Abstract
Several human imaging studies have suggested that anterior cingulate cortex (ACC) is highly active when participants receive competing inputs, and that these signals may be important for influencing the downstream planning of actions. Despite increasing evidence from several neuroimaging studies, no study has examined ACC activity at the level of the single neuron in rodents performing similar tasks. To fill this gap, we recorded from single neurons in ACC while rats performed a stop-change task. We found higher firing on trials with competing inputs (STOP trials), and that firing rates were positively correlated with accuracy and movement speed, suggesting that when ACC was engaged, rats tended to slow down and perform better. Finally, firing was the strongest when STOP trials were preceded by GO trials and was reduced when rats adapted their behavior on trials subsequent to a STOP trial. These data provide the first evidence that activity of single neurons in ACC is elevated when 2 responses are in competition with each other when there is a need to change the course of action to obtain reward.
Keywords: anterior cingulate, conflict adaptation, inhibition, prefrontal, single neuron, stop signal
Introduction
There is a wealth of neuroimaging data implicating anterior cingulate cortex (ACC) in cognitive control. In particular, many studies report that neural activity in ACC is high when there is competition between 2 actions, known as “response conflict.” Some of the first evidence implicating ACC function in this process came from positron emission tomography (PET) studies on participants performing a Stroop task. In the Stroop task, participants are instructed to report the ink color of a written word rather than reading the word itself (Pardo et al. 1990). In this study, and in the many others that followed, when the ink color and the written word were incongruent, or in competition with each other (“red” written in blue ink), neural signals in ACC were high. Many studies have since replicated this basic effect using functional magnetic resonance imaging in participants performing a variety of tasks (Carter et al. 1998, 2000; Brown et al. 1999; Botvinick et al. 2001; Brown and Braver 2005; Curtis et al. 2005; Laird et al. 2005; Nee et al. 2007, 2011; Cole et al. 2009).
Thus, it is clear from the imaging literature that ACC contributes to the processing of response conflict when there is competition between 2 responses. Unfortunately, support for this hypothesis at the single neuron level is still missing; the majority of single-unit recording studies report the absence of response conflict-like signals in ACC (Ito et al. 2003; Nakamura et al. 2005; Emeric et al. 2008; Bryden et al. 2011; Hayden et al. 2011; Cai and Padoa-Schioppa 2012; Ebitz and Platt 2015), with the exception that correlates related to conflict have been observed in single ACC neurons in human patients (Davis et al. 2005; Sheth et al. 2012). To the best of our knowledge, no single neuron recording paper in non-human animals has supported the notion that ACC outputs a response conflict signal.
With the overwhelming interest in ACC’s role in cognitive control and its disruption in numerous disease states (Downar et al. 2015), it is surprising that no study has examined ACC activity at the level of the single neuron during periods of increased competition or conflict. Rat ACC sits in a prime position to mediate cognitive control via its monosynaptic projections to dorsal striatum (Gabbott et al. 2005; Mailly et al. 2013), subthalamic nucleus (Maurice et al. 1998), prefrontal cortex (Hoover and Vertes 2007), and locus coeruleus (Hoover and Vertes 2007). Furthermore, ACC interference impacts performance on 5-choice serial reaction time and stop-signal tasks (Bari et al. 2011) and impairs the ability of rats to make adjustments in cognitive control (Newman et al. 2015). In addition, a PET study in rats showed that activated regions in rats during conflict processing are similar to those described in human imaging (Marx et al. 2012). Currently, it is unknown if the activity of single neurons in rat ACC is modulated when 2 actions are in competition during response conflict.
To address this issue we recorded from single neurons in ACC while rats performed a stop-change task. We found that ACC activity was high when 2 responses were in competition with each other and that activity was positively correlated with performance and movement time. Further, firing in ACC was modulated by greater degrees of competition induced by trial sequence effects; such that firing was the strongest when STOP trials were preceded by GO trials, and was reduced when the rats’ behavior was slowed on trials following STOP trials resulting in enhanced accuracy (commonly referred to as conflict adaptation). This work provides evidence at the single neuron level that ACC in non-human animals can carry response conflict-like signals and opens the door to a number of studies that will allow us to elucidate how changes in actions occur during the processing of competing signals and how changes in ACC firing fosters cognitive control via downstream mechanisms.
Materials and Methods
Subjects
Nine male Long-Evans rats were obtained at 175–200 g from Charles River Labs, and were on average 452 g ± 30 g at the time of surgery. Rats were tested at the University of Maryland in accordance with NIH and IACUC guidelines. One rat did not yield sufficient neural activity as we were unable to isolate any cells due to electrode malfunction; this rat was excluded from all analyses.
Surgical Procedures and Histology
Surgical procedures followed guidelines for aseptic technique. Electrodes were manufactured and implanted as in prior recording experiments (Bryden et al. 2011, 2012; Bryden and Roesch 2015). Rats had a drivable bundle of ten 25 μm diameter FeNiCr wires (Stablohm 675, California Fine Wire, Grover Beach, CA) chronically implanted in the left or right hemisphere dorsal to anterior cingulate cortex (n = 9 rats; 0.2 mm anterior to bregma, 0.5 mm left [n = 4] or right [n = 5] of the midline, and 1 mm ventral to the brain surface). This location was chosen because previous reports have shown that firing here is modulated by errors, response preparation, and attention during learning (Totah et al. 2009; Bryden et al. 2011). Immediately prior to implantation, wires were freshly cut with surgical scissors to extend ~1 mm beyond the cannula and electroplated with platinum (H2PtCl6, Aldrich, Milwaukee, WI) to an impedance of ~300 kOhms. Cephalexin (15 mg/kg p.o.) was administered twice daily for 2 weeks post-operatively to prevent infection.
Behavioral Task
Recording was conducted in aluminum chambers approximately 18″ on each side with downward sloping walls narrowing to an area of 12″ × 12″ at the bottom. On one wall, a central port was located above 2 adjacent fluid wells. Directional lights were located next to the fluid wells. House lights were located above the panel. Task control was implemented via computer. Port entry, licking, and well entry times were monitored by disruption of photobeams.
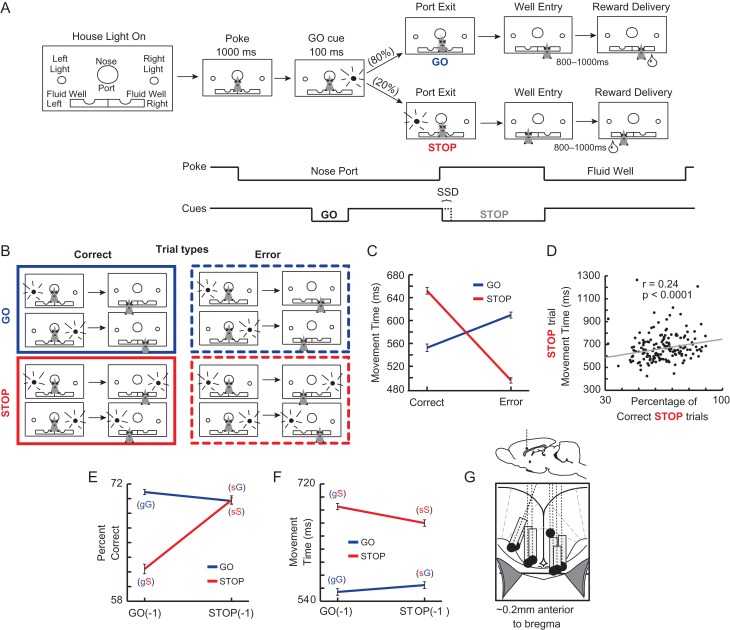
The basic trial design is illustrated in Figure 1A. Each trial began by illumination of house lights that instructed the rat to nose poke into the central port. Nose poking initiated a 1000 ms pre-cue delay period. At the end of this delay, a directional light to the animal’s left (or right) was flashed for 100 ms. The trial was aborted and house lights were extinguished if the rat exited the port at any time prior to offset of the directional cue light. On 80% of trials, presentation of the left (or right) light signaled the direction in which the animal could respond in order to obtain sucrose reward in the corresponding fluid well below. On 20% of trials, after a variable delay between 0 and 100 ms (selected with replacement from a uniform distribution), the light opposite to the location of the originally cued direction turned on and remained illuminated until the behavioral response was made. These trials will be referred to as STOP trials and were randomly interleaved with GO trials. Rats were required to stop the movement signaled by the first light and respond in the direction of the second light. In 37% of sessions, rats experienced no stop-signal delay (SSD) between port exit and illumination of the STOP cue. Sessions where rats experienced no SSD occurred early in the experimental timeline after which SSDs were introduced to reduce STOP trial accuracy. Upon correct responding, rats were required to remain in the fluid well for a variable period between 800 and 1000 ms (pre-fluid delay) before reward delivery (10% sucrose solution). The inter-trial interval (ITI) was a rigid 4 and 7 s for correct and error trials, respectively. Error trials (incorrect direction) were immediately followed by the extinction of house lights and ITI onset.
Figure 1.
Stop-change task and behavior. (A) Rats were required to nose poke and remain in the port for 1000 ms before 1 of the 2 directional lights (left or right) illuminated for 100 ms. The cue light disclosed the response direction in which the animal could retrieve fluid reward (GO trials). On 20% of trials after port withdrawal, the light opposite the first illuminated to instruct the rat to inhibit the current action and redirect behavior to the corresponding well under the second light (STOP trials). (B) Trial types. (C) Average movement time (in ms ± SEM) for GO trials, STOP trials, GO errors, and STOP errors defined as the latency from port exit to fluid well entry. Only sessions where rats made at least 1 error per direction were used (n = 502). (D) Percent correct on STOP trials plotted against average movement time (port exit to well entry) for every session. (E) Average percent correct of STOP (red) and GO (blue) trials (±SEM) when the immediately preceding trial was a STOP trial (STOP-1) or a GO trial (GO-1). (F) Average movement time of correct STOP and GO trials (±SEM) when the immediately preceding trial was a STOP trial or a GO trial. Two-way ANOVAs (P < 0.05) were used to determine movement time (C,G) or percent correct (F) differences with each datum derived from single session means. Sessions used included only those that had at least 1 of each trial type (n = 521). (G) Location of recording sites (Paxinos and Watson). Gray boxes mark the extent of the recording locations.
Trials were presented in a pseudo-random sequence such that left and right trials were presented in equal numbers (±1 over 250 trials). The time necessary to stop and redirect behavior (“stop change reaction time”; SCRT) on STOP trials was computed using the difference between average movement time on correct STOP and GO trials (Bryden et al. 2012, 2016; Bryden and Roesch 2015). There are multiple ways to compute the time necessary to inhibit a behavior when stop-signal delays are fixed (i.e., not titrating to the animal’s performance (Verbruggen and Logan 2009)). This is commonly referred to as the stop-signal reaction time (SSRT), which can be computed via “integration” and “mean” methods (Logan et al. 1984). SCRT was chosen here because we have access to the STOP trial movement time distribution, we did not vary the stop-signal delay on a number of sessions, and SCRT is the most conservative estimate. That is, the mean method SSRT value was 516 ms ± 5.3 and the integration method SSRT calculation was 383.6 ms ± 4.6, whereas the SCRT estimate was only 137.3 ms ± 2.7. By using the most conservative cut-off related the time needed to inhibit movement we guarantee that activity preceding that time is early enough to contribute to the resolution of response conflict prior to action completion. In the peri-event time histogram (line plot) aligned on the SCRT time point (Fig. 4C), a single SCRT value is calculated for each recording session and this duration is added to the time of port exit for each neuron.
Figure 4.
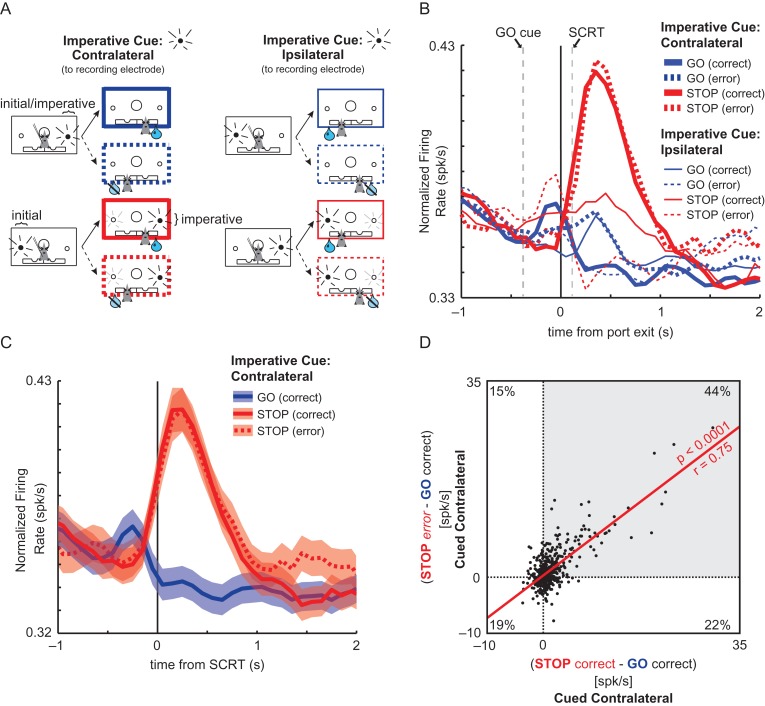
Average population firing over all ACC neurons. (A) Schematic that specifies the criteria for each type of trial. The imperative cue was always the first/only cue on GO trials and the second cue on STOP trials. The imperative cue was presented either contralateral (thick boxes) or ipsilateral (thin boxes) to the recording electrode. Only animals with electrodes in the left hemisphere are presented; cue lights in physical space (i.e., left/right) are opposite in animals with electrodes in the right hemisphere. Solid = correct trials; Dashed = error trials; Blue = GO trials; Red = STOP trials. The color, thickness, and design of the outcome boxes should be used as a guide for lines in population histogram plots (B,C). Faded cue lights represent recently extinguished stimuli. (B) Population histogram of all neurons recorded in sessions where the rat experienced at least 1 of each of the 8 types displayed in A (n = 503). All activity is aligned to port exit. The time necessary to inhibit a response (stop-change reaction time; SCRT) is defined as the difference between STOP trial movement time and GO trial movement time. SCRT is marked as the vertical dotted line labeled “SCRT” at 114.3 ms where line thickness represents the confidence interval (95% CI = 102.6–126). “GO cue” and its associated vertical dashed line indicates the average onset of the GO cue as measured by the latency from port exit (−380 ms; 95% CI =−397.4–362.6). (C) Population histogram from the same neurons as in B. However, only correct GO, correct STOP, and STOP errors (all cued to the contralateral direction) are shown. Activity is aligned to SCRT for every neuron. Ribbons represent standard error of the mean. For unsmoothed activity aligned to the STOP cue and port exit see Supplementary Figures 1 and 2, respectively. (D) Scatter plot of difference scores in firing rate during the response epoch. Y-axis shows difference scores of STOP errors and correct GO trials when the imperative cue was presented contralateral; x-axis shows the difference scores of correct STOP and correct GO trials when the imperative cue was presented contralateral. Each dot represents 1 neuron. Red line marks the least-squares regression line.
Single-unit Recording
Procedures were the same as described previously (Bryden and Roesch 2015). Wires were screened for activity daily; if no activity was detected, the rat was removed and the electrode assembly was advanced 40 or 80 μm. Otherwise, a session was conducted, and the electrode was advanced at the end of the session. Neural activity was recorded using 4 identical Plexon Multichannel Acquisition Processor systems (Dallas, TX). Signals from electrode wires were amplified 20× by an op-amp headstage, located on the electrode array. Immediately outside the training chamber, the signals were passed through a differential pre-amplifier (Plexon Inc, PBX2/16sp-r-G50/16fp-G50) where single-unit signals were amplified 50× and filtered at 150–9000 Hz. The single-unit signals were then sent to the Multichannel Acquisition Processor box, where they were further filtered at 250–8000 Hz, digitized at 40 kHz and amplified at 1–32×. Waveforms (>2.5:1 signal-to-noise) were extracted from active channels and recorded to disk by an associated workstation with event timestamps from the behavior computer.
Data Analysis
Units were sorted via Offline Sorter software from Plexon Inc (Dallas, TX), using a template matching algorithm and analyzed in Neuroexplorer and Matlab. Activity was examined during the period between nose poke exit and well entry (i.e., response epoch). Activity in population histograms was normalized by dividing by the maximal firing rate of each neuron. All statistical procedures were executed using raw firing rates. Unless otherwise specified, behavioral data was analyzed using 2-way ANOVA where each datum is a session average.
For single-unit analysis we used multiple regression to determine the number of cells where firing rate was significantly correlated with either the trial type (STOP/GO), movement time, and/or response direction parameters when variance for the 2 remaining factors was accounted for. To achieve this, we ran the following multiple model during correct trials for each individual cell:
where Y = firing rate (spikes/s) during the response epoch, Movement Time = latency between unpoke and well entry, Direction = coded as (0 = ipsilateral) (1 = contralateral), and Trial Type = coded as (0 = GO) (1 = STOP), as previously described (Bryden and Roesch 2015; Bryden et al. 2016). To determine the significance for each predictor as a function of firing rate during the response epoch, we computed the unique variance of each parameter and divided it by the variance unaccounted for when each respective parameter was not included in the model (partial r2). Significance of each partial r2 was recorded along with the valence of the associated β-value. Counts of positively and negatively correlated cells were compared via binomial sign test (P < 0.05). For clarity, it was possible that a single cell could show a significant partial r2 for all 3 parameters. For analysis of behavioral data, we averaged over multiple sessions, rather than by rat, as this more closely approximates the behavior observed for each single neuron, and better corresponds to the behaviors that occurred during collection of single-unit firing. However, we conducted a secondary analysis and performed a repeated measures ANOVA that looked across rat to confirm the generality of the findings across animals.
To capture activity that differentiated based on the previous trial, we examined firing on STOP and GO trials after either STOP or GO trials. This analysis allows for examination of trials that had the most “conflict” or competition between 2 responses. Abbreviations for trials that are differentiated by the trial type preceding it are labeled as lowercase (“g” or “s”; GO, STOP) which indicates the trial type immediately before the trial marked by the uppercase letter (“G” or “S”; GO, STOP).
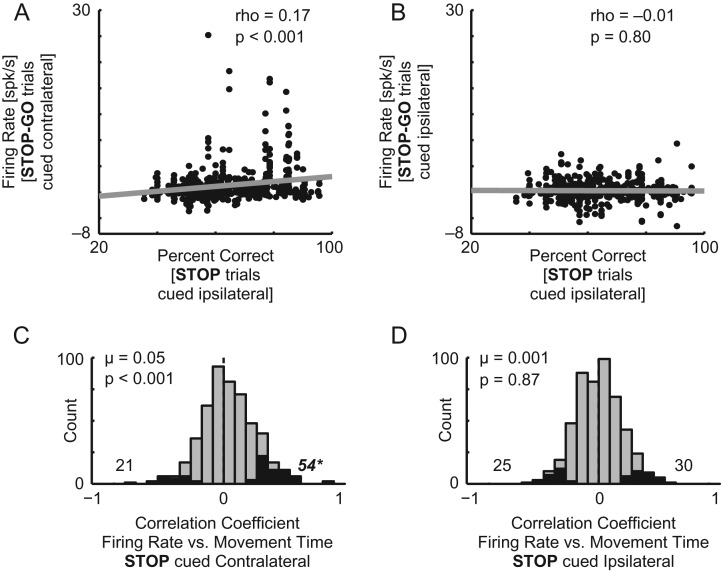
Correlations between firing rate and percent correct were calculated using Spearman’s r after averaging values within each session. Correlation coefficients were determined to be statistically different from one another via Student’s t-test after Fisher’s z-transformation for correlation coefficients. Correlations between firing rate and movement speed (Fig. 5C,D) were computed within each session and correlation coefficients were plotted where black bars represent significantly correlated sessions. Distributions of difference scores always use raw firing rate (spikes/s) for each neuron where there is at least 1 of each of the relevant trial types. Individual distributions were deemed significantly different from zero, and one another, via Wilcoxon sign-rank test.
Figure 5.
Correlations between neuronal activity and behavior. (A,B) Scatter plots showing firing rate for STOP relative GO trials (during the response epoch) against percent correct when the imperative cue was in the contralateral (A) or ipsilateral (B). (C,D) Distributions of correlation coefficients between firing rate and movement time when STOP trials are cued in the contralateral (C) or ipsilateral (D) direction. Black bars (and counts) are significantly (P < 0.05) correlated. Asterisk represents a significantly (χ2; P < 0.05) greater number of neurons for 1 coefficient valence over the other. Distributions are deemed different from zero via Wilcoxon sign-rank test.
Results
Behavior
Rats were trained on a stop-change task (Fig. 1A,B) which required them to maintain their nose poke in the central port during the 1 s pre-cue phase and respond in the direction (right or left) of the cue light to the corresponding fluid well below. Sufficient waiting during the pre-cue phase and correct directional responding led to liquid sucrose reward. These trials are referred to as “GO” trials. Occasionally (20% of trials) rats were required to inhibit their response to the first directional cue at the time when the opposite directional cue light illuminated. These trials are referred to as “STOP” trials.
The low proportion of STOP trials relative to GO trials (20:80) induced a prepotency to respond swiftly to the first directional cue light. Rats were more accurate on GO compared to STOP trials (t502 = 12.86; P < 0.0001) presumably due to the difficulty in inhibiting initiated responses. To determine the impact of both trial type (GO vs. STOP) and correctness on movement speeds (port exit to well entry) we performed a 2-factor ANOVA. Although there was no main effect of trial type (ANOVA; F1,2008 = 2.49; P = 0.12), there was a main effect of correctness (F1,2008 = 105.06; P < 0.0001) and an interaction between trial type and correctness (F1,2008 = 392.05; P < 0.0001; Fig. 1C). Notably, log and square transformed data produced similar results (Log transformed: Trial type, F1,2008 = 1.94, P = 0.16; Correctness, F1,2008 = 194.06; P < 0.0001; Interaction, F1,2008 = 514.56; P < 0.0001; Square root transformed: Trial type, F1,2008 = 3.45, P = 0.06; Correctness, F1,2008 = 160.44; P < 0.0001; Interaction, F1,2008 = 488.25; P < 0.0001). Post hoc t-tests revealed that movement times were faster on correct GO trials than correct STOP trials (t502 = 38.36; P < 0.0001) and the opposite effect was true for error trials (t502 = 25.66; P < 0.0001). These data suggest that rats were not using a “wait-and-see” tactic to distinguish between STOP and GO trial types prior to executing their chosen response. Longer latencies resulted in better STOP trial performance consistent with a speed accuracy tradeoff (Fig. 1D; r = 0.24; P < 0.0001). Compatible with this finding, movement times on STOP error trials were significantly faster than movement times on correctly performed STOP trials (Fig. 1C; t502 = 46.26; P < 0.0001).
In the analysis above we examined behavior over session rather than averaged across sessions. Such an analysis better represents that average behavior that occurs during collection of single neuron activity that will be presented below. Note, however, that if we average over session and perform the same analysis across rats with rat as a repeated measure the results described above hold, with no main effect of trial type (F1,8 = 0.135, P = 0.724), a main effect of correctness (F1,8 = 31.02, P = 0.0008) and an interaction between trial type and correction (F1,8 = 28.47, P = 0.0011).
In many conflict-like tasks it has been shown that performance on the current trial is dependent on the degree of conflict experienced on the previous trial (i.e., conflict adaptation) (Gratton et al. 1992; Botvinick et al. 2001; Mayr et al. 2003). When participants experience high conflict, they tend to exhibit higher control on subsequent trials and perform better. However, if the previous trial had low or no response conflict, the competing irrelevant response has a more detrimental impact on behavior, reducing accuracy during performance of high conflict trials. In our task, the swift and continuous manner in which rats completed trials, in addition to the pseudo-random sequence of the trial types, allowed us to investigate the impact of prior conflict on behavior. That is, the presence of a high competition trial (STOP trial) immediately preceding a STOP trial (sS trial) should positively impact preparation and accuracy—known as conflict adaptation—relative to when a STOP trial is preceded by a low competition, GO trial (gS trial). Behavioral support for this is shown in Figure 1E and F where accuracy and movement times on GO and STOP trials are plotted based on the preceding trial type. There were main effects of both current trial type and previous trial type, as well as a significant interaction between the 2 variables for both percent correct and movement time (F1,2084 > 5.44; P < 0.05). Rats were significantly slower and worse on gS trials relative to sS trials (t521 = 20.20; P < 0.05), demonstrating higher and lower levels of response conflict on gS and sS trials, respectively.
ACC Neurons Fire more Strongly on STOP Trials
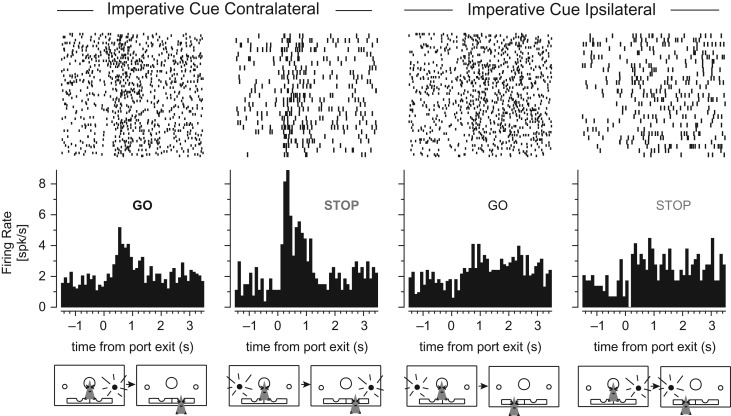
During performance of this task we recorded from 536 neurons from 8 different rats (n’s = 155, 127, 124, 62, 39, 17, 11, 1; Fig. 1G). We found that neural activity in ACC was modulated by both direction (contra- vs. ipsilateral to the recording electrode) and type of trial (GO vs. STOP). Many neurons fired most strongly on STOP trials where the imperative (second) cue was presented contralateral to the recording site. This is illustrated by the firing of the single neuron shown in Figure 2; firing was strongest when the rat stopped the ipsilateral movement and correctly oriented to the contralateral direction.
Figure 2.
Single neuron example of higher firing on STOP trials. Raster plots depict firing on individual trials where every row is a trial and each tick mark represents an action potential. Peri-event time histograms are presented below illustrating the average firing rate across all correct trials aligned to port exit and STOP cue onset (i.e., SSD = 0 in this example).
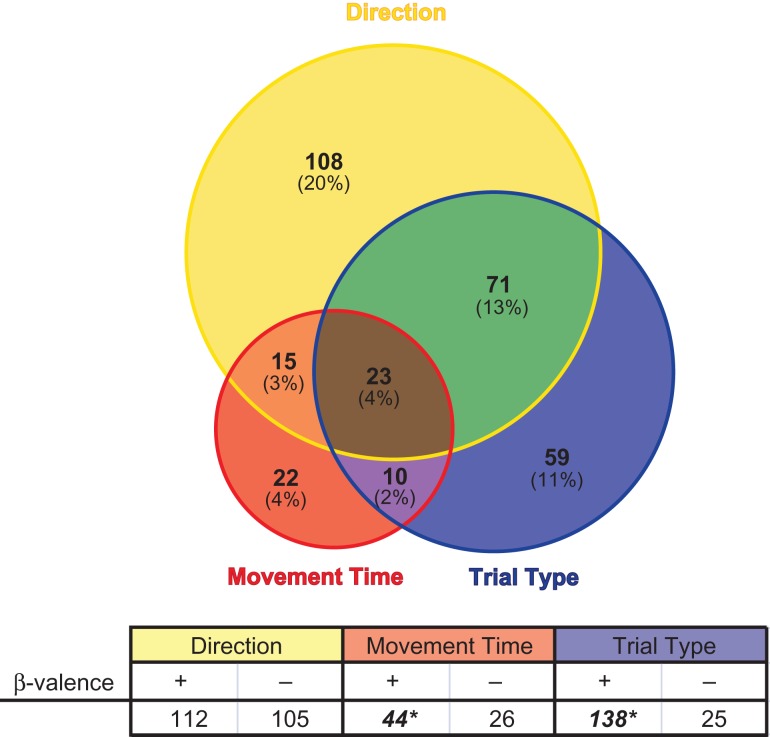
To characterize the firing of each recorded neuron, we used multiple regression to determine the proportion of cells where firing rate during the response epoch (unpoke to well entry) was sensitive to response direction, movement speed, and/or the type of trial (see Method) when rats responded correctly (P < 0.05). Forty percent of neurons (n = 217/536) exhibited a significant partial r2 for direction and of these 217 neurons, 112 β-values were positive (greater firing for the contralateral direction) whereas 105 β-values were negative (Fig. 3; “Direction”; binomial sign test; P = 0.68). The firing of 13% of ACC neurons correlated significantly with the speed of response (Fig. 3; “Movement Time”), with the preponderance of these exhibiting increased firing when movements speeds were slower (Fig. 3; 44 vs. 26; binomial; P < 0.05).
Figure 3.
Multiple regression analysis of single neurons. Circle sizes represent the relative proportions of neurons showing significant (P < 0.05) partial r2 values for the individual task parameters. Top circle encompasses the proportion of neurons that show significant partial r2 values for the direction parameter (yellow). The same conventions apply for the movement time (red circle) and trial type (blue circle) parameters. Non-overlapping portions represent the counts (and percentages) of neurons with significant partial r2 values for 1 parameter. Overlapping portions denote the counts (and percentages) of single cells that exhibited significant partial r2 values for 2 (orange, green, purple) or all 3 (brown) parameters. The table specifies the counts of significant neurons within a variable that have associated positive (“+”) or negative (“−”) β-values. Positive β-values indicate greater firing for the contralateral direction (direction), greater firing for slower movement times (movement time), and greater firing for STOP over GO trials (trial type). Asterisks indicate significantly more neurons with 1 β-valence within a parameter (binomial sign test; P < 0.05). Regression calculation used only correct trials within each neuron. Note, counts of neurons with correlated activity to movement time, direction, and trial type were present throughout the dorsal-ventral extent of the recording sites (Supplementary Fig. 3).
Lastly, the firing of 30% of neurons distinguished STOP from GO trials (Fig. 3; “Trial Type”), with a significantly greater number of neurons firing more during STOP over GO trials than the opposite pattern (Fig. 3; 138 vs. 25; binomial; P < 0.01). This effect was consistent across rats. The number of cells that significantly increased firing on STOP relative to GO trials outnumbering those showing the opposite effect in 6 of the 7 animals (64 vs. 0; 42 vs. 13; 10 vs. 2; 9 vs. 2; 8 vs. 5; and 3 vs. 0) in which multiple recordings occurred. Within 4 of those 6 rats the counts for STOP preferring neurons (i.e., higher firing on STOP vs. GO trials) significantly outnumbered GO preferring neurons (binomial; P < 0.05). In only 1 of the 7 rats did the counts of GO preferring neurons outnumber those that fired more strongly under STOP trials, but these numbers were low (2 STOP neurons and 3 GO neurons) and not significantly different (binomial; P = 1.0).
The above analysis focuses on the response epoch which captures activity that might be involved in stopping the current trial and/or monitoring the competition associated with the behavior being made. If ACC firing can impact the resolution of competition and inhibit behavior during the current trial, the signal must emerge prior to the SCRT. To address this issue we redid the regression analysis described above but divided the response epoch into 2 different analysis epochs: pre-SCRT (port exit to the SCRT) and post-SCRT (SCRT to well entry). We found that the counts of ACC neurons that significantly increased firing on STOP relative to GO trials (i.e., positive correlation) outnumbered those showing significantly higher firing on GO versus STOP trials (i.e., negative correlation) during both pre (34 vs. 8; binomial; P < 0.05) and post-SCRT epochs (134 vs. 31; binomial; P < 0.05). Notably, similar results were obtained using the stop-signal reaction time (SSRT) instead of the SCRT (pre-SSRT: 92 STOP vs. 5 GO; post-SSRT: 57 STOP vs. 5 GO; binomial; P < 0.05). We calculated the SSRT (please see methods) as well as the SCRT to show that estimates related to the time needed to stop did not impact the conclusion of this analysis. We conclude that higher firing of ACC neurons on STOP trials might serve functions related to both resolving and monitoring response conflict, as defined as the competition between 2 responses, and may signal the need to change an action when information is updated during performance of our stop-change task.
The analysis of individual neurons revealed that a substantial number of ACC neurons fired more strongly on STOP compared to GO trials. To determine if this was true across the population of all recorded neurons, we plotted average firing of all cells over time aligned to port exit (Fig. 4B) for which there was at least 1 of each trial type (Fig. 4A; 8 total types: correct and error trials for STOP and GO trials in both directions; n = 503). Within both population line plots (Fig. 4B,C), cued direction, trial type, and correctness are illustrated via thickness, color, and line format, respectively. Line thickness represents the direction (relative to the recording electrode) of the “imperative cue” (i.e., the cue where, if followed, resulted in reward). Line colors designate the type of trial (red = STOP; blue = GO). Solid and dashed lines signify correct and incorrect (i.e., rat moved to the wrong well) trials, respectively. Notable time-points around port exit (Fig. 4B) are indicated by vertical dashed lines; “GO cue” (−380 ms; 95% CI =−397.4–362.6) represents the average time the GO cue illuminated prior to port exit across sessions and “SCRT” (114 ms; 95% CI = 102.6–126) represents the average stop-change reaction time (see Method).
As in the single neuron example, population firing dramatically increased during the behavioral response (response epoch) on correct STOP trials (Fig. 4B; solid red lines) relative to correct GO trials (Fig. 4B; solid blue lines). This differential firing during STOP trials is driven largely by the elevated activity on correct STOP trials when the imperative cue was in the contralateral direction (Fig. 4B; thick solid red); however, activity was also higher for ipsilateral STOP cues (Fig. 4B; thin solid red) relative to GO trials (blue). Firing increased immediately upon port exit and differentiated from the other trial types before the SCRT. Activity was also high on errant STOP trials during which the rat was cued to move into the contralateral direction but, instead, moved in the ipsilateral direction (Fig. 4B; thick red dashed). This is better illustrated in Figure 4C, which isolates STOP trials where the ipsilateral direction was inhibited and the correct contralateral response was made (solid red) versus trials with the same “stimuli”, but the rat moved errantly in the ipsilateral direction (dashed red) aligned to the SCRT. Both trial types elicit nearly identical firing patterns, even though the behavioral responses were in opposite directions. Further, STOP cue induced changes of firing clearly began prior to the SCRT consistent with the pre-SCRT single-unit analysis demonstrating a preponderance of neurons exhibiting higher firing rate on STOP trials early in the response epoch. These results further demonstrate that ACC activity is more closely tied to conflict induced by competing responses as opposed to the ultimate motor response and that firing rate changes that occur on STOP trials occur early enough to impact the change in ongoing behavior.
To determine if high firing on both correct and incorrect cued STOP trials presented on the contralateral side occurred in the same neurons similar to that observed across the population (Fig. 4C), we calculated difference scores of firing on each of these 2 trials types from correct GO trials cued to the contralateral direction. Specifically, we plotted the difference in firing between correct STOP trials and correct GO trials against the difference in firing between errant STOP trials and correct GO trials (Fig. 4D) where the imperative cue in each of these trial types was contralateral. The relevant trial types for this analysis are shown in Figure 4C. The distributions of differences scores were both significantly shifted in the positive direction (Fig. 4D; Correct: mean = 1.34, P < 0.0001; Error: mean = 1.33; P < 0.0001) and were highly correlated (Fig. 4D; r = 0.75; P < 0.0001), with 44% of neurons fired more strongly for STOP errors and correct STOP trials than correct GO trials when the imperative cue was in the contralateral direction (Fig. 4D gray; top right quadrant). We conclude that single ACC neurons increase firing on STOP trials compared to GO trials for both correct and incorrect responses.
Firing Rate was Positively Correlated with Accuracy and Movement Speed
It is clear from the results above that single neuron and population activity in ACC is high when rats must overcome competition and change behavior to obtain reward on STOP trials. If ACC is indeed contributing to behavior, then firing should be correlated with STOP trial performance. To test this prediction, we plotted the average firing rate on STOP trials relative to GO trials where the imperative cue was presented in the contralateral direction against percent correct scores for each neuron. As expected, firing rates were positively correlated with STOP accuracy (Fig. 5A; rho = 0.17; P < 0.001). Thus, higher firing rates in ACC were accompanied by better performance when STOP cues were presented in the contralateral direction. The correlation between percent correct and firing rate was not significant when STOP cues were in the ipsilateral direction (Fig. 5B; rho =−0.01; P = 0.72) and the correlation coefficients between these 2 plots (i.e., ipsi vs. contra) differed significantly (P < 0.05).
The relationship between activity and behavior can be further demonstrated at the single neuron level. We already know that when rats were slower, they were better on STOP trials (Fig. 1D) and that firing rates were positively correlated with accuracy (Fig. 5A), thus within single neurons we predicted that higher firing rates would be correlated with slower movement speeds. Figures 5C and D plot correlation coefficients between firing rate during the response epoch and movement time for each neuron during trials where the STOP cue was presented in the contralateral (Fig. 5C) or ipsilateral (Fig. 5D) direction. The distribution of correlation coefficients was significantly shifted positively for trials when the STOP cue was presented contralateral to the electrode (Fig. 5C; P < 0.001). Furthermore, 54 individual neurons exhibited statistically significant positive correlation coefficients whereas 21 showed significantly negative coefficients (Fig. 5C; black bars; binomial; P < 0.001). For trials in which the STOP cue was presented in the ipsilateral direction, the distribution of correlation coefficients was not significantly shifted (Fig. 5D; Wilcoxon; P = 0.87) and the proportion of neurons showing statistically significant positive correlation coefficients did not differ from those showing negative correlations (Fig. 5D; binomial; P = 0.52). Lastly, the 2 distributions are significantly different from one another (Fig. 5C vs. D; Wilcoxon; P < 0.01).
ACC Firing was Modulated by Degree of Competition Induced by the Previous Trial
From these results it is clear that activity in ACC is modulated by our primary manipulation of (i.e., STOP vs. GO); next we asked if firing in ACC was also modulated by secondary manipulations of conflict that arise from trial sequence. As described above, animals experience varying degrees of competition on STOP trials depending on their experience with the previous trial (Fig. 1F,G); responding on STOP trials was more difficult as demonstrated by elevated error trials and slower movement times when the previous trial was a GO compared to when the previous trial was a STOP trial. Thus, the greatest conflict would be experienced on STOP trials after GO trials (gS trials), followed by 2 consecutive STOP trials (sS trials), and then GO trials.
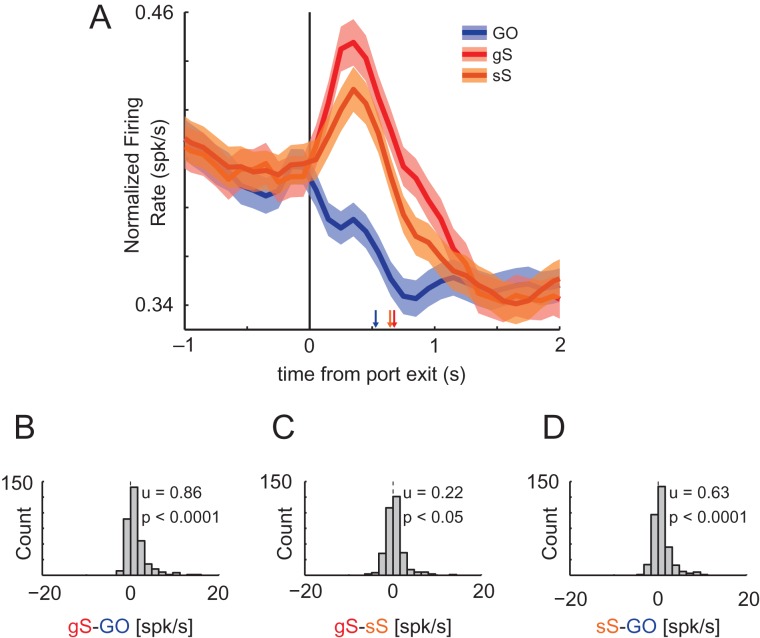
To examine the impact of trial sequence on ACC firing, we plotted the average firing on gS, sS, and GO trials (Fig. 6A). We collapsed activity across the 2 directions for each trial type to maintain statistical power and minimize data attrition caused by splitting STOP trials into sS and gS trials. We found that activity in ACC was modulated by degree of conflict where mean firing was highest on gS (red), reduced on sS (orange), and lowest on GO (blue) trials (Fig. 6A). To quantify this effect across neurons we computed difference scores between relevant trial types for each neuron during the response epoch for correct trials and plotted the distributions. The distributions of neurons comparing gS to GO trials (gS minus GO) and sS to GO trials (sS minus GO) were shifted significantly in the positive direction (Fig. 6B,D; Wilcoxon; P < 0.0001), and the mean of the “gS minus GO” distribution was significantly higher than the “sS minus GO” distribution (Fig. 6B,D; 0.86 vs. 0.63; Wilcoxon; P < 0.05). In addition, when directly comparing gS to sS (gS minus sS) trials, the distribution was significantly shifted in the positive direction, indicating that the majority of ACC neurons fired more strongly for gS compared to sS during the response epoch (Fig. 6C; Wilcoxon; P < 0.05).
Figure 6.
Response of ACC neurons to degrees of conflict induced by the previous trial. (A) Average firing rate over time aligned on port exit for neurons recorded during sessions where there was a SSD and at least 1 of each of the 3 trial types (n = 333). Red lines refer to STOP trials where the preceding trial was a GO trial (gS trials). Orange lines refer to the second of 2 consecutive STOP trials (sS trials). Blue lines refer to GO trials without regard to the previous trial type. Ribbons = SEM. Down-facing arrows mark the average point in time when the well was entered for each trial type during the sessions shown (GO = 528.3 ms; sS = 643.4 ms; gS = 676.3 ms). (B–D) Distributions are the result of difference scores between firing rates on various trial types taken during the response epoch for each neuron. Distributions are deemed to be significantly shifted from zero (and from each other) using the Wilcoxon sign-rank test (P < 0.05).
Discussion
The present work characterizes changes in ACC signaling during performance on a stop-change task. Collectively, we show that neurons in ACC exhibit higher firing when there was competition between 2 responses and a need to change behavior. Increases in firing occur prior to the adjustment of behavior on a task that requires rats to inhibit body movements and redirect them in the opposite direction. Significantly different firing rates between STOP and GO trials was observed in 30% of recorded neurons and was clearly present in population averages that were non-selectively averaged across all neurons. Further, ACC firing rates were positively correlated with percent correct and movement time, indicating that when ACC was engaged on STOP trials, rats tended to perform better. In addition to these primary effects, we showed that firing of ACC neurons scaled with degree of conflict, consistent with predictions from theories of ACC function (Shenhav et al. 2016). Notably these characteristics are unique to ACC, as they have not been observed in other prefrontal areas thought to be involved in response inhibition including medial prefrontal cortex (mPFC) and orbitofrontal cortex (OFC) (Narayanan et al. 2006; Mansouri et al. 2007; Marquis et al. 2007; Bari et al. 2011; Duan et al. 2015; Bryden et al. 2016; Hardung et al. 2017).
While we chose to describe our results in terms of high or low conflict, this is only meant to speak to the nature of the trials where competing signals increase difficulty and the need for cognitive control. We recognize there has been debate surrounding the purpose of ACC and while conflict detection may be an important component of ACC function, it may not be its only function. Recently, discerning the function of ACC has been likened to a Rorschach test for cognitive neuroscientists, as there has been much debate on how best to interpret decades of published evidence on the ACC (Ebitz and Hayden 2016). Two theories in particular have emerged that each claim to offer to a unifying view of ACC function (Kollings et al. 2016; Shenhav et al. 2016). One view, the foraging value theory (FVT) (Shenhav et al. 2016) or behavioral adaptation and persistence view (Kollings et al. 2016) emphasizes ACC function in the context of foraging, suggesting that ACC involvement has broad effects of subsequent behavior, and is responsible for rapidly updating behavioral policies in response to a changing environment (Kollings et al. 2016). This is a broader model, and can encompass findings describing negative surprise as well as conflict monitoring (Kollings et al. 2016). In contrast, the expected value of control theory (EVC) is focused and emphasizes that ACC function can be attributed to a cost/benefit analysis modulated by a task’s degree of involvement and subsequent need for control (Shenhav et al. 2016). Specifically, the EVC model implicates the ACC in both the detection and computation of conflicting inputs (Shenhav et al. 2013, 2014, 2016). In light of these and other viewpoints, it becomes difficult to discern whether our findings of increased ACC activity on high conflict trials are reflective of either a more information updating model such as the FVT or is more in line with EVC theory and more traditional views of “conflict detection”. Our task is not inherently foraging based, and performance is correlated with principles of reward, a context somewhat consistent with the EVC theory. However, the fact that on STOP trials no differences were detected between correct and error responses might be consistent with models of information updating or behavioral adaptation rather than conflict resolution per se. Although, one could argue that if “conflict detection” is defined as the realization of opposing signals, and the use of that information in a behaviorally beneficial way, then this updating of information that occurs on high conflict trials likely also fits with the EVC theory of conflict detection and cognitive control as well. This debate also does not exclude the possibility that differences in firing rate of high and low conflict trials are simply due to differences in arousal stemming from the appearance of either 1 (GO trials) or 2 lights (STOP trials). While, we cannot completely rule out this possibility, it seems unlikely given the directionality of the signal that we observed.
Despite the contention regarding theories of ACC function, there seems to be a general consensus that a primary role of ACC is in modifying behavior and that its signal, regardless of how it’s generated, has great importance for downstream output regions. In addition to the role of ACC in modifying behavior via connections to prefrontal areas, ACC likely impacts ongoing behavior via basal ganglia by modulating stop signals in subthalamic nucleus and directional signals in the dorsal striatum (DS) (Frank et al. 2007; Bryden et al. 2012; Schmidt et al. 2013). We and others have found that DS neurons strongly encoded direction on GO trials but miscode direction STOP trials. At the time of SCRT, neurons in DS exhibited simultaneous activation of both contralateral and ipsilateral movement much like has been described in oculomotor regions during conflict tasks (Nakamura et al. 2005). Importantly, neurons in ACC begin firing immediately after presentation of the stop signal, before the SCRT, thus might serve to directly modify directional tuning in downstream areas like DS so that response conflict can be resolved prior to an error being made. Whether this putative link between increased ACC activity on high conflict trials and subsequent resolution of directional selectivity in downstream regions such as DS is necessary for appropriate responding remains to be tested. Regardless, the ACC correlate we report here fits well with newer integrative views of ACC function in that each can explain similar conflict related signals observed in human studies in the context of their respective models. In part, these theories have arisen from the observation that ACC does more than detect conflict, carrying signals related to action, action-outcome contingencies, error detection and reward to name a few. Indeed, we have shown that firing of neurons in rat ACC are correlated with predictions about future reward, commission and omission errors, and attention during learning (Bryden et al. 2011).
Although there is data from a substantial number of neuroimaging studies showing response conflict signals in ACC, no study has convincingly shown parallel signals in the ACC of non-human animals (Ito et al. 2003; Nakamura et al. 2005; Hayden et al. 2011; Cai and Padoa-Schioppa 2012; Ebitz and Platt 2015). The collective negative findings in the literature are somewhat surprising considering that single neuron work in humans supports the role of ACC in monitoring response conflict (Davis et al. 2005; Sheth et al. 2012). The disconnect between human imaging work and single neuron recording is problematic, as it is unclear whether single neurons in ACC in non-human animals encode information similar to humans (Cole et al. 2009). There are several possible explanations for this disconnect. First, tasks vary based on the species performing them. Non-human primate work typically uses eye movements as the instrumental response, whereas human and rat work more commonly use non-oculomotor body movements (e.g., button presses, nose poke). Saccades differ from whole body movements in that they are ballistic and have fewer degrees of freedom meaning conflict between 2 competing eye responses may not elicit sufficient conflict/arousal to be detected by ACC (Ebitz and Platt 2015). Second, ACC might be more engaged after the behavioral response has already been initiated or when stimuli are not presented simultaneously as in more classic conflict task (e.g., Stroop). This aspect of the task may increase the likelihood of observing response conflict signaling in non-oculomotor tasks. With that said, imaging studies have found conflict-like signals during anti-saccade tasks (Matsuda et al. 2004; Brown et al. 2007), but these results might be better explained by the simultaneous activation of neurons that encode opposite directions (Nakamura et al. 2005) and conflict-like signals were not observed in a single neuron neurophysiology study done in monkeys performing a task-switching anti-saccade task (Johnston et al. 2007). A third possible explanation for these inconsistent results may be that neurons in ACC detect conflict in a task-dependent manner, where specific aspects of the task such as inhibiting full body movements versus inhibiting a saccade may modulate the signal accordingly. Notably, neurons in primate pre-SMA have been shown to increase firing when there is change in the forthcoming direction of an arm movement (Matsuzaka and Tanji 1996), however, unlike neurons in our study, neurons in pre-SMA failed to respond on errors suggesting that firing was more closely tied to the motor response. These results suggest that medial frontal regions may be working together to resolve competition between competing response to change the course of action in these type of tasks.
An alternative possibility explaining why single neuron studies have failed to find similar correlates in ACC might be that those studies were recording from the wrong sub-region of ACC. The advantage of whole brain imaging in humans is that the sampling space is near limitless so that conflict related signals can be more easily localized. Across studies, areas of activation under conflict are highly variable and conflict related activity has been observed in a number of different cingulate and medial frontal sub-regions (Laird et al. 2005; Nee et al. 2007). Thus, it may not be surprising that localized single-unit recording devices have previously failed to uncover correlates of response conflict.
It is also possible that the specific sub-region of ACC dedicated to detecting contrasting events does not exist in non-human animals (Cole et al. 2009); the area critical for conflict, area 32′, appears to be exclusive to humans (Vogt et al. 2013), however recent evidence suggests a potential homologous region in rodents based on a striatal-cortical connectivity analysis (Heilbronner et al. 2016). Rat ACC does have non-limbic connections critical for executive control (Jones et al. 2005), and rodent recording studies have already uncovered neural correlates in ACC that are consistent with other proposed functions derived from human and non-human primate work, such as value encoding, error detection, attention, and surprise processing (Totah et al. 2009; Bryden et al. 2011). Our recording sites in the current study lie near the anterior/mid-cingulate border as opposed to more rostral cingulate regions located above medial prefrontal cortex (Vogt 2016), raising the possibility that this region might be a unique subsection in cingulate cortex dedicated to response conflict processing. With that said, our recordings are still in ACC (area 24) above corpus callosum similar to the recordings performed in primates (Ebitz and Platt) which sampled from the dorsal bank, ventral bank, and fundus of the cingulate sulcus, dorsal to the genu of the corpus callosum (area 24c). Perhaps if primate studies focused on area 24b (more ventral in monkeys) and/or slightly more posterior, they too would observe similar correlates. Whether “conflict-like” signals vary depending on sub-region is highly speculative and requires further study.
The final plausible explanation for the lack of response conflict encoding in non-human ACC lies in the differential engagement of executive control between species. It has been suggested that monkeys use alternative strategies outside of putative conflict adaption to adjust their behavior under certain circumstances (Cole et al. 2009). However behavioral adjustments of control in the presence of prior conflict has been observed in several monkey (Nakamura et al. 2005; Emeric et al. 2007, 2008) and rat studies (Bryden and Roesch 2015; Newman et al. 2015; Bryden et al. 2016). In addition, the results presented here demonstrate that rats can make adjustments in behavioral control comparable to that of human subjects, including speed/accuracy tradeoff, reduced STOP trial accuracy as SSDs increase, and conflict adaptation (Bryden and Roesch 2015; Bryden et al. 2016).
Conclusion
In conclusion, for the first time, we show at the level of the single neuron that ACC activity increases on trials where animals have to inhibit a prepotent response in order to get a reward. This finding opens the door to pursue questions related to how these signals emerge and impact downstream circuits when animals are faced with competing responses, as well as to better understand how this function might be disrupted in animal models of disease (e.g., drug abuse).
Supplementary Material
Notes
Correspondence and requests for materials should be addressed to DB (email: dbryden@umd.edu) or MR (email: mroesch@umd.edu). Conflict of Interest: None declared.
Funding
This work was supported by grants from the NIDA (R01DA031695, MR).
References
- Bari A, Mar AC, Theobald DE, Elands SA, Oganya KC, Eagle DM, Robbins TW. 2011. Prefrontal and monoaminergic contributions to stop-signal task performance in rats. J Neurosci. 31:9254–9263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD. 2001. Conflict Monitoring and Cognitive Control. Psychol Rev. 108:624–652. [DOI] [PubMed] [Google Scholar]
- Brown JW, Braver TS. 2005. Learned predictions of error likelihood in the anterior cingulate cortex. Science. 307:1118–1121. [DOI] [PubMed] [Google Scholar]
- Brown GG, Kindermann SS, Siegle GJ, Granholm E, Wong EC, Buxton RB. 1999. Brain activation and pupil response during covert performance of the Stroop Color Word task. J Int Neuropsychol Soc. 5:308–319. [DOI] [PubMed] [Google Scholar]
- Brown MRG, Vilis T, Everling S. 2007. Frontoparietal activation with preparation for antisaccades. J Neurophysiol. 98:1751–1762. [DOI] [PubMed] [Google Scholar]
- Bryden DW, Burton AC, Barnett BR, Cohen VJ, Hearn TN, Jones EA, Kariyil RJ, Kunin A, Kwak S, Lee J, et al. 2016. Prenatal Nicotine Exposure Impairs Executive Control Signals in Medial Prefrontal Cortex. Neuropsychopharmacology. 41:716–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryden DW, Burton AC, Kashtelyan V, Barnett BR, Roesch MR. 2012. Response inhibition signals and miscoding of direction in dorsomedial striatum. Front Integr Neurosci. 6:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryden DW, Johnson EE, Tobia SC, Kashtelyan V, Roesch MR. 2011. Attention for learning signals in anterior cingulate cortex. J Neurosci. 31:18266–18274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryden DW, Roesch MR. 2015. Executive Control Signals in Orbitofrontal Cortex during Response Inhibition. J Neurosci. 35:3903–3914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai X, Padoa-Schioppa C. 2012. Neuronal Encoding of Subjective Value in Dorsal and Ventral Anterior Cingulate Cortex. J Neurosci. 32:3791–3808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter CS, Braver TS, Barch DM, Botvinick MM, Noll D, Cohen JD. 1998. Anterior cingulate cortex, error detection, and the online monitoring of performance. Science. 280:747–749. [DOI] [PubMed] [Google Scholar]
- Carter CS, Macdonald M, Botvinick M, Ross LL, Stenger V, Noll D, Cohen JD. 2000. Parsing executive processes: strategic vs. evaluative functions of the anterior cingulate cortex. Proc Natl Acad Sci USA. 97:1944–1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole MW, Yeung N, Freiwald WA, Botvinick M. 2009. Cingulate cortex: Diverging data from humans and monkeys. Trends Neurosci. 32:566–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis CE, Cole MW, Rao VY, D’Esposito M. 2005. Canceling planned action: an FMRI study of countermanding saccades. Cereb Cortex. 15:1281–1289. [DOI] [PubMed] [Google Scholar]
- Davis KD, Taylor KS, Hutchison WD, Dostrovsky JO, McAndrews MP, Richter EO, Lozano AM. 2005. Human anterior cingulate cortex neurons encode cognitive and emotional demands. J Neurosci. 25:8402–8406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downar J, Frcpc P, Blumberger DM, Zafiris F, Daskalakis J. 2016. The Neural Crossroads of Psychiatric Illness: An Emerging Brain Stimulation Target. Trends Cog Sci. 20:107–120. [DOI] [PubMed] [Google Scholar]
- Duan C a., Erlich JC, Brody CD. 2015. Requirement of Prefrontal and Midbrain Regions for Rapid Executive Control of Behavior in the Rat. Neuron. 86:1491–1503. [DOI] [PubMed] [Google Scholar]
- Ebitz RB, Platt ML. 2015. Neuronal activity in primate dorsal anterior cingulate cortex signals task conflict and predicts adjustments in pupil-linked arousal. Neuron. 85:628–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebitz RB, Hayden BY. 2016. Dorsal anterior cingulate: a Rorschach test for cognitive neuroscience. Nat Neurosci. 19:1278–1279. [DOI] [PubMed] [Google Scholar]
- Emeric EE, Brown JW, Boucher L, Carpenter RHS, Hanes DP, Harris R, Logan GD, Mashru RN, Pare M, Pouget P, et al. 2007. Influence of history on saccade countermanding performance in humans and macaque monkeys. Vision Res. 47:35–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emeric EE, Brown JW, Leslie M, Pouget P, Stuphorn V, Schall JD. 2008. Performance monitoring local field potentials in the medial frontal cortex of primates: anterior cingulate cortex. J Neurophysiol. 99:759–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MJ, Samantha J, Moustafa AA, Sherman SJ. 2007. Hold Your Horses: Impulsivity, Deep Brain Stimulation, and Medication in Parkinsonism. Science. 318:1309–1312. [DOI] [PubMed] [Google Scholar]
- Gabbott PL a, Warner T a, Jays PRL, Salway P, Busby SJ. 2005. Prefrontal cortex in the rat: projections to subcortical autonomic, motor, and limbic centers. J Comp Neurol. 492:145–177. [DOI] [PubMed] [Google Scholar]
- Gratton G, Coles MG, Donchin E. 1992. Optimizing the use of information: strategic control of activation of responses. J Exp Psychol Gen. 121:480–506. [DOI] [PubMed] [Google Scholar]
- Hardung S, Epple R, Jäckel Z, Eriksson D, Uran C, Senn V, Gibor L, Yizhar O, Diester I. 2017. A Functional Gradient in the Rodent Prefrontal Cortex Supports Behavioral Inhibition. Curr Biol. 27:549–555. [DOI] [PubMed] [Google Scholar]
- Hayden BY, Heilbronner SR, Pearson JM, Platt ML. 2011. Surprise signals in anterior cingulate cortex: neuronal encoding of unsigned reward prediction errors driving adjustment in behavior. J Neurosci. 31:4178–4187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilbronner SR, Rodriguez-Romaguera J, Quirk GJ, Groenewegen HJ, Haber SN. 2016. Circuit-based corticostriatal homologies between rat and primate. Biol Psychiatry. 80(7):509–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoover WB, Vertes RP. 2007. Anatomical analysis of afferent projections to the medial prefrontal cortex in the rat. Brain Struct Funct. 212:149–179. [DOI] [PubMed] [Google Scholar]
- Ito S, Stuphorn V, Brown JW, Schall JD. 2003. Performance monitoring by the anterior cingulate cortex during saccade countermanding. Science. 302:120–122. [DOI] [PubMed] [Google Scholar]
- Johnston K, Levin HM, Koval MJ, Everling S. 2007. Top-Down Control-Signal Dynamics in Anterior Cingulate and Prefrontal Cortex Neurons following Task Switching. Neuron. 53:453–462. [DOI] [PubMed] [Google Scholar]
- Jones BF, Groenewegen HJ, Witter MP. 2005. Intrinsic connections of the cingulate cortex in the rat suggest the existence of multiple functionally segregated networks. Neuroscience. 133:193–207. [DOI] [PubMed] [Google Scholar]
- Kollings N, Wittmann MK, Behrens TEJ, Boorman ED, Mars RB, Rushworth M. 2016. Value, search, persistence and model updating in anterior cingulate cortex. Nat Neurosci. 19:1280–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird AR, McMillan KM, Lancaster JL, Kochunov P, Turkeltaub PE, Pardo JV, Fox PT. 2005. A comparison of label-based review and ALE meta-analysis in the stroop task. Hum Brain Mapp. 25:6–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan GD, Cowan WB, Davis KA. 1984. On the ability to inhibit simple and choice reaction time responses: a model and a method. J Exp Psychol Hum Percept Perform. 10:276–291. [DOI] [PubMed] [Google Scholar]
- Mailly P, Aliane V, Groenewegen HJ, Haber SN, Deniau J-M. 2013. The Rat Prefrontostriatal System Analyzed in 3D: Evidence for Multiple Interacting Functional Units. J Neurosci. 33:5718–5727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansouri FA, Buckley MJ, Tanaka K. 2007. Mnemonic function of the dorsolateral prefrontal cortex in conflict-induced behavioral adjustment. Science. 318:987–990. [DOI] [PubMed] [Google Scholar]
- Marquis JP, Killcross S, Haddon JE. 2007. Inactivation of the prelimbic, but not infralimbic, prefrontal cortex impairs the contextual control of response conflict in rats. Eur J Neurosci. 25:559–566. [DOI] [PubMed] [Google Scholar]
- Marx C, Lex B, Calaminus C, Hauber W, Backes H, Neumaier B, Mies G, Graf R, Endepols H. 2012. Conflict Processing in the Rat Brain: Behavioral Analysis and Functional μPET Imaging Using [F]Fluorodeoxyglucose. Front Behav Neurosci. 6:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda T, Matsuura M, Ohkubo T, Ohkubo H, Matsushima E, Inoue K, Taira M, Kojima T. 2004. Functional MRI mapping of brain activation during visually guided saccades and antisaccades: Cortical and subcortical networks. Psychiatry Res - Neuroimaging. 131:147–155. [DOI] [PubMed] [Google Scholar]
- Matsuzaka Y, Tanji J. 1996. Changing directions of forthcoming arm movements: neuronal activity in the presupplementary and supplementary motor area of monkey cerebral cortex. J Neurophysiol. 76(4):2327–2342. [DOI] [PubMed] [Google Scholar]
- Maurice N, Deniau JM, Glowinski J, Thierry M. 1998. Relationships between the prefrontal cortex and the basal ganglia in the rat: physiology of the corticosubthalamic circuits. J Neurosci. 18:9539–9546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayr U, Awh E, Laurey P. 2003. Conflict adaptation effects in the absence of executive control. Nat Neurosci. 6:450–452. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Roesch MR, Olson CR. 2005. Neuronal activity in macaque SEF and ACC during performance of tasks involving conflict. J Neurophysiol. 93:884–908. [DOI] [PubMed] [Google Scholar]
- Narayanan NS, Horst NK, Laubach M. 2006. Reversible inactivations of rat medial prefrontal cortex impair the ability to wait for a stimulus. Neuroscience. 139:865–876. [DOI] [PubMed] [Google Scholar]
- Nee DE, Kastner S, Brown JW. 2011. Functional heterogeneity of conflict, error, task-switching, and unexpectedness effects within medial prefrontal cortex. Neuroimage. 54:528–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nee DE, Wager TD, Jonides J. 2007. Interference resolution: insights from a meta-analysis of neuroimaging tasks. Cogn Affect Behav Neurosci. 7:1–17. [DOI] [PubMed] [Google Scholar]
- Newman LA, Creer DJ, McGaughy JA. 2015. Cognitive control and the anterior cingulate cortex: How conflicting stimuli affect attentional control in the rat. J Physiol Paris. 109:95–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardo JV, Pardo PJ, Janer KW, Raichle ME. 1990. The anterior cingulate cortex mediates processing selection in the Stroop attentional conflict paradigm. Proc Natl Acad Sci USA. 87:256–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt R, Leventhal DK, Mallet N, Chen F, Berke JD. 2013. Canceling actions involves a race between basal ganglia pathways. Nat Neurosci. 16:1118–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenhav A, Botvinick M, Cohen J. 2013. The expected value of control: An integrative theory of anterior cingulate cortex function. Neuron. 79:217–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenhav A, Cohen JD, Botvinick MM. 2016. Dorsal anterior cingulate cortex and the value of control. Nat Neurosci. 19:1286–1291. [DOI] [PubMed] [Google Scholar]
- Shenhav A, Straccia MA, Cohen JD, Botvinick MM. 2014. Anterior cingulate engagement in a foraging context reflects choice difficulty not foracting value. Nat Neurosci. 17:1249–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheth S a., Mian MK, Patel SR, Asaad WF, Williams ZM, Dougherty DD, Bush G, Eskandar EN. 2012. Human dorsal anterior cingulate cortex neurons mediate ongoing behavioural adaptation. Nature. 488:218–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Totah NKB, Kim YB, Homayoun H, Moghaddam B. 2009. Anterior cingulate neurons represent errors and preparatory attention within the same behavioral sequence. J Neurosci. 29:6418–6426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbruggen F, Logan GD. 2009. Models of response inhibition in the stop-signal and stop-change paradigms. Neurosci Biobehav Rev. 33:647–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt BA. 2016. Midcingulate Cortex: Structure, Connections, Homologies, Functions and Diseases. J Chem Neuroanat. 74:28–46. [DOI] [PubMed] [Google Scholar]
- Vogt BA, Hof PR, Zilles K, Vogt LJ, Herold C, Palomero-Gallagher N. 2013. Cingulate area 32 homologies in mouse, rat, macaque and human: Cytoarchitecture and receptor architecture. J Comp Neurol. 521:4189–4204. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.