Abstract
Background
Delirium is defined as a disturbance in attention, awareness and cognition with reduced ability to direct, focus, sustain and shift attention, and reduced orientation to the environment. Critically ill patients in the intensive care unit (ICU) frequently develop ICU delirium. It can profoundly affect both them and their families because it is associated with increased mortality, longer duration of mechanical ventilation, longer hospital and ICU stay and long‐term cognitive impairment. It also results in increased costs for society.
Objectives
To assess existing evidence for the effect of preventive interventions on ICU delirium, in‐hospital mortality, the number of delirium‐ and coma‐free days, ventilator‐free days, length of stay in the ICU and cognitive impairment.
Search methods
We searched CENTRAL, MEDLINE, Embase, BIOSIS, International Web of Science, Latin American Caribbean Health Sciences Literature, CINAHL from 1980 to 11 April 2018 without any language limits. We adapted the MEDLINE search for searching the other databases. Furthermore, we checked references, searched citations and contacted study authors to identify additional studies. We also checked the following trial registries: Current Controlled Trials; ClinicalTrials.gov; and CenterWatch.com (all on 24 April 2018).
Selection criteria
We included randomized controlled trials (RCTs) of adult medical or surgical ICU patients receiving any intervention for preventing ICU delirium. The control could be standard ICU care, placebo or both. We assessed the quality of evidence with GRADE.
Data collection and analysis
We checked titles and abstracts to exclude obviously irrelevant studies and obtained full reports on potentially relevant ones. Two review authors independently extracted data. If possible we conducted meta‐analyses, otherwise we synthesized data narratively.
Main results
The electronic search yielded 8746 records. We included 12 RCTs (3885 participants) comparing usual care with the following interventions: commonly used drugs (four studies); sedation regimens (four studies); physical therapy or cognitive therapy, or both (one study); environmental interventions (two studies); and preventive nursing care (one study). We found 15 ongoing studies and five studies awaiting classification. The participants were 48 to 70 years old; 48% to 74% were male; the mean acute physiology and chronic health evaluation (APACHE II) score was 14 to 28 (range 0 to 71; higher scores correspond to more severe disease and a higher risk of death). With the exception of one study, all participants were mechanically ventilated in medical or surgical ICUs or mixed. The studies were overall at low risk of bias. Six studies were at high risk of detection bias due to lack of blinding of outcome assessors. We report results for the two most commonly explored approaches to delirium prevention: pharmacologic and a non‐pharmacologic intervention.
Haloperidol versus placebo (two RCTs, 1580 participants)
The event rate of ICU delirium was measured in one study including 1439 participants. No difference was identified between groups, (risk ratio (RR) 1.01, 95% confidence interval (CI) 0.87 to 1.17) (moderate‐quality evidence). Haloperidol versus placebo neither reduced or increased in‐hospital mortality, (RR 0.98, 95% CI 0.80 to 1.22; 2 studies; 1580 participants (moderate‐quality evidence)); the number of delirium‐ and coma‐free days, (mean difference (MD) ‐0.60, 95% CI ‐1.37 to 0.17; 2 studies, 1580 participants (moderate‐quality of evidence)); number of ventilator‐free days (mean 23.8 (MD ‐0.30, 95% CI ‐0.93 to 0.33) 1 study; 1439 participants, (high‐quality evidence)); length of ICU stay, (MD 0.18, 95% CI 0.60 to 0.97); 2 studies, 1580 participants; high‐quality evidence). None of the studies measured cognitive impairment. In one study there were three serious adverse events in the intervention group and five in the placebo group; in the other there were five serious adverse events and three patients died, one in each group. None of the serious adverse events were judged to be related to interventions received (moderate‐quality evidence).
Physical and cognitive therapy interventions (one study, 65 participants)
The study did not measure the event rate of ICU delirium. A physical and cognitive therapy intervention versus standard care neither reduced nor increased in‐hospital mortality, (RR 0.94, 95% CI 0.40 to 2.20, I² = 0; 1 study, 65 participants; very low‐quality evidence); the number of delirium‐ and coma‐free days, (MD ‐2.8, 95% CI ‐10.1 to 4.6, I² = 0; 1 study, 65 participants; very low‐quality evidence); the number of ventilator‐free days (within the first 28/30 days) was median 27.4 (IQR 0 to 29.2) and 25 (IQR 0 to 28.9); 1 study, 65 participants; very low‐quality evidence, length of ICU stay, (MD 1.23, 95% CI ‐0.68 to 3.14, I² = 0; 1 study, 65 participants; very low‐quality evidence); cognitive impairment measured by the MMSE: Mini‐Mental State Examination with higher scores indicating better function, (MD 0.97, 95% CI ‐0.19 to 2.13, I² = 0; 1 study, 30 participants; very low‐quality evidence); or measured by the Dysexecutive questionnaire (DEX) with lower scores indicating better function (MD ‐8.76, 95% CI ‐19.06 to 1.54, I² = 0; 1 study, 30 participants; very low‐quality evidence). One patient experienced acute back pain accompanied by hypotensive urgency during physical therapy.
Authors' conclusions
There is probably little or no difference between haloperidol and placebo for preventing ICU delirium but further studies are needed to increase our confidence in the findings. There is insufficient evidence to determine the effects of physical and cognitive intervention on delirium. The effects of other pharmacological interventions, sedation, environmental, and preventive nursing interventions are unclear and warrant further investigation in large multicentre studies. Five studies are awaiting classification and we identified 15 ongoing studies, evaluating pharmacological interventions, sedation regimens, physical and occupational therapy combined or separately, and environmental interventions, that may alter the conclusions of the review in future.
Plain language summary
Intervention to prevent delirium for critically ill patients in the intensive care unit (ICU)
Background
Delirium is an acutely disturbed state of mind that occurs in critically ill adults in the intensive care unit (ICU). It is associated with a prolonged time on mechanical ventilation to assist breathing, longer stay in the ICU and hospital, and higher risk of death. ICU delirium is also linked with cognitive problems such as loss of memory and attention, difficulty in concentrating and reduced awareness. The risk factors for delirium include old age, alcoholism, vision/hearing impairment and, for critically ill patients, the use of restraints, prolonged pain and some medications.
Review question
Our aim was to assess the existing evidence on the effect of interventions for preventing ICU delirium, reducing in‐hospital death, reducing length of coma/delirium, the need for mechanical ventilation to assist breathing, the length of stay in the ICU and mental problems
Study characteristics
We included 12 randomized controlled trials (3885 participants) in our review. The studies included adults aged 48 to 70 years from surgical and medical ICUs. The studies compared different drugs (three studies) various approaches to sedation (five studies), physical or cognitive therapy or both (one study), noise and light reduction in the ICU (two studies), and preventive nursing care (one study). The studies had mostly small numbers of participants and did not blind the researchers who assessed effects on outcomes. We report the findings regarding the effect of the two most commonly explored approaches for preventing delirium, drug and non‐drug interventions, haloperidol versus a sham drug, and early physical and cognitive therapy versus usual care.
Key results
Our findings suggest that there may be little or no difference between haloperidol and placebo for preventing ICU delirium, but further studies are needed to reduce imprecision and increase our confidence in the findings. More studies of physical and cognitive therapy are needed as there is insufficient evidence to determine whether these non‐pharmacological approaches can prevent delirium in the ICU. Additional research is required to explore the benefits and harms of other approaches to prevent delirium in the ICU such as sedation and changes in the ICU environment, and nursing care tailored to prevent delirium.
Quality of the evidence
We rated the quality of the evidence as moderate to very low. Several studies had quality shortcomings, including their use of small numbers of participants, and lack of blinding of those assessing effects of interventions for preventing delirium and other outcomes. For the interventions testing sedation approaches, physical and cognitive therapy, and changes in the environment, additional research is required to clarify their effectiveness. The five studies in ‘Studies awaiting classification’ and 15 ongoing studies may alter the conclusions of the review once they are completed and assessed.
Summary of findings
Background
Description of the condition
According to the Diagnostic and Statistical Manual of Mental Disorders (DSM 5) (American Psychiatric Association 2013), delirium is defined as a disturbance in attention, awareness and cognition with reduced ability to direct, focus, sustain and shift attention, and reduced orientation to the environment. The disturbance develops over a short period of time, fluctuates and represents an acute change from baseline attention and awareness. Importantly, the disturbances in attention, awareness and cognition are not explained by pre‐existing neurocognitive disorders and do not occur in the context of a severely reduced level of arousal, such as sedation or coma (American Psychiatric Association 2013). There is evidence that the disturbance is a direct physiological consequence of another medical condition, substance intoxication or withdrawal, exposure to a toxin or due to multiple aetiologies (American Psychiatric Association 2013).
Delirium during critical illness is common, with prevalence rates varying from 20% to 84% depending on the severity of the illness and the methods used to diagnose delirium (Girard 2010; Morandi 2009; Pandharipande 2008). The development of delirium symptoms may go unnoticed in the intensive care unit (ICU) (Pandharipande 2005; Shu‐Min Lin 2004), and may even overlap with symptoms of other neuropsychiatric disorders (Morandi 2009).
The genesis of delirium is believed to be multifactorial, and risk factors are traditionally divided into predisposing factors and precipitating factors (Morandi 2008; Morandi 2009; Svenningsen 2009). Predisposing factors are increased age, baseline cognitive impairment, comorbid disease (especially respiratory diseases), increased pain levels and vision or hearing impairment. Precipitating factors specifically for ICU patients include the use of sedative and analgesic medications, specifically benzodiazepines, propofol, dexmedetomidine and fentanyl, medications with anticholinergic properties, steroid administration, dopamine, as well as sleep deprivation, iatrogenic adverse events, severity of illness, severe sepsis, hypoxaemia, dehydration, hypotension, metabolic derangements and anaemia (Hayhurst 2016; Miller 2006; Morandi 2009, Vasilevskis 2010).
Delirium can present as hyperactive or hypoactive states, and may fluctuate between the two as mixed delirium (Hayhurst 2016). Hyperactive delirium is the most recognizable type of delirium (Maldonado 2008), characterized by restlessness and agitation. Hypoactive delirium is characterized by slower thinking and reasoning, lethargy, and decreased movement (Maldonado 2008; Morandi 2009; Peterson 2006).
There are varying reports on the prevalence of ICU delirium depending on the ICU setting, patient mix and methods of detection. A Danish cohort study of adult ICU participants (n = 136) diagnosed 40% of medical and surgical ICU patients with ICU delirium using the Confusion Assessment Method for ICU (CAM‐ICU) (Svenningsen 2009). Others reported 80% of ICU patients developed delirium at some point during the ICU stay (Ely 2004). The distribution of the three subtypes varies across studies. A cohort study of medical ICU participants (n = 610), who were screened for delirium using the CAM‐ICU test, showed that 2% of delirium episodes were of the hyperactive subtype; 44% of the hypoactive subtype; and 55% of the mixed subtype (Peterson 2006). A larger cohort study (n = 1600) with a mixed ICU population screened patients with CAM‐ICU and DSM‐IV criteria (American Psychiatric Association 2000) , and reported that the mixed‐delirium subtype was most common (53%), hypoactive delirium was found in 36% and hyperactive delirium in 11% (van den Boogaard 2012c).
ICU delirium is associated with worsened short‐term outcomes such as increased duration of mechanical ventilation (Ely 2004;van den Boogaard 2012c); prolonged ICU stay (Ely 2001a; Thomason 2005;van den Boogaard 2012c); prolonged length of hospital stay (Ely 2001a; Oimet 2007; Thomason 2005;van den Boogaard 2012c); higher mortality (Ely 2004; Pisani 2009; Shehabi 2010; Shu‐Min Lin 2004; Thomason 2005;van den Boogaard 2012c); removal of tubes and catheters by patients; and increased costs (Maldonado 2008; Milbrandt 2004; Thomason 2005). ICU delirium is also associated with long‐term cognitive impairment as well as impairment of memory, attention, concentration, executive function and motor function (Girard 2010; Jackson 2009; Richter 2006; Salluh 2015;van den Boogaard 2012b). There is a growing body of evidence showing that duration of ICU delirium is an independent predictor of persisting cognitive impairment up to one year after critical illness (Brummel 2014a; Girard 2010; Pandharipande 2013; van den Boogaard 2012b; Wolters 2014). Cognitive impairment may affect the ability to work, and in turn influence living conditions and economic and social status (Rothenhaüsler 2001). Norman and colleagues recently showed that 45% of previously employed ICU survivors experienced decreased employment 12 months after critical illness (Norman 2016). No significant predictors of employment status were identified; however better cognition was marginally associated with a lower risk of decreased employment 12 months after critical illness (Norman 2016).
To our knowledge, ICU delirium and post‐traumatic stress disorder (PTSD) are not associated with one another (Davydow 2008). A prospective cohort study of medical/surgical ICU patients found that depression was at least four times more common than PTSD (Jackson 2014). Seven per cent of patients experienced PTSD at 3‐ and 12‐month follow‐up while 37% and 33% experienced depression, respectively (Jackson 2014). ICU delirium was not associated with depression or PTSD (Jackson 2014).
There are several validated tools for systematic detection of ICU delirium. Among these the CAM‐ICU and the Intensive Care Delirium Screening Checklist (ICDSC) are the most widely studied and used (Bergeron 2001; Ely 2001b; Ely 2001c; Hayhurst 2016; Luetz 2010). Both nurses and physicians can screen patients, and screening should ideally be repeated regularly and considered in the context of clinical symptomatology (Hayhurst 2016). Despite the availability of valid ICU delirium screening tools and the serious ramifications of ICU delirium, surveys show that systematic screening for delirium remains infrequent within ICUs (Devlin 2008; Elliott 2014; Mac Sweeney 2010, Patel 2009; Salluh 2009; Selim 2017). Scepticism among clinicians about the usefulness and the validity of screening tools and the lack of effective evidence‐based interventions for prevention and treatment of delirium may have a role in the lack of routine screening (Oxenbøll‐Collet 2018; Zamoscik 2017).
Treatment rather than prevention of ICU delirium is the most usual approach to delirium management in ICUs. The most common interventions for treating ICU‐delirium are pharmacological, with haloperidol as first choice and atypical antipsychotics and benzodiazepines as secondary options (Mac Sweeney 2010; Patel 2009; Salluh 2009), despite limited evidence to support these practices (Burry 2018; Lonergan 2009; Serafim 2015). Multicomponent interventions are less commonly used in the management of ICU delirium (Mac Sweeney 2010; Patel 2009; Salluh 2009), although there is growing evidence for a preventive effect of multi‐component interventions targeting cognitive impairment, sleep deprivation, immobility, visual and hearing impairment, and dehydration in hospitalized non‐ICU patients in other settings (Siddiqi 2016). Hypothetically, ICU delirium may also be prevented by modifying some of the precipitating factors for delirium, for example sleep deprivation, immobility, visual and hearing impairment.
Description of the intervention
Firm evidence for the exact aetiology of delirium in ICU patients is lacking (Griffiths 2007). As it is widely accepted that the aetiology of ICU delirium is multifactorial, multi‐component preventive interventions seem relevant for the prevention of ICU delirium (Hayhurst 2016; Morandi 2009; Pandharipande 2010). We considered interventions that target one or several of the following risk factors for ICU delirium: immobilization, sensory deprivation, social isolation, sleep deprivation, pain, use of psychoactive medications, iatrogenic adverse events. Interventions can, therefore, include behavioural, cognitive, psychological, environmental and physical; and cognitive training interventions or pharmacological interventions.
How the intervention might work
Interventions that target predisposing and precipitating factors for ICU delirium may reduce the incidence of ICU delirium, thus preventing by treating one or several of its underlying causes.
Why it is important to do this review
In light of the high prevalence of ICU delirium with the severe adverse sequelae for patients and increased costs for society, we consider it relevant to investigate the evidence for an effect of non‐pharmacological as well as pharmacological interventions, combined or in isolation, for preventing delirium in ICU patients.
Objectives
To assess existing evidence for the effect of preventive interventions on ICU delirium, in‐hospital mortality, the number of delirium‐ and coma‐free days, ventilator‐free days, length of stay in the ICU and cognitive impairment.
Methods
Criteria for considering studies for this review
Types of studies
We included randomized controlled trials (RCTs). We excluded non‐randomized controlled trials, controlled before‐and‐after trials, historically‐controlled trials and cohort studies.
Types of participants
We included adult ICU participants aged 18 years and above. We included both intubated and non‐intubated participants. We included ICU participants from medical and surgical ICUs and participants from mixed ICUs. Participants from cardiac ICUs were not included.
Types of interventions
Experimental interventions: any non‐pharmacological intervention, single or multicomponent, including cognitive training i.e. digit span forwards/backwards, letter‐number sequences, sudokus, early mobilization, modification of ICU environment through lighting or ear plugs, physical therapy or pharmacological interventions, or both, aiming to prevent ICU delirium.
Control interventions: standard ICU care defined as not providing any therapy specifically aimed at preventing ICU delirium or placebo, or both.
Types of outcome measures
Primary outcomes
The event rate of ICU delirium (assessed using the Confusion Assessment Method for the ICU (CAM‐ICU) (dichotomous yes/no), the Intensive Care Delirium Screening Checklist (ICDSC) (dichotomous yes/no), or as scores (from 0 to 8) with scores of 4 and higher indicating delirium (Van Den Boogard 2018), the Diagnostic and Statistical Manual of Mental Disorders (DSM‐IV) criteria (dichotomous yes/no), or the Neelon and Champagne Confusion Scale (NEECHAM) (dichotomous yes/no) assessed within the time frames defined in the included studies (see Differences between protocol and review).
In‐hospital mortality, at 30 days or at the longest follow‐up, or both, in the included trials.
Secondary outcomes
Number of delirium‐ and coma‐free days (assessed using CAM‐ICU, the ICDSC, the DSM‐IV criteria or the NEECHAM in combination with a validated sedation monitoring scale, e.g. the Richmond Agitation Sedation Scale (RASS), Ramsay Score, Motor Activity Assessment Scale (MAAS) assessed in the time frame defined by the included studies).
Ventilator‐free days as measured in included trials (see Differences between protocol and review).
Length of stay in the ICU.
Cognitive impairment as measured by Mini Mental State Examination (MMSE) (0 to 30, higher score indicating better cognitive function) or Dysexecutive questionnaire (DEX) (scores range from 0 to 80. Lower scores indicate better functioning at the longest follow‐up in included trials.
Adverse effects of interventions as reported in included trials.
Search methods for identification of studies
Electronic searches
We used the Cochrane Highly Sensitive Search Strategy for identifying RCTs as suggested in the Box 6.4.c, Section 6.4.11.1 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We adapted our subject‐specific MEDLINE search strategy for searching all other databases (see Appendix 1). We performed a systematic and sensitive search strategy to identify relevant RCTs without applying language or date restrictions.
We searched the Cochrane Central Register of Controlled Trials (CENTRAL; 2018, Issue 3) in the Cochrane Library; MEDLINE (OvidSP) (1950 to 11 April 2018); Embase (OvidSP) (1980 to 11 April 2018); BIOSIS (OvidSP) (1993 to 11 April 2018); International Web of Science (1964 to 11 April 2018); Latin American Caribbean Health Sciences Literature (LILACS via BIREME) (1982 to 11 April 2018); and Cumulative Index to Nursing and Allied Health Literature (CINAHL via EBSCO host) (1980 to 11 April 2018).
The search strategy was developed in consultation with the Information Specialist.
Searching other resources
We handsearched the reference list of reviews, randomized and non‐randomized trials, and editorials for additional trials. We contacted the main authors of trials and experts in the field to inquire about any missed, unreported or ongoing trials.
We searched for ongoing clinical trials and unpublished studies on the following Internet sites.
Current Controlled Trials; (Delirium AND ICU AND prevention AND RCT) 24 April 2018
ClinicalTrials.gov; (Delirium AND ICU AND prevention | Interventional Studies | Adult) 24 April 2018
CenterWatch.com.(www.centerwatch.com/clinical‐trials/listings/) 24 April 2018
Data collection and analysis
Selection of studies
Two review authors (SH and TT) independently screened identified titles and abstracts for eligibility. We retrieved the full texts of potentially relevant studies. We were not blinded to authors, institutions or the publication source of trials. We resolved disagreements through discussion. A third review author (IE) acted as arbiter if any disagreements concerning study eligibility could not be resolved by SH and TT through discussion.
Data extraction and management
We (SH and TT) independently extracted and collected data using a standardized data extraction sheet (Appendix 2). We (SH and TT) discussed and resolved any discrepancies. We contacted the corresponding authors of included trials as needed for additional information relevant to the review's outcomes and risk of bias components. For more specific information please see 'Contributions of authors'.
Assessment of risk of bias in included studies
We evaluated the risk of bias in individual studies within the following domains: random sequence generation, allocation concealment, blinding, incomplete outcome data, selective reporting, and other biases. We used the 'Risk of bias' tool described in the Cochrane Handbook for Systematic Reviews of Interventions, Section 8.5 (Higgins 2011), to assess the risk of bias in included studies. We assessed the risk of bias in the domains according to the criteria described in Appendix 3. We display the results by creating a 'Risk of bias' graph. Furthermore, we assessed the quality of the evidence across included studies for all outcomes (see Differences between protocol and review). The quality of the evidence is summarized in 'Summary of findings' tables (Higgins 2011).
Measures of treatment effect
We present the event rate of ICU delirium and in‐hospital mortality as dichotomous outcomes and the intervention effects as risk ratios (RR) with 95% confidence intervals (CI).
We present the number of delirium‐ and coma‐free days, number of ventilator‐free days, length of stay in the ICU and cognitive impairment (as a score) as continuous outcomes, and the intervention effects as mean differences (MD) with 95% CI, where possible.
For treatment effects reported as medians and interquartile ranges (IQR), we calculated means and SDs as suggested in Wan 2014, with the exception of "ventilator‐free days" where we suspected a multi‐modal distribution in the original data. Effects on "ventilator‐free days" are therefore presented as medians and IQRs.
We planned to calculate number needed to treat for an additional benefit (NNTB) for the primary outcomes.
Unit of analysis issues
This review does not include any repeated measurements or include cluster‐randomized or cross‐over trials. We therefore did not encounter any unit of analysis issues.
Dealing with missing data
Intention‐to‐treat analysis (ITT) was considered the least biased way to estimate intervention effects (Higgins 2011). Ideally, ITT should:
keep participants in the interventions to which they are randomized regardless of the intervention they actually receive and regardless of their adherence to the intervention received;
measure outcome data on all participants; and
include all randomized participants in the analysis (Higgins 2011).
The second and third principles can rarely be fulfilled without imputation of data (Higgins 2011).
In the event of missing data, we originally planned to contact the corresponding authors of the trials to potentially retrieve missing data. However this was not relevant as the majority of studies accounted for participants in flowcharts (see Differences between protocol and review).
As there were few missing data in included studies, and missing data were equally distributed across groups, we conducted an available case analysis for all outcomes (see Differences between protocol and review).
The majority of trials had less than 20% dropout. Therefore, we did not conduct sensitivity analyses exploring the effect on effect estimates of trials with high dropout rates (> 20 % dropout) as otherwise planned (see Differences between protocol and review).
Assessment of heterogeneity
We conducted meta‐analysis only if interventions in the included studies were comparable. Otherwise, we presented and summarized results narratively. For meta‐analyses, we assessed heterogeneity among studies using the I² statistic, which describes the percentage of variability in effect estimates that is due to heterogeneity between studies rather than sampling error (chance) (Higgins 2011). We used the following thresholds to guide our assessment of heterogeneity: I² between 0% and 40% might not be important; between 30% and 60% may represent moderate heterogeneity; between 50% to 90 % may represent substantial heterogeneity and 75% to 100% may represent considerable heterogeneity (Higgins 2011). Furthermore, we considered the magnitude and direction of intervention effects and the strength of the evidence for heterogeneity as expressed by the P value from the Chi² test (Higgins 2011).
Assessment of reporting biases
Reporting biases arise when the dissemination of research findings is influenced by the nature and direction of study results (Higgins 2011). We planned to use funnel plots to investigate publication bias; however due to the inclusion of fewer than 10 studies in all meta‐analyses, we refrained from using funnel plots (see Differences between protocol and review).
Data synthesis
We used Review Manager 5 software (RevMan 5.3) as the statistical software. In case of three‐arm trials, where interventions were judged to be comparable, we compared usual care to the combined intervention arms. In case of three‐arm trials where interventions were judged to differ substantially, we first compared arm one with arm two, and subsequently arm one with arm three. For outcomes where we did not encounter heterogeneity, we summarized the effects across studies using the random‐effects meta‐analysis. For outcomes with moderate to substantial heterogeneity, or when studies were incomparable, we summarized effects narratively.
Subgroup analysis and investigation of heterogeneity
We planned to conduct exploratory subgroup analyses of the effects of pharmacological versus non‐pharmacological interventions, medical versus surgical ICU patients and, if possible, of early intervention (defined as initiation of the intervention within 36 hours after ICU admission) versus late intervention (defined as initiation of the intervention 36 hours or later after ICU admission). None of these analyses were possible due to the diversity of interventions in the included studies (see Differences between protocol and review).
Sensitivity analysis
We planned to conduct sensitivity analyses excluding trials with more than 20% dropouts to explore any potential impact of missing data on overall effects (see also 'Dealing with missing data). Furthermore, we planned to conduct a sensitivity analysis excluding trials assessed to be at high risk of bias. However, none of the studies had dropout rates exceeding 20%, and no studies were overall at high risk of bias (see Differences between protocol and review).
'Summary of findings' table and GRADE
We used the principles of the GRADE system (Guyatt 2008), to assess the quality of the body of evidence for the following outcomes.
The event rate of ICU delirium.
In‐hospital mortality.
Number of delirium‐ and coma‐free days.
Ventilator‐free days as measured in included trials.
Length of stay in the ICU.
Cognitive impairment as measured at the longest follow‐up in included trials.
Adverse effects of interventions as reported in included trials.
We constructed 'Summary of findings' tables using the GRADE software for clinically important comparisons (GRADEpro). As haloperidol is a frequently‐used pharmacological intervention, and physical and cognitive therapy is a commonly‐used non‐pharmacological intervention, we constructed 'Summary of findings' tables for those two comparisons (see Differences between protocol and review).
We (TT and SH) used GRADEpro to assess all comparisons. The GRADE approach appraises the quality of the body of evidence based on the extent to which one can be confident that an estimate of effect or association reflects the item being assessed. The quality of the body of evidence was considered according to the following criteria: within study risk of bias (methodologic quality); the directness of the evidence; heterogeneity of the data; precision of effect estimates; and risk of publication bias.
Results
Description of studies
See Included studies and Excluded studies
Results of the search
See Figure 1
1.
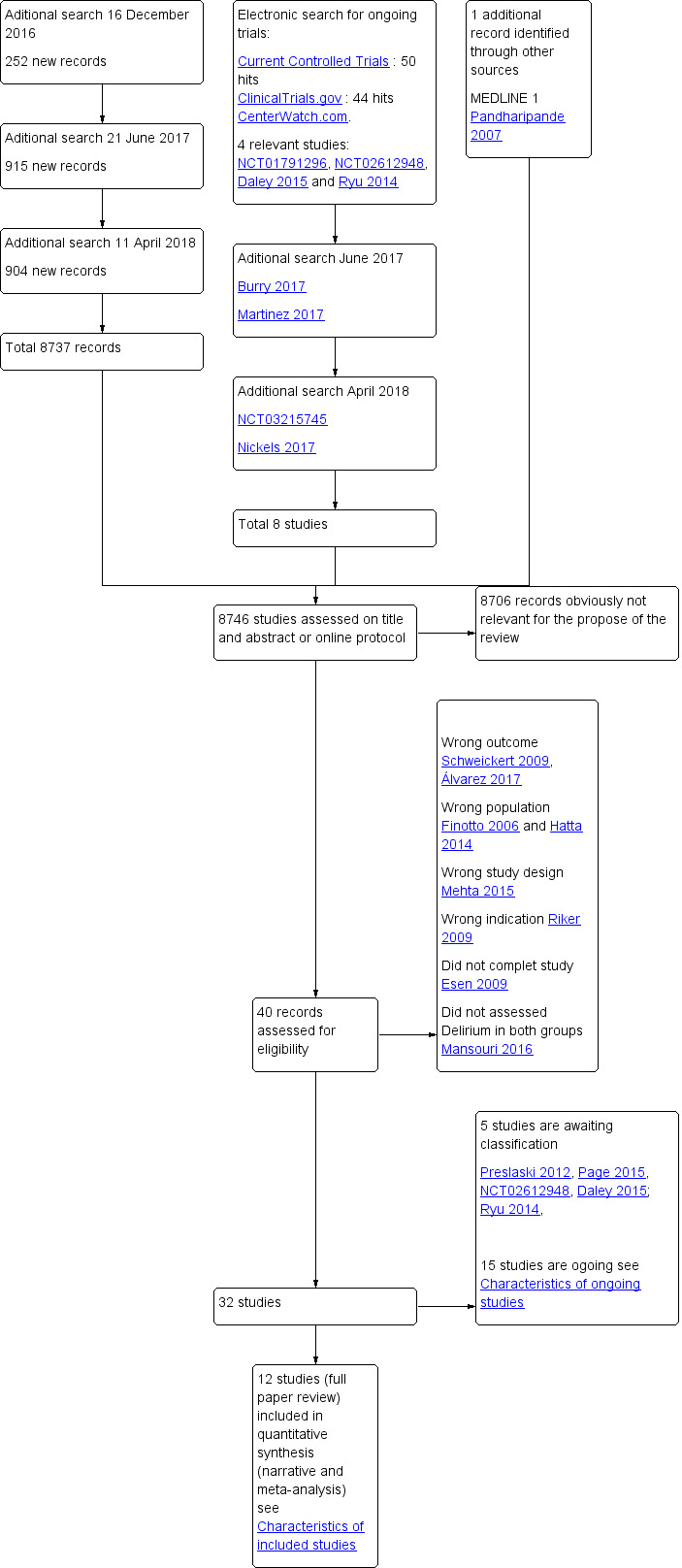
Study flow diagram.
Our electronic search yielded a total of 8746 records. Handsearching of reference lists revealed an additional potentially relevant study (Pandharipande 2007). Two review authors (TT and SH) independently screened the titles and abstracts. After excluding obviously irrelevant records, we retrieved 40 potentially relevant records for full‐text assessment. Following full‐text reading of these records, we excluded a total of eight records for the following reasons.
Riker 2009 evaluated interventions for treating rather than preventing intensive care unit (ICU) delirium.
Mehta 2015 was not a randomized controlled trial (RCT).
Hatta 2014 did not solely include ICU participants.
Finotto 2006 included cardiac ICU patients.
Esen 2009 did not complete the study.
Mansouri 2016 did not assess delirium in both groups.
Schweickert 2009 did not assess any outcomes defined the same as the ones needed for this review.
Álvarez 2017 measured delirium with the confusion assessment method (CAM) not the confusion assessment method for the ICU (CAM‐ICU) or other tools relevant for this review.
Overall, our searches resulted in the inclusion of 12 completed RCTs. We classified a further 15 studies as ongoing, and five studies are awaiting classification due to current lack of study information.
We contacted the authors of all identified ongoing studies and studies awaiting classification, as well as Abdelgalel 2016, Finotto 2006 and Schweickert 2009, in order to clarify methodological issues relevant for potential inclusion of their trials in this review.
Included studies
We included 12 studies based on a total of 3885 randomized participants (Abdelgalel 2016; Brummel 2014b; Mehta 2012; Moon 2015; Nassar 2014; Page 2013; Pandharipande 2007; Shehabi 2013; Simons 2016; Strøm 2010; Van Den Boogard 2018;Van Rompaey 2012).
Study periods and settings
The 12 studies were conducted between 2004 and 2018 and originated in Egypt (Abdelgalel 2016), USA (Brummel 2014a, Pandharipande 2007), USA and Canada (Mehta 2012), the Republic of Korea (Moon 2015), Brazil (Nassar 2014), UK (Page 2013), Australia and New Zealand (Shehabi 2013), the Netherlands (Simons 2016, Van Den Boogard 2018), Denmark (Strøm 2010), and Belgium (Van Rompaey 2012).
Eight of the 12 studies were single centre; three were multicentre, and one was an international multicentre study. Two studies were small feasibility studies.
Interventions
The pharmacological interventions included haloperidol and dexmedetomidine. Sedation interventions included sedation using dexmedetomidine with daily interruption of sedation and a regimen of no sedation. We found studies that tested physical or cognitive therapy interventions or both, environmental interventions with changes in light or sound/hearing (earplugs), and one trial was a preventive nursing care intervention.
Controls
The control interventions also varied and included placebo and standard care; the latter was not described in detail. The event rate of ICU delirium was the primary outcome in four trials (Abdelgalel 2016; Moon 2015; Simons 2016; Van Rompaey 2012); while five trials had the event rate of ICU delirium as a secondary outcome (Mehta 2012; Nassar 2014; Pandharipande 2007; Shehabi 2013; Van Den Boogard 2018). Strøm 2010 also evaluated the event rate of ICU delirium, but this outcome was not predefined.
Types of participants
Eleven studies enrolled critically ill adults who were mechanically ventilated, and one included elderly non‐mechanically ventilated participants (Abdelgalel 2016). The participants were recruited from mixed medical and surgical ICUs (Brummel 2014b; Page 2013; Pandharipande 2007; Simons 2016; Strøm 2010); a mixed ICU including cardiac‐surgical patients (Van Rompaey 2012); mixed ICU including trauma patients (Mehta 2012); a low nurse staffed (nurse‐to‐patient ratio 1:6, and nursing assistant‐to‐patient ratio was 1:2 on all shifts) multidisciplinary six‐bed ICU with participants from the emergency department, surgical room and from the ward (Nassar 2014); and a large ICU with 105 beds covering both medical and surgical participants (Moon 2015). Finally, three studies did not specify the type of ICU from which they recruited (Abdelgalel 2016, Shehabi 2013, Van Den Boogard 2018).
The ages of the study participants across the studies ranged from mean 48 to 70 years; between 48% and 74% were men. The Acute Physiology and Chronic Health Evaluation (APACHE) II score ranged from 14 to 28 (range 0 to 71 with higher scores corresponding to more severe disease and a higher risk of death).
For study‐specific inclusion and exclusion criteria see the Characteristics of included studies
Funding
In four of the 12 included studies (Abdelgalel 2016; Nassar 2014; Simons 2016; Van Rompaey 2012), the source of funding was unclear; two others stated funding of co‐authors of papers, however not of the specific study (Brummel 2014b; Pandharipande 2007). Only four of the 12 studies declared their possible conflicts of interest (Brummel 2014b; Page 2013; Pandharipande 2007; Van Den Boogard 2018). Five studies stated that the authors had no conflicts of interest (Mehta 2012; Nassar 2014; Simons 2016; Strøm 2010; Van Rompaey 2012).
Excluded studies
We excluded eight studies (Álvarez 2017; Esen 2009; Finotto 2006; Hatta 2014; Mansouri 2016; Mehta 2015; Riker 2009; Schweickert 2009).
See Characteristics of excluded studies for more details.
Studies awaiting classification
Five studies are awaiting classification as the information available was insufficient for determining eligibility for inclusion (Daley 2015; NCT02612948; Page 2015, Preslaski 2012; Ryu 2014).
Daley 2015 published a protocol for an RCT testing the effect of 12.5 mg prophylactic quetiapine every 12 hours versus no prophylaxis on the incidence of delirium.
NCT02612948 published a protocol for an RCT testing the effect of dexmedetomidine versus propofol on the duration of mechanical ventilation.
Ryu 2014 published a protocol for an RCT of the effect of low‐dose dexmedetomidine versus placebo on the incidence of delirium.
Page 2015 reported long‐term follow‐up data from the original RCT (Page 2013), testing the effect of haloperidol 2.5 mg every eight hours against placebo (0.9% saline) on cognitive status by the "The Modified Telephone Interview for Cognitive Status" (TICS‐M) and health‐related quality of life.
Preslaski 2012 tested the effect of dexmedetomidine versus midazolam in medical and surgical ICU patients on time to extubation and, secondly, the incidence of delirium. Our attempts to contact the corresponding author or other responsible parties for further information have been unsuccessful so far
See Characteristics of studies awaiting classification for more details
Ongoing studies
We identified 15 ongoing RCTs testing diverse interventions.
Four of the 15 are RCTs of pharmacological interventions (Burry 2017; Jerath 2015; Martinez 2017; NCT01791296). Three studies are testing sedation regimens (Nedergaard 2016; Toft 2014, NCT01739933). Two ongoing studies are testing physical or occupational therapy or combined interventions (Nickels 2017; Thomas 2015). Five of the 15 ongoing studies are testing environmental interventions (Miles 2012; NCT03095443; NCT03215745; NCT03125252; Wassenaar 2017). One study is testing different family visitation models NCT02932358.
We contacted the authors of all ongoing trials for study status; six responded, as follows.
Jerath 2015 is ongoing.
NCT01739933 reported that enrolment was halfway.
NCT01791296 and Nedergaard 2016 reported that recruitment was completed and they were about to begin analysis of data.
Thomas 2015 is completing follow‐up.
Toft 2014 responded that the "Non‐sedation versus sedation with a daily wake‐up trial in critically ill patients receiving mechanical ventilation" (NONSEDA trial) is still recruiting.
See the Characteristics of ongoing studies for more information.
Risk of bias in included studies
Figure 2 summarizes the 'Risk of bias' assessments within and across studies.
2.
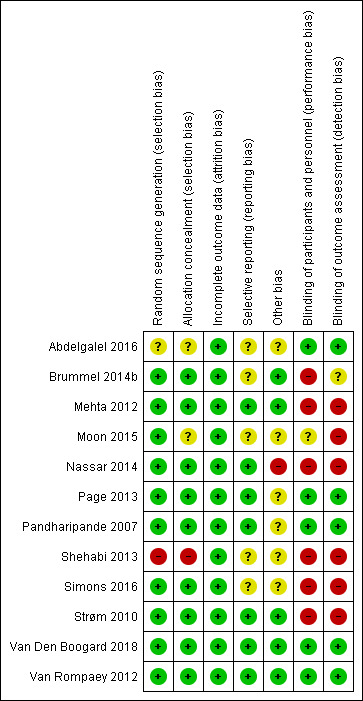
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
The majority of studies were assessed to be at low risk of selection bias with the exception of three (Abdelgalel 2016; Moon 2015; Shehabi 2013). Abdelgalel 2016 provided no description of either sequence generation, or allocation concealment. Moon 2015 used opaque assignment cards indicating assignment to the groups, placed and shuffled them in a large envelope. The leader of the nursing team drew a card from the large envelope for selection and did not replace it afterwards. Theoretically, this could enable prediction of allocation of the last participants recruited into the study. Shehabi 2013 used concealed envelopes, however it was unclear if they were opaque and opened sequentially. Furthermore, they used block randomization in a small sample (n = 37) which we assessed could increase the possibility of foreseeing the next allocation.
Blinding
We assessed the majority of studies to be at high risk of performance or detection bias, or both, due to the difficulty of blinding participants and staff to allocations (specifically in trials testing non‐pharmacological interventions), and lack of blinding of outcome assessors. The risk of performance bias was high in six studies due to lack of blinding of participants and staff (Brummel 2014b; Mehta 2012; Nassar 2014; Shehabi 2013; Simons 2016; Strøm 2010). Moon 2015 did not explicitly state any measures taken to blind participants/staff; we therefore assessed the trial to be at unclear risk of performance bias. Van Rompaey 2012 blinded staff to allocations and was therefore assessed at low risk of performance bias (earplugs versus no earplugs). The risk of detection bias was deemed to be high in six studies due to lack of blinding of outcome assessors (Mehta 2012; Moon 2015; Nassar 2014; Shehabi 2013; Simons 2016; Strøm 2010). We judged Brummel 2014b to be at unclear risk of detection bias because delirium was presumably assessed by unblinded staff while cognitive, functional and health‐related quality of life after discharge was assessed blinded. Five studies were judged at low risk of detection bias due to blinding of outcome assessors (Abdelgalel 2016; Page 2013; Pandharipande 2007; Van Den Boogard 2018; Van Rompaey 2012).
Incomplete outcome data
All included studies were judged to be at low risk of attrition bias. Initially, we judged Van Den Boogard 2018 as unclear risk of other bias due to missing data on delirium outcomes; only 14 of 21 participating sites assessed delirium incidence and coma‐free days (a secondary outcome in the study) due to lack of research staff. Van Den Boogard 2018 informed us that data on delirium were provided for 84.2% of included patients and further forwarded data showing that missing data on the delirium outcomes were evenly distributed between groups. This is why we now judge it to be low risk of bias.No studies exceeded a 20% dropout rate. Overall, dropouts were equally distributed across interventions in all studies. Six studies analysed data using intention‐to‐treat (ITT) analysis (Brummel 2014b; Mehta 2012; Nassar 2014; Page 2013; Pandharipande 2007; Strøm 2010). Simons 2016 and Van Den Boogard 2018 analysed data using both ITT analysis and a per‐protocol analysis. Four studies analysed data using available cases (Abdelgalel 2016;Moon 2015;Shehabi 2013;Van Rompaey 2012).
Selective reporting
We assessed reporting bias by checking available online protocols against the published papers. Six of the included studies fully complied with the online protocols in the published papers and were therefore assessed at low risk of reporting bias (Nassar 2014; Page 2013; Pandharipande 2007; Strøm 2010; Van Den Boogard 2018; Van Rompaey 2012) .
We were unable to locate an online protocol for the study by Abdelgalel 2016, and therefore judged the study to be at unclear risk of selective reporting. Brummel and colleagues described a considerable number of secondary neurocognitive and functional outcomes measures in the online protocol NCT01270269 as well as in a protocol article (Brummel 2012). These were not, however, reported in the published paper (Brummel 2014b). Therefore we judged Brummel 2014b, to be at unclear risk of reporting bias.
Mehta 2012 listed patient recall of their ICU stay as a secondary outcome in the online protocol; these data were reported in a later publication (Burry 2015). Therefore, we judged the risk of reporting bias to be low.
We judged Moon 2015, at unclear risk of reporting bias as they did not have an online protocol.
We assessed Simons 2016 at unclear risk of reporting bias. In the online protocol NCT01274819, Simons and colleagues stated that serum levels of inflammatory markers would be assessed as well as Health‐Related Quality of Life three and six months after discharge; these outcomes were not, however, reported in the published paper (Simons 2016).
We judged Shehabi 2013, at unclear risk of reporting bias as they did not define outcomes in their online protocol.
Other potential sources of bias
Industrial interest could potentially pose a risk of bias in studies testing pharmacological interventions. Three studies (Page 2013 (haloperidol ‒ GSK); Pandharipande 2007 (dexmedetomidine ‒ Orion and lorazepam ‒ Hospira); and Shehabi 2013 (dexmedetomidine ‒ Orion)) listed many honoraria to study authors. Shehabi 2013 stated in the online study report ACTRN 12611000166976 that Hospira Pty Ltd donated an unrestricted grant of USD 100,000.
Three studies disclosed that the industry had no role in design, data collection, analysis and interpretation or publication (Page 2013, Pandharipande 2007; Shehabi 2013). Hospira financed the study drug in Shehabi 2013.
Abdelgalel 2016 (haloperidol ‒ GSK; and dexmedetomidine ‒ Orion) reported no conflict of interest and did not mention any co‐operation with industry despite testing two specific drugs.
We judged that Moon 2015 was at potential risk of bias due to possible contamination or 'spill‐over' between groups. One group was treated with a preventive delirium protocol focusing on environmental factors and early therapeutic nursing interventions while the control group received usual care apparently in the same ICU and, possibly, next to intervention patients. The authors did not describe any measures taken to minimise spill‐over between groups other than placing a small sticker on the corner of the bedside to identify intervention group patients.
Effects of interventions
Summary of findings for the main comparison. Haloperidol compared to placebo for preventing intensive care unit delirium.
| Haloperidol compared to placebo for preventing intensive care unit delirium | ||||||
| Patient or population: critically ill patients 18 years or older demanding mechanical ventilation within the first 72 hours (Page 2013) or anticipated needing ICU admission at least two days (Van Den Boogard 2018). Setting: in a general mixed medical‐surgical ICU in the UK and in 21 (non specified) ICUs in the Netherlands. Intervention: haloperidol 2.0/2.5 mg/ every 8 hours Comparison: placebo (0.9 % saline) every 8 hours | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with haloperidol | |||||
| The event rate of intensive care delirium within 28 days. | Study population | RR 1.01 (0.87 to 1.17) | 1439 (1 RCT) | ⊕⊕⊕⊝
Moderate ,2 |
Only measured in one study Downgraded one level due to imprecision (wide 95% CI) |
|
| 330 per 1000 | 333 per 1000 (287 to 386) | |||||
| In‐hospital mortality within 28 days. | Study population | RR 0.98 (0.80 to 1.22) | 1580 (2 RCTs) | ⊕⊕⊕⊝
Moderate 3 |
Downgraded one level due to imprecision (less than 300 events and wide 95% CI). | |
| 180 per 1000 | 177 per 1000 (144 to 218) | |||||
| Number of delirium ‐ and coma‐free days (within 28 days) measured by CAM‐ICU or ICDSC | The range of mean number of delirium ‐ and coma‐free days was 15 to 24 days. | MD 0.60 lower (1.37 lower to 0.17 higher) | ‐ | 1580 (2 RCTs) | ⊕⊕⊕⊝
Moderate 2 |
Downgraded one level for indirectness (primary outcome was survival and not delirium) |
| Ventilator‐free days (within the first 28 days) | The mean ventilator‐free days (within the first 28 days) was 23.8 | MD 0.30 lower (0.93 lower to 0.33) | 1439 (1 RCT) | ⊕⊕⊕⊕ High | ||
| Length of ICU stay (days) | The range of mean length of ICU stay was 5 to 11 days. | MD 0.25 higher (0.28 lower to 0.77 higher) | ‐ | 1580 (2 RCTs) | ⊕⊕⊕⊕ High | |
| Cognitive impairment | not estimable | (studies) | ‐ | Not measured in any study | ||
| Adverse events | Serious adverse events were reported 3 incidents in the intervention group (fast atrial fibrillation with hypotension (n = 1), readmission to ICU with sepsis (n = 1), failed extubation (n = 1)) and 5 in placebo group (apnoea post treatment for agitation (n = 1), readmission to ICU with sepsis (n = 1), failed extubation (n = 3) in Page 2013. Five serious adverse events were reported, three patients died, one in each group. The events were judged to be unrelated to the study medication. Two patients in the 1 mg haloperidol group and 1 patient in the 2 mg haloperidol group developed momorphic ventricular tachycardia, 1 patient in the 2 mg haloperidol group developed refractory shock, 1 patient in the placebo group developed a suspected malignant neuroleptic syndrome event (Van Den Boogard 2018) |
‐ | 1580 (2 RCTs) | ⊕⊕⊕⊝ Moderate4 | Downgraded one level due imprecision (few events) | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CAM‐ICU: confusion assessment method for the ICU; CI: Confidence interval; ICDSC: Intensive Care Delirium Screening Checklist; MD: mean difference; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1
2 Downgraded due to indirectness: Indirect outcome as the primary outcome of Van den Boogaard study was 28 days survival, not delirium. This focus might have played a part in the missing collection of data on delirium outcomes.
3. Downgraded due to impression (less than 300 events)
4 Downgrade one level due to few events
Summary of findings 2. Physical and cognitive therapy compared to standard care for preventing intensive care unit delirium.
| Physical and cognitive therapy compared to standard care for preventing intensive care unit delirium | ||||||
|
Patient or population: critically ill patients 18 years or older treated for respiratory failure or septic or both, cardiogenic or haemorrhagic shock who resided within 120 miles of the city of hospital. Setting: Nashville, Tennesee, USA Intervention: One daily physical therapy session (passive ROM, active exercise, sit at edge of bed, stand/transfer, ADL training and walk). Duration of physical therapy session is not described combined with one daily physical therapy session and 20 minutes. cognitive therapy sessions twice‐daily during hospitalization. Patients exhibiting impaired executive functioning or impaired functional mobility continued outpatient cognitive therapy for six weeks (6 sessions) using goal management training. Comparison: usual care (approximately physical therapy once every six days) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with standard care | Risk with physical and cognitive therapy | |||||
| The event rate of ICU delirium measured by the CAM‐ICU | not estimable | ‐ | ‐ | Outcome not measured by included study | ||
| In‐hospital mortality at 30 days or at the longest follow‐up or both. | Study population | RR 0.94 (0.40 to 2.20) | 65 (1 RCT) | ⊕⊝⊝⊝ Very Low 1 2 |
Downgraded two levels due to very serious imprecision (one study, few events), and one level due to indirectness (feasibility study of a cognitive therapy protocol). | |
| 273 per 1000 | 256 per 1000 (109 to 600) | |||||
| Number of delirium‐ and coma‐free days (within the first 30 days) measured by the CAM‐ICU | The mean number of delirium‐ and coma‐free days (30 days) was 23.3 days | 2.8 days lower (10.1 lower to 4.6 higher) | ‐ | 65 (1 RCT) | ⊕⊝⊝⊝ Very low 1 2 3 | Downgraded two levels due to very serious imprecision (one study, few events), and one level due to indirectness (feasibility study of a cognitive therapy protocol).Downgraded one level due to risk of bias (not clearly stated whether assessment of outcome was blinded) |
| Ventilator‐free days (within the first 30 days) | median 27.4 (0.0 to 29.2) | median 25.3 (0 to 28.9) | ‐ | 65 (1 RCT) | ⊕⊝⊝⊝ Very low 1 2 3 | Downgraded two levels due to very serious imprecision (one study, few events), and one level due to indirectness (feasibility study of a cognitive therapy protocol). Downgraded one level due to risk of bias (not clearly stated wether assessment of outcome was blinded) |
| Length of ICU stay (days) | The median length of stay in the ICU was 4.0 days | The median length of stay was 7.9 days | MD 1.23 higher (0.68 lower to 3.14 higher) | 65 (1 RCT) | ⊕⊝⊝⊝ Very low 1 2 3 | Downgraded two levels due to very serious imprecision (one study, few events), and one level due to indirectness (feasibility study of a cognitive therapy protocol). Downgraded one level due to risk of bias (not clearly stated wether assessment of outcome was blinded) |
| Cognitive functioning a): Global functioning (MMSE score 0 ‐ 30, higher score indicating better cognitive function) | The mean cognitive status (MMSE) was 28 points | 0.97 points higher (0.19 lower to 2.13 higher) | ‐ | 30 (1 RCT) | ⊕⊝⊝⊝ Very low 1 2 3 | Downgraded two levels due to very serious imprecision (one study, few events), and one level due to indirectness (feasibility study of a cognitive therapy protocol). Downgraded one level due to risk of bias (not clearly stated whether assessment of outcome was blinded) |
| Cognitive functioning b) Executve functioning (DEX score) (scores range from 0 to 80. Lower scores indicate better functioning) (DEX) | The mean cognitive status (DEX) was 18 points | 8.76 points lower (19.06 lower to 1.54 higher) | ‐ | 30 (1 RCT) | ⊕⊝⊝⊝ Very low 1 2 3 | Downgraded two levels due to very serious imprecision (one study, few events), and one level due to indirectness (feasibility study of a cognitive therapy protocol). Downgraded one level due to risk of bias (not clearly stated wether assessment of outcome was blinded) |
| Adverse events | One patient experienced acute back pain accompanied by hypotensive urgency during physical therapy. | ‐ | 65 (1 RCT) | ⊕⊝⊝⊝ Very low 1 2 3 | Downgraded two levels due to very serious imprecision (one study, few events), and one level due to indirectness (feasibility study of a cognitive therapy protocol).Downgraded one level due to risk of bias (not clearly stated wether assessment of outcome was blinded) | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CAM‐ICU: confusion assessment method for the ICU; CI: Confidence interval; DEX: Dysexecutive questionnaire; ICU: intensive care unit; OR: MMSE: Mini‐Mental State Examination; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Downgraded two levels due to serious impression:single study with few events in a small sample.
2 Downgraded one level due to indirectness: this was primarily a feasibility study of a cognitive therapy protocol
3 Downgraded one level due to risk of bias: not clearly stated wether assessment of outcome was blinded
1. Pharmacological interventions
1a. Haloperidol versus placebo
Two studies tested haloperidol against placebo (Page 2013; Van Den Boogard 2018). Page 2013 tested early treatment with intravenous haloperidol 2.5 mg every eight hours initiated within 72 hours of ICU admission in 142 participants, mean age 68 years (58% were male), irrespective of coma or delirium status, and given until discharge from ICU, or until the participants were delirium‐free for two consecutive days, or up to 14 days versus placebo (intravenous 0.9% saline). Van Den Boogard 2018 tested prophylactic treatment with haloperidol three times daily intravenously in a three‐arm parallel RCT. Participants were given either 1 mg (n = 350) or 2 mg (n = 732) of haloperidol or placebo (n = 707) consisting of 0.9% sodium chloride. The first dose of the study medication was administered as soon as possible within 24 hours of ICU admission and continued to day 28, or until ICU discharge (whichever came first), or until delirium occurred. The 1 mg haloperidol group was stopped prematurely due to futility (Van Den Boogard 2018). Therefore, we have included data from the 2 mg haloperidol group (n = 737) and the placebo group (n = 707) in this review.
Event rate of ICU delirium within 28 days
Van Den Boogard 2018 assessed the event rate of ICU delirium in 1439 participants, mean age 66.9 years, 63% males. There was no difference between groups in the event rate of delirium (risk ratio (RR) 1.01, 95% CI 0.87 to 1.17, P = 0.88; Table 1).
In‐hospital mortality within 28 days
There was no difference between groups for in‐hospital mortality (RR 0.98, 95% CI 0.80 to 1.22, P = 0.88, n = 1580, 2 studies; Table 1; Figure 3) .
3.
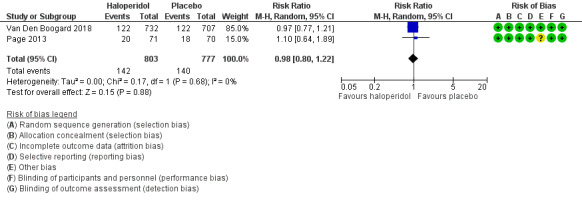
Forest plot of comparison: 1 Haloperidol versus placebo, outcome: 1.2 In‐hospital mortality.
Number of delirium‐ and coma‐free days
There was no difference in number of delirium‐ and coma‐free days (MD ‐0.60, 95% CI ‐1.37 to 0.17, P = 0.13, n = 1580, 2 studies; Table 1).
Ventilator‐free days
Page 2013 found no effect on ventilator‐free days (n = 141), median days 21 (interquartile range (IQR) 0 to 25) in intervention group versus 17 (IQR 0 to 25) in placebo. Van Den Boogard 2018 likewise found no difference between groups in ventilator‐free days (MD −0.30, CI ‐0.93 to 0.33, P = 0.35, n = 1439; Table 3).
1. Effect estimates for orphan studies.
| Comparison | Outcome | n | Statistical method | Effect estimate CI 95%) | P value | Study |
| Dexmedetomidine vs. lorazepam | The event rate of ICU delirium | 103 | Risk ratio | 0.96 (0.76 to 1.16) | 0.65 | Pandharipande 2007 |
| Dexmedetomidine vs. lorazepam | In‐hospital mortality | 103 | Risk ratio | 1.59 (0.75 to 3.33) | 0.22 | Pandharipande 2007 |
| Dexmedetomidine vs. lorazepam | Number of delirium‐ and coma‐free days (within 12 days) | 103 | Mean difference | 2.67 (0.58 to 4.76) | 0.01 | Pandharipande 2007 |
| Dexmedetomidine vs. lorazepam | Length of stay in the ICU | 103 | Mean difference | ‐0.50 (‐3.89 to 2.89) | 0.77 | Pandharipande 2007 |
| Haloperidol vs. placebo | Ventilator‐free days | 1439 | Mean difference | ‐0.30 (‐0.93 to 0.33) | 0.35 | Van Den Boogard 2018 ‐ unpublished data |
| Dexmedetomidine vs. haloperidol | The event rate of ICU delirium | 60 | Risk ratio | 0.3 (0.09 to 0.98) | 0.047 | Abdelgalel 2016 |
| Dexmedetomidine vs. haloperidol | In‐hospital mortality | 60 | Risk ratio | 1.00 (0.15 to 6.64) | 1.0 | Abdelgalel 2016 |
| Dexmedetomidine vs. haloperidol | Length of stay in the ICU | 60 | Mean difference | ‐3.40 (‐3.79 to ‐3.01) | < 0.00001 | Abdelgalel 2016 |
| Dexmedetomidine vs. haloperidol | Adverse events | 60 | Risk ratio | 0.40 (0,08 to 1.90) | 0.25 | Abdelgalel 2016 |
| Dexmedetomidine vs. placebo (saline) | The event rate of ICU delirium | 60 | Risk ratio | 0.23 (0.07 to 0.73) | 0.01 | Abdelgalel 2016 |
| Dexmedetomidine vs. placebo (saline) | In‐hospital mortality | 60 | Risk ratio | 0.67 (0.12 to 3.71) | 0.64 | Abdelgalel 2016 |
| Dexmedetomidine vs. placebo (saline) | Length of stay in the ICU | 60 | Mean difference | ‐3.80 (‐4.25 to ‐3.35) | < 0.00001 | Abdelgalel 2016 |
| Dexmedetomidine vs. placebo (saline) | Adverse events | 60 | Risk ratio | 1.00 (0.15 to 6.64) | 1.0 | Abdelgalel 2016 |
| Early goal directed sedation vs. standard sedation | The event rate of ICU delirium | 37 | Risk ratio | 1.02 (0.44 to 2.34) | 0.97 | Shehabi 2013 |
| Early goal directed sedation vs. standard sedation | In‐hospital mortality | 37 | Risk ratio | 1.14 (0.22 to 6.05) | 0.88 | Shehabi 2013 |
| Early goal directed sedation vs. standard sedation | Ventilator‐free days | 37 | Mean difference | 1.20 (‐5.12 to 7.52) | 0.71 | Shehabi 2013 |
| Early goal directed sedation vs. standard sedation | Length of stay in the ICU | 37 | Mean difference | 0.30 (‐2.97 to 3.57) | 0.86 | Shehabi 2013 |
| Early goal directed sedation vs. standard sedation | Adverse events | 37 | Risk ratio | 3.86 (0.20 to 75.28) | 0.37 | Shehabi 2013 |
| No sedation vs. sedation with daily interruption | The event rate of ICU delirium | 113 | Risk ratio | 2.90 (0.98 to 8.57) | 0.05 | Strøm 2010 |
| No sedation vs. sedation with daily interruption | In‐hospital mortality | 113 | Risk ratio | 0.78 (0.50 to 1.22) | 0.28 | Strøm 2010 |
| No sedation vs. sedation with daily interruption | Ventilator‐free days | 113 | Mean difference | 4.20 (0.32 to 8.08) | 0.034 | Strøm 2010 |
| No sedation vs. sedation with daily interruption | Length of stay in the ICU | 113 | Mean difference | ‐5.20 (‐8.48 to ‐1.92) | 0.002 | Strøm 2010 |
| No sedation vs. sedation with daily interruption | Adverse events | 113 | Risk ratio | 1.23 (0.44 to 3.43) | 0.69 | Strøm 2010 |
| Sedation with daily interruption vs. sedation | Number of delirium‐ and coma‐free days | 60 | Mean difference | 1.00 (‐0.94 to 2.94) | 0.31 | Nassar 2014 |
| ICU‐delirium prevention protocol vs. non preventive nursing care | The event rate of ICU delirium | 123 | Risk ratio | 0.60 (0.32 to 1.11) | 0.10 | Moon 2015 |
| ICU‐delirium prevention protocol vs. non preventive nursing care | In‐hospital mortality | 123 | Risk ratio | 0.38 (0.13 to 1.13) | 0.08 | Moon 2015 |
| ICU‐delirium prevention protocol vs. non preventive nursing care | Length of stay in the ICU | 123 | Mean difference | 0.80 (‐3.01 to 4.61) | 0.68 | Moon 2015 |
| Physical and cognitive therapy vs. standard care | In‐hospital mortality | 65 | Risk ratio | 0.94 (0.40 to 2.20) | 0.88 | Brummel 2014b |
| Physical and cognitive therapy vs. standard care | Number of delirium‐ and coma‐free days (within 30 days) | 65 | Mean difference | ‐2.77 (‐10.09 to 4.55) | 0.46 | Brummel 2014b |
| Physical and cognitive therapy vs. standard care | Length of stay in the ICU | 65 | Mean difference | 1.23 (‐0.68 to 3.14) | 0.21 | Brummel 2014b |
| Physical and cognitive therapy vs. standard care | Cognitive impairment (MMSE score) | 30 | Mean difference | 0.97 (‐0.19 to 2.13) | 0.10 | Brummel 2014b |
| Physical and cognitive therapy vs. standard care | Cognitive impairment (DEX score) | 30 | Mean difference | ‐8.76 (‐19.06 to 1.54) | 0.1 | Brummel 2014b |
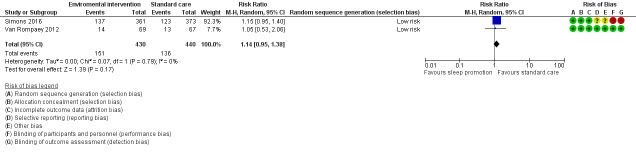
| Enviromental intervention vs standard care | In‐hospital mortality | 734 | Risk ratio | 0.93 (0.96 to 1.26) | 0.66 | Simons 2016 |
| Enviromental intervention vs standard care | Number of delirium‐ and coma‐free days (within 28 days) | 734 | Mean difference | 0.06 (‐1.18 to 1.30) | 0.92 | Simons 2016 |
| Enviromental intervention vs standard care | Length of stay in the ICU | 734 | Mean difference | ‐0.33 (‐1.03 to 0.37) | 0.36 | Simons 2016 |
CI: confidence interval; DEX: Dysexecutive questionnaire; ICU: intensive care unit; MMSE: Mini‐Mental State Examination,
Length of stay in the ICU
The authors found no effect on length of stay in the ICU (MD 0.18, 95% CI ‐0.60 to 0.97, P = 0. 64, n = 1580, 2 studies; Table 1).
Cognitive impairment
The studies did not assess this outcome.
Adverse events
Page 2013 reported prolonged QT intervals, extrapyramidal effects and serious adverse events related to the experimental drug, haloperidol. Three serious adverse events (fast atrial fibrillation with hypotension, readmission to the ICU with sepsis, failed extubation) were reported in the intervention group; and five (apnoea post treatment for agitation, readmission to the ICU due to sepsis and three cases of failed extubation) in the control group.
Van Den Boogard 2018 reported five serious adverse events. Three participants died, one in each group, two participants in the 1 mg haloperidol group and one participant in the 2 mg haloperidol group had monomorphic ventricular tachycardia, one participant in the 2 mg haloperidol group developed refractory shock. One participant in the placebo group had a suspected malignant neuroleptic syndrome event. The adverse events were judged to be unrelated to the study medication.
We judged the quality of the evidence high for ventilator‐free days and length of ICU stay; moderate for in‐hospital mortality,adverse events, the event rate of ICU delirium and number of coma‐ and delirium‐free days. (Table 1). (See Characteristics of included studies).
1b. Dexmedetomidine versus lorazepam
Pandharipande 2007 examined the effect of dexmedetomidine versus lorazepam, both administered as infusions in 106 ICU participants (n = 103 were included in final analysis) with mean age of 60 years (52% were male). The drugs were infused until extubation or for a maximum of 120 hours and titrated to achieve the sedation goal set by the treating clinician using the Richmond Agitation and Sedation scale (RASS) score.
Event rate of ICU delirium
They found no effect on the event rate of ICU delirium (RR 0.96, 95% CI 0.76 to 1.16; P = 0.65, n = 103, 1 study; Table 3).
In‐hospital mortality
They found no effect on in‐hospital mortality (RR 1.59, 95% CI 0.75 to 3.33; P = 0.22, n = 103, 1 study; Table 3).
Number of delirium‐ and coma‐free days
In the dexmedetomidine group, participants (n = 103) had more delirium‐ and coma‐free days within 12 days (MD 2.67, 95% CI 0.58 to 4.76; P = 0.01; Table 3).
Ventilator‐free days
They found no effect on ventilator‐free days (n = 103) (median days 22 (IQR 0 to 24) versus 18 (IQR 0 to 23), (P = 0.22).
Length of stay in the ICU
They found no effect on length of stay in the ICU (n = 103), (MD ‐0.50, 95% CI ‐3.89 to 2.89; P = 0.77; Table 3).
We considered the quality of the evidence low for these outcomes, as there was only one study; the comparison was benzodiazapine, not placebo; and there were few events
Cognitive impairment
Pandharipande 2007 undertook neuropsychological testing of participants within 72 hours of discharge from the ICU if participants were CAM‐ICU negative. Tests included the Mini Mental State Examination (MMSE) and the Trail‐B test administered by research nurses. The dexmedetomidine and lorazepam groups did not differ in cognitive function (n = not specifically stated); median MMSE score of 28 in the dexmedetomidine group versus 27 in lorazepam group (P = 0.23); and median Trail‐B tracking test score 18, versus 19 in the lorazepam group (n = not specifically stated; P = 0.75). We considered the quality of the evidence to be very low, as it is unclear how many participants were assessed.
Adverse events
Adverse events were monitored as seizures, self‐extubations, removal of catheters or other medical devices. They found that seizures were detected in two participants in the dexmedetomidine group and one in the lorazepam group; self‐extubation in four participants in the dexmedetomidine group compared to two in the lorazepam group. In each group, one participant developed bradycardia (slow heart rate) of less than 40 beats per minute at some point during the 120‐hour study drug protocol.
1c Dexmedetomidine versus haloperidol and placebo
Abdelgalel 2016 included 90 ICU participants with a mean age of 50 years, 74% were male, receiving non‐invasive mechanical ventilation and with less severe disease (mean APACHE II Score 16.8) in a three‐arm trial of the effect of 1) dexmedetomidine (0.2 to 0.7 µg/kg/hour), versus 2) haloperidol (0.5 to 2 mg/hour) both administered as continuous infusions with an initial loading dose and subsequently continued to a sedation goal set by a RASS score, versus 3) a saline infusion in less severely ill ICU participants undergoing non‐invasive mechanical ventilation.
Event rate of ICU delirium
They found a reduced event rate of ICU delirium in the dexmedetomidine group compared to the haloperidol group (RR 0.30, 95% CI 0.09 to 0.98; P = 0.047, n = 60, 1 study) corresponding to a number needed to treat for an additional benefit (NNTB) of 3 (95% CI 2 to 13) and compared to the placebo (saline) group (RR 0.23, 95% CI 0.07 to 0. 73; P = 0.01, n = 60, 1 study) (Table 3). The study was small with few events. Moreover, the participants had low APACHE II scores indicating less severe critical illness.
In‐hospital mortality
In‐hospital mortality was similar in the dexmedetomidine and haloperidol group (RR 1.00, 95% CI 0.15 to 6.64; P = 1.0, n = 60, 1 study) and in the placebo group (RR 0.67, 95% CI 0.12 to 3.71; P = 0.64, n = 60, 1 study) (Table 3).
Number of delirium‐ and coma‐free days
The study did not assess this outcome.
Ventilator‐free days
The study did not asses this outcome.
Length of stay in the ICU
The dexmedetomidine group compared to haloperidol group and placebo group had a shorter length of ICU stay MD ‐3.40 (95% CI ‐3.79 to ‐3.01, P < 0.00001, n = 60, 1 study) and (MD ‐3.8 (95% CI ‐4.25 to ‐ 3.35, P < 0.00001,n = 60, 1 study), respectively (Table 3). Moreover, the participants had low APACHE II scores indicating less severe critical illness.
Cognitive impairment
The study did not assess this outcome
Adverse effects
Adverse events were bradycardia, arrhythmia and QTc interval as these are known adverse effects of dexmedetomidine. Bradycardia occurred more frequently in the dexmedetomidine group (n = 8/30) compared to the haloperidol group (n = 2/30) and the saline group (n = 1/30). In the haloperidol group, two participants developed prolonged QTc intervals (Table 3).
We considered the quality of the evidence for these outcomes to be very low as there was no description of the randomization sequence generation or allocation concealment and no online protocol to allow assessment of reporting bias. Moreover, the participants had low APACHE II scores indicating less severe critical illness.
2. Physical and cognitive therapy interventions
2a. Physical and cognitive therapy versus standard care
Brummel 2014b conducted a three‐arm study testing two different interventions with physical therapy versus usual care in 87 ICU participants with a mean age of 61 years, 56% males. The physical therapy interventions were: 1) early once‐daily physical therapy (advancing participants from passive range of motion to independent ambulation; 2) physical therapy (once‐daily session) combined with inpatient cognitive therapy focusing on orientation, memory, attention and problem‐solving twice a day for 20 minutes. Usual care entailed physical therapy as ordered by the treating clinician (typically one to two sessions per week). Those allocated to physical and cognitive therapy displaying impaired executive function and impaired functional mobility at discharge from hospital were additionally offered a 12‐week, 6‐session in‐home cognitive therapy programme.
Event rate of ICU delirium
The study did not measure this outcome.
In‐hospital mortality
Brummel 2014b found no effect of the intervention on in‐hospital mortality (RR 0.94, 95% CI 0.40 to 2.20; P = 0.88, n = 65, 1 study; Table 3).
Number of delirium‐ and coma‐free days within the first 30 days
No effect on the number of delirium‐ and coma‐free days was identified (MD ‐2.77, 95% CI ‐10.09 to 4.55; P = 0.46, n = 65, 1 study; Table 3).
Ventilator‐free days
There was no effect on ventilator‐free days (median days 25.3 versus 27.4; P = 0.81, n = 65, 1 study).
Length of stay in the ICU
There was no effect on length of ICU stay (MD 1.23, 95% CI ‐0.68 to 3.14; P = 0.21, n = 65, 1 study; Table 3).
Cognitive impairment
There was no effect on cognitive functioning assessed by the MMSE score (MD 0.97, 95% CI ‐0.19 to 2.13; P = 0.10, n = 30, 1 study; Table 3).
Likewise, there was no effect on cognitive functioning measured by the Dysexecutive questionnaire (DEX) score (n = 30) (MD ‐8.76, 95% CI ‐19.06 to 1.54; P = 0.1, n = 30, 1 study; Table 3).
Adverse effects
Brummel 2014b reported an adverse event with acute back pain accompanied by hypertensive urgency during a physical therapy session. We considered the quality of the evidence very low as there was only one study with few participants and it was a feasibility study of cognitive therapy protocol; (see Table 3 and Table 2).
3. Sedation interventions
3a. Early goal‐directed sedation versus standard sedation
Shehabi 2013 tested early goal‐directed sedation with a dexmedetomidine‐based algorithm targeted at light sedation (RASS ‐2 to 1) versus standard sedation with propofol or midazolam (without dexmedetomidine) ‐ the latter choice of drug, way of administration, time of cessation and level of sedation left to the discretion of the treating clinician — in 37 ICU participants with the mean age of 63 years (54% were male).
Event rate of ICU delirium
No differences were found between groups in the event rate of delirium (RR 1.02, 95% CI 0.44 to 2.34; P = 0.97, n = 37, 1 study; Table 3).
In‐hospital mortality
The study reported no effect on in‐hospital mortality (RR 1.14, 95% CI 0.22 to 6.05; P = 0.88, n = 37, 1 study; Table 3).
Number of delirium‐ and coma‐free days
The study did not assess this outcome.
Ventilator‐free days
The study found no effect on ventilator‐free days (MD 1.20, 95% CI ‐5.12 to 7.52; P = 0.71, n = 37, 1 study; Table 3).
Length of stay in the ICU
Likewise, length of ICU stay did not differ between groups (MD 0.30, 95% CI ‐2.97 to 3.57; P = 0.86, n = 37, 1 study; Table 3).
Cognitive impairment
The study did not asses this outcome.
Adverse effects
the authors reported one incident of self‐extubation and one incident of removal of devices in the group receiving early goal‐directed sedation with a dexmedetomidine‐based algorithm targeted at light sedation (RASS ‐2 to 1); and no incidents in the group receiving standard sedation with propofol or midazolam.
We considered the quality of evidence to be very low as it was a single study with few events and randomization was done using block randomization with concealed envelopes in a small sample size. We consider that it would be possible to predict the allocation: there was no description of allocation concealment; participants, staff and outcome assessors were not blinded.
3b. No sedation versus sedation with daily interruption
Strøm 2010 included 140 ICU participants with a mean age of 66 years and 67% male in a trial of a protocol of no sedation (intervention) compared to sedation (control) with morphine boluses (doses of 2.5/5 mg) as needed only and six hours of treatment with propofol if the participant was assessed to be uncomfortable versus morphine boluses (doses of 2.5/5 mg) as needed with continuous sedation using propofol (20 mg/mL titrated to a RASS of 3 to 4 and with daily interruption of sedation. The aim of the study was to examine the effect of sedation on ventilator‐free days and delirium was merely assessed and reported descriptively.
Event rate of ICU delirium
They found an increased event rate of hyperactive delirium in the no‐sedation group (RR 2.90, 95% CI 0.98 to 8.57; P = 0.05, n = 113, 1 study; Table 3). Hypoactive delirium was not reported.
In‐hospital mortality
The study found no difference in in‐hospital mortality (RR 0.78, 95% CI 0.50 to 1.22; P = 0.28, n = 113, 1 study; Table 3).
Number of delirium‐ and coma‐free days
The study did not assess this outcome.
Ventilator‐free days
The number of ventilator‐free days was higher in the no‐sedation group (MD 4.20, 95% CI 0.32 to 8.08; P = 0.034, n = 113, 1 study; Table 3).
Length of stay in the ICU
Length of ICU stay was shorter in the no‐sedation group (MD ‐5.20, 95% CI ‐8.48 to ‐1.92; P = 0.002, n = 113, 1 study; Table 3).
Cognitive impairment
The study did not assess this outcome.
Adverse effects
Adverse events were recorded as removal of the endotracheal tube, n = 7 in the intervention group, n = 6 in the control group (Table 3).
We considered the quality of evidence very low for the majority of outcomes and moderate for in‐hospital mortality, as it was a single study with few events, no blinding of participants, staff or outcome assessors, and the research question differed from the research question in this review, which increases the indirectness of the evidence (Higgins 2011).
3c. Sedation with daily interruption versus protocolized sedation
Two trials tested protocolized sedation with benzodiazepine and opioids (Mehta 2012), or midazolam/propofol (Nassar 2014), with daily interruption versus protocolized sedation. Mehta 2012 tested a protocolized sedation intervention with continuous benzodiazepine or opioid infusion,or both, plus daily interruption of sedation (intervention) versus protocolized sedation with continuous benzodiazepine and opioid,or both without daily interruption (control) in 430 ICU participants with a mean age of 59 years and 56% male. Nassar 2014 tested a sedation intervention with daily interruption of continuous sedative (midazolam or propofol) and opioid infusion at the discretion of the treating clinician (intervention) versus intermittent sedation and opioids without daily interruption likewise at the discretion of the treating clinician (control). In both groups the aim was to keep the patient calm, easily arousable or awakened. This study was carried out with 60 ICU patients with a mean age of 49 years, of whom 50% were male.
Treatments in the control groups differed slightly in the two studies and were therefore not entirely comparable. Only two studies were included, one with very few events and hence wide confidence intervals. Although the interventions were not completely identical, we pooled the data from Mehta 2012, and Nassar 2014.
Event rate of ICU delirium
There was no difference between groups in the event rate of ICU delirium (RR 0.96, 95% CI 0.81 to 1.14; P = 0.65, 2 studies, n = 483; Analysis 2.1; Figure 4.) The quality of the evidence was considered very low. There was a high risk of detection bias due to non blinding of participants, staff and outcome assessors.
2.1. Analysis.
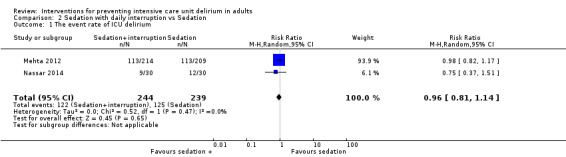
Comparison 2 Sedation with daily interruption vs Sedation, Outcome 1 The event rate of ICU delirium.
4.
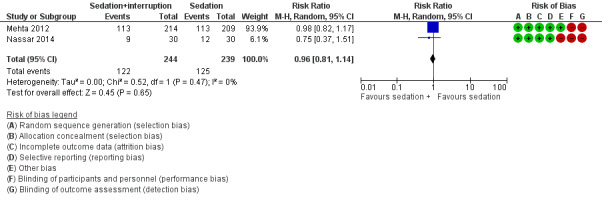
Forest plot of comparison: 8 Sedation with daily interruption vs Sedation, outcome: 8.1 The event rate of ICU delirium .
In‐hospital mortality
There was no effect on in‐hospital mortality (RR 1.05, 95% CI 0.78 to 1.43; P = 0.76, 2 studies, n = 483; Analysis 2.2; Figure 5). We considered the quality of the evidence to be low, due to few participants, few events and wide confidence intervals. Mortality is not considered to be influenced by lack of blinding.
2.2. Analysis.
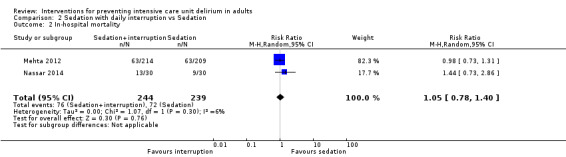
Comparison 2 Sedation with daily interruption vs Sedation, Outcome 2 In‐hospital mortality.
5.
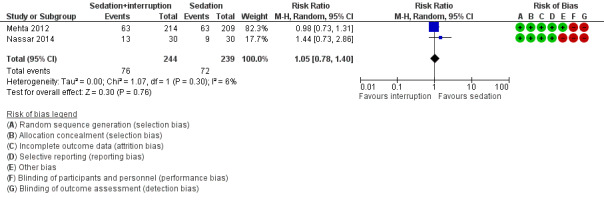
Forest plot of comparison: 8 Sedation with daily interruption vs Sedation, outcome: 8.2 In‐hospital mortality.
Number of delirium‐ and coma‐free days
Nassar 2014 identified similar numbers of delirium‐ and coma‐free days in both groups (MD 1.00, ‐0.94 to 2.94; P = 0.31, 1 study, n = 60; Table 3).
We considered the quality of evidence very low as there was only one study, there was a high risk of detection bias due to non‐blinding of participants, staff and outcome assessors, and few participants and events.
Ventilator‐free days
Nassar 2014 found no effect on ventilator‐free days (median days 24 (IQR 0 to 26) versus 25 (IQR 21 to 27); P = 0.16, 1 study, n = 60) respectively. We considered the quality of evidence to be very low as it was only one study, with few participants, and lack of blinding might have introduced a risk of performance bias.
Length of stay in the ICU
Length of stay in the ICU was likewise similar, irrespective of sedation approach in the pooled studies (MD ‐1.19, 95% CI ‐2.91 to 0.53; P = 0.18, 2 studies, n = 483; Analysis 2.3). We considered the quality of the evidence very low as treatment in control groups differed slightly in the two studies. There was a risk of detection and performance bias due to non‐blinding of participants, staff and outcome assessors, and only 483 participants were included.
2.3. Analysis.
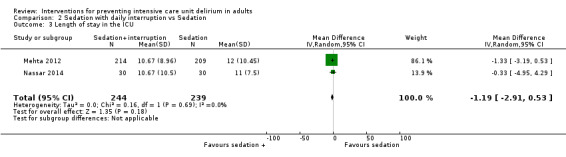
Comparison 2 Sedation with daily interruption vs Sedation, Outcome 3 Length of stay in the ICU.
Cognitive impairment
The study did not assess this outcome.
Adverse effects
Adverse events were similar in the two groups (RR 0.85, 95% CI 0.42 to 1.75; P = 0.66; 2 studies, n = 483; Analysis 2.4), as Mehta 2012 reported unintentional removal of devices and specific removal of endotracheal tubes. The difference was 4.7% (n = 10) in the intervention group versus 5.8% (n = 12) in the control group. Nassar 2014 reported self‐extubations and accidental removal of catheters and found three incidents in both the daily interruption group and the control group.
2.4. Analysis.
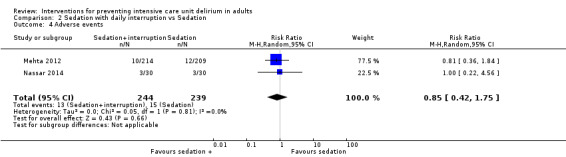
Comparison 2 Sedation with daily interruption vs Sedation, Outcome 4 Adverse events.
We considered the quality of evidence to be very low as treatment in control groups differed slightly in the two studies, there was a risk of detection and performance bias due to non‐blinding of participants, staff and outcome assessors, and only 483 participants were included.
4. Environmental intervention versus standard care
Two RCTs tested environmental interventions versus standard care (Simons 2016; Van Rompaey 2012). Van Rompaey 2012 tested sleeping with earplugs from 10.00 pm to 06.00 am versus sleeping without earplugs in 136 ICU participants with a mean age of 60 years (66% were male). Simons 2016 tested the effect of a dynamic light application in the ICU with alterations in light, colour and temperature over 24‐hour periods versus standard lighting turned on and off for procedures in 734 ICU participants with a mean age of 64 years (59% were males).
Event rate of ICU delirium
Although the interventions differed, we conducted a meta‐analysis on the event rate of delirium as this was the only outcome they had in common. This showed no significant difference between groups in the event rate of delirium (RR 1.14, 95% CI 0.95 to 1.38; P = 0.17; 2 studies, n = 870; Analysis 3.1; Figure 6).
3.1. Analysis.
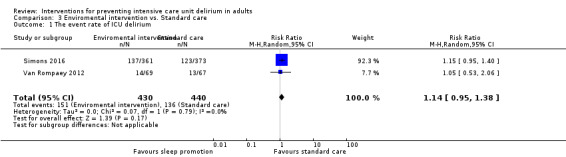
Comparison 3 Enviromental intervention vs. Standard care, Outcome 1 The event rate of ICU delirium.
6.
Forest plot of comparison: 9 Enviromental intervention vs. Standard care, outcome: 9.1 The event rate of ICU delirium.
We considered the quality of the evidence to be low as there was a risk of detection bias due to lack of blinding of outcome assessors in the larger study and one study with wide confidence intervals was included in the meta‐analysis.
In‐hospital mortality
Simons 2016, found no effect on in‐hospital mortality (RR 0.93, 95% CI 0.69 to 1.26; P = 0.66; 1 study, n = 734, Table 3).
We considered the quality of evidence moderate as there was only one study, few events and hence wide confidence intervals.
Number of delirium‐ and coma‐free days
No effect was identified on the number of delirium‐ and coma‐free days within 28 days (MD 0.06, ‐1.18 to 1.30; P = 0.92, n = 734, 1 study).
We considered the quality of evidence to be low as there was only one, non‐blinded study.
Ventilator‐free days
The studies did not assess this outcome.
Length of stay in the ICU,
There was no effect on length of ICU stay (MD ‐0.33, 95% CI 1.03 to 0.37; P = 0.36; n = 734, 1 study; Table 3). We considered the quality of evidence to be low as there was only one, non‐blinded study.
Cognitive impairment
The studies did not assess this outcome.
Adverse effects
The studies did not assess this outcome.
5. Preventive nursing care interventions
ICU‐delirium prevention protocol versus non‐preventive nursing care
Moon 2015 tested a tailored preventive nursing algorithm for the first 7 days after admittance to the ICU versus standard ICU nursing care in 134 ICU participants with the mean age of 70 years, of whom 48% were male. The intervention included monitoring and screening of delirium risk factors, cognitive assessment and orientation, environmental intervention — including provision of glasses and hearing aids, sleep management and comfort — and early therapeutic interventions for nutrition, ambulation, pain management, prevention of infections and hypoxia, removal of unnecessary catheters, and cautious administration of sleeping pills, anticholinergic drugs and opioids versus standard nursing care.
Event rate of ICU delirium
There was no effect on the event rate of ICU delirium (RR 0.60, 95% CI 0.32 to 1.11; P = 0.10, n = 123, 1 study; Table 3).
In‐hospital mortality
There was no effect of in‐hospital mortality (RR 0.38, 95% CI 0.13 to 1.13; P = 0.08, n = 123, 1 study; Table 3).
Number of delirium‐ and coma‐free days
The study did not assess this outcome.
Ventilator‐free days
The study did not assess this outcome.
Length of stay in the ICU
There was no effect on length of ICU stay (MD 0.80, 95% CI ‐3.01 to 4.61, P = 0.68, n = 123, 1 study; Table 3).
Cognitive impairment
The study did not assess this outcome.
Adverse effects
The study did not assess this outcome.
We considered the quality of evidence, for all outcomes, to be very low as it was based on one study with few participants and events, lack of blinding of the outcome assessor and several 'Risk of bias' domains were rated unclear.
Discussion
Summary of main results
The aim of this review was to investigate the evidence for an effect of non‐pharmacological and pharmacological interventions, either combined or in isolation, for preventing delirium in intensive care unit (ICU) patients in medical or surgical ICUs. We conducted a broad search, which retrieved a substantial number of potentially eligible studies, suggesting that there is considerable interest in identifying effective strategies for managing delirium. In total, we included 12 trials encompassing a total of 3885 randomized ICU participants. The studies tested diverse interventions including a variety of pharmacological interventions, sedation regimens, physical and cognitive therapy, environmental interventions and preventive nursing care. Control interventions included placebo, standard sedation protocols and standard care. Overall, the within‐study risk of bias was low. The quality of the evidence was very low to moderate when assessed according to GRADE criteria.
Pharmacological interventions
Overall, pharmacological interventions did not consistently prevent delirium or improve important patient outcomes such as in‐hospital mortality, number of ventilator‐free days, length of ICU stay and cognitive function. We found evidence from one small study indicating that dexmedetomidine opposed to haloperidol and placebo reduced the event rate of delirium, (number needed to treat for an additional benefit (NNTB) was 3) and shortened the duration of ICU stay (Abdelgalel 2016). Similar to this, Pandharipande 2007 reported that dexmedetomidine versus lorazepam increased the number of coma‐free days without, however, reducing the event rate of delirium or length of stay in the ICU. Neither study found an effect of dexmedetomidine on in‐hospital mortality (Abdelgalel 2016; Pandharipande 2007). Likewise, Pandharipande 2007 found no effect on the number of ventilator‐free days or cognitive function. It should be noted that Abdelgalel 2016 included non‐mechanically ventilated participants with relatively low APACHE II scores (higher scores correspond to more severe disease and a higher risk of death), which limits the extent to which the results can be extrapolated to more critically ill ICU patients. The study by Abdelgalel 2016 was moreover assessed to be at unclear risk of bias due to incomplete description of sequence generation and allocation concealment. The study by Pandharipande 2007 was assessed to be at low risk of bias overall; however the study was small with few events, which limits the quality of the evidence. Furthermore, there is evidence that benzodiazepines are associated with an increased risk of delirium (Fraser 2013; Zaal 2015), and this makes it difficult to determine whether dexmedetomidine was in fact effective for preventing delirium or if lorazepam was causal, or both (Serafim 2015).
In line with this, Page 2013 and Van Den Boogard 2018, who tested haloperidol versus placebo for preventing ICU delirium, found no effect on in‐hospital mortality, number of delirium‐ and coma‐free days, length of stay in the ICU or ventilator‐free days. Van Den Boogard 2018, the larger study of the two, was initially assessed to have unclear risk of attrition bias as only 14 of 21 (84 % of total sample) participating sites produced data on delirium outcomes; missing data, however,were evenly distributed between the two groups. This is why it was considered to be of low risk of bias.
There is a need for larger studies testing the robustness of the evidence for a preventive effect of dexmedetomidine on delirium and other patient‐important outcomes in critically ill, mechanically‐ventilated ICU patients. Ongoing studies are currently testing dexmedetomidine against different comparators including placebo. Preferably comparators should be 'non‐deliriogenic' medications as benzodiazepines are considered a risk factor for the development of delirium (Nelson 2015). NCT01791296 is testing the effect of dexmedetomidine versus placebo on the event rate of delirium and quality of sleep in 100 patients, and NCT01739933 the effect of dexmedetomidine versus propofol in 533 septic mechanically ventilated adult ICU patients. NCT03172897 is testing low‐dose dexmedetomidine versus placebo in 260 ICU patients. These results are all expected to be published in 2018.
Currently, Martinez 2017 is testing the preventive effect of melatonin on delirium. Recruitment has started and the ambition is to include a total of 850 patients.
Sedation interventions
Similarly, sedation interventions did not consistently prevent delirium or improve in‐hospital mortality, number of ventilator‐free days, length of ICU stay or cognitive function (Mehta 2012; Nassar 2014; Shehabi 2013; Strøm 2010). Strøm 2010 found that a no‐sedation intervention compared to daily interruption of sedation increased the incidence of hyperactive delirium while reducing the duration of mechanical ventilation and length of ICU and hospital stay. In‐hospital mortality did not differ between interventions (Strøm 2010). Delirium was not a pre‐defined outcome in the study, so the study was not powered to detect a difference in delirium (Strøm 2010). Furthermore, delirium was assessed by study personnel trained to use the Diagnostic and Statistical Manual of Mental disorders, 4th edition (DSM‐IV) criteria (Strøm 2010). Currently, the ideal standard for delirium diagnosis is evaluation by a psychiatric expert using the Diagnostic and Statistical Manual of Mental disorders, 5th edition (DSM‐5) criteria (Hayhurst 2016). We do not know how Strøm 2010 trained the study personnel or if, and how, their delirium assessments were validated.
Sedation studies should be repeated in larger samples and evaluate the effects not only on relevant clinical outcomes but also on delirium and cognitive functioning using appropriate and validated instruments in order to confirm or reject the tentative findings of Strøm 2010. In the future we look forward to the results of Toft and colleagues and Nedergaard and colleagues, who are both currently conducting randomized controlled trials (RCTs) of the effect of no‐sedation interventions on longer‐term cognitive outcomes (Nedergaard 2016), as well as delirium‐ and coma‐free days, length of ICU stay and number of ventilator‐free days (Toft 2014). We hope that these studies will provide more evidence for the effects of no‐sedation on delirium including the potential pros and cons of no‐sedation regimens on other patient‐important outcomes in the ICU. Current limited evidence suggests that no‐sedation may be beneficial for patients on some clinical parameters while potentially increasing the risk of delirium and, possibly, impaired cognitive function.
Physical and cognitive therapy interventions
Physical and cognitive therapy did not prevent delirium or influence in‐hospital mortality, the number of delirium‐ and coma‐free days, ventilator‐free days, length of ICU stay, or cognitive functioning (Brummel 2014b). This evidence comes from a small feasibility study (including 87 participants) not powered to detect significant differences in any outcomes (Brummel 2014b). There are indications, however, that physical, cognitive and occupational therapy interventions may have a potential for preventing or reducing the duration of delirium. Schweickert and colleagues examined the effect of early mobilization and occupational therapy versus usual care in an RCT including 104 ICU participants (Schweickert 2009). They found positive effects of the intervention on the primary outcome — time to return to independent function — and on the secondary outcomes, which were ventilator‐free days and duration of delirium within the first 28 days of hospital stay (Schweickert 2009). The future results from Thomas 2015 (expected n = 157), which is currently testing the effect of early physical therapy with intensive patient mobilization versus usual care on physical function and secondly on mental health, activities of daily living, delirium‐ and ventilator‐free days — will add to the evidence, albeit delirium is evaluated not as a primary but as a secondary outcome. Similarly, Nickels 2017 (expected n= 68) is testing the effect of additional in‐bed cycling versus usual physiotherapy on the primary outcome physical functioning, and on ICU delirium as a secondary outcome. An ongoing Cochrane Review is furthermore examining the evidence for an effect on delirium of mobilization or active exercise, or both in critically ill participants (Doiron 2018).
Environmental interventions
Two trials tested environmental interventions for promoting sleep (earplugs) and circadian rhythm (lighting) on the event rate of delirium without detecting any differences (Simons 2016; Van Rompaey 2012). Pooled data from these studies showed no significant difference between groups in the event rate of delirium. Van Rompaey 2012 included non‐sedated patients and focused on early‐onset delirium, defined as delirium occurring within the first five days of ICU admission. Although there was no difference between groups in the event rate of delirium, patients sleeping with earplugs appeared to develop delirium later than the control group patients. Van Rompaey 2012 did not assess in‐hospital mortality or any of the other secondary outcomes of this review, making conclusions regarding clinical benefit challenging. Moreover, using sedation as an exclusion criterion limits the generalizability of the evidence. Simons 2016 on the other hand, included all consecutive ICU patients and evaluated in‐hospital mortality, number of delirium‐ and coma‐free days within 28 days and length of ICU stay without finding any significant differences between groups in any outcomes. Simons 2016 planned to include 1000 patients but stopped the study prematurely for futility after including 734 patients. ICU patients may be less susceptible to external cues and the applied changes in levels of lighting might not have been sufficient to influence the circadian rhythm. This may explain why Simons 2016 failed to detect any effects. It is also possible that circadian rhythm cannot be affected in critically ill patients, for instance patients with severe sepsis (Verceles 2012). Currently, Miles 2012 is investigating earplugs versus earplugs and noise‐cancelling headphones versus usual care in 45 participants in a three‐arm RCT.
When considering delirium as a syndrome representing decompensation of cerebral function in response to multiple pathophysiological stressors, it may be questioned whether modification of a single environmental cue can influence the development of ICU delirium, and furthermore whether environmental interventions should be tested in isolation or as a part of multi‐component interventions.
Preventive nursing care interventions
Moon 2015 tested an ICU‐delirium preventive nursing care protocol versus standard nursing care and found no effect of the intervention on any of the specified outcomes: the event rate of ICU delirium in‐hospital mortality, or length of ICU stay. Although the study failed to identify an effect of the multi‐component prevention intervention encompassing orientation and information to patients, aiding visual and hearing impairments, prioritizing sleep and reducing pain, reducing noise and bright light, these components may have the potential to prevent ICU delirium. We assessed the Moon 2015 study to be at unclear risk of contamination or 'spill‐over bias', possibly resulting in dilution of any effect of the intervention on outcomes.
Currently, studies involving both multi‐component interventions and preventive nursing care interventions are in the pipeline. There are studies testing family visiting restrictions (NCT02932358), music therapy (NCT03095443), multi‐component interventions of awakening and breathing co‐ordination, delirium monitoring and management (NCT03125252), multisensory stimulation, cognitive stimulation, and active functional and family involvement (NCT03215745), and non‐pharmacological nursing and physical therapy interventions for prevention of ICU delirium (Wassenaar 2017).
Other reviews promote the value of multi‐component interventions for reducing delirium and improving patient outcomes. Morandi 2011, for example, reviewed existing evidence for the individual components of the so‐called ABCDE‐approach which includes airway and breathing, choice of sedation, delirium monitoring and treatment, and early mobility and exercise. Likewise, Siddiqi 2016 in a recently updated review of interventions for preventing delirium in non‐ICU hospitalized patients concluded that there is moderate‐quality evidence that multi‐component interventions reduce delirium incidence, while the effects on mortality and length of hospital stay are uncertain. High‐quality RCTs examining complex multi‐component interventions for preventing ICU delirium are warranted.
Overall completeness and applicability of evidence
The 12 studies included in this review tested a wide variety of interventions ranging from pharmacological interventions, sedation interventions, physical or cognitive therapy and both, environmental interventions and preventive nursing care interventions. Delirium was evaluated in different ways across the studies, i.e. the event rate of delirium, or the number of delirium‐ and coma‐free days, and a variety of instruments were used to screen participants for delirium. Furthermore, the majority of studies evaluated delirium as a secondary or tertiary outcome and were therefore not statistically powered to detect significant effects on delirium. Patient populations, outcomes, definition of secondary outcomes, ways to measure outcomes and follow‐up times varied greatly across the studies, irrespective of the interventions. The included studies overall had low within‐study risk of bias although several were at high risk of detection bias due to lacking blinding of outcome assessors. Moreover, studies were characterized by being primarily small trials, not powered specifically for the primary outcome of this review.
How ICU delirium should ideally be assessed remains a subject of debate (Gusmao‐Flores 2012; Neto 2012; van den Boogaard 2012a). A range of instruments were used to assess delirium in the studies included in this review with the majority using the confusion assessment method for the ICU (CAM‐ICU) (eight studies), one using the Neelon and Champagne confusion (NEECHAM) scale, one using DSM‐IV, one Prediction of delirium in ICU patients (PRE‐DELIRIC) (besides CAM‐ICU (Simons 2016), and finally, one using the Intensive Care Delirium Screening (ICDSC). The use of different instruments with varying psychometric properties naturally limits the strength of the conclusions that can be drawn from this review. The varying definitions of delirium in the studies furthermore prevented meaningful comparison of intervention effects across studies. Delirium needs to be evaluated as a primary outcome in future studies. Consensus with regard to the definition and assessment of delirium in future studies of ICU delirium would facilitate pooling of data and strengthen the evidence for effective preventive interventions. Furthermore, to enable future comparison of effects across studies, consensus with regard to the use of ICU screening tools would be beneficial. In line with other researchers in the field (Brummel 2014b; Nassar 2014; Page 2013; Simons 2016; Pandharipande 2007), we suggest that delirium is defined and assessed as the number of delirium‐ and coma‐free days in future studies. We also acknowledge the ongoing COMET initiative aiming to identify additional core outcomes for critically ill patients as well as acutely hospitalized patients, palliative care patients and older adults (Rose 2017).
When considering the long‐term impact of ICU delirium on cognitive functioning, it is highly relevant to include an assessment of cognitive functioning in studies examining interventions for preventing and treating ICU delirium. Two of the included studies (Brummel 2014b; Pandharipande 2007) evaluated cognitive functioning despite the growing body of evidence for an association between ICU delirium and long‐term cognitive impairment (Pandharipande 2013; van den Boogaard 2012b).
Pandharipande 2013 tested participants with the Mini‐Mental State Examination (MMSE) test at discharge and Brummel 2014b tested cognitive functioning at three months' follow‐up with MMSE and Dysexecutive questionnaire (DEX) test. Assessment of cognitive function may prove more difficult in ICU survivors (Pfoh 2015; Woon 2012). A cross‐sectional analysis of cognitive function in 242 survivors of acute respiratory failure concluded that the MMSE demonstrated moderate to fair agreement in detecting overall cognitive impairment, excellent specificity, but poor sensitivity compared to a detailed neuropsychologic test battery (Pfoh 2015). The authors cautioned against the frequent use of MMSE and highlighted that a negative MMSE does not necessarily rule out cognitive impairment in survivors of acute respiratory failure (Pfoh 2015). The MMSE is characterized by a highly selective coverage of cognitive domains and does not evaluate learning, delayed memory, processing, speed and executive function — all domains relevant to ICU survivors (Pfoh 2015). Researchers in the field recommend trail‐marking tests A and B as these tests more adequately capture executive function (Pandharipande 2013). The Repeatable Battery for the Assessment of Neuropsychological Status (RBANS) is a brief, standardized, cognitive screening instrument that evaluates immediate memory, visuospatial/constructional ability, language, attention and delayed memory, as well as a global measure, and this tool may also be a relevant although it was originally developed for patients with dementia (Sanz 2009).
Cognitive functioning in ICU survivors is the focus of several ongoing studies.
Nedergaard 2016 is testing cognition using a composite cognitive score and several different cognitive tests addressing attention, mental pace, executive function, verbal learning, memory, visual construction, mental flexibility.
Thomas 2015 is testing mental health with the Short Form 36 at hospital discharge and up to six months from study enrolment.
NCT01739933 is testing cognitive impairment within six months.
NCT03125252 is examining outcomes linked to several cognitive and psycho‐behavioural functions including battery for executive functions, Trail Making Test, Digit Span, Stroop and Verbal Fluences and Montgomery's scale (depression).
These studies are interesting given the inclusion of cognitive functioning as a patient‐reported outcome in an intensive care population that potentially may be increasingly able to communicate both during and after ICU treatment as a result of altered sedation practices. It is important to give patients a voice and involve them in eliciting and minimizing the sequelae of ICU treatment and, hopefully, improving health‐related quality of life (HRQoL) after critical illness (Black 2013). It is therefore highly relevant that the effect of intensive care and treatment is evaluated by users to avoid observer (clinician) bias (Black 2013).
This current Cochrane Review focused on interventions for preventing rather than treating ICU delirium. We therefore excluded a number of studies examining treatment interventions. However, we acknowledge that the two dimensions are linked. Given the complex aetiology of ICU delirium, identification of effective interventions for preventing ICU delirium may prove difficult and represent only one side of the coin (Hayhurst 2016). Research into both prevention and treatment should therefore continue simultaneously.
Quality of the evidence
Overall, we found that the quality of evidence was moderate to very low. There was substantial indirectness i.e. different aims, interventions, comparisons across studies. This diversity prevented meaningful meta‐analyses. On the positive side, this diversity can be considered a strength in regard to mapping the existing evidence for delirium prevention in a broad perspective and highlighting gaps in our knowledge and, consequently, identifying areas for future research.
We included several small studies in this review. Pooling of data in meta‐analyses can provide more precise effect estimates and is relevant when evaluating complex problems with conflicting literature (Haidich 2010). However, the diversity of delirium assessments and definitions of outcomes in the included studies prevented meta‐analyses. Small studies with few events also limit the quality of the evidence and thereby the strength of the conclusions of this review.
The inclusion of small studies with insufficient statistical power is a limitation of the review. Brummel 2014b reported a post hoc analysis of power and found that the study was underpowered (38%) to detect a meaningful 1.5% change in executive function (the tower test). Likewise, the trial by Nassar and colleagues was insufficiently powered to conclude that one intervention was superior to the other (Nassar 2014). The majority of studies assessed delirium as a secondary outcome, rendering the studies insufficiently powered to detect differences in the incidence of delirium. Larger multicentre studies are therefore relevant for increasing power and comparing outcomes.
Lack of blinding of outcome assessors introduced a high risk of detection bias, specifically in regard to the delirium outcome in included studies. We acknowledge that blinding of participants, personnel and research staff may be difficult when testing physical/cognitive therapy, environmental and nursing interventions. However, blinded outcome assessment should be incorporated to reduce detection bias.
The included studies used different screening tools for detecting delirium which may introduce heterogeneity and impact the quality of the evidence.
Potential biases in the review process
We evaluated the sources of bias using the 'Risk of bias' tool described in the Cochrane Handbook for Systematic Reviews of Interventions, Section 8.5 (Higgins 2011).
To our knowledge there are no validated screening tools for detecting sub‐syndromal delirium. Therefore we excluded studies focusing on sub‐syndromal delirium in order to reduce the risk of detection bias in the review process.
In the protocol (Greve 2012), we stated that we would include studies that assessed delirium using the CAM‐ICU or ICDSC. We have expanded our criteria to also include delirium assessed using the DSM‐IV criteria or the NEECHAM scale. They are all validated tools for screening of ICU delirium, so we do not consider that the inclusion of these tools introduces bias in the review process.
Originally, we planned to assess duration of mechanical ventilation; however, studies have primarily measured ventilator‐free days, so we changed this outcome accordingly. We consider this reduces the risk of detection bias as it more precisely reflects the need of patients for mechanical ventilation.
We planned to contact the first author or contact persons of the trials to potentially retrieve missing data in the included trials; however this was not necessary as the majority of studies had few missing data and all had less than 20% dropout. Therefore we did not conduct sensitivity analyses exploring the effect on effect estimates of trials with high dropout rates (> 20% dropout), as we had planned. Furthermore, due to missing data we did not conduct 'best‐case' and 'worst‐case' analyses. We do not consider that these changes introduce a risk of bias in the review process.
While writing the protocol (Greve 2012), we did not foresee that treatment effects were reported as medians with interquartile ranges (IQR). For meta‐analyses we therefore calculated means and standard deviations (SDs) as suggested in Wan 2014, with the exception of 'ventilator‐free days' as we suspected a multi‐model distribution in the original data. Effects on 'ventilator‐free days' are therefore presented as medians and IQRs in studies where they were originally reported in this way. The estimates of effects based on the calculated means and SDs do not differ from the estimates of effects based on median and IQRs in the original included studies. Therefore, we do not consider this to have introduced a risk of bias in the review process.
We planned to perform intention‐to‐treat (ITT) analysis using the number of patients initially randomized into the experimental or control intervention as the denominator. For primary outcomes, we also planned to conduct an 'available‐case analysis' in which only those participants on whom data were reported were included in the analysis. In practice, we conducted an available‐case analysis on all variables as there were few missing data in the included studies. We consider the risk of bias of this approach was low.
We believe that not searching in Chinese Biomedical Literature Database constitutes a lack of information about potential interventions for preventing ICU delirium.
The chosen search strategy for databases (Appendix 1) was relevant and revealed a large amount of studies and conference proceedings (Figure 1). When exploring possible drug therapies, more specific drugs other than haloperidol could possibly have been included in the search strategy, potentially allowing for a more extensive search. We do not suspect reporting bias as most of the included studies reported negative results and were published.
Overall, we have complied with the analysis process written in our protocol (Greve 2012).
Agreements and disagreements with other studies or reviews
During the last few years several systematic reviews have been conducted to identify specific interventions, pharmacological and non‐pharmacological, for treating ICU delirium (Al‐Qadheeb 2014; Fraser 2013), and some for preventing ICU delirium (Hu 2015; Nelson 2015), or both (Collinsworth 2016; Trogrlić 2015). The reviews differ in the types of studies included, with some including only RCT studies and others both RCT and non‐RCT studies.
A Cochrane Review from 2015 explored the effect of non‐pharmacological interventions for sleep promotion in the intensive care unit (Hu 2015). The authors included 30 RCTs (n = 29) and one quasi‐RCT involving 1569 ICU participants; one of the studies (Van Rompaey 2012), was also included in our review, although we examined different primary outcomes. The interventions in Hu 2015 included psychological (cognitive or behavioural) interventions, environmental interventions, social support interventions, equipment modification (including mechanical ventilation), complementary and alternative therapies (herbs, acupuncture), and physical therapy modalities. The review found that earplugs and eye masks, alone or in combination, reduced the incidence of ICU delirium as a secondary outcome (RR 0.55, 95% CI 0.38 to 0.80; P = 0.002) (Hu 2015).
Fraser 2013 conducted a systematic review of pharmacological interventions (dexmedetomidine, lorazepam, midazolam) for reducing ICU length of stay, ventilator time, delirium prevalence, and short‐term mortality. They included six RCTs involving 1235 participants; however only two studies (469 participants) assessed delirium. They found no difference in delirium prevalence between the groups (RR = 0.83, 95% CI 0.61 to 1.11; P = 0.19) ( Fraser 2013).
A systematic review explored the effect of interventions aimed at shortening the duration of ICU delirium and reducing short‐term mortality (at hospital discharge or 21, 28, 30, or 45 days after randomization) (Al‐Qadheeb 2014). Seventeen RCTs involving 2849 participants were included in this review. The interventions were diverse: pharmacologic intervention (n = 13) (dexmedetomidine (n = 6), antipsychotic drug (n = 4), rivastigmine (n = 2), and clonidine (n = 1)), a multimodal intervention with spontaneous awakening (n = 2), or a nonpharmacologic intervention (n = 2) with early mobilization (n = 1) and increased perfusion (n = 1). The review found that the average duration of delirium was shorter in the intervention groups when including all 17 studies in a meta‐analysis (difference = –0.64 d; 95% CI –1.15 to –0.13; P = 0.01). Overall (I² = 71%, Phet < 0.001). However, short‐term mortality was not effected (Al‐Qadheeb 2014).
Nelson 2015 conducted a review of dexmedetomidine for preventing delirium. Three RCTs involving 492 participants were included. Comparators were lorazepam, morphine, propofol and midazolam. The specific outcomes were delirium‐ and coma‐free days, delirium incidence within five days and incidence of postoperative delirium (Nelson 2015). Nelson and colleagues found no effect of dexmedetomidine on any outcomes (Nelson 2015).
Trogrlić 2015 evaluated the effect of a multi‐component implementation programme including assessment, prevention and treatment of ICU delirium on the potential to improve knowledge of delirium and delirium incidence, use of antipsychotic drugs, mortality and length of ICU stay (Trogrlić 2015). They included a total of 21 studies, RCTs and non‐randomized studies and found no conclusive effects on any outcomes (Trogrlić 2015). They pointed out that robust data on effectiveness of specific implementation strategies for delirious, critically ill patients are still scarce and there is a lack of data on the association between specific practice changes (for example, delirium screening) and improvements in clinical outcomes.
Collinsworth 2016 reviewed 14 studies, both RCTs and non RCTs, of ICU clinicians’ ability to assess, prevent and treat delirium. Eight studies assessed delirium‐relevant outcomes (incidence of delirium, duration of delirium, days awake and not delirious). Interventions differed across studies and were combined with various therapies including daily breathing trials, reorientation strategies, early rehabilitation, environmental stimulation, pain assessment, and delirium screening. Five of eight studies reported significant reductions in the event rate of delirium, duration of delirium and increases in the number of coma‐ and delirium‐free days. Two controlled trials reported a decrease in the event rate of delirium, while three RCTs did not detect a difference (Collinsworth 2016).
We acknowledge that there is continuing interest in the topic and protocols for future systematic reviews are underway (Bannon 2016; Burry 2016; Foster 2016), as well as an overview of reviews (Barbateskovic 2016).
Authors' conclusions
Implications for practice.
The current, limited evidence suggests that the routine use of haloperidol for preventing intensive care unit (ICU) delirium is not an effective strategy. This information is based on two moderate‐quality studies including 1580 participants. As haloperidol remains one of the most widely used delirium treatment strategies, confirmation or disconfirmation of its potential for preventing ICU delirium is highly relevant. Two studies showed an effect of dexmedetomidine, one reported more coma‐ and delirium‐free days in comparison to treatment with lorazepam, and one reported a reduced event rate of delirium compared to treatment with haloperidol during non‐invasive mechanical ventilation. The quality of the evidence for dexmedetomidine versus lorazepam was low, and very low for dexmedetomidine versus haloperidol. The evidence indicates no preventive effect of physical and cognitive intervention on delirium based on one very small low‐quality study. We found very low‐quality evidence that a regimen of no sedation versus standard sedation increased the incidence of agitated delirium, while decreasing the number of ventilator‐free days and length of ICU and hospital stay. Based on this single study, the benefits of no sedation for preventing delirium therefore appear uncertain. The quality of the evidence for the remaining sedation interventions, physical and cognitive therapy interventions, and nursing care interventions ranged from moderate to very low and none of the interventions prevented delirium or improved or worsened any of the secondary outcomes of this review. The five studies in Studies awaiting classification and 15 ongoing studies may alter the conclusions of the review once they are assessed.
Implications for research.
The clinical benefits of dexmedetomidine, different approaches to sedation interventions such as no sedation, daily interruption of sedation, and of early physical and cognitive therapy and environmental interventions for delirium prevention in ICU patients are unclear and warrant further investigation in large multicentre studies.
In future studies, the comparators in the pharmacological studies should be non "deliriogenic" medications, as benzodiazepines are considered a risk factor for the development of delirium (Nelson 2015). Studies should be designed and powered to assess delirium as well as clinically important and patient‐reported outcomes such as short‐ and long‐term cognitive function, physical function and quality of life. Ideally, studies should include large samples allowing for analysis of effects in subgroups of ICU patients. Furthermore, pharmacological studies should standardize any additional non‐pharmacological preventive interventions in both the intervention and control arms to allow identification of benefit obtained from the medication.
The potential of early physical and cognitive therapy should also be further explored. Future studies should consider cluster randomization to minimize performance bias.
There is a need for consensus regarding definitions of outcomes and time frames for observation of patients. ICU delirium should be defined as the number of coma‐ and delirium‐free days after admission to the ICU. The need for mechanical ventilation should be defined as ventilator‐free days, intuitively this is a patient important variable. Further, we suggest inclusion of cognitive functioning post‐discharge in ICU survivors, both short term and long term, and the identification of a standard instrument for valid assessment of cognitive function in ICU survivors. Health Related Quality of Life ( HRQoL) and Activities of Daily Living (ADL) are important patient reported outcomes to also consider after critical illness. The time to, and ability to, return to previous work status or previous level of functioning in everyday life are also important patient reported outcomes .
We suggest that results are reported, if possible, as means and standard deviations (SDs) for the purpose of pooling data in systematic reviews in future.
What's new
| Date | Event | Description |
|---|---|---|
| 28 January 2019 | Amended | Typo corrected in Plain language summary/ key results section: 'impression' corrected to read 'imprecision'. |
History
Protocol first published: Issue 4, 2012 Review first published: Issue 11, 2018
| Date | Event | Description |
|---|---|---|
| 10 December 2018 | Amended | Editorial team changed to Cochrane Emergency and Critical Care |
| 11 July 2012 | Amended | Contact details updated. |
| 17 April 2012 | Amended | Contact details updated. |
Acknowledgements
We would like to thank Nicola Petrucci (content editor), Marialena Trivella (statistical editor), John Devlin, Jean Kozak, Richard D Griffiths (peer reviewers) and Ann Fonfa (consumer representative) for their help and editorial advice during the preparation of the protocol (Greve 2012) for the systematic review. We would like to thank Nicola Petrucci (content editor), Vibeke Horstmann (statistical editor) Jean‐Francois Kozak, Najma Siddiqi, Imogen Featherstone (peer reviewers), Sandra Doherty (consumer referee) and Harald Herkner (Co‐ordinating Editor) for their help and editorial advice during the preparation of this systematic review.
We would also like to thank Jane Cracknell (Managing Editor of Cochrane Anaesthesia Critical and Emergency Care Group (ACE) for her valuable help with preparing the protocol (Greve 2012) Karen Hovhannisyan (former (Trials Search Co‐ordinator, ACE) and Janne Vendt (Information Specialist ACE) for their substantial support in phrasing the search strategy; Tobias Wihrenfeldt Klausen (MSc, Clinical Research Unit, Department of Haematology, Copenhagen University hospital, Herlev ) for statistical assistance; and finally, Tom Pedersen (Director of the Head and Orthopaedic Center, Rigshospitalet, Copenhagen) for the first reading of the proposal for this protocol (Greve 2012).
Further thanks to Nicola Petrucci for helping with data extraction from a Italien study we excluded.
Appendices
Appendix 1. Search strategy for databases
1Search strategy for CENTRAL, the Cochrane Library
#1 MeSH descriptor Physical Therapy Modalities, this term only #2 MeSH descriptor Occupational Therapy explode all trees #3 MeSH descriptor Combined Modality Therapy, this term only #4 MeSH descriptor Antipsychotic Agents, this term only #5 MeSH descriptor Haloperidol, this term only #6 (Intervention* near (prevent* or multimodal or multi?component)) #7 (Intervention* near (prevent* or multimodal or multi?component)):ti,ab or (therapy near (physical or occupational or drug)):ti,ab or protocol:ti,ab or (antipsychotic* or haloperidol or screening or detect*):ti,ab #8 (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7) #9 MeSH descriptor Delirium explode all trees #10 MeSH descriptor Confusion, this term only #11 delirium* or (acute near (brain and dysfunction)) or confusion:ti,ab or (cognitive near impairment) #12 (#9 OR #10 OR #11) #13 (#8 AND #12)
2 Search strategy for MEDLINE (OvidSP)
1. ((Intervention* adj3 (prevent* or multimodal or multi?component)) or (therapy adj3 (physical or occupational or drug))).mp. or protocol.ti,ab. or (antipsychotic* or haloperidol or screening or detect*).mp. or Physical Therapy Modalities/ or Occupational Therapy/ or Combined Modality Therapy/ or Antipsychotic Agents/ or Haloperidol/ or Drug Therapy/ 2. (delirium* or (acute adj3 (brain and dysfunction))).af. or confusion.mp. or (cognitive adj3 impairment).mp. or exp Delirium/ or exp Confusion/ 3. 1 and 2 4. ((randomized controlled trial or controlled clinical trial).pt. or randomized.ab. or placebo.ab. or clinical trials as topic.sh. or randomly.ab. or trial.ti.) not (animals not (humans and animals)).sh. 5. 3 and 4
3 Search strategy for Embase (OvidSP)
1. ((Intervention* adj3 (prevent* or multimodal or multi?component)) or (therapy adj3 (physical or occupational or drug)) or protocol or (antipsychotic* or haloperidol or screening or detect*)).ti,ab. or physiotherapy/ or occupational therapy/ or neuroleptic agent/ or HALOPERIDOL/ or drug therapy/ 2. (delirium* or (acute adj3 (brain and dysfunction)) or confusion).ti,ab. or (cognitive adj3 impairment).mp. or exp DELIRIUM/ or ACUTE CONFUSION/ 3. 1 and 2 4. (((emergency or intensive or care) adj3 unit*) or ((critical* or acut*) adj (patient* or ill*))).ti,ab. or ICU.mp. or resuscitation.ti,ab. or exp intensive care unit/ or exp critically ill patient/ 5. 3 and 4
4 Search strategy for Biosis (ISI Web of Knowledge) and ISI Web of Science
# 1 TS=(Intervention* SAME (prevent* or multimodal or multi?component)) or TS=(therapy SAME (physical or occupational or drug)) or TS=(protocol or antipsychotic* or haloperidol or screening or detect*) # 2 TS=(delirium* or (acute SAME (brain and dysfunction))) or TI=confusion or TS=(cognitive near impairment) # 3 #2 AND #1 # 4 TS=((emergency or intensive or care) SAME unit*) or TS=((critical* or acut*) SAME (patient* or ill*)) or TS=ICU # 5 #4 AND #3 # 6 TS=(random* or (controlled SAME (trial* or stud*)) or multicenter) or TS=((single or double or triple or treble) or (mask* or blind*)) # 7 #6 AND #5
5 Search strategy for LILACS (BIREME interface)
("DELIRIUM" or "CONFUSION") and ("INTENSIVECARE" or "EMERGENCYCENTERS" or "EMERGENCYSERVICE" or "paciente agudo" or "aguda doente" or "criticamente doentes" or "en estado crítico" or "La terapia física" or "fisioterapia" or "antipsychotic$")
6 Search strategy for CINAHL (EBSCO host)
S1. ((Intervention* and (prevent* or multi modal or multi?component))) OR ((therapy and (physical or occupational or drug)) ) OR ( (MH "Physical Therapy+") OR (MH "Occupational Therapy") OR (MH "Combined Modality Therapy") OR (MH "Antipsychotic Agents") OR (MH "Haloperidol")) S2. ((MH "Delirium") OR (MH "Confusion")) OR ( delirium* or (acute N3 (brain and dysfunction)) or confusion) S3. (MH "Randomized Controlled Trials") OR (MH "Random Assignment") OR (MH "Concurrent Prospective Studies") OR (MH "Prospective Studies") OR (MH "Multicenter Studies") OR (MH "Triple‐Blind Studies") OR (MH "Double‐Blind Studies") OR (MH "Single‐Blind Studies") S4. S1 and S2 and S3
Appendix 2. Data extraction sheet
Interventions for preventing ICU delirium data extraction sheet
Study Selection, Quality Assessment and Data Extraction Form
| First author |
Journal/Conference Proceedings etc | Year |
Study eligibility
| RCT | Relevant participants | Relevant interventions | Relevant outcomes |
| Yes / No / Unclear |
Yes / No / Unclear |
Yes / No / Unclear |
Yes / No* / Unclear |
* Issue relates to selective reporting when authors may have taken measurements for particular outcomes, but not reported these within the paper(s). Reviewers should contact trialists for information on possible non‐reported outcomes & reasons for exclusion from publication. Study should be listed in ‘Studies awaiting assessment’ until clarified. If no clarification is received after three attempts, study should then be excluded.
| Do not proceed if any of the above answers are ‘No’. If study to be included in ‘Excluded studies’ section of there view, record below the information to be inserted into ‘Table of excluded studies. |
| |
| Freehand space for comments on study design and treatment: |
References to trial
Check other references identified in searches. If there are further references to this trial link the papers now & list below. All references to a trial should be linked under one Study ID in RevMan.
| Code each paper | Author(s) | Journal/Conference | Proceedings etc |
Year |
| A | The paper listed above | |||
| B | Further papers | |||
| C |
Participants and trial characteristics
Participant characteristics
| Covariates | Further details |
| Age (mean, median, range, etc) | |
| Sex of participants (numbers / %, etc) | |
| Premorbid cognitive state (as assessed) | |
| Severity of illness (as assessed) | |
| ICU admission diagnosis | |
| BMI (mean, median, range, etc) | |
| Type of ICU from which patients were recruited | |
| Type of sedation received |
Trial characteristics
Methodological quality
Allocation of intervention
| State here method used to generate allocation and reasons for grading | Grade (circle) |
| Low risk of bias (Random) High risk of bias (e.g. alternate) Unclear |
Concealment of allocation
Process used to prevent foreknowledge of group assignment in a RCT, which should be seen as distinct from blinding
| State here method used to conceal allocation and reasons for grading | Grade (circle) |
| Low risk of bias High risk of bias Unclear |
Blinding
Person responsible for participants care Yes/No
Participant Yes/No
Outcome assessor Yes/No
Other (please specify) Yes/No
Incomplete outcome data
Low risk of bias, if the numbers and reasons for dropouts and withdrawals in the intervention groups were described or if it was specified that there were no dropouts or withdrawals Yes/No
High risk of bias, if the number or reasons for dropouts and withdrawals were not described Yes/No
Unclear, if the report gave the impression that there had been no dropouts or withdrawals, but this was not specifically stated Yes/No
Selective reporting
Low risk of bias, if predefined or clinically relevant and reasonably expected outcomes are reported on Yes/No
High risk of bias, one or more clinically relevant and reasonably expected outcomes were not reported on; data on these outcomes were likely to have been recorded Yes/No
Unclear, not all pre‐defined, or clinically relevant and reasonably expected outcomes are reported on or are not reported fully, or it is unclear whether data on these outcomes were recorded or not
Yes/No
Baseline imbalance
Low risk of bias, if there was no baseline imbalance in important characteristics Yes/No
High risk of bias, if there was a baseline imbalance due to chance or due to imbalanced exclusion after randomization Yes/No
Unclear, if the baseline characteristics were not reported Yes/No
Early stopping
Low risk of bias, if sample size calculation was reported and the trial was not stopped, or the trial was stopped early by formal stopping rules at a point where the likelihood of observing an extreme
intervention effect due to chance was low Yes/No
High risk of bias, if the trial was stopped early due to informal stopping rules or the trial was stopped early by a formal stopping rule at a point where the likelihood of observing an extreme intervention effect due to chance was high Yes/No
Unclear, if sample size calculation was not reported and it is not clear whether the trial was stopped early or not Yes/No
Other bias
No risk of other bias, the trial appears to be free of other components that could put it at risk of bias Yes/No
Risk of other bias, there are other factors in the trial that could put it at risk of bias, e.g.’for‐profit involvement, authors have conducted trials on the same topic, etc. Yes/No
Unclear, the trial may or may not be free of other components that could put it at risk of bias
Yes/No
Modified intention‐to‐treat
A modified intention‐to‐treat analysis is one in which all the participants in a trial are analysed according to the intervention to which they were allocated, whether they received it or not.
All participants analysed according to allocated intervention Yes/No
20% or fewer excluded Yes/No
More than 20% excluded Yes/No
Analysed as modified ‘intention‐to‐treat’ Yes/No
Were withdrawals described? Yes ? No ? Unclear?
Discuss if appropriate
Trial characteristics
| Further details |
|
| Single centre / multicentre | |
| Country / Countries | |
| How was participant eligibility defined? | |
| How many participants were randomized? | |
| Number of participants in each intervention group | |
| Number of participants who received allocated intervention | |
| Describe experimental intervention | |
| Describe control intervention | |
| Number of participants who were analysed | |
| Number of mechanically ventilated patients | |
| Time points at which delirium was assessed? | |
| Time points reported in the trial ? | |
| Time points you are using in RevMan | |
| Median (range) length of follow‐up reported in this paper on relevant primary & secondary outcomes (state weeks, months or years or if not stated) |
1 Primary outcomes
1.1 The event rate of delirium
| Intervention | Control |
1.2 Mortality
| Time point | Intervention (No. dead/total No. randomized to intervention) | Control (No. dead/No. randomized to control) |
2. Secondary outcomes
2.1 Number of delirium and coma‐free days (mean, SD)
| Intervention (No. with delirium/total No. randomized to intervention) | Control (No. with delirium/total No. randomized to control)l |
2.2 Duration of mechanical ventilation (mean, SD)
| Intervention | Control |
2.3 Length of stay in the ICU (mean, SD)
| Intervention | Control |
2.4 Cognitive impairment (COGIM)
| Timepoint | Intervention (No.with COGIM/total No. randomized to intervention) | Control (No. with COGIM/No. randomized to control) |
2.5 Adverse events (AE)
| Timepoint | Intervention (No.with AE/total No. randomized to intervention) | Control (No. with AE/No. randomized to control) |
Appendix 3. Criteria for risk of bias evaluation
Criteria for risk of bias evaluation
Random sequence generation
Low risk of bias: use of random sequences, e.g. random number generation, toss of coin.
Unclear risk of bias: no information on random sequence generation available.
High risk of bias: alternate medical record numbers or other non‐random sequence generation.
Allocation concealment
Low risk of bias: use of allocation method that prevents investigators or participants from knowing the next allocation, e.g. central allocation; sealed opaque envelopes; serially‐numbered, sequentially‐numbered but otherwise identical vehicles, including their contents; or other descriptions of convincing concealment of allocation. Unclear risk of bias: no information on allocation method available or the description was insufficient to enable decisive assessment. High risk of bias: use of allocation method that allows investigators or participants to know the next allocation, e.g. alternate medical record numbers; reference to case record numbers or date of birth; an open allocation sequence, unsealed or non‐opaque envelopes or both.
Blinding
Low risk of bias: for non‐pharmacological interventions we consider blinding of patients and staff difficult to uphold. We will therefore consider blinding adequate if outcome assessors were kept unaware of intervention allocations after inclusion of participants into the study. For pharmacological interventions we will consider blinding adequate if patients, staff and outcome assessors were unaware of intervention allocations after inclusion of participants into the study and the method of blinding involved placebo. Unclear risk of bias: blinding not described. High risk of bias: for non‐pharmacological interventions lack of blinded outcome assessment. For pharmacological interventions lack of blinding of patients, staff and outcome assessors or lack of placebo.
Incomplete outcome data
Low risk of bias: if the numbers and reasons for dropouts and withdrawals in the intervention and control groups are described, and dropouts did not exceed 20% of those initially included in the study, or if it was specified that there were no drop‐outs or withdrawals. Unclear risk of bias: if the report gives the impression that there were no dropouts or withdrawals, but this was not specifically stated. High risk of bias: if the number or reasons for dropouts and withdrawals are not described or if dropouts exceeded 20% of those initially included in the study.
Selective outcome reporting
Low risk of bias: if predefined or clinically relevant and reasonably expected outcomes are reported on. Unclear risk of bias: not all pre‐defined, or clinically relevant and reasonably expected outcomes are reported on or are not reported fully, or it is unclear whether data on these outcomes were recorded or not. High risk of bias: one or more clinically relevant and reasonably expected outcomes are not reported on even though data on these outcomes were likely to have been recorded.
Other potential threats to validity
Baseline imbalance between the intervention and control groups. Adequate: if there was no baseline imbalance in important characteristics. Unclear: if the baseline characteristics were not reported. Inadequate: if there was a baseline imbalance due to chance or due to imbalanced exclusion after randomization.
Early stopping
Low risk of bias: if sample size calculation was reported and the trial was not stopped, or the trial was stopped early by a formal stopping rule at a point where the likelihood of observing an extreme intervention effect due to chance was low (Lan 1983). Unclear risk of bias: if sample size calculation was not reported and it is not clear whether the trial was stopped early or not. High risk of bias: if the trial was stopped early due to informal stopping rules or the trial was stopped early by a formal stopping rule at a point where the likelihood of observing an extreme intervention effect due to chance was high (Lan 1983).
Other bias
To report on other bias in addition to the above mentioned (e.g. industry bias, academic bias, etc) one should continue using the following pattern. Low risk of bias: the trial appears to be free of other components that could put it at risk of bias. Unclear risk of bias: the trial may or may not be free of other components that could put it at risk of bias. High risk of bias: there are other factors in the trial that could put it at risk of bias, e.g. ’for‐profit’ involvement, authors have conducted trials on the same topic, etc.
Data and analyses
Comparison 1. Haloperidol versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 The event rate of delirium | 1 | 1439 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.87, 1.17] |
| 2 In‐hospital mortality | 2 | 1580 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.80, 1.22] |
| 3 Coma‐and delirium‐free days ( 28 days) | 2 | 1580 | Mean Difference (IV, Random, 95% CI) | ‐0.60 [‐1.37, 0.17] |
| 4 Length of ICU stay | 2 | 1580 | Mean Difference (IV, Random, 95% CI) | 0.18 [‐0.60, 0.97] |
1.1. Analysis.
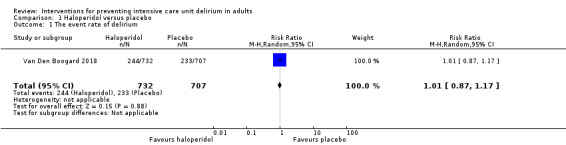
Comparison 1 Haloperidol versus placebo, Outcome 1 The event rate of delirium.
1.2. Analysis.
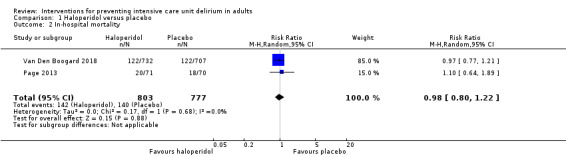
Comparison 1 Haloperidol versus placebo, Outcome 2 In‐hospital mortality.
1.3. Analysis.
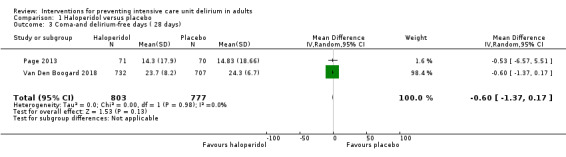
Comparison 1 Haloperidol versus placebo, Outcome 3 Coma‐and delirium‐free days ( 28 days).
1.4. Analysis.
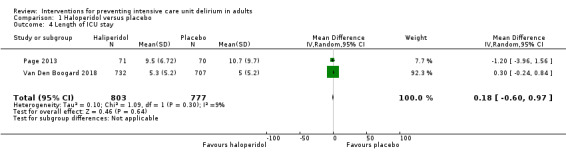
Comparison 1 Haloperidol versus placebo, Outcome 4 Length of ICU stay.
Comparison 2. Sedation with daily interruption vs Sedation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 The event rate of ICU delirium | 2 | 483 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.81, 1.14] |
| 2 In‐hospital mortality | 2 | 483 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.78, 1.40] |
| 3 Length of stay in the ICU | 2 | 483 | Mean Difference (IV, Random, 95% CI) | ‐1.19 [‐2.91, 0.53] |
| 4 Adverse events | 2 | 483 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.42, 1.75] |
Comparison 3. Enviromental intervention vs. Standard care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 The event rate of ICU delirium | 2 | 870 | Risk Ratio (M‐H, Random, 95% CI) | 1.14 [0.95, 1.38] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Abdelgalel 2016.
| Methods | Prospective, randomized, double‐blind controlled trial Setting: ICU Country: Egypt Groups: dexmedetomidine versus haloperidol versus saline infusion Period: January 2014 to October 2015 |
|
| Participants |
Sample size: 90 randomized (30/30/30) Included
Excluded
Missing: none; all are accounted for in the flowchart. |
|
| Interventions | Patients were assigned in a 1:1:1 manner Intervention I: dexmedetomidine continuous intravenous infusion of 0.2 to 0.7 µg/kg/hour preceded by a loading dose of 1.0 µg/kg intravenously over 10 minutes if needed Intervention II: haloperidol continuous intravenous infusion of 0.5 to 2 mg/hour preceded by a loading dose of 2.5 mg intravenously over 10 minutes if needed Control: normal saline continuous intravenous infusion (2 to 8 mL/hour) and loading dose (10 mL) over 10 minutes if needed |
|
| Outcomes |
Primary
Secondary
Measured by: CAM‐ICU and RASS Adverse events: bradycardia occurred significantly more in dexmedetomidine group (8 patients) than in haloperidol group (2 patients) and placebo group (1 patient). 2 patients in haloperidol group developed prolonged QTc‐interval ( > 500 ms). No patients in both placebo and dexmedetomidine groups developed prolonged QTc interval. Three patients developed arrhythmia in haloperidol group compared to 2 patients in both dexmedetomidine and placebo groups. Hypotension occurred in 4 patients in dexmedetomidine group while hypotension occurred in 3 patients in both haloperidol and placebo groups. |
|
| Notes |
Conclusion: incidence of delirium was significantly lower in dexmedetomidine group 3/30 (10%) than haloperidol 10/30 (33.3%) and placebo 13/30 (43.3%) groups. Duration of NIV was significantly shorter in dexmedetomidine group than in placebo group and haloperidol group. The incidence of endotracheal intubation was significantly less in dexmedetomidine group compared to placebo and haloperidol groups. The length of ICU and hospital stay was significantly shorter in dexmedetomidine group compared to the alternatives. Funding: not stated, untypical for a pharmacological study Conflict of interest: not declared, untypical for a pharmacological study Study number: not reported Contact with authors: author emailed 21 December 2016 for more information ‒ awaiting response |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description of how the randomization was performed |
| Allocation concealment (selection bias) | Unclear risk | No description of how the allocation was concealed |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts |
| Selective reporting (reporting bias) | Unclear risk | No online protocol to check |
| Other bias | Unclear risk | Unknown other biases |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding of staff and patients |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of outcome assessor |
Brummel 2014b.
| Methods | Single‐centre, randomized controlled trial with 3‐month follow‐up; a feasibility study Setting: medical and surgical ICU Country: USA Groups: usual care vs. physical therapy only vs cognitive and physical therapy Period: February 2011 to April 2012 |
|
| Participants |
Sample size: 87 randomized (16/16/32 in arms) Included
Excluded
Missing: all are accounted for in flowchart. |
|
| Interventions | Patients were assigned in a 1:1:2 manner Intervention I: 1 daily physical therapy session (passive ROM, active exercise, sit at edge of bed, stand/transfer, ADL training and walk). Duration of physical therapy session is not described. Intervention II: 1 daily physical therapy session and 20 minutes. cognitive therapy sessions twice daily during hospitalization. Patients exhibiting impaired executive functioning or impaired functional mobility continued outpatient cognitive therapy for 6 weeks (6 sessions) using goal management training. Control: usual care (physical therapy approximately once every 6 days). |
|
| Outcomes |
Primary
Secondary
Measured by: CAM‐ICU and RASS Adverse events: 1 patient experienced acute back pain accompanied by hypotensive urgency during physical therapy |
|
| Notes |
Conclusion: delirium‐/coma‐free days did not differ between groups Funding
Conflict of interest
Study number: NCT01270269 Conference proceeding: 1 Contact with authors: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated permuted‐block randomization scheme |
| Allocation concealment (selection bias) | Low risk | Group allocations were printed and placed in sealed opaque envelopes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Flowchart accounts for all patients throughout the study |
| Selective reporting (reporting bias) | Unclear risk | In the online protocol: NCT01270269 and in a protocol paper (Brummel 2012), a considerably large number of secondary outcome measures were listed for follow‐up at both 3 and 6 months. At 3 months, data on Activity‐Specific Balance Confidence, AD8 (assessment of change in cognitive abilities), General and Employment scale, Canadian Study of Health and Aging Frailty scale were planned, but these outcomes were not reported. |
| Other bias | Low risk | A post hoc analysis showed that the study was underpowered (38%) to detect a meaningful 1.5% change in the tower test (executive function) Risk of a type 1 error |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible to blind patients or personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Only outcomes measured at follow‐up were collected by outcome assessor. Data on delirium was presumably collected by study authors. (page 372). |
Mehta 2012.
| Methods | Multi‐national (2), multicentre (16), randomized controlled trial Setting: tertiary care medical and surgical ICUs Countries: USA and Canada Groups: protocolized sedation and daily interruption vs. only protocolized sedation Period: January 2008 to July 2011 |
|
| Participants |
Sample size: 430 critically ill adults (214/209) Included
Excluded
Missing: (4/3); all are accounted for in flowchart |
|
| Interventions |
Intervention: protocolized sedation with daily interruption of benzodiazepines and opioids and hourly assessments of wakefulness Control: protocolized sedation using opioids and benzodiazepines and prioritising pain assessment |
|
| Outcomes |
Primary
Secondary
Delirium measured by: intensive care screening delirium checklist Adverse events: a safety monitoring committee reviewed adverse events: unintentional device removal in specific removal of endotracheal tube. The difference was 4.7% (n = 10) in group with protocolized sedation and interruption vs. 5.8% (n = 12) in control. |
|
| Notes |
Conclusion: median time to successful extubation was 7 days in both groups and prevalence of delirium was identical in both groups Funding: Canadian Institutes for Health Research Conflict of interest: none were reported Study number: NCT00675363 Secondary analysis was published in Mehta 2015 (see Excluded studies) Contact with author: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Automated telephone system that stratified by centre with undisclosed variable block sizes |
| Allocation concealment (selection bias) | Low risk | Adequate allocation concealment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients are accounted for |
| Selective reporting (reporting bias) | Low risk | In the online protocol: NCT00675363 patient recall of ICU stay was listed as a secondary outcome; however it was reported in a later publication (Burry 2015). |
| Other bias | Low risk | Low risk of other biases |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Neither patients, study personnel, clinicians, nor investigators analysing data were masked to group allocation |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Study personnel and investigators analysing data were not blinded for in‐hospital data; however the researchers were blinded for follow‐up data after discharge |
Moon 2015.
| Methods | Single‐blind, randomized controlled trial Setting: ICU (105 beds) in a general hospital with 1049 beds Country: Republic of Korea Groups: preventive delirium protocol according to a nursing algorithm that addressed cognition and orientation, environmental factors and early therapeutic intervention vs. usual care Period: March 2013 to May 2013 |
|
| Participants |
Sample size: 134 (60/63) Included
Excluded
Missing: (5/6); all are accounted for in flowchart. |
|
| Interventions |
Intervention: the first 7 days in the ICU a delirium‐prevention nursing algorithm covering cognitive assessment and orientation, environment intervention (assessment of hearing impairments, sleep management, aroma therapy, comfort) and early therapeutic intervention (nutrition, fluid and electrolyte balance, early mobilization, sleeping pills, early detection of infection, removal of unnecessary catheters, avoidance of hypoxia and pain control) was used. Control: no provision of preventive delirium nursing (i.e. typical nursing care included regular checking of consciousness and orientation without attempting to:
|
|
| Outcomes |
Primary
Secondary
Delirium was measured by: CAM‐ICU Adverse events: none reported |
|
| Notes |
Conclusion: the protocol had no significant effect on delirium incidence, in‐hospital mortality, re‐admission to the ICU, or length of ICU stay. The intervention is described in very general terms and includes 'state of the art' good ICU nursing care, making replication of the study and, if relevant, implementation of the intervention into clinical practice difficult. The control group received standard care entailing no provision of proactive "delirium prevention nursing". No power calculation was conducted prior to the study, the sample is seemingly a result of 3 months of data collection Funding: research fund from College of Nursing, The Catholic University of Korea Conflict of interest: not declared Study number: not stated Contact with author: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Opaque assignment cards indicating assignment to the intervention group (70 cards) or control group (70 cards) were placed in a large envelope and shuffled before the envelope was sealed |
| Allocation concealment (selection bias) | Unclear risk | Leader of nursing team drew a card and did not replace it afterwards: theoretically, the allocation of the last cases can be guessed by the previous allocations before the large envelope was empty |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Flowchart accounts for all patients throughout the study |
| Selective reporting (reporting bias) | Unclear risk | All predefined outcomes listed in the paper were reported. It was not possible to check with an online protocol |
| Other bias | Unclear risk | No discussion related to possible spill‐over from intervention to control patients |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Allegedly patients were unaware of intervention; however we suspect patients could be aware as masking the intervention seems impossible |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcome assessors were not blinded |
Nassar 2014.
| Methods | Single‐centre, randomized controlled trial. Setting: low nurse staffing ICU (developing country) with 6 beds, an academic tertiary hospital Country: Brazil Groups: intermittent sedation vs. daily interruption Period: January 2009 to December 2011 |
|
| Participants |
Sample size: 60 (30/30) Included
Excluded
Missing: not reported, all patients accounted for in flowchart. |
|
| Interventions |
Intervention: daily interruption of continuous sedative and opioid infusion. This group received midazolam/propofol at the discretion of the attending physician to reach the sedation goal which was having the patient awake or easily aroused with gentle stimulus. Every morning at 7.00 am the sedation was stopped. If the patient was agitated or could not follow commands, the infusion was restarted at half dose. Control: intermittent sedation. The intermittent sedation group would be kept without continuous infusion of sedatives. Agitation and pain were treated and delirium was treated with haloperidol. If the patient was still uncomfortable midazolam/propofol infusion was started and interruption of the sedation would be attempted in the next shift In both groups. The goal was to maintain a Sedation Agitation Scale (SAS) level of 3 or 4 (calm, easily aroused or awakened with verbal stimuli). Midazolam or propofol was used at the discretion of the attending physician. |
|
| Outcomes |
Primary
Secondary
Delirium measured by: CAM‐ICU Adverse events: self‐extubations (2/1), accidental removal of catheters (1/2) |
|
| Notes |
Conclusion: there were no differences in ventilator‐free days within 28 days between daily interruption and intermittent sedation; and no difference in the incidence of delirium. Authors calculated that a sample size of 106 patients would be required to detect a mean difference of 2 days of mechanical ventilation. The trial was stopped prematurely due to slow inclusion. The 2 interventions are very similar. Funding: not stated Conflict of interest: the authors declare that they have no competing interests Study number: NCT00824239. Contact with authors: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random selection of opaque sealed envelopes from a box with 120 envelopes |
| Allocation concealment (selection bias) | Low risk | Concealed by random selection of opaque sealed envelopes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Groups were well balanced |
| Selective reporting (reporting bias) | Low risk | All outcomes in the online protocol (NCT00824239) were reported |
| Other bias | High risk | The trial was not sufficiently powered to show 1 intervention superior to the other |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Attending clinicians were aware of which group the included patients were allocated to |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Research staff were aware of which group the included patients were allocated to. |
Page 2013.
| Methods | Single‐centre, double‐blind, placebo‐controlled randomized trial Setting: general mixed medical‐surgical ICU Country: UK Groups: haloperidol vs. placebo Period: November 2010 to September 2012 |
|
| Participants |
Sample size: 142 (71/70) Included
Excluded
Missing: 2 that are accounted for: 1 lost to follow up and 1 discontinued intervention |
|
| Interventions | Interventions: receive haloperidol 2.5 mg every 8 hours Control: 0.9% saline placebo intravenously every 8 hours irrespective of coma or delirium status | |
| Outcomes |
Primary
Secondary
Measured by: CAM‐ICU Adverse events: oversedation was found in 11 patients in the haloperidol group, 6 patients in the placebo group, serious adverse events were 3 reported incidents in the intervention group (fast atrial fibrillation with hypotension (n = 1), readmission to ICU with sepsis (n = 1), failed extubation (n = 1)) and 5 in placebo group (apnoea post treatment for agitation (n = 1), readmission to ICU with sepsis (n = 1), failed extubation (n = 3). |
|
| Notes |
Conclusion: early treatment with haloperidol did not alter the prevalence or duration of delirium or coma, with an average duration of delirium of 5 days in both groups. Haloperidol did not have effect on any secondary outcomes. Funding: National Institute for Health Research, UK Intensive Care Foundation Conflict of interest: VJP has received honoraria from Orion; EWE from Hospira, Orion and Abbott; and DFM and GDP are co‐directors of Research for the UK Intensive Care Foundation. The rest have no conflicts of interest. A health evaluation and cost effectiveness were presented later in a conference proceeding (Page 2015), based on data originating from this study. Study number: ISRCTN83567338 Confrerence proceedings: 2 Contact with authors: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization by an independent nurse in a 1:1 ratio with permuted block sizes of 4 and 6 |
| Allocation concealment (selection bias) | Low risk | A centralized, secure web‐based randomization service was used |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat analysis. All patients were analysed in their randomized group irrespective of treatment actually received. Numbers and reasons for dropouts and withdrawals are described in the trial profile 142 patients randomized/141 contributed outcome data |
| Selective reporting (reporting bias) | Low risk | Authors have reported outcomes described in the online protocol |
| Other bias | Unclear risk | Unknown other biases |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants and personnel were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded data monitoring. Statisticians were not masked to allocation |
Pandharipande 2007.
| Methods | Multicentre (two), double‐blind, randomized controlled trial. Setting: Medical and surgical ICU at a tertiary centre Country: USA Groups: Dexmedetomidine vs. lorazepam Period: August 2004 to April 2006 |
|
| Participants |
Sample size: 106 adults (52/51) Included
Excluded
Missing: 3 were withdrawn by family and accounted for. |
|
| Interventions |
Intervention: sedation with dexmedetomidine as needed or for a maximum of 120 hours Control: sedation with lorazepam for up to 120 hours Study drugs were titrated to achieve the desired level of sedation, using the RASS. Patients in both groups were monitored twice daily for delirium. |
|
| Outcomes |
Primary
Secondary
Measured by: CAM‐ICU Adverse events: seizures were reported for 2 in the dexmedetomidine group and 1 in the lorazepam group and self‐extubation was reported for 4 in the dexmedetomidine group compared to 2 in the lorazepam group. One in each group experienced bradycardia HR < 40 /minute at some point during the 120‐hour study drug protocol. |
|
| Notes |
Conclusion: sedation with dexmedetomidine resulted in 4 more days alive without delirium and coma and significantly more time at the target level of sedation compared to lorazepam. There was no difference in event rate of delirium between groups. Funding: PP, DH, MM, TG, BP have received research grants/honoraria from Hospira. EE has received grants and honoraria from Hospira, Pfizer and Eli Lilly and Aspect Medical Systems. Other authors declared no financial disclosures. Conflict of interest Authors state that the company has not influenced the conduct of the study, the interpretations of data or the publication process. The study had an independent data monitoring and ethics committee monitoring safety. Only statisticians were not masked for allocation, all others were masked. The investigators obtained an Investigational New Drug Approval from the US FDA which permitted the study of dexmedetomidine for longer than the current 24‐hour FDA‐labelled indication use in the ICU and at doses as great as 1.5 mcg/kg/hour, i.e. higher than doses currently approved by the FDA. The FDA demanded that an electrocardiogram and endocrine levels were taken and monitored at baseline. Study number: NCT00095251 Contact with author: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated, permuted block randomizations |
| Allocation concealment (selection bias) | Low risk | Only the investigational pharmacist knew allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low attrition |
| Selective reporting (reporting bias) | Low risk | All predefined outcomes in the online protocol NCT00095251 are reported |
| Other bias | Unclear risk | Unknown other biases |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Patients and study personnel were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The authors do not specifically state who assessed outcomes |
Shehabi 2013.
| Methods | Multicentre (6), randomized controlled trial, unblinded parallel group, feasibility and safety study Setting: tertiary and regional hospital Country: Australia and New Zealand Groups: Early goal directed sedation vs. standard sedation Period: July 2011 to December 2011 |
|
| Participants |
Sample size: n = 37 (21/16) Included
Excluded
Missing: none lost to follow up |
|
| Interventions |
Intervention: early goal‐directed sedation (EGDS) was based on an algorithm including a dexmedetomidine infusion at a starting dose of 1 μg/kg/hour without a loading dose. Bolus administration of dexmedetomidine was strictly prohibited owing to the risk of severe bradycardia and sinus arrest. If required, sedation could be supplemented with propofol. Sedatives were administered to achieve the desired level of light sedation whenever possible. Dexmedetomidine was titrated to the desired level of sedation by the bedside nurses. Propofol could be used as a supplement. Clonidine, remifentanil or benzodiazepines were only to be administered for management of convulsions, palliations, procedural anaesthesia or refractory agitation. Control: standard sedation (type of drug, way of administration, time for cessation and level of sedation) was at the discretion of the treating clinician. |
|
| Outcomes |
Primary
Secondary
Other feasibility outcomes included the average recruitment rate. Safety outcomes included device removal and self‐extubation, the use of physical restraints, major serious adverse events, vasopressor therapy, and haemodynamic instability. Measured by: CAM‐ICU Adverse events: 2 reported in the EGDS group (self‐extubation and removal of devices); none in control group. |
|
| Notes |
Conclusion: delivery of EGDS sedation was feasible, appeared safe, achieved early light sedation, minimized benzodiazepine and propofol use, and decreased the need for physical restraints. An equal proportion of patients (38%) experienced 1 or more positive CAM‐ICU assessments. Funding: Hospira provided the study drug dexmedetomidine at no cost to study sites. Hospira and its employees had no input into the design, protocol, study conduct, data collection, data analysis, manuscript preparation, review or submission. Conflict of interest
Study number: ACTRN 12611000166976 Contact with authors: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Concealed envelopes: unclear whether they were opaque and were opened sequentially, and only after the envelope was irreversibly assigned to the participant |
| Allocation concealment (selection bias) | High risk | Block randomization in a very small sample could have increased the possibility of foreseeing the next allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Flowchart accounts for all patients throughout the study |
| Selective reporting (reporting bias) | Unclear risk | The online protocol ACTRN 12611000166976 does not include information on outcome measures, therefore this cannot be assessed |
| Other bias | Unclear risk | Unknown other biases |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding of patients, clinicians, study personnel |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Unblinded outcome assessor |
Simons 2016.
| Methods | Single‐centre, randomized controlled trial Setting: teaching hospital (730 beds), mixed medical and surgical ICU (16 beds) Country: the Netherlands Groups: High‐intensity dynamic light application (DLA) vs normal lighting Period: July 2011 to 2013 |
|
| Participants |
Sample size: n = 734 (354/ 360) Included
Excluded
Missing: 20 excluded (7/13) all accounted for in the flowchart |
|
| Interventions |
Intervention: for patients in the DLA group lighting level, colour and temperature rose from 7.00 am to 9.00 am. This was maintained to 11.30 am. Until 13.30 pm, lighting was decreased. From 13.30 pm till 16.00 pm, the light was increased again. After 16.00 pm it gradually decreased and lights were switched off automatically at 22.30 pm. Control: standard group was exposed to standard lighting which was turned on and off as usual for procedures |
|
| Outcomes |
Primary
Secondary
Delirium was measured by: PREdiction of DELIRium in ICu patients (PRE‐DELIRIC) and CAM‐ICU Adverse events: none reported |
|
| Notes | Study was terminated prematurely after an interim analysis for futility. Data were analysed as ITT and per protocol analysis. Conclusion: DLA as a single intervention does not reduce the cumulative incidence of delirium. Bright‐light therapy should be assessed as part of a multicomponent strategy. Funding: none Conflict of interest: none declared Study number: NCT01274819 Contact with authors: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A 1:1 ratio randomization, according to a secured computer‐generated randomization list |
| Allocation concealment (selection bias) | Low risk | A secured computer‐generated randomization list was used |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Study was well balanced and all patients are accounted for |
| Selective reporting (reporting bias) | Unclear risk | In the online protocol NCT01274819 it was stated that serum levels of inflammatory markers would be assessed as well as Health‐related Quality of Life 3 and 6 months after discharge; these outcomes were not presented in the paper. |
| Other bias | Unclear risk | Study was terminated prematurely after an interim analysis for futility. The power calculation suggested that 1000 patients should be included to be able to detect a 10% decrease in the incidence of delirium between groups |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcomes assessment was done by ward nurses who were not blinded |
Strøm 2010.
| Methods | Single‐centre, randomized controlled trial Setting: general ICU (18 beds medical and surgical) Country: Denmark Groups: No sedation vs. sedation Period: April 2007 to December 2008 |
|
| Participants |
Sample size: 140 (55/58) Included
Excluded
Missing: 27 excluded after randomization, flowchart accounts for all patients |
|
| Interventions |
Intervention: no sedation and treatment only with analgesics Control: sedation with daily interruption until awake |
|
| Outcomes |
Primary
Secondary
Additional reporting: in‐hospital mortality, delirium, ICU LOS Measured by: twice daily using criteria from diagnostic and statistical manual of mental disorders (DSM‐IV). Adverse events: accidental removal of endotracheal tube n = 7 in intervention group, and n = 6 in control |
|
| Notes |
Conclusions: patients without sedation had significantly more days without ventilation and shorter ICU length of stay, hyperactive delirium was more frequent in the intervention group (20% vs 7%, P = 0.04). Authors discuss that the CAM‐ICU would have enabled assessment of hypoactive delirium as well. Patients in the present study were invited to participate in a follow‐up interview study that aimed to explore long‐term psychological effects of the intervention. Funding: Danish Society of Anesthesiology and Intensive Care Medicine, The Fund of Kirsten Jensa la Cour, The Fund of Danielsen, The Fund of Holger and Ruth Hess. None of these funds affected the trial or the paper according to the authors. Conflict of interest: no conflict declared Study number: NCT00466492 Contact with Author: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random selection of opaque sealed envelopes in a box |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes in a box with 140 envelopes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Flowchart accounts for all patients throughout the study |
| Selective reporting (reporting bias) | Low risk | In the online protocol, NCT00466492, the more long‐term psychological effects were planned to be reported; these outcomes were reported in a later paper Strøm 2011 |
| Other bias | Low risk | Low risk of other potential biases |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding of patients, clinicians, study personnel |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcome assessors were not blinded |
Van Den Boogard 2018.
| Methods | Multicentre, 3‐arm randomized controlled trial Setting: 21 ICUs at university hospitals, teaching and non‐teaching hospitals Country: the Netherlands Groups: 3 arms: 2 arms each with different doses of haloperidol and a placebo arm Period: July 2013 to December 2016 |
|
| Participants |
Sample size: 1789 (353/734/706) Included
Excluded
Missing: 3 + 2 + 2 participants did not receive treatment (documented in flowchart) |
|
| Interventions |
Intervention 1: Haloperidol 1 mg intravenously 3 times daily Intervention 2: Haloperidol 2 mg intravenously 3 times daily. Control: Placebo (0.9 % sodium chloride) intravenously 3 times daily. |
|
| Outcomes |
Primary
Secondary
Measured by: CAM‐ICU and ICDSC Adverse events: 5 serious adverse events were reported, 3 patients died, 1 in each group. The events were judged to be unrelated to the study medication. 2 patients in the 1 mg haloperidol group and 1 patient in the 2 mg haloperidol group developed monomorphic ventricular tachycardia, 1 patient in the 2 mg haloperidol group developed refractory shock, 1 patient in the placebo group developed a suspected malignant neuroleptic syndrome event. |
|
| Notes |
Conclusions: The 1 mg haloperidol study arm was prematurely stopped due to futility. There was no difference in the median days patients survived within 28 days. No differences in effects on the secondary outcomes were found. The number of reported adverse events did not differ between groups. Data on delirium incidence and coma days could be retrieved from a total of 1506 patients (84.2%) from 14 of the 21 sites. Funding: funded partly by the ZonMw programme (dossier no 836031004). They had no influence on study design, conduct or publication. Conflict of interest: All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Dr Brüggemann reported that he received grant support and consultancy and speaker fees from Pfizer, Merck, Sharp, & Dohme, Astellas, and Gilead. Study number: clinicaltrials.gov identifier: NCT01785290 Contact with author: emailed for further information on missing data and data on ventilator‐free days 1 May 2018. Authors provided the data requested. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A permuted block randomization. Patients were allocated to each group in a 1:1:1 ratio |
| Allocation concealment (selection bias) | Low risk | The pharmacist who kept the randomization code and the members of the data and safety management board were the only people who were not blinded. The pharmacist was not involved in the clinical management of the patients. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on delirium incidence and coma days could only be retrieved from a total of 1506 patients (84.2%) or from 14 of the 21 sites. Study authors have produced data to show that missing data were evenly distributed between groups. |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported, with the exception of QoL which will be reported elsewhere in a subsequent publication (1‐ and 6‐month follow‐up) |
| Other bias | Low risk | Low risk of other potential biases |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Pharmacist with the randomization code and the members of the data and safety management board were the only people who were not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors were blinded |
Van Rompaey 2012.
| Methods | Single‐centre, randomized controlled trial Setting: adult ICU Country: Belgium Groups: Sleeping with earplugs vs. without earplugs Period: November 2008 to April 2009 and November 2009 to April 2010 |
|
| Participants |
Sample size: 136 patients (69/67) Included
Excluded
Missing: number of patients assessed the 1st morning until the 4th morning is stated in the flowchart |
|
| Interventions |
Intervention: patients slept with ear‐plugs from 10.00 pm to 6.00 am. Control: patients slept without earplugs during the night for a maximum of 5 days |
|
| Outcomes |
Primary
Secondary
Adverse events: none reported Measured by: the Neelon and Champagne Confusion Scale (NEECHAM) to assess delirium and the Glascow Coma Scale to assess consciousness |
|
| Notes |
Conclusion: earplugs may be a useful instrument in the prevention of confusion or delirium. The beneficial effects seem to be strongest within 48 hours after admission. More cognitive normal patients were in the intervention group (P = 0.006). There were different observation periods for the two groups: mean observation period in the intervention group was 43 hours opposed to 33 hours in control group. Funding: none stated Conflict of interest: authors stated none Study number: ISRCTN36198138 Contact with author: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Assigned in a 1:1 ratio to intervention or control group using a computer program |
| Allocation concealment (selection bias) | Low risk | Assignment to the study was done by an independent nurse researcher |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Flowchart accounts for all patients throughout the study |
| Selective reporting (reporting bias) | Low risk | All predefined outcomes stated in the online protocol ISRCTN36198138 were reported |
| Other bias | Low risk | Low risk of other potential biases |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The researchers were blinded during data collection; patients were not blinded due to study design |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded researcher assessed patients for delirium and sleep perception |
List of acronyms and abbreviations which appear in this table
ADL: activities of daily living; CAM‐ICU: confusion assessment method for the ICU; COPD: chronic obstructive pulmonary disease; CT: computed tomography; DLA: dynamic light application; DSM‐IV: Diagnostic and Statistical Manual of Mental disorders IV; EGDS: early‐goal directed sedation; FDA: Federal Drug Administration;HR: heart rate; ICDSC: Intensive Care Delirium Screening Checklist; ICU: intensive care unit;ITT: intention‐to‐treat; LOS: length of stay; MRI: magnetic resonance imaging; ms: milliseconds; NEECHAM: the Neelon and Champagne confusion scale; NIV: non‐invasive ventilation; PRE‐DELIRIC: PREdiction of DELIRium in ICU patients; QoL: quality of life; RASS: Richmond Agitation and Sedation scale; ROM: range of motion; SAS: Riker Sedation‐Agitation Scale; vs: versus
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Esen 2009 | Trial stopped prematurely because of administrative problems. |
| Finotto 2006 | Cardiac ICU, wrong study population |
| Hatta 2014 | Few ICU participants (24/67) included in overall study population, not solely ICU population. |
| Mansouri 2016 | Delirium data was not measured in the control group. |
| Mehta 2015 | Not an RCT |
| Riker 2009 | Treatment, not prevention; some participants had delirium at inclusion |
| Schweickert 2009 | No outcomes defined the same as the ones needed for this review. Authors contacted November 2016 by email to obtain transformed data; no response, however. |
| Álvarez 2017 | Measured delirium with CAM not CAM‐ICU or other for this review relevant tools |
Acronyms and abbreviations used in this table
CAM: confusion assessment method; CAM‐ICU: confusion assessment method for the ICU; ICU: Intensive care unit; RCT: Randomized controlled trial
Characteristics of studies awaiting assessment [ordered by study ID]
Daley 2015.
| Methods |
Setting Country Period |
| Participants |
Sample size Included excluded Missing |
| Interventions |
Interventions Control |
| Outcomes |
Primary Secondary Measured by Adverse events |
| Notes |
Conclusion Authors have been contacted for more details 5 December 2016. Awaiting response. |
NCT02612948.
| Methods |
Setting Country Period |
| Participants |
Sample size Included excluded Missing |
| Interventions |
Interventions Control |
| Outcomes |
Primary Secondary Measured by Adverse events |
| Notes |
Conclusion. Authors have been contacted 5 December 2016. Awaiting response. |
Page 2015.
| Methods | Double‐blind placebo‐controlled randomized trial (see Page 2013), this study is the follow‐up after 6 months Setting: general ICU Country: UK Groups: haloperidol vs. placebo Period: not stated |
| Participants |
Sample size: 142 Included
Excluded
Missing: not stated how many, however data were imputed |
| Interventions | Interventions: receive haloperidol 2.5 mg every 8 hours Control: 0.9% saline placebo intravenously every 8 hours until delirium free for 48 hours, discharge or death. |
| Outcomes |
Primary
Secondary
Measured by: TICS‐M and EQ‐5D Adverse events: not stated |
| Notes |
Conclusion: TISC‐M scores assessed in 57 survivors (69%) were below normal, however not different between groups. Authors concluded: delirium adversely impacts cognitive function and QoL following critical illness. Funding: not stated Conflict of interest: not stated Study number: not stated, but referred to as HOPE‐ICU trial (ISRCTN83567338) This health evaluation and cost‐effectiveness originated from Page 2015 study data (see previous). Contact with authors: authors were emailed 12 September 2016, asking for further results and full paper reporting — awaiting response. |
Preslaski 2012.
| Methods | Randomized, double‐blind study Setting: Country: USA Period: |
| Participants |
Sample size: 23 (11/12) Included Excluded Missing |
| Interventions |
Interventions: add dexmedetomidine to existing benzodiazepine sedation when patients qualified for daily awakenings Control: add midazolam to existing benzodiazepine sedation |
| Outcomes |
Primary
Secondary
Measured by: ICU Delirium Screening Checklist Adverse events: including haemodynamic profiles and delirium |
| Notes |
Conclusion: need full paper review, but apparently delirium was monitored as an adverse event (ICU Delirium Screening Checklist) Authors have been contacted by email 19 September 2016 — awaiting response. |
Ryu 2014.
| Methods |
Setting Country Period |
| Participants |
Sample size Included excluded Missing |
| Interventions |
Interventions: Control |
| Outcomes |
Primary Secondary Measured by Adverse events |
| Notes |
Conclusion: Authors were contacted 5 December 5, 2016 for more details; awaiting response. |
Acronyms and abbreviations used in this table
EQ‐5D: EuroQol‐5 Domain questionnaire;ICU intensive care unit; QALY: Quality‐adjusted life year; QoL: quality of life; Riker score: Riker Sedation‐Agitation Scale; TICS‐M: The Modified Telephone Interview for Cognitive Status; vs:versus
Characteristics of ongoing studies [ordered by study ID]
Burry 2017.
| Trial name or title | Feasibility of melatonin for prevention of delirium in critically ill patients: a protocol for a multicentre, randomised, placebo‐controlled study Country: Canada |
| Methods | Multicentre (3), randomized 3‐arm placebo‐controlled trial |
| Participants | Adults with expected ICU stay > 48 hours. Expected n = 69 ICU patients. |
| Interventions |
Intervention
Control: placebo |
| Outcomes |
|
| Starting date | July 2017 |
| Contact information | (First author): lisa.burry@sinaihealthsystem.ca, Dep. of Pharmacy. Mount Sinai Hospital, and Leslie Dan Faculty of Pharmacy, University of Toronto, Canada. |
| Notes |
Study number: NCT02615340 Status: not started yet Contact with author: author emailed 30 June 2017 and responded that inclusion was about to start, expected period of recruitment was 12 months |
Jerath 2015.
| Trial name or title | The use of volatile anaesthetic agents for long‐term critical care sedation (VALTS): study protocol for a pilot randomised controlled trial. Country: Canada |
| Methods | Multicentre, pragmatic pilot randomized controlled trial. Safety and feasibility trial. Randomized: 2:1 |
| Participants | Adult ICU patients requiring mechanical ventilation and sedation for more than 48 hours. Expected: 60 (40/20) |
| Interventions |
Intervention
Control
|
| Outcomes |
|
| Starting date | Unknown; trial was registered 2013 |
| Contact information | Angela.Jerath@uhn.ca Department Anesthesia and Pain Medicine, Toronto General Hospital, 200 Elizabeth St, Toronto, Ontario M5G 2C4, Canada |
| Notes |
Study number: NCT01983800 Status: recruiting, expected completion June 2016 Contact with author: email sent 26 September 2016 ‐ the author responded that the study is still ongoing and no results on delirium are published yet. |
Martinez 2017.
| Trial name or title | Prophylactic melatonin for delirium in intensive care (Pro‐MEDIC): study protocol for a randomised controlled trial. Country: Australia |
| Methods | Multicentre, randomized double‐blind placebo‐controlled trial |
| Participants | Adult ICU patient with a ICU LOS of minimum 72 hours of admission. Expected: 850 adult ICU patients |
| Interventions |
Interventions
Control
|
| Outcomes |
CAM‐ICU will be used as measurement of delirium. |
| Starting date | Trial registered 20 December 2015 |
| Contact information | ed.martinez@hnehealth.nsw.gov.au |
| Notes |
Study number: ACTRN12616000436471 Status: Recruiting Contact with author: email sent 5 July 2017 for status ‐ awaiting response from corresponding author |
Miles 2012.
| Trial name or title | A randomised controlled trial of direct noise reduction in the ICU Country: USA |
| Methods | A 3‐arm randomized controlled trial to establish the feasibility of the interventions and their ability to impact noise, sleep, and delirium. |
| Participants | Mechanically ventilated ICU patients. Expected n = 45 |
| Interventions |
Intervention
Control
|
| Outcomes |
|
| Starting date | Unknown |
| Contact information | Dr. Hite. Clevaland Clinic Main Campus, Cleveland, Ohio, USA |
| Notes |
Study number: unknown Status: in 2012 was n = 8 were enrolled Contact with authors: Dr Hite was contacted by post 14 September 2016 for further information and status on the study — we are awaiting response. |
NCT01739933.
| Trial name or title | The MENDSII Study, maximizing the efficacy of sedation and reducing neurological dysfunction and mortality in septic patients with acute respiratory failure (MENDSII) Country: USA |
| Methods | Double‐blind, parallel assignment, multicentre randomized controlled trial, efficacy study |
| Participants | Adults, medical/surgical ICU patients, on mechanical ventilation, requiring sedation and have suspected or known infection. Expected n = 530 |
| Interventions |
Intervention
Control
|
| Outcomes |
|
| Starting date | August 2012 |
| Contact information | Dr. Pandiharpande email: patik.pandiharipande@vanderbilt.eud |
| Notes |
Study number: NCT01739933 Status: enrolment of half of study population is completed and results are expected in 2018. Contact with authors: authors contacted December 2016 and responded. |
NCT01791296.
| Trial name or title | Does nightly dexmedetomidine improve sleep and reduce delirium in ICU patients? (SKY‐DEX) Country: USA and Canada |
| Methods | Double blind, parallel assignment RCT |
| Participants | Adults, requiring ICU treatment for ≥ 48 hours. Expected = 100 patients |
| Interventions |
Intervention: nocturnal protocol with dexmedetomidine Control: placebo |
| Outcomes |
|
| Starting date | January 2011 |
| Contact information | Dr. Yoanna Skrobik email: yoanna.skrobik@umontreal.ca |
| Notes |
Study number: NCT01791296 Status: recruiting completed, and preliminary analysis is in progress. Contacts: authors responded December 2016 |
NCT02932358.
| Trial name or title | Effectiveness and safety of a flexible family visitation model for delirium prevention in the ICU Country: Brazil |
| Methods | A cluster‐randomized, cross‐over trial, multicentre |
| Participants | Adult ICU patients, visitors and ICU workers, expected, n = 1650 participants |
| Interventions |
Intervention
Control
|
| Outcomes |
Incidence of burnout syndrome symptoms among ICU workers (Maslach Burnout Inventory) and any adverse event related to ICU visitation Assessed by confusion assessment method for the ICU 2 times per day |
| Starting date | January 2017 |
| Contact information | Regis G Rosa MD, PHD , regisgoulartrosa@gmail.com |
| Notes | Outcomes are aimed at visitors and ICU workers as well as ICU patients A wash‐out period is planned: after randomization of ICUs to either an RFVM or to an FFVM as the initial intervention and enrolment of 25 ICU patients and 25 family members; there will be a 30‐day period without patient or family member recruitment to avoid spill‐over. After this period, each ICU will be assigned to an intervention contrary to that initially received until the enrolment of 25 more ICU patients and 25 family members. Study number: NCT02932358 Status: recruiting Contact with author: emailed June 2016. The study has completed the recruitment of about 60% of the target population and analysis of the impact of a flexible family visitation model on delirium prevention is realistic by the end of February 2018. |
NCT03095443.
| Trial name or title | Decreasing delirium through Music (DDM) Country: USA |
| Methods | Parallel Assignment, 3‐armed RCT |
| Participants | Adult mechanically ventilated ICU patients, expected: n = 90 |
| Interventions |
Intervention.
Control
The hypothesis is that music therapy is feasible and effective in reducing delirium incidence, duration, and severity among critically ill patients in the ICU. |
| Outcomes |
|
| Starting date | 1 December 2016 |
| Contact information | Amanda M Harrawood aharrawo@iupui.edu |
| Notes |
Study number: NCT03095443 Status: recruiting Contact with author: study status 18 June 2017: the trial is actively enrolling patients and has included n = 40, recruitment will continue for several more months. |
NCT03125252.
| Trial name or title | Impact of non‐pharmacological prevention measures on the incidence of delirium in adult ICUs (DELIREA) Country: France |
| Methods | Parallel assignment multicentre RCT (14 centres) |
| Participants | Adults. Expected n = 952 |
| Interventions |
Intervention
Control
|
| Outcomes |
Assessment will be by CAM‐ICU |
| Starting date | 27 October, 2016, expected completion April 2019 |
| Contact information | Stein Silva MD silva.s@chu‐toulouse.fr |
| Notes |
Study number: NCT03125252 Status: recruiting Contact with author: email sent 14 June 2017 inquiring of study status ‐ awaiting response |
NCT03215745.
| Trial name or title | Delirium prevention in patients from the ICU (DELA) (DELA) Country: Colombia |
| Methods | RCT |
| Participants | Adult without delirium, expected to be in the ICU longer than 24 hours, estimated n = 200 intervention and usual care = 400 participants |
| Interventions |
Intervention
Control
|
| Outcomes |
|
| Starting date | 1 October 2017 to 1 December 2018 |
| Contact information | Claudia C Torres RN, MSc : 57‐7‐6516500 ext 1221, email: claudiaconsuelo@yahoo.com Astrid N Páez Msc, 57‐6516500 ext 1221 email: nathaliapaez1@hotmail.com |
| Notes |
Study number: NCT03215745 Status: recruiting Contact with author: email sent 1 May 2018 asking for study status ‐ awaiting response |
Nedergaard 2016.
| Trial name or title | Non‐sedation versus sedation with a daily wake‐up trial in critically ill patients receiving mechanical ventilation ‐ effects on long‐term cognitive function: Study protocol for a randomised controlled trial, a sub study of the NONSEDA trial Country: Denmark |
| Methods | Randomized clinical parallel group superiority trial (sub‐study of a multinational study see Toft 2014) |
| Participants | Adult ICU patients intubated and expected to be mechanically ventilated more than 24 hours; expected n = 200 |
| Interventions |
Intervention
Control
|
| Outcomes |
|
| Starting date | January 2014 |
| Contact information | Helene Nedergaard (helene.korvenius.nedergaard@rsyd.dk), Department of Anesthesiology and Intensive Care, Lillebaelt Hospital,Skovvangen 2‐8, DK‐6000 Kolding, Denmark |
| Notes |
Study number: NCT02035436 Status: 25 September 2016, 535 of 700 were included. Contact with Authors: emailed 26 September 2016 ‐ Author responded that the NONSEDA‐trail was still recruiting. |
Nickels 2017.
| Trial name or title | Critical care cycling study (CYLIST) trial Study protocol for a randomized controlled trial of usual care plus additional in‐bed cycling sessions versus usual care in the critically ill Country: Australia |
| Methods | 2‐arm parallel randomized controlled trial |
| Participants | Adults, expected to require more than 48 hours of mechanical ventilation, expected n = 68 |
| Interventions |
Intervention
Control
|
| Outcomes |
|
| Starting date | Unclear |
| Contact information | Corresponding author: marc.nickles@health.qld.gov.au |
| Notes |
Funding: no external sponsors. Study number: ACTRN12616000948493 Status: unknown Contact with authors: email sent on 1 May 2018 and study author responded that they expected to have finalized recruitment end of May 2018. |
Thomas 2015.
| Trial name or title | Extra physiotherapy in critical care (EPICC) trial: a randomised controlled trial Country: UK |
| Methods | Multicentre (3) RCT |
| Participants | Adults from Intensive care and high dependency units receiving 48 hours or more invasive or non invasive mechanical ventilation, Expected n =154 (77/77) |
| Interventions |
Intervention
Control
|
| Outcomes |
|
| Starting date | January 2012 |
| Contact information | Dr Simon V Baudouin;simon.baudouin@newcastle.ac.uk |
| Notes |
Study number: ISRCTN20436833 Status: first patient included January 2012 and final patient was recruited December 2014, The trial is currently in follow‐up period. Contact with authors: ‐email 26 September.2016 ‐ awaiting response. |
Toft 2014.
| Trial name or title | Non‐sedation versus sedation with a daily wake‐up trial in critically ill patients receiving mechanical ventilation (NONSEDA Trial): study protocol for a randomized controlled trial Country: Denmark, Norway and Sweden. |
| Methods | Multicentre (six Scandinavian ICUs) randomized parallel group controlled trial |
| Participants | Mechanically‐ventilated patients with expected duration of mechanical ventilation > 24 hours. Expected n = 700 |
| Interventions |
Intervention
Control
|
| Outcomes |
|
| Starting date | Study was registered in 2014 |
| Contact information | Palle.Toft@rsyd.dk, Department Anaesthesiology and Intensive Care, Odense University, Hospital, Sdr. Boulevard 29, DK ‐ 5000 Odense C, Denmark |
| Notes |
Study number: ClinicalTrials.gov NCT0196768 Status: completion is expected 1 January 2017. At July 2014, 55 patients were included. Contact with authors: email 26 September 2016. Author responded that the NONSEDA trial is still recruiting. |
Wassenaar 2017.
| Trial name or title | The Impact of nursing delirium preventive interventions in the ICU (UNDERPIN‐ICU) Country: the Netherlands |
| Methods | Multicentre, stepped wedge RCT (11 ICUs) |
| Participants | Adult, surgical, medical or trauma patients with at high risk for delirium ( > 35% determined with the E‐PRE‐DELIRIC prediction tool) Expected: 1750 |
| Interventions |
Intervention
Control
The hypothesis is that the UNDERPIN‐ICU program will increase the number of delirium‐coma‐free days in 28 days and improve several secondary outcomes, such as delirium incidence, the number of days of survival in 28 and 90 days and delirium‐related outcomes. |
| Outcomes |
Delirium is measured by CAM‐ICU |
| Starting date | December 2018, expected completion December 2019 |
| Contact information | Mark van den Boogaard mark.vandenboogaard@radboudumc.nl |
| Notes |
Study number. NCT03002701 Status: recruiting Contact with author: email sent 14 June 2017 inquiring of study status with the reply that the study is still ongoing and will be finished in 2020, which is why no data or results can be shared at present. |
Acronyms and abbreviations used in this table
ADL: activities of daily living; CAM‐ICU: confusion assessment method for the ICU; EEG: electrography; E‐PRE‐DELIRIC: early PREdiction of DELIRium in ICu patients; FFVM: Flexible Familiy visitation model; HRQoL: health‐related quality of life; ICU: Intensive care unit; ICDSC: Intensive Care Delirium Screening Checklist;LOS: length of stay; NONSEDA: non‐sedation versus sedation with a daily wake‐up trial in critically ill patients receiving mechanical ventilation; PCS: Physical Component Summery; QoL: quality of life; RCT: randomized controlled trial; RFVM: Restrictive Famliy Visitation model; RIFLE criteria: End‐Stage kidney disease;SF36: short form 36 Health survey; UNDERPIN‐ICU: Impact of Nursing Delirium Preventive Interventions in the Intensive Care Unit
Differences between protocol and review
We made the following changes to the published protocol (Greve 2012).
In the protocol we did not specify that we would exclude studies focusing on sub‐syndromal delirium. However, we have done so in the final review.
In the protocol we stated that we would include studies that assessed delirium using the CAM‐ICU or ICDSC. We have expanded our criteria to also include delirium assessed using the DSM‐IV criteria or the NEECHAM scale.
We originally planned to assess duration of mechanical ventilation; however, studies have primarily measured ventilator‐free days, so we changed this outcome accordingly.
We planned to contact the first author or contact persons of the trials to potentially retrieve missing data in the included trials; however this was not necessary as the majority of studies had few missing data.
The majority of trials had less than 20% dropout. Therefore, we did not conduct sensitivity analyses exploring the effect on effect estimates of trials with high dropout rates (> 20% dropout) as otherwise planned.
While writing the protocol, we did not foresee that treatment effects were reported as medians and interquartile ranges (IQR). For meta‐analysis we therefore calculated means and standard deviations (SDs) as suggested in Wan 2014, with the exception of 'ventilator‐free days' as we suspected a multi‐model distribution in the original data. Effects on 'ventilator‐free days' are therefore presented as medians and IQRs.
We planned to perform intention‐to‐treat (ITT) analysis using the number of patients initially randomized into the experimental or control intervention as the denominator. Further for primary outcomes, we also planned to conduct an 'available‐case analysis' in which only those participants on whom data were reported were to be included in the analysis. In practice, we conducted available‐case analysis on all variables as there were few missing data in the included studies.
Originally, we planned to assess the quality of the evidence for the primary outcomes and number of delirium‐ and coma‐free days; however we subsequently considered it relevant to assess all outcomes.
Due to few missing data we did not conduct 'best‐case' and 'worst‐case' analysis.
We did not use funnel plots as planned to investigate publication bias and small‐study effect. Funnel plots require that at least 10 studies are included in the meta‐analysis (Higgins 2011).
We constructed a 'Summary of findings' table for clinically important comparisons as suggested in the protocol. Whilst writing the review, we found it relevant to highlight haloperidol as it is a widely applied pharmacological intervention and, similarly, physical and cogitative intervention as it is a common non‐pharmacological intervention.
We planned to conduct exploratory subgroup analyses of the effects of pharmacological versus non‐pharmacological interventions, medical versus surgical ICU patients and, if possible, of early intervention (defined as initiation of the intervention within 36 hours after ICU admission) versus late intervention (defined as initiation of the intervention 36 hours or later after ICU admission). None of these analyses were possible due to the diversity of interventions.
In the protocol we planned to search the Chinese Biomedical Literature Database and in advanced Google; this was not done in the review phase, due to comprehensive searchs in the major relevant databases.
We excluded studies including only cardiac surgery patients.
Contributions of authors
Conceiving the review: Ingrid Greve (IG), Eduard Vasilevskis (EV), Ingrid Egerod (IE), Helle Svenningsen (HS), Camilla Bekker Mortensen (CBM), Ann M. Møller (AMM), Thordis Thomsen (TT) and Suzanne Herling (SH).
Co‐ordinating the review: SH
Undertaking manual searches: SH, TT
Screening search results: SH, TT, IE
Organizing retrieval of papers: SH
Screening retrieved papers against inclusion criteria: SH, TT
Appraising quality of papers: SH TT,
Abstracting data from papers: SH TT
Writing to authors of papers for additional information: SH
Providing additional data about papers: SH
Obtaining and screening data on unpublished studies: SH, TT
Data management for the review: SH TT
Entering data into Review Manager (RevMan 5.3): SH
RevMan statistical data: SH, TT, Tobias Wihrenfeldt Klausen (TWK)
Other statistical analysis not using RevMan: SH, TT, TWK
Double entry of data: (data entered by person one: SH; data entered by person two: TT)
Interpretation of data: SH, IG, EV, IE, HS, CBM, AMM, TT
Statistical inferences: SH, IG, EV, IE, HS, CBM, AMM, TT
Writing the review: SH, TT
Securing funding for the review: TT
Performing previous work that was the foundation of the present study: TT, EV
Guarantor for the review (one author): TT
Person responsible for reading and checking review before submission: SH, TT
Sources of support
Internal sources
-
National Institutes of Health (K23AG040157), And the Tennessee Valley Geriatric Research, Education and Clinical Center (GRECC), USA.
Funding for Dr Eduard Vasilevskis
External sources
No sources of support supplied
Declarations of interest
Suzanne Forsyth Herling: none known
Ingrid E Greve: none known
Eduard E Vasilevskis was supported by Award Number K23AG040157 from the National Institute On Aging. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute On Aging or the National Institutes of Health see Sources of support. Dr Vasilevskis has also received support for an educational presentation in a topical area relevant to the review. (ICU Mortality Reduction Collaborative. CareFusion Center for Safety and Excellence. ABCDEs of delirium prevention. October 2011.) The content does not reflect the views of the funding body of this educational presentation.
Ingrid Egerod: none known.
Camilla Bekker Mortensen: none known.
Ann Merete Møller: none known.
Helle Svenningsen's PhD study (2009 to 2012) on delirium was supported by several foundations: the Novo Nordics Foundation; Foundation for Psychiatry's encouraging, Risskov; Foundation for Psychiatry's encouraging, Risskov; Region Midts Health Research Foundation;Fundation of Research in Mental Disorders, Aarhus University; Danish Society for Nursing Research; Lippmann Foundation; Færgemans scholarship; Foundation of Psychiatry promotion. None of the foundations had any influence on this review.
Thordis Thomsen: none known.
Edited (no change to conclusions)
References
References to studies included in this review
Abdelgalel 2016 {published data only}
- Abdelgalel EF. Dexmedetomidine versus haloperidol for prevention of delirium during non‐invasive mechanical ventilation. Egyptian Journal of Anaesthesia 2016;32(4):473‐81. [DOI: 10.1016/j.egja.2016.05.008] [DOI] [Google Scholar]
Brummel 2014b {published data only}
- Brummel NE, Girard TD, Ely EW, Pandhariphande PP, Morandi A, Hughes CG, et al. Feasibility and safety of early combined cognitive and physical therapy for critically ill medical and surgical patients: the Activity and cognitive Therapy in ICU (ACT‐ICU) trial. Intensive Care Medicine 2014;40(3):370‐9. [DOI: 10.1007/s00134-013-3136-0; PUBMED: 24257969 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mehta 2012 {published data only}
- Mehta S, Burry L, Cook D, Fergusson D, Steinberg M, Granton, et al. Daily sedation interruption in mechanically ventilated critically ill patients cared for with a sedation protocol: a randomized controlled trial. JAMA 2012;308(19):1985‐92. [PUBMED: 23180503] [DOI] [PubMed] [Google Scholar]
Moon 2015 {published data only}
- Moon KJ, Lee SM. The effects of a tailored intensive care unit delirium prevention protocol : A randomized controlled trial. International Journal of Nursing Studies 2015;52(9):1423‐32. [PUBMED: 26032729] [DOI] [PubMed] [Google Scholar]
Nassar 2014 {published data only}
- Nassar AP, Park M. Daily sedative interruption versus intermittent sedation in mechanically ventilated critically ill patients : a randomized trial. Annals of Intensive Care 2014;4:14. [PUBMED: 24900938 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Page 2013 {published data only}
- Page VJ, Ely EW, Gates S, Zhao XB, Alce T, Shintani A, et al. Effect of intravenous haloperidol on the duration of delirium and coma in critically ill patients (HOPE‐ICU): a randomised, double‐blind, placebo‐controlled trial. The Lancet Respiratory Medicine 2013;1(7):515‐23. [PUBMED: 24461612 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pandharipande 2007 {published data only}
- Pandharipande PP, Pun BT, Herr DL, Maze M, Girard TD, Miller RR, et al. Effect of sedation with dexmedetomidine vs lorazepam on acute brain dysfunction in mechanically ventilated patients: the MENDS randomized controlled trial. JAMA 2007;298(22):2644‐53. [PUBMED: 18073360] [DOI] [PubMed] [Google Scholar]
Shehabi 2013 {published data only}
- Shehabi Y, Bellomo R, Reade MC, Bailey M, Bass F, Howe B, et al. Early goal‐directed sedation versus standard sedation in mechanically ventilated critically ill patients: A pilot study. Critical Care Medicine 2013;41(8):1983‐91. [PUBMED: 23863230] [DOI] [PubMed] [Google Scholar]
Simons 2016 {published data only}
- Simons KS, Laheij RF, Boogaard M, Moviat MM, Paling AJ, Polderman FN, et al. Dynamic light application therapy to reduce the incidence and duration of delirium in intensive‐care patients : a randomised controlled trial. Lancet Respiratory 2016;4(3):194‐202. [PUBMED: 26895652] [DOI] [PubMed] [Google Scholar]
Strøm 2010 {published data only}
- Strøm T, Martinussen T, Toft P. A protocol of no sedation for critically ill patients receiving mechanical ventilation: a randomised trial. Lancet 2010;375(9713):475‐80. [PUBMED: 20116842] [DOI] [PubMed] [Google Scholar]
Van Den Boogard 2018 {published data only}
- Boogaard M, Slooter AJ, Brüggemann RJM, Schoonhoven L, Beishuizen A, Vermeijden JW, et al. Effect of haloperidol on survival among critically ill adults with a high risk of delirium the REDUCE randomized clinical trial. JAMA 2018;319(7):680‐90. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Van Rompaey 2012 {published data only}
- Rompaey B, Elseviers MM, Drom W, Fromont V, Jorens PG. The effect of earplugs during the night on the onset of delirium and sleep perception: a randomised controlled trial in intensive care patients. Critical Care 2012;16(3):R73. [PUBMED: 22559080 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Álvarez 2017 {published data only}
- Álvarez EA, Garrido MA, Tobar EA, Prieto SA, Vergara SO, Briceno CD, et al. Occupational therapy for delirium management in elderly patients without mechanical ventilation in an intensive care unit : A pilot randomized clinical trial. Journal of Critical Care 2017;37:85‐90. [PUBMED: 27660922 ] [DOI] [PubMed] [Google Scholar]
Esen 2009 {unpublished data only}
- Esen F, Senturk E, Erginzcan P, Kiraner E, Dogruel B, Disci R, et al. Effect of magnesium on sepsis associated delirium. 29th International Symposium on Intensive Care and Emergency Medicine Brussels Belgium. 24.03 2009‐27.03.2009.
Finotto 2006 {published data only}
- Finotto S, Artioli G, Davoli L, Barbara B. Nursing interventions for the prevention of the delirium in intensive care unit (ICU): A randomized study [Interventi infermieristici nella prevenzione del delirium in Unita Coronarica: uno studio randomizzata]. Professioni Infermieristiche 2006;59(4):228‐32. [PUBMED: 17320017] [PubMed] [Google Scholar]
Hatta 2014 {published data only}
- Hatta K, Kishi Y, Wada K, Takeuchi T, Odawara T, Usui C, et al. Preventive effects of ramelteon on delirium a randomized placebo‐controlled trial. JAMA Psychiatry 2016;71(4):397‐403. [PUBMED: 24554232] [DOI] [PubMed] [Google Scholar]
Mansouri 2016 {published data only}
- Mansouri P, Javadpour S, Zand F, Ghodsbin F, Sabetian G, Masjedi M, et al. Implementation of a protocol for integrated management of pain, agitation, and delirium can improve clinical outcomes in the intensive care unit: A randomized clinical trial. Journal of Critical Care 2016;28(6):918‐22. [PUBMED: 24011845] [DOI] [PubMed] [Google Scholar]
Mehta 2015 {published data only}
- Mehta S, Cook D, Devlin JW, Skrobik Y, Meade M, Fergusson D, et al. Prevalence, risk factors, and outcomes of delirium in mechanically ventilated adults. Critical Care Medicine 2015;43(3):557‐66. [PUBMED: 25493968] [DOI] [PubMed] [Google Scholar]
Riker 2009 {published data only}
- Riker RR, Shehabi Y, Bokesch PM, Ceraso D, Wisemandle W, Koura F, et al. Dexmedetomidine vs midazolam for sedation of critically ill patients. JAMA 2009;301(5):489‐99. [PUBMED: 19188334] [DOI] [PubMed] [Google Scholar]
Schweickert 2009 {published data only}
- Schweickert WD, Pohlman MC, Pohlman AS, Nigos C, Pawlik AJ, Esbrook CL, et al. Early physical and occupational therapy in mechanically ventilated, critically ill patients: a randomised controlled trial. Lancet 2009;373(9678):1874‐882. [DOI: 10.1016/S0140-6736(09)60658-9; PUBMED: 19446324] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies awaiting assessment
Daley 2015 {unpublished data only}
- Daley B. Dexmedetomidine versus propofol prolonged sedation in critically ill trauma and surgical patients. http://clinicaltrails.gov/show/NCT02548923. [NCT02548923] [DOI] [PubMed]
NCT02612948 {published and unpublished data}
- Quetiapine for delirium prophylaxis in high‐risk critically ill patients. http://cninicaltrials.gov/ct2/show/NCT02612948 2014. [NCT02612948] [DOI] [PubMed]
Page 2015 {published data only}
- Page VJ, Marti J, McAuley DF, Casarin A, Alce T, Zhao XB, et al. Health evaluation and cost‐effectiveness analysis from a randomized trial of haloperidol in the management of delirium in the critically ill (hope‐ICU trial). American Journal of Respiratory and Critical Care Medicine. 2015:191.
Preslaski 2012 {published data only}
- Preslaski C, Mueller S, Kiser T, Fish D, MacLaren R. Dexmedetomidine vs. midazolam for facilitating extubation in medical and surgical ICU patients: A randomized, double‐blind study. Critical Care Medicine. December; Vol. 1:281.
Ryu 2014 {unpublished data only}
- Ryu H. Efficacy of Low‐dose Dexmedetomidine to prevent delirium in liver transplant patients. http: //clinicaltrials.gov.gov/ct2/show/NCT02245256.
References to ongoing studies
Burry 2017 {published data only}
- Burry L, Scales D, Williamson D, Foster J, Mehta S, Guenette M, et al. Feasibility of melatonin for prevention of delirium in critically ill patients: a protocol for a multicentre, randomised, placebo‐controlled study. BMJ Open 2017;7(e015420):e015420. [PUBMED: 28363933] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jerath 2015 {published data only}
- Jerath A, Ferguson ND, Steel A, Wijeysundera D, Macdonald J, Wasowicz M. The use of volatile anaesthetic agents for long‐term critical care sedation (VALTS): study protocol for a pilot randomized controlled trial. Trials 2015;16(506):1‐7. [PUBMED: 26646404 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Martinez 2017 {published data only}
- Martinez FE, Anstey M, Ford A, Roberts B, Hardie M, Palmer R. Prophylactic Melatonin for Delirium in Intensive Care (Pro‐MEDIC ): study protocol for a randomised controlled trial. Trials 2017;18(1):4. [PUBMED: 28061873] [DOI] [PMC free article] [PubMed] [Google Scholar]
Miles 2012 {published data only}
- Miles M, Hite RD. A randomized controlled trial of direct noise reduction in the ICU. Journal of Investigative Medicine. January 2012; Vol. 60:455.
NCT01739933 {published data only}
- NCT01739933. The MENDSII study, maximizing the efficacy of sedation and reducing neurological dysfunction and mortality in septic patients with acute respiratory failure (MENDSII). https://clinicaltrials.gov/ct2/show/NCT01739933?term=01739933&rank=1 first received 4 December 2012. [DOI] [PMC free article] [PubMed]
NCT01791296 {unpublished data only}
- NCT01791296. Does nightly dexmedetomidine improve sleep and reduce delirium in ICU patients? (SKY‐DEX). clinicaltrials.gov/show/NCT01791296 Date first received February 12, 2013. [NCT01791296]
NCT02932358 {published and unpublished data}
- NCT02932358. Effectiveness and safety of a flexible family visitation model in adult intensive care units: a cluster‐randomized, crossover trial (The ICU Visits Study). https://clinicaltrials.gov/ct2/show/NCT02932358 First received 13 October 2016.
NCT03095443 {published data only}
- NCT03095443. Decreasing delirium through music (DDM). https://clinicaltrials.gov/ct2/show/NCT03095443 first received 29 March 2017.
NCT03125252 {unpublished data only}
- NCT03125252. Impact of non‐pharmacological prevention measures on the incidence of delirium in adult intensive care units. clinicaltrials.gov/show/NCT03125252 first received: 5 March 2017. [NCT03125252]
NCT03215745 {unpublished data only}
- NCT03215745. Delirium prevention in patients from the intensive care Uunit (DELA) (DELA). https://clinicaltrials.gov/ct2/show/NCT03215745 first received 12 July 2017. [NCT03215745 ]
Nedergaard 2016 {published data only}
- Nedergaard HK, Jensen HI, Stylsvig M, Lauridsen JT, Toft P. Non‐sedation versus sedation with a daily wake‐up trial in critically ill patients receiving mechanical ventilation ‐ effects on long‐term cognitive function: Study protocol for a randomized controlled trial, a sub study of the NONSEDA trial. Trials 2016;16(310):1‐8. [PUBMED: 26201718 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Nickels 2017 {published data only}
- Nickels MR, Aitken LM, Walsham J, Barnett AG, Mcphail SM. Critical care cycling study (CYCLIST) trial protocol: a randomised controlled trial of usual care plus additional in‐bed cycling sessions versus usual care in the critically ill. BMJ Open 2017;7, e017393:1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Thomas 2015 {published data only}
- Thomas K, Wright SE, Watson G, Baker C, Stafford V, Wade C, et al. Extra Physiotherapy in Critical Care (EPICC) Trial Protocol: a randomised controlled trial of intensive versus standard physical rehabilitation therapy in the critically ill. BMJ Open 2015;5(1):1‐12. [PUBMED: 26009576] [DOI] [PMC free article] [PubMed] [Google Scholar]
Toft 2014 {published data only}
- Toft P, Olsen HT, Jørgensen HK, Strøm T, Nibro HL, Oxlund J, et al. Non‐sedation versus sedation with a daily wake‐up trial in critically ill patients receiving mechanical ventilation (NONSEDA Trial): study protocol for a randomised controlled trial. Trials 2014;15(499):1‐11. [PUBMED: 25528350 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wassenaar 2017 {published data only (unpublished sought but not used)}
- Wassenaar A, Rood P, Schoonhoven L, Teerenstra S, Zegers M, Pickkers P, et al. International Journal of Nursing Studies The impact of nUrsiNg DEliRium Preventive INnterventions in the Intensive Care Unit (UNDERPIN‐ICU): A study protocol for a multi‐centre, stepped wedge randomized controlled trial. International Journal of Nursing Studies 2017;68(Epub 2016 Dec 8):1‐8. [PUBMED: 28013104 ] [DOI] [PubMed] [Google Scholar]
Additional references
Al‐Qadheeb 2014
- Al‐Qadheeb NS, Balk EM, Fraser GL, Skrobik Y, Riker RR, Kress JP, et al. Randomized ICU trials do not demonstrate an association between interventions that reduce delirium duration and short‐term mortality: a systematic review and meta‐analysis. Critical Care Medicine 2014;42(6):1442‐54. [PUBMED: 24557420] [DOI] [PMC free article] [PubMed] [Google Scholar]
American Psychiatric Association 2000
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental disorders DSM‐IV‐TR. 4th Edition. Washington DC: American Psychiatric Association, 2000. [Google Scholar]
American Psychiatric Association 2013
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th Edition. American Psychiatric Association, 2013. [Google Scholar]
Bannon 2016
- Bannon L, Mcgaughey J, Clarke M, Mcauley DF, Blackwood B. Impact of non‐pharmacological interventions on prevention and treatment of delirium in critically ill patients : protocol for a systematic review of quantitative and qualitative research. Systematic Reviews 2016;5(75):1‐9. [PUBMED: 27146132] [DOI] [PMC free article] [PubMed] [Google Scholar]
Barbateskovic 2016
- Barbateskovic M, Larsen LK, Oxenbøll‐Collet M, Jakobsen JC, Perner A, Wetterslev J. Pharmacological interventions for delirium in intensive care patients: a protocol for an overview of reviews. Systematic Reviews 2016;5(1):211. [PUBMED: 27923397] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bergeron 2001
- Bergeron N, Dubois MJ, Dumont M, Dial S, Skrobik Y. Intensive Care Delirium Screening Checklist: evaluation of a new screening tool. Intensive Care Medicine 2001;27(5):859‐64. [DOI: 10.1007/s001340100909; PUBMED: 11430542] [DOI] [PubMed] [Google Scholar]
Black 2013
- Black N. Patient reported outcome measures could help transform healthcare. BMJ 2013;346:167. [PUBMED: 23358487 ] [DOI] [PubMed] [Google Scholar]
Brummel 2012
- Brummel NE, Jackson JC, Girard TD, Pandharipande PP, Schiro E, Work B, et al. A combined early cognitive and physical rehabilitation program for people who are critically ill: the activity and cognitive therapy in the intensive care unit (ACT‐ICU) trial.. Physical Therapy 2012;92(12):1580‐92. [PUBMED: 22577067 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Brummel 2014a
- Brummel NE, Jackson JC, Pandharipande PP, Thompson JL, Shintani AK, Dittus RS, et al. Delirium in the ICU and subsequent long‐term disability among survivors of mechanical ventilation. Critical Care Medicine 2014;42(2):369‐377. [DOI: 10.1097/CCM.0b013e3182a645bd.; PUBMED: 24158172 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Burry 2015
- Burry L, Cook D, Herridge M, Devlin JW, Fergusson D, Meade M, et al. Recall of ICU stay in patients managed with a sedation protocol or a sedation protocol with daily interruption. Critical Care Medicine 2015;43(10):2180‐90. [PUBMED: 26181221] [DOI] [PubMed] [Google Scholar]
Burry 2016
- Burry LD, Hutton B, Guenette M, Williamson D, Mehta S, Egerod I, et al. Comparison of pharmacological and non‐pharmacological interventions to prevent delirium in critically ill patients : a protocol for a systematic review incorporating network meta‐analyses. Systematic Reviews 2016;5(153):1‐8. [PUBMED: 27609018 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Burry 2018
- Burry L, Mehta S, Perreault MM, Luxenberg JS, Siddiqi N, Hutton B, et al. Antipsychotics for treatment of delirium in hospitalised non‐ICU patients. Cochrane Database of Systematic Reviews 2018, Issue 6. [DOI: 10.1002/14651858.CD005594.pub3; PUBMED: 29920656 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Collinsworth 2016
- Collinsworth AW, Priest EL, Campbell CR, Vasilevskis EE, Masica AL. A review of multifaceted care approaches for the prevention and mitigation of delirium in intensive care units. Journal of Intensive Care Medicine 2016;31(2):127‐41. [PUBMED: 25348864 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Davydow 2008
- Davydow DS, Gifford JM, Desai SV, Needham DM, Bienvenu OJ. Posttraumatic stress disorder in general intensive care unit survivors: a systematic review. General Hospital Psychiatry 2008;30(5):421‐34. [DOI: 10.1016/j.genhosppsych.2008.05.006; PUBMED: 18774425 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Devlin 2008
- Devlin JW, Fong JJ, Howard EP, Skrobik Y, McCoy N, Yasuda C, et al. Assessment of delirium in the intensive care unit: nursing practices and perceptions. American Journal of Critical Care 2008;17(6):555‐65. [PUBMED: 18978240 ] [PubMed] [Google Scholar]
Doiron 2018
- Doiron KA, Hoffmann T, Beller EM. Early intervention (mobilization or active exercise) for critically ill adults in the intensive care unit. Cochrane Database of Systematic Reviews 2018, Issue 3. [DOI: 10.1002/14651858.CD010754.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Elliott 2014
- Elliott SR. ICU delirium: a survey into nursing and medical staff knowledge of current practices and perceived barriers towards ICU delirium in the intensive care unit. Intensive and Critical Care Nursing 2014;30(6):333‐8. [DOI: 10.1016/j.ic.cn.2014.06.004; PUBMED: 25201699] [DOI] [PubMed] [Google Scholar]
Ely 2001a
- Ely EW, Gautam S, Margolin R, Francis J, May L, Speroff T, et al. The impact of delirium in the intensive care unit on hospital length of stay. Intensive Care Medicine 2001;27(12):1892‐900. [PUBMED: 11797025] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ely 2001b
- Ely EW, Margolin R, Francis J, May L, Truman B, Dittus R, et al. Evaluation of delirium in critically ill patients: Validation of the Confusion Assessment Method for the Intensive Care Unit (CAM‐ICU). Critical Care Medicine 2001;29(7):1370‐9. [PUBMED: 11445689] [DOI] [PubMed] [Google Scholar]
Ely 2001c
- Ely EW, Inouye SK, Bernard GR, Gordon S, Francis J, May L, et al. Delirium in mechanically ventilated patients: validity and reliability of the Confusion Assessment Method for the Intensive Care Unit (CAM‐ICU). JAMA 2001;286(21):2703‐10. [DOI: 10.1001/jama.286.21.2703; PUBMED: 11730446] [DOI] [PubMed] [Google Scholar]
Ely 2004
- Ely EW, Shintani A, Truman B, Speroff T, Gordon SM, Harell FE, et al. Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA 2004;291(14):1753‐62. [PUBMED: 15082703] [DOI] [PubMed] [Google Scholar]
Foster 2016
- Foster J, Burry LD, Thabane L, Choong K, Menon K. Melatonin and melatonin agonists to prevent and treat delirium in critical illness : a systematic review protocol. Systematic Reviews 2016;5(199):1‐7. [PUBMED: 27881185 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fraser 2013
- Fraser GL, Devlin JW, Worby CP, Alhazzani W, Barr J, Dasta JF, et al. Benzodiazepine versus nonbenzodiazepine‐based sedation for mechanically ventilated, criticallyill adults: a systematic review and meta‐analysis of randomized trials. Critical Care medicine 2013;41(9):30‐8. [PUBMED: 23989093] [DOI] [PubMed] [Google Scholar]
Girard 2010
- Girard TD, Jackson JC, Pandharipande PP, Pun BT, Thompson JL, Shintani AK, et al. Delirium as a predictor of long‐term cognitive impairment in survivors of critical illness. Critical Care Medicine 2010;38(7):1513‐20. [PUBMED: 20473145] [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADEpro [Computer program]
- GRADE Working Group, McMaster University. GRADEpro GDT. Version accessed October 2017. Hamilton (ON): GRADE Working Group, McMaster University, 2014.
Griffiths 2007
- Griffiths RD, Jones C. Delirium, cognitive dysfunction and posttraumatic stress disorder. Current Opinion in Anaesthesiology 2007;20(2):124‐9. [PUBMED: 17413395] [DOI] [PubMed] [Google Scholar]
Gusmao‐Flores 2012
- Gusmao‐Flores D, Ibrain J, Salluh F, Chalhub RÁ, Quarantini LC. The confusion assessment method for the intensive care unit (CAM‐ICU ) and intensive care delirium screening checklist (ICDSC) for the diagnosis of delirium : a systematic review and meta‐analysis of clinical studies. Critical Care 2012;16(4):R115. [PUBMED: 22759376] [DOI] [PMC free article] [PubMed] [Google Scholar]
Guyatt 2008
- Guyatt GH, Oxman AD, Kunz R, Vist GE, Falck‐Ytter Y, Schunemann HJ. What is "quality of evidence" and why is it important to clinicians. BMJ 2008;336(7651):995‐8. [PUBMED: 18456631 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Haidich 2010
- Haidich AB. Meta‐analysis in medical research. Hipokratia 2010;14(Suppl 1):29‐37. [PUBMED: 21487488 ] [PMC free article] [PubMed] [Google Scholar]
Hayhurst 2016
- Hayhurst CJ, Pandharipande PP, Hughes CG. Intensive care unit delirium: a review of diagnosis, prevention, and treatment. Anesthesiology 2016;125(6):1229‐41. [DOI: 10.1097/ALN.0000000000001378; PUBMED: 27748656 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Hu 2015
- Hu R‐F, Jiang X‐Y, Chen J, Zeng Z, Chen XY, Li Y, et al. Non‐pharmacological interventions for sleep promotion in the intensive care unit. Cochrane Database of Systematic Reviews 2015, Issue 10. [DOI: 10.1002/14651858.CD008808.pub2; PUBMED: 26439374] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jackson 2009
- Jackson JC, Mitchell N, Hopkins RO. Cognitive functioning, mental health, and quality of life in ICU survivors: an overview. Critical Care Clinics 2009;25(3):615‐28. [PUBMED: 19576534 ] [DOI] [PubMed] [Google Scholar]
Jackson 2014
- Jackson JC, Pandharipande PP, Girard TD, Brummel NE, Thompson JL, Hughes CG, et al. Depression, post‐traumatic stress disorder, and functional disability in survivors of critical illness in the BRAIN‐ICU study: a longitudinal cohort study. Lancet Respiratory Medicine 2014;2(5):369‐79. [DOI: 10.1016/S2213-2600(14)70051-7; PUBMED: 24815803] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lan 1983
- Lan KG, DeMets DL. Discrete sequential boundaries for clinical trials. Biometrika 1983;70(3):659‐63. [Google Scholar]
Lonergan 2009
- Lonergan E, Luxenberg J, Sastre AA. Benzodiazepines for delirium. Cochrane Database of Systematic Reviews 2009, Issue 4. [DOI: 10.1002/14651858.CD006379.pub3; PUBMED: 19160280 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Luetz 2010
- Luetz A, Heymann A, Radtke FM, Chenitir C, Neuhaus U, Nachtigall I, et al. Different assessment tools for intensive care unit delirium: Which score to use?. Critical Care Medicine 2010;38(2):409‐18. [PUBMED: 20029345] [DOI] [PubMed] [Google Scholar]
Mac Sweeney 2010
- Mac Sweeney R, Barber V, Page V, Ely EW, Perkins GD, Young JD, et al. A national survey of the management of delirium in UK intensive care units. QJM: monthly journal of the Association of Physicians 2010;103(4):243‐51. [PUBMED: 20139102 ] [DOI] [PubMed] [Google Scholar]
Maldonado 2008
- Maldonado JR. Delirium in the acute care setting: characteristics, diagnosis and treatment. Critical Care Clinics 2008;24(4):657‐722. [PUBMED: 18929939] [DOI] [PubMed] [Google Scholar]
Milbrandt 2004
- Milbrandt EB, Deppen S, Harrison PL, Shintani AK, Speroff T, Stiles RA, et al. Costs associated with delirium in mechanically ventilated patients. Critical Care Medicine 2004;32(4):955‐62. [DOI: 10.1097/01.CCM.0000119429.16055.92; PUBMED: 15071384 ] [DOI] [PubMed] [Google Scholar]
Miller 2006
- Miller RR, Ely EW. Delirium and cognitive dysfunction in the intensive care unit. Seminnars in respiratory and critical care medicine 2006;27(3):210‐20. [PUBMED: 16791755] [DOI] [PubMed] [Google Scholar]
Morandi 2008
- Morandi A, Phandharipande P, Trabucchi M, Rozzini R, Mistraletti G, Trompeo AC, et al. Understanding international differences in terminology for delirium and other types of acute brain dysfunction in critically ill patients. Intensive Care Medicine 2008;34(10):1907‐15. [PUBMED: 18563387] [DOI] [PubMed] [Google Scholar]
Morandi 2009
- Morandi A, Jackson JC, Ely EW. Delirium in the intensive care unit. International Review of Psychiatry 2009;21(1):43‐58. [PUBMED: 19219712] [DOI] [PubMed] [Google Scholar]
Morandi 2011
- Morandi A, Brummel NE, Ely EW. Sedation, delirium and mechanical ventilation: the 'ABCDE' approach. Current Opinion in Critical Care 2011;17(1):43‐9. [DOI: 10.1097/MCC.0b013e3283427243; PUBMED: 21169829] [DOI] [PubMed] [Google Scholar]
Nelson 2015
- Nelson S, Muzyk AJ, Bucklin MH, Brudney S, Gagliardi JP. Defining the role of dexmedetomidine in the prevention of delirium in the intensive care unit. BioMed Research International 2015;2015:635737. [PUBMED: 26576429] [DOI] [PMC free article] [PubMed] [Google Scholar]
Neto 2012
- Neto AS, Nassar AP Jr, Cardoso SO, Manetta JA, Pereira VM, Espósito DC, et al. Delirium screening in critically ill patients: A systematic review and meta‐analysis. Critical Care Medicine 2012;40(6):1946‐51. [PUBMED: 22610196] [DOI] [PubMed] [Google Scholar]
Norman 2016
- Norman BC, Jackson JC, Graves JA, Girard TD, Pandharipande PP, Brummel NE, et al. Employment outcomes after critical illness: an analysis of the bringing to light the risk factors and incidence of neuropsychological dysfunction in ICU survivors cohort. Critical Care Medicine 2016;44(11):2003‐9. [PUBMED: 27171492 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Oimet 2007
- Oimet S, Kavanagh BP, Gottfried SB, Skrobik Y. Incidence, risk factors and consequences of ICU delirium. Intensive Care Medicine 2007;33(1):66‐73. [DOI: 10.1007/s00134-006-0399-8; PUBMED: 17102966] [DOI] [PubMed] [Google Scholar]
Oxenbøll‐Collet 2018
- Oxenbøll‐Collet M, Egerod I, Christensen V, Jensen J, Thomsen T. Nurses´ and physicians´ perceptions of Confusion Assessment Method for the intensive care unit for delirium detection: focus group study. Nursing in Critical Care 2018;23(1):16‐22. [PUBMED: 27596941] [DOI] [PubMed] [Google Scholar]
Pandharipande 2005
- Pandharipande P, Jackson J, Ely WE. Delirium: acute cognitive dysfunction in the critically ill. Current Opinion in Critical Care 2005;11(4):360‐8. [PUBMED: 16015117] [DOI] [PubMed] [Google Scholar]
Pandharipande 2008
- Pandharipande P, Cotton BA, Shintani A, Thompson J, Pun BT, Morris JA, et al. Prevalence and risk factors for delirium in surgical and trauma intensive care unit patients. Journal of Trauma, Infection, and Critical Care 2008;65(1):34‐41. [PUBMED: 18580517] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pandharipande 2010
- Pandharipande P, Banerjee A, McGrane S, Ely EW. Liberation and animation for ventilated ICU patients: the ABCDE bundle for the back‐end of critical care. Critical Care 2010;14(3):157. [PUBMED: 20497606 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pandharipande 2013
- Pandharipande PP, Girard TD, Jackson JC, Morandi A, Thompson JL, Pun BT, et al. Long‐Term cognitive impairment after critical illness. New England Journal of Medicine 2013 Oct 3;369(14):1306‐16. [DOI: 10.1056/NEJMoa1301372; PUBMED: 24088092] [DOI] [PMC free article] [PubMed] [Google Scholar]
Patel 2009
- Patel RP, Gambrell M, Speroff T, Scott TA, Pun BT, Okahashi J, et al. Delirium and sedation in the intensive care unit: Survey of behaviours and attitudes of 1384 healthcare professionals. Critical Care Medicine 2009;37(3):825‐32. [PUBMED: 19237884] [DOI] [PMC free article] [PubMed] [Google Scholar]
Peterson 2006
- Peterson JF, Pun BT, Dittus RS, Thomason JW, Jackson JC, Shintani AK, et al. Delirium and its motoric types: a study of 614 critically ill patients. Journal of the American Geriatric Society 2006;54(3):479‐84. [PUBMED: 16551316] [DOI] [PubMed] [Google Scholar]
Pfoh 2015
- Pfoh ER, Chan KS, Dinglas VD, Girard TD, Jackson JC, Morris PE, et al. Cognitive screening among acute respiratory failure survivors: a cross‐sectional evaluation of the Mini‐Mental State Examination. Critical Care 2015;19:220. [PUBMED: 25939482 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pisani 2009
- Pisani MA, Kong SY, Kasl SV, Murphy TE, Araujo KLB, Ness PH. Days of delirium are associated with 1‐year mortality in an older intensive care unit population. American Journal of Respiratory and Critical Care Medicine 2009;180(11):1092‐7. [DOI: 10.1164/rccm.200904-05370C; PUBMED: 19745202 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
RevMan 5.3 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Richter 2006
- Richter JC, Waydhas C, Pajonk FG. Incidence of posttraumatic stress disorder after prolonged surgical intensive care. Psycosomatics 2006;47(3):223‐30. [PUBMED: 16684939 ] [DOI] [PubMed] [Google Scholar]
Rose 2017
- Rose L, Agar M, Burry LD, Campbell N, Clarke M, Lee J, et al. Development of core outcome sets for effectiveness trials of interventions to prevent and / or treat delirium ( Del‐ COrS ): study protocol. BMJ Open 2017;7:1‐9. [PUBMED: 28928181] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rothenhaüsler 2001
- Rothenhaüsler HB, Ehrentraut S, Stoll C, Kampfammer HP. The relationship between cognitive performance and employment and health status in long‐term survivors of the acute respiratory distress syndrome : results of an exploratory study. General Hospital Psychiatry 2001;23(2):90‐6. [PUBMED: 11313077] [DOI] [PubMed] [Google Scholar]
Salluh 2009
- Salluh JIF, Dal‐Pizzol F, Mello PV, Friedman G, Silva E, Teles JM, et al. Delirium recognition and sedation practices in critically ill patients: A survey on the attitudes of 1015 Brazilian critical care physicians. Journal of Critical Care 2009;24(4):556‐62. [PUBMED: 19577412] [DOI] [PubMed] [Google Scholar]
Salluh 2015
- Salluh JF, Wang H, Schneider EB, Nagaraja N, Yenokyan G, Damluji A, et al. Outcome of delirium in critically ill patients : systematic review and meta‐analysis. BMJ 2015;350:1‐10. [PUBMED: 26041151] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sanz 2009
- Sanz JC, Vargas ML, Marin JJ. Battery for assessment of neuropsychological status (RBANS) in schizophrenia: A pilot study in the Spanish population. Acta Neuropsychiatrica 2009;21(1):18‐25. [PUBMED: 25384525 ] [DOI] [PubMed] [Google Scholar]
Selim 2017
- Selim AA, Wesley EE. Delirium the under‐recognised syndrome: survey of healthcare professionals' awareness and practice in the intensive care units. Journal of Clinical Nursing 2017;26(5‐6):813‐24. [DOI: 10.1111/jocn.13517; PUBMED: 27539789 ] [DOI] [PubMed] [Google Scholar]
Serafim 2015
- Serafim RB, Bozza FA, Soares M, do Brasil PE, Tura BR, Ely EW. Pharmacologic prevention and treatment of delirium in intensive care patients: A systematic review. Journal of Critical Care 2015;30(4):799‐807. [PUBMED: 25957498] [DOI] [PubMed] [Google Scholar]
Shehabi 2010
- Shehabi Y, Riker RR, Bokesch BM, Wisemandle W, Shintani A, Ely EW. Delirium duration and mortality in lightly sedated, mechanically ventilated patients. Critical Care Medicine 2010;38(12):2311‐8. [PUBMED: 20838332] [DOI] [PubMed] [Google Scholar]
Shu‐Min Lin 2004
- Lin SM, Liu CY, Wang CH, Lin HC, Huang CD, Huang PY, et al. The impact of delirium on the survival of mechanically ventilated patients. Critical Care Medicine 2004;32(11):2254‐9. [PUBMED: 15640638 ] [DOI] [PubMed] [Google Scholar]
Siddiqi 2016
- Siddiqi N, Harrison JK, Clegg A, Teale EA, Young J, Taylor J, et al. Interventions for preventing delirium in hospitalised non‐ICU patients. Cochrane Database of Systematic Reviews 2016, Issue 3. [DOI: 10.1002/14651858.CD005563.pub3; PUBMED: 10.1002/14651858; PUBMED: 26967259 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Strøm 2011
- Strøm T, Stylsvig M, Toft P. Long‐term psychological effects of a no‐sedation protocol in critically ill patients. Critical Care 2011;15(6):R293. [PUBMED: 22166673 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Svenningsen 2009
- Svenningsen H, Tønnesen E. Incidence of delirium in three Danish intensive care units. Ugeskrift for Læger 2009;171(49):3600‐4. [PUBMED: 19954700] [PubMed] [Google Scholar]
Thomason 2005
- Thomason JW, Shintani A, Peterson JF, Pun BT, Jackson JC, Ely EW. Intensive care unit delirium is an independent predictor of longer hospital stay: a prospective analysis of 261 non‐ventilated patients. Critical Care 2005;9(4):375‐81. [PUBMED: 16137350 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Trogrlić 2015
- Trogrlić Z, Jagt M, Bakker J, Balas MC, Ely EW, Voort PH, et al. A systematic review of implementation strategies for assessment, prevention, and management of ICU delirium and their effect on clinical outcomes. Critical Care 2015;19(1):157. [PUBMED: 25888230] [DOI] [PMC free article] [PubMed] [Google Scholar]
van den Boogaard 2012a
- Boogaard M, Pickkers P, Slooter AJ, Kuiper MA, Spronk PE, Voort PH, et al. Development and validation of PRE‐DELIRIC ( PREdiction of DELIRium in ICUu patients ) delirium prediction model for intensive care patients : observational Multicentre study. BMJ 2012;344:e420. [PUBMED: 22323509 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
van den Boogaard 2012b
- Boogaard M, Schoonhoven L, Evers, AW, Hoeven JG, Achterberg T, Pickkers P. Delirium in critically ill patients: Impact on long‐term health‐related quality of life and cognitive functioning. Critical Care Medicine 2012;40(1):112‐8. [DOI: 10.1097/CCM.0B013e31822e99fc9; PUBMED: 21926597] [DOI] [PubMed] [Google Scholar]
van den Boogaard 2012c
- Boogaard M, Schoonhoven L, Hoeven JG, Achterberg T, Pickkers P. Incidence and short‐term consequences of delirium in critically ill patients : A prospective observational cohort study. International Journal of Nursing Studies 2012;49(7):775‐83. [PUBMED: 22197051] [DOI] [PubMed] [Google Scholar]
Vasilevskis 2010
- Vasilevskis EE, Ely EW, Speroff T, Pun BT, Boehm L, Dittus RS. Reducing iatrogenic risks: ICU‐acquired delirium and weakness ‐ crossing the quality chasm. Chest 2010;138(5):1224‐33. [PUBMED: 21051398 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Verceles 2012
- Verceles AC, Silhan L, Terrin M, Netzer G, Shanholtz C, Scharf SM. Circadian rhythm disruption in severe sepsis : the effect of ambient light on urinary 6‐sulfatoxymelatonin secretion. Intensive Care Medicine 2012;38(5):804‐10. [PUBMED: 22286671 ] [DOI] [PubMed] [Google Scholar]
Wan 2014
- Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and / or interquartile range. BMC Medical Research Methodology 2014;14:135. [PUBMED: 25524443 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wolters 2014
- Wolters AE, Dijk D, Pasma W, Cremer OL, Looije MF, Lange DW, et al. Long‐term outcome of delirium during intensive care unit stay in survivors of critical illness: a prospective cohort study. Critical Care 2014;18(3):R125. [DOI: 10.1186/cc13929; PUBMED: 24942154 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Woon 2012
- Woon FL, Dunn CB, Hopkins RO. Predicting cognitive sequelae in survivors of critical illness with cognitive screening tests. American Journal of Respiratory and Critical Care 2012;186(4):333‐40. [PUBMED: 22700858] [DOI] [PubMed] [Google Scholar]
Zaal 2015
Zamoscik 2017
- Zamoscik K, Godbold R, Freeman P. Intensive care nurses' experiences and perceptions of delirium and delirium care. Intensive and Critical Care Nursing 2017;40:94‐100. [DOI: 10.1016/j.ic.cn.2017.01.003; PUBMED: 28259522 ] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Greve 2012
- Greve I, Vasilevskis EE, Egerod I, Bekker Mortensen C, Møller AM, Svenningsen H, et al. Interventions for preventing intensive care unit delirium. Cochrane Database of Systematic Reviews 2012, Issue 4. [DOI: 10.1002/14651858.CD009783] [DOI] [PMC free article] [PubMed] [Google Scholar]