Abstract
Background:
Fatigue is one of the most common and disabling side effects of cancer and its treatment. Although research has typically focused on fatigue that occurs during and after treatment, patients may experience fatigue even before treatment onset. The current study was designed to identify biobehavioral risk factors associated with fatigue before adjuvant therapy in women with early-stage breast cancer.
Methods:
Patients with Stage 0-IIIA breast cancer (n=270) were recruited before onset of adjuvant or neoadjuvant therapy with radiation, chemotherapy, and/or endocrine therapy. Host factors were identified from an empirically-based, biobehavioral model of fatigue and assessed using self-report questionnaires, medical record review, and blood collection (for genetic data). Fatigue was also assessed by questionnaire. Linear regression analyses were used to assess the association between host factors and dimensions of fatigue, with general fatigue as the primary dimension of interest.
Results:
Fatigue was elevated at the pre-treatment assessment relative to published controls. Bivariate analyses identified a number of demographic, cancer-related, and biobehavioral correlates of fatigue. In the multivariable model, predictors of general fatigue included younger age, lower education, lower cancer stage, and history of childhood maltreatment (all ps < .05), with the full model accounting for 18.4% of the variance in fatigue. Secondary analyses identified common and specific predictors of emotional, mental, and physical dimensions of fatigue.
Conclusion:
Among women who have not yet started treatment for breast cancer, demographic and psychosocial factors are associated with elevated fatigue and could be used to identify at-risk patients for early intervention.
Keywords: Fatigue, Breast Cancer, Host Factors, Childhood Adversity, Depression, Genetic
Precis:
Fatigue is one of the most common side effects of cancer treatment and may be elevated even before treatment onset, setting the stage for more severe and persistent symptoms throughout the cancer trajectory. This study examined biobehavioral risk factors for fatigue in breast cancer patients before adjuvant therapy and identified demographic and psychosocial factors associated with fatigue, including childhoodhood adversity.
BACKGROUND
Fatigue is one of the most common and debilitating side effects of cancer and its treatment.1, 2 Cancer-related fatigue has adverse emotional, social, occupational, and economic consequences for patients and their caregivers,3 and may impact treatment adherence and survival.4 Most studies in this area have focused on fatigue that occurs during and after treatment.5 However, some patients experience significant fatigue even before treatment onset,6 which may portend a more difficult treatment course and slower recovery. Indeed, evidence suggests that pre-treatment fatigue is one of the strongest predictors of persistent fatigue up to 5 years after treatment completion.7–14 To date, there has been minimal examination of factors associated with fatigue at this critical stage of the cancer trajectory. Identifying host factors that contribute to fatigue before adjuvant treatment will help to identify vulnerable patients who would benefit from early, targeted intervention and elucidate underlying mechanisms.
Cancer-related fatigue is multifactorial and can be influenced by a variety of demographic, medical, psychosocial, behavioral, and biological factors.2 An empirically-based biobehavioral model of cancer-related fatigue that identifies key factors associated with fatigue during and after treatment has been proposed.1 However, it is not known whether this model is relevant for patients before adjuvant therapy. Research on other behavioral symptoms (e.g., insomnia) demonstrates that factors involved with symptom initiation may differ from those involved in symptom persistence.15 Similarly, there may be unique predictors of fatigue experienced early in the cancer trajectory, before onset of adjuvant therapy. The few studies to examine correlates of fatigue before treatment onset have identified demographic and medical factors associated with fatigue, including younger age, children in the home, and higher body mass index.16 Fatigue is also correlated with other symptoms before treatment onset, including depressed mood and sleep disturbance,16–18 although it is unclear whether these are a cause or consequence of fatigue. Other host factors that have emerged as predictors of fatigue during and after treatment have rarely been evaluated before treatment onset, including history of depression,19–22 childhood trauma,23–26 and genetic risk factors, particularly variants in genes involved in inflammation and immune response (e.g., IL1, TNFA, IL6).27 Importantly, these host factors are present before cancer diagnosis, which clarifies the temporal nature of their association with fatigue.
The multi-dimensional nature of cancer-related fatigue further complicates the identification of risk factors and development of effective interventions. The defining characteristic of cancer-related fatigue is a subjective feeling of tiredness,28 but it may also include a sense of physical, emotional, and/or cognitive tiredness or exhaustion.29, 30 However, many studies use unidimensional measures of fatigue that focus primarily on fatigue severity or collapse across dimensions for analyses. This limitation is striking given evidence that different dimensions of fatigue have distinct correlates and show different responses to cancer treatment and to intervention.31–33
The goal of the current study was to identify correlates of fatigue among women diagnosed with early-stage breast cancer who had not yet started adjuvant therapy. Drawing from a biobehavioral model of fatigue,1 we tested key demographic, medical, psychosocial, and biological factors that have been linked with fatigue across the cancer continuum. Among the psychosocial and biological risk factors, we focused on stable host factors rather than more transient factors that could be a consequence of fatigue. We were particularly interested in history of depression, childhood adversity, and cytokine genetic polymorphisms which have been linked with fatigue during and after cancer treatment,19–27, 34 Our primary analyses focused on general fatigue, which includes feelings of tiredness and is most comparable to unidimensional measures of fatigue used in previous research. Secondary analyses examined whether these risk factors were also associated with other dimensions of fatigue, including physical, mental, and emotional fatigue.
METHODS
Patients and Procedures:
Patients were recruited from oncology practices in Los Angeles to participate in a longitudinal, observational study of cancer-related fatigue (RISE study). Women were eligible if they had been recently diagnosed with Stage 0-IIIA breast cancer and had not yet started adjuvant or neoadjuvant therapy with radiation, chemotherapy, or endocrine therapy. Primary recruitment sites were UCLA and Cedars Sinai Medical Center (CSMC).
Participants completed assessments at baseline, end of treatment (for those who received radiation and/or chemotherapy), and at 6, 12, and 18 month post-treatment follow-ups; we focus here on the baseline assessment. The Institutional Review Boards at UCLA and CSMC approved the study, and all participants provided written informed consent.
Measures:
Data were collected through self-report questionnaires, interviews, blood collection, and medical chart review.
Fatigue was assessed with the Multidimensional Fatigue Symptom Inventory-Short Form, which includes four subscales assessing distinct dimensions of fatigue.35, 36 General fatigue assesses the degree to which respondents felt tired, worn out, sluggish, and fatigued in the past week, and was the primary outcome of interest. Physical fatigue assesses feelings of weakness, heaviness, and achiness in the past week; mental fatigue assesses trouble remembering things and paying attention, difficulty concentrating, and confusion in the past week; and emotional fatigue assesses feeling upset, nervous, sad, depressed, and tense in the past week. Across subscales, higher scores indicate more fatigue.
Demographic characteristics were obtained from self-report at baseline and included age, race/ethnicity, marital status, income, education, employment status, and presence of children at home.
Disease and treatment-related information was obtained from medical record abstraction and included cancer stage, type of surgery received, and time from diagnosis to baseline assessment.
Pre-cancer medical co-morbidities were assessed with a questionnaire version of the Charlson Co-morbidity Scale, a reliable and valid measure that includes a variety of chronic diseases including heart attack, stroke, cardiovascular disease, asthma, diabetes, autoimmune disease, and dementia.37 Height and weight were measured at baseline for determination of body mass index.
History of childhood maltreatment was assessed with the Childhood Trauma Questionnaire,38 a 28-item measure that includes questions about physical, emotional, and sexual abuse, as well as physical and emotional neglect that occurred during childhood. Women were categorized into one of three maltreatment groups using a scoring algorithm with established sensitivity and specificity: no maltreatment; physical and/or emotional abuse or neglect but no sexual abuse; and sexual abuse with or without physical and/or emotional abuse or neglect.39
History of major depressive disorder (MDD) prior to cancer diagnosis was determined using the Structured Clinical Interview for DSM-IV-TR Axis I Disorders (SCID). The SCID was administered by trained interviewers and interviews were reviewed and scored by a consensus panel, led by a psychiatrist with expertise in depression (MI).
Genomic DNA was extracted from peripheral blood leukocytes and assayed by a commercial TaqMan Genotyping Assay (Applied Biosystems, Foster City, CA) performed on a iCycler real-time polymerase chain reaction instrument (BioRad, Hercules, CA) following manufacturer’s protocols, as previously described.40 We focused on SNPs in genes encoding pro-inflammatory cytokines that have been linked to cancer-related fatigue, including IL1B −511 C>T (rs16944), IL6 −174 G>C (rs1800795), and TNF −308 G>A (rs1800629).40–42
Data and Power Analysis:
Bivariate linear regression analyses assessed the relationship between each predictor and each dimension of fatigue, with general fatigue as the primary outcome. Given our interest in identifying predictors of different dimensions of fatigue (rather than characterizing fatigue groups or cases), fatigue was treated as a continuous variable in all analyses. Variables that were associated with an MFSI-SF scale with bivariate p<0.10 were included in a multivariable model for that scale. Multivariable linear regression models were fit using multiple imputation (20 imputations generated using chained equations)43 to handle missing values, and coefficient estimates and standard errors were obtained using Rubin’s rules. Multivariable models controlled for time since diagnosis. The percent of variance explained, as measured by R2, was obtained by averaging the R2 values from the 20 imputation analyses.44 Analyses were conducted in Stata version 13.1.
The target sample size was based on the prevalence of key predictor variables and the magnitude of the hypothesized association with fatigue. Power analyses determined that 240–280 patients would be required to detect effect sizes of 0.35–0.45.
RESULTS
Enrollment began in 1/2013 and ended in 7/2015. Over this period, 409 women were screened, 302 of whom met initial eligibility criteria and consented for participation. Thirty-two women were later determined to be ineligible (n=5) or failed to complete the baseline questionnaire (n=27). Thus, the final sample consisted of 270 women.
Characteristics of study participants are shown in Table 1. Women were 56 years old on average and primarily White, college educated, and working full- or part-time. The majority had been diagnosed with Stage I (47%) or Stage II (24%) breast cancer and treated with lumpectomy or mastectomy prior to study enrollment. Note that 10% of study participants had not received surgery prior to enrollment, as they were scheduled to undergo neoadjuvant chemotherapy before surgery. The median number of days from diagnosis to study enrollment/baseline assessment was 56; 80% were enrolled within 3 months of diagnosis, though a handful (n=6) were enrolled more than 6 months post-diagnosis, typically because they waited several months before starting adjuvant treatment. Twenty-three percent had a history of major depressive disorder prior to breast cancer diagnosis, which is higher than that reported in breast cancer patients with similar clinical characteristics (17%)21 but lower than that reported in a community sample of midlife women (32%).45 Forty percent reported a history of emotional, physical, or sexual maltreatment as children, comparable to demographically similar samples of women.39
Table 1.
Summary of participant characteristics at baseline (N = 270)
| Mean (SD), min-max or N (%) | |
|---|---|
| Demographic characteristics | |
| Age, years | 56 (11), 26.8-88.5 |
| Race | |
| White, non-Hispanic | 193 (71) |
| Black | 12 (4) |
| Hispanic | 27 (10) |
| Asian | 30 (11) |
| Other | 8 (3) |
| Income (missing n = 4) | |
| Less than $60,000 | 67 (25) |
| $60,000 to $100,000 | 53 (20) |
| $100,000 or more | 146 (55) |
| Educational attainment | |
| Less than college degree | 78 (29) |
| College graduate | 108 (40) |
| Post-graduate degree | 84 (31) |
| Employed (full or part-time) | 161 (60) |
| Married or living as married | 174 (64) |
| Any children living at home | 110 (41) |
| Disease and treatment-related variables | |
| Cancer stage at diagnosis | |
| 0 | 32 (12) |
| 1 | 128 (47) |
| 2 | 64 (24) |
| 3 | 10 (4) |
| Indeterminable (neoadjuvant or missing pathological information) | 36 (13) |
| Surgery | |
| No surgery (neoadjuvant) | 25 (10) |
| Lumpectomy | 159 (59) |
| Mastectomy | 86 (32) |
| Months since diagnosis | 2.2 (1.3), 0.4-9.5 |
| Biobehavioral risk factors | |
| BMI, kg/m2 | 25.4 (5.8), 14.9-45.6 |
| Charlson comorbidity index | |
| 0 | 206 (76) |
| 1 | 49 (18) |
| 2 or 3 | 15 (6) |
| Childhood maltreatment | |
| No maltreatment | 163 (60) |
| Non-sexual maltreatment (physical/emotional abuse or neglect) | 75 (28) |
| Sexual maltreatment | 32 (12) |
| Past history of depression (missing n = 8) | |
| No | 202 (77) |
| Yes | 60 (23) |
| TNF-308 (missing n = 33) | |
| GG | 181 (76) |
| GA | 49 (21) |
| AA | 7 (3) |
| IL6-174 (missing n = 33) | |
| GG | 117 (49) |
| GC | 94 (40) |
| CC | 26 (11) |
| IL1B-511 (missing n = 33) | |
| GG | 38 (16) |
| AG | 112 (47) |
| AA | 87 (37) |
The average score on the MFSI-SF general fatigue scale was 7.8 and ranged from 0–24. In the validation study for this scale,29 the average score among women with no cancer history was 5.06, indicating that fatigue was elevated in our sample even before adjuvant therapy had begun. Mean scores on each of the MFSI-SF subscales, and correlations among subscales, are reported in Table 2.
Table 2.
Means and correlations among MFSI scales (n=270)
| Mean (SD) | General | Emotional | Physical | Mental | |
|---|---|---|---|---|---|
| General | 7.72 (5.79) | ||||
| Emotional | 6.68 (5.30) | 0.50 | |||
| Physical | 3.86 (4.40) | 0.66 | 0.34 | ||
| Mental | 4.65 (4.17) | 0.63 | 0.56 | 0.47 | |
| Cronbach’s alpha | 0.95 | 0.91 | 0.87 | 0.89 |
All correlations are significantly different from 0 with p<0.0001.
Bivariate correlates of fatigue
Bivariate analyses identified a number of significant correlates of fatigue (see Table 3). Primary analyses focusing on general fatigue showed that women of younger age, lower income and/or education, with earlier-stage disease, treated with mastectomy, and who had a history of childhood maltreatment or history of depression reported higher levels of general fatigue (all ps < .05). Income and childhood maltreatment were also associated with significantly higher levels of emotional, physical, and mental fatigue (ps < .05); individual correlates of different fatigue dimensions are reported in Table 3.
Table 3.
Bivariate linear regression results for four MFSI-SF scales
| Primary: | Secondary: | |||||||
|---|---|---|---|---|---|---|---|---|
| General | Emotional | Physical | Mental | |||||
| Coef | P | Coef | P | Coef | P | Coef | P | |
| Demographic characteristics | ||||||||
| Age, 10-year increase | −0.63 | .042 | −0.80 | .004 | −0.04 | .861 | −0.39 | .075 |
| Race (intercept: non-Hispanic white) | 7.99 | 6.76 | 3.66 | 4.65 | ||||
| Black | −2.49 | .151 | −1.68 | .286 | −1.74 | .182 | −1.15 | .356 |
| Hispanic | −0.14 | .910 | 1.35 | .215 | 2.01 | .026 | 0.64 | .456 |
| Asian | −0.95 | .403 | −0.49 | .633 | 0.58 | .503 | −0.05 | .949 |
| Other | −1.11 | .596 | −2.89 | .131 | 0.59 | .708 | −0.40 | .790 |
| Income (intercept: Less than $60,000) | 8.86 | 8.30 | 5.43 | 5.43 | ||||
| $60,000 to $100,000 | −1.01 | .340 | −2.02 | .037 | −1.83 | .021 | −0.17 | .825 |
| $100,000 or more | −1.80 | .035 | −2.29 | .003 | −2.27 | <.001 | −1.40 | .023 |
| Education (intercept: college or less) | 8.21 | 7.17 | 4.20 | 4.83 | ||||
| Post-graduate | −1.57 | .039 | −1.56 | .025 | −1.10 | .058 | −0.60 | .279 |
| Education (intercept: college or less) | 8.21 | 7.17 | 4.20 | 4.83 | ||||
| Employed | 0.44 | .541 | −0.20 | .766 | −0.63 | .249 | −0.11 | .827 |
| Partnered status (intercept: not) | 7.00 | 6.45 | 4.07 | 4.55 | ||||
| Partnered | 1.13 | .125 | 0.36 | .591 | −0.33 | .561 | 0.15 | .779 |
| Children at home (intercept: absent) | 7.38 | 6.51 | 3.60 | 4.54 | ||||
| Present | 0.85 | .238 | 0.43 | .513 | 0.65 | .237 | 0.27 | .600 |
| Cancer and treatment-related variables | ||||||||
| Stage at diagnosis (intercept: 0 or 1) | 8.67 | 6.96 | 4.58 | 5.29 | ||||
| Stage 2 or 3 | −2.21 | .003 | −0.59 | .388 | −1.77 | .002 | −1.72 | .001 |
| Surgery (intercept: none or lumpectomy) | 6.99 | 6.50 | 3.19 | 4.40 | ||||
| Mastectomy | 2.32 | .002 | 0.57 | .411 | 2.11 | <.001 | 0.77 | .157 |
| Biobehavioral risk factors | ||||||||
| BMI, 1-unit increase | 0.04 | .531 | −0.05 | .409 | 0.06 | .169 | −0.01 | .939 |
| Charlson comorbidity index, 1-point increase | 0.92 | .105 | 0.78 | .136 | 1.18 | .006 | 0.73 | .076 |
| Childhood maltreatment (intercept: none) | 6.60 | 5.86 | 3.18 | 3.60 | ||||
| Nonsexual maltreatment | 2.42 | .002 | 1.97 | .007 | 1.50 | .014 | 2.27 | <.001 |
| Sexual maltreatment | 3.81 | .002 | 2.33 | .022 | 2.22 | .008 | 3.52 | <.001 |
| History of major depression (intercept: no) | 7.18 | 6.10 | 3.77 | 4.19 | ||||
| Yes | 2.56 | .003 | 2.63 | .001 | 0.67 | .309 | 2.27 | <.001 |
| TNF, per high expression allele | −1.17 | .122 | 0.13 | .851 | −0.88 | .125 | −0.65 | .237 |
| IL6, per high expression allele | 0.41 | .473 | 0.19 | .716 | 0.71 | .099 | −0.09 | .835 |
| IL1B, per high expression allele | 0.10 | .862 | −0.02 | .962 | 0.29 | .489 | 0.56 | .160 |
Multivariable models of fatigue
We next fit multivariable linear regression models including variables that were associated at p<0.10 in bivariate analyses. Results are shown in Table 4. For general fatigue, younger age, lower education, lower disease stage, and history of childhood maltreatment emerged as significant predictors (all ps < .05). These factors were also associated with other dimensions of fatigue, though childhood maltreatment was the only factor associated with all fatigue dimensions at p< or =0.05. The association between childhood maltreatment and general fatigue is depicted in Figure 1. Other risk factors were associated with specific dimensions of fatigue in the multivariable models. In particular, presence of medical co-morbidities was a significant predictor of physical and mental fatigue, and history of depression was a significant predictor of emotional and mental fatigue. Among the genetic risk factors assessed, only one showed a significant association with fatigue: high-expression variants of the IL6 SNP were associated with significantly higher levels of physical fatigue. Together, the predictors explained 18–22% of the variance in general, physical, and mental fatigue, but only 13.9% in emotional fatigue.
Table 4.
Multivariable linear regression model results for four MFSI-SF scales
| Primary: | Secondary: | |||||||
|---|---|---|---|---|---|---|---|---|
| General | Emotional | Physical | Mental | |||||
| Percent of variance explained (R2) | 18.4% | 13.9% | 22.8% | 20.3% | ||||
| Coef | P | Coef | P | Coef | P | Coef | P | |
| Age, 10-year increase | −0.71 | .016 | −0.87 | .001 | −0.58 | .005 | ||
| Race (ref: white) | ||||||||
| Black | −2.44 | .052 | ||||||
| Hispanic | 0.38 | .645 | ||||||
| Asian | 0.43 | .772 | ||||||
| Other | 1.00 | .238 | ||||||
| Income (ref: Less than $60,000) | ||||||||
| $60,000 to $100,000 | −0.41 | .684 | −1.74 | .061 | −1.13 | .138 | 0.39 | .582 |
| $100,000 or more | −0.88 | .298 | −1.65 | .034 | −1.47 | .027 | −0.74 | .205 |
| Education (ref: college or less) | ||||||||
| Post-graduate | −1.97 | .009 | −1.56 | .025 | −0.93 | .097 | ||
| Stage at diagnosis of 2 or 3 (ref: 0 or 1) | −1.55 | .028 | −1.39 | .010 | −1.32 | .009 | ||
| Mastectomy (ref: no surgery or lumpectomy) | 1.16 | .145 | 1.37 | .020 | ||||
| Charlson, 1-point increase | 0.92 | .025 | 0.83 | .035 | ||||
| Childhood maltreatment (ref: none) | ||||||||
| Nonsexual | 1.76 | .023 | 1.39 | .051 | 1.01 | .079 | 1.73 | .002 |
| Sexual | 3.00 | .009 | 1.72 | .103 | 1.63 | .050 | 2.21 | .005 |
| History of major depression (ref: no) | 1.53 | .063 | 2.22 | .004 | 1.51 | .010 | ||
| IL6, per high expression allele | 0.86 | .036 | ||||||
Results were obtained using multiple imputation (30 imputations) due to missing values. All models control for time since diagnosis.
Figure 1.
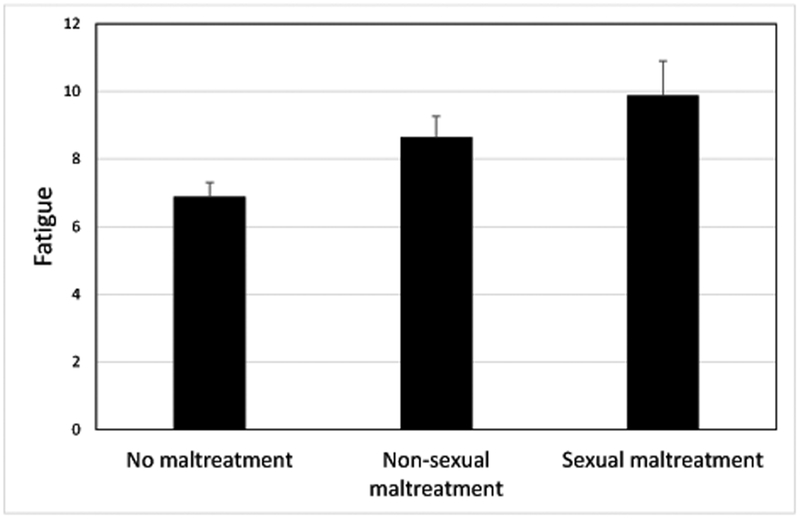
Adjusted mean scores on the MFSI-SF general fatigue subscale for women at each category of childhood maltreatment. Women who had experienced non-sexual maltreatment (physical and/or emotional abuse or neglect) or who had experienced sexual maltreatment (with or without physical and/or emotional abuse or neglect) in childhood reported significantly higher levels of fatigue than women with no history of maltreatment.
CONCLUSION
This study applied a biobehavioral model of cancer-related fatigue to identify risk factors for fatigue before commencement of adjuvant therapy in a large sample of women with breast cancer. This point in the cancer trajectory has received minimal empirical attention, despite evidence that pre-treatment fatigue is one of the strongest and most consistent predictors of post-treatment fatigue and may set the stage for elevated fatigue years after treatment.7, 8 Across general and specific sub-dimensions of fatigue, women of younger age, lower income or education, who had a history of childhood maltreatment reported elevated symptoms, suggesting a vulnerable phenotype. Also notable were those factors that were consistently not associated with fatigue, including partner status, race, and body mass index.
Psychosocial factors emerged as key predictors of fatigue in this sample. Women who had experienced abuse or neglect as children (40% of the sample) reported higher levels of all dimensions of fatigue, and those with a history of depression (23%) reported significantly higher levels of emotional and mental fatigue in bivariate analyses. These factors are known to increase risk for physical and behavioral symptoms in other contexts46, 47 but have only recently been examined in relation to cancer-related fatigue.19–22, 24–26 There are several mechanisms through which these factors may influence fatigue, including alterations in neural, neuroendocrine, immune, and/or behavioral processes.48–51 In particular, both childhood adversity and depression are associated with elevated inflammation51–53 which has linked with cancer-related fatigue,1, 54 suggesting that inflammation may contribute to fatigue even before adjuvant treatment in vulnerable patients. Indeed, one small study found that childhood trauma was associated with elevated fatigue and inflammation before radiation therapy in women with breast cancer.26 Interestingly, childhood adversity and history of depression showed differential associations with specific dimensions of fatigue in multivariable models. Results from these analyses suggested that history of depression and childhood adversity make unique independent contributions to mental fatigue, but that childhood adversity is more strongly associated with general fatigue and history of depression is more strongly associated with emotional fatigue in this sample. These findings highlight the importance of examining dimensions of fatigue and provide insight into their distinct associations with psychosocial vulnerability factors.
Results also demonstrate the importance of socioeconomic status in the experience of fatigue. Women with lower levels of income or education may be less prepared for the demands of diagnosis, surgery, and adjuvant treatment preparation, or may have fewer financial resources to meet those demands. Further, results highlight age as a significant risk factor for general, emotional, and mental fatigue, with younger women at higher risk. These findings are consistent with previous research19, 55, 56 and underline the vulnerability of younger women to behavioral side effects of breast cancer.57
Although certain risk factors were associated with multiple dimensions of fatigue, results also revealed more specific effects. This was particularly evident for physical fatigue, which had several unique predictors in multivariable models, including receipt of mastectomy and high expression variants of the IL6 −174 SNP. These findings suggest that medical and genetic risk factors may play a stronger role in physical fatigue, operationalized by the MFSI-SF as feelings of bodily weakness, achiness, and heaviness. Of note, previous studies have demonstrated associations with variants in the IL6 gene and fatigue before, during, and after cancer treatment.40, 58, 59 In addition to mastectomy, the one disease-related variable to predict fatigue in multivariate models was cancer stage; women with lower stage disease reported higher levels of general, mental, and physical fatigue. In the post-treatment period, cancer stage is typically not strongly associated with fatigue, depression, and other aspects of quality of life.60 However, it is possible that stage may be more salient closer to the time of diagnosis, when women are learning about the treatment and survival-related implications of their cancer. Still, our finding that women with lower stage disease reported more fatigue is unexpected, as previous studies have typically found that lower cancer stage is associated with lower (rather than higher) fatigue, if any association is reported.61 This unexpected finding requires examination in future research.
Factors that were not associated with fatigue in this sample also merit discussion. In particular, we found no association between partner status and fatigue, although previous work has suggested that partnered women report lower levels of fatigue, at least in the post-treatment period.55, 61 It is possible that that the instrumental support provided by a partner may become more important during and after treatment as symptoms increase. Further, body mass index (BMI) was not associated with fatigue, though previous studies have shown that BMI is associated with fatigue before16 and after treatment62 in women with breast cancer. There was also no association between race/ethnicity and fatigue in this sample, other than elevated physical fatigue among Hispanic women. These findings are consistent with our previous research with breast cancer survivors55 and with results from several large samples of mixed cancer survivors,22, 63 although racial differences in fatigue have been observed during cancer treatment.22
Several limitations of the study should be noted. Although this study was designed to evaluate fatigue relatively early in the cancer trajectory, participants were assessed after surgical resection of the primary tumor in most cases (i.e., unless they were scheduled to receive neoadjuvant chemotherapy). Surgery can influence fatigue;64 indeed, we found that treatment with mastectomy was associated with higher levels of physical fatigue. It would be ideal to obtain a pre-surgical measure of fatigue to better capture a true pre-treatment baseline, although there are challenges with recruitment and assessment during the interval between diagnosis and surgery. In addition, our ability to identify socioeconomic correlates of fatigue may have been limited by characteristics of the sample, which was predominantly White and well-educated, although we did find evidence of elevated fatigue among those of lower income and/or education as well as higher levels of physical fatigue among Hispanic women. Further, the majority of women had early-stage (Stage I or II) breast cancer, which limits the generalizability of the results.
There is growing evidence of substantial individual variability in the experience of fatigue during and after treatment, which may be present even before treatment onset. Indeed, a recent study of women with early-stage breast cancer assessed before surgery and at 4 and 8 month follow-ups identified two groups of patients with distinct fatigue trajectories: one with low fatigue before surgery that remained low and stable throughout the assessment period, and one with high fatigue before surgery that increased during treatment then declined by 8 months (though never to pre-surgery levels).65 This suggests that much of the variance in cancer-related fatigue may be driven by factors that predate the cancer experience. The current study identified several of these factors, though note that the combination of medical, demographic, disease, and psychosocial risk factors included here explained roughly 20% of the variance in pre-treatment fatigue. It will be important to determine whether these factors are also associated with increased fatigue in the immediate aftermath of adjuvant treatment and in the subsequent months and years, or if different processes play a more important role at later stages of survivorship. Our findings also offer insight into different dimensions of fatigue and reveal common and unique predictors of these dimensions. From a clinical perspective, several of these risk factors may not be “on the radar” of treating physicians, including childhood maltreatment, but may aid in the identification of vulnerable patients and delivery of early interventions to those most in need.
Acknowledgments
Financial support:
This work was supported by National Institutes of Health R01 CA160427. Crespi and Hurvitz were also supported by P30 CA16042.
Footnotes
Declaration of interests: None
REFERENCES
- 1.Bower JE. Cancer-related fatigue--mechanisms, risk factors, and treatments. Nature reviews Clinical oncology 2014; 11(10): 597–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mitchell SA. Cancer-related fatigue: state of the science. PMR 2010; 2(5): 364–83. [DOI] [PubMed] [Google Scholar]
- 3.Curt GA, Breitbart W, Cella D, et al. Impact of cancer-related fatigue on the lives of patients: new findings from the Fatigue Coalition. Oncologist 2000; 5(5): 353–60. [DOI] [PubMed] [Google Scholar]
- 4.Groenvold M, Petersen MA, Idler E, Bjorner JB, Fayers PM, Mouridsen HT. Psychological distress and fatigue predicted recurrence and survival in primary breast cancer patients. Breast Cancer Res Treat 2007; 105: 209–19. [DOI] [PubMed] [Google Scholar]
- 5.Servaes P, Verhagen C, Bleijenberg G. Fatigue in cancer patients during and after treatment: prevalence, correlates and interventions. EurJ Cancer 2002; 38(1): 27–43. [DOI] [PubMed] [Google Scholar]
- 6.Ancoli-Israel S, Liu L, Rissling M, et al. Sleep, fatigue, depression, and circadian activity rhythms in women with breast cancer before and after treatment: a 1-year longitudinal study. Support Care Cancer 2014; 22(9): 2535–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Geinitz H, Zimmermann FB, Thamm R, Keller M, Busch R, Molls M. Fatigue in patients with adjuvant radiation therapy for breast cancer: long-term follow-up. JCancer ResClinOncol 2004; 130(6): 327–33. [DOI] [PubMed] [Google Scholar]
- 8.Goldstein D, Bennett BK, Webber K, et al. Cancer-related fatigue in women with breast cancer: outcomes of a 5-year prospective cohort study. J Clin Oncol 2012; 30(15): 1805–12. [DOI] [PubMed] [Google Scholar]
- 9.Pertl MM, Hevey D, Boyle NT, et al. C-reactive protein predicts fatigue independently of depression in breast cancer patients prior to chemotherapy. Brain BehavImmun 2013; 34: 108–19. [DOI] [PubMed] [Google Scholar]
- 10.Goedendorp MM, Andrykowski MA, Donovan KA, et al. Prolonged impact of chemotherapy on fatigue in breast cancer survivors: a longitudinal comparison with radiotherapy-treated breast cancer survivors and noncancer controls. Cancer 2012; 118(15): 3833–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Andrykowski MA, Donovan KA, Laronga C, Jacobsen PB. Prevalence, predictors, and characteristics of off-treatment fatigue in breast cancer survivors. Cancer 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Courtier N, Gambling T, Enright S, Barrett-Lee P, Abraham J, Mason MD. A prognostic tool to predict fatigue in women with early-stage breast cancer undergoing radiotherapy. Breast 2013; 22(4): 504–9. [DOI] [PubMed] [Google Scholar]
- 13.Smets EM, Visser MR, Willems-Groot AF, Garssen B, Schuster-Uitterhoeve AL, de Haes JC. Fatigue and radiotherapy: (B) experience in patients 9 months following treatment. BrJCancer 1998; 78(7): 907–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lockefeer JP, De Vries J. What is the relationship between trait anxiety and depressive symptoms, fatigue, and low sleep quality following breast cancer surgery? Psycho-oncology 2013; 22(5): 1127–33. [DOI] [PubMed] [Google Scholar]
- 15.Savard J, Morin CM. Insomnia in the context of cancer: a review of a neglected problem. J Clin Oncol 2001; 19(3): 895–908. [DOI] [PubMed] [Google Scholar]
- 16.Dhruva A, Dodd M, Paul SM, et al. Trajectories of fatigue in patients with breast cancer before, during, and after radiation therapy. Cancer Nurs 2010; 33(3): 201–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smets EM, Visser MR, Willems-Groot AF, et al. Fatigue and radiotherapy: (A) experience in patients undergoing treatment. BrJCancer 1998; 78(7): 899–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu L, Rissling M, Natarajan L, et al. The longitudinal relationship between fatigue and sleep in breast cancer patients undergoing chemotherapy. Sleep 2012; 35(2): 237–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Andrykowski MA, Schmidt JE, Salsman JM, Beacham AO, Jacobsen PB. Use of a case definition approach to identify cancer-related fatigue in women undergoing adjuvant therapy for breast cancer. J Clin Oncol 2005; 23(27): 6613–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fisch MJ, Zhao F, O’Mara AM, Wang XS, Cella D, Cleeland CS. Predictors of significant worsening of patient-reported fatigue over a 1-month timeframe in ambulatory patients with common solid tumors. Cancer 2014; 120(3): 442–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jim HS, Small BJ, Minton S, Andrykowski M, Jacobsen PB. History of major depressive disorder prospectively predicts worse quality of life in women with breast cancer. Annals of behavioral medicine : a publication of the Society of Behavioral Medicine 2012; 43(3): 402–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang XS, Zhao F, Fisch MJ, et al. Prevalence and characteristics of moderate to severe fatigue: a multicenter study in cancer patients and survivors. Cancer 2014; 120(3): 425–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Witek JL, Tell D, Albuquerque K, Mathews HL. Childhood adversity increases vulnerability for behavioral symptoms and immune dysregulation in women with breast cancer. Brain BehavImmun 2013; 30 Suppl: S149–S62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bower JE, Crosswell AD, Slavich GM. Childhood Adversity and Cumulative Life Stress: Risk Factors for Cancer-Related Fatigue. Clin PsycholSci 2014; 2(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fagundes CP, Lindgren ME, Shapiro CL, Kiecolt-Glaser JK. Child maltreatment and breast cancer survivors: social support makes a difference for quality of life, fatigue and cancer stress. EurJ Cancer 2012; 48(5): 728–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Han TJ, Felger JC, Lee A, Mister D, Miller AH, Torres MA. Association of childhood trauma with fatigue, depression, stress, and inflammation in breast cancer patients undergoing radiotherapy. Psycho-oncology 2016; 25(2): 187–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang T, Yin J, Miller AH, Xiao C. A systematic review of the association between fatigue and genetic polymorphisms. Brain Behav Immun 2017; 62: 230–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Berger AM, Mooney K, Alvarez-Perez A, et al. Cancer-Related Fatigue, Version 2.2015. Journal of the National Comprehensive Cancer Network : JNCCN 2015; 13(8): 1012–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stein KD, Martin SC, Hann DM, Jacobsen PB. A multidimensional measure of fatigue for use with cancer patients. Cancer Pract 1998; 6(3): 143–52. [DOI] [PubMed] [Google Scholar]
- 30.Smets EM, Garssen B, Bonke B, De Haes JC. The Multidimensional Fatigue Inventory (MFI) psychometric qualities of an instrument to assess fatigue. J Psychosom Res 1995; 39(3): 315–25. [DOI] [PubMed] [Google Scholar]
- 31.de Raaf PJ, de Klerk C, van der Rijt CC. Elucidating the behavior of physical fatigue and mental fatigue in cancer patients: a review of the literature. Psycho-oncology 2013; 22(9): 1919–29. [DOI] [PubMed] [Google Scholar]
- 32.van Vulpen JK, Peeters PH, Velthuis MJ, van der Wall E, May AM. Effects of physical exercise during adjuvant breast cancer treatment on physical and psychosocial dimensions of cancer-related fatigue: A meta-analysis. Maturitas 2016; 85: 104–11. [DOI] [PubMed] [Google Scholar]
- 33.Schmidt ME, Wiskemann J, Schneeweiss A, Potthoff K, Ulrich CM, Steindorf K. Determinants of physical, affective, and cognitive fatigue during breast cancer therapy and 12 months follow-up. International journal of cancer 2017. [DOI] [PubMed] [Google Scholar]
- 34.Bower JEW J; Petersen L; Irwin MR; Cole SW; Ganz PG. Fatigue after breast cancer treatment: biobehavioral predictors of fatigue trajectories. Health Psychology in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stein KD, Jacobsen PB, Blanchard CM, Thors C. Further validation of the multidimensional fatigue symptom inventory-short form. Journal of Pain and Symptom Management 2004; 27(1): 14–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stein KD, Martin SC, Hann DM, Jacobsen PB. A multidimensional measure of fatigue for use with cancer patients. Cancer Practice 1998; 6(3): 143–52. [DOI] [PubMed] [Google Scholar]
- 37.Katz JN, Chang LC, Sangha O, Fossel AH, Bates DW. Can comorbidity be measured by questionnaire rather than medical record review? MedCare 1996; 34(1): 73–84. [DOI] [PubMed] [Google Scholar]
- 38.Bernstein DPF, L. A.. Childhood Trauma Questionnaire Manual. San Antonio: The Psychological Corporation; 1998. [Google Scholar]
- 39.Walker EA, Unutzer J, Rutter C, et al. Costs of health care use by women HMO members with a history of childhood abuse and neglect. Archives of general psychiatry 1999; 56(7): 609–13. [DOI] [PubMed] [Google Scholar]
- 40.Bower JE, Ganz PA, Irwin MR, Castellon S, Arevalo J, Cole SW. Cytokine genetic variations and fatigue among patients with breast cancer. J Clin Oncol 2013; 31(13): 1656–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Collado-Hidalgo A, Bower JE, Ganz PA, Irwin MR, Cole SW. Cytokine gene polymorphisms and fatigue in breast cancer survivors: Early findings. Brain BehavImmun 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Barsevick A, Frost M, Zwinderman A, Hall P, Halyard M. I’m so tired: biological and genetic mechanisms of cancer-related fatigue. QualLife Res 2010; 19(10): 1419–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van Buuren SG-O, K mice: Multivariate Imputation by Chained Equations in R. Journal of Statistical Software 2011; 45(3): 1–67. [Google Scholar]
- 44.Harel O. The estimation of R^2 and adjusted R^2 in incomplete data sets using multiple imputation. Journal of Applied Statistics 2009; 36: 1109–18. [Google Scholar]
- 45.Cyranowski JM, Bromberger J, Youk A, Matthews K, Kravitz HM, Powell LH. Lifetime depression history and sexual function in women at midlife. Archives of sexual behavior 2004; 33(6): 539–48. [DOI] [PubMed] [Google Scholar]
- 46.Cho HJ, Bower JE, Kiefe CI, Seeman TE, Irwin MR. Early life stress and inflammatory mechanisms of fatigue in the Coronary Artery Risk Development in Young Adults (CARDIA) study. Brain BehavImmun 2012; 26(6): 859–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Heim C, Wagner D, Maloney E, et al. Early adverse experience and risk for chronic fatigue syndrome: results from a population-based study. ArchGenPsychiatry 2006; 63(11): 1258–66. [DOI] [PubMed] [Google Scholar]
- 48.Heim C, Nater UM, Maloney E, Boneva R, Jones JF, Reeves WC. Childhood trauma and risk for chronic fatigue syndrome: association with neuroendocrine dysfunction. ArchGenPsychiatry 2009; 66(1): 72–80. [DOI] [PubMed] [Google Scholar]
- 49.Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat RevNeurosci 2009; 10(6): 434–45. [DOI] [PubMed] [Google Scholar]
- 50.McCrory E, De Brito SA, Viding E. Research review: the neurobiology and genetics of maltreatment and adversity. J Child PsycholPsychiatry 2010; 51(10): 1079–95. [DOI] [PubMed] [Google Scholar]
- 51.Kuhlman KR, Chiang JJ, Horn S, Bower JE. Developmental psychoneuroendocrine and psychoneuroimmune pathways from childhood adversity to disease. Neuroscience and biobehavioral reviews 2017; 80: 166–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chiang JJ, Taylor SE, Bower JE. Early adversity, neural development, and inflammation. Developmental psychobiology 2015; 57(8): 887–907. [DOI] [PubMed] [Google Scholar]
- 53.Miller GE, Chen E, Parker KJ. Psychological stress in childhood and susceptibility to the chronic diseases of aging: moving toward a model of behavioral and biological mechanisms. PsycholBull 2011; 137(6): 959–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fagundes C, LeRoy A, Karuga M. Behavioral Symptoms after Breast Cancer Treatment: A Biobehavioral Approach. Journal of personalized medicine 2015; 5(3): 280–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bower JE, Ganz PA, Desmond KA, Rowland JH, Meyerowitz BE, Belin TR. Fatigue in breast cancer survivors: occurrence, correlates, and impact on quality of life. JClinOncol 2000; 18(4): 743–53. [DOI] [PubMed] [Google Scholar]
- 56.Kober KM, Smoot B, Paul SM, Cooper BA, Levine JD, Miaskowski C. Polymorphisms in Cytokine Genes Are Associated With Higher Levels of Fatigue and Lower Levels of Energy in Women After Breast Cancer Surgery. J Pain Symptom Manage 2016; 52(5): 695–708 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Howard-Anderson J, Ganz PA, Bower JE, Stanton AL. Quality of life, fertility concerns, and behavioral health outcomes in younger breast cancer survivors: a systematic review. J NatlCancer Inst 2012; 104(5): 386–405. [DOI] [PubMed] [Google Scholar]
- 58.Miaskowski C, Dodd M, Lee K, et al. Preliminary Evidence of an Association Between a Functional Interleukin-6 Polymorphism and Fatigue and Sleep Disturbance in Oncology Patients and Their Family Caregivers. J Pain Symptom Manage 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Doong SH, Dhruva A, Dunn LB, et al. Associations between cytokine genes and a symptom cluster of pain, fatigue, sleep disturbance, and depression in patients prior to breast cancer surgery. Biol Res Nurs 2015; 17(3): 237–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bardwell WA, Natarajan L, Dimsdale JE, et al. Objective Cancer-Related Variables Are Not Associated With Depressive Symptoms in Women Treated for Early-Stage Breast Cancer. Journal of Clinical Oncology 2006; 24(16): 2420–7. [DOI] [PubMed] [Google Scholar]
- 61.Abrahams HJ, Gielissen MF, Schmits IC, Verhagen CA, Rovers MM, Knoop H. Risk factors, prevalence, and course of severe fatigue after breast cancer treatment: a meta-analysis involving 12 327 breast cancer survivors. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO 2016; 27(6): 965–74. [DOI] [PubMed] [Google Scholar]
- 62.Donovan KA, Small BJ, Andrykowski MA, Munster P, Jacobsen PB. Utility of a cognitive-behavioral model to predict fatigue following breast cancer treatment. Health Psychol 2007; 26(4): 464–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bevilacqua LA, Dulak D, Schofield E, et al. Prevalence and predictors of depression, pain, and fatigue in older- versus younger-adult cancer survivors. Psycho-oncology 2018; 27(3): 900–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rotonda C, Guillemin F, Bonnetain F, Velten M, Conroy T. Factors associated with fatigue after surgery in women with early-stage invasive breast cancer. The oncologist 2013; 18(4): 467–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bodtcher H, Bidstrup PE, Andersen I, et al. Fatigue trajectories during the first 8 months after breast cancer diagnosis. Quality of life research : an international journal of quality of life aspects of treatment, care and rehabilitation 2015; 24(11): 2671–9. [DOI] [PubMed] [Google Scholar]


