Abstract
Central hypothyrodism (CeH) is a hypothyroid state caused by an insufficient stimulation by thyrotropin (TSH) of an otherwise normal thyroid gland. Several advancements, including the recent publication of expert guidelines for CeH diagnosis and management, have been made in recent years thus increasing the clinical awareness on this condition. Here, we reviewed the recent advancements and give expert opinions on critical issues. Indeed, CeH can be the consequence of various disorders affecting either the pituitary gland or the hypothalamus. Recent data enlarged the list of candidate genes for heritable CeH and a genetic origin may be the underlying cause for CeH discovered in pediatric or even adult patients without apparent pituitary lesions. This raises the doubt that the frequency of CeH may be underestimated. CeH is most frequently diagnosed as a consequence of the biochemical assessments in patients with hypothalamic/pituitary lesions. In contrast with primary hypothyroidism, low FT4 with low/normal TSH levels are the biochemical hallmark of CeH, and adequate thyroid hormone replacement leads to the suppression of residual TSH secretion. Thus, CeH often represents a clinical challenge because physicians cannot rely on the use of the ‘reflex TSH strategy’ for screening or therapy monitoring. Nevertheless, in contrast with general assumption, the finding of normal TSH levels may indicate thyroxine under-replacement in CeH patients. The clinical management of CeH is further complicated by the combination with multiple pituitary deficiencies, as the introduction of sex steroids or GH replacements may uncover latent forms of CeH or increase the thyroxine requirements.
Keywords: thyroxine, thyrotropin, pituitary, thyroid, hormone replacement
Introduction
Central hypothyroidism (CeH) is a rare and heterogenous hypothyroid condition resulting from an insufficient stimulation of an otherwise normal thyroid gland by the hypophyseal thyrotropin hormone (TSH). This loss of central thyroid stimulation can result from a functional or an anatomical disorder of the hypothalamus and/or the pituitary, causing variable TSH secretion modifications (1, 2).
Epidemiology
CeH most frequently occurs as a sporadic form of hypothyroidism and can affect patients of all ages. There is no evidence of male gender prevalence despite the recent finding of X-linked forms (3). CeH incidence was estimated to range from 1:16,000 to about 1:100,000 in different adult or neonatal populations hypothyroid patients (4, 5, 6, 7, 8). This variability in the CeH prevalence seems to be dependent upon several factors, such as the ethnicity and the differences in diagnostic strategy sensitivity. Indeed, the diagnostic strategy used by the Dutch national health system, based on the combined evaluation of total T4 (TT4), thyroxine-binding globulin and TSH (6), has proven to be more sensitive and to uncover milder forms of neonatal CeH that are not detected by the diagnostic strategy used in Japan and USA, based on the combination of low TT4 and normal/low TSH (5, 7). Despite the possible association with life-threatening adrenal crisis in congenital multiple pituitary hormone defeciencies (MPHDs), CeH is not a direct cause of death. The addition of acquired forms of CeH on top of the incidence reported among Dutch newborns raises the suspicion that the prevalence of CeH in the general population is underestimated.
Pathogenesis
The pathogenic mechanisms underlying CeH are still undetermined in several cases although they variably involve both hypothalamic and pituitary cells. CeH can be either congenital or acquired. Congenital CeH is usually manifest in infancy, but has sometimes a delayed onset during childhood or adulthood. Genes causative for CeH can be divided into those leading to isolated form or to combined forms with an MPHD and are listed in Table 1. Nevertheless, more recently, other genes and syndromes have been variably associated with thyrotropin defects (see also Table 1).
Table 1.
Candidate genes for inherited CeH forms and related phenotypes.
| Gene | OMIM | Inheritance | Phenotype |
|---|---|---|---|
| Isolated CeH | |||
| TSHβ | 188540 | AR | Neonatal onset with low TSH, high aGSU and normal PRL circulating levels, pituitary hyperplasia reversible on L-T4 |
| TRHR | 188545 | AR | Normal TSH and low PRL circulating levels, blunted TSH/PRL responses to TRH, male index cases with growth retardation and overweight during childhood; one female proband with prolonged neonatal jaundice |
| TBL1X | 300196 | X-linked | Mild isolated CeH in males with normal TSH circulating levels and normal response to TRH stimulation test (only 1 out of 11 female carriers have CeH); hearing defects |
| IRS4 | 300904 | X-linked | Mild isolated CeH in males with normal TSH circulating levels, blunted TSH response to TRH |
| Multiple pituitary hormone deficiencies | |||
| IGSF1 | 300137 | X-linked | Mild CeH with normal TSH circulating levels and blunted response to TRH stimulation; males are preferentially affected but low FT4 can be found also in a minority of the female carriers, likely due to skewed X-chromosome inactivation; associated with low PRL levels, variable GH deficiency, transient mild hypocortisolism and metabolic syndrome; late adrenarche and delayed rise of testosterone in males, dissociated from testicular growth ending in post-pubertal macrorchidism |
| PUO1F1 | 173110 | AR, AD | Variable age of onset, associated with GH and PRL deficiency, prominent forehead, midface hypoplasia, depressed nose |
| PROP1 | 601538 | AR | Variable age of onset, combined with GH, PRL LH/FSH deficiencies and delayed ACTH defects, small to large pituitary volume |
| HESX1 | 601802 | AR, AD | Hypopituitarism associated with septo-optic dysplasia |
| SOX3 | 313430 | X-linked | Anterior pituitary hypoplasia with ectopic posterior pituitary, persistent cranio-pharyngeal canal and learning difficulties |
| OTX2 | 600037 | AD | Anterior pituitary hypoplasia with ectopic posterior pituitary and ocular defects (ano-/micro-ophthalmia/retinal dystrophy) |
| LHX3 | 600577 | AR | Hypopituitarism with inconstant ACTH defect, small to large pituitary, short and rigid cervical spine and variable hearing defect |
| LHX4 | 602146 | AR, AD | Variable hypopituitarism, anterior pituitary hypoplasia with ectopic posterior pituitary, Arnold–Chiari syndrome, hypoplasia of the corpus callosum |
| LEPR | 601007 | AR | CeH with hyperphagia, obesity and combined with central hypogonadism |
| Genetic defects inconstantly associated with CeH | |||
| SOX2 | 184429 | AD | Variable hypopituitarism, pituitary hypoplasia, microphthalmia, variable learning difficulties |
| NFKB2 | 164012 | AD | Deficient anterior pituitary with variable immune deficiency (DAVID) syndrome associated with ACTH deficiency and variable GH and TSH defects |
| CHD7 | 608892 | AD | CHARGE syndrome (Coloboma, Heart anomaly, choanal Atresia, Retardation, Genital and Ear anomalies) with ectopic posterior pituitary and variable LH/FSH, TSH and GH defects |
| FGFR1 | 136350 | AD | Kallmann’s syndrome (KS) and normosmic congenital hypogonadotropic hypogonadism (nCHH), variable association with defects of other pituitary hormones including TSH, septo-optic dysplasia and ectopic posterior pituitary |
| FGF8 | 600483 | AR | KS and nCHH, variable associations with defects of other pituitary hormones including TSH, holoprosencephaly and corpus callosum agenesia |
| FOXA2 | 600288 | AD | Hypopituitarism with craniofacial and endoderm-derived organ abnormalities and hyperinsulinism |
| PROKR2 | 607123 | AR, AD | Variable hypopituitarism associated with septo-optic dysplasia or pituitary stalk interruption syndrome |
AD, autosomal dominant; AR, autosomal recessive; OMIM, online mendelian inheritance in men (https://www.ncbi.nlm.nih.gov/omim/.
On the other hand, the acquired forms of CeH are mainly related to expansive lesions of the hypothalamic/pituitary region, although head trauma, vascular accidents, autoimmunity, hemochromatosis or iron overload and several iatrogenic causes are also accountable for several CeH cases. Acquired CeH is definitely more common in the adulthood and is prevalently caused by macroadenomas of the pituitary and their treatments. Craniopharyngiomas represent the most prevalent expansive lesion associated with CeH in pediatric patients. The causes of acquired CeH are listed in Table 2 (see also comments in Fig. 1).
Table 2.
Causes of acquired CeH forms.
| Invasive and/or compressive lesion of the sella turcica region | Pituitary macroadenomas |
| Craniopharyngiomas | |
| Meningiomas and gliomas | |
| Rathke cleft cysts | |
| Metastatic seeding | |
| Carotid aneurysm | |
| Iatrogenic causes | Cranial surgery or irradiation |
| Drugs (e.g., rexinoids or mitotane) | |
| Injuries | Head trauma |
| Traumatic delivery | |
| Vascular accidents | Pituitary infarction |
| Sheenan syndrome | |
| Subarachnoid hemorrhage | |
| Autoimmune disease | Lymphocytic hypophysitis (including the forms induced in post-partum or during therapy with check-point inhibitors) |
| Infiltrative lesions | Iron overload |
| Sarcoidosis | |
| Histiocytosis X | |
| Infective diseases | Tuberculosis |
| Mycoses | |
| Syphilis |
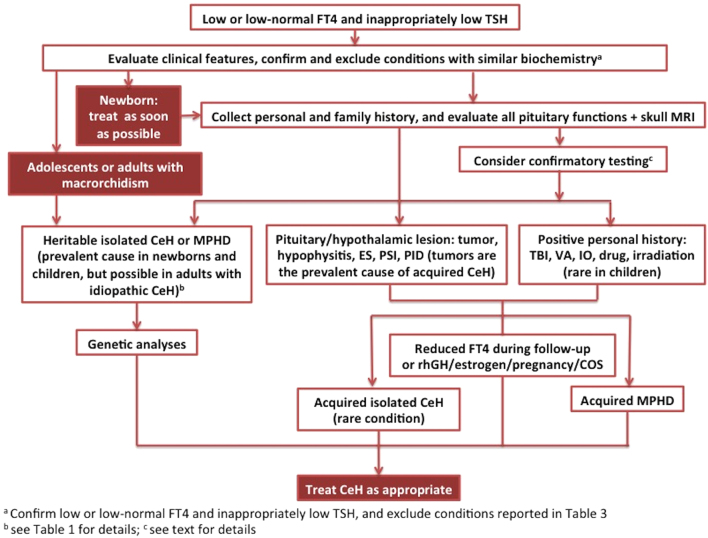
Figure 1.
Flowchart for the diagnosis of CeH. CeH, central hypothyroidism; COS, controlled ovarian stimulation; ES, empty sella; IO, iron overload or hemochromatosis; MPHD, multiple pituitary hormone defect; MRI, magnetic resonance imaging; PID, pituitary infiltrative disease; PSI, pituitary stalk interruption; rhGH, recombinant human growth hormone; TBI, traumatic brain injury; VA, vascular accident.
Altogether these pathological mechanisms can lead to CeH through variable mechanisms:
impaired thyrotrope stimulation by the hypothalamic factors or modifications in the thyroid hormone feedback set-point (e.g. TRH resistance or IGSF1 or TBL1X or IRS4 mutations or hypothalamic lesions) (9, 10, 11, 12);
reduced pituitary TSH reserve (e.g. TSHβ mutations or a deficient number of thyrotrope cells or pituitary lesions causing the loss of the thyrotrope population);
impaired intrinsic bioactivity of the secreted TSH molecules (13, 14, 15, 16, 17).
The three mechanisms are frequently coexisting as a consequence of the expansive lesions of the sella region (16, 17). The impaired bioactivity of circulating TSH has been prevalently demonstrated by in vitro bioassays (15), but this phenomenon can also be supported in vivo by the impaired increment of circulating free T4 and/or T3 following the TSH response upon TRH stimulation test (18, 19).
Clinical presentation
CeH represents a challenging condition in clinical practice as it is characterized by suboptimal accuracy of clinical and biochemical parameters for diagnosis and management. Clinical presentation of CeH may vary depending on the cause. It is worth noting that the typical manifestations of severe congenital hypothyroidism are rarely present at birth in most of the CeH patients since the chorionic gonadotropin could be effective in stimulating the fetal thyroid, differently from a primary thyroid defect, and thyrotrope function is not completely defective in particular when the hypothalamic stimulation is principally affected. Mental retardation can be particularly severe in case of delay in the diagnosis of isolated congenital CeH associated with biallelic TSHβ mutations, due to the false-negative results of the neonatal TSH screening for primary thyroid defects (3, 20, 21, 22, 23, 24, 25). However, when CeH diagnosis is reached in newborns, treatment should be given as soon as possible (Fig. 1).
Genetic CeH can more frequently be part of an MPHD and can be associated with growth retardation, delayed pubertal development and/or variable neurological defects that can be a direct effect of the genetic lesion (Table 1) (1, 26, 27, 28, 29). IGSF1 followed by PROP1 are the genes most frequently accounting for the inheritable forms of CeH. However, a progressive onset of the thyrotrope defect beyond the critical neonatal period can be not infrequently seen in several of these genetic CeH cases (3, 9, 28, 30). On the other hand, some peculiar clinical stigmata illustrated in Table 1 can suggest specific gene defects such as the macrorchidism for IGSF1 or hearing defects for TBL1X (10, 11, 31, 32).
Acquired forms of CeH are usually sporadic and in most cases due to large pituitary macroadenomas with a suprasellar extensions, craniopharyngiomas and suprasellar tumors, head trauma, vascular accident or cranial irradiation (1, 28, 33). In these cases, the tumor size might cause either a defective functionality of the neurohypophysis with an associated diabetes insipidus and/or a compression of the optic chiasm with a direct consequence in the quality of the visual field. Moreover, these lesions usually affect both pituitary and hypothalamus function with a resulting MPHDs clinical picture and hyperprolactinemia secondary to the pituitary stalk resection or compression. Thus, signs and symptoms due to this MHPD condition, such as menstrual disorders, decreased libido, hair loss, galactorrhea, pallor, altered lipid metabolism, visual defect, headache and others might overlap and cover the specific manifestations due to the hypothyroidism. All these manifestations can severely compromise the performance and wellbeing of the patients and generate negative effects on various tissues. Therefore, evaluation for CeH should always be included among the hormone determinations of the patients with diseases of the hypothalamic–pituitary region (34).
Diagnosis
The diagnosis of CeH can be reached by three different means:
clinical manifestations (hypothyroid symptoms) – this is a rare event; a typical example is the poor growth associated with jaundice and muscle hypotonia in infants with congenital CeH;
biochemical findings (low, or even low–normal, free T4 with inappropriately low/normal TSH) – this is the most frequent case possibly occurring at birth because of a positive neonatal screening or during the biochemical workup of patients with known lesions of the hypothalamic–pituitary region or during their follow-up;
genetic investigations following one proband diagnosis – several patients diagnosed with isolated mild CeH in adulthood do not have the perception of being hypothyroid and only the treatment experience can uncover the beneficial effects of thyroid replacement on their wellbeing. The experience with CeH patients diagnosed by genetic testing tells us that gene defects can be a likely cause for mild idiopathic CeH discovered during adolescence or adulthood following an incidental biochemical finding of low free T4 (FT4).
However, diagnostic and clinical management of CeH is still nowadays a challenging condition due to the lack of accurate clinical and biochemical parameters. Very recently, a group of pediatric and adult endocrinologists have produced the 2018 Guidelines European Thyroid Association on the diagnosis and management of CeH (35). In their recommendations, experts agreed that diagnosis of overt CeH should be considered in every subject with low serum concentrations of FT4, measured by reliable immunoassay and low or normal immunoreactive TSH concentration, confirmed on two independent determination (35). A schematic illustration of the diagnostic workup for CeH based on the ETA guidelines is reported in Fig. 1.
Indeed, evidences in a series of CeH patients clearly established that diagnosis of this condition cannot be achieved by the single measurement of TSH. In this respect, the missed diagnosis of CeH represents the most important false-negative result of the ‘reflex TSH’ strategy that is frequently applied for the screening of thyroid function either in newborns or in adults (1, 36). Therefore, the FT4 determination, more than the TT4, which is influenced by the serum-binding protein variation (37, 38) or the FT3, which might be low in some nonthyroidal illness or deiodinase defect rather than in CeH, represents the parameter with the highest diagnostic sensitivity and specificity in this hormone defect (1, 39, 40, 41, 42). As an additional complication, one should remember that slight elevations of serum TSH concentrations can also be found in some CeH patients with a predominant hypothalamic defect. In this subgroup of patients, TSH levels are superimposable to those generally found in subclinical or mild primary hypothyroidism, although the molecule is devoid of full biological activity and the FT4 is already in the hypothyroid range (13, 14, 16, 17). Conditions that can lead to low/normal TSH and low FT4 levels listed in Table 3 should also be ruled out in a correct differential diagnosis. Moreover, the presence of a possible interference in FT4 or TSH measurement should also be considered and excluded (1, 37, 38). Indeed, the FT4 absolute value is related to the assay applied. Although the equilibrium dialysis is the most accurate method for the determination of the FT4 levels, this is not compatible with the routine lab work out, and the automated FT4 assays are commonly used (38). Among these immunoassays, the so-called ‘one-step’ assays are surely less reliable then the ‘two-step’ methods. In these latter methods, an immunoextraction (back-titration) allows the removal of the interfering factors of the serum (e.g., T4/T3-binding auto-antibodies or abnormal-binding proteins). In case of a suspected FT4 assay interference, a ‘two-step’ method or a mass spectrometry should be considered to solve the question. Heterophile antibodies, such as anti-animal antibodies, which might be present in the patient sera, can interfere TSH measurements by immunometric assays, when they are directed against the same species as the assay antibodies. In particular, a heterophile antibody able to block the TSH binding to either capture or detection antibodies will lead to a negative immunoassay interference and a falsely low TSH level determination. This might cause the misdiagnosis of a primary hypothyroidism as a CeH. Due to the frequency of heterophile antibodies, most of the commercial TSH assays contain the pre-immune serum from the source animal in the reagents. Another cause of interference may be represented by the ‘macro-TSH’, which may lead to falsely elevated TSH concentrations. In this case, CeH patients might be interpreted as being affected by primary hypothyroidism. In the real life, a good advise to render less likely a suspected interference is the use of alternative immunoassays including different antibody pairs or the measurement of TSH levels after removal of the interfering immunoglobulins by treating the serum with polyethylene glycol or protein G or by dilution and recovery test (1, 38).
Table 3.
Conditions associated with low/normal TSH and/or low, or even low–normal, free FT4 levels that could lead to erroneous CeH diagnosis or to transient CeH.
| Severe form of nonthyroidal illness or sick euthyroid syndrome |
| Drugs inhibiting TSH secretion: (a) glucocorticoids; (b) dopamine; (c) cocaine; (d) anti-epileptics; (e) anti-psychotics; (f) metformin |
| Thyrotoxicosis-related conditions: Levothyroxine withdrawal syndrome, prolonged TSH suppression after recovery from thyrotoxicosis |
| Pregnancy related conditions: (a) Isolated maternal hypothyroxinemia (to be interpreted in the context of trimester-specific FT4 reference ranges for pregnant women). (b) Premature birth (delayed TSH rise in hypothyroid infants) |
| Genetic conditions: (a) Allen–Herndon–Dudley syndrome (MCT8 gene pathogenic allelic variants); (b) RTHα due to THRA heterozygous mutations; (c) TSHβ allelic variants with conserved bioactivity but lost immunoreactivity of circulating TSH |
Beyond interference, the conditions that can give rise to biochemical results similar to those found in CeH include different groups of conditions listed in Table 3 (see also Fig. 1). In a hospital setting or in the elderly the non-thyroidal illnesses or euthyroid sick syndrome are the most frequent possibility and they are obviously hallmarked by a prevalent and often isolated fall of T3 or free T3 (FT3) levels and by the concomitance of severe or chronic disease states.
Treatments with drugs able to inhibit the TSH secretion or the recovery from a thyrotoxic state can sometimes be confused with a CeH. A careful collection of the personal history, the repetition of the biochemical examination and exclusion of an underlying primary thyroid disease are key to uncover such possibilities. Gestational hypothyroxinemia can come into differential diagnosis with CeH, but this risk may be greatly reduced by the definition of trimester-specific FT4 reference levels. The delayed TSH rise in premature babies can be associated with a transient CeH that is generally of short duration and does not require treatment in most cases. Furthermore, patients with rare inheritable defects of thyroid hormone action can have low FT4 and normal or slightly elevated TSH. However, patients affected with MCT8 or THRA mutations leading to Allan–Herndon–Dudley syndrome or resistance to thyroid hormone α (RTHα) have distinct and typical clinical features and T3 levels at the upper limit of normal range (43). A peculiar confounding condition may be represented by TSHβ allelic variants with conserved bioactivity but lost immunoreactivity of circulating TSH (44). This condition should be suspected when undetectable or low TSH is repeatedly associated with clearly normal and stable thyroid hormone levels and confirmed by an absent TSH rise after TRH stimulation.
In familial, congenital or syndromic CeH cases, genetic analyses should be accomplished (35). The discovery of pathogenic variant(s) in one candidate gene can lead to uncover possible carriers and early diagnosis in the affected families and support the CeH diagnosis in uncertain cases, including those with a delayed evidence or onset of the biochemical abnormalities without an apparent cause. Genetic analysis can be done either with an automated direct sequencing of specific genes, following a phenotype-driven approach or by using a targeted next-generation sequencing technique and thus running in the same time a panel of multiple candidate genes (3, 35).
A still controversial issue is the diagnosis of the hidden or mild forms of CeH usually characterized by FT4 in the lower part of the normal range. Studies performed in patients with primary thyroid disease indicate that replacement therapy of mild or subclinical hypothyroidism may improve the wellbeing and physical or mental performances or prevent cardiovascular morbidities. The same should be true also for mild/hidden forms of CeH, but the lack of a sufficiently sensitive parameter hampers their recognition. In patients at risk, such as those on follow-up for hypothalamic/pituitary lesions or brain cancer survivors, or the carriers of pathogenic variants in novel candidate genes, the diagnosis of CeH can be supported by several investigations or findings (35).
These tests include:
abnormal TSH response to TRH stimulation (17, 45, 46, 47, 48, 49, 50);
blunted nocturnal TSH rise (47);
low TSH index (52);
progressive decrease of FT4 in patients on follow-up (>20% of the initial value) (1, 35, 41);
abnormal findings of parameters of thyroid hormone action (41, 42, 53).
The interpretation of these tests may be controversial and their application depends upon the resources and availability in the different clinical and laboratory settings (1, 35) (Fig. 1).
Therapeutical management
The first-line treatment of central hypothyroidism remains the replacement therapy using levothyroxine (L-T4) (35). Treatment is recommended in all patients receiving the diagnosis once a concomitant cortisol deficit has been excluded. In those presenting adrenal insufficiency or when its presence cannot be excluded, L-T4 supplementation should follow an adequate treatment with glucocorticoid in order to prevent the induction of an adrenal crisis. On the contrary, thyroid hormones enhance GH sensitivity and rise both IGF1 levels and ALS, thus also increasing GH metabolic effect during therapies (54) and allowing a correct evaluation of the somatotrope function.
In adult patients with central diseases, it is recommended to tailor the replacement L-T4 therapy according to the weight and the age of each patient (35). In patients older than 60 years of age and in those with cardiovascular comorbidities, the starting dose should be ranging 1.0–1.2 μg/kg/day. Furthermore, treatment of milder CeH forms (FT4 values within the lower limit of normal range) can be avoided in subjects older than 75 years, as suggested by findings indicating a protective effect of mild or subclinical primary hypothyroidism on cardiovascular mortality risk in the elderly (55). Moreover, in elderly patients, as well as in those with long standing disease and higher cardiovascular risk, the ETA task force advised to start with lower doses of L-T4 and then gradually uptitrate the dosage in subsequent weeks or months (35). It is important to evaluate the adequacy of the replacement after 6–8 weeks measuring FT4 and targeting this parameter above the median values of the reference range. During LT4 replacement, the concomitant determination of TSH is useless in CeH patients with low levels at diagnosis, whereas the lack of TSH secretion suppression in CeH patients with normal TSH concentrations at diagnosis may indicate under replacement. Indeed, all these considerations are valid only if the hormonal examinations are performed before or atleast 4 h after the daily intake of LT4 and are carried out in the same laboratory (1, 35).
Once the therapy has been judged adequate, it should be reevaluated annually measuring serum FT4. Measurement of TSH and T3 could be useful only to exclude the suspect of undertreatment and overtreatment, respectively. An insufficient replacement therapy should be suspected whenever FT4 concentrations are found below or at the lower limit of the normal range, especially when manifestations of hypothyroidism are present or TSH is still within the normal range. In contrast, an excessive LT4 intake should be considered whenever FT4 concentrations are above or at the upper limit of the normal range, in particular when clinical manifestations of thyrotoxicosis and/or high FT3 levels are present (1, 35).
Moreover, an adjustment in levothyroxine dosage may be required in many conditions (listed in Table 4) and FT4 and TSH should be reassessed 4–6 weeks after any change of the regimen.
Table 4.
Conditions requiring a reevaluation and possible adjustment of the replacement therapy.
| Conditions at risk of an uptitration of L-T4 therapy |
| Delay in psychomotor and cognitive development in infants and children |
| Introduction of GH replacement therapy |
| Introduction of estrogen replacement therapy or oral contraceptive |
| Pubertal development |
| Controlled ovarian stimulation |
| Pregnancy |
| Weight gain |
| Introduction of therapies affecting levothyroxine metabolism or absorption |
| Conditions at risk of a downtitration of L-T4 therapy |
| Cardiovascular comorbidities |
| Delivery |
| Menopause |
| Weight loss |
| Discontinuation of GH or estrogen therapies or treatments affecting levothyroxine metabolism or absorption |
Since the thyroid hormone levels are higher during childhood, higher doses of LT4 are required in children (56). In severe congenital CeH, it is mandatory to quickly reach adequate serum FT4 concentrations, and L-T4 treatment should be initiated within 2 weeks from birth at the daily doses commonly used for primary congenital hypothyroidism (10–12 μg/kg/day) (35). In milder congenital forms, the starting doses of the replacement therapy could be lower (L-T4 5–10 μg/kg/day) to avoid an overtreatment and the same holds for diagnoses reached during childhood and adolescence (3.0–5.0 or 2.0–2.4 μg/kg/day, respectively). Once the replacement therapy is started, pediatric patients should be monitored in order to maintain FT4 levels in the reference ranges for age, and their follow-up should be similar to what is done for primary hypothyroidism. In CeH children, LT4 therapy was accompanied by an acceleration of growth velocity thus allowing the attainment of target height (9, 30, 47). A gradual downtitration is obviously required in transition to adulthood (57).
Finally, during pregnancy, it is recommended to increase the hormonal supplementation by 20–50% of the initial dose and to maintain the FT4 levels in the upper quartile of the reference range to compensate the expanding extracellular T4 pool and avoid hypothyroidism in the fetus (58).
Unlike the treatment of primary hypothyroidism, in which TSH is an excellent marker of an adequate replacement, the CeH management is more complex. Even low doses of L-T4 are able to suppress TSH secretion (42, 59). In a comparison between patients with adequately treated primary thyroid disease and other patients presenting hypothalamic–pituitary lesions performed by Koulouri et al. in 2011, it was shown that FT4 levels were significantly lower in central hypothyroidism, suggesting a frequent undertreatment (39). Indeed, in acquired forms of CeH as those following surgery or radiotherapy for pituitary lesions or the start of treatments with an intrinsic risk of CeH, it could prove useful to assess FT4 concentrations before the intervention and LT4 replacement could eventually be targeted to reach prior FT4 levels, but this is impossible in all the other forms of CeH.
The evaluation of biochemical indexes of thyroid hormone metabolism and action at the tissue level, such as SHBG, bone GLA protein for thyrotoxicosis or cholesterol for undertreatment, could sometimes be useful but are too frequently interfered by other hormonal alterations.
Importantly, in MPHDs other major confounders must be taken into account. Both estrogens and GH influence thyroid hormone transport and/or metabolism and consequently interfere CeH management (33, 60). Sex steroid and GH deficiencies can mask an underlying CeH while the introduction of these replacement therapies often requires an uptitration of L-T4; particular attention should also be given to the ovarian stimulation for assisted reproduction procedures because of the associated estrogen rise (61). Controversial is instead the potential influence of glucocorticoid replacement in uncovering a hidden CeH (62). According to these evidences, particular attention should be given to patients with MPHDs whenever new replacement therapies are added or modified.
Other treatments potentially leading to CeH include mitotane, which decreases viability of thyrotrope cells, and the rexinoids (e.g. bexarotene), directly repressing TSHβ expression, as well as novel biological drugs inducing hypophysitis such as ipilimumab or other check-point inhibitors (63). Therefore, TSH and FT4 should be checked before and repeatedly during these treatments. At variance, dopamine agonists and somatostatin analogs exert milder and transient inhibition on thyrotropes.
Interestingly, a prospective study (64), performed on a small number of patients, uncovered a negative metabolic effect of an insufficient treatment of CeH, by evaluating lipid profile and the body fat mass by DEXA scan. These data, besides supporting the necessity of an adequate therapy maintaining FT4 values in the upper range of normality, suggest a negative effect even in the hidden forms of CeH, often undiagnosed.
Whenever there is an insufficient increment of serum FT4 or its decrement during treatment with a given dose of L-T4, malabsorption should be suspected (65).
As for the use of a combined therapy with L-T4 and L-T3 in CeH, it raises the same issues discussed for primary disease. In fact, there is no evidence from the many studies performed both in adults and in children to support a superiority of this treatment over the sole levothyroxine (66, 67, 68). However, it has been demonstrated in the Watts study that patients having a particular polymorphism in DIO2, Thr92Ala, the combined L-T4 and L-T3 therapy leads to a more favorable clinical outcome (69). This suggests that the subjects presenting particular SNPs in genes important for the peripheral regulation of hormones activity (such as deiodinases and thyroid hormone transporters) could benefit from the combined therapy with triiodothyronine according to their genetic background (70). Actually, the above mentioned studies, which failed to find any difference between the two approaches, could lack the power to underline such changes, since the DIO2 polymorphism is relatively infrequent (71). In particular, the combined therapy should be considered in patients lamenting symptoms despite an adequate supplementation with L-T4 alone, following the ETA guidelines (67). However, the lack of a highly reliable parameter to check overtreatment as TSH in primary disease, the combined L-T4/L-T3 replacement may be at a higher risk of thyrotoxicosis in CeH (68).
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of this review.
Funding
This work was partially funded by the Ricerca Corrente funds of the Istituto Auxologico Italiano IRCCS, Milan, Italy (code: 05C303_2013).
References
- 1.Persani L. Clinical review: entral hypothyroidism: pathogenic, diagnostic, and therapeutic challenges. Journal of Clinical Endocrinology and Metabolism 2012. 97 3068–3078. ( 10.1210/jc.2012-1616) [DOI] [PubMed] [Google Scholar]
- 2.Persani L, Beck-Peccoz P. Central hypothyroidism. In Werner and Ingbar’s the Thyroid: A Fundamental and Clinical Text, 10th ed., Chapter 38, pp 560–568. Eds Braverman L. & Cooper D. Philadelphia, PA, USA: Lippincott Williams & Wilkins/Wolters Kluwer Health, 2012. [Google Scholar]
- 3.Persani L, Bonomi M. The multiple genetic causes of central hypothyroidism. Best Practice and Research: Clinical Endocrinology and Metabolism 2017. 31 255–263. ( 10.1016/j.beem.2017.04.003) [DOI] [PubMed] [Google Scholar]
- 4.Price A, Weetman AP. Screening for central hypothyroidism is unjustified. BMJ 2001. 322 798 ( 10.1136/bmj.322.7289.798) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Asakura Y, Tachibana K, Adachi M, Suwa S, Yamagami Y. Hypothalamo-pituitary hypothyroidism detected by neonatal screening for congenital hypothyroidism using measurement of thyroid-stimulating hormone and thyroxine. Acta Paediatrica 2002. 91 172–177. ( 10.1111/j.1651-2227.2002.tb01691.x) [DOI] [PubMed] [Google Scholar]
- 6.Kempers MJE, Lanting CI, Heijst AFJ van, Trotsenburg ASP van, Wiedijk BM, Vijlder JJM de, Vulsma T. Neonatal screening for congenital hypothyroidism based on thyroxine, thyrotropin, and thyroxine-binding globulin measurement: potentials and pitfalls. Journal of Clinical Endocrinology and Metabolism 2006. 91 3370–3376. ( 10.1210/jc.2006-0058) [DOI] [PubMed] [Google Scholar]
- 7.Nebesio TD, McKenna MP, Nabhan ZM, Eugster EA. Newborn screening results in children with central hypothyroidism. Journal of Pediatrics 2010. 156 990–993. ( 10.1016/j.jpeds.2009.12.011) [DOI] [PubMed] [Google Scholar]
- 8.Adachi M, Soneda A, Asakura Y, Muroya K, Yamagami Y, Hirahara F. Mass screening of newborns for congenital hypothyroidism of central origin by free thyroxine measurement of blood samples on filter paper. European Journal of Endocrinology 2012. 166 829–838. ( 10.1530/EJE-11-0653) [DOI] [PubMed] [Google Scholar]
- 9.Bonomi M, Busnelli M, Beck-Peccoz P, Costanzo D, Antonica F, Dolci C, Pilotta A, Buzi F, Persani L. A family with complete resistance to thyrotropin-releasing hormone. New England Journal of Medicine 2009. 360 731–734. ( 10.1056/NEJMbib808557) [DOI] [PubMed] [Google Scholar]
- 10.Sun Y, Bak B, Schoenmakers N, Trotsenburg ASP van, Oostdijk W, Voshol P, Cambridge E, White JK, Tissier P le, Gharavy SNM, et al. Loss-of-function mutations in IGSF1 cause an X-linked syndrome of central hypothyroidism and testicular enlargement. Nature Genetics 2012. 44 1375–1381. ( 10.1038/ng.2453) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heinen CA, Losekoot M, Sun Y, Watson PJ, Fairall L, Joustra SD, Zwaveling-Soonawala N, Oostdijk W, Akker ELT van den, Alders M, et al. Mutations in TBL1X are associated with central hypothyroidism. Journal of Clinical Endocrinology and Metabolism 2016. 101 4564–4573. ( 10.1210/jc.2016-2531) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heinen CA, Vries EM de, Alders M, Bikker H, Zwaveling-Soonawala N, Akker ELT van den, Bakker B, Hoorweg-Nijman G, Roelfsema F, Hennekam RC, et al. Mutations in IRS4 are associated with central hypothyroidism. Journal of Medical Genetics 2018. 55 693–700. ( 10.1136/jmedgenet-2017-105113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Faglia G, Bitensky L, Pinchera A, Ferrari C, Paracchi A, Beck-Peccoz P, Ambrosi B, Spada A. Thyrotropin secretion in patients with central hypothyroidism: evidence for reduced biological activity of immunoreactive thyrotropin. Journal of Clinical Endocrinology and Metabolism 1979. 48 989–998. ( 10.1210/jcem-48-6-989) [DOI] [PubMed] [Google Scholar]
- 14.Beck-Peccoz P, Amr S, Menezes-Ferreira MM, Faglia G, Weintraub BD. Decreased receptor binding of biologically inactive thyrotropin in central hypothyroidism. Effect of treatment with thyrotropin-releasing hormone. New England Journal of Medicine 1985. 312 1085–1090. ( 10.1056/NEJM198504253121703) [DOI] [PubMed] [Google Scholar]
- 15.Persani L, Tonacchera M, Beck-Peccoz P, Vitti P, Mammoli C, Chiovato L, Elisei R, Faglia G, Ludgate M, Vassart G. Measurement of cAMP accumulation in Chinese hamster ovary cells transfected with the recombinant human TSH receptor (CHO-R): a new bioassay for human thyrotropin. Journal of Endocrinological Investigation 1993. 16 511–519. ( 10.1007/BF03348894) [DOI] [PubMed] [Google Scholar]
- 16.Horimoto M, Nishikawa M, Ishihara T, Yoshikawa N, Yoshimura M, Inada M. Bioactivity of thyrotropin (TSH) in patients with central hypothyroidism: comparison between in vivo 3,5,3′-triiodothyronine response to TSH and in vitro bioactivity of TSH. Journal of Clinical Endocrinology and Metabolism 1995. 80 1124–1128. ( 10.1210/jcem.80.4.7714080) [DOI] [PubMed] [Google Scholar]
- 17.Persani L, Ferretti E, Borgato S, Faglia G, Beck-Peccoz P. Circulating thyrotropin bioactivity in sporadic central hypothyroidism. Journal of Clinical Endocrinology and Metabolism 2000. 85 3631–3635. ( 10.1210/jcem.85.10.6895) [DOI] [PubMed] [Google Scholar]
- 18.Beck-Peccoz P, Persani L. Variable biological activity of thyroid-stimulating hormone. European Journal of Endocrinology 1994. 131 331–340. ( 10.1530/eje.0.1310331) [DOI] [PubMed] [Google Scholar]
- 19.Roche EF, McGowan A, Koulouri O, Turgeon MO, Nicholas AK, Heffernan E, El-Khairi R, Abid N, Lyons G, Halsall D, et al. A novel IGSF1 mutation in a large Irish kindred highlights the need for familial screening in the IGSF1 deficiency syndrome. Clinical Endocrinology 2018. 89 813–823. ( 10.1111/cen.13827) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Libri DV, Trettene A, Bonomi M, Beck-Peccoz P, Persani L, Salvatoni A. The unusual adequate development of a child with severe central hypothyroidsm negative at neonatal thyrotropin screening. Journal of Endocrinological Investigation 2013. 36 788–789. ( 10.3275/8963) [DOI] [PubMed] [Google Scholar]
- 21.Ramos HE, Labedan I, Carré A, Castanet M, Guemas I, Tron E, Madhi F, Delacourt C, Maciel RMB, Polak M. New cases of isolated congenital central hypothyroidism due to homozygous thyrotropin beta gene mutations: a pitfall to neonatal screening. Thyroid 2010. 20 639–645. ( 10.1089/thy.2009.0462) [DOI] [PubMed] [Google Scholar]
- 22.Bonomi M, Proverbio MC, Weber G, Chiumello G, Beck-Peccoz P, Persani L. Hyperplastic pituitary gland, high serum glycoprotein hormone alpha-subunit, and variable circulating thyrotropin (TSH) levels as hallmark of central hypothyroidism due to mutations of the TSH beta gene. Journal of Clinical Endocrinology and Metabolism 2001. 86 1600–1604. ( 10.1210/jcem.86.4.7411) [DOI] [PubMed] [Google Scholar]
- 23.Dacou-Voutetakis C, Feltquate DM, Drakopoulou M, Kourides IA, Dracopoli NC. Familial hypothyroidism caused by a nonsense mutation in the thyroid-stimulating hormone beta-subunit gene. American Journal of Human Genetics 1990. 46 988–993. [PMC free article] [PubMed] [Google Scholar]
- 24.Hayashizaki Y, Hiraoka Y, Endo Y, Miyai K, Matsubara K. Thyroid-stimulating hormone (TSH) deficiency caused by a single base substitution in the CAGYC region of the beta-subunit. EMBO Journal 1989. 8 2291–2296. ( 10.1002/j.1460-2075.1989.tb08355.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miyai K, Azukizawa M, Kumahara Y. Familial isolated thyrotropin deficiency with cretinism. New England Journal of Medicine 1971. 285 1043–1048. ( 10.1056/NEJM197111042851902) [DOI] [PubMed] [Google Scholar]
- 26.Pfäffle R, Klammt J. Pituitary transcription factors in the aetiology of combined pituitary hormone deficiency. Best Practice and Research. Clinical Endocrinology and Metabolism 2011. 25 43–60. ( 10.1016/j.beem.2010.10.014) [DOI] [PubMed] [Google Scholar]
- 27.Raivio T, Avbelj M, McCabe MJ, Romero CJ, Dwyer AA, Tommiska J, Sykiotis GP, Gregory LC, Diaczok D, Tziaferi V, et al. Genetic overlap in Kallmann syndrome, combined pituitary hormone deficiency, and septo-optic dysplasia. Journal of Clinical Endocrinology and Metabolism 2012. 97 E694–E699. ( 10.1210/jc.2011-2938) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schoenmakers N, Alatzoglou KS, Chatterjee VK, Dattani MT. Recent advances in central congenital hypothyroidism. Journal of Endocrinology 2015. 227 R51–R71. ( 10.1530/JOE-15-0341) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Castinetti F, Reynaud R, Saveanu A, Jullien N, Quentien MH, Rochette C, Barlier A, Enjalbert A, Brue T. Mechanisms in Endocrinology: an update in the genetic aetiologies of combined pituitary hormone deficiency. European Journal of Endocrinology 2016. 174 R239–R247. ( 10.1530/EJE-15-1095) [DOI] [PubMed] [Google Scholar]
- 30.Collu R, Tang J, Castagné J, Lagacé G, Masson N, Huot C, Deal C, Delvin E, Faccenda E, Eidne KA, et al. A novel mechanism for isolated central hypothyroidism: inactivating mutations in the thyrotropin-releasing hormone receptor gene. Journal of Clinical Endocrinology and Metabolism 1997. 82 1561–1565. ( 10.1210/jcem.82.5.3918) [DOI] [PubMed] [Google Scholar]
- 31.Joustra SD, Schoenmakers N, Persani L, Campi I, Bonomi M, Radetti G, Beck-Peccoz P, Zhu H, Davis TME, Sun Y, et al. The IGSF1 deficiency syndrome: characteristics of male and female patients. Journal of Clinical Endocrinology and Metabolism 2013. 98 4942–4952. ( 10.1210/jc.2013-2743) [DOI] [PubMed] [Google Scholar]
- 32.Joustra SD, Heinen CA, Schoenmakers N, Bonomi M, Ballieux BEPB, Turgeon M-O, Bernard DJ, Fliers E, Trotsenburg ASP van, Losekoot M, et al. IGSF1 deficiency: lessons from an extensive case series and recommendations for clinical management. Journal of Clinical Endocrinology and Metabolism 2016. 101 1627–1636. ( 10.1210/jc.2015-3880) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Beck-Peccoz P, Rodari G, Giavoli C, Lania A. Central hypothyroidism – a neglected thyroid disorder. Nature Reviews Endocrinology 2017. 13 588–598. ( 10.1038/nrendo.2017.47) [DOI] [PubMed] [Google Scholar]
- 34.Feldt-Rasmussen U, Klose M. Central hypothyroidism and its role for cardiovascular risk factors in hypopituitary patients. Endocrine 2016. 54 15–23. ( 10.1007/s12020-016-1047-x) [DOI] [PubMed] [Google Scholar]
- 35.Persani L, Brabant G, Dattani M, Bonomi M, Feldt-Rasmussen U, Fliers E, Gruters A, Maiter D, Schoenmakers N, van Trotsenburg ASP. 2018 European Thyroid Association (ETA) guidelines on the diagnosis and management of central hypothyroidism. European Thyroid Journal 2018. 7 225–237. ( 10.1159/000491388) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.LaFranchi SH. Newborn screening strategies for congenital hypothyroidism: an update. Journal of Inherited Metabolic Disease 2010. 33 S225–S233. ( 10.1007/s10545-010-9062-1) [DOI] [PubMed] [Google Scholar]
- 37.Demers LM, Spencer CA. Laboratory medicine practice guidelines: laboratory support for the diagnosis and monitoring of thyroid disease. Clinical Endocrinology 2003. 58 138–140. ( 10.1046/j.1365-2265.2003.01681.x) [DOI] [PubMed] [Google Scholar]
- 38.Gurnell M, Halsall DJ, Chatterjee VK. What should be done when thyroid function tests do not make sense? Clinical Endocrinology 2011. 74 673–678. ( 10.1111/j.1365-2265.2011.04023.x) [DOI] [PubMed] [Google Scholar]
- 39.Koulouri O, Auldin MA, Agarwal R, Kieffer V, Robertson C, Falconer Smith J, Levy MJ, Howlett TA. Diagnosis and treatment of hypothyroidism in TSH deficiency compared to primary thyroid disease: pituitary patients are at risk of under-replacement with levothyroxine. Clinical Endocrinology 2011. 74 744–749. ( 10.1111/j.1365-2265.2011.03984.x) [DOI] [PubMed] [Google Scholar]
- 40.Yamada M, Mori M. Mechanisms related to the pathophysiology and management of central hypothyroidism. Nature Clinical Practice: Endocrinology and Metabolism 2008. 4 683–694. ( 10.1038/ncpendmebib995) [DOI] [PubMed] [Google Scholar]
- 41.Alexopoulou O, Beguin C, De P, Maiter D. Clinical and hormonal characteristics of central hypothyroidism at diagnosis and during follow-up in adult patients. European Journal of Endocrinology 2004. 150 1–8. ( 10.1530/eje.0.1500001) [DOI] [PubMed] [Google Scholar]
- 42.Ferretti E, Persani L, Jaffrain-Rea ML, Giambona S, Tamburrano G, Beck-Peccoz P. Evaluation of the adequacy of levothyroxine replacement therapy in patients with central hypothyroidism. Journal of Clinical Endocrinology and Metabolism 1999. 84 924–929. ( 10.1210/jcem.84.3.5553) [DOI] [PubMed] [Google Scholar]
- 43.Refetoff S, Bassett JH, Beck-Peccoz P, Bernal J, Brent G, Chatterjee K, De Groot LJ, Dumitrescu AM, Jameson JL, Kopp PA, et al Classification and proposed nomenclature for inherited defects of thyroid hormone action, cell transport, and metabolism. European Thyroid Journal 2014. 3 7–9. ( 10.1159/000358180) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pappa T, Johannesen J, Scherberg N, Torrent M, Dumitrescu A, Refetoff SA. TSHβ variant with impaired immunoreactivity but intact biological activity and its clinical implications. Thyroid 2015. 25 869–876. ( 10.1089/thy.2015.0096) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Costom BH, Grumbach MM, Kaplan SL. Effect of thyrotropin-releasing factor on serum thyroid-stimulating hormone. An approach to distinguishing hypothalamic from pituitary forms of idiopathic hypopituitary dwarfism. Journal of Clinical Investigation 1971. 50 2219–2225. ( 10.1172/JCI106717) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Faglia G. The clinical impact of the thyrotropin-releasing hormone test. Thyroid 1998. 8 903–908. ( 10.1089/thy.1998.8.903) [DOI] [PubMed] [Google Scholar]
- 47.Rose SR. Cranial irradiation and central hypothyroidism. Trends in Endocrinology and Metabolism 2001. 12 97–104. ( 10.1016/S1043-2760(00)00359-3) [DOI] [PubMed] [Google Scholar]
- 48.Yamakita N, Komaki T, Takao T, Murai T, Hashimoto K, Yasuda K. Usefulness of thyrotropin (TSH)-releasing hormone test and nocturnal surge of TSH for diagnosis of isolated deficit of TSH secretion. Journal of Clinical Endocrinology and Metabolism 2001. 86 1054–1060. ( 10.1210/jcem.86.3.7267) [DOI] [PubMed] [Google Scholar]
- 49.Hartoft-Nielsen ML, Lange M, Rasmussen AK, Scherer S, Zimmermann-Belsing T, Feldt-Rasmussen U. Thyrotropin-releasing hormone stimulation test in patients with pituitary pathology. Hormone Research 2004. 61 53–57. ( 10.1159/000075239) [DOI] [PubMed] [Google Scholar]
- 50.Atmaca H, Tanriverdi F, Gokce C, Unluhizarci K, Kelestimur F. Do we still need the TRH stimulation test? Thyroid 2007. 17 529–533. ( 10.1089/thy.2006.0311) [DOI] [PubMed] [Google Scholar]
- 51.Persani L, Borgato S, Romoli R, Asteria C, Pizzocaro A, Beck-Peccoz P. Changes in the degree of sialylation of carbohydrate chains modify the biological properties of circulating thyrotropin isoforms in various physiological and pathological states. Journal of Clinical Endocrinology and Metabolism 1998. 83 2486–2492. ( 10.1210/jcem.83.7.4970) [DOI] [PubMed] [Google Scholar]
- 52.Jostel A, Ryder WDJ, Shalet SM. The use of thyroid function tests in the diagnosis of hypopituitarism: definition and evaluation of the TSH Index. Clinical Endocrinology 2009. 71 529–534. ( 10.1111/j.1365-2265.2009.03534.x) [DOI] [PubMed] [Google Scholar]
- 53.Doin FC, Rosa-Borges M, Martins MRA, Moisés VA, Abucham J. Diagnosis of subclinical central hypothyroidism in patients with hypothalamic–pituitary disease by Doppler echocardiography. European Journal of Endocrinology 2012. 166 631–640. ( 10.1530/EJE-11-0907) [DOI] [PubMed] [Google Scholar]
- 54.Feldt-Rasmussen U. Interactions between growth hormone and the thyroid gland – with special reference to biochemical diagnosis. Current Medicinal Chemistry 2007. 14 2783–2788. ( 10.2174/092986707782360114) [DOI] [PubMed] [Google Scholar]
- 55.Razvi S, Weaver JU, Butler TJ, Pearce SHS. Levothyroxine treatment of subclinical hypothyroidism, fatal and nonfatal cardiovascular events, and mortality. Archives of Internal Medicine 2012. 172 811–817. ( 10.1001/archinternmed.2012.1159) [DOI] [PubMed] [Google Scholar]
- 56.Aleksander PE, Brückner-Spieler M, Stoehr AM, Lankes E, Kühnen P, Schnabel D, Ernert A, Stäblein W, Craig ME, Blankenstein O, et al. Mean high-dose l-thyroxine treatment is efficient and safe to achieve a normal IQ in young adult patients with congenital hypothyroidism. Journal of Clinical Endocrinology and Metabolism 2018. 103 1459–1469. ( 10.1210/jc.2017-01937) [DOI] [PubMed] [Google Scholar]
- 57.Koch CA, Sarlis NJ. The spectrum of thyroid diseases in childhood and its evolution during transition to adulthood: natural history, diagnosis, differential diagnosis and management. Journal of Endocrinological Investigation 2001. 24 659–675. ( 10.1007/BF03343911) [DOI] [PubMed] [Google Scholar]
- 58.Jonklaas J, Bianco AC, Bauer AJ, Burman KD, Cappola AR, Celi FS, Cooper DS, Kim BW, Peeters RP, Rosenthal MS, et al. Guidelines for the treatment of hypothyroidism: prepared by the American Thyroid Association task force on thyroid hormone replacement. Thyroid 2014. 24 1670–1751. ( 10.1089/thy.2014.0028) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shimon I, Cohen O, Lubetsky A, Olchovsky D. Thyrotropin suppression by thyroid hormone replacement is correlated with thyroxine level normalization in central hypothyroidism. Thyroid 2002. 12 823–827. ( 10.1089/105072502760339406) [DOI] [PubMed] [Google Scholar]
- 60.Portes ES, Oliveira JH, MacCagnan P, Abucham J. Changes in serum thyroid hormones levels and their mechanisms during long-term growth hormone (GH) replacement therapy in GH deficient children. Clinical Endocrinology 2000. 53 183–189. ( 10.1046/j.1365-2265.2000.01071.x) [DOI] [PubMed] [Google Scholar]
- 61.Benaglia L, Busnelli A, Somigliana E, Leonardi M, Vannucchi G, De S, Fugazzola L, Ragni G, Fedele L. Incidence of elevation of serum thyroid-stimulating hormone during controlled ovarian hyperstimulation for in vitro fertilization. European Journal of Obstetrics, Gynecology, and Reproductive Biology 2014. 173 53–57. ( 10.1016/j.ejogrb.2013.11.003) [DOI] [PubMed] [Google Scholar]
- 62.Feldt-Rasmussen U, Klose M, Benvenga S. Interactions between hypothalamic pituitary thyroid axis and other pituitary dysfunctions. Endocrine 2018. 62 519–527. ( 10.1007/s12020-018-1738-6) [DOI] [PubMed] [Google Scholar]
- 63.Ryder M, Callahan M, Postow MA, Wolchok J, Fagin JA. Endocrine-related adverse events following ipilimumab in patients with advanced melanoma: a comprehensive retrospective review from a single institution. Endocrine-Related Cancer 2014. 21 371–381. ( 10.1530/ERC-13-0499) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Klose M, Marina D, Hartoft-Nielsen ML, Klefter O, Gavan V, Hilsted L, Rasmussen AK, Feldt-Rasmussen U. Central hypothyroidism and its replacement have a significant influence on cardiovascular risk factors in adult hypopituitary patients. Journal of Clinical Endocrinology and Metabolism 2013. 98 3802–3810. ( 10.1210/jc.2013-1610) [DOI] [PubMed] [Google Scholar]
- 65.Benvenga S, Capodicasa G, Perelli S. L-thyroxine in an oral liquid or softgel formulation ensures more normal serum levels of free T4 in patients with central hypothyroidism. Frontiers in Endocrinology 2017. 8 321 ( 10.3389/fendo.2017.00321) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cassio A, Cacciari E, Cicognani A, Damiani G, Missiroli G, Corbelli E, Balsamo A, Bal M, Gualandi S. Treatment for congenital hypothyroidism: thyroxine alone or thyroxine plus triiodothyronine? Pediatrics 2003. 111 1055–1060. ( 10.1542/peds.111.5.1055) [DOI] [PubMed] [Google Scholar]
- 67.Wiersinga WM, Duntas L, Fadeyev V, Nygaard B, Vanderpump MPJ. 2012 ETA guidelines: the use of L-T4 + L-T3 in the treatment of hypothyroidism. European Thyroid Journal 2012. 1 55–71. ( 10.1159/000339444) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Slawik M, Klawitter B, Meiser E, Schories M, Zwermann O, Borm K, Peper M, Lubrich B, Hug MJ, Nauck M, et al. Thyroid hormone replacement for central hypothyroidism: a randomized controlled trial comparing two doses of thyroxine (T4) with a combination of T4 and triiodothyronine. Journal of Clinical Endocrinology and Metabolism 2007. 92 4115–4122. ( 10.1210/jc.2007-0297) [DOI] [PubMed] [Google Scholar]
- 69.Panicker V, Saravanan P, Vaidya B, Evans J, Hattersley AT, Frayling TM, Dayan CM. Common variation in the DIO2 gene predicts baseline psychological well-being and response to combination thyroxine plus triiodothyronine therapy in hypothyroid patients. Journal of Clinical Endocrinology and Metabolism 2009. 94 1623–1629. ( 10.1210/jc.2008-1301) [DOI] [PubMed] [Google Scholar]
- 70.Bianco AC, Casula S. Thyroid hormone replacement therapy: three ‘simple’ questions, complex answers. European Thyroid Journal 2012. 1 88–98. ( 10.1159/000339447) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Appelhof BC, Peeters RP, Wiersinga WM, Visser TJ, Wekking EM, Huyser J, Schene AH, Tijssen JGP, Hoogendijk WJG, Fliers E. Polymorphisms in type 2 deiodinase are not associated with well-being, neurocognitive functioning, and preference for combined thyroxine/3,5,3′-triiodothyronine therapy. Journal of Clinical Endocrinology and Metabolism 2005. 90 6296–6299. ( 10.1210/jc.2005-0451) [DOI] [PubMed] [Google Scholar]



 This work is licensed under a
This work is licensed under a