Abstract
Background/Aims:
Colorectal cancer (CRC) is a public health issue, and before the initiation of a national cancer screening program, there is a need to examine the acceptance of the public to undergo CRC screening and explore potential barriers.
Materials and Methods:
A nationwide survey was conducted using an electronic platform to collect demographic variables and using the Health Belief Model to assess attitudes and behavior of participants as well as the knowledge about and intent to undergo CRC screening. At the end of the survey, participants from Riyadh were invited to get screened for CRC.
Results:
Responses from 5720 individuals covering all the 13 jurisdictions of Saudi Arabia were collected. Males represented 71.53% of the respondents; the mean age was 43.28 years and 15.24% had already undergone CRC screening using various methods, mostly colonoscopy (72.73%). The mean knowledge score was 11.05 (standard deviation 4.4, range 1–23), with no difference between genders, jurisdictions of the Kingdom, between those who expressed interest in screening and those who did not, and between those who accepted the invitation to undergo CRC screening and those who did not. Participants displayed positive attitudes toward both CRC screening and colonoscopy as a screening tool, and 73% expressed willingness to undergo screening. On multivariate analysis, male gender was the only factor associated with a higher probability of accepting screening, whereas neither knowledge nor willingness to undergo screening predicted accepting the invitation to screening.
Conclusion:
Although the majority of participants were willing to undergo screening, no significant correlation between knowledge and willingness to undergo screening were predictors of screening uptake. Other areas that could be targeted in the promotion of CRC screening uptake to bridge the gap between “knowing” and “doing” should be explored.
Keywords: Colon cancer, colonosocopy, early detection, endoscopy, epidemiology, Saudi Arabia, tumor
INTRODUCTION
Colorectal cancer (CRC) is a public health issue with an estimated 832,000 mortalities globally in 2015,[1] in addition to its associated morbidity, cost, and the productivity loss for those affected. In Saudi Arabia, CRC is the most common cancer in males, with an average annual age standardized rate (ASR) of 11.2/100,000, and it is the third most common cancer in females, with an average annual ASR of 9.1/100,000.[2] Furthermore, CRC tends to affect the Saudi population at a younger age,[3] and the 5-year survival rates are lower than those expected for matching stages in other populations.[4]
The guidelines for CRC screening, including those from Saudi Arabia,[5] vary in the screening method recommended, how frequent to repeat the tests, as well the ages at which to start and stop screening.[6] The Saudi guidelines recommended CRC screening for average risk individuals to start from the age of 45 years, and colonoscopy was the recommended modality for screening; when not available, flexible sigmoidoscopy every 5 years with an annual guaiac fecal occult blood test (FOBT) or fecal immunochemical testing was recommended. This variability in recommendation between guidelines reflects the variability in baseline risks and resources available for such programs. Although the Saudi guidelines for CRC screening have been disseminated, screening has been performed on an opportunistic basis rather than a national level. Also, the Saudi guidelines were based on limited data,[7,8] and assessing the acceptance of the public for CRC screening would be needed prior to any investment in the initiation of a national program.
This study aimed to examine the acceptance of the public to undergo CRC screening and to explore potential barriers to CRC screening using the Health Belief Model (HBM), through a nationwide survey using an electronic platform to assess possible uptake of screening if a national program would be launched in Saudi Arabia.
MATERIALS AND METHODS
Demographic variables collected included the area where the participant resides, sex, age, highest education level obtained, marital status, employment, family history of CRC or having a friend with CRC, and monthly income. We also gathered information with regard to previous screening for CRC. We used the HBM to assess attitudes and behaviors of participants as well as assessing the knowledge and intent to undergo CRC screening and perceived barriers to CRC screening.
Survey tool: The Health Belief Model
The HBM is a sociopsychological model that explains health-seeking behavior of individuals by focusing on the attitudes and behavior that are influenced by perceived susceptibility, severity, benefit, barriers, and cues to actions[9] [Figure 1]. It was adopted in this study because of its extensive use in assessing the construct of health-seeking behavior pertaining to uptake of cancer screening in general and CRC screening in particular, and is one of the most valid tools to measure such an association.[10] Although there is no validated translated version into Arabic, it was converted into an Arabic version and reviewed by two bilingual epidemiologists and a gastroenterologist and used in the original Arabic study that was used in Riyadh[11] and has been reproduced in other publications.[12,13]
Figure 1.
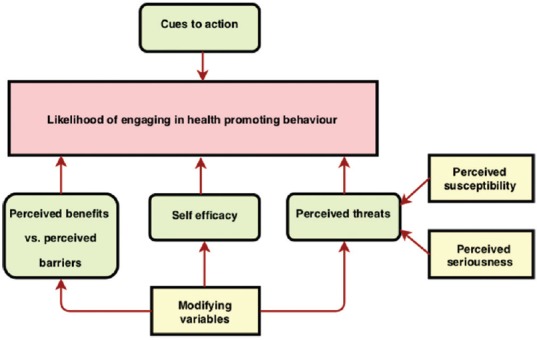
Directed acyclic graph (DAG) of the Health Belief Model
The knowledge section in the survey included questions pertaining to CRC symptoms and risk factors, types of CRC screening tests, perceived risk of CRC, and perceived severity of CRC. A knowledge score according to correct responses to the questionnaire was used as a continuous variable when analyzing the data. Each correct response was appointed a single point and the maximum score that could be achieved was 26.
Attitudes toward CRC and screening were assessed using a 5-point Likert scale ranging from “strongly agree” to “strongly disagree”, and the participants were asked about which screening test would be acceptable to them; each of these screening options was illustrated by figures and text in Arabic in layman terms describing each test, time intervals between each test, and the benefits and limitations of each of them. We included the following CRC screening tests as possible options: FOBT, colonoscopy, flexible sigmoidoscopy, or computed tomographic colonography (CTC).
We also asked the participants about the age at which CRC screening should begin; this was categorized into five age ranges starting at the age of 20 years till the age of 70 years. At the end of the survey, the participants from Riyadh were asked whether they would like to get screened; if they accepted the invitation, they were asked to enter their contact details so that they would be enrolled in a subsequent study using a stool-based test as a screening modality for CRC screening at a later date.
Survey delivery method
The 48-item survey was administered through Qualtrics (Provo, UT, USA).[14] The first screen of the survey after the participant would access the link, explained the reason for the study and was for the purpose of obtaining an informed consent from the participant and was a prerequisite to continue in the survey.
The survey was disseminated through a number of methods: text messaging through a mobile network provider to the general population with the aim of capturing a representative sample from different areas of Saudi Arabia. We also used the snowball sampling method (a non-probability sampling technique) where participants could recruit future subjects through forwarding the link of the survey to other individuals through social media platforms or emails.
Statistical analysis
Descriptive statistics were computed for continuous variables, including minimum and maximum values, means, standard deviations (SDs), as well as 95% confidence intervals (CIs) and frequencies for categorical variables when appropriate. If hypothesis testing was used, Pearson's Chi-square t-test and, where appropriate, Fisher's exact tests were used.[15] A one-way analysis of variance to test for differences among groups when comparing more than one group was performed when appropriate.
Univariate and multivariate logistic regressions were used to examine the possible associations between independent variables and the acceptance to the invitation to undergo CRC screening. Odds ratios and 95% CIs were calculated. A Bonferroni adjustment for multiple comparisons was made. A backward selection method was used to determine the variables to be included in the final model. The goodness of fit for the multivariate model was based on Akaike information criteria.
R Studio[16] was used for analysis using the R statistical language.[17] Numerous statistical packages were used for statistical calculations and data visualization.[18,19,20,21,22,23,24,25,26,27,28,29,30,31,32] A statistical significance threshold of P = 0.05 was adopted. No attempt at imputation was made for missing data.
RESULTS
Demographics of those who responded to the survey
In all, 5720 individuals responded to the survey and were included in the analysis. There were participants from all the 13 jurisdictions of the Kingdom of Saudi Arabia. The composition of the participants from these areas varied in age and occupation. The major demographic features are shown in Table 1. The majority of the respondents were males (71.53% vs. 28.47%), and the mean age of the participants was 43.28 years (95% CI; 42.88–43.69, range 18–78 years) with males being older (47.84) than females (44.69), with P < 0.01. Married individuals constituted 88.94%, 7.6% were single, 2.41% were divorced, while 1.06% were widows.
Table 1.
Basic demographics of the 5720 individuals who participated in the survey by area
| Albaha | Aljawf | Almadinah | Alqaseem | Aseer | Eastern province | Ha’il | Jazan | Makkah | Najran | Northern territories | Riyadh | Tabuk | Total | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age (years) | ||||||||||||||
| Mean | 44.95 | 48.43 | 46.18 | 49.29 | 43.31 | 47.88 | 46.96 | 41.21 | 47.35 | 44.92 | 46.25 | 47.12 | 43.51 | 43.28 |
| Std. dev. | 10.72 | 11.76 | 10.14 | 9.54 | 6.81 | 8.47 | 8.19 | 7.18 | 9.00 | 6.80 | 6.57 | 9.79 | 8.64 | 11.70 |
| Sex | ||||||||||||||
| Female | 20.59 | 10.00 | 20.86 | 18.52 | 13.94 | 36.76 | 28.26 | 7.69 | 24.38 | 9.09 | 18.52 | 34.44 | 21.54 | 28.47 |
| Male | 79.41 | 90.00 | 79.14 | 81.48 | 86.06 | 63.24 | 71.74 | 92.31 | 75.62 | 90.91 | 81.48 | 65.56 | 78.46 | 71.53 |
| Education | ||||||||||||||
| Primary | NA | NA | NA | 3.40 | 0.61 | 1.47 | NA | 9.62 | 1.24 | NA | NA | 1.51 | NA | 1.53 |
| Middle | NA | NA | 2.88 | 5.56 | 4.24 | 4.41 | NA | 3.85 | 3.81 | 4.55 | 11.11 | 2.79 | 9.23 | 3.62 |
| High school | 14.71 | 10.00 | 20.86 | 21.30 | 23.64 | 26.23 | 23.91 | 28.85 | 17.90 | 13.64 | 18.52 | 17.83 | 35.38 | 19.73 |
| College/university | 61.76 | 80.00 | 58.27 | 57.72 | 60.00 | 55.64 | 65.22 | 46.15 | 55.62 | 63.64 | 48.15 | 58.01 | 49.23 | 57.06 |
| Higher studies | 23.53 | 10.00 | 17.99 | 12.04 | 11.52 | 12.25 | 10.87 | 11.54 | 21.43 | 18.18 | 22.22 | 19.86 | 6.15 | 18.06 |
| Employment | ||||||||||||||
| Governmental sector | 64.71 | 50.00 | 47.48 | 47.22 | 44.24 | 29.90 | 41.30 | 59.62 | 36.48 | 40.91 | 33.33 | 40.94 | 38.46 | 39.91 |
| Housewife | 2.94 | NA | 7.19 | 6.17 | 4.24 | 10.78 | 6.52 | 1.92 | 9.14 | 4.55 | NA | 10.51 | 12.31 | 9.15 |
| Military sector | 5.88 | 10.00 | 6.47 | 4.01 | 19.39 | 12.01 | 10.87 | 11.54 | 6.95 | 31.82 | 22.22 | 7.72 | 24.62 | 8.66 |
| Private sector | 5.88 | 10.00 | 9.35 | 10.49 | 7.27 | 17.16 | 10.87 | 9.62 | 18.67 | NA | 11.11 | 14.23 | 7.69 | 14.54 |
| Retired | 11.76 | 30.00 | 17.99 | 24.07 | 16.97 | 24.26 | 23.91 | 7.69 | 21.43 | 9.09 | 18.52 | 19.40 | 13.85 | 20.35 |
| Self-employment | 2.94 | NA | 5.76 | 3.70 | 4.24 | 3.43 | 2.17 | NA | 3.71 | 9.09 | 3.70 | 2.61 | NA | 3.20 |
| Student | 2.94 | NA | 4.32 | 0.93 | 1.21 | 1.47 | 4.35 | 5.77 | 1.52 | 4.55 | 7.41 | 2.56 | 1.54 | 2.14 |
| Unemployed | 2.94 | NA | 1.44 | 3.40 | 2.42 | 0.98 | NA | 3.85 | 2.10 | NA | 3.70 | 2.03 | 1.54 | 2.04 |
| Income | ||||||||||||||
| Less than 5000 | 11.76 | 10.00 | 12.95 | 15.12 | 10.91 | 12.75 | 15.22 | 17.31 | 15.90 | 18.18 | 14.81 | 13.76 | 16.92 | 14.30 |
| 5000-9000 | 32.35 | 20.00 | 24.46 | 20.68 | 20.00 | 22.79 | 17.39 | 28.85 | 18.00 | 9.09 | 11.11 | 18.64 | 18.46 | 19.44 |
| 10,000-19,000 | 47.06 | 50.00 | 47.48 | 51.23 | 54.55 | 40.20 | 56.52 | 44.23 | 43.05 | 40.91 | 29.63 | 39.84 | 56.92 | 43.01 |
| 20,000-29,0000 | 8.82 | 20.00 | 9.35 | 8.95 | 10.91 | 12.75 | 8.70 | 5.77 | 12.95 | 13.64 | 18.52 | 14.63 | 4.62 | 12.87 |
| 30,000-39,000 | NA | NA | 1.44 | 2.16 | 2.42 | 4.90 | 2.17 | 3.85 | 4.67 | 9.09 | 11.11 | 6.39 | 1.54 | 4.95 |
| More than 40,000 | NA | NA | 4.32 | 1.85 | 1.21 | 6.62 | NA | NA | 5.43 | 9.09 | 14.81 | 6.74 | 1.54 | 5.44 |
| Marital status | ||||||||||||||
| Divorced | NA | NA | 0.72 | 0.93 | NA | 2.94 | NA | NA | 3.24 | 4.55 | NA | 2.50 | 6.15 | 2.41 |
| Married | 91.18 | 80.00 | 90.65 | 94.14 | 92.73 | 89.71 | 84.78 | 86.54 | 87.81 | 81.82 | 88.89 | 88.27 | 89.23 | 88.95 |
| Single | 8.82 | 20.00 | 7.91 | 4.32 | 7.27 | 6.62 | 15.22 | 11.54 | 7.62 | 9.09 | 7.41 | 8.13 | 3.08 | 7.58 |
| Widow | NA | NA | 0.72 | 0.62 | NA | 0.74 | NA | 1.92 | 1.33 | 4.55 | 3.70 | 1.10 | 1.54 | 1.06 |
Most of the respondents were college or university graduates (57.06%), whereas those with postgraduate degrees and high school degrees comprised similar proportions (18.06% vs. 19.73%) respectively. Those with a middle school education were 3.62%, and 1.53% had a primary school education level. Government employees represented 39.91% of the respondents, followed by retired individuals (20.35%), employees in the private sector (14.54%), military personnel (8.66%), self-employed individuals (3.2%), whereas students were 2.14%. Housewives were 9.15%, and 2.04% were unemployed. The participants in the survey represented different strata of the community with regard to monthly income, as demonstrated in Table 1.
Baseline CRC screening rates
Of the participants who responded to the survey, 15.24% had already undergone CRC screening using various methods, with the most frequently used method being colonoscopy (72.73%), whereas stool-based screening was 13.94%, CTC 8.48%, and flexible sigmoidoscopy was used by 4.85%.
Knowledge score
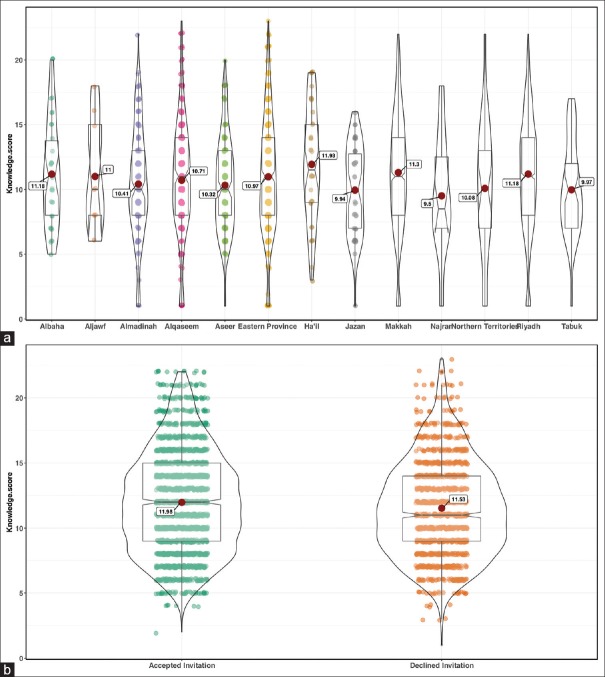
The mean knowledge score in our current survey was 11.05 (SD 4.4, range 1–23), with no difference between males and females. Also, the average knowledge score was similar across all jurisdictions of the Kingdom of Saudi Arabia [Figure 2a] and was not different between those who expressed interest in screening versus those who did not, neither between those who accepted the invitation to undergo CRC screening versus those who did not, in Riyadh [Figure 2b].
Figure 2.
Violin plot of the knowledge score between (a) all jurisdictions of Saudi Arabia and (b) comparing those who accepted the invitation to undergo CRC screening with those who did not in Riyadh
Attitudes toward CRC, its screening, and colonoscopies
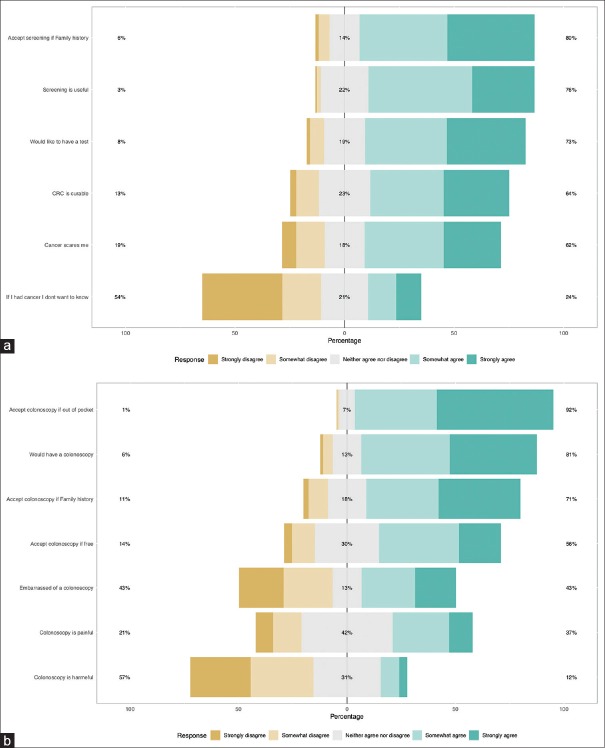
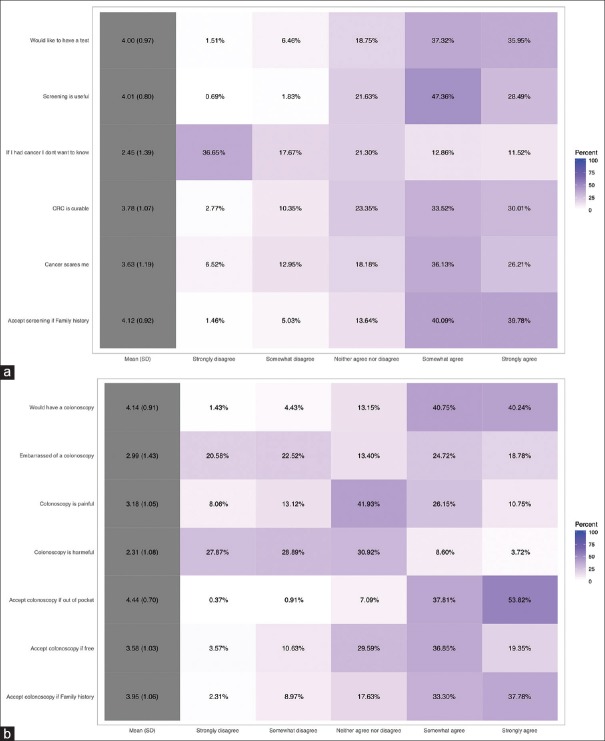
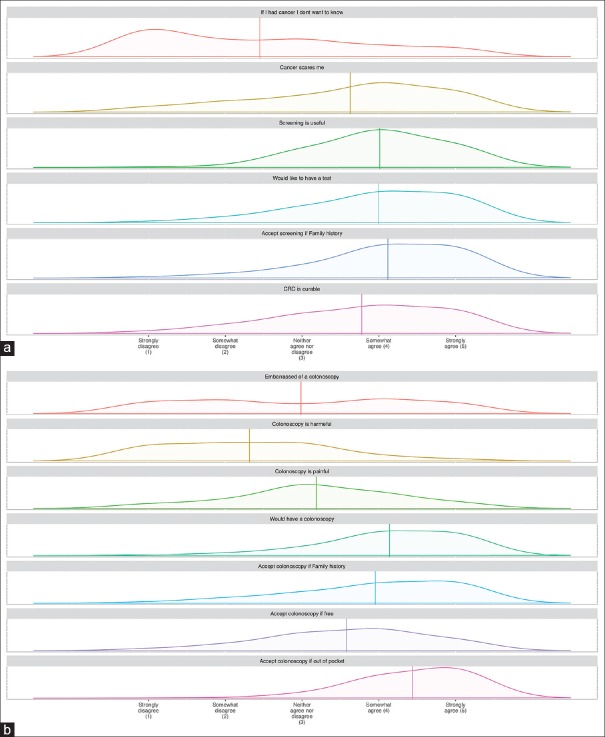
A Likert scale was used to measure the attitudes of participants. All the factors that have been explored by the HBM tended to point towards a positive attitude towards both CRC screening and colonoscopy as a screening tool [Figure 3a and b]. This is even depicted in the heat plot of participants’ answers to those questions [Figure 4a and b] where statements like “If I had cancer I would not want to know” or “Colonoscopy is harmful” achieved low median scores, whereas statements like “Would undergo colonoscopy even if out-of-pocket” achieved high median scores. Figure 5a and b demonstrate these details further, including not only the median and percentages of responses to these statements but also the distribution of answers obtained from the participants.
Figure 3.
Stack bars of answers to questions pertaining to the attitudes of participants toward (a) colorectal cancer and its screening and (b) colonoscopy
Figure 4.
Heat plot of answers to questions pertaining to the attitudes of participants toward (a) colorectal cancer and its screening and (b) colonoscopy
Figure 5.
Kernel plot distribution of answers to questions pertaining to the attitudes of participants toward (a) colorectal cancer and its screening and (b) colonoscopy
Appropriate age to initiate CRC screening
Most of those surveyed thought that screening for CRC should start at the age of 40–49 years (49.6%) followed by the age range of 30–39 years (22.3%), and 16.4% thought it should start from 50 to 59 years of age. A minority thought that it should start between 60 and 69 years of age (2.6%), and 9% thought it should start between 20 and 29 years of age.
Which screening test participants would choose?
After adequate explanation of the screening methods with figures as well as the advantages and disadvantages of each modality of screening, the majority of participants chose colonoscopy as the screening modality of choice (34.6%) followed by stool-based testing (30.83%) and CTC (24.52%), and the least chosen option was flexible sigmoidoscopy with only 10.05% of the participants opting for that.
Willingness to undergo CRC screening
The majority of the surveyed population was willing to undergo a screening test for CRC (73%), and the proportion increased to 80% if there was a family history of CRC. Colonoscopy was accepted as a screening test by most individuals (81%) and had a higher acceptance if the participant had to pay out of pocket (92%) as opposed to being free (56%).
Response to invitations to undergo screening for CRC
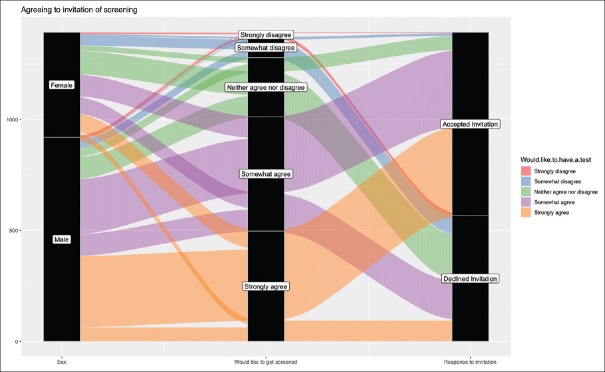
Interestingly, participants had a change of mind when they were invited to undergo CRC screening where people who strongly disagreed to undergo CRC screening accepted the invitation and others who had claimed that they strongly wanted to undergo screening declined [Figure 6].
Figure 6.
The respondents from Riyadh were invited to undergo screening for colorectal cancer and how they responded and how that correlated with how they answered in the questionnaire if they would like to get tested
Univariate analysis
Factors that were associated with accepting the invitation to undergo screening for CRC were being a male, employed in the private sector and those who were retired, and those who thought that CRC was common, could present without symptoms, was curable, and was preventable. It was interesting that those who wanted to know whether they had cancer also were more likely to accept the invitation to undergo CRC screening. Also, those who said that they would not undergo screening colonoscopy if it were offered for free and those who were neutral were more likely to accept the invitation. In addition, those who thought that it was not useful were also more likely to accept the invitation. Factors associated with an increased probability of declining the invitation for screening for CRC included being a student, those who claimed that they would like to know whether they had cancer, those who somewhat agreed to undergo a colonoscopy if it was free of charge, and those who chose a CTC or a stool-based test as a method of screening [Table 2].
Table 2.
Probability of declining a screening test for CRC on univariate modeling
| Variable | Univariate model | |
|---|---|---|
| Odds ratio | 95% CI | |
| Age | 0.99 | 0.97-1.00 |
| Sex (male) | 0.41* | 0.33-0.52* |
| Highest education level attained | ||
| Intermediate | 0.82 | 0.43-1.56 |
| High school | 1.05 | 0.60-1.83 |
| College | 0.65 | 0.37-1.12 |
| Postgraduate | 1.14 | 0.76-1.75 |
| Occupation | ||
| Military sector | 0.83 | 0.55-1.26 |
| Private sector | 0.68* | 0.46-0.98* |
| Retired | 0.70* | 0.51-0.94* |
| Self-employed | 0.54 | 0.24-1.11 |
| Housewife | 1.13 | 0.78-1.64 |
| Unemployed | 0.64 | 0.27-1.42 |
| Student | 3.15* | 1.47-7.32* |
| Monthly income (in Saudi Riyals) | ||
| 5000-9000 | 0.74 | 0.51-1.07 |
| 10,000-19,000 | 0.97 | 0.69-1.34 |
| 20,000-29,000 | 1.00 | 0.71-1.40 |
| 30,000-39,000 | 1.01 | 0.73-1.40 |
| >40,000 | 1.14 | 0.88-1.49 |
| Marital status | ||
| Married | 1.06 | 0.53-2.19 |
| Single | 1.86 | 0.85-4.21 |
| Widow | 3.63 | 0.97-15.75 |
| Heard of CRC | 0.81 | 0.56-1.17 |
| Think CRC is common | 0.70* | 0.56-0.87* |
| CRC can present | 0.78* | 0.62-0.97* |
| without symptoms | ||
| At what age should CRC screening start? | ||
| 30-39 years | 1.49 | 0.94-2.40 |
| 40-49 years | 1.19 | 0.78-1.86 |
| 50-59 years | 1.43 | 0.89-2.34 |
| 60-69 years | 1.99 | 0.87-4.63 |
| Think CRC is curable | 0.62* | 0.42-0.91* |
| Think CRC is preventable | 0.70* | 0.50-0.99* |
| Think CRC is fatal | 0.89 | 0.70-1.12 |
| If I had cancer I don’t want to know* | ||
| Somewhat disagree | 1.84* | 1.39-2.43* |
| Neither agree nor disagree | 0.79 | 0.61-1.04 |
| Somewhat agree | 1.06 | 0.79-1.41 |
| Strongly agree | 1.23 | 0.95-1.61 |
| Cancer scares me | ||
| Somewhat disagree | 1.14 | 0.83-1.57 |
| Neither agree nor disagree | 0.99 | 0.73-1.34 |
| Somewhat agree | 1.00 | 0.76-1.30 |
| Strongly agree | 1.17 | 0.90-1.51 |
| I would accept colonoscopy if it were for free* | ||
| Somewhat disagree | 0.22* | 0.15-0.34* |
| Neither agree nor disagree | 0.54* | 0.37-0.79* |
| Somewhat agree | 1.87* | 1.35-2.61* |
| Strongly agree | 0.86 | 0.67-1.11 |
| Screening is useful* | ||
| Somewhat disagree | 0.21* | 0.03-0.71* |
| Neither agree nor disagree | 1.94 | 0.70-9.58 |
| Somewhat agree | 0.53 | 0.19-1.21 |
| Strongly agree | 1.16 | 0.70-2.01 |
| Which screening test would you chose?* | ||
| CTC | 2.05* | 1.51-2.78* |
| Stool-based testing | 2.87* | 2.17-3.81* |
| Flexible sigmoidoscopy | 1.49 | 0.99-2.22 |
| Knowledge score* | 0.96* | 0.93-0.99* |
CRC: Colorectal cancer; CI: Confidence interval; CTC: Computed tomographic colonography. *Statistically significant
Multivariate analysis
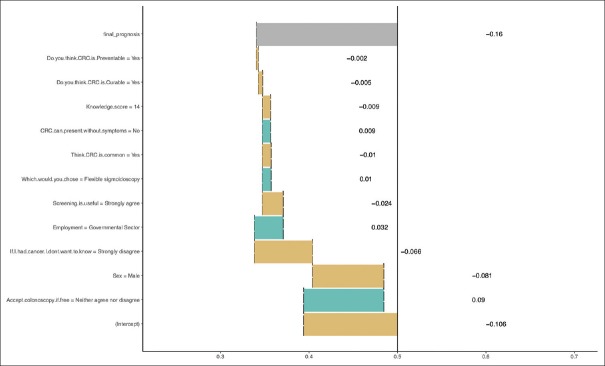
On multivariate analysis, being a male was the only factor that was associated with a higher probability of accepting screening for CRC. Factors associated with an increased probability of declining the invitation for screening for CRC included those who claimed that they would like to know whether they had cancer and those who somewhat agreed to undergo a colonoscopy if it was free of charge [Table 3]. The relative contribution of each variable in the multivariate model is shown in Figure 7.
Table 3.
Probability of declining a screening test for CRC on multivariate modeling
| Variable | Multivariate model | |
|---|---|---|
| Odds ratio | 95% CI | |
| Sex (male) | 0.53* | 0.38-0.73* |
| Employment | ||
| Military sector | 1.12 | 0.69-1.83 |
| Private sector | 0.77 | 0.50-1.19 |
| Retired | 0.93 | 0.65-1.32 |
| Self-employed | 0.80 | 0.31-1.90 |
| Housewife | 0.74 | 0.45-1.20 |
| Unemployed | 2.14 | 0.88-5.49 |
| Student | 0.50 | 0.18-1.27 |
| Think CRC is common | 1.03 | 0.79-1.35 |
| CRC can present without symptoms | 0.89 | 0.68-1.17 |
| Think CRC is curable | 0.77 | 0.45-1.31 |
| Think CRC is preventable | 0.84 | 0.55-1.28 |
| If I had cancer I don’t want to know* | ||
| Somewhat disagree | 1.78* | 1.29-2.46* |
| Neither agree nor disagree | 0.91 | 0.66-1.25 |
| Somewhat agree | 0.93 | 0.67-1.30 |
| Strongly agree | 1.14 | 0.83-1.55 |
| Accept colonoscopy if free* | ||
| Somewhat disagree | 0.89 | 0.51-1.55 |
| Neither agree nor disagree | 0.81 | 0.51-1.31 |
| Somewhat agree | 1.58* | 1.06-2.35* |
| Strongly agree | 0.84 | 0.62-1.14 |
| Screening is useful | ||
| Somewhat disagree | 0.36 | 0.05-1.54 |
| Neither agree nor disagree | 2.73 | 0.79-15.07 |
| Somewhat agree | 0.47 | 0.15-1.27 |
| Strongly agree | 1.50 | 0.81-2.86 |
| Which screening test would you chose? | ||
| CTC | 1.31 | 0.92-1.86 |
| Stool-based testing | 1.35 | 0.96-1.88 |
| Flexible sigmoidoscopy | 1.46 | 0.92-2.29 |
| Knowledge score | 0.98 | 0.94-1.02 |
CRC: Colorectal cancer; CI: Confidence interval; CTC: Computed tomographic colonography. *Statistically significant
Figure 7.
The relative contribution of each variable in the multivariate model
Subgroup analysis
A subgroup analysis that was limited to those between the ages of 45 years and below 75 years showed no difference in the results. We also did a second subgroup analysis excluding those who had undergone a screening test for CRC in the past and again there was no difference.
DISCUSSION
Saudi guidelines for CRC screening have been published[5] and have been used on an opportunistic basis. The implementation of a national program has not been undertaken yet because there are major infrastructure and organizational resources that have to be addressed.[33] In addition, the age-adjusted rate for neoplasia in general,[34] and CRC incidence specifically,[35] is low; the adenoma detection rates on colonoscopy[7] are relatively low when compared with other populations, and there are no formal cost-effectiveness studies on screening for the country. These challenges have also limited the rollout of such a program.
Even then, for such a screening program to be effective, the uptake of screening should be high as whatever health resources are allocated to the program, if the uptake in the targeted population is low, it would not be able to achieve the hoped benefit and return on investment. Uptake of CRC screening varies between the method that is implemented for screening, whether colonoscopy or a stool-based test, as well as between populations. For instance, the participation rate in a screening program in the Netherlands was reported to be 68.2% as opposed to some areas of Canada, which had a rate of 16%.[36] Compounding this matter is the multistage process of some of these screening tests; for example, although fecal immunochemical testing (FIT) has a high uptake when compared with endoscopic screening methods, a study from South Korea found that only 31.4% of those with a positive test underwent a colonoscopy,[37] thus it might not achieve the benefit of screening. This not only represents a lost opportunity for a potentially positive intervention but also a lost investment by governments with no impact.
The knowledge, behavior, and beliefs of the public are expected to predict the response to a national CRC screening program. A few attempts of identifying the obstacles and challenges in establishing a CRC screening program in Saudi Arabia have been made,[38] but most of these were limited to a number of cities,[11,15] while some targeted healthcare professionals[13] or undergraduate students in universities[12] which might not necessarily reflect the target population. It has been demonstrated that the targeted population's perceived severity and susceptibility to CRC and knowledge of guidelines increased the odds of screening intention,[39] thus it would be pertinent to examine acceptance of the public to undergo CRC screening and to explore potential barriers. Thus, the current survey is timely and much needed especially when considering initiation of a national program for CRC screening.
The HBM is widely used to assess the likelihood of engaging in health promotion behavior. In this model, it is thought that engaging in health promotion behavior is effected by perceived benefits when compared with barriers, perceived threats, self-efficacy, cues to action, and modifying variables that could modulate all the prior variables. In addition, the perceived threats are affected by the perceived susceptibility and seriousness of the issue at hand [Figure 1]. The questionnaire that was used in this study covers these aspects in addition to exploring the relationship between participants’ behaviour and their knowledge about CRC.
There are numerous challenges in obtaining a representative sample of the general population for such a survey given the absence of a defined sampling frame as well as the time-consuming and cost-prohibitive traditional household surveys. In addition, the wide geography of Saudi Arabia compounds such challenges, thus we opted to use mobile phone surveys. Mobile phone surveys include interactive voice responses, SMS, human operators, or computer-assisted telephone interviews. The multiple dissemination tools that were used to obtain this national survey aimed to increase the representation of the sample population and the diversity of the participants given the high penetration rate for mobile phones and smart phones in Saudi Arabia.
Although there are some concerns when using mobile phone surveys which include the possibility of obtaining imbalanced responses because of limited access of some segments of the population to mobile phones secondary to income discrepancies,[40] the penetrance of mobile telecommunications services in Saudi Arabia was 138.7%, while 77% of the population was using the Internet, and 88% had mobile broadband subscriptions in the third quarter of 2017.[41] Also, the penetrance of social media tools in Saudi Arabia is high.[42]
This study is by far the largest to address this question till date (5720 respondents) and has covered all administrative regions of the Kingdom and cuts through different social strata in terms of age, gender, education, vocation, and income. Although the percentage of males outweighed females, given the absolute number of participants, we believe that the representation of females in the survey was sufficient.
Knowledge and motivation on their own, despite their clear effect,[43,44] are the only facets in the continuum from health literacy to action, and numerous other factors, including those in the HBM, are important in the prediction of an individual undergoing health promotion behavior. This is clear by the finding that the mean knowledge score in our current survey was 11.05 (SD 4.4, range 1–23), which is similar to the one performed in Riyadh city in 2015[11] and the knowledge score was not associated with the expression of interest in CRC screening neither was it associated with the actual acceptance of the invitation to undergo CRC screening for those located in Riyadh. Interestingly, 15.24% of the respondents had already undergone screening for CRC with the most frequently used modality being colonoscopy (72.73%) that is higher than that reported earlier.[11]
Although the participants in the survey expressed interest in undergoing screening tests for CRC, when those in the Riyadh area were invited to undergo screening a proportion of those who had expressed interest declined the invitation [Figure 6]. Even on multivariate analysis, neither knowledge nor beliefs about CRC had a major influence on the acceptance of the invitation for CRC screening. Even in previous studies, despite a reported 70.7% willingness to undergo a screening test for CRC,[11] which indicates a positive attitude toward CRC screening,[11,12,45] the uptake of CRC screening was low in those surveyed (about 6%). Whether that is a result of limited access or a lack of a national program is yet to be clarified.
This gap between intention and actual behavior has been well-documented.[46] Numerous obstacles have been identified in the literature including limited access to physicians, the setting and organization of the screening program, access to the healthcare delivery system, lack of time for those intended to be screened, transportation, financial barriers, as well as fear from receiving unwanted results, and embarrassment or shame.[47,48] Other barriers that have been identified in qualitative studies include lack of trust in physicians, lack of symptoms, and absence or presence of a physicians’ recommendation to undergo screening. In addition, competing priorities, such as psychosocial stressors or comorbid medical illness, could be barriers to screening.[49]
Even from the side of healthcare providers, although primary healthcare providers had positive attitudes toward CRC screening, this did not translate into a better adherence to guidelines[13,50] which suggests that there is a need to bridge the gap between knowledge and implementation.
In a randomized trial from China, a multifaceted intervention that included an interaction with a health educator, bilingual materials (a video, a motivational pamphlet, an informational pamphlet, and FOBT instructions), and giving the intervention arm three stool-based test cards resulted in increase uptake of CRC cancer screening.[51] This study emphasizes the effect that cues to action and modifiers can play a role in increasing the uptake of CRC screening.
Motivational factors that predict attendance behavior for screening test have been studied and are related to the theory of planned behavior[52,53] and the theory of reasoned action.[54] In a meta-analysis that quantified how well the theories of reasoned action and planned behavior can predict intentions to attend screening programs and actual attendance behavior, attitudes appeared to be the best predictor of intentions to attend screening tests.[54] Thus, it might be more appropriate to disseminate positive attitudes toward screening rather than just focusing on knowledge enhancing messages. Also, studies have found that the responses to invitations to undergo screening vary based on the proposed screening test and the inviting authority. For example, invitations for CRC screening from a hospital were perceived as a “have to undergo” test as opposed to a checkup invitation from a general practitioner's office which was perceived as a “should do” test.[54]
Having implementation intentions in the form of specifying when, where, and how a person would make arrangements to undergo a screening test has been found to increase the probability of undergoing a screening test even when the motivation to undergo that test was equivalent between those who attended and those who did not.[46] This also might indicate that a simple invitation to undergo a screening test might not be sufficient on its own to increase the uptake of screening within the targeted population.[46]
Some of the limitations of this study are the use of social media as a medium of disseminating the survey. The use of social media as a source of information including validating patient-reported outcomes is an area of ongoing research and has been identified by numerous healthcare authorities as having a huge potential with the advantages of being asynchronous, not requiring face-to-face interviews, rapid, cheap, and able to be administered on a large scale.[55,56] All these factors were drivers for the methodology of this study. Nonetheless, there are limitations to the use of social media including whether there should be any adjustments in the analysis to account for the method of data generation;[55] also, the recruited subjects might not be representative of populations that would be recruited through a traditional clinic-based approach[57] or household surveys. Nonetheless, the use of digital interventions has been shown to increase uptake of CRC screening in randomized trials and such patient engagement through these low-cost platforms are gaining interest.[58] Another challenge that this study could not address was the willingness of participants to undergo a confirmatory test if initial screening was positive. This shall be addressed in a following study where those who accepted screening would be enrolled in a study using a stool-based screening test.
In conclusion, although a majority of Saudis expressed the will to undergo screening, a substantially lower number accepted the invitation to undergo screening. Interestingly, the gap between “Saying yes to screening” and “Doing it” is because of multiple factors other than knowledge. We believe that this represents an opportunity to borrow concepts from behavioral economics and possible “nudge” factors that might bridge the gap between “knowing” and “doing.”
Financial support and sponsorship
This project was supported by the National Cancer Center from the Saudi Health Council.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Fitzmaurice C, Allen C, Barber RM, Barregard L, Bhutta ZA, Brenner H, et al. Global Burden of Disease Cancer C. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015: A systematic analysis for the global burden of disease study. JAMA Oncol. 2017;3:524–48. doi: 10.1001/jamaoncol.2016.5688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rufibach K. reporttools: R Functions to generate LaTeX tables of descriptive statistics. J Statis Softw. 2009;31:1–7. [Google Scholar]
- 3.Alsanea N, Abduljabbar AS, Alhomoud S, Ashari LH, Hibbert D, Bazarbashi S. Colorectal cancer in Saudi Arabia: Incidence, survival, demographics and implications for national policies. Ann Saudi Med. 2015;35:196–202. doi: 10.5144/0256-4947.2015.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Al-Ahwal MS, Shafik YH, Al-Ahwal HM. First national survival data for colorectal cancer among Saudis between 1994 and 2004: What's next? BMC Public Health. 2013;13:73. doi: 10.1186/1471-2458-13-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alsanea N, Almadi MA, Abduljabbar AS, Alhomoud S, Alshaban TA, Alsuhaibani A. National Guidelines for Colorectal Cancer Screening in Saudi Arabia with strength of recommendations and quality of evidence. Ann Saudi Med. 2015;35:189–95. doi: 10.5144/0256-4947.2015.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benard F, Barkun AN, Martel M, von Renteln D. Systematic review of colorectal cancer screening guidelines for average-risk adults: Summarizing the current global recommendations. World J Gastroenterol. 2018;24:124–38. doi: 10.3748/wjg.v24.i1.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Almadi MA, Alharbi O, Azzam N, Wadera J, Sadaf N, Aljebreen AM. Prevalence and characteristics of colonic polyps and adenomas in 2654 colonoscopies in Saudi Arabia. Saudi J Gastroenterol. 2014;20:154–61. doi: 10.4103/1319-3767.132986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Almadi MA, Barkun AN. Initial guidelines for colorectal cancer screening in Saudi Arabia: A beginning. Ann Saudi Med. 2015;35:341–2. doi: 10.5144/0256-4947.2015.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sung JJ, Choi SY, Chan FK, Ching JY, Lau JT, Griffiths S. Obstacles to colorectal cancer screening in Chinese: A study based on the health belief model. Am J Gastroenterol. 2008;103:974–81. doi: 10.1111/j.1572-0241.2007.01649.x. [DOI] [PubMed] [Google Scholar]
- 10.Kiviniemi MT, Bennett A, Zaiter M, Marshall JR. Individual-level factors in colorectal cancer screening: A review of the literature on the relation of individual-level health behavior constructs and screening behavior. Psychooncology. 2011;20:1023–33. doi: 10.1002/pon.1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Almadi MA, Mosli MH, Bohlega MS, Al Essa MA, AlDohan MS, Alabdallatif TA, et al. Effect of public knowledge, attitudes, and behavior on willingness to undergo colorectal cancer screening using the health belief model. Saudi J Gastroenterol. 2015;21:71–7. doi: 10.4103/1319-3767.153814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Imran M, Sayedalamin Z, Alsulami SS, Atta M, Baig M. Knowledge and Awareness of colorectal cancer among undergraduate students at King Abdulaziz University, Jeddah, Saudi Arabia: A survey-based study. Asian Pac J Cancer Prev. 2016;17:2479–83. [PubMed] [Google Scholar]
- 13.Mosli M, Alnahdi Y, Alghamdi A, Baabdullah M, Hadadi A, Khateery K, et al. Knowledge, attitude, and practices of primary health care physicians toward colorectal cancer screening. Saudi J Gastroenterol. 2017;23:330–6. doi: 10.4103/sjg.SJG_1_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.The output for this paper was generated using Qualtrics software, Version [research CORE] of Qualtrics. Copyright ©[2018] Qualtrics. Qualtrics and all other Qualtrics product or service names are registered trademarks or trademarks of Qualtrics, Provo, UT, USA. Available from: https://www.qualtrics.com.
- 15.Zubaidi AM, AlSubaie NM, AlHumaid AA, Shaik SA, AlKhayal KA, AlObeed OA. Public awareness of colorectal cancer in Saudi Arabia: A survey of 1070 participants in Riyadh. Saudi J Gastroenterol. 2015;21:78–83. doi: 10.4103/1319-3767.153819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.RStudio Team. RStudio: Integrated Development for R. Boston, MA: RStudio, Inc; 2016. [Google Scholar]
- 17.Vienna, Austria: R Foundation for Statistical Computing; 2017. R Core Team. R: A language and environment for statistical computing. [Google Scholar]
- 18.Wickham H. New York: Springer-Verlag; 2016. ggplot2: Elegant Graphics for Data Analysis. [Google Scholar]
- 19.Wickham H, François R, Henry L, Müller K. dplyr: A Grammar of Data Manipulation. R package version 0.7.6. 2018. Available from: https://CRAN.R-project.org/package=dplyr .
- 20.Wickham H. lazyeval: Lazy (Non-Standard) Evaluation. R package version 0.2.1. 2017. Available from: https://CRAN.R-project.org/ package=lazyeval .
- 21.Dominic Comtois. summarytools: Tools to Quickly and Neatly Summarize Data. R package version 0.8.7. 2018. Available from: https://CRAN.R-project.org/package=summarytools .
- 22.Casas P. funModeling: Exploratory Data Analysis and Data Preparation Tool-Box Book. R package version 1.6.8. 2018. Available from: https:// CRAN.R-project.org/package=funModeling .
- 23.Harrell FE., Jr with contributions from Charles Dupont and many others. Hmisc: Harrell Miscellaneous. R package version 4.1-1. 2018. Available from: https://CRAN.R-project.org/package=Hmisc .
- 24.Kassambara A. ggpubr: “ggplot2” Based Publication Ready Plots. R package version 0.1.7. 2018. Available from: https:// CRAN.R-project.org/package=ggpubr .
- 25.Plotly Technologies Inc. Collaborative Data Science Montréal, QC. 2015. Available from: https://plot.ly .
- 26.Carstensen B, Plummer M, Laara E, Hills M. Epi: A Package for Statistical Analysis in Epidemiology. R package version 2.30. 2018. Available from: https://CRAN.R-project.org/package=Epi .
- 27.Wilke CO. ggridges: Ridgeline Plots in “ggplot2.” R package version 0.5.0. 2018. Available from: https://CRAN.R-project.org/ package=ggridges .
- 28.Wickham H. The Split-Apply-Combine Strategy for Data Analysis. J Stat Softw. 2011;40:1–29. [Google Scholar]
- 29.Wickham H. scales: Scale Functions for Visualization. R package version 1.0.0. 2018. Available from: https://CRAN.R-project.org/ package=scales .
- 30.Revelle W. psych: Procedures for Personality and Psychological Research. Evanston, IL, USA: Northwestern University; 2018. Available from: https://CRAN.R-project.org/package=psych Version=1.8.4 . [Google Scholar]
- 31.Bryer J, Speerschneider K. likert: Analysis and Visualization Likert Items. R package version 1.3.5. 2016. Available from: https:// CRAN.R-project.org/package=likert .
- 32.Patil I. ggstatsplot: “ggplot2” Based Plots with Statistical Details. R package version 0.0.5. 2018. Available from: https://CRAN.R-project. org/package=ggstatsplot .
- 33.Aziz MA, Allah-Bakhsh H. Colorectal cancer: A looming threat, opportunities, and challenges for the Saudi population and its healthcare system. Saudi J Gastroenterol. 2018;24:196–7. doi: 10.4103/sjg.SJG_164_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rahim HF, Sibai A, Khader Y, Hwalla N, Fadhil I, Alsiyabi H, et al. Non-communicable diseases in the Arab world. Lancet. 2014;383:356–67. doi: 10.1016/S0140-6736(13)62383-1. [DOI] [PubMed] [Google Scholar]
- 35.Institute for Health Metrics and Evaluation (IHME). GBD Compare Data Visualization. Seattle, WA: IHME, University of Washington; 2017. [Last accessed on 2018 Jun 17]. Available from: http://vizhub.healthdata.org/ gbd-compare . [Google Scholar]
- 36.Navarro M, Nicolas A, Ferrandez A, Lanas A. Colorectal cancer population screening programs worldwide in 2016: An update. World J Gastroenterol. 2017;23:3632–42. doi: 10.3748/wjg.v23.i20.3632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Choi KS, Lee HY, Jun JK, Shin A, Park EC. Adherence to follow-up after a positive fecal occult blood test in an organized colorectal cancer screening program in Korea, 2004-2008. J Gastroenterol Hepatol. 2012;27:1070–7. doi: 10.1111/j.1440-1746.2011.06944.x. [DOI] [PubMed] [Google Scholar]
- 38.Aljumah AA, Aljebreen AM. Policy of screening for colorectal cancer in Saudi Arabia: A prospective analysis. Saudi J Gastroenterol. 2017;23:161–8. doi: 10.4103/sjg.SJG_468_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tsoh JY, Tong EK, Sy AU, Stewart SL, Gildengorin GL, Nguyen TT. Knowledge of colorectal cancer screening guidelines and intention to obtain screening among nonadherent Filipino, Hmong, and Korean Americans. Cancer. 2018;124(Suppl 7):1560–7. doi: 10.1002/cncr.31097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gibson DG, Pereira A, Farrenkopf BA, Labrique AB, Pariyo GW, Hyder AA. Mobile phone surveys for collecting population-level estimates in low- and middle-income countries: A literature review. J Med Internet Res. 2017;19:e139. doi: 10.2196/jmir.7428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kingdom of Saudi Arabia information and communication technologies (ICT) indicators, Communication and Information Technology Commission in Saudi Arabia, 2017. [Last accessed on 2018 Jun 17]. Available from: http://www.citc.gov.sa/en/Reportsandstudies/Indicators/Pages/ CITCICTIndicators.aspx .
- 42.Penetration of leading social networks in Saudi Arabia as of 3rd quarter 2017, Statista. 2018. [Last accessed on 2018 Jun 17]. Available from https://www.statista.com/ statistics/284451/saudi-arabia-social-network-penetration/
- 43.Berkowitz Z, Hawkins NA, Peipins LA, White MC, Nadel MR. Beliefs, risk perceptions, and gaps in knowledge as barriers to colorectal cancer screening in older adults. J Am Geriatr Soc. 2008;56:307–14. doi: 10.1111/j.1532-5415.2007.01547.x. [DOI] [PubMed] [Google Scholar]
- 44.von Wagner C, Steptoe A, Wolf MS, Wardle J. Health literacy and health actions: A review and a framework from health psychology. Health Educ Behav. 2009;36:860–77. doi: 10.1177/1090198108322819. [DOI] [PubMed] [Google Scholar]
- 45.Alsanea NA. The acceptability of screening for colorectal cancer in Saudi Arabia: Myths busted. Saudi J Gastroenterol. 2015;21:59. doi: 10.4103/1319-3767.153806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sheeran P, Orbell S. Using implementation intentions to increase attendance for cervical cancer screening. Health Psychol. 2000;19:283–9. doi: 10.1037//0278-6133.19.3.283. [DOI] [PubMed] [Google Scholar]
- 47.Ma GX, Wang MQ, Toubbeh J, Tan Y, Shive S, Wu D. Factors associated with colorectal cancer screening among Cambodians, Vietnamese, Koreans and Chinese living in the United States. N Am J Med Sci. 2012;5:1–8. doi: 10.7156/v5i1p001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Senore C, Malila N, Minozzi S, Armaroli P. How to enhance physician and public acceptance and utilisation of colon cancer screening recommendations. Best Pract Res Clin Gastroenterol. 2010;24:509–20. doi: 10.1016/j.bpg.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 49.Lasser KE, Ayanian JZ, Fletcher RH, Good MJ. Barriers to colorectal cancer screening in community health centers: A qualitative study. BMC Family Pract. 2008;9:15. doi: 10.1186/1471-2296-9-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Demyati E. Knowledge, attitude, practice, and perceived barriers of colorectal cancer screening among family physicians in National Guard Health Affairs, Riyadh. Int J Family Med. 2014;2014:457354. doi: 10.1155/2014/457354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tu SP, Taylor V, Yasui Y, Chun A, Yip MP, Acorda E, et al. Promoting culturally appropriate colorectal cancer screening through a health educator: A randomized controlled trial. Cancer. 2006;107:959–66. doi: 10.1002/cncr.22091. [DOI] [PubMed] [Google Scholar]
- 52.Sheeran P, Conner M, Norman P. Can the theory of planned behavior explain patterns of health behavior change? Health Psychol. 2001;20:12–9. doi: 10.1037//0278-6133.20.1.12. [DOI] [PubMed] [Google Scholar]
- 53.Godin G, Kok G. The theory of planned behavior: A review of its applications to health-related behaviors. Am J Health Promot. 1996;11:87–98. doi: 10.4278/0890-1171-11.2.87. [DOI] [PubMed] [Google Scholar]
- 54.Cooke R, French DP. How well do the theory of reasoned action and theory of planned behaviour predict intentions and attendance at screening programmes? A meta-analysis. Psychol Health. 2008;23:745–65. doi: 10.1080/08870440701544437. [DOI] [PubMed] [Google Scholar]
- 55.Rothman M, Gnanaskathy A, Wicks P, Papadopoulos EJ. Can we use social media to support content validity of patient-reported outcome instruments in medical product development? Value Health. 2015;18:1–4. doi: 10.1016/j.jval.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 56.Tonia T. Social media in public health: Is it used and is it useful? Int J Public Health. 2014;59:889–91. doi: 10.1007/s00038-014-0615-1. [DOI] [PubMed] [Google Scholar]
- 57.Admon L, Haefner JK, Kolenic GE, Chang T, Davis MM, Moniz MH. Recruiting pregnant patients for survey research: A head to head comparison of social media-based versus clinic-based approaches. J Med Internet Res. 2016;18:e326. doi: 10.2196/jmir.6593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Miller DP, Jr, Denizard-Thompson N, Weaver KE, Case LD, Troyer JL, Spangler JG, et al. Effect of a digital health intervention on receipt of colorectal cancer screening in vulnerable patients: A randomized controlled trial. Ann Intern Med. 2018;168:550–7. doi: 10.7326/M17-2315. [DOI] [PMC free article] [PubMed] [Google Scholar]