi: 10.1523/JNEUROSCI.06-10-03031.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Mey J, Thanos S. Intravitreal injections of neurotrophic factors support the survival of axotomized retinal ganglion cells in adult rats in vivo. Brain Res. 1993;602(2):304–317. doi: 10.1016/0006-8993(93)90695-J. [DOI] [PubMed] [Google Scholar]
- 141.Mansour-Robaey S, Clarke DB, Wang YC, Bray GM, Aguayo AJ. Effects of ocular injury and administration of brain-derived neurotrophic factor on survival and regrowth of axotomized retinal ganglion cells. Proc Natl Acad Sci USA. 1994;91(5):1632–1636. doi: 10.1073/pnas.91.5.1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Peinado-Ramón P, Salvador M, Villegas-Pérez MP, Vidal-Sanz M. Effects of axotomy and intraocular administration of NT-4, NT-3, and brain-derived neurotrophic factor on the survival of adult rat retinal ganglion cells. A quantitative in vivo study. Invest Ophthalmol Vis Sci. 1996;37(4):489–500. [PubMed] [Google Scholar]
- 143.Harper MM, Grozdanic SD, Blits B, Kuehn MH, Zamzow D, Buss JE, et al. Transplantation of BDNF-secreting mesenchymal stem cells provides neuroprotection in chronically hypertensive rat eyes. Invest Ophthalmol Vis Sci. 2011;52(7):4506–4515. doi: 10.1167/iovs.11-7346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Jung G, Sun J, Petrowitz B, Riecken K, Kruszewski K, Jankowiak W, et al. Genetically modified neural stem cells for a local and sustained delivery of neuroprotective factors to the dystrophic mouse retina. Stem Cells Transl Med. 2013;2(12):1001–1010. doi: 10.5966/sctm.2013-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Gregory-Evans K, Chang F, Hodges MD, Gregory-Evans CY. Ex vivo gene therapy using intravitreal injection of GDNF-secreting mouse embryonic stem cells in a rat model of retinal degeneration. Mol Vis. 2009;15:962–973. [PMC free article] [PubMed] [Google Scholar]
- 146.Levkovitch-Verbin H, Sadan O, Vander S, Rosner M, Barhum Y, Melamed E, et al. Intravitreal injections of neurotrophic factors secreting mesenchymal stem cells are neuroprotective in rat eyes following optic nerve transection. Invest Ophthalmol Vis Sci. 2010;51(12):6394–6400. doi: 10.1167/iovs.09-4310. [DOI] [PubMed] [Google Scholar]
- 147.Mackay DS, Henderson RH, Sergouniotis PI, Li Z, Moradi P, Holder GE, et al. Novel mutations in MERTK associated with childhood onset rod–cone dystrophy. Mol Vis. 2010;16:369–377. [PMC free article] [PubMed] [Google Scholar]
- 148.Shahzadi A, Riazuddin SA, Ali S, Li D, Khan SN, Husnain T, et al. Nonsense mutation in MERTK causes autosomal recessive retinitis pigmentosa in a consanguineous Pakistani family. Br J Ophthalmol. 2010;94(8):1094–1099. doi: 10.1136/bjo.2009.171892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Ostergaard E, Duno M, Batbayli M, Vilhelmsen K, Rosenberg T. A novel MERTK deletion is a common founder mutation in the Faroe Islands and is responsible for a high proportion of retinitis pigmentosa cases. Mol Vis. 2011;17:1485–1492. [PMC free article] [PubMed] [Google Scholar]
- 150.Dowling JE, Sidman RL. Inherited retinal dystrophy in the rat. J Cell Biol. 1962;14:73–109. doi: 10.1083/jcb.14.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Herron WL, Riegel BW, Myers OE, Rubin ML. Retinal dystrophy in the rat—a pigment epithelial disease. Invest Ophthalmol. 1969;8(6):595–604. [PubMed] [Google Scholar]
- 152.LaVail MM, Battelle BA. Influence of eye pigmentation and light deprivation on inherited retinal dystrophy in the rat. Exp Eye Res. 1975;21(2):167–192. doi: 10.1016/0014-4835(75)90080-9. [DOI] [PubMed] [Google Scholar]
- 153.LaVail MM, Sidman M, Rausin R, Sidman RL. Discrimination of light intensity by rats with inherited retinal degeneration: a behavioral and cytological study. Vis Res. 1974;14(8):693–702. doi: 10.1016/0042-6989(74)90066-2. [DOI] [PubMed] [Google Scholar]
- 154.Vollrath D, Feng W, Duncan JL, Yasumura D, D’Cruz PM, Chappelow A, et al. Correction of the retinal dystrophy phenotype of the RCS rat by viral gene transfer of Mertk. Proc Natl Acad Sci USA. 2001;98(22):12584–12589. doi: 10.1073/pnas.221364198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Smith AJ, Schlichtenbrede FC, Tschernutter M, Bainbridge JW, Thrasher AJ, Ali RR. AAV-mediated gene transfer slows photoreceptor loss in the RCS rat model of retinitis pigmentosa. Mol Ther. 2003;8(2):188–195. doi: 10.1016/S1525-0016(03)00144-8. [DOI] [PubMed] [Google Scholar]
- 156.Tschernutter M, Schlichtenbrede FC, Howe S, Balaggan KS, Munro PM, Bainbridge JWB, et al. Long-term preservation of retinal function in the RCS rat model of retinitis pigmentosa following lentivirus-mediated gene therapy. Gene Ther. 2005;12(8):694–701. doi: 10.1038/sj.gt.3302460. [DOI] [PubMed] [Google Scholar]
- 157.Buch PK, MacLaren RE, Durán Y, Balaggan KS, MacNeil A, Schlichtenbrede FC, et al. In contrast to AAV-mediated CNTF expression, AAV-mediated GDNF expression enhances gene replacement therapy in rodent models of retinal degeneration. Mol Ther. 2006;14(5):700–709. doi: 10.1016/j.ymthe.2006.05.019. [DOI] [PubMed] [Google Scholar]
- 158.Deng W-T, Dinculescu A, Li Q, Boye SL, Li J, Gorbatyuk MS, et al. Tyrosine-mutant AAV8 delivery of human MERTK provides long-term retinal preservation in RCS rats. Invest Ophthalmol Vis Sci. 2012;53(4):1895–1904. doi: 10.1167/iovs.11-8831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Asokan A, Schaffer DV, Jude SR. The AAV vector toolkit: poised at the clinical crossroads. Mol Ther. 2012;20(4):699–708. doi: 10.1038/mt.2011.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Vandenberghe LH, Bell P, Maguire AM, Cearley CN, Xiao R, Calcedo R, et al. Dosage thresholds for AAV2 and AAV8 photoreceptor gene therapy in monkey. Sci Transl Med. 2011;3(88):88ra54. doi: 10.1126/scitranslmed.3002103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Petit L, Ma S, Cheng S-Y, Gao G, Punzo C. Rod outer segment development influences AAV-mediated photoreceptor transduction after subretinal injection. Hum Gene Ther. 2017;28(6):464–481. doi: 10.1089/hum.2017.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Bhattacharya SS, Wright AF, Clayton JF, Price WH, Phillips CI, McKeown CME, et al. Close genetic linkage between X-linked retinitis pigmentosa and a restriction fragment length polymorphism identified by recombinant DNA probe L1.28. Nature. 1984;309(5965):253–255. doi: 10.1038/309253a0. [DOI] [PubMed] [Google Scholar]
- 163.Schwahn U, Lenzner S, Dong J, Feil S, Hinzmann B, van Duijnhoven G, et al. Positional cloning of the gene for X-linked retinitis pigmentosa 2. Nat Genet. 1998;19(4):327–332. doi: 10.1038/1214. [DOI] [PubMed] [Google Scholar]
- 164.Hosch J, Lorenz B, Stieger K. RPGR: role in the photoreceptor cilium, human retinal disease, and gene therapy. Ophthalmic Genet. 2011;32(1):1–11. doi: 10.3109/13816810.2010.535889. [DOI] [PubMed] [Google Scholar]
- 165.Zahid S, Khan N, Branham K, Othman M, Karoukis AJ, Sharma N, et al. Phenotypic conservation in patients with X-linked retinitis pigmentosa caused by RPGR mutations. JAMA Ophthalmol. 2013;131(8):1016–1025. doi: 10.1001/jamaophthalmol.2013.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Fischer MD, McClements ME, Martinez-Fernandez de la Camara C, Bellingrath J-S, Dauletbekov D, Ramsden SC, et al. Codon-optimized RPGR improves stability and efficacy of AAV8 gene therapy in two mouse models of X-linked retinitis pigmentosa. Mol Ther. 2017;25:1854–1865. doi: 10.1016/j.ymthe.2017.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Hong D-H, Pawlyk BS, Adamian M, Sandberg MA, Li T. A single, abbreviated RPGR-ORF15 variant reconstitutes RPGR function in vivo. Invest Ophthalmol Vis Sci. 2005;46(2):435–441. doi: 10.1167/iovs.04-1065. [DOI] [PubMed] [Google Scholar]
- 168.Deng W-T, Dyka FM, Dinculescu A, Li J, Zhu P, Chiodo VA, et al. Stability and safety of an AAV vector for treating RPGR-ORF15 X-linked retinitis pigmentosa. Hum Gene Ther. 2015;26(9):593–602. doi: 10.1089/hum.2015.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Wu Z, Hiriyanna S, Qian H, Mookherjee S, Campos MM, Gao C, et al. A long-term efficacy study of gene replacement therapy for RPGR-associated retinal degeneration. Hum Mol Genet. 2015;24(14):3956–3970. doi: 10.1093/hmg/ddv134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Beltran WA, Cideciyan AV, Lewin AS, Iwabe S, Khanna H, Sumaroka A, et al. Gene therapy rescues photoreceptor blindness in dogs and paves the way for treating human X-linked retinitis pigmentosa. Proc Natl Acad Sci USA. 2012;109(6):2132–2137. doi: 10.1073/pnas.1118847109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Cai X, Conley SM, Nash Z, Fliesler SJ, Cooper MJ, Naash MI. Gene delivery to mitotic and postmitotic photoreceptors via compacted DNA nanoparticles results in improved phenotype in a mouse model of retinitis pigmentosa. FASEB J. 2010;24(4):1178–1191. doi: 10.1096/fj.09-139147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Chadderton N, Millington-Ward S, Palfi A, O’Reilly M, Tuohy G, Humphries MM, et al. Improved retinal function in a mouse model of dominant retinitis pigmentosa following AAV-delivered gene therapy. Mol Ther. 2009;17(4):593–599. doi: 10.1038/mt.2008.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Georgiadis A, Tschernutter M, Bainbridge JWB, Robbie SJ, McIntosh J, Nathwani AC, et al. AAV-mediated knockdown of peripherin-2 in vivo using miRNA-based hairpins. Gene Ther. 2010;17(4):486–493. doi: 10.1038/gt.2009.162. [DOI] [PubMed] [Google Scholar]
- 174.Gorbatyuk M, Justilien V, Liu J, Hauswirth WW, Lewin AS. Preservation of photoreceptor morphology and function in P23H rats using an allele independent ribozyme. Exp Eye Res. 2007;84(1):44–52. doi: 10.1016/j.exer.2006.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Gorbatyuk MS, Pang JJ, Thomas J, Hauswirth WW, Lewin AS. Knockdown of wild-type mouse rhodopsin using an AAV vectored ribozyme as part of an RNA replacement approach. Mol Vis. 2005;11:648–656. [PubMed] [Google Scholar]
- 176.Millington-Ward S, Chadderton N, O’Reilly M, Palfi A, Goldmann T, Kilty C, et al. Suppression and replacement gene therapy for autosomal dominant disease in a murine model of dominant retinitis pigmentosa. Mol Ther. 2011;19(4):642–649. doi: 10.1038/mt.2010.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.O’Reilly M, Palfi A, Chadderton N, Millington-Ward S, Ader M, Cronin T, et al. RNA interference-mediated suppression and replacement of human rhodopsin in vivo. Am J Hum Genet. 2007;81(1):127–135. doi: 10.1086/519025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Sullivan JM, Pietras KM, Shin BJ, Misasi JN. Hammerhead ribozymes designed to cleave all human rod opsin mRNAs which cause autosomal dominant retinitis pigmentosa. Mol Vis. 2002;8:102–113. [PubMed] [Google Scholar]
- 179.Palfi A, Millington-Ward S, Chadderton N, O’Reilly M, Goldmann T, Humphries MM, et al. Adeno-associated virus-mediated rhodopsin replacement provides therapeutic benefit in mice with a targeted disruption of the rhodopsin gene. Hum Gene Ther. 2010;21(3):311–323. doi: 10.1089/hum.2009.119. [DOI] [PubMed] [Google Scholar]
- 180.Mao H, James T, Schwein A, Shabashvili AE, Hauswirth WW, Gorbatyuk MS, et al. AAV delivery of wild-type rhodopsin preserves retinal function in a mouse model of autosomal dominant retinitis pigmentosa. Hum Gene Ther. 2011;22(5):567–575. doi: 10.1089/hum.2010.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Gorbatyuk MS, Knox T, LaVail MM, Gorbatyuk OS, Noorwez SM, Hauswirth WW, et al. Restoration of visual function in P23H rhodopsin transgenic rats by gene delivery of BiP/Grp78. Proc Natl Acad Sci USA. 2010;107(13):5961–5966. doi: 10.1073/pnas.0911991107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Current status of the use of gene therapy in ophthalmology. Retinal Physician. https://www.retinalphysician.com/issues/2013/april-2013/current-status-of-the-use-of-gene-therapy-in-ophth. Cited 6 Jan 2018.
- 183.A clinical trial of retinal gene therapy for X-linked retinitis pigmentosa—full text view—ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT03116113. Cited 6 Jan 2018.
- 184.Safety and efficacy study in patients with retinitis pigmentosa due to mutations in PDE6B gene—full text view—ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT03328130. Cited 6 Jan 2018.
- 185.Gene therapy for X-linked retinitis pigmentosa (XLRP) retinitis pigmentosa GTPase regulator (RPGR)—full text view—ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT03252847. Cited 6 Jan 2018.
- 186.RetroSense therapeutics product development. http://retrosense.com/development.html. Cited 6 Jan 2018.
- 187.RST-001 Phase I/II trial for advanced retinitis pigmentosa—full text view—ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT02556736. Cited 6 Jan 2018.
- 188.Package Insert—LUXTURNA (voretigene neparvovec-rzyl). FDA; 2017. https://www.fda.gov/downloads/BiologicsBloodVaccines/CellularGeneTherapyProducts/ApprovedProducts/UCM589541.pdf. Accessed 6 Jan 2018.
- 189.Chalberg TW, Vankov A, Molnar FE, Butterwick AF, Huie P, Calos MP, et al. Gene transfer to rabbit retina with electron avalanche transfection. Invest Ophthalmol Vis Sci. 2006;47(9):4083–4090. doi: 10.1167/iovs.06-0092. [DOI] [PubMed] [Google Scholar]
- 190.Horbinski C, Stachowiak MK, Higgins D, Finnegan SG. Polyethyleneimine-mediated transfection of cultured postmitotic neurons from rat sympathetic ganglia and adult human retina. BMC Neurosci. 2001;2:2. doi: 10.1186/1471-2202-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All articles and resources referenced herein were accessed between 1 May 2017 and 5 April 2018 and located through PubMed/MEDLINE database and Google searches using the relevant keywords.