Abstract
Cortical resting state networks have been consistently identified in infants using resting state-functional connectivity magnetic resonance imaging (rs-fMRI). Comparable studies in adults have demonstrated cerebellar components of well-established cerebral networks. However, there has been limited investigation of early cerebellar functional connectivity. We acquired non-sedated rs-fMRI data in the first week of life in 57 healthy, term-born infants and at term-equivalent postmenstrual age in 20 very preterm infants (mean birth gestational age 27 ± 2 weeks) without significant cerebral or cerebellar injury. Seed correlation analyses were performed using regions of interests spanning the cortical and subcortical gray matter and cerebellum. Parallel analyses were performed using rs-fMRI data acquired in 100 healthy adults. Our results demonstrate that cortico-cerebellar functional connectivity is well-established by term. Intra- and cortico-cerebellar functional connectivity were largely similar in infants and adults. However, infants showed more functional connectivity structure within the cerebellum, including stronger homotopic correlations and more robust anterior–posterior anticorrelations. Prematurity was associated with reduced correlation magnitudes, but no alterations in intra- and cortico-cerebellar functional connectivity topography. These results add to the growing evidence that the cerebellum plays an important role in shaping early brain development during infancy.
Keywords: cerebellum, developmental neuroimaging, functional MRI, prematurity, resting state networks
Introduction
The cerebellum plays an increasingly recognized role in brain function and development (Steinlin 2007; Stoodley 2012; Buckner 2013). Beyond its well-established involvement in motor function and coordination, the cerebellum contributes to higher cognitive functions including executive control, language, visuospatial reasoning, social cognition, and working memory (for a review, see Stoodley and Schmahmann 2009). Recent investigations of the cerebellum have increasingly included resting state-functional connectivity magnetic resonance imaging (rs-fMRI), a neuroimaging modality that measures the temporal correlation of spontaneous, low frequency fluctuations of the blood oxygen level dependent (BOLD) signal (Fox and Raichle 2007). In adults, rs-fMRI has consistently revealed multiple resting state networks (RSNs) incorporating both cerebellar and cortical regions, the topography of which corresponds to the known representation of function across the brain. For example, motor and somatosensory RSNs are represented in the anterior cerebellum, while RSNs associated with “higher-order” functions, e.g., the default mode network (DMN) and the salience network (SAL), are represented in posterior cerebellar lobes (Habas et al. 2009; O’Reilly et al. 2010; Buckner et al. 2011; Bernard et al. 2012). Similar findings have been obtained in older children and adolescents (Khan et al. 2015; Bernard et al., 2016; Wang et al. 2016). However, little is known regarding cerebellar RSNs in infants.
The basic morphology of the cerebellum is established around 20 weeks of gestation. Major volumetric expansion and surface foliation develop over the last trimester of pregnancy (Rakic and Sidman 1970; Volpe 2009; Leto et al. 2015). The cerebellum is particularly vulnerable to injury during this later period, potentially leading to impaired structural and functional development (Volpe 2009; Poretti et al. 2016). Multiple studies have shown that prematurity predisposes to structural cerebellar injury, with risk progressively increasing as gestational age decreases (Limperopoulos et al. 2005a, 2005b, 2007; Volpe 2009). Several recent studies have shown strong associations between cerebellar injury and under-development of the contralateral supratentorial hemisphere (Limperopoulos et al. 2005a, 2005b, 2010). Further, domain-specific motor and cognitive deficits are associated with cerebellar injury and impaired development in both term- and preterm-born populations (Castellanos et al. 2002; Allin et al. 2005; Messerschmidt et al. 2008; Nicolson and Fawcett 2011; Bolduc et al. 2012; Stoodley and Stein 2013; Limperopoulos et al. 2014; Brossard-Racine et al. 2015; Poretti et al. 2016). Taken together, these findings indicate that evaluating the cerebellum during the neonatal period is necessary in order to fully understand its role in early brain development and function.
rs-fMRI has been used to study functional cerebral development in pediatric populations during infancy and early childhood (De Asis-Cruz et al. 2015; Smyser and Neil 2015). Despite differing acquisition and analysis techniques, these investigations have consistently demonstrated canonical RSNs throughout the brain in infants with strong similarity to adult RSNs (Fransson et al. 2007; Doria et al. 2010; Smyser et al. 2010, 2016; Toulmin et al. 2015). These studies have shown RSN developmental patterns often parallel known rates of cortical maturation based upon histological investigation. Furthermore, quantitative group- and individual-level alterations in RSN architecture attributable to premature birth suggest that functional network architecture undergoes a critical developmental period prior to term-equivalent postmenstrual age (PMA; 37–41 weeks; Fransson et al. 2007; Doria et al. 2010; Smyser et al. 2010). Some studies have demonstrated interhemispheric correlation between homotopic regions of the cerebellum (Fransson et al. 2007; Doria et al. 2010; Smyser et al. 2010). However, the anatomic specificity of cerebellar RSNs and their relationship to cortical RSNs have not been studied in the neonatal period.
In this study, we used rs-fMRI to explore the intrinsic cerebellar and cortico-cerebellar functional organization of the developing brain in 57 full-term born (FT) infants. A group of 20 very preterm-born infants (VPT; born at <30 weeks gestation) were studied at term-equivalent PMA to evaluate the effects of prematurity on early cerebellar RSN development. In addition, a group of 100 healthy adults were studied to assess the effects of later development on cerebellar RSNs. Low-motion rs-fMRI data were available for all subjects. Equally sized regions of interest (ROIs), spanning the entire cerebellum and cortical and subcortical gray matter, were used as seeds to delineate the cerebellar RSNs and cortical and subcortical gray matter structures in each group. We hypothesized that 1) multiple intrinsic cerebellar functional networks with distinct cortico-cerebellar rs-fMRI patterns are identifiable during the neonatal period in FT infants, 2) prematurity manifests as altered functional connectivity in VPT infants scanned at comparable PMA, and 3) a similar intrinsic cerebellar and cortico-cerebellar functional connectivity architecture is present in adults, though with network-specific differences in comparison to neonates.
Materials and Methods
Subjects
Fifty-seven FT infants (31 females, gestational age at birth 37–41 weeks) were recruited from the Barnes-Jewish Hospital Newborn Nursery. All infants had no evidence of cerebral injury on MRI and no history of in utero illicit substance exposure or evidence of acidosis (pH < 7.20) on umbilical cord or arterial blood gas assessments during the first hour of life. Twenty VPT infants (9 female, gestational age at birth 23–29 weeks) were prospectively recruited from the St. Louis Children’s Hospital Neonatal Intensive Care Unit (NICU). VPT infants were excluded if the following structural abnormalities were noted on MRI in reviews by a neuroradiologist (J.S.S.) and pediatric neurologist (C.D.S.): grade II–IV intraventricular hemorrhage, cystic periventricular leukomalacia, lesions in the cortical or deep nuclear gray matter, and any hemorrhagic lesions in the cerebellum (Kidokoro et al. 2013). These criteria were chosen to provide a study population in which the effects of common forms of neuropathology found in VPT infants, including cerebellar injury, were minimized. Infants were also excluded if found to have chromosomal abnormality or suspected or proven congenital infection (e.g., HIV, sepsis, toxoplasmosis, rubella, cytomegalovirus, herpes simplex virus). Demographic information for both groups is provided in Table 1. Parental informed consent was obtained for each subject prior to participation. All aspects of the study were approved by the Washington University School of Medicine’s Human Studies Committee.
Table 1.
Demographic information for term and preterm infants
| Clinical variable | Preterm infants (N = 20) | Term infants (N = 57) | P-value |
|---|---|---|---|
| Gestational age at birth (weeks)—mean standard deviation (SD) | 27 (2) | 39 (1) | <0.001a |
| Female—N (%) | 9 (45%) | 31 (54%) | 0.470b |
| African-American—N (%) | 10 (50%) | 37 (65%) | 0.239b |
| Postmenstrual age at scan (weeks)—mean (SD) | 38 (2) | 39 (1) | 0.02a |
| Birthweight (g)—mean (SD) | 1008 (206) | 3315 (417) | <0.001a |
| Multiple gestation—N (%) | 7 (35%) | 0 (0%) | <0.001b |
| Antenatal steroids—N (%) | 18 (90%) | ||
| Inotropic support—N (%) | 5 (25%) | ||
| Postnatal steroids—N (%) | 3 (15%) | ||
| Inbutated/ventilated—N (%) | 15 (75%) | ||
| Total ventilator days—mean (SD) | 9 (16) | ||
| Total parenteral nutrition (days)—mean (SD) | 20 (17) | ||
| Patent ductus arteriosus treated medically—N (%) | 6 (30%) |
aResults from two-sample, two-tailed t-test.
bResults from chi-square test.
In addition, data for a group of 100 clinically healthy adults (56 female, age at scan 18–35 years, mean age 21.4 ± 2.4 years) studied through the Harvard-MGH Brain Genomics Superstruct Project were included in the analyses (sample described in Yeo et al. 2011). Adult participants provided written informed consent in accordance with the guidelines set by the institutional review boards of Harvard University or Partners Healthcare.
MRI Data Acquisition
FT infants were scanned within the first 4 days of life (mean PMA at scan 39 ± 1 weeks). VPT infants were scanned at term-equivalent age (mean PMA at scan 38 ± 2 weeks). All imaging data were acquired on a Siemens Trio 3 T scanner (Erlangen, Germany) using an infant-specific quadrature head coil (Advanced Imaging Research, Cleveland, OH, USA). All infants were scanned during natural sleep or while resting quietly without the use of sedating medications (Mathur et al. 2008). For a description of procedures used to prepare infants for scanning, please see Smyser et al. (2010).
Structural images were acquired using a T2-weighted sequence (TR = 8600 ms, TE = 160 ms, voxel size 1 mm3, echo train length 17 ms). rs-fMRI data were collected using a gradient-echo echo planar image (EPI) sequence sensitized to T2* BOLD contrast (TR = 2910 ms, TE = 28 ms, voxel size 2.4 mm3, flip angle 90°). Whole brain coverage was obtained with 44 contiguous slices. Each fMRI run consisted of ~200 volumes (9.6 min), and a minimum of one run was obtained in each infant. Additional runs were acquired in a subset of participants depending on subject tolerance.
Adult data were downloaded from the Harvard-MGH Brain Genomics Superstruct Project (http://neuroinformatics.harvard.edu/gsp/). These data were collected on Siemens Trio 3 T scanners (Erlangen, Germany) using a 12-channel phased-array head coil. Structural data were acquired using a high-resolution multiecho T1-weighted magnetization-prepared gradient-echo sequence (TR = 2200 ms, TI = 1100 ms, TE = 1.54 ms for image 1 to 7.01 for image 4, voxel size 1.2 mm3). Functional data were collected using a gradient-echo EPI sequence sensitized to T2* BOLD contrast (TR = 3000 ms, TE = 30 ms, voxel size 3 mm3, flip angle 85°). Whole brain coverage was obtained with 47 interleaved slices with no gap between slices. Two fMRI runs with 124 volumes per run (6.2 min) were acquired per subject; for more details on the rs-fMRI acquisition, please see Yeo et al. (2011).
Functional MRI Preprocessing
Infant rs-fMRI data were preprocessed as previously described (Smyser et al. 2010). These procedures were implemented using the 4dfp suite of tools (ftp://imaging.wustl.edu/pub/raichlab/4dfp_tools/). Preprocessing included correction of asynchronous slice timing and rigid body correction of head movement. EPI distortions in the BOLD data were corrected using the FUGUE module in FSL (Jenkinson et al. 2012). Magnetization inhomogeneity-related distortions were corrected using a mean field map technique (Gholipour et al. 2008). Following completion of these procedures, atlas transformation was computed using a PMA-specific infant target (Smyser et al. 2010). The volumetric time series were resampled to a representative adult 711-2B (Talairach) atlas space target (subject → PMA-specific infant atlas target → adult Talairach atlas space), combining motion correction and atlas transformation in a single re-sampling step. Thus, the atlas transformation applied to each volume (frame) of infant fMRI was computed by transform composition: frame → individual fMRI data mean → PMA-specific target → adult target, producing 3 mm isotropic voxels. The resampled rs-fMRI data were intensity scaled to obtain a whole brain mode value of 1000 (one constant applied to all voxels and all volumes for each rs-fMRI run; Smyser et al. 2010). For each subject, registration of the structural and functional images was manually inspected to ensure accuracy of registration, including the cerebellum.
rs-fMRI preprocessing additionally included removal by regression of nuisance waveforms derived from retrospective motion correction, sampling regions in white matter and cerebrospinal fluid, and the global signal averaged over the whole brain. The data were low-pass filtered retaining frequencies below 0.08 Hz and spatially smoothed (6 mm full-width at half-maximum in each direction). Volumes corrupted by head motion (volume-to-volume head displacement ≥0.5 mm) were identified by analysis of the fully preprocessed data and subsequently excluded from functional connectivity measures (Power et al. 2012). A minimum of 5 min of low-motion rs-fMRI data was required for inclusion in the present analysis.
Adult rs-fMRI data were preprocessed comparably to the infant data except that the temporal high-pass limit was 0.1 Hz (as opposed to 0.08 Hz). Additional preprocessing details regarding the adult data are provided in Hacker et al. (2013).
Cerebellar, Cortical, and Subcortical Gray Matter ROIs
ROIs were defined in an infant brain template image in 711-2B atlas space, the representative adult template used at the Washington University Neuroimaging Laboratory (Smyser et al. 2010). This target is based on data from 12 normal individuals and conforms to the Talairach atlas (Talairach and Tournoux 1988) as defined by spatial normalization procedure (Lancaster et al. 1995). All supratentorial gray matter and the cerebellum of the template were divided into ROIs consisting of (6 mm)3 cubes (Fig. 1). The template was created by averaging T2-weighted images acquired in 66 healthy, term-born infants (42 females; GA at birth 39 ± 1, 37–41 weeks; PMA at scan 39 ± 1, 37–41 weeks) satisfying the current inclusion criteria. The T2-weighted images were individually segmented in a semi-automated fashion using Advanced Normalization Tools (ANTs) software (http://www.picsl.upenn.edu/ANTS) followed by manual correction using ITK-Snap software tools (http://www.itksnap.org/). The segmented images were averaged to create probabilistic and subsequently binarized masks of cortical and subcortical gray matter. The cerebellum was cubed as a whole because insufficient gray/white matter contrast resulting from immature myelination during the neonatal period precluded segmentation. ROIs with mean BOLD fMRI intensities outside the typical range for gray matter in either the FT or VPT groups due to individual differences in susceptibility inhomogeneity-related signal voids (or incomplete coverage) were excluded from further analyses. Thus, functional connectivity was assessed in a total of 1810 ROIs (1311 cortical ROIs, 201 subcortical ROIs, 298 cerebellar ROIs).
Figure 1.
Cortical and subcortical gray matter and cerebellar cubes used for seed correlation analysis of resting state-functional connectivity MRI data.
To enable comparisons between infants and adults in a common atlas space, the adult 711-2B space atlas-representative template was registered to the infant 711-2B space atlas-representative template using the linear image registration tool (FLIRT) in FSL (FMRIB’s Linear Image Registration Tool; Jenkinson and Smith 2001; Jenkinson et al. 2002). Owing to the relative under-development of the neonatal cerebellum, the infant-to-adult 711-2B space template registration was computed separately for the infra- and supratentorial compartments (compare Fig. 2 and S1). The ROIs defined in the infant 711-2B space template were then resampled in adult 711-2B space to enable accurate between group comparisons.
Figure 2.
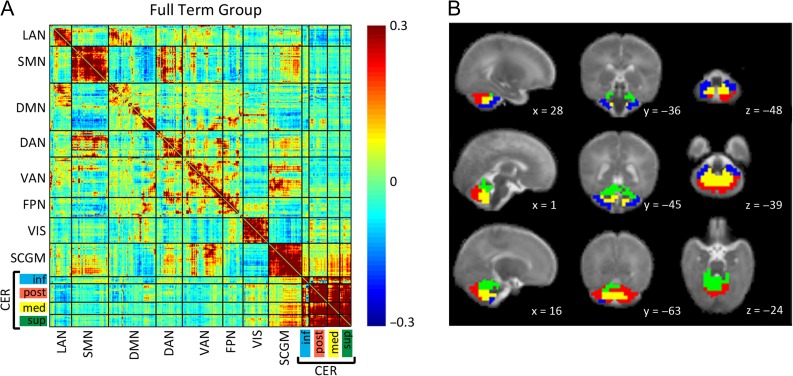
(A) Full-term infant group mean Fisher’s z-transformed correlation coefficient matrix depicting relationships between ROI pairs covering the entire brain. Warm colors indicate positive correlation coefficients, while cool colors represent negative correlation coefficients. The matrix is sorted into 7 cortical, 1 subcortical, and 4 cerebellar RSNs with hierarchical clustering applied for within network sorting of ROIs. Block structure along the diagonal corresponds to networks and black lines denote borders between networks. LAN = language network; SMN = somatomotor network; DMN = default mode network; DAN = dorsal attention network; VAN = ventral attention network; FPN = frontoparietal network; VIS = visual network; SCGM = subcortical gray matter; CER inf = inferior cerebellum; CER post = posterior lateral cerebellum; CER med = medial cerebellum; CER sup = superior cerebellum/vermis. (B) Localization of cerebellar networks identified by hierarchical clustering of cerebellar ROIs in the full-term infant group overlaid on an infant atlas brain in sagittal, coronal, and axial views. Planes are identified by atlas x, y, and z coordinates. Blue = inferior cerebellum; Red = posterior lateral cerebellum; Yellow = medial cerebellum; Green = superior cerebellum/vermis.
Functional Connectivity Analyses
BOLD rs-fMRI time series were extracted from all 1810 ROIs. Pearson correlations were then computed for all ROI pairs (excluding censored volumes). These results were then Fisher z-transformed and averaged over subjects to generate mean z(r) correlation matrices. Sorting strategies were applied to the ROIs in the correlation matrix: probability maps of 7 RSNs were used to sort cortical ROIs (Hacker et al. 2013) and subcortical ROIs were identified based on their anatomical location using the automated anatomical labeling (AAL) map from the UNC Infant 0-1-2 atlas (Shi et al. 2011). To identify networks in the cerebellum, hierarchical clustering using Matlab’s clustergram function (Bioinformatics Toolbox; 2015a; MathWorks, Natick, MA, USA) was applied to the correlation matrix of the cerebellar ROIs (Fig. 2; Table 2). This function was applied to FT, VPT, and adult groups separately with similar network topography evident in all groups. For subsequent analyses, the sorted results obtained from the FT group were used (see Supplementary Figs. S1–3 for adult and VPT data sorting results).
Table 2.
Number of ROIs per RSN
| RSN | ROI |
|---|---|
| Cortical | |
| Language (LAN) | 131 |
| Somatomotor (SMN) | 221 |
| Default mode (DMN) | 284 |
| Dorsal attention (DAN) | 157 |
| Ventral attention (VAN) | 244 |
| Frontoparietal control (FPN) | 120 |
| Visual (VIS) | 154 |
| Subcortical gray matter (SCGM) | 201 |
| Cerebellar | |
| Inferior | 40 |
| Posterior lateral | 111 |
| Medial | 77 |
| Superior/vermis | 70 |
Statistical Analyses
Across-group comparisons were conducted at the RSN level using the composite score strategy described in Brier et al. (2012). Thus, the z(r) values were aggregated over the matrix blocks illustrated in Figure 2 to obtain within-RSN (diagonal blocks) and across-RSN (off-diagonal blocks) composite scores. This strategy reduces the dimensionality of the data space, thereby increasing the power of statistical comparisons between groups. Statistical analyses were performed using SPSS version 23 (Chicago, IL, USA). First, localization and functional connectivity patterns of the cerebellar networks in the FT group were identified using descriptive statistics. Next, independent sample t-tests were used for FT to adult comparisons to assess the effects of later development. Finally, a multivariate analysis of covariance (MANCOVA), controlling for PMA at scan, and subsequent ANCOVAs were conducted for FT to VPT group comparisons to assess the effect of prematurity on early cerebellar RSN development. For these analyses, the Bonferroni multiple comparisons corrected threshold for a significance level of α = 0.05 was 0.005.
Results
Intrinsic Cerebellar Functional Connectivity in FT Group
Hierarchical clustering of the cerebellar ROIs in the mean correlation matrix for FT infants produced 4 bilateral symmetric networks with distinct intrinsic and cortico-cerebellar correlation patterns. The location of the ROIs assigned to each network revealed an anterior–posterior and lateral-medial segregation (Fig. 2). Network 1 (40 ROIs) was located in the inferior cerebellum with an anterior to lateral sorting of the ROIs. ROIs in the posterior lateral cerebellum were assigned to network 2 (111 ROIs). Network 3 (77 ROIs) included the medial cerebellar regions most likely covering the cerebellar nuclei. Network 4 (70 ROIs) combined regions of the superior cerebellar hemispheres and vermis.
Examination of correlation values between cerebellar networks showed that, overall, inferiorly located network 1 was positively correlated with posteriorly located network 2 (z(r) = 0.08); however, of note, the anterior portions of network 1 were negatively correlated with network 2 (Fig. 3). Network 1 was also positively correlated with medial network 3 (mean z(r) = 0.25), with lesser positive correlations with superiorly located network 4 (mean z(r) = 0.15). Posterior network 2 and medial network 3 were strongly positively correlated (mean z(r) = 0.36), with similar positive correlation with superior network 4 (mean z(r) = 0.32).
Figure 3.
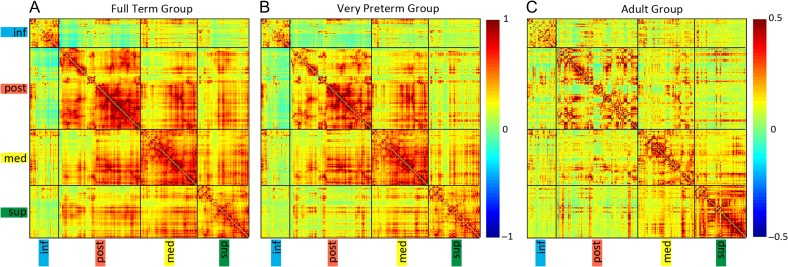
Group mean Fisher’s z-transformed correlation coefficient matrices representing all ROI pairs within the cerebellum sorted using results from the hierarchical clustering of full-term infant group. Black lines indicate borders between networks. (A) Full-term infants, (B) Very preterm infants, and (C) Adults. inf = inferior cerebellum; post = posterior lateral cerebellum; med = medial cerebellum; sup = superior cerebellum/vermis.
Cortico-Cerebellar Functional Connectivity
Cerebellar networks derived through hierarchical clustering yielded regionally specific cortico-cerebellar functional connectivity patterns. Figure 2 shows the Fisher’s z-transformed correlation matrix of the FT group encompassing cortical, subcortical, and cerebellar ROIs. A robust cortical RSN structure was identified similar to previous reports (Doria et al. 2010; Smyser et al. 2010, 2016). Cortico-cerebellar correlation magnitudes were lower compared with cortico-cortical correlation values, resembling findings obtained in studies of older subjects (Buckner et al. 2011; Wang et al. 2016). Network 1 in the inferior cerebellum was positively correlated with the cortical somatomotor (SMN) and dorsal attention (DAN) networks, and negatively correlated with the cortical DMN, language (LAN), and visual (VIS) networks (Fig. 4). In contrast, network 2 in the posterior cerebellum was positively correlated with the VIS and parts of the DMN. Further inspection of the ROIs in the DMN revealed that the medial prefrontal cortex (mPFC) showed the opposite correlation pattern for cerebellar networks 1 and 2. Weaker negative correlations were evident between the cortical RSNs and cerebellar networks 3 and 4 (medial and superior, respectively).
Figure 4.
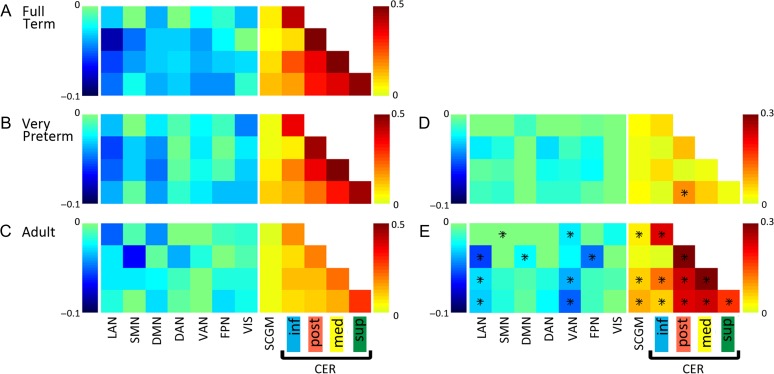
Group mean Fisher’s z-transformed correlation coefficient matrices demonstrating composite cortico-, subcortico-, and cerebello-cerebellar connectivity patterns for all ROI pairs grouped by RSN for (A) full-term infants, (B) very preterm infants, and (C) adults. Difference matrices are displayed in (D) for full-term minus very preterm groups and (E) full-term minus adult groups. Significant group differences after multiple comparisons correction are indicated by black stars. Only those rows which included cerebellar ROIs were selected from larger correlation coefficient matrices for display. Warm colors indicate positive correlation coefficients, while cool colors represent negative correlation coefficients.
FT Versus Adult Cerebellar Functional Connectivity
Similar topography of cerebellar networks was evident between the FT and adult group. However, intrinsic cerebellar functional connectivity was greater in the infant group. Correlation coefficient matrices corresponding to all ROIs within the cerebellum for both FT and adults are shown in Figure 3. Comparison of the cerebellar network composite correlation values between the 2 groups revealed lower correlation values in adults within and between all cerebellar networks (P ≤ 0. 001 for all comparisons), except between inferior and posterior networks (P = 0.59), and for all subcortical-cerebellar networks (P ≤ 0. 001 for all comparisons) except the posterior cerebellar network (P = 0.22; Fig. 4). In the adult group, the cortico-cerebellar network composite correlation matrix revealed the strongest positive correlations were between inferior cerebellar network 1 and the cortical DAN and strongest negative correlations were with the DMN, similar to the FT group (Supplementary Fig. S1). In contrast to infants, adults showed negative correlation between cerebellar network 1 and the SMN and reduced negative correlations between the cortical LAN and cerebellar networks 2, 3, and 4. Further, the cortical ventral attention network (VAN) and frontoparietal network (FPN) both revealed more developed correlation patterns with cerebellar networks in adults compared with the FT group.
FT Versus VPT Cerebellar Functional Connectivity
A similar topography of cerebellar networks was evident in VPT infants compared with the FT infants, with reduced magnitudes of positive and negative correlations within and between RSNs in the VPT group, similar to prior reports in these populations (Toulmin et al. 2015; Smyser et al. 2016). Cerebellar correlation coefficient matrices for the VPT group are shown in Figure 3. Cortico- and subcortico-cerebellar functional correlation patterns were also similar between groups, but again showed reduced correlation magnitudes in VPT subjects (Fig. 4 and Supplementary Fig. S2). Using network composite correlation values, the MANCOVA revealed differences between groups (P = 0.006). Separate consideration of the ANCOVA for each dependent variable revealed reduced positive correlations between the posterior and superior cerebellar networks in the VPT group (P = 0.005; Fig. 4).
Discussion
Summary of Findings
Using rs-fMRI, we identified multiple cerebellar functional networks with specific intrinsic and cortico- and subcortico-cerebellar connectivity patterns in healthy, term-born neonates. Intrinsic cerebellar networks demonstrated strong positive interhemispheric correlation within networks located in the inferior, posterior, medial, and superior regions. Cortico-cerebellar correlation magnitudes were low in comparison to intra-cerebellar and cortico-cortical values, as in previous reports (Buckner et al. 2011; Bernard et al. 2012; Wang et al. 2016). Overall, negative correlations were evident along the anterior–posterior axis for networks within the cerebellum. Positive correlations were revealed between subcortical and cerebellar networks, and distinct cortico-cerebellar correlation behavior was identified for networks in the inferior and posterior, medial, and superior cerebellum: cortical SMN and DAN were positively correlated with the former and negatively correlated with the 3 remaining cerebellar networks. A similar network structure was found in adults, although with reduced cerebellar intrinsic and subcortico-cerebellar correlation magnitudes. The generally greater intrinsic cerebellar functional connectivity in infants as compared with adults may reflect systematic differences in the magnitude of BOLD signal fluctuations (Supplementary Fig. S4). Further, cortico-cerebellar correlation patterns in adults were different from infants for the cortical SMN and inferior cerebellar network and for correlations between cerebellar networks and the cortical LAN, FPN, and VAN, suggesting a more specialized functional correlation pattern. Similar to prior reports, a comparable network architecture with overall reduced correlation magnitudes was identified in the preterm infants, with regionally specific differences in intra-cerebellar correlations.
Relation to Previous rs-fMRI Studies of the Cerebellum
Recent rs-fMRI studies in adults and older children using diverse methodologies have revealed converging evidence regarding the parcellation of the cerebellum into homotopic functional networks along the anterior–posterior axis (Habas et al. 2009; O’Reilly et al. 2010; Buckner et al. 2011; Bernard et al. 2012, 2016; Khan et al. 2015; Wang et al. 2016). Within these intrinsic cerebellar networks, spatially adjacent regions in the cerebellar hemispheres and vermis, as well as homotopic counterparts, are positively correlated. Here, most anterior and posterior regions were weakly correlated with one exception: the inferiorly located cerebellar lobule IX showed strong positive correlations with the posteriorly located Crus I and Crus II, and both regions showed positive correlations with the DMN. Across studies employing seed correlation analysis, the number of identified cerebellar networks has depended on the number of networks included and the cortical parcellation (O’Reilly et al. 2010; Buckner et al. 2011; Khan et al. 2015; Bernard et al. 2016), with the resulting functional topography confirmed by studies using more data-driven approaches (Habas et al. 2009; Bernard et al. 2012; Wang et al. 2016). Importantly, these intrinsic cerebellar RSNs demonstrate distinct, regionally specific correlation patterns with cortical RSNs, with inferior and superior anterior regions positively correlated with the sensorimotor cortex and posterior regions generally positively correlated with association cortices, including the inferior parietal cortex and mPFC.
Overall, our results demonstrate a similar functional organization of the cerebellum during the neonatal period with the exception of correlation patterns with the cortical SMN. Previous studies in adults describe the cerebellar sensorimotor network as located in lobules I–V (anterior superior cerebellum) and VIII (inferior cerebellum) (O’Reilly et al. 2010; Buckner et al. 2011; Bernard et al. 2012). During middle childhood, however, the correlation pattern between cerebellar and cortical motor areas appears to be more regionally specific: the inferior cerebellum (lobule VIII) shows stronger associations with medial and superior motor network regions, while stronger correlation with lateral somatomotor cortical regions are evident for the anterior superior cerebellum (lobules I–V) (Khan et al. 2015; Wang et al. 2016). Our study similarly revealed the inferior cerebellum to be positively correlated with the somatomotor cortex in neonates, though we did not find evidence for a superior cerebellar motor representation, despite the somatomotor RSN being well-established (Fransson et al. 2007; Doria et al. 2010; Smyser et al. 2010). In our adult group, positive correlations between the cortical SMN and the superior and inferior cerebellar networks were evident. Prior results indicate that the SMN matures throughout the first years of life (Lin et al. 2008), paralleling motor development (Onis 2007). This finding suggests that this may include establishment of additional somatomotor cerebellar representations.
In the adult literature, lateral posterior cerebellar regions (Crus I/II) are positively correlated with the DMN (mPFC, precuneus/posterior cingulate, angular gyrus), while more medial posteriorly located cerebellar regions (lobules VII and VIII) show associations with the executive control or SAL networks (dorsomedial and dorsolateral prefrontal cortex, superior parietal cortex) (Habas et al. 2009; Bernard et al. 2012; Khan et al. 2015; Wang et al. 2016). In accordance with these results, we found positive associations between the posterior cerebellar network and the DMN in adults, but negative correlation in the composite correlation network analysis in infants. Further inspection revealed that ROIs covering the mPFC in the DMN are positively correlated with the posterior cerebellar network in our FT group. This may reflect the immature status of DMN, with anterior and posterior regions not yet fully functionally integrated (Fransson et al. 2007; Smyser Snyder and Neil 2011; De Asis-Cruz et al. 2015). Previous studies in adults reported cerebellar lobule IX to be correlated with the DMN, but this finding was absent in our neonatal data (Habas et al. 2009; Lu et al. 2011; Bernard et al. 2012; Wang et al. 2016).
Finally, prior cerebellar functional connectivity studies showed vermal regions to be largely indistinguishable from lateral regions (O’Reilly et al. 2010; Buckner et al. 2011; Bernard et al. 2012), with specificity only between the posterior vermis (vermian lobules VI and VIIa/b) and limbic regions, thalamus and insular cortex (Habas et al. 2009; Bernard et al. 2012). Our data demonstrated strong positive associations between all cerebellar and subcortical gray matter networks.
Of note, many previous studies in older pediatric populations and adults did not consider patterns of negative correlation for both intrinsic and cortico-cerebellar networks. For example, negative cortico-cerebellar correlations were found between the somatosensory cortices and posterior cerebellum and between combined supramodal cortical (i.e., prefrontal, parietal, and temporal) and anterior cerebellar regions (Khan et al. 2015), patterns also evident in our neonatal results. These findings appear to extend supratentorial relationships between RSNs (both positive and negative) to the cerebellum. Though investigation of negative correlations has been much debated, multiple studies support the view that these relationships have a biological origin and provide valuable information in the interpretation of cortical networks across age groups and clinical populations (Fox et al. 2005, 2009; Fox and Raichle 2007; Chai et al. 2012; Smyser et al. 2016; Murphy and Fox 2017). Accordingly, we suggest consideration of these patterns is warranted when interpreting functional connectivity results both within the cerebellum and between the cerebellum and cerebral cortex.
Developmental Implications
Increasing evidence points to reciprocal involvement of the cerebellum in maturation of cortical areas and establishment and modification of cortical functional networks (Limperopoulos et al. 2005a, 2005b; Ito 2008; Steinlin 2008; Bolduc et al. 2012; Stoodley 2012, 2015; Wang et al. 2014). Structural maturation of the cerebellum commences in the embryonic period, with neuronal migration and differentiation resulting in an established basic morphology by around 20 weeks gestation (Rakic and Sidman 1970; Volpe 2009; Leto et al. 2015). The subsequent period up to 40 weeks gestation is characterized by major increases in volume and foliation, with neuronal differentiation ongoing throughout the first postnatal year (Volpe 2009). Afferent connections between the cortex and spinal cord with the cerebellum via the middle and inferior cerebellar peduncles, as well as efferent connections to the brainstem and cortex via the superior cerebellar peduncles, establish cortico-cerebellar circuits (Volpe 2009). Clinical studies have shown the consequences of cerebellar impairments and compensation of such depend on the time and localization of injury, suggesting that cerebellar development occurs in a regionally specific manner that may be subject to sensitive periods (Wang et al. 2014). Considering the early structural maturation of the cerebellum compared with the cortex and evidence for the cerebellum’s role in learning, it has been argued that the cerebellum might shape and refine the eventual organization of cortical brain structure and function through cerebro-cerebellar loops, influencing processes such as pruning and white matter growth (Steinlin 2007; Wang et al. 2014; Stoodley and Limperopoulos 2016).
Increasing understanding of regional differentiation of the cerebellum derives from investigations of focal cerebellar lesions: while motor dysfunction is associated with lesions in the anterior cerebellum, vermal lesions have been associated with social-emotional behavioral abnormalities; damage to posterior regions results in language delay and deficits in verbal and visual-spatial reasoning, working memory, decision making, and planning (Steinlin 2008; Wang et al. 2014; Brossard-Racine et al. 2015; Stoodley and Limperopoulos 2016). Our results reflect this regional specialization through the specific cortico-cerebellar correlation patterns of individual networks, suggesting these processes begin early in life. For instance, inferior cerebellar networks showed strong positive correlation with the motor cortices, but not the DMN. Differences in our infant results as compared with those in the adult literature (e.g., the lack of an additional representation of the motor network in the superior cerebellum) indicate this functional differentiation continues beyond the neonatal period, potentially at different rates across cerebellar regions. Thus, disruptions of early maturation consequent to acquired cerebellar injury, malformations and/or premature birth may have long-lasting effects on neurodevelopmental outcomes.
Impact of Prematurity on Cerebellar Functional Connectivity
Previous studies of functional connectivity in preterm-born infants have shown preserved but immature topography of RSNs with reduced correlation magnitude, predominantly in higher-order RSNs (Fransson et al. 2007; Smyser et al. 2010, 2016; Toulmin et al. 2015). These inquiries have included only limited investigations of cerebellar functional connectivity. In agreement with these past reports, we demonstrate similar topography for intra- and cortico-cerebellar connectivity between FT and VPT infants, with overall reduced positive and negative correlation magnitude in the VPT group. This result may relate to suppressed intrinsic BOLD signal fluctuations as a consequence of prematurity (Supplementary Fig. S4; see also Smyser et al. 2016). Differences between these groups were modulated in a RSN-dependent manner, with more prominent differences between networks incorporating higher-order association than established primary sensorimotor cortices. For example, similar positive correlation patterns were found between the inferior cerebellum and SMN. Weaker correlations between DAN, FPN, and VAN and cerebellar networks indicate less developed networks in the VPT group. These findings support and extend prior reports on the deleterious effects of premature birth on RSN development and suggest these effects are brain-wide.
The last trimester of pregnancy is a critical period for cerebellar development. Hence, premature birth poses a significant risk for cerebellar injury and impaired development (Limperopoulos et al. 2007; Volpe 2009). Preterm-born infants show considerably higher rates of neurodevelopmental disability and psychopathology (Marlow et al. 2009; Volpe 2009; Woodward et al. 2009; Dean et al. 2014; Brossard-Racine et al. 2015; Glass et al. 2015; Stoodley and Limperopoulos 2016). Most prior studies have been limited to assessment of cerebellar structure visible on ultrasound or MRI during the neonatal period. However, premature birth could result in additional deleterious effects on development of the cerebellum during this early sensitive period not evident on ultrasound or structural MRI. Our findings suggest differences in intrinsic and cortico-cerebellar functional connectivity may also play an important role in mediating disability in the VPT population.
Caveats and Limitations
Our rigorous data quality criteria resulted in a sample of 57 term- and 20 preterm-born infants, a sample size comparable to prior neonatal investigations. However, rs-fMRI investigations of the cerebellum pose unique challenges. The cerebellum’s anatomical location adjacent to the vascular and ventricular systems makes this area susceptible to physiological artifacts and magnetization inhomogeneities. Further, normalization and atlas registration algorithms are often not optimized for the cerebellum, potentially resulting in suboptimal image registration. To account for these difficulties, we employed qualitative and quantitative procedures to verify the accuracy of cerebellar registration and the location of ROIs. However, these procedures may not capture subtle differences across individual subjects or between groups. In addition, the superior cerebellum is anatomically close to visual cortices and subcortical gray matter structures. Accordingly, our cubing approach excluded ROIs spanning the tentorium. As detailed, the ROIs used in the comparisons of infant and adult data were generated using infant data. Generation of a comparable set of regions using adult data may differ, particularly in the cerebellum where myelination in adults would allow differentiation of gray and white matter. As such, for an analysis with a primary focus on adult cerebellar functional connectivity, use of different segmenting and cubing procedures would need to be considered. However, because the primary focus of our investigation was on infant cerebellar functional connectivity, we elected to use methods optimized to study infant data in our analyses of adult data in order to improve comparability.
We excluded infants with parenchymal and cerebellar injury on structural imaging in order to focus on the primary effect of prematurity on cerebellar functional connectivity. However, our cohort of VPT infants demonstrated medical comorbidities common in this clinical population (as detailed Table 1). While no relationships between these variables and key results were identified in the VPT subjects, their effects may be incompletely assessed using the current analysis approaches.
Finally, differences in technical factors (e.g., head coils, acquisition parameters) exist between data for infants and adults. As detailed, we have undertaken several measures designed to ensure that reported differences maximally reflect the basic phenomenology of the findings between these populations rather than being driven by methodological differences. However, it is impossible to completely eliminate all technical factors that potentially could contribute to observed adult versus infant functional connectivity differences. Further, the impact of the fact that infants must be scanned while asleep on rs-fMRI measures has yet to be carefully explored. Therefore, these differences must also be taken into account in interpretation of adult versus infant comparisons.
Conclusions
The definition of the functional organization of the neonatal cerebellum with its intrinsic and cortico-cerebellar associations adds to the growing body of research on the cerebellum’s role in the RSN architecture and development. Our results reveal that cerebellar regions with distinct functional correlations to cortical and subcortical networks are already established in the neonatal period. Further, we find an overall similar functional topography of the cerebellum in healthy, term-born infants and adults. However, adults showed reduced positive correlations for cerebellar intrinsic and subcortico-cerebellar regions and greater differentiation in cortico-cerebellar associations, suggesting the existence of maturational effects. Preterm-born infants showed reduced correlation magnitude of cerebellar intrinsic and cortico-cerebellar regions in agreement with other studies. Further investigation of these functional networks within the cerebellum and their cortico-cerebellar associations is needed to better define the role of the cerebellum in neurobehavioral development.
Supplementary Material
Notes
The authors thank Joseph J. Ackerman, Jr. and Karen Lukas for assistance with data collection, Tara A. Smyser for assistance with data analysis, and Alison G. Cahill and Amit M. Mathur for providing data. The authors declare no competing financial interests. Conflict of Interest: None declared.
Abbreviations
- AAL
automated anatomical labeling
- ANCOVA
analysis of covariance
- ANTs
Advanced Normalization Tools
- BOLD
blood oxygen level dependent
- DAN
dorsal attention network
- DMN
default mode network
- FLIRT
FMRIB linear image registration tool
- FPN
frontoparietal network
- FT
full term
- LAN
language network
- MANCOVA
multivariate analysis of covariance
- mPFC
medial prefrontal cortex
- NICU
Neonatal Intensive Care Unit
- PMA
postmenstrual age
- ROI
region of interest
- rs-fMRI
resting state functional magnetic resonance imaging
- RSN
resting state network
- SAL
salience network
- SMN
somatomotor network
- VAN
ventral attention network
- VIS
visual network
- VPT
very preterm
Funding
This work was supported by the National Institutes of Health (grant numbers K02 NS089852 to C.D.S., UL1 TR000448 to C.D.S. and C.E.R., K23 MH105179 to C.E.R., 1 P30 NS098577, R01 HD061619, U54 HD087011, and R01 HD057098); Child Neurology Foundation (to C.D.S.); Cerebral Palsy International Research Foundation (to C.D.S.); and The Dana Foundation [to C.D.S.]. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
References
- Allin MPG, Salaria S, Nosarti C, Wyatt J, Rifkin L, Murray RM. 2005. Vermis and lateral lobes of the cerebellum in adolescents born very preterm. Neuroreport. 16:1821–1824. [DOI] [PubMed] [Google Scholar]
- Bernard JA, Orr JM, Mittal VA. 2016. Differential motor and prefrontal cerebello-cortical network development: evidence from multimodal neuroimaging. Neuroimage. 124:591–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard JA, Seidler RD, Hassevoort KM, Benson BL, Welsh RC, Wiggins JL, Jaeggi SM, Buschkuehl M, Monk CS, Jonides J, et al. . 2012. Resting state cortico-cerebellar functional connectivity networks: a comparison of anatomical and self-organizing map approaches. Front Neuroanat. 6:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolduc ME, du Plessis AJ, Sullivan N, Guizard N, Zhang X, Robertson RL, Limperopoulos C. 2012. Regional cerebellar volumes predict functional outcome in children with cerebellar malformations. Cerebellum. 11:531–542. [DOI] [PubMed] [Google Scholar]
- Brier MR, Thomas JB, Snyder AZ, Benzinger TL, Zhang D, Raichle ME, Holtzman DM, Morris JC, Ances BM. 2012. Loss of intranetwork and internetwork resting state functional connections with Alzheimer’s disease progression. J Neurosci. 32:8890–8899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brossard-Racine M, du Plessis AJ, Limperopoulos C. 2015. Developmental cerebellar cognitive affective syndrome in ex-preterm survivors following cerebellar injury. Cerebellum. 14:151–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL. 2013. The cerebellum and cognitive function: 25 years of insight from anatomy and neuroimaging. Neuron. 80:807–815. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Krienen FM, Castellanos A, Diaz JC, Yeo BTT. 2011. The organization of the human cerebellum estimated by intrinsic functional connectivity. J Neurophysiol. 106:2322–2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellanos FX, Lee PP, Sharp W, Jeffries NO, Greenstein DK, Clasen LS, Blumenthal JD, James RS, Ebens CL, Walter JM, et al. . 2002. Developmental trajectories of brain volume abnormalities in children and adolescents with attention-deficit/hyperactivity disorder. JAMA. 288:1740–1748. [DOI] [PubMed] [Google Scholar]
- Chai XJ, Castañón AN, Ongür D, Whitfield-Gabrieli S. 2012. Anticorrelations in resting state networks without global signal regression. Neuroimage. 59:1420–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Asis-Cruz J, Bouyssi-Kobar M, Evangelou I, Vezina G, Limperopoulos C. 2015. Functional properties of resting state networks in healthy full-term newborns. Sci Rep. 5:17755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean JM, Bennet L, Back SA, McClendon E, Riddle A, Gunn AJ. 2014. What brakes the preterm brain? An arresting story. Pediatr Res. 75:227–233. [DOI] [PubMed] [Google Scholar]
- Doria V, Beckmann CF, Arichi T, Merchant N, Groppo M, Turkheimer FE, Counsell SJ, Murgasova M, Aljabar P, Nunes RG, et al. . 2010. Emergence of resting state networks in the preterm human brain. Proc Natl Acad Sci USA. 107:20015–20020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Raichle ME. 2007. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 8:700–711. [DOI] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. 2005. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci USA. 102:9673–9678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Zhang D, Snyder AZ, Raichle ME. 2009. The global signal and observed anticorrelated resting state brain networks. J Neurophysiol. 101:3270–3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fransson P, Skiöld B, Horsch S, Nordell A, Blennow M, Lagercrantz H, Aden U. 2007. Resting-state networks in the infant brain. Proc Natl Acad Sci USA. 104:15531–15536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gholipour A, Kehtarnavaz N, Gopinath K, Briggs R, Panahi I. 2008. Average field map image template for echo-planar image analysis. Conf Proc IEEE Eng Med Biol Soc. 2008:94–97. [DOI] [PubMed] [Google Scholar]
- Glass HC, Costarino AT, Stayer SA, Brett CM, Cladis F, Davis PJ. 2015. Outcomes for extremely premature infants. Anesth Analg. 120:1337–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habas C, Kamdar N, Nguyen D, Prater K, Beckmann CF, Menon V, Greicius MD. 2009. Distinct cerebellar contributions to intrinsic connectivity networks. J Neurosci. 29:8586–8594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hacker CD, Laumann TO, Szrama NP, Baldassarre A, Snyder AZ, Leuthardt EC, Corbetta M. 2013. Resting state network estimation in individual subjects. Neuroimage. 82:616–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito M. 2008. Control of mental activities by internal models in the cerebellum. Nat Rev Neurosci. 9:304–313. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. 2002. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 17:825–841. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Beckmann CF, Behrens TEJ, Woolrich MW, Smith SM. 2012. FSL. Neuroimage. 62:782–790. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Smith S. 2001. A global optimisation method for robust affine registration of brain images. Med Image Anal. 5:143–156. [DOI] [PubMed] [Google Scholar]
- Khan AJ, Nair A, Keown CL, Datko MC, Lincoln AJ, Müller RA. 2015. Cerebro-cerebellar resting-state functional connectivity in children and adolescents with autism spectrum disorder. Biol Psychiatry. 78:625–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidokoro H, Neil JJ, Inder TE. 2013. New MR imaging assessment tool to define brain abnormalities in very preterm infants at term. Am J Neuroradiol. 34:2208–2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster JL, Glass TG, Lankipalli BR, Downs H, Mayberg H, Fox PT. 1995. A modality-independent approach to spatial normalization of tomographic images of the human brain. Hum Brain Mapp. 3:209–223. [Google Scholar]
- Leto K, Arancillo M, Becker EBE, Buffo A, Chiang C, Ding B, Dobyns WB, Dusart I, Haldipur P, Hatten ME, et al. . 2015. Consensus paper: cerebellar development. Cerebellum. 1–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limperopoulos C, Bassan H, Gauvreau K, Robertson RL, Sullivan NR, Benson CB, Avery L, Stewart J, Soul JS, Ringer SA, et al. . 2007. Does cerebellar injury in premature infants contribute to the high prevalence of long-term cognitive, learning, and behavioral disability in survivors? Pediatrics. 120:584–593. [DOI] [PubMed] [Google Scholar]
- Limperopoulos C, Chilingaryan G, Guizard N, Robertson RL, Du Plessis AJ. 2010. Cerebellar injury in the premature infant is associated with impaired growth of specific cerebral regions. Pediatr Res. 68:145–150. [DOI] [PubMed] [Google Scholar]
- Limperopoulos C, Chilingaryan G, Sullivan N, Guizard N, Robertson RL, du Plessis AJ. 2014. Injury to the premature cerebellum: outcome is related to remote cortical development. Cereb Cortex. 24:728–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limperopoulos C, Soul JS, Gauvreau K, Huppi PS, Warfield SK, Bassan H, Robertson RL, Volpe JJ, du Plessis AJ. 2005. a. Late gestation cerebellar growth is rapid and impeded by premature birth. Pediatrics. 115:688–695. [DOI] [PubMed] [Google Scholar]
- Limperopoulos C, Soul JS, Haidar H, Huppi PS, Bassan H, Warfield SK, Robertson RL, Moore M, Akins P, Volpe JJ, et al. . 2005. b. Impaired trophic interactions between the cerebellum and the cerebrum among preterm infants. Pediatrics. 116:844–850. [DOI] [PubMed] [Google Scholar]
- Lin W, Zhu Q, Gao W, Chen Y, Toh CH, Styner M, Gerig G, Smith JK, Biswal B, Gilmore JH. 2008. Functional connectivity MR imaging reveals cortical functional connectivity in the developing brain. AJNR Am J Neuroradiol. 29:1883–1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, Liu H, Zhang M, Wang D, Cao Y, Ma Q, Rong D, Wang X, Buckner RL, Li K. 2011. Focal pontine lesions provide evidence that intrinsic functional connectivity reflects polysynaptic anatomical pathways. J Neurosci. 31:15065–15071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marlow N, Wolke D, Bracewell MA, Samara M, EPICure Study Group . 2009. Neurologic and developmental disability at six years of age after extremely preterm birth. N Engl J Med. 352:9–19. [DOI] [PubMed] [Google Scholar]
- Mathur AM, Neil JJ, McKinstry RC, Inder TE. 2008. Transport, monitoring, and successful brain MR imaging in unsedated neonates. Pediatr Radiol. 38:260–264. [DOI] [PubMed] [Google Scholar]
- Messerschmidt A, Fuiko R, Prayer D, Brugger PC, Boltshauser E, Zoder G, Sterniste W, Weber M, Birnbacher R. 2008. Disrupted cerebellar development in preterm infants is associated with impaired neurodevelopmental outcome. Eur J Pediatr. 167:1141–1147. [DOI] [PubMed] [Google Scholar]
- Murphy K, Fox MD. 2017. Towards a consensus regarding global signal regression for resting state functional connectivity MRI. Neuroimage. 154:169–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolson RI, Fawcett AJ. 2011. Dyslexia, dysgraphia, procedural learning, and the cerebellum. Cortex. 47:117–127. [DOI] [PubMed] [Google Scholar]
- Onis M. 2007. WHO motor development study: windows of achievement for six gross motor development milestones. Acta Paediatr. 95:86–95. [DOI] [PubMed] [Google Scholar]
- O’Reilly JX, Beckmann CF, Tomassini V, Ramnani N, Johansen-Berg H. 2010. Distinct and overlapping functional zones in the cerebellum defined by resting state functional connectivity. Cereb Cortex. 20:953–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poretti A, Boltshauser E, Huisman TA. 2016. Prenatal cerebellar disruptions: neuroimaging spectrum of findings in correlation with likely mechanisms and etiologies of injury. Neuroimaging Clin N Am. 26:359–372. [DOI] [PubMed] [Google Scholar]
- Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. 2012. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 59:2142–2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakic P, Sidman RL. 1970. Histogenesis of cortical layers in human cerebellum, particularly the lamina dissecans. J Comp Neurol. 139:473–500. [DOI] [PubMed] [Google Scholar]
- Shi F, Yap PT, Wu G, Jia H, Gilmore JH, Lin W, Shen D. 2011. Infant brain atlases from neonates to 1- and 2-year-olds. PLoS ONE. 6:e18746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyser CD, Inder TE, Shimony JS, Hill JE, Degnan AJ, Snyder AZ, Neil JJ. 2010. Longitudinal analysis of neural network development in preterm infants. Cereb Cortex. 20:2852–2862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyser CD, Neil JJ. 2015. Use of resting-state functional MRI to study brain development and injury in neonates. Semin Perinatol. 39:130–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyser CD, Snyder AZ, Neil JJ. 2011. Functional connectivity MRI in infants: exploration of the functional organization of the developing brain. Neuroimage. 56:1437–1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyser CD, Snyder AZ, Shimony JS, Mitra A, Inder TE, Neil JJ. 2016. Resting-state network complexity and magnitude are reduced in prematurely born infants. Cereb Cortex. 26:322–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinlin M. 2007. The cerebellum in cognitive processes: supporting studies in children. Cerebellum. 6:237–241. [DOI] [PubMed] [Google Scholar]
- Steinlin M. 2008. Cerebellar disorders in childhood: cognitive problems. Cerebellum. 7:607–610. [DOI] [PubMed] [Google Scholar]
- Stoodley CJ. 2012. The cerebellum and cognition: evidence from functional imaging studies. Cerebellum. 11:352–365. [DOI] [PubMed] [Google Scholar]
- Stoodley CJ. 2015. The cerebellum and neurodevelopmental disorders. Cerebellum. 15:34–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoodley CJ, Limperopoulos C. 2016. Structure-function relationships in the developing cerebellum: evidence from early-life cerebellar injury and neurodevelopmental disorders. Semin Fetal Neonatal Med. 21:356–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoodley CJ, Schmahmann JD. 2009. Functional topography in the human cerebellum: a meta-analysis of neuroimaging studies. Neuroimage. 44:489–501. [DOI] [PubMed] [Google Scholar]
- Stoodley CJ, Stein JF. 2013. Cerebellar function in developmental dyslexia. Cerebellum. 12:267–276. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. 1988. Co-planar seterotaxic atlas of the human brain: 3-D proportional system: an approach to cerebral imaging In: Thieme Medical. New York. [Google Scholar]
- Toulmin H, Beckmann CF, O’Muircheartaigh J, Ball G, Nongena P, Makropoulos A, Ederies A, Counsell SJ, Kennea N, Arichi T, et al. . 2015. Specialization and integration of functional thalamocortical connectivity in the human infant. Proc Natl Acad Sci USA. 112:6485–6490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpe JJ. 2009. Cerebellum of the premature infant: rapidly developing, vulnerable, clinically important. J Child Neurol. 24:1085–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Kipping J, Bao C, Ji H, Qiu A. 2016. Cerebellar functional parcellation using sparse dictionary learning clustering. Front Neurosci. 10:188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang SSH, Kloth AD, Badura A. 2014. The cerebellum, sensitive periods, and autism. Neuron. 83:518–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward LJ, Moor S, Hood KM, Champion PR, Foster-Cohen S, Inder TE, Austin NC. 2009. Very preterm children show impairments across multiple neurodevelopmental domains by age 4 years. Arch Dis Child Fetal Neonatal Ed. 94:F339–F344. [DOI] [PubMed] [Google Scholar]
- Yeo BTT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M, Roffman JL, Smoller JW, Zöllei L, Polimeni JR, et al. . 2011. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol. 106:1125–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.