Abstract
Cognitive impairment is highly prevalent in patients with Parkinson’s disease (PD) and causes adverse health outcomes. Novel procognitive therapies are needed to address this unmet need. It is now established that there is an increased risk of dementia in patients with type 2 diabetes mellitus (T2DM) and, moreover, T2DM and PD may have common underlying biological mechanisms. As such, T2DM medications are emerging as potential therapies in the context of PD dementia (PDD). In this review, we provide an update on pathophysiological mechanisms underlying cognitive impairments and PDD, focusing on diabetes-related pathways. Finally, we have conducted a review of ongoing clinical trials in PD patients with dementia, highlighting the multiple pharmacological mechanisms that are targeted to achieve cognitive enhancement.
Key Points
| Mild cognitive impairment and Parkinson’s disease (PD) dementia (PDD) are common disorders and are associated with severe morbidity and increased mortality. |
| PDD is treated with cholinesterase inhibitors (ChIs). Ongoing trials against PDD target glutamate, monoamines, kinases, glucocerebrosidase and glucagon-like peptide-1 alone or together with ChIs. |
| Type 2 diabetes mellitus shares pathophysiological mechanisms with PD and neurodegenerative dementias, such as central and peripheral insulin resistance that in turn results in altered autophagy, cell proliferation and increased inflammation. |
| Antidiabetic drugs have shown positive effects on cognitive outcomes in PD animal models, as well as in early-phase clinical trials. |
Introduction
Parkinson’s disease (PD) has traditionally been considered a movement disorder and its diagnosis relies on the presence of bradykinesia along with rigidity and/or rest tremor [1]. Non-motor symptoms have recently gained attention and quality-of-life studies have shown that PD patients are severely affected by its non-motor aspects. Indeed, increasing recognition has been given to non-motor manifestations; these are incorporated into both the latest Movement Disorder Society (MDS) criteria for PD [1] and particularly into separate MDS criteria for prodromal PD [2]. Among the non-motor symptoms, cognitive impairment and dementia are especially debilitating and they are the strongest component leading to nursing home placement of PD patients.
Prevalence of Cognitive Impairments in Parkinson’s Disease (PD)
Cognitive deficits are common in PD, but vary in both quality and severity in different stages of the disease. The spectrum ranges from subjective cognitive decline [3] to mild cognitive impairment (MCI) [4] and PD dementia (PDD) [5]. Subjective cognitive decline does not have established criteria, but it is generally defined as a mild impairment noted by the patient, family or caregivers, although performance in formal cognitive testing is within normal range. Little is known about the occurrence of subjective cognitive decline in PD, but it is assumed to precede future progression to MCI and dementia [6, 7]. The main difference between PD–MCI and PDD is the degree to which the cognitive decline affects everyday life functioning, which is minimal in the case of PD–MCI but substantial in PDD [4, 5]. The cognitive decline often hides under more profound motor deficits and only detailed neurocognitive evaluation reveals the grade to which these deficits extend [8]. Among patients with PD, 25–30% have PD–MCI [4, 9], while an additional 30% have a PDD diagnosis [10]. An 8-year cumulative prevalence of 78% for getting a diagnosis of PDD has been reported in a Norwegian cohort [11] and a cumulative incidence of 83% 20 years after initial diagnosis was reported in the Sydney multicentre study of PD [12].
PD–Mild Cognitive Impairment (MCI)
MCI can affect one or more cognitive domains, and is further subdivided into amnestic MCI, when memory is the predominantly affected domain, and non-amnestic MCI, when domains other than memory are more impaired [13]. Previously, MCI diagnosis was based on subjective complaints from the patient and/or observations from a caregiver or clinician about cognitive difficulties that cannot be explained by age alone and do not interfere with everyday living [14]. However, recently, the MDS Task Force has redefined PD–MCI and established criteria to facilitate the use of a common definition, additionally stating that the deficits should be attributed to PD, excluding other conditions, and that detailed neuropsychological testing should confirm any subjective observation [4].
Older age, longer disease duration, concomitant depression and a higher motor burden are positively correlated with PD–MCI [9]. While MCI most often develops into PDD, amelioration and return to normal cognitive functioning is not uncommon [15]. Non-amnestic, single-domain PD–MCI is the most common according to some studies [9], while others report that multi-domain MCI is the most common [16]. Executive functioning, visuospatial abilities, verbal fluency, memory and language have all been reported to be affected [9, 15]. Accumulating evidence suggest that there are two distinct categories of cognitive dysfunction in PD with different prognoses. The first one is a frontal executive dysfunction that correlates with dopaminergic loss, which is affected by dopaminergic therapy and has a better prognosis. The second type is characterised by visuospatial and semantic fluency deficits attributed to posterior and temporal dysfunction related to cholinergic loss. Prognosis in the latter is more severe, as the patients commonly progress to PDD [17, 18].
PD with Dementia
Dementia is the most severe cognitive syndrome, defined as acquired objective cognitive impairment affecting multiple cognitive domains that is severe enough to affect activities of daily life (ADL) [19]. PDD affects multiple cognitive domains, including attention, memory, executive and/or visuospatial ability [5]. In addition to this, PDD is characterised by neuropsychiatric features such as hallucinations and delusions, apathy, depression and anxiety [5]. PDD shares a lot of similarities with dementia with Lewy bodies (DLB), the latter presenting in close chronological proximity to the initiation of motor symptoms as opposed to PDD where parkinsonian semiology must precede cognitive deficits by at least 1 year [20]. Progression from PD or PD–MCI to PDD cannot be predicted but several risk factors have been identified [15]. These includes PD diagnosis at an older age, akinetic–rigid motor subtype, psychiatric manifestations, loss of postural control, dysautonomia, and rapid eye movement (REM) behaviour disorder (RBD) [5, 21].
Multiple mechanisms seem to be involved in the genesis of cognitive decline in PD. Limbic and cortical Lewy body pathology, as well as amyloid plaque and tau pathologies, are the most common contributors to PDD [22]. Additionally, the role of vascular disease [23] and neuroinflammation [24] has been emphasised. In order to explain the clinical heterogeneity between PDD patients, other mechanisms have also been examined such as the role of synaptic proteins [25], neurotransmitters [26] and genetic factors [26, 27].
Studies on glucosylceramidase beta (GBA) mutations have indeed highlighted a high risk for carriers to develop PD with a more rapid cognitive decline [27]. Moreover, several autosomal dominant α-synuclein mutations cause PD with a worse cognitive prognosis [28]. On the other hand, leucine-rich repeat kinase 2 (LRRK2) and parkin mutations have been described to lead to a milder PD phenotype with less cognitive decline [15, 28]. The role of polymorphisms in genes such as microtubule-associated protein tau (MAPT), apolipoprotein E (APOE) and catechol-O-methyltransferase (COMT) on cognitive outcomes has been examined, but results are not yet conclusive [15, 18, 28].
With regards to wet biomarkers, reduced cerebrospinal fluid (CSF) amyloid β 1–42 (Aβ1–42) concentration is consistently associated with cognitive impairment and predicts dementia development [29], while high levels of neurofilament (NFL) [30], inflammatory glycoprotein YKL-40 [31] and neurogranin [25] have been associated with cognitive decline in PD. Furthermore, it has been reported that PDD patients have increased CSF levels of total and phosphorylated tau and α-synuclein; however, there are still inconsistencies between different studies [32].
In the field of neuroimaging, several modalities have been used to investigate PD–MCI and PDD. Magnetic resonance imaging (MRI) with various structural and functional techniques along with positron emission tomography (PET) and single-photon emission computerised tomography (SPECT) of various tracers (glucose metabolism, cholinergic, amyloid and tau PET) have mainly been used [15, 33, 34]. Structural MRI studies have shown that there is an early cortical thinning in PDD [35] and PD–MCI patients have parietal cortical thinning as well as lower left orbitofrontal cortex grey matter volume as compared to non-demented PD patients [36]. It has also been reported that PD patients have reduced glucose metabolism compared to controls in a range of regions including the parietal and prefrontal cortices [37–39]. Moreover, PD patients with cognitive impairment have reduced metabolism in the temporal, parietal and premotor cortices as compared to PD patients without cognitive decline [38, 39]. Additionally, electroencephalography and magnetoencephalography studies have shown promising results in terms of predicting cognitive deterioration in PD [40].
Role of Diabetes-Related Pathways and Antidiabetic Drugs in PD Dementia (PDD)
Various mechanisms are involved in the underlying pathology of cognitive decline in PD. These include limbic and cortical Lewy body pathology, amyloid plaque and tau pathology, neuroinflammation, synaptic plasticity dysregulation, neurotransmitter alterations as well as genetic factors [15, 18, 22–26, 28]. The complex roles of these in the underlying pathology of PDD has been previously reviewed in depth [41, 42]. There is accumulating evidence that diabetes-related pathways are involved in cognitive decline in PD and here we focus on these mechanisms.
PD and Diabetes
Although PD and diabetes are clinically different, an association between the two disorders was described in the 1960s, with the assessment of glucose tolerance in PD patients [43]. It was later suggested that alterations in similar pathways and common underlying mechanisms exist between the two disorders [44, 45].
Several epidemiological studies have demonstrated an association between type 2 diabetes mellitus (T2DM) and an increased risk of developing PD [46–50]. More specifically, a large proportion of PD and PDD patients have impaired insulin signalling and insulin resistance [44, 51]. A prospective epidemiological study in a Finnish population found an association between T2DM and the risk of developing PD [46] and, furthermore, T2DM has been associated with a younger onset of PD and more severe symptoms [47, 48]. However, in contrast, some studies did not find diabetes to be a risk factor for PD and even reported a lower prevalence of diabetes in PD patients than in controls [45, 52–54]. Despite these conflicting reports, it has been suggested that the diseases may have common underlying biological mechanisms [44, 45]. Mitochondrial dysfunction, autophagy, inflammation and impaired insulin signalling deficits and resistance have all been associated with PD, PDD and diabetes [44]. In addition to shared biological pathways, whole-genome transcriptome profiling of the substantia nigra of PD patients has provided evidence of genetic links between PD and diabetes [55]. It was found that 892 dysregulated priority genes were altered, and various ‘hub’ genes with multiple interactions with other genes were identified, including those encoding glycogen synthase kinase-3β (GSK-3β) and insulin-like growth factor-1 (IGF-1) receptor (IGF-1R). Furthermore, diabetes was among the top three diseases showing the strongest probable relationship to the top upregulated priority genes [55]. Network-based approaches provide further evidence for a molecular link between diabetes and PD [56]. Interactome mapping revealed more than 400 genes linking both T2DM and PD and identified insulin receptor and lipid signalling, activation of the immune response, mitogen-activated protein (MAP) kinase (MAPK) cascade and protein serine–threonine kinase activity as convergent pathways [57].
PD, Diabetes and Dementia
The link between diabetes and PDD was noted back in 1992 when Sandyk and Awerbuch [58] reported an association between the two conditions in a small study of 12 PD patients. In their study, all five PD patients with diabetes also had dementia while the seven diabetes-free PD patients did not exhibit dementia at the time [58]. Since then the field investigating diabetes and PDD has grown. Bosco et al. [51] reported that PD patients with dementia are two times more likely to be insulin resistant than PD patients without dementia [51].
Diabetes and Dementia
In addition to lifestyle factors, such as obesity and physical inactivity, increased longevity and population aging are crucial factors contributing to the increasing prevalence of T2DM worldwide [59]. Similar trends are observed in the prevalence of dementia, resulting in co-occurrence of the two diseases [60]. It is now established that there is an increased risk of dementia in patients with T2DM [61, 62]. Diabetes is also linked to less severe forms of cognitive dysfunction, including MCI [63, 64] and subtle cognitive changes reported under the term diabetes-associated cognitive decrements [65]. Diabetes-associated cognitive decrements in adults with T2DM are commonly subtle changes in cognitive function that may be bothersome, but do not affect ADL [65]. These cognitive decrements affect one or several domains, including processing speed, executive function, visual and verbal memory, as well as motor function [66]. It has been suggested that diabetes-associated cognitive decline develops during the pre-diabetic stages, and advances very slowly, over many years [67]. Cognitive decrements do not represent a pre-dementia stage in patients younger than 60 years, whereas older patients may progress to MCI and dementia [65].
An increasing body of evidence suggests that neurodegenerative and vascular dementias share some common underlying pathologies, such as insulin dysregulation, that act both through disease-specific and general mechanisms [68]. In a meta-analysis of 14 studies including over 2 million people, T2DM was associated with a 60% increased risk for dementia of all types, and for vascular dementia the additional risk was 19% higher in women than in men [69]. Radiological signs of vascular brain injury such as lacunes and white matter hyperintensities are common findings in patients with T2DM [70]; however, neuropathologic studies do not confirm increased occurrence of large artery infarcts or microinfarcts in these patients [71]. Also, although the risk for Alzheimer’s dementia is increased in patients with T2DM, neither Alzheimer’s pathology in the brain [72] nor in vivo biomarkers of amyloid β deposition and tau pathology [73, 74] are more common in patients with diabetes than in non-diabetic individuals. These findings may indicate that T2DM accelerates neurodegeneration by other, non-Alzheimer’s-specific mechanisms, which may also contribute in other dementia syndromes, such as PDD. One such mechanism is insulin resistance, which appears in T2DM and neurodegenerative dementias including PD [75, 76]. Insulin resistance is a core sign of T2DM that, besides hyperglycaemia, also contributes in oxidative stress, inflammation, atherosclerosis and hyperlipidaemia. Brain insulin resistance has been defined as the failure of nerve cells to respond normally to insulin, with subsequent disturbances in synaptic, metabolic and immune response functions [77]. Systemic insulin resistance and T2DM are associated with brain insulin resistance and with neurodegeneration; however, it is unclear whether the inter-relation of these conditions depends on mechanistic links or co-occurrence of processes of aging [75].
Insulin, Insulin-Like Growth Factor (IGF)-1 and Insulin Resistance in Relation to PD
Insulin is thought to have neuroprotective functions in the central nervous system (CNS) [78]. Animal studies have shown that downregulating insulin receptor expression in the rat hippocampus impairs hippocampal plasticity, spatial learning and long-term potentiation [79]. Conversely, insulin treatment has been shown to improve memory and cognition in rats [80] and promote protective effects in rat models of PD [81]. IGF-1 is a neuronal survival factor and is closely related to insulin (Fig. 1). IGF-1 can bind to both the insulin receptor and IGF-1R with similar outcomes, and animal studies have demonstrated that IGF-1 can exert a neuroprotective effect on neurons in in vitro and in vivo models of PD and reduce α-synuclein aggregation [82–84]. IGF-1 levels are increased in the serum and CSF of PD patients [85–87], possibly as an endogenous neuroprotective response. Lower serum IGF-1 levels in PD patients have been associated with poor executive function [88] and found to predict poor verbal memory performance [89].
Fig. 1.
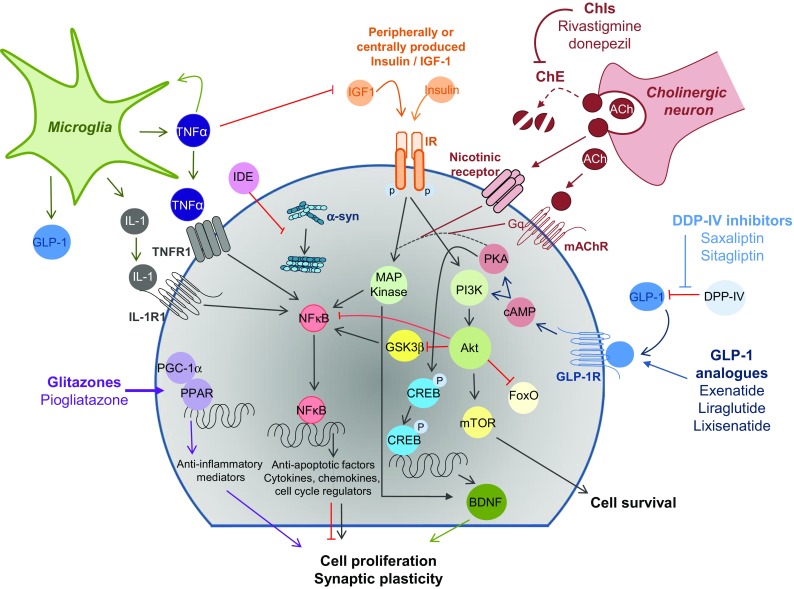
Potential neuroprotective mechanisms of antidiabetic drugs in the context of Parkinson’s disease dementia. ChIs block ACh degradation by ChE allowing ACh to bind to either the mAChR or nicotinic receptors resulting in MAP kinase signalling. IGF-1 or insulin bind to the IR activating MAP kinase signalling, promoting cell growth and synaptic plasticity via BDNF, or the PI3 K/AKT signalling pathway, resulting in cell proliferation and survival via modulating multiple downstream targets including mTOR, GSK-3β, FoxO and NFκB. DPP-IV inhibitors block the degradation of GLP-1 by DDP-IV, promoting signalling, while GLP-1 analogues directly promote GLP-1R signalling, which increases cAMP activating of the aforementioned signalling pathways and also activates the PKA signalling pathway resulting in the activation of CREB and resultant gene transcription. Proinflammatory cytokines, which are elevated in Parkinson’s disease, such as TNFα and IL-1 promote pro-inflammatory cytokine release via NFκB signalling and can also inhibit insulin and IGF-1 signalling, resulting in the disruption of MAP kinase and PI3 K signalling. In contrast to this, glitazones can activate PPARγ, resulting in gene transcription of neuroprotective mediators. PGC-1α can bind to various transcription factors including PPARγ and exerts a neuroprotective effect. The insulin degrading enzyme can target α-syn, binding to α-syn oligomers inhibiting the formation of fibrils. α-syn alpha-synuclein, ACh acetylcholine, AKT protein kinase B, ChE cholinesterase, ChI cholinesterase inhibitor, BDNF brain-derived neurotrophic factor, cAMP cyclic adenosine monophosphate, CREB cyclic adenosine monophosphate response element-binding protein, DPP-IV dipeptidyl peptidase IV, FoxO forkhead box O, GLP-1 glucagon-like peptide-1, GLP1R glucagon-like peptide-1 receptor, GSK3β glycogen synthase kinase-3β, IDE insulin degrading enzyme, IGF-1 insulin growth factor-1, IL-1 interleukin-1, IR insulin receptor, mAChR muscarinic acetylcholine receptors, MAP mitogen-activated protein, mTOR mammalian target of rapamycin, NFκB nuclear factor-κB, PI3 K phosphatidylinositol 3 kinase pathway, PGC-1α PPARγ coactivator 1α, PKA protein kinase A, PPARγ proliferator-activated receptor-γ, TNFα tumour necrosis factor-α
Peripheral insulin and IGF-1 can cross the blood–brain barrier (BBB) and animal studies have shown that they are also produced in regions of the CNS associated with cognition and dementia, including the hippocampus, cortex and olfactory bulb [76, 90–92]. The insulin receptor is widely expressed in the brain—in the olfactory bulb, cerebral cortex, hippocampus, hypothalamus, amygdala [93] and substantia nigra [76, 94]. It is more concentrated in neurons than in glial cells and is present in post-synaptic neuron terminals [93]. To induce signalling, IGF-1 or insulin bind to the insulin receptor, causing autophosphorylation of the intracellular unit. This causes a signalling cascade resulting in the activation of either the mammalian MAPK/extracellular signal-regulated kinases (ERK) signalling pathway, resulting in cell growth, or the phosphoinositide 3-kinase (PI3 K)/protein kinase B (AKT) signalling pathway for metabolic functions, resulting in cell proliferation and survival, and the synthesis of lipids and proteins [95] (Fig. 1). The activation of these pathways can modulate multiple downstream targets [76, 96] (Fig. 1). This results in the modulation of pathways in PD and PDD, such as apoptosis (mammalian target of rapamycin [mTOR], GSK-3β and forkhead box O [FoxO]), autophagy (mTOR), inflammation (GSK-3β and nuclear factor-κB [NFκB]) and synaptic plasticity (GSK-3β, and cyclic adenosine monophosphate [cAMP] response element-binding protein [CREB]). GSK-3β has been widely researched in neurogenerative disorders, inflammation and cognition as well as diabetes, playing a role in each. Of note, α-synuclein, a key player in both PD and PDD can activate GSK-3β directly and indirectly by inhibiting insulin receptor substrate 1, thereby altering insulin signalling [76, 97–99].
Dysfunctional insulin signalling due to insulin resistance, diabetes or excessive inflammation [100] may result in activated GSK-3β and NFκΒ and inactivated mTOR and CREB signalling, among others, resulting in altered autophagy, cell proliferation and increased inflammation (Fig. 1). Indeed, loss of insulin sensitivity centrally in animal models of Alzheimer’s disease (AD) has resulted in increased formation of AD-related pathology [101]. Willette et al. [102] have shown that insulin resistance is associated with higher frontal and temporal amyloid levels in normoglycaemic participants. In contrast, activation of the insulin degrading enzyme (IDE) results in degradation of amyloid β under physiological conditions [103], which is of particular interest due to the fact that amyloid β levels have been associated with memory decline and cognitive impairment in PD [104–106]. Furthermore, upon activation, IDE can target α-synuclein, the main component of Lewy bodies, binding to α-synuclein oligomers, inhibiting the formation of fibrils [107].
In addition to the aforementioned pathways, it has been suggested that low concentrations of insulin together with reduced receptor levels, and thus reduced signalling, would result in decreased acetylcholine [108]. Reductions in cortical choline acetyltransferase correlate with cognitive impairment in PD [109] and there is widespread loss of cortical acetylcholine in PDD [110, 111]. Taken together, it is evident that insulin plays a role in many of the proposed pathological mechanisms in PDD, including autophagy, inflammation, apoptosis, synaptic plasticity and neurotransmitter alteration.
Glucagon-Like Peptide-1 (GLP-1), GLP-1 Analogues and Dipeptidyl Peptidase IV (DPP-IV) Inhibitors
Glucagon-like peptide-1 (GLP-1) is an incretin hormone that maintains glucose homeostasis and facilitates insulin signalling by stimulating insulin secretion and biosynthesis as well as inhibiting glucagon secretion [112]. GLP-1 is primarily produced in the small intestine and is also thought to be produced in the CNS in the lower brainstem and in the axons of the hypothalamic nuclei as well as by microglia [113, 114]. GLP-1 binds to the G-protein coupled receptor (GPCR) GLP-1R, which has been shown to be expressed widely in the brain [115, 116]. In addition to its role in regulating glucose homeostasis, GLP-1 is also involved in cell proliferation, neuronal growth, inhibiting apoptosis and reducing oxidative stress in the CNS [117]. Interestingly, Kappe and colleagues [113] have demonstrated that GLP-1 expression is decreased in response to a lipopolysaccharide (LPS)-mediated inflammation challenge in vitro. The structure of GLP-1R in complex with a Gs protein has recently been revealed [118]. In general, this study has facilitated the integration of a large body of biochemical and biophysical data towards understanding the activation of GPCRs through peptide binding. GLP-1 binding to GLP-1R increases cAMP, and, similarly to IGF-1 and insulin signalling, activates the PI3 K signalling pathway resulting in the activation of AKT signalling pathways [119] (Fig. 1). The dipeptidyl peptidase IV (DPP-IV) enzyme degrades GLP-1, thus inhibiting downstream signalling. As such, GLP-1 analogues and DPP-IV inhibitors have been developed as T2DM drugs, and have been found to have protective functions in PD animal models, protecting against cell death and having anti-inflammatory effects [112, 119]. Indeed, it has been suggested that the protective effect of antidiabetic drugs observed in PD may partly be mediated by their anti-inflammatory effects [44]. GLP-1 analogues and DPP-IV inhibitors can cross the BBB, and affect neuroinflammation, mitochondrial function and cellular proliferation, among others. Moreover, because GLP-1R stimulation activates similar signalling pathways that are ‘de-activated’ as a consequence of insulin resistance, it has been suggested that the ability to restore brain insulin sensitivity also underlies the neuroprotective effect of GLP-1 analogues. Using a population-based register study, we have recently found that patients with T2DM treated with GLP-1R agonists or DPP-4 inhibitors have a lower incidence of future PD [120]. GLP-1 analogues, such as exenatide, liraglutide and lixisenatide, and DPP-IV inhibitors, including saxagliptin, vildagliptin and sitagliptin, have also been found to have neuroprotective effects in in vitro and in vivo animal models of both PD and AD. This has been comprehensively reviewed by Athauda and Foltynie [76, 112, 117] and by Hölscher [121, 122]. Of particular note here is that GLP-1R activation using exendin-4 has been shown to promote acetylcholine production in NSC-19 neuroblastoma cells in vitro [123], which has specific relevance in relation to the loss of cortical acetylcholine in PDD [110, 111]. Moreover, exendin-4 stimulates subventricular zone neurogenesis in the adult rodent brain and induces behavioural and histological recovery in an animal model of Parkinsonism [124].
Glitazones
Insulin-sensitizing thiazolidinediones (TZDs) are another group of antidiabetic drugs that have been investigated in relation to PD. TZDs increase insulin sensitivity by targeting peroxisome proliferator-activated receptor (PPAR)-γ. PPARs have been identified centrally in non-human primates in regions including the ventral tegmental nucleus, putamen and substantia nigra [125]. Furthermore, PPARs are expressed in adult human brain in an isotype- and cell-specific manner; PPARγ is found in neurons and astrocytes but not in microglia in postmortem superior frontal gyrus [126]. However, PPARγ is expressed in mouse microglia after an LPS challenge [126]. PPARs are ligand-dependent transcription factors and PPARγ agonists inhibit proinflammatory cytokine production [127, 128]. As neuroinflammation plays a role in many neurodegenerative disorders, investigation of PPAR agonists has gained momentum. Indeed, pioglitazone has been found to have protective properties in rodent models of PD, reducing mediators of oxidative stress including nitric oxides and mitochondrial outer membrane protein [129], decreasing glial activation and NFκB signalling, and protecting dopaminergic neurons [130, 131]. Furthermore, pioglitazone had neuroprotective effects in experimental parkinsonism in rhesus monkeys [132], and in a retrospective register study, Brauer and colleagues [133] reported that diabetic patients also being treated with glitazones had a lower incidence of PD. However, a phase II multicentre double-blind randomized trial of pioglitazone with PD patients did not find protective effects of the drug (ClinicalTrials.gov identifier NCT01280123). This could be due to the relatively short follow-up time (44 weeks) of early diagnosed PD patients, which is not consistent with large changes in cognitive scores even in the natural course of the disease. In early PD patients the sensitivity to evaluate early and mild cognitive impairment is low [134]. It could also be attributed to inefficient penetration of the drug in the human brain, contrary to previous positive results in animal models [135]. Despite this, more recently pioglitazone was shown to protect against the risk of dementia in patients with T2DM [136]. Recently, attention has been drawn to the PPARγ coactivator 1α (PGC-1α). PGC-1α is involved in gluconeogenesis and mitochondrial biogenesis and, interestingly, GLP-1 has been found to increase PGC-1α in rat pancreatic β cell lines [137]. Polymorphisms in PGC-1α have been associated with the risk and age of onset of PD [138], while overexpression of PGC-1α has a neuroprotective effect on dopaminergic neurons in the 1-methyl-4-pheny-1,2,3,6-tetrahydropyridine (MPTP) model of PD, while also protecting against α-synuclein-induced dopaminergic cell death [139, 140].
Current Treatment of PDD
Cholinergic dysfunction is one of the pathophysiological mechanisms that play a critical role in cognitive impairment in PD, and is targeted by cholinesterase inhibitors (ChIs) for the treatment of Lewy body disorders (PD, PD–MCI, PDD and DLB) [17, 141–148]. In a large meta-analysis assessing the efficacy and safety of 17 clinical trials for PDD and DLB, rivastigmine and donezepil were reported to have a beneficial effect on cognitive function, behavioural disturbances, ADL and global function. Furthermore, there were no detrimental effects on motor function compared with placebo [149]. There was, however, a higher rate of all-cause discontinuation of treatment and discontinuation due to adverse events (AEs) in the ChIs group than in controls. Nausea and tremor were common in all ChIs and vomiting and dizziness were more often associated with rivastigmine. It is concluded that ChIs are beneficial in the treatment of Lewy body disorders, including PDD.
An experimental medicine study [150] with a rivastigmine patch used functional MRI evaluations to assess precognitive effectiveness. Despite a rather small number of participants, the study results implicate the use of rivastigmine with frontal function amelioration. Rivastigmine has also been tested against MCI in PD patients and had some beneficial actions, but failed to meet its primary outcome on global clinical improvement [151].
Apart from ChIs, there have also been studies that evaluated the effect of N-methyl-D-aspartate (NMDA) receptor antagonists in PDD. Memantine affects the glutamatergic neuronal transmission and prevents toxicity from high concentrations of glutamate. Based on previous findings of altered glutamergic markers in DLB, scientists have conducted studies on the effect of memantine on DLB and PDD [152–154]. The studies were multicentre and double-blind and medication was titrated to 20 mg. Pooled data presented in a meta-analysis confirmed a significant effect of memantine on the Clinician’s Global Impression of Change scale and good safety outcomes. However, no effect on cognitive function, behavioural symptoms or ADL was reported [155].
Recent and Ongoing Drug Studies Against PD-MCI and PDD
The electronic databases ClinicalTrials.gov and the European Union Clinical Trials Register were searched using the terms “Parkinson’s disease” and “dementia or cognitive impairment”. Cross-references from the identified articles were also scrutinised for relevant studies. Litvan and colleagues [156] recently reviewed previous clinical trials in PD-MCI. As well as critically reviewing previous trials, they also highlight important factors to be considered for future PD-MCI trials including clarity on which cognitive syndrome investigators are addressing (for trial design), improved outcome measure choice and meaningful biomarkers [156]. As such, in this section we discuss ongoing trials.
Drugs Primarily Acting on Neurotransmitter Mechanisms
Glutamatergic Mechanisms
Sarcosine is a glycine transporter I inhibitor that blocks glycine uptake and enforces the function of the NMDA receptor. Glycine works as a potentiator via the NR1 subunit of the NMDA receptors. A placebo-controlled, double-blind study has shown that sarcosine has a positive effect on neuropsychiatric symptoms of PDD (NCT01785628) [157]. Patients received placebo or 2 mg of sarcosine daily for 8 weeks. Efficacy regarding neuropsychiatric symptoms and safety regarding AEs were the primary endpoints and the Unified Parkinson’s Disease Rating Scale (UPDRS) and cognitive assessment were the secondary endpoints. Temporary dizziness was the only AE reported in the sarcosine group and temporary neuropsychiatric amelioration was achieved in the treatment group, but no differences in cognition or motor function were reported. The researchers indicate a possible tolerance to sarcosine after a relatively short period of treatment and a need for larger studies.
Ceftriaxone is a sterile, semisynthetic, broad-spectrum cephalosporin antibiotic that has been shown to reduce glutamatergic hyperactivity and excitotoxicity and exert neuroprotective properties in preclinical PD models [158–160]. A phase II trial of ceftriaxone in PDD in which efficacy and safety are going to be investigated is ongoing in Taiwan (NCT03413384; Table 1). Patients will receive ceftriaxone 1 g or placebo on days 1, 3 and 5 per cycle on a 2-weekly cycle.
Table 1.
Clinical trials investigating novel treatments for Parkinson’s disease dementia
| ClinicalTrials.gov/EudraCT identifier | Study design | Estimated enrolment (n) | Agent | Mechanism of action | Comparison | Relevant outcome measures |
|---|---|---|---|---|---|---|
| NCT02415062 | R, OL prospective, paralleled study | 150 | Donepezil | ChI | Donepezil 25 mg (high dose) vs. donepezil 10 mg (standard dose) | General cognitive function assessed with Korean MMSE-2. Timeframe: change from baseline to week 24 |
| NCT03413384 | R, DB, PC, phase II study | 106 | Ceftriaxone | Cephalosporin antibiotic | Ceftriaxone vs. placebo | Assessment of memory, orientation, language, and praxis measured with ADAS-Cog. Timeframe: change from baseline to weeks 17 and 33 |
| 2017-004335-36 | R, DB, PC, phase II study | 120 | ANAVEX2-73 | Sigma-1 receptor agonist | ANAVEX2-73 vs. placebo | Continuity of attention measured by CDR computerised assessment system continuity of attention test |
| NCT01738191 | R, DB, PC | 30 | Atomoxetine | Norepinephrine reuptake inhibitor | Atomoxetine vs. placebo | Assessment of 7 atomoxetine-sensitive neuropsychological measures of attention, set-shifting, information processing speed and working memory. Timeframe: change from baseline to 10 weeks |
| NCT02258152 | R, DB, PC, POC | 82 | SYN120 | Dual 5-HT6/5-HT2A antagonist | SYN120 vs. placebo | Assessment of CDR cognition battery continuity of attention. Timeframe: baseline to week 16 |
| NCT02708186 | R, DB, PC | 20 | Nelotanserin | Selective 5-HT2A inverse agonist | Nelotanserin vs. placebo | Assessment of the frequency of REM sleep behaviours. Timeframe: baseline to day 28 |
| NCT02871427 | MC, OL long-term study | 80 | Nelotanserin | Selective 5-HT2A inverse agonist | Nelotanserin 20, 40, 60 or 80 mg | Assessment of the frequency and severity of hallucinations, and REM sleep behaviours. Timeframe: 24 weeks |
| NCT02910102 | R, DB, PC, phase II crossover study | 38 | Intepirdine | Selective 5-HT6 receptor antagonist | Intepirdine vs. placebo | Quantitative gait measurements assessed by computerized gait assessment tools. Timeframe: baseline to week 12 |
| NCT03305809 | R, DB, PC, phase II study | 340 | LY3154207 | Dopamine receptor D1 enhancer | LY3154207 vs. placebo | Assessment of Continuity of Attention measured by CDR computerized cognition battery continuity of attention composite score. Timeframe: baseline to week 12 |
| NCT02562768 | R, DB, PC, multiple-ascending dose study | 80 | LY3154207 | Dopamine receptor D1 enhancer | LY3154207 vs. placebo | Assessment of serious adverse events and pharmacokinetic properties. Timeframe: baseline through day 15 |
| NCT01256905 | OL | 20 | Armodafinil | Dopamine reuptake inhibitor | Armodafinil | Evaluation of striatal–thalamo–cortical network disturbances assessed with EEG. Timeframe: 2 h |
| 2017-001673-17 | R, DB, PC, MC, phase IIa study | 40 | IRL752 | 5-HT7 and α-adrenergic receptor antagonist | IRL752 vs. placebo | Safety, tolerability and efficacy on motor and non-motor symptoms including cognitive function measured with CANTAB and EEG pattern changes. Timeframe: baseline to day 28 |
| 2010-024424-26 | Prospective, MC, R, DB, PC, parallel group, phase II study | 45 | Masitinib | Non-selective tyrosine kinase receptor inhibitor | Masitinib vs. placebo | Assessment of cognition with ADCS instruments. Timeframe: baseline to week 48 |
| NCT02954978 | R, DB, PC | 75 | Nilotinib | C-Abelson tyrosine kinase inhibitor | Nilotinib 300 mg vs. nilotinib 150 mg vs. placebo | Assessment of adverse events and pharmacokinetic properties and measurement of biomarker of dopamine metabolism in cerebrospinal fluid. Timeframe: baseline to 12 months |
| NCT02914366 | R, DB, PC | 75 | Ambroxol | Chaperone, glucocerebrosidase stabiliser | Ambroxol 1050 mg vs. ambroxol 525 mg vs. placebo | Assessment of cognition with ADAS-Cog and ADCS–Clinician’s Global Impression of Change. Timeframe: baseline to week 26 and week 52 |
| NCT02906020 | R, DB, PC | 243 | GZ/SAR402671 (ibiglustat) | Glucosylceramide synthase inhibitor | GZ/SAR402671 vs. placebo | Assessment of MDS-UPDRS and Parkinson’s disease Cognitive Rating Scale. Timeframe: baseline to week 8 and at week 52 |
| NCT03456687 | OL | 20 | Exenatide | GLP-1 receptor agonist | Exenatide | MRI and fMRI-based markers of free-water accumulation in substantia nigra, blood oxygen level-dependent signal in the posterior putamen, in M1 and in the supplementary motor areas. Timeframe: baseline to 1 year |
| NCT03439943 | MC, R, PC, DB parallel arm, POC | 158 | Lixisenatide | GLP-1 receptor agonist | Placebo | Assessment of MDS-UPDRS III motor score in the best ON condition. Timeframe: baseline to 12 months |
| NCT03659682 | R, DB, PC | 270 or 120 | Semaglutide | GLP-1 analogue | Placebo | MDS-UPDRS III in OFF medication state, MoCA and MMSE scores. Timeframe: baseline and at 12, 24, 36 and 48 months |
5-HT 5-hydroxytriptamine, ADAS-Cog Alzheimer’s Disease Assessment Scale–Cognitive Subscale, ADCS Alzheimer’s Disease Cooperative Study, CANTAB Cambridge Neuropsychological Test Automated Battery, CDR Cognitive Drug Research, ChI cholinesterase inhibitors, DB double-blind, EEG electroencephalography, fMRI functional magnetic resonance imaging, GLP-1 glucagon-like peptide 1, MC multicentre, MDS-UPDRS Movement Disorder Society Unified Parkinson’s Disease Rating Scale, MMSE Mini-Mental State Examination, MoCA Montreal Cognitive Assessment, MRI magnetic resonance imaging, OL open-label, PC placebo-controlled, POC proof-of-concept, R randomised, REM rapid eye movements
A phase I dose-finding clinical trial of NYX-458, a modulator of NMDA receptors, has been initiated. The aim is to optimize conditions for a phase II trial in PDD. A press release has reported that a preclinical study on a non-human primate model of PD-related cognitive impairment showed that NYX-458 significantly increased sustained attention, improved cognitive flexibility and enhanced working memory. These effects were present as early as 2 h following a single oral dose and remained 3 weeks post-dosing. NYX-458 resulted in complete reversal of cognitive impairment and performance back to baseline levels. No issues of tolerability were observed.
Cholinergic Mechanisms and their Interplay with Sigma 1 Receptors
ANAVEX2-73 (EudraCT: 2017-004335-36; Table 1) acts on the muscarinic M1 receptors and reverses scopolamine-induced learning impairments in rodents. A phase II trial was recently initiated in Spain to evaluate the efficacy, tolerability and safety of ANAVEX2-73 for cognitive impairment in patients with PDD. ANAVEX2-73 is also a sigma-1 receptor agonist. The sigma-1 receptor is located on mitochondria-associated endoplasmic reticulum (ER) membranes and modulates the ER stress response and local calcium exchanges with the mitochondria. Previous preclinical studies on AD models have shown that ANAVEX2-73 acts synergistically on sigma-1 and muscarinic receptors and alleviates amyloid load in AD models [161]. ANAVEX2-73 has also been found to prevent learning impairment in mice injected with Aβ25–35 by blocking hippocampal oxidative stress [162]. Finally, a synergistic protective effect between sigma-1 receptor agonists and donepezil on learning outcomes has been demonstrated in a non-transgenic mouse model of AD. This may be due to an inter-related mechanism involving α7 nicotinic acetylcholine receptors and sigma-1 receptor [163].
Monoaminergic Mechanisms
Atomoxetine is a selective norepinephrine reuptake inhibitor that affects dopaminergic and noradrenergic transmission in frontal areas of the brain and is approved for the treatment of attention deficit disorder. It has also been investigated with positive results in non-demented PD patients with executive dysfunction in an 8-week, pilot, open-label, flexible-dose study [164]. The study reported improved executive dysfunction and only mild to moderate AEs, including sleep and gastrointestinal disturbances and hypomania. Another randomised, double-blind, placebo-controlled trial (NCT01738191; Table 1) on the potential benefit of atomoxetine on PD-MCI has just been completed, but the results are yet to be published.
Serotonin receptors have received increased attention in the context of non-motor symptoms in neurodegenerative disorders and the use of pimavanserin, a serotonin 5-HT2A antagonist, has already been approved for PD psychosis [165]. Furthermore, 5-HT6 and 5-HT7 receptor antagonists have been shown to produce promnesic and anti-amnesic effects in preclinical models, as well as in humans with schizophrenia, PD or AD [166]; however, early results from phase III studies in AD have failed to demonstrate significant impact on cognition [167]. SYNAPSE is a proof-of-concept study that assessed the safety, tolerability and efficacy of a dual 5-HT6/5-HT2A antagonist (SYN120) in patients with PDD and ongoing treatment with ChI (NCT02258152; Table 1). Recruitment was completed in October 2017 but no results are available yet. Nelotanserin is a selective 5-HT2A inverse agonist that can be administered orally and has been tested on sleep-maintenance insomnias [168]. There is an ongoing clinical trial on the effect of nelotanserin against RBD in patients with PDD and DLB (NCT02708186; Table 1). It is investigating the safety and efficacy of nelotanserin, based on motor scales and clinical evaluation of the REM sleep behaviours. Additionally, another study (NCT02871427; Table 1) is evaluating the long-term safety, tolerability and effectiveness of nelotanserin in DLB and PDD, targeting mainly psychiatric semiology and RBD.
The potentially beneficial role of intepirdine (RVT-101) in cognition has already been highlighted in AD [169]. It is a 5-HT6 antagonist that has been implicated as playing an important role in multiple pathways. Intepirdine has also been investigated in DLB and there has been a study on AD, DLB and PDD (NCT02910102; Table 1) that assessed its effect on gait and balance. To date, no results have been published.
LY3154207 is an orally administered enhancer of dopamine D1 receptors that is under investigation in a multicentre, phase II trial in the USA for patients with PDD (NCT03305809; Table 1). In addition, the pharmacokinetic properties of LY3154207 are going to be assessed in the Multiple-Ascending Dose, Safety, Tolerability, and Pharmacokinetic Study in healthy subjects and PD patients (NCT02562768; Table 1).
Armodafinil is a dopamine reuptake inhibitor that acts as a wake-promoting agent, and has been shown to have positive effects on cognition, daytime sleepiness and visual hallucinations in patients with narcolepsy [170]. It has also been evaluated in a small, open-label, single-dose study in nine patients with DLB and PDD with positive results on attention and global mental status [171]. Another pilot study of 20 patients with PDD and DLB reported improvement in hypersomnia and wakefulness and reasonable safety and tolerability of armodafinil during 12 weeks of treatment [172]. In the next phase, a larger, open-label study (NCT01256905; Table 1) will evaluate the effect of armodafinil on attention modulation in PDD and DLB patients.
IRL752 belongs to a new class of CNS-active agents called psychomotor stabilisers. Such compounds modify psychomotor activity depending on the initial level of activity. In preclinical models, IRL752 potentiates cortical transmission of dopamine, noradrenaline and acetylcholine and has procognitive effects. This provided the rationale for developing IRL752 as a potential therapy against PDD. A multicentre phase IIa study evaluating the safety and tolerability of IRL752 in PDD patients is currently ongoing in Sweden and Finland (EudraCT: 2017-001673-17; Table 1). A press release has reported positive data on cognition and apathy, but no results have been published.
Drugs Primarily Acting on Intracellular Enzymes
Protein Kinase Inhibitors
Masitinib is a non-selective tyrosine kinase receptor (TKR) inhibitor currently being tested against cognitive impairment in PD in a phase II study assessing safety and efficacy (EudraCT: 2010-024424-26; Table 1). The primary target of masitinib is the TKR c-Kit, but it also exerts weak inhibitory activity in fibroblast growth factor receptor 3, lymphocyte-specific kinase (Lck), Lck/Yes-related protein, focal adhesion kinase and the Fyn TKR. It also targets platelet-derived growth factor receptor and is currently being used in the treatment of mast cell (MC) tumours in dogs, and in oncology for the treatment of unresectable or metastatic gastrointestinal stromal tumours [173]. Clinical use of masitinib in non-oncological applications seems reasonable, based on the involvement of c-Kit receptors in MC-mediated inflammatory pathogenesis.
Another biochemical pathway of increasing interest in PD is that of the C-Abelson (C-Abl) tyrosine kinase enzyme. C-Abl is phosphorylated and activated upon mitochondrial dysfunction, resulting in increased oxidative stress and dopamine neuron degeneration supposedly through parkin inactivation, α-synuclein aggregation and impaired autophagy of toxic elements [174]. The C-Abl inhibitor nilotinib, which is used in much higher doses in leukaemia treatment, has been investigated in a small, open-label, proof-of-concept trial on 12 patients with advanced PD or DLB with good results on safety and tolerability during 24 weeks of treatment; however, one patient had a myocardial infarction and two had a corrected QT (QTc) interval prolongation on electrocardiogram [175]. Secondary objectives included the determination of the ability of nilotinib to cross the BBB, and to determine target engagement through measurements of phosphorylation of Abl in CSF. The study had very high coverage by the media when initially presented and a great impact on decision-making among patients and physicians. This led to a great escalation of off-label prescription of nilotinib, which in turn led to call for further, larger studies to include a control arm [176]. A larger phase II study is now underway (NCT02954978; Table 1) and is estimated to be completed by 2020.
Glucocerebrosidase Targeting
There are currently two clinical trials investigating drugs targeting glucocerebrosidase (GCase). Targeting the function of the GBA-encoded enzyme, GCase is a relatively new therapeutic approach in PDD. NCT02914366 is a currently recruiting study on the safety, tolerability and efficacy of ambroxol, a GCase chaperone, in PDD patients (Table 1). It is thought that as ambroxol will increase lysosomal function and has been shown to improve GCase functions it may reduce α-synuclein levels [177]. The study will investigate cognitive and motor scales as well as changes in GCase in lymphocytes and the pharmacokinetics of ambroxol. Another study on PD patients carrying a GBA mutation is also currently ongoing and aims to assess the drug dynamics, efficacy and safety of the substrate inhibitor GZ/SAR402671 (MOVES-PD, NCT02906020; Table 1).
GLP-1 Receptor Agonists
A phase II clinical trial is currently underway evaluating the GLP-1R agonist lixisenatide (NCT03439943; Table 1) as an add-on therapy for early-stage PD patients. However, the primary endpoint for this study is motor improvement with no specific interest in the cognitive aspects of the disease. In addition to this a phase II clinical trial with the GLP-1 analogue semaglutide and newly diagnosed PD patients is planned for early 2019 (NCT03659682; Table 1). In this trial cognitive assessment is also going to be examined among other parameters. Both of these drugs have demonstrated protective effects in preclinical studies [178, 179]. In addition to this the GLP-1 receptor agonist exenatide has been investigated in an open-label, randomised controlled trial of 45 patients with moderate PD [180]. In this study, exenatide was well-tolerated, although weight loss was a common concern. The single-blinded ratings of motor and cognitive performance suggested clinically relevant improvements in the treatment group compared with control. Patients who had previously been exposed to exenatide showed persistent advantage in motor and cognitive performance 1 year after study completion [181]. Following these encouraging results, a phase II randomised, double-blind, placebo-controlled study was conducted including 60 patients with PD who were allocated to exenatide or placebo for 48 weeks, followed by 12 weeks of washout period [182]. The study reported positive results on practically defined off-medication motor scores, which were sustained after the exposure period. However, no significant differences were observed in cognitive performance between exenatide and placebo. This may be due to the short observation period, combined with the mixed population including both early and more advanced PD patients. The observation period may not have been long enough to show significant changes in cognitive outcome [134]. Interestingly, in a post hoc analysis of the Exenatide-PD trial, the authors report improvement in cognition in the subgroups of patients with insulin resistance and those with obesity [183]. Another open-label study of exenatide in PD is currently ongoing in the USA and aims to investigate how the brain and motor behaviour changes in response to treatment (NCT03456687; Table 1). It is also important to note that clinical trials are also evaluating GLP-1 analogues in AD, such as a randomised, placebo-controlled phase II trial that is assessing the safety and efficacy of liraglutide in 206 patients with early AD (NCT018430755). Another trial that aimed to evaluate exenatide in patients with AD or MCI (NCT01255163) was terminated due to insufficient recruitment.
Conclusions and Future Perspective
ChIs are being used for the treatment of PDD. However, there is a strong medical need for additional procognitive therapies. Several distinct pharmacological mechanisms are being targeted alone or together with ChIs to counteract MCI and PDD. T2DM appears to be associated with PD and neurodegenerative dementias, possibly through peripheral and cerebral insulin resistance that in turn results in altered autophagy, mitochondrial function, cell proliferation and increased inflammation. Drugs used in diabetes treatment have shown positive effects on neurodegenerative processes and on clinical outcome, regarding memory and cognition, and could, hopefully, be developed into novel therapies against PDD and related conditions. Since these drugs have primarily been developed for the treatment of T2DM, it is likely that a next generation of compounds with more BBB penetrance may be better neuroprotective agents. The fact that the structure of GLP-1R has been solved will likely facilitate the development of non-peptidergic GLP-1 compounds that may turn out to be favourable against neurodegeneration.
Funding
The work was supported by the Stockholm County Council (Grant number 20160372) and King’s College London. Per Svenningsson is a Wallenberg Clinical Scholar. Dag Aarsland is a Royal Society Wolfson Research Merit Award Holder and would like to thank the Wolfson Foundation for their support. The open access fee was paid by Professor Svenningson's funding from the Karolinska Institutet.
Conflict of interest
PS and PT are co-investigators on MOVES-PD and have been co-investigators in a clinical trial with IRL75 against dementia in Parkinson’s disease. PS has also received grants from Servier and honoraria from IRLAB, AbbVie and Shire. DA has received research support and/or honoraria from AstraZeneca, H. Lundbeck, Novartis Pharmaceuticals and GE Health, and serves as a paid consultant for H. Lundbeck, Eisai and Heptares. HG and IM have no conflicts of interest directly relevant to the content of this article.
Contributor Information
Holly Green, Email: holly.green@kcl.ac.uk.
Per Svenningsson, Email: per.svenningsson@ki.se.
References
- 1.Postuma RB, Berg D, Stern M, Poewe W, Olanow CW, Oertel W, et al. MDS clinical diagnostic criteria for Parkinson’s disease. Mov Disord. 2015;30(12):1591–1601. doi: 10.1002/mds.26424. [DOI] [PubMed] [Google Scholar]
- 2.Berg D, Postuma RB, Adler CH, Bloem BR, Chan P, Dubois B, et al. MDS research criteria for prodromal Parkinson’s disease. Mov Disord. 2015;30(12):1600–1611. doi: 10.1002/mds.26431. [DOI] [PubMed] [Google Scholar]
- 3.Hong JY, Lee Y, Sunwoo MK, Sohn YH, Lee PH. Subjective cognitive complaints and objective cognitive impairment in Parkinson’s disease. J Clin Neurol. 2018;14(1):16–21. doi: 10.3988/jcn.2018.14.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Litvan I, Goldman JG, Troster AI, Schmand BA, Weintraub D, Petersen RC, et al. Diagnostic criteria for mild cognitive impairment in Parkinson’s disease: movement Disorder Society Task Force guidelines. Mov Disord. 2012;27(3):349–356. doi: 10.1002/mds.24893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Emre M, Aarsland D, Brown R, Burn DJ, Duyckaerts C, Mizuno Y, et al. Clinical diagnostic criteria for dementia associated with Parkinson’s disease. Mov Disord. 2007;22(12):1689–1707. doi: 10.1002/mds.21507. [DOI] [PubMed] [Google Scholar]
- 6.Jessen F, Wiese B, Bachmann C, Eifflaender-Gorfer S, Haller F, Kolsch H, et al. Prediction of dementia by subjective memory impairment: effects of severity and temporal association with cognitive impairment. Arch Gen Psychiatry. 2010;67(4):414–422. doi: 10.1001/archgenpsychiatry.2010.30. [DOI] [PubMed] [Google Scholar]
- 7.Erro R, Santangelo G, Barone P, Picillo M, Amboni M, Longo K, et al. Do subjective memory complaints herald the onset of mild cognitive impairment in Parkinson disease? J Geriatr Psychiatry Neurol. 2014;27(4):276–281. doi: 10.1177/0891988714532015. [DOI] [PubMed] [Google Scholar]
- 8.Roheger M, Kalbe E, Liepelt-Scarfone I. Progression of cognitive decline in Parkinson’s disease. J Parkinsons Dis. 2018;8(2):183–193. doi: 10.3233/JPD-181306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aarsland D, Bronnick K, Williams-Gray C, Weintraub D, Marder K, Kulisevsky J, et al. Mild cognitive impairment in Parkinson disease: a multicenter pooled analysis. Neurology. 2010;75(12):1062–1069. doi: 10.1212/WNL.0b013e3181f39d0e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Svenningsson P, Westman E, Ballard C, Aarsland D. Cognitive impairment in patients with Parkinson’s disease: diagnosis, biomarkers, and treatment. Lancet Neurol. 2012;11(8):697–707. doi: 10.1016/S1474-4422(12)70152-7. [DOI] [PubMed] [Google Scholar]
- 11.Aarsland D, Andersen K, Larsen JP, Lolk A, Kragh-Sorensen P. Prevalence and characteristics of dementia in Parkinson disease: an 8-year prospective study. Arch Neurol. 2003;60(3):387–392. doi: 10.1001/archneur.60.3.387. [DOI] [PubMed] [Google Scholar]
- 12.Hely MA, Reid WG, Adena MA, Halliday GM, Morris JG. The Sydney multicenter study of Parkinson’s disease: the inevitability of dementia at 20 years. Mov Disord. 2008;23(6):837–844. doi: 10.1002/mds.21956. [DOI] [PubMed] [Google Scholar]
- 13.Csukly G, Siraly E, Fodor Z, Horvath A, Salacz P, Hidasi Z, et al. The differentiation of amnestic type MCI from the non-amnestic types by structural MRI. Front Aging Neurosci. 2016;8:52. doi: 10.3389/fnagi.2016.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256(3):183–194. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- 15.Aarsland D, Creese B, Politis M, Chaudhuri KR, Ffytche DH, Weintraub D, et al. Cognitive decline in Parkinson disease. Nat Rev Neurol. 2017;13(4):217–231. doi: 10.1038/nrneurol.2017.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marras C, Armstrong MJ, Meaney CA, Fox S, Rothberg B, Reginold W, et al. Measuring mild cognitive impairment in patients with Parkinson’s disease. Mov Disord. 2013;28(5):626–633. doi: 10.1002/mds.25426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kehagia AA, Barker RA, Robbins TW. Cognitive impairment in Parkinson’s disease: the dual syndrome hypothesis. Neurodegener Dis. 2013;11(2):79–92. doi: 10.1159/000341998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Williams-Gray CH, Evans JR, Goris A, Foltynie T, Ban M, Robbins TW, et al. The distinct cognitive syndromes of Parkinson’s disease: 5 year follow-up of the CamPaIGN cohort. Brain. 2009;132(Pt 11):2958–2969. doi: 10.1093/brain/awp245. [DOI] [PubMed] [Google Scholar]
- 19.Association AP. Diagnostic and statistical manual of mental disorders, fourth edition (DSM-IV) 4. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 20.Jellinger KA, Korczyn AD. Are dementia with Lewy bodies and Parkinson’s disease dementia the same disease? BMC Med. 2018;16(1):34. doi: 10.1186/s12916-018-1016-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hanagasi HA, Tufekcioglu Z, Emre M. Dementia in Parkinson’s disease. J Neurol Sci. 2017;15(374):26–31. doi: 10.1016/j.jns.2017.01.012. [DOI] [PubMed] [Google Scholar]
- 22.Irwin DJ, Lee VM, Trojanowski JQ. Parkinson’s disease dementia: convergence of alpha-synuclein, tau and amyloid-beta pathologies. Nat Rev Neurosci. 2013;14(9):626–636. doi: 10.1038/nrn3549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Malek N, Lawton MA, Swallow DM, Grosset KA, Marrinan SL, Bajaj N, et al. Vascular disease and vascular risk factors in relation to motor features and cognition in early Parkinson’s disease. Mov Disord. 2016;31(10):1518–1526. doi: 10.1002/mds.26698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lindqvist D, Hall S, Surova Y, Nielsen HM, Janelidze S, Brundin L, et al. Cerebrospinal fluid inflammatory markers in Parkinson’s disease–associations with depression, fatigue, and cognitive impairment. Brain Behav Immun. 2013;33:183–189. doi: 10.1016/j.bbi.2013.07.007. [DOI] [PubMed] [Google Scholar]
- 25.Bereczki E, Bogstedt A, Hoglund K, Tsitsi P, Brodin L, Ballard C, et al. Synaptic proteins in CSF relate to Parkinson’s disease stage markers. NPJ Parkinsons Dis. 2017;3:7. doi: 10.1038/s41531-017-0008-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O’Callaghan C, Lewis SJG. Cognition in Parkinson’s disease. Int Rev Neurobiol. 2017;133:557–583. doi: 10.1016/bs.irn.2017.05.002. [DOI] [PubMed] [Google Scholar]
- 27.Winder-Rhodes SE, Evans JR, Ban M, Mason SL, Williams-Gray CH, Foltynie T, et al. Glucocerebrosidase mutations influence the natural history of Parkinson’s disease in a community-based incident cohort. Brain. 2013;136(Pt 2):392–399. doi: 10.1093/brain/aws318. [DOI] [PubMed] [Google Scholar]
- 28.Collins LM, Williams-Gray CH. The genetic basis of cognitive impairment and dementia in Parkinson’s disease. Front Psychiatry. 2016;7:89. doi: 10.3389/fpsyt.2016.00089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lleó A, Cavedo E, Parnetti L, Vanderstichele H, Herukka SK, Andreasen N, et al. Cerebrospinal fluid biomarkers in trials for Alzheimer and Parkinson diseases. Nat Rev Neurol. 2015;11(1):41–55. doi: 10.1038/nrneurol.2014.232. [DOI] [PubMed] [Google Scholar]
- 30.Backstrom DC, Eriksson Domellof M, Linder J, Olsson B, Ohrfelt A, Trupp M, et al. Cerebrospinal fluid patterns and the risk of future dementia in early, incident Parkinson disease. JAMA Neurol. 2015;72(10):1175–1182. doi: 10.1001/jamaneurol.2015.1449. [DOI] [PubMed] [Google Scholar]
- 31.Hall S, Surova Y, Ohrfelt A, Blennow K, Zetterberg H, Hansson O. Longitudinal measurements of cerebrospinal fluid biomarkers in Parkinson’s disease. Mov Disord. 2016;31(6):898–905. doi: 10.1002/mds.26578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lin CH, Wu RM. Biomarkers of cognitive decline in Parkinson’s disease. Parkinsonism Relat Disord. 2015;21(5):431–443. doi: 10.1016/j.parkreldis.2015.02.010. [DOI] [PubMed] [Google Scholar]
- 33.Brooks DJ, Tambasco N. Imaging synucleinopathies. Mov Disord. 2016;31(6):814–829. doi: 10.1002/mds.26547. [DOI] [PubMed] [Google Scholar]
- 34.Lanskey JH, McColgan P, Schrag AE, Acosta-Cabronero J, Rees G, Morris HR, et al. Can neuroimaging predict dementia in Parkinson’s disease? Brain. 2018;141(9):2545–2560. doi: 10.1093/brain/awy211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pereira JB, Svenningsson P, Weintraub D, Brønnick K, Lebedev A, Westman E, et al. Initial cognitive decline is associated with cortical thinning in early Parkinson disease. Neurology. 2014;82(22):2017–2025. doi: 10.1212/WNL.0000000000000483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kunst J, Marecek R, Klobusiakova P, Balazova Z, Anderkova L, Nemcova-Elfmarkova N, et al. Patterns of grey matter atrophy at different stages of Parkinson’s and Alzheimer’s diseases and relation to cognition. Brain Topogr. 2018 doi: 10.1007/s10548-018-0675-2. [DOI] [PubMed] [Google Scholar]
- 37.Juh R, Pae C-U, Lee C-U, Yang D, Chung Y, Suh T, et al. Voxel based comparison of glucose metabolism in the differential diagnosis of the multiple system atrophy using statistical parametric mapping. Neurosci Res. 2005;52(3):211–219. doi: 10.1016/j.neures.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 38.Hosokai Y, Nishio Y, Hirayama K, Takeda A, Ishioka T, Sawada Y, et al. Distinct patterns of regional cerebral glucose metabolism in Parkinson’s disease with and without mild cognitive impairment. Mov Disord. 2009;24(6):854–862. doi: 10.1002/mds.22444. [DOI] [PubMed] [Google Scholar]
- 39.Schindlbeck KA, Eidelberg D. Network imaging biomarkers: insights and clinical applications in Parkinson’s disease. Lancet Neurol. 2018;17(7):629–640. doi: 10.1016/S1474-4422(18)30169-8. [DOI] [PubMed] [Google Scholar]
- 40.Olde Dubbelink KT, Stoffers D, Deijen JB, Twisk JW, Stam CJ, Berendse HW. Cognitive decline in Parkinson’s disease is associated with slowing of resting-state brain activity: a longitudinal study. Neurobiol Aging. 2013;34(2):408–418. doi: 10.1016/j.neurobiolaging.2012.02.029. [DOI] [PubMed] [Google Scholar]
- 41.Jellinger KA. Dementia with Lewy bodies and Parkinson’s disease-dementia: current concepts and controversies. J Neural Transm (Vienna) 2018;125(4):615–650. doi: 10.1007/s00702-017-1821-9. [DOI] [PubMed] [Google Scholar]
- 42.Halliday GM, Leverenz JB, Schneider JS, Adler CH. The neurobiological basis of cognitive impairment in Parkinson’s disease. Mov Disord. 2014;29(5):634–650. doi: 10.1002/mds.25857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Barbeau A, Giguere R, Hardy J. Experience clinique avec le tolbutamide dans la maladie de Parkinson. Union Med Can. 1961;90:147–151. [PubMed] [Google Scholar]
- 44.Santiago JA, Potashkin JA. Shared dysregulated pathways lead to Parkinson’s disease and diabetes. Trends Mol Med. 2013;19(3):176–186. doi: 10.1016/j.molmed.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 45.Driver JA, Smith A, Buring JE, Gaziano JM, Kurth T, Logroscino G. Prospective cohort study of type 2 diabetes and the risk of Parkinson’s disease. Diabetes Care. 2008;31(10):2003–2005. doi: 10.2337/dc08-0688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hu G, Jousilahti P, Bidel S, Antikainen R, Tuomilehto J. Type 2 diabetes and the risk of Parkinson’s disease. Diabetes Care. 2007;30(4):842–847. doi: 10.2337/dc06-2011. [DOI] [PubMed] [Google Scholar]
- 47.Cereda E, Barichella M, Cassani E, Caccialanza R, Pezzoli G. Clinical features of Parkinson disease when onset of diabetes came first: a case-control study. Neurology. 2012;78(19):1507–1511. doi: 10.1212/WNL.0b013e3182553cc9. [DOI] [PubMed] [Google Scholar]
- 48.Schernhammer E, Hansen J, Rugbjerg K, Wermuth L, Ritz B. Diabetes and the risk of developing Parkinson’s disease in Denmark. Diabetes Care. 2011;34(4):1102–1008. doi: 10.2337/dc10-1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xu Q, Park Y, Huang X, Hollenbeck A, Blair A, Schatzkin A, et al. Diabetes and risk of Parkinson’s disease. Diabetes Care. 2011;34(4):910–915. doi: 10.2337/dc10-1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yue X, Li H, Yan H, Zhang P, Chang L, Li T. Risk of Parkinson disease in diabetes mellitus: an updated meta-analysis of population-based cohort studies. Medicine. 2016;95(18):e3549. doi: 10.1097/MD.0000000000003549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bosco D, Plastino M, Cristiano D, Colica C, Ermio C, De Bartolo M, et al. Dementia is associated with insulin resistance in patients with Parkinson’s disease. J Neurol Sci. 2012;315(1–2):39–43. doi: 10.1016/j.jns.2011.12.008. [DOI] [PubMed] [Google Scholar]
- 52.Powers KM, Smith-Weller T, Franklin GM, Longstreth W, Jr, Swanson PD, Checkoway H. Diabetes, smoking, and other medical conditions in relation to Parkinson’s disease risk. Parkinsonism Relat Disord. 2006;12(3):185–189. doi: 10.1016/j.parkreldis.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 53.Scigliano G, Musicco M, Soliveri P, Piccolo I, Ronchetti G, Girotti F. Reduced risk factors for vascular disorders in Parkinson disease patients: a case-control study. Stroke. 2006;37(5):1184–1188. doi: 10.1161/01.STR.0000217384.03237.9c. [DOI] [PubMed] [Google Scholar]
- 54.Lu L, Fu D-L, Li H-Q, Liu A-J, Li J-H, Zheng G-Q. Diabetes and risk of Parkinson’s disease: an updated meta-analysis of case-control studies. PLoS One. 2014;9(1):e85781. doi: 10.1371/journal.pone.0085781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Moran LB, Graeber MB. Towards a pathway definition of Parkinson’s disease: a complex disorder with links to cancer, diabetes and inflammation. Neurogenetics. 2008;9(1):1–13. doi: 10.1007/s10048-007-0116-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Santiago JA, Potashkin JA. System-based approaches to decode the molecular links in Parkinson’s disease and diabetes. Neurobiol Dis. 2014;72:84–91. doi: 10.1016/j.nbd.2014.03.019. [DOI] [PubMed] [Google Scholar]
- 57.Santiago JA, Potashkin JA. Integrative network analysis unveils convergent molecular pathways in Parkinson’s disease and diabetes. PLoS One. 2013;8(12):e83940. doi: 10.1371/journal.pone.0083940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sandyk R, Awerbuch GI. The association of diabetes mellitus with dementia in Parkinson’s disease. Int J Neurosci. 1992;64(1–4):209–212. doi: 10.3109/00207459209000547. [DOI] [PubMed] [Google Scholar]
- 59.van Dieren S, Beulens JW, van der Schouw YT, Grobbee DE, Neal B. The global burden of diabetes and its complications: an emerging pandemic. Eur J Cardiovasc Prev Rehabil. 2010;17(Suppl 1):S3–S8. doi: 10.1097/01.hjr.0000368191.86614.5a. [DOI] [PubMed] [Google Scholar]
- 60.Prince M, Ali GC, Guerchet M, Prina AM, Albanese E, Wu YT. Recent global trends in the prevalence and incidence of dementia, and survival with dementia. Alzheimers Res Ther. 2016;8(1):23. doi: 10.1186/s13195-016-0188-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Biessels GJ, Staekenborg S, Brunner E, Brayne C, Scheltens P. Risk of dementia in diabetes mellitus: a systematic review. Lancet Neurol. 2006;5(1):64–74. doi: 10.1016/S1474-4422(05)70284-2. [DOI] [PubMed] [Google Scholar]
- 62.Riederer P, Korczyn AD, Ali SS, Bajenaru O, Choi MS, Chopp M, et al. The diabetic brain and cognition. J Neural Transm (Vienna). 2017;124(11):1431–1454. doi: 10.1007/s00702-017-1763-2. [DOI] [PubMed] [Google Scholar]
- 63.Luchsinger JA, Reitz C, Patel B, Tang MX, Manly JJ, Mayeux R. Relation of diabetes to mild cognitive impairment. Arch Neurol. 2007;64(4):570–575. doi: 10.1001/archneur.64.4.570. [DOI] [PubMed] [Google Scholar]
- 64.Knopman DS, Gottesman RF, Sharrett AR, Tapia AL, DavisThomas S, Windham BG, et al. Midlife vascular risk factors and midlife cognitive status in relation to prevalence of mild cognitive impairment and dementia in later life: the Atherosclerosis Risk in Communities Study. Alzheimers Dement. 2018;14(11):1406–1415. doi: 10.1016/j.jalz.2018.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Koekkoek PS, Kappelle LJ, van den Berg E, Rutten GE, Biessels GJ. Cognitive function in patients with diabetes mellitus: guidance for daily care. Lancet Neurol. 2015;14(3):329–340. doi: 10.1016/S1474-4422(14)70249-2. [DOI] [PubMed] [Google Scholar]
- 66.Palta P, Schneider AL, Biessels GJ, Touradji P, Hill-Briggs F. Magnitude of cognitive dysfunction in adults with type 2 diabetes: a meta-analysis of six cognitive domains and the most frequently reported neuropsychological tests within domains. J Int Neuropsychol Soc. 2014;20(3):278–291. doi: 10.1017/S1355617713001483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Biessels GJ, Strachan MW, Visseren FL, Kappelle LJ, Whitmer RA. Dementia and cognitive decline in type 2 diabetes and prediabetic stages: towards targeted interventions. Lancet Diabetes Endocrinol. 2014;2(3):246–255. doi: 10.1016/S2213-8587(13)70088-3. [DOI] [PubMed] [Google Scholar]
- 68.Craft S, Watson GS. Insulin and neurodegenerative disease: shared and specific mechanisms. Lancet Neurol. 2004;3(3):169–178. doi: 10.1016/S1474-4422(04)00681-7. [DOI] [PubMed] [Google Scholar]
- 69.Chatterjee S, Peters SA, Woodward M, Arango MS, Batty GD, Beckett N, et al. Type 2 diabetes as a risk factor for dementia in women compared with men: a pooled analysis of 2.3 million people comprising more than 100,000 cases of dementia. Diabetes Care. 2016;39(2):300–307. doi: 10.2337/dc15-1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Biessels GJ, Reijmer YD. Brain changes underlying cognitive dysfunction in diabetes: what can we learn from MRI? Diabetes. 2014;63(7):2244–2252. doi: 10.2337/db14-0348. [DOI] [PubMed] [Google Scholar]
- 71.Abner EL, Nelson PT, Kryscio RJ, Schmitt FA, Fardo DW, Woltjer RL, et al. Diabetes is associated with cerebrovascular but not Alzheimer’s disease neuropathology. Alzheimers Dement. 2016;12(8):882–889. doi: 10.1016/j.jalz.2015.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Arvanitakis Z, Schneider JA, Wilson RS, Li Y, Arnold SE, Wang Z, et al. Diabetes is related to cerebral infarction but not to AD pathology in older persons. Neurology. 2006;67(11):1960–1965. doi: 10.1212/01.wnl.0000247053.45483.4e. [DOI] [PubMed] [Google Scholar]
- 73.Moran C, Beare R, Phan TG, Bruce DG, Callisaya ML, Srikanth V, et al. Type 2 diabetes mellitus and biomarkers of neurodegeneration. Neurology. 2015;85(13):1123–1130. doi: 10.1212/WNL.0000000000001982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Vemuri P, Lesnick TG, Przybelski SA, Knopman DS, Lowe VJ, Graff-Radford J, et al. Age, vascular health, and Alzheimer disease biomarkers in an elderly sample. Ann Neurol. 2017;82(5):706–718. doi: 10.1002/ana.25071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Arnold SE, Arvanitakis Z, Macauley-Rambach SL, Koenig AM, Wang HY, Ahima RS, et al. Brain insulin resistance in type 2 diabetes and Alzheimer disease: concepts and conundrums. Nat Rev Neurol. 2018;14(3):168–181. doi: 10.1038/nrneurol.2017.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Athauda D, Foltynie T. Insulin resistance and Parkinson’s disease: a new target for disease modification? Prog Neurobiol. 2016;145–146:98–120. doi: 10.1016/j.pneurobio.2016.10.001. [DOI] [PubMed] [Google Scholar]
- 77.Hoyer S. Is sporadic Alzheimer disease the brain type of non-insulin dependent diabetes mellitus? A challenging hypothesis. J Neural Transm (Vienna) 1998;105(4–5):415–422. doi: 10.1007/s007020050067. [DOI] [PubMed] [Google Scholar]
- 78.Nguyen TTL, Chan LC, Borreginne K, Kale RP, Hu C, Tye SJ. A review of brain insulin signaling in mood disorders: from biomarker to clinical target. Neurosci Biobehav Rev. 2018;92:7–15. doi: 10.1016/j.neubiorev.2018.05.014. [DOI] [PubMed] [Google Scholar]
- 79.Grillo CA, Piroli GG, Lawrence RC, Wrighten SA, Green AJ, Wilson SP, et al. Hippocampal insulin resistance impairs spatial learning and synaptic plasticity. Diabetes. 2015;64(11):3927–3936. doi: 10.2337/db15-0596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Park CR, Seeley RJ, Craft S, Woods SC. Intracerebroventricular insulin enhances memory in a passive-avoidance task. Physiol Behav. 2000;68(4):509–514. doi: 10.1016/s0031-9384(99)00220-6. [DOI] [PubMed] [Google Scholar]
- 81.Pang Y, Lin S, Wright C, Shen J, Carter K, Bhatt A, et al. Intranasal insulin protects against substantia nigra dopaminergic neuronal loss and alleviates motor deficits induced by 6-OHDA in rats. Neuroscience. 2016;318:157–165. doi: 10.1016/j.neuroscience.2016.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Offen D, Shtaif B, Hadad D, Weizman A, Melamed E, Gil-Ad I. Protective effect of insulin-like-growth-factor-1 against dopamine-induced neurotoxicity in human and rodent neuronal cultures: possible implications for Parkinson’s disease. Neurosci Lett. 2001;316(3):129–132. doi: 10.1016/s0304-3940(01)02344-8. [DOI] [PubMed] [Google Scholar]
- 83.Kao SY. Rescue of α-synuclein cytotoxicity by insulin-like growth factors. Biochem Biophys Res Commun. 2009;385(3):434–438. doi: 10.1016/j.bbrc.2009.05.089. [DOI] [PubMed] [Google Scholar]
- 84.El Ayadi A, Zigmond MJ, Smith AD. IGF-1 protects dopamine neurons against oxidative stress: association with changes in phosphokinases. Exp Brain Res. 2016;234(7):1863–1873. doi: 10.1007/s00221-016-4572-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mashayekhi F, Mirzajani E, Naji M, Azari M. Expression of insulin-like growth factor-1 and insulin-like growth factor binding proteins in the serum and cerebrospinal fluid of patients with Parkinson’s disease. J Clin Neurosci. 2010;17(5):623–627. doi: 10.1016/j.jocn.2009.08.013. [DOI] [PubMed] [Google Scholar]
- 86.Godau J, Knauel K, Weber K, Brockmann K, Maetzler W, Binder G, et al. Serum insulinlike growth factor 1 as possible marker for risk and early diagnosis of Parkinson disease. Arch Neurol. 2011;68(7):925–931. doi: 10.1001/archneurol.2011.129. [DOI] [PubMed] [Google Scholar]
- 87.Bernhard FP, Heinzel S, Binder G, Weber K, Apel A, Roeben B, et al. Insulin-like growth factor 1 (IGF-1) in Parkinson’s disease: potential as trait-, progression-and prediction marker and confounding factors. PLoS One. 2016;11(3):e0150552. doi: 10.1371/journal.pone.0150552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Picillo M, Pivonello R, Santangelo G, Pivonello C, Savastano R, Auriemma R, et al. Serum IGF-1 is associated with cognitive functions in early, drug-naïve Parkinson’s disease. PLoS One. 2017;12(10):e0186508. doi: 10.1371/journal.pone.0186508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pellecchia M, Santangelo G, Picillo M, Pivonello R, Longo K, Pivonello C, et al. Insulin-like growth factor-1 predicts cognitive functions at 2-year follow-up in early, drug-naïve Parkinson’s disease. Eur J Neurol. 2014;21(5):802–807. doi: 10.1111/ene.12137. [DOI] [PubMed] [Google Scholar]
- 90.Devaskar SU, Giddings SJ, Rajakumar PA, Carnaghi LR, Menon RK, Zahm DS. Insulin gene expression and insulin synthesis in mammalian neuronal cells. J Biol Chem. 1994;269(11):8445–8454. [PubMed] [Google Scholar]
- 91.Kuwabara T, Kagalwala MN, Onuma Y, Ito Y, Warashina M, Terashima K, et al. Insulin biosynthesis in neuronal progenitors derived from adult hippocampus and the olfactory bulb. EMBO Mol Med. 2011;3(12):742–754. doi: 10.1002/emmm.201100177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Werner H, LeRoith D. Insulin and insulin-like growth factor receptors in the brain: physiological and pathological aspects. Eur Neuropsychopharmacol. 2014;24(12):1947–1953. doi: 10.1016/j.euroneuro.2014.01.020. [DOI] [PubMed] [Google Scholar]
- 93.Unger JW, Livingston JN, Moss AM. Insulin receptors in the central nervous system: localization, signalling mechanisms and functional aspects. Prog Neurobiol. 1991;36(5):343–362. doi: 10.1016/0301-0082(91)90015-s. [DOI] [PubMed] [Google Scholar]
- 94.Takahashi M, Yamada T, Tooyama I, Moroo I, Kimura H, Yamamoto T, et al. Insulin receptor mRNA in the substantia nigra in Parkinson’s disease. Neurosci Lett. 1996;204(3):201–204. doi: 10.1016/0304-3940(96)12357-0. [DOI] [PubMed] [Google Scholar]
- 95.Saltiel AR, Kahn R. Insulin signalling and the regulation of glucose and lipid metabolism. Nature. 2001;414:799–806. doi: 10.1038/414799a. [DOI] [PubMed] [Google Scholar]
- 96.Vanhaesebroeck B, Stephens L, Hawkins P. PI3 K signalling: the path to discovery and understanding. Nat Rev Mol Cell Biol. 2012;13(3):195–203. doi: 10.1038/nrm3290. [DOI] [PubMed] [Google Scholar]
- 97.Eldar-Finkelman H, Krebs EG. Phosphorylation of insulin receptor substrate 1 by glycogen synthase kinase 3 impairs insulin action. Proc Natl Acad Sci USA. 1997;94(18):9660–9664. doi: 10.1073/pnas.94.18.9660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Duka T, Duka V, Joyce JN, Sidhu A. α-Synuclein contributes to GSK-3β-catalyzed Tau phosphorylation in Parkinson’s disease models. FASEB J. 2009;23(9):2820–2830. doi: 10.1096/fj.08-120410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gao S, Duan C, Gao G, Wang X, Yang H. Alpha-synuclein overexpression negatively regulates insulin receptor substrate 1 by activating mTORC1/S6K1 signaling. Int J Biochem Cell Biol. 2015;64:25–33. doi: 10.1016/j.biocel.2015.03.006. [DOI] [PubMed] [Google Scholar]
- 100.Spielman LJL, Klegeris A. Inflammation and insulin/IGF-1 resistance as the possible link between obesity and neurodegeneration. J Neuroimmunol. 2014;273:8–21. doi: 10.1016/j.jneuroim.2014.06.004. [DOI] [PubMed] [Google Scholar]
- 101.Ho L, Qin W, Pompl PN, Xiang Z, Wang J, Zhao Z, et al. Diet-induced insulin resistance promotes amyloidosis in a transgenic mouse model of Alzheimer’s disease. FASEB J. 2004;18(7):902–904. doi: 10.1096/fj.03-0978fje. [DOI] [PubMed] [Google Scholar]
- 102.Willette AA, Johnson SC, Birdsill AC, Sager MA, Christian B, Baker LD, et al. Insulin resistance predicts brain amyloid deposition in late middle-aged adults. Alzheimers Dement. 2015;11(5):504.e1–510.e1. doi: 10.1016/j.jalz.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Qiu WQ, Folstein MF. Insulin, insulin-degrading enzyme and amyloid-β peptide in Alzheimer’s disease: review and hypothesis. Neurobiol Aging. 2006;27(2):190–198. doi: 10.1016/j.neurobiolaging.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 104.Terrelonge M, Marder KS, Weintraub D, Alcalay RN. CSF β-amyloid 1-42 predicts progression to cognitive impairment in newly diagnosed Parkinson disease. J Mol Neurosci. 2016;58(1):88–92. doi: 10.1007/s12031-015-0647-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Alves G, Brønnick K, Aarsland D, Blennow K, Zetterberg H, Ballard C, et al. CSF amyloid-β and tau proteins, and cognitive performance, in early and untreated Parkinson’s disease: the Norwegian ParkWest study. J Neurol Neurosurg Psychiatry. 2010;81(10):1080–1086. doi: 10.1136/jnnp.2009.199950. [DOI] [PubMed] [Google Scholar]
- 106.Hepp DH, Vergoossen DL, Huisman E, Lemstra AW, Bank NB, Berendse HW, et al. Distribution and load of amyloid-β pathology in Parkinson disease and dementia with Lewy bodies. J Neuropathol Exp Neurol. 2016;75(10):936–945. doi: 10.1093/jnen/nlw070. [DOI] [PubMed] [Google Scholar]
- 107.Sharma SK, Chorell E, Steneberg P, Vernersson-Lindahl E, Edlund H, Wittung-Stafshede P. Insulin-degrading enzyme prevents α-synuclein fibril formation in a nonproteolytical manner. Sci Rep. 2015;5:12531. doi: 10.1038/srep12531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hoyer S. The aging brain. Changes in the neuronal insulin/insulin receptor signal transduction cascade trigger late-onset sporadic Alzheimer disease (SAD). A mini-review. J Neural Transm (Vienna) 2002;109(7–8):991–1002. doi: 10.1007/s007020200082. [DOI] [PubMed] [Google Scholar]
- 109.Mattila PM, Röyttä M, Lönnberg P, Marjamäki P, Helenius H, Rinne JO. Choline acetyltransferase activity and striatal dopamine receptors in Parkinson’s disease in relation to cognitive impairment. Acta Neuropathol. 2001;102(2):160–166. doi: 10.1007/s004010100372. [DOI] [PubMed] [Google Scholar]
- 110.Yarnall A, Rochester L, Burn DJ. The interplay of cholinergic function, attention, and falls in Parkinson’s disease. Mov Disord. 2011;26(14):2496–2503. doi: 10.1002/mds.23932. [DOI] [PubMed] [Google Scholar]
- 111.Bohnen NI, Albin RL. The cholinergic system and Parkinson disease. Behav Brain Res. 2011;221(2):564–573. doi: 10.1016/j.bbr.2009.12.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Athauda D, Foltynie T. The glucagon-like peptide 1 (GLP) receptor as a therapeutic target in Parkinson’s disease: mechanisms of action. Drug Discov Today. 2016;21(5):802–818. doi: 10.1016/j.drudis.2016.01.013. [DOI] [PubMed] [Google Scholar]
- 113.Kappe C, Tracy LM, Patrone C, Iverfeldt K, Sjöholm Å. GLP-1 secretion by microglial cells and decreased CNS expression in obesity. J Neuroinflammation. 2012;9(1):276. doi: 10.1186/1742-2094-9-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Trapp S, Cork SC. PPG neurons of the lower brain stem and their role in brain GLP-1 receptor activation. Am J Physiol Regul Integr Comp Physiol. 2015;309(8):R795–R804. doi: 10.1152/ajpregu.00333.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Alvarez E, Martínez MD, Roncero I, Chowen JA, García-Cuartero B, Gispert JD, et al. The expression of GLP-1 receptor mRNA and protein allows the effect of GLP-1 on glucose metabolism in the human hypothalamus and brainstem. J Neurochem. 2005;92(4):798–806. doi: 10.1111/j.1471-4159.2004.02914.x. [DOI] [PubMed] [Google Scholar]
- 116.Merchenthaler I, Lane MPS. Distribution of pre-proglucagon and glucagon-like peptide-1 receptor messenger RNAs in the rat central nervous system. J Comp Neurol. 1990;403(2):261–280. doi: 10.1002/(sici)1096-9861(19990111)403:2<261::aid-cne8>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 117.Athauda D, Foltynie T. Protective effects of the GLP-1 mimetic exendin-4 in Parkinson’s disease. Neuropharmacology. 2018;136(Pt B):260–270. doi: 10.1016/j.neuropharm.2017.09.023. [DOI] [PubMed] [Google Scholar]
- 118.Zhang Y, Sun B, Feng D, Hu H, Chu M, Qu Q, et al. Cryo-EM structure of the activated GLP-1 receptor in complex with a G protein. Nature. 2017;8(546):248–253. doi: 10.1038/nature22394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kim DS, Choi HI, Wang Y, Luo Y, Hoffer BJ, Greig NH. A new treatment strategy for Parkinson’s disease through the gut–brain axis: the glucagon-like peptide-1 receptor pathway. Cell Transplant. 2017;26(9):1560–1571. doi: 10.1177/0963689717721234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Svenningsson P, Wirdefeldt K, Yin L, Fang F, Markaki I, Efendic S, et al. Reduced incidence of Parkinson’s disease after dipeptidyl peptidase-4 inhibitors—a nationwide case-control study. Mov Disord. 2016;31(9):1422–1423. doi: 10.1002/mds.26734. [DOI] [PubMed] [Google Scholar]
- 121.Hölscher C. Insulin, incretins and other growth factors as potential novel treatments for Alzheimer’s and Parkinson’s diseases. Biochem Soc Trans. 2014;42(2):593–599. doi: 10.1042/BST20140016. [DOI] [PubMed] [Google Scholar]
- 122.Hölscher C. Novel dual GLP-1/GIP receptor agonists show neuroprotective effects in Alzheimer’s and Parkinson’s disease models. Neuropharmacology. 2018;136(Pt B):251–259. doi: 10.1016/j.neuropharm.2018.01.040. [DOI] [PubMed] [Google Scholar]
- 123.Li Y, Chigurupati S, Holloway HW, Mughal M, Tweedie D, Bruestle DA, et al. Exendin-4 ameliorates motor neuron degeneration in cellular and animal models of amyotrophic lateral sclerosis. PLoS One. 2012;7(2):e32008. doi: 10.1371/journal.pone.0032008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Bertilsson G, Patrone C, Zachrisson O, Andersson A, Dannaeus K, Heidrich J, et al. Peptide hormone exendin-4 stimulates subventricular zone neurogenesis in the adult rodent brain and induces recovery in an animal model of Parkinson’s disease. J Neurosci Res. 2008;86(2):326–338. doi: 10.1002/jnr.21483. [DOI] [PubMed] [Google Scholar]
- 125.Swanson C, Emborg M. Expression of peroxisome proliferator-activated receptor-gamma in the substantia nigra of hemiparkinsonian nonhuman primates. Neurol Res. 2014;36(7):634–646. doi: 10.1179/1743132813Y.0000000305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Warden A, Truitt J, Merriman M, Ponomareva O, Jameson K, Ferguson LB, et al. Localization of PPAR isotypes in the adult mouse and human brain. Sci Rep. 2016;6:27618. doi: 10.1038/srep27618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Daynes RA, Jones DC. Emerging roles of PPARs in inflammation and immunity. Nat Rev Immunol. 2002;2(10):748–759. doi: 10.1038/nri912. [DOI] [PubMed] [Google Scholar]
- 128.Jiang C, Ting AT, Seed B. PPAR-γ agonists inhibit production of monocyte inflammatory cytokines. Nature. 1998;391(6662):82–86. doi: 10.1038/34184. [DOI] [PubMed] [Google Scholar]
- 129.Hunter RL, Choi D-Y, Ross SA, Bing G. Protective properties afforded by pioglitazone against intrastriatal LPS in Sprague-Dawley rats. Neurosci Lett. 2008;432(3):198–201. doi: 10.1016/j.neulet.2007.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Breidert T, Callebert J, Heneka M, Landreth G, Launay J, Hirsch E. Protective action of the peroxisome proliferator-activated receptor-γ agonist pioglitazone in a mouse model of Parkinson’s disease. J Neurochem. 2002;82(3):615–624. doi: 10.1046/j.1471-4159.2002.00990.x. [DOI] [PubMed] [Google Scholar]
- 131.Dehmer T, Heneka MT, Sastre M, Dichgans J, Schulz JB. Protection by pioglitazone in the MPTP model of Parkinson’s disease correlates with IκBα induction and block of NFκB and iNOS activation. J Neurochem. 2004;88(2):494–501. doi: 10.1046/j.1471-4159.2003.02210.x. [DOI] [PubMed] [Google Scholar]
- 132.Swanson CR, Joers V, Bondarenko V, Brunner K, Simmons HA, Ziegler TE, et al. The PPAR-γ agonist pioglitazone modulates inflammation and induces neuroprotection in parkinsonian monkeys. J Neuroinflammation. 2011;8(1):91. doi: 10.1186/1742-2094-8-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Brauer R, Bhaskaran K, Chaturvedi N, Dexter DT, Smeeth L, Douglas I. Glitazone treatment and incidence of Parkinson’s disease among people with diabetes: a retrospective cohort study. PLoS Med. 2015;12(7):e1001854. doi: 10.1371/journal.pmed.1001854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Matteau E, Dupré N, Langlois M, Provencher P, Simard M. Clinical validity of the Mattis dementia rating scale-2 in Parkinson disease with MCI and dementia. J Geriatr Psychiatry Neurol. 2012;25(2):100–106. doi: 10.1177/0891988712445086. [DOI] [PubMed] [Google Scholar]
- 135.NINDS Exploratory Trials in Parkinson Disease (NET-PD) FS-ZONE Investigators Pioglitazone in early Parkinson’s disease: a phase 2, multicentre, double-blind, randomised trial. Lancet Neurol. 2015;14(8):795–803. doi: 10.1016/S1474-4422(15)00144-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lu CH, Yang CY, Li CY, Hsieh CY, Ou HT. Lower risk of dementia with pioglitazone, compared with other second-line treatments, in metformin-based dual therapy: a population-based longitudinal study. Diabetologia. 2018;61(3):562–573. doi: 10.1007/s00125-017-4499-5. [DOI] [PubMed] [Google Scholar]
- 137.Kang MY, Oh TJ, Cho YM. Glucagon-like peptide-1 increases mitochondrial biogenesis and function in INS-1 rat insulinoma cells. Endocrinol Metab (Seoul). 2015;30(2):216–220. doi: 10.3803/EnM.2015.30.2.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Clark J, Reddy S, Zheng K, Betensky RA, Simon DK. Association of PGC-1alpha polymorphisms with age of onset and risk of Parkinson’s disease. BMC Med Genet. 2011;12(1):69. doi: 10.1186/1471-2350-12-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Zheng B, Liao Z, Locascio JJ, Lesniak KA, Roderick SS, Watt ML, et al. PGC-1α, a potential therapeutic target for early intervention in Parkinson’s disease. Sci Transl Med. 2010;2(52):52ra73. doi: 10.1126/scitranslmed.3001059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Mudo G, Mäkelä J, Di Liberto V, Tselykh TV, Olivieri M, Piepponen P, et al. Transgenic expression and activation of PGC-1α protect dopaminergic neurons in the MPTP mouse model of Parkinson’s disease. Cell Mol Life Sci. 2012;69(7):1153–1165. doi: 10.1007/s00018-011-0850-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.van Laar T, De Deyn PP, Aarsland D, Barone P, Galvin JE. Effects of cholinesterase inhibitors in Parkinson’s disease dementia: a review of clinical data. CNS Neurosci Ther. 2011;17(5):428–441. doi: 10.1111/j.1755-5949.2010.00166.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.O’Brien JT, Holmes C, Jones M, Jones R, Livingston G, McKeith I, et al. Clinical practice with anti-dementia drugs: a revised (third) consensus statement from the British Association for Psychopharmacology. J Psychopharmacol. 2017;31(2):147–168. doi: 10.1177/0269881116680924. [DOI] [PubMed] [Google Scholar]
- 143.Emre M, Aarsland D, Albanese A, Byrne EJ, Deuschl G, De Deyn PP, et al. Rivastigmine for dementia associated with Parkinson’s disease. N Engl J Med. 2004;351(24):2509–2518. doi: 10.1056/NEJMoa041470. [DOI] [PubMed] [Google Scholar]
- 144.Emre M, Poewe W, De Deyn PP, Barone P, Kulisevsky J, Pourcher E, et al. Long-term safety of rivastigmine in parkinson disease dementia: an open-label, randomized study. Clin Neuropharmacol. 2014;37(1):9–16. doi: 10.1097/WNF.0000000000000010. [DOI] [PubMed] [Google Scholar]
- 145.Aarsland D, Laake K, Larsen JP, Janvin C. Donepezil for cognitive impairment in Parkinson’s disease: a randomised controlled study. J Neurol Neurosurg Psychiatry. 2002;72(6):708–712. doi: 10.1136/jnnp.72.6.708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Leroi I, Brandt J, Reich SG, Lyketsos CG, Grill S, Thompson R, et al. Randomized placebo-controlled trial of donepezil in cognitive impairment in Parkinson’s disease. Int J Geriatr Psychiatry. 2004;19(1):1–8. doi: 10.1002/gps.993. [DOI] [PubMed] [Google Scholar]
- 147.Ravina B, Putt M, Siderowf A, Farrar JT, Gillespie M, Crawley A, et al. Donepezil for dementia in Parkinson’s disease: a randomised, double blind, placebo controlled, crossover study. J Neurol Neurosurg Psychiatry. 2005;76(7):934–939. doi: 10.1136/jnnp.2004.050682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Dubois B, Tolosa E, Katzenschlager R, Emre M, Lees AJ, Schumann G, et al. Donepezil in Parkinson’s disease dementia: a randomized, double-blind efficacy and safety study. Mov Disord. 2012;27(10):1230–1238. doi: 10.1002/mds.25098. [DOI] [PubMed] [Google Scholar]
- 149.Matsunaga S, Kishi T, Yasue I, Iwata N. Cholinesterase inhibitors for Lewy body disorders: a meta-analysis. Int J Neuropsychopharmacol. 2015;19(2):pyv086. doi: 10.1093/ijnp/pyv086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Possin KL, Kang GA, Guo C, Fine EM, Trujillo AJ, Racine CA, et al. Rivastigmine is associated with restoration of left frontal brain activity in Parkinson’s disease. Mov Disord. 2013;28(10):1384–1390. doi: 10.1002/mds.25575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Mamikonyan E, Xie SX, Melvin E, Weintraub D. Rivastigmine for mild cognitive impairment in Parkinson disease: a placebo-controlled study. Mov Disord. 2015;30(7):912–918. doi: 10.1002/mds.26236. [DOI] [PubMed] [Google Scholar]
- 152.Aarsland D, Ballard C, Walker Z, Bostrom F, Alves G, Kossakowski K, et al. Memantine in patients with Parkinson’s disease dementia or dementia with Lewy bodies: a double-blind, placebo-controlled, multicentre trial. Lancet Neurol. 2009;8(7):613–618. doi: 10.1016/S1474-4422(09)70146-2. [DOI] [PubMed] [Google Scholar]
- 153.Emre M, Tsolaki M, Bonuccelli U, Destee A, Tolosa E, Kutzelnigg A, et al. Memantine for patients with Parkinson’s disease dementia or dementia with Lewy bodies: a randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2010;9(10):969–977. doi: 10.1016/S1474-4422(10)70194-0. [DOI] [PubMed] [Google Scholar]
- 154.Leroi I, Overshott R, Byrne EJ, Daniel E, Burns A. Randomized controlled trial of memantine in dementia associated with Parkinson’s disease. Mov Disord. 2009;24(8):1217–1221. doi: 10.1002/mds.22495. [DOI] [PubMed] [Google Scholar]
- 155.Wang HF, Yu JT, Tang SW, Jiang T, Tan CC, Meng XF, et al. Efficacy and safety of cholinesterase inhibitors and memantine in cognitive impairment in Parkinson’s disease, Parkinson’s disease dementia, and dementia with Lewy bodies: systematic review with meta-analysis and trial sequential analysis. J Neurol Neurosurg Psychiatry. 2015;86(2):135–143. doi: 10.1136/jnnp-2014-307659. [DOI] [PubMed] [Google Scholar]
- 156.Litvan I, Kieburtz K, Tröster AI, Aarsland D. Strengths and challenges in conducting clinical trials in Parkinson’s disease mild cognitive impairment. Mov Disord. 2018;33(4):520–527. doi: 10.1002/mds.27345. [DOI] [PubMed] [Google Scholar]
- 157.Tsai CH, Huang HC, Liu BL, Li CI, Lu MK, Chen X, et al. Activation of n-methyl-d-aspartate receptor glycine site temporally ameliorates neuropsychiatric symptoms of parkinson’s disease with dementia. Psychiatry Clin Neurosci. 2014;68(9):692–700. doi: 10.1111/pcn.12175. [DOI] [PubMed] [Google Scholar]
- 158.Hsu CY, Hung CS, Chang HM, Liao WC, Ho SC, Ho YJ. Ceftriaxone prevents and reverses behavioral and neuronal deficits in an MPTP-induced animal model of Parkinson’s disease dementia. Neuropharmacology. 2015;91:43–56. doi: 10.1016/j.neuropharm.2014.11.023. [DOI] [PubMed] [Google Scholar]
- 159.Kaur B, Prakash A. Ceftriaxone attenuates glutamate-mediated neuro-inflammation and restores BDNF in MPTP model of Parkinson’s disease in rats. Pathophysiology. 2017;24(2):71–79. doi: 10.1016/j.pathophys.2017.02.001. [DOI] [PubMed] [Google Scholar]
- 160.Weng JC, Tikhonova MA, Chen JH, Shen MS, Meng WY, Chang YT, et al. Ceftriaxone prevents the neurodegeneration and decreased neurogenesis seen in a Parkinson’s disease rat model: an immunohistochemical and MRI study. Behav Brain Res. 2016;15(305):126–139. doi: 10.1016/j.bbr.2016.02.034. [DOI] [PubMed] [Google Scholar]
- 161.Lahmy V, Meunier J, Malmstrom S, Naert G, Givalois L, Kim SH, et al. Blockade of tau hyperphosphorylation and Abeta(1)(-)(4)(2) generation by the aminotetrahydrofuran derivative ANAVEX2-73, a mixed muscarinic and sigma(1) receptor agonist, in a nontransgenic mouse model of Alzheimer’s disease. Neuropsychopharmacology. 2013;38(9):1706–1723. doi: 10.1038/npp.2013.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Villard V, Espallergues J, Keller E, Vamvakides A, Maurice T. Anti-amnesic and neuroprotective potentials of the mixed muscarinic receptor/sigma 1 (sigma1) ligand ANAVEX2-73, a novel aminotetrahydrofuran derivative. J Psychopharmacol. 2011;25(8):1101–1117. doi: 10.1177/0269881110379286. [DOI] [PubMed] [Google Scholar]
- 163.Maurice T. Protection by sigma-1 receptor agonists is synergic with donepezil, but not with memantine, in a mouse model of amyloid-induced memory impairments. Behav Brain Res. 2016;1(296):270–278. doi: 10.1016/j.bbr.2015.09.020. [DOI] [PubMed] [Google Scholar]
- 164.Marsh L, Biglan K, Gerstenhaber M, Williams JR. Atomoxetine for the treatment of executive dysfunction in Parkinson’s disease: a pilot open-label study. Mov Disord. 2009;24(2):277–282. doi: 10.1002/mds.22307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Stahl SM. Mechanism of action of pimavanserin in Parkinson’s disease psychosis: targeting serotonin 5HT2A and 5HT2C receptors. CNS Spectr. 2016;21(4):271–275. doi: 10.1017/S1092852916000407. [DOI] [PubMed] [Google Scholar]
- 166.Meneses A. Memory formation and memory alterations: 5-HT6 and 5-HT7 receptors, novel alternative. Rev Neurosci. 2014;25(3):325–356. doi: 10.1515/revneuro-2014-0001. [DOI] [PubMed] [Google Scholar]
- 167.Khoury R, Grysman N, Gold J, Patel K, Grossberg GT. The role of 5 HT6-receptor antagonists in Alzheimer’s disease: an update. Expert Opin Investig Drugs. 2018;27(6):523–533. doi: 10.1080/13543784.2018.1483334. [DOI] [PubMed] [Google Scholar]
- 168.Al-Shamma HA, Anderson C, Chuang E, Luthringer R, Grottick AJ, Hauser E, et al. Nelotanserin, a novel selective human 5-hydroxytryptamine2A inverse agonist for the treatment of insomnia. J Pharmacol Exp Ther. 2010;332(1):281–290. doi: 10.1124/jpet.109.160994. [DOI] [PubMed] [Google Scholar]
- 169.Calhoun A, Ko J, Grossberg GT. Emerging chemical therapies targeting 5-hydroxytryptamine in the treatment of Alzheimer’s disease. Expert Opin Emerg Drugs. 2017;22(1):101–105. doi: 10.1080/14728214.2017.1293651. [DOI] [PubMed] [Google Scholar]
- 170.Harsh JR, Hayduk R, Rosenberg R, Wesnes KA, Walsh JK, Arora S, et al. The efficacy and safety of armodafinil as treatment for adults with excessive sleepiness associated with narcolepsy. Curr Med Res Opin. 2006;22(4):761–774. doi: 10.1185/030079906X100050. [DOI] [PubMed] [Google Scholar]
- 171.Varanese S, Perfetti B, Gilbert-Wolf R, Thomas A, Onofrj M, Di Rocco A. Modafinil and armodafinil improve attention and global mental status in Lewy bodies disorders: preliminary evidence. Int J Geriatr Psychiatry. 2013;28(10):1095–1097. doi: 10.1002/gps.3952. [DOI] [PubMed] [Google Scholar]
- 172.Lapid MI, Kuntz KM, Mason SS, Aakre JA, Lundt ES, Kremers W, et al. Efficacy, safety, and tolerability of armodafinil therapy for hypersomnia associated with dementia with Lewy bodies: a pilot study. Dement Geriatr Cogn Disord. 2017;43(5–6):269–280. doi: 10.1159/000471507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Nishida T, Doi T, Naito Y. Tyrosine kinase inhibitors in the treatment of unresectable or metastatic gastrointestinal stromal tumors. Expert Opin Pharmacother. 2014;15(14):1979–1989. doi: 10.1517/14656566.2014.937707. [DOI] [PubMed] [Google Scholar]
- 174.Abushouk AI, Negida A, Elshenawy RA, Zein H, Hammad AM, Menshawy A, et al. C-Abl inhibition; a novel therapeutic target for Parkinson’s disease. CNS Neurol Disord Drug Targets. 2018;17(1):14–21. doi: 10.2174/1871527316666170602101538. [DOI] [PubMed] [Google Scholar]
- 175.Pagan F, Hebron M, Valadez EH, Torres-Yaghi Y, Huang X, Mills RR, et al. Nilotinib effects in Parkinson’s disease and dementia with Lewy bodies. J Parkinsons Dis. 2016;6(3):503–517. doi: 10.3233/JPD-160867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Wyse RK, Brundin P, Sherer TB. Nilotinib—differentiating the hope from the hype. J Parkinsons Dis. 2016;6(3):519–522. doi: 10.3233/JPD-160904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Balestrino R, Schapira AHV. Glucocerebrosidase and Parkinson disease: molecular, clinical, and therapeutic implications. Neuroscientist. 2018;24(5):540–559. doi: 10.1177/1073858417748875. [DOI] [PubMed] [Google Scholar]
- 178.Liu W, Jalewa J, Sharma M, Li G, Li L, Hölscher C. Neuroprotective effects of lixisenatide and liraglutide in the 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine mouse model of Parkinson’s disease. Neuroscience. 2015;303:42–50. doi: 10.1016/j.neuroscience.2015.06.054. [DOI] [PubMed] [Google Scholar]
- 179.Zhang L, Zhang L, Li L, Hölscher C. Neuroprotective effects of the novel GLP-1 long acting analogue semaglutide in the MPTP Parkinson’s disease mouse model. Neuropeptides. 2018;71:70–80. doi: 10.1016/j.npep.2018.07.003. [DOI] [PubMed] [Google Scholar]
- 180.Aviles-Olmos I, Dickson J, Kefalopoulou Z, Djamshidian A, Ell P, Soderlund T, et al. Exenatide and the treatment of patients with Parkinson’s disease. J Clin Invest. 2013;123(6):2730–2736. doi: 10.1172/JCI68295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Aviles-Olmos I, Dickson J, Kefalopoulou Z, Djamshidian A, Kahan J, Ell P, et al. Motor and cognitive advantages persist 12 months after exenatide exposure in Parkinson’s disease. J Parkinsons Dis. 2014;4(3):337–344. doi: 10.3233/JPD-140364. [DOI] [PubMed] [Google Scholar]
- 182.Athauda D, Maclagan K, Skene SS, Bajwa-Joseph M, Letchford D, Chowdhury K, et al. Exenatide once weekly versus placebo in Parkinson’s disease: a randomised, double-blind, placebo-controlled trial. Lancet. 2017;390(10103):1664–1675. doi: 10.1016/S0140-6736(17)31585-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Athauda D, Maclagan K, Budnik N, Zampedri L, Hibbert S, Aviles-Olmos I, et al. Post hoc analysis of the Exenatide-PD trial—factors that predict response. Eur J Neurosci. 2018 doi: 10.1111/ejn.14096. [DOI] [PubMed] [Google Scholar]


