Abstract
Like virtually all age-related chronic diseases, late-onset Alzheimer’s disease (AD) develops over an extended preclinical period and is associated with modifiable lifestyle and environmental factors. We hypothesize that multimodal interventions that address many risk factors simultaneously and are individually tailored to patients may help reduce AD risk. We describe a novel clinical methodology used to evaluate and treat patients at two Alzheimer’s Prevention Clinics. The framework applies evidence-based principles of clinical precision medicine to tailor individualized recommendations, follow patients longitudinally to continually refine the interventions, and evaluate N-of-1 effectiveness (trial registered at ClinicalTrials.gov NCT03687710). Prior preliminary results suggest that the clinical practice of AD risk reduction is feasible, with measurable improvements in cognition and biomarkers of AD risk. We propose using these early findings as a foundation to evaluate the comparative effectiveness of personalized risk management within an international network of clinician researchers in a cohort study possibly leading to a randomized controlled trial.
Keywords: Alzheimer’s disease prevention, Clinical precision medicine, Alzheimer’s precision medicine, Multidomain interventions, Alzheimer’s Prevention Clinic, Personalized medicine, APOE, Preclinical Alzheimer’s disease
1. Introduction
Alzheimer’s disease (AD) is the most common form of dementia and the sixth leading cause of death in Western societies, presenting a significant public health challenge [1]. It is now recognized that late-onset AD begins decades before a diagnosis of dementia, with a long prodromal phase often beginning in midlife [2]. The earliest part of this prodromal phase is called preclinical AD and involves no observable cognitive symptoms but offers a large window of opportunity for early intervention [3,4].
Evolving evidence has helped define target age groups for implementing risk reduction interventions for AD [5,6]. Among people aged 85 years (an age at which more than 30% have developed dementia due to AD), brain pathology began between the ages of 55 and 65 years [7]. Similarly, in people aged 65 years (an age at which about 10% have developed dementia due to AD), brain pathology began between the ages of 35 and 45 years [7]. Thus, AD may be more aptly termed a younger and middle-aged persons’ disease. Early intervention is especially important as recent estimates have found that more than 46 million Americans currently have preclinical AD [8].
Considering recent setbacks in drug development, new approaches for early detection of AD and intervention geared toward prevention are necessary [9]. As such, over the last several years, it has become more common for health care providers to deliver direct clinical care in the subspecialty of AD risk reduction, with a number of clinics focusing on both risk assessment and early intervention [10]. Lifestyle and environmental interventions differ in terms of level of evidence for effectiveness, and published studies have used a variety of interventions. However, the general clinic approach is to recommend interventions that have minimal to no risk, along with empirical evidence of efficacy—without overpromising on the expected results (see Appendix A for more information).
In this article, we describe a clinical approach used since 2013 to evaluate and treat patients at risk for AD at the Alzheimer’s Prevention Clinic (APC) at Weill Cornell Medicine and NewYork-Presbyterian, and since 2016 at the Alzheimer’s Prevention Clinic and Research Center in San Juan, Puerto Rico.
With this approach, clinical care begins by evaluating AD risk and then providing a comprehensive plan toward risk reduction. Longitudinal follow-up occurs every 6 months to evaluate the N-of-1 effectiveness of the approach, while continually refining the precision interventions [10,11]. N-of-1 trial design considers the individual patient as the sole unit for observation, comparing the patient to himself or herself at baseline and then adjusting the management plan to achieve specific goals [12].
Our experience thus far suggests that patients will engage in outpatient risk reduction care and remain committed over an extended period of time. Therefore, the clinical practice of AD risk reduction can be a viable construct in medical practice. Preliminary analyses have also demonstrated measurable improvements in cognition and biomarkers of AD risk [13–17] with differential effects associated with a variety of factors such as patient compliance and genotype. Additional analyses are ongoing [13–15]. We propose using these early findings as a stepping stone to accomplish four key goals: (1) more rigorous study of the comparative effectiveness of personalized risk management to help improve quality of life and eventually reduce the global burden of disease; (2) establish a network of clinician researchers who can apply and continually refine this framework for AD preventive care; (3) support the design of a large multisite international study to validate clinical effectiveness; and (4) advocate for public and private funding to move health services research into the realm of precision medicine clinical trials.
2. Background
Several known modifiable factors are associated with increased risk for AD development, such as hypertension and physical inactivity [18,19]. In fact, findings from population-attributable risk models estimate that one in every three cases of AD may be related to modifiable risk factors [20]. Targeting modifiable AD risk factors [21] through a comprehensive approach incorporating exercise and nutrition counseling, micronutrient supplementation, and pharmacological treatment of conditions such as insulin resistance [22–26] and hypertension represents a practical method for potentially reducing AD risk [27]. Each of these categories of risk factors may influence pathological pathways leading to AD (e.g., amyloid burden, dysregulation of glucose metabolism, inflammation, oxidative stress, trophic factor release, calcium toxicity) and are consequently targeted [20,28]. Several randomized controlled trials (RCTs) [29–31] have provided persuasive evidence that lifestyle and dietary interventions can help people at risk for developing AD maintain cognitive function and potentially delay cognitive decline.
Of particular importance is the Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability (FINGER) [29]. This 2-year, multidomain randomized controlled trial found that a combined evidence-based program of a brain-healthy diet, exercise, cognitive training, and stimulating social activity, teamed with careful monitoring of vascular risk, helped improve or maintain cognitive function in an elderly cohort at risk for AD [29]. Response to this multidomain intervention proved beneficial regardless of participants’ baseline characteristics, which gives greater impetus for implementation across the general population at increased risk for dementia [32].
3. Intervention design
APC’s mission is to care for patients at risk for AD and provide personalized therapeutic interventions, based on individual risk factors, through clinical precision medicine. The National Institutes of Health defines precision medicine as “an emerging approach for disease treatment and prevention that takes into account individual variability in genes, environment, and lifestyle for each person” [33,34]. A term used for adaptation of this approach in an APC setting is clinical precision medicine, whereby the use of an expanded clinical history (e.g., neurodevelopment, academic trajectory, past and current lifestyle patterns, environmental exposures, and life course events) is combined with past medical history and physical/neurological examination and then interpreted in conjunction with anthropometrics, blood biomarkers (including genetics), and cognitive performance [16,17]. A multimodal management plan is then crafted by evaluating each point of data within the context of other data points and then following the patient longitudinally to evaluate the effectiveness of, and further refine, this clinical precision medicine intervention. We simultaneously consider multiple data points to maximize potential reliability of the medical decision-making process (while also evaluating potential synergistic effects or interactions between different risk factors). In addition, we examine overall patterns that are indicative of a specific pathological pathway and use that information to guide management. For example, if a single cholesterol marker is borderline elevated (e.g., low-density lipoprotein [LDL]) but not to the degree where outright intervention is required, we will rely on other data points (e.g., coronary calcium scan if available, advanced cholesterol markers such as LDL-P, and calculated cardiovascular risk scale profile) to decide whether to intervene, thus maintaining a comprehensive personalized approach informed by the totality of data.
4. Methodology
4.1. Initial evaluation
The initial APC visit includes an assessment carried out by a member of our clinical team who has extensive training in the practice of AD risk reduction, including a board-certified neurologist and/or family nurse practitioner in New York and a neuropsychologist and board-certified internist in Puerto Rico. Patients are given the option to consent to having their clinical data added to the APC Comparative Effectiveness Dementia & Alzheimer’s Registry (CEDAR), an Institutional review board–approved observational data repository (Weill Cornell Medicine Protocol #1408015423), and enroll in the trial registered at ClinicalTrials.gov (NCT03687710). The registry facilitates the longitudinal study of outcomes based on multimodal precision interventions. Extensive counseling and patient education are given in person by the treating clinician during the visit, with a focus on clinical recommendations and genetic counseling (see Appendix A for additional details on clinic visit structure and Table 1 for a comprehensive list of data points assessed and follow-up time points).
Table 1.
Clinical assessments and timelines
| Measure category | Data points | Frequency/duration |
|---|---|---|
| Behavioral assessment (patients reported via online previsit baseline survey; several validated scales; and other questionnaires) | •Medical history (including expanded education history and family history initially, along with past medical history, social history [e.g., alcohol, tobacco, ownership of weapons], review of systems, and allergies) •Review of systems and detailed concussion, cardiovascular, and learning disability questionnaire •Modified Global Cognitive Complaints scale (subjective cognitive complaints) •Rapid Assessment of Physical Activity (RAPA) •MIND diet questionnaire and Food Frequency questionnaire •PROMIS measures (e.g., sleep disturbance, anxiety, depression, alcohol use, social isolation and perceived stress scale) and Fear of Alzheimer’s scale •Cardiovascular risk scales, including the MultiEthnic Study of Atherosclerosis (MESA) and the American College of Cardiology/American Heart Association (ACC-AHA) Assessment of Cardiovascular Risk Scale) •Berlin Questionnaire (sleep) |
Every 6 months/35 min |
| Neuropsychological assessment (at home via AlzU.org) | Neurotrack, Cognitive Function Test, Face Name Associative Memory Test | Every 6 months/20–25 min |
| Clinical visit | Visit with care provider, clinical history, physical examination; generalized recommendations provided at new patient visit; recommendations refined based on laboratory/anthropometric/ neuropsychological measures at follow-up visits | Every 6 months/1.5 h baseline; 1 h follow-up |
| Anthropometrics (InBody) | Vital signs, height, weight, waist, hip, fat, BMI, lean dry mass, total body water, intracellular water, extracellular water, phase angle | Every 6 months |
| Laboratory blood biomarkers & genetics (Boston Heart Diagnostics or True Health Diagnostics) | APOE status, MTHFR (C677T & A1298C), lipids (total cholesterol, ldl-c, hdl-c, triglycerides, lipid ratios), ApoB, LDL-P, sdLDL-C, Lp(a), ApoA-I, fibrinogen, Lp-PLA2, hs-CRP, myeloperoxidase, HbAlc, HOMA-IR, glucose, GSP, adiponectin, insulin, C-peptide, NT-proBNP, homocysteine, TSH, estimated glomerular filtration rate, cystatin C, vitamin D, vitamin B12, RBC folate, sitosterol ratio, campesterol ratio, desmosterol ratio, fatty acids (saturated total, trans total, cis- monounsaturated total, unsaturated/saturated ratio index, omega-3, omega-3 EPA, omega-3 DHA, ALA, omega-6, linoleic, arachidonic, AA/EPA, omega-6/3) | Every 6 months |
| Neuropsychological assessment (in clinic) | •Paper-and-pencil tests: MMSE, FAS, ANT, Trails B, Boston Naming, Logical Memory •NIH Toolbox-Cognition Battery tests: RAVLT Auditory Verbal Learning (Trials 1–3), RAVLT Delayed Recall & Recognition, Dimensional Change Card Sort (DDCS), Flanker Inhibitory Control and Attention, Pattern Comparison Process Speed, Odor Identification, Oral Symbol Digit (OSD), Picture Vocabulary, Oral Reading Recognition |
Every 6 months/1.5 h |
| • Other Computer-based tests: CogState (Detection, Identification, One Card Learning, One Back Speed, One Back Accuracy), A4 Face Name Test, Neurotrack | ||
| Timeline | |
|---|---|
| Timepoint 1 (before visit) | Complete previsit baseline survey and online neuropsychological testing via AlzU before coming to the APC (1hr); complete two online educational modules on AlzU before coming to the APC (20 min) |
| Timepoint 2 (day 1) | Arrive to APC and undergo 1.5 h new patient visit, 1.5 hr baseline neuropsychological testing. New patient visit consists of ~45 minutes obtaining the clinical history and physical examination, followed by 45 minutes of one-on-one counseling and patient education about the variety of multimodal interventions suggested and detailed genetic counseling. |
| Timepoint 3 (day 2–4) | Return to APC for fasting laboratory and anthropometric assessment (30 min) [baseline neuropsychological testing may also occur at this time, instead]; complete additional lesson content covering a broad overview of relevant information on AD risk reduction and early intervention (including genetic testing considerations) on AlzU before next in-person visit (2 hours) |
| Timepoint 4 (month 1–2) | Return to APC for informing clinical visit (1 hr). Extensive discussion of the anthropometric, blood biomarker, and cognitive testing results occurs, combined with genetic counseling. Additional counseling about the precision medicine recommendations suggested is also provided. |
| Timepoint 5 (month 5) | Return to APC for follow-up laboratory, anthropometric, and neuropsychological assessments (2–2.5 h) |
| Timepoint 6 (month 6)* | Return to APC for follow-up clinical visit (1 h). Discussion regarding interim clinical history and data review, followed by one-on- one counseling by the treating clinician occurs (including discussion of compliance barriers, when present). |
Abbreviations: AD, Alzheimer’s disease; APC, Alzheimer’s Prevention Clinic.
Repeat every 6 months or as ordered by physician.
4.2. Clinical history and physical examination
The cornerstone of developing a comprehensive AD risk management plan is the patient’s clinical history, which is obtained at the initial clinical encounter (Appendix A, Table 2). This information provides the framework for the treatment plan and allows the clinician to target specific areas of concern (see Appendix A for more details).
Table 2.
Components of an Alzheimer’s disease prevention clinical history
| Area of focus | Data points |
|---|---|
| Educational trajectory | Birth place and high school attended, rank in high school, standardized test scores, college attended, major in college and GPA, graduate school and associated GPA. Career achievements throughout life |
| Dietary patterns | Red meat consumption (frequency and source), fish consumption (frequency and type), poultry (frequency), vegetables (frequency), berry consumption (frequency and type), sweets (frequency), dairy products (frequency and type), total carbohydrate intake (type and frequency), coffee intake (frequency), organic foods, olive oil consumption and type used, period of fasting between dinner and breakfast |
| Exercise patterns | Exercise frequency, type of exercise (cardiovascular vs. resistance training), duration of exercise, physical trainer guidance, exercise patterns in the past, sit for extended periods of time |
| Sleep | Number of hours per night, troubles initiating sleep, troubles staying asleep, any changes to sleep patterns, dreams and remembering dreams, vivid dreams (acting out or talking in sleep), change in the ability to remember dreams over time, snore or diagnosed with sleep apnea, sleep aids used (frequency and type), use of electronic devices in bed or before |
| Cognitive engagement activities | Stress reduction techniques (yoga, mindfulness based stress reduction, meditation, etc.), hobbies, speak a different language, play a musical instrument, listen to music (type), cognitive training activities |
| Other | Waist size in college compared with present waist size; changes in hearing, taste, or smell; constipation; skin disorders (e.g., dandruff); past head trauma; depression or anxiety in the past; frequency of dental visits. |
During the visit, clinical staff take the patient’s vital signs and perform a physical and focused neurological examination. Careful attention is paid to mildly elevated blood pressure, as prehypertension in midlife has been associated with increased dementia risk [35]. Focal deficits or asymmetries identified on examination may also suggest otherwise subclinical cerebrovascular pathology.
4.3. Clinical data
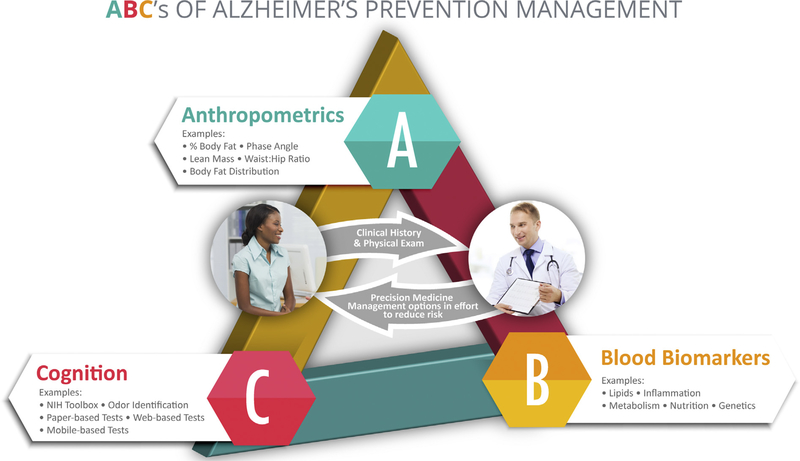
Clinical management decisions are evidence based and rely significantly on the “ABCs” of AD prevention (Fig. 1). This method allows for the stratification and consideration of key factors including (A) anthropometrics (e.g., % body fat, lean muscle mass, waist-to-hip ratio); (B) blood biomarkers (e.g., genetic analysis; lipid profile; inflammatory, metabolic, and nutritional biomarkers); and (C) cognition (via computer-based and traditional neuropsychological testing).
Fig. 1.
ABCs of Alzheimer’s Prevention Management.
These factors are used, in combination with clinical history, to more definitively assess risk and devise an initial intervention plan by implementing the emerging clinical precision medicine practice of “deep phenotyping” [36]. This approach provides clinicians with the knowledge needed to prioritize specific treatments and the requisite data to evaluate a patient’s progress over time in an N-of-1 fashion [10].
General intervention categories informed by the “ABCs” include targeted cardiovascular risk factor management, physical exercise, nutrition (dietary patterns and/or single nutrients or multinutrients), sleep, cognitive engagement, cognitive enhancement, social interaction, sense of purpose, stress management, oral hygiene, and ongoing care with a primary care physician, among other areas (such as clinical trials).
An essential strategy that provides the foundation for this multimodal treatment approach is to “triangulate” the interpretation of specific categories of data (clinical history, anthropometrics, blood biomarkers, genetics, cognition) within the context of other key data points. Management decisions are then made via interpretation of several subjective and objective measures across domains. For example, when blood biomarkers of AD risk are borderline, and cognitive function across domains is lower than expected based on norms and/or based on that individual’s level of crystallized intelligence, then the clinician may use cognitive performance as an indicator of whether to be more attentive to evidence-based low-risk modifiable risk factor interventions (and/or referral to a subspecialist physician) that would otherwise not have been considered. This novel approach of using cognitive measures to inform management decisions of clinical data (e.g., lipids) is similar to the approach in preventive cardiology, where a coronary calcium scan may be used to better stratify risk and intervene against asymptomatic cardiovascular disease [37] (see Appendix B for further discussion). To facilitate medical communication between the treating clinician and the patient, these data are discussed in person with the patient, as well as family members, when present. Clinical notes are also shared with the patients’ treating physicians.
From a practical clinical perspective, the concept that traditional reference ranges (usually defined as the set of values that 95 percent of the healthy population falls within) can be broadly applied in the management of AD risk reduction may not be well suited for optimal preventative care. It may be more prudent to instead rely on setting individual targets for each patient based on his or her overall constellation of risk, while considering a surrogate marker of end-organ function of the brain (e.g., performance on cognitive testing). Incorporating this concept into the development of the clinical management plan is further discussed in Appendix B.
AD diagnosis can be improved by the use of biomarkers, particularly in a research setting [38]. The wide array of biological measures of functional impairment, neuronal loss, and protein accumulation that may be assessed by brain imaging (e.g., magnetic resonance imaging with special attention to hippocampal volumes and regional atrophy; amyloid and/or fluorodeoxyglucose-positron emission tomography) and/or cerebral spinal fluid analysis are increasingly being used, particularly in research settings [39]. In clinical practice today, however, there are many barriers including cost, limited availability in some practice settings, invasive nature of the tests, and unclear applicability of test results to clinical management. In addition, the clinical usefulness of these biomarkers is not yet clearly established, resulting in limited reimbursement by insurance providers. In addition, considering the broad age range of APC patients thus far (age 27–86 years, mean 59.6), traditional AD biomarkers may be less applicable in our young and middle-aged patients.
5. Clinical precision medicine intervention
A successful AD risk-reduction program must include evidence-based interventions for which potential benefits are more likely to outweigh any potential risks. The general categories of therapies include patient education and counseling and pharmacologic (medications, vitamins, supplement) and nonpharmacologic approaches (see Appendix C for additional details).
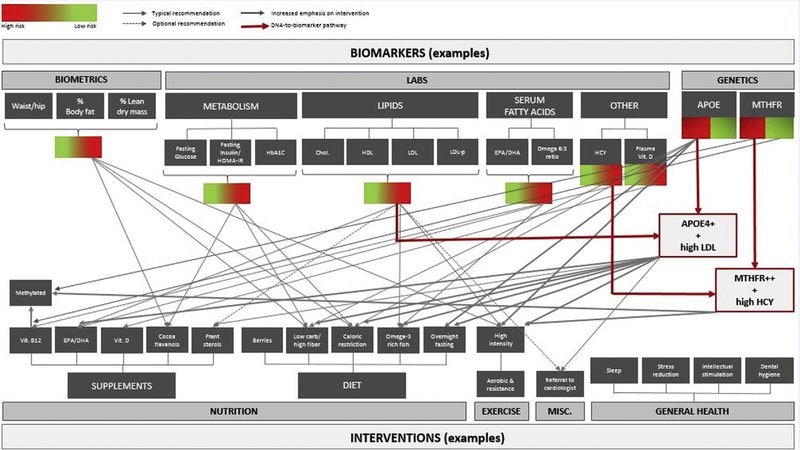
For example, a sedentary, postmenopausal 60-year-old woman (apolipoprotein E4 [APOE ε4/ε4] homozygote) with no subjective cognitive complaints and a past medical history of high cholesterol and abdominal obesity who is found to have elevated visceral body fat, insulin resistance, and normal (albeit below optimal) memory function will receive comprehensive recommendations. These may include patient education about the potential risks and benefits of long-term hormone replacement therapy, physical exercise counseling that includes a targeted amount and type of aerobic versus resistance training (geared for body-fat reduction), nutrition advice focusing on the Mediterranean-style dietary pattern (with special attention to extra-virgin olive oil and fatty fish consumption to address elevated LDL and low HDL-cholesterol) while limiting high-glycemic foods (based on insulin resistance) and supplementing with cocoa flavanols (considering insulin resistance and lower than expected memory performance), as well as a host of other detailed recommendations such as sleep hygiene, cognitive engagement strategies, stress management, ongoing care with her primary care physician (Fig. 2), and information on ongoing AD prevention clinical trials (e.g., Generation 1 and 2), which she may qualify for based on genotype and/or age [17,20,40–48]. An introductory course on AD prevention that has been shown to increase knowledge and willingness to participate in an AD prevention clinical trial will also be suggested via the online learning portal AlzU.org [49]. (See Appendix C for additional details on the precision approach). These recommendations are likely to differ from clinician to clinician in different practice settings and depending on availability of resources, yet it is essential to study the comparative effectiveness of these varied approaches and let the outcomes data inform future practice patterns.
Fig. 2.
Example biomarker: Intervention paradigm.
Although medical practice is both an art and science that cannot be confined to any one specific algorithm, the therapeutic interventions described here focus primarily on the most common recommendations given in our clinical practice [50]. Each recommendation in Appendix C lists which of the key categories of data (clinical history, anthropometrics, blood biomarkers, genetics, cognition) are considered when making a management decision. Gender considerations are also made (e.g., greater attention to anthropometric/serum metabolic risk markers and the APOE4 genotype in women, vs. greater attention to muscle mass in men), but a full discussion of these emerging data is beyond the scope of this article [40,51,52].
6. Challenges in the practice of AD risk reduction
Clinical practice in the field of AD risk reduction has not been without challenges. Determination of which objective measures to track over time required input from a team of multidisciplinary experts. For example, selection of cognitive instruments that would be sensitive to change in an asymptomatic cohort required extensive consultation with neuropsychologists and other clinicians, as well as initial experimentation in diverse patient groups. Traditional cognitive assessment methods and composite batteries utilized initially had ceiling effects that made it difficult to adequately evaluate response to therapy.
Incorporating the continually evolving evidence into daily practice, including disconfirming evidence, also poses challenges in medical decision-making. Although a detailed review is beyond the scope of this article, some studies have provided inconsistent data for the use of certain pharmacologic and nonpharmacologic interventions for improving brain health. For example, in the recent case of the Multidomain Alzheimer Preventive Trial (MAPT), the lack of positive findings may have been a result of factors such as study design, subjects recruited, outcomes measured, and specific interventions tested. Consideration of these factors is essential when deciding how to incorporate such findings into clinical practice [53,54]. Further studies are warranted to more accurately understand why these interventions failed; however, it seems prudent to focus on a younger population with precision medicine interventions of a longer duration (see Appendix D for additional discussion).
Another challenge is the lack of well-defined mechanisms for reimbursement of medical care related to preventative interventions, which can pose fiscal challenges. APC providers accept most major United States medical insurance plans and use the traditional evaluation and management (E/M) billing codes for visits that conform to the modifiable AD risk factors that clinicians treat. Notwithstanding, there are also a wide array of costs (e.g., time, money) to the patient, family members, health care providers, clinic, as well as health care system, including opportunity costs (which are difficult to measure and track). Recent estimates have found that early AD diagnosis may lead to $7 trillion in savings in the US alone, due to the long degenerative stage requiring extensive medical management [55,56]. This very large potential benefit would need to be weighed against the cost of broadly implementing risk-reducing interventions in a clinical setting before indiscriminate implementation.
Patient demand for risk reduction services may vary, depending on a variety of factors (e.g., practice setting, clinic location). For clinicians already practicing in the area of dementia care, a natural first step is to offer preventative services to family members at risk. Other outreach initiatives that initially generated interest included community lectures by clinic staff, hospital announcements (which led to referrals from other physicians), and postings on social media as well as traditional media. Referral sources are tracked, and the most common sources generally have included newspaper articles, physician referrals, community lectures, and “word of mouth.” Our public education clinical trial (www.AlzU.org, NCT03149380) includes links to several established clinics and has generated a steady source of interest, yet with more than 1,200,000 unique visits (from 56 countries) in the last few years, it has not been possible to accommodate in-person appointments for all those who have subsequently expressed interest. AlzU.org has helped to increase patient demand and increase willingness to participate in AD prevention clinical trials [49] (see Appendix E for additional discussion).
Given the likelihood of continued growth in AD prevention research in clinical settings, health care providers should be mindful of the ethical implications. Although the Risk Evaluation and Education for Alzheimer’s Disease (REVEAL) study demonstrated that APOE4 disclosure to adult children of AD patients did not result in significant short-term psychological risks, it remains important to use careful clinical judgment and counsel patients accordingly, before ordering genetic testing [57,58]. Clinician researchers should also weigh the potential risks and benefits of disclosing certain clinical data, such as cortical amyloid deposition [59,60]. Finally, issues of distributive justice should be considered to avoid disproportionately reallocating resources away from those already diagnosed with dementia due to AD to those who are currently in need of therapeutic advances.
7. Next steps and future directions
Accumulating evidence suggests that modifiable risk factors for AD can be addressed in an effort to delay onset [20,61,62]. However, to date, a comprehensive and feasible clinical framework for risk reduction and comparative effectiveness research for complex multimodal interventions has been lacking. The paradigm described in this article presents an evidence-based, structured and novel framework for risk assessment and early intervention that has been feasibly applied at two APC centers [17]. From a practical clinical perspective, this approach would be applicable to the tens of millions of patients worldwide at risk for, or already experiencing, the earliest pre-symptomatic (stage 1) and mildly symptomatic (stage 2) predementia phases of AD [8].
To date, our programs have enrolled more than 600 patients, and preliminary analyses demonstrate measurable benefits on a host of cognitive measures and blood biomarkers related to AD risk. Differential effects have also been found based on patient compliance and genotype [13–16]. Additional analyses are currently ongoing, and further study is warranted to determine which strategies, if any, are most effective. It also will be necessary to evaluate the most optimal study population in which to intervene. For example, although the exact critical period of intervention is still unclear, this framework has been applied by APC to asymptomatic patients ranging from their third to ninth decade of life in an effort to achieve both primary and secondary AD prevention.
Establishing a larger network of clinics will increase the number and diversity of patients studied, and an initial step toward building an international consortium of related programs is currently underway. This collaborative approach, which includes resource sharing, collegial mentorship, and ongoing peer-to-peer communication, will increase the likelihood for success. Harmonization of thevaried assessments, outcome measures, and evaluation time points will also help strengthen the validity of research results.
As the field evolves, clinician researchers should continue to learn from each other and continually modify their approaches. To accomplish this, rigorous data collection methods will be needed to cross-compare individual risk-reduction paradigms that may be practiced across different clinical specialties and locations, depending on available resources and/or subspecialty expertise. Collecting these data in a comparable way will allow fair comparisons to be made to assess outcomes of different strategies, allow for prioritization and paring down of measures formally assessed as the body of evidence grows, and enable deeper understanding of the predictability and repeatability of approaches.
Dissemination of successful risk-reduction approaches to a broad and diverse range of specialties (e.g., Internal Medicine, Family Medicine, Neurology, Psychiatry, Geriatric Psychiatry, Preventative Cardiology, Geriatrics) will provide additional incentive for patients to implement healthy lifestyle changes and help lay the groundwork for establishing this field as an area of medicine practiced across primary care and/or subspecialty practice. Building upon these experiences, and through continued collaborative efforts across medical specialties, the next logical step is to replicate AD risk reduction clinical practice in additional cohorts globally. It will be necessary for international advocacy initiatives to generate public and private support and funding for this realm of health services outcomes research, with the goal of conducting a broad-scale, multisite study powered to definitively evaluate clinical efficacy of the different types of comparative effectiveness intervention paradigms. Ultimately, the creation of a robust and well-characterized data set (both genotypically and phenotypically) will enable predictive analytics, involving traditional statistical learning and artificial neural networks, to help automate patient recommendations, improve access to care, and optimize clinician workflow [63,64]. Moreover, the practical application of clinical care augmented by predictive analytics will most likely fall within the continuum of entirely human-guided versus fully machine-guided patient care [64].
Similar to research efforts in the fields of cardiovascular disease and stroke prevention, it will soon be possible to determine whether risk assessment and early intervention using a clinical precision medicine approach can effectively mitigate AD risk and improve patient outcomes [65,66].
Supplementary Material
RESEARCH IN CONTEXT.
Systematic review: The authors reviewed ClinicalTrials.gov and WHO’s International Clinical Trial Registry Platform up to June 1, 2018, along with traditional sources such as PubMed, meeting abstracts, and presentations. While clinical precision medicine interventions to delay cognitive decline in patients at risk for AD have not been well studied, there have been several publications regarding a “one-size-fits-all” multidomain approach. These relevant citations are appropriately cited.
Interpretation: The application of evidence-based principles of clinical precision medicine to tailor individualized recommendations addressing AD risk factors is feasible in a clinical setting. Patients may be followed longitudinally to continually refine the interventions and evaluate the N-of-1 effectiveness.
Future directions: This manuscript provides a clinical framework toward AD risk reduction through an evidence-based, multidomain precision medicine approach. We propose using this approach to evaluate the comparative effectiveness of personalized risk management within an international network of clinician researchers.
Acknowledgments
The authors thank Dr. Juan Melendez, Dr. Neil Smith, Dr. Hannah Gardener, Dr. Tanja Rundek, Dr. Octavio Rodriguez, Dr. Islon Woolf, Dr. Arthur Agatston, Dr. Yakir Kaufman, Dr. Dharma Khalsa, Dr. Peilin Lu, Jack Hodes, Michael Woodbury, Nabeel Saif, Dr. Laurie Glimcher, and Dr. Matthew Fink for their support and/or contributions to the development of this methodology over time. The authors are also grateful to all the patients who have consented to donate their time and clinical data to our longitudinal registry. The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of the United States Department of Health and Human Services or any of its agencies.
Funding statement: Study funded by philanthropic support (Zuckerman Family Foundation, proceeds from the Annual Memories for Mary fundraiser organized by David and Kathy Twardock, the Annual Aces for Alzheimer’s fundraiser organized by Abby Owen and Jane Smoltz, the Rimora foundation, the Washkowitz Family in Memory of Alan Washkowitz, and contributions from grateful patients of the Alzheimer’s Prevention Clinic, Weill Cornell Memory Disorders Program), the Women’s Alzheimer’s Movement, Hilarity for Charity, the Weill Cornell Medical College Clinical and Translational Science Center (NIH/NCATS #UL1TR002384), and NIH PO1AG026572. The funders had no role in the study design, data collection or analysis, decision to publish, or preparation of the manuscript.
Footnotes
The authors have declared that no conflict of interest exists.
Data availability: The authors are happy to share all study-related materials, previsit/follow-up visit questionnaires, surveys, and other research tools (when possible); and data can be made available through a Data Use Agreement with Weill Cornell Medicine and NewYork-Presbyterian. A full CME/CE-accredited course for health care professionals based on the content of this article is also available for free at www.AlzU.org/HCP (6 credit hours).
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jalz.2018.08.004.
References
- [1].Alzheimer’s Disease: Facts and Figures. Alzheimer’s Association (online); 2017. Accessed online February 20th, 2018. [Google Scholar]
- [2].Frisoni GB, Winblad B, O’Brien JT. Revised NIA-AA criteria for the diagnosis of Alzheimer’s disease: a step forward but not yet ready for widespread clinical use. Int psychogeriatrics 2011;23:1191–6. [DOI] [PubMed] [Google Scholar]
- [3].McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Kawas CH, et al. The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s Demen 2011;7:263–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Mortimer JA, Borenstein AR, Gosche KM, Snowdon DA. Very early detection of Alzheimer neuropathology and the role of brain reserve in modifying its clinical expression. J Geriatr Psychiatry Neurol 2005;18:218–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Gonneaud J, Arenaza-Urquijo EM, Mézenge F, Landeau B, Gaubert M, Bejanin A, et al. Increased florbetapir binding in the temporal neocortex from age 20 to 60 years. Neurology 2017:10. [DOI] [PubMed] [Google Scholar]
- [6].Reiman EM, Chen K, Alexander GE, Caselli RJ, Bandy D, Osborne D, et al. Functional brain abnormalities in young adults at genetic risk for late-onset Alzheimer’s dementia. Proc Natl Acad Sci 2004;101:284–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Matura S, Fleckenstein J, Deichmann R, Engeroff T, Füzéki E, Hattingen E, et al. Effects of aerobic exercise on brain metabolism and grey matter volume in older adults: results of the randomised controlled SMART trial. Transl Psychiatry 2017;7:e1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Brookmeyer R, Abdalla N, Kawas CH, Corrada MM. Forecasting the prevalence of preclinical and clinical Alzheimer’s disease in the United States. Alzheimer’s Dement 2017;14:121–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Jobke B, McBride T, Nevin L, Peiperl L, Ross A, Stone C, et al. Setbacks in Alzheimer research demand new strategies, not surrender. PLoS Med 2018;15:e1002518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Isaacson R Is Alzheimer’s Prevention Possible Today? J Am Geriatr Soc 2017;65:2153–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Galvin JE. Prevention of Alzheimer’s Disease: Lessons Learned and Applied. J Am Geriatr Soc 2017;65:2128–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Lillie EO, Patay B, Diamant J, Issell B, Topol EJ, Schork NJ, et al. The n-of-1 clinical trial: the ultimate strategy for individualizing medicine? Personalized Med 2011;8:161–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Isaacson RS, Krikorian R, Hackett K, Shish C, Chen J, Melendez-Cabrero J, et al. , A Clinical Precision Medicine Approach Reduces Alzheimer’s, Dementia and Vascular Risk and Improves Cognition: A Prospective Cohort Study from the Alzheimer’s Prevention Clinic at Weill Cornell Medicine and NewYork-Presbyterian. The Lancet Neurology Conference, October 2016. [Google Scholar]
- [14].Isaacson RS, Krikorian R, Hackett K, Shish C, Chen J, Melendez-Cabrero J, et al. , A Clinical Precision Medicine Approach Reduces Alzheimer’s, Dementia and Vascular Risk and Improves Cognition: A Prospective Cohort Study from the Alzheimer’s Prevention Clinic at Weill Cornell Medicine and NewYork-Presbyterian. Clinical Trials in Alzheimer’s Disease meeting, December 2016. [Google Scholar]
- [15].Isaacson RS, Caesar E, Hackett K, Shish C, Hristov H, Melendez-Cabrero J, et al. , A Clinical Precision Medicine Approach Reduces Alzheimer’s, Dementia and Vascular Risk and Improves Cognition: A Prospective Cohort Study. American Academy of Neurology Annual Meeting, April 2017. [Google Scholar]
- [16].Schelke MW, Hackett K, Chen JL, Shih C, Shum J, Montgomery ME, et al. Nutritional interventions for Alzheimer’s prevention: a clinical precision medicine approach. Ann New York Acad Sci 2016; 1367:50–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Seifan A, Isaacson R. The Alzheimer’s prevention clinic at Weill Cornell Medical College/New York-Presbyterian Hospital: risk stratification and personalized early intervention. J Prev Alzheimers Dis 2015; 2:254–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Gottesman RF, Schneider AL, Zhou Y, Coresh J, Green E, Gupta N, et al. Association between midlife vascular risk factors and estimated brain amyloid deposition. JAMA 2017;317:1443–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Norton S, Matthews FE, Barnes DE, Yaffe K, Brayne K. Potential for primary prevention of Alzheimer’s disease: an analysis of populationbased data. Lancet Neurol 2014;13:788–94. [DOI] [PubMed] [Google Scholar]
- [20].Livingston G, Sommerlad A, Orgeta V, Costafreda SG, Huntley J, Ames D, et al. Dementia prevention, intervention, and care. Lancet 2017;390:2673–734. [DOI] [PubMed] [Google Scholar]
- [21].Kloppenborg RP, Van Den Berg E, Kappelle LJ, Biessels GJ. Diabetes and other vascular risk factors for dementia: which factor matters most? A systematic review. Eur J Pharmacol 2008;585:97–108. [DOI] [PubMed] [Google Scholar]
- [22].Craft S Insulin resistance and Alzheimer’s disease pathogenesis: potential mechanisms and implications for treatment. Curr Alzheimer Res 2007;4:147–52. [DOI] [PubMed] [Google Scholar]
- [23].Karimi M, Vedin I, Freund Levi Y, Basun H, Faxen Irving G, Eriksdotter M, et al. DHA-rich n-3 fatty acid supplementation decreases DNA methylation in blood leukocytes: the OmegAD study. Am J Clin Nutr 2017;106:1157–65. [DOI] [PubMed] [Google Scholar]
- [24].Llamas-Velasco S, Contador I, Villarejo-Galende A, Lora-Pablos D, Bermejo-Pareja F. Physical Activity as Protective Factor against Dementia: A Prospective Population-Based Study (NEDICES). J Int Neuropsychol Soc 2015;21:861–7. [DOI] [PubMed] [Google Scholar]
- [25].Schelke MW, Shapiro SD, Hackett K, Chen J, Simchon-Steinhof S, Ganzer CA, et al. Diagnosis of developmental learning and attention disorders in adults: A review of clinical modalities. Neurol Psychiatry Brain Res 2017;23:27–35. [Google Scholar]
- [26].Zilberter Y, Zilberter M. The vicious circle of hypometabolism in neurodegenerative diseases: Ways and mechanisms of metabolic correction. J Neurosci Res 2017;95:2217–35. [DOI] [PubMed] [Google Scholar]
- [27].National Academies of Sciences, Engineering, and Medicine, Preventing cognitive decline and dementia: A way forward 2017. Washington, DC: The National Academies Press; 2017. [PubMed] [Google Scholar]
- [28].Schelke MW, Attia P, Palenchar DJ, Kaplan B, Mureb M, Ganzer CA, et al. Mechanisms of Risk Reduction in the Clinical Practice of Alzheimer’s Disease Prevention. Front Aging Neurosci 2018;10:96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Kivipelto M, Solomon A, Ahtiluoto S, Ngandu T, Lehtisalo J, Antikainen R, et al. The Finnish geriatric intervention study to prevent cognitive impairment and disability (FINGER): study design and progress. Alzheimer’s Demen 2013;9:657–65. [DOI] [PubMed] [Google Scholar]
- [30].Krikorian R, Boespflug EL, Dudley JA, Norris MM, Chu W, Summer S, et al. Enhanced cerebral bioenergetics with dietary ketosis in Mild Cognitive Impairment. Nutr Aging 2014;2:223–32. [Google Scholar]
- [31].Sachs BC, Skinner JS, Sink KM, Craft S, Baker LD. High intensity aerobic exercise improves performance on computer tests of executive function in adults with mild cognitive impairment: implications for cognitive assessment in clinical trials. Alzheimer’s Demen The J Alzheimer’s Assoc 2016;12:P428. [Google Scholar]
- [32].Rosenberg A, Ngandu T, Rusanen M, Antikainen R, Backman L, Havulinna S. Multidomain lifestyle intervention benefits a large elderly population at risk for cognitive decline and dementia regardless of baseline characteristics: The FINGER trial. Alzheimer’s Dement 2017;14:263–70. [DOI] [PubMed] [Google Scholar]
- [33].Kraus N Biological impact of music and software-based auditory training. J Commun Disord 2012;45:403–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].What is precision medicine?. Available at: https://ghr.nlm.nih.gov/primer/precisionmedicine/definition Accessed February 20, 2018.
- [35].Gottesman RF, Albert MS, Alonso A, Coker LH, Coresh J, Davis SM. Associations between midlife vascular risk factors and 25-year incident dementia in the Atherosclerosis Risk in Communities (ARIC) Cohort. JAMA Neurol 2017;74:1246–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Jack CR, Bennett DA, Blennow K, Carrillo MC, Dunn B, Haeberlein SB, et al. Deep phenotyping for precision medicine. Hum Mutat 2012;33:777–80. [DOI] [PubMed] [Google Scholar]
- [37].Nasir K, Rubin J, Blaha MJ, Shaw LJ, Blankstein R, Rivera JJ. Interplay of Coronary Artery Calcification and Traditional Risk Factors for the Prediction of All-Cause Mortality in Asymptomatic Individuals-Clinical Perspective. Circ Cardiovasc Imaging 2012;5:467–73. [DOI] [PubMed] [Google Scholar]
- [38].Jack CR, Bennett DA, Blennow K, Carrillo MC, Dunn B, Haeberlein SB, et al. NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimer’s & Dementia 2018; 14:535–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Frisoni GB, Boccardi M, Barkhof F, Blennow K, Cappa S, Chiotis K. Strategic roadmap for an early diagnosis of Alzheimer’s disease based on biomarkers. Lancet Neurol 2017;16:661–76. [DOI] [PubMed] [Google Scholar]
- [40].Rettberg JR, Dang H, Hodis HN, Henderson VW, St John JA, Mack WJ. Identifying postmenopausal women at risk for cognitive decline within a healthy cohort using a panel of clinical metabolic indicators: potential for detecting an at-Alzheimer’s risk metabolic phenotype. Neurobiol Aging 2016;40:155–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Desideri G, Kwik-Uribe C, Grassi D, Necozione S, Ghiadoni L, Mastroiacovo D. Benefits in cognitive function, blood pressure, and insulin resistance through cocoa flavanol consumption in elderly subjects with mild cognitive impairment: the Cocoa, Cognition, and Aging (CoCoA) study. Hypertension 2012;60:794–801. [DOI] [PubMed] [Google Scholar]
- [42].Isaacson RS, Ochner CN. The Alzheimer’s Prevention & Treatment Diet. Garden City: Square One Publishers; 2016. [Google Scholar]
- [43].Bayer-Carter JL, Green PS, Montine TJ, VanFossen B, Baker LD, Watson GS. Diet intervention and cerebrospinal fluid biomarkers in amnestic mild cognitive impairment. Arch Neurol 2011;68:743–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Scarmeas N, Stern Y, Mayeux R, Manly JJ, Schupf N, Luchsinger JA. Mediterranean diet and mild cognitive impairment. Arch Neurol 2009; 66:216–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Eugene AR, Masiak J. The neuroprotective aspects of sleep. MEDtube Sci 2015;3:35. [PMC free article] [PubMed] [Google Scholar]
- [46].Kliegel M, Zimprich D, Rott C. Life-long intellectual activities mediate the predictive effect of early education on cognitive impairment in centenarians: a retrospective study. Aging Ment Health 2004;8:430–7. [DOI] [PubMed] [Google Scholar]
- [47].Khalsa DS. Stress, meditation, and Alzheimer’s disease prevention: where the evidence stands. J Alzheimer’s Dis 2015;48:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Taylor MK, Sullivan DK, Swerdlow RH, Vidoni ED, Morris JK, Mahnken JD. A high-glycemic diet is associated with cerebral amyloid burden in cognitively normal older adults. Am J Clin Nutr 2017; 106:1463–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Isaacson R, Haynes N, Seifan A, Larsen D, Christiansen S, Berger JC. Alzheimer’s Prevention Education: If We Build It, Will They Come? J Prev Alzheimer’s Dis:91, www.AlzU.org, 2014;1. [PMC free article] [PubMed] [Google Scholar]
- [50].Panda S Medicine: science or art? Mens sana Monogr 2006;4:127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Laws KR, Irvine K, Gale TM. Sex differences in cognitive impairment in Alzheimer’s disease. World J Psychiatry 2016;6:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Mosconi L, Berti V, Quinn C, McHugh P, Petrongolo G, Varsavsky I. Sex differences in Alzheimer risk Brain imaging of endocrine vs chronologic aging. Neurology 2017;89:1382–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Andrieu S, Guyonnet S, Coley N, Cantet C, Bonnefoy M, Bordes S. Effect of long-term omega 3 polyunsaturated fatty acid supplementation with or without multidomain intervention on cognitive function in elderly adults with memory complaints (MAPT): a randomised, placebo-controlled trial. Lancet Neurol 2017;16:377–89. [DOI] [PubMed] [Google Scholar]
- [54].Robinson L, Dickinson C, Magklara E, Newton L, Prato L, Bamford C. Lessons from the Multidomain Alzheimer Preventive Trial. Lancet Neurol 2017;16:585–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Alzheimer’s Association. 2018 Alzheimer’s disease facts and figures. Alzheimer’s Demen 2018;14:367–429. [Google Scholar]
- [56].Robinson L, et al. Proactive approaches to identifying dementia and dementia risk; a qualitative study of public attitudes and preferences. BMJ open 2018;8:e018677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Cupples LA, Fareer LA, Sadovnick AD, Relkin N, Whitehouse P, Green RC. Estimating risk curves for first-degree relatives of patients with Alzheimer’s disease: the REVEAL study. Genet Med 2004;6:192. [DOI] [PubMed] [Google Scholar]
- [58].Green RC, Roberts JS, Cupples LA, Relkin NR, Whitehouse PJ, Brown T. Disclosure of APOE genotype for risk of Alzheimer’s disease. New Engl J Med 2009;361:245–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Stites SD. Cognitively Healthy Individuals Want to Know Their Risk for Alzheimer’s Disease: What Should We Do? J Alzheimer’s Dis 2018;62:499–502. [DOI] [PubMed] [Google Scholar]
- [60].Burns JM, Johnson DK, Liebmann EP, Bothwell RJ, Morris JK, Vidoni ED. Safety of disclosing amyloid status in cognitively normal older adults. Alzheimer’s Demen J Alzheimer’s Assoc 2017;13:1024–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Ngandu T, Lehitsalo J, Solomon A, Levalahti E, Ahtiluoto S, Antikainen R. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): a randomised controlled trial. Lancet 2015;385:2255–63. [DOI] [PubMed] [Google Scholar]
- [62].Solomon A, Turunen H, Ngandu T, Peltonen M, Levalahti E, Helisalmi S. Effect of the Apolipoprotein E Genotype on Cognitive Change During a Multidomain Lifestyle Intervention: A Subgroup Analysis of a Randomized Clinical Trial. JAMA Neurol 2018;75:462–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Feero W, Wicklund CA, Veenstra D. Precision medicine, genome sequencing, and improved population health. JAMA 2018;319:1979–80. [DOI] [PubMed] [Google Scholar]
- [64].Beam AL, Kohane IS. Big data and machine learning in health care. JAMA 2018;319:1317–8. [DOI] [PubMed] [Google Scholar]
- [65].Antman EM, Loscalzo J. Precision medicine in cardiology. Nat Rev Cardiol 2016;13:591. [DOI] [PubMed] [Google Scholar]
- [66].Kim J, Thrift AG, Nelson MR, Bladin CF, Cadilhac DA. Personalized medicine and stroke prevention: where are we? Vasc Health Risk Manag 2015;11:601. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.