Abstract
An end‐stage renal failure patient who was planned for a left brachioaxillary arteriovenous graft required an implantable cardioverter‐defibrillator for secondary prevention of ventricular tachycardia and a pacemaker for complete heart block but was found to have a right subclavian venous occlusion. Due to the lack of vascular access, we performed a successful subcutaneous implantable cardioverter‐defibrillator (S‐ICD) and leadless pacemaker implantation. There was no interaction between the devices at the time of implantation, during defibrillation testing and following an appropriate defibrillation therapy.
Keywords: emblem, leadless pacemaker, micra, subcutaneous ICD, vascular occlusion
1. INTRODUCTION
Patients with limited upper extremity venous access who have indications for device‐based therapies are often faced with the technical challenge of obtaining venous access. There have been reports of the use of venoplasty to facilitate transvenous lead implantation, transiliac, and epicardial approaches for patients with vascular access issues.1, 2 A leadless pacemaker (LP) and subcutaneous implantable cardioverter‐defibrillator (S‐ICD) circumvents vascular issues by obviating the need for upper extremity venous access but runs the limitation of losing atrioventricular (AV) synchrony and not having antitachycardia pacing (ATP). There have been previous case reports of a combined LP and S‐ICD system demonstrating safety and efficacy and we present our experience on such a case.3, 4, 5
2. CASE REPORT
A 64‐year‐old male with a background of end‐stage renal failure, hypertension, and paroxysmal atrial fibrillation presented to us with a non‐ST elevation myocardial infarction complicated by cardiogenic shock and ventricular arrhythmias (VA). Coronary angiogram revealed triple vessel disease while an echocardiogram showed left ventricular ejection fraction of 18%. As he was deemed to be a high surgical risk candidate, he underwent complete revascularization percutaneously. He developed intermittent complete heart block and had three more episodes of pulseless VA requiring external cardioversion. An initial plan was made to implant a right‐sided dual chamber ICD as the patient was planned for a left arteriovenous graft creation for hemodialysis. However, a right upper extremity venogram demonstrated right subclavian venous occlusion. After extensive discussion with the patient and family, a decision was made to proceed with a combined LP (Micra VR TCP; Medtronic Inc, Minneapolis, MN, USA) and S‐ICD (Emblem; Boston Scientific Corp, St Paul, MN, USA). A left‐sided implant was not used due to the potential risk of cardiovascular implantable electronic device (CIED) infection. Also, given the risks of CIED infection and potential of requiring device explant in the future,6, 7 the patient opted for a combined LP and S‐ICD instead. The patient passed the S‐ICD screening in the primary and secondary vectors but not the alternate vector. As he was deemed to be high surgical risk, an epicardial device was not considered.
The S‐ICD was implanted using a two‐incision technique. Device testing revealed adequate sensing of electrograms in the primary and secondary but not alternate vectors.
The LP was then implanted in the right ventricular midseptum via a left femoral venous delivery sheath. Interrogation of the device showed satisfactory parameters. We performed induction of ventricular fibrillation (VF) using the S‐ICD with 50 Hz burst and the device successfully detected VF and defibrillated back to paced rhythm with a single 65 J shock with an appropriate charge time of under 10 seconds and impedance of 48 ohms (Figure 1A). The LP was reinterrogated and found to have stable parameters. The LP was paced at maximum output and determined to have no oversensing issues on the S‐ICD. Post‐procedural chest radiographs demonstrate satisfactory LP and S‐ICD positions (Figure 2). The patient was discharged with oral bisoprolol. The S‐ICD was programmed with shock zone of 220 bpm and conditional shock zone of 170 bpm with post shock pacing and SMART Pass on. The LP was programmed to VVI pacing at 2.50 V amplitude at 0.24 milliseconds pulse width, as the capture threshold was 1 V at 0.24 milliseconds pulse width. The patient presented 1 month later with ventricular tachycardia which was appropriately and successfully cardioverted back to his baseline rhythm through the S‐ICD (Figure 1B). Repeat interrogation of the LP showed stable parameters. The patient was followed‐up until his demise 2 months later due to progression of heart failure.
Figure 1.
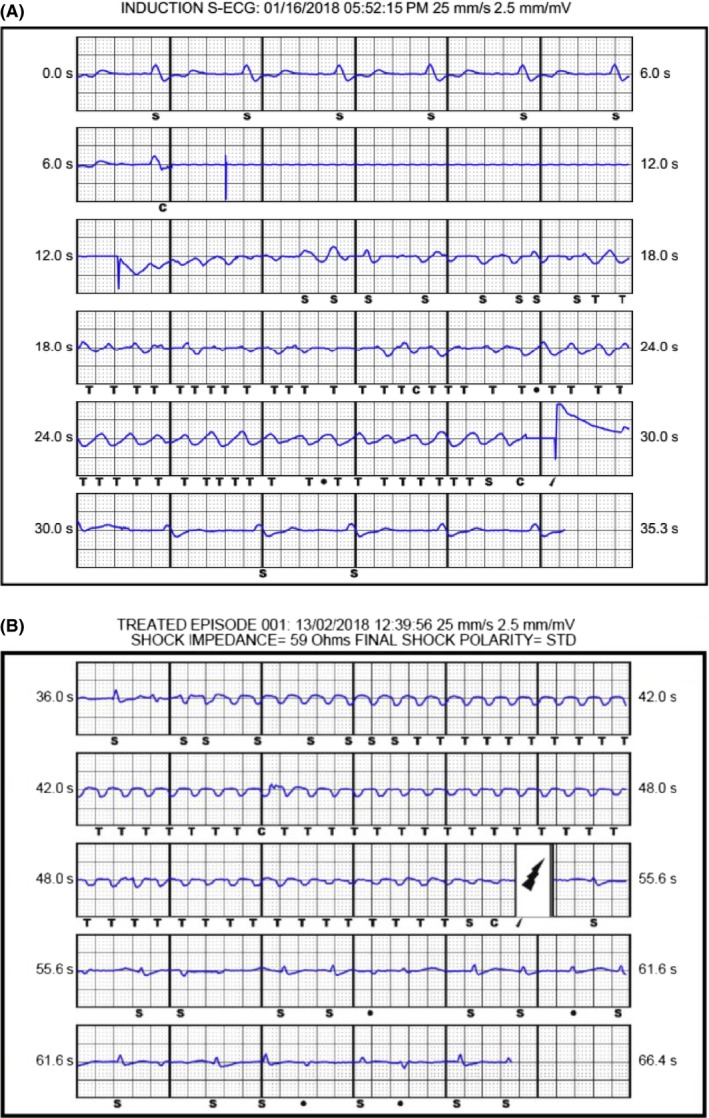
A, Induction of VF and successful defibrillation following implant. B, Ventricular tachycardia which was successfully cardioverted 1 month following implant
Figure 2.
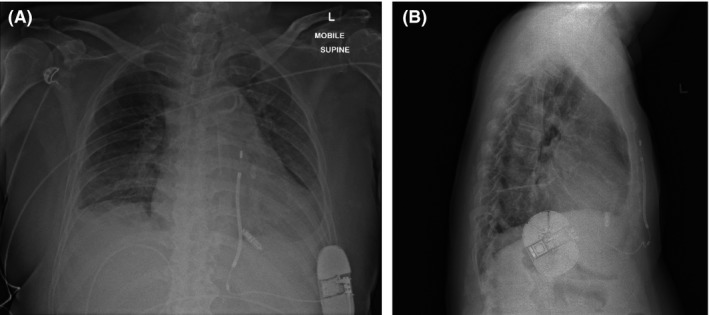
A, chest radiograph of the anteroposterior view and B, chest radiograph of the lateral view following implant
3. DISCUSSION
We present a patient with limited venous access who meets both pacing and defibrillation indications and offer our experience on the simultaneous use of S‐ICD and LP. There were no interactions detected between the LP and the S‐ICD. In addition, the LP continued to function normally after two high output shocks from the S‐ICD proving the integrity of both systems. A complex venoplasty to relieve the right subclavian occlusion was deemed to be high risk. We avoided an epicardial system which would require a thoracotomy for a patient at a high cardiovascular risk for general anesthesia. As our patient had an overall low pacing burden, we accepted the risk of losing AV synchrony. A limitation of the S‐ICD is that it is unable to detect and treat tachyarrhythmias below 170 beats per minute, and is unable to perform antitachycardia pacing. The preliminary result of a modular pacing system (Empower; Boston Scientific Corp) leveraging on wireless communication between a LP and S‐ICD allowing ATP for VA is promising and we eagerly await future trials on this system.
Even though the patient passed S‐ICD screening, it is possible that QRS (refers to the QRS complex of the cardiac electrogram) double counting or T wave oversensing could have occurred during paced rhythm. One way to overcome this would be to perform pacing with a diagnostic catheter prior to S‐ICD implant, or to implant the LP prior to implanting the S‐ICD. However, due to the requirement for heparin administration during LP implant, the decision was made to implant the S‐ICD first, and ensure adequate hemostasis prior to LP implant. Also, diagnostic pacing was not performed to minimize procedure time and risk of infection.
To our knowledge, this is the first case of a simultaneous LP and S‐ICD implantation in the same setting demonstrating preserved integrity in both systems post defibrillation testing and shock for spontaneous VA. This is a viable alternative for patients with vascular access issues requiring pacing for bradycardia as well as defibrillation for VA. Simultaneous implant was preferred over separate implant procedures to avoid the patient having to return for a separate procedure, as well as to minimize infection risk.
CONFLICT OF INTERESTS
The authors declare no conflict of interests for this article.
Ng JB, Chua K, Teo WS. Simultaneous leadless pacemaker and subcutaneous implantable cardioverter‐defibrillator implantation—When vascular options have run out. J Arrhythmia. 2019;35:136–138. 10.1002/joa3.12140
REFERENCES
- 1. Worley SJ, Gohn DC, Pulliam RW, Raifsnider MA, Ebersole BI, Tuzi J. Subclavian venoplasty by the implanting physicians in 373 patients over 11 years. Heart Rhythm. 2011;8:526–33. [DOI] [PubMed] [Google Scholar]
- 2. Ching CK, Elayi CS, Di Biase L, et al. Transiliac ICD implantation: defibrillation vector flexibility produces consistent success. Heart Rhythm. 2009;6:978–83. [DOI] [PubMed] [Google Scholar]
- 3. Mondésert B, Dubuc M, Khairy P, Guerra PG, Gosselin G, Thibault B. Combination of a leadless pacemaker and subcutaneous defibrillator: First in‐human report. Heart Rhythm Case Rep. 2015;1:469–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ahmed FZ, Cunnington C, Motwani M, Zaidi AM. Totally leadless dual‐device implantation for combined spontaneous ventricular tachycardia defibrillation and pacemaker function: a first report. Can J Cardiol. 2017;33:1066.e1065–7. [DOI] [PubMed] [Google Scholar]
- 5. Montgomery JA, Orton JM, Ellis CR. Feasibility of defibrillation and pacing without transvenous leads in a combined MICRA and S‐ICD system following lead extraction. J Cardiovasc Electrophysiol. 2017;28:233–4. [DOI] [PubMed] [Google Scholar]
- 6. Saad TF, Hentschel DM, Koplan B, et al. Cardiovascular implantable electronic device leads in CKD and ESRD patients: review and recommendations for practice. Semin Dial. 2013;26(1):114–23. [DOI] [PubMed] [Google Scholar]
- 7. Lin Y‐S, Chen T‐H, Lin M‐S, et al. Impact of chronic kidney disease on short‐term cardiac implantable electronic device related infection: a nationwide population‐based cohort study. Yue‐Chun. L, ed. Medicine. 2016;95(5):e2587. [DOI] [PMC free article] [PubMed] [Google Scholar]


