Abstract
This study aimed to determine whether prenatal stress, measured by the number of stressful life events during the first 20 weeks of pregnancy, might relate to mood dysregulation and altered brain structure in young adulthood. Participants included 93 young adults from a community-based birth cohort from the Czech Republic. Information on prenatal stress exposure was collected from their mothers in 1990−1992. Magnetic resonance imaging (MRI) and mood-related data were collected from the young adults in 2015. MRI analyses focused on overall gray matter (GM) volume and GM volume of cortical regions previously associated with major depression. Higher prenatal stress predicted more mood dysregulation, lower overall GM volume, and lower GM volume in mid-dorsolateral frontal cortex, anterior cingulate cortex, and precuneus in young adulthood. We observed no prenatal stress by sex interactions for any of the relations. We conclude that prenatal stress is an important risk factor that relates to worse mood states and altered brain structure in young adulthood irrespective of sex. Our results point to the importance and long-lasting effects of prenatal programming and suggest that offspring of mothers who went through substantial stress during pregnancy might benefit from early intervention that would reduce the odds of mental illness in later life.
Keywords: European longitudinal study of pregnancy and childhood mood dysregulation, gray matter volume, magnetic resonance imaging, prenatal stress
Introduction
Prenatal period is critical for brain development (Stiles and Jernigan 2010). Exposure to stress during this period seems to have long-lasting effects on health in adulthood (Sandman et al. 2012; Scheinost et al. 2017). Recent reports indicate that exposure to prenatal stress is a global public-health problem (Kinney et al. 2008; Janssen et al. 2016; Kertes et al. 2016; Rubin 2016). Approximately, 10–35% of children worldwide are exposed prenatally to stress (Maselko et al. 2015). Approximately, 8–23% of children are prenatally exposed to maternal depression (Yonkers et al. 2009; Sawyer et al. 2010; Fisher et al. 2012; Mapayi et al. 2013; Sandman et al. 2015). Moreover, since depression was not diagnosed in half of the pregnant women experiencing depression (United States data, Ko et al. 2012), these numbers might underestimate the problem.
Maternal stress during pregnancy (Van Os et al. 1997; Watson et al. 1999; Brown et al. 2000) has been identified as a risk for mental illness in adulthood. Prenatal stress “re-programs” the fetal hypothalamic-pituitary-adrenal (HPA) axis (Mairesse et al. 2007; O’Donnell et al. 2012; Li et al. 2012; Bock et al. 2014), and produces long-term hyper-responsiveness to stress with high levels of circulating glucocorticoids (Maniam et al. 2014). Female infants whose mothers suffered from major depressive disorder (MDD) during pregnancy had higher cortisol baseline levels as well as higher cortisol response to stress than female infants whose mothers did not suffer from MDD (Stroud et al. 2016).
Studies of prenatal origins of anxiety and mood dysregulation suggest that adverse prenatal exposures disrupt fetal development. Prenatal stress has been associated with intrauterine growth restriction, reduced fetal head growth, and preterm birth (Engel et al. 2005; Brand et al. 2006; Sandman et al. 2012). Prenatal stress induces changes in neurogenesis (Pryce et al. 2011) and corticogenesis (Bock et al. 2014; Lussier and Stevens 2016) of the developing brain. Literature on the effects of prenatal stress on brain structure in humans is sparse but Sandman et al. (2015) showed that maternal depression at 25th week of gestation predicted lower cortical thickness in the frontal lobes of the offspring.
Clinically depressed patients, who are known to suffer from severe mood dysregulation, were repeatedly found to have lower gray matter (GM) volume than healthy controls (Vythilingam et al. 2002; Frodl et al. 2010). Drevets et al. (2008) showed that depressed patients had also altered glucose metabolism in the brain regions with altered structural variation. A recent meta-analysis of FDG-PET studies (Jensen et al. 2015) identified a set of cortical regions with consistently reduced glucose metabolism in depressed patients vs. healthy controls.
Sex differences in the prevalence of stress-related psychiatric disorders such as major depression or post-traumatic stress disorder seem well established (Bangasser and Valentino 2014). It has been suggested that fetal sex modulates the responsiveness to prenatal stress (Buss et al. 2012; Goldstein et al. 2014; Bock et al. 2015; Constantinof et al. 2016; Gilman et al. 2016). The proposed mechanisms might include the sex-specific placental adaptation to stress exposure (Clifton 2010) or increased susceptibility of the female brain due to the more rapid neurodevelopmental trajectory in females compared with males (Buss et al. 2009; Nathanielsz et al. 2003). So far, however, only one study reported sex-specific effects of prenatal stress on magnetic resonance imaging (MRI) outcome measures (Buss et al. 2012).
In this study, we aim to determine whether prenatal stress, defined as a high number of stressful life events during the first 20 weeks of pregnancy, predicts mood dysregulation and brain structure in young adult offspring, and whether these effects differ by sex. Since, differences in GM volume of depressed patients vs. healthy controls are well established (Vythilingam et al. 2002; Frodl et al. 2010), we focus here on the volumes of cortical GM and test whether stressful life events during pregnancy might be related to lower GM volume, particularly in regions that were identified as hypometabolic by a meta-analysis of depressed patients versus healthy controls (Jensen et al. 2015). Based on the results of Jensen et al. (2015) who studied a sample of young men recruited from a birth cohort and found a negative relation between early childhood adversity and GM volume in a number of these cortical regions, we predict that higher stress during pregnancy will be associated with lower GM volume in anterior cingulate cortex and superior frontal gyrus, and higher GM volume in the precuneus.
Materials and Methods
Participants
Typically developing young adults from the European longitudinal study of pregnancy and childhood, the Czech Republic (ELSPAC-CZ; Golding 1989; Piler et al. 2016) were invited to participate in a neuroimaging study VULDE at Central European Institute of Technology, Masaryk University. All members from this prenatal birth cohort were born in the South Moravian Region of the Czech Republic between 1991 and 1992. A total of 93 individuals (40 males and 53 females) had both prenatal stress and adult neuroimaging data and were thus included in the current research on prenatal antecedents of mood dysregulation. All participants were 23–24 years old and of White Caucasian background. Further characteristics of the sample are provided in Supplementary Table 1. After the procedures had been fully explained, all participants provided written informed consent including the agreement to merge data from VULDE with their historic data from ELSPAC-CZ. Ethical approval for the VULDE study was obtained from ELSPAC Ethics Committee.
Procedures
Between 1990 and 1992, mothers of our participants answered a questionnaire about stressful life events during pregnancy (20th week of gestation). This questionnaire included 40 questions on stressful events such as break up or divorce with the partner, consideration of abortion, violence, serious illness or death in the family or financial difficulties answered by a 5-point Likert scale. See Supplementary Methods for the complete list of questions. Prenatal stress was estimated as the mean score on the stressful life events questionnaire. In 2015, all young adults were scanned using a 3 T Siemens Prisma MRI scanner and answered the long version of the Profile of Mood States questionnaire (POMS; McNair et al. 1971). The POMS questionnaire measures the following components of current mood state: depression/dejection, tension/anxiety, fatigue/inertia, anger/hostility, confusion/bewilderment, and vigor/activity.
MRI Acquisition
T1-weighted images were acquired on 3 T Siemens Prisma MRI scanner with 64 channel head/neck coil using the following acquisition parameters: voxel size 1 × 1 × 1 mm, 240 slices per slab, repetition time (TR) 2300 ms, echo time (TE) 2.34 >ms, inversion time (TI) 900 ms, flip angle 8°.
Analyses
T1-weighted data were processed using Freesurfer version 5.3.0 (Fischl and Dale 2000). Using the same pipeline as in Jensen et al. (2015), we calculated GM volumes (thickness by surface area) in a set of regions of interest (ROIs) that were obtained by projecting 30 ROIs identified by Activation Likelihood Estimation (ALE) meta-analysis of F-fluorodeoxyglucose Positron Emission Tomography (FDG PET) studies describing lower resting state glucose metabolism in 271 depressed patients compared with 193 healthy controls (Jensen et al. 2015). The list of these 30 ROIs is provided in Table 1 of Jensen et al. (2015). As explained in Jensen et al. (2015), we believe that glucose metabolism is a useful index of the probability of a functional engagement of these regions in a given individual (and disease state); over time, group differences in such cumulative measure of functional “underutilization”may lead to group differences in cortical thickness/volume in the same regions. Such an overlap between altered glucose metabolism and structural variation in the same brain regions of depressed patients was reported by Drevets et al. (2008). More information on the calculation and extraction of GM volume, surface area and cortical thickness is available in the eAppendix 3 of Jensen et al. (2015).
Questionnaire-based variables (both POMS-based and prenatal stress variables) were log-transformed to follow normal distribution and statistical analyses were done in JMP version 10.0.0 (SAS Institute Inc., Cary, NC). We used 2-sided hypothesis tests and significance level of 0.05. First, multivariate analysis of variance (MANOVA) assessed the association between prenatal stress and the 5 mood domains assessed by the POMS questionnaire in young adulthood. Next, linear regression assessed the association between prenatal stress and the average volume of cortical GM (corrected for the total brain volume) in young adulthood. Next, MANOVA assessed associations between prenatal stress and GM volume (corrected for the total brain volume) in the 30 ROIs. Prenatal stress (continuous variable) was entered as the only between-group parameter and GM volume and GM volume by prenatal stress interaction were entered as 2 within-group parameters. Post hoc linear regressions are reported. Finally, all these analyses were repeated considering also the potential interactions with sex. In addition, since previous research suggested that surface area and cortical thickness are 2 developmentally independent components of cortical GM volume (Wierenga et al. 2014), we carried out additional exploratory analyses to determine whether the observed associations between prenatal stress and regional GM volumes were driven by variations in surface area or cortical thickness. Since, as expected, the average cortical thickness did not vary as a function of the total brain volume (beta = −0.008, P = 0.94), we corrected for the total brain volume only in the analyses of surface area.
Results
Prenatal Stress and Mood
In the offspring, MANOVA showed a main effect of prenatal stress on mood (F(1,91) = 6.4, P = 0.01) and no interaction (F(5,455) = 1.99, P = 0.08) between prenatal stress and the type of POMS domain (anxiety, depression, anger, fatigue, vigor). Experience of higher prenatal stress was associated with more dysregulated mood. These findings remained significant even when correcting for sex or other possible confounders such as prenatal smoking, maternal education, maternal age, birth weight, or offspring’s education (main effect of prenatal stress on mood: F(1,83) = 5.6, P = 0.02; interaction between prenatal stress and type of POMS domain: F(5,415) = 1.99, P = 0.08).
Prenatal Stress and GM Volume in Young Adulthood
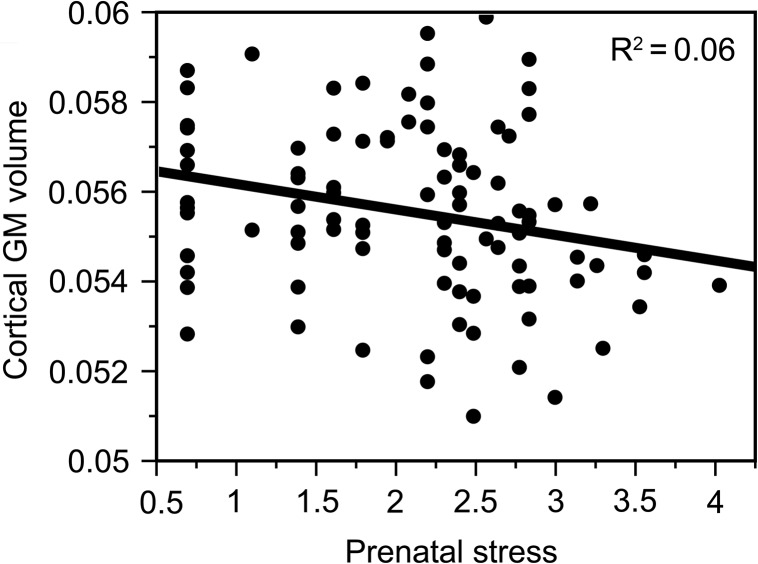
Greater exposure to prenatal stress was associated with lower brain-size corrected overall volume of cortical GM in the young adult offspring (beta = −0.24, P = 0.02, 95% CI [−0.001, −0.00009], R2 = 0.06; Fig. 1). Again, these findings remained significant even when correcting for sex or other possible confounders such as prenatal smoking, maternal education, maternal age, birth weight, or offspring’s education (beta = −0.25, P = 0.03, 95% CI [−0.001, −0.00007]).
Figure 1.
Prenatal stress and its effect on overall cortical GM volume (corrected for the total brain volume) in young adulthood (beta = −0.24, P = 0.02, 95% CI [−0.001, −0.00009], R2 = 0.06) (Prenatal stress refers to the log-transformed mean score on the stressful life events questionnaire).
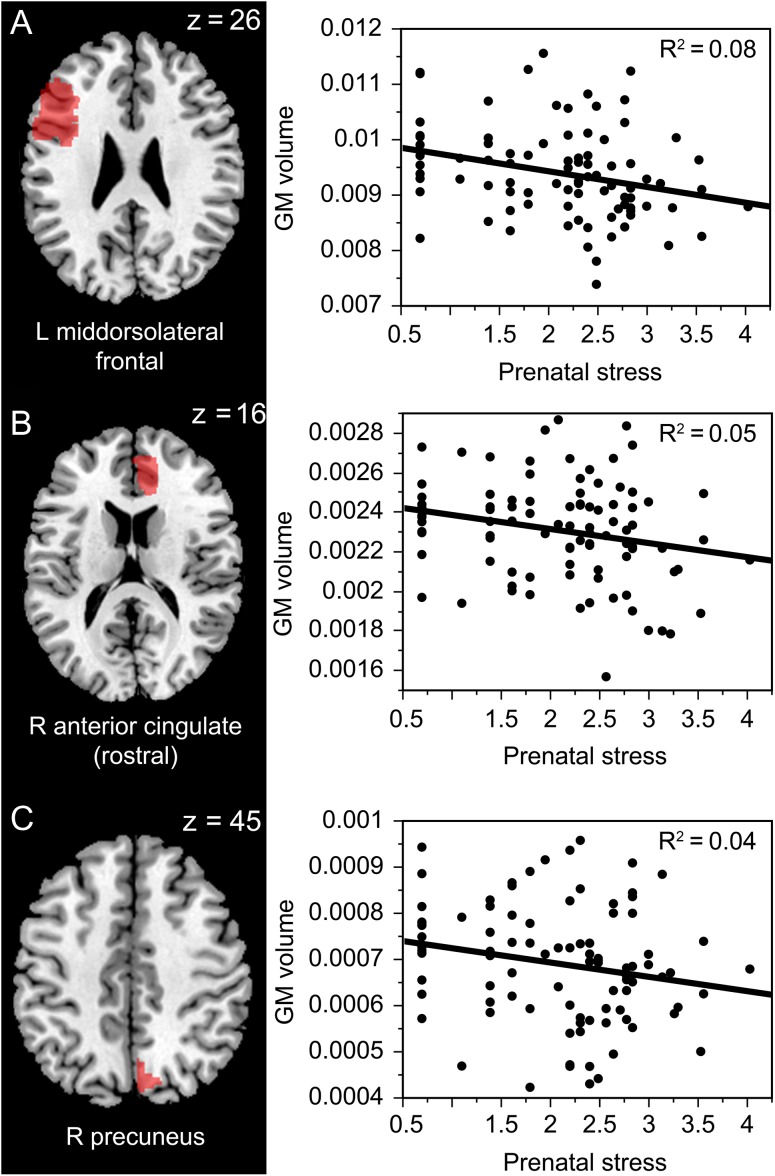
Region-based analyses using MANOVA showed an interaction between prenatal stress and ROIs (F(29,2639) = 2.78, P < 0.0001). This interaction remained significant (F(29,2581) = 2.68, P < 0.0001) even when we included sex in the model. Post hoc analyses revealed that higher prenatal stress was associated with lower total brain volume- corrected GM volume of left mid-dorsolateral frontal cortex (beta = −0.29, P = 0.005, 95% CI [−682.78, −128.27], R2 = 0.08), right anterior cingulate rostral (beta = −0.21, P = 0.04, 95% CI [−177.29, −6.01], R2 = 0.05), and right precuneus (beta = −0.20, P = 0.05, 95% CI [−84.69, −0.004], R2 = 0.04) (see Fig. 2). There was no effect of prenatal stress on total brain volume-corrected GM volume in the remaining ROIs (see Supplementary Table 2). For the relationships between prenatal stress and gray matter volume not corrected for total brain volume, see Supplementary Results.
Figure 2.
Prenatal stress and its effect on GM volume (corrected for the total brain volume) in regions identified by Jensen et al. (2015)’s meta-analysis as hypometabolic in depressed patients vs. healthy controls—left mid-dorsolateral frontal cortex ( A; beta = −0.29, P = 0.005, 95% CI [−682.78, −128.27], R2 = 0.08), right anterior cingulate rostral (B; beta = −0.21, P = 0.04, 95% CI [−177.29, −6.01], R2 = 0.05) and right precuneus ( C; beta = −0.20, P = 0.05, 95% CI [−84.69, −0.004], R2 = 0.04) in young adulthood (Prenatal stress refers to the log-transformed mean score on the Stressful life events questionnaire).
In order to determine the relative contribution of cortical thickness and surface with regards to the above volumetric findings, our exploratory analyses revealed the following. For the overall measures, there was no association between prenatal stress and average cortical thickness (beta = −0.12, P = 0.26, 95% CI [−0.04, 0.009]) or total brain-size corrected cortical area (beta = −0.14, P = 0.17, 95% CI [−0.002, 0.0004]). For the 3 ROIs, namely left mid-dorsolateral frontal cortex, right anterior cingulate and right precuneus, we observed only an association between prenatal stress and brain-size corrected cortical area of the left mid-dorsolateral frontal cortex (beta = −0.28, P = 0.006, 95% CI [−211.35, −35.61], R2 = 0.08). The relationships between prenatal stress and brain-size corrected cortical area of the right anterior cingulate (beta = −0.13, P = 0.23, 95% CI [−47.59, 11.42]) and right precuneus (beta = −0.16, P = 0.12, 95% CI [−19.38, 2.39]) did not reach significance. There were no associations between prenatal stress and cortical thickness of the 3 regions. See Supplementary Results for the relationships between prenatal stress and cortical area not corrected for total brain volume.
Discussion
This study examined the extent to which prenatal stress relates to cortical structure and mood in young adulthood, and whether these relations differ by sex. We found that higher prenatal stress predicted more mood dysregulation in young adulthood. Our findings also demonstrated that young adults with greater exposure to stress prenatally had lower overall cortical GM volume (corrected for the total brain volume) and lower regional GM volume (corrected for the total brain volume) in mid-dorsolateral frontal cortex, anterior cingulate cortex and precuneus. Sex did not modulate any of these relations.
The positive relation between prenatal stress and dysregulated mood in young adulthood is in agreement with Kingsbury et al. (2016) who analyzed data from the Avon Longitudinal Study of Parents and Children (ALSPAC), and showed that exposure to prenatal stress was associated with more depressive symptoms in the adolescent offspring. It is also in agreement with Dipietro et al. (2006) who showed a relationship between pregnancy specific stress and infant emotional regulation. According to Teicher et al. (2003), the psychiatric consequences associated with early exposure to stress are mediated by stress-induced modification of brain development. According to Seckl and colleagues (Seckl 1998; Seckl et al. 2000; Welberg and Seckl 2001), these organizing effects on development are driven by altered levels of glucocorticoids.
The negative relation between prenatal stress and GM volume is in agreement with animal research reporting an impact of prenatal stress on neurogenesis (Pryce et al. 2011) and corticogenesis (Bock et al. 2014) of the developing brain. The lower GM volume in frontal cortex of individuals with higher exposure to stress prenatally is consistent with a number of studies reporting the lower GM volume in frontal cortex in depressed individuals (Drevets et al. 2008; Frodl et al. 2010; Kempton et al. 2011). It is also consistent with the findings of Sandman et al. (2015) who showed that the maternal depression at 25th week of gestation predicted cortical thinning in the frontal lobes of the offspring. According to Teicher et al. (2003), early stress impacts particularly brain regions with protracted postnatal development, some degree of postnatal neurogenesis, and high density of glucocorticoid receptors. The most delayed ontogeny of all cortical regions is characteristic particularly for prefrontal cortex (Teicher et al. 2003). It is important to note, however, that different timing of the prenatal exposures might have different effects on the gray matter volume. Buss et al. (2010) showed that while maternal anxiety at mid-gestation (19 weeks) was associated with gray matter volume reduction in 6–9-year-old offspring, this was not the case for maternal anxiety at 25 or 31 weeks of gestation.
The lower GM volume in ACC and precuneus is consistent with research reporting lower GM volume in ACC of individuals with adverse childhood events (Cohen et al. 2006; Jensen et al. 2015) and harsh parenting (Tomoda et al. 2011) and lower thickness of precuneus in maltreated children (Kelly et al. 2013). The anterior cingulate cortex (ACC) lies at the intersection of regulatory and executive networks, thus allowing to overcome inertia or innate tendencies and initiating a willed control of action (Paus 2001). Lesions of the ACC are known to interfere with initiation of action, resistance to automatic subroutines and acquisition of novel behavior (Paus 2001). This functional characteristic of the anterior cingulate helps to explain why this study found impact of prenatal stress on both lower gray matter volume in this ROI and more pronounced mood dysregulation (including the fatigue/inertia domain measured by the POMS). Precuneus is known for its engagement in voluntary attention shifts between targets, self-processing, perspective-taking and experience of agency (Cavanna and Trimble 2006). It has a widespread network of subcortical and cortical connections, including mid-dorsolateral prefrontal cortex and anterior cingulate cortex (Cavanna and Trimble 2006), which also showed significant relations with prenatal stress in the present study.
Our study did not find any modulatory effects of sex on the relations between prenatal stress and mood or prenatal stress and GM volume in young adulthood. This is in agreement with Scheinost et al. (2016) who reviewed the effects of prenatal stress on MRI outcome measures and showed that only one study so far reported sex differences in MRI outcome measures, namely the relation between higher cortisol at 15 weeks of gestation on larger volume of the right amygdala in female but not male offspring (Buss et al. 2012). The lack of sex-specific effects of prenatal stress found in this study might be also related to the timing of prenatal stress exposure. Sexual differentiation of the brain happens mainly during the second and third trimester (Goldstein et al. 2014) but the current study assessed the impact of stressful life events during the first half of pregnancy.
Our post hoc exploratory analyses suggest that the associations between prenatal stress and GM volume were driven by surface area rather than cortical thickness. This is in agreement with studies suggesting that cortical area and cortical thickness are developmentally independent (Wierenga et al. 2014) and thus might vary in timing of sensitivity toward adverse environments (Jensen et al. 2015). It goes in line with studies of nonhuman primates reporting earlier expansion of cortical surface area (Rakic 1995; Selemon et al. 2013). Selemon et al. (2013) compared the effects of X-ray radiation during early (before corticogenesis) vs. mid (peak of corticogenesis) gestation of Rhesus macaque and showed that while both early and midgestational radiation diminished the surface area of dorsolateral prefrontal cortex, only the midgestational radiation reduced cortical thickness. Selemon et al. (2013) concluded that while early gestation induced subtle cytoarchitectonic alterations without loss of neurons, midgestational exposure induced selective elimination of neurons and cortical thinning. Longitudinal studies in humans report substantial expansion of cortical surface area but only a moderate increase in cortical thickness within the first 2 years of life (Li et al. 2013; Gilmore et al. 2010). We speculate that stress experienced in utero by our participants interfered with the overall growth of the cerebral cortex during early brain development, with some regional differences in such relations.
Since the effect sizes reported in this study are relatively small, future research with larger samples are required to evaluate replicability of the current findings. Future research might also use other measures of depression in young adulthood and consider other factors such as prenatal stress experienced during the second half of pregnancy or postnatal stress (e.g. stress experienced during childhood or adolescence). While the hypothesis-based ROI approach could be seen as a strength, for both conceptual and statistical reasons, by definition, it cannot detect possible associations outside these regions, as would be the case with voxel-wise analyses.
Overall, our study has 2 main strengths: 1) prospective evaluation of the relation between prenatal stress (measured in pregnancy) and GM volumes assessed in young adulthood, in the context of a birth cohort and 2) the focus on ROIs that were identified as hypometabolic in depressed patients vs. healthy controls by a meta-analysis, and evaluated in a similar fashion in a different birth cohort by Jensen et al. (2015). We conclude that higher stress during pregnancy was associated with greater mood dysregulation and lower cortical GM volume in the young adult offspring, particularly in mid-dorsolateral frontal cortex, anterior cingulate, and precuneus. These results extend the findings of Jensen et al. (2015) who found a relation between childhood adversity and cortical GM volume in young men and support the importance of prenatal programming.
Supplementary Material
Notes
We also thank 3 anonymous reviewers for their constructive comments and suggestions. Conflict of interest: None declared.
Funding
This work was supported by the European Union (Marie Curie Intra-European Fellowship for Career Development FP7-IEF-2013), the Ministry of Education, Youth and Sports of the Czech Republic/MEYS (CEITEC 2020, LQ1601, LM2015051) and Canadian Institutes of Health Research (MOP125892 to T.P.).
References
- Bangasser DA, Valentino RJ.. 2014. Sex differences in stress-related psychiatric disorders: neurobiological perspectives. Front Neuroendocrinol. 35(3):303–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bock J, Rether K, Gröger N, Xie L, Braun K.. 2014. Perinatal programming of emotional brain circuits: an integrative view from systems to molecules. Front Neurosci. 8:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bock J, Wainstock T, Braun K, Segal M.. 2015. Stress in utero: prenatal programming of brain plasticity and cognition. Biol Psychiatry. 78(5). 10.1016/j.biopsych.2015.02.036. [DOI] [PubMed] [Google Scholar]
- Brand SR, Engel SM, Canfield RL, Yehuda R.. 2006. The effect of maternal PTSD following in utero trauma exposure on behavior and temperament in the 9-month-old infant. Ann N Y Acad Sci. 1071:454–458. [DOI] [PubMed] [Google Scholar]
- Brown AS, van Os J, Driessens C, Hoek HW, Susser ES.. 2000. Further evidence of relation between prenatal famine and major affective disorder. Am. Psychiatry. 157(2):190–195. [DOI] [PubMed] [Google Scholar]
- Buss C, Davis EP, Muftuler LT, Head K, Sandman CA.. 2010. High pregnancy anxiety during mid-gestation is associated with decreased gray matter density in 6–9 year old children. Psychoneuroendocrinology. 35(1):141–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss C, Davis EP, Class QA, Gierczak M, Pattillo C, Glynn LM, Sandman CA. 2009. Maturation of the human fetal startle response: Evidence for sex-specific maturation of the human fetus. Early Hum Dev. 85:633–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss C, Davis EP, Shahbaba B, Pruessner JC, Head K, Sandman CA.. 2012. Maternal cortisol over the course of pregnancy and subsequent child amygdala and hippocampus volumes and affective problems. Proc Natl Acad Sci USA. 109:E1312–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanna AE, Trimble MR.. 2006. The precuneus: a review of its functional anatomy and behavioral correlates. Brain. 129:564–583. [DOI] [PubMed] [Google Scholar]
- Clifton VL. 2010. Review: Sex and the human placenta: mediating differential strategies of fetal growth and survival. Placenta. 31(Suppl):S33–S39. [DOI] [PubMed] [Google Scholar]
- Cohen RA, Grieve S, Hoth KF, Paul RH, Sweet L, Tate D, Gunstad J, Stroud L, McCaffery J, Hitsman B, et al. . 2006. Early life stress and morphometry of the adult anterior cingulate cortex and caudate nuclei. Biol Psychiatry. 59(10):975–982. [DOI] [PubMed] [Google Scholar]
- Constantinof A, Moisiadis VG, Matthews SG.. 2016. Programming of stress pathways: a transgenerational perspective. J Steroid Biochem Mol Biol. 160:175–180. [DOI] [PubMed] [Google Scholar]
- Dipietro JA, Novak MF, Costigan KA, Atella LD, Reusing SP.. 2006. Maternal psychological distress during pregnancy in relation to child development at age two. Child Dev. 77:573–587. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Price JL, Furey ML.. 2008. Brain structural and functional abnormalities in mood disorders: implications for neurocircuitry models of depression. Brain Struct Funct. 213(1–2):93–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel SM, Berkowitz GS, Wolff MS, Yehuda R.. 2005. Psychological trauma associated with the World Trade Center attacks and its effect on pregnancy outcome. Paediatr Perinat Epidemiol. 19:334–41. [DOI] [PubMed] [Google Scholar]
- Fischl B, Dale AM.. 2000. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci U S A. 97(20):11050–11055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher J, Cabral de Mello M, Patel V, Rahman A, Tran T, Holton S, Holmes W.. 2012. Prevalence and determinants of common perinatal mental disorders in women in low- and lowermiddle income countries: a systematic review. Bull World Health Organ. 90:139G–49G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frodl T, Reinhold E, Koutsouleris N, Reiser M, Meisenzahl EM.. 2010. Interaction of childhood stress with hippocampus and prefrontal cortex volume reduction in major depression. J Psychiatr Res. 44(13):799–807. [DOI] [PubMed] [Google Scholar]
- Gilman SE, Cherkerzian S, Buka SL, Hahn J, Hornig M, Goldstein JM.. 2016. Prental immune programming of the sex -dependent risk for major depression. Transl Psychiatry. 6(5):e822 10.1038/tp.2016.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore JH, Schmitt JE, Knickmeyer RC, Smith JK, Lin W, Styner M, Gerig G, Neale MC.. 2010. Genetic and environmental contributions to neonatal brain structure: a twin study. Hum Brain Mapp. 31(8):1174–1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golding J. 1989. European Longitudinal Study of Pregnancy and Childhood (ELSPAC). Paediatr Perinat Epidemiol. 3(4):460–469. [DOI] [PubMed] [Google Scholar]
- Goldstein JM, Handa RJ, Tobet SA.. 2014. Disruption of fetal hormonal programming (prenatal stress) implicates shared risk for sex differences in depression and cardiovascular disease. Front Neuroendocrinol. 35(1):140–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen AB, Kertes DA, McNamara GI, Braithwaite EC, Creeth HD, Glover VI, John RM.. 2016. A role for the placenta in programming role for the placenta in programming maternal mood and childhood behavioural disorders. J Neuroendocrinol. 28(8). 10.1111/jne.12373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen SKG, Dickie EW, Schwartz DH, Evans JC, Dumontheil I, Paus T, Barker ED.. 2015. Effect of early adversity and childhood internalizing symptoms on brain structure in young men. JAMA Pediatr. 169(10):938–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kertes DA, Kamin HS, Hughes DA, Rodney NC, Bhatt S, Mulligan CJ.. 2016. Prenatal maternal stress predicts methylation of genes regulating the hypothalamic-pituitary-adrenocortical system in mothers and newborns in the Democratic Republic of Congo. Child Dev. 87:61–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly PA, Viding E, Wallace GL, Schaer M, De Brito SA, Robustelli B, McCrory EJ.. 2013. Cortical thickness, surface area, and gyrification abnormalities in children exposed to maltreatment: neural markers of vulnerability? Biol Psychiatry. 74(11):845–852. [DOI] [PubMed] [Google Scholar]
- Kempton MJ, Salvador Z, Munafò MR, Geddes JR, Simmons A, Frangou S, Williams SC.. 2011. Structural neuroimaging studies in major depressive disorder: meta-analysis and comparison with bipolar disorder. Arch Gen Psychiatry. 68(7):675–690. [DOI] [PubMed] [Google Scholar]
- Kingsbury M, Weeks M, MacKinnon N, Evans J, Mahedy L, Dykxhoorn J, Colman I.. 2016. Stressful life events during pregnancy and offspring depression: evidence from a prospective cohort study. J Am Acad Child Adolesc Psychiatry. 55(8):709–716. [DOI] [PubMed] [Google Scholar]
- Kinney DK, Munir KM, Crowley DJ, Miller AM.. 2008. Prenatal stress and risk for autism. Neurosci Biobehav Rev. 32:1519–1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko JY, Farr SL, Dietz PM, Robbins CL.. 2012. Depression and treatment among US pregnant and nonpregnant women of reproductive age, 2005–2009. J Womens Health (Larchmt). 21:830–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, Nie J, Wang L, Shi F, Lin W, Gilmore JH, Shen D.. 2013. Mapping region-specific longitudinal cortical surface expansion from birth to 2 years of age. Cereb Cortex. 23(11):2724–2733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Gonzalez P, Zhang L.. 2012. Fetal stress and programming of hypoxic/ischemic-sensitive phenotype in the neonatal brain: mechanisms and possible interventions. Prog Neurobiol. 98(2):145–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lussier SJ, Stevens HE.. 2016. Delays in GABAergic interneuron development and behavioral inhibition after prenatal stress. Dev Neurobiol. 76:1078–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mairesse J, Lesage J, Breton C, Bréant B, Hahn T, Darnaudéry M, Dickson SL, Seckl J, Blondeau B, Vieau D, et al. . 2007. Maternal stress alters endocrine function of the feto-placental unit in rats. Am J Physiol Endocrinol Metab. 292:E1526–E1533. [DOI] [PubMed] [Google Scholar]
- Maniam J, Antoniadis C, Morris MJ.. 2014. Early-life stress, HPA axis adaptation, and mechanisms contributing to later health outcomes. Front Endocrinol (Lausanne). 5–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mapayi B, Makanjuola RO, Mosaku SK, Adewuya OA, Afolabi O, Aloba OO, Akinsulore A.. 2013. Impact of intimate partner violence on anxiety and depression amongst women in Ile-Ife, Nigeria. Arch Womens Ment Health. 16:11–8. [DOI] [PubMed] [Google Scholar]
- Maselko J, Sikander S, Bhalotra S, Bangash O, Ganga N, Mukherjee S, Egger H, Franz L, Bibi A, Liaqat R, et al. . 2015. Effect of an early perinatal depression intervention on long-term child development outcomes: follow-up of the Thinking Healthy Programme randomised controlled trial. Lancet Psychiatry. 2:609–617. [DOI] [PubMed] [Google Scholar]
- McNair DM, Lorr M, Droppleman LF.. 1971. Manual for the Profile of Mood States. San Diego, CA: Educational and Industrial Testing Service. [Google Scholar]
- Nathanielsz PW, Berghorn KA, Derks JB, Giussani DA, Docherty C, Unno N, Davenport A, Kutzlers M, Koenen S, Visser GH, et al. . 2003. Life before birth: Effects of cortisol on future cardiovascular and metabolic function. Acta Paediatr. 92:766–772. [PubMed] [Google Scholar]
- O’Donnell KJ, Bugge Jensen A, Freeman L, Khalife N, O’Connor TG, Glover V.. 2012. Maternal prenatal anxiety and downregulation of placental 11β-HSD2. Psychoneuroendocrinology. 37:818–826. [DOI] [PubMed] [Google Scholar]
- Paus T. 2001. Primate anterior cingulate cortex: where motor control, drive, and cognition interface. Nature Reviews Neuroscience. 2:417–424. [DOI] [PubMed] [Google Scholar]
- Piler P, Kandrnal V, Kukla L, Andrýsková L, Švancara J, Jarkovský J, Dušek L, Pikhart H, Bobák M, Klánová J.. 2016. Cohort profile: European Longitudinal Study of Pregnancy and Childhood (ELSPAC) in the Czech Republic. International Journal of Epidemiology. 10.1093/ije/dyw091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pryce R, Aubert Y, Maier C, Pearce PC, Fuchs E.. 2011. The developmental impact of prenatal stress, prenatal dexamethasone and postnatal social stress on physiology, behaviour, and neuroanatomy of primate offspring: studies in rhesus macaque and common marmoset. Psychopharmacology. 214(1):33–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakic P. 1995. A small step for the cell, a giant leap for mankind: a hypothesis of neocortical expansion during evolution. Trends Neurosci. 18(9):383–388. [DOI] [PubMed] [Google Scholar]
- Rubin LP. 2016. Maternal and pediatric health and disease: integrating biopsychosocial models and epigenetics. Pediatr Res. 79:127–135. [DOI] [PubMed] [Google Scholar]
- Sandman CA, Buss C, Head K, Davis EP.. 2015. Fetal exposure to maternal depressive symptoms is associated with cortical thickness in late childhood. Biol Psychiatry. 77:324–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandman CA, Davis EP, Buss C, Glynn LM.. 2012. Exposure to prenatal psychobiological stress exerts programming influences on the mother and her fetus. Neuroendocrinology. 95:7–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawyer A, Ayers S, Smith H.. 2010. Pre- and postnatal psychological wellbeing in Africa: a systematic review. J Affect Disord. 123:17–29. [DOI] [PubMed] [Google Scholar]
- Scheinost D, Sinha R, Cross SN, Kwon HS, Sze G, Constable RT, Ment LR.. 2017. Does prenatal stress alter the developing connectome? Pediatric Research. 81:214–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheinost D, Kwon SH, Lacadie C, Sze G, Sinha R., Constable RT, Ment LR.. 2016. Prenatal stress alters amygdala functional connectivity in preterm neonates. Neuroimage Clin. 12:381–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seckl JR. 1998. Physiologic programming of the fetus. Clin Perinatol. 25(4):939–962. [PubMed] [Google Scholar]
- Seckl JR, Cleasby M, Nyirenda MJ.. 2000. Glucocorticoids, 11betahydroxysteroid dehydrogenase, and fetal programming. Kidney Int. 57(4):1412–1417. [DOI] [PubMed] [Google Scholar]
- Selemon LD, Ceritoglu C, Ratnanather JT, Wang L, Harms MP, Aldridge K, Begović A, Csernansky JG, Miller MI, Rakic P.. 2013. Distinct abnormalities of the primate prefrontal cortex caused by ionizing radiation in early or midgestation. J Comp Neurol. 521(5):1040–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiles J, Jernigan TL.. 2010. The basics of brain development. Neuropsychol Rev. 20(4):327–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroud LR, Papandonatos GD, Parade SH, Salisbury AL, Phipps MG, Lester BM, Padbury JF, Marsit CJ.. 2016. Prenatal major depressive disorder, placenta glucocorticoid and serotonergic signalling, and infant cortisol response. Psychosom Med. 78(9):979–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teicher MH, Andersen SL, Polcari A, Anderson CM, Navalta CP, Kim DM.. 2003. The neurobiological consequences of early stress and childhood maltreatment. Neurosci Biobehav Rev. 27:33–44. [DOI] [PubMed] [Google Scholar]
- Tomoda A, Sheu YS, Rabi K, Suzuki H, Navalta CP, Polcari A, Teicher MH.. 2011. Exposure to parental verbal abuse is associated with increased gray matter volume in superior temporal gyrus. Neuroimage. 54:S280–S286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Os J, Jones P, Lewis G, Wadsworth M, Murray R.. 1997. Developmental precursors of affective illness in a general population birth cohort. Arch Gen Psychiatry. 54(7):625–631. [DOI] [PubMed] [Google Scholar]
- Vythilingam M, Heim C, Newport J, Miller AH, Anderson E, Bronen R, Brummer M, Staib L, Vermetten E, Charney DS, et al. . 2002. Childhood trauma associated with smaller hippocampal volume in women with major depression. Am J Psychiatry. 159(12):2072–2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson JB, Mednick SA, Huttunen M, Wang X.. 1999. Prenatal teratogens and the development of adult mental illness. Dev Psychopathol. 11(3):457–466. [DOI] [PubMed] [Google Scholar]
- Welberg LA, Seckl JR.. 2001. Prenatal stress, glucocorticoids and the programming of the brain. J Neuroendocrinol. 13(2):113–128. [DOI] [PubMed] [Google Scholar]
- Wierenga LM, Langen M, Oranje B, Durston S.. 2014. Unique developmental trajectories of cortical thickness and surface area. Neuroimage. 87:120–126. [DOI] [PubMed] [Google Scholar]
- Yonkers KA, Wisner KL, Stewart DE, Oberlander TF, Dell DL, Stotland N, Ramin S, Chaudron L, Lockwood C.. 2009. The management of depression during pregnancy: a report from the American Psychiatric Association and the American College of Obstetricians and Gynecologists. Gen Hosp Psychiatry. 31:403–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.