Abstract
Purpose
The Western Ontario and McMaster Universities Arthritis Index (WOMAC) is a commonly used outcome measure for osteoarthritis. There are different versions of the WOMAC (Likert, visual analogue or numeric scales). A previous review of trials published before 2010 found poor reporting and inconsistency in how the WOMAC was used. This review explores whether these problems persist.
Methods
This systematic review included randomised trials of hip and/or knee osteoarthritis published in 2016 that used the WOMAC. Data were extracted on the version used, score range, analysis and results of the WOMAC, and whether these details were clearly reported.
Results
This review included 62 trials and 41 reported the WOMAC total score. The version used and item range for the WOMAC total score were unclear in 44% (n = 18/41) and 24% (n = 10/41) of trials, respectively. The smallest total score range was 0–10 (calculated by averaging 24 items scored 0–10); the largest was 0–2400 (calculated by summing 24 items scored 0–100). All trials reported the statistical analysis methods but only 29% reported the between-group mean difference and 95% confidence interval.
Conclusion
Details on the use and scoring of the WOMAC were often not reported. We recommend that trials report the version of the WOMAC and the score range used. The between-group treatment effect and corresponding confidence interval should be reported. If all the items of the WOMAC are collected, the total score and individual subscale scores should be presented. Better reporting would facilitate the interpretation, comparison and synthesis of the WOMAC score in trials.
Electronic supplementary material
The online version of this article (10.1007/s11136-018-1978-1) contains supplementary material, which is available to authorized users.
Keywords: WOMAC, Osteoarthritis, Randomised trial, Reporting
Introduction
A lack of clarity and transparency in how outcome measurement tools are used in clinical research can make it difficult to interpret study results [1, 2]. It can be hard to decipher whether a clinically meaningful effect has occurred when it is unclear how the outcome scores have been calculated. Additionally, inconsistency in how an outcome measure is used can make it more difficult to compare the results with previous research findings. A systematic review published in 2012 identified issues of inconsistency and poor reporting of the methods and results of the WOMAC measure in knee osteoarthritis trials, including poor reporting of the type of scale used and how the WOMAC measure was administered [3].
The Western Ontario and McMaster Universities Arthritis Index (WOMAC) is a patient-reported outcome measure for the assessment of lower limb osteoarthritis [4]. The WOMAC measure has been used for decades [5–7] and is one of the most commonly used outcome measures in hip and knee osteoarthritis research [8–10]. The WOMAC measure has been used in clinical trials to evaluate the efficacy of surgical treatments [11, 12], medicinal and biological products [13, 14], devices [15] and physical therapies [16]. The WOMAC has been recommended as a trial endpoint by the Food and Drug Administration (FDA) [13] and is noted as a potential measure for efficacy in recommendations for updates of FDA and European Medicines Agency (EMA) guidance [17–19] and other working groups [20, 21]. In addition, the WOMAC has been recommended as one of the highest-performing outcome measures for knee and hip osteoarthritis, in terms of reliability, validity, responsiveness and interpretability [22–24]. The WOMAC is often used as a comparator to assess the measurement properties of other outcome measures [25, 26]. However, there is variation in how the WOMAC outcome score is collected and calculated.
The WOMAC is composed of 24 items over 3 subscales (5 for pain, 2 for stiffness and 17 for physical function). Participants rate their difficulty for each item, for example, their pain level going up and down stairs or their difficulty rising from a chair. The seminal paper did not provide the wording for the individual questions used in the questionnaire. A user guide for the WOMAC is not freely available. The user guide includes information on how the WOMAC was derived, calculation of scores and specific clinimetric and statistical issues. To obtain the user guide, users (researchers or clinicians) are required to submit a request to the developer of the WOMAC via the website http://www.womac.org, including their personal details and information on the intended use of the WOMAC measure [27]. As well as being translated into over 90 languages, different versions of the WOMAC measure exist [28]. Items can be rated on a five-level Likert scale (no difficulty to extremely difficult) or using a 0–100 mm visual analogue scale (VAS) or an 11-point numerical rating scale (NRS from 0 to 10). These different approaches mean that the range of the WOMAC scores can be unclear if it is not explicitly stated [3]. There are also variations in how item scores are combined, for example, using the total or average of the items. The effect on the total score range of the different options for scoring and combining individual items of the WOMAC is shown in Table 1.
Table 1.
Score range for the WOMAC measure using different versions and methods to combine 24 individual item scores
| Combine 24 item scores using | |||
|---|---|---|---|
| Total | Average | Percentage | |
| Version used for item scores | |||
| Likert scale (item 0–4) | 0–96 | 0–4 | 0–100 |
| NRS (item 0–10) | 0–240 | 0–10 | 0–100 |
| VAS (item 0–100) | 0–2400 | 0–100 | 0–100 |
A lack of clarity in the use and scoring of the WOMAC, including the version used and the score range, can hinder the interpretation of study results. It is difficult to interpret the importance of a 10 point difference between the treatment groups when it is unclear whether this relates to a score range of 0–96 or 0–2400. This also causes problems when combining and comparing results across different trials if it is unclear whether trials have measured and combined the items of the WOMAC differently.
The statistical analysis methods used and how the trial results are reported can also make it more difficult to compare and synthesise results across different trials. The use of different techniques, dichotomisation of continuous measures, adjusting for different covariates or how missing data are handled can impact on the overall treatment effect. Therefore, it is important to be transparent in the methodology used to analyse trial outcomes. Poor reporting of the results of the analysis can also make it more difficult to interpret the trial results. For example, reporting only the p value from the analysis and omitting the between-group mean difference mean that the reader cannot assess the clinical significance of the treatment effect on the original scale of the outcome measure.
The previous review that found poor reporting on how the WOMAC was used included trials of physical therapies published prior to 2010 [3]. Since then, there have been considerable efforts to improve the reporting of clinical trials [29]. To our knowledge, no previous review has examined the use and scoring of all of the WOMAC subscales (including pain, stiffness and function) or the statistical analysis of the WOMAC measure.
This review aims to examine the clarity and consistency of the scoring, analysis and reporting of the WOMAC measure and its subscales in two-arm randomised trials of hip and/or knee osteoarthritis published in 2016.
Methods
Identification of studies
This review utilised a systematic review which identified a cohort of osteoarthritis trials published in 2016 [30].
In the previous review, the cohort of trials were identified by searching seven databases for clinical trials of osteoarthritis from inception to 31 March 2017: Medline, Cochrane Central Register of Controlled Trials (CENTRAL), CINAHL, EMBASE, AMED, PsycINFO and PEDro. Searches were limited to trials published in 2016. An example of the search strategy developed for the previous review of osteoarthritis trials is given in Online Resource 1 [30].
Selection of studies
Eligibility criteria for the cohort of trials
The cohort of trials included in the previous review were two-arm parallel-group randomised controlled trials in a sample of people living with hip and/or knee osteoarthritis published in 2016. The cohort did not include pilot or feasibility studies, since the main outcomes could relate to, for example, feasibility of recruitment, rather than measuring the efficacy of the intervention.
Additional screening criteria
We restricted the eligibility to include only those trials that measured the WOMAC or one of its subscales (pain, stiffness or physical function) as a trial outcome, whether primary or secondary. Trials that only measured the WOMAC at baseline were excluded since we expected these trials to report fewer details on the use of the WOMAC when it was not used as a trial outcome to measure treatment efficacy. Eligibility screening was conducted independently by pairs of reviewers.
Data extraction
Data extraction on study characteristics included the study design, population, sample size and follow-up assessment time points. To further describe the trial samples, data were extracted on the baseline demographics for the WOMAC (e.g. mean score) and whether the WOMAC was used to restrict eligibility to participate in the trial.
For the WOMAC measure and its subscales, data were extracted on the version of the WOMAC used (VAS, NRS or Likert scale) and the scoring system (range of individual items and overall scale or subscale). It was noted when these details were unclear or not reported.
Data on the statistical methods used to analyse the WOMAC, including covariate adjustment and dichotomisation of the WOMAC score, were also extracted and summarised. This would impact the effect estimates reported for the WOMAC score. Summarising this information on the analysis of the WOMAC will allow future trials to utilise consistent methodology to facilitate the comparison of trials results. Data were extracted on how trials examined the impact of missing data on the WOMAC to explore the likelihood of bias in the treatment effects.
Finally, data were extracted on how the results of the WOMAC measure were reported, for example, whether and how the within-group WOMAC scores and the between-group treatment effect were presented. The reporting of the results of the WOMAC will influence the ease with which trial results can be interpreted, compared and combined together.
Data synthesis
Categorical data were summarised using the number and proportion within each category. For continuous outcomes, data were summarised using the median and interquartile range. Data were summarised separately for the WOMAC total score and each individual subscale.
Results
Characteristics of included studies
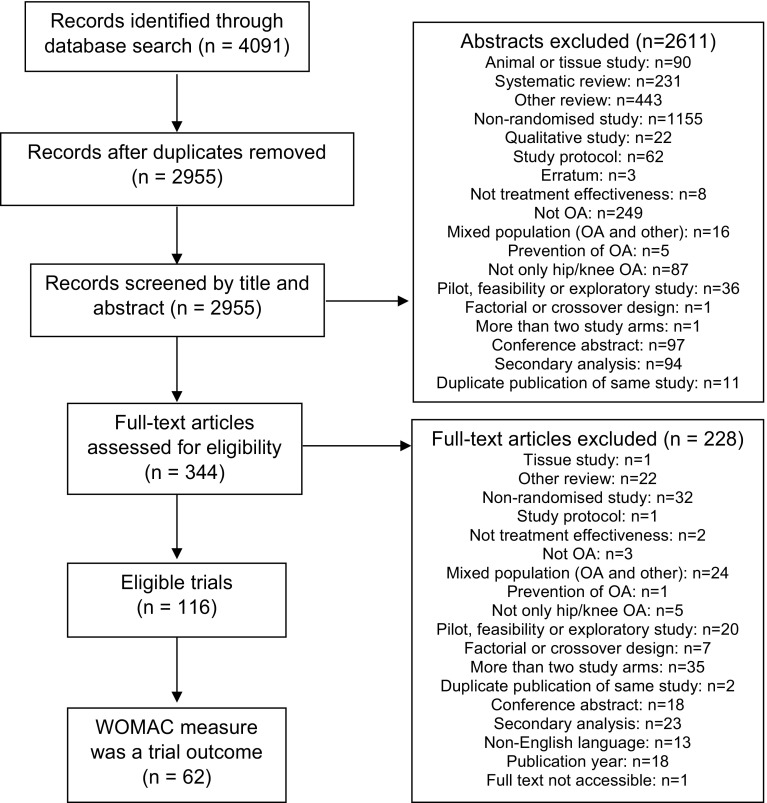
The original cohort of trials included 116 studies, which were two-arm randomised trials of hip and/or knee osteoarthritis (Fig. 1). Of these 116 trials, 62 reported using the WOMAC as an outcome measure and were included in this review. The majority of the included trials were single-centre, superiority trials of knee osteoarthritis (Table 2). The median sample size was 75 randomised participants (IQR 50–148). The smallest trial randomised 20 participants and the largest randomised 606 participants. The median follow-up period was 4.5 months (IQR 1.5–6). Some trials assessed participants only immediately after a single session of an intervention. The maximum length of the follow-up period was 3 years.
Fig. 1.
Flow of studies
Table 2.
Characteristics of included studies (n = 62)
| n | % | |
|---|---|---|
| Study design | ||
| Individually randomised | 61 | 98 |
| Cluster-randomised | 1 | 2 |
| Study hypothesis | ||
| Superiority | 40 | 65 |
| Non-inferiority | 3 | 5 |
| Multiple | 1 | 2 |
| Unclear | 18 | 29 |
| Allocation ratio | ||
| 1:1 | 61 | 98 |
| Unclear | 1 | 2 |
| Study centres | ||
| Single centre | 46 | 74 |
| Multi-centre | 9 | 15 |
| Unclear | 7 | 11 |
| Funding source | ||
| Industry | 8 | 13 |
| Non-industry | 25 | 40 |
| Combination | 3 | 5 |
| No funding | 4 | 6 |
| Not reported | 22 | 35 |
| Population | ||
| Knee OA | 57 | 92 |
| Hip OA | 4 | 6 |
| Hip or knee OA | 1 | 2 |
| Intervention | ||
| Drug | 19 | 31 |
| Surgery | 4 | 6 |
| Exercise | 14 | 23 |
| Other | 25 | 40 |
| Comparator | ||
| Active treatment | 41 | 66 |
| Usual care | 8 | 13 |
| Placebo or sham | 13 | 21 |
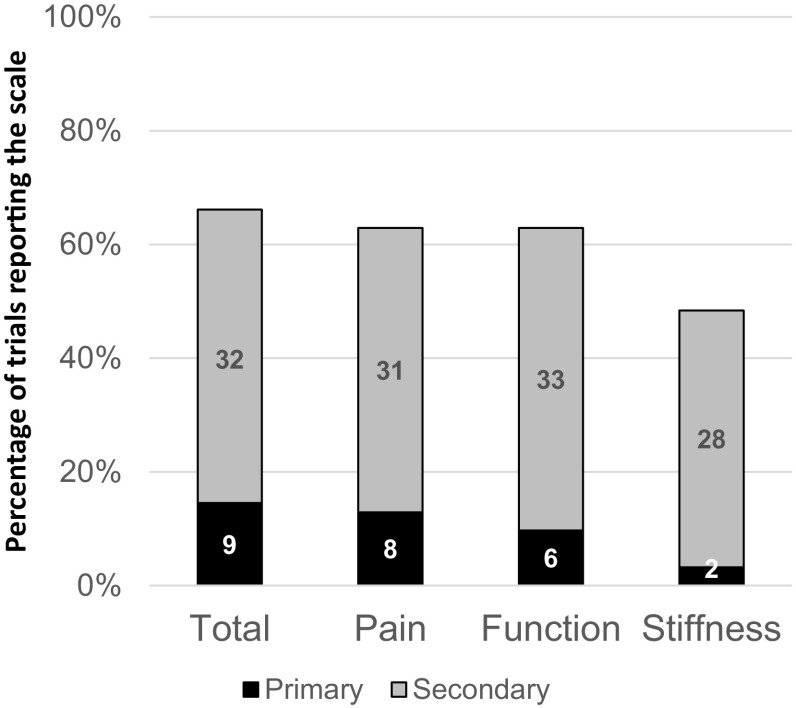
The WOMAC total score was reported in 41 trials (66%, n = 41/62) and it was used as the primary outcome in 22% of these trials (n = 9/41) (Fig. 2). The pain and function subscales were reported in 39 trials (63%, n = 39/62). However, only 30 trials reported the stiffness subscale (48%, n = 30/62). Of the 41 trials reporting the WOMAC total score, less than half reported the results for all three individual subscales (pain, stiffness and function) (37%, n = 15/41) (Table 3).
Fig. 2.
Use of WOMAC total and subscales as a trial outcome measure (n = 62) (Number of trials shown in graph bars, percentage of trials shown on y-axis)
Table 3.
Reporting of WOMAC total and individual subscales (n = 62)
| n (%) | |
|---|---|
| Reported total score | 41 (66) |
| Total and all three subscales | 15 (24) |
| Total and two subscales (pain and function) | 4 (6)a |
| Total only | 22 (35) |
| Did not report total score | 21 (34) |
| Three individual subscales | 15 (24) |
| Two subscales (pain and function) | 4 (6) |
| One subscale | 2 (3)b |
aFor one study, the total score used was the sum of the pain and function subscales only. For three studies, the stiffness subscale was used to calculate the total score but results on the stiffness subscale were not reported separately
bOne study reported pain subscale only and one study reported the function subscale only
Few trials restricted eligibility of the trial participants using the WOMAC measure (6%, n = 4/62). For example, one trial only included participants > 300 on a 0–500 scale for the WOMAC pain subscale.
An alternative version of the WOMAC was used in 12 trials, most commonly using a translated version (n = 8), a shortened version with reduced items or combining only two subscales (n = 3) or alternative item weightings (n = 1). None of the trials made the WOMAC questionnaire available or provided details of the wording of individual items as part of the results publication. Of the 10 trials that referred to a published protocol, one trial provided the WOMAC questionnaire used as an appendix to the study protocol [31].
Scoring of the WOMAC
The version of the WOMAC most commonly used was 5-point Likert scale with a range of 0–96 (41%, n = 17/41) (Table 4). The smallest range used was 0–10 and the largest range used was 0–2400.
Table 4.
Version used and scoring of the WOMAC total (n = 41)
| n | % | |
|---|---|---|
| Scoring version | ||
| Likert scale | 17 | 41 |
| NRS | 3 | 7 |
| VAS | 3 | 7 |
| Unclear | 18 | 44 |
| Item range | ||
| 0–4 | 21 | 51 |
| 0–10 | 7 | 17 |
| 0–100 | 2 | 5 |
| 1–4 | 1 | 2 |
| Unclear | 10 | 24 |
| Method to combine subscales | ||
| Sum | 31 | 76 |
| Average | 3 | 7 |
| Sum and convert to percentage | 1 | 2 |
| Sum with equal weighting for subscales | 1 | 2 |
| Unclear | 5 | 12 |
In most trials, the range for the total score was not reported and it was unclear how the individual items were combined. The range of the total score was often assumed from the mean score or range of a single item; however, it was still unclear for 20% of trials (n = 8/41).
For the individual WOMAC subscales, the scoring version and item range were reported more clearly than for the WOMAC total score. For example, the scoring version was unclear for 44% of trials reporting the WOMAC total score (n = 18/41), compared to 21% reporting the pain subscale (n = 8/39). Around ¾ of trials reported the version used and item range for each subscale (Online Resource 2). The range for the subscale was apparent for all trials; half of trials reported the range and for the other half, the range could be assumed based on the mean score or the item range.
In the trial results, four trials dichotomised participants based on the WOMAC total score (n = 2) or WOMAC pain score (n = 2) and none used the same cut-off points. Two additional studies categorised participants as ‘treatment responders’ using a combination of the WOMAC post-treatment score, WOMAC change score and the patient global assessment score according to the OARSI criteria [32]. No studies reported the WOMAC as a subset of the items from the KOOS score.
Trials reported that the WOMAC was completed by the participant (34%, n = 21/62) or by the participant with a clinician assessor (34%, n = 21/62). The data collection for the WOMAC was unclear for the remaining 20 studies (32%, n = 20/62). No trial reported that the WOMAC was collected by proxy for the participant. When an outcome assessor was involved, it was often unclear whether the participant completed a written version of the WOMAC questionnaire that was checked by the assessor or whether the assessor delivered the questionnaire verbally. One trial noted that ‘the questionnaire was read by the investigator for illiterate patients’ [33].
Analysing the WOMAC
To analyse the WOMAC measure, the most commonly used methods were a t test, repeated-measures ANOVA or a mixed effects model (Table 5). The majority of trials used complete case analysis (24%, n = 10/41) and few trials used multiple imputation to assess the impact of missing data (7%, n = 3/41). Statistical analysis methods and handling of missing data was similar for the WOMAC total score and the individual subscales.
Table 5.
Analysis of the WOMAC total score (n = 41)
| n | % | |
|---|---|---|
| Statistical analysis method | ||
| t test | 13 | 33 |
| Repeated-measures ANOVA | 9 | 23 |
| Mixed model | 8 | 20 |
| ANCOVA | 3 | 8 |
| Mann–Whitney U test | 6 | 15 |
| Other: Kruskal–Wallis H test | 1 | 3 |
| Adjusted for covariates | ||
| Yes | 11 | 27 |
| Unclear | 4 | 10 |
| No | 26 | 63 |
| Method to handle missing data | ||
| Complete case | 10 | 24 |
| Multiple imputation | 3 | 7 |
| Single imputation (e.g. LVCF) | 3 | 7 |
| Mixed model without imputation | 5 | 12 |
| No missing data | 4 | 10 |
| Unclear | 16 | 39 |
For the WOMAC total score, around ¼ of trials reported using covariate adjustment in the analysis (27%, n = 11/41). Covariate adjustment was more common for analysis on the individual WOMAC subscales (46% for the pain subscale, n = 18/39). For adjusted analyses, trials most commonly adjusted for baseline score (13 trials), BMI (7 trials), sex (6 trials) and age (5 trials).
Interpreting results from the WOMAC
The majority of studies reported the within-group mean post-treatment score (88%, n = 36/41 for WOMAC total) and the corresponding standard deviation (68%, n = 28/41 for WOMAC total) (Table 6). For the WOMAC total, the between-group mean difference with the corresponding 95% confidence interval was reported in less than 1/3 of trials (29%, n = 12/41). The proportion of studies reporting the between-group difference was higher for the WOMAC subscales but still remained below half (46%, n = 18/39 for the pain and function subscales). For the majority of trials, the results of the between-group analyses were reported using only the p value (49%, n = 20/41).
Table 6.
Reporting of results for the WOMAC total score (n = 41)
| n | % | |
|---|---|---|
| Summary score | ||
| Mean post-treatment score | 28 | 68 |
| Mean change score | 2 | 5 |
| Mean post-treatment and change scores | 8 | 20 |
| Median post-treatment score | 1 | 2 |
| Multiple reported | 1 | 3 |
| None reported | 1 | 3 |
| Within-group variation | ||
| Standard deviation | 25 | 61 |
| 95% confidence interval | 5 | 12 |
| Range | 1 | 2 |
| Standard deviation and 95% confidence interval | 2 | 5 |
| Multiple reported | 1 | 2 |
| None reported | 7 | 17 |
| Between-group score | ||
| Mean difference | 13 | 32 |
| None reported | 28 | 68 |
| Between-group variation | ||
| 95% confidence interval and p value | 10 | 24 |
| 95% confidence interval | 3 | 7 |
| p value | 20 | 49 |
| p value interval (e.g. p < 0.05) | 6 | 15 |
| None reported | 2 | 5 |
Of the 4 trials where dichotomisation of the WOMAC score was used, all reported the proportion of participants achieving the cut-off score, one reported the odds ratio and two reported the corresponding p value.
Discussion
Summary of findings
The WOMAC is a commonly used outcome measure in trials of hip and knee osteoarthritis. It was used to assess self-reported patient outcomes in around half of hip and knee osteoarthritis trials, predominantly as a secondary outcome measure. However, there was substantial variation in the way the WOMAC measure was implemented and analysed. The scoring range for the WOMAC total score was 0–96 for most trials; however, it was as small as 0–10 for some trials and as large as 0–2400 for others. Randomised trials often did not adequately report the version of the WOMAC used, how individual item scores were combined and the range of the score.
Most studies used a t test, repeated-measures ANOVA or mixed effects model to analyse the WOMAC score. The majority of studies did not adjust for baseline covariates in their analysis. In the study results, the mean score and variation within the treatment groups was well reported. However, interpretation of the results of between-group analyses was hindered by poor reporting, with less than half of trials reporting the between-group mean difference for the WOMAC score.
Comparison with existing literature
Only one previous review has looked at the use of the WOMAC [3]. The results of this review align with and build upon the findings of Woolacott et al. Both reviews found that the type of scale and the score range were unclear for 20% and 10% of trials, respectively, with these values being explicitly reported in only around half of trials. Despite the passage of time and increasing awareness of, and initiatives to address, the need for improvement in the reporting of clinical trials, we found little evidence of improvement in reporting on essential characteristics of the WOMAC measure. There was also no indication of improvement in the consistency across trials on how the WOMAC is measured. Additionally, restricting focus to a particular clinical condition did not lead to greater consistency being observed. This suggests that the previously observed variation in implementation and poor reporting is a generic issue, irrespective of the clinical condition.
There were some differences between this review’s findings and that of Woolacott and colleagues. In this review, most trials reporting the WOMAC pain subscale also reported the function and stiffness subscales, whereas the previous review found that the function subscale was rarely reported. It is unclear whether this is due to changes over time or the differences of the interventions and clinical area being considered. However, both reviews found that many trials report the results using the WOMAC total score without reporting the results of the component subscales.
Strengths and limitations
This review examines the reporting of the WOMAC in an up-to-date sample of trials identified using a systematic search strategy. This is the first review to examine the reporting of the function and stiffness subscales of the WOMAC, as well as the pain subscale and total score. This review did not restrict eligibility based on the intervention, including trials of physical therapies, surgery and pharmacological treatments.
This review is limited in that it only includes trials published during a 1-year period. Therefore, the results do not provide information on the trends in the use and reporting of the WOMAC over time. It also does not consider the use of the WOMAC in trials of conditions other than knee or hip osteoarthritis, such as ankle osteoarthritis [34, 35] or rheumatoid arthritis [7]. An additional limitation is that the use of the WOMAC may be different in observational or multi-arm studies, compared to two-arm randomised trials.
Implications
This review demonstrates that problems persist in the poor reporting and inconsistency in the measurement of the WOMAC total score and the WOMAC subscale scores. This makes it difficult to interpret trial results and hinders comparisons between trials, for instance, where the range of the scale is unknown. The clinical importance of a 20-point difference between treatments varies greatly depending on whether a 0–96 scale or 0–2400 scale was used. Poor reporting of the effect estimates from between-group analyses could also hinder the interpretation of study results.
Analysing only the WOMAC total score without examining the individual subscales may hide intricacies in the study results. For example, there may be an improvement in pain but worsening function, which would not be evident since the total score remains unchanged. The presentation of the individual subscale results would be important if the changes in specific domains are seen as more desirable than others due to patient preference or the hypothesised treatment mechanisms [36]. Reporting the results of individual subscales also makes it easier to compare the trial results with other trials where only individual subscales are reported without the total score.
Future research
Because there are multiple versions of the WOMAC measure in use, this means that research into the measurement properties of the WOMAC can be of uncertain applicability. Although the different versions have been shown to be correlated, it is unclear whether the psychometric properties are similar [37]. When assessing validity and responsiveness, the sensitivity of a 0–4 Likert scale, 0–10 NRS and 0–100 VAS are likely to be different. Future research should compare the different versions of the WOMAC measure, in terms of the validity, responsiveness and usability. The findings of this research could be used to make recommendations on which version of the WOMAC measure is most appropriate for particular settings and how it should be measured and analysed.
Further studies could also examine whether, or when, it is appropriate to ‘translate’ the results from one version of the WOMAC into another version. For instance, it is unclear whether results for the WOMAC Likert pain subscale (range 0–20) can be multiplied to translate the results onto the WOMAC VAS pain subscale (range 0–500). If it is appropriate to standardise different versions of the WOMAC to the same scale (e.g. converting all to a 0–100 scale), this would make it easier to compare the results of different trials and combine results in meta-analyses.
Recommendations for using the WOMAC
When publishing the results of osteoarthritis trials, trialists should clearly report the version of the WOMAC measure that was used and the associated score range. Unless there is sufficient justification to do otherwise, trialists should favour the 5-point Likert scale version and combine scores by summation of the individual items. Since the Likert scale version is the most commonly used, this would make it easier to compare the results with the findings of previous trials.
Trialists using the WOMAC total score should also report results for the individual subscales for pain, function and stiffness. This would allow readers to see whether the treatment effect (or lack of it) is consistent across the three domains.
Trialists should also assess the robustness of the results based on adjustment for baseline covariates and the handling of missing data.
Conclusion
While the WOMAC measure is commonly used in trials of hip and knee osteoarthritis, there is wide variation on how the WOMAC is implemented and analysed. Relevant details are often poorly reported and, as such, the ranges of the outcome scales are often unclear. This inhibits the interpretation of findings and comparisons with other studies. The interpretation of results on the WOMAC measure was also hindered by other limitations, such as not using an analysis method that estimates an effect size, not reporting an effect size estimate or not exploring the effects of assumptions on missing data mechanisms.
Trials should report the version of the WOMAC used, the score range and how item scores were combined. Future research should examine which version of the WOMAC measure has optimal properties. Improved consistency and transparency in how the WOMAC is measured would make it easier to interpret trial results and facilitate the comparison of results across trials.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Funding
This project is funded by a doctoral studentship from the EPSRC (Engineering and Physical Sciences Research Council, Grant No. MR/K501256/1) and Nuffield Department of Orthopaedics, Rheumatology and Musculoskeletal Sciences via the Medical Sciences Division of the University of Oxford. The funders had no input into the study design, the collection, analysis and interpretation of data, the writing of the report or in the decision to submit the article for publication. Sarah E Lamb, Usama Ali, Raymond Fitzpatrick and Bethan Copsey receive funding from the National Institute for Health Research (NIHR) Collaboration for Leadership in Applied Health Research and Care Oxford at Oxford Health NHS Foundation Trust. Jonathan Cook receives support from the National Institute for Health Research (NIHR) Oxford Biomedical Research Centre. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Boers M, Kirwan JR, Wells G, Beaton D, Gossec L, d’Agostino MA, et al. Developing core outcome measurement sets for clinical trials: OMERACT filter 2.0. Journal of Clinical Epidemiology. 2014;67(7):745–753. doi: 10.1016/j.jclinepi.2013.11.013. [DOI] [PubMed] [Google Scholar]
- 2.Kyte, D. G. (2015). The methodological and ethical issues associated with patient-reported outcome measurement in clinical trials. Ph.D., University of Birmingham.
- 3.Woolacott NF, Corbett MS, Rice SJ. The use and reporting of WOMAC in the assessment of the benefit of physical therapies for the pain of osteoarthritis of the knee: Findings from a systematic review of clinical trials. Rheumatology. 2012;51(8):1440–1446. doi: 10.1093/rheumatology/kes043. [DOI] [PubMed] [Google Scholar]
- 4.Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: A health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. The Journal of Rheumatology. 1988;15(12):1833–1840. [PubMed] [Google Scholar]
- 5.Bellamy N, Kean WF, Buchanan WW, Gerecz-Simon E, Campbell J. Double blind randomized controlled trial of sodium meclofenamate (Meclomen) and diclofenac sodium (Voltaren): Post validation reapplication of the WOMAC Osteoarthritis Index. The Journal of Rheumatology. 1992;19(1):153–159. [PubMed] [Google Scholar]
- 6.McGrory BJ, Harris WH. Can the western Ontario and McMaster Universities (WOMAC) osteoarthritis index be used to evaluate different hip joints in the same patient? The Journal of Arthroplasty. 1996;11(7):841–844. doi: 10.1016/S0883-5403(96)80184-7. [DOI] [PubMed] [Google Scholar]
- 7.Wolfe F, Kong SX. Rasch analysis of the Western Ontario MacMaster questionnaire (WOMAC) in 2205 patients with osteoarthritis, rheumatoid arthritis, and fibromyalgia. Annals of the Rheumatic Diseases. 1999;58(9):563–568. doi: 10.1136/ard.58.9.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bond M, Davis A, Lohmander S, Hawker G. Responsiveness of the OARSI-OMERACT osteoarthritis pain and function measures. Osteoarthritis and Cartilage. 2012;20(6):541–547. doi: 10.1016/j.joca.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 9.Collins NJ, Misra D, Felson DT, Crossley KM, Roos EM. Measures of knee function: International Knee Documentation Committee (IKDC) Subjective Knee Evaluation Form, Knee Injury and Osteoarthritis Outcome Score (KOOS), Knee Injury and Osteoarthritis Outcome Score Physical Function Short Form (KOOS-PS), Knee Outcome Survey Activities of Daily Living Scale (KOS-ADL), Lysholm Knee Scoring Scale, Oxford Knee Score (OKS), Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC), Activity Rating Scale (ARS), and Tegner Activity Score (TAS) Arthritis Care & Research. 2011;63(0 11):S208–S228. doi: 10.1002/acr.20632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kersten P, White PJ, Tennant A. The visual analogue WOMAC 3.0 scale—internal validity and responsiveness of the VAS version. BMC Musculoskeletal Disorders. 2010;11:80–80. doi: 10.1186/1471-2474-11-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Katz JN, Brophy RH, Chaisson CE, de Chaves L, Cole BJ, Dahm DL, et al. Surgery versus physical therapy for a meniscal tear and osteoarthritis. New England Journal of Medicine. 2013;368(18):1675–1684. doi: 10.1056/NEJMoa1301408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Strange S, Whitehouse MR, Beswick AD, Board T, Burston A, Burston B, et al. One-stage or two-stage revision surgery for prosthetic hip joint infection–the INFORM trial: A study protocol for a randomised controlled trial. Trials. 2016;17:90. doi: 10.1186/s13063-016-1213-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smith PA. Intra-articular autologous conditioned plasma injections provide safe and efficacious treatment for knee osteoarthritis: An FDA-sanctioned, randomized, double-blind, placebo-controlled clinical trial. The American Journal of Sports Medicine. 2016;44(4):884–891. doi: 10.1177/0363546515624678. [DOI] [PubMed] [Google Scholar]
- 14.Underwood M, Ashby D, Carnes D, Castelnuovo E, Cross P, Harding G, et al. Topical or oral ibuprofen for chronic knee pain in older people. The TOIB study. Health Technology Assessment. 2008 doi: 10.3310/hta12220. [DOI] [PubMed] [Google Scholar]
- 15.Palmer S, Domaille M, Cramp F, Walsh N, Pollock J, Kirwan J, et al. Transcutaneous electrical nerve stimulation as an adjunct to education and exercise for knee osteoarthritis: A randomized controlled trial. Arthritis Care & Research. 2014;66(3):387–394. doi: 10.1002/acr.22147. [DOI] [PubMed] [Google Scholar]
- 16.Monaghan B, Grant T, Hing W, Cusack T. Functional exercise after total hip replacement (FEATHER): A randomised control trial. BMC Musculoskeletal Disorders. 2012;13:237. doi: 10.1186/1471-2474-13-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.FDA . Clinical development programs for drugs, devices and biological products intended for the treatment of OA. Draft guidance. Bethesda: FDA; 1999. [Google Scholar]
- 18.Goldberg VM, Buckwalter J, Halpin M, Jiranek W, Mihalko W, Pinzur M, et al. Recommendations of the OARSI FDA Osteoarthritis Devices Working Group. Osteoarthritis Cartilage. 2011;19(5):509–514. doi: 10.1016/j.joca.2011.02.017. [DOI] [PubMed] [Google Scholar]
- 19.Reginster JY, Reiter-Niesert S, Bruyere O, Berenbaum F, Brandi ML, Branco J, et al. Recommendations for an update of the 2010 European regulatory guideline on clinical investigation of medicinal products used in the treatment of osteoarthritis and reflections about related clinically relevant outcomes: Expert consensus statement. Osteoarthritis Cartilage. 2015;23(12):2086–2093. doi: 10.1016/j.joca.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 20.Cooper C, Adachi JD, Bardin T, Berenbaum F, Flamion B, Jonsson H, et al. How to define responders in osteoarthritis. Current Medical Research and Opinion. 2013;29(6):719–729. doi: 10.1185/03007995.2013.792793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mithoefer K, Saris DBF, Farr J, Kon E, Zaslav K, Cole BJ, et al. Guidelines for the design and conduct of clinical studies in knee articular cartilage repair: International cartilage repair society recommendations based on current scientific evidence and standards of clinical care. Cartilage. 2011;2(2):100–121. doi: 10.1177/1947603510392913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ahmad MA, Xypnitos FN, Giannoudis PV. Measuring hip outcomes: Common scales and checklists. Injury. 2011;42(3):259–264. doi: 10.1016/j.injury.2010.11.052. [DOI] [PubMed] [Google Scholar]
- 23.Gagnier JJ, Mullins M, Huang H, Marinac-Dabic D, Ghambaryan A, Eloff B, et al. A systematic review of measurement properties of patient-reported outcome measures used in patients undergoing total knee arthroplasty. The Journal of Arthroplasty. 2017;32(5):1688–1697. doi: 10.1016/j.arth.2016.12.052. [DOI] [PubMed] [Google Scholar]
- 24.Harris K, Dawson J, Gibbons E, Lim CR, Beard DJ, Fitzpatrick R, et al. Systematic review of measurement properties of patient-reported outcome measures used in patients undergoing hip and knee arthroplasty. Patient Related Outcome Measures. 2016;7:101–108. doi: 10.2147/PROM.S97774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gossec L, Hawker G, Davis AM, Maillefert JF, Lohmander LS, Altman R, et al. OMERACT/OARSI initiative to define states of severity and indication for joint replacement in hip and knee osteoarthritis. The Journal of Rheumatology. 2007;34(6):1432–1435. [PubMed] [Google Scholar]
- 26.Howe TE, Dawson LJ, Syme G, Duncan L, Reid J. Evaluation of outcome measures for use in clinical practice for adults with musculoskeletal conditions of the knee: A systematic review. Manual Therapy. 2012;17(2):100–118. doi: 10.1016/j.math.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 27.Norman R, King MT, Clarke D, Viney R, Cronin P, Street D. Does mode of administration matter? Comparison of online and face-to-face administration of a time trade-off task. Quality of Life Research. 2010;19(4):499–508. doi: 10.1007/s11136-010-9609-5. [DOI] [PubMed] [Google Scholar]
- 28.Noyes FR. Noyes’ knee disorders: Surgery, rehabilitation, clinical outcomes e-book. Philadelphia: Elsevier; 2016. [Google Scholar]
- 29.Schulz KF, Altman DG, Moher D. CONSORT 2010 statement: Updated guidelines for reporting parallel group randomised trials. BMJ. 2010;340:c332. doi: 10.1136/bmj.c332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Copsey B, Dutton S, Fitzpatrick R, Lamb SE, Cook JA. Current practice in methodology and reporting of the sample size calculation in randomised trials of hip and knee osteoarthritis: A protocol for a systematic review. Trials. 2017;18(1):466. doi: 10.1186/s13063-017-2209-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arden NK, Cro S, Sheard S, Dore CJ, Bara A, Tebbs SA, et al. The effect of vitamin D supplementation on knee osteoarthritis, the VIDEO study: A randomised controlled trial. Osteoarthritis Cartilage. 2016;24(11):1858–1866. doi: 10.1016/j.joca.2016.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dougados M, Leclaire P, van der Heijde D, Bloch DA, Bellamy N, Altman RD. Response criteria for clinical trials on osteoarthritis of the knee and hip: A report of the Osteoarthritis Research Society International Standing Committee for Clinical Trials response criteria initiative. Osteoarthritis Cartilage. 2000;8(6):395–403. doi: 10.1053/joca.2000.0361. [DOI] [PubMed] [Google Scholar]
- 33.Moorthy S, Codi SR, Surendher R, Manimekalai K. Comparison of the efficacy and safety of tramadol versus tapentadol in acute osteoarthritic knee pain: A randomized, controlled trial. Asian Journal of Pharmaceutical and Clinical Research. 2016;9(3):1–4. [Google Scholar]
- 34.Angers M, Svotelis A, Balg F, Allard J-P. Cross-cultural adaptation and validation of the Ankle Osteoarthritis Scale for use in French-speaking populations. Canadian Journal of Surgery. 2016;59(2):123–127. doi: 10.1503/cjs.010415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Golightly YM, DeVellis RF, Nelson AE, Hannan MT, Lohmander LS, Renner JB, et al. Psychometric properties of the foot and ankle outcome score in a community-based study of adults with and without osteoarthritis. Arthritis Care & Research. 2014;66(3):395–403. doi: 10.1002/acr.22162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hauber AB, Arden NK, Mohamed AF, Johnson FR, Peloso PM, Watson DJ, et al. A discrete-choice experiment of United Kingdom patients’ willingness to risk adverse events for improved function and pain control in osteoarthritis. Osteoarthritis Cartilage. 2013;21(2):289–297. doi: 10.1016/j.joca.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 37.Bolognese JA, Schnitzer TJ, Ehrich EW. Response relationship of VAS and Likert scales in osteoarthritis efficacy measurement. Osteoarthritis Cartilage. 2003;11(7):499–507. doi: 10.1016/S1063-4584(03)00082-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.