Abstract
Context
Maternal metabolic status reflects underlying physiological changes in the maternal-placental-fetal unit that may help identify contributors to adverse pregnancy outcomes associated with infertility and treatments used.
Objective
To determine if maternal metabolomic profiles differ between spontaneous pregnancies and pregnancies conceived with fertility treatments that may explain the differences in pregnancy outcomes.
Design
Metabolon metabolomic analysis and ELISAs for 17-β-estradiol and progesterone were performed during the late first trimester of pregnancy.
Setting
Academic institution.
Subjects
Women in the Spontaneous/Medically Assisted/Assisted Reproductive Technology cohort (N = 409), 208 of whom conceived spontaneously and 201 with infertility [non in vitro fertilization treatments (NIFT), n=90; in vitro fertilization (IVF), n=111].
Intervention
Mode of conception.
Main Outcome Measures
Levels of of 806 metabolites within eight superpathways, 17-β-estradiol, and progesterone in maternal plasma in the late first trimester.
Results
Metabolomic differences in the lipid superpathway (i.e., steroid metabolites, lipids with docosahexaenoyl acyl chains, acyl cholines), and xanthine and benzoate metabolites (P < 0.05) were significant among the spontaneous and two infertility groups, with greatest differences between the spontaneous and IVF groups. 17-β-estradiol and progesterone levels were significantly elevated in the infertility groups, with greatest differences between the spontaneous and IVF groups.
Conclusion
Metabolomic profiles differ between spontaneous and infertility pregnancies, likely driven by IVF. Higher levels of steroids and their metabolites are likely due to increased hormone production from placenta reprogrammed from fertility treatments, which may contribute to adverse outcomes associated with infertility and the treatments used.
Levels of lipid metabolites and xenobiotics differed significantly in maternal plasma from pregnancies conceived spontaneously vs through infertility treatment, largely driven by IVF.
Approximately 15% of couples experience infertility. Fertility treatments include in vitro fertilization (IVF), accounting for 1.5% of live births in the United States and non-IVF treatments (NIFTs), accounting for 4.6% (1, 2). NIFT includes ovarian stimulation and/or intrauterine insemination, in which fertilization occurs in the reproductive tract, whereas with IVF, fertilization and embryo development occur in the laboratory. Treatments used lead to supraphysiologic levels of estrogen and progesterone, plus additional hormone supplementation is administered through early gestation to maintain the pregnancy (3, 4). Pregnancies conceived through IVF are at increased risk of low-birth-weight, small-for-gestational-age infants; birth defects; preeclampsia; retained placenta; placenta previa; and preterm labor and delivery (5–9). NIFT also leads to increased risk of placental abruption, low birth weight, and fetal loss. The diagnosis of infertility alone leads to increased risk of pregnancy complications, and even time to conception increases the risk of birth defects (6, 10–12). It is unclear if adverse outcomes are due to the actual infertility or the treatments used. Many of the adverse outcomes are associated with placental defects, including small-for-gestational-age infants, preeclampsia, placental abruption, placenta accreta, and placenta previa, which likely develop early in gestation during placentation (13).
Pregnancy leads to substantial changes in maternal metabolic profiles, compared with the nonpregnant state (14, 15). These changes are a culmination of the maternal-fetal-placental unit. Lipoprotein and lipid levels are increased, with greatest elevations in levels of lipoprotein triglycerides (TAGs), which are important for placental and fetal development. During the first trimester, normal placental development is critical because it lays the foundation for normal placental function, including steroidogenesis, that can affect maternal and fetal well-being throughout gestation. Despite this critical metabolic state in early gestation, where preconception environmental influences such as the mode of conception and infertility affect pregnancy, little is known about how these variables affect metabolism early in gestation, which can have long-term implications throughout gestation. Because pregnancies conceived in couples with infertility are at increased risk of adverse outcomes, we set out to determine if the metabolomic profiles are influenced as a result of infertility as well as the treatments used.
Materials and Methods
Subject selection
Subjects were identified from the Cedars Sinai Medical Center (CSMC) Prenatal Biorepository (16) in accordance with the institutional review board’s guidelines at the CSMC under Institutional Review Board Protocol Pro00006806. Between May 2008 and July 2017, 409 women were recruited to the Spontaneous/Medically Assisted/Assisted Reproductive Technology (SMAART) cohort, of whom 208 had conceived spontaneously and 201 had infertility and used fertility treatments [non in vitro fertilization treatments (NIFT), n = 90; in vitro fertilization (IVF), n = 111]. Of the 111 women who underwent IVF, 91 had fresh embryo transfers and 20 had frozen embryo transfers (FETs). Most subjects were no longer receiving any hormone supplementation. Of the 409 women, 11 who conceived spontaneously, 9 who used NIFT, and 34 who used IVF were still receiving hormone supplementation at the time of enrollment. These women were excluded in analyses where noted. All women were enrolled at the time of chorionic villus sampling, which takes place between 10 and 13 weeks of gestation. All pregnancies were genetically normal singletons that were delivered.
A subset of women from the SMAART cohort were recruited into this metabolome study: 67 who conceived spontaneously and 51 with infertility (n = 25 who used NIFT; n = 26 who used IVF). Of those who conceived with IVF, 18 had fresh embryo transfers and eight had FETs. From the SMAART cohort, a subset of 325 patients, including those recruited in the metabolome study, were recruited for the plasma hormone ELISA analysis. Of these, 161 conceived spontaneously and 164 with infertility treatment (NIFT, n = 72; IVF, n = 92).
Plasma collection
Blood was collected in Vacutainer tube (BD Biosciences, San Jose, CA) containing K2EDTA and centrifuged at 1600g for 10 minutes to separate buffy coat from plasma. Plasma was transferred to cryovial tubes, flash frozen, and stored at −80°C in the CSMC Prenatal Biorepository.
Quantification of metabolites
Biochemical profiles were determined by Metabolon, Inc. (Durham, NC), from 200 µL of plasma, as described previously (17). Samples were prepared using the automated Microlab STAR system (Hamilton). Each sample was divided into five fractions: two for analysis by two separate reverse phase/ultraperformance liquid chromatography–tandem mass spectrometry (UPLC-MS/MS) methods with positive ion–mode electrospray ionization (ESI), one for analysis by reverse phase /UPLC-MS/MS with negative ion–mode ESI, one for analysis by hydrophilic-interaction chromatography/UPLC-MS/MS with negative ion–mode ESI, and one was reserved for backup. Proteins and organic solvent were removed from the samples to recover chemically diverse metabolites before preparation for analysis. Quality controls included pooled matrix samples, extracted water samples as process blanks, and a cocktail of quality-control standards for monitoring instrument performance.
A total of 806 known compounds were quantified. Each metabolite was classified into one of the following super pathways: lipid, carbohydrate, amino acid, xenobiotics, nucleotide, energy, peptide, cofactors, or vitamins. A fill value of ≥50% in all groups was considered valid, unless otherwise noted. After log transformation with the minimum observed values for each compound and imputation of missing values, analysis of covariance contrasts were used to identify metabolites that differed significantly between the groups. Graphical representations of enriched pathways were generated in Cytoscape (18), as implemented by Metabolon.
17-β-Estradiol and Progesterone ELISA
Plasma was thawed on ice and aliquoted in triplicates onto 96-well plates of 17-β-estradiol or progesterone ELISA kits (catalog no. ab108667, ab108670; Abcam, Cambridge, MA). Assays were performed according to the manufacturer’s instructions. Both kits had low inter- and intra-assay variability, with coefficient of variation (CV) ≤10% (interassay) and CV ≤9% (intra-assay) for 17-β-estradiol ELISA kit and CV ≤9.3% (interassay) and CV≤4% (intra-assay) for progesterone ELISA kit. Briefly, samples were aliquoted onto 17-β-estradiol or progesterone ELISA plates coated with the capture antibody. Horseradish peroxidase conjugate was added and detected with tetramethylbenzidine substrate solution. Absorbance of the sample at 450 nm was measured using CLARIOstar plate reader (BMG Labtech, Cary, NC). A standard curve was generated from mean background–subtracted absorbance of each standard of known concentration using Four Parameter Logistic fit. The sample concentrations were interpolated from the standard curve.
Statistical analysis
For demographic analyses, Student t test and ANOVA were used for continuous variables among the groups and χ2 test was used for categorical variables. Data are reported as mean ± SD. For metabolome data analyses, unsupervised principle component analysis was applied to assess the distribution of the dataset. From principle component analysis, we deduced that body mass index (BMI) represented a potential confounding factor contributing to statistically significant differences between the groups. As a result, a least square means statistical comparison adjusting for BMI was conducted to control for this affect. Analysis of covariance was performed in ArrayStudio on log-transformed data to compare the spontaneous conception (hereafter, spontaneous), NIFT, and IVF groups, using BMI as a covariate. P < 0.05 was considered statistically significant and 0.05 < P < 0.1 was considered marginally significant. Pathway enrichment analysis was conducted within Cytoscape implemented by Metabolon to highlight important changes at the biological pathway level, using the formula: , with k being the number of significant metabolites in the pathway; m, the number of detected metabolites in the pathway; n, the number of significant metabolites in both the study and pathway library; and, N, the total number of metabolites in both the study and pathway library. The pathway enrichment displays the amount of significantly different compounds relative to all the detected compounds in the pathway, compared with the total number of significantly different compounds relative to all the detected compounds in the study. P < 0.05 was considered significant for pathway enrichment analysis. ANOVA was used for ELISAs, with P < 0.05 considered statistically significant.
Results
Demographics
In the SMAART cohort, maternal age of women in the infertility group (i.e., IVF and NIFT groups) was statistically older than those in the spontaneous group (P = 0.019; Table 1). When the infertility group was separated into those who conceived with IVF and those who conceived using NIFT, the maternal age of the IVF group was statistically older than that of the spontaneous group (40 ± 3.0 years vs 39 ± 2.7 years; P = 0.023); however, the difference was small and likely not clinically significant (Table 2). Paternal age was similar between the spontaneous group and infertility group, but it was significantly older in the IVF group than in the NIFT group (42 ± 9.4 years vs 39 ± 6.3 years; P = 0.003). Most women were white, although the racial composition differed between the spontaneous group and infertility group (P = 0.034) and also among all three groups (P = 0.012). Overall, the paternal and fetal race and ethnicity, as well as the fetal sex were not significantly different in both comparisons. Maternal conditions at the time of chorionic villus sampling did not differ between the infertility group and spontaneous group, except the former had a higher rate of thyroid disease (20% vs 13%, respectively; P = 0.042). This difference was no longer significant when the infertility group was separated into NIFT and IVF groups. Maternal BMI was not different in both comparisons.
Table 1.
SMAART Cohort Baseline Demographics and Pregnancy Outcomes (Spontaneous Group vs Infertility Group)
| Spontaneous Group (n = 208) | Infertility Group (n = 201) | P Value | |
|---|---|---|---|
| Baseline demographics | |||
| Maternal age, years | 39 ± 2.7 | 40 ± 3.0 | 0.019a |
| Paternal age, years (n = 207,193) | 40 ± 5.0 | 41 ± 8.3 | 0.472 |
| Maternal race | 0.034a | ||
| White | 181 (87) | 165 (82) | |
| Asian | 21 (10) | 24 (12) | |
| Black | 5 (2.4) | 2 (1) | |
| Biracial (white/Asian) | 1 (0.48) | 2 (1) | |
| Biracial (other) | 0 | 8 (4) | |
| Paternal race | 0.492 | ||
| Sex of fetus | 0.348 | ||
| Male | 109 (52) | 96 (48) | |
| Female | 99 (48) | 105 (52) | |
| Fetal race | 0.135 | ||
| White | 170 (82) | 154 (77) | |
| Asian | 6 (2.9) | 6 (3) | |
| Black | 5 (2.4) | 1 (0.5) | |
| Biracial (white/Asian) | 23 (11) | 28 (14) | |
| Biracial (other) | 4 (1.9) | 10 (5) | |
| Multiracial | 0 | 2 (1) | |
| Maternal BMI, kg/m2 (n = 205,193) | 23 ± 4.1 | 24 ± 4.9 | 0.133 |
| Hypertension | 5 (2.4) | 5 (2.5) | 0.956 |
| Diabetes | 3 (1.4) | 8 (4) | 0.113 |
| Thyroid disease | 26 (13) | 40 (20) | 0.042a |
| Other | 9 (4.3) | 5 (2.5) | 0.306 |
| Gestational age at CVS, days | 82 ± 6.6 | 82 ± 6.5 | 0.832 |
| Crown rump length, mm (n = 203,195) | 54 ± 12 | 53 ± 11 | 0.619 |
| Pregnancy outcomes | |||
| Gestational age at delivery, days (n = 197,184) | 273 ± 12 | 272 ± 14 | 0.499 |
| Birth weight, g (n = 183,171) | 3350 ± 548 | 3303 ± 582 | 0.436 |
| Mode of delivery (n = 200,182) | <0.001a | ||
| Vaginal, spontaneous | 126 (63) | 76 (42) | |
| Cesarean section | 74 (37) | 106 (58) | |
| Pregnancy complications (n = 202,190) | 30 (15) | 44 (23) | 0.036a |
| Hypertension | 13 (6.3) | 14 (7) | 0.771 |
| Diabetes | 18 (8.7) | 26 (13) | 0.162 |
| Coagulation disorders | 0 | 2 (1) | 0.149 |
| Placenta previa | 3 (1.4) | 5 (2.5) | 0.445 |
| Placental abruption | 1 (0.48) | 1 (0.5) | 0.981 |
| Other placental complication | 0 | 4 (2) | 0.041a |
Data given as no. (%) or mean ± SD.
Abbreviation: CVS, chorionic villus sampling.
P < 0.05. Number of observations, if different, is noted.
Table 2.
SMAART Cohort Baseline Demographics and Pregnancy Outcomes (Groups: Spontaneous vs NIFT vs IVF)
| Spontaneous Group (n = 208) | NIFT Group (n = 90) | IVF Group (n = 111) | P Value | P Value | |||
|---|---|---|---|---|---|---|---|
| Spontaneous vs NIFT |
Spontaneous vs IVF
|
NIFT vs IVF
|
|||||
| Baseline demographics | |||||||
| Maternal age, years | 39 ± 2.7 | 39 ± 2.9 | 40 ± 3.0 | 0.029a | 0.879 | 0.023a | 0.604 |
| Paternal age, years (n = 207;83;110) | 40 ± 5.0 | 39 ± 6.3 | 42 ± 9.4 | 0.004a | 0.371 | 0.055 | 0.003a |
| Maternal race | 0.012a | ||||||
| White | 181 (87) | 73 (81) | 92 (83) | ||||
| Asian | 21 (10) | 11 (12) | 13 (12) | ||||
| Black | 5 (2.4) | 0 | 2 (1.8) | ||||
| Biracial (white/Asian) | 1 (0.48) | 0 | 2 (1.8) | ||||
| Biracial (other) | 0 | 6 (6.7) | 2 (1.8) | ||||
| Paternal race | 0.357 | ||||||
| Sex of fetus | 0.234 | ||||||
| Male | 109 (52) | 48 (53) | 48 (43) | ||||
| Female | 99 (48) | 42 (47) | 63 (57) | ||||
| Fetal race | 0.053 | ||||||
| White | 170 (82) | 67 (74) | 87 (78) | ||||
| Asian | 6 (2.9) | 1 (1.1) | 5 (4.5) | ||||
| Black | 5 (2.4) | 0 | 1 (0.9) | ||||
| Biracial (white/Asian) | 23 (11) | 13 (14) | 15 (14) | ||||
| Biracial (other) | 4 (1.9) | 8 (8.9) | 2 (1.8) | ||||
| Multiracial | 0 | 1 (1.1) | 1 (0.9) | ||||
| Maternal BMI, kg/m2 (n = 205;90;103) | 23 ± 4.1 | 24 ± 5.8 | 23 ± 3.9 | 0.055 | |||
| Hypertension | 5 (2.4) | 4 (4.4) | 1 (0.9) | 0.270 | |||
| Diabetes | 3 (1.4) | 3 (3.3) | 5 (4.5) | 0.250 | |||
| Thyroid disease | 26 (13) | 15 (17) | 25 (23) | 0.067 | |||
| Other | 9 (4.3) | 2 (2.2) | 3 (2.7) | 0.582 | |||
| Gestational age at CVS, days | 82 ± 6.6 | 81 ± 6.3 | 82 ± 6.6 | 0.683 | |||
| Crown rump length, mm (n = 203;86;109) | 54 ± 12 | 53 ± 11 | 53 ± 12 | 0.825 | |||
| Pregnancy outcomes | |||||||
| Gestational age at delivery, days (n = 197;82;102) | 273 ± 12 | 273 ± 11 | 271 ± 16 | 0.507 | |||
| Birth weight, g (n = 183;80;91) | 3350 ± 548 | 3328 ± 583 | 3281 ± 587 | 0.634 | |||
| Mode of delivery (n = 200;82;100) | <0.001a | ||||||
| Vaginal, spontaneous | 126 (63) | 42 (51) | 34 (34) | ||||
| Cesarean section | 74 (37) | 40 (49) | 66 (66) | ||||
| Pregnancy complications (n = 202;86;104) | 30 (15) | 18 (21) | 26 (25) | 0.085 | |||
| Hypertension | 13 (6.3) | 7 (7.8) | 7 (6.3) | 0.878 | |||
| Diabetes | 18 (8.7) | 12 (13) | 14 (13) | 0.372 | |||
| Coagulation disorders | 0 | 1,1.1) | 1 (0.9) | 0.346 | |||
| Placenta previa | 3 (1.4) | 0 | 5 (4.5) | 0.054 | |||
| Placental abruption | 1 (0.48) | 0 | 1 (0.9) | 0.660 | |||
| Other placental complication | 0 | 1 (1.1) | 3 (2.7) | 0.065 | |||
Data given as no. (%) or mean ± SD.
Abbreviation: CVS, chorionic villus sampling.
P < 0.05. Number of observations, if different, is noted.
Comparing pregnancy outcomes, the rate of delivery by cesarean section was significantly higher in the infertility group (P < 0.001), with the higher rate in the IVF group. There was a significantly higher rate of pregnancy complications in the infertility group compared with the spontaneous group, and this was driven by the significantly higher rate of placental abnormalities, which included velamentous cord insertion, vasa previa, hemorrhage, and retained placenta (Table 1).
In the subset of patients in the metabolomic analyses (n = 118), differences in maternal age, maternal race, and pregnancy complications were no longer present between the spontaneous group and infertility group. Maternal BMI and rate of diabetes became significantly higher in the infertility group. When IVF and NIFT were separated, results for paternal age, maternal race, and the mode of delivery were similar to those of the SMAART cohort. The mean BMI of the spontaneous group was significantly lower than that of the IVF and NIFT groups. There was no difference in maternal conditions and pregnancy complications among the three groups.
In the subset of patients in the hormone ELISA assay (n = 325), demographics were similar to those of the SMAART cohort. Pregnancy complications, however, were no longer different. When the infertility cohort was separated into IVF and NIFT groups, the only differences seen relative to the SMAART cohort was fetal race.
Metabolomic differences among spontaneous, NIFT, and IVF pregnancies
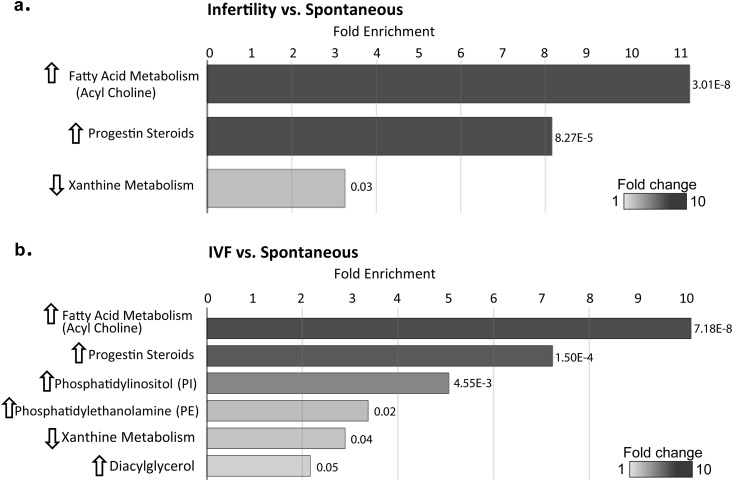
Of 806 measured compounds from eight super pathways (namely, lipid, carbohydrate, amino acid, xenobiotics, nucleotide, energy, peptide, cofactors, and vitamins), 730 had a fill value >50% in all groups. The most differences between groups were found in the lipid superpathway, within which the greatest differences were seen in acyl choline, steroid metabolite, phospholipid (PL), and diacylglycerol (DAG) subpathways (Fig. 1). Pathway enrichment analysis identified the acyl cholines and progestin steroids to be most enriched when comparing the infertility group with the spontaneous group, with increased levels in the infertility group (Fig. 1a). When the infertility group was divided into NIFT and IVF groups, few differences were seen overall in the NIFT group vs spontaneous group or the IVF group vs NIFT group. However, there were differences between the IVF vs spontaneous groups (Fig. 1b) in the acyl cholines and progestin steroids, similar to the infertility group vs spontaneous group, suggesting that the IVF group is driving the differences in outcomes between spontaneously conceived pregnancies and those conceived with infertility treatment. In addition, within the lipid superpathway, differences in the phosphatidylinositols, phosphatidylethanolamines, and DAGs were also seen between the IVF group and spontaneous group. Pathway enrichment analysis identified xanthine metabolism, as a result of caffeine ingestion, as the only subpathway that was decreased among the infertility group compared with the spontaneous group, as well as the IVF group vs spontaneous group.
Figure 1.
Pathway enrichment analysis at the subpathway level. (a) Subpathways that were significantly different between the spontaneous group and infertility group. (b) Subpathways that were significantly different between the spontaneous group and IVF group. The length of the bars shows the fold enrichment for each subpathway, with P values noted. Graphical representations of enriched pathways were generated in Cytoscape to highlight important changes at the biological pathway level.
Acyl cholines
Acyl cholines are choline conjugates of fatty acids and our understanding of their functions is still limited (19). Levels of all seven measured acyl cholines were significantly higher in the infertility group compared with the spontaneous group (Table 3), with an even greater difference in the IVF group compared with spontaneous group. There were no differences between the NIFT and spontaneous groups. Similar differences were seen in the IVF group vs NIFT group, suggesting that NIFT was similar to the spontaneous group, with the IVF group driving differences between the spontaneous and infertility groups.
Table 3.
Significantly Different, Nonsteroid Metabolites Within the Lipid Superpathway, Between Groups
| Superpathway | Subpathway | Biochemical Name | Groups | |||
|---|---|---|---|---|---|---|
| Infertility vs Spontaneous | IVF vs Spontaneous a | NIFT vs Spontaneous | IVF vs NIFT | |||
| Lipid | Fatty acid metabolism (acyl choline) | Palmitoylcholine | 1.50a | 1.74 | 1.02 | 1.70a |
| Lipid | Fatty acid metabolism (acyl choline) | Oleoylcholine | 1.49a | 1.74 | 0.97 | 1.79a |
| Lipid | Fatty acid metabolism (acyl choline) | Dihomo-linolenoyl-choline | 1.82a | 2.13 | 1.19 | 1.80a |
| Lipid | Fatty acid metabolism (acyl choline) | Linoleoylcholine | 1.38a | 1.58 | 0.97 | 1.63a |
| Lipid | Fatty acid metabolism (acyl choline) | Stearoylcholine | 1.50a | 1.78 | 0.94 | 1.90a |
| Lipid | Fatty acid metabolism (acyl choline) | Docosahexaenoylcholineb | 1.65a | 1.93 | 1.08 | 1.79a |
| Lipid | Fatty acid metabolism (acyl choline) | Arachidonoylcholine | 1.48a | 1.74 | 0.97 | 1.80a |
| Lipid | Phosphatidylinositol | 1,2-Dipalmitoyl-GPI (16:0/16:0) | 1.65a | 1.94 | 1.07 | 1.81c |
| Lipid | Phosphatidylinositol | 1-Palmitoyl-2-oleoyl-GPI (16:0/18:1) | 1.16c | 1.24 | 1.01 | 1.23c |
| Lipid | Phosphatidylinositol | 1-Palmitoyl-2-arachidonoyl-GPI (16:0/20:4) | 1.23a | 1.33 | 1.03 | 1.29c |
| Lipid | Phosphatidylinositol | 1-Stearoyl-2-oleoyl-GPI (18:0/18:1) | 1.10 | 1.16 | 0.99 | 1.18c |
| Lipid | Phosphatidylethanolamine | 1,2-Dipalmitoyl-GPE (16:0/16:0) | 1.29c | 1.38 | 1.12 | 1.23 |
| Lipid | Phosphatidylethanolamine | 1-Palmitoyl-2-docosahexaenoyl-GPE (16:0/22:6)b | 1.25a | 1.30 | 1.14 | 1.13 |
| Lipid | Phosphatidylethanolamine | 1-Stearoyl-2-docosahexaenoyl-GPE (18:0/22:6)b | 1.32a | 1.38 | 1.18c | 1.17 |
| Lipid | Phosphatidylethanolamine | 1-Oleoyl-2-docosahexaenoyl-GPE (18:1/22:6)b | 1.24a | 1.29 | 1.14 | 1.14 |
| Lipid | Diacylglycerol | Stearoyl-arachidonoyl-glycerol (18:0/20:4) [1]d | 1.14 | 1.25 | 0.93 | 1.34a |
| Lipid | Diacylglycerol | Stearoyl-arachidonoyl-glycerol (18:0/20:4) [2]d | 1.10 | 1.20 | 0.90 | 1.34a |
| Lipid | Diacylglycerol | Oleoyl-arachidonoyl-glycerol (18:1/20:4) [1]d | 1.19c | 1.27 | 1.01 | 1.26c |
| Lipid | Diacylglycerol | Oleoyl-arachidonoyl-glycerol (18:1/20:4) [2]d | 1.19c | 1.29 | 1.00 | 1.28c |
| Lipid | Diacylglycerol | Linoleoyl-arachidonoyl-glycerol (18:2/20:4) [2]d | 1.23a | 1.30 | 1.09 | 1.20 |
| Lipid | Diacylglycerol | Linoleoyl-docosahexaenoyl-glycerol (18:2/22:6)b [2]d | 1.46c | 1.59 | 1.20 | 1.33 |
Fold changes of members in the subpathways of acyl cholines, phosphatidylinositol, phosphatidylethanolamine, and DAG between the following groups: infertility vs spontaneous, the IVF vs spontaneous, NIFT vs spontaneous, and IVF vs NIFT. Analysis of covariance was performed using BMI as a covariate.
P < 0.05.
Contains the DHA acyl chain.
0.05 < P < 0.1.
Isomer.
PLs and glycerolipids, including docosahexaenoyl-containing lipids
PLs form the surface layer of lipoprotein particles, which are the major source of PLs in the plasma. Levels of several PL subpathway members were significantly increased in the infertility group compared with the spontaneous group, including phosphatidylinositols and phosphatidylethanolamines (Table 3). More members were different in the IVF group than in the infertility group, compared with the spontaneous group. Again, there were no differences between the NIFT and spontaneous groups, and there were small differences in the IVF group vs NIFT group, suggesting that the IVF group drove differences between the spontaneous group and infertility group. Of interest, three of four phosphatidylethanolamines with significantly elevated levels in the IVF group contained a docosahexaenoyl (DHA) acyl chain (Table 3).
DAGs are important for synthesis of TAGs. TAGs serve as the main storage of fatty acids and are within the core of lipoprotein particles [i.e., chylomicrons, low-density lipoproteins (LDLs), high-density lipoproteins (HDLs), very low-density lipoproteins (VLDLs)], together with cholesteryl esters in the circulation. Although there were few differences between the infertility vs spontaneous group, levels of six of eight measured DAGs were significantly higher in the IVF group compared with those of the spontaneous group (Table 3).
In addition, levels of 1-palmitoyl-2-docosahexaenoyl-GPC (16:0/22:6), a member in the phosphatidylcholine subpathway, and 1-docosahexaenoylglycerol (22:6), a member in the monoacylglycerol subpathway, which contain a DHA acyl chain, were also significantly elevated in the infertility group, compared with the spontaneous group, again driven by IVF, because levels of these were all significantly elevated in the IVF group vs spontaneous group but not other groups. Although levels of only one polyunsaturated fatty acid, nisinate (24:6n3), in the DHA synthesis pathway (one of 15 measured polyunsaturated fatty acids) were significantly elevated, it was among the most significantly elevated metabolites measured among the IVF group vs spontaneous group [FC (fold change) 2.07]. Levels of the majority of DHA acyl chain–containing lipids (seven of 10) were elevated in the IVF group, regardless of the subpathway, with five being present in significant pathways (Table 3).
Steroid metabolites
Steroidogenesis is critical to support a pregnancy. During fertility treatment, steroid hormone levels increase, either endogenously from treatment or through exogenous supplementation. At approximately 7 to 9 weeks’ gestation, the syncytiotrophoblast (STB) cells of the placenta become the steroid-producing cells to support the pregnancy (20, 21) and all exogenous hormone supplementation is typically discontinued (21, 22).
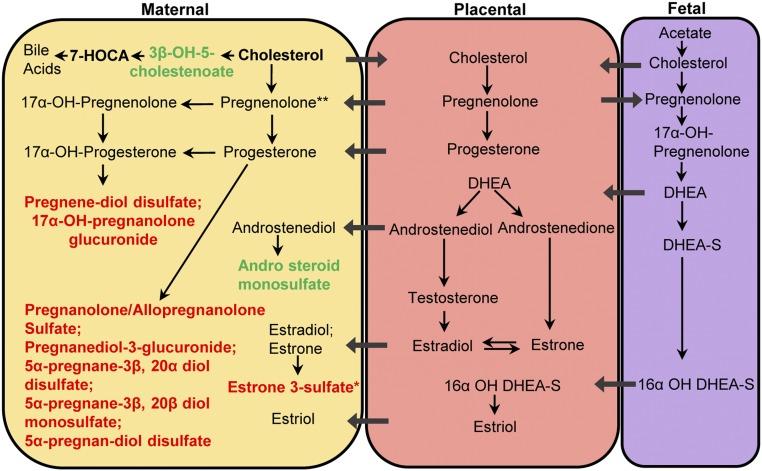
Progestin steroids were one of the most enriched in pathway enrichment analysis (Fig. 1a, 1b). Levels of five of seven measured progestin metabolites were increased in the infertility group compared with the spontaneous group. They were all increased in the IVF group compared with the spontaneous group, with few differences among the other groups (Table 4). Compared with the spontaneous group, levels of two pregnenolone steroids, pregnenediol disulfate (FC1.36) and 17α-OH-pregnanolone glucuronide (FC1.63; spontaneous fill value 33%), were increased in the infertility group, as well as specifically in the IVF group compared with the spontaneous group. The level of estrone 3-sulfate, the only measured estrogenic steroid metabolite, was 1.76-fold higher in the IVF group compared with the spontaneous group but did not reach significance in the infertility group compared with the spontaneous group. Levels of cortisol, a glucocorticoid hormone, were higher in the infertility group and IVF group compared with the spontaneous group. Levels of the sterol metabolites 3β-OH-5-cholestenoate and campesterol were significantly lower in the infertility group compared with the spontaneous group. Levels of the androgenic steroid metabolite androsteroid monosulfate (C19H28O6S) were decreased in the infertility group compared with the spontaneous group. The metabolites in the steroidogenic pathway are summarized in Fig. 2. Differences between the infertility group vs spontaneous group and IVF group vs spontaneous group are highlighted.
Table 4.
Significantly Different Steroid Metabolites Within the Lipid Superpathway, Between Groups
| Superpathway | Subpathway | Biochemical Name | Groups | |||
|---|---|---|---|---|---|---|
| Infertility vs Spontaneous | IVF vs Spontaneous | NIFT vs Spontaneous | IVF vs NIFT | |||
| Lipid | Progestin steroid | 5α-Pregnan-3β,20β-diol monosulfate [1]a | 1.45b | 1.54b | 1.27c | 1.21 |
| Lipid | Progestin steroid | 5α-Pregnan-3β,20α-diol disulfate | 1.53b | 1.70b | 1.19 | 1.44b |
| Lipid | Progestin steroid | 5α-Pregnan-diol disulfate | 1.99b | 2.31b | 1.33 | 1.74b |
| Lipid | Progestin steroid | Pregnanediol-3-glucuronide | 1.28b | 1.26b | 1.31b | 0.96 |
| Lipid | Progestin steroid | Pregnanolone/allopregnanolone sulfate | 1.52b | 1.68b | 1.20 | 1.40c |
| Lipid | Pregnenolone steroid | 17α-OH-pregnanolone glucuronide | 1.63b | 1.67b | 1.54b | 1.09 |
| Lipid | Pregnenolone steroid | Pregnenediol disulfate (C21H34O8S2) | 1.36b | 1.51b | 1.06 | 1.42c |
| Lipid | Corticosteroid | Cortisol | 1.23b | 1.29b | 1.11 | 1.16 |
| Lipid | Estrogenic | Estrone 3-sulfate | 1.54c | 1.76b | 1.10 | 1.60 |
| Lipid | Sterol | 3β-OH-5-cholestenoate | 0.84d | 0.84e | 0.85e | 0.98 |
| Lipid | Sterol | Campesterol | 0.83d | 0.84e | 0.82e | 1.03 |
| Lipid | Androgenic steroid | Androsteroid monosulfate (C19H28O6S) [1]a | 0.63d | 0.66e | 0.57d | 1.14 |
In the metabolome cohort, fold changes of members from subpathways of progestin steroids, pregnenolone steroids, corticosteroids, estrogenic, sterols, and androgenic steroids were shown between the following groups: infertility vs spontaneous, the IVF vs spontaneous, NIFT vs spontaneous and IVF vs NIFT. Analysis of covariance was performed, using BMI as a covariate.
Isomer.
P < 0.05 for increased levels of metabolites.
0.05 < P < 0.1 for increased levels of metabolites.
P < 0.05 for decreased levels of metabolites.
0.05 < P < 0.1 for decreased levels of metabolites.
Figure 2.
Model of steroid hormones and metabolites within the maternal-placental-fetal unit. Bold text indicates metabolites that were detected from maternal plasma by Metabolon; black and bold indicates metabolites that were detected but not different among groups; red indicates metabolites that were increased in both IVF and infertility groups when compared with the spontaneous group; green indicates metabolites that were downregulated in the infertility group compared with the spontaneous group; *metabolites that were increased only in the IVF group compared with spontaneous group; **increased pregnenolone sulfate (pregnenolone metabolite) in fresh IVF group compared with spontaneous group that is derived directly from pregnenolone. DHEA, dehydroepiandrosterone; DHEA-S, dehydroepiandrosterone sulfate.
Plasma hormone validation study
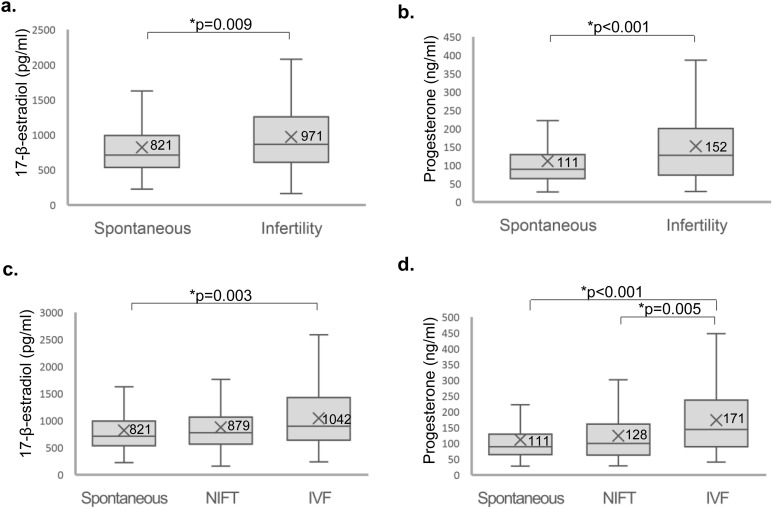
To validate findings from the metabolomics study that the increased steroid metabolite levels were the result of elevated hormones in infertile patients, we assessed the concentrations of plasma 17-β-estradiol and progesterone in the spontaneous group compared with those in the infertility group. Levels of 17-β-estradiol and progesterone were significantly elevated in the infertility group compared with the spontaneous group (17-β-estradiol levels: 971 ± 562 pg/mL vs 821 ± 456 pg/mL, respectively, P = 0.009; progesterone: 152 ± 101 ng/mL vs 111 ± 71 ng/mL, respectively, P < 0.001; Fig. 3).
Figure 3.
Measurement of 17-β-estradiol and progesterone levels using ELISA. (a, c) 17-β-Estradiol and (b, d) progesterone in maternal plasma at the time of chorionic villus sampling were measured in (a, b) spontaneous vs infertility pregnancies and (c, d) spontaneous vs NIFT vs IVF pregnancies of all participants in the ELISA cohort. P < 0.05 was considered significantly different.
When we separated the infertility group into NIFT and IVF groups, the IVF group had significantly higher levels of 17-β-estradiol and progesterone than did the spontaneous group (Fig. 3). The mean 17-β-estradiol level was 821 ± 456 pg/mL in the spontaneous group, 879 ± 552 pg/mL in the NIFT group, and 1042 ± 562 pg/mL in the IVF group (for spontaneous group vs IVF group, P = 0.003), and the mean progesterone level was 111 ± 71 ng/mL in the spontaneous group, 128 ± 86 ng/mL in the NIFT group, and 171 ± 109 ng/mL in the IVF group (P = 0.005 for the NIFT vs IVF groups; P < 0.001 for the spontaneous group vs IVF group).
When 58 women who received or possibly received hormone supplementation were removed, similar results were found. Levels of 17-β-estradiol and progesterone were significantly elevated in the infertility group compared with the spontaneous group (17-β-estradiol: 978 ± 601 pg/mL vs 822 ± 447 pg/mL, respectively, P = 0.015; progesterone: 140 ± 87 ng/mL vs 110 ± 70 ng/mL, respectively, P = 0.002). When we separated the infertility group into NIFT and IVF groups, the IVF group had significantly higher levels of 17-β-estradiol and progesterone than the spontaneous group. The mean 17-β-estradiol level was 822 ± 447 pg/mL in the spontaneous group, 923 ± 570 pg/mL in the NIFT group, and 1042 ± 634 pg/mL in the IVF group (for the spontaneous group vs IVF group, P = 0.024) and the mean progesterone level was 110 ± 70 ng/mL in the spontaneous group, 125 ± 79 ng/mL in the NIFT group, and 157 ± 94 ng/mL in the IVF group (for the spontaneous group vs IVF group, P = 0.001).
Our results indicate that although heterogeneity in hormonal states within groups exists, levels of circulating 17-β-estradiol and progesterone are persistently elevated in the late first trimester in mothers of pregnancies conceived with infertility treatment, which is likely driven by IVF compared with those who conceived spontaneously even after discontinuation of hormone supplementation, consistent with our metabolomics results demonstrating increased metabolites of estrogen and progesterone.
Xanthine and benzoate metabolites
Xenobiotics consist of food component/plant, clinical drug, chemical, benzoate metabolism, and xanthine metabolism subpathways. Levels of four compounds in xanthine metabolism that are also involved in caffeine metabolism—namely paraxanthine, 1,3,7-trimethylurate (40% fill value for the IVF group), 1-methylxanthine (43% fill value for the IVF group), and 5-acetylamino-6-formylamino-3-methyluracil—were significantly decreased in the infertility group. This difference was also observed when the IVF group was compared with the spontaneous group. There were significant increases in the benzoate metabolism pathway, as well as salicyluric glucuronide, a byproduct of aspirin (fill values were low: spontaneous group, 25%; IVF group, 40%; NIFT group, 27%), in the infertility group compared with the spontaneous group. These differences were partly replicated when the IVF group was compared with the spontaneous group. Table 5 lists these results.
Table 5.
Significantly Different Metabolites Within the Xenobiotic Superpathway, Between Groups
| Subpathway | Biochemical Name | Groups | |||
|---|---|---|---|---|---|
| Infertility vs Spontaneous | IVF vs Spontaneous | NIFT vs Spontaneous | IVF vs NIFT | ||
| Xanthine metabolism | Paraxanthine | 0.59a | 0.53a | 0.72 | 0.74 |
| Xanthine metabolism | 1,3,7-Trimethylurate | 0.57a | 0.55a | 0.63b | 0.86 |
| Xanthine metabolism | 1-Methylxanthine | 0.59a | 0.54a | 0.70 | 0.77 |
| Xanthine metabolism | 5-Acetylamino-6-formylamino-3-methyluracil | 0.54a | 0.48a | 0.66 | 0.73 |
| Drug (analgesics, anesthetics) | Salicyluric glucuronide | 1.72c | 1.96c | 1.24 | 1.58 |
| Benzoate metabolism | 2-Hydroxyhippurate (salicylurate) | 1.71c | 1.98c | 1.16 | 1.72 |
| Benzoate metabolism | 3-Hydroxyhippurate | 1.69d | 2.06c | 0.95 | 2.18c |
| Benzoate metabolism | 4-Hydroxyhippurate | 1.34 | 1.53c | 0.96 | 1.59c |
| Benzoate metabolism | 3-(3-Hydroxyphenyl) propionate sulfate | 1.47c | 1.66c | 1.09 | 1.51d |
In the metabolome cohort, fold changes of members from subpathways of xanthine metabolism and benzoate metabolism are shown between the following groups: infertility vs spontaneous, the IVF vs spontaneous, NIFT vs spontaneous, and IVF vs NIFT. Analysis of covariance was performed, using BMI as a covariate.
P < 0.05 for decreased levels of metabolites.
0.05 < P < 0.1 for decreased levels of metabolites.
P < 0.05 for increased levels of metabolites.
0.05 < P < 0.1 for increased levels of metabolites.
Differences attributed to IVF treatment: fresh embryo transfer IVF vs FETs
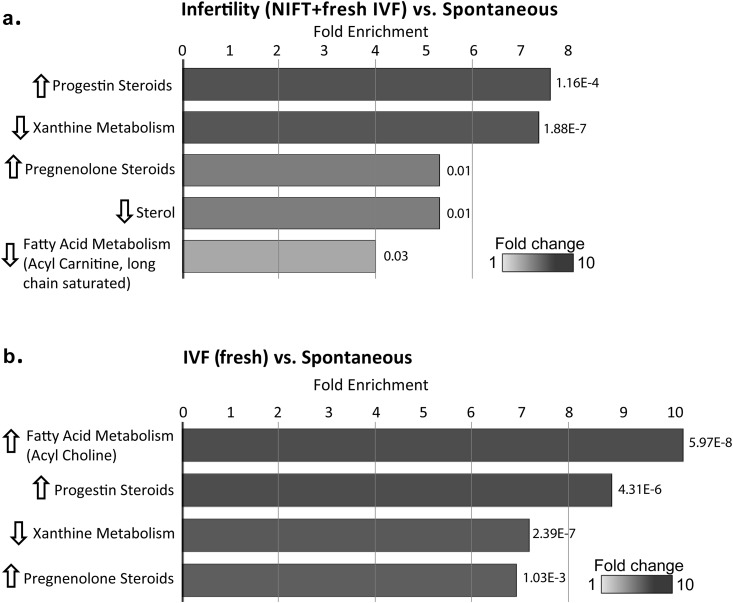
Because differences in hormonal regimens and outcomes may be present in IVF with fresh embryo transfers (hereafter, fresh IVF) compared with FETs, additional analysis was performed using only fresh IVF, excluding FETs, to look at a more homogenous population. When FET cycles were excluded, significant differences in 17-β-estradiol levels between the spontaneous and IVF groups remained, as did significant differences in progesterone levels between the spontaneous and IVF groups, as well as between NIFT and IVF groups. Levels of 17-β-estradiol and progesterone were significantly elevated in the infertility group (NIFT plus fresh IVF) compared with the spontaneous group (17-β-estradiol: 972 ± 568 pg/mL vs 821 ± 456 pg/mL, respectively, P = 0.011; progesterone: 155 ± 102 ng/mL vs 111 ± 71 ng/mL, respectively, P < 0.001). When we separated the infertility group into NIFT and fresh IVF groups, the fresh IVF group had significantly higher levels of 17-β-estradiol and progesterone than did the spontaneous group and significantly higher levels of progesterone than did the NIFT group. The mean 17-β-estradiol level was 821 ± 456 pg/mL in the spontaneous group, 879 ± 552 pg/mL in the NIFT group, and 1061 ± 573 pg/mL in the fresh IVF group (for the spontaneous group vs fresh IVF group, P = 0.006), and the mean progesterone level was 111 ± 71 ng/mL in the spontaneous group, 128 ± 86 ng/mL in the NIFT group, and 182 ± 109 ng/mL in the IVF fresh group (for the spontaneous group vs fresh IVF group, P < 0.001; and for the NIFT group vs fresh IVF group, P = 0.003).
Pathway enrichment analysis again identified progestin steroids and xanthine metabolism as the most enriched when comparing the infertility and spontaneous groups. In addition, pregnenolone steroids, sterols, and fatty acid metabolism (i.e., acyl carnitine, long-chain saturated) were enriched (Fig. 4a). When we compared fresh IVF with spontaneous conceptions, pathway enrichment analysis still identified the acyl choline, progestin steroid, and xanthine metabolism subpathways, with the addition of the pregnenolone steroid subpathway (Fig. 4b).
Figure 4.
Pathway enrichment analysis at the subpathway level when the fresh IVF group was used for comparison. (a) Subpathways that were significantly different between the spontaneous and infertility groups (i.e., NIFT plus fresh IVF groups). (b) Subpathways that were significantly different between the spontaneous and fresh IVF groups. The length of the bars shows the fold enrichment for each subpathway, with P values noted. Graphical representations of enriched pathways were generated in Cytoscape to highlight important changes at the biological pathway level.
Levels of five of seven measured progestin metabolites remained increased in the infertility group compared with the spontaneous group. They also remained increased in the IVF group compared with the spontaneous group, and the level of 5α-pregnan-3β,20α-diol monosulfate was also now increased (Table 6). Compared with the spontaneous group, levels of two pregnenolone steroids, pregnenediol disulfate (FC1.35) and 17α-OH-pregnanolone glucuronide (FC2.1), remained increased in the infertility group. In addition, pregnenediol sulfate (C21H34O5S) level was also increased (FC1.34). Levels of these three pregnenolone metabolites were also increased in the fresh IVF group compared with the spontaneous group with the addition of pregnenolone sulfate (FC1.64). Levels of estrone 3-sulfate, which were significantly higher in the IVF group than the spontaneous group (P < 0.05; FC1.76), became marginally significant (0.05 < P < 0.1; FC1.67) when only the fresh IVF group was included. Cortisol levels remained higher in the infertility group and IVF group compared with the spontaneous group. Levels of the sterol metabolites 3β-OH-5-cholestenoate and campesterol remained significantly lower in the infertility group compared with the spontaneous group and significantly lower in fresh IVF group vs the spontaneous group. β-Sitosterol levels were also significantly lower in the infertility group vs the spontaneous group. Levels of the androgenic steroid metabolite androsteroid monosulfate (C19H28O6S) remained decreased in the infertility group compared with the spontaneous group and levels of androstenediol (3β,17β) monosulfate were also now significantly decreased.
Table 6.
Significantly Different Steroid Metabolites Within the Lipid Superpathway When Data From All Women Using IVF or Fresh IVF Were Used to Compare Infertility Group vs Spontaneous Group and IVF Group vs Spontaneous Group
| Subpathway | Biochemical Name | Groups | |||
|---|---|---|---|---|---|
| Infertility vs Spontaneous | NIFT Plus Fresh IVF vs Spontaneous | IVF vs Spontaneous | Fresh IVF vs Spontaneous | ||
| Progestin steroid | 5α-Pregnan-3β,20β-diol monosulfate [1]a | 1.45b | 1.53b | 1.54b | 1.78b |
| Progestin steroid | 5α-Pregnan-3β,20α-diol disulfate | 1.53b | 1.40b | 1.70b | 1.60b |
| Progestin steroid | 5α-Pregnan-diol disulfate | 1.99b | 1.79b | 2.31b | 2.27b |
| Progestin steroid | Pregnanediol-3-glucuronide | 1.28b | 1.40b | 1.26b | 1.45b |
| Progestin steroid | Pregnanolone/allopregnanolone sulfate | 1.52b | 1.58b | 1.68b | 1.98b |
| Progestin steroid | 5α-Pregnan-3β,20α-diol monosulfate [2]a | 1.11 | 1.21c | 1.11 | 1.32b |
| Pregnenolone steroid | 17α-OH-pregnanolone glucuronide | 1.63b | 2.10b | 1.67b | 2.66b |
| Pregnenolone steroid | Pregnenediol disulfate (C21H34O8S2) | 1.36b | 1.35b | 1.51b | 1.67b |
| Pregnenolone steroid | Pregnenolone sulfate | 1.14 | 1.32c | 1.20 | 1.64b |
| Pregnenolone steroid | Pregnenediol sulfate (C21H34O5S) | 1.17 | 1.34b | 1.22 | 1.61b |
| Sterol | 3β-OH-5-cholestenoate | 0.84d | 0.80d | 0.84e | 0.75d |
| Sterol | β-Sitosterol | 0.91 | 0.78d | 0.95 | 0.74e |
| Sterol | Campesterol | 0.83d | 0.71d | 0.84e | 0.62d |
| Corticosteroid | Cortisol | 1.23b | 1.18b | 1.29b | 1.26b |
| Estrogenic steroid | Estrone 3-sulfate | 1.54c | 1.36 | 1.76b | 1.67c |
| Androgenic steroid | Androstenediol (3β,17β) monosulfate [2]a | 0.73e | 0.62d | 0.82 | 0.70 |
| Androgenic steroid | Androsteroid monosulfate (C19H28O6S) [1]a | 0.63d | 0.61d | 0.66e | 0.65e |
In the metabolome cohort, fold changes of members from subpathways of progestin steroids, pregnenolone steroids, sterol, corticosteroids, and estrogenic and androgenic steroids were shown between the infertility group vs spontaneous group, and the IVF group vs spontaneous group when only data from women who underwent fresh IVF or all women who underwent IVF were used. Analysis of covariance was performed, using BMI as a covariate.
Isomer.
P < 0.05 for increased levels of metabolites.
0.05 < P < 0.1 for increased levels of metabolites.
P < 0.05 for decreased levels of metabolites.
0.05 < P < 0.1 for decreased levels of metabolites.
Discussion
In maternal plasma from pregnancies conceived in couples with and without infertility, we identified differences in the metabolomic profile that were largely driven by the differences between the IVF group and the spontaneous group. Specifically, there were differences in the lipid superpathway, including the acyl cholines, PLs, glycerolipids, and steroid metabolites, with increased levels in the infertility group and the IVF group, specifically, compared with the spontaneous group. Additional studies in which we looked at levels of 17-β-estradiol and progesterone validated these results. In addition, within the xenobiotics superpathway, there were increases in the level of benzoate (salicylate) metabolites in the infertility group compared with the spontaneous group, and these were largely driven by the differences between the IVF and spontaneous groups, whereas the xanthine metabolite levels were significantly decreased in the infertility group compared with the spontaneous group, again largely driven by the differences between the IVF and spontaneous groups.
Normally, throughout gestation, levels of TAGs, cholesterol (total, LDL, HDL), and PLs increase dramatically in response to estrogen stimulation and insulin resistance that contribute to hyperlipidemia and lipogenesis (14, 23). Maternal metabolism is largely anabolic during early pregnancy and becomes catabolic as pregnancy progresses, providing the fetus with glucose and fatty acid as primary and alternative fuels, respectively (23). The higher levels of DAGs in the IVF group compared with the spontaneous group may be in response to estrogen or perhaps higher energy requirements. Regardless, elevated levels of lipids such as TAGs in the first trimester are associated with gestational hypertension, preeclampsia, and preterm birth (24), all of which have been associated with IVF.
In addition, in the pregnant state, cholesterol, the level of which is elevated, is available for the fetus for cell membrane development and production of steroid hormones or bile acids. As main carriers of TAGs and cholesteryl esters in the circulation, lipoproteins (i.e., chylomicrons, LDL, HDL, VLDL) have a surface layer composed of PLs that are interspersed with cholesterols and apoproteins. The hydrolysis of TAGs releases fatty acids as energy fuels, structural components, and precursors for eicosanoids. Elevated PLs in the infertility group, largely due to the IVF group, may be the result of early exposure to elevated estradiol levels from fertility treatments. These elevated PLs and TAGs in the infertility and IVF groups may be a reflection of a larger pool of cholesterol and TAGs transported to the placenta for steroid production; these were also significantly increased in the infertility and IVF groups compared with the spontaneous group.
Steroid production is critical for establishment and maintenance of a pregnancy. Early in gestation, the corpus luteum is the main source of estrogen and progesterone production until approximately 6 to 7 weeks, when the placenta starts to become the main source of hormone production to maintain the pregnancy (21). This process requires the mother, fetus, and placenta to work as one unit, the maternal-fetal-placental unit. Cholesterol, the steroid precursor, is transported to the STBs of the placenta to produce pregnenolone, which is then converted to progesterone. Pregnenolone is also used by the fetal adrenal to produce dehydroepiandrosterone sulfate and with the use of the fetal liver, provides precursors for the placenta to produce estrogens, including estrone, estradiol, and estriol (25).
Our metabolomics studies identified increased levels of metabolites of pregnenolone and progesterone, 17α-OH pregnanolone glucuronide and pregnenediol disulfate, in the infertility and the IVF group compared with the spontaneous group. Elevations of steroids were validated using ELISAs. Levels of 3β-OH-5-cholestenoate, a precursor for bile acids, were decreased in the infertility group compared with the spontaneous group, suggesting that cholesterol is being shunted toward the steroidogenic pathway and not the bile acid pathway. These results suggest that the supraphysiologic hormonal state during fertility treatments and hormone supplementation is driving increased PL and glycerol lipid production, providing increased cholesterol to the placenta for hormone production, not bile acid production, to maintain these pregnancies in a high hormonal state. In addition, early elevations of estrogen from fertility treatments and supplementation may also increase cholesterol uptake in trophoblast cells (26), stimulate the cholesterol production in fetal liver (27), and increase the placental P450 enzyme activity (28), all of which enhance the production of progesterone. Thus, the placenta may be reprogrammed postimplantation, ultimately leading to increased STB hormone production.
Estrogen steroids are downstream of pregnenolone and, normally, their levels are significantly elevated in pregnancy due to placental production, cooperatively between mother and fetus. In addition to increased progesterone production and metabolism in the infertility group, largely due to the IVF group, levels of the only estrogen metabolite measured, estrone 3-sulfate, also were increased in IVF, suggesting that estrogen is increased in the IVF group. This was validated in our hormone studies, with 17-β-estradiol significantly increased in the infertility group and most pronounced in the IVF group. Furthermore, the androstenedione metabolite androsteroid monosulfate was decreased in the infertility group, suggesting that androgenic precursors are being shunted toward estrogen production in pregnancies resulting from infertility treatments, working toward maintenance of an elevated estrogenic state, similar to exposure during early gestation.
Fertility treatments lead to increased estrogen and progesterone levels during implantation and early pregnancy, either due to treatment or supplementation (3, 4). These elevated hormonal states have been associated with adverse obstetric and perinatal outcomes, including low birth weight, fetal growth restriction, preeclampsia, and abnormal placentation (4, 29–31). Although fertility treatments and supplementation were common in the infertility group, supplementation was discontinued in our cohort, suggesting that the placenta was the main source of hormone production, and this likely was due to reprogramming as a result of exposure to a high hormonal state early in gestation. Thus, high hormonal states early in gestation may lead to placental reprogramming and subsequent development of adverse outcomes. GATA binding protein 3, a transcription factor critical in trophectoderm differentiation, is regulated by estradiol and important for trophoblast migration and invasion, which affects overall placentation (32). Mainigi et al. (33) also identified the GRB10 gene as differentially regulated by estradiol and important for placental function. Elevated progesterone levels, like elevated estradiol levels also affect placentation, which, in conjunction with estrogen, induces first-trimester trophoblast tubulogenesis through the lysophosphatidic acid pathway (34).
Cortisol levels were significantly increased in the IVF group compared with the spontaneous group. This is likely due to maternal production, because maternal cortisol is normally three times higher in the mother compared with the fetus and the placenta expresses the enzyme 11β-hydroxylase, which inactivates cortisol to cortisone (25). Cortisol and the hypothalamic pituitary adrenal axis have been implicated in contributing to infertility in women (35).
There were significant increases in benzoate metabolite levels within the IVF group. This may have been due to the widespread use of low-dose aspirin in IVF (36). The metabolites found in IVF plasma, levels of which were significantly lower than those in the spontaneous group are metabolites involved in caffeine metabolism. The decreased levels of multiple metabolites involved in caffeine metabolism are suggestive of lower caffeine intake in women conceived with IVF. This may be due to greater preconception counseling and earlier prenatal care in women who undergo IVF (37).
When we performed analysis using only women who had undergone fresh IVF, significant differences between groups remained similar to when we included all women who had undergone IVF, suggesting little contribution from the heterogeneity of IVF treatments to the hormonal differences between the infertility group (or specifically the IVF group) and spontaneous group in our study. Similarly, metabolomic analysis with fresh IVF overall revealed similar enriched subpathways. However, certain subpathways lost significance when only the fresh IVF group was included in the IVF group for comparison, likely because of decreased sample size, because more individual metabolites lost statistical significance although the direction of change remained. As more study participants are removed, there is potentially larger variation, which may undervalue the real biological differences that exist between the IVF group and spontaneous group.
There are some limitations to our analyses. Pathway enrichment analysis was used to highlight the most representative pathways. However, it can be affected by the number of metabolites detected in each pathway. Therefore, pathway enrichment analysis results need to be interpreted with caution. In addition, we were unable to include our entire SMAART study cohort for metabolomic and hormone studies, because maternal plasma in the late first trimester was not available for all subjects. Another limitation is that we did not have infertility etiology available for our study participants. Although infertility etiology may have an impact, our intent was to determine the effect of fertility treatments independent of underlying infertility etiology. Additional studies will be necessary to look at the impact of infertility etiology on outcomes.
In conclusion, in this study, we looked at metabolomic profile differences in women who conceived with infertility treatment; specifically, we compared conceptions via NIFT vs IVF and compared these with spontaneous conceptions. We found that levels of many members of the lipid superpathway are elevated in infertility, largely driven by IVF. This may be in response to estrogen stimulation early in gestation, due to underlying treatment. This, in turn, leads to increased cholesterol for steroid hormone production by the STBs of the placenta, with shunting away from bile acid production. In addition, this early estrogen exposure may increase cholesterol uptake in trophoblast cells (26), which leads to increased hormone production by the placenta. Thus, the placenta may be reprogrammed postimplantation, leading to increased STB hormone production to maintain these pregnancies in a high hormonal state, which has been validated with our hormone studies. The elevation in steroids leads to dysfunction in trophoblast cells, thus these elevated states may be contributors to adverse outcomes associated with infertility and the treatments used. Further mechanistic and additional population-based studies are necessary to determine the exact mechanisms leading to these outcomes.
Acknowledgments
We thank Rae A. Buttle, BA; Erica J. Sauro, BS; and Kerlly Castellano, BS, for patient recruitment and sample collection; and Lauren W. Sundheimer, MD, MS, FACOG, and Nikhil V. Joshi, MD for discussing and providing clinical insights.
Financial Support: This work was supported by the National Institutes of Health, Eunice Kennedy Shriver National Institute of Child Health and Human Development R01 Grant R01HD074368 (to M.D.P.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The funding agency was not involved in the design, analysis, or interpretation of the data reported.
Disclosure Summary: The authors have nothing to disclose.
Glossary
Abbreviations:
- BMI
body mass index
- CSMC
Cedars Sinai Medical Center
- DAG
diacylglyerol
- DHA
docosahexaenoyl
- ESI
electrospray ionization
- FC
fold change
- FET
frozen embryo transfer
- HDL
high-density lipoprotein
- IVF
in vitro fertilization
- LDL
low-density lipoprotein
- NIFT
non–in vitro fertilization
- SMAART
Spontaneous/Medically Assisted/Assisted Reproductive Technology
- STB
syncytiotrophoblast
- TAG
triglyceride
- UPLC-MS/MS
ultraperformance liquid chromatography–tandem mass spectrometry
- VLDL
very low-density lipoprotein
References
- 1. Schieve LA, Devine O, Boyle CA, Petrini JR, Warner L. Estimation of the contribution of non-assisted reproductive technology ovulation stimulation fertility treatments to US singleton and multiple births. Am J Epidemiol. 2009;170(11):1396–1407. [DOI] [PubMed] [Google Scholar]
- 2. Sunderam S, Kissin DM, Crawford SB, Folger SG, Boulet SL, Warner L, Barfield WD. doi: 10.15585/mmwr.ss6703a1. Assisted Reproductive Technology Surveillance - United States, 2015. MMWR Surveill Sum. 2018;67(3):1–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dickey RP, Taylor SN, Lu PY, Sartor BM, Rye PH, Pyrzak R. Relationship of follicle numbers and estradiol levels to multiple implantation in 3,608 intrauterine insemination cycles. Fertil Steril. 2001;75(1):69–78. [DOI] [PubMed] [Google Scholar]
- 4. Pereira N, Reichman DE, Goldschlag DE, Lekovich JP, Rosenwaks Z. Impact of elevated peak serum estradiol levels during controlled ovarian hyperstimulation on the birth weight of term singletons from fresh IVF-ET cycles. J Assist Reprod Genet. 2015;32(4):527–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jackson S, Hong C, Wang ET, Alexander C, Gregory KD, Pisarska MD. Pregnancy outcomes in very advanced maternal age pregnancies: the impact of assisted reproductive technology. Fertil Steril. 2015;103(1):76–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wang ET, Ramos L, Vyas N, Bhasin G, Simmons CF, Pisarska MD. Maternal and neonatal outcomes associated with infertility. J Matern Fetal Neonatal Med. 2018:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shevell T, Malone FD, Vidaver J, Porter TF, Luthy DA, Comstock CH, Hankins GD, Eddleman K, Dolan S, Dugoff L, Craigo S, Timor IE, Carr SR, Wolfe HM, Bianchi DW, D’Alton ME. Assisted reproductive technology and pregnancy outcome. Obstet Gynecol. 2005;106(5 Pt 1):1039–1045. [DOI] [PubMed] [Google Scholar]
- 8. Dhalwani NN, Boulet SL, Kissin DM, Zhang Y, McKane P, Bailey MA, Hood ME, Tata LJ. Assisted reproductive technology and perinatal outcomes: conventional versus discordant-sibling design. Fertil Steril. 2016;106(3):710–716.e2. [DOI] [PubMed] [Google Scholar]
- 9. Boulet SL, Kirby RS, Reefhuis J, Zhang Y, Sunderam S, Cohen B, Bernson D, Copeland G, Bailey MA, Jamieson DJ, Kissin DM; States Monitoring Assisted Reproductive Technology (SMART) Collaborative. Assisted reproductive technology and birth defects among liveborn infants in Florida, Massachusetts, and Michigan, 2000-2010. JAMA Pediatr. 2016;170(6):e154934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Davies MJ, Moore VM, Willson KJ, Van Essen P, Priest K, Scott H, Haan EA, Chan A. Reproductive technologies and the risk of birth defects. N Engl J Med. 2012;366(19):1803–1813. [DOI] [PubMed] [Google Scholar]
- 11. Wang ET, Sundheimer LW, Spades C, Quant C, Simmons CF, Pisarska MD. Fertility treatment is associated with stay in the neonatal intensive care unit and respiratory support in late preterm infants. J Pediatr. 2017;187:309–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhu JL, Basso O, Obel C, Bille C, Olsen J. Infertility, infertility treatment, and congenital malformations: Danish national birth cohort. BMJ. 2006;333(7570):679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kroener L, Wang ET, Pisarska MD. Predisposing factors to abnormal first trimester placentation and the impact on fetal outcomes. Semin Reprod Med. 2016;34(1):27–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wang Q, Würtz P, Auro K, Mäkinen VP, Kangas AJ, Soininen P, Tiainen M, Tynkkynen T, Jokelainen J, Santalahti K, Salmi M, Blankenberg S, Zeller T, Viikari J, Kähönen M, Lehtimäki T, Salomaa V, Perola M, Jalkanen S, Järvelin MR, Raitakari OT, Kettunen J, Lawlor DA, Ala-Korpela M. Metabolic profiling of pregnancy: cross-sectional and longitudinal evidence. BMC Med. 2016;14(1):205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Orczyk-Pawilowicz M, Jawien E, Deja S, Hirnle L, Zabek A, Mlynarz P. Metabolomics of human amniotic fluid and maternal plasma during normal pregnancy. PLoS One. 2016;11(4):e0152740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pisarska MD, Akhlaghpour M, Lee B, Barlow GM, Xu N, Wang ET, Mackey AJ, Farber CR, Rich SS, Rotter JI, Chen YI, Goodarzi MO, Guller S, Williams J III. Optimization of techniques for multiple platform testing in small, precious samples such as human chorionic villus sampling. Prenat Diagn. 2016;36(11):1061–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yousri NA, Fakhro KA, Robay A, Rodriguez-Flores JL, Mohney RP, Zeriri H, Odeh T, Kader SA, Aldous EK, Thareja G, Kumar M, Al-Shakaki A, Chidiac OM, Mohamoud YA, Mezey JG, Malek JA, Crystal RG, Suhre K. Whole-exome sequencing identifies common and rare variant metabolic QTLs in a Middle Eastern population. Nat Commun. 2018;9(1):333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. doi: 10.1101/gr.1239303. Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, Amin N, Schwikowski B, Ideker T. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13(11):2498–2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Audet-Delage Y, Villeneuve L, Grégoire J, Plante M, Guillemette C. Identification of metabolomic biomarkers for endometrial cancer and its recurrence after surgery in postmenopausal women. Front Endocrinol (Lausanne). 2018;9:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Csapo AI, Pulkkinen MO, Ruttner B, Sauvage JP, Wiest WG. The significance of the human corpus luteum in pregnancy maintenance. I. Preliminary studies. Am J Obstet Gynecol. 1972;112(8):1061–1067. [DOI] [PubMed] [Google Scholar]
- 21. Yen SSC, Jaffe RB, Barbieri RL. Reproductive Endocrinology: Physiology, Pathophysiology, and Clinical Management. 5th ed.Philadelphia, PA: Saunders. [Google Scholar]
- 22. Yanushpolsky EH. Luteal phase support in in vitro fertilization. Semin Reprod Med. 2015;33(2):118–127. [DOI] [PubMed] [Google Scholar]
- 23. Lain KY, Catalano PM. Metabolic changes in pregnancy. Clin Obstet Gynecol. 2007;50(4):938–948. [DOI] [PubMed] [Google Scholar]
- 24. Vrijkotte TGM, Krukziener N, Hutten BA, Karlijn C, Vollebregt KC, van Eijsden M, Twickler MB. Maternal lipid profile during early pregnancy and pregnancy complications and outcomes: the ABCD Study. J Clin Endocrinol Metab. 2012;97(11):3917–3925. [DOI] [PubMed] [Google Scholar]
- 25. Morel Y, Roucher F, Plotton I, Goursaud C, Tardy V, Mallet D. Evolution of steroids during pregnancy: maternal, placental and fetal synthesis. Ann Endocrinol (Paris). 2016;77(2):82–89. [DOI] [PubMed] [Google Scholar]
- 26. Grimes RW, Pepe GJ, Albrecht ED. Regulation of human placental trophoblast low-density lipoprotein uptake in vitro by estrogen. J Clin Endocrinol Metab. 1996;81(7):2675–2679. [DOI] [PubMed] [Google Scholar]
- 27. Carr BR, Simpson ER. Cholesterol synthesis by human fetal hepatocytes: effects of hormones. J Clin Endocrinol Metab. 1984;58(6):1111–1116. [DOI] [PubMed] [Google Scholar]
- 28. Babischkin JS, Grimes RW, Pepe GJ, Albrecht ED. Estrogen stimulation of P450 cholesterol side-chain cleavage activity in cultures of human placental syncytiotrophoblasts. Biol Reprod. 1997;56(1):272–278. [DOI] [PubMed] [Google Scholar]
- 29. Kalra SK, Barnhart KT. In vitro fertilization and adverse childhood outcomes: what we know, where we are going, and how we will get there. A glimpse into what lies behind and beckons ahead. Fertil Steril. 2011;95(6):1887–1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Imudia AN, Awonuga AO, Doyle JO, Kaimal AJ, Wright DL, Toth TL, Styer AK. Peak serum estradiol level during controlled ovarian hyperstimulation is associated with increased risk of small for gestational age and preeclampsia in singleton pregnancies after in vitro fertilization. Fertil Steril. 2012;97(6):1374–1379. [DOI] [PubMed] [Google Scholar]
- 31. Wennerholm UB, Henningsen AK, Romundstad LB, Bergh C, Pinborg A, Skjaerven R, Forman J, Gissler M, Nygren KG, Tiitinen A. Perinatal outcomes of children born after frozen-thawed embryo transfer: a Nordic cohort study from the CoNARTaS group. Hum Reprod. 2013;28(9):2545–2553. [DOI] [PubMed] [Google Scholar]
- 32. Lee B, Kroener LL, Xu N, Wang ET, Banks A, Williams J III, Goodarzi MO, Chen YI, Tang J, Wang Y, Gangalapudi V, Pisarska MD. Function and hormonal regulation of GATA3 in human first trimester placentation. Biol Reprod. 2016;95(5):113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mainigi MA, Sapienza C, Butts S, Coutifaris C. A molecular perspective on procedures and outcomes with assisted reproductive technologies. Cold Spring Harb Perspect Med. 2016;6(4):a023416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Beltrame JS, Sordelli MS, Cañumil VA, Alonso CAI, Perez Martinez S, Ribeiro ML. Steroid hormones induce in vitro human first trimester trophoblast tubulogenesis by the lysophosphatidic acid pathway. Mol Cell Endocrinol. 2018;478:126–132. [DOI] [PubMed] [Google Scholar]
- 35. Damti OB, Sarid O, Sheiner E, Zilberstein T, Cwikel J. Stress and distress in infertility among women [in Hebrew]. Harefuah. 2008;147(3):256–260, 276. [PubMed] [Google Scholar]
- 36. Wang L, Huang X, Li X, Lv F, He X, Pan Y, Wang L, Zhang X. Efficacy evaluation of low-dose aspirin in IVF/ICSI patients evidence from 13 RCTs: a systematic review and meta-analysis. Medicine (Baltimore). 2017;96(37):e7720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gaskins AJ, Chavarro JE. Diet and fertility: a review. Am J Obstet Gynecol. 2018;218(4):379–389. [DOI] [PMC free article] [PubMed] [Google Scholar]