Abstract
Purpose
The treatment decisions of melanoma patients are poorly understood. Most research on cancer patient decision-making focuses on limited components of specific treatment decisions. This study aimed to holistically characterize late-stage melanoma patients’ approaches to treatment decision-making in order to advance understanding of patient influences and supports.
Methods
(1) Exploratory analysis of longitudinal qualitative data to identify themes that characterize patient decision-making. (2) Pattern analysis of decision-making themes using an innovative method for visualizing qualitative data: a hierarchically-clustered heatmap. Participants were 13 advanced melanoma patients at a large academic medical center.
Results
Exploratory analysis revealed eight themes. Heatmap analysis indicated two broad types of patient decision-makers. “Reliant outsiders” relied on providers for medical information, demonstrated low involvement in decision-making, showed a low or later-in-care interest in clinical trials, and expressed altruistic motives. “Active insiders” accessed substantial medical information and expertise in their networks, consulted with other doctors, showed early and substantial interest in trials, demonstrated high involvement in decision-making, and employed multiple decision-making strategies.
Conclusion
We identified and characterized two distinct approaches to decision-making among patients with late-stage melanoma. These differences spanned a wide range of factors (e.g., behaviors, resources, motivations). Enhanced understanding of patients as decision-makers and the factors that shape their decision-making may help providers to better support patient understanding, improve patient-provider communication, and support shared decision-making.
Keywords: Decision making, Ethnography, Ethnoarray approach, Melanoma
Introduction
Melanoma is currently the fifth most common cancer in the USA and its incidence is steadily rising [1]. Approximately 2.2% of individuals in the USA will be diagnosed with the disease during their lifetime [1]. Melanoma is more common among men than among women (29.2% vs. 17.3% of new cases), and among white and non-Hispanic men (34.4% and 33.1%) and women (20.9% and 19.9%) than among other groups (≤ 5% of new cases) [1, 2]. Metastatic melanoma is particularly deadly, with only 20% patients surviving at least 5 years post-diagnosis [1]. In recent years, however, the prognosis of patients with advanced melanoma has improved with the development of novel immunotherapies and targeted therapies [3–5]. These complement longer-standing treatments such as systemic chemotherapy, palliative surgery, or palliative radiation [3, 4].
Like all late-stage cancer patients, late-stage melanoma patients face difficult decisions in the course of their medical care. These decisions may be difficult technically (e.g., understanding different therapies), emotionally (e.g., deciding when to curtail curative treatment), and ethically (e.g., deciding whether to join a clinical trial). In recent years, decision-making has grown even more complex. Novel immunotherapies and targeted agents have greatly expanded the number of treatment and experimental options available for late-stage melanoma patients [6], each of which has its unique mix of potential therapeutic benefits, side effects, and costs.
Relatively little is known about decision-making among melanoma patients, particularly at later stages. Some studies have examined patients’ preferences regarding treatment options, or their preferred or experienced level of involvement in treatment decision-making [7–9]. These investigations have focused on, for example, German melanoma patients’ preferred role in treatment decisions [8], and US melanoma patients’ preferences regarding adjuvant treatments [9]. We found no studies of decision-making among melanoma patients with advanced disease, though some studies examine related issues such as coping [10].
Far more is known about influences on and levels of involvement in decision-making among cancer patients with breast or prostate cancer, or earlier-stage cancer patients more broadly [11–16]. However, even in this literature, most research focuses on a single influence on, or a narrow aspect of, cancer patients’ decision-making. These include the amount of control or involvement the patient wants in decision-making [8, 13, 17, 18], the patient-provider relationship [19–21], assessments of quantity versus quality of life [7, 9], or decisional satisfaction or regret [22, 23].
While many studies examine one or more decision-making domains, our review found few that sought to characterize this complex process holistically—examining how decision-making reflected the simultaneous interplay of a variety of factors such as patient-provider interactions, patients’ information-seeking behaviors, logistics, and patient preferences [14, 15, 24–26]. Studies of cancer screening among diverse patient populations illustrate that reductionist frameworks for analyzing decision-making may limit our ability to detect the broader holistic frameworks within which patients make specific treatment decisions [27–29]. Given the expanding treatment options for melanoma patients and calls for research that can support patient-centered care and shared decision-making [11, 30–32], a holistic research approach that captures the broad frameworks that patients bring to the decision-making process may provide novel insights.
This study aims to characterize late-stage melanoma patients holistically as treatment decision-makers. To do this, we draw on prospectively-collected ethnographic data and use two analytic approaches that support complex multifactorial inquiries. These analyses are centered on the person, and the broader relational and informational context in which they make decisions, rather than on any single decision event or process [30, 33].
Methods
This paper draws on the Cancer Patient Deliberation Study, a multi-site ethnographic study of decision-making among late-stage cancer patients. We overview the study’s methods here. Additional details of the study design and procedures are available elsewhere [34–37].
Data
We use data from late-stage melanoma patients who participated in the Cancer Patient Deliberation Study at a West Coast academic medical center. The study recruited and prospectively followed patients with metastatic disease as they exhausted standard therapies. Trained fieldworkers observed patient-oncologist clinical encounters, conducted in-depth semi-structured interviews with patients and their caregivers about treatment and clinical trial decision-making, and collected structured demographic data from each patient. All interviews were digitally recorded and professionally transcribed. Fieldworkers sought follow-up observations and interviews with patients after 6 and 12 months. Many patients had incomplete follow-up due to advancing disease or death. Informed consent was obtained from all individual participants included in the study.
As data collection proceeded, we developed a codebook that reflected theoretically-important concepts and behaviors from the literature as well as themes and concepts that emerged during data collection. We entered all observational and interview data into ATLAS.ti qualitative data analysis software (ATLAS.ti version 7.5, Berlin, Germany). We trained a team of three coders to code all of the qualitative data in ATLAS.ti and achieved inter-coder reliability kappa scores above 0.80, which indicates high agreement [38].
In this analysis, we use data from the 13 melanoma patients in the study. They participated in 1 to 3 clinic observations (n = 26) and 1 to 3 interviews (n = 29) between Fall 2011 and Fall 2013. The in-depth semi-structured patient interviews typically lasted 60–90 min and occurred at a location of the patient’s preference (e.g., their home, a private office at the cancer clinic, or the clinic’s infusion center). All interviews addressed a variety of topics that the literature and investigators’ preliminary work indicated were relevant to treatment decision-making, including the participant’s: cancer diagnosis, treatment history, and treatment experiences; understanding and evaluation of treatment options; decisions and motivations related to their cancer care; social support; information-seeking; spirituality; and others’ involvement in decision-making. Second and third interviews sought to capture updates on these same topics. As the study was designed to allow for the naturalistic investigation of patient knowledge of and decision-making about early-phase trials, field workers asked about clinical trials only after the participant raised the topic themselves.
Analyses
Inductive qualitative analysis
For the inductive qualitative analysis, we reviewed patients’ case summaries and coded data from ATLAS.ti. We focused on observational data on treatment discussion in clinics and on the patient’s interview data about treatment decisions, information-seeking activities, preferences and priorities, and interactions with providers. We employed a constant comparative approach to iteratively identify and characterize analytic themes and patient narratives in the data [39, 40].
Heatmap analysis
To investigate patterns of themes within the ethnographic data, we used an innovative heatmapping procedure—called an “ethnoarray”—that we developed previously during the Cancer Patient Deliberation Study [34, 35]. It provides a platform on which to visually analyze patterns both within and across cases in the sample. Heatmaps have long been deployed as an effective visual tool in the biological and social sciences. The ethnoarray approach employed here is unique in that it combines the systematic quantification and color-coding of multi-level ethnographic data with the use of primarily inductive statistical techniques [35, 41].
An ethnoarray heatmap is a column-by-row grid in which each individual patient case is represented as a column, and in which rows represent the qualitative data that has been thematically coded in ATLAS.ti. The cells of the grid are shaded to capture the presence/absence of a particular coded theme in a given patient’s data. The ethnoarray facilitates identification of patterns in complex ethnographic data in two ways. First, analysts can visually inspect the heatmap to examine how themes or patient experiences cluster together. Second, patterns within the heatmap can be identified using computational techniques found in inductive statistical approaches and data science. The ethnoarray methodology has been described in detail elsewhere [34, 35].
We constructed an ethnoarray that included 13 columns (one for each melanoma patient) and 19 rows (one for each ATLAS.ti code that captured a theme or subtheme identified in inductive analysis), totaling 104 cells. Using the R statistical platform, we applied complete-link hierarchical clustering in order to group patients based on observed similarities in the data [35]. We held the ordering of the rows constant so that related themes remained grouped together. Using this clustered ethnoarray, we examined which qualitative themes were salient for which patients and noted the extent to which patients clustered into distinct groups.
Finally, we checked the clustering results against the narrative data and drew on both sources iteratively to characterize the resultant groups. We used self-reported demographic data to further describe them.
Results
Sample characteristics
The sample consisted of 13 melanoma patients with metastatic disease, evenly split with respect to gender (Table 1). All patients self-identified as White, non-Hispanic; one patient indicated he was also American Indian. Most patients had less than a college degree (8 of the 13) and age ranged from 33 to 72 years, with a mean of 59.
Table 1.
Characteristics of study participants
| Age range (years) | Gender | Education | Race and ethnicity | Relationship status | Annual household income ($) | |
|---|---|---|---|---|---|---|
| P1 | 50–60 | M | Some college | Non-Hispanic White | Divorced/separated | 60–80k |
| P2 | 60–70 | M | H.S. degree | Non-Hispanic White and American Indian | Married/partnered | 60–80k |
| P3 | 40–50 | F | Some college | Non-Hispanic White | Married/partnered | 40–60k |
| P4 | 60–70 | F | Adv. degree | Non-Hispanic White | Married/partnered | 80–100k |
| P5 | 60–70 | M | Assoc. degree | Non-Hispanic White | Married/partnered | 40–60k |
| P6 | 70–80 | M | Adv. degree | Non-Hispanic White | Married/partnered | 100k+ |
| P7 | 50–60 | M | Assoc. degree | Non-Hispanic White | Married/partnered | [missing] |
| P8 | 30–40 | F | H.S. degree | Non-Hispanic White | Divorced/separated | 40–60k |
| P9 | 70–80 | F | Assoc. degree | White [ethnicity missing] | Divorced/separated | 100k+ |
| P10 | 70–80 | F | H.S. degree | Non-Hispanic White | Married/partnered | [missing] |
| P11 | 50–60 | F | H.S. degree | Non-Hispanic White | Married/partnered | 40–60k |
| P12 | 60–70 | M | Adv. degree | Non-Hispanic White | Married/partnered | 100k+ |
| P13 | 40–50 | M | Some college | Non-Hispanic White | Married/partnered | 60–80k |
F female, M male, Adv. advanced, Assoc. associates, H.S. high school
Inductive analysis: exploring themes in decision-making
Our analysis identified eight broad themes that were salient in patients’ decision-making processes (see Table 2). Some of these were similar across patients in the sample, such as conducting personal research about the disease, but most varied considerably. We describe the themes here and present examples from the data in Table 2.
Table 2.
Thematic definitions and examples
| Definition | Example | |
|---|---|---|
| 1. Involvement in treatment discussions and decisions | ||
| High involvement* | In patient-provider discussions leading up to treatment decisions and in treatment decision-making itself, the patient (P) performed an evaluating or collaborating role. Evaluating Ps regularly evaluated, even scrutinized, oncologist (O) recommendations or decisions; P had own interpretations of opinions, symptoms, and recommendations. Collaborator Ps provided regular and substantive (informational or opinion) input into the patient-provider considerations of treatments and other medical decisions. | P4 (F, 60s, adv.) readily provided her own analysis of symptoms and side effects in discussions with her provider. |
| P3 (F, 40s, some college) says understanding why her O recommended a treatment is a “huge part” of her wellbeing “I want to know that what I’m doing is really the best opinion for me... Is there something else out there?” | ||
| Low involvement | In patient-provider discussions leading up to treatment decisions and in treatment decision-making itself, the P demonstrated or described a limited role, e.g., no involvement; approving the provider’s recommendation; or only occasionally contributing information or opinion. | “It was always our decision but I would always ask him, you know, which way does he want to go. ‘Cause I do not see how you ask a patient which direction you want to go, you know… It makes no sense… So we pretty much followed his lead” (P7, M, 50s, Assoc.). |
| “[Providers] decided to put me on this trial” (P10, F, 70s, H.S.). | ||
| 2. Intensive information-seeking | ||
| Multiple consults* | P reported actively researching and evaluating different Os for their care. | “I took a trip to see Dr. [x] at, what is it, is it [institute]... And I also went to see another melanoma physician, and I’m drawing a blank now on his name, in [x] institution” (P6, M, 70s, adv.). |
| Second opinion* | P reported getting a second opinion from another medical provider about their disease and/or treatments. | “It wasn’t like I was going to [other medical center] to find the magic bullet. It was my way of sort of getting away from out here, getting ...someone else to look at it and step back and say, ‘Okay, this is what we are going to do’” (P1, M, 50s, some college). |
| 3. Access to medical knowledge | ||
| Patient* | P had substantial experience, formal training, or substantial informal training in medicine, health or science. | P9 (F, 70s, Assoc.) worked as specialized support staff in an oncology ward. |
| P3 (F, 40s, some college) worked for years for an oncologist. | ||
| P6 (70s, M, adv.) had formal training in biological sciences and had been an executive for a pharmaceutical company. | ||
| Network* | P had substantial access to medical expertise or advice through a very close contact (e.g., family member) and/or a contact that was accessed regularly, and/or multiple contacts. | P11’s (F, 50s, H.S.) son-in-law is an aspiring medical researcher and her daughter is a nurse. Both frequently provided information, advice, and research support. |
| 4. Interest in clinical trials | ||
| Sought out trial(s)* | P talked about or described being interested in cancer clinical trials early in their care (e.g., brought it up to O, researched available trials). There is evidence that O was not the first to alert or attract P to clinical trials. | P4 (F, 60s, adv.) came to [cancer center] with the hope of participating on a phase II clinical trial, and described herself as “roaring down” from her out-of-region home to determine if she was eligible. |
| Considered starting trial(s)* | P talked about plans to start, or interest in starting, a specific clinical trial. | “Yeah, [other clinic] is doing a clinical trial that I would be open to doing... they are getting some good results… [O] is finding out if there were spots up there for me” (P8, F, 30s, H.S.). |
| 5. Optimism | ||
| Positive outlook* | P indicated optimism or positivity in their general outlook toward cancer, treatment, or illness. | “I am just a very lucky person. I have been so sick. I don’t know how I have survived” (P2, M, 60s, H.S.). |
| “I know how bad my disease is. But I will be perfectly honest. I feel good. I do not have this sense of like an impending doom. I just know that it’s going to have a good outcome” (P3, F, 40s, some college). | ||
| Hope in decision* | P indicated hope or optimism about a decision. | “We are doing this because we hope it will help...it’s better than not getting anything” (P13, M, 40s, some college). |
| 6. Relationship with oncologist | ||
| Very positive* | P explicitly described liking, respecting and/or trusting their O. | “I think he is a very good doctor. I think he really cares about me… And he’s got a good sense of humor, which is nice, and I like him, I respect him” (P8, F, 30s, H.S.). |
| Good communication* | P described O as communicating well with them, having good communication skills in general, or P and O understanding each other. | From observational fieldnotes: P6 (M, 70s, adv.) “felt that [O] was always open to questions and that he felt there was always plenty of time to ask and receive answers to whatever he wanted.” |
| 7. Decision strategies | ||
| Partner with doctor* | P reported or demonstrated that they made a decision in partnership with their physician. | “Dr. [x] always offered opinions and then we would decide together which way we would go” (P7, M, 50s, Assoc.). |
| Partner with family* | P reported or demonstrated that they made a decision in partnership with one or more family members. | “We make all decisions together and in consultation with our son, who’s a doctor also” (P12, M, 60s, adv.). |
| Intuitive* | P described or demonstrated using an emotional or intuitive style, e.g., “feeling” or “just knowing” something is the right thing to do, or “knowing” the right course of action. | In choosing [cancer center]: “I do do [research] on the Internet but I did make some phone calls and you know, just, I don’t know. I just felt like this was where I needed to go” (P3, F, 40s, some college). |
| Rational* | P described or demonstrated using an instrumentally rational style, e.g., weighing pros and cons, drawing on facts to decide. | “I looked at [this drug] really hard and I decided that it was not a drug that I would ever want to try. I do not like the autoimmune side effects that it has and the successes that they are having are just not worth, it’s just not worth it” (P8, F, 30s, H.S.). |
| Had no choice* | P described or demonstrated not having any choices for a given decision. | On whether “scary” risks of a clinical trial changed P’s thinking about whether or not he would do it: “No... Well, because I do not have any other alternative” (P10, F, 70s, H.S.). |
| 8. Motives | ||
| Longer life/survival* | P indicated staying alive, prolonging life, or getting rid of cancer was a key motive in decision-making. | This [‘scary’ treatment] is just what you do because you want to live (P8, F, 30s, H.S.). |
| “I definitely want to live for another year or 2...” (P2, M, 60s, H.S.). | ||
| Quality of life* | P indicated improving or maintaining quality of life was a key motive in treatment/trial decision-making. | On deciding against brain radiation: “It makes you have no memory... it kind of crispy critters your brain….I like to know who I am and who everybody around me is, and if I do not have that, what are you saving?” (P2, M, 60s, H.S.). |
| Altruism* | P indicated helping improve society or the lives of others as a key motive in decision-making. | “Just wanting to know that someday what I did made a difference...whether it’s my children or grandchildren or some girl in Haiti that I do not even know.... that instantly they would know that there was something that could be done and they do not have to become a statistic... That was definitely part of my thinking when I was considering a clinical trial...” (P3, F, 40s, some college). |
P patient, O oncologist(s) involved in P’s care, F female, M male, Adv. advanced degree, Assoc. associate’s degree, H.S. high school degree
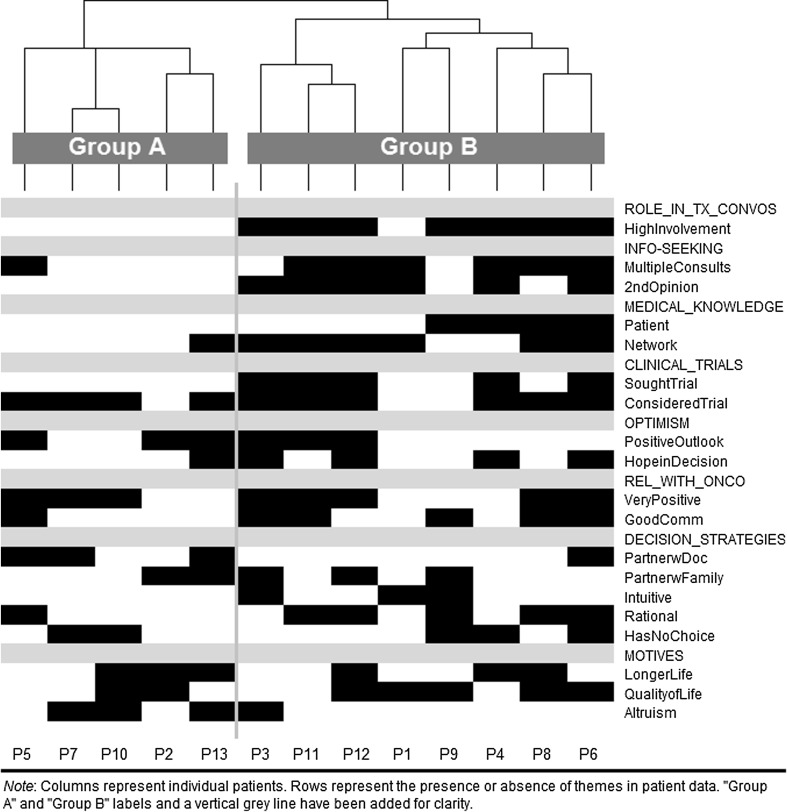
*These themes appear as rows in the ethnoarray (Fig. 1)
Involvement in treatment discussions and decisions
Many patients in the sample demonstrated high levels of involvement in discussions and decisions about their treatment. For example, some regularly evaluated, even scrutinized, their provider’s recommendations. Other patients interacted collaboratively with their provider, contributing substantive information and ideas in discussions of treatment options. Other patients, however, demonstrated non-involvement or very limited involvement in treatment decisions. Most of these patients described their decisions about treatment or clinical trials as led by their provider. When they described having a role in decision-making, it was typically to endorse something their doctor suggested.
Intensive information seeking
All patients in the sample reported that they or their caregiver looked up information about their disease or treatments, mostly via online research from home. A subset of patients also made particularly intensive efforts to gather information about their care options, including consulting with multiple oncologists, seeking a second opinion about a specific treatment, or both. These “high burden” activities provided information and sometimes gave patients a different perspective on their disease or treatments.
Access to medical knowledge
Patients varied substantially regarding their access to medical or scientific expertise. The information that some patients brought to clinic discussions reflected their own medical background, e.g., as someone who had provided physical therapy to oncology patients. Other patients accessed medical information from their social network, such as a family member or former physician-employer. Other patients, however, lacked medical expertise themselves and in their personal networks, and primarily relied on their providers for medical information and counsel.
Interest in clinical trials
Several patients were interested in clinical trials, and many came to an academic center with experimental therapy in mind. Many described seeking out or favoring an academic center because of its reputation as a research facility on “the cutting edge” of cancer care. Other patients expressed little interest in trials at the debut of their care. Many of these patients nevertheless became interested clinical trials in the course of their treatment.
Optimism
Some patients emphasized a feeling of optimism about an available treatment option or treatment decision. Some described optimism or hopefulness about their general outlook or their disease trajectory more broadly. Still other patients indicated they made one or more specific treatment decisions based on their feeling hopeful about it. This was particularly true of those who elected to participate in a clinical trial.
Relationship with oncologist
All patients who mentioned their relationship with their oncologist indicated they were satisfied with the care their oncologist provided. A subset of patients described their oncologist in particularly positive terms, often related to trust, respect or affection. Some individuals also reported having a particularly good communication dynamic with their oncologist, for example citing the doctor’s clear explanations or commitment to discussing all of the patient’s questions.
Decision strategies
We identified four strategies, used alone or in combination, that patients employed for making individual treatment decisions. Several patients partnered with others, such as their family caregiver or their oncologist, to make decisions. Some patients used an intuitive strategy, basing their decisions on what “felt right” and “following their gut.” Others used an instrumentally rational strategy, weighing pros and cons and drawing on facts to decide between different options. Finally, some patients faced decisions over which they felt they had no choice because the path ahead was self-evident or the only one available. In these situations, the patients often described their role as merely going along with the “only option.”
Decision-making motives
Finally, patients expressed a variety of motivations or priorities when making a particular decision. These fell primarily into three categories. Prolonging life manifested as the wish to extend time until death, to forestall disease progression, and even to find a cure so as not to die of advanced cancer. Additionally, some patients discussed a desire to enhance or maintain the quality of their lives, and they raised these concerns in the context of considering specific treatments or trials. Some individuals even rejected a given treatment option because they believed it would diminish their quality of life. Finally, a number of patients indicated that altruism—a desire to help others—influenced their decision-making. These considerations arose specifically when they were considering a trial offer or discussing hypothetical trial participation.
Ethnoarray analysis: identifying patterns among themes
For each patient, the ethnoarray depicts the presence or absence of themes and subthemes identified above (Table 2), and it highlights how these varied across patients. Hierarchical clustering sorted patients (columns) into two major groupings, as indicated by the dendogram “brackets” across the top of the graphic (Fig. 1). These groups differ substantially in several regards.
Fig. 1.
Clustered ethnoarray of factors in advanced melanoma patients’ treatment decision-making (n = 13)
For patients in group A, there is little indication of high involvement in decision-making, high-burden actions to inform decisions, access to insider medical/scientific knowledge, or early trials seeking. A higher proportion of group A patients expressed altruistic motivations and cooperated with doctors on decisions than did group B members.
Nearly all patients in group B demonstrated high involvement in decision-making discussions, participated in high-burden information-seeking activities, had access to medical or scientific expertise through themselves or a network contact, and sought out trials early in their care. A higher proportion of group B patients reported good communication with their providers, made decisions based on hope or hopefulness, and used an instrumentally rational decision-making style (e.g., relying on facts, weighing pros and cons) than did group A.
The groups were similar in terms of patient relationship with the provider and their interest in or plans for starting a clinical trial. Patterns for other factors were too subtle to interpret.
Characterizing decision-making types
Drawing on both the narrative data and the ethnoarray clustering, we identify two broad approaches to treatment decision-making, which we label “reliant outsiders” and “active insiders.” Case examples of these types are presented in Table 3.
Table 3.
Exemplar case descriptions from group A (reliant outsider) and group B (active insider)
| Reliant outsider | Active insider |
|---|---|
| Dennis (P7) is a white carpenter in his 50s who was referred to the cancer center after a lengthy diagnosis process. After learning he had melanoma, “there was only one option. It was go to [cancer center] and start treatment with [oncologist].” He tells the interviewer that he does not know much about the different treatments available to him: “it’s all mumbo jumbo to me… I am not big into reading up on stuff like that.” His caregiver wife, a cashier, keeps track of this information. In the course of this experience Dennis did not look for second opinions. He described his decision-making process as: “always our decision, but I would always ask [oncologist], you know, which way does he want to go. ‘Cause I do not see how you ask a patient which direction you want to go, you know, when a doctor’s been doing this for however many years. It makes no sense. So we pretty much just kind of let him -- I mean, we agreed to everything that he wanted to do so we let, you know, pretty much followed his lead.” He could not remember any point when he and his wife second-guessed a decision made that way. After a year of care at [cancer center] he was told there were no remaining anti-cancer options for him unless he was willing to travel outside of California for trials, something he was not willing to do. | Gary (P12) is a white semi-retired engineer in his 60s. When he was diagnosed he and his wife did not know anyone with melanoma, so they relied on research they did, their MD son’s insights, and information and referrals they secured from doctors at three different institutions. Gary and his wife were proactive and effective in pushing for the care they wanted with the providers they wanted. Gary described his approach to decision-making as relatively “cautious”: “We make sure we got the whole picture before we make a decision.” He described weighing the pros and cons of different courses of action. Gary, his caregiver wife and MD son used information they found via research and medical networking to make decisions together as a “team.” Throughout his care, Gary scrutinized oncologists’ descriptions of the risks of different treatments, as his MD son had told him that oncologists tended to minimize the severity of treatment side effects. From early on Gary saw PD1 trials as the only potentially efficacious option available to him: “They have given me the distinct impression that this is a much better, although still experimental drug, both for minimizing side effects and chances of success… I want to get into [it] very badly and… as soon as possible…We have never wavered from that approach.” |
Reliant outsiders viewed their role in treatment decision-making as relatively limited. They saw their providers as an appropriate and desirable source of guidance and leadership on most medical decisions. For most, their provider was their only source of medical information germane to their care. Despite having sought care at a research institution, this group entered their therapeutic relationship with little or no demonstrated interest in clinical trials. However, nearly all later considered experimental involvement. When they felt they had a decision to make, they did so in partnership with family or a provider. Many considered the possibility of helping others (e.g., future patients) when making their decisions.
Active insiders (group B) were more engaged in discussions around treatment decisions. Nearly all demonstrated early and enthusiastic interest in clinical trials, had access to considerable medical or scientific expertise outside the clinical setting, and participated in intensive information-seeking activities. These patients were diverse in their decision strategies. Nearly all indicated that intuition or hope had played a part in their decisions, but many also drew on facts and considered the pros and cons of their options.
Demographically, the groups are similar in terms of age ranges and mean age (62 vs. 58), and there is virtually no variation in race or ethnicity in the sample. The proportion of women, however, is considerably higher among active insiders (5 of 8) than among reliant outsiders (1 of 5, see Table 4). Additionally, none of the reliant outsiders had a college degree, while nearly half (3/8) of active insiders had a master’s degree or higher.
Table 4.
Characteristics of study participants and decision approach type
| Age range (years) | Gender | Education | Race and ethnicity | Relationship status | Annual household income ($) | Decision approach type | |
|---|---|---|---|---|---|---|---|
| P1 | 50–60 | M | Some college | Non-Hispanic White | Divorced/separated | 60–80k | Active insider |
| P2 | 60–70 | M | H.S. degree | Non-Hispanic White and American Indian | Married/partnered | 60–80k | Reliant outsider |
| P3 | 40–50 | F | Some college | Non-Hispanic White | Married/partnered | 40–60k | Active insider |
| P4 | 60–70 | F | Adv. degree | Non-Hispanic White | Married/partnered | 80–100k | Active insider |
| P5 | 60–70 | M | Assoc. degree | Non-Hispanic White | Married/partnered | 40–60k | Reliant outsider |
| P6 | 70–80 | M | Adv. degree | Non-Hispanic White | Married/partnered | 100k+ | Active insider |
| P7 | 50–60 | M | Assoc. degree | Non-Hispanic White | Married/partnered | [missing] | Reliant outsider |
| P8 | 30–40 | F | H.S. degree | Non-Hispanic White | Divorced/separated | 40–60k | Active insider |
| P9 | 70–80 | F | Assoc. degree | White [ethnicity missing] | Divorced/separated | 100k+ | Active insider |
| P10 | 70–80 | F | H.S. degree | Non-Hispanic White | Married/partnered | [missing] | Reliant outsider |
| P11 | 50–60 | F | H.S. degree | Non-Hispanic White | Married/partnered | 40–60k | Active insider |
| P12 | 60–70 | M | Adv. degree | Non-Hispanic White | Married/partnered | 100k+ | Active insider |
| P13 | 40–50 | M | Some college | Non-Hispanic White | Married/partnered | 60–80k | Reliant outsider |
F female, M male, Adv. advanced, Assoc. associates, H.S. high school
Discussion and conclusion
Discussion
This ethnographic investigation identified several factors that characterize how patients with late-stage melanoma approached their treatment decision-making. Using an innovative analytic approach, we discovered that these factors clustered into two groups, which we characterized as reliant outsider and active insider approaches to decision-making. These differ particularly in terms of patient-provider dynamics, patient access to “insider” medical information, and the degree to which patients assumed an active role inside medical institutions. We observed patterns relating certain demographic characteristics to the groups (gender, educational attainment), but membership was not determined solely by these factors. These analyses highlight the complex ways social, behavioral, and individual factors shape treatment decision-making.
These findings add to the literature on decision-making by providing in-depth information on how some late-stage melanoma patients are approaching their decisions amid increasingly complex treatment regimens. In addition to systemic chemotherapy, palliative radiation, and palliative surgery, patients with advanced melanoma may now use immunotherapies, targeted therapies, or combinations of them, either in standard usage or in the clinical trial pipeline. Research on melanoma patients’ perspectives is particularly timely as the annual incidence rates for melanoma in the USA have increased significantly and may affect as many as 112,000 new patients per year by 2030 [42, 43]. These trends suggest an increased need to understand how melanoma patients make decisions and how these decision-making approaches may relate to patient care and outcomes.
The study may also contribute to the understanding of cancer patients’ decision-making more broadly. Most analyses on this topic have focused on one or a narrow set of patient considerations, interactional styles, or specific decisions. Such studies provide partial glimpses into a dynamic and complex process. To understand cancer patients as decision-makers in real-world settings, with their disparate resources and social contexts, a holistic and person-centered analysis is required [14, 33]. This study joins a small field of such studies [14, 15, 24, 25, 44] and contributes to it an innovative analytic approach that facilitates the rigorous and transparent analysis of complex qualitative data. Ethnoarray analysis enabled us both to consider how diverse factors shaped individual patients as decision-makers and to identify differences in how groups of patients approached decision-making as a whole. This approach can be applied to decision-making in other late- or early-stage cancer patient populations.
Finally, enhanced understanding of patients as decision-makers, and the factors that shape their decision-making process, is necessary to develop effective supports for shared decision-making and patient-centered care [11, 33]. Existing scholarship has documented associations between patient demographic characteristics, preferences, and decision-making behaviors [11–13, 16, 45]. Ethnographic patient-centered research, however, can illuminate the context and nuance of these associations. For example, our findings reveal different approaches to decision-making even within a racially homogenous group, and they reveal how these differences are enacted. Additionally, we find that among patients with lower levels of education in our sample, substantial access to medical or scientific expertise differentiates between those with high and low levels of involvement in treatment decisions. All of the active insiders with less than a college degree had access to such “insider” knowledge via their own background (2 of 5) or their immediate social network (4 of 5), whereas only one of the five reliant outsider patients had such a resource. This suggests that enhancing vulnerable patients’ access to individuals with medical expertise (e.g., via health navigators, lay health educators, and other health literacy mediators [46–49]) may be a promising intervention target for supporting their involvement in treatment decision-making. Additional research is needed to confirm this. Overall, we posit that greater awareness of such stylistic differences, and of their diversity within even demographically-similar populations, may help providers to more effectively evaluate and tailor their communication efforts with their patients.
Limitations
The study has several limitations, mostly reflecting the limited number of late-stage melanoma patients who participated, single-site recruitment from an academic medical center, and limited diversity within the sample. The inclusion of other sites and other racial and ethnic groups would have strengthened the analysis. More research is needed to assess the generalizability of our findings to other populations of melanoma and late-stage cancer patients. The clustered ethnoarray also has limitations. Analyses of larger samples may identify more and more nuanced approaches than could be identified here. Additionally, clustering results cannot be used uncritically; they must be interpreted and checked in conversation with the associated narrative data. Finally, as with all classificatory analyses, the characteristics of the resulting groups may not perfectly describe the characteristics of each of its members. Responsible and transparent interpretation of clustered ethnoarrays must acknowledge these complexities.
Conclusion
These findings contribute a nuanced and holistic perspective on treatment decision-making among late-stage melanoma patients that advances scholarly understanding of the process. These findings may provide helpful targets for interventions designed to support cancer patients as decision-makers. Moreover, this work illustrates the potential utility of ethnoarray analysis for pursuing rigorous person-centered analysis of medical decisions. Future research must investigate the applicability of these findings to other settings and discover if the identified decision-making approaches are tied to important patient outcomes.
Acknowledgments
We are grateful to the participants of the Cancer Patient Deliberation Study for contributing time and energy to the study. We thank study co-investigators and research staff for their many contributions. Finally, we thank Jacqueline Joslyn, MA, for helping to create the ethnoarrays presented here, and Thea Matthews, BA, for her careful editorial assistance.
Funding
Research reported in this article was partially funded through a Patient-Centered Outcomes Research Institute® (PCORI®) Award (ME-1409-22996). The statements presented in this article are solely the responsibility of the authors and do not necessarily represent the views of PCORI®, its Board of Governors or Methodology Committee. Financial support for this study was also provided in part by a grant from the National Cancer Institute (R01 CA152195; Daniel Dohan, principal investigator). The funding agreement ensured the authors’ independence in designing the study, interpreting the data, writing, and publishing the report. The content presented here is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health.
Conflict of interest
The authors declare that they have no conflicts of interest.
Study data
The authors have full control of all primary data. Interested parties may contact the authors to inquire about data access.
References
- 1.National Cancer Institute Cancer Stat Facts: Melanoma of the skin. In: NCI Surveillance, Epidemiol. End Results Progr. https://seer.cancer.gov/statfacts/html/melan.html. Accessed 2 Jun 2018
- 2.Wang Y, Zhao Y, Ma S. Racial differences in six major subtypes of melanoma: descriptive epidemiology. BMC Cancer. 2016;16:691. doi: 10.1186/s12885-016-2747-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.PDQ Adult Treatment Editorial Board (2018) Melanoma treatment (PDQ®): health professional version. In: PDQ cancer information summaries [Internet]. National Cancer Institute (US), Bethesda, 2002–2018
- 4.Miller KD, Siegel RL, Lin CC, Mariotto AB, Kramer JL, Rowland JH, Stein KD, Alteri R, Jemal A. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin. 2016;66:271–289. doi: 10.3322/caac.21349. [DOI] [PubMed] [Google Scholar]
- 5.Lang BM, Peveling-Oberhag A, Faidt D, Hötker AM, Weyer-Elberich V, Grabbe S, Loquai C. Long-term survival with modern therapeutic agents against metastatic melanoma—vemurafenib and ipilimumab in a daily life setting. Med Oncol. 2018;35:24. doi: 10.1007/s12032-018-1084-9. [DOI] [PubMed] [Google Scholar]
- 6.Michielin O, Hoeller C. Gaining momentum: new options and opportunities for the treatment of advanced melanoma. Cancer Treat Rev. 2015;41:660–670. doi: 10.1016/j.ctrv.2015.05.012. [DOI] [PubMed] [Google Scholar]
- 7.Krammer R, Heinzerling L. Therapy preferences in melanoma treatment - willingness to pay and preference of quality versus length of life of patients, physicians and healthy controls. PLoS One. 2014;9:e111237. doi: 10.1371/journal.pone.0111237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Albrecht KJ, Nashan D, Meiss F, Bengel J, Reuter K. Shared decision making in dermato-oncology: preference for involvement of melanoma patients. Melanoma Res. 2014;24:68–74. doi: 10.1097/CMR.0000000000000030. [DOI] [PubMed] [Google Scholar]
- 9.Beusterien K, Middleton M, Feng Wang P et al (2017) Patient and physician preferences for treating adjuvant melanoma: a discrete choice experiment. J Cancer Ther. 10.4236/jct.2017.81004
- 10.Kasparian NA, McLoone JK, Butow PN, et al. Psychological responses and coping strategies among patients with malignant melanoma. Arch Dermatol. 2009;145:1453–1457. doi: 10.1001/archdermatol.2009.308. [DOI] [PubMed] [Google Scholar]
- 11.Zafar SY, Alexander SC, Weinfurt KP, Schulman KA, Abernethy AP. Decision making and quality of life in the treatment of cancer: a review. Support Care Cancer. 2009;17:117–127. doi: 10.1007/s00520-008-0505-2. [DOI] [PubMed] [Google Scholar]
- 12.Tariman JD, Berry DL, Cochrane B, Doorenbos A, Schepp K. Preferred and actual participation roles during health care decision making in persons with cancer: a systematic review. Ann Oncol. 2010;21:1145–1151. doi: 10.1093/annonc/mdp534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schuler M, Schildmann J, Trautmann F, et al. Cancer patients’ control preferences in decision making and associations with patient-reported outcomes: a prospective study in an outpatient cancer center. Support Care Cancer. 2017;25(9):2753–2760. doi: 10.1007/s00520-017-3686-8. [DOI] [PubMed] [Google Scholar]
- 14.Weber KM, Solomon DH, Meyer BJF. A qualitative study of breast cancer treatment decisions: evidence for five decision-making styles. Health Commun. 2013;28:408–421. doi: 10.1080/10410236.2012.713775. [DOI] [PubMed] [Google Scholar]
- 15.Balneaves LG, Truant TLO, Kelly M, Verhoef MJ, Davison BJ. Bridging the gap: decision-making processes of women with breast cancer using complementary and alternative medicine (CAM) Support Care Cancer. 2007;15:973–983. doi: 10.1007/s00520-007-0282-3. [DOI] [PubMed] [Google Scholar]
- 16.Rose JH, O’Toole EE, Dawson NV, et al. Perspectives, preferences, care practices, and outcomes among older and middle-aged patients with late-stage cancer. J Clin Oncol. 2004;22:4907–4917. doi: 10.1200/JCO.2004.06.050. [DOI] [PubMed] [Google Scholar]
- 17.Degner LF, Kristjanson LJ, Bowman D, Sloan JA, Carriere KC, O'Neil J, Bilodeau B, Watson P, Mueller B. Information needs and decisional preferences in women with breast cancer. JAMA J Am Med Assoc. 1997;277:1485–1492. doi: 10.1001/jama.1997.03540420081039. [DOI] [PubMed] [Google Scholar]
- 18.Singh JA, Sloan JA, Atherton PJ, Smith T, Hack TF, Huschka MM, Rummans TA, Clark MM, Diekmann B, Degner LF. Preferred roles in treatment decision making among patients with cancer: a pooled analysis of studies using the control preferences scale. Am J Manag Care. 2010;16:688–696. [PMC free article] [PubMed] [Google Scholar]
- 19.Albrecht TL, Eggly SS, Gleason MEJ, Harper FWK, Foster TS, Peterson AM, Orom H, Penner LA, Ruckdeschel JC. Influence of clinical communication on patients’ decision making on participation in clinical trials. J Clin Oncol. 2008;26:2666–2673. doi: 10.1200/JCO.2007.14.8114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Salkeld G, Solomon M, Short L, Butow PN. A matter of trust - patient’s views on decision-making in colorectal cancer. Health Expect. 2004;7:104–114. doi: 10.1111/j.1369-7625.2004.00257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Comis RL, Miller JD, Colaizzi DD, Kimmel LG. Physician-related factors involved in patient decisions to enroll onto cancer clinical trials. J Oncol Pract. 2009;5:50–56. doi: 10.1200/JOP.0922001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Soran A, Ibrahim A, Kanbour M, McGuire K, Balci FL, Polat AK, Thomas C, Bonaventura M, Ahrendt G, Johnson R. Decision making and factors influencing long-term satisfaction with prophylactic mastectomy in women with breast cancer. Am J Clin Oncol. 2015;38:179–183. doi: 10.1097/COC.0b013e318292f8a7. [DOI] [PubMed] [Google Scholar]
- 23.Lantz PM, Janz NK, Fagerlin A, Schwartz K, Liu L, Lakhani I, Salem B, Katz SJ. Satisfaction with surgery outcomes and the decision process in a population-based sample of women with breast cancer. Health Serv Res. 2005;40:745–768. doi: 10.1111/j.1475-6773.2005.00383.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pierce P. Deciding on breast cancer treatment: a description of decision behavior. Nurs Res. 1993;42:22–28. doi: 10.1097/00006199-199301000-00005. [DOI] [PubMed] [Google Scholar]
- 25.Howard AF, Balneaves LG, Bottorff JL, Rodney P. Preserving the self: the process of decision making about hereditary breast cancer and ovarian cancer risk reduction. Qual Health Res. 2011;21:502–519. doi: 10.1177/1049732310387798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ejaz A, Spolverato G, Bridges JF, Amini N, Kim Y, Pawlik TM. Choosing a cancer surgeon: analyzing factors in patient decision making using a best–worst scaling methodology. Ann Surg Oncol. 2014;21:3732–3738. doi: 10.1245/s10434-014-3819-y. [DOI] [PubMed] [Google Scholar]
- 27.Pasick RJ, Burke NJ, Barker JC, Joseph G, Bird JA, Otero-Sabogal R, Tuason N, Stewart SL, Rakowski W, Clark MA, Washington PK, Guerra C. Behavioral theory in a diverse society: like a compass on Mars. Health Educ Behav. 2009;36:11S–35S. doi: 10.1177/1090198109338917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de Heer HD, Balcazar HG, Castro F, Schulz L. A path analysis of a randomized Promotora de Salud cardiovascular disease–prevention trial among at-risk Hispanic adults. Health Educ Behav. 2012;39:77–86. doi: 10.1177/1090198111408720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Afable-Munsuz A, Pasick R, Nguyen KH, Pérez-Stable EJ (2011) Understanding Filipina women’s health orientation and the implications for colorectal cancer screening. Diversity in Health & Care 8(3)
- 30.Whitney SN, Holmes-Rovner M, Brody H, Schneider C, McCullough LB, Volk RJ, McGuire AL. Beyond shared decision making: an expanded typology of medical decisions. Med Decis Mak. 2008;28:699–705. doi: 10.1177/0272989X08318465. [DOI] [PubMed] [Google Scholar]
- 31.Makoul G, Clayman ML. An integrative model of shared decision making in medical encounters. Patient Educ Couns. 2006;60:301–312. doi: 10.1016/j.pec.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 32.Stiggelbout AM, Van der Weijden T, De Wit MPT, et al. Shared decision making: really putting patients at the centre of healthcare. Br Med J. 2012;344:e256. doi: 10.1136/bmj.e256. [DOI] [PubMed] [Google Scholar]
- 33.Clayman ML, Gulbrandsen P, Morris MA. A patient in the clinic; a person in the world. Why shared decision making needs to center on the person rather than the medical encounter. Patient Educ Couns. 2017;100:600–604. doi: 10.1016/j.pec.2016.10.016. [DOI] [PubMed] [Google Scholar]
- 34.Abramson CM, Dohan D. Beyond text. Sociol Methodol. 2015;45:272–319. doi: 10.1177/0081175015578740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Abramson CM, Joslyn J, Rendle KA, Garrett SB, Dohan D. The promises of computational ethnography: improving transparency, replicability, and validity for realist approaches to ethnographic analysis. Ethnography. 2018;19(2):254–284. doi: 10.1177/1466138117725340. [DOI] [Google Scholar]
- 36.Dunn LB, Wiley J, Garrett S, et al (2016) Interest in initiating an early phase clinical trial: results of a longitudinal study of advanced cancer patients. Psychooncology. 10.1002/pon.4179 [DOI] [PubMed]
- 37.Garrett SB, Koenig CJ, Trupin L, Hlubocky FJ, Daugherty CK, Reinert A, Munster P, Dohan D. What advanced cancer patients with limited treatment options know about clinical research: a qualitative study. Support Care Cancer. 2017;25:3235–3242. doi: 10.1007/s00520-017-3734-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.MacPhail C, Khoza N, Abler L, Ranganathan M. Process guidelines for establishing Intercoder Reliability in qualitative studies. Qual Res. 2016;16:198–212. doi: 10.1177/1468794115577012. [DOI] [Google Scholar]
- 39.Glaser BG, Strauss AL. The discovery of grounded theory: strategies for qualitative research. New York: Aldine Publishing Company; 1967. [Google Scholar]
- 40.Strauss AL, Corbin JM. Basics of qualitative research: grounded theory procedures and techniques. Thousand Oaks: Sage Publications; 1990. [Google Scholar]
- 41.Dohan D, Abramson CM, Miller S (2013) Beyond text: using arrays of ethnographic data to identify causes and construct narratives. In: Small M (ed) Am J Sociol "Causal Think Ethnogr Res Symp 19
- 42.Guy GP, Jr, Thomas CC, Thompson T, et al. Vital signs: melanoma incidence and mortality trends and projections—United States, 1982–2030. MMWR Morb Mortal Wkly Rep. 2015;64(21):591–596. [PMC free article] [PubMed] [Google Scholar]
- 43.Jemal A, Clegg LX, Ward E, et al. Annual report to the nation on the status of cancer, 1975–2001, with a special feature regarding survival. Cancer. 2004;101(1):3–27. doi: 10.1002/cncr.20288. [DOI] [PubMed] [Google Scholar]
- 44.Abramson CM. The end game: how inequality shapes our final years. Cambridge: Harvard University Press; 2015. [Google Scholar]
- 45.Maly RC, Umezawa Y, Ratliff CT, Leake B. Racial/ethnic group differences in treatment decision-making and treatment received among older breast carcinoma patients. Cancer. 2006;106:957–965. doi: 10.1002/cncr.21680. [DOI] [PubMed] [Google Scholar]
- 46.Edwards M, Wood F, Davies M, Edwards A. “Distributed health literacy”: longitudinal qualitative analysis of the roles of health literacy mediators and social networks of people living with a long-term health condition. Health Expect. 2013;18:1180–1193. doi: 10.1111/hex.12093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dohan D, Schrag D. Using navigators to improve care of underserved patients. Cancer. 2005;104:848–855. doi: 10.1002/cncr.21214. [DOI] [PubMed] [Google Scholar]
- 48.Sudore RL, Schillinger D. Interventions to improve care for patients with limited health literacy. J Clin Outcomes Manag. 2009;16:20–29. [PMC free article] [PubMed] [Google Scholar]
- 49.Natale-Pereira A, Enard KR, Nevarez L, Jones LA. The role of patient navigators in eliminating health disparities. Cancer. 2011;117:3541–3550. doi: 10.1002/cncr.26264. [DOI] [PMC free article] [PubMed] [Google Scholar]