Abstract
Maize ear rot is a common disease found worldwide, caused by several toxigenic Fusarium species. Maize ears and kernels infected by Fusarium subglutinans contained significant amounts of beauvericin, fusaproliferin, moniliformin, and enniatins. In 2011, F. subglutinans sensu lato has been divided into two species: Fusarium temperatum sp. nov. and F. subglutinans sensu stricto, showing different phylogeny and beauvericin production within the populations of maize pathogens in Belgium. Isolates of the new species—F. temperatum—were also identified and characterized in Spain, Argentina, Poland, France, and China as one of the most important pathogens of maize. Moreover, F. temperatum was proved to be pathogenic to maize seedlings and stalks. We identified Fusarium isolates obtained from diseased maize ears collected between 2013 and 2016 in Poland (321 isolates). Based on morphological analyses, six Fusarium species were identified. Molecular identification performed on the set of selected isolates (42 isolates) revealed 34 isolates to be F. temperatum and only five to be F. subglutinans. Interestingly, the phylogenetic analysis showed that the population of F. temperatum infecting maize in Poland remained quite uniform for over 30 years with only a few exceptions. For the first time, a single isolate of Fusarium ramigenum was detected from the area of Poland. Significant amounts of BEA were found in Fusarium-damaged kernels. The same kernel samples contained also enniatins A1, A, B1, and B. The results clearly demonstrate the occurrence of F. temperatum as maize pathogen in Poland for over the last three decades.
Keywords: Fusarium species, Beauvericin, Enniatins, Maize ear rot, Translation elongation factor tef-1α
Introduction
Maize ear rot is a common disease found worldwide, caused by several toxigenic Fusarium species and leading to the accumulation of several mycotoxins in kernels. The prevailing pathogenic species can vary over the years, depending on various factors such as the continent and region, agro-ecological conditions (Bottalico and Perrone 2002), and insect damage (Lew et al. 1991). Disease severity can also be influenced by other stress factors and susceptibility of cultivars (hybrids) to the infection by Fusarium species and to the accumulation of mycotoxins in kernels (Pascale et al. 2002).
The research on toxigenic fungal species and mycotoxins contaminating maize kernels has been conducted in Poland since 1984 in 15 growing seasons. F. subglutinans sensu lato was estimated as the prevailing species from 1985 until 1991, and it was accompanied by seven other species. Since 1995, the frequency of Fusarium verticillioides increased in most years, and this species outcompeted F. subglutinans, which frequency decreased, particularly in 1993 and 2006 seasons (Gromadzka et al. 2016). Mycotoxin moniliformin was the first toxin identified in the cultures of F. subglutinans isolates and in infected maize kernels from Poland (Sharman et al. 1991; Lew et al. 1996). Maize ears and kernels infected by F. subglutinans contained significant amounts of beauvericin (BEA), fusaproliferin (FP), moniliformin (MON), and enniatins (ENNs) (Logrieco et al. 1998; Gromadzka et al. 2016). MON has been shown to be toxic to animals and plants, whereas BEA and FP to insects, human cell lines, and chicken embryos and they play important roles in plant and animal diseases. BEA is entomopathogenic and highly toxic to insects (Logrieco et al.1998).
F. subglutinans is a member of the Fusarium fujikuroi species complex (FFSC) mating population E, known for many years as cosmopolitan species in agricultural and global environments, particularly being a pathogen of maize. The species was described for the first time by Nelson et al. (1983) in their manual, but at that time, no morphologically distinct subspecies could be found (Leslie and Summerell 2006). The number of recognized species of FFSC increased after over 15 years of DNA assays application and now over 50 distinct species are recognized (Aoki et al. 2014).
Isolates originating from Europe produced several mycotoxins: e.g., moniliformin, beauvericin, and fusaproliferin, both under laboratory and field conditions (Sharman et al. 1991; Logrieco et al. 1998; Kostecki et al. 1995, 1999; Krska et al. 1996; Lew et al. 1996).
Steenkamp et al. (2002) found two groups of F. subglutinans in a South African population of the species, showing distinct PCR-RFLP patterns. Additionally, isolates belonging to group 1 produced beauvericin (BEA) and isolates from group 2 were non-producers of BEA.
Moretti et al. (2008) examined the collection of 87 European F. subglutinans isolates using RFLP method and found two cryptic subspecies in these populations, which originated from Poland, Germany, Slovakia, Portugal, Italy, and former Yugoslavia. BEA was produced by 77% of isolates in amounts of 10–532 μg/g in rice cultures under laboratory conditions. Scauflaire et al. (2011) distinguished F. temperatum Scaufl. & Munaut species from F. subglutinans sensu lato within FFSC using the translation elongation factor gene (tef-1α) and β-tubulin (tub-2) DNA sequence analyses, mating compatibility and metabolite profiling, and described the species formally.
Fumero et al. (2015) isolated both F. temperatum and F. subglutinans from maize in Argentina. F. temperatum strains were BEA and fusaproliferin producers. All F. subglutinans isolates were fusaproliferin producers but none produced BEA.
Furthermore, both species were found in Spain, Poland, and France (Pintos et al. 2013; Czembor et al. 2014, 2015; Boutigny et al. 2017), and F. temperatum was proved to be pathogenic to maize seedlings and stalks. Wang et al. (2014) isolated F. temperatum from maize in China in 2009 and suggested that species produced fumonisins in infected ears under field conditions.
The aim of the present paper was to re-examine the isolates of F. subglutinans sensu lato from maize maintained 1984–1990 in the KF collection of pathogenic fungi at the Institute of Plant Genetics, Polish Academy of Sciences, Poznań, Poland (7 isolates), and to compare their identity and diversity to the 42 new isolates collected between 2013 and 2016 growing seasons using molecular analyses. Until recently, the balance between the frequencies of both species (F. subglutinans sensu stricto and F. temperatum) was not clear. Therefore, we tried to get a deep insight in the Polish population of the species and confront the data with the historical results. Additionally, Fusarium mycotoxin accumulation was examined in maize kernels.
Materials and methods
Fungal isolation and identification
Maize ear samples were collected in October 2013 (80 maize ear samples), 2014 (100 maize ear samples), 2015 (83 maize ear samples), and 2016 (58 maize ear samples) from maize fields in main maize-growing areas in Poland. Ears with significant ear rot symptoms were scored for the Fusarium ear rot rating (1–100% for moldy, discolored, and shrunken kernels), placed in separate paper bags, transported to the laboratory, and dried at room temperature. Then, surface mycelium and small pieces of kernels from each ear were placed in duplicate on agar plates with a low nutrient SNA medium to identify Fusarium species (Kostecki et al. 1995; Kwaśna et al. 1991). A tip of hyphae from each culture was transferred to both potato dextrose agar and synthetic SNA low nutrient agar. Fusarium species were identified according to Kwaśna et al. (1991) and Leslie and Summerell (2006).
Fusarium species were identified in all collected maize ears (321 samples) between 2013 and 2016. Based on this morphological identification, Table 1 was prepared. Then, F. subglutinans sensu lato isolates were selected for further molecular studies (2013, 1 isolate; 2014, 10 isolates; 2015, 20 isolates; 2016, 11 isolates). Additionally, we re-examined the isolates of F. subglutinans sensu lato from maize, maintained in the culture collection and isolated between 1984 and 1990 (7 isolates).
Table 1.
Fusarium species isolated from maize with ear rot symptoms in 2013–2016 seasons in Poland based on morphological identification
| Year | Frequency of Fusarium species [%] | |||||
|---|---|---|---|---|---|---|
| F. cul | F. prolif | F. gram | F. poae | F. sub | F. vert | |
| 2013* | 0 | 0 | 7.1 | 45.7 | 3.1 | 44.1 |
| 2014* | 0 | 0 | 13.2 | 14.0 | 26.3 | 46.5 |
| 2015 | 2.7 | 4.0 | 5.3 | 20.0 | 28.0 | 40.0 |
| 2016 | 0 | 4.5 | 7.2 | 26.0 | 27.6 | 34.7 |
*According to Gromadzka et al. (2016)
F. prolif, Fusarium proliferatum (Matsushima) Nirenberg; F. cul, Fusarium culmorum (W.G. Smith) Saccardo; F. gram, Fusarium graminearum Schwabe; F. poae, Fusarium poae (Peck) Wollenw; F. sub, Fusarium subglutinans sensu lato (Wollenw. & Reinking) Nelson, Toussoun & Marasas; F. vert, Fusarium verticillioides (Saccardo) Nirenberg (F. moniliforme Sheldon)
New isolates from harvest seasons 2013–2014 (accessions with “K” designation) and isolates originating from maize kernels from harvest seasons 1984–1990, deposited in the culture collection of the Institute of Plant Genetics, Poznań, Poland (accessions with “KF” designation) and the Institute of Sciences of Food Production, CNR, Bari, Italy (accessions with “ITEM” designation), were used to examine their genetic diversity based on partial tef-1α sequence analysis.
Fungal isolates were stored on SNA slants in the refrigerator and in sterile 18% glycerol at − 75 °C.
The frequency of F. subglutinans occurrence (1985–2014) was based partly on previous studies (Gromadzka et al. 2016) and partly on present studies (2015–2016) for comparison.
Molecular and phylogenetic analyses
Growing mycelia of individual fungal isolates were maintained in pure cultures for 7 days on PDA medium. Genomic DNA was extracted using a modified CTAB (hexadecyltrimethylammonium bromide) (method described earlier by Stępień et al. (2013)). The DNA extracts were stored at − 20 °C. Species identification was done on the basis of the sequences of the translation elongation factor 1 alpha (tef-1α). Polymerase chain reactions were performed as described earlier by Stępień et al. (2013), using DreamTaq Green DNA polymerase (Thermo Scientific, Espoo, Finland). Amplicons of ca. 600 bp in length were electrophoresed in 1.5% agarose gel (Invitrogen) with GelGreen Nucleic Acid Stain (Biotium, Inc.). For sequence analysis, PCR-amplified DNA fragments were labeled using forward primer and the BigDyeTerminator 3.1 kit (Applied Biosystems, Foster City, CA, USA), according to producer’s recommendations, and precipitated with 96% ethanol. Sequence reading was performed using Applied Biosystems equipment. Sequences were analyzed using BLASTn algorithm. The dendrogram was calculated using MEGA 4 software package (Tamura et al. 2007) using F. oxysporum (GenBank sequence) and F. nygamai KF 337 (home made sequence) as an outgroup. Maximum parsimony heuristics were used with “fill gaps” function. Bootstrapping was done using 1000 iterations. Only branches with at least 50% of support were considered and presented.
Mycotoxin analyses
Mycotoxins were analyzed in maize ear samples, which were collected in the years 2013–2016. Kernels of ears were manually separated into two fractions: Fusarium-damaged kernels (FDK) and healthy-looking kernels (HLK—symptomless kernels). Then kernels of the FDK fraction of samples naturally colonized by F. subglutinans sensu lato were subjected to chemical analyses of mycotoxins using methodologies described below.
In the case of maize ears number K116, K308.1, K308.2, K343, K419, and K429, the amounts of the FDK fractions were insufficient to determine mycotoxins.
Chemicals and reagents
Mycotoxin standards (MON; Enniatins: A, A1, B, and B1; BEA) and all chemicals were supplied by Sigma-Aldrich (Steinheim, Germany). Water of HPLC grade from our own Millipore water purification system was used for analyses.
Sample preparation, extraction, and HPLC analysis
Mycotoxin content was determined using the chromatographic system: a Waters 2695 high-performance liquid chromatograph, a Waters 2475 Multi λ Fluorescence Detector, and/or a Waters 2996 Array Detector.
Enniatins and beauvericin were identified and quantified as reported by Jestoi et al. (2004). The MON content was analyzed according to the method described by Kostecki et al. (1999).
Results and discussion
Diversity of the Fusarium isolates
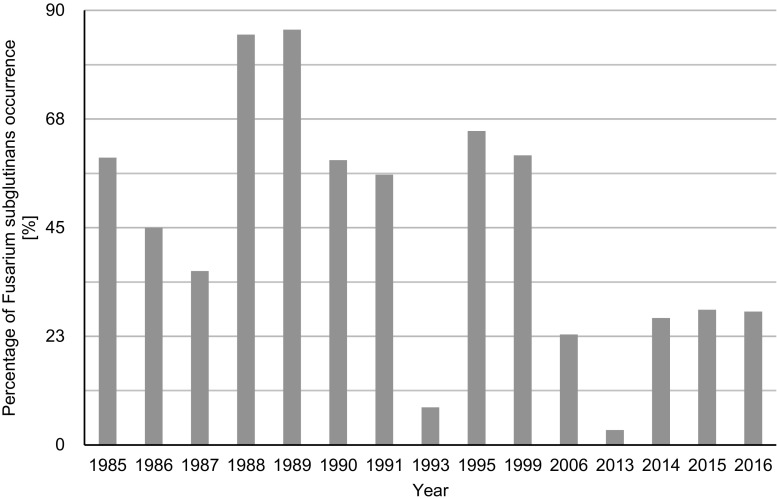
Among all isolates obtained during the 4-year study, at least six species were identified based on morphological analyses (Table 1). Three Fusarium species were identified in moldy maize ears in two localities studied with the high frequencies: F. verticillioides (34.7–46.5%), F. subglutinans sensu lato (3.1–28.0%), and F. poae (14.0–45.7%). F. subglutinans sensu lato was found in maize ear samples on the area of Poland in 15 seasons since 1984 until 2016, and the species frequencies were ranging from 3.1 up to 86% of the total Fusarium isolates (Fig. 1).
Fig. 1.
Percentage of Fusarium subglutinans sensu lato (F. subglutinans sensu stricto and F. temperatum) occurrence [%] in Poland in the years 1985–2016
Frequency of F. subglutinans sensu lato was particularly high in harvest years 1985–1991 and also in 1995 and 1999 seasons. High content of moniliformin up to 4 mg/kg was found in the fraction of F. subglutinans–damaged kernels of those samples (Lew et al. 1996). In the last year, the frequency of F. subglutinans sensu lato increased (3.1–28.0%) with the decreasing frequency of F. verticillioides (44.1–34.7%). F. proliferatum was found in 2015 and 2016 seasons with the frequencies of 4.0 and 4.5%, respectively (Table 1). Considering F. subglutinans and F. verticillioides, their frequencies significantly changed (Gromadzka et al. 2016). In the years 1988–1990, the discussed species were at the level of 85–56% and 3%, respectively.
Scauflaire et al. (2011) have divided F. subglutinans sensu lato into the two species, F. temperatum sp. nov. and F. subglutinans, showing different phylogeny and beauvericin production within the populations of maize pathogens in Belgium. Isolates of the new species—F. temperatum—were also identified and characterized in Spain (Pintos et al. 2013), Argentina (Fumero et al. 2015), Poland (Czembor et al. 2014, 2015), France (Boutigny et al. 2017), and China (Wang et al. 2014) among the most important pathogens of maize.
That is why we decided to check how the population of these two species is shaped in Poland and which of the two discussed species dominates nowadays compared to the 1985–1999 period.
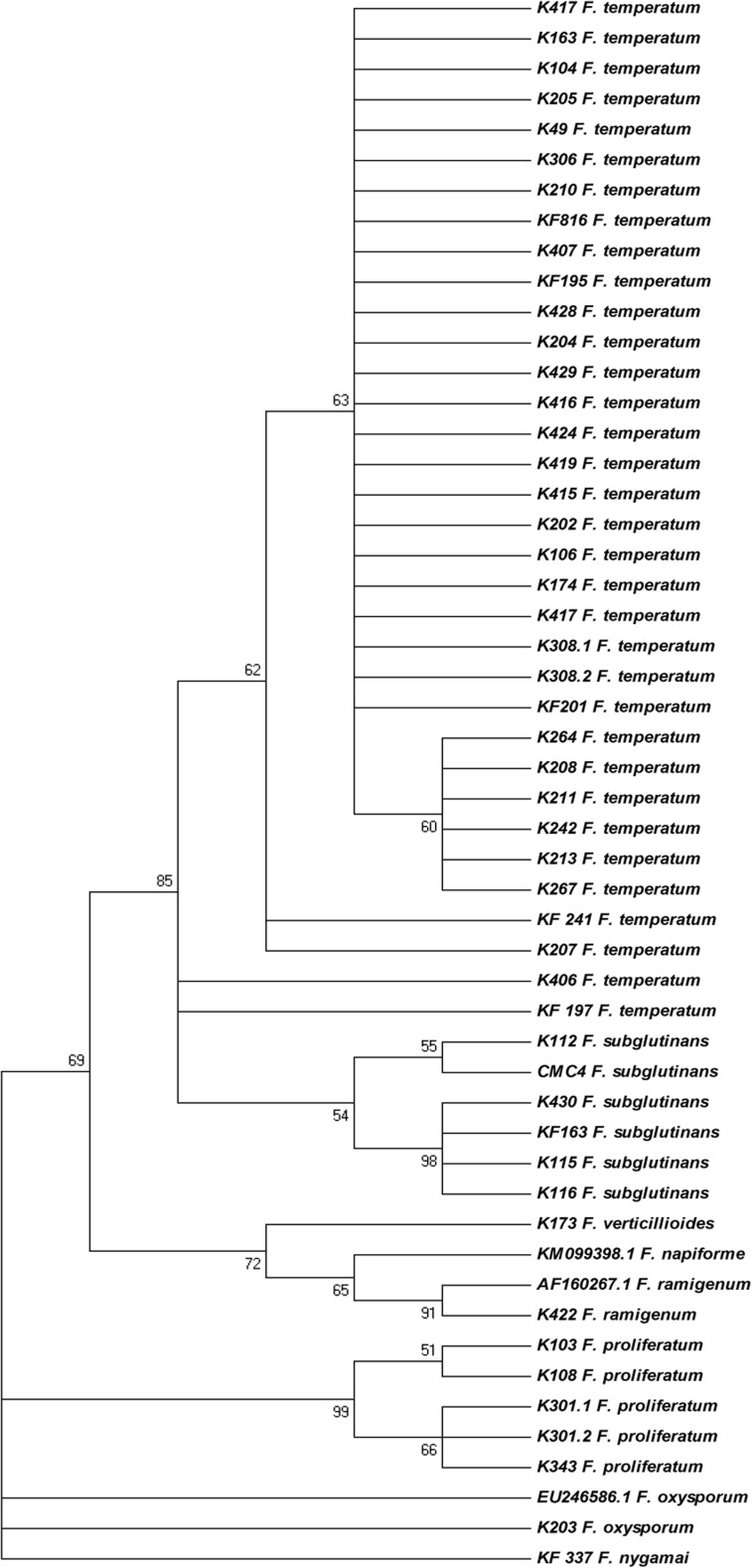
Moretti et al. (2008) examined 43 isolates of F. subglutinans sensu lato originating from maize in Poland from harvest seasons between 1984 and 1990. Using RFLP method and testing their ability to produce BEA, two groups of isolates were distinguished: 34 were proven to be BEA and fusaproliferin producers belonging to the group 1 (and corresponding to F. temperatum, according to Scauflaire et al. 2011). Three isolates were BEA non-producers and belonged to the group 2, corresponding to F. subglutinans sensu stricto. Those results were confirmed in our study by the identification of 34 isolates (2013–2016) as representing group 1, actually to be F. temperatum (Fig. 2, Table 2). Additionally, five isolates from our collection (KF 816, KF 1955, KF 197, KF 201, and KF 241), which were previously described as F. subglutinans by Kostecki et al. (1995, 1999), were confirmed to be F. temperatum using the analysis of the tef-1α gene sequence (Table 2). Only one isolate corresponded to F. subglutinans sensu stricto (KF 163).
Fig. 2.
Maximum parsimony dendrogram of Fusarium temperatum and F. subglutinans isolates obtained during 2013–2016 seasons from maize ear diseased samples from two localities in Poland. Additionally, KF collection isolates were included, along with some other species for reference (F. proliferatum, F. verticillioides, F. oxysporum, and F. ramigenum)
Table 2.
Fusarium subglutinans, F. temperatum, and F. proliferatum isolates analyzed in this study
| Year of isolation | Origin | Isolate code and number | Species identified using tef-1α gene sequence analysis |
|---|---|---|---|
| 1984 | Poland | KF 816 nt | F. temperatum |
| 1984 | Poland | KF 163 (ITEM 1348) f, a | F. subglutinans |
| 1984 | Poland | KF 195 (ITEM 1349) b, f, a | F. temperatum |
| 1984 | Radzików, Poland | KF 197 (ITEM 1422) b, f, a | F. temperatum |
| 1984 | Poland | KF 201 (ITEM 1352) b, f, a | F. temperatum |
| 1984 | Poland | KF 241 (ITEM 1353) f, a | F. temperatum |
| Unknown | USA | KF 337 (NRRL 25312) c | F. nygamai |
| 2013 | Złotniki, Poland | K 49 bn | F. temperatum |
| 2014 | Złotniki, Poland | K 103 bn | F. proliferatum |
| 2014 | Złotniki, Poland | K 104 bn | F. temperatum |
| 2014 | Złotniki, Poland | K 106 bn | F. temperatum |
| 2014 | Złotniki, Poland | K 108 bn | F. proliferatum |
| 2014 | Złotniki, Poland | K 112 bn | F. subglutinans |
| 2014 | Złotniki, Poland | K 115 bn, b | F. subglutinans |
| 2014 | Złotniki, Poland | K 116 bn, b | F. subglutinans |
| 2014 | Kobierzyce, Poland | K 163 bn | F. temperatum |
| 2014 | Kobierzyce, Poland | K 173 bn | F. verticillioides |
| 2014 | Kobierzyce, Poland | K 174 bn | F. temperatum |
| 2015 | Swadzim, Poland | K 201 bn | F. temperatum |
| 2015 | Swadzim, Poland | K 202 bn | F. temperatum |
| 2015 | Swadzim, Poland | K 203 bn | F. oxysporum |
| 2015 | Swadzim, Poland | K 204 bn | F. temperatum |
| 2015 | Swadzim, Poland | K 205 bn | F. temperatum |
| 2015 | Swadzim, Poland | K 207 bn | F. temperatum |
| 2015 | Swadzim, Poland | K 208 bn | F. temperatum |
| 2015 | Swadzim, Poland | K 210 bn | F. temperatum |
| 2015 | Swadzim, Poland | K 211 bn | F. temperatum |
| 2015 | Swadzim, Poland | K 213 bn | F. temperatum |
| 2015 | Kobierzyce, Poland | K 242 bn | F. temperatum |
| 2015 | Kobierzyce, Poland | K 264 bn | F. temperatum |
| 2015 | Kobierzyce, Poland | K 267 bn | F. temperatum |
| 2015 | Kobierzyce, Poland | K 270 bn | F. proliferatum |
| 2015 | Kwieciszewo, Poland | K 301.1 bn | F. proliferatum |
| 2015 | Kwieciszewo, Poland | K 301.2 bn | F. proliferatum |
| 2015 | Kwieciszewo, Poland | K 306 bn | F. temperatum |
| 2015 | Kwieciszewo, Poland | K 308.1 bn | F. temperatum |
| 2015 | Kwieciszewo, Poland | K 308.2 bn | F. temperatum |
| 2015 | Komalwy, Poland | K 343 bn | F. proliferatum |
| 2016 | Złotniki, Poland | K 406 bn | F. temperatum |
| 2016 | Złotniki, Poland | K 407 bn | F. temperatum |
| 2016 | Złotniki, Poland | K 415 bn | F. temperatum |
| 2016 | Złotniki, Poland | K 416 bn | F. temperatum |
| 2016 | Złotniki, Poland | K 417 bn | F. temperatum |
| 2016 | Złotniki, Poland | K 419 bn | F. temperatum |
| 2016 | Złotniki, Poland | K 424 bn | F. temperatum |
| 2016 | Złotniki, Poland | K 428 bn | F. temperatum |
| 2016 | Złotniki, Poland | K 429 bn | F. temperatum |
| 2016 | Złotniki, Poland | K 430 bn | F. subglutinans |
| 2016 | Złotniki, Poland | K 422 bn | F. ramigenum |
a - according to Logrieco et al. (1996)
b - isolates proved to be BEA producers under laboratory conditions (Kostecki et al. 1995)
c - according to Azor et al. (2009)
bn - BEA present in ears exhibiting Fusarium ear rot under field conditions
f - isolates proved to be fusaproliferin producers (Kostecki et al. 1999)
Interestingly, the phylogenetic analysis showed that the population of F. temperatum infecting maize in Poland remains quite uniform (Fig. 2), since most of the collection strains and newly isolated genotypes of the species grouped on the same branch, which means that the sequences were identical. There were only few exceptions that were genetically distinct from that group: K406 and KF 197, KF 241, and K207 grouped outside of the main group. There was also a small group of isolates very similar to each other: K264, K208, K211, K242, K213, and K267. It can be concluded that there are no significant differences between the F. temperatum populations present in Poland some 20 years ago and those present now. This may suggest that the population propagates clonally with the maize seed material. However, it is not possible to draw a similar conclusion regarding F. subglutinans sensu stricto, since there were too few isolates to be analyzed. Detailed analysis of this species requires additional strain isolation and studies. Interestingly, detailed molecular identification procedure allowed to detect a single isolate of F. ramigenum. To our best knowledge, this is the first time this species was identified in Poland. The strain K270 of F. proliferatum gave the sequence read of poor quality; therefore, we decided to exclude it from the phylogenetic analysis.
To improve clarity and put emphasis on the F. subglutinans and F. temperatum species, only one F. verticillioides isolate was included in the phylogenetic analysis for reference, along with single isolates of F. oxysporum and F. ramigenum and five isolates of F. proliferatum (Fig. 2).
The frequency of F. temperatum was significantly higher than that of F. subglutinans in Poland and in other countries as well. The frequency of F. proliferatum increased in 2015 and 2016 seasons, comparing to the earlier years. This finding may be particularly important because of high toxigenicity of F. proliferatum, producing the same mycotoxins as F. temperatum but additionally fumonisins. The spread of Fusarium species caused recently increase in frequencies of species such as F. graminearum and F. verticillioides in other countries of moderate climate, including Poland and Austria (Adler et al. 2002; Gromadzka et al. 2016).
The description of F. subglutinans isolates found in manuals available before 2011 was not detailed enough to distinguish F. subglutinans sensu stricto from F. temperatum using morphological characters only (Nelson et al. 1983; Leslie and Summerell 2006). Implementing molecular identification, in particular, the analysis of the sequence of tef-1α gene, provided a method to identify closely related species such as about 15 members of FFSC species complex (Stępień et al. 2011, 2013; Aoki et al. 2014). According to Aoki et al. (2014), FFSC comprises of 13 species that are known to reproduce sexually and are heterothallic. On the other hand, molecular phylogenetic studies have revealed that FFSC is represented by over 50 phylogenetically distinct species. Over 300 species of Fusarium have been recognized using DNA assays; however, fewer than a half of this number have been formally described. Continuous changes in Fusarium species identification and nomenclature require careful edition of new modern reviews and manuals for verification and dissemination of this knowledge. F. temperatum Scaufl. & Munaut has not yet been included in the manuals on Fusarium taxonomy; however, it was already accepted in several scientific papers mentioned above.
Mycotoxins production by F. temperatum and F. subglutinans sensu stricto
Of all the known mycotoxins, beauvericin, moniliformin, and enniatins were accumulated in maize kernels infected by F. temperatum.
Beauvericin production under laboratory conditions on solid substrate (polished rice) is often helpful in confirming the identification of species (Kostecki et al. 1999; Fumero et al. 2015). Until now, only few isolates of F. temperatum were studied in detail concerning their potential to synthesize BEA and enniatins in vitro (Stępień and Waśkiewicz 2013). One of them produced only BEA, one BEA and all four enniatin analogs measured (KF 3321 from pineapple), and some produced mixtures of BEA and some enniatins but not all. Therefore, the production of other mycotoxins (such as enniatins) by F. temperatum isolates should be examined in future for a larger number of isolates to understand the profiles of these mycotoxins for F. temperatum and F. subglutinans fully.
BEA and MON were produced by 19 isolates of F. subglutinans sensu lato examined in Poland in 1993 and, under laboratory conditions, the efficiencies ranged between 201 and 4000 mg/kg for BEA and 0.9–381 mg/kg for MON. The strain KF 534 (ITEM 1434)—considered to be the model strain—was found to produce the highest amounts of MON (4 g/kg) and also FP and BEA on polished rice under laboratory conditions (Kostecki et al. 1995, 1999) This strain was confirmed to be F. subglutinans group 1, later known to be F. temperatum.
Isolate KF 555 was previously found to produce high amounts of BEA in kernels of nine maize hybrids inoculated under field conditions (Krska et al. 1996). Fusarium-damaged kernels accumulated 6.58–15.22 mg/g of BEA, and the lowest amounts were accumulated by the hybrid “Anna,” which was found to be of the lowest susceptibility to infection and mycotoxin accumulation. Healthy-looking kernels from the same ears accumulated very low amounts of BEA (Krska et al. 1996).
Significant amounts and frequencies of BEA contamination were found in Fusarium-damaged kernels in our survey of field maize samples harvested in 2013–2016 seasons and colonized by F. subglutinans sensu lato (Table 3). The same kernel samples contained also enniatins A1, A, B1, and B.
Table 3.
Moniliformin, beauvericin, and enniatins identified in kernels colonized with Fusarium temperatum, F. subglutinans, and F. proliferatum species under field conditions in 2013, 2014, 2015, and 2016 seasons (nd, not detected; na, not analyzed)
| Species | Sample number | MON (μg/g) | BEA (μg/g) | Enniatins | |||
|---|---|---|---|---|---|---|---|
| B (μg/g) | B1 (μg/g) | A (μg/g) | A1 (μg/g) | ||||
| F. temperatum | K49 | nd | 16.60 | nd | nd | nd | 47.82 |
| F. proliferatum | K103 | 12.05 | 51.45 | nd | nd | 24.90 | nd |
| F. temperatum | K104 | 7.84 | 71.17 | nd | nd | nd | 42.93 |
| F. temperatum | K106 | 0.65 | 33.79 | 104.39 | nd | nd | 24.78 |
| F. proliferatum | K108 | 8.80 | 34.07 | 58.98 | nd | nd | 34.49 |
| F. subglutinans | K112 | 9.93 | 98.94 | nd | nd | nd | 16.89 |
| F. subglutinans | K115 | 4.36 | 106.34 | nd | nd | 10.78 | 24.46 |
| F. subglutinans | K116 | na | na | na | na | na | na |
| F. temperatum | K163 | 13.52 | 91.75 | nd | nd | nd | nd |
| F. temperatum | K174 | 118.48 | nd | nd | nd | nd | nd |
| F. temperatum | K201 | 4.32 | 9.95 | nd | 14.08 | 14.86 | nd |
| F. temperatum | K202 | 5.62 | 19.04 | nd | nd | 38.49 | nd |
| F. temperatum | K204 | 3.69 | 48.31 | nd | nd | 17.08 | nd |
| F. temperatum | K205 | 3.24 | 33.56 | nd | 13.92 | 9.44 | nd |
| F. temperatum | K207 | 0.03 | 52.26 | nd | 29.67 | 11.23 | nd |
| F. temperatum | K208 | 0.15 | 6.58 | nd | 7.48 | 16.28 | nd |
| F. temperatum | K210 | 3.75 | 71.56 | nd | 26.79 | 13.53 | 5.55 |
| F. temperatum | K211 | 0.02 | 49.56 | nd | 47.21 | 73.73 | nd |
| F. temperatum | K213 | nd | nd | nd | 45.13 | 15.66 | 46.03 |
| F. temperatum | K242 | 1.26 | 126.08 | nd | 8.36 | 39.14 | nd |
| F. temperatum | K264 | 2.91 | 457.31 | 130.63 | nd | 46.51 | 325.35 |
| F. temperatum | K267 | 0.03 | 392.72 | 109.68 | 8.61 | 48.90 | 7.66 |
| F. proliferatum | K270 | 0.06 | 240.30 | 124.35 | nd | 50.97 | 44.71 |
| F. proliferatum | K301 | 0.04 | 1.70 | 60.17 | nd | nd | 3883.28 |
| F. temperatum | K306 | 0.03 | 7.50 | 42.02 | nd | 13.29 | 972.45 |
| F. temperatum | K308.1 | na | na | na | na | na | na |
| F. temperatum | K308.2 | na | na | na | na | na | na |
| F. proliferatum | K343 | na | na | na | na | na | na |
| F. temperatum | K406 | 90.53 | 64.33 | nd | nd | 38.43 | nd |
| F. temperatum | K407 | 68.21 | 40.11 | nd | nd | 120.86 | nd |
| F. temperatum | K415 | 14.26 | 151.69 | nd | nd | 29.78 | nd |
| F. temperatum | K416 | 3.21 | 164.10 | nd | nd | 390.48 | nd |
| F. temperatum | K417 | 3.30 | 48.70 | nd | nd | 80.19 | nd |
| F. temperatum | K419 | na | na | na | na | na | na |
| F. temperatum | K424 | 9.85 | 55.08 | nd | 36.84 | 111.59 | nd |
| F. temperatum | K428 | 75.55 | 76.88 | nd | nd | 132.27 | nd |
| F. temperatum | K429 | na | na | na | na | na | na |
| F. subglutinans | K430 | 10.50 | 37.42 | nd | nd | 110.52 | nd |
| F. ramigenum | K422 | 24.76 | 37.71 | nd | nd | 315.15 | nd |
Comparing the amounts of mycotoxins synthesized in maize kernels by F. temperatum and F. subglutinans, high differences were noticed, in particular regarding moniliformin and enniatin A1. In FDK fraction infected by F. temperatum, the average amount of MON (17.22 mg/kg) was almost twice as high as in kernels (8.26 mg/kg) from which F. subglutinans was identified. In the case of the enniatin A1, the differences were even higher and amounts of the toxin accumulated in kernels infected by F. temperatum and F. subglutinans were on the average at the levels of 58.90 mg/kg and 13.78 mg/kg, respectively. The analysis of mycotoxins also showed that in the case of F. subglutinans, no enniatins B and B1 were found, whereas, in the case of kernels infected by F. temperatum, they were on average at the level of 15.47 and 9.52 mg/kg, respectively. In addition, both species were characterized by a similar amount of synthesized beauvericin (about 80 mg/kg) as well as enniatin A (about 45 mg/kg).
Both in rice cultures and under field conditions, F. temperatum was found to be BEA, MON, and FP producer and contributed to the contamination of maize kernels with the abovementioned mycotoxins and also enniatins A1, A, B, and B1 (Tables 2 and 3).
The results clearly demonstrate the occurrence of F. temperatum as maize pathogen in Poland for over the last three decades (since 1984) and recent literature has reported the species also in several other countries mentioned above.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
References
- Adler A, Lew H, Brunner S, Oberforster M, Hinterholzer J, Kulling-Gradinger CM, Mach RL, Kubicek CP. Fusaria in Austrian cereals – change in species and toxins spectrum. J Appl Genet. 2002;43A:11–16. [Google Scholar]
- Aoki T, O’Donnell K, Geiser D. Systematics of key phytopathogenic Fusarium species: current status and future challenges. J Gen Plant Pathol. 2014;80(3):189–201. doi: 10.1007/s10327-014-0509-3. [DOI] [Google Scholar]
- Azor M, Gene J, Cano J, Manikandan P, Venkatapathy N, Guarro J. Less-frequent Fusarium species of clinical interest: correlation between morphological and molecular identification and antifungal susceptibility. J Clin Microbiol. 2009;47(5):1463–1468. doi: 10.1128/JCM.02467-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottalico A, Perrone G. Toxigenic Fusarium species and mycotoxins associated with head blight in small-grain cereals in Europe. Eur J Plant Pathol. 2002;108:611–624. doi: 10.1023/A:1020635214971. [DOI] [Google Scholar]
- Boutigny AL, Scauflaire J, Ballois N, Ioos R. Fusarium temperatum isolated from maize in France. Eur J Plant Pathol. 2017;148:997–1001. doi: 10.1007/s10658-016-1137-x. [DOI] [Google Scholar]
- Czembor E, Stępień Ł, Waśkiewicz A. Fusarium temperatum as new species causing ear rot on maize in Poland. Plant Dis. 2014;98(7):1001. doi: 10.1094/PDIS-11-13-1184-PDN. [DOI] [PubMed] [Google Scholar]
- Czembor E, Stępień Ł, Waśkiewicz A. Effect of environmental factors on Fusarium species and associated mycotoxins in maize grain grown in Poland. PLoS One. 2015;10(7):e0133644. doi: 10.1371/journal.pone.0133644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fumero MV, Reynoso MM, Chulze S. Fusarium temperatum and Fusarium subglutinans isolated from maize in Argentina. Int J Food Microbiol. 2015;199:86–92. doi: 10.1016/j.ijfoodmicro.2015.01.011. [DOI] [PubMed] [Google Scholar]
- Gromadzka K, Górna K, Chełkowski J, Waśkiewicz A. Mycotoxins and related Fusarium species in preharvest maize ear rot in Poland. PSE. 2016;62(8):348–354. [Google Scholar]
- Jestoi M, Rokka M, Yli-Matilla T, Parikkas P, Rizzo A, Peltonen K. Presence and concentrations of the Fusarium-related mycotoxins beauvericin, enniatins and moniliformin in Finish grain samples. Food Addit Contam. 2004;21:794–802. doi: 10.1080/02652030410001713906. [DOI] [PubMed] [Google Scholar]
- Kostecki M, Grabarkiewicz-Szczęsna J, Chełkowski J, Wiśniewska H. Beauvericin and moniliformin production by Polish isolates of Fusarium subglutinans and natural cooccurrence of both mycotoxins in maize samples. Microbiol Aliment Nutr. 1995;13:67–70. [Google Scholar]
- Kostecki M, Wiśniewska H, Perrone G, Ritieni A, Goliński P, Chełkowski J, Logrieco A. The effects of cereal substrate and temperature on production of beauvericin, moniliformin and fusaproliferin by Fusarium subglutinans ITEM-1434. Food Addit Contam. 1999;16:361–365. doi: 10.1080/026520399283849. [DOI] [PubMed] [Google Scholar]
- Krska R, Lemmens M, Schuhmacher R, Grasserbauer M, Prończuk M, Wiśniewska H, Chelkowski J. Accumulation of the mycotoxin beauvericin in kernels of corn hybrids inoculated with Fusarium subglutinans. J Agric Food Chem. 1996;44:3665–3667. doi: 10.1021/jf960064m. [DOI] [Google Scholar]
- Kwaśna H, Chełkowski J, Zajkowski P. Fusarium (Sierpik), Vol. XXII: Mycota. Warszawa, Kraków: Institute of Botany, Polish Academy of Sciences; 1991. [Google Scholar]
- Leslie JF, Summerell BA. The Fusarium laboratory manual. Ames: Blackwell Publishing; 2006. [Google Scholar]
- Lew H, Adler AA, Edinger W. Moniliformin and European corn borer (Ostrinia nubilalis) Mycotoxin Res. 1991;7A:71–76. doi: 10.1007/BF03192189. [DOI] [PubMed] [Google Scholar]
- Lew H, Chełkowski J, Prończuk P, Edinger W. Occurrence of the mycotoxin moniliformin in maize (Zea mays L.) ears infected by Fusarium subglutinans (Wollenw. & Reinking) Food Addit Contam. 1996;13:321–324. doi: 10.1080/02652039609374414. [DOI] [PubMed] [Google Scholar]
- Logrieco A, Moretti A, Fornelli F, Fogliano V, Ritieni A, Caiaffa MF, Randazzo G, Bottalico A, Macchia L. Fusaproliferin production by Fusarium subglutinans and its toxicity to Artemia salina, SF-9 insect cells, and IARC/LCL 171 human B lymphocytes. Appl Environ Microbiol. 1996;62(9):3378–3384. doi: 10.1128/aem.62.9.3378-3384.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logrieco A, Moretti A, Castella G, Kostecki M, Goliński P, Ritieni A, Chełkowski J. Beauvericin production by Fusarium species. Appl Environ Microbiol. 1998;64:3084–3088. doi: 10.1128/aem.64.8.3084-3088.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moretti A, Mulé G, Laday M, Stubnya V, Hornok L, Logerico A. Cryptic subspecies and beauvericin production by Fusarium subglutinans from Europe. Int J Food Microbiol. 2008;127:312–315. doi: 10.1016/j.ijfoodmicro.2008.08.003. [DOI] [PubMed] [Google Scholar]
- Nelson PE, Toussoun TA, Marasas WFO. Fusarium species: an illustrated manual for identification. London: Pennsylvania State University Press; 1983. [Google Scholar]
- Pascale M, Visconti A, Chełkowski J. Ear rot susceptibility and mycotoxin contamination of maize hybrids inoculated with Fusarium species under field conditions. Eur J Plant Pathol. 2002;108:645–651. doi: 10.1023/A:1020622812246. [DOI] [Google Scholar]
- Pintos VC, Aguín OC, Chaves PM, Ferreiroa Martinez V, Sainz Oses MJ, Scauflaire J, Munaut F, Bande Castro MJ, Mansilla Vázquez JP. First report of Fusarium temperatum causing seedling blight and stalk rot on maize in Spain. Plant Dis. 2013;97(9):1252. doi: 10.1094/PDIS-02-13-0167-PDN. [DOI] [PubMed] [Google Scholar]
- Scauflaire J, Gourgue M, Munaut F. Fusarium temperatum sp. nov. from maize, an emergent species closely related to Fusarium subglutinans. Mycologia. 2011;103:586–597. doi: 10.3852/10-135. [DOI] [PubMed] [Google Scholar]
- Sharman M, Gilbert J, Chełkowski J. A survey of the occurrence of the mycotoxin moniliformin in cereal samples from sources worldwide. Food Addit Contam. 1991;8:459–466. doi: 10.1080/02652039109373996. [DOI] [PubMed] [Google Scholar]
- Steenkamp ET, Desjardins AE, Marasas WFO, Wingfield M. Cryptic speciation in Fusarium subglutinans. Mycologia. 2002;94:1032–1043. doi: 10.1080/15572536.2003.11833158. [DOI] [PubMed] [Google Scholar]
- Stępień Ł, Waśkiewicz A. Sequence divergence of the enniatin synthase gene in relation to production of beauvericin and enniatins in Fusarium species. Toxins. 2013;5:537–555. doi: 10.3390/toxins5030537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stępień Ł, Koczyk G, Waśkiewicz A. FUM cluster divergence in fumonisins-producing Fusarium species. Fungal Biol. 2011;115:112–123. doi: 10.1016/j.funbio.2010.10.011. [DOI] [PubMed] [Google Scholar]
- Stępień Ł, Jestoi M, Chełkowski J. Cyclic hexadepsipeptides in wheat field samples and esyn1 gene divergence among enniatin producing Fusarium avenaceum strains. World Mycotoxin J. 2013;6:399–409. doi: 10.3920/WMJ2012.1464. [DOI] [Google Scholar]
- Tamura K, Dudley J, Nei M, Kumar S. MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol Biol Evol. 2007;24:1596–1599. doi: 10.1093/molbev/msm092. [DOI] [PubMed] [Google Scholar]
- Wang JH, Zhang JB, Li HP, Gong AD, Xue S, Agboola RS, Liao YC. Molecular identification, mycotoxin production and comparative pathogenicity of Fusarium temperatum isolated from maize in China. J Phytopathol. 2014;162(3):147–157. doi: 10.1111/jph.12164. [DOI] [Google Scholar]