ABSTRACT
Background
Formate is an important metabolite that serves as a donor of one-carbon groups to the intracellular tetrahydrofolate pool. However, little is known of its circulating concentrations or of their determinants.
Objective
This study aimed to define formate concentrations and their determinants in a healthy young population.
Design
Serum formate was measured in 1701 participants from the Trinity Student Study. The participants were men and women, aged 18 to 28 y, enrolled at Trinity College, Dublin. Formate concentrations were compared with other one-carbon metabolites, vitamin status, potential formate precursors, genetic polymorphisms, and lifestyle factors.
Results
Serum formate concentrations ranged from 8.7 to 96.5 µM, with a mean of 25.9 µM. Formate concentrations were significantly higher in women than in men; oral contraceptive use did not further affect them. There was no effect of smoking or of alcohol ingestion, but the TT genotype of the methylenetetrahydrofolate reductase (MTHFR) 677C→T (rs1801133) polymorphism was associated with a significantly decreased formate concentration. Formate was positively associated with potential metabolic precursors (serine, methionine, tryptophan, choline) but not with glycine. Formate concentrations were positively related to serum folate and negatively related to serum vitamin B-12.
Conclusions
Formate concentrations were sensitive to the concentrations of metabolic precursors. In view of the increased susceptibility of women with the TT genotype of MTHFR to give birth to infants with neural tube defects as well as the effectiveness of formate supplementation in decreasing the incidence of folate-resistant neural tube defects in susceptible mice, it will be important to understand how this genotype decreases the serum formate concentration. This trial was registered at www.clinicaltrials.gov as NCT03305900.
Keywords: folate, vitamin B-12, methylenetetrahydrofolate reductase, one-carbon metabolism, Trinity Student Study
INTRODUCTION
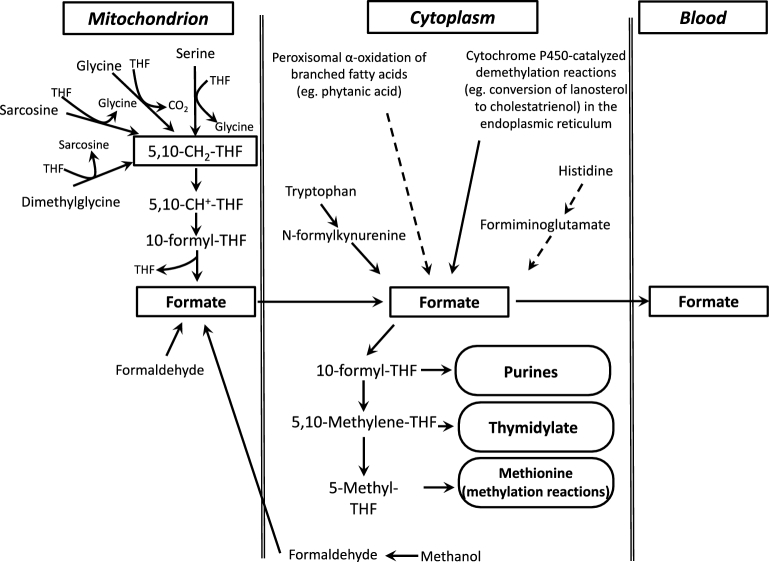
Formate, the smallest carboxylic acid, plays a critical role in one-carbon metabolism, primarily as a source of one-carbon groups for the synthesis of 10-formyl-tetrahydrofolate (THF). Thus, formate plays a role in the provision of one-carbon groups for purine synthesis, thymidylate synthesis, and methylation reactions. Formate metabolism may also serve as a source of cytoplasmic NAD(P)H (1). Formate is synthesized by a variety of pathways, but principally via the mitochondrial metabolism of serine, glycine, and 2 metabolites of choline catabolism (dimethylglycine and sarcosine), which results in the production of mitochondrial 5,10-methylene-THF (Figure 1). This 5,10-methylene-THF is then oxidized to 10-formyl-THF by the combined action of 5,10-methylene-THF dehydrogenase and 5,10-methenyl-THF cyclohydrolase (2). There are 2 mitochondrial enzymes that act on 10-formyl-THF. 10-Formyl-THF dehydrogenase [mitochondrial NAD(P)-dependent 10-formyl-THF dehydrogenase (ALDH1L2)] oxidizes 10-formyl-THF to carbon dioxide, with the accompanying reduction of NAD(P)+ to NAD(P)H (3). 10-Formyl-THF is converted to formate plus THF by mitochondrial monofunctional 10-formyl-THF synthetase (MTHFD1L); this conversion may be accompanied by the synthesis of ATP from ADP and inorganic phosphate. Formate then leaves the mitochondria by an unknown mechanism, whereupon it may be converted in the cytoplasm to 10-formyl-THF, which has 3 principal fates: it may be directly used to provide one-carbon groups for the synthesis of purine nucleotides, it may be reduced to 5,10-methylene-THF to be used for thymidylate synthesis, or it may be further reduced by methylenetetrahydrofolate reductase (MTHFR) to 5-methyl-THF, which provides methyl groups for the remethylation of homocysteine to methionine (2). Formate may also enter the nucleus, where it can serve as a one-carbon source for nuclear thymidylate synthesis (4). Additional sources of formate include the catabolism of tryptophan and histidine, cytochrome P450–dependent demethylation reactions, α-oxidation of branched-chain fatty acids such as phytanic acid, and methanol metabolism, which involves the oxidation of methanol to formaldehyde and then to formate (5). Methanol may also be produced by the gut microbiota during the metabolism of pectin, and this may be a source of formate (6). Despite these many sources of formate, it should be noted that, in humans, serine is the dominant source of methyl groups for the methylation of homocysteine to methionine (7) and much of this serine metabolism occurs in mitochondria (8), producing formate.
FIGURE 1.
Outline of one-carbon metabolism with particular reference to formate. THF, tetrahydrofolate; 5,10-CH2-THF, 5,10-methylene-tetrahydrofolate; 5,10-CH+-THF, 5,10-methenyl-tetrahydrofolate.
Cytoplasmic formate may also exit cells (mechanism unknown) and is found in serum from humans (9), sheep (10), and rats (11) at concentrations of 25–50 µM. In fetal sheep, plasma and amniotic fluid concentrations are much higher (200 and 300 µM, respectively) (10). Little is known with regard to the determinants of circulating formate. Lamarre et al. (12) reported that plasma formate increased markedly in rats that were made either folate- or vitamin B-12–deficient, but was not affected by vitamin B-6 deficiency even when accompanied by a methionine load that caused profound hyperhomocysteinemia. They concluded that formate accumulation in these experiments was a result of impaired remethylation (12). Dow and Green (13) found that trichloroethylene, a common commercial degreaser, and some of its metabolites caused increased formic acid excretion in rats; methylmalonate was also increased in both plasma and urine, indicating a functional vitamin B-12 deficiency. Recently, Katre et al. (14) examined markers of one-carbon metabolism in a clinically healthy group of young women from Pune (India) compared with a similar group from Cleveland, Ohio. The group from Pune was predominantly vegetarian and had significantly lower concentrations of folate, vitamin B-6, and vitamin B-12, as well as higher homocysteine concentrations than did the Cleveland group; they also had appreciably elevated plasma formate concentrations (180 µM in the Pune group, 40 µM in the Cleveland group), possibly as a result of impaired remethylation (14).
Circulating formate concentrations can, therefore, provide pertinent information concerning one-carbon metabolism. As far as we are aware, there has been no study of the metabolic or genetic determinants of the circulating formate concentration in humans. The aim of this study was to examine the factors that influence serum concentrations of formate in a cohort of healthy young adults (n = 1701; median age: 22 y) in order to establish the baseline range and to explore the influence of other metabolic, genetic, and lifestyle characteristics, such as oral contraceptive use, alcohol intake, and smoking on formate concentration.
METHODS
Participants
The Trinity Student Study (TSS) is a cross-sectional design study that aims to examine genotype-phenotype associations of blood metabolites in healthy young adults of an ethnically homogeneous background. The study enrolled students attending the University of Dublin, Trinity College, between February 2003 and 2004. Eligibility criteria included being between ages 18 and 28 y, having no current serious medical condition, and having Irish ethnicity based on origins of grandparents. In total, 2524 individuals were eligible to participate. Ethical approval was obtained from the Dublin Federated Hospitals Research Ethics Committee, which is affiliated with Trinity College Dublin, and participants gave written informed consent. The study was reviewed by the Office of Human Subjects Research at the NIH, and the formate analysis was approved by the Health Research Ethics Board of the Province of Newfoundland and Labrador. Further details relating to the TSS have been published elsewhere (15–18). Fifteen participants with no questionnaire data and one duplicate sample were excluded, which left 2508 eligible participants whose samples were assigned to analysis. Participants were not required to fast. Each participant provided 30 mL blood for preparation of plasma in EDTA-coated tubes, or serum. All of the samples were processed within 3 h of collection, and aliquots were stored at −80°C. Blood samples and questionnaire data were coded and made anonymous before analysis. Questionnaire data included details on lifestyle choices, including smoking status, reported alcohol beverage intake converted to grams of ethanol per day, and contraceptive use among women.
This trial was registered at clinicaltrials.gov as NCT03305900.
Biochemical analyses
For formate analysis, serum stored in liquid nitrogen was used. Serum formate was analyzed in duplicate by the gas chromatography–mass spectrometry isotope-dilution method of Lamarre et al. (9). We divided the samples into 5 groups, each covering 20% of the samples, and analyzed 10 samples from each group per day. In this way, we staggered the analyses such that any excess variability would not be concentrated in a particular group of samples. In addition, as a daily measure of quality control, we froze a single plasma sample in aliquots and analyzed an aliquot on each analysis day. Day-to-day variation in the formate concentration of this sample was 7.4% (n = 38). Because of apparent contamination with formic acid of sealing O-rings in one brand of cryopreservation tubes, we had to exclude samples from 807 participants. The formate data set for the current study therefore includes 1701 participants.
Analyses of serum choline, betaine, and dimethylglycine were carried out by Bevital, Norway (www.bevital.no), by using a liquid chromatography–tandem mass spectrometry method (19). The interassay CV was <10% for all 3 metabolites. Serum methylmalonic acid, methionine, cysteine, cystathionine, serine, glycine, sarcosine, cotinine, tryptophan, and plasma total homocysteine were also assayed by Bevital with the use of an automated isotope-dilution gas chromatography–mass spectrometry method (20). The between-day CVs were ≤8.1% for all analytes.
Serum folate, red blood cell folate, and serum vitamin B-12 were measured by microbiological assays, as previously described (21, 22). The between-assay CVs were <11.0%. Holotranscobalamin was measured by using an Abbott AxSYM analyzer (23). The between-assay CV was <11.1%. Serum-based liver and kidney function tests were carried out commercially by Claymon Laboratories (Dublin, Ireland) by using an automated Abbott architect autoanalyzer platform with standard kits (Abbott Ireland Diagnostics Division). Inter- and intra-assay CVs were <4.8%. Hemoglobin was measured by using a Sysmex F-800 cell counter calibrated with CBC-ST Plus hematology controls (low, normal, and high) (R&D Systems, Inc.).
Genotyping
Genotyping, quality control, and data preparation have been described by Molloy et al. (18). Briefly, DNA for each sample was extracted from peripheral blood. Genomewide single nucleotide polymorphism (SNP) genotyping was carried out by using Illumina 1M HumanOmni1-Quad_v1–0_B chips at the Center for Inherited Disease Research (Baltimore, Maryland). Genotypes were generated for 2438 TSS samples, 14 blind duplicates, and HapMap controls. Blind duplicates had a concordance rate of 99.997%, and the HapMap samples had a 99.71% concordance rate. After quality-control filters were applied, ∼758,443 genotyped SNPs were available for each participant.
Statistical analysis
We performed all of the metabolite statistical analyses with the use of SAS software (version 9.4; SAS Institute). Independent Student's t tests were used to determine significant differences between groups, shown in Tables 1 and 2. One-factor ANOVA was used to determine differences in metabolite concentrations by MTHFR 677C→T genotype. Formate concentrations had non-normal distributions, and so inverse normal rank transformations were applied to the formate to satisfy the normality assumption before examination of associations with metabolite variables through linear regression analysis. The inverse normal rank-transformed formate data were regressed on each of methionine, serine, glycine, tryptophan, serum folate, homocysteine, serum total vitamin B-12, choline, cysteine, cystathionine, red blood cell folate, holotranscobalamin, and methylmalonic acid for association analysis. Multiple linear regression analysis was performed to examine the associations of formate with the metabolite variables adjusted for sex, smoking, alcohol intake, and oral contraceptive use. Correlation analysis was performed with the use of Spearman correlation coefficients. To explore nonlinear relations between metabolites, generalized additive models (GAMs) were constructed by using R statistical software (version 3.1.1). Significance was set at P < 0.05.
TABLE 1.
Characteristics of the study population1
| Total (n = 1701) | Men (n = 638) | Women (n = 1063) | P | |
|---|---|---|---|---|
| Age, y | 22.2 ± 1.8 | 22.3 ± 1.9 | 22.1 ± 1.8 | 0.022 |
| BMI, kg/m2 | 22.9 ± 3.1 | 23.2 ± 3.0 | 22.7 ± 3.2 | 0.002 |
| Alcohol, g/d | 23.1 ± 20.5 | 31.7 ± 24.8 | 18.0 ± 15.2 | <0.0001 |
| Creatinine, µmol/L | 65.2 ± 13.3 | 74.8 ± 12.0 | 59.5 ± 10.3 | <0.0001 |
| CRP, mg/L | 1.6 ± 3.2 | 1.5 ± 4.0 | 1.7 ± 2.6 | 0.30 |
| Cystatin C, mg/L | 0.76 ± 0.14 | 0.82 ± 0.14 | 0.73 ± 0.13 | <0.0001 |
| Hemoglobin, g/dL | 14.2 ± 1.8 | 15.4 ± 1.5 | 13.4 ± 1.5 | <0.0001 |
| AST, units/L | 21.3 ± 10.4 | 24.6 ± 14.9 | 19.4 ± 5.6 | <0.0001 |
| ALT, units/L | 16.4 ± 9.936 | 19.8 ± 12.0 | 14.5 ± 7.8 | <0.0001 |
| GGT, units/L | 18.0 ± 10.0 | 21.6 ± 11.5 | 15.8 ± 8.2 | <0.0001 |
| Bilirubin, µmol/L | 9.6 ± 5.7 | 12.1 ± 6.5 | 8.2 ± 4.6 | <0.0001 |
| Urea mmol/L | 4.4 ± 1.1 | 4.9 ± 1.1 | 4.0 ± 0.9 | <0.0001 |
| Uric acid umol/L | 265 ± 79 | 331 ± 67 | 226 ± 56 | <0.0001 |
| Current smokers, n (%) | 522 (31) | 197 (31) | 325 (31) | |
| Oral contraceptive use, n (%) | — | — | 268 (25.2) | |
| MTHFR 677C→T, n (%) | ||||
| CC | 742 (44.8) | 260 (42.3) | 482 (46.3) | |
| CT | 716 (43.2) | 277 (45.0) | 439 (42.2) | |
| TT | 198 (12.0) | 78 (12.7) | 120 (11.8) | |
| Total valid genotypes | 1656 | 615 | 1041 |
1Values are means ± SDs unless otherwise indicated. Student's t test was used to determine significant differences between male and female participants. ALT, alanine aminotransferase; AST, aspartate aminotransferase; CRP, C-reactive protein; GGT, γ-glutamyltransferase; MTHFR, methylenetetrahydrofolate reductase.
TABLE 2.
Metabolites and biomarkers in the study population1
| Total (n = 1701) | Men (n = 638) | Women (n = 1063) | P | |
|---|---|---|---|---|
| Serum folate, nmol/L | 33.3 ± 18.0 | 31.8 ± 16.3 | 34.2 ± 18.9 | 0.007 |
| Red blood cell folate, nmol/L | 1046 ± 427 | 1064 ± 400 | 1035 ± 442 | 0.16 |
| Serum vitamin B-12, pmol/L | 339 ± 150 | 365 ± 147 | 324 ± 150 | <0.0001 |
| Homocysteine, µmol/L | 8.7 ± 3.1 | 9.6 ± 3.6 | 8.2 ± 2.7 | <0.0001 |
| Holotranscobalamin, pmol/L | 59.3 ± 31.8 | 64.3 ± 30.4 | 56.2 ± 32.3 | <0.0001 |
| Methylmalonic acid, µmol/L | 0.19 ± 0.09 | 0.19 ± 0.08 | 0.19 ± 0.09 | 0.27 |
| Methionine, µmol/L | 29.2 ± 8.2 | 32.1 ± 8.5 | 27.4 ± 7.4 | <0.0001 |
| Serine, µmol/L | 148 ± 24 | 144 ± 23 | 150 ± 25 | <0.0001 |
| Glycine, µmol/L | 296 ± 64 | 305 ± 52 | 290 ± 70 | <0.0001 |
| Sarcosine, µmol/L | 1.3 ± 0.6 | 1.7 ± 0.7 | 1.1 ± 0.5 | <0.0001 |
| Tryptophan, µmol/L | 70.5 ± 12.9 | 74.6 ± 12.8 | 68.1 ± 12.4 | <0.0001 |
| Cystathionine, µmol/L | 0.14 ± 0.07 | 0.16 ± 0.07 | 0.14 ± 0.07 | <0.0001 |
| Cysteine, µmol/L | 242 ± 23.8 | 248 ± 23.4 | 238 ± 23.2 | <0.0001 |
| Choline, µmol/L | 7.6 ± 1.8 | 8.0 ± 1.8 | 7.4 ± 1.8 | <0.0001 |
| Betaine, µmol/L | 37.4 ± 14.2 | 45.0 ± 12.4 | 32.8 ± 13.3 | <0.0001 |
| Dimethylglycine, µmol/L | 4.2 ± 1.2 | 4.6 ± 1.1 | 4.0 ± 1.1 | <0.0001 |
| Formate, µmol/L | 25.9 ± 7.7 | 25.0 ± 7.8 | 26.5 ± 7.7 | <0.0001 |
1Values are means ± SDs. Student's t test was used to determine significant differences between male and female participants. There are some missing values for several metabolites; therefore, the n values for metabolites range from 628 to 638 in men and from 1057 to 1063 in women.
SNPs were tested for association with log10-transformed formate concentrations by using the simple linear regression model executed in PLINK (10.1086/519795) version 1.07 under the assumption of an additive genetic model. The genomewide significance level was set at P < 5 × 10−8. We also tested for associations between variants in preselected candidate genes. Bonferroni corrections for these tests were based on the number of SNPs tested for all candidate genes.
RESULTS
Characteristics of the selected population
Table 1 shows the general demographic and clinical characteristics of the selected population. The clinical variables were within normal ranges and the differences between men and women were expected and have been reported previously for the full TSS cohort (15–18). Alcohol intake was higher among men, and 31% of participants reported that they were current smokers. One-quarter of the women used oral contraceptives. The frequency of the genotypes for the MTHFR 677C→T polymorphism was in accord with the Hardy-Weinberg equilibrium, with an allele frequency of 0.664 C and 0.336 T.
Table 2 shows the relevant B-vitamin and one-carbon metabolites in the study population. Red blood cell folate was not affected by sex. Otherwise, serum vitamin B-12, homocysteine, methionine, glycine, choline, betaine, dimethylglycine, sarcosine, and tryptophan were higher in men, whereas serum folate, serine, and formate were higher in women. There was no difference in formate concentrations (P = 0.51) between women who used (mean ± SD: 26.8 ± 7.0 µmol/L; n = 267) and did not use (26.4 ± 7.9 µmol/L; n = 794) oral contraceptives.
Distribution and associations of formate in the TSS cohort
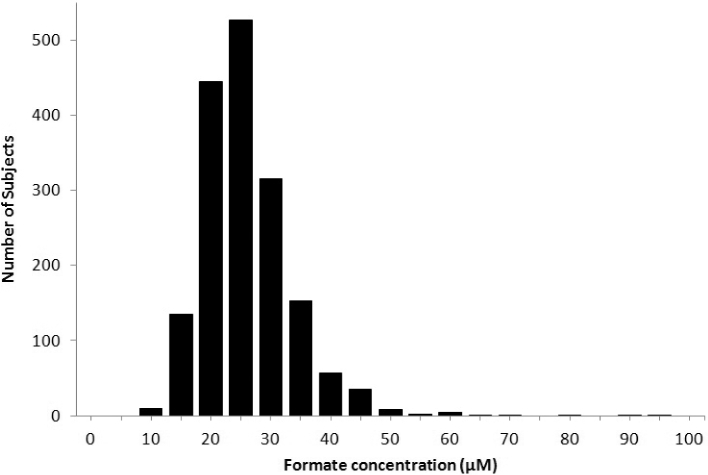
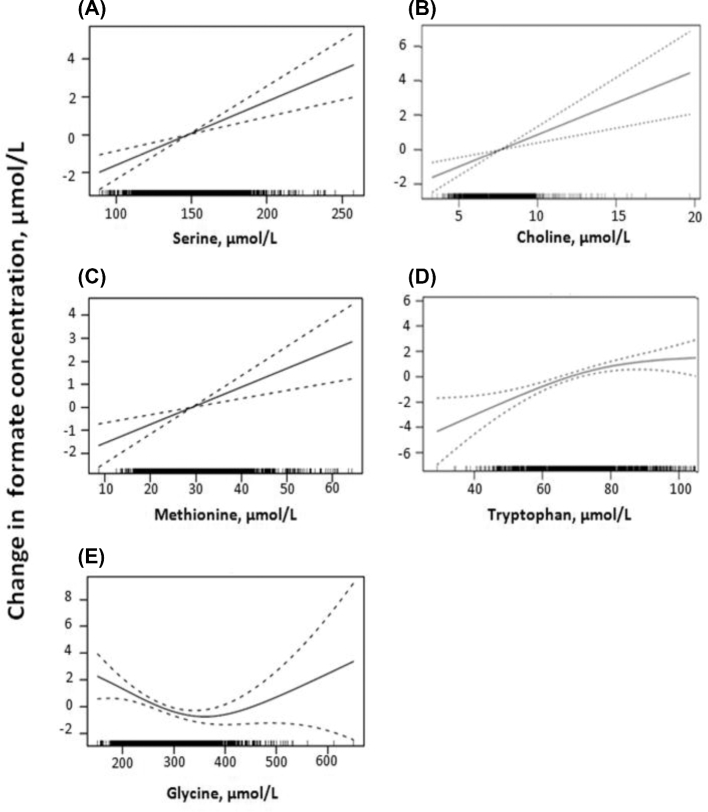
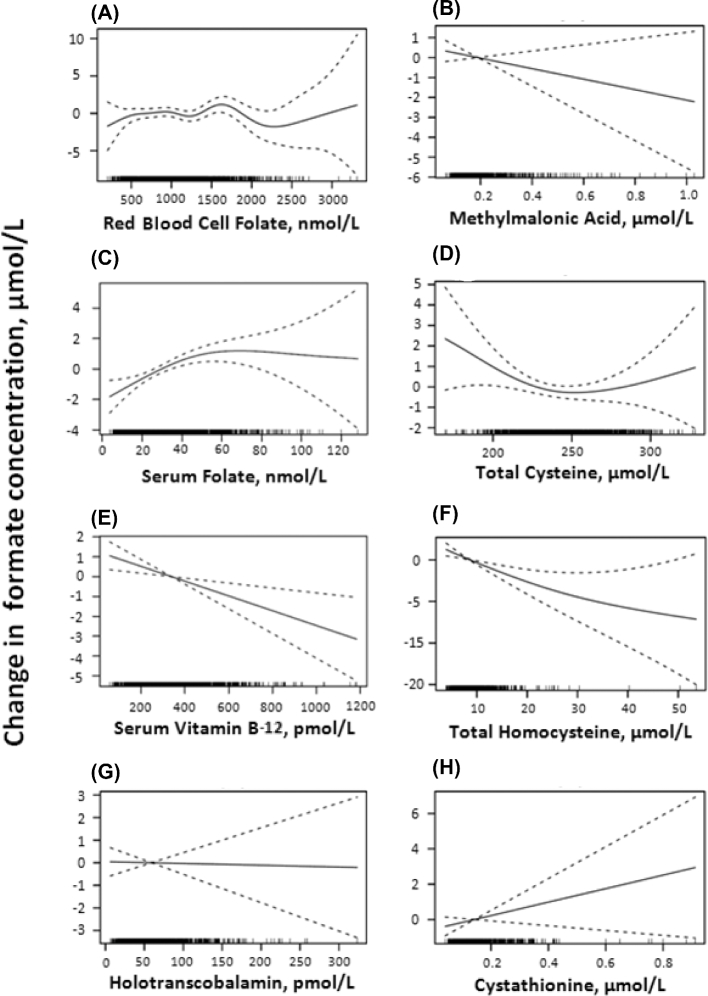
Figure 2 shows that the distribution of formate concentrations in the 1701 participants in the current study ranged from 8.74 to 96.5 µM, with a mean ± SD of 25.94 ± 7.7 µM. There were no significant associations between formate concentrations and BMI, alcohol ingestion (grams of alcohol per day), and serum cotinine, a marker for smoking (data not shown). Spearman correlation coefficients and log-rank–transformed regression P values showed that formate concentrations were positively correlated with methionine, serine, tryptophan, serum folate, and choline and negatively correlated with glycine, homocysteine, and serum total vitamin B-12. There were no significant correlations with cysteine, cystathionine, red blood cell folate, holotranscobalamin, or methylmalonic acid (Table 3). The relations between formate and the potential formate precursors are shown graphically as GAM plots in Figure 3, and the relations with other variables (vitamins and metabolites of the trans-sulfuration pathway) are shown in Figure 4.
FIGURE 2.
Distribution of serum formate concentrations in the Trinity Student Study cohort, n = 1701.
TABLE 3.
Spearman correlation coefficients and regression P values for log-rank–transformed formate data on blood metabolites in the study population
| Metabolite | Formate, Spearman’s r | P 1 |
|---|---|---|
| Methionine | 0.088 | 1.20 × 10−4 |
| Serine | 0.130 | 1.5 × 10−7 |
| Glycine | −0.082 | 0.016 |
| Tryptophan | 0.122 | 5.82 × 10−7 |
| Serum folate | 0.124 | 1.67 × 10−5 |
| Homocysteine | −0.081 | 6.03 × 10−5 |
| Serum total vitamin B-12 | −0.081 | 7.61 × 10−4 |
| Choline | 0.091 | 1.07 × 10−4 |
| Cysteine | −0.037 | 0.150 |
| Cystathionine | 0.035 | 0.105 |
| Red blood cell folate | 0.000 | 0.921 |
| Holotranscobalamin | −0.031 | 0.674 |
| Methylmalonic acid | 0.006 | 0.268 |
1 P values are based on the following models: y = α0 + α1x + ε, where y = inverse normal rank transformation of formate and x is one of the metabolites. The P value is for the null H0: α1 = 0.
FIGURE 3.
GAM plots of potential formate precursors against formate. GAM plots shown are between formate and serine (A), choline (B), methionine (C), tryptophan (D), and glycine (E). The area between the dotted lines shows the 95% CI of the fitted model. The x axis shows the concentration of the variable; the black bars along the x axis represent the sample density. The y axis shows the distribution of formate relative to the median value (represented as 0). n = 1701. GAM, generalized additive model.
FIGURE 4.
GAM plots of indexes of vitamin concentrations and sulfur amino acid metabolism against formate. GAM plots shown are between formate and red blood cell folate (A), methylmalonic acid (B), serum folate (C), total cysteine (D), vitamin B-12 (E), total homocysteine (F), holotranscobalamin (G), and cystathionine (H). The area between the dotted lines shows the 95% CI of the fitted model. The x axis shows the concentration of the variable; the black bars along the x axis represent the sample density. The y axis shows the distribution of formate relative to the median value (represented as 0). n = 1701. GAM, generalized additive model.
Effect of genetic polymorphisms on formate concentrations
We carried out a genomewide association study analysis of log10-transformed formate concentrations and tested for an association between functional polymorphisms in a number of genes that are involved in one-carbon metabolism. One SNP reached the established genomewide significance level of P < 5 × 10−8 (rs10819587; P = 3.03 × 10−8). This and the next most-associated SNP (rs2297603; P = 1.53 × 10−7) are located in collagen type XV α1 chain (COL15A1), a gene without an obvious role in formate metabolism. Due to the limited availability of additional samples, this finding has not been replicated.
After this analysis, we performed a more focused association analysis of serum formate with specific candidate genes. These genes were cytoplasmic NAD(P)-dependent 10-formyl-THF dehydrogenase (ALDH1L1), ALDH1L2, cytoplasmic trifunctional 10-formyl-THF synthase (MTHFD1), MTHFD1L, mitochondrial bifunctional NAD-dependent methylene-THF dehydrogenase/methenyl-THF cyclohydrolase (MTHFD2), NAD-dependent methylenetetrahydrofolate dehydrogenase 2-like (MTHFD2L), MTHFR, and serine hydroxymethyltransferase 2 (SHMT2). No significant relation between formate concentrations and the genetic variants tested was observed for these genes.
We also examined the relation between formate concentrations and the MTHFR 677C→T genotype, determined separately as an independent variable. This analysis showed a significant effect of the MTHFR 677C→T polymorphism. Participants with the CC genotype had higher formate concentrations (by 10%) than did those with the TT genotype. CT participants had intermediate concentrations (Table 4). As expected, both serum folate and red blood cell folate were higher in CC participants than in TT participants, with CT participants exhibiting intermediate concentrations. Homocysteine was higher (by 13%) in TT participants than in CC participants, with CT participants exhibiting intermediate concentrations.
TABLE 4.
Effect of the MTHFR 677C→T polymorphism1
| Genotype | ||||
|---|---|---|---|---|
| CC (n = 742) | CT (n = 716) | TT (n = 198) | P 2 | |
| Serum folate, nmol/L | 34.7 ± 18.0 | 33.3 ± 17.2 | 28.6 ± 19.7 | <0.0001 |
| Red blood cell folate, nmol/L | 1085 ± 427 | 1046 ± 416 | 898 ± 437 | <0.0001 |
| Total homocysteine, µmol/L | 8.3 ± 2.1 | 8.6 ± 2.9 | 10.6 ± 5.8 | <0.0001 |
| Formate, µmol/L | 26.6 ± 7.3 | 25.8 ± 7.7 | 24.1 ± 9.0 | <0.0001 |
1Values are means ± SDs. MTHFR, methylenetetrahydrofolate reductase.
2One-factor ANOVA was used to determine differences in metabolite concentrations by MTHFR 677C→G genotype.
Multiple regression analysis
A multiple regression analysis (Table 5) identified the most important determinants of serum formate. By using a stepwise selection, sex, glycine, serum vitamin B-12, serine, serum folate, tryptophan, and choline were found to be significantly associated with the formate concentration (P < 0.05). Smoking, alcohol intake, red blood cell folate, plasma total homocysteine, oral contraceptive use, methionine, cysteine, cystathionine, holotranscobalamin, and methylmalonic acid were not significantly associated with formate and are not included in the final model.
TABLE 5.
Multiple linear regression analysis of inverse normal transformed formate concentration against metabolites adjusted for sex. The t-values are t-distributed statistic values to test the regression coefficients to be 0 and the (Pr > |t|) are the related P-values1
| Variable | Estimate | SE | t Value | Pr > |t| |
|---|---|---|---|---|
| Intercept | −0.9433087411 | 0.23224020 | −4.06 | <0.0001 |
| Sex | −0.1693615946 | 0.05434786 | −3.12 | 0.0019 |
| Glycine | −0.0016462631 | 0.00044307 | −3.72 | 0.0002 |
| Serum vitamin B-12 | −0.0006902293 | 0.00016918 | −4.08 | <0.0001 |
| Serine | 0.0062076509 | 0.00123529 | 5.03 | <0.0001 |
| Serum folate | 0.0071649768 | 0.00167058 | 4.29 | <0.0001 |
| Tryptophan | 0.0085507021 | 0.00198087 | 4.32 | <0.0001 |
| Choline | 0.0342071342 | 0.01331375 | 2.57 | 0.0103 |
1The formate concentration is transformed by inverse normal rank transformation to satisfy normality. Smoking, alcohol intake, red blood cell folate, total homocysteine, and oral contraceptive use were found not to be significantly associated with the inverse normal transformed formate concentrations; hence, they are not included in the final models.
DISCUSSION
As far as we are aware, this is the first study that examines the determinants of formate concentrations in a human population. The principal results are as follows: 1) the strong, positive relations between formate concentration and most of its metabolic precursors; 2) the effect of sex; and 3) the relation with the MTHFR 677C→T genotype.
Table 3 shows a strong positive relation between formate concentration and concentrations of methionine, serine, tryptophan, and choline. Tryptophan catabolism produces formate when N-formylkynurenine is converted to kynurenine and formate by the cytosolic enzyme formamidase. The metabolism of methionine, serine, and glycine may all produce 5,10-methylene-THF within mitochondria, which may be converted to formate (Figure 1) by the combined actions of 5,10-methylene-THF reductase, 5,10-methenyl-THF-cyclohydrolase, and MTHFD1L; the first 2 of these activities occur as a bifunctional enzyme, MTHFD2. Choline catabolism produces both sarcosine and dimethylglycine, which are metabolized within mitochondria by sarcosine dehydrogenase and dimethylglycine dehydrogenase, respectively, to give rise to 5,10-methylene-THF. Methionine may be converted to S-adenosylmethionine (SAM) by SAM synthase. MAT III, one of the hepatic isoforms of SAM synthase, exhibits the unusual property of positive feedback (24). Thus, when in abundance in the liver, SAM activates MAT III, thereby further increasing SAM concentrations. SAM, via glycine:N-methyltransferase, may then methylate glycine to produce sarcosine, a precursor for mitochondrial formate production, as outlined above (5). Serine is generally thought to be the major precursor for the production of one-carbon groups (7); the mitochondrial isoform of SHMT2 initiates mitochondrial serine metabolism, which produces formate (2).
The GAM plot of formate against glycine was U-shaped (Figure 3). Increasing the glycine concentration to ∼370 µM was associated with decreasing formate, whereas further increases in glycine (from 370 to 700 µM) were associated with increasing formate concentrations. A careful study of diurnal variations in plasma amino acid concentrations in young, healthy men as a function of protein intake showed an inverse relation between glycine concentration and protein intake (i.e., glycine concentrations were lowest at high protein intakes) (25). This was true throughout the day in both the postabsorptive and postprandial phases. Notably, serine, methionine, and tryptophan behaved differently in that their plasma concentrations were directly associated with protein intake. Therefore, it is possible that the positive associations between formate and a number of dietary formate precursors (serine, tryptophan, methionine) may not be inconsistent with the negative association between formate and glycine concentrations. The mechanism whereby glycine concentrations decrease as a function of protein intake is not fully understood, but studies in rats have shown a marked activation of the glycine cleavage system on feeding either a high-protein diet or a single high-protein meal (26). This has been attributed to the action of glucagon (27). Regardless of the mechanism, the inverse relation between glycine and formate concentrations may be attributed to decreased glycine concentrations in individuals who ingested a protein-rich meal and who would also have had elevated concentrations of serine, methionine, and tryptophan. The positive association between glycine and formate, at high formate concentrations, may simply reflect a response of the glycine cleavage system to higher substrate concentrations.
The glycine cleavage system appears to be critical for the provision of one-carbon units during neural tube formation in the mouse embryo, because knock-out of one of its subunits (Gldc) results in a marked incidence of neural tube defects, which can be eliminated by the provision of sodium formate in the maternal drinking water (28).
Figure 4 shows that formate concentrations respond differently to changes in folate or vitamin B-12 concentrations. Increased serum vitamin B-12 was associated with decreased formate, whereas increased serum folate (particularly at low folate concentrations) was associated with increased formate. Because these 2 vitamins work in tandem to effect remethylation, it is more usual for their deficiencies to display common outcomes. Because there is no relation between transcobalamin and formate, the effect of serum vitamin B-12 may then be due to haptocorrin, which carries ∼80% of the total vitamin B-12 in serum. The fate of vitamin B-12 associated with haptocorrin is unclear; it is transported into the liver but not into other tissues (29). It is possible that this vitamin B-12 may influence formate metabolism. It should be borne in mind that folate is involved in both the production of one-carbon groups (via mitochondrial formate synthesis) and the cytoplasmic utilization of these groups, whereas vitamin B-12 is only involved in the cytoplasmic utilization of one-carbon groups. Figure 4 also shows an inverse relation between homocysteine and formate. The explanation for this is not immediately apparent. Our studies with folate and vitamin B-12 in rats found that deficiencies of either of these vitamins resulted in increases in both formate and homocysteine. It is, however, possible that the observed relation may reflect the effect of the TT or CT MTHFR genotype on both decreasing formate concentrations (possibly as a consequence of decreasing folate concentrations) and increasing homocysteine concentrations as a consequence of impaired remethylation.
There was a significant effect of sex. Formate concentrations were ∼10% higher in women than in men. There was no effect of oral contraceptive use on formate concentrations. The reasons for the sexual dimorphism are not immediately apparent, although there may be an effect of estrogen. Certainly, uterine transcript levels for MTHFD2 are increased after treatment of mice with ethinyl estradiol (30). However, if the difference in formate concentrations between women and men is due to the action of estrogen in women, this effect must be saturated because no further effect was evident in the oral contraceptive users. There is little information with regard to the effects of estrogen on other genes that govern one-carbon metabolism other than phosphatidylethanolamine methyltransferase, the gene that codes for the hepatic enzyme that synthesizes phosphatidylcholine (31). The relative resistance of premenopausal women to choline deficiency has been attributed to the induction of this gene by estrogen (32).
There was a clear but modest effect of MTHFR 677C→T genotype on formate concentrations; formate was 10% lower in participants with the TT genotype than in those with the CC genotype. We suggest that this may be a function of the reduced MTHFR activity in TT participants. There is evidence that decreased MTHFR activity may result in decreased formate concentrations. Krebs and Hems (33) and Waydhas et al. (34) found that the addition of methionine to either isolated rat hepatocytes or to isolated perfused rat liver resulted in a marked increase in formate oxidation to carbon dioxide. They interpreted this to be a result of the allosteric inhibition of MTHFR by an increased SAM concentration. The subsequent accumulation of 10-formyl-THF increases its rate of oxidation to carbon dioxide by the dehydrogenase domain of MTHFD1. Krebs and Hems (33) and Waydhas et al. (34) assumed that ALDH1L1 was responsible for the oxidation of 10-formyl-THF to carbon dioxide. More recently, however, the mitochondrial isoform of ALDH1L1 (ALDH1L2) has been discovered (3), which must also be considered to play a role in the oxidation of the formyl group of 10-formyl-THF to carbon dioxide.
The MTHFR 677C→T genotype has been identified as a risk factor for neural tube defects (35, 36). The catalytic activity of MTHFR is greatly reduced, to ∼30%, in participants with the TT genotype, because of increased loss of its flavin prosthetic group, particularly in a low-folate environment (37, 38). In addition, many flavoproteins, including MTHFR, that have lost their flavin prosthetic group are catabolized more rapidly than are the holo-enzymes (39). We therefore suggest that decreased MTHFR activity in individuals homozygous for the 677 C→T MTHFR genotype may similarly increase the oxidation of the formyl group to carbon dioxide and decrease serum formate concentrations. In support of this suggestion, we used a computer simulation of folate metabolism (40) to assess the effect of reducing the MTHFR activity by 66%; the simulation predicted an increased flux through 10-formyl-THF dehydrogenase of 9% and a reduction in formate concentrations of 10%. Because maternal formate supplementation reduces the incidence of neural tube defects in a number of susceptible mouse models (24, 41, 42), it will be of interest to consider whether decreased formate concentrations may play a role in the etiology of neural tube defects in women homozygous for the MTHFR 677 C→T genotype.
Formate has been described as the neglected member of one-carbon metabolism. It occurs in serum at considerable concentrations and has been suggested to play a role in distributing one-carbon groups to different organs (5). The present study describes for the first time, to our knowledge, some of the determinants of serum formate concentration in a healthy human population and provides the foundation for future studies on formate metabolism in humans.
Acknowledgements
The authors responsibilities were as follows—JTB, JLM, MEB, and AMM: designed the research; AMM, TP, PMU, FP, and LCB: collected and analyzed the data; RF and C-YC: performed the statistical analysis; JTB, AMM, and JLM: wrote the initial draft of the manuscript; BS, FP, LCB, PMU, MEB, and RF: critically revised the manuscript; and all authors: had responsibility for the accuracy of the final content and read and approved the final manuscript. None of the authors reported a conflict of interest related to the study.
Notes
The Canadian Institutes of Health Research (MOP14321) supported the development and measurement of formate concentrations; the Intramural Research Programs of the National Human Genome Research Institute supported all genotyping analysis, and the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the Health Research Board, Dublin, supported the recruitment of the Trinity Student Study and the analysis of all other metabolites.
Abbreviations used: ALDH1L1, cytoplasmic NAD(P)-dependent 10-formyl-tetrahydrofolate dehydrogenase; ALDH1L2, mitochondrial NAD(P)-dependent 10-formyl-tetrahydrofolate dehydrogenase; GAM, generalized additive model; MTHFD1L, mitochondrial monofunctional 10-formyl-tetrahydrofolate synthetase; MTHFD2 mitochondrial bifunctional NAD-dependent methylene-tetrahydrofolate dehydrogenase/methenyl-tetrahydrofolate cyclohydrolase; MTHFR, methylenetetrahydrofolate reductase; SAM, S-adenosylmethionine; SNP, single nucleotide polymorphism; THF, tetrahydrofolate; TSS, Trinity Student Study.
REFERENCES
- 1. Fan J, Ye J, Kamphorst JJ, Shlomi T, Thompson CB, Rabinowitz JD. Quantitative flux analysis reveals folate-dependent NADPH production. Nature 2014;510:298–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tibbetts AS, Appling DR. Compartmentalization of mammalian folate-mediated one-carbon metabolism. Annu Rev Nutr 2010;30:57–81. [DOI] [PubMed] [Google Scholar]
- 3. Krupenko NI, Dubard ME, Strickland KC, Moxley KM, Oleinik NV, Krupenko SA. ALDH1L2 is the mitochondrial homolog of 10-formyltetrahydrofolate dehydrogenase. J Biol Chem 2010;285:23056–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Field MS, Kamynina E, Agunloye OC, Liebenthal RP, Lamarre SG, Brosnan ME, Brosnan JT, Stover PJ. Nuclear enrichment of folate cofactors and methylenetetrahydrofolate dehydrogenase 1 (MTHFD1) protect de novo thymidylate biosynthesis during folate deficiency. J Biol Chem 2014;289:29642–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brosnan ME, Brosnan JT. Formate: the neglected member of one-carbon metabolism. Annu Rev Nutr 2016;36:369–88. [DOI] [PubMed] [Google Scholar]
- 6. Brosnan ME, MacMillan L, Stevens JR, Brosnan JT. Division of labour: how does folate metabolism partition between one-carbon metabolism and amino acid oxidation? Biochem J 2015;472:135–46. [DOI] [PubMed] [Google Scholar]
- 7. Davis SR, Stacpoole PW, Williamson J, Kick LS, Quinlivan EP, Coats BS, Shane B, Bailey LB, Gregory JF III. Tracer-derived total and folate-dependent homocysteine remethylation and synthesis rates in humans indicate that serine is the main one-carbon donor. Am J Physiol Endocrinol Metab 2004;286:E272–9. [DOI] [PubMed] [Google Scholar]
- 8. Gregory JF III, Cuskelly GJ, Shane B, Toth JP, Baumgartner TG, Stacpoole PW. Primed, constant infusion with [2H3]serine allows in vivo kinetic measurement of serine turnover, homocysteine remethylation, and transsulfuration processes in human one-carbon metabolism. Am J Clin Nutr 2000;72:1535–41. [DOI] [PubMed] [Google Scholar]
- 9. Lamarre SG, MacMillan L, Morrow GP, Randell E, Pongnopparat T, Brosnan ME, Brosnan JT. An isotope-dilution, GC-MS assay for formate and its application to human and animal metabolism. Amino Acids 2014;46:1885–91. [DOI] [PubMed] [Google Scholar]
- 10. Washburn SE, Caudill MA, Malysheva O, MacFarlane AJ, Behan NA, Harnett B, MacMillan L, Pongnopparat T, Brosnan JT, Brosnan ME. Formate metabolism in fetal and neonatal sheep. Am J Physiol Endocrinol Metab 2015;308:E921–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Morrow GP, MacMillan L, Lamarre SG, Young SK, MacFarlane AJ, Brosnan ME, Brosnan JT. In vivo kinetics of formate metabolism in folate-deficient and folate-replete rats. J Biol Chem 2015;290:2244–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lamarre SG, Molloy AM, Reinke SN, Sykes BD, Brosnan ME, Brosnan JT. Formate can differentiate between hyperhomocysteinemia due to impaired remethylation and impaired transsulfuration. Am J Physiol Endocrinol Metab 2012;302:E61–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dow JL, Green T. Trichloroethylene induced vitamin B(12) and folate deficiency leads to increased formic acid excretion in the rat. Toxicology 2000;146:123–36. [DOI] [PubMed] [Google Scholar]
- 14. Katre P, Joshi S, Bhat DS, Deshmukh M, Gurav N, Pandit S, Lubree H, Marczewski S, Bennett C, Gruca L, et al. Effect of multi-nutrient insufficiency on markers of one carbon metabolism in young women: response to a methionine load. Eur J Clin Nutr 2016;70:687–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mills JL, Carter TC, Scott JM, Troendle JF, Gibney ER, Shane B, Kirke PN, Ueland PM, Brody LC, Molloy AM. Do high blood folate concentrations exacerbate metabolic abnormalities in people with low vitamin B-12 status? Am J Clin Nutr 2011;94:495–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Deac OM, Mills JL, Shane B, Midttun O, Ueland PM, Brosnan JT, Brosnan ME, Laird E, Gibney ER, Fan R, et al. Tryptophan catabolism and vitamin B-6 status are affected by gender and lifestyle factors in healthy young adults. J Nutr 2015;145:701–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Deac OM, Mills JL, Gardiner CM, Shane B, Quinn L, Midttun Ø, McCann A, Meyer K, Ueland PM, Fan R, et al. Serum immune system biomarkers neopterin and interleukin-10 are strongly related to tryptophan metabolism in healthy young adults. J Nutr 2016;146:1801–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Molloy AM, Pangilinan F, Mills JL, Shane B, O'Neill MB, McGaughey DM, Velkova A, Abaan HO, Ueland PM, McNulty H, et al. A common polymorphism in HIBCH influences methylmalonic acid concentrations in blood independently of cobalamin. Am J Hum Genet 2016;98:869–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Holm PI, Ueland PM, Kvalheim G, Lien EA. Determination of choline, betaine, and dimethylglycine in plasma by a high-throughput method based on normal-phase chromatography-tandem mass spectrometry. Clin Chem 2003;49:286–94. [DOI] [PubMed] [Google Scholar]
- 20. Windelberg A, Arseth O, Kvalheim G, Ueland PM. Automated assay for the determination of methylmalonic acid, total homocysteine, and related amino acids in human serum or plasma by means of methylchloroformate derivatization and gas chromatography-mass spectrometry. Clin Chem 2005;51:2103–9. [DOI] [PubMed] [Google Scholar]
- 21. Molloy AM, Scott JM. Microbiological assay for serum, plasma, and red cell folate using cryopreserved, microtiter plate method. Methods Enzymol 1997;281:43–53. [DOI] [PubMed] [Google Scholar]
- 22. Kelleher BP, Broin SD. Microbiological assay for vitamin B12 performed in 96-well microtiter plates. J Clin Pathol 1991;44:592–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Brady J, Wilson L, McGregor L, Valente E, Orning L. Active B12: a rapid, automated assay for holotranscobalamin on the Abbott AxSYM analyzer. Clin Chem 2008;54:567–73. [DOI] [PubMed] [Google Scholar]
- 24. Lu SC, Mato JM. S-adenosylmethionine in liver health, injury, and cancer. Physiol Rev 2012;92:1515–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fernstrom JD, Wurtman RJ, Hammarstrom-Wiklund B, Rand WM, Munro HN, Davidson CS. Diurnal variations in plasma concentrations of tryptophan, tyrosine, and other neutral amino acids: effect of dietary protein intake. Am J Clin Nutr 1979;32:1912–22. [DOI] [PubMed] [Google Scholar]
- 26. Ewart HS, Jois M, Brosnan JT. Rapid stimulation of the hepatic glycine-cleavage system in rats fed on a single high-protein meal. Biochem J 1992;283:441–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jois M, Hall B, Fewer K, Brosnan JT. Regulation of hepatic glycine catabolism by glucagon. J Biol Chem 1989;264:3347–51. [PubMed] [Google Scholar]
- 28. Pai YJ, Leung KY, Savery D, Hutchin T, Prunty H, Heales S, Brosnan ME, Brosnan JT, Copp AJ, Greene ND. Glycine decarboxylase deficiency causes neural tube defects and features of non-ketotic hyperglycinemia in mice. Nat Commun 2015;6:6388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Alpers DH. Absorption and blood/cellular transport of folate and cobalamin: pharmacokinetic and physiological considerations. Biochimie 2016;126:52–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Boverhof DR, Burgoon LD, Williams KJ, Zacharewski TR. Inhibition of estrogen-mediated uterine gene expression responses by dioxin. Mol Pharmacol 2008;73:82–93. [DOI] [PubMed] [Google Scholar]
- 31. Resseguie M, Song J, Niculescu MD, da Costa KA, Randall TA, Zeisel SH. Phosphatidylethanolamine N-methyltransferase (PEMT) gene expression is induced by estrogen in human and mouse primary hepatocytes. FASEB J 2007;21:2622–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zeisel SH. Nutritional genomics: defining the dietary requirement and effects of choline. J Nutr 2011;141:531–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Krebs HA, Hems R. The regulation of the degradation of methionine and of the one-carbon units derived from histidine, serine and glycine. Adv Enzyme Regul 1976;14:493–513. [DOI] [PubMed] [Google Scholar]
- 34. Waydhas C, Weigl K, Sies H. The disposition of formaldehyde and formate arising from drug N-demethylations dependent on cytochrome P-450 in hepatocytes and in perfused rat liver. Eur J Biochem 1978;89:143–50. [DOI] [PubMed] [Google Scholar]
- 35. Kirke PN, Mills JL, Whitehead AS, Molloy A, Scott JM. Methylenetetrahydrofolate reductase mutation and neural tube defects. Lancet 1996;348:1037–8. [DOI] [PubMed] [Google Scholar]
- 36. Van der Put NM, Steegers-Theunissen RP, Frosst P, Trijbels FJ, Eskes TK, van den Heuvel LP, Mariman EC, den Heyer M, Rozen R, Blom HJ. Mutated methylenetetrahydrofolate reductase as a risk factor for spina bifida. Lancet 1995;346:1070–1. [DOI] [PubMed] [Google Scholar]
- 37. Frosst P, Blom HJ, Milos R, Goyette P, Sheppard CA, Matthews RG, Boers GJH, den Heijer M, Kluijtmans LAJ, van den Heuve LP, et al. A candidate genetic risk factor for vascular disease: a common mutation in methylenetetrahydrofolate reductase. Nature Genet 1995;10:111–3. [DOI] [PubMed] [Google Scholar]
- 38. Yamada K, Chen Z, Rozen R, Matthews RG. Effects of common polymorphisms on the properties of recombinant human methylenetetrahydrofolate reductase. Proc Natl Acad Sci USA 2001;98:14853–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Martinez-Limón A, Alriquet M, Lang WH, Calloni G, Wittig I, Vabulas RM. Recognition of enzymes lacking bound cofactor by protein quality control. Proc Natl Acad Sci USA 2016;113:12156–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Nijhout HF, Reed MC, Ulrich CM. Mathematical models of folate-mediated one-carbon metabolism. Vitam Horm 2008;79:45–82. [DOI] [PubMed] [Google Scholar]
- 41. Momb J, Lewandowski JP, Bryant JD, Fitch R, Surman DR, Vokes SA, Appling DR. Deletion of Mthfd1l causes embryonic lethality and neural tube and craniofacial defects in mice. Proc Natl Acad Sci USA 2013;110:549–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sudiwala S, De Castro SC, Leung KY, Brosnan JT, Brosnan ME, Mills K, Copp AJ, Greene ND. Formate supplementation enhances folate-dependent nucleotide biosynthesis and prevents spina bifida in a mouse model of folic acid-resistant neural tube defects. Biochimie 2016;126:63–70. [DOI] [PMC free article] [PubMed] [Google Scholar]