Abstract
Purpose
Olaratumab is a recombinant human IgG1 monoclonal antibody against PGDFRα. Olaratumab plus doxorubicin improved survivalversus doxorubicin in an open-label, randomised phase 2 soft tissue sarcoma (STS) trial. We characterised the olaratumab exposure–response relationship for progression-free survival (PFS), overall survival (OS), and safety.
Methods
PFS and OS data from the 133 patients enrolled in the phase 2 study were analysed using time-to-event modelling. The effect of olaratumab on PFS/OS was explored using the trough serum concentration after cycle 1 (Cmin1) and the average concentration throughout treatment (Cavg). The rate of treatment-emergent adverse events (TEAEs) was compared across olaratumab exposure quartiles.
Results
PFS and OS were described by models with an exponential hazard function and inhibitory EMAX functions to describe the effect of olaratumab, regardless of the PK endpoint. The olaratumab EC50s for PFS (ECmin150 = 82.0 µg/mL, ECavg50 = 179 µg/mL) and OS (ECmin150 = 66.1 µg/mL, ECavg50 = 134 µg/mL) corresponded to the median and 25th percentile of Cmin1/Cavg in the study, respectively. Maximum predicted improvement in the hazard ratio for OS and PFS was approximately 75% and 60%, respectively. There was no change in the rate of TEAEs with increasing olaratumab serum levels.
Conclusions
PFS/OS benefits occurred without a rate change in TEAEs across quartiles. Maximum benefit in OS was achieved in the upper three quartiles and a potential of early disease progression in the lower quartile of olaratumab serum exposure. These results prompted a loading dose strategy in the ongoing phase 3 STS trial.
Keywords: Olaratumab, Doxorubicin, Exposure response, Outcome, Soft tissue sarcomas
Introduction
Soft tissue sarcomas (STS) are a group of rare tumours of mesenchymal origin, accounting for approximately 1% of all adult cancers [1–3]. For most histological subtypes, the standard management of localised disease consists of complete surgical resection with or without radiation. Despite optimal management, however, high-risk patients will develop recurrent locally advanced inoperable or metastatic disease. The outcome for patients with advanced inoperable/metastatic STS is poor with a median overall survival (OS) in the range of 12–18 months [4–8]. There are few treatment options available, and these have historically included doxorubicin with or without ifosfamide. Over the last few years, a number of other drugs have emerged including gemcitabine/docetaxel, trabectedin, pazopanib, and eribulin [6, 9–12].
Olaratumab is a recombinant human immunoglobulin G1 monoclonal antibody to platelet-derived growth factor receptor alpha (PDGFRα) [13]. A randomised phase 2 trial of doxorubicin with or without olaratumab in patients with advanced STS demonstrated a significantly longer median OS for the combination of doxorubicin and olaratumab compared to doxorubicin alone (26.5 and 14.7 months, respectively, hazard ratio [HR] 0.46, p = 0.0003) [14]. The increase in progression-free survival (PFS) was also significant (6.6 months and 4.1 months, respectively, HR 0.67, p = 0.0615) and the combination of olaratumab with doxorubicin led to a slight increase in toxicity but remained well-tolerated. Based on this phase 2 STS trial, olaratumab was granted accelerated/conditional approval by a number of regulatory agencies.
A matched case–control analysis [15] performed on the phase 2 PFS and OS survival data stratified by quartiles of olaratumab serum exposure indicated that patients in the lowest quartile may not have received optimal level of clinical benefit [14]. A population pharmacokinetic (PopPK) analysis subsequently performed using PK data combined from four phase 2 studies, including that in STS, indicated that the dose of 15 mg/kg administered on Days 1 and 8 of a 21-day cycle yields olaratumab serum levels likely to achieve full target saturation [16]. In light of these findings, it seems necessary to better define the therapeutic window of olaratumab and determine whether the dose of 15 mg/kg used in the phase 2 study represents the optimal dose to be used in combination with doxorubicin in STS patients. The aim of this study was therefore to characterise the exposure–response relationship of olaratumab in combination with doxorubicin for PFS, OS, and safety for patients with advanced STS.
Materials and methods
Clinical trial and data
OS, PFS, and safety data were obtained for the 133 patients enrolled in a randomised, open-label, multicenter, phase 2 trial where the efficacy of olaratumab in combination with doxorubicin was tested in patients with histologically confirmed locally advanced or metastatic STS (NCT01185964) [14]. Patients were randomly assigned in a 1:1 ratio to receive olaratumab (15 mg/kg) intravenously on Day 1 and Day 8 plus doxorubicin (75 mg/m2) (n = 66) or doxorubicin alone (75 mg/m2) on Day 1 of each 21-day cycle for up to eight cycles (n = 67). Randomization was stratified according to number of previous lines of treatment (0 versus 1 + lines), histological tumour type (that is, leiomyosarcoma versus synovial sarcoma versus other tumour type), Eastern Cooperative Oncology Group performance status (ECOG PS) (0, 1 versus 2), number of prior lines of treatment (0 versus ≥ 1), and PDGFRα expression (positive versus negative). Throughout the study, patients were assessed for tumour response every 6 weeks according to RECIST 1.1. Patients on the investigational arm without disease progression could continue to receive olaratumab until the development of unacceptable toxicity, noncompliance or withdrawal of consent by the patient, or investigator decision.
The study was conducted in accordance with the Declaration of Helsinki and the International Conference on Harmonisation Guidelines for Good Clinical Practice. The local institutional review boards at each participating study site approved the study protocol, and all patients in the study provided written informed consent to participate.
Olaratumab PK endpoints
The effect of olaratumab serum exposure on clinical outcome was described using two PK endpoints: the trough concentration at the end of the first cycle of treatment (Cmin1), and the average concentration over each patient’s entire treatment (Cavg). These endpoints were selected primarily because they allow the description of olaratumab serum exposure in two different methods: Cmin1 describes the intended serum exposure prior to any dose reductions, whereas Cavg summarises serum exposure retrospectively and captures the impact of dose reductions during the course of treatment. Individual estimates of Cmin1 and Cavg for each patient in the experimental arm were obtained from the population PK model developed using PK data combined from a total of four phase 2 studies, including that in STS patients [16].
Survival models for OS and PFS
OS and PFS were described by means of parametric time-to-event modeling, where survival was calculated as the inverse of the exponent of the cumulative hazard over time during the study. Both OS and PFS were best described by a time-to-event model where the baseline hazard remains constant throughout the study, so that the time to event (survival duration) is exponentially distributed over time. The effect of olaratumab Cmin1 and Cavg was incorporated into the OS and PFS models as a fractional decrease to the hazard function. Upon establishment of the appropriate survival model, the intrinsic and extrinsic patient factors listed in Table 1 were tested as covariates for their influence on OS and PFS.
Table 1.
Patient factors assessed in the population pharmacodynamic analysis
| Covariate | Type | Parameters tested |
|---|---|---|
| ECOG group | Categorical | BASE_HAZ, EMAX, EC50 |
| Tumour size | Continuous | BASE_HAZ, EMAX, EC50 |
| Tumour histology | Categorical | BASE_HAZ, EMAX, EC50 |
| Age group | Categorical | BASE_HAZ, EMAX, EC50 |
| Gender | Categorical | BASE_HAZ, EMAX, EC50 |
| Race | Categorical | BASE_HAZ, EMAX, EC50 |
| Body weight | Categorical | BASE_HAZ, EMAX, EC50 |
| Prior treatment | Categorical | BASE_HAZ, EMAX, EC50 |
| Hemoglobin | Continuous | BASE_HAZ, EMAX, EC50 |
| Albumin | Continuous | BASE_HAZ, EMAX, EC50 |
ECOG Eastern Cooperative Oncology Group, BASE_HAZ baseline hazard, EMAX maximum response achievable from a dose, EC50 concentration of a drug that gives half-maximal response
Exposure–response for safety
The overall incidence of treatment-emergent adverse events (TEAEs) in the investigational arm of the study was stratified by grade (≤ grade 2, > grade 2, ≥ grade 4) and by quartile of olaratumab Cmin1 and Cavg in a tabular format and compared to that in the control arm. The rates of neutropenia and mucositis were also examined in a similar manner.
Results
Overall survival model
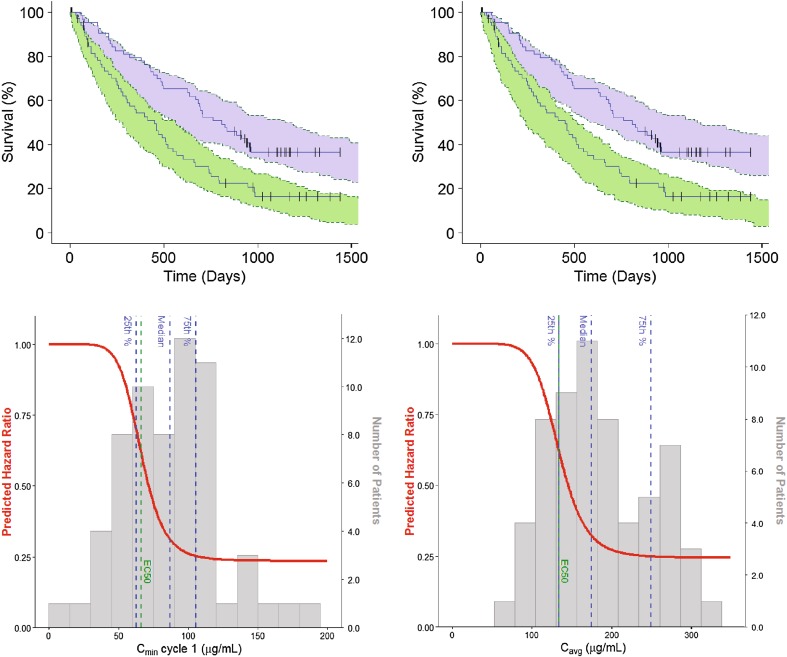
OS in the study was best described by a time-to-event model with an exponential hazard function. In both the Cmin1- and Cavg-based models, an inhibitory EMAX drug effect model on the hazard function performed the best to describe the effect of olaratumab. The visual predictive check (VPC) showed good agreement between observed data and model prediction (Fig. 1a). The precision of the parameter estimates was verified by bootstrap analysis, except for the Hill coefficient, which was fixed to allow model stability. All parameter estimates for both OS models are listed in Table 2. Cmin1 and Cavg had a similar predictive role for drug effect, so that the half-maximum effective Cmin1 and Cavg (ECmin150 and ECavg50) estimates corresponded to the 25th percentile of Cmin1 and Cavg in the study, respectively. The maximum effect (EMAX) estimates corresponded to a predicted improvement in the hazard ratio of approximately 75% (Fig. 1b). Consistent with the high value for the Hill coefficient, there was no notable change predicted in the HR for patients in the lowest quartile of olaratumab exposure, whereas EMAX was reached within the range of olaratumab serum concentrations achieved in the study. The ECOG PS and the number of prior treatments were found to be significant covariates affecting the baseline hazard for both the Cmin1- and Cavg-based models. Patients with an ECOG PS ≥ 1 have a predicted 86.2% increase in the baseline hazard and patients who had received no prior lines of treatment had a 58.3% decrease in the baseline hazard.
Fig. 1.
Visual predictive check and model prediction of the overall survival models. a Left graph is the VPC of the Cmin1-based OS model. Right graph is the VPC of the Cavg-based OS model. The shaded areas indicate the predicted OS in the control (green) and experimental (blue) arm; the solid blue lines describe the corresponding observed OS signals. b Overall survival as predicted by Cmin1-based model (left panel) and the Cavg-based model (right panel). The solid red lines describe the change in HR as a function of Cmin1 and Cavg; the grey histograms describe the distribution of olaratumab Cmin1 and Cavg in the study JGDG experimental arm together with their quartiles (dashed blue lines); the green dashed lines indicate ECmin150 and ECavg50. Cavg = average concentration over patient’s entire treatment. Cmin1 = trough serum concentration at the end of Cycle 1. ECavg50 = olaratumab Cavg yielding a 50% decrease in the baseline hazard. ECmin150=olaratumab Cmin1 yielding a 50% decrease in the baseline hazard. HR hazard ratio, OS overall survival, VPC visual predictive check
Table 2.
Parameter estimates and bootstrap results for the OS models
| Parameters | Population estimate (%SEE) | Bootstrap parameter results (5–95 percentile) | ||
|---|---|---|---|---|
| Cmin1 model | Cavg model | Cmin1 model | Cavg model | |
| Baseline hazard | ||||
| BaseHazard | 0.00205 (2.18) | 0.00203 (2.26) | 0.00206 (0.00158–0.00280) |
0.00204 (0.00156–0.00277) |
| Olaratumab effect | ||||
| EMAX | 0.765 (8.63) | 0.756 (9.07) | 0.771 (0.625–0.884) | 0.761 (0.607–0.876) |
| ECmin150 (µg/mL) | 66.1 (12.1) | – | 65.9 (50.9–80.3) | – |
| ECavg50 (µg/mL) | – | 134 (6.72) | – | 135 (115–163) |
| Hill | 8 (fixed) | 8 (fixed) | 8 (fixed) | 8 (fixed) |
| Covariate effects | ||||
| EGRPBase | 0.862 (42.1) | 0.802 (44.0) | 0.925 (0.273–1.83) | 0.857 (0.220–1.72) |
| PRVTRTBase | − 0.583 (15.9) | − 0.535 (19.1) | − 0.57 − (0.740–0.345) | − 0.528 − (0.706–0.282) |
OS overall survival, SEE standard error of the estimate, Cmin1 trough serum concentration at the end of Cycle 1, Cavg average concentration over patient’s entire treatment, EMAX maximum response achievable from a dose, ECmin150 olaratumab Cmin1 yielding a 50% decrease in the baseline hazard, ECavg50 olaratumab Cavg yielding a 50% decrease in the baseline hazard, EGRPBase covariate effect of ECOG status on the baseline hazard, PRVTRTBase covariate effect of the number of prior treatment on the baseline hazard
Progression-free survival model
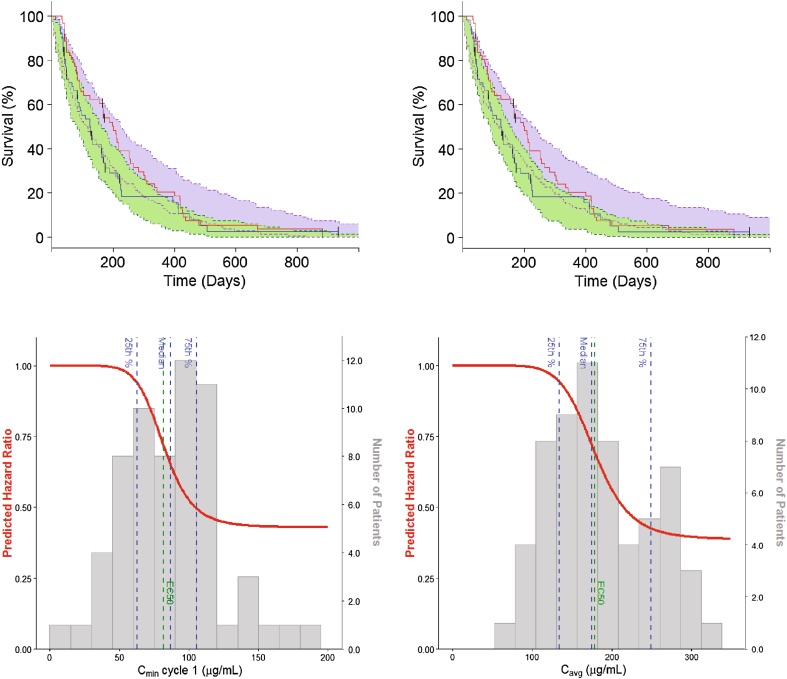
PFS was best described by a model with a structure similar to that used for OS. Parameter estimates are listed in Table 3 and the model VPC is shown in Fig. 2a. Again, Cmin1 and Cavg had a similar predictive role for drug effect. The ECmin150 and ECavg50 estimates corresponded to the median of Cmin1 and Cavg in the study, rather than their 25th percentile (Fig. 2b). The maximum predicted effect of olaratumab on PFS was also lower compared to OS, with EMAX estimates of approximately 0.60. None of the covariates explored were found to have a significant effect on PFS in our analysis.
Table 3.
Parameter estimates and bootstrap results for the PFS models
| Parameters | Population estimate (%SEE) | Bootstrap parameter results (5–95 Percentile) | ||
|---|---|---|---|---|
| Cmin1 model | Cavg model | Cmin1 model | Cavg model | |
| Baseline hazard | ||||
| BaseHazard | 0.00604 (2–52) | 0.00616 (2.63) | 0.00607 (0.00473–0.00786) |
0.00619 (0.00476–0.00805) |
| Olaratumab effect | ||||
| EMAX | 0.571 (15–4) | 0.614 (14.5) | 0.567 (0.363–0.723) | 0.617 (0.435–0.770) |
| ECmin150 (µg/mL) | 82.0 (6–15) | – | 82.3 (69.3–95.4) | |
| ECavg50 (µg/mL) | – | 179 (9.33) | 182 (149–241) | |
| Hill | 8 (fixed) | 8 (fixed) | 8 (fixed) | 8 (fixed) |
PFS progression-free survival, SEE standard error of the estimate, Cmin1 trough serum concentration at the end of Cycle 1, Cavg average concentration over patient’s entire treatment, EMAXmaximum response achievable from a dose, ECmin150 olaratumab Cmin1 yielding a 50% decrease in the baseline hazard, ECavg50 olaratumab Cavg yielding a 50% decrease in the baseline hazard
Fig. 2.
Visual predictive check and model prediction of the progression-free survival models. a Left graph is the VPC of the Cmin1-based PFS model. Right graph is the VPC of the Cavg-based PFS models. The shaded areas indicate the predicted PFS in the control (green) and experimental (blue) arm; the solid blue lines describe the corresponding observed PFS signals. Cavg = average concentration over patient’s entire treatment. b Progression-free survival as predicted by Cmin1-based model (left panel) and the Cavg-based model (right panel). The solid red lines describe the change in HR as a function of Cmin1 and Cavg; the grey histograms describe the distribution of olaratumab Cmin1 and Cavg in the study JGDG experimental arm together with their quartiles (dashed blue lines); the green dashed lines indicate ECmin150 and ECavg50. Cmin1 = trough serum concentration at the end of Cycle 1. ECavg50 = olaratumab Cavg yielding a 50% decrease in the baseline hazard. ECmin150 olaratumab Cmin1 yielding a 50% decrease in the baseline hazard, HR hazard ratio, PFS progression-free survival, VPC visual predictive check
Exposure–response for safety
The incidence of TEAEs in the phase 2 trial stratified by grade and by olaratumab Cmin1 and Cavg quartiles are listed in Table 4. The addition of olaratumab to doxorubicin led to a moderate increase in the rate of TEAEs compared to doxorubicin alone, consistent with previous reports. However, when examined across quartiles of olaratumab serum exposure, there was no change in the rate of TEAEs with increasing olaratumab serum concentrations in the investigational arm, regardless of the PK endpoint considered.
Table 4.
Treatment-related adverse events stratified by olaratumab concentration
| Olaratumab (Cmin1) | Olaratumab (Cavg) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dox | Overall | Q1 | Q2 | Q3 | Q4 | Dox | Overall | Q1 | Q2 | Q3 | Q4 | |
| n = 65 | n = 62 | n = 15 | n = 16 | n = 15 | n = 16 | n = 65 | n = 62 | n = 15 | n = 16 | n = 15 | n = 16 | |
| Cmin1 range (µg/mL) |
– | 12.3–188.1 | 12.3–<62.8 | 62.8–<86.9 | 86.9–<105.6 | 105.6–188.1 | – | 56.0–347.3 | 56.0–<134.4 | 134.4–<175.2 | 175.2–<249.9 | 249.9–347.3 |
| Overall TEAEs | ||||||||||||
| Grade ≤ 2, % | 19 (29.2) | 12 (19.4) | 1 (6.7) | 5 (31.3) | 3 (20.0) | 3 (18.8) | 19 (29.2) | 12 (19.4) | 1 (6.7) | 5 (31.3) | 1 (6.7) | 5 (31.3) |
| Grade > 2, % | 45 (69.2) | 49 (79.0) | 13 (86.7) | 11 (68.8) | 12 (80.0) | 13 (81.3) | 45 (69.2) | 49 (79.0) | 13 (86.7) | 11 (68.8) | 14 (93.3) | 11 (68.8) |
| Grade ≥ 4, % | 20 (30.8) | 25 (40.3) | 7 (46.7) | 5 (31.3) | 7 (46.7) | 6 (37.5) | 20 (30.8) | 25 (40.3) | 6 (40.0) | 8 (50.0) | 6 (40.0) | 5 (31.3) |
| Neutropenia | ||||||||||||
| Grade ≤ 2, %* | 3 (4.6) | 3 (4.8) | 0 | 2 (12.5) | 1 (6.7) | 0 | 3 (4.6) | 3 (4.8) | 0 | 2 (12.5) | 1 (6.7) | 0 |
| Grade > 2, %* | 22 (33.8) | 35 (56.5) | 9 (60.0) | 6 (37.5) | 11 (73.3) | 9 (56.3) | 22 (33.8) | 35 (56.5) | 9 (60.0) | 8 (50.0) | 11 (73.3) | 7 (43.8) |
| Grade ≥ 4, %* | 17 (26.2) | 23 (37.1) | 6 (40.0) | 5 (31.3) | 6 (40.0) | 6 (37.5) | 17 (26.2) | 23 (37.1) | 5 (33.3) | 7 (43.8) | 6 (40.0) | 5 (31.3) |
| Mucositis | ||||||||||||
| Grade ≤ 2, %* | 20 (30.8) | 32 (51.6) | 8 (53.3) | 6 (37.5) | 9 (60.0) | 9 (56.3) | 20 (30.8) | 32 (51.6) | 6 (40.0) | 8 (50.0) | 8 (53.3) | 10 (62.5) |
| Grade > 2, %* | 3 (4.6) | 2 (3.2) | 0 | 2 (12.5) | 0 | 0 | 3 (4.6) | 2 (3.2) | 0 | 2 (12.5) | 0 | 0 |
| Grade ≥ 4, %* | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Doxdoxorubicin, Q quartile, Cmin1 trough serum concentration at the end of Cycle 1, Cavg average concentration over patient’s entire treatment, TEAEs treatment-emergent adverse events
Discussion
The objective of this analysis was to characterise the exposure–response relationship of olaratumab for survival outcomes and safety when combined with doxorubicin in patients with advanced STS. The combined exposure–response information for efficacy and safety was then used to optimise the dosing strategy of olaratumab and better target its therapeutic window in the ongoing confirmatory phase 3 study (NCT02451943) after accelerated approval by Food and Drug Administration and the conditional approval by the European Medicines Agency.
The exposure–response relationship of olaratumab was first characterised for survival outcomes. OS in the study was best described by a model with a constant baseline hazard, and a sigmoidal relationship for the effect of olaratumab. The Cmin1-based model yielded an ECmin150 estimate (66 µg/mL) corresponding to the 25th percentile of the Cmin1 distribution in the study, and a Hill coefficient indicative of a steep exposure–response relationship. The EMAX estimate corresponded to a maximum decrease in the HR of approximately 75%, and was reached within the range of olaratumab serum concentration achieved in the study. Importantly, a similar exposure–response relationship was identified with the Cavg-based model: the baseline hazard, EMAX, and Hill coefficient estimates were similar to those obtained in the Cmin1-based model, and the ECavg50 estimate (134 µg/mL) also corresponded to the 25th percentile of the Cavg distribution in the study. Both PK variables therefore seem to be similarly predictive of the effect of olaratumab on OS.
These findings indicate that a small increase in Cmin1 or Cavg in the vicinity of the EC50 is expected to lead to a dramatic change from low to near maximal OS benefit. Since the EC50 estimates correspond to the 25th percentile of olaratumab exposure in the study population, the dose of 15 mg/kg, administered on Days 1 and 8 of a 21-day cycle, therefore provides the majority of the study population with near maximum OS benefit. This is consistent with results from the PopPK analysis, where the linear clearance used to describe the disposition of olaratumab suggested that the dose of 15 mg/kg achieves serum levels leading to full target saturation [16]. This is also in line with the results of a previously published matched-case control (MCC) analysis on the same data [14] which indicated that: (1) patients in the upper three Cmin1 and Cavg quartiles showed an improvement in OS; (2) there was no consistent difference in OS benefit across the upper three Cmin1 and Cavg quartiles; and (3) HR values observed in the upper quartiles were in line with the model-predicted EMAX. It should also be pointed out that the ECOG PS and the number of prior lines of treatment were found to have a significant influence on OS, which is in line with the current understanding of clinical prognostic factors in STS and further supports the validity of our findings.
PFS in the study was also best described by a model with a constant baseline hazard and a sigmoidal relationship for the effect of olaratumab. The ECmin150 and ECavg50 estimates corresponded, respectively, to the median Cmin1 and Cavg in the study population, and the predicted EMAX was lower than that for OS. In addition, the HR for PFS was not predicted to improve until the Cmin1 or Cavg reaches values corresponding to the 25th percentile of their distribution in the study. These findings are line with the lower activity on PFS compared with OS previously reported for olaratumab and with the previous MCC analysis on PFS which suggested that patients in the lowest exposure quartile tend to experience disease progression within the first two to three cycles of treatment.
Patients who received olaratumab in combination with doxorubicin did experience an increase in the rate of TEAEs when compared to doxorubicin alone, consistent with the toxicity profile of doxorubicin. There was, however, no apparent additional increase in the rate of TEAEs with increasing olaratumab serum levels, regardless of the TEAEs examined in the patients examined thus far. The exposure–response relationship of olaratumab for toxicity is thus very shallow, so that an increase in clinical benefit may be achieved without an increase in serious (high grade) TEAEs. The findings from the safety assessment should be interpreted with caution due to the limited number of patients that experienced TEAEs. Safety data from the ongoing confirmatory phase 3 study will provide more conclusive results.
Altogether, our analysis indicates that olaratumab has a wide therapeutic window, characterised by a steep exposure–response relationship for efficacy and a shallow exposure–response relationship for toxicity. It also indicates that the therapeutic window of olaratumab was effectively targeted by the dose of 15 mg/kg tested in the randomised phase 2 study where approximately 75% of the population were exposed to olaratumab serum levels associated with OS benefit and maximum OS benefit was potentially reached. Finally, our analysis suggests that an olaratumab Cmin1 of 66 µg/mL or Cavg of 134 µg/mL may represent a minimum threshold for delaying disease progression and providing OS benefit in STS.
This hypothesis was used to further optimise the dosing strategy for the ongoing randomised phase 3 study of olaratumab combined with doxorubicin (ANNOUNCE). Simulations using the PopPK model previously developed for olaratumab indicate that the use of a loading dose of 20 mg/kg on Days 1 and 8 of Cycle 1 would achieve olaratumab serum levels comparable to those observed at steady state with 15 mg/kg. The dosing strategy for the randomised phase 3 study therefore consists of a loading dose of 20 mg/kg of olaratumab during Cycle 1 followed by 15 mg/kg in ensuing cycles. This dosing strategy is expected to better target the therapeutic window of olaratumab by (1) minimising the number of patients whose Cmin1 falls below 66 µg/mL at the start of treatment; (2) replicating olaratumab steady-state serum levels associated with OS benefit; and (3) preserving the positive benefit–risk ratio of olaratumab by maintaining olaratumab serum levels with the same total range as in the randomised phase 2 olaratumab trial.
Conclusions
The exposure–response relationship of olaratumab for PFS and OS are best described by time-to-event models with exponential hazard functions, and the effects of olaratumab on PFS and OS were well-characterized by inhibitory EMAX functions with Hill coefficients. Both PK endpoints, Cmin1 and Cavg, were equally predictive of the effect of olaratumab on OS and PFS. The model estimated maximum OS benefit was achieved by 75% of the patients in the trial, whereas only 50% of the patients are estimated to have achieved maximum benefit in PFS. The therapeutic window of olaratumab appears wide as increasing olaratumab serum concentration was not associated with increased incidence rate of TEAEs. Based on the analysis presented here and the evidence previously reported on this clinical study, a loading cycle of 20 mg/kg of olaratumab was incorporated into the confirmatory phase 3 study with the aim to prevent early disease progression and increase the number of patients that could potentially experience OS benefit.
Acknowledgements
We thank Anastasia Perkowski for editorial assistance.
Funding
The study was designed by the sponsor, Eli Lilly and Company, with input from sarcoma experts. The data were analysed and interpreted by Eli Lilly and Company in collaboration with the academic authors. All authors had access to all of the data and vouch for the accuracy and completeness of the data and analyses reported and for the fidelity of the study to the study protocol. All authors had final responsibility for the decision to submit for publication. The corresponding author prepared the initial draft of the manuscript with editorial assistance, and all authors contributed to the subsequent drafts.
Conflict of interest
RLJ reports consulting work with Blueprint, Deciphera, Immune Design, Merck, Clinigen, Pharmamar, and Eisai. GM, RLI, IC, and DMC report a patent pending for dosing regimen assigned to ImClone LLC, a wholly owned subsidiary of Eli Lilly and Company. WDT reports a patent pending for Companion Diagnostic for CDK4 inhibitors − 14/854,329 for the identification and development of biomarkers for CDK4 inhibition in cancer. WDT reports Advisory Board, consulting, and travel expense reimbursement for Eli Lilly and Company, EMD Serono, Novartis, Eisai, Janssen, Immune Design, Adaptimmune, Daiichi Sankyo, Blueprint, and Loxo for the purposes of discussing cancer drug development, all outside the submitted work. WDT also reports serving as IDMC chair for sarcoma clinical trial with Morphotek, outside the submitted work. GM and PMP are full-time employees of Eli Lilly and Company; RLI, IC, DMC, and JRB are former employees of Eli Lilly and Company; GM, PMP, RLI, IC, and DMC are current shareholders of Eli Lilly and Company.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all the individual participants included in the study.
Footnotes
John R. Baldwin retired from Eli Lilly and Company.
References
- 1.D’Angelo SP, Tap WD, Schwartz GK, Carvajal RD (2014) Sarcoma immunotherapy: past approaches and future directions. Sarcoma 2014:391967 [DOI] [PMC free article] [PubMed]
- 2.Sharma S, Takyar S, Manson SC, Powell S, Penel N. Efficacy and safety of pharmacological interventions in second- or later-line treatment of patients with advanced soft tissue sarcoma: a systematic review. BMC Cancer. 2013;13:385. doi: 10.1186/1471-2407-13-385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Singer S, Demetri GD, Baldini EH, Fletcher CD. Management of soft-tissue sarcomas: an overview and update. Lancet Oncol. 2000;1:75–85. doi: 10.1016/S1470-2045(00)00016-4. [DOI] [PubMed] [Google Scholar]
- 4.Ryan CW, Merimsky O, Agulnik M, et al. PICASSO III: A phase III, placebo-controlled study of doxorubicin with or without palifosfamide in patients with metastatic soft tissue sarcoma. J Clin Oncol. 2016;34:3898–3905. doi: 10.1200/JCO.2016.67.6684. [DOI] [PubMed] [Google Scholar]
- 5.Tap WD, Papai Z, Van Tine BA, et al. Doxorubicin plus evofosfamide versus doxorubicin alone in locally advanced, unresectable or metastatic soft-tissue sarcoma (TH CR-406/SARC021): an international, multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2017;18:1089–1103. doi: 10.1016/S1470-2045(17)30381-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seddon B, Strauss SJ, Whelan J, et al. Gemcitabine and docetaxel versus doxorubicin as first-line treatment in previously untreated advanced unresectable or metastatic soft-tissue sarcomas (GeDDiS): a randomised controlled phase 3 trial. Lancet Oncol. 2017;18:1397–1410. doi: 10.1016/S1470-2045(17)30622-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Judson I, Verweij J, Gelderblom H, European Organisation and Treatment of Cancer Soft Tissue and Bone Sarcoma Group et al. Doxorubicin alone versus intensified doxorubicin plus ifosfamide for first-line treatment of advanced or metastatic soft-tissue sarcoma: a randomised controlled phase 3 trial. Lancet Oncol. 2014;15:415–423. doi: 10.1016/S1470-2045(14)70063-4. [DOI] [PubMed] [Google Scholar]
- 8.Lorigan P, Verweij J, Papai Z, European Organisation for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group Study et al. Phase III trial of two investigational schedules of ifosfamide compared with standard-dose doxorubicin in advanced or metastatic soft tissue sarcoma: a European Organisation for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group Study. J Clin Oncol. 2007;25:3144–3150. doi: 10.1200/JCO.2006.09.7717. [DOI] [PubMed] [Google Scholar]
- 9.Maki RG, Wathen JK, Patel SR, et al. Randomized phase II study of gemcitabine and docetaxel compared with gemcitabine alone in patients with metastatic soft tissue sarcomas: results of sarcoma alliance for research through collaboration study 002 [corrected] J Clin Oncol. 2007;25:2755–2763. doi: 10.1200/JCO.2006.10.4117. [DOI] [PubMed] [Google Scholar]
- 10.Demetri GD, von Mehren M, Jones RL, et al. Efficacy and safety of trabectedin or dacarbazine for metastatic liposarcoma or leiomyosarcoma after failure of conventional chemotherapy: results of a phase III randomized multicenter clinical trial. J Clin Oncol. 2016;34:786–793. doi: 10.1200/JCO.2015.62.4734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van der Graaf WT, Blay JY, Chawla SP, EORTC Soft Tissue and Bone Sarcoma Group; PALETTE study group et al. Pazopanib for metastatic soft-tissue sarcoma (PALETTE): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2012;379:1879–1886. doi: 10.1016/S0140-6736(12)60651-5. [DOI] [PubMed] [Google Scholar]
- 12.Schöffski P, Chawla S, Maki RG, et al. Eribulin versus dacarbazine in previously treated patients with advanced liposarcoma or leiomyosarcoma: a randomised, open-label, multicentre, phase 3 trial. Lancet. 2016;387:1629–1637. doi: 10.1016/S0140-6736(15)01283-0. [DOI] [PubMed] [Google Scholar]
- 13.Loizos N, Xu Y, Huber J, et al. Targeting the platelet-derived growth factor receptor alpha with a neutralizing human monoclonal antibody inhibits the growth of tumor xenografts: implications as a potential therapeutic target. Mol Cancer Ther. 2005;4:369–379. doi: 10.1158/1535-7163.MCT-04-0114. [DOI] [PubMed] [Google Scholar]
- 14.Tap WD, Jones RL, Van Tine BA, et al. Olaratumab and doxorubicin versus doxorubicin alone for treatment of soft-tissue sarcoma: an open-label phase 1b and randomised phase 2 trial. Lancet. 2016;388:488–497. doi: 10.1016/S0140-6736(16)30587-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang J, Zhao H, Garnett C, et al. The combination of exposure-response and case-control analyses in regulatory decision making. J Clin Pharmacol. 2013;53:160–166. doi: 10.1177/0091270012445206. [DOI] [PubMed] [Google Scholar]
- 16.Mo G, Baldwin JR, Luffer-Atlas D, Ilaria RL, Jr, Conti I, Heathman M, Cronier DM. Population pharmacokinetic modeling of olaratumab, an anti-PDGFRα human monoclonal antibody, in patients with advanced and/or metastatic cancer. Clin Pharmacokinet. 2018;57:355–365. doi: 10.1007/s40262-017-0562-0. [DOI] [PMC free article] [PubMed] [Google Scholar]