Abstract
Objective
To determine the association between demographic, psychosocial, and injury-related characteristics and traumatic brain injury (TBI) occurring prior to a moderate or severe TBI requiring rehabilitation.
Design
Secondary data analysis.
Setting
TBI Model System inpatient rehabilitation facilities.
Participants
Persons (N = 4464) 1, 2, 5, 10, 15, or 20 years after TBI resulting in participation in the TBI Model System National Database.
Interventions
Not applicable.
Main Outcome Measures
History of TBI prior to the TBI Model System Index injury, pre-Index injury demographic and behavioral characteristics, Index injury characteristics, post-Index injury behavioral health and global outcome.
Results
Twenty percent of the cohort experienced TBIs preceding the TBI Model System Index injuryd80% of these were mild and 40% occurred before age 16. Pre- and post-Index injury behavioral issues, especially substance abuse, were highly associated with having had a prior TBI. Greater severity of the pre-Index injury as well as occurrence before age 6 often showed stronger associations. Unexpectedly, pre-Index TBI was associated with less severe Index injuries and better functioning on admission and discharge from rehabilitation.
Conclusions
Findings suggest that earlier life TBI may have important implications for rehabilitation after subsequent TBI, especially for anticipating behavioral health issues in the chronic stage of recovery. Results provide additional evidence for the potential consequences of early life TBI, even if mild.
Keywords: Anxiety, Brain injuries, Craniocerebral trauma, Depression, Rehabilitation
Although clinicians have long thought that sustaining >1 traumatic brain injury (TBI) is associated with poorer outcomes, research on repeated TBI has been limited1 and largely focused on sports-related concussions.2 However, even these sparse findings suggest that a history of TBI prior to the injury that brought the individual to the attention of the researcher or clinician (hereafter termed Index TBI) is associated with decreased life satisfaction,3 depressed mood and anxiety,4 and increased risk for subsequent TBIs.1,5
In population-based studies of persons referred for an Index TBI, medical record reviews have shown that 4% sustained TBIs prior to the Index injury,1 and 7% sustained TBIs subsequent to it.1,6 In contrast, population-based studies using 1 or 2 items querying TBI history provide estimates of TBI occurring prior to a person’s Index injury (hereafter referred to as prior TBIs) as high as 25% to 29%.7,8 The large discrepancy in rates is likely the result of limitations inherent in methods, whether medical record review9 or retrospective self-report.10–12 A structured interview conducted by an informed professional can mitigate many of the difficulties of self-report and remains the best method available for measuring lifetime TBI.12
Based on the Centers for Disease Control and Prevention recommendations for surveillance of TBI, the Ohio State University Traumatic Brain Injury Identification Method (OSU TBI-ID)13,14 is a standardized structured interview designed to elicit a lifetime history of TBI, including the presence, severity, and nature of altered consciousness and the age(s) at which injury occurred. The interview uses validated injury recall methods,11,15 avoids the need for knowledge of TBI terminology, and can be administered to the person or a proxy. TBIs reported by the OSU TBI-ID have shown associations between important outcomes and age at first TBI with loss of consciousness (LOC), worst injury, number of TBIs with LOC, and other summary indices of lifetime TBI history.13,14 For example, in a study of prisoners, age at first TBI with LOC was associated with self-reported cognitive symptoms, whereas the number of TBIs with LOC predicted working memory, risk taking, and disinhibition.14 A recent cluster analysis identified patterns of lifetime history (eg, people first injured between ages 6e10y, who were also more likely to be hospitalized; people who sustained a number of mild injuries) that were predictive of current functioning.13
The OSU TBI-ID was recently added to the Traumatic Brain Injury Model Systems (TBIMS) National Database, replacing a single yes or no question about prior TBI requiring hospitalization, which had revealed a rate of 7%.16 The purpose of the current study was to use the data on lifetime TBI, gathered with the OSU TBI-ID method, to describe relations between TBI prior to the Index TBI, and other variables in the TBIMS National Database16 that capture both case mix and outcomes relative to the Index injury (ie, demographics, injury characteristics, rehabilitation characteristics, postrehabilitation functioning). The TBIMS National Database variables chosen for examination were the most commonly used to operationalize these domains. To somewhat limit the number of comparisons, we did not examine scale items and included only those variables that are typically the best representation of a construct (eg, days to follow commands and length of posttraumatic amnesia are superior to Glasgow Coma Scale scores because of the number of subjects who do not have the latter because of intubation or chemical paralysis).
We examined relations associated with the presence of at least 1 prior TBI, age at first TBI, and the worst severity of injury. Given the paucity of previous literature, we had no a priori hypotheses beyond the expectation that events preceding the Index injury (hereafter referred to as pre-Index injury) TBIs would further exacerbate negative influences of experiencing a TBI. Specifically, we expected that (1) patients with a TBI that preceded their TBIMS Index injury would have worse pre- and post-Index injury sequelae; (2) the worse the pre-Index injury, the worse the pre- and post-Index injury sequelae; and (3) early childhood pre-Index injuries would result in worse pre- and post-Index injury sequelae than pre-Index injuries that occurred later in life.
Methods
Participants
The sample was drawn from the TBIMS National Database. As such, all participants were over age 15 years, incurred a moderate or severe TBI, and received comprehensive rehabilitation in a TBIMS site. Complete inclusion criteria can be found on the website for the TBIMS National Data and Statistical Center.16 The sample consisted of 4464 TBIMS participants who received a follow-up interview between April 1, 2010, when the OSU TBI-ID was adopted, and March 31, 2012. Eligible participants or their proxies could have been interviewed at any of the following follow-up time points: 1, 2, 5, 10, 15, or 20 years post-Index injury. If >1 interview had been conducted using the OSU TBI-ID, data from the most recent interview were used. All TBIMS participants provide informed consent directly or by proxy, and the study is overseen at all TBIMS centers by institutional review boards. The overall follow-up rate for all years in the TBIMS National Database is 79%. The TBIMS data collection protocol uses a best source policy16 for interviewing a proxy when the individual is not able to provide valid information. Measures that require reporting of a subjective state (eg, life satisfaction, emotional state) are only collected from the individual with a TBI.
The sample was composed of 73.5% men, with 69.1% non-Hispanic whites, 18.4% non-Hispanic blacks, 8.4% Hispanic, and 4.1% all other racial/ethnic categories. The average age±SD at injury was 39±18.2 years (range, 16−94y). The proportion of participants interviewed at each follow-up was 17% at 1 year post-Index injury, 31% at 2 years post-Index injury, 28% at 5 years post-Index injury, 18% at 10 years post-Index injury, 4% at 15 years post-Index injury, and 3% at 20 years post-Index injury.
Measures
OSU TBI-ID Short Form
The OSU TBI-ID Short Form is a structured interview designed to elicit lifetime history of TBI. Interrater and test-retest reliability are acceptable, and construct validity has also been supported.13,14 The respondent is first asked questions designed to prompt recall of injuries to the head or neck, then elicit information about LOC/ alteration of consciousness after each event and age of occurrence. Summary indices include the number of TBIs with LOC, the number of TBIs with LOC >30 minutes, age at first TBI with LOC, whether a TBI with LOC occurred before the age of 15, and the worst injury severity. In this study, the Index injury was not included in the summary indices.
As shown in tables 1 through 3, a large number of variables in the TBIMS National Database were examined for their association to the OSU TBI-ID summary indices. These variables are described in detail in the TBIMS Syllabus found on the website of the National Data and Statistical Center16 and will be summarized very briefly here.
Table 1.
Demographic and pre-Index injury behavioral issues
| Worst |
Age at First TBI (y) |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Parameter | No Prior TBI | Prior TBI | Mild/AC Only | Mild/LOC | Moderate or Severe | 0–5 | 6–10 | 11–15 | ≥16 |
| Age(N=4464) | |||||||||
| Mean± SD | 39.56±18.628* | 36.64±16.181* | 36.41±16.927* | 36.13±15.552* | 38.21±15.780 | 33.44±16.152‡ | 34.00±13.409‡ | 32.99±13.917‡ | 38.86±17.160 |
| Sex (N=4464) | |||||||||
| Male | 72.2 | 78.6* | 72.3 | 82.7† | 70.3 | 75.5 | 80.9† | 79.8* | 70.3 |
| Female | 27.8 | 21.4* | 27.7 | 17.3† | 29.7 | 24.5 | 19.1† | 20.2* | 29.7 |
| Race/ethnicity (N=4464) | |||||||||
| White, non-Hispanic | 68.6 | 70.9 | 71.8 | 73.6* | 63.3* | 75.0* | 67.3 | 72.0 | 71.0 |
| Black, non-Hispanic | 18.3 | 18.7 | 18.3 | 16.5 | 24.3* | 17.2 | 21.8 | 16.6 | 18.7 |
| Hispanic | 8.8 | 6.7 | 6.7 | 6.8 | 6.5 | 3.1† | 5.4* | 8.9 | 6.9 |
| Asian Pacific-Islander | 2.7 | 2.0 | 1.6 | 2.0 | 3.0 | 4.7 | 2.0 | 1.3 | 1.9 |
| All other | 1.5 | 1.7 | 1.6 | 1.1 | 3.0 | 0.0 | 3.4 | 1.3 | 1.5 |
| Marital status (n=4460) | |||||||||
| Single, never-married | 47.3 | 52.2 | 53.5* | 51.0 | 52.1 | 60.9† | 53.7* | 55.4* | 49.8 |
| Married | 32.7 | 29.7 | 32.3 | 29.1 | 25.4* | 29.7 | 27.9* | 29.9 | 30.2 |
| Single, previously married | 19.9 | 18.0 | 14.2* | 19.9 | 22.5 | 9.4‡ | 18.4 | 14.6* | 20.0 |
| Other | 0.1 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Education (n=4403) | |||||||||
| <High school completion | 24.7 | 23.9 | 23.5 | 23.6 | 25.2 | 23.8 | 22.1 | 26.9 | 23.4 |
| High school diploma or GED | 35.1 | 34.8 | 33.2 | 35.7 | 36.2 | 27.0* | 33.1 | 35.3 | 36.0 |
| Some college | 24.6 | 27.3 | 28.1 | 26.2 | 27.6 | 38.1† | 30.3* | 26.9 | 25.2 |
| ≥Bachelors degree | 15.6 | 14.1 | 15.1 | 14.4 | 11.0* | 11.1* | 14.5 | 10.9* | 15.3 |
| Employment (n=4439) | |||||||||
| Employed | 65.3 | 68.4 | 70.0* | 67.8 | 66.3 | 68.8 | 73.3* | 70.5* | 66.4 |
| Unemployed | 11.1 | 13.5 | 10.8 | 15.4* | 15.7* | 14.1 | 11.6 | 14.1 | 13.8 |
| Student | 6.9 | 7.8 | 9.7* | 7.4 | 4.2* | 9.4 | 11.0* | 9.0 | 6.3 |
| Retired | 13.7 | 7.4† | 7.6† | 6.3† | 7.8* | 1.4§ | 5.8† | 9.6* | |
| Other | 3.0 | 2.8 | 1.9 | 3.1 | 4.2 | 0.0* | 2.7 | 0.6* | 3.8 |
| Primary person living with (n=4455) | |||||||||
| Alone | 17.2 | 17.4 | 15.6 | 17.7 | 20.8 | 9.4† | 17.0 | 14.0 | 19.5 |
| Spouse or significant other | 39.3 | 38.1 | 41.8 | 36.8 | 32.7* | 35.9 | 38.1 | 36.9 | 38.7 |
| Parents, siblings, other family | 35.1 | 34.5 | 31.5 | 36.2 | 37.5 | 45.3† | 40.1* | 30.6 | 32.8 |
| All else | 8.4 | 10.0 | 11.1 | 9.4 | 8.9 | 9.4 | 4.8* | 18.5‡ | 9.0 |
| Primary payer acute care (n=4367) | |||||||||
| Medicaid | 20.6 | 23.0 | 20.9 | 22.6 | 28.7* | 14.3* | 21.9 | 27.6* | 23.0 |
| Medicare | 12.1 | 7.0* | 7.1* | 6.1† | 8.5* | 11.1 | 1.4‡ | x3.9† | 9.0* |
| Private insurance | 26.5 | 23.8 | 25.8 | 23.2 | 20.7* | 20.6* | 30.8 | 26.3 | 21.5* |
| Self-pay or private pay | 7.9 | 4.1* | 3.3* | 4.3* | 5.5 | 3.2* | 5.5 | 1.3† | 4.7* |
| All else | 32.9 | 42.0* | 42.9† | 43.8† | 36.6 | 50.8‡ | 40.4* | 40.8* | 41.8* |
| Primary payer rehabilitation(n=4421) | |||||||||
| Medicaid | 21.5 | 24.7 | 23.0 | 22.9 | 31.9† | 20.3 | 23.8 | 28.6* | 24.3 |
| Medicare | 12.3 | 7.7* | 7.6* | 7.2* | 9.0* | 10.9 | 2.0‡ | 4.5† | 9.8 |
| Private insurance | 26.1 | 24.1 | 26.0 | 24.1 | 19.9* | 20.3* | 31.3* | 26.6 | 21.8* |
| Self or private pay | 5.5 | 2.8* | 2.2* | 3.4 | 3.0* | 1.6* | 4.1 | 1.3* | 3.1 |
| All else | 34.5 | 40.7* | 41.2* | 42.4* | 36.1 | 49.9† | 46.9† | 38.8 | 39.0 |
| Felony incarceration(n=4309) | 8.5 | 12.7* | 8.7 | 13.1* | 20.5‡ | 6.7 | 15.0† | 13.2* | 12.6* |
| Psychiatric hospitalization(n=2116) | 4.9 | 7.9* | 8.0* | 9.4* | 4.1 | 7.1 | 8.7* | 9.0* | 7.5* |
| Suicide attempt(n=2112 | 3.9 | 6.0 | 2.9 | 8.2* | 8.2* | 0.0* | 7.2* | 9.0* | 5.5 |
| Received mental healthcare(n=2116) | 18.1 | 24.6* | 24.6* | 23.4* | 27.4† | 32.1‡ | 26.1* | 17.9* | 25.1* |
| Use of illicit drugs(n=4316 | 20.3 | 27.9* | 29.3† | 24.2 | 32.7† | 35.6‡ | 32.6† | 29.8† | 25.1* |
| Alcohol misuse(n=3887) | 31.3 | 42.4† | 41.6† | 41.8† | 45.8† | 53.6§ | 41.0† | 41.5† | 41.7† |
| Problem use(n=4075) | 41.5 | 54.9† | 54.0† | 54.0† | 58.4‡ | 59.6‡ | 57.7‡ | 53.5† | 53.9† |
NOTE. All values are in percentages or as otherwise indicated.
Abbreviations: AC, altered consciousness; GED, General Educational Development.
Modest effect size: 0.1≤h/d≤0.2.
Important effect size: 0.2<h/d≤0.3.
Very important effect size: 0.3<h/d<0.4.
Substantial effect size: 0.4<h/d.
Demographic and preinjury characteristics
Demographic and preinjury characteristics included age, sex, race, education, and several variables capturing the social situation at the time of Index injury. At the time of enrollment into the TBIMS National Database, participants or proxies were asked about pre- injury emotional and behavioral issues, shown in table 1. Illicit drug use was coded positive for any use of illicit drugs during the year prior to Index TBI. Alcohol misuse was coded positive for heavy consumption during the month prior to Index TBI (≥14 drinks per week for men, ≥7 drinks per week for women, or at least 1 occurrence of binge drinking [≥5 drinks during a single day]). Problem use was coded as positive for either illicit drug use or alcohol misuse.
Injury, acute care, and rehabilitation characteristics
Injury, acute care, and rehabilitation characteristics are listed in table 2 and include the etiology and severity of the Index TBI and the measures of functional status on admission and discharge from inpatient rehabilitation (FIM17 and Disability Rating Scale18).
Table 2.
Index injury characteristics
| Worst |
Age at First TBI (y) |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Parameter | No Prior TBI | Prior TBI | Mild/AC Only | Mild/LOC | Moderate or Severe | 0–5 | 6–10 | 11–15 | ≥16 |
| Index TBI etiology(%) (n=4457) | |||||||||
| Vehicular | 53.7 | 54.5 | 52.2 | 59.5* | 49.1 | 58.7* | 50.3 | 59.2* | 53.7 |
| Falls | 24.2 | 19.5* | 21.2 | 17.4* | 20.1 | 19.0* | 23.1 | 14.6† | 20.0* |
| Violence | 11.2 | 13.1 | 12.6 | 11.7 | 17.2* | 11.1 | 12.2 | 13.4 | 13.5 |
| Other | 10.9 | 12.9 | 14.0 | 11.4 | 13.6 | 11.1 | 14.3* | 12.7 | 12.8 |
| BAC(n=3227) Mean±SD | 64.91±99.866 | 79.27±111.852* | 69.96±105.628 | 80.66±111.824* | 95.37±122.559‡ | 76.30±112.809* | 74.79±112.781 | 66.33±102.368 | 84.40±114.031* |
| BAC grouping (n=4337) Negative | 41.9 | 39.6 | 42.7 | 39.3 | 33.5* | 36.1* | 42.4 | 40.0 | 39.1 |
| 1–100mg/dL | 1.3 | 1.5 | 0.8 | 1.2 | 3.7 | 0.0 | 0.7 | 3.3 | 1.4 |
| >100mg/dc | 30.9 | 34.3 | 30.5 | 34.1 | 43.3† | 36.1* | 32.6 | 29.3 | 36.0* |
| Positive but unknown | 0.3 | 0.7 | 0.3 | 1.2 | 0.6 | 0.0 | 0.7 | 2.0 | 0.4 |
| Not tested | 25.5 | 23.9 | 25.8 | 24.3 | 18.9* | 27.9 | 23.6 | 25.3 | 23.1 |
| Acute care LOS (n=4462) Mean±SD | 21.13±16.590 | 19.01±13.669* | 18.98±14.434* | 18.69±12.924* | 19.72±13.501 | 18.63±12.419* | 17.06±10.017† | 19.11±14.039* | 19.56±14.543 |
| Rehabilitation LOS (n=4459) Mean±SD | 28.24±27.241 | 25.89±23.536 | 25.81±26.490 | 25.69±21.309 | 26.46±21.020 | 27.59±18.273 | 24.14±19.355* | 28.10±35.551 | 25.50±20.473* |
| Days in PTA (n=4293) Mean±SD | 34.02±35.079 | 25.83±24.648† | 25.14±25.846† | 25.391±23.915† | 28.28±23.401* | 24.78±20.684† | 23.79±25.386† | 24.55±23.213† | 26.92±25.311† |
| Days to follow commands (n=4324) Mean±SD | 9.10±14.789 | 5.87±9.699† | 5.99±10.329† | 5.36±9.071† | 6.68±9.538* | 4.75±7.947† | 5.49±9.271† | 6.02±10.349† | 6.07±9.828† |
| FIM Total admit (n=4321) Mean±SD | 49.00±23.493 | 56.06±23.840† | 57.23±24.231‡ | 57.15±23.419‡ | 51.06±23.341 | 52.27±23.850* | 60.43±23.566§ | 57.17±24.875‡ | 54.94±23.478† |
| FIM Motor admit (n=4329) Mean±SD | 34.27±17.934 | 39.64±18.853† | 40.60±19.011‡ | 40.39±18.691‡ | 35.84±18.484 | 36.68±19.808* | 43.25±18.363§ | 40.46±19.309‡ | 38.71±18.629† |
| FIM Cognitive admit (n=4416) Mean±SD | 14.75±7.761 | 16.43±7.170† | 16.58±7.338† | 16.84±7.139† | 15.25±6.767 | 15.83±6.795* | 17.31±7.049‡ | 16.72±7.570† | 16.17±7.122* |
| FIM total discharge (n=4295) Mean±SD | 90.40±23.267 | 98.30±17.629‡ | 98.71±18.678‡ | 98.66±17.067‡ | 96.57±16.308† | 100.11±17.972§ | 101.37±14.511§ | 99.13±17.385‡ | 96.95±18.353† |
| FIM motor discharge (n=4309) Mean±SD | 66.82 ±18.615 | 72.94±14.589‡ | 73.29±15.071‡ | 73.17±14.302‡ | 71.67±14.105† | 74.43±15.244§ | 75.70±11.884§ | 73.49±14.083‡ | 71.80±15.255† |
| FIM cognitive discharge (n=4406) Mean±SD | 23.60±6.914 | 25.42±5.551† | 25.52±5.791† | 25.54±5.507† | 24.92±5.076* | 25.68±5.538‡ | 25.88±5.189‡ | 25.60±5.824† | 25.20±5.571† |
| DRS admit (n=4368) Mean±SD | 12.55±5.678 | 10.98±4.801 | 10.65±4.904‡ | 10.78±4.690‡ | 12.13±4.654 | 11.49±4.624* | 10.41±4.641‡ | 10.78±4.738‡ | 11.14±4.880† |
| DRS discharge (n=4367) Mean±SD | 6.38±3.876 | 5.39±2.696 † | 5.316±2.882 † | 5.32±2.500† | 5.70±2.66* | 5.35±2.882† | 5.12±2.156‡ | 5.13±2.815‡ | 5.55±2.767† |
| Discharged to private residence (%) (n=4459) | 84.5 | 85.4 | 89.0* | 83.0 | 82.7 | 95.3‡ | 87.1 | 85.4 | 83.8 |
| Primary person living with at discharge (%) (n=4448) | |||||||||
| Alone | 3.3 | 2.9 | 1.6 | 4.0 | 3.6 | 0.0* | 2.0 | 1.9 | 3.8 |
| Spouse or significant other | 31.2 | 29.1 | 31.5 | 27.5 | 26.9 | 29.7 | 25.9* | 30.8 | 29.4 |
| Parents, siblings, other family | 47.8 | 51.5 | 54.6* | 49.0 | 49.7 | 64.1 ‡ | 55.8* | 51.9 | 48.6 |
| All else | 17.6 | 16.6 | 12.4* | 19.5 | 19.8 | 6.3 ‡ | 16.3 | 15.4 | 18.2 |
Abbreviations:AC,alteredconsciousness;DRS,DisabilityRatingScale; FIM, Functional Independence Measure; LOS, length of stay; PTA, posttraumatic amnesia.
Modest effect size: 0.1≤h/d≤0.2.
Important effect size: 0.2<h/d≤0.3.
Very important effect size: 0.3<h/d≤0.4.
Substantial effect size: 0.4<h/d.
Follow-up outcomes
The follow-up outcomes, shown in table 3, are variables collected via telephone interviews (form II in the TBIMS National Database Syllabus). Depression was measured using the Patient Health Questionnaire-9,19 a self-report instrument that assesses the frequency of each of the 9 symptoms of depression listed in the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition.20 Anxiety was measured using a parallel instrument assessing each of the 7 Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition symptoms of the Generalized Anxiety Disorder,21 the Generalized Anxiety Disorder 7- Item Scale. Alcohol and drug use at follow-up focus on the previous month as the reference point for alcohol use, and the previous 12 months for illicit drug use. Problem use was again defined as being positive for either illicit drug use or alcohol misuse.
Table 3.
Post-Index injury follow-up on functional and behavioral status
| Worst |
Age at First TBI (y) |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Parameter | No Prior TBI | Prior TBI | Mild/AC Only | Mild/LOC | Moderate or Severe | 0–5 | 6–10 | 11–15 | ≥16 |
| PHQ-9 (n=3586) Mean±SD | 5.02±5.634 | 6.78±6.297‡ | 6.30±5.964y | 6.86±6.230‡ | 7.69±7.072§ | 5.61±5.986* | 7.71 6.156§ | 6.72±6.603† | 6.67±6.258† |
| GAD-7 (n=3587) Mean±SD | 3.89±5.102 | 5.08±5.522† | 4.89±5.389* | 4.98±5.463† | 5.73 ±5.929‡ | 4.48±4.809* | 6.09 5.774§ | 5.16±5.410† | 4.82±5.542* |
| Use of illicit drugs (n=4419) | 8.1 | 17.8† | 17.3† | 18.6‡ | 17.5† | 18.8‡ | 23.3§ | 21.8‡ | 15.0† |
| Alcohol misuse (n=4377) | 13.5 | 22.8† | 23.3† | 24.1† | 19.1* | 28.1‡ | 22.8† | 26.9‡ | 21.0† |
| Problem use (n=4385) | 18.7 | 32.8‡ | 31.9‡ | 35.7‡ | 28.4† | 37.5§ | 34.9‡ | 35.9‡ | 30.6† |
| FIM Total score Mean±SD | 114.76±18.959 | 116.56±15.310* | 116.79±16.063* | 116.37±15.801 | 116.45±12.338 | 118.67±12.056† | 119.74±6.800† | 117.74±12.048* | 115.03±17.921 |
| FIM Motor score (n=4337) Mean±SD | 83.95±14.746 | 85.28±12.249 | 85.30±12.903 | 85.16±12.782 | 85.51±9.328* | 86.97±9.903† | 87.72± 5.406† | 86.28±9.810* | 84.07±14.287 |
| FIM Cognitive score (n=4344) Mean SD | 30.77±5.447 | 31.28±4.267* | 31.50±4.073* | 31.21±4.350 | 30.93±4.499 | 31.70±3.736* | 32.02±2.531† | 31.46±3.800* | 30.96±4.799 |
| GOS-E (n=4394) Mean±SD | 5.82±1.802 | 6.03±1.533* | 6.08±1.543* | 6.01±1.522* | 5.98±1.542 | 6.16±1.405* | 6.20±1.296† | 6.19±1.413† | 5.92±1.636 |
| GOS-E categories (%) | |||||||||
| Good recovery | 38.9 | 37.0 | 39.3 | 35.7 | 34.5 | 38.1 | 36.7 | 38.5 | 36.5 |
| Moderate disability | 33.3 | 45.4† | 44.0† | 46.6† | 45.8† | 47.6† | 52.4‡ | 47.4† | 42.5* |
| Severe disability or vegetative (%) | 27.7 | 17.6† | 16.7† | 17.7† | 19.6* | 14.3‡ | 10.9§ | 14.1‡ | 21.0* |
Abbreviations: AC, altered consciousness; FIM, Functional Independence Measure; GAD-7, Generalized Anxiety Disorder 7-Item Scale; GOS-E, Glasgow Outcome Score-Extended; PHQ-9, Patient Health Questionnaire-9.
Modest effect size: 0.1≤h/d≤0.2.
Important effect size: 0.2<h/d≤0.3.
Very important effect size: 0.3<h/d≤0.4.
Substantial effect size: 0.4<h/d.
Analyses
Because of the large sample size, we anticipated that a number of comparisons would be statistically significant, but not necessarily clinically meaningful. Therefore, we determined a priori that standardized effect sizes would be used to determine the relative importance of findings, rather than performing statistical tests. Effect sizes <.10 were considered immaterial, those between .10 and .20 were considered modest, those >.20 were considered important, those >.30 were considered very important, and those >.40 were substantial. These thresholds were chosen because they corresponded to nonstandardized differences that the authors felt were clinically meaningful. For example, when the lowest proportion in a comparison was 50%, a modest difference between proportions was 5%, an important difference was 10%, a very important difference was 15%, and a substantial difference was 20%.
Cohen’s d, calculated using a weighted pooled SD of both groups,22,23 was used to calculate the effect size for mean differences, whereas Cohen’s h (based on the arcsine transformation of proportions22,24) was used for differences between proportions. Although multiple methods for determining standardized effect sizes exist, Cohen’s d is the standard method for examining mean differences. Some variants of Cohen’s d have been developed for different situations (eg, Hedges g for use with small sample sizes); however, these variants were not warranted for the current study. Cohen’s h was used to compare proportions because it normalizes the sampling distributions of proportions and stabilizes the variance (due to the observation that when proportions approach 0, differences become easier to detect when compared with proportions in the midrange).23,25 In addition, Cohen’s h provides an effect size estimate that is comparable with Cohen’s d, allowing for the use of the same interpretive thresholds across all variables. The referent group for all comparisons consisted of persons without a pre-Index TBI.
Results
Twenty percent of the sample had experienced a TBI prior to Index injury. Of those, 64 reported 1 pre-Index TBI, 20% reported 2, and 16% reported ≥3. The worst pre-Index TBIs were most often mild (42% had altered consciousness only, and 39% with LOC), though 19% experienced at least 1 moderate or severe pre- Index TBI. For almost half of those with a pre-Index TBI, their first occurred in childhood: 6% of those with a prior TBI had a TBI before age 6 years; 15% between ages 6 and 10 years, inclusive; 16% between ages 11 and 15 years, inclusive; and 63% after the age of 15 years.
Demographic characteristics
As shown in table 1, individuals with a pre-Index TBI were younger (mean±SD, 36.64±16.18y) than those without a pre- Index TBI (mean±SD, 39.56±18.63y). Overall, this was a modest effect size; however, very important associations were evident for all groups who had a pre-Index TBI before age 16 years. Individuals with a pre-Index TBI tended to include more men (78.6% vs 72.2%), with the most marked differences related to severity of the worst pre-Index injury. Individuals with more severe worst injuries were more likely to be men (82.7% and 84% for mild with LOC and moderate/severe, respectively). There were few meaningful differences for race/ethnicity, though higher proportions of non-Hispanic whites and blacks had more severe pre-Index injuries (modest effect size). There were several modest associations with an important one between never having been married and having a first TBI between ages 0 and 5 (60.9% vs 47.3%). Those with the earliest pre-Index injuries were also far less likely to be single, yet previously married (9.4% vs 19.9%).
Pre-Index injury emotional and behavioral issues
Except for suicide attempts, greater rates of pre-Index injury problems were reported by individuals with a pre-Index history of TBI. These associations were modest, except for pre-Index injury history of alcohol misuse, which was important. Rates of preinjury mental health problems were particularly marked for individuals who sustained their first pre-Index TBI in early childhood. Compared with individuals with no pre-Index TBI, those with their first pre-Index TBIs between ages 0 and 5 reported very important differences in rates of treatment for mental health problems (18% and 32%, respectively), use of illicit drugs (20% and 36%, respectively), and problem alcohol use (42% and 60%, respectively); a substantial association was noted with alcohol misuse (31% and 54%, respectively). The associations between pre-Index injury alcohol misuse and pre-Index TBI were some of the most consistently large effect sizes among all variables reflecting a person’s status prior to their Index injury.
Index injury characteristics
As shown in table 2, there were some modest associations among Index injury etiology, intoxication at time of Index injury, and length of stay for the Index injury acute and rehabilitation stays. One exception was the relation between severity of pre-Index TBI and intoxication at Index injury. Although mean blood alcohol content (BAC) was modestly higher for those with pre-Index TBI (79.27 vs 64.31), this figure increased with greater severity of worst injury (69.96 mild/altered consciousness only vs 80.66 mild/LOC vs 95.37 moderate/severe). It is not clear that each increment is clinically meaningful; however, mean BAC for the moderate/severe group showed a very important effect size. Mean BAC assessed as BAC categories were not significantly different for those with or without pre-Index TBI, though those with BAC >100mg/dL were more likely to have had a pre-Index moderate/severe TBI (important effect size).
Multiple indices, reflecting the severity of the Index injury, showed important to substantial associations with characteristics of pre-Index TBI. With a few exceptions, pre-Index TBI was associated with less severe Index injury indices. One notable exception was evident for those with moderate/severe pre-Index injuries. They were more similar to the no pre-Index injury group in terms of injury severity and admission functional status. Also, those with their first injury at 0 to 5 years only showed modestly better functional abilities at rehabilitation admission. Despite those with pre-Index injuries appearing to have less severe Index TBIs and better functional status on admission and discharge, their rehabilitation lengths of stay were not different and only a modestly shorter acute care length of stay was observed.
The status at discharge from rehabilitation also showed few differences between groups, with no difference in discharge to private residence or primary person living with. Those with a first pre-Index injury aged between 0 and 5 were much more likely to be discharged to a private residence (95.3% vs 84.5%, a very important effect size), and were more likely to be discharged to live with parents, siblings, or other family members who were not a spouse (64.1% vs 47.8%, a very important effect size).
Follow-up outcomes
As shown in table 3, the effects of pre-Index TBI, overall, were important for anxiety and very important for depression. The more severe the worst pre-Index TBI, the greater the effect on depression scores: important for mild pre-Index TBI, very important for mild injury with LOC, and substantial for moderate/severe pre- Index injury. A similar trend with increasing severity was seen for anxiety, although the effects were slightly less pronounced: modest for mild pre-Index TBI, important for mild TBI with LOC, and very important for moderate/severe pre-Index TBI. For both anxiety and depression, the largest effects were observed for age at first injury between 6 and 10 years (substantial). Age 0 to 5 showed modest effects on both emotional measures, and ages 11 to 15 and ≥16 showed modest to important effect sizes. Figure 1 shows the dose relation between post-Index injury anxiety and depression and severity of pre-Index injury.
Fig 1.
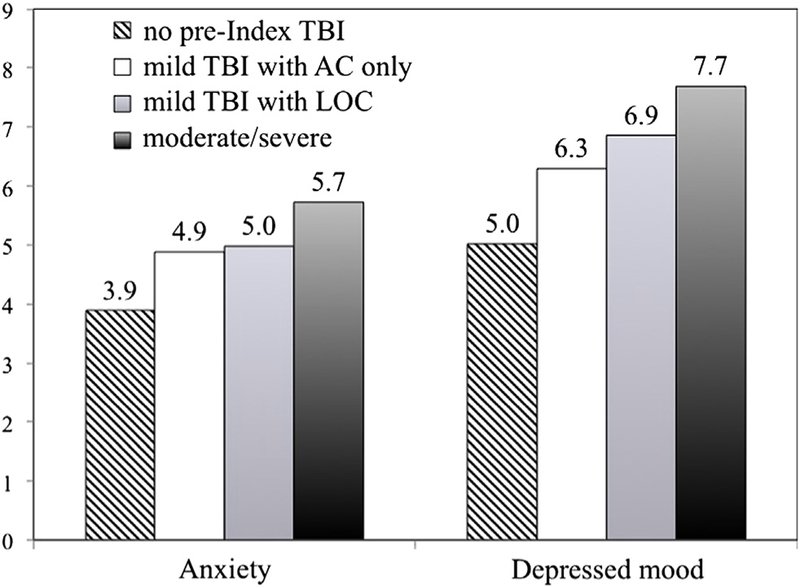
Severity of pre-Index TBI and mood disorders on follow-up. Abbreviation: AC, altered consciousness.
Problematic substance use at follow-up showed very important effects of pre-Index TBI, overall; nearly twice as many previously injured participants reported problematic use of drugs and/or alcohol compared with their counterparts without pre-Index TBI (33% vs 19%). Severity of worst pre-Index injury did not appear to be closely associated with problem substance use, although the proportion of positive cases was slightly lower in the most severe group. More participants injured before age 16 years tended to report problematic use (very important to substantial effect sizes), although effects for those injured after age 15 years were still important. Figure 2 graphically displays the 3-way interaction of pre-Index and post-Index problem use and age at which first pre- Index injury occurred. Although not tested statistically, there is an apparent greater tendency for those youngest at first injury to have pre- and/or post-Index problems with substance misuse.
Fig 2.
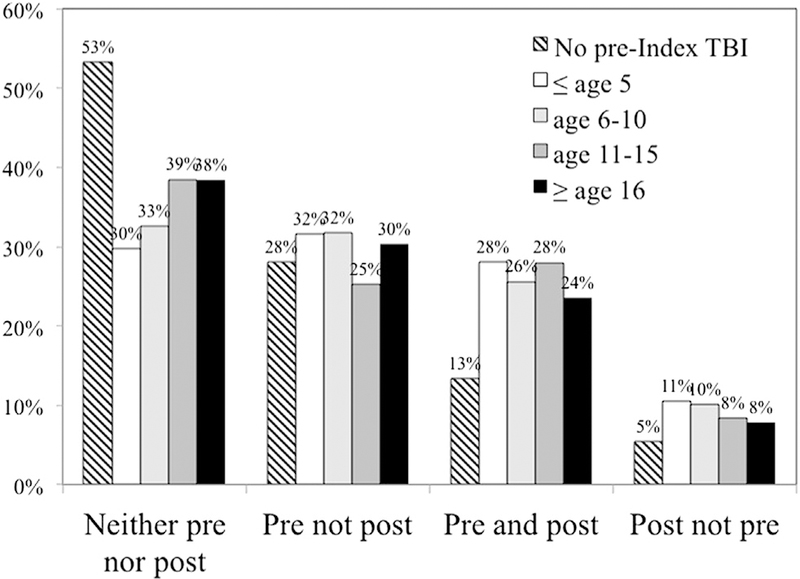
Age at first TBI and pre- and post-Index injury problem substance use.
There were modest effects of pre-Index TBI on FIM and Glasgow Outcome Scale-Extended26 scores. With respect to age at first pre-Index TBI, there was an important increase in FIM total score for individuals with first pre-Index TBI at <10 years of age. FIM motor scores were not different between those with a pre- Index TBI (mean±SD, 85.28±12.25) and those without a pre- Index TBI (mean±SD, 83.95±14.75); however, there were important increases in FIM motor scores for those who experienced their first injury at ≤10 years. FIM cognitive scores showed modest to important increases for those injured ≤15 years. For the more severe Glasgow Outcome Scale-Extended26 categories, moderate disability and severe disability/vegetative, there were important differences, which were also evident in all categories of worst pre-Index TBI and for those first injured before age 16 years.
Discussion
The current study suggests a relatively large proportion (20%) of patients who enter rehabilitation with a primary diagnosis of TBI may have had another TBI earlier in life. Most (81%) earlier injuries were mild; 40% of all pre-Index TBIs occurred prior to age 16 years. Without population data, it is not possible to determine whether persons who qualify for the TBIMS National Database are more likely to have had prior TBIs than same-aged peers. However, greater likelihood of repeat injury, or repeat injury that is more severe, would be consistent with previous studies2,5 and conceptions about the cumulative effects of TBI.27,28
In most, but not all, respects, our findings were consistent with the expectation that prior TBI will exacerbate pre- and post-Index sequelae, especially those that are more severe or were experienced at a younger age. A consistent and large association was observed between pre-Index injuries and emotional and behavioral issues, particularly substance use problems. Alcohol and other drug abuse were strongly associated with pre-Index injuries, regardless of their severity or age at first occurrence. Having a pre- Index injury of moderate or severe TBI was associated with the likelihood of pre-Index felony incarceration and psychiatric hospitalization. A pre-Index injury before age 6 years increased the likelihood of psychiatric hospitalization and substance abuse. Given the young age, it is nearly certain that the TBIs preceded the behavioral problems. This study joins a growing number of investigations that suggest (1) TBI in childhood increases the likelihood of adolescent and adult behavioral health problems; and (2) the younger the age at injury, the greater the effect appears to be.29,30 These results provide further evidence of the potential importance of expanding longitudinal research on children and youth who incur TBI.
Post-Index injury depression, anxiety, and substance abuse also showed large and consistent associations with pre-Index injury TBIs. Beyond the magnitude of the relation, evidence for a causative relation is supported by both the temporal order of events and the dose-effect relations evident in figure 1 between pre-Index injury severity and both anxiety and depression post-Index injury. For substance abuse, the chances of experiencing both pre- and post-Index injury problem use were almost doubled by the presence of childhood injuries. These findings may be useful for identifying candidates for preventive interventions as part of a chronic disease management approach.31
The most unexpected findings were the consistent pattern that those with pre-Index injuries had less severe Index TBIs, and that they both entered and left rehabilitation at higher levels of function than those without a pre-Index injury. These relations were not consistent with our a priori expectation that pre-Index injuries will result in worse sequelae of the Index injury. It is possible that pre-Index emotional and behavioral problems led to these patients’ being viewed as complicated and contributed to the perceived need for rehabilitation care despite the effects of the TBI itself being less severe. A similar phenomenon has been observed with older adults admitted to rehabilitation with a primary diagnosis of TBI—functional deficits associated with greater age help drive the perceived need for acute rehabilitation. At the same time, brain injury rehabilitation patients with pre- Index injuries did not have shorter rehabilitation lengths of stay, despite better entering functioning at admission.
Study limitations
Although the large sample size increases the likely robustness of the current findings, generalization of proportions to a larger population of persons in rehabilitation for a primary diagnosis of TBI should not be attempted. Corrigan,31 Cuthbert,32 and colleagues have published comparisons of the TBIMS National Database cohort with the U.S. population receiving rehabilitation for a primary diagnosis of TBI and have recommended weighting cases to assure representativeness.
Pre-Index TBI is based on self-report of TBIs. Despite reliability and validity data supporting the OSU TBI-IDas a self-report measure, more objective indicators of actual history of TBI, a biomarker of history of TBI, or the mechanism of the active ingredient (eg, extent of executive function impairment) would increase confidence in and insights about the nature and extent of the association.
Conclusions
Newly available data from the TBIMS National Database indicate that TBIs occurring earlier in life, prior to the Index injury resulting in TBIMS enrollment, are frequent (20%) and significantly associated with pre-Index injury characteristics, Index injury rehabilitation status, and post-Index injury outcomes. The associations between prior TBI and behavioral health problems (ie, anxiety, depression, substance misuse) are important relations that should be studied further for the ability to denote sentinel, developmental events. Additionally, lifetime history of TBI appears to be an important individual difference when predicting both the acute and postacute rehabilitation course. Finally, relations observed between early life TBIs and adult consequences underscore the necessity for eliminating discontinuities between pediatric and adult TBI research.
Acknowledgments
Supported by the National Institute on Disability and Rehabilitation Research, Office of Special Education and Rehabilitative Services, U.S. Department of Education, Traumatic Brain Injury Model System Centers (grant nos. H133A070029, H133A070022, H133A070033, H113A070040, H133A070038, H113A070074, H133A0120085).
List of abbreviations:
- BAC
blood alcohol content
- LOC
loss of consciousness
- OSU TBI-ID
Ohio State University Traumatic Brain Injury Identification Method
- TBI
traumatic brain injury
- TBIMS
Traumatic Brain Injury Model Systems
Glossary
Terms used to reference injuries:
- Index injury
the TBI requiring rehabilitation and qualifying a person for the TBI NDB
- Pre-index injury or prior TBI
TBIs occurring prior to the Index injury
- Pre-injury
events preceding the Index injury
- Post-index injury
TBIs occurring after the Index injury
Terms used to describe effect sizes
- Immaterial
effect sizes with values of Cohen’s h or d less than .10
- Modest
effect sizes =.10 but =.20
- Important
effect sizes >.20 but =.30
- Very Important
effect sizes >.30 but =.40
- Substantial
effect sizes >.40
Footnotes
No commercial party having a direct financial interest in the results of the research supporting this article has or will confer a benefit on the authors or on any organization with which the authors are associated.
References
- 1.Saunders LL, Selassie AW, Hill EG, et al. A population-based study of repetitive traumatic brain injury among persons with traumatic brain injury. Brain Inj 2009;23:866–72. [DOI] [PubMed] [Google Scholar]
- 2.Guskiewicz KM, McCrea M, Marshall SW, et al. Cumulative effects associated with recurrent concussion in collegiate football players: the NCAA concussion study. JAMA 2003;290:2549–55. [DOI] [PubMed] [Google Scholar]
- 3.Davis LC, Sherer M, Sander AM, et al. Preinjury predictors of life satisfaction at 1 year after traumatic brain injury. Arch Phys Med Rehabil 2012;93:1324–30. [DOI] [PubMed] [Google Scholar]
- 4.Horner MD, Selassie AW, Lineberry L, Ferguson PL, Labbate LA. Predictors of psychological symptoms 1 year after traumatic brain injury: a population-based, epidemiological study. J Head Trauma Rehabil 2008;23:74–83. [DOI] [PubMed] [Google Scholar]
- 5.Dams-O’Connor K, Gibbons LE, Bowen JD, McCurry SM, Larson EB, Crane PK. Risk for late-life re-injury, dementia and death among individuals with traumatic brain injury: a population-based study. J Neurol Neurosurg Psychiatry 2013;84:177–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Annegers JF, Hauser WA, Coan SP, Rocca WA. A population-based study of seizures after traumatic brain injury. New Engl J Med 1998;338:20–4. [DOI] [PubMed] [Google Scholar]
- 7.Pickelsimer EE, Selassie AW, Gu JK, Langlois JA. A population- based outcomes study of persons hospitalized with traumatic brain injury: operations of the South Carolina Traumatic Brain Injury Follow-Up Registry. J Head Trauma Rehabil 2006;21: 491–504. [DOI] [PubMed] [Google Scholar]
- 8.Whiteneck G, Melick D, Brooks C, Harrison-Felix C, Nobel K, Sendroy TM, editors. Colorado traumatic brain injury and follow-up system Englewood: Craig Hospital; 2001. [Google Scholar]
- 9.Powell JM, Ferraro JV, Dikmen SS, Temkin NR, Bell KR. Accuracy of mild traumatic brain injury diagnosis. Arch Phys Med Rehabil 2008; 89:1550–5. [DOI] [PubMed] [Google Scholar]
- 10.Diamond PM, Harzke AJ, Magaletta PR, Cummins AG, Frankowski R. Screening for traumatic brain injury in an offender sample: a first look at the reliability and validity of the traumatic brain injury questionnaire. J Head Trauma Rehabil 2007;22:330–8. [DOI] [PubMed] [Google Scholar]
- 11.Warner M, Schenker N, Heinen MA, Fingerhut LA. The effects of recall on reporting injury and poisoning episodes in the national health interview survey. Inj Prev 2005;11:282–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Corrigan JD, Bogner J. Screening and identification of TBI. J Head Trauma Rehabil 2007;22:315–7. [DOI] [PubMed] [Google Scholar]
- 13.Corrigan JD, Bogner J. Initial reliability and validity of the Ohio State University TBI Identification Method. J Head Trauma Rehabil 2007; 22:318–29. [DOI] [PubMed] [Google Scholar]
- 14.Bogner J, Corrigan JD. Reliability and predictive validity of the Ohio State University TBI Identification Method with prisoners. J Head Trauma Rehabil 2009;24:279–91. [DOI] [PubMed] [Google Scholar]
- 15.Warner M, Barnes PM, Fingerhut LA, Centers for Disease Control and Prevention/National Center for Health Statistics. Injury and poisoning episodes and conditions: National Health Interview Survey, 1997. Vital Health Stat 10 2000;(202):1–38. [PubMed] [Google Scholar]
- 16.Traumatic Brain Injury Model Systems National Data and Statistical Center. Syllabus 2013. Available at: www.tbindsc.org/Syllabus.aspx. Accessed February 4, 2013.
- 17.Hamilton BB, Granger CV, Sherwin FS, Zielezny M. A Uniform National Data System for medical rehabilitation. In: Fuhrere MJ, editor. Rehabilitation outcomes: analysis and measurement Baltimore: Paul H. Brookes Publishing; 1987. p 137–47. [Google Scholar]
- 18.Rappaport M, Hall KM, Hopkins K, Belleza T, Cope N. Disability rating scale for severe head trauma patients: coma to community. Arch Phys Med Rehabil 1982;63:118–23. [PubMed] [Google Scholar]
- 19.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med 2001;16:606–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.American Psychiatric Association. Diagnostic and statistical manual of mental disorders, fourth edition text revision (DSM-IV TR) Washington (DC): American Psychiatric Association; 2000. [Google Scholar]
- 21.Spitzer RL, Kroenke K, Williams JB, Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med 2006;166:1092–7. [DOI] [PubMed] [Google Scholar]
- 22.Turner HM, Bernard RM. Calculating and synthesizing effect sizes. Contemp Issues Commun Sci Disord 2006;33:42–55. [Google Scholar]
- 23.Rossi JS. Tables of effect size for z score tests of differences between proportions and between correlation coefficients. Educ Psychol Meas 1985;45:737–43. [Google Scholar]
- 24.Green RE, Colella B, Christensen B, et al. Examining moderators of cognitive recovery trajectories after moderate to severe traumatic brain injury. Arch Phys Med Rehabil 2008;89(12 Suppl):S16–24. [DOI] [PubMed] [Google Scholar]
- 25.Cohen J Statistical power analysis for the behavioral sciences 2nd ed. Hillsdale: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- 26.Wilson JT, Pettigrew LE, Teasdale GM. Structured interviews for the Glasgow Outcome Scale and the Extended Glasgow Outcome Scale: guidelines for their use. J Neurotrauma 1998;15:573–85. [DOI] [PubMed] [Google Scholar]
- 27.Satz P, Cole MA, Hardy DJ, Rassovsky Y. Brain and cognitive reserve: mediator(s) and construct validity, a critique. J Clin Exp Neuropsychol 2011;33:121–30. [DOI] [PubMed] [Google Scholar]
- 28.McKinlay A, Grace RC, Horwood LJ, Fergusson DM, MacFarlane MR. Long-term behavioural outcomes of pre-school mild traumatic brain injury. Child Care Health Dev 2010;36:22–30. [DOI] [PubMed] [Google Scholar]
- 29.Karver CL, Wade SL, Cassedy A, et al. Age at injury and long-term behavior problems after traumatic brain injury in young children. Rehabil Psychol 2012;57:256–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Corrigan JD, Hammond FM. Traumatic brain injury as a chronic health condition. Arch Phys Med Rehabil 2013;94:1199–201. [DOI] [PubMed] [Google Scholar]
- 31.Corrigan JD, Cuthbert JP, Whiteneck GG, et al. Representativeness of the Traumatic Brain Injury Model Systems National Database. J Head Trauma Rehabil 2012;27:391–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cuthbert J, Corrigan JD, Whiteneck GG, et al. Extension of the representativeness of the Traumatic Brain Injury Model Systems National Database: 2001 to 2010. J Head Trauma Rehabil 2012;27: E15–27. [DOI] [PMC free article] [PubMed] [Google Scholar]


