Summary
Understanding how epithelial progenitors within exocrine glands establish specific cell lineages and form complex functional secretory units is vital for organ regeneration. Here we identify the transcription factor Sox10 as essential for both the maintenance and differentiation of epithelial KIT+FGFR2b+ progenitors into secretory units, containing acinar, myoepithelial, and intercalated duct cells. The KIT/FGFR2b-Sox10 axis marks the earliest multi-potent and tissue-specific progenitors of exocrine glands. Genetic deletion of epithelial Sox10 leads to loss of secretory units, which reduces organ size and function, but the ductal tree is retained. Intriguingly, the remaining duct progenitors do not compensate for loss of Sox10 and lack plasticity to properly form secretory units. However, overexpression of Sox10 in these ductal progenitors enhances their plasticity toward KIT+ progenitors and induces differentiation into secretory units. Therefore, Sox10 controls plasticity and multi-potency of epithelial KIT+ cells in secretory organs, such as mammary, lacrimal, and salivary glands.
Keywords: SOX10, secretory unit, cell fate, stem/progenitor cell, exocrine glands, salivary gland, mammary gland, lacrimal gland, KIT
Graphical Abstract
Highlights
-
•
Sox10 marks the initial multi-potent KIT+ progenitors of exocrine glandular tissues
-
•
KIT+ progenitors require Sox10 for their maintenance
-
•
Sox10 is necessary for proper lineage development of secretory units
-
•
Sox10 is sufficient to induce cell plasticity in non-KIT+ epithelial cells
In this manuscript, Lombaert and colleagues discovered that transcription factor Sox10 is not only essential to maintain epithelial progenitors in exocrine glands, but also acts as a master regulator to induce plasticity toward secretory units. These results provide new avenues for directed differentiation and engineering of secretory units in vitro and/or in vivo.
Introduction
Cellular plasticity is an important feature within adult organs facilitating rapid adaptation of progenitors to injury or environmental changes. Previously, it was thought that progenitors within organs would respond to injury in a uni-directional cell lineage manner. A series of differentiation steps producing multiple cell intermediates, each with a more restricted lineage potential than the last, would eventually lead to specialized cells. Recent reports challenge this paradigm showing that cells can acquire characteristics of other cell types beyond their proposed lineage (Tata and Rajagopal, 2016) by converting into earlier cell types (de-differentiation), more distant phenotypes (trans-differentiation), or interchange between different progenitors (trans-determination). Each of these three cellular processes may occur in different settings and to various degrees (Donati and Watt, 2015). Interestingly, both cell-autonomous and non-cell-autonomous mechanisms are proposed to contribute to this plasticity. Environmental factors, such as cell-cell contact or ligand presentation, contribute to non-cell-autonomous induction. Whereas transcription factors (TFs) are part of the autonomous mechanism, playing a major role in regulating cell fate, stability, and conversion. However, very little is known about how plasticity is regulated, and how it varies among cell types and different conditions.
Exocrine glands, such as the mammary, lacrimal, and salivary glands, all share a similar secretory function that entails production and secretion of milk, tears, and saliva, respectively (Wang and Laurie, 2004). They all undergo branching morphogenesis to create a ductal tree structure distally ending in secretory units whereby fluid can be produced, modified, transported, and released from the gland. These secretory units include secretory acinar cells, associated intercalated ducts that connect larger ducts to the acini, and contractile myoepithelial cells surrounding the acini (Lombaert et al., 2017). As such, it was postulated that various signaling pathways and cellular mechanisms must overlap among these exocrine glands (Wang and Laurie, 2004). For example, the TF MIST1 was identified as a “scaling factor” to induce and maintain the secretory cell architecture of mature acinar cells in multiple exocrine tissues (Lo et al., 2017). However, which specific TFs control and direct epithelial progenitors to form the secretory units, and whether common TFs are involved in different organs are unknown.
TFs whose activity is necessary and sufficient to direct specific cell lineage commitment, and that can also re-specify the fate of cells destined to become other lineages, are termed core master regulators (Chan and Kyba, 2013). Master regulators promote gene transcription to initiate or maintain the desired cell fate, and repress gene expression that oppose this decision; ultimately stabilizing cell fate decisions. Here we identify Sox10 as a master regulator to maintain and direct KIT+ progenitors into secretory units of exocrine glands. SOX proteins have previously been described as mediators of both stemness and cell differentiation (Abdelalim et al., 2014), and Sox10 is well-known for its role in neural crest stem cell maintenance and their differentiation into oligodendrocytes and glia cells (Reiprich and Wegner, 2015). Surprisingly, more recent studies reported SOX10 in epithelial cell types of exocrine mammary, lacrimal, and salivary glands (Chen et al., 2014, Dravis et al., 2015, Lombaert et al., 2013). Using salivary glands as our primary model system, we report that Sox10 is an exocrine gland-specific core master regulator that is sufficient to induce plasticity and multi-potency of tissue-specific progenitors to form functional secretory units.
Results
The KIT/FGFR2b-Sox10 Axis Defines Initial Tissue-Specific Cells
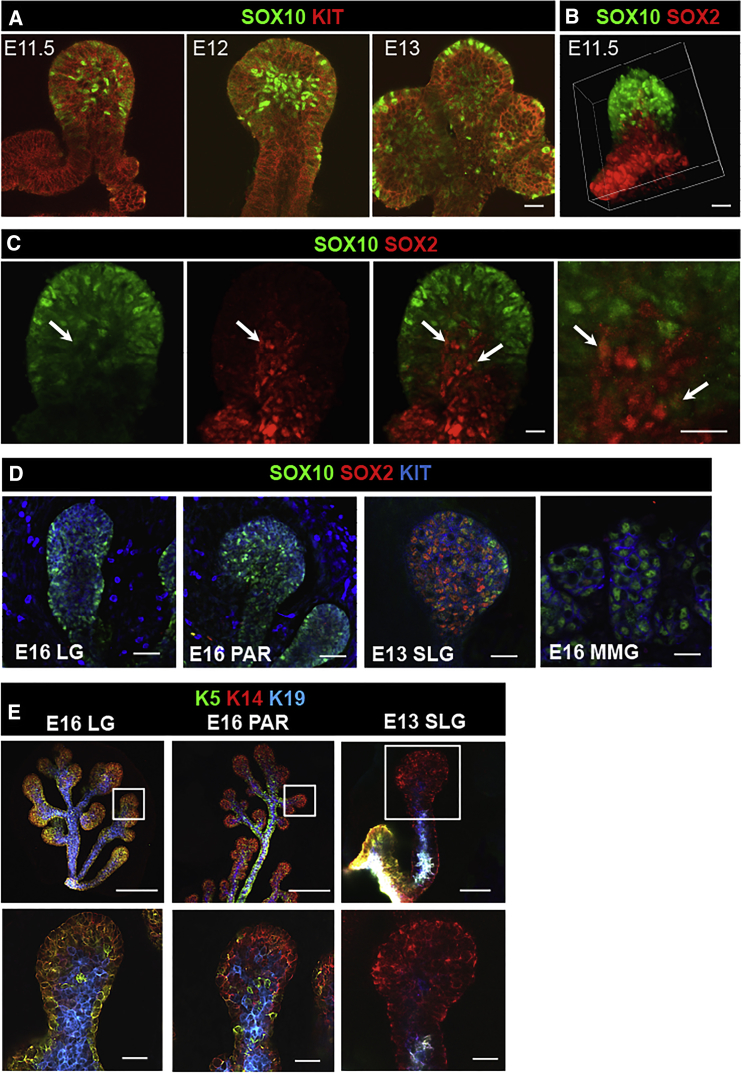
To identify tissue-specific progenitors, we analyzed protein expression of known markers of adult and fetal salivary submandibular gland (SMG) progenitors. Adult SMG progenitors expressing CD117 (KIT, c-Kit) were previously shown to regenerate radiation-damaged mouse SMGs in vivo by differentiating into saliva-secreting acinar and saliva-transporting duct cells (Lombaert et al., 2008). However, their presence and function at SMG ontogenesis (embryonic day 11.5 [E11.5]) remained unclear. SMGs, such as the parotid (PAR) and sublingual (SLG) salivary glands, derive from an invagination and thickening of oral epithelium (Knosp et al., 2012). This thickened epithelium forms a single endbud, termed cap or tip cells in other exocrine glands, which clefts to generate multiple distal endbuds on a lengthening proximal duct. We found that KIT+ cells are present at SMG initiation, as protein staining of enzymatically isolated epithelia from E11.5–E12 embryos showed membrane localization of KIT on the oral epithelial lining, initial single SMG endbud, and main duct (Figures 1A and S1A). By E13, however, KIT expression becomes restricted to endbuds only (Figure S1A) (Lombaert et al., 2013). These KIT+ progenitors require FGFR2b signaling for cell survival, cell proliferation, and initiation of SOX10 expression to become uniquely distinct from the SOX2+KIT− main ducts (Lombaert et al., 2013, Lombaert and Hoffman, 2010). Thus, as oral epithelial cells express KIT at gland initiation, we hypothesized that KIT/FGFR2b-regulated TFs specify the initial tissue-specific progenitors. We show that, during the initial oral budding, SOX10+ cells are localized in the distal epithelia while proximal layers expressed SOX2+ (Figures 1A–1C). Sporadically, a SOX2+SOX10+ cell was found at the border of both cell layers (Figure 1C, arrows), suggesting a potential transitioning cell. The oral epithelium is known to express Sox2, ΔNp63, Fgfr2b, and intracellular cytokeratins KRT14 (K14) and KRT5 (K5) (Jones and Klein, 2013, Rice et al., 2004). Protein analysis of KRT's in E11.5–E12 isolated epithelia revealed two distinct layers, in a similar manner to SOX2 and SOX10 expression. Proximal cells co-expressed K14, K5, and K19, while distal cells were enriched for K14+ (Figures S1B and S1C). SOX2 expression overlapped with K14+K5+ proximal cells and SOX10 was co-expressed in K14+ distal cells (Figure S1D); confirming the positioning of two distinct epithelial layers at SMG ontogenesis.
Figure 1.
The KIT/FGFR2b-Sox10 Axis Defines Initial Tissue-Specific Cells
(A) Confocal images of E11.5, E12, and E13 isolated SMG epithelia stained for SOX10 and KIT. Scale bars, 20 μm.
(B) E11.5 isolated epithelium stained for SOX10 and SOX2. Scale bars, 20 μm.
(C) SOX10 and SOX2 expression in E11.5 epithelium. Arrows outline SOX10+SOX2+. Scale bars, 20 μm.
(D and E) Confocal images of E16 LG, E16 PAR, E13 SLG, and E16 MMG. Tissue was stained for SOX10, SOX2, and KIT, or K14, K5, and K19. Scale bars, 100 μm (D) and 20 μm (E).
To investigate the role of FGFR2b signaling in specifying the tissue-specific distal epithelial progenitors, we analyzed the initiating glands of Fgf10−/− murine embryos, which lack the ligand for FGFR2b and die at birth due to severe abnormalities in multiple organs. E11.5 Fgf10−/− isolated SMG epithelia expressed SOX2 but failed to express SOX10, even though surrounding neuronal cells (CDH1/E-cadherin-negative) clearly expressed SOX10 (Figure S1E, arrow). As FGF10/FGFR2b signaling is the primary signal to initiate Sox10+ cells, we isolated and cultured wild-type E12 epithelia for 2 h in basal medium +/− FGF10. Within this time frame, Sox2 expression was downregulated and Sox10 was upregulated (Figure S1F), suggesting that FGF10/FGFR2b signaling induces the switch from SOX2+ into SOX10+ cells.
To confirm that the KIT/FGFR2b-Sox10 axis was important in other exocrine glands, we evaluated distal cells in lacrimal, PAR, SLG, and mammary glands (MMGs). The SLG was the only exception where SOX2 was expressed in distal KIT+ cells. The other exocrine glands exclusively expressed KIT and SOX10 (Figure 1D), and all salivary glands shared a similar epithelial KRT-expressing cell population (Figure 1E).
Thus, we identified two distinct KIT+ epithelial cell layers present at SMG initiation: proximal SOX2+ oral epithelial cells and distal SOX10+ cells that initiate in an Fgf10-dependent manner. Collectively, these data suggest that SOX10 defines the initial endbud cells of exocrine glands.
Glandular Tissues Originate from Oral Epithelial Sox2 Cells
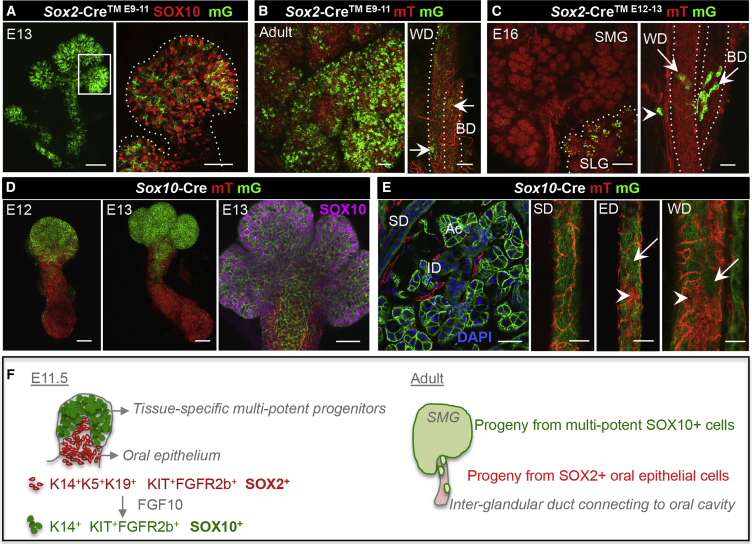
To elucidate the contribution of SOX2+ oral epithelial and SOX10+ cells to tissue formation, we used lineage tracing to visualize their progeny. Sox2-Cre mice crossed with Rosa26-floxmTomatoflox-mGFP (mTmG) mice showed that the SMG is mGFP+, demonstrating that SMGs are offspring of Sox2 cells (Figure S2A). This is consistent with data that SOX2 is first expressed by pluripotent embryonic stem cells at E2.5–E3.5, and thus expected to give rise to all glands (Avilion et al., 2003). Next, tamoxifen-inducible Sox2-Cre mice allowed us to specifically track progeny of E9–E11 oral epithelial cells. Consistent with previous literature (Rothova et al., 2012, Tucker, 2007), we found that all (E13) epithelial cells, including SOX10+ cells, arose from E9 to E11 Sox2 oral epithelia (Figure 2A). The E9–E11 Sox2 cells also contributed to epithelial cells in adult SMGs, as well as their inter-glandular ducts, which connect the organ to the oral cavity (Figure 2B, arrows). Inter-glandular ducts are comprised of two types of ducts that transport saliva outside the organ: excretory ducts (EDs) that connect with one main Wharton's duct (WD, SMG) or Bartholin's duct (BD, SLG).
Figure 2.
Initial Tissue-Specific SOX10+ Cells Are Multi-potent Progenitors
Sox2-Cre™ or Sox10-Cre mice were crossed with Rosa26-mTmG mice for lineage tracing. mGFP+ (mG) cells are lineage-derived cells, mTomato (mT) cells are not.
(A and B) E9–E11 induced lineage tracing seen in isolated E13 epithelia (A) or adult SMG (B). E13 epithelium was co-stained with SOX10. Scale bars: (A, left and B, left) 100 μm; (A, right and B, right) 20 μm. WD, SMG Wharton's duct; BD, SLG Bartholin's duct. Arrows outline mG+ cells.
(C) Confocal images of E16 SMG with WD, and SLG with BD. Tissue was lineage traced from E12–13. Scale bar, 100 μm.
(D) Isolated epithelia were analyzed by confocal microscopy at E12 and E13. E13 epithelium was co-stained for SOX10. Scale bars, 100 μm (left and middle) and 20 μm (right).
(E) Confocal imaging of adult SMG intra-glandular striated ducts (SD), inter-glandular excretory ducts (EDs) and WDs. Arrowheads and arrows represent mT and mG epithelial cells, respectively. Acinar cell (Ac), intercalated duct (ID). Scale bars, 20 μm (left) and 200 μm (centers and right).
(F) Graphical cartoon depicting the presence of two epithelial cell types after E11.5 SMG initiation: SOX2+ oral epithelial cells co-expressing K14, K5, and K19, and distal SOX10+ tissue-specific SMG cells solely co-expressing K14. Both cell types express KIT and FGFR2b, but contribute differentially to adult glands. Once tissue-specific cells are formed, oral epithelial cells only contribute to parts of the inter-glandular ducts connecting the secretory organ with the oral cavity. Instead, Sox10 cells form all epithelial cells in the adult SMG, as well cells parts in inter-glandular ducts.
We hypothesized that SOX2+ oral epithelial cells would not contribute to organ formation after initiation of the SOX10+ cells in the distal endbud. Indeed, induction of Sox2-Cre at E12–E13, after SMG initiation, supported our hypothesis, as Sox2 cells no longer contributed to SMG development (Figures 2C and S2B). In addition, the intra-glandular ducts, such as striated ducts (SD), were mGFP-negative. However, various portions of the larger inter-glandular ducts as well as cells in SLGs did derive from E12 to E13 Sox2+ cells (Figure S2B, arrows). This result supports our previous data that basal cells in the main duct of developing SMGs remain SOX2+ (Lombaert et al., 2011), and that SOX2 is present in distal cells of SLGs (Figure 1D). These data thus suggest that all salivary glandular tissue is derived from the Sox2+ oral epithelium at E9–E11, but thereafter more restricted tissue-specific progenitor cell contributes to organ development.
The Initial Sox10+ Cells Are Multi-potent Progenitors of Secretory Units
We next investigated the contribution of Sox10 epithelial cells during development. Lineage tracing with a constitutive Sox10-Cre mouse confirmed the unique location of SOX10+ cells in distal epithelial endbuds (E12–E13) (Figure 2D). Up to 99.9% ± 0.1% and 98.3% ± 0.6% of all myoepithelial and acinar cells were Sox10-derived (Figure S2C), respectively, as quantified by co-expression with mGFP in adult Sox10-Cre mice. On the other hand, cells of the inter-glandular ducts (EDs and WDs) were not entirely Sox10 derived, and these non-Sox10-derived offspring became more prominent in the WD (Figure 2E, arrowheads). Interestingly, distal parts of these inter-glandular ducts, were Sox10 derived (Figure 2E, arrows), suggesting that inter-glandular ducts may be derived from both oral epithelium and SOX10+ cells. This pattern was apparent during development (E14) where distal parts of the duct remain Sox10+ (Figure S2D). However, adult intra-glandular epithelia were exclusively mGFP+ (Figure 2E), which could be observed by co-staining with an epithelial marker K8, myoepithelial marker alpha-smooth muscle actin (ACTA2), and acinar cell-specific water channel aquaporin5 (AQP5) (Figure S2D). In fact, the parenchyma and inter-glandular ducts of adult lacrimal and PAR glands were also entirely Sox10 derived (Figure S2E), while the SLG epithelial cells were both Sox2 and Sox10 derived. When Sox10-rtTA;Tet-Cre mice were induced at E9–12, the analysis confirmed the exclusive contribution of the initial Sox10 cells to adult SMG organ formation. The acinar (84.3% ± 1.2%), myoepithelial (85.4% ± 4.1%) and ductal (97.3% ± 4.5%) cells co-expressed mGFP, suggesting that initial Sox10 cells form the entire SMG epithelium (Figure S2F). Taken together, these data identify Sox10 as an early marker of multi-potent tissue-specific epithelial progenitors of exocrine glands (Figure 2F).
Loss in Sox10 Reduces Exocrine Gland Development
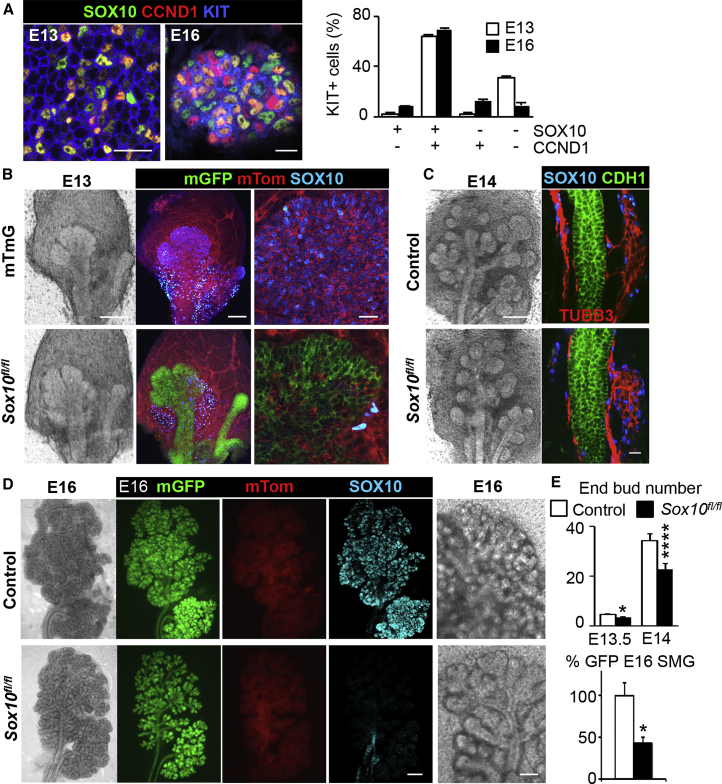
We next identified mechanisms through which Sox10 regulates cellular processes. There was ∼64% overlapping protein expression of SOX10 with CCND1 or KI67 in distal E13 KIT+ progenitors, suggesting that Sox10 could regulate cell proliferation (Figures 3A and S3A). At E16, secretory cell differentiation begins and KIT+ progenitors could lose potency by either downregulating Sox10 or becoming quiescent. Quantification of SOX10 and/or CCND1 in E16 KIT+ progenitors indicated that SOX10 remained highly expressed in proliferating cells.
Figure 3.
Loss in Sox10 Negatively Impacts Exocrine Gland Formation
(A) Confocal pictures of KIT, SOX10, and CCND1 co-staining in E13 and E16 SMG endbuds. Scale bars, 20 μm. Graph represents KIT+ subpopulations counted on multiple sections through endbuds at each time point. N > 3, Mean ± SEM.
(B and C) Bright-field and confocal pictures of E13 (B) and E14 (C) control (Krt14-Cre;Rosa26-mTmG;Sox10flox/+ or Rosa26-mTmG or Sox10flox/flox mice) and Sox10fl/fl (Krt14-Cre;Rosa26-mTmG;Sox10flox/flox) SMGs. SMGs were labeled for SOX10, TUBB3, and CDH1. Scale bars, 100 and 20 μm.
(D) Bright-field and fluorescent images of E16 control and Sox10fl/fl SMGs. SMGs were stained for SOX10. Scale bars, 500 and 250 μm.
(E) Quantification of endbud number in E13.5 and E14 SMGs from control and Sox10fl/fl mice. Mean ± SEM, N > 3, unpaired t test. ∗p < 0.05, ∗∗∗∗p < 0.0001. Control E13 (4.5 ± 0.4) versus Sox10fl/fl (3.4 ± 0.2), control E14 (34.2 ± 2.8) versus Sox10fl/fl (22.7 ± 2.4). Graph depicting GFP expression in E16 control and Sox10fl/fl SMGs. Data are normalized to control, mean ± SEM, N > 3, unpaired t test. ∗p < 0.05. Control (100.0% ± 15.8%) versus Sox10fl/fl (43.1 ± 7.2%).
To determine the function of Sox10 during organ formation, we analyzed glands from Sox10flox/flox mice crossed with epithelial-specific Krt14-Cre (referred as Sox10fl/fl) and/or Rosa26-mTmG mice (Figure S3B). Fetal (E13, E14, and E16) SMGs were evaluated for mGFP and loss of SOX10 (Figures 3B–3D and S3C). Non-epithelial cells remained mTomato+, and, as expected, SOX10 was only detected in TUBB3+ neuronal cells (Figure 3C). These data suggested that the Krt14-Cre;Sox10fl/fl system specifically and efficiently targets epithelial Sox10.
We next measured the impact of Sox10 loss on branching morphogenesis in SMG (Figures 3D and 3E). There was a significant reduction in endbud number at both E13.5 and E14 (40%), and 50% at E16 (Figure S3D). Notably, endbud counts becomes less accurate at E16; however, the remaining ductal tree structure became visible with reduced endbuds, as noticed in higher magnifications (Figure 3D). There was a 50% reduction in mGFP intensity in E16 Krt14-Cre;Sox10fl/lf;mTmG glands (Figure 3E), which reflected the reduction in epithelial size. This morphology was similar in post-natal SMGs (Figure S3E). Similar impacts on organ formation of lacrimal, PAR, and SLG glands were observed, where distal branching and/or endbud numbers were significantly decreased (Figures S3F and S3G). These data highlight that Sox10 is necessary for proper organ formation induced by KIT+ endbud progenitors.
Sox10 Is Essential for KIT+ Progenitor Maintenance and Differentiation into Secretory Units
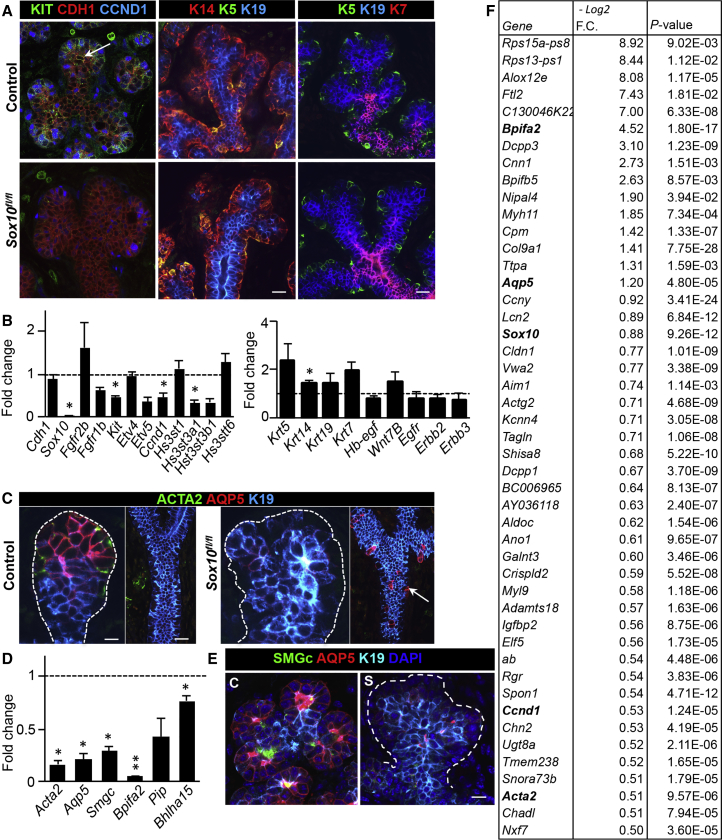
To identify how Sox10 influences progenitors, we performed protein and transcriptome profiling of known markers that define SMG progenitors in both control and Sox10fl/fl E16 SMGs. Proliferating KIT+ progenitors are present in distal endbuds, but the remaining distal cells in Sox10fl/fl SMGs did not show KIT and had reduced CCND1 (Figures 4A and 4B). While cell apoptosis occurs in developing organs as part of size expansion and differentiation (Teshima et al., 2016), no differences in cleaved caspase-3 were observed in epithelia or surrounding cells of Sox10fl/fl SMGs (Figure S4A). This suggests that Sox10 directly affects KIT+ cell maintenance via proliferation. Alternatively, K14 and K5 expression, which mark basal and supra-basal duct cells that comprise a subpopulation of KIT+ cells (Lombaert et al., 2013), were unaffected by Sox10 loss, nor were their ductal K19+ and/or K7+ offspring or the Wnt/Egfr-related signaling pathway (Figures 4A and 4B). The expression of the KIT/FGFR2b signaling pathway (Lombaert et al., 2013), including receptors Kit and Fgfr1b along with downstream targets Etv5, Sox10, and heparan sulfate (Figure 4B), were reduced in Sox10fl/fl SMGs. In parallel, surface markers integrin alpha 6 (Itga6, CD49f), beta 1 (Itgb1, CD29), and CD24, which can enrich for adult SMG progenitors, remained unaltered, as did TF Sox9 (Figure S4B). SOX9 is expressed by distal and proximal cells in salivary and lacrimal glands, and is required for SOX10 expression (Chatzeli et al., 2017, Chen et al., 2014). In wild-type SMGs, Sox9 is regulated by KIT/FGFR2b signaling, as stimulation of isolated E13 epithelia with FGF10 (F10) and/or KIT ligand (K) rapidly upregulated Sox9 in 3 h (Figure S4C), confirming the Chatzeli et al. study in which Sox9 was absent in the SLG/SMG of Fgf10−/− mice. Nonetheless, SOX9 protein expression remained present in basal epithelial and surrounding cell types in Sox10fl/fl SMGs (Figure S4D), suggesting that loss of Sox10 and the subsequent reduction in KIT/FGFR2b signaling did not directly impact Sox9. Lastly, progenitors that form later in development were also evaluated. One progenitor population expressing the TF Ascl3, is detectable at E16 and is bipotent as it contributes to a subpopulation of adult SMG ductal and acinar cells (Bullard et al., 2008). To our surprise, Ascl3 expression was upregulated in Sox10fl/fl SMGs (Figure S4B), which may be due to either decreased distal to proximal cell ratio or direct compensation in response to loss of Sox10. Overall, these data illustrate that Sox10 is essential for the maintenance and proliferation of KIT+ progenitors.
Figure 4.
Sox10 Induces Plasticity By Regulating fetal KIT+ Progenitor Maintenance and Differentiation
(A) Confocal imaging of control and Sox10fl/fl E16 SMGs endbuds. Tissue was stained for KIT (arrow), CDH1, CCND1, K14, K5, K19, and/or K7. Scale bars, 20 μm.
(B) Graphs show fold changes in gene expression of the Fgfr2b/Kit signaling pathway in epithelial (Cdh1) cells of Sox10fl/fl E16 SMGs, Ccnd1, heparan sulfates, as well as ductal-related markers (Krt's) and correlated signaling pathways (Egf, Wnt). Data were normalized to Rps29 and control (dotted line). Mean ± SEM, N > 3, multiple comparison t test. ∗p < 0.05.
(C) Confocal imaging of stained E16 control and Sox10fl/fl SMGs with ACTA2, AQP5, and K19. Arrow represents mislocated AQP5 expression in ducts. Scale bars, 20 μm.
(D) Fold changes in gene expression of proteins expressed by myoepithelial, acinar, and/or ID cells. Data was normalized to Rps29 and control (dotted line). Mean ± SEM, N > 3, multiple comparison t test. ∗p < 0.05. ∗∗p < 0.01.
(E) Sox10fl/fl and control E16 SMGs were stained for SMGc, AQP5, K19, and DAPI and analyzed by confocal microscopy. Dotted line outlines the distal area. Scale bar, 20 μm.
(F) RNA sequencing data of E16 Sox10fl/fl SMGs versus control. The list of downregulated genes was generated after bioinformatic analysis of next-generation sequencing data and cutoff at log2 fold change (FC) of −0.25. Genes outlined in bold were validated by qPCR analysis.
The observation that E16 Sox10fl/fl SMGs had aberrant morphology led us to hypothesize that Sox10 affects differentiation. At E16, the differentiation of proacinar, intercalated duct, and myoepithelial cells begins, and continues up to post-natal day 20 (Larsen et al., 2011). The proacinar and intercalated duct cells both express AQP5 and are surrounded by an outer layer of myoepithelial cells (Figure 4C). This differentiation process only occurs in distal endbuds and not in the ducts. Consistent with our hypothesis, ACTA2 and AQP5 were absent in distally located cells of Sox10fl/fl SMGs (Figure 4C). Moreover, acinar-specific secretory proteins, such as Bpifa2 (parotid secretory protein, Psp), submandibular gland protein C (Smgc), and TF Bhlha15 (Mist1) were downregulated (Figures 4D and 4E), illustrating a loss in initial differentiation of multiple cell types comprising the secretory unit of adult glands. Surprisingly, AQP5+ cells emerged proximally along the ducts of Sox10fl/fl SMGs (Figure 4C), suggesting a potential compensation mechanism of the remaining Sox10fl/fl ducts to differentiate into AQP5+ pro-acinar and/or intercalated duct (ID) cells.
Next, we performed RNA sequencing on E16 control and Sox10fl/fl SMGs to identify molecular pathways related to Sox10 in organ development. We obtained expression profiles from independent (N ≥ 3) biological samples and identified 81 genes that were downregulated more than −0.25 log2-fold change in Sox10fl/fl SMGs (Figures 4F and S4E). We used qPCR to validate Sox10, Aqp5, Ccnd1, Bpifa2, and Acta2. The set of downregulated genes were generally epithelial cell specific, as suggested by the mouse gene atlas network (Enrichr program [Chen et al., 2013], Figure S4F). Kyoto Encyclopedia of Genes and Genomes network pathway analysis identified downregulated genes as part of the vascular smooth muscle contraction, focal adhesion, and tight junction pathways (Figure S4G). Genes in cGMP-PKG signaling reflect decreases in pathways using intracellular cGMP-dependent physiological processes that may show alterations of cytosolic calcium concentrations. Also associated with this is the neuropeptide oxytocin pathway, which is mediated via the oxytocin receptor, and exerts stimulation of milk release during lactation and myoepithelial contraction (Crowley, 2015). Other genes are related to the overall salivary secretion and hormone release/secretory protein synthesis, including prolactin signaling, mucin type O-glycan biosynthesis, glycolysis/gluconeogenesis, and insulin secretion. Overall, these results indicate a regulatory network by which Sox10 stimulates KIT-dependent maintenance and their initiation toward multiple cell types of the secretory unit.
Non-SOX10+ Adult Duct Progenitors Fail to Initiate Proper Secretory Organ Function
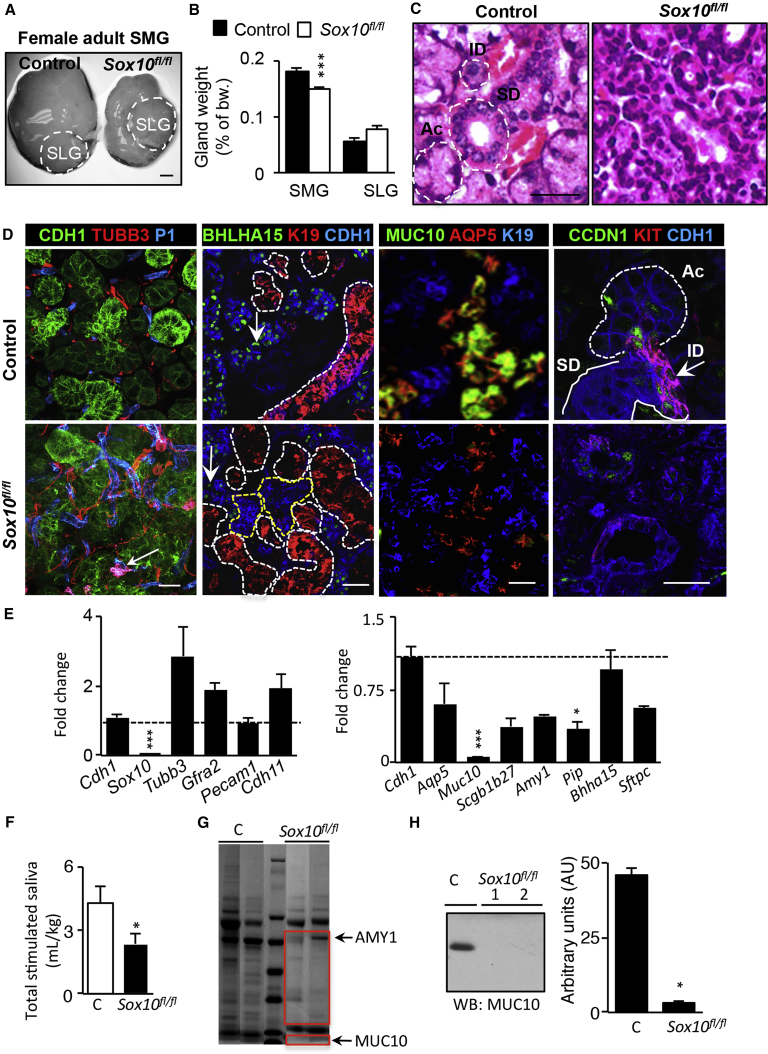
While the loss of Sox10 during development reduced secretory unit differentiation, we predicted that post-natal salivary glands would also have reduced secretory function. A significant decrease was noticed in adult SMG size and weight in both sexes (Figures 5A and 5B and S5A). Although the SLG weight was not affected, which is expected since its development has been shown to be SOX2 dependent (Emmerson et al., 2017), we examined adult male and female SMGs via H&E staining, which showed disrupted cellular morphology. Acinar (Ac), ID, SD, and granular convoluted tubule cells are well defined in controls based on nuclear/cytoplasmic deposition, but their unique characteristics were not visible in Sox10fl/fl SMGs (Figures 5C and S5A). Cells became disorganized within the SMG, and some ductal structures were observed. The epithelial compartment of the adult SMG showed aberrant morphology, with an apparent increase in the amount of blood vessels (PECAM1, P1) and neuronal cells (TUBB3) (Figure 5D). mRNA analysis via qPCR confirmed increased trends of neuronal Tubb3 and Gfra2 (Figure 5E). Interestingly, changes in the mesenchymal and neuronal microenvironment were not noticeable during developmental stages E13 and E16 (Figures S5B and S5C). The parasympathetic ganglia (TUBB3+) remained closely associated with the main duct, appeared similar in size, and innervated the endbuds similar to control. In addition, endothelial networks (PECAM1+) and stromal (Cdh11) cells appeared similar to control, suggesting that loss of the secretory units does not affect innervation or blood vessel formation.
Figure 5.
Sox10 Induces Plasticity by Regulating Fetal KIT+ Progenitor Maintenance and Differentiation
(A) Bright-field picture of SMGs from female adult control or Sox10fl/fl mice. Scale bar, 1 mm.
(B) Graph represents weight of female SMGs as a percentage of body weight (bw) (control, 0.18% ± 0.01%; Sox10fl/fl, 0.13% ± 0.02%). Female SLG (control, 0.06% ± 0.01%; Sox10fl/fl, 0.07% ± 0.01%). Mean ± SEM, N > 3, unpaired t test. ∗∗∗p < 0.005.
(C) H&E staining on paraffin sections of adult female SMGs of control or Sox10fl/fl mice. Ac, ID, and SDs are outlined by white dotted line. Scale bar, 25 μm.
(D) Confocal imaging of adult control and Sox10fl/fl SMGs stained for CDH1, TUBB3, PECAM1 (P1), BHLHA15, K19, MUC10, AQP5, KIT, and CCND1. Scale bars, 20 μm.
(E) qPCR analysis of genes comparing adult control and Sox10fl/fl SMGs. Mean ± SEM, N > 3, multiple comparison t test. ∗∗∗p < 0.001, ∗p < 0.05.
(F) Saliva production of adult control and Sox10fl/fl mice, normalized to their body weight. Mean ± SEM, N > 3, unpaired t test. ∗p < 0.05.
(G) Secretory proteins from saliva of adult control and Sox10fl/fl mice were separated using SDS-PAGE. Gels were stained with Coomassie blue.
(H) MUC10 protein analysis in saliva of adult control and Sox10fl/fl mice via western blot (WB). Graph shows quantification of MUC10 western using densitometry analysis. Mean ± SEM, N ≥ 3, unpaired t test. ∗p > 0.05.
Next, we investigated the cell fate of remaining epithelial cells in adult Sox10fl/fl SMGs. As predicted, Sox10fl/fl SMGs had fewer secretory units and significantly more ducts (K19+, white dotted line) that were, surprisingly, surrounded by non-duct epithelia (CDH1+ K19–, yellow dotted line) (Figure 5D). However, only a few of the non-ductal epithelial cells expressed adult acinar-specific TF BHLHA15 (MIST1), AQP5, and/or secretory mucin protein MUC10 (Figures 5D and 5E). Interestingly, while the overall protein expression of AQP5 decreased (Figure 5D), its specific apical expression on the cells was reduced and the membrane localization remained limited only to the lateral border (Figure S5D). The absence of apical AQP5, as seen in control SMGs (Larsen et al., 2011), would be predicted to reduce fluid secretion. In addition, using qPCR, there was a reduction in the mRNA expression of other secretory proteins observed that are produced by acinar cells, including Secretoglobin b1b27 (Scgb1b27, Abpa), amylase-1 (Amy1), prolactin-induced protein (Pip), and surfactant-associated protein C (Sftpc) (Figure 5E).
Importantly, adult epithelial cells that did not fully differentiate into secretory unit cells were confirmed to be the progeny of Krt14-Cre;Sox10flox/flox cells, as all adult epithelia expressed mGFP (Figure S5E). To outline ID cells, we analyzed KIT expression. Typical KIT+ ID cells were absent in Sox10fl/fl SMGs, and overall proliferation (Ccnd1) was reduced (Figure 5D). In contrast, myoepithelial cells co-expressing ACTA2, K14, and K5 were more abundant in Sox10fl/fl SMGs, surrounding the prominent ductal compartment (Figure S5F). Interestingly, these data suggest that non-Sox10 duct cells also have the potential to form myoepithelial cells in the absence of KIT+SOX10+ progenitors, suggesting a second source of myoepithelial progenitors.
To confirm the secretory deficiency of the Sox10fl/fl SMGs, we measured total saliva production after pilocarpine stimulation, which includes secretions from SMG, PAR, and SLGs. We measured ∼50% reduction in saliva, likely reflecting reduced PAR and SMG function, since the SLGs appeared unaffected by loss of Sox10 (Figure 5F, N ≥ 4). Subsequently, protein analysis of the saliva by SDS-PAGE and Coomassie staining highlighted that there are quantitative differences in salivary proteins (e.g., AMY1), as well as in low-molecular-weight proteins (Figure 5G, red box). Western blot analysis of an acinar-specific mucin, MUC10, confirmed its loss in saliva (Figure 5H). Overall, these observations suggest that the remaining non-Sox10 duct cells attempt to compensate for the loss in secretory unit formation. However, in the adult environment the duct cells cannot alter their fate to form functional secretory units, which reduces organ function.
In a similar manner, the MMGs of lactating Sox10fl/fl mice were smaller and showed white discoloration (Figure S5G). Morphologically, Sox10fl/fl MMGs showed few to no secretory units, which normally consist of ductal (KRT8+CDH1+), alveolar (KRT8−CDH1+), and myoepithelial (ACTA2+) cells. In addition, pups from Sox10fl/fl mice did not survive the first 24 h post-birth unless fostered by control mice, confirming the inability of MMGs in adult Sox10fl/fl mice to properly produce milk.
Overall, these data indicate that the effects of SOX10 loss affect multiple branching organs in their secretory function.
Expression of Sox10 Increases Plasticity of Ductal Cells and Drives KIT+ Progenitor Formation
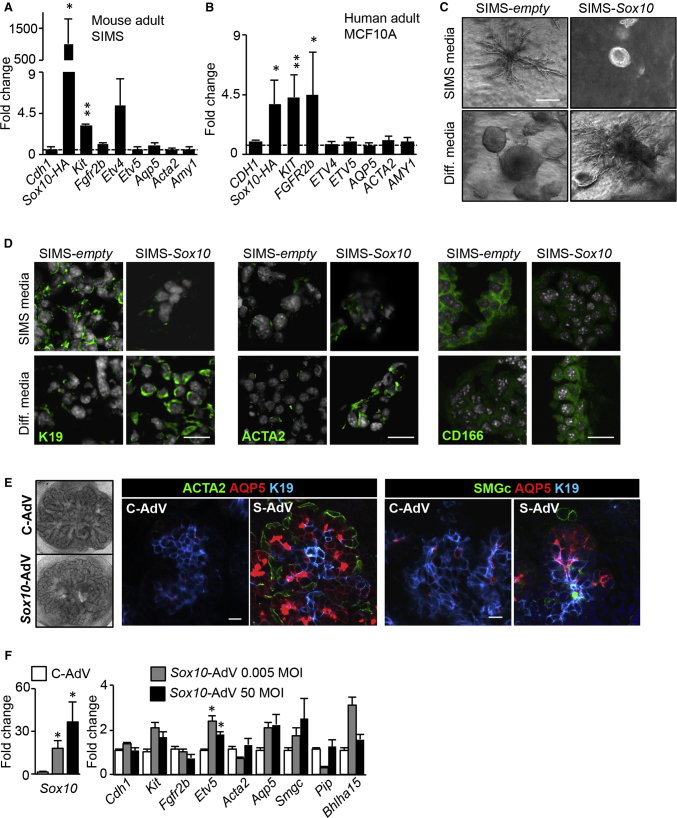
In complementary in vitro and ex vivo experiments, we used virus-mediated overexpression of Sox10 in a mouse and human cell line and in primary mouse fetal cells as proof-of-principle experiments to determine Sox10’s capacity to alter the cell fate in non-KIT duct cells. We first transfected mouse salivary gland epithelial duct (SIMS) and human adult MMG luminal duct (MCF10A) cell lines. Cells were transduced with lentivirus particles expressing Sox10 with human influenza hemagglutinin tag (Sox10-HA), a mutated Sox10 that did not generate SOX10 protein (Figure S6A) and/or CMV-mCherry. There was ∼97% infection efficiency, which was confirmed on protein level after transduction with the SOX10+-mCherry+ vector, the empty-CMV-mCherry vector, or the mutated Sox10-CMV-mCherry vector by immunofluorescence and western blot quantification (Figures S6A–S6C). The mCherry fluorescence was enhanced by RFP staining, which detected all mCherry+ cells (Figure S6B). Interestingly, after transduction with Sox10 there was a significant increased gene expression of Kit in MCF10A and SIMS cells (Figures 6A and 6B). Ffgr2b was significantly upregulated in MCF10A and showed an increased trend in SIMS, as well as its downstream target Etv4. However, genes related to differentiation, such as Acta2, Aqp5, and/or Amy1 remained unaltered during the limited time of passaging. Thus, in a minimal environment of 2D epithelial cell culture, Sox10 expression can induce KIT+FGFR2b+SOX10+ cell-like type in adult ductal cells.
Figure 6.
Sox10 Induces Plasticity by Regulating Fetal KIT+ Progenitor Self-Renewal and Differentiation
(A and B) Fold changes in gene expression of SIMS and MCF10A transduced with lentivirus-expressing Sox10, grown in 2D. Data were normalized to empty lentivirus-transduced cells and Rps29 (dotted line). Mean ± SEM, N > 3, multiple comparison t test. ∗p < 0.05. ∗∗p < 0.01.
(C) Bright-field pictures of SIMS-empty or SIMS-Sox10 in 3D, and cultured for 7 days in SIMS medium or differentiation (diff) medium. Scale bar, 250 μm.
(D) Confocal images of K19, ACTA2, and CD166 on cells in the various conditions seen in (C). Scale bars, 30 μm.
(E) Bright-field pictures of recombined fetal SMGs. Control or Sox10fl/fl SMG E13 epithelia was transfected with control eGFP-Adv (C-Adv) or Sox10-Adv (S-Adv) at 0.005 or 50 MOI before being recombined with its original mesenchyme, blood vessels, and parasympathetic ganglia. Recombined glands were cultured for an additional 6 days. Tissue was stained for ACTA2, AQP5, K19, or SMGc, and analyzed by confocal microscopy. Scale bars, 20 μm.
(F) Fold changes in expression of Sox10, and genes related to Fgfr2b/Kit signaling, myoepithelial, acinar, and ID markers from tissue in (E). Data were normalized to Rps29 and C-Adv recombined tissue. Mean ± SEM, N > 3, multiple comparison t test. ∗p < 0.05.
As proper secretory unit formation may require 3D settings, we placed SIMS-empty or SIMS-Sox10 cells in 3D. We used SIMS medium, as used in 2D, or differentiation medium, to stimulate secretory unit formation (Figures 6C, 6D, S6D, and S6E). By 7 days in SIMS medium, SIMS-empty cells showed branched structures containing K19+ duct cells and cells expressing CD116, a marker for adult acinar cells (Maria et al., 2012). In contrast, SIMS-Sox10 cells formed round spheroids consisting of cells with low protein expression of K19, ACTA2, or CD166, suggesting that Sox10 prevents differentiation. However, the opposite outcome was observed in differentiation medium. SIMS-empty cells grew as round spheroids with cells expressing low levels of K19, ACTA, and CD166, whereas SIMS-Sox10 cells formed organoid-like structures with K19+ ductal, ACTA2+ myoepithelial, and CD166+ acinar cells. Thus, while SOX10- SIMS-empty cells are able to form ducts and/or acini in 3D, overexpression of Sox10 enables either cell maintenance or enhances differentiation into all secretory unit cell types. Moreover, this Sox10-driven cell fate decision seems to be dictated by different growth factors.
To evaluate whether Sox10 alone can induce plasticity of fetal KIT+SOX10-primary duct cells, we enzymatically isolated E13 epithelia of control and Sox10fl/fl SMGs and transduced them with adenovirus (Adv)-expressing mouse Sox10. The fetal SMG is easily dissociated into epithelium and mesenchyme, which is the endogenous fetal microenvironment containing stromal, endothelial, and neuronal cells. These compartments can be genetically manipulated with viral vectors before they get recombined and cultured further. Adv-eGFP was used as a control to show the high transduction efficiency of Adv in SMG epithelia (Figure S6F). Consequently, transduced epithelia were recombined with mesenchyme and cultured ex vivo for 6 days to reach an E16 SMG equivalent stage, as determined by the expression of cellular differentiation markers of myoepithelial, Ac, and ID cells (Figure S6G). Recombined Sox10fl/fl SMGs treated with control Adv had a ductal morphology, while Sox10-Adv treated explants formed prominent endbuds (Figures 6E and S6H). Two different doses of Adv were used; MOI 0.005 and 50. Both MOIs resulted in increased expression of Sox10 ∼20–40-fold, and qPCR analysis also showed increased expression of Kit, Etv5, Aqp5, Smgc, and Bhlha15 (Figure 6F). Thus, gene expression changes suggested there was increased secretory unit differentiation with Sox10 overexpression. Immunostaining highlighted a striking change in endbud morphology. There was a reappearance of distally located ACTA2+ myoepithelial, AQP5+ acinar/ID cells, and SMGc+ acinar cells (Figure 6E), which confirmed that Sox10 is sufficient to induce cell fate changes in fetal non-KIT duct cells and regain the characteristics of multi-potent KIT+SOX10+ progenitors in a normal developing environment.
Overall, these data indicate that expression of the TF Sox10 can induce plasticity to form KIT+SOX10+ progenitors in adult epithelial duct cells. Moreover, in 3D ex vivo settings, Sox10 expression increases the plasticity of non-SOX10 expressing duct cells to either induce maintenance or differentiation into secretory units.
Discussion
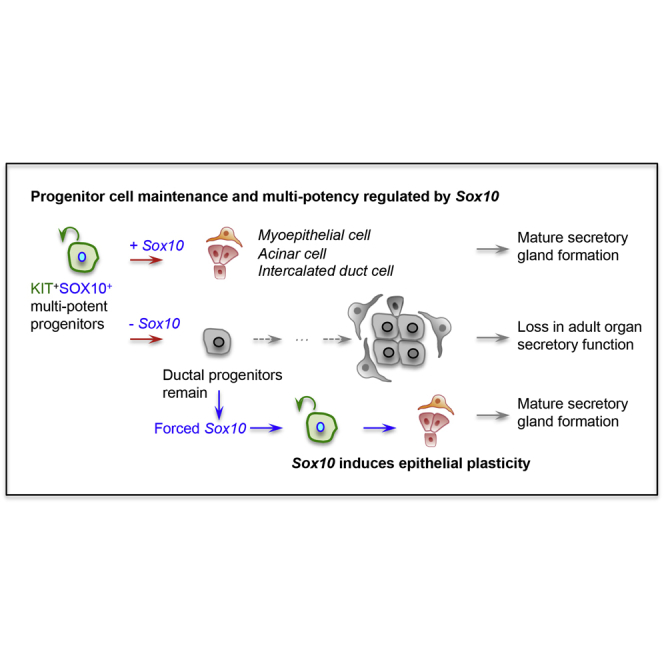
Our studies have identified an essential role for Sox10 in the formation of multi-potent progenitors within multiple exocrine glands. First, Sox10 is essential for the maintenance and proliferation of KIT+ progenitors. An additional role of Sox10 is to initiate the differentiation of KIT+ progenitors toward myoepithelial, acinar, and ID cells, all of which form the secretory unit of exocrine glandular tissues. Lastly, we show that Sox10 expression can alter the plasticity of non-KIT epithelial cells by driving their cell fate toward a multi-potent KIT+ progenitor; all which indicate that SOX10 is a universal master regulator in exocrine glands.
There are striking similarities in the role of Sox10 in multiple exocrine glands. Our initial findings that Sox10 is regulated by KIT/FGFR2b signaling (Lombaert et al., 2013) was later, in part, confirmed in both lacrimal and MMGs (Chen et al., 2014, Dravis et al., 2015), in which FGF10/FGFR2b activation or loss influenced Sox10 expression. We show that KIT and SOX10 are present in distal lacrimal gland endbuds. Similarly, both SOX10 and KIT expression were correlated with mammary epithelial progenitors (Dravis et al., 2015, Regan et al., 2012).
Also, in vitro mammary spheroid assays determined that SOX10+ stem/progenitors were three times more potent than the classical CD24hiCD49f+ cells, which long defined MMG stem cells (Dravis et al., 2015). In addition, SOX10+ tumors from both salivary (Ohtomo et al., 2013) and MMGs (Hsieh et al., 2016) show characteristics of acinar, ID, and/or myoepithelial cells. Together with our data showing that SOX10 marks multi-potent progenitors during the initiation of lacrimal and salivary glands, we conclude that Sox10 is a master regulator of secretory unit differentiation in multiple exocrine glands.
The function of Sox10 correlates to its expression levels (Dravis et al., 2015). For example, ectopic Sox10 overexpression in isolated primary mammary organoids results in a mesenchymal-like phenotype. The high expressing Sox10 cells failed to organize into secondary organoids, whereas lower Sox10 levels enabled the formation of secondary organoids. In contrast, our ectopic expression experiments were performed by ex vivo recombination assays allowing the Sox10-transduced epithelium to interact with its endogenous multi-cell fetal microenvironment. We concluded that Sox10 expression drives the formation of the KIT+SOX10+ progenitor state, which can differentiate into multiple cell types. Even at high Sox10 expression levels, myoepithelial cell differentiation occurs, which involves an epithelial-mesenchymal transition in glandular tissues (Zhao et al., 2012). Myoepithelial cells in the MMG share a transcriptional profile with a breast cancer subtype, which was proposed to derive from a highly migratory myoepithelial progenitor (Zhao et al., 2012). These data are thus not contradictory with our results, as plasticity is defined by both cell-autonomous and non-cell-autonomous settings. The microenvironment therefore plays a critical role in the level to which plasticity is induced. Both studies cultured mammary myoepithelial progenitors or Sox10-expressing epithelial cells in Matrigel, a basement membrane extract, which resulted in loss of polarization and elevated migratory properties. Our data show how Sox10 overexpression in non-Sox10 fetal epithelial cells cultured in their endogenous multi-cell-type fetal microenvironment results in epithelial differentiation. This endogenous 3D fetal tissue microenvironment is as important to direct cell plasticity as the intracellular master regulator(s). Secreted factors from other cell types may be required in combination with Sox10 to induce plasticity and direct the various cell lineages in normal, deprived, diseased, or injured environments. This idea is supported by the fact that Sox10 overexpression in adult duct cells in 2D induced plasticity toward a KIT-like cell, but drove maintenance or differentiation in 3D settings. However, we speculate that Sox10 might need additional master regulators to induce plasticity in cells that are of a more distant cell lineage.
An interesting finding in our study was the inability of remaining epithelial duct cells in Sox10fl/fl SMGs to differentiate into functional secretory units. Multiple progenitors have been identified that participate during salivary gland development. At various time-points during organogenesis multi-potent and more restricted progenitors are formed. One late-stage bipotent progenitor expressing TF Ascl3 is detected in the SMG at E16. From lineage-tracing studies, Ascl3 cells give rise to some, but not all, adult ductal and mucous-secreting acinar cells (Bullard et al., 2008). Ablation of Ascl3 cells did not reveal any impact on glandular formation, likely due to compensation of other progenitors present (Arany et al., 2011). In contrast, loss of KIT progenitors by reducing Sox10 had a great impact on gland morphology. Surprisingly, higher Ascl3 expression was observed at E16. Yet, Ascl3-expressing cells and/or other non-KIT progenitors were not sufficient to produce fully functional secretory units. Interestingly, myoepithelial cell formation did occur from non-KIT duct progenitors, which supports a novel concept that myoepithelial cells can arise from two independent cell sources; KIT+ progenitors and non-KIT progenitors.
Another issue requiring further investigation is whether an altered microenvironment is created after loss of KIT+ progenitors and their immediate progeny post-E16. Multi-cell-type crosstalk occurs between glandular epithelial progenitors, neuronal, stromal, and endothelial cells (Kwon et al., 2017, Lombaert, 2017). It is possible that the absence of KIT+ progenitors and their progeny affects paracrine signals that influence blood vessel and neuronal cell formation. The control of secretory function in adult tissues is dependent on innervation. Whether lack of secretory units influences the amount or type of innervation remains to be investigated. It also remains to be tested whether SOX10– duct cells can form mature acinar cells postnatally in a SOX10-independent manner. Similarly, it still needs to be determined whether SOX10 regulates the expression of Kit and Fgfr2b directly or indirectly. At a minimum, our data suggest that SOX10 plays a role in the positive feedback loop of KIT/FGFR2b signaling.
In conclusion, our results suggest that Sox10 is a master regulator in epithelial KIT+ progenitors of exocrine glands, including lacrimal, salivary, and MMGs, thus making it a potential target to induce secretory unit formation for in vitro glandular tissue engineering purposes and/or regenerative strategies.
Experimental Procedures
Animal Care and Use
All experiments were approved by the animal care and use committees at the University of Michigan and the National Institute of Dental and Craniofacial Research at the NIH. More information is given in the Supplemental Information.
Ex Vivo Organ Culture, Recombination Assays, and Adenovirus Transduction
Isolation of fetal tissue, epithelial dissection, fetal tissue culture, growth factor concentrations, and recombination assays were described previously (Lombaert et al., 2013). More information is given in the Supplemental Information.
Immunohistochemistry/Fluorescence
Fetal tissue was fixed, blocked, stained, and labeled with primary antibodies according to previously described protocols (Lombaert et al., 2013). More information is given in the Supplemental Information.
qPCR
Real-time PCR was performed as described previously (Lombaert et al., 2013). More information is given in the Supplemental Information.
Saliva Collection
Animals were placed in a restraining device 5 min after pilocarpine injection (2.5 mg/kg, subcutaneously). Saliva was then collected for 15 min and quantified as a volume to body weight ratio, as outlined in (Lombaert et al., 2008). More information is given in the Supplemental Information.
Western Blot Analysis and Coomassie Staining
Protein from saliva was quantified with a BCA test, and resolved on Bis-Tris gels. More information is given in the Supplemental Information.
RNA Sequencing and Bioinformatics Analysis
Total RNA was extracted from E16 SMGs using the Ambion microRNA kit. More information is given in the Supplemental Information.
Lentiviral Overexpression and 3D Culture
SIMS and MCF10a cells were cultured and grown in their respective growth media (Laoide et al., 1996, Qu et al., 2015). More information is given in the Supplemental Information.
Statistical Analysis and Data Availability
Statistical parameters included N ≥ 3. More information is given in the Supplemental Information.
Author Contributions
H.K.A., G.M., E.T., A.C., E.H., K.Y., and E.B. performed the experiments and analyzed the data. M.P.H. and I.M.A.L. conceptualized the project. I.M.A.L. designed, coordinated the work, and wrote the article. All authors revised the manuscript for intellectual content, agreed to be accountable for all aspects of the work, and gave final approval for submission.
Acknowledgments
This work was supported by NIH R00 DE022557 grant and the Intramural Program of the National Institute of Dental and Craniofacial Research at the NIH. We thank Dr. Wegner for providing the Sox10fl/fl mice, and Drs. Robert Morell and Daniel Martin of the NIDCR/NIDCD Genomics and Computational Biology Core for the RNA sequencing data and the NIDCR Veterinary Resources Core at NIH.
Published: January 31, 2019
Footnotes
Supplemental Information includes Supplemental Experimental Procedures and six figures and can be found with this article online at https://doi.org/10.1016/j.stemcr.2019.01.002.
Accession Numbers
The accession number for the RNA-seq data reported in this paper is GEO: GSE123341.
Supplemental Information
References
- Abdelalim E.M., Emara M.M., Kolatkar P.R. The SOX transcription factors as key players in pluripotent stem cells. Stem Cells Dev. 2014;23:2687–2699. doi: 10.1089/scd.2014.0297. [DOI] [PubMed] [Google Scholar]
- Arany S., Catalan M.A., Roztocil E., Ovitt C.E. Ascl3 knockout and cell ablation models reveal complexity of salivary gland maintenance and regeneration. Dev. Biol. 2011;353:186–193. doi: 10.1016/j.ydbio.2011.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avilion A.A., Nicolis S.K., Pevny L.H., Perez L., Vivian N., Lovell-Badge R. Multipotent cell lineages in early mouse development depend on SOX2 function. Genes Dev. 2003;17:126–140. doi: 10.1101/gad.224503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullard T., Koek L., Roztocil E., Kingsley P.D., Mirels L., Ovitt C.E. Ascl3 expression marks a progenitor population of both acinar and ductal cells in mouse salivary glands. Dev. Biol. 2008;320:72–78. doi: 10.1016/j.ydbio.2008.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan S.S., Kyba M. What is a master regulator? J. Stem Cell Res. Ther. 2013;3 doi: 10.4172/2157-7633.1000e114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatzeli L., Gaete M., Tucker A.S. Fgf10 and Sox9 are essential for the establishment of distal progenitor cells during mouse salivary gland development. Development. 2017;144:2294–2305. doi: 10.1242/dev.146019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen E.Y., Tan C.M., Kou Y., Duan Q., Wang Z., Meirelles G.V., Clark N.R., Ma'ayan A. Enrichr: interactive and collaborative HTML5 gene list enrichment analysis tool. BMC Bioinformatics. 2013;14:128. doi: 10.1186/1471-2105-14-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z., Huang J., Liu Y., Dattilo L.K., Huh S.H., Ornitz D., Beebe D.C. FGF signaling activates a Sox9-Sox10 pathway for the formation and branching morphogenesis of mouse ocular glands. Development. 2014;141:2691–2701. doi: 10.1242/dev.108944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowley W.R. Neuroendocrine regulation of lactation and milk production. Compr. Physiol. 2015;5:255–291. doi: 10.1002/cphy.c140029. [DOI] [PubMed] [Google Scholar]
- Donati G., Watt F.M. Stem cell heterogeneity and plasticity in epithelia. Cell Stem Cell. 2015;16:465–476. doi: 10.1016/j.stem.2015.04.014. [DOI] [PubMed] [Google Scholar]
- Dravis C., Spike B.T., Harrell J.C., Johns C., Trejo C.L., Southard-Smith E.M., Perou C.M., Wahl G.M. Sox10 regulates stem/progenitor and mesenchymal cell states in mammary epithelial cells. Cell Rep. 2015;12:2035–2048. doi: 10.1016/j.celrep.2015.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmerson E., May A.J., Nathan S., Cruz-Pacheco N., Lizama C.O., Maliskova L., Zovein A.C., Shen Y., Muench M.O., Knox S.M. SOX2 regulates acinar cell development in the salivary gland. Elife. 2017;6 doi: 10.7554/eLife.26620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh M.S., Lee Y.H., Chang Y.L. SOX10-positive salivary gland tumors: a growing list, including mammary analogue secretory carcinoma of the salivary gland, sialoblastoma, low-grade salivary duct carcinoma, basal cell adenoma/adenocarcinoma, and a subgroup of mucoepidermoid carcinoma. Hum. Pathol. 2016;56:134–142. doi: 10.1016/j.humpath.2016.05.021. [DOI] [PubMed] [Google Scholar]
- Jones K.B., Klein O.D. Oral epithelial stem cells in tissue maintenance and disease: the first steps in a long journey. Int. J. Oral Sci. 2013;5:121–129. doi: 10.1038/ijos.2013.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knosp W.M., Knox S.M., Hoffman M.P. Salivary gland organogenesis. Wiley Interdiscip. Rev. Dev. Biol. 2012;1:69–82. doi: 10.1002/wdev.4. [DOI] [PubMed] [Google Scholar]
- Kwon H.R., Nelson D.A., DeSantis K.A., Morrissey J.M., Larsen M. Endothelial cell regulation of salivary gland epithelial patterning. Development. 2017;144:211–220. doi: 10.1242/dev.142497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laoide B.M., Courty Y., Gastinne I., Thibaut C., Kellermann O., Rougeon F. Immortalised mouse submandibular epithelial cell lines retain polarised structural and functional properties. J. Cell Sci. 1996;109(Pt 12):2789–2800. doi: 10.1242/jcs.109.12.2789. [DOI] [PubMed] [Google Scholar]
- Larsen H.S., Aure M.H., Peters S.B., Larsen M., Messelt E.B., Kanli Galtung H. Localization of AQP5 during development of the mouse submandibular salivary gland. J. Mol. Histol. 2011;42:71–81. doi: 10.1007/s10735-010-9308-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo H.G., Jin R.U., Sibbel G., Liu D., Karki A., Joens M.S., Madison B.B., Zhang B., Blanc V., Fitzpatrick J.A. A single transcription factor is sufficient to induce and maintain secretory cell architecture. Genes Dev. 2017;31:154–171. doi: 10.1101/gad.285684.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombaert I., Movahednia M.M., Adine C., Ferreira J.N. Concise review: salivary gland regeneration: therapeutic approaches from stem cells to tissue organoids. Stem Cells. 2017;35:97–105. doi: 10.1002/stem.2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombaert I.M., Abrams S.R., Li L., Eswarakumar V.P., Sethi A.J., Witt R.L., Hoffman M.P. Combined KIT and FGFR2b signaling regulates epithelial progenitor expansion during organogenesis. Stem Cell Reports. 2013;1:604–619. doi: 10.1016/j.stemcr.2013.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombaert I.M., Brunsting J.F., Wierenga P.K., Faber H., Stokman M.A., Kok T., Visser W.H., Kampinga H.H., de Haan G., Coppes R.P. Rescue of salivary gland function after stem cell transplantation in irradiated glands. PLoS One. 2008;3:e2063. doi: 10.1371/journal.pone.0002063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombaert I.M., Hoffman M.P. Epithelial stem/progenitor cells in the embryonic mouse submandibular gland. Front. Oral Biol. 2010;14:90–106. doi: 10.1159/000313709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombaert I.M., Knox S.M., Hoffman M.P. Salivary gland progenitor cell biology provides a rationale for therapeutic salivary gland regeneration. Oral Dis. 2011;17:445–449. doi: 10.1111/j.1601-0825.2010.01783.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombaert I.M.A. Implications of salivary gland developmental mechanisms for the regeneration of adult damaged tissues. In: Cha S., editor. Salivary Gland Development and Regeneration. Springer; 2017. pp. 3–22. [Google Scholar]
- Maria O.M., Maria A.M., Cai Y., Tran S.D. Cell surface markers CD44 and CD166 localized specific populations of salivary acinar cells. Oral Dis. 2012;18:162–168. doi: 10.1111/j.1601-0825.2011.01858.x. [DOI] [PubMed] [Google Scholar]
- Ohtomo R., Mori T., Shibata S., Tsuta K., Maeshima A.M., Akazawa C., Watabe Y., Honda K., Yamada T., Yoshimoto S. SOX10 is a novel marker of acinus and intercalated duct differentiation in salivary gland tumors: a clue to the histogenesis for tumor diagnosis. Mod. Pathol. 2013;26:1041–1050. doi: 10.1038/modpathol.2013.54. [DOI] [PubMed] [Google Scholar]
- Qu Y., Han B., Yu Y., Yao W., Bose S., Karlan B.Y., Giuliano A.E., Cui X. Evaluation of MCF10A as a reliable model for normal human mammary epithelial cells. PLoS One. 2015;10:e0131285. doi: 10.1371/journal.pone.0131285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regan J.L., Kendrick H., Magnay F.A., Vafaizadeh V., Groner B., Smalley M.J. c-Kit is required for growth and survival of the cells of origin of Brca1-mutation-associated breast cancer. Oncogene. 2012;31:869–883. doi: 10.1038/onc.2011.289. [DOI] [PubMed] [Google Scholar]
- Reiprich S., Wegner M. From CNS stem cells to neurons and glia: Sox for everyone. Cell Tissue Res. 2015;359:111–124. doi: 10.1007/s00441-014-1909-6. [DOI] [PubMed] [Google Scholar]
- Rice R., Spencer-Dene B., Connor E.C., Gritli-Linde A., McMahon A.P., Dickson C., Thesleff I., Rice D.P. Disruption of Fgf10/Fgfr2b-coordinated epithelial-mesenchymal interactions causes cleft palate. J. Clin. Invest. 2004;113:1692–1700. doi: 10.1172/JCI20384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothova M., Thompson H., Lickert H., Tucker A.S. Lineage tracing of the endoderm during oral development. Dev. Dyn. 2012;241:1183–1191. doi: 10.1002/dvdy.23804. [DOI] [PubMed] [Google Scholar]
- Tata P.R., Rajagopal J. Cellular plasticity: 1712 to the present day. Curr. Opin. Cell Biol. 2016;43:46–54. doi: 10.1016/j.ceb.2016.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teshima T.H., Wells K.L., Lourenco S.V., Tucker A.S. Apoptosis in early salivary gland duct morphogenesis and lumen formation. J. Dent. Res. 2016;95:277–283. doi: 10.1177/0022034515619581. [DOI] [PubMed] [Google Scholar]
- Tucker A.S. Salivary gland development. Semin. Cell. Dev. Biol. 2007;18:237–244. doi: 10.1016/j.semcdb.2007.01.006. [DOI] [PubMed] [Google Scholar]
- Wang J., Laurie G.W. Organogenesis of the exocrine gland. Dev. Biol. 2004;273:1–22. doi: 10.1016/j.ydbio.2004.05.025. [DOI] [PubMed] [Google Scholar]
- Zhao X., Malhotra G.K., Band H., Band V. Derivation of myoepithelial progenitor cells from bipotent mammary stem/progenitor cells. PLoS One. 2012;7:e35338. doi: 10.1371/journal.pone.0035338. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.