Abstract
Background
The aim of this study was to assess and compare peripapillary retinal nerve fiber layer (RNFL) thickness in patients with Alzheimer’s disease (AD), primary open-angle glaucoma (POAG), preperimetric glaucoma (PPG), and healthy controls with the use of Spectral Domain Optical Coherence Tomography (SD-OCT).
Material/Methods
Thirty patients with AD, 30 patients with POAG, 30 patients with PPG, and 30 healthy controls were enrolled in this cross-sectional study. Only 1 randomly selected eye of each patient was analyzed. Every subject underwent a thorough ophthalmological examination and OCT of the optic disc. The peripapillary RNFL thickness in each of the 6 sectors and globally was analyzed.
Results
The RNFL was thinnest in patients with POAG. The mean RNFL thickness value was 60.97±12.97 μm and it was significantly lower than in healthy controls (106.30±8.95 μm), patients with PPG (93.20±12.04 μm), and AD patients (95.73±13.52 μm). Mean RNFL thickness in patients with AD was significantly lower when compared to healthy controls, and was higher compared to eyes with POAG, while there were no significant differences compared to patients with PPG.
Conclusions
Neuronal damage in the central nervous system (CNS) also affects to retinal axons. A major problem is to distinguish the cause for a moderate decrease in the RNFL thickness. This is particularly true for patients with glaucoma who have not been diagnosed with changes in the visual field. It is not possible to distinguish the cause of a mild decrease in the RNFL thickness based on the SD-OCT. This may result in misdiagnosis of glaucoma, unnecessary use of anti-glaucoma eye drops, and a delayed diagnosis of AD.
MeSH Keywords: Alzheimer Disease; Glaucoma, Open-Angle; Tomography, Optical Coherence
Background
Alzheimer’s disease (AD) is the most frequent cause of dementia across the world, and with the aging of population the prevalence of the disease is rising. In 2006, 26.6 million people were diagnosed with AD. It is estimated that by 2050 there will be a 4-fold increase in the incidence of the disease [1,2].
In AD there is a gradual dying-back of nerve fibers, which is preceded by the formation of extracellular β-amyloid (Aβ) deposits and intracellular neurofibrillary tangles (NFTs) of tau protein [3,4]. The disease is characterized by a progressive decline in cognitive function, resulting in change of behavior. A diagnosis by exclusion is used to detect AD. This diagnostic method allows the clinician to identify the disease entity’s specific neuropsychological and neurological characteristics and at the same time to eliminate other possible causes of dementia. A final diagnosis can be reached following a histopathological examination of the brain tissue attesting to the presence of the amyloid deposits [5,6]. New diagnostic methods are constantly being developed with the use of brain imaging, such as, for example PET, SPECT, and fMRI [7]. However, these diagnostic technics are costly and not usually available, and there are doubts about whether they are sensitive enough to enable final diagnosis of AD.
In 1986, Hinton et al. were the first to report that neurodegenerative changes in the brain also involve the optic nerve and retina [8]. Examinations carried out with optical coherence tomography (OCT) showed that the peripapillary retinal nerve fiber layer (RNFL) thickness is significantly reduced in patients with AD comparing to healthy controls. The difference in the OCT examination is from 16.64 μm to 8.25 μm and is the biggest in the superior quadrant [9]. The correlation between the clinical stage of AD and the average RNFL thickness is not clear. Most research papers deny a correlation between the Mini-Mental State Examination (MMSE) results and the RNFL thickness [10–14]. Changes in the RNFL thickness are not only due to AD, but can also be linked to other neurodegenerative diseases of the central nervous system (CNS), most importantly, to glaucoma. Primary open-angle glaucoma (POAG) is the most common cause of blindness and the incidence of the disease in people over 40 years of age is 1%. It could be presumed that due to their prevalence, AD and POAG are often coexisting medical conditions, making the diagnosis of both conditions even more difficult. Accordingly, a comparison of the 2 populations of patients and an attempt to find differences regarding the extent and topography of the RNFL damage could be of clinical importance.
The aim of this study was to assess and compare the RNFL thickness in the eyes of patients with AD, POAG, preperymetric glaucoma (PPG), and healthy controls in the individual segments around the optic nerve disc.
Material and Methods
This cross-sectional study, conducted in the years 2016–2018, included patients from the Oftalmika Eye Hospital, Bydgoszcz, Poland. Each patient was examined by MMSE screening for cognitive impairment in order to exclude them if they did not belong to the AD group, and underwent eye examination by an ophthalmologist, including the measurement of visual acuity, tonometry (Icare TAO1i, USA), assessment of the fundus with the Volk lens, and biomicroscopy with anterior angle assessment and also SD-OCT (Spectralis OCT, Heidelberg Engineering, Germany). The common inclusion criteria for the project were the best corrected visual acuity (BCVA) ≥0.6, refractive error between ±3.0 Dsph, and age ≥50 years old. The exclusion criteria were any previous surgical eye procedures (except for uncomplicated cataract surgery), advanced stage of cataract, health condition after head injuries, and other eye and neurological conditions. This study followed the tenets of the Declaration of Helsinki. Informed written consent was obtained from all subjects after explaining the nature and possible consequences of the study. The research was approved by the Bioethics Committee of Nicolaus Copernicus University in Toruń at Collegium Medicum in Bydgoszcz.
A group of patients with POAG was defined based on the diagnostic characteristics of glaucomatous optic neuropathy (focal or diffuse neuroretinal rim thinning, hemorrhage on the edge of the optic nerve disc abnormal cup-to-disc (c/d ratio >0.6, the c/d asymmetry between the 2 eyes 0.2) accompanied by a decreased peripapillary RNFL thickness corresponding to a loss of visual field in perimetry with an open-angle. Glaucomatous losses in the visual field were identified with a computerized threshold perimetry (SITA Standard 24-2, Humphrey Field Analyzer II; Carl Zeiss Meditec). Only reliable visual field tests (fixation loss ≤20%, false-positive rate ≤15% and false-negative rate ≤33%) were included. One of the following changes observed in the 2 consecutive visual field tests were used as a criterion for glaucomatous damage, namely a cluster of 3 or more adjacent points in a typical localization for glaucoma, with p<5% in PSD, and for one of them with p<1% in PSD and/or glaucoma hemifield test (GHT) outside normal limits and/or average PSD value calculated for the entire tested area found in less than 5% of healthy eyes. Patients in moderate stage of glaucoma were included in the study [15,16].
A sample of individuals with PPG included patients with glaucomatous defects of the optic disc detected in ophthalmoscopy, a decreased peripapillary RNFL thickness detected in SD-OCT, without characteristic glaucoma scotoma detected in the visual field testing.
A sample of individuals with AD included patients from Department of Psychiatry, Collegium Medicum, Nicolaus Copernicus University, Bydgoszcz, Poland. A diagnosis of AD was made by a psychiatrist base on the DSM-IV (Diagnostic and Statistical Manual of Mental Disorders) and the criteria of the National Institute on Aging and the Alzheimer’s Association (NIA/AA) confirmed by a positive result from a PET scan [16]. Patients with mild dementia (with the Mini-Mental State Examination score ranging from 11 to 18 points) were qualified for the study. Additional inclusion criteria for the participants were intraocular pressure (IOP) <21 mmHg, absence of changes in the optic nerve head that would suggest glaucoma. Visual field testing requires cooperation, significant concentration and it may be impossible, to perform in patients with AD [17]. Trick et al. performed a study where evaluated visual field test in 61 people with AD at various stages of the disease. The reliable result was obtained only in 55.7% of people [18]. Therefore, we decided that, due to the low reliability of the visual field test, we will not perform in the AD group.
Healthy controls had IOP less than 21 mmHg, healthy optic disc without asymmetry, the RNFL thickness within normal limits, and no abnormalities in visual exam.
The peripapillary RNFL thickness was measured using SD-OCT with a 3.46 mm in diameter scan circle centered on the optic disc (Spectralis, Heidelberg, Germany). The Spectralis OCT combines confocal Scanning Laser Ophthalmoscopy (cSLO) that allows for tracking eye movement and averaging of several OCT B-scans formed in the same retinal location in real time. Spectralis provides peripapillary RNFL thickness values for 4 quadrants (N – nasal, T – temporal, S – superior and I – inferior), 6 sectors (N – nasal, NS – nasal-superior, T – temporal, TS – temporal-superior, NI – nasal-inferior), and global mean values (360 degrees).
Only 1 randomly selected eye of each patient was analyzed.
Statistical calculations were conducted with Statistica 12. The results obtained in the studied groups and in the control group were analyzed using one-way analysis of variance ANOVA or in the absence of normal distribution the Kruskal-Wallis test by ranks. For the remaining variables, the t test was used or in the absence of normal distribution of the compared parameters the Mann-Whitney U test was administered. Unless otherwise indicated, the data are given are ± mean values and standard deviation (SD) with the p-value threshold for statistical significance of less than 0.05.
Results
The study groups consisted of 30 patients with AD, 30 patients with POAG, 30 patients with PPG, and 30 healthy controls. The groups were well matched by age and sex distribution. Demographic data are presented in Table 1.
Table 1.
Demographic and clinical data of the groups.
| AD | POAG | PPG | Controls | p-Value* | |
|---|---|---|---|---|---|
| Number of participants | 30 | 30 | 30 | 30 | – |
| Women/Men | 18/12 | 15/15 | 17/13 | 17/13 | – |
| Age** | 69.97±7.07 | 69.20±8.58 | 69.10±5.69 | 69.70±7.48 | 0.962 |
| Best corrected visual acuity** (Snellen) | 0.94±0.12 | 0.91±0.13 | 0.97±0.07 | 0.96±0.07 | 0.174 |
| Intraocular pressure** (mmHg) | 16.97±2.49 | 19.07±3.87 | 17.81±2.52 | 16.09±2.31 | 0.001 |
AD – Alzheimer’s disease; POAG – primary open-angle glaucoma; PPG – preperymetric glaucoma.
ANOVA test, P-value <0.05 was considered to be statistically significant;
M ±SD.
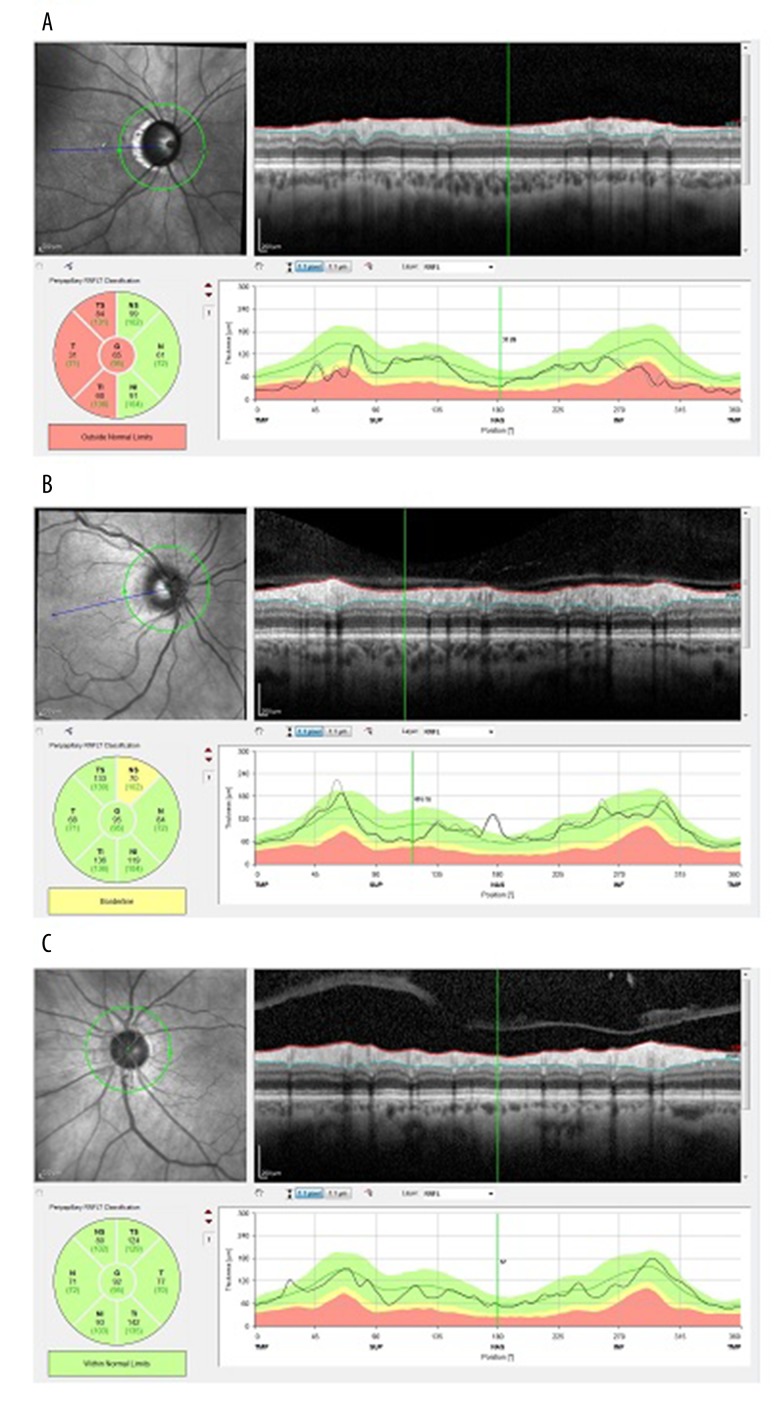
Age differences were not statistically significant (p>0.05). BCVA of the study subjects was not statistically different between the groups (p>0.05), while intraocular pressure values, despite the treatment used in individuals with glaucoma, were higher in the POAG group despite the treatment. Figure 1 shows examples of peripapillary RNFL thickness exam in eyes from subsequent groups.
Figure 1.
Examples of peripapillary RNFL measurement in the 3 examined groups: (A) Significant reduction of RNFL thickness in all temporal sectors in patient with diagnosis of POAG. The red color indicates significant difference in respect to normative database. (B) In eye with diagnosis of PPG, only mild reduction of RNFL thickness compared to normative database in nasal temporal sector is visible. (C) In the eye of patient with AD, the peripapillary RNFL thickness is within normal limits.
The thinnest peripapillary RNFL was observed in patients with POAG. The global mean value for all quadrants was 60.97±12.97 μm and was significantly lower when compared with healthy eyes (106.30±8.95 μm), eyes with PPG (93.20±12.04 μm) and eyes of patients with AD (95.73±13.52 μm). Comparison of global peripapillary RNFL thickness is presented in Figure 2.
Figure 2.
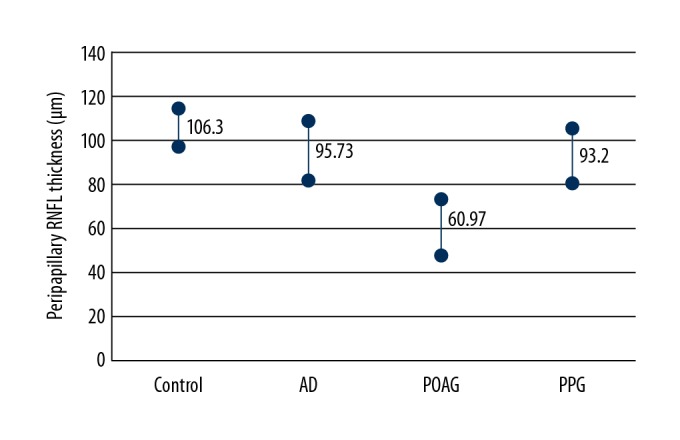
Comparison of the global average peripapillary RNFL thickness. The values in AD groups were significantly lower compared to normal control and higher than in eyes from the POAG group. The differences between AD and PPG were not statistically significant. AD – Alzheimer’s disease; POAG – primary open-angle glaucoma; PPG – preperymetric glaucoma.
The average global peripapillary RNFL thickness value in patients with AD was significantly lower when compared with healthy controls and significantly higher than in patients with POAG. However, the differences were statistically insignificant compared with eyes of patient with PPG. Comparison of peripapillary RNFL thickness in each sector is presented in Figure 3.
Figure 3.
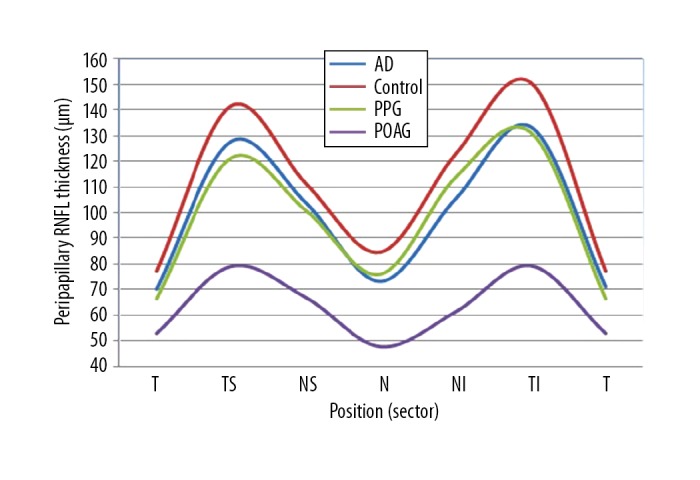
Graphical presentation demonstrates reduction of average peripapillary RNFL in all sectors in POAG, PPG, and AD compared to normal controls. AD – Alzheimer’s disease; POAG – primary open-angle glaucoma; PPG – preperymetric glaucoma; T – temporal sector; TS – temporal-superior sector; NS – nasal-superior sector; N – nasal sector; NI – nasal-inferior sector; TI – temporal-inferior sector.
In all the studied groups, the RNFL thickness value was highest in the temporal-inferior and temporal-superior quadrants. The lowest value, regardless of the reason for RNFL thinning, was reported in the nasal and temporal quadrants. The differences between AD and POAG group regarding average peripapillary RNFL thickness were significant in all sectors and between AD and controls in all sectors except for the nasal-superior. Comparison of average peripapillary RNFL thickness within sectors between AD and PPG did not reveal significant changes in all sectors; however, in the temporal sector this difference was close to the statistical significance. The values of RNFL thickness within sector in subsequent groups are presented in Table 2.
Table 2.
Comparison of average peripapillary RNFL thickness in subsequent sectors and globally between AD patients and POAG, PPG and healthy controls.
| AD | POAG | p-Value | AD | PPG | p-Value | AD | Controls | p-Value* | |
|---|---|---|---|---|---|---|---|---|---|
| RNFL T (μm) | 71.23± 10.1 | 52.93± 22.53 | <0.001 | 71.23± 10.1 | 66.20± 12.75 | 0.051 | 71.23± 10.1 | 77.17± 14.59 | 0.039 |
| RNFL TS (μm) | 127.57± 23,86 | 78.97± 22.39 | <0.001 | 127.57± 23,86 | 121.30± 20.61 | 0.144 | 127.57± 23,86 | 141.17± 15.85 | 0.006 |
| RNFL NS (μm) | 103.67± 23.84 | 66.83± 24.41 | <0.001 | 103.67± 23.84 | 100.70± 18.38 | 0.299 | 103.67± 23.84 | 111.27± 16.44 | 0.081 |
| RNFL N (μm) | 73.27± 18.01 | 47.77± 16.49 | <0.001 | 73.27± 18.01 | 75.77± 14.69 | 0.283 | 73.27± 18.01 | 84.60± 16.19 | 0.007 |
| RNFL NI (μm) | 105.30± 29.28 | 61.33± 24.74 | <0.001 | 105.30± 29.28 | 113.73± 29.28 | 0.117 | 105.30± 29.28 | 122.77± 26.55 | 0.025 |
| RNFL TI (μm) | 133.27± 22.29 | 79.23± 33.35 | <0.001 | 133.27± 22.29 | 131.33± 24.59 | 0.309 | 133.27± 22.29 | 150.63± 15.95 | <0.001 |
| RNFL G (μm) | 95.73± 13.52 | 60.97± 12.97 | <0.001 | 95.73± 13.52 | 93.20± 12.04 | 0.184 | 95.73± 13.52 | 106.30± 8.95 | <0.001 |
AD – Alzheimer’s disease; POAG – primary open-angle glaucoma; PPG – preperymetric glaucoma; T – temporal sector; TS – temporal-superior sector; NS – nasal-superior sector; N – nasal sector; NI – nasal-inferior sector; TI – temporal-inferior sector; G – global.
Independent t-test, P-value < 0.05 was considered to be statistically significant.
Discussion
Our study confirmed the differences in the peripapillary RNFL thickness among the groups of healthy controls, patients with POAG, and patients with AD. Previous research regarding the changes in RNFL in patients with AD suggested that that RNFL thickness analysis may be considered a diagnostic biomarker of this disease [19–21]. In 2001, Parisi et al. used Stratus OCT 3 to study individuals with a diagnosed AD. They compared the results obtained in the study with the values obtained in healthy controls. The analysis showed a decrease in peripapillary RNFL thickness (P<0.01) in all 4 quadrants [13]. Some analyses showed that statistically significant differences occur in the superior quadrants only [22–24] or in the superior and inferior ones [10,25]. It was suggested that the likely reason for the variation in the above results was the stage of the disease assessed with the Mini-Mental State Examination (MMSE) test. Kelsey et al. showed a correlation between the MMSE results and the decreased peripapillary RNFL thickness, suggesting that retinal nerve fiber degeneration and the damage to the CNS are simultaneous [10]. In studies in which patients scored low on the MMSE test (Parisini et al. and Iseri el al.), statistically significant decreases in RNFL thickness were observed in all the quadrants [13,26]. Studies by Berisha et al. and Paquet et al., in which patients with AD scored high on the MMSE test, reported decreased RNFL thickness only in the superior quadrants, postulating this to be the location of early retinal damage in patients with AD [14,27].
AD and POAG are multifactorial, chronic, and age-related neurodegenerative diseases. It is important to note that the optic nerve and retina develop as a direct extension of the diencephalon during the embryonic development stage; therefore, abnormalities that occur in the CNS in the case of AD can also be observed at the fundus of the eye [28]. It is believed that nerve cell damage may have a common pathogenesis, so the topics of common risk factors and mediators responsible for the emergence and development of AD and POAG are increasingly raised [29]. Yoneda et al. [30], in their investigation of the pathogenesis of glaucoma, identified a significant decrease in Aβ as well as an increase in the abnormal tau protein in the vitreous humor. Similar changes in the amount of these proteins occur in the cerebrospinal fluid (CSF) of people with AD [31]. McKinnon et al., in an animal models study, suggested that the cause of retinal ganglion cells (RGC) death in patients with ocular hypertension may be chronic neurotoxicity due to Aβ induced by increased of IOP, which at the molecular level resembles AD [32]. These results support the hypothesis that neurodegenerative changes in the eye with glaucoma may have the same pathogenesis as in the case of AD. In addition to specific neuropathological changes caused by abnormal proteins, impairment of vascular are also strictly associated neurodegenerative diseases [33]. The most important modifiable risk factor contributing to the development and progression of AD and POAG is a high-level of blood pressure variability (BPV) [34–37]. Lattanzi et al. conducted a study that evaluated visit-to-visit BPV in a cohort of patients with AD and healthy controls, finding that AD patients had significantly higher-level BPV compared with age-matched cognitive normal controls, which also confirms the influence of BPV on the pathogenesis and progression of AD [38]. In a postmortem study of 291 brains, significantly fewer neuritic plaques and neurofibrillary tangles were found in the group of people with treated hypertension than in the nonhypertensive group, suggesting that antihypertensive agents may interfere with AD-associated neuropathology [39]. Research results also suggest that reducing BPV by antihypertensive therapy can effectively decrease the risk and delay the development of cognitive impairment [40,41]. Similarly, obstructive sleep apnea syndrome and impairment of cerebrovascular reactivity are other equally important risk factors for the occurrence and progression of glaucomatous neuropathy and dementia [42–46]. BPV, obstructive sleep apnea syndrome, and impaired cerebrovascular reactivity are vascular risk factors resulting in impaired blood flow in the form of ischemia-reperfusion injury. Death of nerve cells via apoptosis occurs as a result of metabolic disturbances, impaired retrograde transport of neurotrophins, increased vascular permeability, oxidative stress, and increased inflammatory cytokine synthesis [47–50].
In AD and POAG, we deal with axonal damage in the retina. A review of publications available in PubMed showed that only Eraslan et al. compared peripapillary RNFL thickness in normal tension glaucoma (NTG) patients with AD patients and the control group [51]. Contrary to our analysis, the result of that study did not show statistically significant differences in RNFL thickness of patients diagnosed with glaucoma and those with AD (p>0.05). Research to date has not assessed the correlation of changes in RNFL thickness in patients with AD and individuals diagnosed with glaucoma despite the absence of losses in the visual field. Our data indicate that there are no significant differences in BCVA, IOP, and peripapillary RNFL thickness among the groups of AD patients and patients with PPG. We believe that this may lead to the misdiagnosis of PPG in patients who in fact suffer from dementia.
Thinning of RNFL is also observed in other neurological diseases, such as Parkinson’s disease [52–54], multiple sclerosis, dementia with Lewy bodies [55], inflammation of the optic nerve [56], and migraines [57]. Further research is necessary to find a biomarker specific for Alzheimer’s disease. In vivo imaging of extracellular amyloid deposits in retina layers is emerging as the most appropriate way to achieve this. Maya Koronyo-Hamaoui et al. identified post mortem Aβ deposits in the retina of patients with suspected or diagnosed AD, and the image corresponded with the histological brain examination [58].
Conclusions
RNFL thickness measured with OCT can be an additional diagnostic tool for AD. Analyses of RNFL thickness prove that neural damage to the CNS also involve axonal damage of the cells in the retina. A major difficulty is to distinguish the cause of mild reduction in RNFL thickness. This is particularly true for glaucoma patients with no changes in the visual field. This may result in misdiagnosis of glaucoma, unnecessary use of anti-glaucoma eye drops, and a delayed diagnosis of AD. In cases of decreased RNFL thickness, it seems particularly important to pay attention to symptom suggesting dementia.
Footnotes
Source of support: Departmental sources
Conflicft of interests
None.
References
- 1.Brookmeyer R, Johnson E, Ziegler-Graham K, Arrighi HM. Forecasting the global burden of Alzheimer’s disease. Alzheimers Dement. 2007;3(3):186–91. doi: 10.1016/j.jalz.2007.04.381. [DOI] [PubMed] [Google Scholar]
- 2.Sandeep CS, Sukesh KA. A review on the early diagnosis of Alzheimer’s disease (AD) through different tests, techniques and databases. AMSE Journals Modelling C. 2015;76(1):1–22. [Google Scholar]
- 3.Dickson DW. Apoptotic mechanisms in Alzheimer neurofibrillary degeneration: cause or effect? J Clin Invest. 2004;114(1):23–27. doi: 10.1172/JCI22317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fernández-Albarral JA, Salobrar-García E, Martínez-Páramo R, et al. Retinal glial changes in Alzheimer’s disease – A review. J Optom. :2018. doi: 10.1016/j.optom.2018.07.001. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Takeda S, Sato N, Morishita R. Systemic inflammation, blood-brain barrier vulnerability and cognitive/non-cognitive symptoms in Alzheimer disease: Relevance to pathogenesis and therapy. Front Aging Neurosci. 2014;6:171. doi: 10.3389/fnagi.2014.00171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.American Psychiatric Association, & American Psychiatric Association. DSM-IV-TR: Diagnostic and statistical manual of mental disorders, text revision. Vol. 75. Washington, DC: American Psychiatric Association; 2000. pp. 78–85. [Google Scholar]
- 7.Jack CR, Jr, Lowe VJ, Senjem ML, et al. 11C PiB and structural MRI provide complementary information in imaging of Alzheimer’s disease and amnestic mild cognitive impairment. Brain. 2008;131(3):665–80. doi: 10.1093/brain/awm336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hinton DR, Sadun AA, Blanks JC, Miller CA. Optic-nerve degeneration in Alzheimer’s disease. N Engl J Med. 1986;315(8):485–87. doi: 10.1056/NEJM198608213150804. [DOI] [PubMed] [Google Scholar]
- 9.Thomson KL, Yeo JM, Waddell B, et al. A systematic review and meta-analysis of retinal nerve fiber layer change in dementia, using optical coherence tomography. Alzheimers Dement. 2015;1(2):136–43. doi: 10.1016/j.dadm.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kesler A, Vakhapova V, Korczyn AD, et al. Retinal thickness in patients with mild cognitive impairment and Alzheimer’s disease. Clin Neurol Neurosurg. 2011;113(7):523–26. doi: 10.1016/j.clineuro.2011.02.014. [DOI] [PubMed] [Google Scholar]
- 11.Günes A, Demirci S, Tok L, et al. Evaluation of retinal nerve fiber layer thickness in Alzheimer’s disease using spectral-domain optical coherence tomography. Turk J Med Sci. 2013;54:5953–58. [PubMed] [Google Scholar]
- 12.Kirbas S, Turkyilmaz K, Anlar O, et al. Retinal nerve fiber layer thickness in patients with Alzheimer disease. J Neuroophthalmol. 2013;33(1):58–61. doi: 10.1097/WNO.0b013e318267fd5f. [DOI] [PubMed] [Google Scholar]
- 13.Parisi V, Restuccia R, Fattapposta F, et al. Morphological and functional retinal impairment in Alzheimer’s disease patients. Clin Neurophysiol. 2001;112(10):1860–67. doi: 10.1016/s1388-2457(01)00620-4. [DOI] [PubMed] [Google Scholar]
- 14.Berisha F, Feke GT, Trempe CL, et al. Retinal abnormalities in early Alzheimer’s disease. Invest Ophthalmol Vis Sci. 2007;48(5):2285–89. doi: 10.1167/iovs.06-1029. [DOI] [PubMed] [Google Scholar]
- 15.Susanna R, Jr, Vessani RM. Staging glaucoma patient: Why and how? Open Ophthalmol J. 2009;3:59–64. doi: 10.2174/1874364100903010059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee EJ, Lee KM, Lee SH, Kim TW. OCT angiography of the peripapillary retina in primary open-angle glaucoma. Invest Ophthalmol Vis Sci. 2016;57(14):6265–70. doi: 10.1167/iovs.16-20287. [DOI] [PubMed] [Google Scholar]
- 16.McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):263–69. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Diniz-Filho A, Delano-Wood L, Daga FB, et al. Association between neurocognitive decline and visual field variability in glaucoma. JAMA Ophthalmol. 2017;135(7):734–39. doi: 10.1001/jamaophthalmol.2017.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Trick GL, Trick LR, Morris P, Wolf M. Visual field loss in senile dementia of the Alzheimer’s type. Neurology. 1995;45(1):68–74. doi: 10.1212/wnl.45.1.68. [DOI] [PubMed] [Google Scholar]
- 19.Polo V, Garcia-Martin E, Bambo MP, et al. Reliability and validity of Cirrus and Spectralis optical coherence tomography for detecting retinal atrophy in Alzheimer’s disease. Eye. 2014;28(6):680–90. doi: 10.1038/eye.2014.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kang BH, Kim JI. Decreased retinal thickness in patients with Alzheimer’s disease. Journal of the Korean Neurological Association. 2013;31(3):173–77. [Google Scholar]
- 21.Ascaso FJ, Cruz N, Modrego PJ, et al. Retinal alterations in mild cognitive impairment and Alzheimer’s disease: An optical coherence tomography study. J Neurol. 2014;261(8):1522–30. doi: 10.1007/s00415-014-7374-z. [DOI] [PubMed] [Google Scholar]
- 22.Chi Y, Wang YH, Yang L. [The investigation of retinal nerve fiber loss in Alzheimer’s disease]. Zhonghua Yan Ke Za Zhi. 2010;46(2):134–39. [in Chinese] [PubMed] [Google Scholar]
- 23.Shen Y, Shi Z, Jia R, et al. The attenuation of retinal nerve fiber layer thickness and cognitive deterioration. Front Cell Neurosci. 2013;7:142. doi: 10.3389/fncel.2013.00142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kromer R, Serbecic N, Hausner L, et al. Detection of retinal nerve fiber layer defects in Alzheimer’s disease using SD-OCT. Front Psychiatry. 2014;5:22. doi: 10.3389/fpsyt.2014.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lu Y, Li Z, Zhang X, et al. Retinal nerve fiber layer structure abnormalities in early Alzheimer’s disease: evidence in optical coherence tomography. Neurosci Lett. 2010;480(1):69–72. doi: 10.1016/j.neulet.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 26.Iseri PK, Altinas Ö, Tokay T, Yüksel N. Relationship between cognitive impairment and retinal morphological and visual functional abnormalities in Alzheimer disease. J Neuroophthalmol. 2006;26(1):18–24. doi: 10.1097/01.wno.0000204645.56873.26. [DOI] [PubMed] [Google Scholar]
- 27.Paquet C, Boissonnot M, Roger F, et al. Abnormal retinal thickness in patients with mild cognitive impairment and Alzheimer’s disease. Neurosci Lett. 2007;420(2):97–99. doi: 10.1016/j.neulet.2007.02.090. [DOI] [PubMed] [Google Scholar]
- 28.Cheung CYL, Ong YT, Ikram MK, et al. Retinal microvasculature in Alzheimer’s disease. J Alzheimers Dis. 2014;42(s4):S339–52. doi: 10.3233/JAD-141596. [DOI] [PubMed] [Google Scholar]
- 29.Bayer AU, Keller ON, Ferrari F, Maag KP. Association of glaucoma with neurodegenerative diseases with apoptotic cell death: Alzheimer’s disease and Parkinson’s disease. Am J Ophthalmol. 2002;133(1):135–37. doi: 10.1016/s0002-9394(01)01196-5. [DOI] [PubMed] [Google Scholar]
- 30.Yoneda S, Hara H, Hirata A, et al. Vitreous fluid levels of β-amyloid (1–42) and tau in patients with retinal diseases. Jpn J Ophthalmol. 2005;49(2):106–8. doi: 10.1007/s10384-004-0156-x. [DOI] [PubMed] [Google Scholar]
- 31.Engelborghs S, De Vreese K, Van de Casteele T, et al. Diagnostic performance of a CSF-biomarker panel in autopsy-confirmed dementia. Neurobiol Aging. 2008;29(8):1143–59. doi: 10.1016/j.neurobiolaging.2007.02.016. [DOI] [PubMed] [Google Scholar]
- 32.McKinnon SJ. Glaucoma: Ocular Alzheimer’s disease. Front Biosci. 2003;8(Suppl):1140–56. doi: 10.2741/1172. [DOI] [PubMed] [Google Scholar]
- 33.Hays CC, Zlatar ZZ, Wierenga CE. The utility of cerebral blood flow as a biomarker of preclinical Alzheimer’s disease. Cell Mol Neurobiol. 2016;36(2):167–79. doi: 10.1007/s10571-015-0261-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lattanzi S, Luzzi S, Provinciali L, Silvestrini M. Blood pressure variability predicts cognitive decline in Alzheimer’s disease patients. Neurobiol Aging. 2014;35(10):2282–87. doi: 10.1016/j.neurobiolaging.2014.04.023. [DOI] [PubMed] [Google Scholar]
- 35.Lee NY, Jung Y, Han K, Park CK. Fluctuation in systolic blood pressure is a major systemic risk factor for development of primary open-angle glaucoma. Sci Rep. 2017;7:43734. doi: 10.1038/srep43734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lattanzi S, Brigo F, Vernieri F, Silvestrini M. Visit-to-visit variability in blood pressure and Alzheimer’s disease. J Clin Hypertens. 2018;20(5):918–24. doi: 10.1111/jch.13290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lattanzi S, Luzzi S, Provinciali L, Silvestrini M. Blood pressure variability in Alzheimer’s disease and frontotemporal dementia: The effect on the rate of cognitive decline. J Alzheimers Dis. 2015;45(2):387–94. doi: 10.3233/JAD-142532. [DOI] [PubMed] [Google Scholar]
- 38.Lattanzi S, Viticchi G, Falsetti L, et al. Visit-to-visit blood pressure variability in Alzheimer disease. Alzheimer Dis Assoc Disord. 2014;28(4):347–51. doi: 10.1097/WAD.0000000000000040. [DOI] [PubMed] [Google Scholar]
- 39.Hoffman LB, Schmeidler J, Lesser GT, et al. Less Alzheimer disease neuropathology in medicated hypertensive than nonhypertensive persons. Neurology. 2009;72(20):1720–26. doi: 10.1212/01.wnl.0000345881.82856.d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lattanzi S, Vernieri F, Silvestrini M. Blood pressure variability and neurocognitive functioning. J Clin Hypertens. 2018;20(4):645–47. doi: 10.1111/jch.13232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yasar S, Xia J, Yao W, et al. Antihypertensive drugs decrease risk of Alzheimer disease Ginkgo Evaluation of Memory Study. Neurology. 2013;81(10):896–903. doi: 10.1212/WNL.0b013e3182a35228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang S, Xie Y, Yang J, et al. Reduced cerebrovascular reactivity in posterior cerebral arteries in patients with primary open-angle glaucoma. Ophthalmology. 2013;120(12):2501–7. doi: 10.1016/j.ophtha.2013.05.017. [DOI] [PubMed] [Google Scholar]
- 43.Lattanzi S, Carbonari L, Pagliariccio G, et al. Neurocognitive functioning and cerebrovascular reactivity after carotid endarterectomy. Neurology. 2018;90(4):e307–15. doi: 10.1212/WNL.0000000000004862. [DOI] [PubMed] [Google Scholar]
- 44.Chaitanya A, Pai VH, Mohapatra AK, Ve RS. Glaucoma and its association with obstructive sleep apnea: a narrative review. Oman J Ophthalmol. 2016;9(3):125–34. doi: 10.4103/0974-620X.192261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lattanzi S, Brigo F, Silvestrini M. Obstructive sleep apnea syndrome and the nocturnal blood pressure profile. J Clin Hypertens (Greenwich) 2018;20(6):1036–38. doi: 10.1111/jch.13316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lattanzi S, Brigo F, Silvestrini M. Blood pressure profile and nocturnal oxygen desaturation. J Clin Hypertens. 2018;20(4):656–58. doi: 10.1111/jch.13259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sun MH, Pang JHS, Chen SL, et al. Retinal protection from acute glaucoma-induced ischemia-reperfusion injury through pharmacologic induction of heme oxygenase-1. Invest Ophthalmol Vis Sci. 2010;51(9):4798–808. doi: 10.1167/iovs.09-4086. [DOI] [PubMed] [Google Scholar]
- 48.Forman MB, Puett DW, Virmani R. Endothelial and myocardial injury during ischemia and reperfusion: Pathogenesis and therapeutic implications. J Am Coll Cardiol. 1989;13(2):450–59. doi: 10.1016/0735-1097(89)90526-3. [DOI] [PubMed] [Google Scholar]
- 49.De la Torre JC. Alzheimer disease as a vascular disorder: Nosological evidence. Stroke. 2002;33(4):1152–62. doi: 10.1161/01.str.0000014421.15948.67. [DOI] [PubMed] [Google Scholar]
- 50.Silvestrini M, Pasqualetti P, Baruffaldi R, et al. Cerebrovascular reactivity and cognitive decline in patients with Alzheimer disease. Stroke. 2006;37(4):1010–15. doi: 10.1161/01.STR.0000206439.62025.97. [DOI] [PubMed] [Google Scholar]
- 51.Eraslan M, Cerman E, Cekic O, et al. Neurodegeneration in ocular and central nervous systems: Optical coherence tomography study in normal-tension glaucoma and Alzheimer disease. Turk J Med Sci. 2015;45(5):1106–14. doi: 10.3906/sag-1406-145. [DOI] [PubMed] [Google Scholar]
- 52.Moschos MM, Tagaris G, Markopoulos I, et al. Morphologic changes and functional retinal impairment in patients with Parkinson disease without visual loss. Eur J Ophthalmol. 2011;21(1):24–29. doi: 10.5301/ejo.2010.1318. [DOI] [PubMed] [Google Scholar]
- 53.Albrecht P, Müller AK, Südmeyer M, et al. Optical coherence tomography in parkinsonian syndromes. PLoS One. 2012;7(4):e34891. doi: 10.1371/journal.pone.0034891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Adam CR, Shrier E, Ding Y, et al. Correlation of inner retinal thickness evaluated by spectral-domain optical coherence tomography and contrast sensitivity in Parkinson disease. J Neuroophthalmol. 2013;33(2):137–42. doi: 10.1097/WNO.0b013e31828c4e1a. [DOI] [PubMed] [Google Scholar]
- 55.Moreno-Ramos T, Benito-León J, Villarejo A, Bermejo-Pareja F. Retinal nerve fiber layer thinning in dementia associated with Parkinson’s disease, dementia with Lewy bodies, and Alzheimer’s disease. J Alzheimers Dis. 2013;34(3):659–64. doi: 10.3233/JAD-121975. [DOI] [PubMed] [Google Scholar]
- 56.Ratchford JN, Quigg ME, Conger A, et al. Optical coherence tomography helps differentiate neuromyelitisoptica and MS optic neuropathies. Neurology. 2009;73(4):302–8. doi: 10.1212/WNL.0b013e3181af78b8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Martinez A, Proupim N, Sanchez M. Retinal nerve fiber layer thickness measurements using optical coherence tomography in migraine patients. Br J Ophthalmol. 2008;92(8):1069–75. doi: 10.1136/bjo.2008.137471. [DOI] [PubMed] [Google Scholar]
- 58.Koronyo-Hamaoui M, Koronyo Y, Ljubimov AV, et al. Identification of amyloid plaques in retinas from Alzheimer’s patients and noninvasive in vivo optical imaging of retinal plaques in a mouse model. Neuroimage. 2011;54:204–17. doi: 10.1016/j.neuroimage.2010.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]