Abstract
Viruses encode a variety of mechanisms to evade host immune pathways. Large DNA viruses (herpesviruses and poxviruses) encode proteins that mimic chemokines and chemokine receptors. Also, some viruses encode secreted proteins that bind chemokines and have structure unrelated to host proteins. Recent research in this area has led to the identification of new viral proteins that modulate the chemokine system, has provided information on the molecular mechanisms leading to interference of chemokine signaling, and has shed light into the function of these proteins in the context of infection. The therapeutic value of these viral proteins to inhibit immune responses that cause pathology has been explored further. Finally, a new family of chemokine binding proteins identified in ticks expands this strategy of immune modulation beyond the virus world.
Introduction
Infection with pathogens triggers signals that initiate the immune response and the recruitment of immune cells to sites of infection. Chemokines are a family of chemoattractant cytokines that mediate the migration of leukocytes to tissues during homeostasis and following injury and infection [1,2]. Chemokines are classified into C, CC, CXC and CX3C subfamilies according to the relative positioning of the N-terminal cysteine residues. Chemokines interact with glycosaminoglycans (GAGs) and this interaction seems to be required for a proper presentation of the chemokine to specific G-protein-coupled receptors (GPCRs) present on the surface of target cells [3]. Dysregulation of the chemokine network is observed in inflammatory and autoimmune diseases [4].
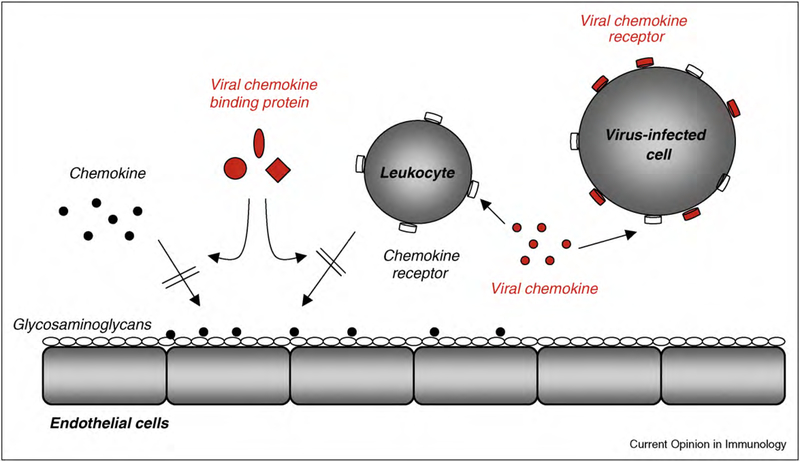
Viruses have evolved numerous strategies to evade innate and acquired immune responses that may eliminate them in the infected host. Large DNA viruses, such as herpesviruses and poxviruses, encode homologs of chemokine ligands and chemokine receptors, and secreted chemokine binding proteins (CKBPs) to modulate chemokine activity [5] (Figure 1). More recently, several examples of CKBPs have been identified in other pathogens different to viruses.
Figure 1.
Chemokine modulators encoded by herpesviruses and poxviruses. Chemokines interact with glycosaminoglycans (GAGs) and activate the migration of leukocytes that express specific chemokine receptors. Herpesviruses and poxviruses encode homologs of chemokines, which may function as agonists or antagonists, and chemokine receptors, which are expressed on the surface of infected cells and may be constitutively activated. Viral chemokine binding proteins (CKBPs) are secreted proteins of structure unrelated to host chemokine receptors that bind chemokines and may block the interaction of chemokines with GAGs or chemokine receptors.
Here we review recent findings that increase our understanding of the properties of these virus-encoded chemokine modulators, their mechanism to interfere with the chemokine system and their contribution to pathogenesis. We also discuss a new family of CKBPs recently identified in ticks.
Viruses encode chemokine receptors
The best studied viral chemokine receptors are ORF74 (or viral GPCR, vGPCR) encoded by human herpesvirus 8 (HHV-8, also known as Kaposi’s sarcoma associated herpesvirus, KSHV), and US28 encoded by human cytomegalovirus (HCMV or HHV-5). Both chemokine receptors are constitutively active and signal through a variety of pathways depending on the cellular background in which they are expressed.
vGPCR has been shown to activate both autocrine and paracrine mechanisms leading to tumor formation when expressed in transgenic mice [6]. The ability of vGPCR to cause these changes appears to depend on its expression on endothelial cell progenitors [6,7]. vGPCR affects the biology of both vascular and lymphatic endothelium, by activating several pathways including NFkB [8]. Recently the phosphatase Shp has been shown to be required for vGPCR-mediated activation of MEK, NFkappaB, and AP-1 [9].
Increased expression of Notch signaling pathway components is observed in Kaposi sarcoma (KS). Recent work has provided some clues into these changes and points to vGPCR as one of the possible mediators. In vascular endothelial cells vGPCR upregulates the expression of several components of the Notch pathway (the receptor Notch2, the ligand Jagged1, and Notch downstream targets such as Hey1), via the ERK pathway [8]. In lymphatic endothelial cells vGPCR upregulates the Notch ligand DLL4 through a mechanism also dependent on ERK, and promotes Notch signaling through Notch4. Gene expression profiling showed that JAG1-stimulated or DLL4-stimulated signaling results in the suppression of genes associated with the cell cycle in adjacent lymphatic endothelial cells, indicating a role for Notch signaling in inducing cellular quiescence in these cells. Upregulation of JAG1 and DLL4 by HHV-8 could therefore alter the expression of cell cycle components in neighboring uninfected cells during latent and lytic phases of viral infection, influencing cellular quiescence and plasticity [10].
Recent progress in understanding the mechanisms whereby vGPCR affects vascular biology includes the demonstration that vGPCR can promote expression of angiogenin [11•] and activate expression of cyclooxygenase-2 (COX-2) in vascular cells [12] which could explain the increased levels of COX-2 detected in KS tissue sections [11•].
A recent and provocative study suggests that vGPCR signaling can be regulated by another HHV-8 encoded molecule, K7. Biochemical and confocal microscopy studies indicate that K7, a membrane protein, retains vGPCR in the endoplasmic reticulum and induces vGPCR proteasomal degradation. Knockdown of K7 by shRNA-mediated silencing increases vGPCR protein expression in BCBL-1 cells. Interestingly, K7 expression significantly reduces vGPCR tumorigenicity in nude mice [13•].
Recent work on US28 provides a molecular link between HCMV and cancer. US28 stably transfected NIH-3T3 cells give origin to tumors when transfected into nude mice [14]. Microarray analysis of such cells suggested that expression of COX-2, a key mediator of inflammatory diseases and major determinant in several forms of cancer, was highly upregulated. Targeting COX-2 in vivo with Celecoxib led to a marked delay in the onset of tumor formation in nude mice injected with US28-transfected NIH-3T3 cells suggesting that tumorigenic properties of US28 in this setting depends on the ability of US28 to activate COX-2 [15•].
Viruses encode chemokine ligands
HHV-8 and HCMV also encode chemokine ligands. HHV-8 encodes three chemokine ligands, known as vMIP-I, vMIP-II and vMIP-III (or vCCL1, vCCL22 and vCCL3, respectively). vCCL1 is a selective agonist for CCR8, whereas vCCL2 is an antagonist for CCR1, CCR2, CCR5, CX3CR1 and CXCR4, and an agonist for CCR3 (for an extended review see [16]). Recently it has been shown that vCCL2 can also antagonize the XCR1 receptor and that vCCL3 can act as an agonist of this receptor. The full biological meaning of these interactions is not known at present. It has been speculated that vCCL2 may facilitate immune evasion by blocking recruitment of leukocytes expressing some of these receptors, whereas vCCL1 and vCCL3 facilitate virus spread by regulating influx or activation of other cell types. Besides their potential role in immune evasion, vCCL1 and vCCL2 may have direct effects on endothelial survival and virus replication and contribute to the pathogenesis of KS [17].
HCMV encodes at least two CXC ligands, encoded by the genes UL146 and UL147. The gene product of UL147 has not yet been characterized, but the UL146 gene encodes the protein vCXCL1, which functions as an agonist not only for CXCR2 but also for CXCR1, although with lower affinity and potency [18].
Viruses encode CKBPs
Poxviruses and herpesviruses encoded secreted proteins that bind chemokines in order to control the activity of chemokines during infection [19]. In contrast to other virus-encoded cytokine receptor homologs that share amino acid sequence similarity to host receptor and may have a common evolutionary origin, viral CKBPs are unrelated to host receptors and may have evolved independently. New CKBPs have been identified in recent years and of special interest is the identification of CKBPs encoded by ticks.
Structural studies
Because CKBPs are unrelated to membrane-anchored GPCRs or other proteins in the human and mouse genomes, a better knowledge of their structure is of great interest to understand how a secreted protein may interact with chemokines with high affinity.
The M3 protein encoded by murine gammaherpesvirus 68 (MHV-68) was the first CKBP crystallized as a complex with chemokines, in this case CCL2 [20]. Dimerization of the M3 protein was found to generate two chemokine binding sites. A recent study describing M3 complexed to CL1 illustrated the flexibility of this protein that binds a broad range of chemokines [21]. Most viral CKBPs prevent the interaction of chemokine with their cellular receptors and the subsequent triggering of intracellular signaling. However, both MHV-68 M3 and gG from alphaherpesviruses were found to use an additional mechanism, inhibiting the interaction of chemokines with both chemokine receptors and GAGs [22,23]. The structural basis for the inhibition of chemokine–GAG interaction by M3 was uncovered in the crystal structure of the M3–chemokine complex [21].
The A41 protein from VACV has immunomodulatory activity and sequence similarity to the 35-kDa CKBP from VACV and other poxviruses [24,25]. However, none of these studies demonstrated chemokine binding activity for A41. Two research groups carried out an extensive screening of chemokines by Surface Plasmon Resonance for their potential binding to recombinant A41 from VACV and ectromelia virus, and recently reported the identification of a set of CC and CXC chemokines that interact with the A41 protein [26•,27•]. It was proposed that the A41-mediated inhibition of leukocyte migration in vivo could be related to its ability to block the correct interaction of chemokines with GAGs, which is required for appropriate chemokine function in vivo [3,28]. The crystal structure of the VACV A41 protein confirmed its similarity to the 35-kDa CKBP [29,30], but A41 has notable structural differences in surface loops and electrostatic charge distribution [26•]. Although the structure of A41 bound to chemokines was not determined, structural modeling suggested that the interaction of A41 and 35-kDa proteins with chemokines involves the same domains [26•]. The binding site on chemokines for GAGs and chemokine receptors frequently overlap and the high affinity of the 35-kDa protein is sufficient to interfere with receptor binding while the lower affinity of A41 for chemokines can block GAG binding but not receptor binding. Thus poxviruses encode related proteins that inhibit the chemokine system in different ways.
Glycoprotein G (gG) from alphaherpesviruses represents another example of a CKBP unrelated to other viral CKBPs [23]. The expression of overlapping 100 amino acid-long peptides identified a domain of equine herpesvirus 1 gG involved in chemokine binding, which corresponds to a hypervariable region located in the extracellular domain adjacent to the transmembrane domain [31•]. The lack of chemokine binding activity in EHV-4 gG offered the possibility of testing chimeric gGs for activity. Replacement of the hypervariable region of EHV-1 gG with the one of EHV-4 rendered a gG unable to bind chemokines. Conversely, an EHV-4 gG containing the hypervariable region of EHV-1 gG bound chemokines, although this protein was unable to inhibit chemokine-mediated migration [31•].
Role of CKBPs in viral pathogenesis and immune modulation
CKBPs are likely to contribute to virus virulence but this has not been demonstrated for all of them. gG from a variety of alphaherpesviruses was shown to bind chemokines and inhibit their activity [23]. The identification of chemokine binding activity in gG from pseudorabies virus [32], a virus that may cause economic problems in pigs, and in infectious laryngotracheitis virus [33], the causative agent of a severe respiratory disease in poultry, emphasizes the importance of this protein for alphaherpesvirus pathogenesis. A role for gG in immune modulation was supported by in vivo experiments showing that chickens infected with a gG-deficient infectious laryngotracheitis virus had altered tracheal leukocyte populations and lower serum antibody levels compared with those infected with the parent virus. Additional evidence for the immunomodulatory role of gG was provided in a mouse model of infection with EHV-1. Mice infected with low doses of a mutant EHV-1 lacking gG showed exacerbated respiratory disease, high virus titers and more pronounced inflammatory response in the lungs, compared to wild type infections [34]. EHV-1 gG also reduced the infiltration of mouse neutrophils and macrophages into the lungs of infected mice and the chemotactic function of CCL3 in mice [35].
Orf virus (ORFV) encodes a CKBP related to the VACV 35-kDa protein. ORFV is a zoonotic parapoxvirus that induces acute pustular skin lesions in sheep and humans. Using a mouse model, it was demonstrated that ORFV CKBP inhibits both inflammatory and constitutive mouse chemokines, blocks the recruitment of immature and mature dendritic cells to the skin and lymph nodes, and inhibits T cell responsiveness in lymph nodes [36,37•].
Additional evidence for the immune modulatory activity of virus-encoded CKBPs was illustrated by studies with the 35-kDa CKBP from monkeypox virus (MPXV), a poxvirus that causes a smallpox-like disease in humans. The MPXV CKBP was found to bind rhesus CCL3 and to inhibit its activity both in vitro and in rhesus macaques [38]. Moreover, it was demonstrated that the MPXV CKBP inhibits relapsing experimental autoimmune encephalitis disease in mice.
Therapeutic application of CKBPs
The role that chemokines play in controlling the migration of immune cells to sites of infection and injury has led to an interest to develop chemokine inhibitors to treat autoimmune and inflammatory diseases [4]. The generation of genetically modified mice expressing the chemokine inhibitor M3 has been used as an elegant approach to improve our understanding of the immunomodulatory role of CKBPs in vivo and their therapeutic value [39]. The expression of chemokine inhibitors will shed light into the role of chemokines during homeostasis and inflammation. This approach demonstrated the ability of M3 to block chemokine function in vivo [40,41]. Moreover, M3 expression prevented development of disease in bilateral femoral arterial injury [42] and in autoimmune diabetes [43,44], and inhibited leukocyte trafficking to the intestine [45].
Evasins, a family of CKBPs encoded by ticks
One of the most relevant findings in recent years was the identification of a family of CKBPs in ticks [46,47••], extending the description of CKBPs beyond viruses. Another example is the CKBP encoded by the human parasite Schistosoma mansoni [48]. This family of CKBPs, termed Evasins, comprises four members of small proteins of 7–12 kDa that are produced in the tick saliva. Ticks are blood-sucking parasites that feed on their hosts for several days but cause no inflammatory response. It has been suggested that chemokine inhibition by Evasins may prevent innate responses that protect from parasites. In contrast to the broad binding specificity of many viral CKBPs (M-T7, 35-kDa or M3 proteins), Evasins bind a reduced set of chemokines: Evasin-1 binds CCL3, CCL4 and CCL18, Evasin-3 binds CXCL8 and CXCL1, and Evasin-4 binds CCL5 and CCL11 [46,47••]. Evasins inhibit the interaction of chemokines with their cellular receptors and the chemokine-induced recruitment of leukocytes in vitro and in vivo. The crystal structure of Evasin-1 and Evasin-3 has been determined and reveal novel protein folds [47••]. Both proteins are unrelated in their secondary and tertiary structures, and interact with host chemokines presumably in different ways. The recent determination of the structure of Evasin-1 complexed with chemokines has defined the structural basis for the Evasin selectivity of chemokines [49•]. Interestingly, the structure of Evasins is different to the protein fold observed in viral CKBPs and the sequestration of chemokines by Evasin-1 differs from that of other CKBPs complexed with chemokines (35 kDa and M3). The immunomodulatory activity of Evasins and their therapeutic value have recently been demonstrated in animal models [50,51].
Conclusions
The mimicry of components of the host chemokine system by viruses was discovered many years ago, and since then many large DNA viruses have been found to encode homologs of chemokines and their receptors, and CKBPs. However, we still do not fully understand the function of this diverse family of viral proteins. The fact that viral chemokines and chemokine receptor homologs may activate or inhibit chemokine signaling illustrates the variety of functions that lead to immune evasion or to hijacking of the chemokine networks for the interest of the virus. The function of viral CKBPs is easier to predict, but we do not understand why viruses encode CKBPs with distinct chemokine specificity, indicating that viruses adapt CKBPs to inhibit different aspects of immunity. Although recent studies have increased our understanding of viral mimics of the chemokine system, more research is needed to uncover their specific roles in the context of infection and how they contribute to the pathology caused by viruses. The therapeutic use of these viral proteins is worth exploring further since they will provide us with new immune modulatory strategies that may lead to new medicaments to treat inflammatory diseases. Finally, it is likely that similar strategies are utilized by complex pathogens and future studies are likely to uncover more chemokine modulators outside viruses.
Acknowledgements
AA was funded by the Spanish Ministry of Science and Innovation, and Comunidad de Madrid.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Mackay CR: Chemokines: immunology’s high impact factors. Nat Immunol 2001, 2:95–101. [DOI] [PubMed] [Google Scholar]
- 2.Zlotnik A, Yoshie O, Nomiyama H: The chemokine and chemokine receptor superfamilies and their molecular evolution. Genome Biol 2006, 7:243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Handel TM, Johnson Z, Crown SE, Lau EK, Proudfoot AE: Regulation of protein function by glycosaminoglycans — as exemplified by chemokines. Annu Rev Biochem 2005, 74:385–410. [DOI] [PubMed] [Google Scholar]
- 4.Proudfoot AE: Chemokine receptors: multifaceted therapeutic targets. Nat Rev Immunol 2002, 2:106–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alcami A: Viral mimicry of cytokines, chemokines and their receptors. Nat Rev Immunol 2003, 3:36–50. [DOI] [PubMed] [Google Scholar]
- 6.Grisotto MG, Garin A, Martin AP, Jensen KK, Chan P, Sealfon SC, Lira SA: The human herpesvirus 8 chemokine receptor vGPCR triggers autonomous proliferation of endothelial cells. J Clin Invest 2006, 116:1264–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mutlu AD, Cavallin LE, Vincent L, Chiozzini C, Eroles P, Duran EM, Asgari Z, Hooper AT, La Perle KM, Hilsher C et al. : In vivo-restricted and reversible malignancy induced by human herpesvirus-8 KSHV: a cell and animal model of virally induced Kaposi’s sarcoma. Cancer Cell 2007, 11:245–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu R, Li X, Tulpule A, Zhou Y, Scehnet JS, Zhang S, Lee JS, Chaudhary PM, Jung J, Gill PS: KSHV-induced notch components render endothelial and mural cell characteristics and cell survival. Blood 2010, 115:887–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bakken T, He M, Cannon ML: The phosphatase Shp2 is required for signaling by the Kaposi’s sarcoma-associated herpesvirus viral GPCR in primary endothelial cells. Virology 2010, 397:379–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Emuss V, Lagos D, Pizzey A, Gratrix F, Henderson SR, Boshoff C: KSHV manipulates Notch signaling by DLL4 and JAG1 to alter cell cycle genes in lymphatic endothelia. PLoS Pathog 2009, 5:e1000616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sharma-Walia N, Paul AG, Bottero V, Sadagopan S, Veettil MV, Kerur N, Chandran B: Kaposi’s sarcoma associated herpes virus (KSHV) induced COX-2: a key factor in latency, inflammation, angiogenesis, cell survival and invasion. PLoS Pathog 2010, 6:e1000777.• This report underscores the role that activation of COX-2, mediated by vGPCR, has in the induction of inflammatory cytokines and angiogenic factors that contribute to the pathogenesis of HHV-8.
- 12.Shelby BD, LaMarca HL, McFerrin HE, Nelson AB, Lasky JA, Sun G, Myatt L, Offermann MK, Morris CA, Sullivan DE: Kaposi’s sarcoma associated herpesvirus G-protein coupled receptor activation of cyclooxygenase-2 in vascular endothelial cells. Virol J 2007, 4:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feng H, Dong X, Negaard A, Feng P: Kaposi’s sarcoma-associated herpesvirus K7 induces viral G protein-coupled receptor degradation and reduces its tumorigenicity. PLoS Pathog 2008, 4:e1000157.• An interesting observation showing that the HHV-8 vGPCR signaling and tumorigenesis is regulated by another viral protein, K7, that induces vGPCR proteasomal degradation.
- 14.Maussang D, Verzijl D, van Walsum M, Leurs R, Holl J, Pleskoff O, Michel D, van Dongen GA, Smit MJ: Human cytomegalovirusencoded chemokine receptor US28 promotes tumorigenesis. Proc Natl Acad Sci U S A 2006, 103:13068–13073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maussang D, Langemeijer E, Fitzsimons CP, Stigter-van Walsum M, Dijkman R, Borg MK, Slinger E, Schreiber A, Michel D, Tensen CP et al. : The human cytomegalovirus-encoded chemokine receptor US28 promotes angiogenesis and tumor formation via cyclooxygenase-2. Cancer Res 2009, 69:2861–2869.• This publication describes a mechanism by which a viral chemokine receptor promotes angiogenesis and tumor development.
- 16.Luttichau HR: The herpesvirus 8 encoded chemokines vCCL2 (vMIP-II) and vCCL3 (vMIP-III) target the human but not the murine lymphotactin receptor. Virol J 2008, 5:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Choi YB, Nicholas J: Autocrine and paracrine promotion of cell survival and virus replication by human herpesvirus 8 chemokines. J Virol 2008, 82:6501–6513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Luttichau HR: The cytomegalovirus UL146 gene product vCXCL1 targets both CXCR1 and CXCR2 as an agonist. J Biol Chem 2010, 285:9137–9146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alcami A, Saraiva M: Chemokine binding proteins encoded by pathogens. Adv Exp Med Biol 2009, 666:167–179. [DOI] [PubMed] [Google Scholar]
- 20.Alexander JM, Nelson CA, van Berkel V, Lau EK, Studts JM, Brett TJ, Speck SH, Handel TM, Virgin HW, Fremont DH: Structural basis of chemokine sequestration by a herpesvirus decoy receptor. Cell 2002, 111:343–356. [DOI] [PubMed] [Google Scholar]
- 21.Alexander-Brett JM, Fremont DH: Dual GPCR and GAG mimicry by the M3 chemokine decoy receptor. J Exp Med 2007, 204:3157–3172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Webb LM, Smith VP, Alcami A: The gammaherpesvirus chemokine binding protein can inhibit the interaction of chemokines with glycosaminoglycans. FASEB J 2004, 18:571–573. [DOI] [PubMed] [Google Scholar]
- 23.Bryant NA, Davis-Poynter N, Vanderplasschen A, Alcami A: Glycoprotein G isoforms from some alphaherpesviruses function as broad-spectrum chemokine binding proteins. EMBO J 2003, 22:833–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ng A, Tscharke DC, Reading PC, Smith GL: The vaccinia virus A41L protein is a soluble 30 kDa glycoprotein that affects virus virulence. J Gen Virol 2001, 82:2095–2105. [DOI] [PubMed] [Google Scholar]
- 25.Clark RH, Kenyon JC, Bartlett NW, Tscharke DC, Smith GL: Deletion of gene A41L enhances vaccinia virus immunogenicity and vaccine efficacy. J Gen Virol 2006, 87:29–38. [DOI] [PubMed] [Google Scholar]
- 26.Bahar MW, Kenyon JC, Putz MM, Abrescia NG, Pease JE, Wise EL, Stuart DI, Smith GL, Grimes JM: Structure and function of A41, a vaccinia virus chemokine binding protein. PLoS Pathog 2008, 4:e5.• See annotation to Ref. [27•].
- 27.Ruiz-Arguello MB, Smith VP, Campanella GS, Baleux F, Arenzana-Seisdedos F, Luster AD, Alcami A: An ectromelia virus protein that interacts with chemokines through their glycosaminoglycan binding domain. J Virol 2008, 82:917–926.• Along with Ref. [26•], this paper describes a new CKBP encoded by poxviruses that interacts with the GAG binding domain of chemokines, and Ref. [26•] reports the crystal structure of the protein.
- 28.Johnson Z, Proudfoot AE, Handel TM: Interaction of chemokines and glycosaminoglycans: a new twist in the regulation of chemokine function with opportunities for therapeutic intervention. Cytokine Growth Factor Rev 2005, 16:625–636. [DOI] [PubMed] [Google Scholar]
- 29.Carfi A, Smith CA, Smolak PJ, McGrew J, Wiley DC: Structure of a soluble secreted chemokine inhibitor vCCI (p35) from cowpox virus. Proc Natl Acad Sci U S A 1999, 96:12379–12383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arnold PL, Fremont DH: Structural determinants of chemokine binding by an Ectromelia virus-encoded decoy receptor. J Virol 2006, 80:7439–7449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Van de Walle GR, Kaufer BB, Chbab N, Osterrieder N: Analysis of the herpesvirus chemokine-binding glycoprotein G residues essential for chemokine binding and biological activity. J Biol Chem 2009, 284:5968–5976.• The authors identify a region of the alphaherpesvirus CKBP that interacts with chemokines, an interesting observation considering that the CKBP is unrelated to host chemokine receptors.
- 32.Viejo-Borbolla A, Munoz A, Tabares E, Alcami A: Glycoprotein G from pseudorabies virus binds to chemokines with high affinity and inhibits their function. J Gen Virol 2010, 91:23–31. [DOI] [PubMed] [Google Scholar]
- 33.Devlin JM, Viejo-Borbolla A, Browning GF, Noormohammadi AH, Gilkerson JR, Alcami A, Hartley CA: Evaluation of immunological responses to a glycoprotein G deficient candidate vaccine strain of infectious laryngotracheitis virus. Vaccine 2010, 28:1325–1332. [DOI] [PubMed] [Google Scholar]
- 34.Van de Walle GR, May ML, Sukhumavasi W, von Einem J, Osterrieder N: Herpesvirus chemokine-binding glycoprotein G (gG) efficiently inhibits neutrophil chemotaxis in vitro and in vivo. J Immunol 2007, 179:4161–4169. [DOI] [PubMed] [Google Scholar]
- 35.Van de Walle GR, Sakamoto K, Osterrieder N: CCL3 and viral chemokine-binding protein gg modulate pulmonary inflammation and virus replication during equine herpesvirus 1 infection. J Virol 2008, 82:1714–1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lateef Z, Baird MA, Wise LM, Mercer AA, Fleming SB: Orf virusencoded chemokine-binding protein is a potent inhibitor of inflammatory monocyte recruitment in a mouse skin model. J Gen Virol 2009, 90:1477–1482. [DOI] [PubMed] [Google Scholar]
- 37.Lateef Z, Baird MA, Wise LM, Young S, Mercer AA, Fleming SB: The chemokine-binding protein encoded by the poxvirus orf virus inhibits recruitment of dendritic cells to sites of skin inflammation and migration to peripheral lymph nodes. Cell Microbiol 2010, 12:665–676.• This study identifies dendritic cell migration as one of the immune functions that is targeted by the poxvirus 35-kDa, representing an advantage for the virus.
- 38.Jones JM, Messauodi I, Estep RD, Orzechowska B, Wong SW: Monkeypox virus viral chemokine inhibitor (MPV vCCI), a potent inhibitor of rhesus macrophage inflammatory protein-1. Cytokine 2008, 43:220–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lira SA, Viejo-Borbolla A, Shang L, Martin AP: The chemokine-binding protein M3 as a tool to understand the chemokine network in vivo. Methods Enzymol 2009, 460:193–207. [DOI] [PubMed] [Google Scholar]
- 40.Jensen KK, Chen SC, Hipkin RW, Wiekowski MT, Schwarz MA, Chou CC, Simas JP, Alcami A, Lira SA: Disruption of CCL21-induced chemotaxis in vitro and in vivo by M3, a chemokine-binding protein encoded by murine gammaherpesvirus 68. J Virol 2003, 77:624–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Martin AP, Canasto-Chibuque C, Shang L, Rollins BJ, Lira SA: The chemokine decoy receptor M3 blocks CC chemokine ligand 2 and CXC chemokine ligand 13 function in vivo. J Immunol 2006, 177:7296–7302. [DOI] [PubMed] [Google Scholar]
- 42.Pyo R, Jensen KK, Wiekowski MT, Manfra D, Alcami A, Taubman MB, Lira SA: Inhibition of intimal hyperplasia in transgenic mice conditionally expressing the chemokine-binding protein M3. Am J Pathol 2004, 164:2289–2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Martin AP, Alexander-Brett JM, Canasto-Chibuque C, Garin A, Bromberg JS, Fremont DH, Lira SA: The chemokine binding protein M3 prevents diabetes induced by multiple low doses of streptozotocin. J Immunol 2007, 178:4623–4631. [DOI] [PubMed] [Google Scholar]
- 44.Martin AP, Grisotto MG, Canasto-Chibuque C, Kunkel SL, Bromberg JS, Furtado GC, Lira SA: Islet expression of M3 uncovers a key role for chemokines in the development and recruitment of diabetogenic cells in NOD mice. Diabetes 2008, 57:387–394.•• This study illustrates how CKBPs may be used in mouse models to uncover the contribution of chemokines to pathological conditions, in this case diabetes.
- 45.Shang L, Thirunarayanan N, Viejo-Borbolla A, Martin AP, Bogunovic M, Marchesi F, Unkeless JC, Ho Y, Furtado GC, Alcami A et al. : Expression of the chemokine binding protein M3 promotes marked changes in the accumulation of specific leukocytes subsets within the intestine. Gastroenterology 2009, 137:1006–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Frauenschuh A, Power CA, Deruaz M, Ferreira BR, Silva JS, Teixeira MM, Dias JM, Martin T, Wells TN, Proudfoot AE: Molecular cloning and characterization of a highly selective chemokine-binding protein from the tick Rhipicephalus sanguineus. J Biol Chem 2007, 282:27250–27258. [DOI] [PubMed] [Google Scholar]
- 47.Deruaz M, Frauenschuh A, Alessandri AL, Dias JM, Coelho FM, Russo RC, Ferreira BR, Graham GJ, Shaw JP, Wells TN et al. : Ticks produce highly selective chemokine binding proteins with antiinflammatory activity. J Exp Med 2008, 205:2019–2031.•• It describes a new family of CKBPs with diverse structure and narrow binding specificity, which are used by ticks to inhibit the inflammatory response and provides a second example of CKBPs identified outside the virus world.
- 48.Smith P, Fallon RE, Mangan NE, Walsh CM, Saraiva M, Sayers JR, McKenzie AN, Alcami A, Fallon PG: Schistosoma mansoni secretes a chemokine binding protein with antiinflammatory activity. J Exp Med 2005, 202:1319–1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dias JM, Losberger C, Deruaz M, Power CA, Proudfoot AE, Shaw JP: Structural basis of chemokine sequestration by a tick chemokine binding protein: the crystal structure of the complex between Evasin-1 and CCL3. PLoS One 2009, 4:e8514.• One of the few available reports describing the crystal structure of a CKBP complexed to chemokines. It identifies the molecular interactions that allow a small, secreted protein to interact and neutralize chemokines.
- 50.Vieira AT, Fagundes CT, Alessandri AL, Castor MG, Guabiraba R, Borges VO, Silveira KD, Vieira EL, Goncalves JL, Silva TA et al. : Treatment with a novel chemokine-binding protein or eosinophil lineage-ablation protects mice from experimental colitis. Am J Pathol 2009, 175:2382–2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Castor MG, Rezende B, Resende CB, Alessandri AL, Fagundes CT, Sousa LP, Arantes RM, Souza DG, Silva TA, Proudfoot AE et al. : The CCL3/macrophage inflammatory protein-1alpha-binding protein evasin-1 protects from graft-versus-host disease but does not modify graft-versus-leukemia in mice. J Immunol 2010, 184:2646–2654. [DOI] [PubMed] [Google Scholar]