Abstract
Ginkgo biloba seeds and leaves have been used as a traditional herbal remedy for thousands of years, and its leaf extract has been consumed as a botanical dietary supplement for decades. Ginkgo biloba extract is a complex mixture with numerous components, including flavonol glycosides and terpene lactones, and is one of the most widely sold botanical dietary supplements worldwide. Concerns about potential health risks for the general population have been raised because of the widespread human exposure to Ginkgo biloba and its potential toxic and carcinogenic activities in rodents. The National Toxicology Program conducted 2-year gavage studies on one Ginkgo biloba leaf extract and concluded that there was clear evidence of carcinogenic activity of this extract in mice based on an increased incidence of hepatocellular carcinoma and hepatoblastoma. Recently, Ginkgo biloba leaf extract has been classified as a possible human carcinogen (Group 2B) by the International Agency for Research on Cancer. This review presents updated information on the toxicological effects from experimental studies both in vitro and in vivo to human case reports (caused by ginkgo seeds or leaves), and also summarizes the negative results from relatively large clinical trials.
Keywords: Case report, clinical trial, Ginkgo biloba leaf extract, genotoxicity, ginkgo seeds, toxicity
Introduction
Dietary supplements have been placed in a special category under the general umbrella of foods in the United States since the US Congress passed the Dietary Supplement Health and Education Act (DSHEA) in 1994.[1,2] Under the DSHEA, dietary supplements can be marketed without proof of effectiveness or safety and without the approval from the US Food and Drug Administration (FDA). Among the category of dietary supplements, botanical dietary supplements (sometimes referred to as herbals or herbal dietary supplements) are classified as nonvitamin, nonmineral, natural products, and are available on the market as plants, plant parts, or plant extracts.[3] According to the 2012 National Health Interview Survey, about 18% of Americans used botanical dietary supplements,[4] the result of which has created a US $35 billion dietary supplement industry.[5] It is estimated that up to 3000 species of plants are included in the formulations of at least 55,000 different dietary supplement products in the United States.[6,7] Dietary supplements can be found in many forms, including tablets, capsules, liquids, soft gels, gel caps, and powders, and are widely used across all ages worldwide. Therefore, the concerns regarding the potential public health risks relating to the concentration, composition, and contaminants of individual dietary supplements, as well as their interactions with drugs or other dietary supplements, have been increasing.[8,9] Testing of dietary supplements for efficacy and safety is important and needs to be conducted scientifically.
To assess the safety of botanical dietary supplements, the US National Toxicology Program (NTP) held an International Workshop to Evaluate Research Needs on the Use and Safety of Medicinal Herbs[9] and initiated a number of studies to evaluate the carcinogenicity of botanical dietary supplements and their constituents in mice and rats. Numerous botanical dietary supplements were nominated to the NTP by US government agencies, including the FDA and the National Cancer Institute. The studies completed by the NTP have indicated that Aloe vera, Ginkgo biloba, goldenseal, and kava showed different levels of evidence of carcinogenic activity in rats, mice, or both species.[3]
The Ginkgo biloba (ginkgo) tree is believed to be one of the world’s oldest species of trees living today. This tree originates from China and has been cultivated around the world as an ornamental tree.[10] The Chinese name for this plant is “Yin-xing” in mandarin, meaning “silver fruit”; it is called “Icho” in Japanese. The ginkgo tree is a deciduous plant with green leaves turning golden in autumn and the ginkgo seeds are contained in ginkgo fruits born on the female trees. The nut-like gametophytes inside the seeds have been used in traditional Chinese medicine for cough, asthma, enuresis, pyogenic skin infections, and intestinal tract worm infections,[11] and have been cooked in traditional Chinese and Japanese foods. Ginkgo leaves have also been used to treat memory loss and cognitive disorders, arrhythmias and ischemic heart disease, cancer, diabetes, and thromboses.[12] Ginkgo biloba leaf extracts in various forms are widely available in herbal supplement stores and online in the United States[13] and can be purchased in many countries as a dietary supplement. Ginkgo biloba is one of the best-selling botanical dietary supplements in the United States at US $94 million in 2012,[14] while total worldwide sales of ginkgo products were US $1.26 billion in 2012, with US $578 million being sold in China, US $152 million in Germany, and US $40 to 61 million in Australia, France, Brazil, and Korea.[15]
Over the past few decades, Ginkgo biloba leaf extract and some of its components have been reported to have cardioprotective effects, neuroprotection against neurovascular insults, and anticancer activities,[16,17] and ginkgo appears a promising botanical dietary supplement with therapeutic benefits. In order to determine the current pharmacological and/or toxicological research status on ginkgo, we performed a literature search in PubMed using “ginkgo biloba” alone or combined with “toxicity” as the keywords. A total of 3603 publications were identified when using “ginkgo biloba” alone as keyword in “All Fields,” while there were 275 publications (7.6%) related to “toxicity” when “ginkgo biloba and toxicity” were combined. Out of the 3603 publications, 2953 papers (82.0%) were published in the period of 2000–2016. Figure 1 shows the details from 2000 to 2016 (searches conducted on July 20 and November 7 of 2016), indicating a great increase in the interests of ginkgo study since 2000. About 8.3% of these articles investigated ginkgo-related safety issue and/or toxicity. Data on the toxic effects of Ginkgo biloba, including genotoxicity and carcinogenicity, have been reported.
Figure 1.
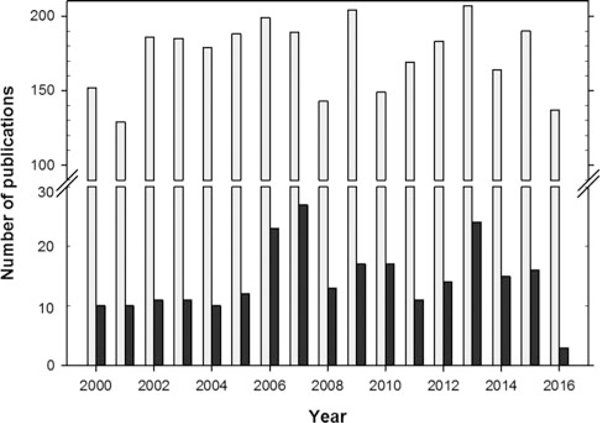
Pharmacological and toxicological research on Ginkgo biloba based on PubMed online search. Open bars represent numbers of all research on ginkgo and black bars indicate the research related to toxicity (searches conducted on November 7 of 2016).
Ginkgo biloba leaf extract was nominated to the NTP for a 2-year carcinogenicity bioassay[18] for the following reasons: (1) Ginkgo biloba leaf extract and its active ingredients have biological activities, (2) ginkgo extracts can be consumed in large doses over an extended period of time, and (3) some of ginkgo’s ingredients possess mutagenic activities. The NTP 2-year bioassay indicated that administration of Ginkgo biloba leaf extract caused carcinogenic activity in livers of mice.[19] Recently, Ginkgo biloba leaf extract has been classified as a possible human carcinogen (Group 2B) by the International Agency for Research on Cancer,[15,20] along with other natural products such as Aloe vera extract[21] and kava extract.[22] The beneficial properties of Ginkgo biloba have been addressed widely as more than 90% publications are related to the beneficial effects (Figure 1), which have been reviewed comprehensively[10,12,16,23]; however, less is known about the toxic properties of Ginkgo biloba. In this review, Ginkgo biloba-induced toxicity from experimental studies, both in vitro and in vivo, to human case reports (caused by ginkgo seeds or leaves) are reviewed, and the outcomes from relatively large clinical trials are also summarized.
Chemical components of Ginkgo biloba
The chemical analysis and quality control of ginkgo leaves, ginkgo extracts, phytopharmaceuticals, and some herbal supplements have been comprehensively reviewed.[10,11,15,24] Ginkgo biloba leaf extracts are made from dried ginkgo leaves with an acetone-water mixture or other suitable solvents; it is a multistep procedure with the enrichment of preferred components and elimination of unwanted substances. The ratio of the crude plant material to powdered extract is between 35~76:1.[25] Ginkgo biloba leaf extract contains a mixture of known and possible active compounds. Using a capillary HPLC/MS method, more than 70 components were identified from the ginkgo products.[26] This method also successfully detected the active components of ginkgo in human urine after ingesting ginkgo extract.[27]
The commercial products of Ginkgo biloba leaf extracts can vary in chemical analysis from one manufacturer to another depending on many variable factors, for example, strain, climate, growth phase, and processing. Several initiatives have been made to establish standards regarding preparative procedures, analysis, and contents of these products. By mixing different preparations with high or low contents of components, a constant quality of ginkgo product can be achieved.[11] A standardized preparation of Ginkgo biloba leaf extract, called EGb761, which is commercially available in Europe, contains two major components, flavonol glycosides (24%) and terpene lactones (6%), along with less than 5 parts-per-million (ppm, i.e., μ.g/g) of ginkgolic acids (Table 1) and other constituents, including proanthocyanadins, glucose, rhamnose, organic acids, and D-glucaric.[12] Synthesis of EGb761 requires a 27-step extraction process, and the liquid extract is dried to give 1 part extract from approximately 50 parts raw ginkgo leaves.[10] Of the flavonoids, glycosylated quercetin, kaempferol, and isorhamnetin are the most abundant.
Table 1.
Chemical components in Ginkgo biloba leaf extracts.
| Reference | Component class |
Survey |
|||
|---|---|---|---|---|---|
| Flavonol glycosides (%) |
Terpene lactones (%) |
Ginkgolic acids (ppm) |
Commercial samples |
Location | |
| EGb761 (2003)[12] | 24 | 6 | <5 | ||
| Health Canada (2009)[17] | 22–27 | 5–7 | n/a | ||
| USP (2011)[25] | 22–27 | 5.4–12 | n/a | ||
| Kressmann et al. (2002)[28] | 24–36 | 4–11 | <500~90,000 | 27 | United States |
| Ding et al. (2006)[29] | 28–35 | 6–11 | n/a | 5 | United Kingdom |
| Gawron-Gzella et al. (2010)[30] | 0.5–28 | 0.2–7 | 2–8053 | 11 | Poland |
| Fransen et al. (2010)[31] | 0.9–4.2 fold* | 0.3–3.6 fold* | n/a | 29 | Netherlands |
| Yin et al. (2004)[69] | 23 | 6.8 | n/a | 1 | China |
Data presented as fold-changes when compared to the declared amount on the label.
The standardization of ginkgo products on the market, however, is far from ideal. A survey on 27 Ginkgo biloba leaf extracts in the US market was conducted from ginkgo products purchased in health-food stores and supermarkets.[28] When the fingerprints of products were compared, the spectra of lipophilic and hydrophilic substances in the extracts and the quantity of substances were quite different. Seventeen of the 27 products were not in accordance with the specified standard range. Overall, the percentages in the extracts showed variations of 24%−36% for flavonol glycosides and 4%—11% for terpene lactones, with a wide range from < 500 (below the limit of quantification) to 90,000 ppm for ginkgolic acids (Table 1). Another study analyzed the compositions of five commercial ginkgo products; two were collected from a local market in the United Kingdom, one from a nationwide health food store, and two from a local research company. Using a traditional quality control method of HPLC separation with UV detection, the five samples had 6%—11% terpene lactones and 28%−35% flavonoids; however, the individual amounts of specific compounds within each class varied by as much as 200% between the different commercial samples.[29]
Considerable variations in components also exist in more regulated products involving ginkgo extract. Three pharmaceutical products and eight dietary supplements containing ginkgo extract in Poland were analyzed using chromatography (TLC and HPLC).[30] Most of samples contained 21%−28% flavonoids, except one sample only had 0.53%. For terpene lactones, nine of eleven samples had 4.6%-7.3%, while two samples contained 0.17% and 0.73%. The extracts of three herbal pharmaceuticals and four dietary supplements were found to contain less than 5 ppm of ginkgolic acids, whereas its concentration in the other four dietary supplements was much greater, ranging from 392 to 8053 ppm (Table 1). Twenty-nine commercially available ginkgo products were randomly collected in the Netherlands and analyzed for their content.[31] Among the 29 products, only 2 and 7 met the requirement of pharmaceutical guideline for terpene lactones and flavonoids, and only one product met both requirements simultaneously. Variations were seen when compared to the declared amount on the label: the amount varied between 27% and 358% for terpene lactones and 86% and 418% for flavonoids (Table 1).
Clinical trials of Ginkgo biloba
Many clinical trials designed to assess the efficacy of Ginkgo biloba leaf extract have been conducted in patients with different diseases; however, these clinical trials have not produced consistent evidence of benefits. The positive effects of ginkgo found in several early case reports or small trials have not been confirmed in the recent studies with relatively larger number of patients, longer duration, and better study designs.
Three commonly claimed health effects of Ginkgo biloba leaf extract are improved blood circulation, improved memory, and beneficial effects on symptoms of old age. However, Fransen and colleagues[31] reanalyzed 26 original studies conducted in healthy volunteers or patients, and 10 meta-analysis and systematic reviews, and concluded that these health benefits could not be substantiated; in addition, neither safety nor efficacy could be guaranteed at the recommended daily dose. Since country differences may exist in the guidelines and procedures of clinical trials, and in the components of the ginkgo products used, hereafter we review large scale clinical trials and studies based on regions.
Clinical trials in France
In France, a large study named “the GuidAge” was conducted with ginkgo leaf extract for the prevention of Alzheimer disease. A total of 2854 participants (mean age = 76.8 years and 67% female) with spontaneous memory complaint were enrolled and randomized to receive either EGb761 120 mg twice a day (n = 1419) or a matching placebo (n = 1435) in a double-blinded trial conducted by general practitioners and hospital practitioners specializing in memory disorders. Within 5-year period, 61 and 73 participants were diagnosed with probable Alzheimer disease in the ginkgo-treated and placebo groups, respectively; 76 and 82 participants died in the ginkgo and placebo groups; 65 and 60 participants suffered a stroke in each group. The results from this 5-year study indicate that long-term use of a standardized Ginkgo biloba leaf extract did not reduce the risk of progression to Alzheimer disease compared with placebo.[34]
Clinical trials in the United Kingdom
In the United Kingdom, the Cochrane Library with a collection of six databases has published a series of systematic reviews on the effect of ginkgo extract on a variety of diseases.[35–39] As a vasoactive agent, ginkgo extract has been used to relieve intermittent claudication, the main symptom of peripheral arterial disease with leg pain while walking. Fourteen randomized controlled trials of ginkgo extract versus placebo with a total of 739 patients were reviewed. Although the patients using ginkgo extract could walk 65 meters further than the patients in the placebo group, there was no significant difference between the two groups. Therefore, there is no evidence in general that ginkgo extract can clinically benefit patients with peripheral arterial disease.[35]
Age-related macular degeneration is a pathological change affecting the central area of the retina. Since vascular factors and oxidative damage are believed to be two potential mechanisms and ginkgo extract has been suggested to be a helpful agent to slow down the progression of this disease, two clinical trials, one in France with 20 people, and the other in Germany with 99 patients, were conducted with different daily doses (60–240 mg) for six months. Due to their short durations and small numbers of participants, these studies had insufficient power to answer the question whether or not ginkgo extract is beneficial to people suffering from age-related macular degeneration.[37]
People with tinnitus can hear sounds in the absence of external acoustic stimulation and currently there is no specific therapy that is satisfactory for all tinnitus patients. Ginkgo leaf extract has been suggested in the management of tinnitus. Four randomized controlled trials with 1543 participants were analyzed and three trials enrolled 1143 patients with a primary complaint of tinnitus. The results indicated that there was no evidence that ginkgo extract was effective for the patients who are troubled by tinnitus.[36]
Ginkgo extract has been widely used for the treatment of cerebral dysfunction. In a review of 36 randomized, double-blinded trials, most studies were small and ≤ 3 months in length, except for nine trials that were 6 months in duration with 2016 patients. The results of the studies are inconsistent regarding the efficacy of ginkgo extract for dementia or cognitive decline. Among the four most recent trials, three found no difference between ginkgo-treated group and placebo group and one reported positive treatment effects while using ginkgo.[38]
In order to determine whether or not ginkgo extract improves functional outcome in the patients with acute ischemic stroke, 10 randomized controlled trials with 792 patients were reanalyzed, with the conclusion that there was no convincing evidence for the routine use of ginkgo extract in the treatment of acute ischemic stroke.[39]
Clinical trials in the United States
In the United States, the Ginkgo Evaluation of Memory (GEM) Study is the largest clinical trial in evaluating ginkgo’s effect on dementia over the course of 8 years at four clinical sites in California, Maryland, North Carolina, and Pennsylvania. More than 20 research articles have been published regarding GEM study data (https://nccih.nih.gov/research/results/gems). In this study, 3069 participants aged ≥75 years (mean age = 79 years and 46% female) with normal cognitive (n = 2587) or mild cognitive impairment (n = 482) were enrolled and randomly assigned to two groups, one group (n = 1545) receiving 120 mg of ginkgo extract twice a day, and the other group (n = 1524) having an identical-appearing placebo. These participants were assessed every 6 months for incident dementia and followed for an average of 6 years. During this period, 523 participants (47% in the placebo group and 53% in the ginkgo group) were diagnosed with dementia. The ginkgo extract showed no overall effect on the rate of progression to dementia in participants with mild cognitive impairment. The results indicate that ginkgo extract at 120 mg twice a day does not reduce the overall incidence rate of dementia or Alzheimer disease in older adults.[40] In addition, there were no differences between ginkgo extract and placebo groups in any neuropsychological domains, including memory, attention, visual-spatial abilities, language, and executive functions. There were also no differences in rates of change over time for the Modified Mini-Mental State Examination and the cognitive subscale of the Alzheimer Disease Assessment Scale although rates of change varied by baseline cognitive status, and no significant effect modification of treatment on rate of decline when stratified by age, sex, race, education, or baseline mild cognitive impairment. These results indicate that ginkgo extract does not slow the rates of cognitive decline in elderly individuals with normal cognition or with mild cognitive impairment.[41]
In order to investigate whether or not Ginkgo biloba leaf extract is cardioprotective through its vasodilatory and antihypertensive properties, the effects of ginkgo extract on blood pressure and incidence of hypertension were determined. Among the 3069 volunteers at baseline, 17% were normotensive, 28% were pre-hypertensive, and 54% were hypertensive. During the study, all participants assigned to ginkgo and placebo groups showed similar changes in blood pressure and pulse pressure, and there were no differences in the rate of hypertension incidence in either group. Therefore, ginkgo extract does not appear to reduce blood pressure or the incidence of hypertension in elderly people.[42]
The differences on cardiovascular disease between ginkgo and placebo groups were also evaluated using Cox proportional hazards regression adjusted for age and sex. Between ginkgo and placebo groups, there were no differences on the number of total death (n = 355) and the death due to coronary heart diseases (n = 87), and there were no differences in the incident of myocardial infarction (n = 164), angina pectoris (n = 207), or stroke (n = 151). These results suggest that ginkgo extract cannot be recommended for preventing cardiovascular disease.[43] There were, however, more peripheral vascular disease events in the placebo group (1.5% vs. 0.8%).
The GEM study analyzed cancer as a secondary endpoint and found little evidence of a protective role of Ginkgo biloba leaf extract in cancer occurrence.[44] Among the participants in both groups, the demographic, lifestyle, and medical characteristics were comparable. During the follow-up period (median = 6.1 years), there were 162 cancer hospitalizations in the ginkgo-treated group and 148 cases in the placebo group. Compared to the placebo group, higher incidence rate of breast and colorectal cancers and lower incidence rate of prostate cancer were observed in the ginkgo-treated group. These data should be cautiously viewed due to several study limitations, such as insufficient follow-up time for cancer occurrences; nonetheless, the findings do not support the hypothesis that consumption of ginkgo reduces the risk of cancer.[44]
Many studies have reported the beneficial properties of Ginkgo biloba leaf extract for treatment of other diseases, either used alone or as an adjuvant therapy with prescription drugs and some other dietary supplements; however, the sample size used in these trials was generally small. To confirm these claims, additional high-quality studies, with more participants, longer duration, and randomized double-blinded design conducted in multiple clinical sites are needed.[45]
Adverse clinical effects of Ginkgo biloba in humans
Recently, Restani and colleagues reviewed adverse effects of plant food supplements and botanical preparations, and found that 39 plants, 59% of all plants searched, resulted in adverse effects in humans.[46] Of the 492 publications reviewed, direct association between the adverse effects and the use of botanicals was reported in 82% of the publications, while the remaining 18% reported indirect associations, such as the interaction with conventional drugs. Ginkgo biloba was ranked as the fourth highest plant associated with adverse effects, with 28 papers related to adverse effects and 14 reporting an interaction with conventional drugs.[46] In the following sections, we review some case reports of the adverse effects observed in humans from the intake of ginkgo seeds and leaf extracts.
Ginkgo biloba seeds
Food poisoning by ginkgo seeds have been reported in Japan and China,[47,48] probably due to the fact that Japanese and Chinese have been consuming ginkgo seeds/nuts as an ordinary food since ancient times. The main symptoms of ginkgo seed poisoning are tonic and/or clonic convulsion, vomiting, and loss of consciousness.[48–51] Ginkgo seed poisoning is primarily due to the neurotoxic compound 4’-O-methylpyridoxine (MPN, also known as ginkgotoxin)[47,52] and MPN glucoside in ginkgo seeds.[53,54] MPN is chemically related to vitamin B6 and interferes with its biosynthesis, metabolism, and function. MPN inhibits enzymatic activation of vitamin B6 by pyridoxal kinase in vitro[55–57] and in vivo,[58] resulting in vitamin B6 deficiency and leading to diminished γ -aminobutyric acid (GABA) synthesis.[57] Ginkgo seeds contain MPN at concentrations of 170–404 ppm.[51,59]
In 1881, the first Japanese case report indicated that the death of two boys was caused by ginkgo seed poisoning.[60] Figure 2 shows the number of patients with ginkgo seed poisoning in Japan by decades based on the previous survey.[48] The first peak of the incidence occurred in 1940s and 1950s after World War II when food was scarce. Soon afterwards a transient decrease of the numbers was observed before the second peak of the incidence appeared after 2000. The decrease might be due to the improvement in nutrition and the changes in data collection of intoxications in Japan. “Food name,” such as ginkgo seed, was previously recorded as the source of food poisoning in food poisoning database. However, “food category,” such as nuts and seeds, has been recorded as the source of food poisoning, and “food name” has not been recorded after around 1970. Therefore, there was a possibility of under-reporting ginkgo poisoning cases during this period. Potential reasons of the second peak after 2000 might be the recognition of MPN in ginkgo seeds as the toxic principle[52] and the spread of information about food poisoning by ginkgo seed over the Internet. The mortality is about 13% (22 out of 170) in Japan and no deaths caused by the poisoning have been reported since 1969 (Figure 2). The most effective treatment of the food poisoning by ginkgo seed is the administration of vitamin B6,[50,51,61] to overcome the vitamin B6 deficiency caused by the poisoning. Most cases of ginkgo seed poisoning resolve without sequelae.
Figure 2.
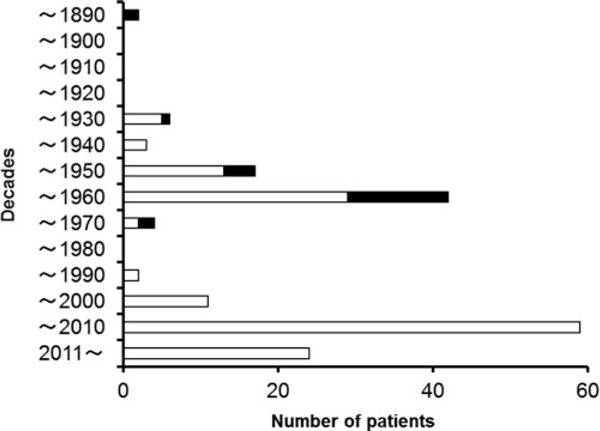
The number per decade of patients with food poisoning from Ginkgo biloba seeds in Japan. Closed bars and open bars represent number of deaths and survivals, respectively.
A number of case reports have reported adverse effects and/or the presence of MPN in patients suffering from ginkgo poisoning. A 36-year-old Japanese woman experienced frequent vomiting and generalized convulsions 4 hours after consuming 70–80 ginkgo nuts cooked in a microwave oven.[49] HPLC analysis showed that the serum levels of MPN ranged from 240 to 1280 ng/ml in five patients with ginkgo seed poisoning.[51] A 2-year-old Japanese girl presented with vomiting, diarrhea, and irritability 7 hours after the ingestion of 50–60 roasted ginkgo seeds and the concentration of MPN was 360 ng/ml in her serum drawn immediately after an afebrile convulsion, about 9 hours after ingestion (values of MPN are usually below the detectable limit of 15 ng/ml).[61] Another 2-year-old Japanese boy had vomiting and afebrile convulsion 4 hours after eating about 50 ginkgo nuts, and the concentrations of MPN were 37–157 ng/ml in his serum within 12 hours after the admission and 397 ng/ml in the urine at18 hours after admission.[50] Recently, a 23-month-old boy in Switzerland experienced two afebrile tonic-clonic seizures after the consumption of an unknown amount of ginkgo seeds and the poisoning was confirmed by measuring MPN levels in both blood and urine.[59] A 51-year-old Korean woman had overconsumed large amounts of ginkgo nuts (1 kg) within 1 hour and about half a day later, she experienced tonic-clonic seizure and postictal confusion with a decreased blood vitamin B6 level of 2.2 μ.g/l (normal range: 5–50 μ.g/l).[62]
Ginkgo biloba leaf extracts
MPN is also found in Ginkgo biloba leaves[63] and in fruit pulp of Ginkgo biloba (Kobayashi et al., unpublished data); however, ginkgo leaves contain only small amount of MPN that maybe insufficient to exert detrimental effects.[63] Even measured with new methodology, MPN was below the limit of detection (9 ppm) in ginkgo leaf extract[64]; nonetheless, the possibility of contamination by seeds or fruits of Ginkgo biloba in all products of ginkgo leaf extract cannot be completely excluded. At least 37 components have been identified in ginkgo leaf extracts,[19] and some of these constituents may be responsible for the adverse effects observed in humans, as discussed hereafter.
In 2001, seven reports of seizures related to Ginkgo biloba leaf extract (three associated with single-ingredient ginkgo preparations and four with multi-ingredient products) were listed in the US FDA’s Special Nutritionals Adverse Event Monitoring System.[65] In the United Kingdom, two patients (a 78-year-old man and an 84-year-old woman) with epilepsy well-controlled by valproate presented with recurrent seizures within 2 weeks of taking 120 mg/day ginkgo leaf extract.[66] A fatal case of a 55-year-old male patient who suffered a breakthrough seizure with no evidence of noncompliance with his anticonvulsant medications was also reported. Following his death, his physician discovered that he had started taking herbal supplements (Ginkgo biloba, ginseng, and saw palmetto extracts) and nonprescription vitamins a year prior to his death. The autopsy report revealed subtherapeutic serum levels of the anticonvulsants, valproate, and phenytoin. Since both anticonvulsants are metabolized by cytochrome P-450 2C9 (CYP2C9) and 2C19 (CYP2C19), and ginkgo can increase the activity of CYP2C19, extensive metabolism of the anticonvulsants due to CYP2C19 induction and the effect of potent neurotoxin MPN on the seizure could be a plausible explanation.[67] Samuels and colleagues summarized case reports of herb-induced seizures, and three mechanisms of ginkgo-induced seizures were proposed: involvement of MPN, inhibition of platelet activating factor, and CYP2C19 induction.[68]
Several reports have described the induction of CYP enzymes in humans by Ginkgo biloba leaf extracts, which shed light on potential interactions between ginkgo and conventional drugs. Eighteen healthy male Chinese, who were previously genotyped for CYP2C19, received 280 mg/day Ginkgo biloba extract (two 70-mg tablets twice a day) for 12 days and then received 40 mg omeprazole (a CYP2C19 substrate); plasma and urine concentrations of omeprazole and its metabolites were determined by an HPLC method. Ginkgo extract induced omeprazole hydroxylation in a CYP2C19 genotype-dependent manner and reduced the renal clearance of 5-hydroxyomeprazole, suggesting that ginkgo extract may reduce the effects of medications, such as omeprazole or other drugs that are substrates for CYP2C19.[69] Ten healthy male Japanese volunteers were administered 360 mg/day Ginkgo biloba leaf extract orally (2 tablets of 60 mg and 3 times a day) for 28 days; they then received 125 mg tolbutamide (CYP2C9 probe), 8 mg midazolam (CYP3A4 probe), and 75 g glucose. Plasma drug concentrations and blood glucose levels were then measured to determine the area-under-the-plasma-concentration-time curve (AUC).[70] Compared to the drug concentrations before ginkgo extract intake, the AUC for tolbutamide after ginkgo intake was significantly decreased (16%) while the AUC for midazolam was increased (25%); ginkgo treatment also slightly attenuated the AUC of blood glucose-lowering effect of tolbutamide, indicating the potential risk of ginkgo extract with prescribed drugs.[70] Nonetheless, the effect of Ginkgo biloba leaf extract on CYP3A4 remains controversial. It has been suggested that consumption of Ginkgo biloba extracts should be monitored in patients receiving drugs metabolized by CYP2C19 and CYP2C9, and more studies are needed to investigate the combination of ginkgo extract and drugs known to be CYP3A4 or P-gp substrates.[71]
Another adverse effect associated with the use of Ginkgo biloba leaf extract is spontaneous bleeding, although contradictory research findings also exist. For example, a 59-year-old Korean man underwent a liver transplant and a routine postoperative ultrasound revealed probable subcapsular hematoma around the dome of the liver, with a vitreous hemorrhage in his right eye.[72] His physicians then found that the patient consumed ginkgo extract prior to transplantation and during his recovery in the hospital. A review paper with 15 published case reports (with patients ranging from 33- to 78-year-old) suggested a possible causal relationship between Ginkgo biloba extract and bleeding events. Three case reports indicated an increased bleeding time while taking ginkgo, and 6 case reports stated that bleeding terminated when ginkgo was stopped.[73] In contrast, a review article of 21 cases, including 14 cases reported in Bent’s study, concluded that Ginkgo biloba extract did not significantly impact hemostasis or adversely affect the safety of co-administered aspirin or warfarin.[74] In addition, a meta-analysis of 18 randomized controlled trials with 1985 adults did not find a higher bleeding risk associated with standardized Ginkgo biloba leaf extract, based on the parameters of hemostasis and a comparison of mean difference between treatment and placebo groups.[75]
When 12 healthy male subjects received a single dose of 25 mg warfarin with or without pre-exposure to multiple dose of ginkgo extract (2 tablets, 3 times a day) for 1 week, there were no differences in pharmacokinetics and pharmacodynamics, such as international normalized ratio of prothrombin time or platelet aggregation, between the two groups.[76] Although the literature on ginkgo and its bleeding complications has been controversial, additional case reports continue to be published, such as the association of ginkgo with cerebral bleeding in a 38-year-old woman[77] and a diffuse alveolar hemorrhage in a 46-year-old woman.[78] Recently, a study used a large medical record database, the Veterans Administration Informatics and Computing Infrastructure database in the United States, and collected the information of 11,003 patients who used ginkgo with warfarin and 796,396 patients who used warfarin alone.[79] This study indicated that Ginkgo biloba leaf extract significantly increased patients’ risk of bleeding when taking concurrently with warfarin, suggesting an increased risk of bleeding associated with the consumption of ginkgo and warfarin.
Other adverse effects associated with Ginkgo biloba leaf extracts have been reported as well. For example, a patient with electrocardiographic evidence of ventricular arrhythmia that resolved upon the discontinuation of ginkgo[80]; a case of acute generalized exanthematous pustulosis caused by oral ginkgo medication[81]; a randomized placebo-controlled trial of ginkgo showed that there were more ischemic stroke and transient ischemic attack cases observed among the ginkgo group[82]; a 35-year-old woman complaining frequent nocturnal palpitations, which was attributed to a pro-arrhythmic effect of ginkgo[83]; and a 66-year-old woman developed allergic contact dermatitis after picking ginkgo tree fruit among ginkgo leaves.[84] In addition, it has been suggested that Ginkgo biloba (including seeds and leaves) should be used with caution during pregnancy, particularly around labor, and during lactation, due to its anti-platelet properties.[85]
In vivo toxicity of Ginkgo biloba
In addition to clinical trials, the toxicity of Ginkgo biloba has been assessed in vivo in mice and rats. Acute toxicity studies on the standardized extract EGb761 demonstrated LD50 (lethal dose caused the death of 50%) values of 7700, 1100, or 1900 mg/kg (ppm) in mice and >10,000, 1100, or 2100 mg/kg in rats when administered by oral, intravenous, or isoelectric point routes, respectively.[10] Next we summarize major findings regarding the toxicity of ginkgo in these rodent models, including genotoxic and carcinogenic potential, effects on embryonic development, and other observed abnormality.
In the safety assessment of a substance, the evaluation of genotoxic and carcinogenic potential is a major toxicity endpoint.[86] The NTP performed 3-month and 2-year toxicity and carcinogenicity studies with ginkgo leaf extract (obtained from Shanghai Xing Ling Science and Technology Pharmaceutical Company, Shanghai, China) in corn oil administered by oral gavage to F344/N rats and B6C3F1/N mice.[19] The liver, thyroid, and nose were found to be the targets of the treatment and these targets were consistent across sex, species, and exposure period, although with varied degrees of effect.[87]
A 3-month treatment of ginkgo increased the liver weights and incidences of hepatocytic hypertrophy in rats and mice in both sexes. A dose-related increase in pigmentation of the olfactory epithelium of the nose was observed in rats and mice; histopathological lesions, such as hyaline droplet accumulation in the respiratory and olfactory epithelium and hyaline droplet atrophy in the olfactory epithelium, were observed in the nose of male and female mice.[19]
A 2-year treatment significantly reduced the survival in the 1000 mg/kg male rats and in the 600 and 2000 mg/kg male mice as compared to controls, when groups of rats were gavaged with 0, 100, 300, or 1000 mg/kg ginkgo leaf extract and groups of mice were administered 0, 200, 600, or 2000 mg/kg. Incidences of hepatocellular adenoma were slightly increased in male rats, whereas there were significantly increased incidences of hepatocellular adenoma, carcinoma, and hepatoblastoma in both sexes of mice. The incidences of thyroid follicular cell hypertrophy and follicle hyperplasia were significantly increased, whereas follicular cell adenoma and carcinoma were observed occasionally in ginkgo treated mice and rats. In the nose, respiratory epithelium adenomas were found in two 300 mg/kg female rats. The incidences of transitional epithelium and respiratory epithelium hyperplasia were significantly increased, as well as atrophy, metaplasia, nerve atrophy, and pigmentation in the olfactory epithelium in rats.[19]
Microarray analysis on gene expression changes in the liver of male B6C3F1 mice revealed significant alteration in the expression of genes associated with drug metabolic enzymes, the Nrf2-mediated oxidative stress, and the Myc gene-centered network named “cell cycle, cellular movement, and cancer.”[88] A mechanistic study of hepatogenesis illustrated that although morphologically indistinguishable, significant differences were observed in molecular changes between ginkgo-induced and spontaneous hepatocellular carcinoma (HCC) in B6C3F1 mice.[89] Compared to spontaneous tumors, ginkgo-induced tumors showed statistically significant increases in Ctnnb1 mutations and decreases in H-ras mutations with increasing ginkgo doses. Significant differences in gene expression profiles were found between ginkgo-induced and spontaneous HCC and the pathway analysis using Ingenuity Pathway Analysis software indicated overrepresentation of genes associated with cancer signaling, HCC development, xenobiotic metabolism, and oxidative stress pathways.
Reproductive and developmental toxicities of ginkgo extract were evaluated in rodents. A 90-day subchronic treatment with commercial ginkgo extract tablets (obtained from General Nutrition Corporation, Pittsburgh, PA, USA) (25–100 mg/kg/day) by oral gavage caused significant increases in the weight of caudae epididymis and prostate, induced pre-implantation loss, and decreased the percentage of pregnancy in male Swiss albino mice.[90] Percent sperm motility, count, and morphology of the spermatozoa were not affected.
The standardized ginkgo extract EGb761, which has defined concentrations of active ingredients (e.g., 0.4 ppm ginkgolic acids) showed no effects on embryo-fetal development, pre- and post-implantation losses in CD-1 mice orally administered 100–1225 mg/kg/day EGb761 daily from day 6 to day 15 of pregnancy (the days are critical days for organogenesis).[91] No EGb761-related malformations, variations, and retardations were observed in skeletal and soft tissues at any tested dose level, indicating that EGb761 tested in this study was not embryotoxic.
Adult Wistar rats were treated with ginkgo extracts (Origin: China, but prepared by JR Pharma, Juiz de Fora, Minas Gerais, Brazil) daily at 3.5, 7, and 14 mg/kg/day from the first to eighth day of pregnancy, which is the one-cell-to-blastocyst period for tubal transit and implantation.[92] These doses were equivalent to one, two, and four times the maximum human dose (240 mg/day, that is, 3.5 mg/kg/day for a human weighing 70 kg). When the rats were killed on the fifteenth day of pregnancy, no significant maternal and embryonic toxicities were observed as evaluated by maternal liver, kidney, and ovary weights, number of corpora lutea, pre- and post-implantation loss, live fetuses, fetus and placenta weight, and fetal external malformation. However, when the same extract was administered to Wistar rats at the same doses from the eighth to twentieth day of pregnancy, the middle- and high-dose treatments caused significant decrease in the fetuses mean weights, indicating fetal intra-uterine growth retardation.[93] Similarly, when dams were given ginkgo capsules (obtained from General Nutrition Corporation, Pittsburgh, PA, USA) at a dose of 100 mg/kg/day during pregnancy, mice fetuses exhibited a high frequency of malformations, including round shaped eye and orbits, syndactyly, malformed pinnae, nostrils, lips, and jaws, suggesting a teratogenic effect.[94]
Increases in sperm abnormalities, which mainly resulted in broken tail and amorphous head, were reported in F1 male Wister rats when dams were exposed to ginkgo extracts (obtained from JR Pharma, Juiz de Fora, Minas Gerais, Brazil) at doses of 25–100 mg/kg/day from the sixteenth to the twentieth day of pregnancy.[95] The administration of ginkgo extract at a dose of 3.5 mg/kg/day to nursing Wistar rats showed no toxic effects on mothers or on the physical, motor, and sensory development of the pups.[96] Ginkgolide B, one of the major active components of ginkgo extracts, was reported to trigger significant injury of oocyte maturation, fertilization, and sequential embryonic development in ICR mice administered 1–6 μ.M ginkgolide B in drinking water for 4 days.[97]
Ginkgo extracts showed immunotoxic properties and cardiovascular toxicity in treated rodents. Two milligrams of a crude aqueous-alcoholic preparation obtained by extracting dried green ginkgo leaves with 60% w/w ethanol were injected subplantar into the paw of male C57B1I/6 mice.[98] A clear and significant lymphoproliferative reaction was observed in the ipsilateral popliteal lymph node of treated mice when compared with control mice. Chromatography studies indicated that ginkgolic acids were the main constituents in the crude preparation leading to the allergenic reaction.
When a diet containing 0.5% ginkgo (obtained from Tama Biochemical Co., Tokyo, Japan) was administered to aged male spontaneously hypertensive (SH) rats for 4 weeks, heart rate and blood flow velocity in tail arteries of aged SH rats were significantly reduced as compared to control rats. The impaired peripheral circulation may result from the inhibition of cardiac function in aged SH rats. In addition, ginkgo feeding significantly increased liver weight, the alanine aminotransferase level in serum, and the protein expression level of CYP2B in the liver.[99] EGb761 was also reported to enhance amikacin-induced ototoxicity as measured by distortion product otoacoustic emissions in Sprague-Dawley (SD) rats. These rats received amikacin 600 mg/kg/day intramuscularly between postnatal days (PND) 30 and PND 44 and EGb761 100 mg/kg/day orally between PND 30 and PND 50, indicating an herb-drug interaction.[100]
Quercetin, a flavonoid, is the most investigated component of ginkgo extracts. An NTP 2-year feeding study showed that quercetin (1000–40,000 ppm, the estimated dose delivered was approximately 40–1900 mg/kg/day) caused dose-related increases in the severity of chronic nephropathy and in the incidence of renal tubule hyperplasia.[101] Some evidence of carcinogenic activity was indicated by an increased incidence of renal tubule cell adenomas in male F344 rats. Quercetin was reported to induce apoptosis in both the liver and the brain in mice after an 18-day administration at doses of 0–250 mg/kg body weight, as evidenced by increased caspase-3 expression and activity.[102] The brain was found to be more susceptible to apoptosis than the liver.
Polyprenols, a class of chemicals accounting for 0.35% in dried ginkgo leaves, are natural active lipids that showed no acute or subchronic toxicity in rodents.[103] In the acute toxicity study, ICR mice were given polyprenols orally at doses of 5000–21,500 mg/kg body weight twice and were observed during 14 days. In the subchronic study, female SD rats were dosed with 500, 1000, and 2000 mg/kg body weight/ day for 91 days, which was equal to 60, 120, and 240 times the daily dosage for a human weighing 60 kg, respectively. Both treatments showed no significant changes on the weight, food intake, hematological and biochemical indexes, or on all clinical observation indexes.
In vitro toxicity of Ginkgo biloba
Extensive research work has been carried out in vitro to assess the toxicity of Ginkgo biloba and decipher the underlying molecular mechanisms. A Ginkgo biloba leaf extract was cytotoxic and genotoxic, and induced oxidative stress and apoptosis in a variety of normal and carcinoma cells, as summarized in the following section.
In human HCC cell lines (HepG2 and Hep3B), a 24-hour treatment with a standard Ginkgo biloba extract solution EGb761 (Cerenin®, Karlsruhe, Germany) at concentrations of 0.05–1 mg/ml significantly inhibited cell growth and caused lactate dehydrogenase (LDH) release, indicating suppressed cell proliferation and increased cell cytotoxicity.[104] These observations were further confirmed by Western blot analysis showing this extract solution decreased proliferating cell nuclear antigen (PCNA, a cell proliferation marker) and increased p53 expression (an apoptosis biomarker) in HepG2 cells. In another study, EGb761 (obtained from Yuyu Pharmaceutical Co., Seoul, Korea) in 100% ethanol inhibited the proliferation of a human retromolar trigone squamous carcinoma cell (SCC-1483) and caused apoptosis in a time- and concentration-dependent manner, which was confirmed by DNA fragmentation, increased caspase-3 activity, and poly ADP ribose polymerase (PARP) cleavage.[105]
In the neutral red assay, a Ginkgo biloba leaf extract (obtained from the National Institute of Standards and Technology, Gaithersburg, MD, USA) was demonstrated to be cytotoxic in SCC-1483 cells, human oral cavity squamous carcinoma cells (HSC-2), human salivary gland carcinoma cells (HSG), and human normal gingival fibroblasts (HF-1).[106] This study also demonstrated that ginkgo induced morphological abnormalities and apoptosis in HSC-2 cells. A linear increase in peroxide (hydrogen peroxide and superoxide anion) was found in cell culture medium incubated with 0.25–1 mg/ml ginkgo for 2 hours, accompanied by time- and concentration-dependent glutathione (GSH) depletion. In addition, ginkgo leaf extract (obtained from Tian-Yi Bio-Tech Inc., Xi’an, China), at concentrations of 25–100 μ.g/ml, induced significant morphological alterations and increased cell fragility in isolated human red blood cells.[107] Dose-dependent GSH depletion and increases in cellular methaemoglobin were also observed upon co-incubation of red blood cells with the extract.
The genotoxicity of a Ginkgo biloba leaf extract (obtained from Shanghai Xing Ling Science and Technology Pharmaceutical Co., Shanghai, China), containing comparable components to the marketed products, was investigated in the bacterial gene mutation assays by the NTP.[19] The extract (1–10 mg/plate) was mutagenic in Salmonella typhimurium strains TA98 and TA100 and in Escherichia coli strain WP2 uvrA/pKM101, either in the presence or absence of metabolic activation (10% S9), causing frame shift and base substitution mutations. Using the same test material, ginkgo leaf extract (0.2–1.2 mg/ml) induced concentration-dependent increases in cytotoxicity, mutant frequency, and DNA double-strand breaks in mouse lymphoma cells following a 4-hour treatment without metabolic activation.[108] The extract also increased intracellular reactive oxygen species levels and decreased GSH levels, suggesting pro-oxidant effects. The extract-induced DNA damage was further confirmed in human hepatoma HepG2 cells as measured by the Comet assay and by the induction of phosphorylation of histone H2A.X (γ -H2A.X), a hallmark of DNA damage.[109] A mechanistic study showed that the inhibition of topoisomerase II (Topo II) contributed to the extract-induced DNA damage. This ginkgo extract was also genotoxic and cytotoxic to human lymphoblast TK6 cells. In a GreenScreen HC assay, a 46-hour treatment increased GFP fluorescence and caused the relative cell survival of less than 90% of the vehicle control.[109]
As mentioned above, Ginkgo biloba leaves contain numerous chemical constituents (Table 1). The flavonol glycosides and terpene lactones are the two major classes of components. The major flavonol glycosides are quercetin, kaempferol, and isorhamnetin, which were identified to be the major contributors to Ginkgo biloba leaf extract-induced genotoxicity.[108,109] Various research groups have used in vitro approaches to investigate the toxicity of these components individually.
The toxic effect of quercetin has been well-documented in multiple cell lines. Quercetin at a concentration of 100 μ.M significantly inhibited cell growth and cell cycle progression (sub-G1 arrest) in human breast cancer MDA-MB-453 cells.[110] Quercetin also caused apoptosis with the involvement of B-cell lymphoma 2 family proteins and caspase-3 and PARP cleavage.
Quercetin was mutagenic in a number of Salmonella typhimurium strains, including TA100, TA98, and TA1538, both with and without metabolic activation,[101,111,112] and induced DNA strand breaks in L5178Y mouse lymphoma cells, HepG2 cells, HeLa cells, and human peripheral blood lymphocytes.[108,113] Quercetin was cytotoxic and mutagenic in L5178Y cells at concentrations ≥30 μ.M,[108] and increased frequencies of micronuclei, sister chromatid exchange and chromosomal aberrations in Chinese hamster lung V79 cells, Chinese hamster ovary (CHO) cells, and human lymphocytes with and without S9.[101,114‘115]
Kaempferol was mutagenic in the Salmonella typhimurium TA98 reversion assay in the presence of S9[116] and decreased relative survival, induced chromosomal aberrations, and increased mutation frequencies at the Tk locus in CHO-AT3–2 cells with and without S9 activation.[115] Kaempferol also increased Tk gene mutations and induced DNA damage in L5178Y cells, HepG2 cells, and human peripheral blood lymphocytes.[108,109,117] At concentrations of 17–104 μ.M in the presence of S9, kaempferol induced significant increases in chromosomal aberrations and micronuclei in V79 cells in a concentration-dependent manner.[116] Kaempferol and quercetin, at concentrations of 40 μ.M inhibited cellular proliferation by inducing caspase-3-dependent apoptosis and PARP cleavage in various oral cancer cell lines (SCC-1483, SCC-25, and SCC-QLL1).[118]
Isorhamnetin was cytotoxic but not mutagenic in the mouse lymphoma assay both in the presence and absence of S9[108];however, it induced a moderate increase in DNA strand breakages in HepG2 cells in a concentration-dependent manner when compared to quercetin and kaempferol.[109] DNA damaging effects of these flavonol glycosides have shown to be the result of Topo II inhibition. Topo II inhibition by these flavonol glycosides was demonstrated by both in silico predication and biochemical analysis. Molecular docking indicated that quercetin and kaempferol have the greatest potential for interacting with Topo II. Using purified human Topo II, quercetin, kaempferol, and isorhamnetin showed inhibitory effects as measured by the ability to convert double-stranded catenated kinetoplast DNA (kDNA) to decatenated relaxed products.[109] Importantly, DNA damage triggered by quercetin was decreased in Topo II knockdown cells, indicating that DNA damage is directly linked to Topo II.
The class of terpene lactones in ginkgo leaves includes ginkgolide A, ginkgolide B, ginkgolide C, bilobalide, and other ginkgolides. Some of these ginkgolides showed teratogenic effects during mammalian embryonic development.[119] Ginkgolide A and B decreased the viability of mouse blastocysts by inducing apoptosis and caused developmental retardation in post-implantation mouse embryos in vitro, leading to embryonic death. Following a 24-hour treatment, ginkgolide B at concentrations of 1–6 μ.M reduced the rate of oocytes maturation, in vitro fertilization, cleavage to 2-cell stage embryo, and blastocyst development, in a dose-dependent manner.[97] Ginkgolides have structures similar to the convulsant picrotoxin, a classic γ-aminobutyric acid A (GABAA) receptor antagonist.[120] An amino acid alignment of insect and human GABAA receptor subunits shows that the 2’ residue differs between subunits: GABA-activated RDL (resistant to dieldrin) subunits have an Ala, while human GABAA receptor α subunits have Val. The single amino acid substitution of Val for Ala at the 2’ position into the RDL receptor pore caused dramatic decreases of ginkgolide cytotoxic potency (up to 10,000-fold), indicating the importance of this position in determining the toxicity of ginkgo extracts.
Biflavonoids and organic acids are the two minor classes of components in ginkgo leaf extracts. Ginkgetin (a biflavonoid) caused morphological changes in different human cells and their nuclei, and increased intracellular levels of hydrogen peroxide within 30 min.[121] The EC50 of ginkgetin (half maximal effective concentration) in ovarian adenocarcinoma (OVCAR-3) cells, cervical carcinoma (HeLa) cells, and foreskin fibroblasts (FS-5) was 3.0, 5.2, and 8.3 μ.g/ml, respectively.
Ginkgolic acids are alkylphenols found in the fruits and leaves of Ginkgo biloba. The cytotoxicity of two ginkgo extracts containing less than 3 ppm ginkgolic acids, one crude ginkgo extract having 2.2% ginkgolic acids, and two alkylphenol-containing fractions with a concentration of 26.6% or 58% ginkgolic acids was tested in human and animal cell lines using the neutral red assay.[122] A close correlation was observed between the concentration of ginkgolic acids and the cytotoxicity of the extracts—the extract containing the lowest concentration of ginkgolic acids displayed the highest IC50 (half maximal inhibition concentration) values in treated cells, indicating a low toxic potential. The IC50 values of the extracts showed 22–63-fold differences between the extracts with the lowest and highest ginkgolic acid content. Thus, ginkgolic acids have been recognized as potential hazardous compounds. Based on safety concerns, a maximal concentration of ≤5 ppm of ginkgolic acids was specified by the Monograph of the Commission E of the former German Federal Health Agency (Table 1).[123]
Three major ginkgolic acids with different alkyl or alkenyl groups (13:0, 15:1, and 17:1) were cytotoxic at concentrations ≥ 50 μ.M in male Chinese hamster lung fibroblasts (V79 cells) as measured by the resazurin reduction assay.[124] In the human keratinocyte cell line HaCaT and the rhesus monkey kidney tubular epithelial cell line LLC-MK2, the IC50 values of ginkgolic acids were significantly lower than those of EGb761, 21.8 vs. 889 mg/l in HaCaT, and 4.6 vs. 1481 mg/l in LLC-MK2, measured by a neutral red uptake assay, indicating a higher cytotoxicity.[125] In addition, ginkgolic acids at concentrations of 1–100 mg/l induced a dose-dependent release of LDH in LLC-MK2 and increased the proportion of apoptotic cells in HaCaT cells. Morphological evaluations by electron microscopy revealed that ginkgolic acids induced formation of myelinosomes in the cytoplasma of HaCaT cells, while transformation of mitochondria resulting from uncoupled oxidative phosphorylation was observed in LLC-MK2 cells. Ginkgolic acids also induced DNA strand breaks in primary rat hepatocytes[126] and showed neurotoxic effects manifested by inducing concentration-dependent death in primary cultures of neurons from chick embryo telencephalons, in the presence or absence of serum.[127] Features of apoptosis, such as chromatin condensation and shrinkage of the nucleus, were observed in chick neurons treated with 250 μ.M ginkgolic acids for 24 hours. A follow-up mechanistic study showed that protein phosphatase 2Cα was highly activated, which may contribute to ginkgolic acid-induced neuronal death. The embryotoxic effects of ginkgolic acid were confirmed by comparing the LD50 values in fresh fertile Leghorn eggs of three Ginkgo extract fractions containing different percentages of ginkgolic acids.[123] Eliminating ginkgolic acids during the manufacturing process significantly reduced the toxic potential of the extracts.
Concluding remarks
Traditional and complementary medicine (T&CM) is widely used around the world and in many cultures; medicinal botanicals have long history as a major component of T&CM. Currently, there is an increasing global interest in the use of botanicals or derivative products because people believe that their sources are found in nature and such products as “natural” maybe beneficial to health.[7,128] Despite this common belief, many botanical dietary supplements have been reported to have potential health risks. In addition, a great number of supplement-related adverse events remain unreported.[129] In the United States, botanical supplement sales have continually increased from 2003 to 2014, and products containing ginkgo were among the 20 top-selling botanical supplements in the US mainstream outlets (e.g., supermarkets, drugstores, and mass-market retailers) and natural outlets (e.g., co-ops and natural product retail stores) as surveyed in 2014.[130]
It is clear that there are a number of difficulties faced by the national and international agencies regarding the regulatory issues related to botanical dietary supplement products, such as lack of research data and lack of appropriate mechanisms to control herbal products and to monitor the safety of these products.[131] Currently, there are several important safety-related challenges that need to be addressed. Botanical dietary supplements are usually a mixture of chemicals, and it is not known which components are responsible for the pharmacological or toxicological effects. The complex chemical nature of dietary supplements makes it difficult to evaluate their efficacy and safety.[132,133] Ginkgo products contain more than 70 components.[26] The NTP animal studies indicated that ginkgo extract induced liver tumors and also targeted thyroid and nose.[19,87] Human clinical studies also showed that cancer incidences for breast and colon were significantly increased.[44] The components of ginkgo extract that may be responsible for ginkgo’s toxicity, genotoxicity, and carcinogenicity have not been definitively identified; therefore, more studies on the mechanism of toxicity and genotoxicity of ginkgo extract are needed.
Another challenge is a high frequency of adulteration due to popularity and market value of ginkgo. Recently, an analysis of 65 commercial ginkgo extracts from Asia, Europe, and North America, reported ginkgo extract adulteration in the global market, and growing evidence has showed substandard and adulterated ginkgo extracts in the international supply chain.[134] With well-developed analytic methods, adulterated and authentic supplements can be easily distinguished. When 18 commercially available ginkgo products were analyzed, three of these commercial products were adulterated with rutin, four with quercetin, and one with an unidentified flavonoid.[135] In another study, 42 commercial ginkgo samples were analyzed and only three of them were determined to be authentic while evidence of adulteration was found in the other samples.[136] Three leading nonprofit organizations in the United States have recently initiated a large-scale program—the Botanical Adulterants Program, and a botanical adulterants bulletin on ginkgo extract adulteration is under development.[134]
Botanical dietary supplements are often used in combination with some therapeutic drugs and this raises concern about the potential toxicity resulting from herb-drug interactions.[137] According to the majority of systematic reviews, there are minor or moderate interactions between herbal medicinal products and synthetic drugs; however, some herbal medicinal products can result in severe health threats, even death.[138] Ginkgo may interact with aspirin, warfarin, trazodone, omeprazole, antihypertensive agents, and antihyperglycaemics.[139,140] Since herb-drug interactions are much more difficult to characterize, monitoring of potential adverse events when herbal botanicals are co-administered with drugs is very important.
In summary, current published data demonstrate both beneficial and adverse effects of Ginkgo biloba extracts and it is critical to consider which specific extract was applied. Generally, research on toxicological effects of ginkgo is much less extensive compared to the research on pharmaceutical effects (Figure 1). From a toxicological perspective, this review updated available data on Ginkgo biloba-induced toxicity from in vitro and in vivo experimental studies and human case reports, with some negative results from relatively large clinical trials. More investigations regarding ginkgo’s safety and efficacy are warranted.
Acknowledgments
The authors thank Drs. Page McKinzie, Yuanfeng Wu, and Frederick Beland for their critical review of this manuscript. The information in this manuscript is not a formal dissemination of information by the United States Food and Drug Administration (US FDA) and does not represent agency position or policy.
Funding
ZR was supported by the appointment to the Postgraduate Research Program at the National Center for Toxicological Research administered by the Oak Ridge Institute for Science Education through an interagency agreement between the US Department of Energy and the US FDA.
References
- [1].Food and Drug Administration. Dietary supplement health and education act of 1994. 1994. http://www.fda.gov/regulatoryinformation/legislation/significantamendmentsto-thefdcact/ucm148003.htm (accessed July 20, 2016).
- [2].Young AL, Bass IS. The Dietary Supplement Health and Education Act. Food Drug Law J. 1995;50:285–292. [PubMed] [Google Scholar]
- [3].National Toxicology Program. NTP botanical dietary supplements program. 2016. http//www.niehs.nih.gov/health/materials/ntp_botanical_fact_new_508.pdf (accessed November 7, 2016).
- [4].Clarke TC, Black LI, Stussman BJ, Barnes PM, Nahin RL. Trends in the use of complementary health approaches among adults: United States, 2002–2012. Natl Health Stat Report. 2015;79:1–16. [PMC free article] [PubMed] [Google Scholar]
- [5].National Toxicology Program. Challenges in studying hazards of botanicals explored at NTP workshop 2016. http://ntp.niehs.nih.goV/update/2016/6/botanicals/index.html. (accessed November 7, 2016). [Google Scholar]
- [6].Zurer P, Hanson D. Chemistry puts herbal supplements to the test. Chemical & Engineering News. 2004;82:16. [Google Scholar]
- [7].Abdel-Rahman A, Anyangwe N, Carlacci L, Casper S, Danam RP, Enongene E, Erives G, Fabricant D, Gudi R, Hilmas CJ, Hines F, Howard P, Levy D, Lin Y, Moore RJ, Pfeiler E, Thurmond TS, Turujman S, Walker NJ. The safety and regulation of natural products used as foods and food ingredients. Toxicol Sci. 2011;123:333–348. [DOI] [PubMed] [Google Scholar]
- [8].Petroczi A, Taylor G, Naughton DP. Mission impossible? Regulatory and enforcement issues to ensure safety of dietary supplements. Food Chem Toxicol. 2011;49:393–402. [DOI] [PubMed] [Google Scholar]
- [9].Matthews HB, Lucier GW, Fisher KD. Medicinal herbs in the United States: research needs. Environ Health Perspect. 1999;107:773–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Chan PC, Xia Q, Fu PP. Ginkgo biloba leave extract: biological, medicinal, and toxicological effects. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2007;25: 211–244. [DOI] [PubMed] [Google Scholar]
- [11].van Beek TA, Montoro P. Chemical analysis and quality control of Ginkgo biloba leaves, extracts, and phytopharmaceuticals. J ChromatogrA. 2009;1216:2002–2032. [DOI] [PubMed] [Google Scholar]
- [12].EGb. EGb 761: ginkgo biloba extract, Ginkor. Drugs R D. 2003;4:188–193. [DOI] [PubMed] [Google Scholar]
- [13].Lindstrom A, Ooyen C, Lynch ME, Bluementhal M. Herb supplement sales increase 5.5% in 2012: Herbal supplement sales rise for 9th consecutive source; turmeric sales jump 40% in natural channel. HerbalGram. 2013;99:60–65. [Google Scholar]
- [14].Nutrition Business Journal. Supplement Business Report 2013. Boulder, CO: Penton Media Inc; 2013. [Google Scholar]
- [15].International Agency for Research on Cancer. Some drugs and herbal products. 2015. http://monographs.iarc.fr/ENG/Monographs/vol108/index.php(accessed July 20, 2016).
- [16].Mahadevan S, Park Y. Multifaceted therapeutic benefits of Ginkgo biloba L.: chemistry, efficacy, safety, and uses. J Food Sci. 2008;73:R14–19. [DOI] [PubMed] [Google Scholar]
- [17].Health Canada. Monograph: Ginkgo Biloba. 2009. http://webprod.hc-sc.gc.ca/nhpid-bdipsn/monoReq.do?id=100 (accessed July 20, 2016).
- [18].National Toxicology Program. Nomination of Ginkgo biloba extract. 1998. http://ntp.niehs.nih.gov/ntp/htdocs/chem_background/exsumpdf/ginkgo_gbe_508.pdf (accessed July 20, 2016).
- [19].National Toxicology Program. Toxicology and carcinogenesis studies of Ginkgo biloba extract (CAS No. 90045–36-6) in F344/N rats and B6C3F1/N mice (Gavage studies). Natl Toxicol Program Tech Rep Ser. 2013;578:1–183. [PubMed] [Google Scholar]
- [20].Grosse Y, Loomis D, Lauby-Secretan B, El Ghissassi F, Bouvard V, Benbrahim-Tallaa L, Guha N, Baan R, Mattock H, Straif K, International Agency for Research on Cancer Monograph Working G. Carcinogenicity of some drugs and herbal products. Lancet Oncol. 2013;14:807–808. [DOI] [PubMed] [Google Scholar]
- [21].Guo X, Mei N. Aloe vera: A review of toxicity and adverse clinical effects. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2016;34:77–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Fu PP, Xia Q, Guo L, Yu H, Chan PC. Toxicity of kava kava. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2008;26:89–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Yoshikawa T, Naito Y, Kondo M. Ginkgo biloba leaf extract: review of biological actions and clinical applications. Antioxid Redox Signal. 1999;1:469–480. [DOI] [PubMed] [Google Scholar]
- [24].van Beek TA. Chemical analysis of Ginkgo biloba leaves and extracts. J Chromatogr A. 2002;967:21–55. [DOI] [PubMed] [Google Scholar]
- [25].US Pharmacopeial Convention. Powdered ginkgo extract. 2011. http://www.usp.org/sites/default/files/usp_pdf/EN/USPNF/revisions/m34982-powdered_ginkgo_extract.pdf (accessed July 20, 2016).
- [26].Ding S, Dudley E, Plummer S, Tang J, Newton RP, Brenton AG. Fingerprint profile of Ginkgo biloba nutritional supplements by LC/ESI-MS/MS. Phytochemistry. 2008;69:1555–1564. [DOI] [PubMed] [Google Scholar]
- [27].Ding S, Dudley E, Chen L, Plummer S, Tang J, Newton RP, Brenton AG. Determination of active components of Ginkgo biloba in human urine by capillary high-performance liquid chromatography/mass spectrometry with on-line column-switching purification. Rapid Commun Mass Spectrom. 2006;20:3619–3624. [DOI] [PubMed] [Google Scholar]
- [28].Kressmann S, Muller WE, Blume HH. Pharmaceutical quality of different Ginkgo biloba brands. J Pharm Pharmacol 2002;54:661–669. [DOI] [PubMed] [Google Scholar]
- [29].Ding S, Dudley E, Plummer S, Tang J, Newton RP, Brenton AG. Quantitative determination of major active components in Ginkgo biloba dietary supplements by liquid chromatography/mass spectrometry. Rapid Commun Mass Spectrom. 2006;20:2753–2760. [DOI] [PubMed] [Google Scholar]
- [30].Gawron-Gzella A, Marek P, Chanaj J, Matlawska I. Comparative analysis of pharmaceuticals and dietary supplements containing extracts from the leaves of Ginkgo biloba L. Acta Pol Pharm. 2010;67:335–343. [PubMed] [Google Scholar]
- [31].Fransen HP, Pelgrom SM, Stewart-Knox B, de Kaste D, Verhagen H. Assessment of health claims, content, and safety of herbal supplements containing Ginkgo biloba. Food Nutr Res. 2010;54:1–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Kleijnen J, Knipschild P. Ginkgo biloba. Lancet. 1992;340:1136–1139. [DOI] [PubMed] [Google Scholar]
- [33].Kleijnen J, Knipschild P. Ginkgo biloba for cerebral insufficiency. Br J Clin Pharmacol. 1992;34:352–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Vellas B, Coley N, Ousset PJ, Berrut G, Dartigues JF, Dubois B, Grandjean H, Pasquier F, Piette F, Robert P, Touchon J, Garnier P, Mathiex-Fortunet H, Andrieu S, GuidAge Study G. Long-term use of standardised Ginkgo biloba extract for the prevention of Alzheimer’s disease (GuidAge): a randomised placebo-controlled trial. Lancet Neurol. 2012;11:851–859. [DOI] [PubMed] [Google Scholar]
- [35].Nicolai SP, Kruidenier LM, Bendermacher BL, Prins MH, Stokmans RA, Broos PP, Teijink JA. Ginkgo biloba for intermittent claudication. Cochrane Database Syst Rev. 2013;6:CD006888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Hilton MP, Zimmermann EF, Hunt WT. Ginkgo biloba for tinnitus. Cochrane Database Syst Rev. 2013;3:CD003852. [DOI] [PubMed] [Google Scholar]
- [37].Evans JR. Ginkgo biloba extract for age-related macular degeneration. Cochrane Database SystRev. 2013;1:CD001775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Birks J, Grimley Evans J. Ginkgo biloba for cognitive impairment and dementia. Cochrane Database Syst Rev. 2009;1:CD003120. [DOI] [PubMed] [Google Scholar]
- [39].Zeng X, Liu M, Yang Y, Li Y, Asplund K. Ginkgo biloba for acute ischaemic stroke. Cochrane Database SystRev. 2005;4:CD003691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].DeKosky ST, Williamson JD, Fitzpatrick AL, Kronmal RA, Ives DG, Saxton JA, Lopez OL, Burke G, Carlson MC, Fried LP, Kuller LH, Robbins JA, Tracy RP, Woolard NF, Dunn L, Snitz BE, Nahin RL, Furberg CD, Ginkgo Evaluation of Memory Study I. Ginkgo biloba for prevention of dementia: a randomized controlled trial. JAMA. 2008;300:2253–2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Snitz BE, O’Meara ES, Carlson MC, Arnold AM, Ives DG, Rapp SR, Saxton J, Lopez OL, Dunn LO, Sink KM, DeKosky ST, Ginkgo Evaluation of Memory Study I. Ginkgo biloba for preventing cognitive decline in older adults: a randomized trial. JAMA. 2009;302:2663–2670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Brinkley TE, Lovato JF, Arnold AM, Furberg CD, Kuller LH, Burke GL, Nahin RL, Lopez OL, Yasar S, Williamson JD. Effect of Ginkgo biloba on blood pressure and incidence of hypertension in elderly men and women. Am J Hypertens. 2010;23:528–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Kuller LH, Ives DG, Fitzpatrick AL, Carlson MC, Mercado C, Lopez OL, Burke GL, Furberg CD, DeKosky ST, Ginkgo Evaluation of Memory Study I. Does Ginkgo biloba reduce the risk of cardiovascular events? Circ Cardiovasc Qual Outcomes. 2010;3:41–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Biggs ML, Sorkin BC, Nahin RL, Kuller LH, Fitzpatrick AL. Ginkgo biloba and risk of cancer: secondary analysis of the Ginkgo Evaluation of Memory (GEM) Study. Pharmacoepidemiol Drug Saf. 2010;19:694–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Chen X, Hong Y, Zheng P. Efficacy and safety of extract of Ginkgo biloba as an adjunct therapy in chronic schizophrenia: A systematic review of randomized, double-blind, placebo-controlled studies with meta-analysis. Psychiatry Res. 2015;228:121–127. [DOI] [PubMed] [Google Scholar]
- [46].Di Lorenzo C, Ceschi A, Kupferschmidt H, Lude S, De Souza Nascimento E, Dos Santos A, Colombo F, Frigerio G, Norby K, Plumb J, Finglas P, Restani P. Adverse effects of plant food supplements and botanical preparations: a systematic review with critical evaluation of causality. Br J Clin Pharmacol. 2015;79:578–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Wada K, Ishigaki S, Ueda K, Take Y, Sasaki K, Sakata M, Haga M. Studies on the constitution of edible and medicinal plants. I. Isolation and identification of 4-O-methylpyridoxine, toxic principle from the seed of Ginkgo biloba L. Chem Pharm Bull (Tokyo). 1988;36:1779–1782. [DOI] [PubMed] [Google Scholar]
- [48].Wada K Ginkgo seed food poisoning. Chudoku Kenkyu. 2005;18:11–16. [PubMed] [Google Scholar]
- [49].Miwa H, Iijima M, Tanaka S, Mizuno Y. Generalized convulsions after consuming a large amount of gingko nuts. Epilepsia. 2001;42:280–281. [PubMed] [Google Scholar]
- [50].Hasegawa S, Oda Y, Ichiyama T, Hori Y, Furukawa S. Ginkgo nut intoxication in a 2-source-old male. Pediatr Neurol. 2006;35:275–276. [DOI] [PubMed] [Google Scholar]
- [51].Hori Y, Fujisawa M, Shimada K, Oda A, Katsuyama S, Wada K. Rapid analysis of 4-O-methylpyridoxine in the serum of patients with Ginkgo biloba seed poisoning by ion-pair high-performance liquid chromatography. Biol Pharm Bull. 2004;27:486–491. [DOI] [PubMed] [Google Scholar]
- [52].Wada K, Ishigaki S, Ueda K, Sakata M, Haga M. An antivitamin B6,4’-methoxypyridoxine, from the seed of Ginkgo biloba L. Chem Pharm Bull (Tokyo). 1985;33:3555–3557. [DOI] [PubMed] [Google Scholar]
- [53].Yoshimura T, Udaka N, Morita J, Jinyu Z, Sasaki K, Kobayashi D, Wada K. High performance liquid chromatographic determination of ginkgotoxin and ginkgotoxin-5’-glucoside in Ginkgo biloba seeds. JLiq Chromatogr Relat Technol. 2006;29:605–616. [Google Scholar]
- [54].Kobayashi D, Yoshimura T, Johno A, Sasaki K, Wada K. Toxicity of 4’-O-methylpyridoxine-5’-glucoside in Ginkgo biloba seedstion. Food Chemistry. 2011;126:1198–1202. [Google Scholar]
- [55].Mizuno N, Kawakami K, Morita E. Competitive inhibition between 4’-substituted pyridoxine analogues and pyridoxal for pyridoxal kinase from mouse brain. J Nutr Sci Vitaminol (Tokyo). 1980;26:535–543. [DOI] [PubMed] [Google Scholar]
- [56].Kastner U, Hallmen C, Wiese M, Leistner E, Drewke C. The human pyridoxal kinase, a plausible target for ginkgotoxin from Ginkgo biloba. FEBS J. 2007;274:1036–1045. [DOI] [PubMed] [Google Scholar]
- [57].Leistner E, Drewke C. Ginkgo biloba and ginkgotoxin. J Nat Prod. 2010;73:86–92. [DOI] [PubMed] [Google Scholar]
- [58].Kobayashi D, Yoshimura T, Johno A, Ishikawa M, Sasaki K, Wada K. Decrease in pyridoxal-5’-phosphate concentration and increase in pyridoxal concentration in rat plasma by 4’-O-methylpyridoxine administration. Nutr Res. 2015;35:637–642. [DOI] [PubMed] [Google Scholar]
- [59].Di Lorenzo C, Ceschi A, Colombo F, Frigerio G, Bianchetti MG, Lude S, von Dechend M, Valoti E, Restani P. Identification and quantification of biomarkers to confirm the poisoning by Ginkgo biloba seeds in a 2-source-old boy. Toxicol Res. 2015;4:922–930. [Google Scholar]
- [60].Kudo K Does the ginkgo seed contain large amounts of cyanogenetic glycosides? Tokyo Iji Shinshi. 1881;149:19–21. [Google Scholar]
- [61].Kajiyama Y, Fujii K, Takeuchi H, Manabe Y. Ginkgo seed poisoning. Pediatrics. 2002;109:325–327. [DOI] [PubMed] [Google Scholar]
- [62].Jang HS, Roh SY, Jeong EH, Kim BS, Sunwoo MK. Ginkgotoxin induced seizure caused by vitamin B6 deficiency. JEpilepsyRes. 2015;5:104–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Arenz A, Klein M, Fiehe K, Gross J, Drewke C, Hemscheidt T, Leistner E. Occurrence of neurotoxic 4’-O-methylpyridoxine in Ginkgo biloba leaves, Ginkgo medications and Japanese Ginkgo food. Planta Med. 1996;62:548–551. [DOI] [PubMed] [Google Scholar]
- [64].Liu Y, Chen SN, McAlpine JB, Klein LL, Friesen JB, Lankin DC, Pauli GF. Quantification of a botanical negative marker without an identical standard: ginkgotoxin in Ginkgo biloba. J Nat Prod. 2014;77:611–617. [DOI] [PubMed] [Google Scholar]
- [65].Gregory PJ. Seizure associated with Ginkgo biloba? Ann Intern Med. 2001;134:344. [DOI] [PubMed] [Google Scholar]
- [66].Granger AS. Ginkgo biloba precipitating epileptic seizures. Age Ageing. 2001;30:523–525. [DOI] [PubMed] [Google Scholar]
- [67].Kupiec T, Raj V. Fatal seizures due to potential herb-drug interactions with Ginkgo biloba. JAnal Toxicol 2005;29:755–758. [DOI] [PubMed] [Google Scholar]
- [68].Samuels N, Finkelstein Y, Singer SR, Oberbaum M. Herbal medicine and epilepsy: proconvulsive effects and interactions with antiepileptic drugs. Epilepsia. 2008;49:373–380. [DOI] [PubMed] [Google Scholar]
- [69].Yin OQ, Tomlinson B, Waye MM, Chow AH, Chow MS. Pharmacogenetics and herbdrug interactions: experience with Ginkgo biloba and omeprazole. Pharmacogenetics. 2004;14:841–850. [DOI] [PubMed] [Google Scholar]
- [70].Uchida S, Yamada H, Li XD, Maruyama S, Ohmori Y, Oki T, Watanabe H, Umegaki K, Ohashi K, Yamada S. Effects of Ginkgo biloba extract on pharmacokinetics and pharmacodynamics of tolbutamide and midazolam in healthy volunteers. J Clin Pharmacol. 2006;46:1290–1298. [DOI] [PubMed] [Google Scholar]
- [71].Shi S, Klotz U. Drug interactions with herbal medicines. Clin Pharmacokinet. 2012;51:77–104. [DOI] [PubMed] [Google Scholar]
- [72].Hauser D, Gayowski T, Singh N. Bleeding complications precipitated by unrecognized Gingko biloba use after liver transplantation. Transpl Int. 2002;15:377–379. [DOI] [PubMed] [Google Scholar]
- [73].Bent S, Goldberg H, Padula A, Avins AL. Spontaneous bleeding associated with ginkgo biloba: a case report and systematic review of the literature: a case report and systematic review of the literature. J Gen Intern Med. 2005;20:657–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Bone KM. Potential interaction of Ginkgo biloba leaf with antiplatelet or anticoagulant drugs: what is the evidence? Mol Nutr Food Res. 2008;52:764–771. [DOI] [PubMed] [Google Scholar]
- [75].Kellermann AJ, Kloft C. Is there a risk of bleeding associated with standardized Ginkgo biloba extract therapy? A systematic review and meta-analysis. Pharmacotherapy. 2011;31:490–502. [DOI] [PubMed] [Google Scholar]
- [76].Jiang X, Williams KM, Liauw WS, Ammit AJ, Roufogalis BD, Duke CC, Day RO, McLachlan AJ. Effect of ginkgo and ginger on the pharmacokinetics and pharmacodynamics of warfarin in healthy subjects. Br J Clin Pharmacol. 2005;59:425–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Pedroso JL, Henriques Aquino CC, Escorcio Bezerra ML, Baiense RF, Suarez MM, Dutra LA, Braga-Neto P, Povoas Barsottini OG. Ginkgo biloba and cerebral bleeding: a case report and critical review. Neurologist. 2011;17:89–90. [DOI] [PubMed] [Google Scholar]
- [78].Carlile PV. Unexplained alveolar hemorrhage associated with Ginkgo and ginseng use. JBronchology Interv Pulmonol. 2015;22:170–172. [DOI] [PubMed] [Google Scholar]
- [79].Stoddard GJ, Archer M, Shane-McWhorter L, Bray BE, Redd DF, Proulx J, Zeng-Treitler Q. Ginkgo and Warfarin Interaction in a Large Veterans Administration Population. AMIA Annu SympProc. 2015;2015:1174–1183. [PMC free article] [PubMed] [Google Scholar]
- [80].Cianfrocca C, Pelliccia F, Auriti A, Santini M. Ginkgo biloba-induced frequent ventricular arrhythmia. Ital Heart J. 2002;3:689–691. [PubMed] [Google Scholar]
- [81].Pennisi RS. Acute generalised exanthematous pustulosis induced by the herbal remedy Ginkgo biloba. Med J Aust. 2006;184:583–584. [DOI] [PubMed] [Google Scholar]
- [82].Dodge HH, Zitzelberger T, Oken BS, Howieson D, Kaye J. A randomized placebocontrolled trial of Ginkgo biloba for the prevention of cognitive decline. Neurology. 2008;70:1809–1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Russo V, Rago A, Russo GM, Calabro R, Nigro G. Ginkgo biloba: an ancient tree with new arrhythmic side effects. J Postgrad Med. 2011;57:221. [DOI] [PubMed] [Google Scholar]
- [84].Hotta E, Tamagawa-Mineoka R, Katoh N. Allergic contact dermatitis due to ginkgo tree fruit and leaf. Eur J Dermatol. 2013;23:548–549. [DOI] [PubMed] [Google Scholar]
- [85].Dugoua JJ, Mills E, Perri D, Koren G. Safety and efficacy of ginkgo (Ginkgo biloba) during pregnancy and lactation. Can J Clin Pharmacol. 2006;13:e277–284. [PubMed] [Google Scholar]
- [86].Luijten M, Olthof ED, Hakkert BC, Rorije E, van der Laan JW, Woutersen RA, van Benthem J. An integrative test strategy for cancer hazard identification. Crit Rev Toxicol. 2016;46:615–639. [DOI] [PubMed] [Google Scholar]
- [87].Rider CV, Nyska A, Cora MC, Kissling GE, Smith C, Travlos GS, Hejtmancik MR, Fomby LM, Colleton CA, Ryan MJ, Kooistra L, Morrison JP, Chan PC. Toxicity and carcinogenicity studies of ginkgo biloba extract in rat and mouse: liver, thyroid, and nose are targets. Toxicol Pathol. 2013;42:830–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Guo L, Mei N, Liao W, Chan PC, Fu PP. Ginkgo biloba extract induces gene expression changes in xenobiotics metabolism and the Myc-centered network. OMICS. 2010;14:75–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Hoenerhoff MJ, Pandiri AR, Snyder SA, Hong HH, Ton TV, Peddada S, Shockley K, Witt K, Chan P, RiderC, KooistraL,NyskaA, Sills RC. Hepatocellular carcinomas inB6C3F1 mice treated with Ginkgo biloba extract for two sources differ from spontaneous liver tumors in cancer gene mutations and genomic pathways. Toxicol Pathol. 2013;41:826–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Al-Yahya AA, Al-Majed AA, Al-Bekairi AM, Al-Shabanah OA, Qureshi S. Studies on the reproductive, cytological and biochemical toxicity of Ginkgo Biloba in Swiss albino mice. J Ethnopharmacol. 2006;107:222–228. [DOI] [PubMed] [Google Scholar]
- [91].Koch E, Noldner M, Leuschner J. Reproductive and developmental toxicity of the Ginkgo biloba special extract EGb 761(R) in mice. Phytomedicine. 2013;21:90–97. [DOI] [PubMed] [Google Scholar]
- [92].Fernandes ES, Pinto RM, de Paula Reis JE, de Oliveira Guerra M, Peters VM. Effects of Ginkgo biloba extract on the embryo-fetal development in Wistar rats. Birth Defects Res B Dev Reprod Toxicol. 2010;89:133–138. [DOI] [PubMed] [Google Scholar]
- [93].Pinto RM, Fernandes ES, Reis JE, Peters VM, Guerra Mde O. Intra-uterine growth retardation after prenatal administration of Ginkgo biloba to rats. Reprod Toxicol. 2007;23:480–485. [DOI] [PubMed] [Google Scholar]
- [94].Zehra U, Tahir M, Lone KP. Ginkgo biloba induced malformations in mice. J Coll Physicians SurgPak. 2010;20:117–121. [PubMed] [Google Scholar]
- [95].Bezerra JC, Rocha ERS, Teixeira JR, Bellei PM, Sa RC, Reis JE, Peters VM, Guerra MO. Effect of Ginkgo biloba extract on F1 generation of male Wistar rats during fetogenesis. JMed Plant Res. 2015;9:1081–1088. [Google Scholar]
- [96].de Faria DE, Borges LV, Peters VM, Reis JE, Ribeiro LC, de Cassia da Silveira ESR, Guerra Mde O. Postnatal development of pups from nursing rats treated with Gingko biloba. PhytotherRes. 2008;22:185–189. [DOI] [PubMed] [Google Scholar]
- [97].Shiao NH, Chan WH. Injury effects of ginkgolide B on maturation of mouse oocytes, fertilization, and fetal development in vitro and in vivo. Toxicol Lett. 2009;188:63–69. [DOI] [PubMed] [Google Scholar]
- [98].Koch E, Jaggy H, Chatterjee SS. Evidence for immunotoxic effects of crude Ginkgo biloba L. leaf extracts using the popliteal lymph node assay in the mouse. Int J Immunopharmacol. 2000;22:229–236. [DOI] [PubMed] [Google Scholar]
- [99].Tada Y, Kagota S, Kubota Y, Nejime N, Nakamura K, Kunitomo M, Shinozuka K. Longterm feeding of Ginkgo biloba extract impairs peripheral circulation and hepatic function in aged spontaneously hypertensive rats. Biol Pharm Bull. 2008;31:68–72. [DOI] [PubMed] [Google Scholar]
- [100].Miman MC, Ozturan O, Iraz M, Erdem T, Olmez E. Amikacin ototoxicity enhanced by Ginkgo biloba extract (EGb 761). Hear Res. 2002;169:121–129. [DOI] [PubMed] [Google Scholar]
- [101].National Toxicology Program. Toxicology and carcinogenesis studies of quercetin (CAS No. 117–39-5) in F344 rats (feed studies). Natl Toxicol Program Tech Rep Ser. 1992;409:1–171. [PubMed] [Google Scholar]
- [102].Choi EJ, Kim GH. Quercetin accumulation by chronic administration causes the caspase-3 activation in liver and brain of mice. Biofactors. 2010;36:216–221. [DOI] [PubMed] [Google Scholar]
- [103].Wang CZ, Yuan JJ, Li WJ, Zhang HY, Ye JZ. In vivo and in vitro toxicity evaluation of polyprenols extracted from Ginkgo biloba L. leaves. Molecules. 2015;20:22257–22271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Chao JC, Chu CC. Effects of Ginkgo biloba extract on cell proliferation and cytotoxicity in human hepatocellular carcinoma cells. World J Gastroenterol. 2004;10:37–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Kim KS, Rhee KH, Yoon JH, Lee JG, Lee JH, Yoo JB. Ginkgo biloba extract (EGb 761) induces apoptosis by the activation of caspase-3 in oral cavity cancer cells. Oral Oncol. 2005;41:383–389. [DOI] [PubMed] [Google Scholar]
- [106].Babich H, Ackerman NJ, Burekhovich F, Zuckerbraun HL, Schuck AG. Gingko biloba leaf extract induces oxidative stress in carcinoma HSC-2 cells. Toxicol In Vitro. 2009;23:992–999. [DOI] [PubMed] [Google Scholar]
- [107].He J, Lin J, Li J, Zhang JH, Sun XM, Zeng CM. Dual effects of Ginkgo biloba leaf extract on human red blood cells. Basic Clin Pharmacol Toxicol. 2009;104:138–144. [DOI] [PubMed] [Google Scholar]
- [108].Lin H, Guo X, Zhang S, Dial SL, Guo L, Manjanatha MG, Moore MM, Mei N. Mechanistic evaluation of Ginkgo biloba leaf extract-induced genotoxicity in L5178Y cells. Toxicol Sci. 2014;139:338–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Zhang Z, Chen S, Mei H, Xuan J, Guo X, Couch L, Dobrovolsky VN, Guo L, Mei N. Ginkgo biloba leaf extract induces DNA damage by inhibiting topoisomerase II activity in human hepatic cells. Sci Rep. 2015;5:14633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Choi EJ, Bae SM, Ahn WS. Antiproliferative effects of quercetin through cell cycle arrest and apoptosis in human breast cancer MDA-MB-453 cells. Arch Pharm Res. 2008;31:1281–1285. [DOI] [PubMed] [Google Scholar]
- [111].BjeldanesLF Chang GW. Mutagenic activity of quercetin and related compounds. Science. 1977;197:577–578. [DOI] [PubMed] [Google Scholar]
- [112].MacGregor JT, Jurd L. Mutagenicity of plant flavonoids: structural requirements for mutagenic activity in Salmonella typhimurium. Mutat Res. 1978;54:297–309. [DOI] [PubMed] [Google Scholar]
- [113].Duthie SJ, Johnson W, Dobson VL. The effect of dietary flavonoids on DNA damage (strand breaks and oxidised pyrimdines) and growth in human cells. Mutat Res. 1997;390:141–151. [DOI] [PubMed] [Google Scholar]
- [114].Caria H, Chaveca T, Laires A, Rueff J. Genotoxicity of quercetin in the micronucleus assay in mouse bone marrow erythrocytes, human lymphocytes, V79 cell line and identification of kinetochore-containing (CREST staining) micronuclei in human lymphocytes. Mutat Res. 1995;343:85–94. [DOI] [PubMed] [Google Scholar]
- [115].Carver JH, Carrano AV, MacGregor JT. Genetic effects of the flavonols quercetin, kaempferol, and galangin on Chinese hamster ovary cells in vitro. Mutat Res. 1983;113:45–60. [DOI] [PubMed] [Google Scholar]
- [116].Silva ID, Rodrigues AS, Gaspar J,Maia R, Laires A, Rueff J. Involvement of rat cytochrome 1A1 in the biotransformation of kaempferol to quercetin: relevance to the genotoxicity of kaempferol. Mutagenesis. 1997;12:383–390. [DOI] [PubMed] [Google Scholar]
- [117].Rusak G, Piantanida I, Masic L, Kapuralin K, Durgo K, Kopjar N. Spectrophotometric analysis of flavonoid-DNA interactions and DNA damaging/protecting and cytotoxic potential of flavonoids in human peripheral blood lymphocytes. Chem Biol Interact. 2010;188:181–189. [DOI] [PubMed] [Google Scholar]
- [118].Kang JW, Kim JH, Song K, Kim SH, Yoon JH, Kim KS. Kaempferol and quercetin, components of Ginkgo biloba extract (EGb 761), induce caspase-3-dependent apoptosis in oral cavity cancer cells. Phytother Res. 2010;24 Suppl 1:S77–82. [DOI] [PubMed] [Google Scholar]
- [119].Chan WH. Ginkgolides induce apoptosis and decrease cell numbers in mouse blastocysts. Biochem Biophys Res Commun. 2005;338:1263–1267. [DOI] [PubMed] [Google Scholar]
- [120].Thompson AJ, McGonigle I, Duke R, Johnston GA, Lummis SC. A single amino acid determines the toxicity of Ginkgo biloba extracts. FASEB J. 2012;26:1884–1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Su Y, Sun CM, Chuang HH, Chang PT. Studies on the cytotoxic mechanisms of ginkgetin in a human ovarian adenocarcinoma cell line. Naunyn Schmiedebergs Arch Pharmacol. 2000;362:82–90. [DOI] [PubMed] [Google Scholar]
- [122].Siegers CP. Cytotoxicity of alkylphenols from Ginkgo biloba. Phytomedicine. 1999;6:281–283. [DOI] [PubMed] [Google Scholar]
- [123].Baron-Ruppert G, Luepke NP. Evidence for toxic effects of alkylphenols from Ginkgo biloba in the hen’s egg test (HET). Phytomedicine. 2001;8:133–138. [DOI] [PubMed] [Google Scholar]
- [124].Berg K, Braun C, Krug I, Schrenk D. Evaluation of the cytotoxic and mutagenic potential of three ginkgolic acids. Toxicology. 2015;327:47–52. [DOI] [PubMed] [Google Scholar]
- [125].Hecker H, Johannisson R, Koch E, Siegers CP. In vitro evaluation of the cytotoxic potential of alkylphenols from Ginkgo biloba L. Toxicology. 2002;177:167–177. [DOI] [PubMed] [Google Scholar]
- [126].Westendorf J, Regan J. Induction of DNA strand-breaks in primary rat hepatocytes by ginkgolic acids. Pharmazie. 2000;55:864–865. [PubMed] [Google Scholar]
- [127].Ahlemeyer B, Selke D, Schaper C, Klumpp S, Krieglstein J. Ginkgolic acids induce neuronal death and activate protein phosphatase type-2C. Eur J Pharmacol. 2001;430:1–7. [DOI] [PubMed] [Google Scholar]
- [128].Fu PP, Xia Q, Zhao Y, Wang S, Yu H, Chiang HM. Phototoxicity of herbal plants and herbal products. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2013;31:213–255. [DOI] [PubMed] [Google Scholar]
- [129].Cohen PA. American roulette—contaminated dietary supplements. N Engl J Med. 2009;361:1523–1525. [DOI] [PubMed] [Google Scholar]
- [130].Smith T, Lynch ME, Johnson J, Kawa K, Bauman H, Blumenthal M. Herbal dietary supplement sales in US increase 6.8% in 2014. HerbalGram. 2015;107:52–59. [Google Scholar]
- [131].World Health Organization. WHO traditional medicine strategy 2014–2023. 2013. http//apps.who.int/iris/bitstream/10665/92455/1/9789241506090_eng.pdf?ua=1, (accessed July 20, 2016).
- [132].Ning B, Su Z, Mei N, Hong H, Deng H, Shi L, Fuscoe JC, Tolleson WH. Toxicogenomics and cancer susceptibility: advances with next-generation sequencing. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2014;32:121–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Ceccaroli C, Pulliero A, Geretto M, Izzotti A. Molecular fingerprints of environmental carcinogens in human cancer. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2015;33:188–228. [DOI] [PubMed] [Google Scholar]
- [134].Gafner S Ginkgo extract aduleration in the global market: A brief review. HerbalGram. 2016;109:58–59. [Google Scholar]
- [135].Harnly JM, Luthria D, Chen P. Detection of adulterated Ginkgo biloba supplements using chromatographic and spectral fingerprints. JAOAC Int. 2012;95:1579–1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Ma YC, Mani A, Cai Y, Thomson J, Ma J, Peudru F, Chen S, Luo M, Zhang J, Chapman RG, Shi ZT. An effective identification and quantification method for Ginkgo biloba flavonol glycosides with targeted evaluation of adulterated products. Phytomedicine. 2016;23:377–387. [DOI] [PubMed] [Google Scholar]
- [137].Ruan J, Gao H, Li N, Xue J, Chen J, Ke C, Ye Y, Fu PP, Zheng J, Wang J, Lin G. Blood Pyrrole-Protein Adducts-A Biomarker of Pyrrolizidine Alkaloid-Induced Liver Injury in Humans. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2015;33:404–421. [DOI] [PubMed] [Google Scholar]
- [138].Posadzki P, Watson L, Ernst E. Herb-drug interactions: an overview of systematic reviews. Br J Clin Pharmacol. 2013;75:603–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Hu Z, Yang X, Ho PC, Chan SY, Heng PW, Chan E, Duan W, Koh HL, Zhou S. Herb-drug interactions: a literature review. Drugs. 2005;65:1239–1282. [DOI] [PubMed] [Google Scholar]
- [140].Izzo AA, Ernst E. Interactions between herbal medicines and prescribed drugs: a systematic review. Drugs. 2001;61:2163–2175. [DOI] [PubMed] [Google Scholar]


