Abstract
Background
Stable angina pectoris is a chronic medical condition with significant impact on mortality and quality of life; it can be macrovascular or microvascular in origin. Ranolazine is a second‐line anti‐anginal drug approved for use in people with stable angina. However, the effects of ranolazine for people with angina are considered to be modest, with uncertain clinical relevance.
Objectives
To assess the effects of ranolazine on cardiovascular and non‐cardiovascular mortality, all‐cause mortality, quality of life, acute myocardial infarction incidence, angina episodes frequency and adverse events incidence in stable angina patients, used either as monotherapy or as add‐on therapy, and compared to placebo or any other anti‐anginal agent.
Search methods
We searched CENTRAL, MEDLINE, Embase and the Conference Proceedings Citation Index ‐ Science in February 2016, as well as regional databases and trials registers. We also screened reference lists.
Selection criteria
Randomised controlled trials (RCTs) which directly compared the effects of ranolazine versus placebo or other anti‐anginals in people with stable angina pectoris were eligible for inclusion.
Data collection and analysis
Two authors independently selected studies, extracted data and assessed risk of bias. Estimates of treatment effects were calculated using risk ratios (RR), mean differences (MD) and standardised mean differences (SMD) with 95% confidence intervals (CI) using a fixed‐effect model. Where we found statistically significant heterogeneity (Chi² P < 0.10), we used a random‐effects model for pooling estimates. Meta‐analysis was not performed where we found considerable heterogeneity (I² ≥ 75%). We used GRADE criteria to assess evidence quality and the GRADE profiler (GRADEpro GDT) to import data from Review Manager 5.3 to create 'Summary of findings' tables.
Main results
We included 17 RCTs (9975 participants, mean age 63.3 years). We found very limited (or no) data to inform most planned comparisons. Summary data were used to inform comparison of ranolazine versus placebo. Overall, risk of bias was assessed as unclear.
For add‐on ranolazine compared to placebo, no data were available to estimate cardiovascular and non‐cardiovascular mortality. We found uncertainty about the effect of ranolazine on: all‐cause mortality (1000 mg twice daily, RR 0.83, 95% CI 0.26 to 2.71; 3 studies, 2053 participants; low quality evidence); quality of life (any dose, SMD 0.25, 95% CI ‐0.01 to 0.52; 4 studies, 1563 participants; I² = 73%; moderate quality evidence); and incidence of non‐fatal acute myocardial infarction (AMI) (1000mg twice daily, RR 0.40, 95% CI 0.08 to 2.07; 2 studies, 1509 participants; low quality evidence). Add‐on ranolazine 1000 mg twice daily reduced the fervour of angina episodes (MD ‐0.66, 95% CI ‐0.97 to ‐0.35; 3 studies, 2004 participants; I² = 39%; moderate quality evidence) but increased the risk of non‐serious adverse events (RR 1.22, 95% CI 1.06 to 1.40; 3 studies, 2053 participants; moderate quality evidence).
For ranolazine as monotherapy compared to placebo, we found uncertain effect on cardiovascular mortality (1000 mg twice daily, RR 1.03, 95% CI 0.56 to 1.88; 1 study, 2604 participants; low quality evidence). No data were available to estimate non‐cardiovascular mortality. We also found an uncertain effect on all‐cause mortality for ranolazine (1000 mg twice daily, RR 1.00, 95% CI 0.81 to 1.25; 3 studies, 6249 participants; low quality evidence), quality of life (1000 mg twice daily, MD 0.28, 95% CI ‐1.57 to 2.13; 3 studies, 2254 participants; moderate quality evidence), non‐fatal AMI incidence (any dose, RR 0.88, 95% CI 0.69 to 1.12; 3 studies, 2983 participants; I² = 50%; low quality evidence), and frequency of angina episodes (any dose, MD 0.08, 95% CI ‐0.85 to 1.01; 2 studies, 402 participants; low quality evidence). We found an increased risk for non‐serious adverse events associated with ranolazine (any dose, RR 1.50, 95% CI 1.12 to 2.00; 3 studies, 947 participants; very low quality evidence).
Authors' conclusions
We found very low quality evidence showing that people with stable angina who received ranolazine as monotherapy had increased risk of presenting non‐serious adverse events compared to those given placebo. We found low quality evidence indicating that people with stable angina who received ranolazine showed uncertain effect on the risk of cardiovascular death (for ranolazine given as monotherapy), all‐cause death and non‐fatal AMI, and the frequency of angina episodes (for ranolazine given as monotherapy) compared to those given placebo. Moderate quality evidence indicated that people with stable angina who received ranolazine showed uncertain effect on quality of life compared with people who received placebo. Moderate quality evidence also indicated that people with stable angina who received ranolazine as add‐on therapy had fewer angina episodes but increased risk of presenting non‐serious adverse events compared to those given placebo.
Plain language summary
Ranolazine for people with stable angina pectoris
Review question
We wanted to find out if ranolazine (a drug to prevent angina) was more effective than a fake drug (placebo) or other drugs to treat stable angina.
Background
Angina pectoris is sudden chest pain caused when the heart does not get enough oxygen or from other stresses. People with stable angina have a predictable pattern of when they experience angina symptoms. Angina is made worse by physical effort and relieved by rest or some medications. Ranolazine is a relatively new drug for people with angina pectoris who are already taking other drugs to treat angina.
Search date
The evidence is current to February 2016.
Study funding sources
Most studies were either fully (9/17) or partly (3/17) funded by drug companies, two received no external funding, and three did not declare sources of funding.
Study characteristics
We included 17 studies that involved a total of 9975 adult participants and lasted between 1 and 92 weeks.
Key results
We only compared ranolazine and placebo because there were few data for other comparisons. The evidence was uncertain about the effect of ranolazine 1000 mg given alone twice daily to people with stable angina pectoris on the chance of dying from heart‐related causes. There was no evidence about whether ranolazine changed the risk of dying from causes that were not heart‐related.
Although the evidence was uncertain about the effect of ranolazine 1000 mg twice daily on the chance of dying from any cause, quality of life, the possibility of heart attack or the frequency of angina attacks (for ranolazine taken alone), ranolazine did modestly reduce the numbers of angina attacks per week when given with other anti‐angina drugs. Ranolazine 1000 mg twice daily increased the risk for experiencing dizziness, nausea and constipation from taking the drug (mild adverse events).
Quality of evidence
Overall, evidence quality was assessed as very low for the chance of mild adverse events (for people who took ranolazine alone). Evidence was also low for estimating the chance of death from heart‐related (when ranolazine is taken alone) or any causes, having a heart attack, and how often angina attacks occur (when ranolazine is taken alone). We found moderate quality evidence about quality of life, frequency of angina attacks and the chance of experiencing mild adverse events (for people who took ranolazine together with other anti‐angina drugs),
Low evidence quality related to problems and reporting of study methods and too few data to calculate precise estimates.
Summary of findings
Summary of findings for the main comparison. Ranolazine (add‐on therapy) versus placebo for stable angina pectoris.
| Ranolazine (add‐on therapy) versus placebo for stable angina pectoris* | ||||||
| Patient or population: patients with stable angina pectoris Settings: not specified Intervention: ranolazine (add‐on therapy) Comparison: placebo (add‐on therapy) | ||||||
| Outcomes | Illustrative comparative risks** (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Ranolazine | |||||
| Cardiovascular mortality ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | No data were available for this outcome |
| Non‐cardiovascular mortality ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | No data were available for this outcome |
| All‐cause mortality Follow‐up: 42 to 84 days | 6 per 1000 | 5 per 1000 (2 to 16) | RR 0.83 (0.26 to 2.71) | 2053 (3 studies) | ⊕⊕⊝⊝ low¹ ² | Ranolazine 1000 mg twice daily |
| Quality of life Scale: 0 to 100. Follow‐up: 28 to 56 days | Mean quality of life in control group participants was 44.3 points | Mean quality of life in intervention group participants was 0.25 standard deviations higher (0.01 lower to 0.52 higher) | 1563 (4 studies) | ⊕⊕⊕⊝ moderate³ | Ranolazine any dose (SMD 0.25, 95% CI ‐0.01 to 0.52) |
|
| AMI incidence Follow‐up: 42 to 56 days | 7 per 1000 | 3 per 1000 (1 to 14) | RR 0.40 (0.08 to 2.07) | 1509 (2 studies) | ⊕⊕⊝⊝ low⁴ | Ranolazine 1000 mg twice daily |
| Angina episodes frequency Follow‐up: 42 to 84 days | Mean angina episode frequency in control group participants was 4.1 episodes per week | Mean angina episodes frequency in intervention group participants was 0.66 lower (0.97 to 0.35 lower) | 2004 (3 studies) | ⊕⊕⊕⊝ moderate¹ | Ranolazine 1000 mg twice daily (MD ‐0.66, 95% CI ‐0.97 to ‐0.35) |
|
| Adverse events incidence Follow‐up: 42 to 84 days | 241 per 1000 | 294 per 1000 (256 to 337) | RR 1.22 (1.06 to 1.4) | 2123 (3 studies) | ⊕⊕⊕⊝ moderate⁵ | Ranolazine 1000 mg twice daily |
| *Add‐on therapy: refers to the addition of ranolazine to an antianginal regimen already in course. The results reported correspond to the comparisons (data and analyses) 3 and 4 of the review (involving ranolazine given at 1000mg twice daily or any dosage); this is specified in the Comments column. **The assumed risk is based on the median control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
¹ Quality of evidence was downgraded one level due to unclear risk of bias regarding blinding of outcome assessment and incomplete outcome data ² Quality of evidence was downgraded one level due to insufficient number of events (less than 300), and the 95% confidence interval around the pooled effect includes both 1) no effect and 2) appreciable benefit/harm ³ Quality of evidence was downgraded one level due to substantial heterogeneity ⁴ Quality of evidence was downgraded two levels due to insufficient number of events (less than 300), and the 95% confidence interval around the pooled effect includes both 1) no effect and 2) appreciable benefit/harm ⁵ Quality of evidence was downgraded one level due to unclear risk of bias regarding blinding of outcome assessment and selective reporting
Summary of findings 2. Ranolazine (monotherapy) versus placebo for stable angina pectoris.
| Ranolazine (monotherapy) versus placebo for stable angina pectoris* | ||||||
| Patient or population: patients with stable angina pectoris Settings: not specified Intervention: ranolazine (monotherapy) Comparison: placebo (monotherapy) | ||||||
| Outcomes | Illustrative comparative risks** (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Ranolazine | |||||
| Cardiovascular mortality Follow‐up: mean 643 days | 16 per 1000 | 16 per 1000 (9 to 29) | RR 1.03 (0.56 to 1.88) | 2604 (1 study) | ⊕⊕⊝⊝ low¹ ² | Ranolazine 1000 mg twice daily |
| Non‐cardiovascular mortality ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | No data were available for this outcome |
| All‐cause mortality Follow‐up: 37 to 643 days | 49 per 1000 | 49 per 1000 (39 to 61) | RR 1.00 (0.81 to 1.25) | 6249 (3 studies) | ⊕⊕⊝⊝ low² ³ | Ranolazine 1000 mg twice daily |
| Quality of life Scale: 0 to 100 Follow‐up: 14 to 643 days | Mean quality of life in control group participants was 68.6 points | Mean quality of life in intervention group participants was 0.28 higher (1.57 lower to 2.13 higher) | 2256 (3 studies) | ⊕⊕⊕⊝ moderate⁴ | Ranolazine 1000 mg twice daily (MD 0.28. 95% CI ‐1.57 to 2.13) |
|
| AMI incidence Follow‐up: 7 to 643 days | 85 per 1000 | 75 per 1000 (59 to 96) | RR 0.88 (0.69 to 1.12) | 2983 (3 studies) | ⊕⊕⊝⊝ low | Ranolazine any dose |
| Angina episodes frequency Follow‐up: 14 to 28 days | Mean angina episode frequency in control group participants was 2.08 episodes per week | Mean angina episode frequency in intervention group participants was 0.08 higher (0.85 lower to 1.01 higher) | 402 (2 studies) | ⊕⊕⊝⊝ low³ ⁵ | Ranolazine any dose (MD 0.08, 95% CI ‐0.85 to 1.01) |
|
| Adverse events incidence Follow‐up: 7 to 14 days | 131 per 1000 | 197 per 1000 (147 to 262) | RR 1.50 (1.12 to 2) | 947 (3 studies) | ⊕⊝⊝⊝ very low³ ⁵ ⁶ | Ranolazine any dose |
| *Monotherapy: refers to the administration of ranolazine as the only antianginal drug. The results reported correspond to the comparisons (data and analyses) 1 and 2 of the review (involving ranolazine given at 1000mg twice daily or any dosage); this is specified in the Comments column. **The assumed risk is based on the median control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
¹ Quality of evidence was downgraded one level due to unclear risk of bias regarding allocation concealment and high risk of bias regarding selective reporting ² Quality of evidence was downgraded one level due to insufficient numbers of events (< 300), and the 95% CI around the pooled effect includes both 1) no effect and 2) appreciable benefit/harm ³ Quality of evidence was downgraded one level because a group of participants (not quantified) in one or more included studies received ranolazine in addition to usual anti‐anginals (i.e. not as monotherapy) ⁴ Quality of evidence was downgraded one level because the 95% CI around the pooled effect included both 1) no effect and 2) appreciable benefit/harm ⁵ Quality of evidence was downgraded one level due to unclear risk of bias for most criteria ⁶ Quality of evidence was downgraded one level due to insufficient numbers of events (< 300)
Background
Description of the condition
Stable angina pectoris is a chronic medical condition which is generally regarded as the main symptomatic manifestation of coronary artery disease (CAD) (NICE 2011). It has been estimated that stable angina affects 58% of people with CAD (Ohman 2016), with an annual mortality rate ranging between 1.2% and 2.4% (ESC 2013). Apart from its associated risk of cardiovascular death and recurrent myocardial infarction, stable angina pectoris has a significant impact on functional capacity and quality of life (Scirica 2009). Mortality is higher among people with angina than those with no history of CAD at baseline (O'Toole 2008). Factors associated with a poorer prognosis include more severe symptoms, male sex, abnormal resting electrocardiogram (ECG) and previous myocardial infarction (O'Toole 2008).
A universal definition for stable angina has not been agreed internationally, but it is usually recognised clinically by its character, location and relationship to provocative stimuli (NICE 2010). Angina pain is identified by: constricting discomfort in the chest or neck, shoulders, jaw or arms; precipitated by physical exertion; and relieved by rest or nitrates within about 5 to 10 minutes. Typical angina is defined by the presence of all of these features (NICE 2010). The underlying cause is of angina pectoris is usually macrovascular CAD, but may be microvascular in some people (ESC 2013). Importantly, other (non CAD) cardiac conditions may be responsible for typical anginal pain, including aortic valve disease and hypertrophic cardiomyopathy (NICE 2010). Macrovascular CAD refers to dysfunction of the coronary arteries and their main branches, as opposed to microvascular CAD in which dysfunction involves the small coronary arterioles (< 500 μm) (Jones 2012).
Diagnosis of stable angina due to CAD can be established based solely on clinical assessment or with the aid of additional diagnostic testing (NICE 2010). Basic tests usually involve biochemical tests, resting ECG, echocardiography, etc. Non‐invasive diagnostic tests include exercise ECG, stress imaging testing and coronary computed‐tomography angiography (CTA). The only invasive test is invasive coronary angiography (ESC 2013). The choice of diagnostic test (functional or structural, invasive or non‐invasive) is guided by the estimated likelihood of CAD (from clinical assessment) and consideration of coronary revascularisation (NICE 2010). Although current NICE guidelines do not recommend exercise ECG to evaluate people with suspected stable angina, it remains a useful option because of its simplicity and widespread availability (ESC 2013). Furthermore, according to American (ACC/AHA 2012) and European (SIGN 2007; ESC 2013) guidelines, exercise ECG is recommended as an option to impose stress during imaging for people with intermediate pre‐test probability of CAD. Some people with stable angina have microvascular coronary disorders, which can be detected on normal coronary angiography only (Di Fiore 2013). Since evaluation of a person with stable angina does not always include coronary angiography (either invasive or non‐invasive), people with microvascular coronary dysfunction would remain unidentified using this approach.
Description of the intervention
Management options for people with stable angina include lifestyle modifications, pharmacological therapy, and revascularisation interventions. Treatment is aimed at improving prognosis (by preventing myocardial infarction and death) and minimising or abolishing symptoms. All management options have potential to meet both treatment aims (ESC 2013). However, the main aim of anti‐anginal drug treatment is to prevent episodes of angina; the secondary aim is to prevent cardiovascular events such as heart attack and stroke (NICE 2011). Anti‐anginal drugs are classified as first‐line (adrenergic beta antagonists, calcium channel blockers) or second‐line (long‐acting nitrates, ivabradine, nicorandil, ranolazine, trimetazidine) (Tarkin 2012). Anti‐angina treatment is recommended to begin using one of the first‐line drugs as monotherapy. If symptoms are not controlled satisfactorily, a combination of two first‐line drugs is recommended. Second‐line drugs are recommended as add‐on therapy when a combination of two first‐line drugs cannot be accomplished, or as monotherapy when none of the first‐line drugs can be used (ESC 2013; NICE 2011). Adding a third anti‐angina drug can be considered only when revascularisation is not an option or as a temporary measure while the patient awaits revascularisation (NICE 2011). However, since ranolazine is the only second‐line drug approved by the US Food and Drug Administration (FDA) (Hawwa 2013), American guidelines (ACC/AHA 2012) recommend use of ranolazine in a similar way to second‐line drugs in European guidelines (ESC 2013).
Ranolazine was approved by the US FDA in 2007 for use in a maximum dose (extended release) of 1000 mg twice daily (FDA 2016), and by the European Medicines Agency (EMA) in 2008 for use in a maximum dose (prolonged release) of 750 mg twice daily (EMA 2008). The immediate release presentation shows peak plasma concentrations within one hour, with an estimated half‐life of 1.4 hours to 1.9 hours (Jerling 2006); for the extended release presentation, values are 2 hours to 6 hours and 7 hours, respectively (Cattaneo 2015; Jerling 2006). The ranolazine extended release preparation reduces the frequency of angina episodes, improves exercise performance, and delays the development of exercise‐induced angina and ST‐segment depression (ACC/AHA 2012). Although these effects are considered to be dose‐related (Chaitman 2011), they have been observed to be modest (EMA 2008) and of uncertain clinical significance (NICE 2011). Furthermore, there is no evidence about the effects of ranolazine on long‐term outcomes in people with stable angina, or for the addition of ranolazine to a calcium channel blocker (NICE 2011). Conversely, an advantage of ranolazine is that it does not cause significant haemodynamic changes, with an average of less than 2 beats per minute reduction in heart rate and less than 3 mm Hg decrease in systolic blood pressure (ACC/AHA 2012). However, ranolazine is associated with a dose‐dependant increase in QT‐interval, with a mean increase of 6 ms at the maximum recommended dosing (ACC/AHA 2012). More recently, an anti‐arrhythmic (antifibrillatory) effect of ranolazine has been proposed, but current evidence is based on small, non‐controlled trials (Hawwa 2013). Contra‐indications to ranolazine are prolonged QT‐interval and co‐administration with other QT‐prolonging drugs, previous history of ventricular tachycardia and moderate to severe kidney impairment or severe liver failure (Tarkin 2012). The most common adverse events related to use of ranolazine are headache (5.5%), dizziness (1% to 6%), constipation (5%) and nausea (≤ 4%; dose related). Although there is concern about QT prolongation on ECG, its prevalence has been estimated to be less than 1% (Ranexa PI 2013).
How the intervention might work
Ranolazine is a selective inhibitor of the late sodium current (INaL) in cardiomyocytes, which is thought to be an important contributor to the pathogenesis of angina through calcium overload and increase in oxygen consumption in the cardiomyocytes (Codolosa 2014). Although most studies have focused on its role in macrovascular angina, some findings suggest that ranolazine also has anti‐inflammatory or antioxidant effects which may improve glycometabolic homeostasis, which are more important in microvascular angina (Cattaneo 2015).
Early studies on the effects of ranolazine in people with stable angina have been undertaken using the drug's immediate release formulation. However, given that its action was deemed significant only in the peak measurements, an extended release formulation was developed, which was approved for use in people with stable angina (Keating 2008). Overall, ranolazine has been shown to improve exercise tolerance test (ETT) parameters and angina frequency in people with stable angina without substantial haemodynamic effect (Savarese 2013). However, ranolazine has also been related to prolongation of the QTc interval, although not pro‐arrhythmic at therapeutic doses (Thadani 2012). Moreover, ranolazine has been shown to have anti‐arrhythmic effects by reducing atrial and ventricular arrhythmias (Hawwa 2013).
A number of subgroup analyses have been performed for ranolazine in people with stable angina. The effects of ranolazine on ETT parameters have been found to be greater among women. However, the effects of ranolazine on decreased angina frequency and nitroglycerin consumption were comparable among the gender groups (Wenger 2007). Differences among age groups have been evaluated. Although ranolazine efficacy is similar among people aged 70 years or older and patients younger than 70 years, its safety profile was better in the younger age group (Rich 2007). Differences between diabetic and non‐diabetic patients have also been sought. Although ranolazine has not been found to have a different effect for people with diabetes regarding ETT parameters, angina frequency and nitroglycerin consumption, it was related to a significant reduction in HbA1c levels (Patel 2008).
Why it is important to do this review
Although ranolazine reduces angina episodes and improves ETT parameters, its impact on the long‐term prognosis in people with stable angina remains unclear. Moreover, the clinical significance of those effects is a matter of debate (NICE 2011). Even though the main indication for ranolazine in people with stable angina is as add‐on therapy, the evidence regarding its use in combination with some first‐line drugs is lacking (NICE 2011). More evidence is needed on the use of ranolazine as monotherapy, given that it may provide an option for people who cannot use any of the first‐line drugs (ESC 2013; NICE 2011) or recommended as a first‐line drug given its apparently better side effects profile compared with classical anti‐anginal agents (ACC/AHA 2012). In view of these gaps in the knowledge of the role of ranolazine for the management of people with stable angina, a systematic analysis of relevant, high quality, and up‐to‐date evidence was needed.
Objectives
To assess the effects of ranolazine on cardiovascular and non‐cardiovascular mortality, all‐cause mortality, quality of life, acute myocardial infarction incidence, angina episodes frequency and adverse events incidence in stable angina patients, used either as monotherapy or as add‐on therapy, and compared to placebo or any other anti‐anginal agent.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised, controlled, parallel‐group and cross‐over trials, with double blinding (of participants and trial personnel) that assessed the effects of ranolazine in the management of stable angina pectoris, irrespective of the number of groups and the length of follow‐up. However, for safety outcomes, we also included trials regardless of blinding so long as other criteria were met. We included studies reported as full‐text, those published only as abstracts, and unpublished data.
Types of participants
We included adults (aged 18 years or over) diagnosed with stable angina pectoris, irrespective of gender, country of enrolment, setting, previous treatment status, comorbidities and symptom severity. The diagnosis of stable angina pectoris could be established based on clinical history, myocardial ischaemia demonstrated by functional tests or significant obstructive coronary artery disease (CAD) demonstrated by angiography. We considered studies which included a subset of relevant participants if results were reported separately for people with stable angina.
Types of interventions
We included trials comparing ranolazine (given orally for at least one week as either monotherapy or add‐on therapy, irrespective of dose, presentation (immediate or extended release) and daily frequency) with placebo or other anti‐anginal agent. We included the following co‐interventions provided they were not part of the randomised treatment: other anti‐anginal agents (long‐acting nitrates, adrenergic beta antagonists and/or calcium channel blockers), statins, antiplatelet agents, antihypertensive agents and surgical interventions for CAD. We included trials that applied the following designs.
Monotherapy
Ranolazine versus placebo.
Ranolazine versus first‐line anti‐anginal drugs, grouped by class: 1) adrenergic beta antagonists and 2) calcium channel blockers.
Ranolazine versus other second‐line anti‐anginal drugs, grouped as: 1) long‐acting nitrates, 2) ivabradine, 3) nicorandil and 4) trimetazidine.
Add‐on therapy
Ranolazine added to first‐line anti‐anginal drugs (grouped by class as for monotherapy) versus placebo added to first‐line anti‐anginal drugs (grouped by class).
Ranolazine added to other second‐line anti‐anginal drugs (grouped as for monotherapy) versus placebo added to other second‐line anti‐anginal drug (grouped).
Ranolazine added to first‐line anti‐anginal drugs (grouped as for monotherapy) versus other second‐line anti‐anginal drugs (grouped as mentioned before) added to first‐line anti‐anginal drugs (grouped).
Ranolazine added to other second‐line anti‐anginal drugs (grouped as for monotherapy) versus first‐line anti‐anginal drugs (grouped) added to other second‐line anti‐anginal drugs (grouped).
Types of outcome measures
We considered effectiveness and safety outcome measures, and only measures taken at the longest follow‐up within each study. For the outcomes considered, we included only results measured with a follow‐up of at least one week. Studies were included irrespective of whether or not they assessed the outcomes listed below.
Primary outcomes
Effectiveness
Cardiovascular mortality, expressed as a proportion of the total study population.
Safety
Non‐cardiovascular mortality, expressed as a proportion of the total study population.
Secondary outcomes
Effectiveness
All‐cause mortality, expressed as a proportion of the total study population.
Quality of life, measured using general scales: Medical Outcomes Study Short Form‐36 (SF‐36), World Health Organization Quality of Life tool (WHOQOL), Illness Perception Questionnaire (IPQ) and Nottingham Health Profile (NHP) (Silva 2011); or specific scales: Seattle Angina Questionnaire (SAQ), MacNew Heart Disease Health‐Related QoL Questionnaire, Ferrans and Powers QoL Index and Speak from the Heart Chronic Angina Checklist (Young 2013); all were expressed as mean differences (MDs).
Acute myocardial infarction (AMI) incidence (fatal and non‐fatal), defined as the proportion of participants who experienced one or more AMI episodes, expressed separately for fatal and non‐fatal AMI.
Need for revascularisation procedure, expressed as a proportion of the total study population.
Angina episodes frequency, measured as a weekly average, expressed as MDs.
Costs of health care. We considered any information regarding costs of study interventions and related medical care (hospitalisations, additional interventions and outpatient health care).
Time to 1‐mm ST‐segment depression on exercise electrocardiogram (ECG) at peak, measured in seconds, expressed as MDs.
Safety
Adverse events incidence, defined as the proportion of participants who experienced one or more serious (non‐cardiac life‐threatening) or non‐serious events, expressed as a whole but separately for each category. Serious adverse events were defined as those that threaten life, require or prolong hospitalisation, result in permanent disability, or cause birth defects (Cochrane Glossary).
Search methods for identification of studies
Electronic searches
We searched the following databases:
Cochrane Central Register of Controlled Trials (CENTRAL) in the Cochrane Library (Issue 1, 2016, searched 9 February 2016);
MEDLINE In‐Process and Other Non‐Indexed Citations and MEDLINE (Ovid, 1946 to 9 February 2016);
Embase (Ovid, 1980 to 2016 week 6, searched 9 February 2016); and
Conference Proceedings Citation Index ‐ Science (CPCI‐S Web of Science, 1990 to 9 February 2016).
The detailed MEDLINE search strategy is presented in Appendix 1. We adapted the MEDLINE search strategy for other databases (Appendix 1). The Cochrane highly sensitive search strategy for identifying randomised trials, sensitivity‐maximising version, was applied to MEDLINE and adapted for Embase and CPCI‐S (Lefebvre 2011). We searched these databases from dates of inception to 9 February 2016 and did not apply language restrictions.
Searching other resources
In an effort to identify further ongoing, unpublished and published trials (Van Enst 2012) we also searched the following resources (Higgins 2011):
-
National and regional databases:
African Index Medicus (AIM, Africa) (http://indexmedicus.afro.who.int/) (1966 to 24 April 2016);
Informit Health Collection (Australasia) (http://www.informit.com.au/health.html) (1846 to 24 April 2016);
VIP Information/Chinese Scientific Journals Database (CSJD‐VIP, China) (http://www.cqvip.com/) (from inception to 24 April 2016);
Index Medicus for the Eastern Mediterranean Region (IMEMR, Eastern Mediterranean); (http://www.emro.who.int/information‐resources/imemr‐database/) (1966 to 24 April 2016);
IndMED (India) (http://indmed.nic.in/indmed.html) (1980 to 24 April 2016);
KoreaMed (Korea) (http://www.koreamed.org/SearchBasic.php) (1959 to 24 April 2016);
LILACS (Latin America and the Caribbean) (http://lilacs.bvsalud.org/es/) (1980 to 24 April 2016);
Index Medicus for South‐East Asia Region (IMSEAR, DSpace, South‐East Asia) (http://imsear.hellis.org/) (1871 to 24 April 2016); and
Western Pacific Region Index Medicus (WPRIM, Western Pacific) (http://www.wprim.org/) (1950 to 24 April 2016).
-
Grey literature databases:
OpenGrey (Europe, formerly OpenSIGLE (Stock 2011)) (http://www.opengrey.eu/) (1973 to 24 April 2016); and
National Technical Information Service (NTIS, U.S.) (http://www.ntis.gov/) (1851 to 24 April 2016).
-
Prospective trial registers search portals:
WHO International Clinical Trials Registry Platform (WHO ICTRP) (http://www.who.int/ictrp/en/) (1 January 1990 to 24 April 2016);
MetaRegister of Current Controlled Trials (mRCT) (http://www.controlled‐trials.com/mrct/) (6 April 2000 to 24 April 2016); and
ClinicalTrials.gov (http://www.clinicaltrials.gov/) (3 November 1999 to 24 April 2016).
-
Conference abstracts:
American Heart Association Scientific Sessions from 2009 to February 2016 (http://my.americanheart.org/professional/Sessions/ScientificSessions/Archive/Archive‐Scientific‐Sessions_UCM_316935_SubHomePage.jsp); and
European Society of Cardiology Congresses from 2007 to February 2016 (http://www.escardio.org/congresses/past_congresses/Pages/Past‐Congresses.aspx).
-
Other reviews: checking studies included in other relevant reviews retrieved from searches of:
Database of Abstracts of Reviews of Effects (DARE) through the Centre for Reviews and Dissemination (CRD) (http://www.crd.york.ac.uk/CRDWeb/) (1975 to 24 April 2016);
NHS Economic Evaluation Database (NHS EED) through the CRD (http://www.crd.york.ac.uk/CRDWeb/) (1975 to 24 April 2016); and
Health Technology Assessment Database (HTA Database) through the CRD (http://www.crd.york.ac.uk/CRDWeb/) (1975 to 24 April 2016).
Approval documents from the US Food and Drrug Administration (FDA) (http://www.fda.gov/) and the European Medcines Agency (EMA) (http://www.ema.europa.eu/), checked on 24 April 2016.
Checking reference lists of included studies and other relevant papers identified through the search process.
The website of Gilead Sciences (http://www.gilead.com/), the company which discovered, developed and commercialised ranolazine, checked on 24 April 2016.
Data collection and analysis
Selection of studies
Two review authors (LV, JM) independently screened titles and abstracts for inclusion identified from searches, and coded them as 'retrieve' (eligible or potentially eligible/unclear) or 'do not retrieve'. Disagreements were resolved by arbitration involving a third review author (JB). We retrieved full‐text study reports and publications; two review authors (LV, JM) then independently screened the studies for inclusion, and recorded reasons for exclusion of ineligible studies. Disagreements were resolved through discussion, or if required, the participation of a third review author (JB or CS). We identified and excluded duplicates and collated multiple reports of the same study so that each study, rather than each report, was the unit of interest in this Cochrane review. We recorded the selection process in sufficient detail to complete a PRISMA flow diagram (Figure 1) and Characteristics of excluded studies table.
1.
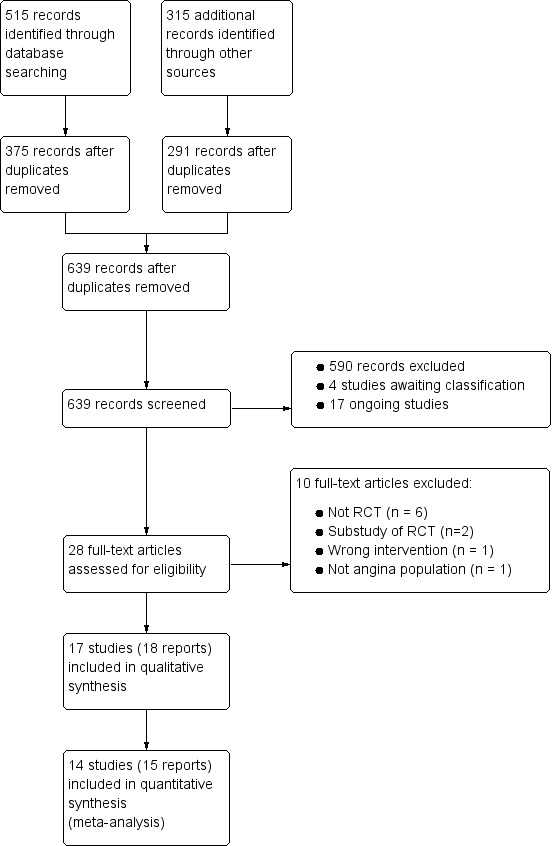
Study flow diagram
†Two included articles report data from the RIVER‐PCI trial
Data extraction and management
We used a data collection form for study characteristics and outcome data which had been piloted on one study included in the review. Four review authors (JB, LV, JM, DR) were involved in both processes so that two review authors independently analysed each included study. We resolved disagreements by consensus or by involving a fifth review author (CS). One review author (JB) entered data into RevMan 2014. We double‐checked that study characteristics and outcome data were entered correctly by comparing the data presented in the systematic review with the study reports. We extracted the following study characteristics:
Methods: date of study, study design, method of randomisation, method of concealment of allocation, blinding, power calculation, duration of follow‐up, number of patients randomised, exclusions post‐randomisation, withdrawals (and reasons).
Participants: N, countries of enrolment, setting/location, mean age/age range, gender (male %), severity of condition, diagnostic criteria, comorbidities, inclusion and exclusion criteria.
Interventions: intervention (including type of formulation), comparison, concomitant medications, excluded medications and duration of treatment.
Outcomes: primary and secondary outcomes (efficacy and safety) specified and collected, and time points reported. For each outcome: outcome definition, method of measurement and unit of measurement. Results: number of patients analysed (according to type of analysis) and main results.
Notes: source of funding and notable conflicts of interest of trial authors.
Assessment of risk of bias in included studies
Two review authors (JB, LV) independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved any disagreement by discussion or by involving a third review author (CS). We assessed the risk of bias according to the following domains:
Random sequence generation (selection bias).
Allocation concealment (selection bias).
Blinding of participants and personnel (performance bias).
Blinding of outcome assessment (detection bias).
Incomplete outcome data (attrition bias).
Selective outcome reporting (reporting bias).
Other bias: source of funding.
We graded each potential source of bias as high, low or unclear and provided a quote from the study report together with a justification for our judgment in 'Risk of bias' tables. We took into account the recommendations of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) regarding 'Risk of bias' assessment of cross‐over studies. We summarised the risk of bias judgements across different studies for each domain. We did not obtain information on risk of bias related to unpublished data or correspondence with trial authors. We performed an additional handsearch to identify published study protocols to check for selective reporting bias. When considering treatment effects, we took into account the risk of bias for the studies that contributed to that outcome.
Assessment of bias in conducting the systematic review
We conducted this Cochrane Review according to the published protocol and reported deviations in Differences between protocol and review.
Measures of treatment effect
We analysed dichotomous data as risk ratios (RRs) with 95% confidence intervals (CIs) and continuous data as mean differences (MDs) with 95% CIs. We used standardised mean differences (SMD) for quality of life meta‐analyses if included data were measured using different tools. We entered data presented as a scale with a consistent direction of effect (with higher scores indicating better quality of life). We did not use skewed data for the quantitative analysis.
Unit of analysis issues
We included randomised controlled trials (RCTs) with either parallel‐group or cross‐over designs. Cross‐over studies were suitable for this Cochrane review because stable angina pectoris is a relatively stable chronic manifestation of disease and the interventions we assessed have only a temporal effect. We took into account the recommendations of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) regarding statistical analysis of cross‐over studies.
Dealing with missing data
We contacted trial authors to obtain missing numerical outcome data where possible. Where this was not possible, we performed analyses only with the available data.
Assessment of heterogeneity
We statistically assessed the presence of heterogeneity among study results by means of the Chi² test with a P value < 0.10 as cut‐off point. We further assessed the degree of heterogeneity by using the I² statistic (McNamara 2015), considering the following thresholds for interpretation: 0% to 40%: not important heterogeneity; 30% to 60%: moderate heterogeneity; 50% to 90%: substantial heterogeneity; and 75% to 100%: considerable heterogeneity (in which case meta‐analyses were not performed) (Higgins 2011).
Assessment of reporting biases
We planned to perform statistical tests for funnel plot asymmetry only for those meta‐analyses which included 10 or more studies (Sterne 2011). Since none of the meta‐analyses performed met this criterion, we used only visual inspection of funnel plots to assess for publication bias.
Data synthesis
We used Review Manager software, version 5.3 (RevMan 2014) for data synthesis and analysis. We undertook meta‐analyses only where this was meaningful, that is, if the treatments, participants and underlying clinical question were sufficiently similar for pooling to make sense. We used fixed‐effect meta‐analyses to calculate effect estimates if there was no statistically significant heterogeneity (Chi² P < 0.10). For results with statistically significant but not considerable heterogeneity (I² ≥ 75%), we used random‐effects meta‐analyses to calculate effect estimates.
Subgroup analysis and investigation of heterogeneity
We planned to carry out subgroup analyses based on the following variables:
age;
gender;
previous AMI status;
patients undergoing percutaneous coronary intervention (PCI); and
number of revascularisation procedures.
Subgroup analyses for these variables could be carried out because we found insufficient data. However, we decided to add another variable: type of stable angina diagnosis (macrovascular versus microvascular). This subgroup analysis was performed for incidence of non‐serious adverse events for ranolazine given as monotherapy compared to placebo, and for quality of life for add‐on ranolazine compared to placebo.
Sensitivity analysis
We undertook sensitivity analyses to explore the effects of decisions we made throughout the review process, including:
Restriction to trials with low risk of bias (those which had at least three domains graded as low risk of bias).
Exchanging the statistical approach for data synthesis (random‐effects versus fixed‐effect models).
Changing the measures of treatment effects for dichotomous (RRs to ORs) and continuous data (SMDs to MDs and vice versa).
Changing the method of dealing with missing data (ignoring versus imputing with replacement values for poor outcomes). This sensitivity analysis was not performed because we decided not to impute any missing data; however, we calculated some data included in the quantitative synthesis from the available information published in reports of the included studies.
Those relevant issues identified during the analyses of studies: we decided to pool the available data irrespective of the duration of follow‐up and perform an additional sensitivity analysis based on this variable (< 6 weeks versus ≥ 6 weeks).
Summary of Findings table and quality of evidence (GRADE)
We created 'Summary of findings' tables for the following outcomes: cardiovascular mortality, non‐cardiovascular mortality, all‐cause mortality, quality of life, AMI incidence, angina episodes frequency and adverse events incidence. We used the five GRADE considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of the body of evidence as it relates to the studies which contributed data to the meta‐analyses for the prespecified outcomes. We used the methods and recommendations described in Section 8.5 and Chapter 12 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) using GRADEpro software (GRADEpro GDT 2015). We provided justifications for all decisions to downgrade the quality of evidence in footnotes and included comments to aid readers' understanding of the review where necessary.
Reaching conclusions
We based our conclusions only on findings from the quantitative or narrative synthesis of included studies for this Cochrane review. The Implications for research sections suggests priorities for future research and outlines remaining uncertainties in the area.
Results
Description of studies
See the Characteristics of included studies; Characteristics of excluded studies; Characteristics of ongoing studies and Characteristics of studies awaiting classification tables for detailed descriptions.
Results of the search
We identified 515 records through searching electronic databases and 315 additional records from other sources. After removing duplicates, 639 records remained for screening of titles and abstracts. We deemed 611 to be irrelevant and the remaining 28 records were obtained in full‐text for eligibility assessment. We excluded 10 reports. We included 17 RCTs (18 records) in the qualitative analysis; of these, 14 studies (15 reports) were also included in the quantitative synthesis (CARISA 2004; ERICA 2006; MARISA 2004; Mehta 2011; MERLIN‐TIMI 36 2007; Pelliccia 2012; Pepine 1999; RAN080 2005; RIVER‐PCI 2016; RWISE 2016; Shammas 2015; TERISA 2013; Thadani 1994; Villano 2013) (see Figure 1).
The MERLIN‐TIMI 36 2007 trial included patients with non ST‐elevation acute coronary syndrome; a subgroup of those patients had also a history of stable angina, and the results regarding these patients were reported in the sub study included in this review. The RIVER‐PCI 2016 trial considered three sub studies in its protocol, all of which were of interest for this review; however, only the results of two have been published in separate reports included in this review.
Included studies
We included 17 randomised controlled trials (RCTs) that enrolled a total of 9975 participants. Two RCTs (MERLIN‐TIMI 36 2007; RIVER‐PCI 2016) provided data for 61.8% of participants.
Of the 17 RCTs, 11 were parallel‐group and six were cross‐over design studies. Most studies were performed in high‐income regions such as North America, Europe and Australia; five studies included participants from Asia, Russia, Israel, India (CARISA 2004; MERLIN‐TIMI 36 2007; RIVER‐PCI 2016; Sandhiya 2015; TERISA 2013) and Africa (MERLIN‐TIMI 36 2007). All but three studies included mostly female participants; Mehta 2011, RWISE 2016 and Villano 2013 reported percentages of male participants as 0%, 4% and 19.6%, respectively. Most participants' ages ranged from 60 years to 80 years. Although all studies included people with angina, some considered additional inclusion criteria that enabled discrimination between people with macrovascular angina (Babalis 2015; CARISA 2004; ERICA 2006; MARISA 2004; RAN080 2005; Sandhiya 2015; Shammas 2015; TERISA 2013) and microvascular angina (Mehta 2011; RWISE 2016; Tagliamonte 2015; Villano 2013). Notably, three of four studies that included people with microvascular angina were also those that included mostly female participants. Only four studies enrolled participants with comorbidities such as acute coronary syndrome (ACS) (MERLIN‐TIMI 36 2007), incomplete revascularisation post‐percutaneous coronary intervention (PCI) (RIVER‐PCI 2016) and type 2 diabetes mellitus (Sandhiya 2015; TERISA 2013). Intervention durations ranged from 1 to 92 weeks.
Ranolazine was used as both extended and immediate release formulations; however, the formulation was not specified in nine studies (Babalis 2015; Mehta 2011; Pelliccia 2012; RIVER‐PCI 2016; Sandhiya 2015; Shammas 2015; Tagliamonte 2015; Thadani 1994; Villano 2013). Ranolazine was administered as add‐on therapy in seven studies. Co‐medications included adrenergic beta antagonists (Shammas 2015), calcium channel blockers (ERICA 2006) or both (Babalis 2015; CARISA 2004; Pepine 1999; TERISA 2013; Villano 2013). However, several other studies (Mehta 2011; MERLIN‐TIMI 36 2007; Pelliccia 2012; RAN080 2005; RIVER‐PCI 2016; RWISE 2016) permitted concomitant anti‐angina medications to be administered to some participants.
Most studies compared ranolazine only with placebo; other comparators included atenolol (RAN080 2005), ivabradine (Villano 2013) and trimetazidine (Sandhiya 2015).
Seven included studies evaluated mainly parameters related to exercise electrocardiogram (ECG) (Babalis 2015; CARISA 2004; MARISA 2004; MERLIN‐TIMI 36 2007; Pepine 1999; RAN080 2005; Thadani 1994), which were added (time to 1‐mm ST‐segment depression at peak) to the review outcomes. Three studies reported data relevant only for a secondary safety outcome (incidence of adverse events) (Babalis 2015; MARISA 2004; Pepine 1999). Only one study reported data for the primary outcomes of this review and collected data on the costs of health care (a secondary outcome). However, results are not yet published (RIVER‐PCI 2016).
Most included studies (n = 12) reported commercial sources of funding including: CV Therapeutics (CARISA 2004; ERICA 2006; MARISA 2004; MERLIN‐TIMI 36 2007; RAN080 2005; RWISE 2016); Syntex Research (Pepine 1999; Thadani 1994); Gilead Sciences (RIVER‐PCI 2016; Shammas 2015; TERISA 2013); and other (Mehta 2011). Two studies reported no external sources of funding (Sandhiya 2015; Pelliccia 2012) and three did not state sources of funding (Babalis 2015; Tagliamonte 2015; Villano 2013).
Excluded studies
We excluded 10 studies after retrieving and assessing full‐text reports. Six studies were excluded because their design did not correspond to a RCT. Four of these studies were economic analyses for which the health economics data provided did not come from studies conducted alongside a RCT (Coleman 2015; Hidalgo‐Vega 2014; Kohn 2014; Lucioni 2009). Another of these studies was a safety study on ranolazine without comparator (ROLE 2007). The remaing study was a one‐group cross‐over trial and it did not state if the treatment order was randomised (Jain 1990). Two studies were excluded because of corresponding to substudies of already included studies (Arnold 2014, Rich 2007). One study was excluded because the duration of the intervention was shorter than the 1‐week period established as a minimum for inclusion (Cocco 1992). Another study was excluded because its population did not match inclusion criteria for participants (Rehberger‐Likozar 2015).
Studies awaiting classification
Four studies await classification (Characteristics of studies awaiting classification). Available data were insufficient to determine if these studies met the inclusion criteria for this review. In three studies, population characteristics were not described in sufficient detail to determine if they were restricted to people with stable angina (NCT01304095; Tagarakis 2013; Tian 2012). The fourth study (Wang 2012) did not provide information about randomisation and blinding. The full‐text reports for three studies (Tagarakis 2013; Tian 2012; Wang 2012) could not be obtained.
Ongoing studies
We identified 17 ongoing trials which met the review eligibility criteria (Characteristics of ongoing studies). Of those, 15 studies are parallel‐group designs and two are cross‐over studies (NCT01754259, NCT01495520). Most studies are being performed in high‐income regions, such as North America and Europe, two studies in Asia (India) (CTRI/2014/01/004332; Gupta 2014); and six did not state locations (Calcagno 2014; Calcagno 2015; NCT02147067; NCT02252406; NCT02423265; Šebeštjen 2014). All but one study includes a mixed population regarding gender; Šebeštjen 2014 includes only males. Seven studies restricted the population to people with macrovascular angina (EUCTR 2011‐001278‐24; EUCTR 2012‐001584‐77; NCT01495520; NCT01754259; NCT01948310; NCT02252406; NCT02423265) and two restricted participants to people with microvascular angina (NCT02052011; NCT02147067). Some studies consider comorbidities or important antecedents such as PCI‐stent implantation (Calcagno 2014; Calcagno 2015; NCT02423265), sustained STEMI (CTRI/2014/01/004332), diabetes mellitus (Gupta 2014; NCT01754259), metabolic syndrome (NCT02252406) and other cardiac conditions (NCT01558830). Intervention durations range from 4 weeks to 12 months.
The evaluation of ranolazine as add‐on therapy is explicitly stated in two studies (Gupta 2014; NCT02423265); the remainder provide insufficient information to determine how ranolazine is being administered. Ranolazine doses range from 375 mg twice daily to 1000 mg twice daily. The comparator for most studies is placebo or no treatment, with only three studies comparing ranolazine with other second‐line anti‐angina treatments such as ivabradine (Calcagno 2015) and trimetazidine (CTRI/2014/01/004332; Šebeštjen 2014).
Five studies assess quality of life (NCT02052011; NCT02147067; NCT02147834; NCT02265796; NCT02423265); two assess frequency of angina episodes (EUCTR 2011‐001278‐24; Gupta 2014); two assess need for revascularisation procedure (NCT02147834; NCT02265796); five assess exercise ECG parameters (Calcagno 2014; Calcagno 2015; EUCTR 2011‐001278‐24; NCT02147067; NCT02423265). Eight studies do not assess any of the effectiveness outcomes of this review (CTRI/2014/01/004332; EUCTR 2012‐001584‐77; NCT01495520; NCT01558830; NCT01754259; NCT01948310; NCT02252406; Šebeštjen 2014). Eight studies state commercial sources of funding (at least partially) from pharmaceutical companies such as Gilead Sciences (NCT01558830; NCT01948310; NCT02052011; NCT02147067; NCT02147834; NCT02265796) and Menarini International Operations (EUCTR 2011‐001278‐24; EUCTR 2012‐001584‐77).
Risk of bias in included studies
Risk of bias is illustrated in Figure 2 and Figure 3. Also see Characteristics of included studies.
2.
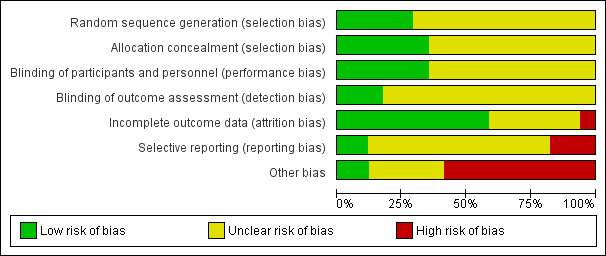
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies
3.
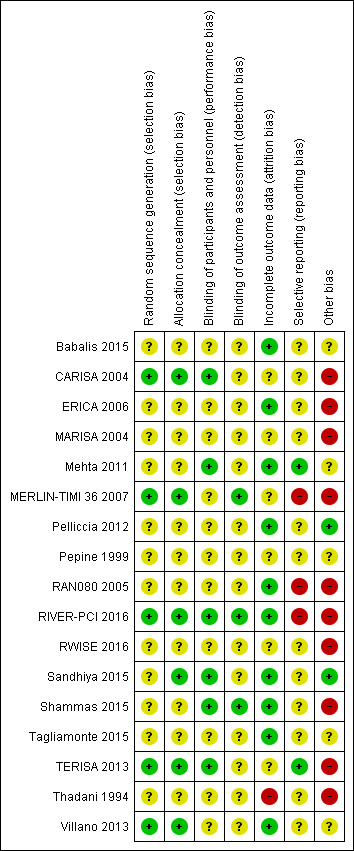
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Other bias criteria: we considered the source of funding in this section, we scored high risk of bias if the source of funding was solely from private organisations, unclear risk of bias if it was mixed (private and public) and low risk of bias if it was solely not external or from public organisations.
Allocation
All included studies randomly assigned participants to treatment groups using either computer‐generated sequences (CARISA 2004; MERLIN‐TIMI 36 2007; RIVER‐PCI 2016; TERISA 2013; Villano 2013) or other methods that were described with insufficient detail to enable assessment (Babalis 2015; ERICA 2006; MARISA 2004; Mehta 2011; Pelliccia 2012; Pepine 1999; RAN080 2005; RWISE 2016; Sandhiya 2015; Shammas 2015; Tagliamonte 2015; Thadani 1994).
Adequate concealment of allocation methods were described in four studies (CARISA 2004; Sandhiya 2015; TERISA 2013; Villano 2013). Two additional studies (MERLIN‐TIMI 36 2007; RIVER‐PCI 2016) did not describe in detail their method for allocation concealment, but it was considered to be adequate given the use of a centralised randomization system. One study stated that investigators had been blinded to treatment allocation (Shammas 2015); this was not considered to be sufficiently detailed to inform assessment.
Blinding
Most studies reported using a double‐blind design for the treatment phase. However, only one study explicitly indicated who were blinded (Shammas 2015). This was established from information provided in five other studies (CARISA 2004; Mehta 2011; RIVER‐PCI 2016; Sandhiya 2015; TERISA 2013). Blinding was not stated in two study reports (Babalis 2015; Villano 2013).
Blinding of outcome assessment was reported in seven studies (CARISA 2004; MARISA 2004; Mehta 2011; MERLIN‐TIMI 36 2007; RIVER‐PCI 2016; Shammas 2015; Villano 2013). However, for outcomes considered in this review, only three studies reported blinding measures for outcome assessment (MERLIN‐TIMI 36 2007; RIVER‐PCI 2016; Shammas 2015).
Incomplete outcome data
Six studies did not report any withdrawals or exclusions of participants, but of these, only three explicitly stated that no participants withdrew or were excluded (Babalis 2015; Mehta 2011; Tagliamonte 2015). Five studies did not describe reasons for exclusions or withdrawal in sufficient detail to inform assessment (CARISA 2004; MERLIN‐TIMI 36 2007; Pepine 1999; RWISE 2016; TERISA 2013). One study did not report the allocated groups of excluded or withdrawn participants (MARISA 2004). The five studies in which numbers, reasons and allocated group of participants who withdrew or were excluded were reported, inconsistencies in data were identified for one or two participants in three studies (ERICA 2006; RAN080 2005; Shammas 2015). The number of exclusions and withdrawals approached 10% of the total study population in one study (Thadani 1994).
The type of analysis was not stated in eight studies (Babalis 2015; ERICA 2006; RWISE 2016; Sandhiya 2015; Shammas 2015; Tagliamonte 2015; TERISA 2013; Villano 2013). Seven studies reported performing intention‐to‐treat analyses (CARISA 2004; MARISA 2004, Mehta 2011; MERLIN‐TIMI 36 2007; Pelliccia 2012; RAN080 2005; RIVER‐PCI 2016); two studies reported performing intention‐to‐treat and per‐protocol analyses (Pepine 1999; Thadani 1994). Pepine 1999 and Thadani 1994 reported results from intention‐to‐treat analyses, and stated that no significant differences were found among intention‐to‐treat and per‐protocol analyses.
Selective reporting
We assessed selective reporting by cross‐checking study outcomes with published protocols. We found protocols for only five included studies (Mehta 2011; MERLIN‐TIMI 36 2007; RIVER‐PCI 2016; RWISE 2016; TERISA 2013). The protocol for the MERLIN‐TIMI 36 trial (Morrow 2006) did not consider the sub study (MERLIN‐TIMI 36 2007) we included in this review (post‐hoc analyses), and thus was considered to be at high risk of bias. Of note, the report of this sub study met our pre‐specified inclusion criteria, and it takes part of only one of our analyses (Analysis 1.2), whose result do not change if the MERLIN TIMI 36 sub study is not considered.
1.2. Analysis.
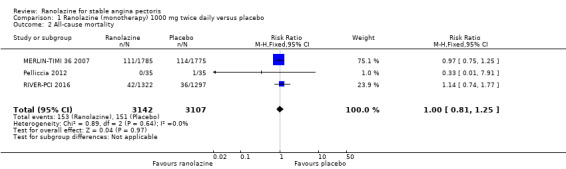
Comparison 1 Ranolazine (monotherapy) 1000 mg twice daily versus placebo, Outcome 2 All‐cause mortality.
We used information presented in studies' Methods sections as a proxy for protocols. We found that three studies did not report data for some outcomes (Babalis 2015; MERLIN‐TIMI 36 2007; RIVER‐PCI 2016) and four studies reported data for additional outcomes (CARISA 2004; MERLIN‐TIMI 36 2007; RAN080 2005; RIVER‐PCI 2016). Selective reporting affected outcomes considered in this review in three studies (CARISA 2004; RAN080 2005; RIVER‐PCI 2016).
Other potential sources of bias
We considered the source of funding and conflicts of interest as potential sources of bias. Most studies reported funding from commercial pharmaceutical companies. Sources of funding were reported to be partially supported by commercial pharmaceutical companies in three studies, no conflicts of interest were reported in two of these (Mehta 2011, Pepine 1999) and conflicts of interest were reported for some of the authors in the other one (RWISE 2016). Three studies (Babalis 2015; Tagliamonte 2015; Villano 2013) reported neither funding source nor authors' conflicts of interest. Two studies reported no external source of funding and absence of conflicts of interest (Pelliccia 2012; Sandhiya 2015).
Effects of interventions
Table 1 presents ranolazine compared to placebo (add‐on therapy) and Table 2 presents ranolazine compared to placebo (monotherapy).
Primary outcomes
Cardiovascular mortality
Only RIVER‐PCI 2016 reported data on cardiovascular mortality for ranolazine 1000 mg twice daily administered as monotherapy compared to placebo. We observed uncertain effect on cardiovascular mortality from the 20/1287 cardiovascular deaths in the placebo group and 21/1317 cardiovascular deaths in the ranolazine group (RR 1.03, 95% CI 0.56 to 1.88; low quality evidence) (Analysis 1.1).
1.1. Analysis.
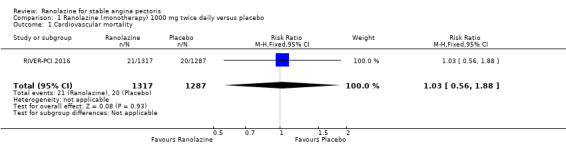
Comparison 1 Ranolazine (monotherapy) 1000 mg twice daily versus placebo, Outcome 1 Cardiovascular mortality.
Non‐cardiovascular mortality
None of the included studies reported non‐cardiovascular mortality.
Secondary outcomes
Effectiveness
The main results are summarised in analyses for ranolazine as monotherapy compared to placebo (Analysis 1.2; Analysis 1.3; Analysis 1.4; Analysis 1.5; Analysis 2.1; Analysis 2.2) and as add‐on therapy compared to placebo (Analysis 3.1; Analysis 3.2; Analysis 3.3; Analysis 3.4; Analysis 3.5; Analysis 4.1; Analysis 4.2).
1.3. Analysis.
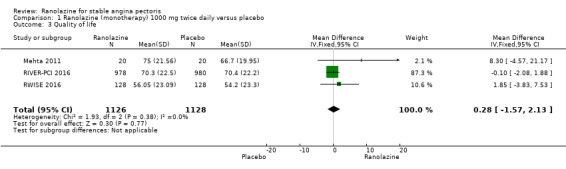
Comparison 1 Ranolazine (monotherapy) 1000 mg twice daily versus placebo, Outcome 3 Quality of life.
1.4. Analysis.
Comparison 1 Ranolazine (monotherapy) 1000 mg twice daily versus placebo, Outcome 4 AMI incidence.
1.5. Analysis.
Comparison 1 Ranolazine (monotherapy) 1000 mg twice daily versus placebo, Outcome 5 Need for revascularisation procedure.
2.1. Analysis.
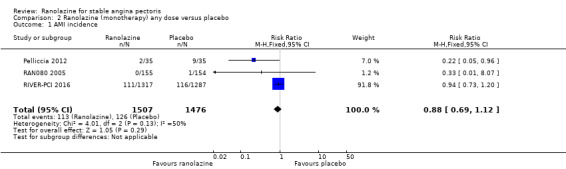
Comparison 2 Ranolazine (monotherapy) any dose versus placebo, Outcome 1 AMI incidence.
2.2. Analysis.
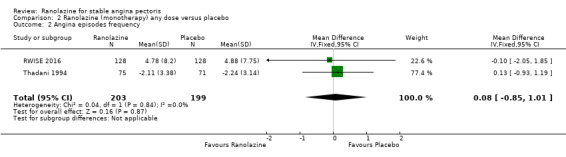
Comparison 2 Ranolazine (monotherapy) any dose versus placebo, Outcome 2 Angina episodes frequency.
3.1. Analysis.
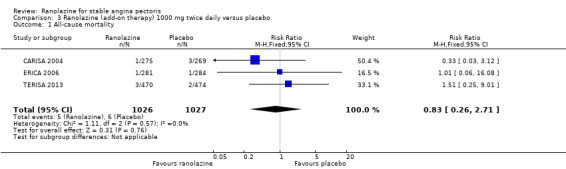
Comparison 3 Ranolazine (add‐on therapy) 1000 mg twice daily versus placebo, Outcome 1 All‐cause mortality.
3.2. Analysis.
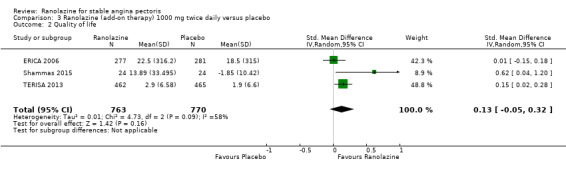
Comparison 3 Ranolazine (add‐on therapy) 1000 mg twice daily versus placebo, Outcome 2 Quality of life.
3.3. Analysis.
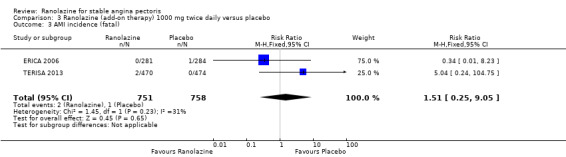
Comparison 3 Ranolazine (add‐on therapy) 1000 mg twice daily versus placebo, Outcome 3 AMI incidence (fatal).
3.4. Analysis.
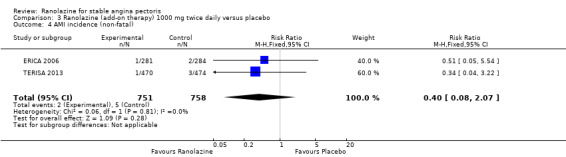
Comparison 3 Ranolazine (add‐on therapy) 1000 mg twice daily versus placebo, Outcome 4 AMI incidence (non‐fatal).
3.5. Analysis.
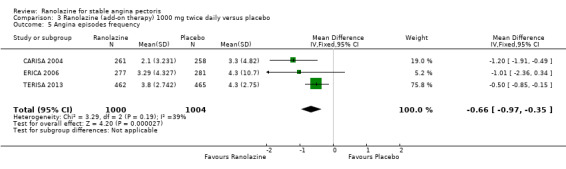
Comparison 3 Ranolazine (add‐on therapy) 1000 mg twice daily versus placebo, Outcome 5 Angina episodes frequency.
4.1. Analysis.
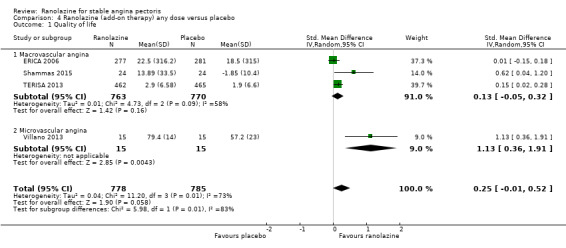
Comparison 4 Ranolazine (add‐on therapy) any dose versus placebo, Outcome 1 Quality of life.
4.2. Analysis.
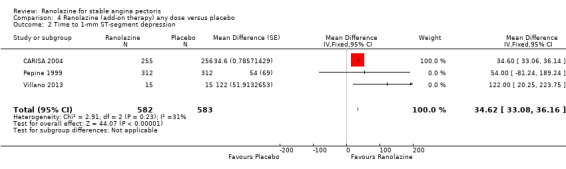
Comparison 4 Ranolazine (add‐on therapy) any dose versus placebo, Outcome 2 Time to 1‐mm ST‐segment depression.
Data were reported for ranolazine as monotherapy compared to placebo for quality of life (Tagliamonte 2015) and frequency of angina episodes (RAN080 2005; Thadani 1994). These data could not be pooled in a meta‐analysis due to incompleteness.
Ranolazine was compared to other first‐ (RAN080 2005, atenolol 100 mg once daily) and second‐line anti‐anginals (Sandhiya 2015; Villano 2013; trimetazidine 35 mg twice daily and ivabradine 5 mg twice daily respectively), either as monotherapy (RAN080 2005; Sandhiya 2015) or add‐on therapy (Villano 2013). Data could not be meta‐analysed because, for any outcome, only one trial provided data. Study authors were contacted to obtain missing data. Additional data were provided by Dr Noel Bairey Merz (Mehta 2011) and Dr Nicolas W Shammas (Shammas 2015) and included in the quantitative synthesis.
All‐cause mortality
Three studies reported all‐cause mortality for ranolazine 1000 mg monotherapy administered twice daily compared to placebo (MERLIN‐TIMI 36 2007; Pelliccia 2012; RIVER‐PCI 2016). Low quality evidence showed that intervention and placebo group participants were at similar risk of death from all causes (RR 1.00, 95% CI 0.81 to 1.25; 3 studies, 6249 participants; Analysis 1.2). There was no heterogeneity (Chi² P = 0.64, I² = 0%).
Three studies reported all‐cause mortality for ranolazine 1000 mg as add‐on therapy administered twice daily (co‐medications: adrenergic beta antagonists and calcium channel blockers) compared to placebo (CARISA 2004; ERICA 2006; TERISA 2013). Low quality evidence showed that intervention and placebo group participants were at similar risk of death from all causes (RR 0.83, 95% CI 0.26 to 2.71; 3 studies, 2053 participants; Analysis 3.1). There was no heterogeneity (Chi² P = 0.57, I² = 0%).
Quality of life
Three studies evaluated quality of life for ranolazine 1000 mg monotherapy administered twice daily compared to placebo (Mehta 2011; RIVER‐PCI 2016, RWISE 2016). Moderate quality evidence showed that quality of life did not differ between intervention and placebo group participants (MD 0.28, 95% CI ‐1.57 to 2.13; 3 studies, 2254 participants; Analysis 1.3). There was no heterogeneity (Chi² P = 0.38, I² = 0%). Data were assessed using the quality of life dimension of the Seattle Angina Questionnaire (SAQ) in the three trials. The score for this dimension ranged between 0 and 100, with a higher score indicating a better quality of life.
Three studies evaluated quality of life for ranolazine 1000 mg as add‐on therapy administered twice daily (co‐medications: adrenergic beta antagonists and calcium channel blockers) compared to placebo (ERICA 2006; Shammas 2015; TERISA 2013). Moderate quality evidence showed that quality of life did not differ between intervention and control group participants (SMD 0.13, 95% CI ‐0.05 to 0.32; 3 studies, 1533 participants; Analysis 3.2). Since there was statistically significant, but not considerable, heterogeneity (Chi² P = 0.09, I² = 58%) we used a random‐effects model to calculate the pooled estimate. We observed that studies differed in risk of selection and detection bias and population size (fewer than 30 participants versus more than 400 participants), which may explain heterogeneity. Pooled data were reported for quality of life assessed using different scales: the angina frequency and quality of life dimensions of the SAQ, and the physical component of SF‐36. Scores range from 0 to 100, with higher scores indicating better quality of life.
One additional study evaluated quality of life for ranolazine given as add‐on therapy (to adrenergic beta antagonists and calcium channel blockers) compared to placebo (Villano 2013), making a total of four trials for the ranolazine any dose (375mg twice daily and 1000mg twice daily) comparison. Moderate quality evidence showed that quality of life did not differ between intervention and placebo group participants (ranolazine any dose, SMD 0.25, 95% CI ‐0.01 to 0.52; 4 studies, 1563 participants, Analysis 4.1). Since there was statistically significant, but not considerable, heterogeneity (Chi² P = 0.01, I² = 73%) we used a random‐effects model to calculate the pooled estimate. We observed that trials in this analysis differed in risk of selection and detection bias and population size (fewer than 40 versus more than 400) and type of stable angina diagnosis (macrovascular angina versus microvascular angina), which may explain heterogeneity.
Acute myocardial infarction (AMI) incidence
Fatal AMI incidence
We found no data on fatal AMI for ranolazine given as monotherapy compared to placebo. Two studies reported data on fatal AMI events for ranolazine 1000 mg twice daily given as add‐on therapy compared to placebo (ERICA 2006; TERISA 2013). Low quality evidence showed uncertain effect for the risk of fatal AMI between intervention and placebo group participants (RR 1.51, 95% CI 0.25 to 9.05; 2 studies, 1509 participants; Analysis 3.3). There was no statistically significant heterogeneity (Chi² P = 0.23, I² = 31%).
Non‐fatal AMI incidence
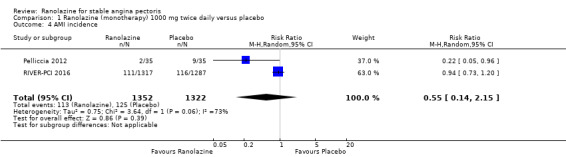
Two studies reported non‐fatal AMI incidence for ranolazine 1000 mg twice daily given as monotherapy compared to placebo (Pelliccia 2012; RIVER‐PCI 2016). Very low quality evidence showed that participants in both intervention and control groups were at similar risk of non‐fatal AMI (RR 0.55, 95% CI 0.14 to 2.15; 2 studies, 2674 participants; Analysis 1.4). Since there was statistically significant, but not considerable, heterogeneity (Chi² P = 0.06, I² = 73%) we used a random‐effects model to calculate the pooled estimate. We observed that studies in this analysis differed in risk of performance and detection bias and duration of follow‐up (30 days versus 643 days), which may explain heterogeneity.
One study reported non‐fatal AMI incidence for ranolazine given as monotherapy compared to placebo (RAN080 2005), making a total of three studies for ranolazine any dose (400 mg three times daily and 1000 mg twice daily) comparison. Low quality evidence showed that intervention and control group participants were at a similar risk of non‐fatal AMI (RR 0.88, 95% CI 0.69 to 1.12; 2983 participants, Analysis 2.1). There was no statistically significant heterogeneity (Chi² P = 0.13, I² = 50%).
Two other studies reported non‐fatal AMI incidence for ranolazine 1000 mg twice daily given as add‐on therapy compared to placebo (ERICA 2006; TERISA 2013). Low quality evidence showed that participants in both groups were at a similar risk of suffering a non‐fatal AMI (RR 0.40, 95% CI 0.08 to 2.07, 1509 participants, Analysis 3.4). There was no heterogeneity (Chi² P = 0.81, I² = 0%).
Need for revascularisation procedure
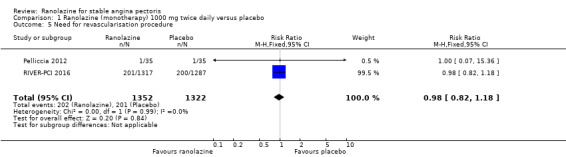
Two studies reported incidence of revascularisation procedures for ranolazine 1000 mg twice daily given as monotherapy compared to placebo (Pelliccia 2012; RIVER‐PCI 2016). Moderate quality evidence showed that ranolazine has no effect on the risk of undergoing a revascularisation procedure (RR 0.98, 95% CI 0.82 to 1.18, 2674 participants, Analysis 1.5). There was no heterogeneity (Chi² P = 0.99, I² = 0%).
Angina episodes frequency
Two studies evaluated angina episodes frequency for ranolazine any dose (120 mg 3 times daily and 1000 mg twice daily) given as monotherapy compared to placebo (RWISE 2016; Thadani 1994). Low quality evidence showed that the average number of angina episodes per week did not differ in the participants in both groups (MD 0.08, 95% CI ‐0.85 to 1.01, 402 participants, Analysis 2.2). There was no heterogeneity (Chi² P = 0.84, I² = 0%).
Three studies evaluated angina episodes frequency for ranolazine 1000 mg twice daily given as add‐on therapy (to adrenergic beta antagonists and calcium channel blockers) compared to placebo (CARISA 2004; ERICA 2006; TERISA 2013). Moderate quality evidence showed that the average number of angina episodes per week was lower in the participants who received ranolazine (MD ‐0.66, 95% CI ‐0.97 to ‐0.35, 2004 participants, Analysis 3.5). There was no statistically significant heterogeneity (Chi² P = 0.19, I² = 39%).
Costs of healthcare
None of the included trials reported data on costs and resource use of the management of stable angina participants. We found that only one trial (RIVER‐PCI 2016) reported a planned health economics sub‐study (Weisz 2013), but results have not yet been published.
Time to 1‐mm ST‐segment depression
Three studies evaluated time to 1‐mm ST‐segment depression in exercise ECG at peak for ranolazine any dose (375 mg twice daily, 400 mg three times daily and 1000 mg twice daily) given as add‐on therapy compared to placebo (CARISA 2004; Pepine 1999; Villano 2013). Moderate quality evidence showed that the average time to 1‐mm ST‐segment depression in seconds was higher in participants who received ranolazine (MD 34.62, 95% CI 33.08 to 36.16, 1198 participants, Analysis 4.2). There was no statistically significant heterogeneity (Chi² P = 0.23, I² = 31%).
Data were also available for ranolazine given as monotherapy compared to placebo; however, pooled estimates showed substantial heterogeneity (I² = 90% to 99%) which precluded us from including those results in the quantitative synthesis. We observed that studies included in this analysis differed notably in design (parallel‐group versus cross‐over), duration of follow‐up (< 1 month versus ˜12 months), number of participants (< 200 versus > 3000), and baseline 1‐mm ST‐segment depression (< 260 s versus > 430 s).
Safety
The main results are summarised in forest plots for ranolazine given as monotherapy compared to placebo (Analysis 1.6, Analysis 2.3) and as add‐on therapy compared to placebo (Analysis 3.6). Data from other trials are also reported for ranolazine given as monotherapy compared to placebo for adverse events incidence (Pepine 1999; Thadani 1994) which could not be pooled for meta‐analysis due to incompleteness. Similarly, data for ranolazine given as add‐on therapy compared to placebo for adverse events incidence (Shammas 2015, Villano 2013) could not be pooled for meta‐analysis due to incompleteness. Missing data have been requested from the study contact authors. Although no quantitative analysis could be performed for specific events, it was observed that the most frequently reported events were dizziness, nausea and constipation. 'Other' which included peripheral oedema, headache, asthenia, palpitations, dyspepsia, weakness and postural hypotension, was the most commonly re[ported category.
1.6. Analysis.
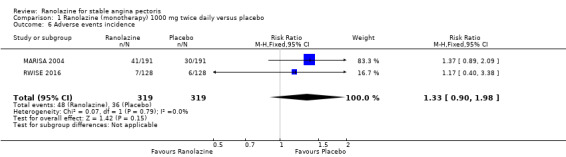
Comparison 1 Ranolazine (monotherapy) 1000 mg twice daily versus placebo, Outcome 6 Adverse events incidence.
2.3. Analysis.
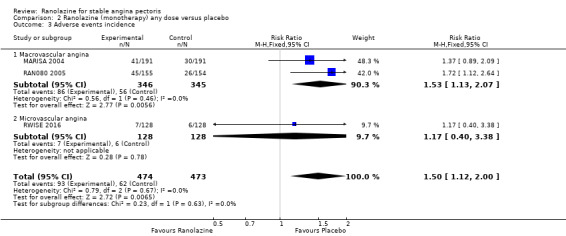
Comparison 2 Ranolazine (monotherapy) any dose versus placebo, Outcome 3 Adverse events incidence.
3.6. Analysis.
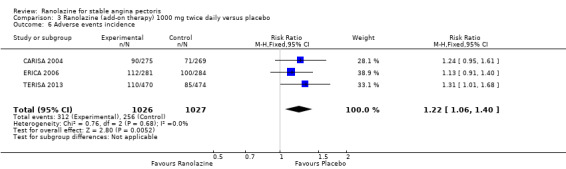
Comparison 3 Ranolazine (add‐on therapy) 1000 mg twice daily versus placebo, Outcome 6 Adverse events incidence.
Adverse events incidence
Serious adverse events
We found insufficient data on serious adverse events to perform quantitative synthesis. Although the included studies reported types of serious adverse events inconsistently, we summarised data into three categories:
Cerebrovascular events: Two studies (RIVER‐PCI 2016; RWISE 2016) reported data for ranolazine 1000 mg twice daily given as monotherapy compared to placebo. RIVER‐PCI 2016 reported 22/1317 and 20/1287 events of stroke and 13/1317 and 3/1287 events of transitory ischaemic attack among participants who received ranolazine and placebo respectively. RWISE 2016 reported 2/128 and 0/128 events of pre‐syncope and 1/128 and 0/128 events of syncope among participants who received ranolazine and placebo respectively. Three other studies (ERICA 2006; Shammas 2015; TERISA 2013) reported data for ranolazine 1000 mg twice daily given as add‐on therapy compared to placebo. ERICA 2006 reported that there were no events of stroke in any treatment groups. Shammas 2015 reported 1/24 and 0/24 events of stroke among participants given ranolazine and placebo respectively. TERISA 2013 reported 1/470 and 4/474 events of stroke among participants given ranolazine and placebo respectively. Pooling data resulted in RR of 0.56 (95% CI 0.12 to 2.60, 3 studies, 1557 participants, Chi² P = 0.21, I² = 38%).
Heart failure: Only RIVER‐PCI 2016 reported data for ranolazine 1000 mg twice daily given as monotherapy compared to placebo. RIVER‐PCI 2016 reported heart failure events requiring hospitalisation (further classified as ischaemia and non‐ischemia‐related) in 38/1317 and 25/1287 of participants given ranolazine and placebo respectively.
Arrhythmias: None of the included studies reported arrhythmia‐related hospitalisations. However two trials reported symptomatic documented arrhythmias for ranolazine 1000 mg twice daily given as monotherapy (MERLIN‐TIMI 36 2007) and as add‐on therapy (ERICA 2006) compared to placebo. MERLIN‐TIMI 36 2007 (stable angina patients subgroup) reported 52/1785 and 52/1775 symptomatic documented arrhythmias among participants given ranolazine and placebo respectively. ERICA 2006 reported 8/281 and 10/284 arrhythmias (ventricular extrasystoles, sinus bradycardias, sinus tachycardias and atrioventricular blockages) among participants given ranolazine and placebo respectively. Of note, Shammas 2015 and MERLIN‐TIMI 36 2007 conducted separate recordings of arrhythmias over short periods of the total study duration, which we considered did not fit the purposes of this review.
Some other events were labelled as major or serious adverse events in some trials (RIVER‐PCI 2016; TERISA 2013) but these were not reported in sufficient detail to determine their suitability to meet the definition for this outcome.
Non‐serious adverse events
Two studies reported the incidence of non‐serious adverse events for ranolazine 1000 mg twice daily given as monotherapy compared to placebo (MARISA 2004; RWISE 2016). Very low quality evidence showed that participants in both groups were at similar risk of presenting non‐serious adverse events (RR 1.33, 95% CI 0.90 to 1.98, 638 participants, Analysis 1.6). There was no heterogeneity (Chi² P = 0.79, I² = 0%).
RAN080 2005 reported non‐fatal AMI incidence for ranolazine given as monotherapy compared to placebo. Very low quality evidence showed that the participants treated with ranolazine were at a higher risk of presenting non‐serious adverse events (RR 1.50, 95% CI 1.12 to 2.00, 947 participants, Analysis 2.3). There was no heterogeneity (Chi² P = 0.67, I² = 0%).
Three studies reported incidence of non‐serious adverse events for ranolazine 1000 mg twice daily given as add‐on therapy (to adrenergic beta antagonists and calcium channel blockers) compared to placebo (CARISA 2004; ERICA 2006; TERISA 2013). Moderate quality evidence showed that participants treated with ranolazine were at higher risk of presenting non‐serious adverse events (RR 1.22, 95% CI 1.06 to 1.40; 3 studies, 2053 participants, Analysis 3.6). There was no heterogeneity (Chi² P = 0.68, I² = 0%). Two studies reported incidence of non‐serious adverse events for ranolazine given as add‐on therapy (to adrenergic beta antagonists and calcium channel blockers) compared to placebo (Babalis 2015; Villano 2013). However, since these studies reported no events for each treatment group, individual RRs were not estimable, and could not be pooled with the other studies. Therefore, meta‐analysis for these five studies (Babalis 2015; CARISA 2004; ERICA 2006; TERISA 2013. Villano 2013) was not performed.
Subgroup analysis
We performed subgroup analysis for type of stable angina diagnosis. There were insufficient data to conduct other planned subgroup analyses. This subgroup analysis was performed for non‐serious adverse events incidence for ranolazine given as monotherapy compared to placebo (Analysis 2.3) and for quality of life for ranolazine given as add‐on therapy compared to placebo (Analysis 4.1). The direction and magnitude of the treatment effects for the macrovascular angina subgroups were similar to those of the overall pooled estimates. We observed difference (Chi² P = 0.01) in the pooled estimates between subgroups only for quality of life (Analysis 4.1).
Sensitivity analysis
We performed sensitivity analyses to assess the effects of including only studies at low risk of bias, by switching statistical models for data synthesis (fixed‐effect to random‐effects and vice versa) and changing measures of treatment effects (RRs to ORs, MDs to SMDs and vice versa).
We could not perform sensitivity analysis for change of the measure of treatment effect (MD to SMD) for time to 1‐mm ST‐segment depression because there were insufficient data to inform calculation. We were unable to conduct sensitivity analysis for restriction to trials with low risk of bias for some outcomes because none of the studies initially included was regarded as having low risk of bias. Such outcomes are: adverse events incidence (for ranolazine given as monotherapy at 1000mg twice daily or any dosage versus placebo) and quality of life (for any dose ranolazine given as monotherapy versus placebo).
Overall, we found no major differences in either the direction or magnitude of treatment effects except for the quality of life outcome for ranolazine 1000 mg twice daily given as add‐on therapy versus placebo (Analysis 6.10) or any dose ranolazine given as add‐on therapy versus placebo (Analysis 6.15). For these two analyses, the measure of treatment effect became statistically significant after changing the model (random‐effects to fixed‐effect).
6.10. Analysis.
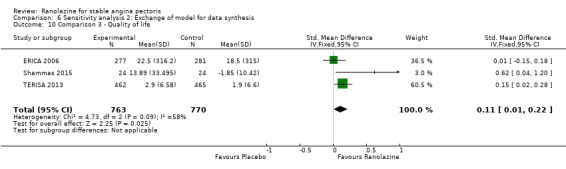
Comparison 6 Sensitivity analysis 2: Exchange of model for data synthesis, Outcome 10 Comparison 3 ‐ Quality of life.
6.15. Analysis.
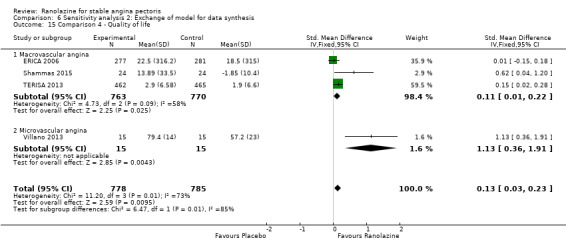
Comparison 6 Sensitivity analysis 2: Exchange of model for data synthesis, Outcome 15 Comparison 4 ‐ Quality of life.
We also performed a sensitivity analysis to assess the effect of including only studies with follow‐up duration of at least six weeks. We were unable to conduct this sensitivity analysis for some outcomes because none of the studies initially included reported results from follow‐up ≥ 6 weeks (leaving 0 trials for analysis). Such outcomes are: adverse events incidence (for ranolazine given as monotherapy at 1000mg twice daily or any dosage versus placebo), quality of life (for any dose ranolazine given as monotherapy versus placebo) and time to 1‐mm ST‐segment depression (Microvascular angina subgroup, for any dose ranolazine given as add‐on therapy versus placebo).
We found no major differences in either the direction or magnitude of treatment effects except for quality of life with ranolazine 1000 mg monotherapy administered twice daily versus placebo (Analysis 8.2). For this analysis, the measure of treatment effect changed direction from favouring ranolazine to favouring placebo, but remained not statistically significant. We observed that heterogeneity in Analysis 1.4 (2 studies) may be explained by differences in duration of follow‐up (1 week versus 643 days). Heterogeneity in Analysis 4.1 (4 studies) was not explained by differences in duration of follow‐up.
8.2. Analysis.
Comparison 8 Sensitivity analysis 4: follow‐up ≥ 6 weeks, Outcome 2 Comparison 1 ‐ Quality of life.
Discussion
Summary of main results
We included 17 randomised controlled trials (RCTs). We found evidence on the effects of ranolazine compared to placebo given as monotherapy and as add‐on therapy for people with stable angina pectoris from 14 RCTs. Three studies did not provide data for quantitative analysis.
For ranolazine given as add‐on therapy (Table 1), we found no evidence for the effect on cardiovascular and non‐cardiovascular mortality. We also found low quality evidence of uncertain effect on all‐cause mortality (for ranolazine 1000mg twice daily), moderate quality evidence of uncertain effect on quality of life (for any dose ranolazine), and low quality evidence of uncertain effect on AMI incidence (for ranolazine 1000mg twice daily). We assessed moderate quality evidence for reduced frequency of angina episodes with the use of ranolazine 1000mg twice daily. There was moderate quality evidence for increased time to 1‐mm ST‐segment depression in exercise electrocardiogram (ECG) associated with the use of any dose ranolazine. There was moderate quality evidence of increased risk of non‐serious adverse events with the use of ranolazine 1000mg twice daily.
In relation to ranolazine as monotherapy (Table 2), we found low quality evidence of uncertain effect on cardiovascular mortality (for ranolazine 1000mg twice daily) and no evidence of the effect on non‐cardiovascular mortality. We also found low quality evidence of uncertain effect on all‐cause mortality (for ranolazine 1000mg twice daily), moderate quality evidence of uncertain effect on quality of life (for ranolazine 1000mg twice daily), low quality evidence of uncertain effect on non‐fatal acute myocardial infarction (AMI) incidence (for any dose ranolazine), and frequency of angina episodes (for any dose ranolazine). We found moderate quality evidence of no effect on need for revascularisation procedures (for ranolazine 1000mg twice daily), and very low quality evidence for increased risk of non‐serious adverse events (for any dose ranolazine).
Overall, we found evidence of clinical benefit from the use of ranolazine as add‐on therapy by reducing the frequency of angina episodes and increasing the time to 1‐mm ST‐segment depression. However, we found also evidence of clinical harm from the use of ranolazine as either monotherapy or add‐on therapy by increasing the risk of non‐serious adverse events.
We found evidence on the effects of ranolazine compared to other anti‐angina agents (atenolol, ivabradine and trimetazidine) for people with stable angina from three RCTs, but these data were insufficient to perform quantitative synthesis.
We found evidence of differential effect on quality of life for any dose ranolazine given as add‐on therapy compared to placebo according to the type of stable angina (macrovascular versus microvascular).
The sensitivity analyses generally showed no major differences in the results we obtained. For any dose ranolazine given as monotherapy, no trials left after restricting to studies with low risk of bias or follow‐up ≥ 6 weeks for angina episodes frequency and adverse events incidence. For ranolazine given as add‐on therapy, a modest increase in quality of life was obtained with a fixed‐effect model (exchange of model for data synthesis).
Overall completeness and applicability of evidence
Several gaps in the evidence remain. Data were available for the primary effectiveness outcome (cardiovascular mortality) from only one study (RIVER‐PCI 2016). None of the included studies reported results on the primary safety outcome (non‐cardiovascular mortality). Similarly, no data were available for healthcare costs; RIVER‐PCI 2016 included a sub study on health economics but results have not yet been published. No data from head‐to‐head comparisons on ranolazine versus other anti‐anginals were available for quantitative synthesis. Only three trials reported data on these later comparisons (ranolazine versus other anti‐anginals); all had small population sizes (40 participants to 158 participants). Notably, we found no studies that compared ranolazine with a calcium channel blocker, long‐acting nitrate or nicorandil. We therefore present findings for ranolazine compared to placebo only.
Assessment of external validity of our results should consider the following.
The included studies varied in several important aspects: (a) ranolazine dosage and type of formulation, (b) the presence of comorbidity (type 2 diabetes mellitus, acute coronary syndrome, incomplete revascularisation); (c) concomitant medication (permitted), with some participant groups labelled as 'monotherapy' actually receiving concomitant anti‐anginal drugs at varying proportions; and (d) duration of follow‐up ranged from one week to more than two years.
The diagnostic criteria for stable angina varied among included studies. Three studies included in the quantitative analysis restricted study populations to people with microvascular angina. Diagnostic criteria roughly fell into either of two categories for the remaining studies: clinical diagnosis (history of exertional angina) and angiographic diagnosis (evidence of macrovascular coronary disease). Studies applying clinical diagnosis enabled enrolment of people with macrovascular and microvascular angina.
Taken together, the included studies enrolled participants from multiple sites mainly in North America, Europe and Australia, with less contribution from people in Asia and Africa, and no representation of Central and South American region peoples.
The marked heterogeneity among the included studies regarding the characteristics listed above, along with paucity of data for most planned comparisons and subgroup analyses meant that some components of review questions remain unresolved.
Quality of the evidence
We included data from 14 RCTs (9292 participants) to the 'Summary of findings' tables. Although studies were heterogeneous in individual quality assessments, most shared important characteristics such as a parallel‐group (8/14), double‐blind design (11/14), macrovascular angina population (6/14), 1000 mg twice daily dosage for ranolazine (10/14) and intention‐to‐treat analysis approach (9/14, explicitly stated). Fewer than 10% of participants randomised to all studies were lost to follow‐up. However, allocation concealment and blinding of study personnel were not described for most studies, rendering unclear risk of bias. Of note, an important part of the evidence in this review came from trials deemed at high risk of bias related to the source of funding and conflicts of interest, since every analysis we report includes at least one trial with that characteristic.
None of the results obtained were deemed to be high quality. Evidence quality was low for the primary outcomes (cardiovascular mortality). In relation to secondary outcomes, evidence quality was lower for ranolazine given as monotherapy than as add‐on therapy. This was due in part to the indirectness of the comparison for ranolazine given as monotherapy (a group of participants actually received ranolazine as add‐on therapy).
Overall, evidence quality was low for all‐cause mortality, moderate for quality of life, low for non‐fatal AMI incidence, low to moderate for frequency of angina episodes (low for ranolazine as monotherapy and moderate for ranolazine as add‐on therapy) and very low to moderate for non‐serious adverse events incidence (very low for ranolazine as monotherapy and moderate for ranolazine as add‐on therapy).
We downgraded quality of life evidence by one level due to indirectness concerns or substantial heterogeneity. We downgraded evidence quality for non‐fatal AMI incidence due to risk of bias concerns (by one level) or imprecision of the estimate (by one or two levels). We downgraded evidence quality for frequency of angina episodes due to risk of bias or indirectness concerns. We also downgraded evidence quality for non‐serious adverse events incidence due to risk of bias and indirectness concerns or small number of events.
Potential biases in the review process
The risk of having introduced bias throughout the review process was limited given that study selection, data extraction and assessment of risk of bias were performed by pairs of authors who worked independently to reach consensus. Comprehensive electronic and other resources searches were performed to identify all potentially relevant studies for this review. Nevertheless, there are a number of potential biases in the review process given the presence of some limitations: 1) few additional data were obtained apart from published data; 2) all available data were summarised irrespective of study quality; and 3) insufficient data to perform planned subgroup analyses. We also made some deviations from the published protocol during the review process (see Differences between protocol and review) which we considered to be appropriate and did not change the conclusions of the review.
Agreements and disagreements with other studies or reviews
The available evidence regarding the use of ranolazine in people with stable angina pectoris has been systematically analysed in some studies. Two systematic reviews (Banon 2014; Savarese 2013) studied the effects of ranolazine given as monotherapy or as add‐on therapy for people with stable angina. Another systematic review (Belsey 2015) focused on the effects of ranolazine given as add‐on therapy for people with stable angina. These reviews focused on exercise ECG parameters (duration, time to angina, time to ST‐segment depression). Of these, two also studied weekly frequency of angina attacks and nitroglycerin use (Banon 2014; Savarese 2013). Banon 2014 also assessed quality of life and incidence of adverse events. Although differing in numbers of included studies, these reviews arrived at similar conclusions about the effects of ranolazine compared to placebo for people with stable angina. Results from these reviews show beneficial effect for ranolazine (mainly given as add‐on therapy) on quality of life (data not pooled), frequency of angina attacks, exercise ECG parameters and a harmful effect on adverse events incidence.
Our results regarding quality of life showed that ranolazine (either as monotherapy or add‐on therapy) had uncertain effects for people with stable angina. In relation to other outcomes, our results show a similar direction and magnitude of treatment effect for ranolazine given as add‐on therapy. Notably, our results include data from a greater number of studies, especially for ranolazine given as monotherapy, and data on other relevant outcomes, such as all‐cause mortality, AMI incidence, and need for revascularisation procedure.
Authors' conclusions
Implications for practice.
There was uncertain evidence of the effect from treatment with ranolazine given as monotherapy compared to placebo in people with stable angina pectoris in regard to cardiovascular mortality. Similarly, there was uncertain evidence of the effect from treatment with ranolazine (given either as monotherapy or add‐on therapy) compared to placebo in people with stable angina pectoris in regard to all‐cause mortality, quality of life and non‐fatal AMI incidence. There was also uncertain evidence of the effect from treatment with ranolazine given as monotherapy in regard to the weekly frequency of angina episodes.
There is evidence of clinical benefit from treatment with ranolazine given as add‐on therapy compared to placebo in people with stable angina pectoris in regard to the weekly frequency of anginal episodes.
There is evidence of clinical harm from ranolazine treatment (either as monotherapy or add‐on therapy) compared to placebo in people with stable angina pectoris in regard to non‐serious adverse events incidence.
There was insufficient evidence of clinical benefit or harm from ranolazine treatment compared to placebo in people with stable angina pectoris in regard to non‐cardiovascular mortality and healthcare costs. Similarly, there was limited evidence of clinical benefit or harm from treatment with ranolazine compared to other anti‐anginals.
Implications for research.
Future RCTs should consider aiming to:
Further determine the effectiveness and safety of ranolazine compared to first‐line anti‐anginals, particularly calcium channel blockers, and other second‐line anti‐anginals in a population of people with stable angina pectoris.
Further determine differences in the effect of ranolazine in subgroups of people with stable angina pectoris with macrovascular and microvascular angina.
Provide more long‐term data (beyond two years) on mortality, quality of life, acute myocardial infarction incidence, need for revascularisation procedures and cost‐effectiveness of treatment with ranolazine in people with stable angina pectoris.
What's new
| Date | Event | Description |
|---|---|---|
| 24 January 2019 | Amended | Minor correction in Characteristics of included studies table ‐ intervention for CARISA 2004 corrected to 'Ranolazine 1000 mg group'. |
Acknowledgements
We thank Diane Horsley, Nicole Martin and the peer review editors of the Cochrane Heart Group for their help in developing the protocol and performing the review. Finally, we are especially grateful to Juan Nivin for his contributions to the initial design and development of the review.
Appendices
Appendix 1. Search strategies
CENTRAL
#1 MeSH descriptor: [Angina Pectoris] explode all trees #2 angina*:ti,ab,kw (Word variations have been searched) #3 stenocardia*:ti,ab,kw (Word variations have been searched) #4 angor pectoris:ti,ab,kw (Word variations have been searched) #5 #1 or #2 or #3 or #4 #6 ranolazine:ti,ab,kw (Word variations have been searched) #7 ranexa:ti,ab,kw (Word variations have been searched) #8 latixa:ti,ab,kw (Word variations have been searched) #9 (rs next "43285"):ti,ab,kw (Word variations have been searched) #10 #6 or #7 or #8 or #9 #11 #5 and #10
MEDLINE OVID
1. exp Angina Pectoris/ 2. angina*.tw. 3. stenocardia*.tw. 4. angor pectoris.tw. 5. or/1‐4 6. ranolazine.mp. 7. ranexa.mp. 8. latixa.mp. 9. (rs adj "43285").mp. 10. 110445‐25‐5.rn. 11. or/6‐10 12. randomized controlled trial.pt. 13. controlled clinical trial.pt. 14. randomized.ab. 15. placebo.ab. 16. drug therapy.fs. 17. randomly.ab. 18. trial.ab. 19. groups.ab. 20. 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 21. exp animals/ not humans.sh. 22. 20 not 21 23. 5 and 11 24. 22 and 23
EMBASE OVID
1. exp Angina Pectoris/ 2. angina*.tw. 3. stenocardia*.tw. 4. angor pectoris.tw. 5. or/1‐4 6. ranolazine.mp. 7. ranexa.mp. 8. latixa.mp. 9. (rs adj "43285").mp. 10. ranolazine/ 11. or/6‐10 12. random$.tw. 13. factorial$.tw. 14. crossover$.tw. 15. cross over$.tw. 16. cross‐over$.tw. 17. placebo$.tw. 18. (doubl$ adj blind$).tw. 19. (singl$ adj blind$).tw. 20. assign$.tw. 21. allocat$.tw. 22. volunteer$.tw. 23. crossover procedure/ 24. double blind procedure/ 25. randomized controlled trial/ 26. single blind procedure/ 27. 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 28. (animal/ or nonhuman/) not human/ 29. 27 not 28 30. 5 and 11 and 29
Web of Science
#12 #11 AND #10 Indexes=CPCI‐S Timespan=All years
#11 TOPIC: ((random* or blind* or allocat* or assign* or trial* or placebo* or crossover* or cross‐over*)) Indexes=CPCI‐S Timespan=All years
#10 #9 AND #4 Indexes=CPCI‐S Timespan=All years
#9 #8 OR #7 OR #6 OR #5 Indexes=CPCI‐S Timespan=All years
#8 TOPIC: (("rs 43285")) Indexes=CPCI‐S Timespan=All years
#7 TOPIC: (latixa) Indexes=CPCI‐S Timespan=All years
#6 TOPIC: (ranexa) Indexes=CPCI‐S Timespan=All years
#5 TOPIC: (ranolazine) Indexes=CPCI‐S Timespan=All years
#4 #3 OR #2 OR #1 Indexes=CPCI‐S Timespan=All years
#3 TOPIC: ("angor pectoris") Indexes=CPCI‐S Timespan=All years
#2 TOPIC: (stenocardia*) Indexes=CPCI‐S Timespan=All years
#1 TOPIC: (angina*) Indexes=CPCI‐S Timespan=All years
Other sources
Since several of the searched databases did not have an "advanced search" tool, we used only the term "ranolazine" as the search strategy.
Data and analyses
Comparison 1. Ranolazine (monotherapy) 1000 mg twice daily versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Cardiovascular mortality | 1 | 2604 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.56, 1.88] |
| 2 All‐cause mortality | 3 | 6249 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.81, 1.25] |
| 3 Quality of life | 3 | 2254 | Mean Difference (IV, Fixed, 95% CI) | 0.28 [‐1.57, 2.13] |
| 4 AMI incidence | 2 | 2674 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.14, 2.15] |
| 5 Need for revascularisation procedure | 2 | 2674 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.82, 1.18] |
| 6 Adverse events incidence | 2 | 638 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.90, 1.98] |
Comparison 2. Ranolazine (monotherapy) any dose versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 AMI incidence | 3 | 2983 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.69, 1.12] |
| 2 Angina episodes frequency | 2 | 402 | Mean Difference (IV, Fixed, 95% CI) | 0.08 [‐0.85, 1.01] |
| 3 Adverse events incidence | 3 | 947 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.50 [1.12, 2.00] |
| 3.1 Macrovascular angina | 2 | 691 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.53 [1.13, 2.07] |
| 3.2 Microvascular angina | 1 | 256 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.40, 3.38] |
Comparison 3. Ranolazine (add‐on therapy) 1000 mg twice daily versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 All‐cause mortality | 3 | 2053 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.26, 2.71] |
| 2 Quality of life | 3 | 1533 | Std. Mean Difference (IV, Random, 95% CI) | 0.13 [‐0.05, 0.32] |
| 3 AMI incidence (fatal) | 2 | 1509 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.51 [0.25, 9.05] |
| 4 AMI incidence (non‐fatal) | 2 | 1509 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.40 [0.08, 2.07] |
| 5 Angina episodes frequency | 3 | 2004 | Mean Difference (IV, Fixed, 95% CI) | ‐0.66 [‐0.97, ‐0.35] |
| 6 Adverse events incidence | 3 | 2053 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [1.06, 1.40] |
Comparison 4. Ranolazine (add‐on therapy) any dose versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Quality of life | 4 | 1563 | Std. Mean Difference (IV, Random, 95% CI) | 0.25 [‐0.01, 0.52] |
| 1.1 Macrovascular angina | 3 | 1533 | Std. Mean Difference (IV, Random, 95% CI) | 0.13 [‐0.05, 0.32] |
| 1.2 Microvascular angina | 1 | 30 | Std. Mean Difference (IV, Random, 95% CI) | 1.13 [0.36, 1.91] |
| 2 Time to 1‐mm ST‐segment depression | 3 | 1165 | Mean Difference (Fixed, 95% CI) | 34.62 [33.08, 36.16] |
Comparison 5. Sensitivity analysis 1: Studies at low risk of bias.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Comparison 1 ‐ All‐cause mortality | 2 | 6179 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.81, 1.26] |
| 2 Comparison 1 ‐ Quality of life | 2 | 1998 | Mean Difference (IV, Fixed, 95% CI) | 0.09 [‐1.86, 2.05] |
| 3 Comparison 1 ‐ AMI incidence | 1 | 2604 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.73, 1.20] |
| 4 Comparison 1 ‐ Need for revascularisation procedure | 1 | 2604 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.82, 1.18] |
| 5 Comparison 2 ‐ AMI incidence | 1 | 2604 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.73, 1.20] |
| 6 Comparison 3 ‐ All‐cause mortality | 2 | 1488 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.22, 2.95] |
| 7 Comparison 3 ‐ Quality of life | 2 | 975 | Std. Mean Difference (IV, Random, 95% CI) | 0.30 [‐0.13, 0.73] |
| 8 Comparison 3 ‐ AMI incidence (fatal) | 1 | 944 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.04 [0.24, 104.75] |
| 9 Comparison 3 ‐ AMI incidence (non‐fatal) | 1 | 944 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.04, 3.22] |
| 10 Comparison 3 ‐ Angina episodes frequency | 2 | 1446 | Mean Difference (IV, Fixed, 95% CI) | ‐0.64 [‐0.96, ‐0.32] |
| 11 Comparison 3 ‐ Adverse events incidence | 2 | 1488 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.28 [1.06, 1.53] |
| 12 Comparison 4 ‐ Quality of life | 3 | 1005 | Std. Mean Difference (IV, Random, 95% CI) | 0.54 [‐0.03, 1.10] |
| 12.1 Macrovascular angina | 2 | 975 | Std. Mean Difference (IV, Random, 95% CI) | 0.30 [‐0.13, 0.73] |
| 12.2 Microvascular angina | 1 | 30 | Std. Mean Difference (IV, Random, 95% CI) | 1.13 [0.36, 1.91] |
| 13 Comparison 4 ‐ Time to 1‐mm ST‐segment depression | 2 | Mean Difference (Fixed, 95% CI) | 34.62 [33.08, 36.16] |
5.1. Analysis.
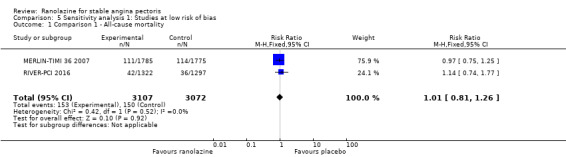
Comparison 5 Sensitivity analysis 1: Studies at low risk of bias, Outcome 1 Comparison 1 ‐ All‐cause mortality.
5.2. Analysis.
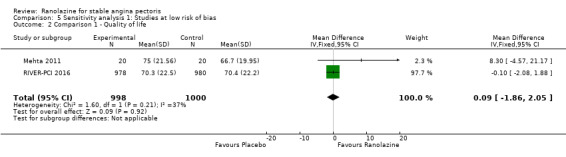
Comparison 5 Sensitivity analysis 1: Studies at low risk of bias, Outcome 2 Comparison 1 ‐ Quality of life.
5.3. Analysis.
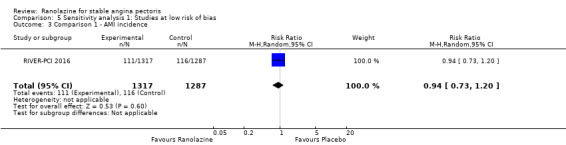
Comparison 5 Sensitivity analysis 1: Studies at low risk of bias, Outcome 3 Comparison 1 ‐ AMI incidence.
5.4. Analysis.
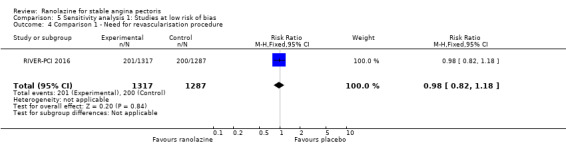
Comparison 5 Sensitivity analysis 1: Studies at low risk of bias, Outcome 4 Comparison 1 ‐ Need for revascularisation procedure.
5.5. Analysis.
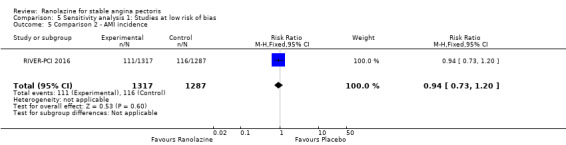
Comparison 5 Sensitivity analysis 1: Studies at low risk of bias, Outcome 5 Comparison 2 ‐ AMI incidence.
5.6. Analysis.
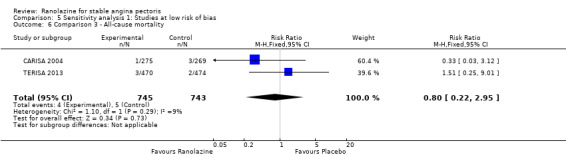
Comparison 5 Sensitivity analysis 1: Studies at low risk of bias, Outcome 6 Comparison 3 ‐ All‐cause mortality.
5.7. Analysis.
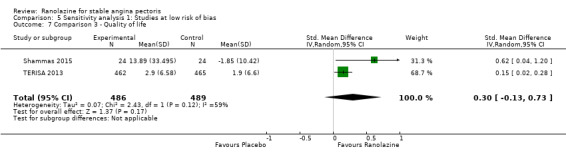
Comparison 5 Sensitivity analysis 1: Studies at low risk of bias, Outcome 7 Comparison 3 ‐ Quality of life.
5.8. Analysis.
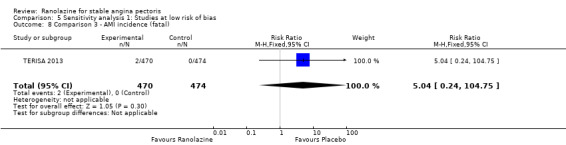
Comparison 5 Sensitivity analysis 1: Studies at low risk of bias, Outcome 8 Comparison 3 ‐ AMI incidence (fatal).
5.9. Analysis.
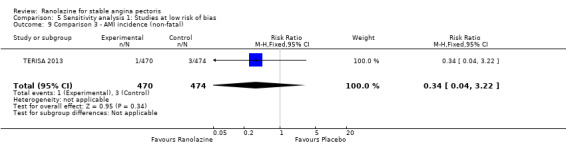
Comparison 5 Sensitivity analysis 1: Studies at low risk of bias, Outcome 9 Comparison 3 ‐ AMI incidence (non‐fatal).
5.10. Analysis.
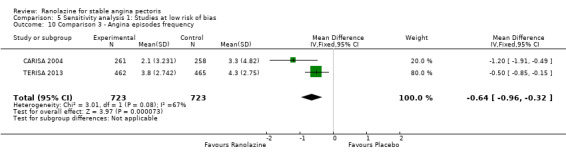
Comparison 5 Sensitivity analysis 1: Studies at low risk of bias, Outcome 10 Comparison 3 ‐ Angina episodes frequency.
5.11. Analysis.
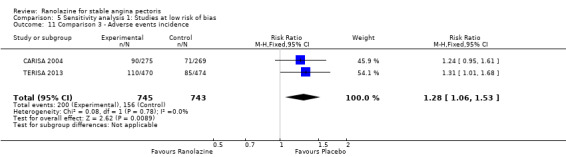
Comparison 5 Sensitivity analysis 1: Studies at low risk of bias, Outcome 11 Comparison 3 ‐ Adverse events incidence.
5.12. Analysis.
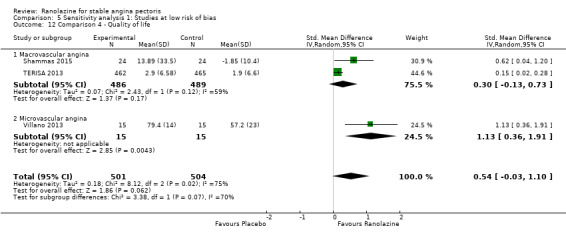
Comparison 5 Sensitivity analysis 1: Studies at low risk of bias, Outcome 12 Comparison 4 ‐ Quality of life.
5.13. Analysis.
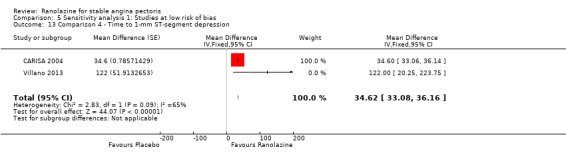
Comparison 5 Sensitivity analysis 1: Studies at low risk of bias, Outcome 13 Comparison 4 ‐ Time to 1‐mm ST‐segment depression.
Comparison 6. Sensitivity analysis 2: Exchange of model for data synthesis.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Comparison 1 ‐ All‐cause mortality | 3 | 6249 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.81, 1.25] |
| 2 Comparison 1 ‐ Quality of life | 3 | 2254 | Mean Difference (IV, Random, 95% CI) | 0.28 [‐1.57, 2.13] |
| 3 Comparison 1 ‐ AMI incidence | 2 | 2674 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.69, 1.13] |
| 4 Comparison 1 ‐ Need for revascularisation procedure | 2 | 2674 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.82, 1.18] |
| 5 Comparison 1 ‐ Adverse events incidence | 2 | 638 | Risk Ratio (M‐H, Random, 95% CI) | 1.34 [0.90, 1.99] |
| 6 Comparison 2 ‐ AMI incidence | 3 | 2983 | Risk Ratio (M‐H, Random, 95% CI) | 0.56 [0.19, 1.63] |
| 7 Comparison 2 ‐ Angina episodes frequency | 2 | 402 | Mean Difference (IV, Random, 95% CI) | 0.08 [‐0.85, 1.01] |
| 8 Comparison 2 ‐ Adverse events incidence | 3 | 947 | Risk Ratio (M‐H, Random, 95% CI) | 1.50 [1.12, 2.01] |
| 8.1 Macrovascular angina | 2 | 691 | Risk Ratio (M‐H, Random, 95% CI) | 1.53 [1.13, 2.07] |
| 8.2 Microvascular angina | 1 | 256 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [0.40, 3.38] |
| 9 Comparison 3 ‐ All‐cause mortality | 3 | 2053 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.25, 3.04] |
| 10 Comparison 3 ‐ Quality of life | 3 | 1533 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.11 [0.01, 0.22] |
| 11 Comparison 3 ‐ AMI incidence (fatal) | 2 | 1509 | Risk Ratio (M‐H, Random, 95% CI) | 1.37 [0.10, 19.46] |
| 12 Comparison 3 ‐ AMI incidence (non‐fatal) | 2 | 1509 | Risk Ratio (M‐H, Random, 95% CI) | 0.41 [0.08, 2.11] |
| 13 Comparison 3 ‐ Angina episodes frequency | 3 | 2004 | Mean Difference (IV, Random, 95% CI) | ‐0.78 [‐1.28, ‐0.27] |
| 14 Comparison 3 ‐ Adverse events incidence | 3 | 2053 | Risk Ratio (M‐H, Random, 95% CI) | 1.21 [1.06, 1.39] |
| 15 Comparison 4 ‐ Quality of life | 4 | 1563 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.13 [0.03, 0.23] |
| 15.1 Macrovascular angina | 3 | 1533 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.11 [0.01, 0.22] |
| 15.2 Microvascular angina | 1 | 30 | Std. Mean Difference (IV, Fixed, 95% CI) | 1.13 [0.36, 1.91] |
| 16 Comparison 4 ‐ Time to 1‐mm ST‐segment depression | 3 | Mean Difference (Random, 95% CI) | 51.05 [4.05, 98.04] |
6.1. Analysis.
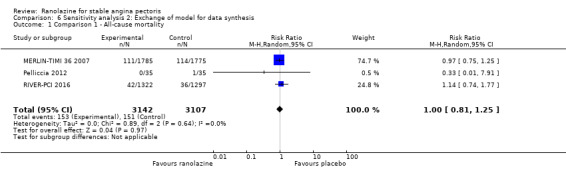
Comparison 6 Sensitivity analysis 2: Exchange of model for data synthesis, Outcome 1 Comparison 1 ‐ All‐cause mortality.
6.2. Analysis.
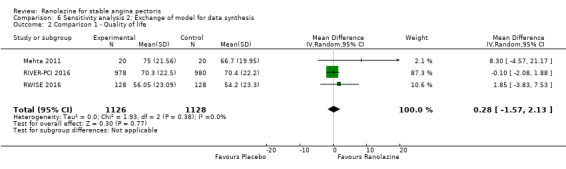
Comparison 6 Sensitivity analysis 2: Exchange of model for data synthesis, Outcome 2 Comparison 1 ‐ Quality of life.
6.3. Analysis.
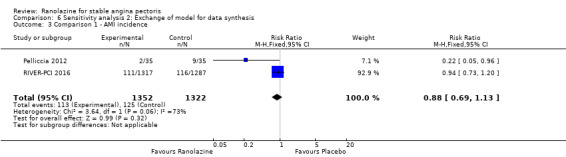
Comparison 6 Sensitivity analysis 2: Exchange of model for data synthesis, Outcome 3 Comparison 1 ‐ AMI incidence.
6.4. Analysis.
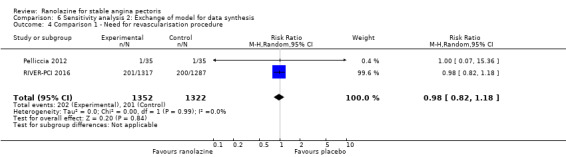
Comparison 6 Sensitivity analysis 2: Exchange of model for data synthesis, Outcome 4 Comparison 1 ‐ Need for revascularisation procedure.
6.5. Analysis.
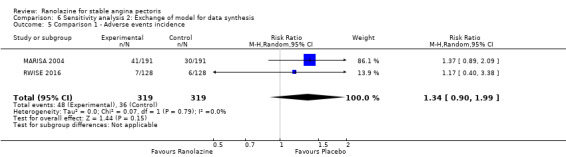
Comparison 6 Sensitivity analysis 2: Exchange of model for data synthesis, Outcome 5 Comparison 1 ‐ Adverse events incidence.
6.6. Analysis.
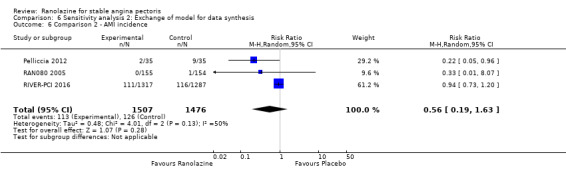
Comparison 6 Sensitivity analysis 2: Exchange of model for data synthesis, Outcome 6 Comparison 2 ‐ AMI incidence.
6.7. Analysis.
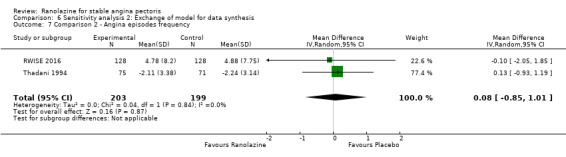
Comparison 6 Sensitivity analysis 2: Exchange of model for data synthesis, Outcome 7 Comparison 2 ‐ Angina episodes frequency.
6.8. Analysis.
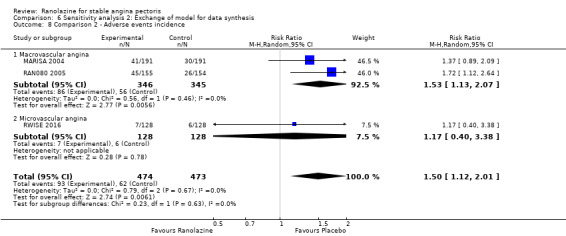
Comparison 6 Sensitivity analysis 2: Exchange of model for data synthesis, Outcome 8 Comparison 2 ‐ Adverse events incidence.
6.9. Analysis.
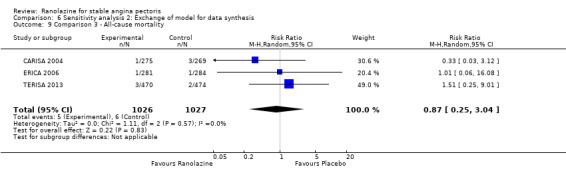
Comparison 6 Sensitivity analysis 2: Exchange of model for data synthesis, Outcome 9 Comparison 3 ‐ All‐cause mortality.
6.11. Analysis.
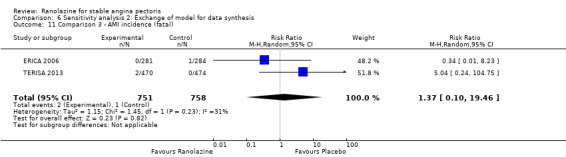
Comparison 6 Sensitivity analysis 2: Exchange of model for data synthesis, Outcome 11 Comparison 3 ‐ AMI incidence (fatal).
6.12. Analysis.
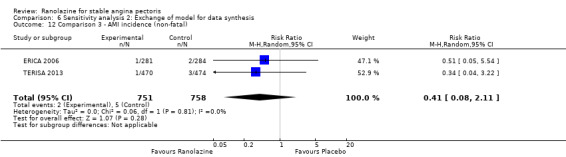
Comparison 6 Sensitivity analysis 2: Exchange of model for data synthesis, Outcome 12 Comparison 3 ‐ AMI incidence (non‐fatal).
6.13. Analysis.
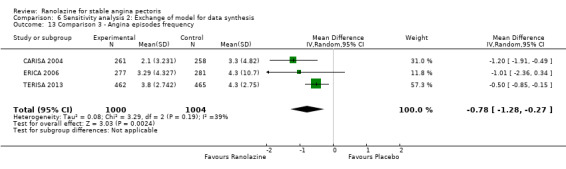
Comparison 6 Sensitivity analysis 2: Exchange of model for data synthesis, Outcome 13 Comparison 3 ‐ Angina episodes frequency.
6.14. Analysis.
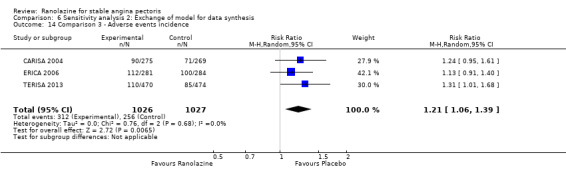
Comparison 6 Sensitivity analysis 2: Exchange of model for data synthesis, Outcome 14 Comparison 3 ‐ Adverse events incidence.
6.16. Analysis.
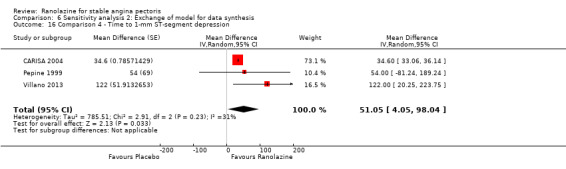
Comparison 6 Sensitivity analysis 2: Exchange of model for data synthesis, Outcome 16 Comparison 4 ‐ Time to 1‐mm ST‐segment depression.
Comparison 7. Sensitivity analysis 3: Change of the measure of treatment effect.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Comparison 1 ‐ All‐cause mortality | 3 | 6249 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.80, 1.27] |
| 2 Comparison 1 ‐ Quality of life | 3 | 2254 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.01 [‐0.07, 0.09] |
| 3 Comparison 1 ‐ AMI incidence | 2 | 2674 | Odds Ratio (M‐H, Random, 95% CI) | 0.49 [0.10, 2.41] |
| 4 Comparison 1 ‐ Need for revascularisation procedure | 2 | 2674 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.79, 1.21] |
| 5 Comparison 1 ‐ Adverse events incidence | 2 | 638 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.41 [0.88, 2.26] |
| 6 Comparison 2 ‐ AMI incidence | 3 | 2983 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.67, 1.13] |
| 7 Comparison 2 ‐ Angina episodes frequency | 2 | 402 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.01 [‐0.19, 0.20] |
| 8 Comparison 2 ‐ Adverse events incidence | 3 | 947 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.64 [1.15, 2.35] |
| 8.1 Macrovascular angina | 2 | 691 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.71 [1.17, 2.49] |
| 8.2 Microvascular angina | 1 | 256 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.38, 3.60] |
| 9 Comparison 3 ‐ All‐cause mortality | 3 | 2053 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.25, 2.73] |
| 10 Comparison 3 ‐ Quality of life | 3 | 1533 | Mean Difference (IV, Random, 95% CI) | 5.91 [‐5.52, 17.34] |
| 11 Comparison 3 ‐ AMI incidence (fatal) | 2 | 1509 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.52 [0.25, 9.09] |
| 12 Comparison 3 ‐ AMI incidence (non‐fatal) | 2 | 1509 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.40 [0.08, 2.08] |
| 13 Comparison 3 ‐ Angina episodes frequency | 3 | 2004 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.19 [‐0.28, ‐0.11] |
| 14 Comparison 3 ‐ Adverse events incidence | 3 | 2053 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.32 [1.09, 1.61] |
| 15 Comparison 4 ‐ Quality of life | 4 | 1563 | Mean Difference (IV, Random, 95% CI) | 11.17 [‐2.54, 24.87] |
| 15.1 Macrovascular angina | 3 | 1533 | Mean Difference (IV, Random, 95% CI) | 5.91 [‐5.52, 17.34] |
| 15.2 Microvascular angina | 1 | 30 | Mean Difference (IV, Random, 95% CI) | 22.20 [8.57, 35.83] |
7.1. Analysis.
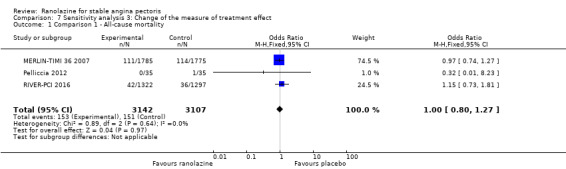
Comparison 7 Sensitivity analysis 3: Change of the measure of treatment effect, Outcome 1 Comparison 1 ‐ All‐cause mortality.
7.2. Analysis.
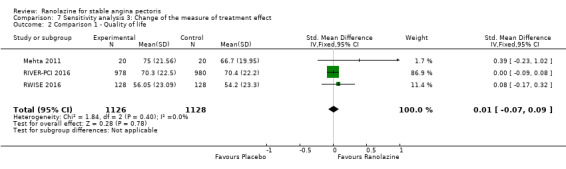
Comparison 7 Sensitivity analysis 3: Change of the measure of treatment effect, Outcome 2 Comparison 1 ‐ Quality of life.
7.3. Analysis.
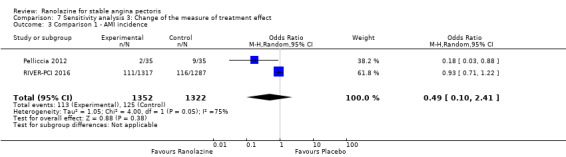
Comparison 7 Sensitivity analysis 3: Change of the measure of treatment effect, Outcome 3 Comparison 1 ‐ AMI incidence.
7.4. Analysis.
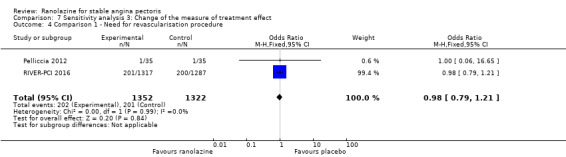
Comparison 7 Sensitivity analysis 3: Change of the measure of treatment effect, Outcome 4 Comparison 1 ‐ Need for revascularisation procedure.
7.5. Analysis.
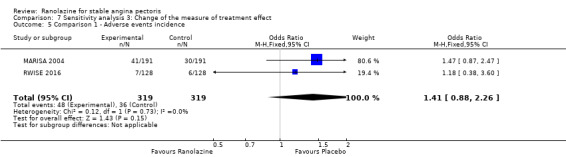
Comparison 7 Sensitivity analysis 3: Change of the measure of treatment effect, Outcome 5 Comparison 1 ‐ Adverse events incidence.
7.6. Analysis.
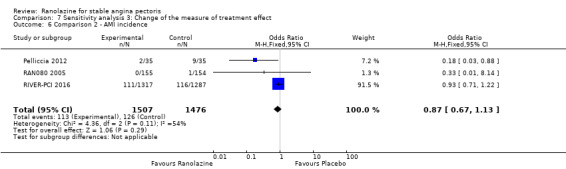
Comparison 7 Sensitivity analysis 3: Change of the measure of treatment effect, Outcome 6 Comparison 2 ‐ AMI incidence.
7.7. Analysis.
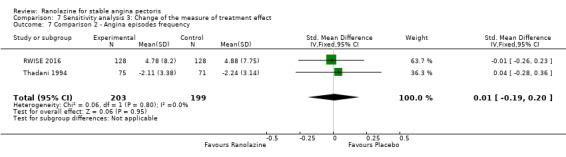
Comparison 7 Sensitivity analysis 3: Change of the measure of treatment effect, Outcome 7 Comparison 2 ‐ Angina episodes frequency.
7.8. Analysis.
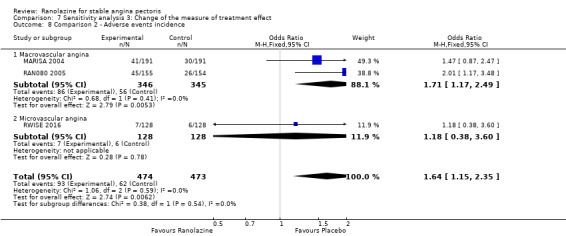
Comparison 7 Sensitivity analysis 3: Change of the measure of treatment effect, Outcome 8 Comparison 2 ‐ Adverse events incidence.
7.9. Analysis.
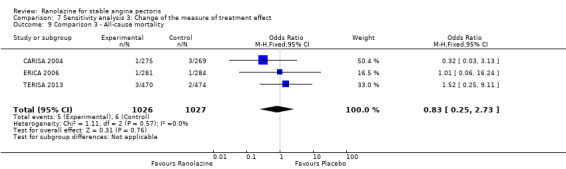
Comparison 7 Sensitivity analysis 3: Change of the measure of treatment effect, Outcome 9 Comparison 3 ‐ All‐cause mortality.
7.10. Analysis.
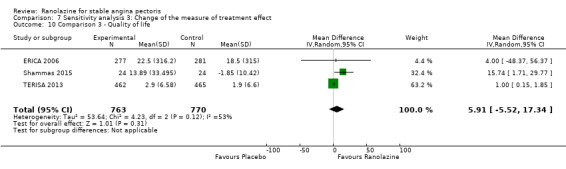
Comparison 7 Sensitivity analysis 3: Change of the measure of treatment effect, Outcome 10 Comparison 3 ‐ Quality of life.
7.11. Analysis.
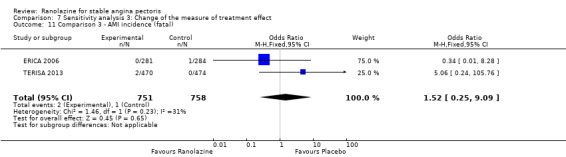
Comparison 7 Sensitivity analysis 3: Change of the measure of treatment effect, Outcome 11 Comparison 3 ‐ AMI incidence (fatal).
7.12. Analysis.
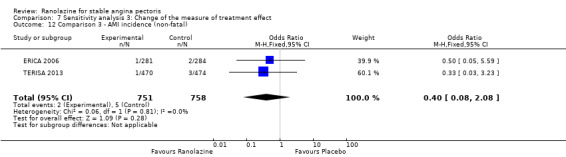
Comparison 7 Sensitivity analysis 3: Change of the measure of treatment effect, Outcome 12 Comparison 3 ‐ AMI incidence (non‐fatal).
7.13. Analysis.
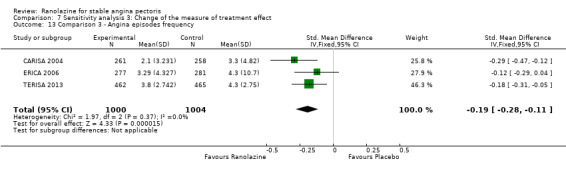
Comparison 7 Sensitivity analysis 3: Change of the measure of treatment effect, Outcome 13 Comparison 3 ‐ Angina episodes frequency.
7.14. Analysis.
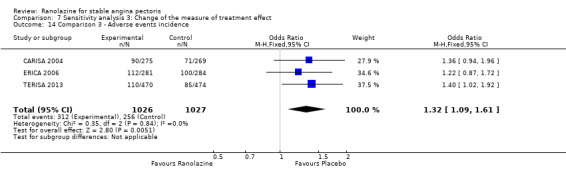
Comparison 7 Sensitivity analysis 3: Change of the measure of treatment effect, Outcome 14 Comparison 3 ‐ Adverse events incidence.
7.15. Analysis.
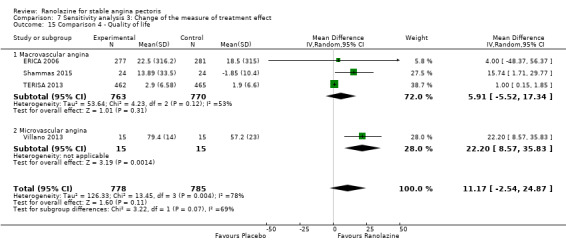
Comparison 7 Sensitivity analysis 3: Change of the measure of treatment effect, Outcome 15 Comparison 4 ‐ Quality of life.
Comparison 8. Sensitivity analysis 4: follow‐up ≥ 6 weeks.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Comparison 1 ‐ All‐cause mortality | 2 | 6179 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.81, 1.26] |
| 2 Comparison 1 ‐ Quality of life | 1 | 1958 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐2.08, 1.88] |
| 3 Comparison 1 ‐ AMI incidence | 1 | 2604 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.73, 1.20] |
| 4 Comparison 1 ‐ Need for revascularisation procedure | 1 | 2604 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.82, 1.18] |
| 5 Comparison 2 ‐ AMI incidence | 1 | 2604 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.73, 1.20] |
| 6 Comparison 3 ‐ All‐cause mortality | 3 | 2053 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.26, 2.71] |
| 7 Comparison 3 ‐ Quality of life | 3 | 1533 | Std. Mean Difference (IV, Random, 95% CI) | 0.13 [‐0.05, 0.32] |
| 8 Comparison 3 ‐ AMI incidence (fatal) | 2 | 1509 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.51 [0.25, 9.05] |
| 9 Comparison 3 ‐ AMI incidence (non‐fatal) | 2 | 1509 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.40 [0.08, 2.07] |
| 10 Comparison 3 ‐ Angina episodes frequency | 3 | 2004 | Mean Difference (IV, Fixed, 95% CI) | ‐0.66 [‐0.97, ‐0.35] |
| 11 Comparison 3 ‐ Adverse events incidence | 3 | 2053 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [1.06, 1.40] |
| 12 Comparison 4 ‐ Quality of life | 3 | 1533 | Std. Mean Difference (IV, Random, 95% CI) | 0.13 [‐0.05, 0.32] |
| 12.1 Macrovascular angina | 3 | 1533 | Std. Mean Difference (IV, Random, 95% CI) | 0.13 [‐0.05, 0.32] |
| 13 Comparison 4 ‐ Time to 1‐mm ST‐segment depression | 1 | Mean Difference (Fixed, 95% CI) | 34.6 [33.06, 36.14] |
8.1. Analysis.
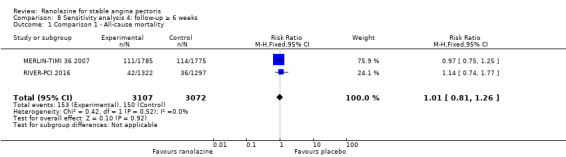
Comparison 8 Sensitivity analysis 4: follow‐up ≥ 6 weeks, Outcome 1 Comparison 1 ‐ All‐cause mortality.
8.3. Analysis.
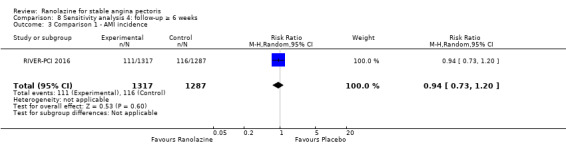
Comparison 8 Sensitivity analysis 4: follow‐up ≥ 6 weeks, Outcome 3 Comparison 1 ‐ AMI incidence.
8.4. Analysis.
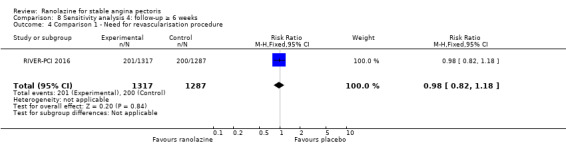
Comparison 8 Sensitivity analysis 4: follow‐up ≥ 6 weeks, Outcome 4 Comparison 1 ‐ Need for revascularisation procedure.
8.5. Analysis.
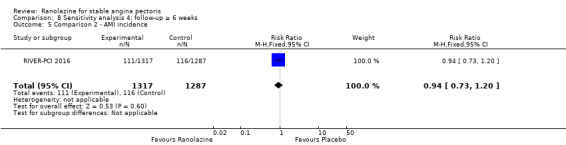
Comparison 8 Sensitivity analysis 4: follow‐up ≥ 6 weeks, Outcome 5 Comparison 2 ‐ AMI incidence.
8.6. Analysis.
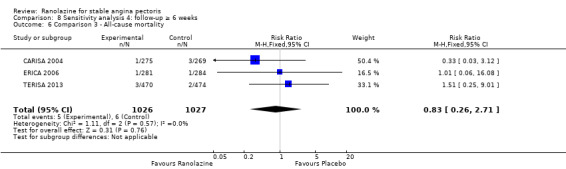
Comparison 8 Sensitivity analysis 4: follow‐up ≥ 6 weeks, Outcome 6 Comparison 3 ‐ All‐cause mortality.
8.7. Analysis.
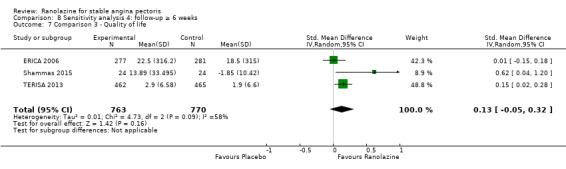
Comparison 8 Sensitivity analysis 4: follow‐up ≥ 6 weeks, Outcome 7 Comparison 3 ‐ Quality of life.
8.8. Analysis.
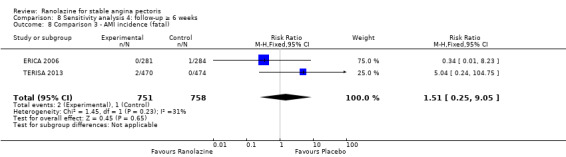
Comparison 8 Sensitivity analysis 4: follow‐up ≥ 6 weeks, Outcome 8 Comparison 3 ‐ AMI incidence (fatal).
8.9. Analysis.
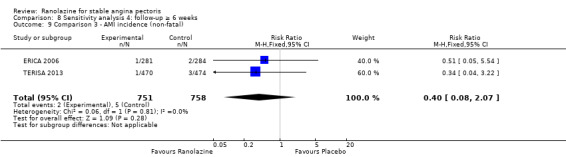
Comparison 8 Sensitivity analysis 4: follow‐up ≥ 6 weeks, Outcome 9 Comparison 3 ‐ AMI incidence (non‐fatal).
8.10. Analysis.
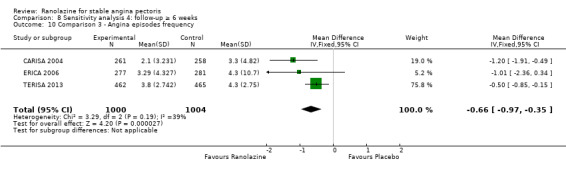
Comparison 8 Sensitivity analysis 4: follow‐up ≥ 6 weeks, Outcome 10 Comparison 3 ‐ Angina episodes frequency.
8.11. Analysis.
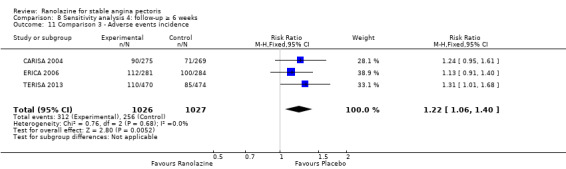
Comparison 8 Sensitivity analysis 4: follow‐up ≥ 6 weeks, Outcome 11 Comparison 3 ‐ Adverse events incidence.
8.12. Analysis.
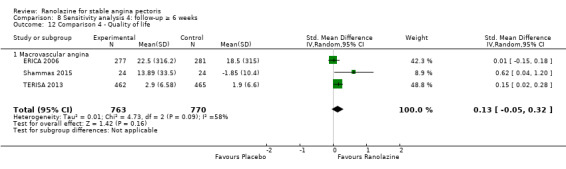
Comparison 8 Sensitivity analysis 4: follow‐up ≥ 6 weeks, Outcome 12 Comparison 4 ‐ Quality of life.
8.13. Analysis.
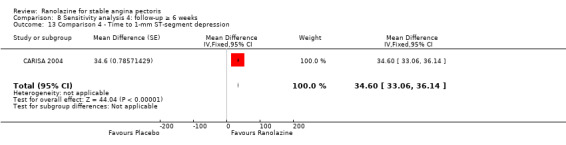
Comparison 8 Sensitivity analysis 4: follow‐up ≥ 6 weeks, Outcome 13 Comparison 4 ‐ Time to 1‐mm ST‐segment depression.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Babalis 2015.
| Methods | Study design: parallel‐group trial Total study duration: 3 months Duration of follow‐up: no follow‐up beyond treatment phase Method of randomisation: not described Method of concealment of allocation: not mentioned Blinding: not mentioned Power calculation: not mentioned Phases of the study: 1 (treatment phase) Number of patients randomised: 40 (20/20 for placebo/ranolazine group) Exclusions post‐randomisation: not reported Withdrawals (and reasons): not reported |
|
| Participants | Total number: 40 Country of enrolment: Greece Setting/location: not specified Diagnostic criteria (stable angina pectoris): symptoms of stable angina and coronary artery disease (CAD) established by coronary stenosis > 70% in one or more vessels documented by angiography (macrovascular angina) Comorbidities: none (non suitability for invasive treatment) Age (mean): 69 ± 7 years Gender (male %): 75% Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Number of intervention groups: 2 Concomitant medications: optimised anti‐anginal treatment (not further specified) Excluded medications: none Placebo group Intervention: placebo Duration of intervention: 3 months Ranolazine group Intervention: ranolazine (type of formulation not specified) 500 mg twice daily Duration of intervention: 3 months |
|
| Outcomes | Total number of outcomes: 1
OUTCOMES Adverse events incidence
RESULTS Adverse events incidence
|
|
| Notes | Relevant observations for the data provided before: It is worth mentioning that two ranolazine group participants and four placebo group participants were not eligible for revascularisation because of complicated coronary anatomy or lack of grafts Source of funding: not stated Notable conflicts of interest: the authors declare that they have no conflict of interest |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation is stated but described only as "in a 1:1 ratio" |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No exclusion or withdrawal is reported, it is stated that no patient withdrew from the study because of ranolazine‐related adverse reactions. We assume that all patients completed the study |
| Selective reporting (reporting bias) | Unclear risk | There is no published protocol. Exercise capacity and duration and time to appearance of angina results were not reported in spite they were mentioned among the study benchmarks |
| Other bias | Unclear risk | The authors declare that they have no conflict of interest. However, the source of funding is not stated. Furthermore, editorial assistance for the preparation of the manuscript was provided by Luca Giacomelli, PhD, on behalf of Content Ed Net; this assistance was funded by Menarini International, an Italian pharmaceutical company. |
CARISA 2004.
| Methods | Study design: parallel‐group trial Total study duration: 14 weeks plus follow‐up (around 14 months). Patients were enrolled from July 1999 to August 2001 Duration of follow‐up: from August 2001 to 31 October 2002 Method of randomisation: randomisation schedules generated by Quintiles (UK) Limited in SAS version 6.12, stratified by the 3 background anti‐anginal therapies using a block size of 6 Method of concealment of allocation: drug packaging with code break envelopes provided by Brecon Pharmaceuticals Ltd. Blinding: double‐blind, not described but presumably referred to participants and study personnel Power calculation: 90% to detect a difference of 30s in the primary end point Phases of the study: 3 (qualifying phase, treatment phase, open‐label follow‐up phase) Number of patients randomised: 823 (269/279/275 for placebo/ranolazine 750 mg/ranolazine 1000 mg group) Exclusions post‐randomisation (for each phase): Qualifying phase: 32 (11/7/14 from placebo/ranolazine 750 mg/ranolazine 1000 mg group). Treatment phase: 54 (14/18/22 from placebo/ranolazine 750 mg/ranolazine 1000 mg group) Withdrawals (and reasons): not reported (excluded patients were reported only as "dropped out") |
|
| Participants | Total number: 823 Country of enrolment: 15 (Canada, Czech Republic, Georgia, Greece, Ireland, Israel, Italy, New Zealand, Poland, Romania, Russia, Spain, United Kingdom, United States) Setting/location: ambulatory outpatient Diagnostic criteria (stable angina pectoris): minimum 3 month history of exertional angina with CAD confirmed by angiography, documented prior myocardial infarction, or a diagnostic stress myocardial imaging study (macrovascular angina) Comorbidities: none Age (mean, SD): 63.7(8.9)/64.3(9.3)/63.9(9.3) for placebo/ranolazine 750 mg/ranolazine 1000 mg group Gender (male %): 75.1/77.8/79.6 for placebo/ranolazine 750 mg/ranolazine 1000 mg group Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Number of intervention groups: 3 Concomitant medications: background anti‐anginal treatment (atenolol 50 mg once daily, diltiazem 180 mg once daily, or amlodipine 5m g once daily) Excluded medications: none Placebo group Intervention: placebo twice daily Duration of intervention: 12 weeks Ranolazine 750 mg group Intervention: ranolazine ER 750 mg twice daily Duration of intervention: 12 weeks Ranolazine 1000 mg group Intervention: ranolazine ER 1000 mg twice daily Duration of intervention: 12 weeks |
|
| Outcomes | Total number of outcomes:
All‐cause mortality Outcome definition: number of deaths Method and unit of measurement: absolute frequency, survival rate Time points reported: 12 weeks (plus the 14‐day safety follow‐up), 17 months (including follow‐up phase) Angina episodes frequency
Adverse events incidence
RESULTS All‐cause mortality
Angina episodes frequency
Adverse events incidence
|
|
| Notes | Relevant observations for the data provided before: none Source of funding: CV Therapeutics Inc. Notable conflicts of interest: six of the authors have financial relationships with CV Therapeutics |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random sequence. Randomisation schedules generated by Quintiles (UK) Limited in SAS version 6.12, stratified by the 3 background anti‐anginal therapies using a block size of 6 |
| Allocation concealment (selection bias) | Low risk | Drug packaging with code break envelopes made by Brecon Pharmaceuticals Ltd. The medication assignment was provided to the principal investigator in a sealed envelope. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Treatment phase is declared to be double‐blinded, but no description is provided. Drug packages were made with code break envelopes and provided to the clinical units, so patients and personnel were not aware of the treatment assigned. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Exercise treadmill test ECGs were interpreted by a core EGC laboratory blinded to treatment assignment using customised software. Although this is stated only for the single‐blind qualifying phase, we assume it also applies for the double‐blind treatment phase. However, for outcomes such as all‐cause mortality, angina frequency and adverse events incidence blinding measures have not been described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Number of patients dropped out during the qualifying and treatment phases are reported, but reasons and explanations are not provided |
| Selective reporting (reporting bias) | Unclear risk | There is no published protocol. Results for all the outcomes stated in the "Methods" section of the paper are reported, but results for some additional outcomes (haemodynamics) are also reported. |
| Other bias | High risk | The study was supported by CV Therapeutics Inc. Furthermore, six of the authors have financial relationships with CV Therapeutics. |
ERICA 2006.
| Methods | Study design: parallel‐group trial Total study duration: 9 weeks; recruitment from July 30, 2004 to February 16, 2005 Duration of follow‐up: no follow‐up beyond treatment phase Method of randomisation: randomisation in a 1:1 ratio, centralised and not stratified by centre Method of concealment of allocation: not mentioned Blinding: double‐blind, not described Power calculation: not mentioned Phases of the study: 2 (qualifying phase, treatment phase), treatment phase made up by 1‐week run‐in phase and 6‐week full‐dose treatment phase Number of patients randomised: 565 (284/281 for placebo/ranolazine group) Exclusions post‐randomisation: run‐in phase: 1 withdrawal before study drug treatment (placebo group), 3 exclusions from placebo group because not beginning full‐dose treatment phase, 4 exclusions from ranolazine group, 1 because not beginning full‐dose treatment phase, 3 because not having any diary data in the full‐dose treatment phase Withdrawals (and reasons): ranolazine group (3 adverse events, 1 death, 3 withdrew consent), placebo group (5 adverse events (4 according to the text), 1 death) |
|
| Participants | Total number: 564 Country of enrolment: Eastern Europe, United States, Canada Setting/location: not specified Diagnostic criteria (stable angina pectoris): history of chronic stable angina ≥3 months, documented history of CAD (macrovascular angina) Comorbidities: none Age (mean ± SD): 61.3±9.0 / 62.0±8.7 for placebo / ranolazine group Gender (male %): 73% / 72% for placebo / ranolazine group Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Number of intervention groups: 2 Concomitant medications: amlodipine 10 mg/day; LANs and sublingual nitroglycerin as required Excluded medications: inhibitors of cytochrome P450‐3A4, digitalis preparation, perhexiline, trimetazidine, beta‐blockers, calcium cannel blockers other than amlodipine Placebo group
Ranolazine group
|
|
| Outcomes | Total number of outcomes:
All‐cause mortality
Quality of life
Acute myocardial infarction incidence (fatal and non‐fatal)
Angina episodes frequency
Adverse events incidence
RESULTS All‐cause mortality
Quality of life
Acute myocardial infarction incidence (fatal and non‐fatal)
Angina episodes frequency
Adverse events incidence
|
|
| Notes | Relevant observations for the data provided before: given that 4 placebo patients and 3 ranolazine patients discontinued the study because of adverse events but 5 placebo patients are reported not to have terminated the trial because of adverse events and 3 more ranolazine patients are reported not to have terminated the trial because of withdrawing consent, it is not clear which patients were finally included in the efficacy analysis Source of funding: CV Therapeutics Notable conflicts of interest: all the authors have received some kind of reward or support for participating in this trial from several pharmaceutical companies |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation is stated but described only as "in a 1:1 ratio, centralized but not stratified" |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Treatment phase is declared to be double‐blinded, but not description is provided |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Treatment phase is declared to be double‐blinded, but not description is provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Patients who did not complete the trial are described clearly, however, there is an inconsistency in the data provided for terminating patients in the placebo group for only one case and it is not clear which patients were finally included in the efficacy analysis |
| Selective reporting (reporting bias) | Unclear risk | There is no published protocol. Results for all the outcomes mentioned in the "Methods" section of the paper are reported |
| Other bias | High risk | The study was supported by CV Therapeutics Inc. All the authors have received some kind of reward or support for participating in this trial from several pharmaceutical companies |
MARISA 2004.
| Methods | Study design: cross‐over trial Total study duration: 6 weeks plus follow‐up, the study began in December 1997 and ended in May 1999 Duration of follow‐up: about 2 years; to October 15, 2001 Method of randomisation: not described Method of concealment of allocation: not mentioned Blinding: double‐blind, not described Power calculation: not mentioned Phases of the study: 3 (qualifying phase, treatment phase, open‐label follow‐up phase) Number of patients randomised: 191 Exclusions post‐randomisation: 16 (treatment phase, those who did not complete at least three treatment periods) Withdrawals (and reasons): 23 patients (12%) discontinued the study before completing all treatment periods: 15 patients (7.9%) for adverse events, 4 patients (2.1%) by elective withdrawal, 2 patients (1.0%) for other reasons, 1 patient (˂ 1%) because of death and 1 patient (˂ 1%) because of inappropriate enrolment |
|
| Participants | Total number: 191 Country of enrolment: 4 (Canada, Czech Republic, Poland, United States) Setting/location: not specified Diagnostic criteria (stable angina pectoris): at least a three‐month history of effort angina responding to beta‐blockers, calcium channel blockers and/or long‐acting nitrates with well‐documented CAD (macrovascular angina) Comorbidities: none Age (mean ± SD): 64.3 ± 9.4 years Gender (male %): 73.3% Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Number of intervention groups: 4 Concomitant medications: sublingual nitroglycerin Excluded medications: anti‐anginal treatment except sublingual nitroglycerin Placebo group Intervention: placebo twice daily Duration of intervention: 1 week Ranolazine 500 mg group Intervention: ranolazine SR 500 mg twice daily Duration of intervention: 1 week Ranolazine 1000 mg group Intervention: ranolazine SR 1000 mg twice daily Duration of intervention: 1 week Ranolazine 1500 mg group Intervention: ranolazine SR 1500 mg twice daily Duration of intervention: 1 week |
|
| Outcomes | Total number of outcomes:
Adverse events incidence Outcome definition: number of patients who report any adverse event Method and unit of measurement: percentage Time points reported: 1 week RESULTS Adverse events incidence Sample size: 191 (intention‐to‐treat analysis) Missing participants: none Summary data: 15.6%/16.0%/21.7%/34.2% for placebo/ranolazine 500 mg/ranolazine 1000 mg/ranolazine 1500 mg group. Over a total of 191 participants, the adverse events most frequently reported were dizziness (2/2/10/23), nausea (0/1/2/16), asthenia (4/0/3/12), constipation (0/0/3/8), angina (10/10/3/6), headache (4/1/2/5) and sweating (0/0/0/5) for placebo/ranolazine 500 mg/ranolazine 1000 mg/ranolazine 1500 mg group. Subgroup analyses: not performed |
|
| Notes | Relevant observations for the data provided before: none Source of funding: CV Therapeutics Notable conflicts of interest: three of the authors have financial relationships with CV Therapeutics |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation is stated but not described |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Study is declared to be double‐blinded, but no description is provided |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Exercise treadmill test ECGs were interpreted by a core EGC laboratory blinded to treatment assignment using customised software. However, for outcomes such as adverse events incidence, blinding measures have not been described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Patients who did not complete the trial are described, but the treatment they were receiving is not specified |
| Selective reporting (reporting bias) | Unclear risk | There is no published protocol. Results for all the outcomes stated in the "Methods" section of the paper are reported |
| Other bias | High risk | The study was supported by CV Therapeutics. Three of the authors have financial relationships with CV Therapeutics |
Mehta 2011.
| Methods | Study design: cross‐over trial Total study duration: 10 weeks plus up to 24 months of qualifying phase Duration of follow‐up: no follow‐up beyond treatment phase Method of randomisation: not described Method of concealment of allocation: not mentioned Blinding: double‐blind, not described Power calculation: not mentioned Phases of the study: 2 (qualifying phase, treatment phase) Number of patients randomised: 20 Exclusions post‐randomisation: not reported Withdrawals (and reasons): not reported |
|
| Participants | Total number: 20 Country of enrolment: United States Setting/location: not specified Diagnostic criteria (stable angina pectoris): chest pain and abnormal routine stress testing without obstructive CAD (< 50% epicardial coronary stenosis in all epicardial coronary arteries) on clinically indicated coronary angiography (microvascular angina) Comorbidities: none Age (mean): 57 ± 11 years Gender (male %): 0% Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Number of intervention groups: 2 Concomitant medications: usual anti‐anginal medication Excluded medications: none (apart from those mentioned in the exclusion criteria) Placebo group Intervention: placebo twice daily Duration of intervention: 4 weeks (plus 2 weeks of washout) Ranolazine group Intervention: ranolazine (type of formulation not specified) 500/1000 mg twice daily (the dose was increased from 500 mg to 1000 mg twice daily during the second half of treatment period if tolerated) Duration of intervention: 4 weeks (plus 2 weeks of washout) |
|
| Outcomes | Total number of outcomes:
Quality of life 1. Seattle Angina Questionnaire (SAQ) Outcome definition: score in the 5 sub‐scales, reported separately (For scales) upper and lower limits and whether a high or low score is good: higher scores are better, upper and lower limits are not described Method and unit of measurement: score Time points reported: 4 weeks 2. Duke Activity Status Index (DASI) Outcome definition: functional capacity scale, not further described (For scales) upper and lower limits and whether a high or low score is good: not described Method and unit of measurement: score Time points reported: 4 weeks RESULTS Quality of life 1. Seattle Angina Questionnaire (SAQ) Sample size: 47 (intention‐to‐treat analysis) Missing participants: none Summary data: reported as mean (minimum, maximum) i) Physical functioning: 83.3 (66.6, 97.2) /91.7 (79.2, 97.9) for placebo/ranolazine group; ii) Angina stability: 50.0 (25.0, 75.0)/75.0 (50.0, 100.0) for placebo/ranolazine group; iii) angina frequency: 75.0 (60.0, 87.5)/80.0 (50.0, 100.0) for placebo/ranolazine group; iv) Treatment satisfaction: 93.8 (75.0, 100.0)/87.5 (75.0, 100.0) for placebo/ranolazine group v) Quality of life: 66.7 (58.3, 75.0)/75.0 (60.4, 83.3) for placebo/ranolazine group Subgroup analyses: not performed 2. Duke Activity Status Index (DASI) Sample size: 20 (intention‐to‐treat analysis) Missing participants: none Summary data: 8.9 (5.4 minimum, 12.1 maximum) METS / 8.6 (3.7 minimum, 11.5 maximum) METS for placebo/ranolazine group Subgroup analyses: not performed |
|
| Notes | Relevant observations for the data provided before: details about the qualifying phase are not provided Source of funding: grants and contracts from several public and private organisations in the United States. Notable conflicts of interest: the authors reported they have no relationships to disclose. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation is stated, but no description is provided |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Study is declared to be double‐blinded. Participants and investigators. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Cardiac magnetic resonance imaging outcomes were measured by readers blinded to treatment assignment. However, for outcomes such as quality of life, blinding measures have not been described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients completed the study. |
| Selective reporting (reporting bias) | Low risk | There is published protocol in clinical trials. Results for all the outcomes stated in the "Methods" section of the paper are reported. |
| Other bias | Unclear risk | The study received grants and contracts from several public and private organisations in the United States. The authors reported they have no relationships to disclose. |
MERLIN‐TIMI 36 2007.
| Methods | Study design: parallel‐group trial Total study duration: mean of 350 days Duration of follow‐up: no follow‐up beyond treatment phase Method of randomisation: via a central computerised telephone system with stratification by the responsible physician’s intended management strategy (early invasive versus conservative) Method of concealment of allocation: not mentioned Blinding: double‐blind, not described Power calculation: 90% to detect a significant difference between treatment groups at 2‐sided 5% significance level Phases of the study: 1, treatment phase Number of patients randomised: 6560 in the original trial, 3565 in the sub‐study on stable angina patients (1789/1776 for ranolazine/placebo group) Exclusions post‐randomisation: 5 lost to follow‐up Withdrawals (and reasons): 8.1%/4.1% discontinued ranolazine due to an adverse event in ranolazine/placebo group |
|
| Participants | Total number: 3565 Country of enrolment: 17 (Australia, France, Germany, Poland, United Kingdom, United States, Spain, Israel, Austria, Switzerland, Italy, The Netherlands, South Africa, Hungary, Czech Republic, Canada, Belgium) Setting/location: hospitalisation Diagnostic criteria (stable angina pectoris): history of prior stable angina before and separate from the presenting ACS Comorbidities: Acute coronary syndrome: clinical presentation consistent with an ACS with at least 1 indicator of moderate to high risk of death or recurrent ischaemic events Age (mean (25th, 75th)): 65 (57,73)/66 (56/73) for ranolazine/placebo group Gender (male %): 1149/1789(64.2%)‐1083/1776(61.0%) for ranolazine‐placebo group Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Number of intervention groups: 2 Concomitant medications: intravenous ranolazine 12 hours to 96 hours before intervention Excluded medications: none (apart from those mentioned in the exclusion criteria) Placebo group Intervention: placebo twice daily Duration of intervention: mean of 350 days Ranolazine group Intervention: ranolazine ER 1000 mg twice daily (500 mg twice daily for renal insufficiency patients) Duration of intervention: mean of 350 days |
|
| Outcomes | Total number of outcomes:
All‐cause mortality Outcome definition: number of deaths Method and unit of measurement: absolute frequency Time points reported: overall study duration (mean of 350 days) RESULTS All‐cause mortality Sample size: 3560 (1785 + 1775) (intention‐to‐treat analysis) Missing participants: 5 Summary data: 114/1775 ‐ 111/1785 for placebo‐ranolazine group Subgroup analyses: not performed Adverse events The most common adverse effects that were more frequent in the ranolazine group compared with placebo were dizziness (12.4% versus 7.4%), nausea (9.7% versus 6.1%) and constipation (8.5% versus 3,3%). |
|
| Notes | Relevant observations for the data provided before: sub‐study of the MERLIN TIMI 36 trial not considered in the protocol Source of funding: CV Therapeutics (CVT), Inc. Notable conflicts of interest: five of the authors have financial relationships with CVT |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Via a central computerised telephone system |
| Allocation concealment (selection bias) | Low risk | Not mentioned. However, given the use of a centralised telephone system for randomization (and allocation), it can be assumed that study personnel was blinded until the moment of assignment. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Study is declared to be double‐blinded, but no description is provided |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All elements of the primary composite and major secondary efficacy end points as well as hospitalisation for new or worsening heart failure were adjudicated by members of a Clinical Events Committee blinded to treatment allocation. Exercise treadmill test results were also interpreted by a core laboratory blinded to treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Patients who did not complete the trial are described, but the treatment they were receiving or the reasons for withdrawal are not specified |
| Selective reporting (reporting bias) | High risk | This sub‐study of the MERLIN TIMI 36 trial included in this review was not pre‐specified in the published protocol. Furthermore, results for some outcomes (laboratory abnormalities) included in the protocol are not reported in the paper while results for some other outcomes (worsening angina, need for an increase of anti‐anginal therapy) not mentioned in the protocol are reported in the paper. |
| Other bias | High risk | The study was supported by CV Therapeutics (CVT), Inc. Five of the authors have financial relationships with CVT |
Pelliccia 2012.
| Methods | Study design: parallel‐group trial Total study duration: 37 days Duration of follow‐up: 30 days Method of randomisation: not described Method of concealment of allocation: not mentioned Blinding: double‐blind, not described Power calculation: not mentioned Phases of the study: 2 (treatment phase, 30‐day follow‐up phase) Number of patients randomised: 70 (35/35 for placebo/ranolazine group) Exclusions post‐randomisation: not reported Withdrawals (and reasons): not reported |
|
| Participants | Total number: 70 Country of enrolment: Italy Setting/location: not specified Diagnostic criteria (stable angina pectoris): typical stable effort angina with positive stress test, with indication for PCI Comorbidities: none Age (mean ± SD): 60 ± 18/64 ± 17 for placebo/ranolazine group Gender (male %): 57%/63% for placebo/ranolazine group Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Number of intervention groups: 2 Concomitant medications: aspirin 100 mg/d, loading dose of clopidogrel 600 mg or ticlopidine 250 mg bid (before the procedure), and clopidogrel 75 mg/d or ticlopidine 250 mg bid for 1 or 12 months. Other medications such as β‐blockers, calcium antagonists, statins, and angiotensin‐converting enzyme inhibitors given as appropriate Excluded medications: none Placebo group Intervention: placebo as pretreatment for PCI Duration of intervention: 1 week Ranolazine group Intervention: ranolazine (type of formulation not specified) 1000 mg twice daily as pretreatment for PCI Duration of intervention: 1 week |
|
| Outcomes | Total number of outcomes:
All‐cause mortality Outcome definition: number of deaths Method and unit of measurement: absolute frequency Time points reported: 37 days Acute myocardial infarction incidence (fatal and non‐fatal) Outcome definition: (periprocedural) postprocedural increase of CK‐MB ≥ 3 times above the upper limit of normal, number of cases Method and unit of measurement: absolute frequency Time points reported: 7 days (periprocedural), 37 days (periprocedural plus spontaneous) Need for revascularisation procedure Outcome definition: target‐vessel revascularisation, number of cases Method and unit of measurement: absolute frequency Time points reported: 37 days RESULTS All‐cause mortality Sample size: 70 (intention‐to‐treat analysis) Missing participants: none Summary data: 1/35‐0/35 for placebo‐ranolazine group Subgroup analyses: not performed Acute myocardial infarction incidence (fatal and non‐fatal) Sample size: 70 (intention‐to‐treat analysis) Missing participants: none Summary data: 9/35‐2/35 for placebo/ranolazine group (37 days) Subgroup analyses: not performed Need for revascularisation procedure Sample size: 70 (intention‐to‐treat analysis) Missing participants: none Summary data: 1/35‐1/35 for placebo‐ranolazine group Subgroup analyses: not performed |
|
| Notes | Relevant observations for the data provided before: none Source of funding: no extramural funding Notable conflicts of interest: no conflicts of interest to declare. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation is stated but not described |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Study declared to be double‐blinded, but no description is provided |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Study declared to be double‐blinded, but no description is provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No exclusion or withdrawal is reported, we assume that all patients completed the study |
| Selective reporting (reporting bias) | Unclear risk | There is no published protocol. Results for all the outcomes stated in the "Methods" section of the paper are reported |
| Other bias | Low risk | The authors declare that the study was not supported by any external source of funding. Furthermore, there were no conflicts of interest to declare. |
Pepine 1999.
| Methods | Study design: cross‐over trial Total study duration: 8 weeks Duration of follow‐up: no follow‐up beyond treatment phase Method of randomisation: not described Method of concealment of allocation: not mentioned Blinding: double‐blind (with "double dummy" technique) Power calculation: not mentioned Phases of the study: 2 (qualifying phase, treatment phase) Number of patients randomised: 318 Exclusions post‐randomisation: 6 (not described) Withdrawals (and reasons): premature withdrawals due to adverse events are declared to have been very similar for all treatments, but no details are provided |
|
| Participants | Total number: 312 Country of enrolment: United States, Canada. Setting/location: not specified Diagnostic criteria (stable angina pectoris): chronic (≥ 3 months) stable angina pectoris that had responded to conventional anti‐anginal therapy Comorbidities: none Age (mean, range): 64.3 (33‐85) years Gender (male %): 226 (72%) Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Number of intervention groups: 4 Concomitant medications: β‐blockers and/or calcium antagonists (minimum medication needed during qualifying phase) Excluded medications: long‐acting nitrates Placebo group Intervention: placebo Duration of intervention: 1 week (5 double‐blind treatment periods in an extended period Latin square design) Ranolazine 400 mg bid group Intervention: ranolazine IR 400 mg twice daily Duration of intervention: 1 week (5 double‐blind treatment periods in an extended period Latin square design) Ranolazine 267 mg tid group Intervention: ranolazine IR 267 mg thrice daily Duration of intervention: 1 week (5 double‐blind treatment periods in an extended period Latin square design) Ranolazine 400 mg tid group Intervention: ranolazine IR 400 mg thrice daily Duration of intervention: 1 week (5 double‐blind treatment periods in an extended period Latin square design) |
|
| Outcomes | Total number of outcomes:
Adverse events incidence Outcome definition: number of patients experiencing an adverse event Method and unit of measurement: percentage Time points reported: 1 week RESULTS Adverse events incidence Sample size: 312 (intention‐to‐treat) Missing participants: 6 Summary data: it is stated that adverse events rates were similar for all ranolazine and placebo regimens and approximately 25%, but data for each group is not reported. It is stated that only minor gastrointestinal complaints tended to occur more often with ranolazine (6.6% to 10.7%) than with placebo (3,2%). Subgroup analyses: not performed |
|
| Notes | Relevant observations for the data provided before: "all patients" (intention‐to‐treat) (N = 312) and per‐protocol (N = 260) analysis were performed for ETT variables Source of funding: in part by a grant from Syntex Research Notable conflicts of interest: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation is stated but no described |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Study is declared to be double‐blinded, but no description is provided |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Study is declared to be double‐blinded, but no description is provided |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Exclusions and withdrawals are reported, but no reasons or explanations are provided |
| Selective reporting (reporting bias) | Unclear risk | There is no published protocol. Results for all the outcomes stated in the "Methods" section of the paper are reported |
| Other bias | Unclear risk | The study was supported in part by a grant from Syntex Research. The authors did not stated any conflicts of interest. |
RAN080 2005.
| Methods | Study design: cross‐over trial Total study duration: 28 to 40 days Duration of follow‐up: no follow‐up beyond treatment phase Method of randomisation: not described Method of concealment of allocation: not mentioned Blinding: double‐blind (with 'double‐dummy' technique), not described Power calculation: not mentioned Phases of the study: 2 (qualifying phase, treatment phase) Number of patients randomised: 158 Exclusions post‐randomisation: 4 (did not perform ≥ 1 exercise test in the treatment phase) Withdrawals (and reasons): 6, 4 withdrawals attributed to adverse events (2 during ranolazine therapy, 1 because of hematologic abnormality, 1 because of asthenia, nausea and chest pain; 2 during placebo therapy, due to exacerbation of angina) |
|
| Participants | Total number: 158 Country of enrolment: Europe and Canada Setting/location: not specified Diagnostic criteria (stable angina pectoris): symptoms and exercise test results that support the diagnosis of chronic angina with evidence of CAD (macrovascular angina) Comorbidities: none Age (mean ± SD): 59 ± 8 years Gender (male %): 89% Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Number of intervention groups: 3 Concomitant medications: (permitted) short‐acting nitrates, calcium cannel blockers (except those that are cardiodepressants) Excluded medications: β‐blockers, verapamil Placebo group Intervention: Placebo Duration of intervention: 7 to 10 days Atenolol group Intervention: atenolol 100 mg/d Duration of intervention: 7 to 10 days Ranolazine group Intervention: ranolazine IR 400 mg thrice daily Duration of intervention: 7 to 10 days |
|
| Outcomes | Total number of outcomes:
Acute myocardial infarction incidence (fatal and non‐fatal) Outcome definition: mentioned as “serious adverse events”, number of cases Method and unit of measurement: absolute frequency Time points reported: 28 to 40 days (total study duration) Angina episodes frequency Outcome definition: average number of episodes per week Method and unit of measurement: number per week Time points reported: 28 to 40 days (total study duration) Adverse events incidence Outcome definition: number of patients that reported ≥ 1 adverse event Method and unit of measurement: absolute frequency Time points reported: 28 to 40 days RESULTS Acute myocardial infarction incidence (fatal and non‐fatal) Sample size: 155 (intention‐to‐treat analysis) Missing participants: 3 Summary data (for each intervention group) (according to type of analysis) (for the largest time point): 1/154 – 0/154 – 0/155 for placebo – atenolol – ranolazine group Subgroup analyses: not performed Angina episodes frequency Sample size: not specified (presumably 154) Missing participants: not specified (presumably 4) Summary data: numerical data not reported Subgroup analyses: not performed Adverse events incidence Sample size: 155 (intention‐to‐treat analysis) Missing participants: 3 Summary data: 26/154 – 39/154 – 45/155 for placebo – atenolol – ranolazine group. The most frequently reported adverse events were asthenia (19/26/4), dizziness (2/9/4), headache (6/0/5), nausea (6/0/2), palpitations (4/2/3), dyspepsia (7/0/2), pain (1/2/2), constipation (5/0/1), malaise (1/1/2) and dyspnoea (0/1/3) for ranolazine/atenolol/placebo group. Subgroup analyses: not performed |
|
| Notes | Relevant observations for the data provided before: none Source of funding: CV Therapeutics, Inc. Notable conflicts of interest: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation is stated but not described |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Study declared to be double‐blinded, but no description is provided |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Study declared to be double‐blinded, but no description is provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Six patients are reported not to have completed the study. Reasons for withdrawal and treatment assigned are not described for two of them |
| Selective reporting (reporting bias) | High risk | There is no published protocol. Results for all the outcomes stated in the "Methods" section of the paper are reported, but results for some additional outcomes (haemodynamics and safety and adverse events) are also reported |
| Other bias | High risk | The study was supported by CV Therapeutics, Inc. Conflicts of interest were not stated. |
RIVER‐PCI 2016.
| Methods | Study design: parallel‐group trial Total study duration: about 3.5 years Duration of follow‐up: mean of 643 days Method of randomisation: interactive web‐based block randomisation system (block sizes of 10), with randomisation stratified by diabetes history (presence versus absence) and acute coronary syndrome presentation (acute versus non‐acute) Method of concealment of allocation: not described in enough detail, it is just mentioned that investigators and patients were masked to treatment allocation Blinding: double‐blind, referred to participants, clinicians ("masked to treatment allocation"), data collectors (independent Data Safety Monitoring Board, independent on‐site clinical monitors, independent angiographic core laboratory), outcome adjudicators (independent Clinical Endpoint Committee) and data analysts (independent statistical data analysis group, Duke Clinical Research Institute for quality of life and economic analyses) Power calculation: 85% power using a 2‐sided log‐rank test at the 5% significance level, with regard to the primary end point events Phases of the study: 1 (treatment phase, including a 7‐day run‐in period) Number of patients randomised: 2651 (1319/1332 for placebo/ranolazine group) Exclusions post‐randomisation: 32 exclusions in the placebo group (19 not treated, 3 scientific misconduct, 10 no qualifying PCI), 15 exclusions in the ranolazine group (7 not treated, 3 scientific misconduct, 5 no qualifying PCI). Additionally, for the quality of life sub‐study, there were 105 exclusions in the placebo group (97 questionnaires invalid, 8 questionnaires not done) and 110 exclusions in the ranolazine group (103 questionnaires invalid, 7 questionnaires not done) Withdrawals (and reasons): 463/1287 ‐ 529/1317 for placebo ‐ ranolazine group, reasons detailed in the appendix of the study report |
|
| Participants | Total number: 2651 Country of enrolment: 15 countries (Europe, Israel, Russia, USA) Setting/location: inpatient and outpatient Diagnostic criteria (stable angina pectoris): symptoms of stable angina Comorbidities: incomplete revascularisation (ICR) post‐PCI Age (mean): 63.3±10 / 63.3±10.4 years for placebo/ranolazine group Gender (male %): 80.3% / 79.7% for placebo/ranolazine group Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Number of intervention groups: 2 Concomitant medications: per the discretion of the investigator Excluded medications: those mentioned in exclusion criteria Placebo group Intervention: placebo Duration of intervention: mean (IQR) of 642 (575‐561) days Ranolazine group Intervention: ranolazine (type of formulation not specified) 1000 mg twice daily Duration of intervention: mean (IQR) of 644 (575‐757) days |
|
| Outcomes | Total number of outcomes: 1
Cardiovascular mortality
All‐cause mortality
Quality of life 1. Seattle Angina Questionnaire (SAQ)
2. Duke Activity Satuts Index (DASI)
3. Mental Health Inventory‐5 (MHI‐5)
4. European QOL Five Dimension Three‐Level Scale (EuroQOL‐5D‐3 L)
5. Rose Dyspnea Scale (RDS)
Acute myocardial infarction incidence (fatal and non‐fatal)
Need for revascularisation procedure
RESULTS Cardiovascular mortality
All‐cause mortality
Quality of life 1182 1207 1. Seattle Angina Questionnaire (SAQ)
2. Duke Activity Satuts Index (DASI)
3. Mental Health Inventory‐5 (MHI‐5)
4. European QOL Five Dimension Three‐Level Scale (EuroQOL‐5D‐3 L)
5. Rose Dyspnea Scale (RDS)
Acute myocardial infarction incidence (fatal and non‐fatal)
Need for revascularisation procedure
Adverse events Dizziness, constipation, nausea, hypotension, vomiting and vertigo were reported more often in the ranolazine group than in the placebo group. |
|
| Notes | Relevant observations for the data provided before: none Source of funding: Gilead Sciences, Menarini Group Notable conflicts of interest: seven of the authors declare current of past financial relationships with Gilead Sciences |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random sequence. Random sequence generated by an interactive web‐based block randomisation system with block sizes of ten |
| Allocation concealment (selection bias) | Low risk | Described for participants and clinicians as "masked to treatment allocation". The use of a web‐based system for randomization (and allocation) can be considered sufficient to mantain blinded the study personnel until the momment of assignment. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Treatment phase is declared to be double‐blinded, but no description for participants and personnel is provided. However, the subjects idea about study arm was measured, and study data was collected by independent groups, then presumably both participants and investigators were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Data for events and quality of life/economic analyses were collected by independent outcome adjudication groups |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Exclusions and withdrawals are described, and reason are reported |
| Selective reporting (reporting bias) | High risk | There are two sub studies (quality of life and health economics) besides the main study for the RIVER‐PCI trial, and all the main endpoints considered in the protocol were reported (the health economics sub study has not yet been published). Of note, for the events under study, time to event occurrence was stated to be the endpoint rather than the rate/incidence of the event; however, results for the secondary endpoint events were reported only as rate/incidence, and no data about time to event occurrence was provided |
| Other bias | High risk | The study was supported by Gilead Sciences and Menarini Group. Seven of the authors declare current or past financial relationships with Gilead Sciences. |
RWISE 2016.
| Methods | Study design: cross‐over trial Total study duration: recruitment was undertaken since 12 May 2011 to 10 Aug 2015, treatment phase duration was of 6 weeks (including 2 periods of treatment of 2 weeks and 1 period of washout of 1 week) Duration of follow‐up: 2 weeks Method of randomisation: performed at a 1:1 ratio blocked by clinical site, not further described Method of concealment of allocation: not mentioned Blinding: double‐blind, not described Power calculation: 90% power to detect a mean difference of 15 in SAQ score using a two‐sided t‐test at the 0.017 Holm‐Bonferroni corrected level of significance Phases of the study: 1 (treatment phase) Number of patients randomised: 142 Exclusions post‐randomisation: 4 participants were excluded because they received incomplete treatment Withdrawals (and reasons): number differ between the text (subject characteristics) and figure 1, data from the later was deemed to be more coherent. Five participants dropped‐out during ranolazine treatment and 4 during placebo washout (no dropouts during ranolazine washout), reasons not described |
|
| Participants | Total number: 128 Country of enrolment: USA Setting/location: not specified Diagnostic criteria (stable angina pectoris): chronic angina or its equivalent with coronary angiogram revealing coronary microvascular dysfunction (CMD) with no obstructive CAD (microvascular angina) Comorbidities: none Age (mean ± SD): 55.2 ± 9.2 years Gender (male %): 4% Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Number of intervention groups: 2 Concomitant medications: (permitted) anti‐anginals, antihypertensives, statins, hormone replacement therapy Excluded medications: those mentioned in exclusion criteria Placebo group Intervention: placebo twice daily Duration of intervention: 2 weeks Ranolazine group Intervention: ranolazine ER 500/1000 mg twice daily Duration of intervention: 2 weeks |
|
| Outcomes | Total number of outcomes:
Quality of life 1. Seattle Angina Questionnaire (SAQ and SAQ‐7)
2. Duke Activity Satuts Index (DASI)
3. QOL
Angina episodes frequency
Adverse events incidence
RESULTS Quality of life 1. Seattle Angina Questionnaire (SAQ)
2. Duke Activity Satuts Index (DASI)
3. QOL
Angina episodes frequency
Adverse events incidence
|
|
| Notes | Relevant observations for the data provided before: none Source of funding: unrestricted research grant from Gilead and contracts from several public (USA) entities Notable conflicts of interest: seven of the authors declare financial relationships with private pharmaceutical organisations (Gilead among others) and public (USA) entities |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation is stated but described only as "in a 1:1 ratio, blocked by clinical site" |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Study declared to be double‐blinded, but no description is provided |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Study declared to be double‐blinded, but no description is provided |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Exclusions and withdrawals are reported, but no reasons or explanations are provided. Furthermore, inconsistencies among the data provided were observed |
| Selective reporting (reporting bias) | Unclear risk | Protocol published in clinicaltrials.gov and as Online Exhibit adjoined to the results publication. Healthcare costs and biochemical parameters were not reported, adverse events incidence was reported but not included among the study outcomes in protocol |
| Other bias | High risk | The study was supported by Gilead and several public organisations. Seven authors declared financial relationships with private pharmaceutical organisations |
Sandhiya 2015.
| Methods | Study design: parallel‐group trial Total study duration: from 1 January 2012 to 11 April 2013 Duration of follow‐up: no follow‐up beyond treatment phase Method of randomisation: not described, performed in a 1:1 ratio Method of concealment of allocation: drugs distributed using sequentially numbered opaque sealed envelopes Blinding: not mentioned Power calculation: not mentioned Phases of the study: 1 (treatment phase) Number of patients randomised: 47 (24/23 for trimetazidine/ranolazine group) Exclusions post‐randomisation: not reported Withdrawals (and reasons): not reported |
|
| Participants | Total number: 40 Country of enrolment: Greece Setting/location: outpatient Diagnostic criteria (stable angina pectoris): history of exertional angina and CAD documented by coronary angiography (macrovascular angina) Comorbidities: diabetes mellitus Age (mean): 57.4 ± 9.1 / 58 ± 8.1 years for trimetazidine/ranolazine group Gender (male %): 83% (87.5/78.3 for trimetazidine/ranolazine group) Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Number of intervention groups: 2 Concomitant medications: statins Excluded medications: those mentioned in exclusion criteria Trimetazidine group
Ranolazine group
|
|
| Outcomes | Total number of outcomes: 1
Angina episodes frequency
RESULTS Angina episodes frequency
Adverse events The adverse events reported for the ranolazine group included angina, constipation, postural hypotension, headache, dizziness, nausea and weakness; for the trimetazidine group were constipation, weakness, palpitations, angina, dizziness, nausea, dyspepsia, headache, gastric discomfort and joint pain. |
|
| Notes | Relevant observations for the data provided before: none Source of funding: no external source of funding Notable conflicts of interest: the authors declare that they have no conflict of interest |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation is stated but described only as "in a 1:1 ratio" |
| Allocation concealment (selection bias) | Low risk | Study drugs were provided in sequentially numbered opaque sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Study declared to be double‐blinded, but no description is provided. However, it can be presumed that participants and personnel were blinded since drugs were provided in sealed envelopes |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Study declared to be double‐blinded, but no description is provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No exclusion or withdrawal is reported, we assume that all patients completed the study |
| Selective reporting (reporting bias) | Unclear risk | There is no published protocol. Results for all the outcomes stated in the "Methods" section of the paper are reported |
| Other bias | Low risk | The authors declare that the study was not supported by external source of funding. Also, they declared that they had no conflict of interest. |
Shammas 2015.
| Methods | Study design: cross‐over trial Total study duration: 16 weeks Duration of follow‐up: no follow‐up beyond treatment phase Method of randomisation: not described Method of concealment of allocation: not described Blinding: double‐blind, it is stated that the investigators and the patient remained blinded to the treatment until the completion of the trial, but no details are provided Power calculation: not performed (pilot study) Phases of the study: 1 (treatment phase) Number of patients randomised: 28 Exclusions post‐randomisation: 4 Withdrawals (and reasons): 5 (3 withdrew voluntarily, 1 lost to follow‐up, 1 withdrew involuntarily) (only 4 patients were excluded from the analysis) |
|
| Participants | Total number: 28 Country of enrolment: United States Setting/location: not specified Diagnostic criteria (stable angina pectoris): symptomatic (exertional angina or dyspnoea) ischaemic cardiomyopathy (ICM) with angiographic documentation of CAD (macrovascular angina) Comorbidities: none (not treatable by further revascularisation) Age (mean ± SD): 71.5 ± 8.4 years Gender (male %): 82.1% Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Number of intervention groups: 2 Concomitant medications: angiotensin‐converting enzyme inhibitor (ACEI) or angiotensin receptor blocker (ARB), a beta blocker, and at least one additional anti‐ischaemic drug (amlodipine or long‐acting nitrate) Excluded medications: none Placebo group Intervention: placebo Duration of intervention: 6 weeks (plus 2 weeks of washout) Ranolazine group Intervention: ranolazine (type of formulation not specified) 500 mg/1000 mg twice daily Duration of intervention: 6 weeks (plus 2 weeks of washout) |
|
| Outcomes | Total number of outcomes:
Quality of life 1. Seattle Angina Questionnaire (SAQ)
2. Rose Dyspnea Scale (RDS)
Adverse events incidence
RESULTS Quality of life 1. Seattle Angina Questionnaire (SAQ)
2. Rose Dyspnea Scale (SAQ)
Adverse events incidence
|
|
| Notes | Relevant observations for the data provided before: none Source of funding: research grant from Gilead Notable conflicts of interest: Dr Shammas is a speaker for and is on the advisory board of Gilead |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation is stated but not described |
| Allocation concealment (selection bias) | Unclear risk | It is stated that the investigators remained blinded to the treatment (allocation) until the completion of the trial, but no details are provided |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Study declared to be double‐blinded, with blinding corresponding to investigators and patients |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | A core laboratory blinded to patient treatment determined the SAQ scores |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Withdrawals and reasons are described; however, there is an inconsistency in the number of patients who did not complete the trial (5) and the number of patients excluded from the analysis (4) |
| Selective reporting (reporting bias) | Unclear risk | There is no published protocol. Results for all the outcomes stated in the "Methods" section of the paper are reported |
| Other bias | High risk | The study was supported by a research grant from Gilead Science. Dr Shammas is a speaker for and is on the advisory board of Gilead. |
Tagliamonte 2015.
| Methods | Study design: parallel‐group trial Total study duration: 8 weeks Duration of follow‐up: no follow‐up beyond treatment phase Method of randomisation: not described Method of concealment of allocation: not mentioned Blinding: double‐blind, not described Power calculation: not mentioned Phases of the study: 1 (treatment phase) Number of patients randomised: 58 (29/29 for placebo/ranolazine group) Exclusions post‐randomisation: none Withdrawals (and reasons): none |
|
| Participants | Total number: 58 Country of enrolment: Italy Setting/location: not specified Diagnostic criteria: sings and symptoms of myocardial ischaemia without obstructive CAD (microvascular angina) Comorbidities: none Age (mean ± SD): 66 ± 10 years Gender (male %): 67% Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Number of intervention groups: 2 Concomitant medications: aspirin Excluded medications: those mentioned in exclusion criteria Placebo group
Ranolazine group
|
|
| Outcomes | Total number of outcomes: 3 According to study protocol: no published protocol; according to the "Methods" section: 3 (coronary flow reserve, left ventricular ejection fraction, SAQ score) Reported: 3 Quality of life
RESULTS Quality of life
|
|
| Notes | Relevant observations for the data provided before: none Source of funding: not stated Notable conflicts of interest: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation is stated but not described |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Study declared to be double‐blinded, but no description is provided |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Study declared to be double‐blinded, but no description is provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | It is reported that no patient withdrew the study |
| Selective reporting (reporting bias) | Unclear risk | There is no published protocol. Results for all the outcomes stated in the "Methods" section of the paper are reported |
| Other bias | Unclear risk | The source of funding was not stated. Conflicts of interest were not stated. |
TERISA 2013.
| Methods | Study design: parallel‐group trial Total study duration: 12 weeks Duration of follow‐up: no follow‐up beyond treatment phase Method of randomisation: Interactive Voice/Web Response System (IVRS/IWRS) Method of concealment of allocation: blinded study drug bottle assigned by the IVRS/IWRS Blinding: double‐blind, participants and clinicians were blinded by using study drug bottles assigned by the IVRS/IWRS Power calculation: 90% to show a relative reduction of 20% in weekly angina frequency Phases of the study: 2 (qualifying phase, treatment phase) Number of patients randomised: 949 (476/473 for placebo/ranolazine group) Exclusions post‐randomisation: 22 (11 in the ranolazine arm, 11 in the placebo arm) Withdrawals (and reasons): 20 (9 withdrawals, 3 deaths in the ranolazine group, 11 withdrawals, 2 deaths in the placebo group) |
|
| Participants | Total number: 949 Country of enrolment: 14 (United States, Belarus, Bulgaria, Canada, Czech Republic, Georgia, Germany, Israel, Poland, Russian Federation, Serbia, Slovakia, Slovenia, Ukraine) Setting/location: not specified Diagnostic criteria (stable angina pectoris): at least a three‐month history of chronic stable angina that remain symptomatic despite treatment with 1 or 2 anti‐anginal, with documented history of CAD (macrovascular angina) Comorbidities: type 2 diabetes mellitus Age (mean ± SD): 64 ± 8.5 years Gender (male %): 61% Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Number of intervention groups: 2 Concomitant medications: Anti‐anginal: beta blockers, calcium channel blockers, long‐acting nitrates; other cardiovascular medications: statins, antiplatelet agents, ACE‐I/ARB; antidiabetic medications: glucose‐lowering medications, insulin Excluded medications: none (apart from those mentioned in the exclusion criteria) Placebo group
Ranolazine group
|
|
| Outcomes | Total number of outcomes:
All‐cause mortality
Quality of life 1. Medical Outcomes Short Form‐36 (SF‐36)
2. Patient’s Global Impression of Change (PGIC)
Acute myocardial infarction incidence (non‐fatal)
Angina episodes frequency
Adverse events incidence
RESULTS All‐cause mortality
Quality of life 1. Medical Outcomes Short Form‐36 (SF‐36)
2. Patient’s Global Impression of Change (PGIC)
Acute myocardial infarction incidence (non‐fatal)
Angina episodes frequency
Adverse events incidence:
|
|
| Notes | Relevant observations for the data provided before: none Source of funding: Gilead Sciences Notable conflicts of interest: all the authors have financial relationships with Gilead Sciences |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation undertaken by the IVRS/IWRS |
| Allocation concealment (selection bias) | Low risk | Intervention was provided to personnel in blinded study drug bottles assigned by the IVRS/IWRS |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Study declared to be double‐blinded, but no description is provided. Intervention was provided to personnel and patients in blinded drug bottles, so they were unaware of the treatment assigned |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Study declared to be double‐blinded, but no description about the blinding of data collectors/outcome adjudicators is provided |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Withdrawals are reported, but no description is provided |
| Selective reporting (reporting bias) | Low risk | According to the protocol published in Clinicaltrials.gov, results for all the outcomes are reported |
| Other bias | High risk | The study was supported by Gilead Sciences. All the authors have financial relationships with Gilead Sciences |
Thadani 1994.
| Methods | Study design: parallel‐group trial Total study duration: 35‐40 days Duration of follow‐up: no follow‐up beyond treatment phase Method of randomisation: not described Method of concealment of allocation: not mentioned Blinding: double‐blind, not described Power calculation: not mentioned Phases of the study: 2 (qualifying phase, treatment phase) Number of patients randomised: 319 (79, 81, 81 and 78 for placebo, ranolazine 30 mg, ranolazine 60 mg and ranolazine 120 mg groups) Exclusions post‐randomisation: 20 Withdrawals (and reasons): 31 (15 because of adverse events, new intercurrent illnesses, or new laboratory abnormalities, 4 because of unsatisfactory therapeutic response, 2 because of study administration problems, 10 for 'other' reasons (4 did not take study medication in compliance with the protocol, 2 elected to have surgical intervention, 2 declined to finish the study, 1 violated the protocol, and 1 required medication to control ventricular ectopy)). Withdrawals were similarly distributed among the study groups: 9/79, 9/81, 7/81 and 6/78 for placebo, ranolazine 30 mg, ranolazine 60 mg and ranolazine 120 mg groups |
|
| Participants | Total number: 319 Country of enrolment: United States Setting/location: not specified Diagnostic criteria: at least a 3‐month history of symptomatic chronic stable angina triggered by physical effort and relieved by rest or nitroglycerin Comorbidities: none Age (mean ± SD): 65 ± 8 years Gender (male %): 74.7%/80.2%/81.5%/79.5% for placebo/ranolazine 30 mg/ranolazine 60 mg/ranolazine 120 mg group Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Number of intervention groups: 4 Concomitant medications: (permitted) sublingual nitroglycerin (0.4mg tablets), taken for anginal pain and not as a prophylactic agent; hydrochlorothiazide and potassium supplementation for the treatment of hypertension Excluded medications: all anti‐anginal medication with the exception of sublingual nitroglycerin Placebo group
Ranolazine 30 mg group
Ranolazine 60 mg group
Ranolazine 120 mg group
|
|
| Outcomes | Total number of outcomes: 6 According to study protocol: no published protocol; according to the "Methods" section: 10 (change from baseline in ETT total exercise duration, time to onset of angina and time to 1‐mm ST segment depression (peak and through), change from baseline in the number and duration of ST segment depression episodes (by Holter monitoring), change from baseline in the weekly rate of NTG consumption and anginal attacks, adverse events incidence) Reported: 12 (including circulatory data at peak and through) Angina episodes frequency
Adverse events incidence
RESULTS Angina episodes frequency
Adverse events incidence
|
|
| Notes | Relevant observations for the data provided before: 'all patients' (intention‐to‐treat) (N = 299) and per‐protocol (N = 258) analysis were performed for ETT variables Source of funding: Syntex Research, Palo Alto, CA, USA Notable conflicts of interest: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation is stated but not described |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Study declared to be double‐blinded, but no description is provided |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Study declared to be double‐blinded, but no description is provided |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Withdrawals and reasons are provided and similarly distributed among the study groups, however, number of withdrawals is nearly 10% of total number of randomised patients |
| Selective reporting (reporting bias) | Unclear risk | There is no published protocol. Results for all the outcomes stated in the "Methods" section of the paper are reported |
| Other bias | High risk | The study was supported by Syntex Research. Authors did not state conflicts of interest. |
Villano 2013.
| Methods | Study design: parallel‐group trial Total study duration: 4 weeks Duration of follow‐up: no follow‐up beyond treatment phase Method of randomisation: computer‐generated table of random numbers Method of concealment of allocation: drugs were given to patients in anonymous drug packages by three of the authors who were not involved in the clinical assessment of patients. Cardiologists involved in the clinical and laboratory assessment of patients and/or analyses of data were blinded to the allocation of treatment Blinding: not mentioned, but presumably involving participants and data collectors/outcome adjudicators Power calculation: 90% to detect a significant difference of 15 points (SD 10) between each active drug versus placebo in any SAQ item and in the EuroQoL score Phases of the study: 1 (treatment phase) Number of patients randomised: 46 Exclusions post‐randomisation: not reported Withdrawals (and reasons): not reported |
|
| Participants | Total number: 46 Country of enrolment: Italy Setting/location: ambulatory patients Diagnostic criteria (stable angina pectoris): history of typical effort angina with exercise‐induced ST‐segment depression ≥ 1 mm, normal coronary angiography and absence of any specific cardiac disease including vasospastic angina (microvascular angina) which remains symptomatic despite anti‐ischaemic therapy Comorbidities: none Age (mean, interval): 57 ± 12/57 ± 11/60 ± 9 years for ivabradine/ranolazine/placebo group Gender (male %): 2/16, 3/15, 4/15 for ivabradine‐ranolazine‐placebo group Inclusion criteria:
Exclusion criteria: Not described |
|
| Interventions | Number of intervention groups: 3 Concomitant medications: anti‐anginals, antihypertensives, anti‐aggregants, statins Excluded medications: none Placebo group
Ivabradine group
Ranolazine group
|
|
| Outcomes | Total number of outcomes:
Quality of life 1. Seattle Angina Questionnaire (SAQ)
2. EuroQoL visual analogue scale (VAS)
Adverse events incidence
RESULTS Quality of life 1. Seattle Angina Questionnaire (SAQ)
2. EuroQoL visual analogue scale (VAS)
Adverse events incidence
|
|
| Notes | Relevant observations for the data provided before: none Source of funding: not stated Notable conflicts of interest: no conflicts of interest to disclose |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated table of random numbers |
| Allocation concealment (selection bias) | Low risk | Drugs were given to patients in anonymous drug packages by three of the authors who were not involved in the clinical assessment of patients. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding design is not stated. It is mentioned that study drugs were provided in anonymous packages, so it could be assumed that patients and personnel were blinded to the allocation of treatment |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding design is not stated. It is mentioned that the cardiologists involved in the clinical and laboratory assessment of patients and/or analyses of data were blinded to the allocation of treatment. However, for outcomes such as quality of life, blinding measures have not been described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No exclusions or withdrawals were reported. We assumed that all patients completed the study |
| Selective reporting (reporting bias) | Unclear risk | There was no published protocol. Results for all outcomes in the 'Methods' section were reported |
| Other bias | Unclear risk | Funding source not stated. The authors declared no conflicts of interest |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Arnold 2014 | Substudy of RCT: substudy of the TERISA 2013 trial on quality of life |
| Cocco 1992 | Wrong intervention: ranolazine given in single dose |
| Coleman 2015 | Not RCT: health economics study not conducted alongside a RCT |
| Hidalgo‐Vega 2014 | Not RCT: health economics study not conducted alongside a RCT |
| Jain 1990 | Not RCT: three‐period cross‐over trial with only one group of participants, no randomisation method stated |
| Kohn 2014 | Not RCT: health economics study not conducted alongside a RCT |
| Lucioni 2009 | Not RCT: health economics study not conducted alongside a RCT |
| Rehberger‐Likozar 2015 | No angina population: condition studied did not meet inclusion criteria |
| Rich 2007 | Substudy of RCT: subgroup analysis of the CARISA 2004 and ERICA 2006 trials |
| ROLE 2007 | Not RCT: open‐label follow‐up study of the CARISA 2004 and MARISA 2004 trials, including only participants treated with ranolazine (without comparator) |
Characteristics of studies awaiting assessment [ordered by study ID]
NCT01304095.
| Methods | Study design: parallel‐group trial Duration of follow‐up: 6 months Method of randomisation: not described Method of concealment of allocation: not described Blinding: open‐label Power calculation: not mentioned Phases of the study: 1 (treatment phase) |
| Participants | Total number: 160 (estimated) Country of enrolment: USA Setting/location: not specified Diagnostic criteria (stable angina pectoris): symptoms of angina with evidence of stable CAD (macrovascular angina) Comorbidities: metabolic syndrome Inclusion criteria:
|
| Interventions | Number of intervention groups: 2 Concomitant medications: standard medical therapy Excluded medications: those mentioned in exclusion criteria Control group Intervention: no treatment Duration of intervention: 6 months Ranolazine group Intervention: ranolazine (type of formulation not specified) 500/1000 mg twice daily Duration of intervention: 6 months |
| Outcomes | Total number of outcomes: 6 (ETT parameters, fasting glucose, angina (SAQ scale), concomitant medications, lipid profile, HbA1c) OUTCOMES No outcome meets inclusion criteria |
| Notes |
Tagarakis 2013.
| Methods | Study design: parallel‐group trial Duration of follow‐up: not reported Method of randomisation: not described Method of concealment of allocation: not described Blinding: single‐blind (outcome assessors) Power calculation: not mentioned Phases of the study: 1 (treatment phase) |
| Participants | Country of enrolment: Greece Setting/location: inpatient Diagnostic criteria (stable angina pectoris): not described Comorbidities: patients scheduled for elective on‐pump CABG Inclusion criteria: not described Exclusion criteria: not described |
| Interventions | Number of intervention groups: 2 Concomitant medications: not described Excluded medications: not described Control group Intervention: no treatment Duration of intervention: not reported Ranolazine group Intervention: ranolazine (type of formulation not specified) 375 mg twice daily for 3 days prior to surgery and until discharge Duration of intervention: not reported |
| Outcomes | Total number of outcomes: 3 (post‐operative atrial fibrillation, left atrial diameter, left ventricular ejection fraction) OUTCOMES No outcome meets the inclusion criteria |
| Notes |
Tian 2012.
| Methods | Study design: parallel‐group trial Duration of follow‐up: Method of randomisation: not described Method of concealment of allocation: not described Blinding: not mentioned Power calculation: not mentioned Phases of the study: 1 (treatment phase) |
| Participants | Total number: 86 Country of enrolment: China Setting/location: not specified Diagnostic criteria (stable angina pectoris): not described Comorbidities: none Inclusion criteria: not described Exclusion criteria: not described |
| Interventions | Number of intervention groups: 2 Concomitant medications: diltiazem Excluded medications: not described Control group Intervention: no treatment Duration of intervention: not reported Ranolazine group Intervention: ranolazine (type of formulation not specified) (dosage not reported) Duration of intervention: not reported |
| Outcomes | Total number of outcomes: 2 (ECG total effective rate, adverse events incidence) OUTCOMES Adverse events incidence Outcome definition: not described Method and unit of measurement: absolute frequency Time points to report: not reported |
| Notes |
Wang 2012.
| Methods | Study design: parallel‐group trial Duration of follow‐up: 8 weeks Method of randomisation: not described Method of concealment of allocation: not described Blinding: not mentioned Power calculation: not mentioned Phases of the study: 1 (treatment phase) |
| Participants | Country of enrolment: China Setting/location: not specified Diagnostic criteria (stable angina pectoris): stable angina with coronary heart disease (macrovascular angina) Comorbidities: none Inclusion criteria: not described Exclusion criteria: not described |
| Interventions | Number of intervention groups: 3 Concomitant medications: conventional therapy (aspirin, cholesterol lowering agents, metoprolol) Excluded medications: not described Placebo group Intervention: placebo Duration of intervention: 8 weeks Ranolazine 500 mg group Intervention: ranolazine SR 500 mg twice daily Duration of intervention: 8 weeks Ranolazine 1000 mg group Intervention: ranolazine SR 1000 mg twice daily Duration of intervention: 8 weeks |
| Outcomes | Total number of outcomes: 5 (angina frequency, nitroglycerin consumption frequency, ECG total effective rate, ADR, liver/kidney function) OUTCOMES Angina episodes frequency Outcome definition: not described Method and unit of measurement: not described Time points to report: 8 weeks |
| Notes |
Characteristics of ongoing studies [ordered by study ID]
Calcagno 2014.
| Trial name or title | Not stated |
| Methods | Study design: parallel‐group trial Duration of follow‐up: 12 months Method of randomisation: not described Method of concealment of allocation: not described Blinding: not mentioned Power calculation: not mentioned Phases of the study: 2 (treatment phase, follow‐up phase) |
| Participants | Country of enrolment: not described Setting/location: not specified Diagnostic criteria (stable angina pectoris): not described Comorbidities: percutaneous coronary intervention plus stent implantation Inclusion criteria: not described Exclusion criteria: not described |
| Interventions | Number of intervention groups: 2 Concomitant medications: medical therapy (not described) Excluded medications: not mentioned No treatment group Intervention: none Duration of intervention: 30 days Ranolazine group Intervention: ranolazine (type of formulation not specified) (dose not reported) Duration of intervention: 30 days |
| Outcomes | Total number of outcomes: 5 (ETT parameters, symptoms, arrhythmia, angina during moderate exercises, re‐hospitalisation) OUTCOMES No outcome appears to meet the inclusion criteria |
| Starting date | Not reported |
| Contact information | Not provided |
| Notes |
Calcagno 2015.
| Trial name or title | Not stated |
| Methods | Study design: parallel‐group trial Duration of follow‐up: 12 months Method of randomisation: not described Method of concealment of allocation: not described Blinding: not mentioned Power calculation: not mentioned Phases of the study: 2 (treatment phase, follow‐up phase) |
| Participants | Country of enrolment: not described Setting/location: not specified Diagnostic criteria (stable angina pectoris): not described Comorbidities: percutaneous coronary intervention plus stent implantation Inclusion criteria: not described Exclusion criteria: not described |
| Interventions | Number of intervention groups: 3 Concomitant medications: standard therapy (not described) Excluded medications: not mentioned No treatment group Intervention: none Duration of intervention: 30 days Ivabradine group Intervention: ivabradine (dose not reported) Duration of intervention: 30 days Ranolazine group Intervention: ranolazine (type of formulation not specified) (dose not reported) Duration of intervention: 30 days |
| Outcomes | Total number of outcomes: 3 (ETT parameters, weekly angina during daily moderate exercises, re‐hospitalisation) OUTCOMES No outcome meets the inclusion criteria |
| Starting date | Not reported |
| Contact information | Not provided |
| Notes |
CTRI/2014/01/004332.
| Trial name or title | CTRI/2014/01/004332 |
| Methods | Study design: parallel‐group trial Duration of follow‐up: 8 weeks Method of randomisation: computer generated randomisation Method of concealment of allocation: sequentially numbered, sealed, opaque envelopes Blinding: not specified ("Investigator Blinded") Power calculation: not mentioned Phases of the study: 1 (treatment phase) |
| Participants | Total number: 50 (estimated) Country of enrolment: India Setting/location: not specified Diagnostic criteria (stable angina pectoris): not described Comorbidities: sustained STEMI Inclusion criteria:
Exclusion criteria:
|
| Interventions | Number of intervention groups: 2 Concomitant medications: those indicated by the patient's cardiologist Excluded medications: not mentioned Trimetazidine group Intervention: trimetazidine 35 mg twice daily Duration of intervention: 8 weeks Ranolazine group Intervention: ranolazine (type of formulation not specified) 500 mg twice daily Duration of intervention: 8 weeks |
| Outcomes | Total number of outcomes: 3 (changes in LV systolic and diastolic function, improvement in angina symptoms, ADR) OUTCOMES No outcome meets the inclusion criteria |
| Starting date | 28/10/2013 |
| Contact information | Dr Melvin George Assistant Professor Cardiac Research SRM Medical College Hospital Dept of Cardiology SRM MCH RC Kattankulathur Kancheepuram Kancheepuram TAMIL NADU 603203 India 9894133697 melvingeorge2003@gmail.com |
| Notes | Source of funding: SRM Medical College Hospital |
EUCTR 2011‐001278‐24.
| Trial name or title | 2011‐001278‐24 / MEIN/10/Ran‐Cad/003 |
| Methods | Study design: parallel‐group trial Duration of follow‐up: 24 weeks Method of randomisation: not described Method of concealment of allocation: not described Blinding: double‐blind Power calculation: not mentioned Phases of the study: 1 (treatment phase) |
| Participants | Total number: 1460 (estimated) Country of enrolment: Greece, Switzerland Setting/location: not specified Diagnostic criteria (stable angina pectoris): exercise angina in patients with CAD (macrovascular angina) Comorbidities: none Inclusion criteria:
Exclusion criteria:
|
| Interventions | Number of intervention groups: 2 Concomitant medications: not described Excluded medications: those mentioned in exclusion criteria Placebo group Intervention: placebo Duration of intervention: 24 weeks Ranolazine group Intervention: ranolazine (type of formulation not specified) 750 mg twice daily Duration of intervention: 24 weeks |
| Outcomes | Total number of outcomes: 5 (ETT parameters, angina frequency, nitroglycerin consumption frequency, adverse events incidence, laboratory findings) OUTCOMES Angina episodes frequency Outcome definition: weekly frequency Method and unit of measurement: number/week Time points to report: 4, 12 and 24 weeks Adverse events incidence Outcome definition: not described Method and unit of measurement: absolute frequency Time points to report: 4, 12 and 24 weeks |
| Starting date | Not reported |
| Contact information | Study Medical Expert (SME) Via Walter Tobagi, 8 Peschiera Borromeo ‐ Italy 003902516555236 dzava@lusofarmaco.it |
| Notes | Source of funding: Menarini International Operations Luxembourg SA |
EUCTR 2012‐001584‐77.
| Trial name or title | EUCTR2012‐001584‐77‐DE / MEIN/10/Ran‐PCI/005 |
| Methods | Study design: parallel‐group trial Duration of follow‐up: 5 weeks Method of randomisation: not described Method of concealment of allocation: not described Blinding: double‐blind Power calculation: not mentioned Phases of the study: 1 (treatment phase) |
| Participants | Country of enrolment: Germany Setting/location: not specified Diagnostic criteria (stable angina pectoris):history of chronic stable angina and coronary stenosis by angiography (macrovascular angina) Comorbidities: none Inclusion criteria:
Exclusion criteria:
|
| Interventions | Number of intervention groups: 2 Concomitant medications: standard therapy (beta‐blocker and/or calcium channel blocker) Excluded medications: those mentioned in exclusion criteria Placebo group Intervention: film‐coated tablet Duration of intervention: 5 weeks Ranolazine group Intervention: ranolazine ER (dose not reported) Duration of intervention: 5 weeks |
| Outcomes | Total number of outcomes: 2 (changes of the wall motion abnormalities, heart’s perfusion deficit and related variables) OUTCOMES No outcome meets the inclusion criteria |
| Starting date | Not reported |
| Contact information | Dr. Notghi Contract Research GmbH Zimmerstraße 55 ‐ Berlin 004903046064780 eraser@notghi.com |
| Notes | Source of funding: Menarini International Operations Luxembourg S.A. Reported as Prematurely ended |
Gupta 2014.
| Trial name or title | Not stated |
| Methods | Study design: parallel‐group Duration of follow‐up: 6 weeks Method of randomisation: not described Method of concealment of allocation: not described Blinding: not specified Power calculation: not mentioned Phases of the study: 1 (treatment phase) |
| Participants | Total number: 185 Country of enrolment: India Setting/location: not specified Diagnostic criteria (stable angina pectoris): not described Comorbidities: type 2 diabetes Inclusion criteria: not described Exclusion criteria: not described |
| Interventions | Number of intervention groups: 2 Concomitant medication: 1 or 2 anti‐anginals Excluded medications: not described Placebo group Intervention: placebo Duration of intervention: 6 weeks Ranolazine group Intervention: ranolazine (type of formulation not specified) (dose not reported) Duration of intervention: 6 weeks |
| Outcomes | Total number of outcomes: 2 (weekly angina frequency, weekly sublingual nitrate use) OUTCOMES Angina episodes frequency Outcome definition: not described Method and unit of measurement: number per week Time points reported: 6 weeks |
| Starting date | Not reported |
| Contact information | Not provided |
| Notes |
NCT01495520.
| Trial name or title | NCT01495520 |
| Methods | Study design: cross‐over trial Duration of follow‐up: 30 days Method of randomisation: not described Method of concealment of allocation: not described Blinding: double‐blind (Subject, Caregiver, Investigator) Power calculation: not mentioned Phases of the study: 1 (treatment phase) |
| Participants | Total number: 100 (estimated) Country of enrolment: Italy Setting/location: not specified Diagnostic criteria (stable angina pectoris): coronary artery disease (macrovascular angina) Comorbidities: none Inclusion criteria:
Exclusion criteria:
|
| Interventions | Number of intervention groups: 2 Concomitant medications: not described Excluded medications: not described Placebo group Intervention: placebo Duration of intervention: 30 days Ranolazine group Intervention: ranolazine (type of formulation not specified) 750 mg twice daily Duration of intervention: 30 days |
| Outcomes | Total number of outcomes: 2 (occurrence of symptoms of palpitations, occurrence of arrhythmia in case of symptoms of palpitations) OUTCOMES No outcome meets the inclusion criteria |
| Starting date | January 2014 |
| Contact information | Francesco Pelliccia, MD +39064997 f.pelliccia@mclink.it |
| Notes | Source of funding: University of Roma La Sapienza |
NCT01558830.
| Trial name or title | NCT01558830 |
| Methods | Study design: parallel‐group Duration of follow‐up: 3 months Method of randomisation: not described Method of concealment of allocation: not described Blinding: single‐blind (subject) Power calculation: not mentioned Phases of the study: 1 (treatment phase) |
| Participants | Country of enrolment: United States Setting/location: not specified Diagnostic criteria (stable angina pectoris): history of stable angina Comorbidities: other cardiac conditions (such as atrial fibrillation) Inclusion criteria:
Exclusion criteria:
|
| Interventions | Number of intervention groups: 2 Concomitant medications: amiodarone Excluded medications: not described Placebo group Intervention: sugar pill Duration of intervention: 3 months Ranolazine group Intervention: ranolazine (type of formulation not specified) 500/1000 mg twice daily Duration of intervention: 3 months |
| Outcomes | Total number of outcomes: 6 (ventricular arrhythmia burden, atrial arrhythmia burden, QTc interval measurement, hospitalisation rate, syncope hospitalisation rate, liver function assay) OUTCOMES No outcomes meets inclusion criteria |
| Starting date | Not reported |
| Contact information | Erik J Sirulnick, MD 702‐731‐8224 erikmd@me.com |
| Notes | Source of funding: Cardiovascular Consultants of Nevada, Gilead Sciences |
NCT01754259.
| Trial name or title | NCT01754259 |
| Methods | Study design: cross‐over trial Duration of follow‐up: 4 weeks Method of randomisation: not described Method of concealment of allocation: "labelled" bottles provided by the sponsor Blinding: double‐blind (Subject, Investigator) Power calculation: not mentioned Phases of the study: 1 (treatment phase) |
| Participants | Total number: 70 (estimated) Country of enrolment: United States Setting/location: not specified Diagnostic criteria (stable angina pectoris): symptoms of stable angina and CAD (criteria for diagnosis not described) (macrovascular angina) Comorbidities: type 1 and 2 diabetes mellitus Inclusion criteria:
Exclusion criteria:
|
| Interventions | Number of intervention groups: 2 Concomitant medications: anti‐anginals (not described) Excluded medications: those mentioned in exclusion criteria Placebo group Intervention: placebo Duration of intervention: 4 weeks Ranolazine group Intervention: ranolazine (type of formulation not specified) (dosage not reported) Duration of intervention: 4 weeks |
| Outcomes | Total number of outcomes: 9 (post‐exercise coronary vasodilator reserve, symptoms of exertional angina and/or dyspnoea (SAQ/RDS scales), left ventricular systolic function, post‐exercise global myocardial blood flow, post‐exercise global coronary vascular resistance, serum biomarkers of myocardial strain, LV diastolic function, correlation between multimodality imaging parameters) OUTCOMES No outcome meets the inclusion criteria |
| Starting date | April 2013 |
| Contact information | Ron Blankstein, MDBrigham and Women's Hospital |
| Notes | Source of funding: Brigham and Women's Hospital |
NCT01948310.
| Trial name or title | NCT01948310 |
| Methods | Study design: parallel‐group Duration of follow‐up: not stated Method of randomisation: not described Method of concealment of allocation: not described Blinding: double‐blind (Subject, Caregiver, Investigator, Outcomes Assessor) Power calculation: not mentioned Phases of the study: 1 (treatment phase) |
| Participants | Total number: 40 (estimated) Country of enrolment: United States Setting/location: not specified Diagnostic criteria (stable angina pectoris): stable angina for at least 3 months with documented CAD (macrovascular angina) Comorbidities: none Inclusion criteria:
Exclusion criteria:
Contraindicated medications
|
| Interventions | Number of intervention groups: 2 Concomitant therapy: aerobic exercise three times per week, 45 minutes per session at an intensity of 10‐20 beats per minute below the angina threshold Excluded medications: those mentioned in exclusion criteria Placebo group Intervention: placebo Duration of intervention: 13 weeks (not explicitly stated) Ranolazine group Intervention: ranolazine (type of formulation not specified) 1000 mg twice daily Duration of intervention: 13 weeks (not explicitly stated) |
| Outcomes | Total number of outcomes: 3 (change in peak oxygen consumption, change in treatment satisfaction (SAQ scale), change in total daily energy expenditure) Adverse events incidence No outcome meets the inclusion criteria |
| Starting date | December 2013 |
| Contact information | Leslie H Willis, MS 9196606782 leslie.willisduke.edu |
| Notes | Source of funding: Duke University, Gilead Sciences |
NCT02052011.
| Trial name or title | NCT02052011 |
| Methods | Study design: parallel‐group trial Duration of follow‐up: 4 weeks Method of randomisation: not described Method of concealment of allocation: not described Blinding: double‐blind (Subject, Caregiver, Investigator, Outcomes Assessor) Power calculation: not mentioned Phases of the study: 1 (treatment phase) |
| Participants | Total number: 30 (estimated) Country of enrolment: United States Setting/location: emergency patients Diagnostic criteria (stable angina pectoris): microvascular angina Comorbidities: none Inclusion criteria:
Exclusion criteria:
|
| Interventions | Number of intervention groups: 2 Concomitant medications: not mentioned Excluded medications: those mentioned in exclusion criteria Placebo group Intervention: placebo Duration of intervention: 4 weeks Ranolazine group Intervention: ranolazine ER 1000 mg twice daily (up‐titrated from 500 mg twice daily for 1 week) Duration of intervention: 4 weeks |
| Outcomes | Total number of outcomes: 3 (Coronary Flow Reserve, quality of life (SAQ scale), return visits) OUTCOMES Quality of life Outcome definition: not described Method and unit of measurement: score Time points reported: 4 weeks |
| Starting date | April 2014 |
| Contact information | Matthew Naftilan, MS 203‐785‐4676 matthew.naftilan@yale.edu |
| Notes | Source of funding: Yale University, Gilead Sciences |
NCT02147067.
| Trial name or title | NCT02147067 |
| Methods | Study design: parallel‐group trial Duration of follow‐up: 12 weeks Method of randomisation: not described Method of concealment of allocation: not described Blinding: double‐blind (Subject, Caregiver, Investigator, Outcomes Assessor) Power calculation: not mentioned Phases of the study: 1 (treatment phase) |
| Participants | Total number: 50 (estimated) Country of enrolment: not described Setting/location: not specified Diagnostic criteria (stable angina pectoris): symptoms of stable angina and no evidence of CAD (microvascular angina) Comorbidities: none Inclusion criteria:
Exclusion criteria:
|
| Interventions | Number of intervention groups: 2 Concomitant medications: not described Excluded medications: not described Placebo group Intervention: placebo Duration of intervention: 12 weeks Ranolazine group Intervention: ranolazine (type of formulation not specified) 1000 mg twice daily Duration of intervention: 12 weeks |
| Outcomes | Total number of outcomes: 5 (quality of life (SAQ scale), peak rate of oxygen consumption, ETT parameters, Coronary Flow Velocity Reserve (CFR), Hyperemic Microcirculatory Resistance (HMR)) OUTCOMES Quality of life Outcome definition: Seattle Angina Questionnaire score regarding angina frequency, physical functioning, treatment satisfaction, angina stability, and quality of life Method and unit of measurement: score (change from baseline) Time points reported: 12 weeks |
| Starting date | September 2014 |
| Contact information | Habib Samady, MD (404) 778‐1237 hsamady@emory.edu |
| Notes | Source of funding: Emory University, Gilead Sciences |
NCT02147834.
| Trial name or title | NCT02147834 |
| Methods | Study design: parallel‐group trial Duration of follow‐up: 4 months Method of randomisation: not described Method of concealment of allocation: not described Blinding: double‐blind (Subject, Caregiver, Investigator, Outcomes Assessor) Power calculation: not mentioned Phases of the study: 1 (treatment phase) |
| Participants | Total number: 250 (estimated) Country of enrolment: United States Setting/location: not specified Diagnostic criteria (stable angina pectoris): not described, patients deferred from having a PCI Comorbidities: none Inclusion criteria:
Exclusion criteria:
|
| Interventions | Number of intervention groups: 2 Concomitant medications: not described Excluded medications: those mentioned in exclusion criteria Placebo group Intervention: sugar pill twice daily Duration of intervention: 16 weeks Ranolazine group Intervention: ranolazine (type of formulation not specified) 1000 mg twice daily (up‐titrated from 500 mg twice daily for 1 week) Duration of intervention: 16 weeks |
| Outcomes | Total number of outcomes: 3 (quality of life (SAQ scale), subjective well being, ischemia‐driven revascularisation or hospitalisation) OUTCOMES Quality of life Outcome definition: the Seattle Angina Questionnaire is a valid and reliable instrument that measures five clinically important dimensions of health in patients with CAD (physical limitation, anginal stability, anginal frequency, treatment satisfaction, and disease perception) Method and unit of measurement: score (change from baseline) Time points reported: 4 months Need for revascularisation procedure Outcome definition: frequency of the number of reported adverse events for ischaemia driven revascularisation Method and unit of measurement: absolute frequency Time points reported: 4 months |
| Starting date | August 2015 |
| Contact information | Anthony A Bavry, MD MPH 352‐376‐1611 ext 4726 anthony.bavry@va.gov |
| Notes | Source of funding: North Florida Foundation for Research and Education, Gilead Sciences, University of Florida |
NCT02252406.
| Trial name or title | NCT02252406 |
| Methods | Study design: parallel‐group Duration of follow‐up: 24 weeks Method of randomisation: not described Method of concealment of allocation: not described Blinding: double‐blind (Subject, Caregiver, Investigator, Outcomes Assessor) Power calculation: not mentioned Phases of the study: 1 (treatment phase) |
| Participants | Total number: 40 (estimated) Country of enrolment: not described Setting/location: not specified Diagnostic criteria (stable angina pectoris): symptoms of chronic stable angina and evidence of CAD (macrovascular angina) Comorbidities: Metabolic Syndrome Inclusion criteria:
Exclusion criteria:
Additional exclusion
|
| Interventions | Number of intervention groups: 2 Concomitant medications: not described Excluded medications: those mentioned in exclusion criteria Placebo group Intervention: placebo Duration of intervention: 24 weeks Ranolazine group Intervention: ranolazine (type of formulation not specified) 500 mg twice daily Duration of intervention: 24 weeks |
| Outcomes | Total number of outcomes: (angina frequency (SAQ scale), biomarkers) OUTCOMES No outcome meets the inclusion criteria |
| Starting date | September 2015 |
| Contact information | Gladys Velarde, MD 904‐244‐43095 gladys.velarde@jax.ufl.edu |
| Notes | Source of funding: University of Florida |
NCT02265796.
| Trial name or title | NCT02265796 |
| Methods | Study design: parallel‐group trial Duration of follow‐up: 16 weeks Method of randomisation: not described Method of concealment of allocation: not described Blinding: double‐blind (Subject, Caregiver, Investigator, Outcomes Assessor) Power calculation: not mentioned Phases of the study: 1 (treatment phase) |
| Participants | Total number: 50 (estimated) Country of enrolment: United States Setting/location: not specified Diagnostic criteria (stable angina pectoris): not described Comorbidities: none Inclusion criteria:
Exclusion criteria:
|
| Interventions | Number of intervention groups: 2 Concomitant medications: not described Excluded medications: those mentioned in exclusion criteria Placebo group Intervention: sugar pill twice daily Duration of intervention: 16 weeks Ranolazine group Intervention: ranolazine (type of formulation not specified) 1000 mg twice daily (up‐titrated from 500 mg twice daily for 1 week) Duration of intervention: 16 weeks |
| Outcomes | Total number of outcomes: 3 (quality of life (SAQ scale), subjective well being, ischemia‐driven revascularisation or hospitalisation) OUTCOMES Quality of life Outcome definition: the Seattle Angina Questionnaire is a valid and reliable instrument that measures five clinically important dimensions of health in patients with coronary artery disease (physical limitation, anginal stability, anginal frequency, treatment satisfaction, and disease perception) Method and unit of measurement: score (change from baseline) Time points reported: 16 weeks Need for revascularisation procedure Outcome definition: frequency of the number of reported adverse events for ischaemia driven revascularisation Method and unit of measurement: absolute frequency Time points reported: 16 weeks |
| Starting date | September 2014 |
| Contact information | Anthony A Bavry, MD, MPH 352‐376‐1611 ext 4726 anthon.bavry@va.gov |
| Notes | Source of funding: North Florida Foundation for Research and Education, Gilead Sciences |
NCT02423265.
| Trial name or title | NCT02423265 |
| Methods | Study design: parallel‐group trial Duration of follow‐up: 9 weeks Method of randomisation: not described Method of concealment of allocation: not described Blinding: Double‐blind (Subject, Investigator, Outcomes Assessor) Power calculation: not mentioned Phases of the study: 2 (treatment phase, follow‐up phase) |
| Participants | Country of enrolment: not described Setting/location: not specified Diagnostic criteria (stable angina pectoris): not described Comorbidities: percutaneous coronary intervention plus stent implantation Inclusion criteria:
Exclusion criteria:
|
| Interventions | Number of intervention groups: 3 Concomitant medications: standard therapy (not described) Excluded medications: not mentioned Placebo group Intervention:placebo with up‐titration after 1 week Duration of intervention: 9 weeks Ranolazine group Intervention: ranolazine (type of formulation not specified) 1000 mg twice daily (up‐titrated after 1‐week 500 mg twice daily) Duration of intervention: 9 weeks |
| Outcomes | Total number of outcomes: 4 (cardiac MRI strain, dobutamine wall motion scoring index, quality of life, ETT parameters) OUTCOMES Quality of life Outcome definition: measured with 3 scales (SAQ, DASI, SF‐12) Method and unit of measurement: total score Time points to report: 9 weeks |
| Starting date | June 2015 |
| Contact information | Ashesh N Buch, MB.ChB, M.D. bucha@ecu.edu |
| Notes |
Šebeštjen 2014.
| Trial name or title | |
| Methods | Study design: parallel‐group trial Duration of follow‐up: 12 weeks Method of randomisation: not described Method of concealment of allocation: not described Blinding: double‐blind Power calculation: not mentioned Phases of the study: 1 (treatment phase) |
| Participants | Total number: 52 Country of enrolment: not mentioned Setting/location: not specified Diagnostic criteria (stable angina pectoris): not described Comorbidities: none Inclusion criteria: not described Exclusion criteria: not described |
| Interventions | Number of intervention groups: 2 Concomitant medications: not described Excluded medications: not described Trimetazidine group Intervention: trimetazidine 35 mg twice daily Duration of intervention: 12 weeks Ranolazine group Intervention: ranolazine (type of formulation not specified) 375/500 mg twice daily Duration of intervention: 12 weeks |
| Outcomes | Total number of outcomes: 2 (flow‐mediated (endothelium‐dependent) dilation (FMD) of brachial artery, nitroglycerin‐induced (endothelium‐independent) (GTN) dilation of brachial artery) OUTCOMES No outcomes meets inclusion criteria |
| Starting date | Not reported |
| Contact information | Not provided |
| Notes |
Differences between protocol and review
We changed the minimal duration of follow‐up of outcome measures for inclusion in analysis to one week from six weeks in the protocol, and performed an additional sensitivity analysis restricting to results of outcomes measured with a follow‐up of at least six weeks. We included an additional outcome regarding exercise electrocardiogram (time to 1‐mm ST‐segment depression, measured at peak) as a secondary effectiveness outcome. We added type of stable angina diagnosis (macrovascular versus microvascular) as an additional variable for subgroup analysis. We calculated some missing data using formulae from the Cochrane Handbook, but did not perform imputation of any missing data. Thus, we did not perform sensitivity analysis regarding the method of dealing with missing data.
Contributions of authors
CS: selection of studies, data extraction, risk of bias assessment, data analysis, writing the final review.
JB: selection of studies, data extraction, risk of bias assessment, data analysis, writing the final review.
JM: selection of studies, data extraction.
LV: selection of studies, data extraction, risk of bias assessment, writing the final review.
DR: data extraction.
CL: data analysis.
Sources of support
Internal sources
-
None, Other.
No sources of support supplied
External sources
-
None, Other.
No sources of support supplied
Declarations of interest
None of the authors received any payment or service from a third party (government, private foundation, other) for any aspect of the submitted work. Relationships that were present during the 36 months prior to publication are reported. None of the authors have any patent, either planned, pending or issued, broadly relevant to the work or have any other relationships or activities that readers could perceive to have influenced, or that give the appearance of potentially influencing, this review.
Edited (no change to conclusions)
References
References to studies included in this review
Babalis 2015 {published data only (unpublished sought but not used)}
- Babalis D, Tritakis V, Floros G, Mouzarou A, Kafkas N, Bampali K, et al. Effects of ranolazine on left ventricular diastolic and systolic function in patients with chronic coronary disease and stable angina. Hellenic Journal of Cardiology 2015;56(3):237‐41. [PubMed] [Google Scholar]
CARISA 2004 {published data only (unpublished sought but not used)}
- Chaitman BR, Pepine CJ, Parker JO, Skopal J, Chumakova G, Kuch J, et al Combination Assessment of Ranolazine In Stable Angina (CARISA) Investigators. Effects of ranolazine with atenolol, amlodipine, or diltiazem on exercise tolerance and angina frequency in patients with severe chronic angina: a randomized controlled trial. JAMA 2004;291(3):309‐16. [DOI] [PubMed] [Google Scholar]
ERICA 2006 {published data only (unpublished sought but not used)}
- Stone PH, Gratsiansky NA, Blokhin A, Huang IZ, Meng L. ERICA Investigators. Antianginal efficacy of ranolazine when added to treatment with amlodipine: the ERICA (Efficacy of Ranolazine in Chronic Angina) trial. Journal of the American College of Cardiology 2006;48(3):566‐75. [DOI] [PubMed] [Google Scholar]
MARISA 2004 {published data only (unpublished sought but not used)}
- Chaitman BR, Skettino SL, Parker JO, Hanley P, Meluzin J, Kuch J, et al. MARISA Investigators. Anti‐ischemic effects and long‐term survival during ranolazine monotherapy in patients with chronic severe angina. Journal of the American College of Cardiology 2004;43(8):1375‐82. [DOI] [PubMed] [Google Scholar]
Mehta 2011 {published data only (unpublished sought but not used)}
- Mehta PK, Goykhman P, Thomson LE, Shufelt C, Wei J, Yang Y, et al. Ranolazine improves angina in women with evidence of myocardial ischemia but no obstructive coronary artery disease. JACC. Cardiovascular Imaging 2011;4(5):514‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
MERLIN‐TIMI 36 2007 {published data only (unpublished sought but not used)}
- Wilson SR, Scirica BM, Braunwald E, Murphy SA, Karwatowska‐Prokopczuk E, Buros JL, et al. Efficacy of ranolazine in patients with chronic angina observations from the randomized, double‐blind, placebo‐controlled MERLIN‐TIMI (Metabolic Efficiency With Ranolazine for Less Ischemia in Non‐ST‐Segment Elevation Acute Coronary Syndromes) 36 Trial. Journal of the American College of Cardiology 2009;53(17):1510‐6. [DOI] [PubMed] [Google Scholar]
Pelliccia 2012 {published data only (unpublished sought but not used)}
- Pelliccia F, Pasceri V, Marazzi G, Rosano G, Greco C, Gaudio C. A pilot randomized study of ranolazine for reduction of myocardial damage during elective percutaneous coronary intervention. American Heart Journal 2012;163(6):1019‐23. [DOI] [PubMed] [Google Scholar]
Pepine 1999 {published data only (unpublished sought but not used)}
- Pepine CJ, Wolff AA, on behalf of the Ranolazine Study Group. A controlled trial with a novel anti‐ischemic agent, ranolazine, in chronic stable angina pectoris that is responsive to conventional antianginal agents. American Journal of Cardiology 1999;84(1):46‐50. [DOI] [PubMed] [Google Scholar]
RAN080 2005 {published data only (unpublished sought but not used)}
- Rousseau MF, Pouleur H, Cocco G, Wolff AA. Comparative efficacy of ranolazine versus atenolol for chronic angina pectoris. American Journal of Cardiology 2005;95(3):311‐6. [DOI] [PubMed] [Google Scholar]
RIVER‐PCI 2016 {published data only (unpublished sought but not used)}
- Alexander KP, Weisz G, Prather K, James S, Mark DB, Anstrom KJ, et al. Effects of ranolazine on angina and quality of life after percutaneous coronary intervention with incomplete revascularization: results from the Ranolazine for Incomplete Vessel Revascularization (RIVER‐PCI) Trial. Circulation 2016;133(1):39‐47. [DOI] [PubMed] [Google Scholar]
- Weisz G, Généreux P, Iñiguez A, Zurakowski A, Shechter M, Alexander KP, et al. Ranolazine in patients with incomplete revascularisation after percutaneous coronary intervention (RIVER‐PCI): a multicentre, randomised, double‐blind, placebo‐controlled trial. Lancet 2016;387(10014):136‐45. [DOI] [PubMed] [Google Scholar]
RWISE 2016 {published data only (unpublished sought but not used)}
- Bairey Merz CN, Handberg EM, Shufelt CL, Mehta PK, Minissian MB, Wei J, et al. A randomized, placebo‐controlled trial of late Na current inhibition (ranolazine) in coronary microvascular dysfunction (CMD): impact on angina and myocardial perfusion reserve. European Heart Journal 2016;37(19):1504‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sandhiya 2015 {published data only (unpublished sought but not used)}
- Sandhiya S, Steven AD, Ajith AP, Melvin G, Balachander J, Adithan C. Comparison of ranolazine and trimetazidine on glycemic status in diabetic patients with coronary artery disease – a randomized controlled trial. Journal of Clinical and Diagnostic Research 2015;9(1):OC01–OC05. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shammas 2015 {published data only (unpublished sought but not used)}
- Shammas NW, Shammas GA, Keyes K, Duske S, Kelly R, Jerin M. Ranolazine versus placebo in patients with ischemic cardiomyopathy and persistent chest pain or dyspnea despite optimal medical and revascularization therapy: randomized, double‐blind crossover pilot study. Therapeutics and Clinical Risk Management 2015;11:469‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tagliamonte 2015 {published data only (unpublished sought but not used)}
- Tagliamonte E, Rigo F, Cirillo T, Astarita C, Quaranta G, Marinelli U, et al. Effects of ranolazine on noninvasive coronary flow reserve in patients with myocardial ischemia but without obstructive coronary artery disease. Echocardiography 2015;32(3):516‐21. [DOI] [PubMed] [Google Scholar]
TERISA 2013 {published data only (unpublished sought but not used)}
- Kosiborod M, Arnold SV, Spertus JA, McGuire DK, Li Y, Yue P, et al. Evaluation of ranolazine in patients with type 2 diabetes mellitus and chronic stable angina: results from the TERISA randomized clinical trial (Type 2 Diabetes Evaluation of Ranolazine in Subjects With Chronic Stable Angina). Journal of the American College of Cardiology 2013;61(20):2038‐45. [DOI] [PubMed] [Google Scholar]
Thadani 1994 {published data only (unpublished sought but not used)}
- Thadani U, Ezekowitz M, Fenney L, Chiang YK. Double‐blind efficacy and safety study of a novel anti‐ischemic agent, ranolazine, versus placebo in patients with chronic stable angina pectoris. Circulation 1994;90(2):726‐34. [DOI] [PubMed] [Google Scholar]
Villano 2013 {published data only (unpublished sought but not used)}
- Villano A, Franco A, Nerla R, Sestito A, Tarzia P, Lamendola P, et al. Effects of ivabradine and ranolazine in patients with microvascular angina pectoris. American Journal of Cardiology 2013;112(1):8‐13. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Arnold 2014 {published data only}
- Arnold SV, Kosiborod M, McGuire DK, Li Y, Yue P, Ben‐Yehuda O, et al. Effects of ranolazine on quality of life among patients with diabetes mellitus and stable angina. JAMA Internal Medicine 2014;174(8):1403‐5. [DOI] [PubMed] [Google Scholar]
Cocco 1992 {published data only}
- Cocco G, Rousseau MF, Bouvy T, Cheron P, Williams G, Detry JM, et al. Effects of a new metabolic modulator, ranolazine, on exercise tolerance in angina pectoris patients treated with beta‐blocker or diltiazem. Journal of Cardiovascular Pharmacology 1992;20(1):131‐8. [PubMed] [Google Scholar]
Coleman 2015 {published data only}
- Coleman CI, Freemantle N, Kohn CG. Ranolazine for the treatment of chronic stable angina: a cost‐effectiveness analysis from the UK perspective. BMJ Open 2015;5(11):e008861. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hidalgo‐Vega 2014 {published data only}
- Hidalgo‐Vega A, Ramos‐Goñi JM, Villoro R. Cost‐utility of ranolazine for the symptomatic treatment of patients with chronic angina pectoris in Spain. European Journal of Health Economics 2014;15(9):917‐25. [DOI] [PubMed] [Google Scholar]
Jain 1990 {published data only (unpublished sought but not used)}
- Jain D, Dasgupta P, Hughes LO, Lahiri A, Raftery EB. Ranolazine (RS‐43285): a preliminary study of a new anti‐anginal agent with selective effect on ischaemic myocardium. European Journal of Clinical Pharmacology 1990;38(2):111–4. [DOI] [PubMed] [Google Scholar]
Kohn 2014 {published data only}
- Kohn CG, Parker MW, Limone BL, Coleman CI. Cost‐effectiveness of ranolazine added to standard‐of‐care treatment in patients with chronic stable angina pectoris. American Journal of Cardiology 2014;113(8):1306‐11. [DOI] [PubMed] [Google Scholar]
Lucioni 2009 {published data only}
- Lucioni C, Mazzi S. Economic evaluation of add‐on ranolazine in the treatment of chronic stable angina [Una valutazione economica di ranolazina add‐on nel trattamento dell’angina stabile cronica]. Pharmacoeconomics Italian Research Articles 2009;11(3):141‐52. [Google Scholar]
Rehberger‐Likozar 2015 {published data only}
- Rehberger‐Likozar A, Šebeštjen M. Influence of trimetazidine and ranolazine on endothelial function in patients with ischemic heart disease. Coronary Artery Disease 2015;26(8):651‐6. [DOI] [PubMed] [Google Scholar]
Rich 2007 {published data only}
- Rich MW, Crager M, McKay CR. Safety and efficacy of extended‐release ranolazine in patients aged 70 years or older with chronic stable angina pectoris. American Journal of Geriatric Cardiology 2007;16(4):216‐21. [DOI] [PubMed] [Google Scholar]
ROLE 2007 {published data only}
- Koren MJ, Crager MR, Sweeney M. Long‐term safety of a novel antianginal agent in patients with severe chronic stable angina: the Ranolazine Open Label Experience (ROLE). Journal of the American College of Cardiology 2007;49(10):1027‐34. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
NCT01304095 {published data only}
- NCT01304095. Ranolazine, ethnicity and the metabolic syndrome (REMS). https://clinicaltrials.gov/ct2/show/NCT01304095?term=NCT01304095&rank=1 (first received 16 February 2011).
Tagarakis 2013 {published data only}
- Tagarakis GI, Aidonidis I, Daskalopoulou SS, Simopoulos V, Liouras V, Daskalopoulos ME, et al. Effect of ranolazine in preventing postoperative atrial fibrillation in patients undergoing coronary revascularization surgery. Current Vascular Pharmacology 2013;11(6):988‐91. [DOI] [PubMed] [Google Scholar]
Tian 2012 {published data only}
- Tian C, Liu Y. Effect of ranolazine and diltiazem on patients with stable angina pectoris [雷诺拉嗪联合地尔硫对稳定性心绞痛的疗效评价]. 中国现代医生 (Chinese Modern Physician) 2012;50(5):122‐3. [Google Scholar]
Wang 2012 {published data only}
- Wang G, Zhou C. Clinical observation of ranolazine hydrochloride sustained‐release tablets in the treatment of stable angina pectoris [盐酸雷诺嗪缓释片治疗稳定型心绞痛的临床观察]. 中国药房 (Chinese Pharmacy) 2012;23(26):2455‐8. [Google Scholar]
References to ongoing studies
Calcagno 2014 {published data only}
- Calcagno S, Taccheri T, Carnesale R, Nicol S, Lembo M, Bruno P, et al. Complete coronary revascularization in different clinical settings is not enough: role of ranolazine. Journal of the American College of Cardiology. 2014; Vol. 63:12 S.
Calcagno 2015 {published data only}
- Calcagno S, Severino P, Taccheri T, Placentino F, Ceccacci A, Cinque A, et al. Complete coronary revascularization in some clinical settings is not enough: comparison of ivabradine, ranolazine and standard medical therapy in term of efficacy and tolerability. Journal of the American College of Cardiology. 2015; Vol. 65:10 S.
CTRI/2014/01/004332 {published data only}
- CTRI/2014/01/004332. Effect of ranolazine on improvement of heart function [Effect of ranolazine on improvement of left ventricular dysfunction in patients with chronic stable angina – a randomized controlled clinical trial]. http://ctri.nic.in/Clinicaltrials/showallp.php?mid1=7785&EncHid=&userName=CTRI/2014/01/0043 (first received 22 January 2014).
EUCTR 2011‐001278‐24 {published data only}
- EUCTR 2011‐001278‐24. Efficacy of ranolazine in patients with coronary artery disease (CAD). www.clinicaltrialsregister.eu/ctr‐search/search?query=Efficacy+of+ranolazine+in+patients+with+coronary+artery+disease+%28CAD%29 (first received 18 July 2011).
EUCTR 2012‐001584‐77 {published data only}
- EUCTR 2012‐001584‐77. Effect of ranolazine in ischemic patients with indication of staged interventional therapy. www.clinicaltrialsregister.eu/ctr‐search/search?query=A+study+to+investigate+the+effect+of+a+drug+to+treat+angina+pectoris (first received 18 July 2012).
Gupta 2014 {published data only}
- Gupta G, Varma CM, Thakur R, Bhardwaj RPS, Ahmad M, Bansal RK, et al. Effect of ranolazine on angina frequency in patients with type 2 DM and chronic stable angina. Indian Heart Journal. 2014; Vol. 66 (Suppl 2):S11.
NCT01495520 {published data only}
- NCT01495520. Ranolazine for improving symptoms of palpitations (RYPPLE). https://clinicaltrials.gov/ct2/show/NCT01495520 (first received 15 December 2011).
NCT01558830 {published data only}
- NCT01558830. Safety of amiodarone and ranolazine together in patients with angina (SARA). https://clinicaltrials.gov/ct2/show/NCT01558830 (first received 18 March 2012).
NCT01754259 {published data only}
- NCT01754259. Effects of ranolazine on coronary flow reserve in symptomatic diabetic patients and CAD (RAND‐CFR). https://clinicaltrials.gov/ct2/show/NCT01754259 (first received 12 December 2012).
NCT01948310 {published data only}
- NCT01948310. Effects of ranolazine and exercise on daily physical activity trial (EREDA). https://clinicaltrials.gov/ct2/show/NCT01948310 (first received 17 September 2013).
NCT02052011 {published data only}
- NCT02052011. Ranolazine and microvascular angina by PET in the emergency department (RAMP‐ED). https://clinicaltrials.gov/ct2/show/NCT02052011 (first received 30 January 2014).
NCT02147067 {published data only}
- NCT02147067. Microvascular assessment of ranolazine in non‐obstructive atherosclerosis (MARINA). https://clinicaltrials.gov/ct2/show/NCT02147067 (first received 22 May 2014).
NCT02147834 {published data only}
- NCT02147834. Effectiveness of ranolazine on reducing chest pain in patients with blockage but no stents (IMWELL3). https://clinicaltrials.gov/ct2/show/NCT02147834 (first received 22 May 2014).
NCT02252406 {published data only}
- NCT02252406. Impact of ranolazine in blood markers in women with angina and metabolic syndrome (IRMA). https://clinicaltrials.gov/ct2/show/NCT02252406 (first received 11 August 2014).
NCT02265796 {published data only}
- NCT02265796. Improvement of subjective well‐being by ranolazine among unrevascularized chronic stable coronary artery disease patients (IMWELL). https://clinicaltrials.gov/ct2/show/NCT02265796 (first received 10 October 2014).
NCT02423265 {published data only}
- NCT02423265. Efficacy of ranolazine in patients with chronic total occlusions of coronary arteries. https://clinicaltrials.gov/ct2/show/NCT02423265 (first received 12 April 2015).
Šebeštjen 2014 {published data only}
- Šebeštjen M, Rehberjer‐Likozar A, Vrtovec B, Poglajen G. Both trimetazidine and ranolazine improve arterial vasoreactivity in patients with ischemic heart disease (Abstract 14057). Circulation. 2014; Vol. 130:A14057.
Additional references
ACC/AHA 2012
- Fihn SD, Gardin JM, Abrams J, Berra K, Blankenship JC, Dallas AP, et al. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. Circulation 2012;126(25):e354‐471. [DOI] [PubMed] [Google Scholar]
Banon 2014
- Banon D, Filion KB, Budlovsky T, Franck C, Eisenberg MJ. The usefulness of ranolazine for the treatment of refractory chronic stable angina pectoris as determined from a systematic review of randomized controlled trials. American Journal of Cardiology 2014;113(6):1075‐82. [DOI] [PubMed] [Google Scholar]
Belsey 2015
- Belsey J, Savelieva I, Mugelli A, Camm AJ. Relative efficacy of antianginal drugs used as add‐on therapy in patients with stable angina: A systematic review and meta‐analysis. European Journal of Preventive Cardiology 2015;22(7):837‐48. [DOI] [PubMed] [Google Scholar]
Cattaneo 2015
- Cattaneo M, Porretta AP, Gallino A. Ranolazine: Drug overview and possible role in primary microvascular angina management. International Journal of Cardiology 2015;181:376‐81. [DOI] [PubMed] [Google Scholar]
Chaitman 2011
- Chaitman BR, Laddu AA. Stable angina pectoris: antianginal therapies and future directions. Nature Reviews. Cardiology 2011;9(1):40‐52. [DOI] [PubMed] [Google Scholar]
Cochrane Glossary
- Cochrane Community. Glossary. https://community‐archive.cochrane.org/glossary (accessed 31 October 2016).
Codolosa 2014
- Codolosa JN, Acharjee S, Figueredo VM. Update on ranolazine in the management of angina. Vascular Health and Risk Management 2014;10:353‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Di Fiore 2013
- Fiore DP, Beltrame JF. Chest pain in patients with 'normal angiography': could it be cardiac?. International Journal of Evidence‐Based Healthcare 2013;11(1):56‐68. [DOI] [PubMed] [Google Scholar]
EMA 2008
- European Medicines Agency. Ranexa (previously Latixa). www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/000805/human_med_001009.jsp&mid=WC0b01ac058001d124 (accessed prior to 30 January 2017).
ESC 2013
- Montalescot G, Sechtem U, Achenbach S, Andreotti F, Arden C, Budaj A, et al. 2013 ESC guidelines on the management of stable coronary artery disease: the Task Force on the management of stable coronary artery disease of the European Society of Cardiology. European Heart Journal 2013;34(38):2949‐3003. [DOI] [PubMed] [Google Scholar]
FDA 2016
- US Food, Drug Administration. Orange Book: Approved Drug Products with Therapeutic Equivalence Evaluations. www.accessdata.fda.gov/scripts/cder/ob/default.cfm. U.S. Food and Drug Administration, Accessed prior to 30 January 2017.
GRADEpro GDT 2015
- GRADEpro Guideline Development Tool [software]. Available from gradepro.org. McMaster University (developed by Evidence Prime, Inc.), 2015.
Hawwa 2013
- Hawwa N, Menon V. Ranolazine: clinical applications and therapeutic basis. American Journal of Cardiovascular Drugs 2013;13(1):5‐16. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Jerling 2006
- Jerling M. Clinical pharmacokinetics of ranolazine. Clinical Pharmacokinetics 2006;45(5):469‐91. [DOI] [PubMed] [Google Scholar]
Jones 2012
- Jones E, Eteiba W, Merz NB. Cardiac syndrome X and microvascular coronary dysfunction. Trends in Cardiovascular Medicine 2012;22(6):161‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Keating 2008
- Keating GM. Ranolazine: a review of its use in chronic stable angina pectoris. Drugs 2008;68(17):2483‐503. [DOI] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org. The Cochrane Collaboration.
McNamara 2015
- McNamara DA, Goldberger JJ, Berendsen MA, Huffman MD. Implantable defibrillators versus medical therapy for cardiac channelopathies. Cochrane Database of Systematic Reviews 2015, Issue 10. [DOI: 10.1002/14651858.CD011168.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Morrow 2006
- Morrow DA, Scirica BM, Karwatowska‐Prokopczuk E, Skene A, McCabe CH, Braunwald E, MERLIN‐TIMI 36 Investigators. Evaluation of a novel anti‐ischemic agent in acute coronary syndromes: design and rationale for the Metabolic Efficiency with Ranolazine for Less Ischemia in Non‐ST‐elevation acute coronary syndromes (MERLIN)‐TIMI 36 trial. Am Heart J. 2006 Jun;151(6):1186.e1‐9. [DOI] [PubMed] [Google Scholar]
NICE 2010
- National Institute for Health and Care Excellence (NICE). Chest pain of recent onset: Assessment and diagnosis (CG95) (Updated 2016). http://guidance.nice.org.uk/CG95 (accessed prior to 30 January 2017). [PubMed]
NICE 2011
- National Institute for Health and Clinical Excellence. Stable angina (CG126) (Updated 2016). http://guidance.nice.org.uk/CG126 2011; Vol. (accessed prior to 30 January 2017).
O'Toole 2008
- O'Toole L. Angina (chronic stable). Systematic review 213. BMJ Clinical Evidence http://clinicalevidence.bmj.com/x/systematic‐review/0213/overview.html 2008; Vol. (accessed prior to 30 January 2017). [PMC free article] [PubMed]
Ohman 2016
- Ohman EM. Clinical practice. Chronic stable angina. New England Journal of Medicine 2016;374(12):1167‐76. [DOI] [PubMed] [Google Scholar]
Patel 2008
- Patel PD, Arora RR. Utility of ranolazine in chronic stable angina patients. Vascular Health and Risk Management 2008;4(4):819‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ranexa PI 2013
- Ranexa (product information). Gilead Sciences Inc, Foster City, CA, USA December 2013.
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Savarese 2013
- Savarese G, Rosano G, D'Amore C, Musella F, Della Ratta GL, Pellegrino AM, et al. Effects of ranolazine in symptomatic patients with stable coronary artery disease. A systematic review and meta‐analysis. International Journal of Cardiology 2013;169(4):262‐70. [DOI] [PubMed] [Google Scholar]
Scirica 2009
- Scirica BM. Chronic angina: definition, prevalence, and implications for quality of life. Reviews in Cardiovascular Medicine 2009;10(Suppl 1):S3‐S10. [DOI] [PubMed] [Google Scholar]
SIGN 2007
- Scottish Intercollegiate Guidelines Network. Management of stable angina. A national clinical guideline (guideline n°96). http://www.sign.ac.uk/guidelines/fulltext/96/ (accessed 19 March 2015).
Silva 2011
- Silva SA, Passos SR, Carballo MT, Figueiró M. Quality of life assessment after acute coronary syndrome: systematic review [Avaliação da qualidade de vida após síndrome coronariana aguda: revisão sistemática]. Arquivos Brasileiros de Cardiologia 2011;97(6):526‐40. [DOI] [PubMed] [Google Scholar]
Sterne 2011
- Sterne JA, Sutton AJ, Ioannidis JP, Terrin N, Jones DR, Lau J, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta‐analyses of randomised controlled trials. BMJ 2011;343:d4002. [DOI: 10.1136/bmj.d4002] [DOI] [PubMed] [Google Scholar]
Stock 2011
- Stock C, Henrot N. From OpenSIGLE to OpenGrey: Change and Continuity. Grey Journal 2011; Vol. 7, issue 2:93‐7.
Tarkin 2012
- Tarkin JM, Kaski JC. Pharmacological treatment of chronic stable angina pectoris. Clinical Medicine 2012;13(1):63‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Thadani 2012
- Thadani U. Should ranolazine be used for all patients with ischemic heart disease or only for symptomatic patients with stable angina or for those with refractory angina pectoris? A critical appraisal. Expert Opinion on Pharmacotherapy 2012;13(17):2555‐63. [DOI] [PubMed] [Google Scholar]
Van Enst 2012
- Enst WA, Scholten RJ, Hooft L. Identification of additional trials in prospective trial registers for Cochrane systematic reviews. PLoS One 2012;7(8):e42812. [DOI] [PMC free article] [PubMed] [Google Scholar]
Weisz 2013
- Weisz G, Farzaneh‐Far R, Ben‐Yehuda O, Debruyne B, Montalescot G, Lerman A, et al. Use of ranolazine in patients with incomplete revascularization after percutaneous coronary intervention: design and rationale of the Ranolazine for Incomplete Vessel Revascularization Post‐Percutaneous Coronary Intervention (RIVER‐PCI) trial. American Heart Journal 2013;166(6):953‐9. [DOI] [PubMed] [Google Scholar]
Wenger 2007
- Wenger NK, Chaitman B, Vetrovec GW. Gender comparison of efficacy and safety of ranolazine for chronic angina pectoris in four randomized clinical trials. American Journal of Cardiology 2007;99(1):11‐8. [DOI] [PubMed] [Google Scholar]
Young 2013
- Young JW Jr, Melander S. Evaluating symptoms to improve quality of life in patients with chronic stable angina. Nursing Research and Practice 2013;2013:504915. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Salazar 2015
- Salazar CA, Basilio Flores JE, Mejia Dolores JW, Veramendi Espinoza LE, Rey Rodriguez DE, Loza Munárriz C. Ranolazine for stable angina pectoris. Cochrane Database of Systematic Reviews 2015, Issue 6. [DOI: 10.1002/14651858.CD011747] [DOI] [PMC free article] [PubMed] [Google Scholar]


