Abstract
Quantifying the genetic architecture of the cerebral cortex is necessary for understanding disease and changes to the brain across the lifespan. Prior work shows that both surface area (SA) and cortical thickness (CT) are heritable. However, we do not yet understand the extent to which region-specific genetic factors (i.e., independent of global effects) play a dominant role in the regional patterning or inter-regional associations across the cortex. Using a population sample of young adult twins (N = 923), we show that the heritability of SA and CT varies widely across regions, generally independent of measurement error. When global effects are controlled for, we detected a complex pattern of genetically mediated clusters of inter-regional associations, which varied between hemispheres. There were generally weak associations between the SA of different regions, except within the occipital lobe, whereas CT was positively correlated within lobar divisions and negatively correlated across lobes, mostly due to genetic covariation. These findings were replicated in an independent sample of twins and siblings (N = 698) from the Human Connectome Project. The different genetic contributions to SA and CT across regions reveal the value of quantifying sources of covariation to appreciate the genetic complexity of cortical structures.
Keywords: cortical thickness, genetics, magnetic resonance imaging, surface area
Individual variation within the cerebral cortex can be related to both normal and abnormal behavior (Bakken et al. 2012; Geschwind and Rakic 2013; Schnack et al. 2015). This variation arises due to environmental or genetic factors and the interplay between the 2, and their relative contributions may be crucial to elucidating the etiology of mental illness and neurodegenerative diseases (Kendler 2013; Zhao and Castellanos 2016). Twin and family studies can partition the variance in a phenotype among individuals into genetic and environmental sources; they show that the proportion of variance attributed to genetic factors (heritability) for cortical structure (surface area [SA] and cortical thickness [CT]) varies substantially across different cortical regions (Schmitt et al. 2008; Winkler et al. 2010; Eyler et al. 2012).
Several studies have investigated the degree to which genetic effects on different cortical structures/parcellations are correlated. Generally high genetic correlations have been reported between corresponding left/right hemisphere regions (Schmitt et al. 2008; Eyler et al. 2014; Docherty et al. 2015). Examining CT in 54 neuroanatomical regions of interest (ROIs) (Schmitt et al. 2008) demonstrated strong and positive genetic correlations across the cortex. However, when whole brain mean CT was included as a covariate, regionally specific patterns of genetic influence emerged, showing there is genetic specificity, with distinct genetic effects across brain regions. Genetic covariance across SA measures of neuroanatomical ROIs has not been examined, though a wide range of genetic correlations across genetically identified cortical regions (corrected for total SA) has been reported (Peng et al. 2016). Perhaps surprisingly, the genetic factors that affect SA and CT are largely independent (Panizzon et al. 2009; Winkler et al. 2010), and this may be due to differences in the cellular processes that influence each measure during corticogenesis (Rakic 2009).
For all the evidence of a strong genetic influence on SA and CT, the question remains whether genetic factors are the dominant force behind regional patterning or inter-regional associations across the cortex. Significant estimates of environmental variance (Schmitt et al. 2008; Kremen et al. 2010) and evidence of cortical variation associated with activity and behavior (McEwen and Morrison 2013; Erickson et al. 2014; Kuhn et al. 2014) suggest that neuroplasticity may underlie some aspects of cortical organization. Even so, it can be hard to differentiate environmental variance from measurement error—the 2 are attributed to the same factor in twin models as they are unrelated to twin similarity. However, by calculating the reliability of SA/CT measures, estimates of the variance attributed to measurement unreliability can be considered in conjunction with estimates of genetic and environmental variance to better evaluate differences between variance components. Past studies of cortical variation have not examined the role of measurement reliability, nor the contribution of environmental factors to phenotypic associations across different cortical regions.
In this study, we use the QTIM dataset (one of the largest in neuroimaging genetics, including brain scans from 157 monozygotic (MZ) and 194 dizygotic (DZ) twin pairs) to estimate region-specific genetic and environmental influence (i.e., independent of global effects) on SA and CT in 34 ROIs. We then examine associations between regions, and the strength of genetic and environmental contributions to these associations. Using this large dataset, we seek to determine (1) whether regional differences in estimates of variance components across the cortex primarily reflect differences in measurement reliability and (2) whether genetic factors contribute predominantly to phenotypic associations across different cortical neuroanatomical regions. For generalizability, we undertake the same analyses using an independent sample (237 twin pairs) from the Human Connectome Project (HCP).
Materials and Methods
Participants
Participants were from the Queensland Twin IMaging (QTIM) study of brain structure and function (de Zubicaray et al. 2008; Chiang et al. 2009; Joshi et al. 2011; Blokland et al. 2014; Whelan et al. 2016). Here we included 923 healthy, right-handed young adults (597 females, 326 males, mean age 22.27 ± 3.37 years, age range 15.40–30.11 years), consisting of 157 MZ pairs (106 females, 51 males), 194 DZ pairs (88 females, 30 males, 76 opposite sex), and 221 unpaired twins (75 MZ, 146 DZ; 133 females, 88 males). In addition, 53 participants were scanned a second time (mean duration between first and second scan was 113.36 ± 52.25 days) to assess the test–retest reliability of imaging measures. Sample demographics are described in Table 1. Prior to scanning, participants were screened for neurological and psychiatric conditions, including loss of consciousness for more than 5 min, and general MRI contraindications. Zygosity of same-sex twin pairs was determined using a commercial kit (AmpFISTR Profiler Plus Amplification Kit, ABI) and later confirmed by genome-wide single nucleotide polymorphism genotyping (Illumina 610K chip). The study was approved by the Human Research Ethics Committees of the University of Queensland, QIMR Berghofer Medical Research Institute, and UnitingCare Health. Written informed consent was obtained from all participants, including a parent or guardian for those aged under 18 years. Participants received an honorarium for their time and to cover any transport expenses.
Table 1.
Demographic characteristics (mean ± SD) of the QTIM samplea.
| Females | Males | Total | |
|---|---|---|---|
| Full sample | (n = 597 individuals) | (n = 326 individuals) | (n = 923 individuals) |
| Age (years) | 22.20 ± 3.32 | 22.39 ± 3.46 | 22.27 ± 3.37 |
| FIQb | 111.76 ± 12.14 | 116.79 ± 13.11 | 113.55 ± 12.72 |
| Gestational age (weeks)c | 36.94 ± 2.69 | 37.21 ± 2.62 | 37.04 ± 2.66 |
| Birth weight (kg) | 2.43 ± 0.50 | 2.64 ± 0.58 | 2.51 ± 0.54 |
| Socioeconomic index | 61.06 ± 23.73 | 63.77 ± 24.40 | 62.03 ± 23.99 |
| Total surface area (mm2) | 164 049.03 ± 13 046.33 | 184 379.82 ± 14 713.69 | 171 229.79 ± 16 759.10 |
| Mean cortical thickness (mm) | 2.51 ± 0.09 | 2.51 ± 0.09 | 2.51 ± 0.09 |
| Retest subsample | (n = 31 individuals) | (n = 22 individuals) | (n = 53 individuals) |
| Age (years) | 24.15 ± 2.09 | 23.19 ± 2.33 | 23.75 ± 2.22 |
| FIQ | 111.94 ± 11.88 | 120.91 ± 11.52 | 115.66 ± 12.45 |
| Gestational age (weeks) | 37.92 ± 2.02 | 37.88 ± 2.43 | 37.90 ± 2.23 |
| Birth weight (kg) | 2.60 ± 0.30 | 3.13 ± 0.51 | 2.80 ± 0.46 |
| Socioeconomic index | 52.59 ± 24.04 | 72.48 ± 25.94 | 61.17 ± 26.56 |
| Total surface area (mm2) | 160 742.11 ± 10 684.84 | 188 993.09 ± 12 223.13 | 172 468.93 ± 17 991.77 |
| Mean cortical thickness (mm) | 2.52 ± 0.08 | 2.47 ± 0.11 | 2.50 ± 0.10 |
aThe QTIM sample (N = 923 individuals) consisted of 157 MZ pairs, 194 DZ pairs, 75 unpaired MZ twins, and 146 unpaired DZ twins. Unpaired twins were included to improve estimates of means and variances. The retest sample (i.e., participants scanned twice) consisted of a subsample of 53 participants, including 11 MZ pairs, 12 DZ pairs, and 7 unpaired twins.
bFull-scale intelligence quotient (FIQ) measured using the Multidimensional Aptitude Battery (Jackson 1984) as close as possible to participants’ 16th birthday.
cGestational age, birth weight and socioeconomic status (McMillan et al. 2009) were obtained from parental reports when participants were 12 or 16 years of age.
Image Acquisition and Processing
Imaging was conducted on a 4T Bruker Medspec (Bruker, Germany) whole-body MRI system paired with a transverse electromagnetic (TEM) head coil. Structural T1-weighted 3D images were acquired ([TR = 1500 ms, TE = 3.35 ms, TI = 700 ms, 230 mm FOV], 0.9 mm slice thickness, 256 or 240 slices depending on acquisition orientation (86% coronal [256 slices], 14% sagittal [240 slices]). SA and CT were measured using FreeSurfer (v5.3; http://surfer.nmr.mgh.harvard.edu/) previously reported in depth (Fischl and Dale 2000). Prior to FreeSurfer analysis, the raw T1-weighted images were corrected for intensity inhomogeneity with SPM12 (Wellcome Trust Center for Neuroimaging, London, UK; http://www.fil.ion.ucl.ac.uk/spm). Total SA and mean CT were extracted for 34 ROI per hemisphere from the Desikan–Killiany atlas (Desikan et al. 2006) (Supplementary Fig. S1) contained within FreeSurfer. Measures for whole brain, global variables (total SA, mean CT) were also extracted. Cortical reconstructions and ROI labeling were checked using the procedures of the ENIGMA consortium (enigma.ini.usc.edu), with incorrectly delineated cortical structures excluded from the analysis. Prior to analysis, raw scores were standardized (i.e., converted to z-scores) and outliers at ±3.29 SD from the mean were replaced by the relevant threshold value (±3.29; see Supplementary Table S1 for the number of excluded and replaced values).
Heritabilities of SA and CT
The heritabilities of SA and CT were estimated for each ROI in a series of univariate ACE models using maximum-likelihood structural equation modeling using the OpenMx 2.7.11 package (Neale et al. 2016) in R 3.2.2 (R Core Team 2015). Briefly, the classic twin model partitions the variance within a phenotype into additive genetic (A), common or shared environment (C) and unique or nonshared environment (E) sources (Neale and Cardon 1992) (Supplementary Fig. S2a). Correlations between additive genetic factors (A) are fixed to 1 for MZ and 0.5 for DZ twins as MZ and DZ twins share 100% and (on average) 50% of their genetic material respectively. For common environment factors (C), correlations are fixed at 1 for both MZ and DZ twins (the model assumes MZ and DZ twins raised together experience similar environments), and the unique environment factors (E) are uncorrelated between twin pairs as this represents environmental influence affecting one twin only. Estimates of unique environment also include measurement error, as it is random and unrelated to twin similarity.
Univariate models included a simultaneous means regression to adjust for effects of whole brain total SA/mean CT, sex, linear and nonlinear age effects (modeled through normal splines with 3 degrees of freedom), interactions between age and sex, and MRI acquisition orientation. To determine the most parsimonious model, nested models containing AE, CE, or E sources of variance were compared with the fully saturated ACE decomposition. We assessed the fit of the constrained models by examining the −2 log likelihood difference between the ACE model and the reduced model (AE, CE, or E). The difference in the maximum likelihood (assessed through the −2 log likelihood difference) is distributed as a chi-squared statistic for a given number of degrees of freedom (equal to the difference in the number of free parameters estimated), which denotes whether the parameter is significant. If a reduced model is significant, this indicates that the parameter removed from the model accounted for a significant proportion of the phenotypic variance. Correlations between pairs of MZ and DZ twin were estimated in the model, and maximum-likelihood 95% confidence intervals were estimated for all model parameters. The significance of covariate effects was tested by fitting ACE models in which the covariate of interest was dropped from the model (i.e., the regression coefficient was set to zero), but all other covariates remained, and assessing the significance of the covariate via likelihood ratio test.
Test–Retest Reliability
Test–retest reliability was estimated by calculating the Pearson correlation coefficient between SA/CT measures from time one and time 2 scans (covariate effects were removed by using regression residuals; same covariates as for heritability estimates). The square of the Pearson correlation coefficient (r2) can be used to make direct comparisons between the portion of variability not explained by the repeats (1 − r2 test–retest) and e2 (variance due to nonshared environment and measurement error). Where e2 exceeds unreliability (1 − r2 test–retest), nonshared environmental influences are greater than measurement error. Conversely, measurement error is greater than nonshared environment when unreliability (1 − r2 test–retest) is greater than e2. While unreliability can be modeled to disassociate the unique environmental variance component (“E”) from measurement error (Luciano et al. 2001), the size of our retest sample is too small to provide an accurate estimate of measurement unreliability (i.e., the retest sample of 53 individuals is substantially smaller than the twin sample of 351 pairs; hence, estimates of measurement error will be much rougher, and have wider confidence intervals than estimates of genetic/environmental variance).
Associations Between Left/Right Homologous ROIs and Across Regions
Prior studies (Winkler et al. 2010; McKay et al. 2014) have combined left and right hemisphere ROIs to form 34 bilateral brain phenotypes, reducing the number of statistical tests and possibly increasing power. Here, before combining measures from left and right regions, we estimated correlations between corresponding left/right ROIs, and then tested whether the genetic variance to left and right regions was the same. Bivariate analyses extended the univariate design to decompose the variance in a trait, and also the covariance between 2 traits, into genetic and unique environmental sources (Supplementary Fig. S2b). As effects of common environment (C) were not significant in univariate models, they were not assessed in bivariate analyses. From the covariance decompositions, we further estimated genetic, unique or nonshared environmental (from this point forward referred to as environmental) and phenotypic correlations. Genetic (environmental) correlations indicate the extent to which 2 phenotypes share genetic (environmental) variance. Both genetic and unique environmental correlations underlie the phenotypic correlation. As a high genetic correlation between 2 traits can be observed, even if the traits themselves have low heritability, a high genetic correlation can be misleading when genes explain only a small portion of the phenotypic variance. Hence, we examined shared genetic influence between ROIs by calculating the genetic contribution to the phenotypic correlation (rph-a):
rph-a is easily conceptualized as the phenotypic correlation (rph) between 2 traits based only on the shared genetic variance. We similarly calculated the environmental contribution to the phenotypic correlation (rph-e). Both rph-a and rph-e were computed using variance estimates from the bivariate model in which the genetic (environmental) correlations were estimated. The significance of the genetic or environmental contribution to the phenotypic correlation was assessed by fitting a reduced model in which the genetic or environmental covariance between ROIs was set to zero and assessing model fit. The significance of the phenotypic correlation was assessed by setting both the genetic and environmental covariances between ROIs to zero.
The bivariate design specifies 2 genetic factors, and assumes that the first genetic factor explains genetic variance in the first phenotype and the second phenotype. Any genetic variance in the second phenotype (i.e., specific variance) which is not due to the first latent factor is assumed to be due to a second latent factor. We fitted a reduced decomposition in which the genetic factor specific to one hemisphere (i.e., the second latent factor) was dropped to assess whether this significantly reduced model fit. There were 2 brain regions for SA (parahippocampal gyrus, superior temporal gyrus) and 4 for CT (superior frontal gyrus, pars opercularis, supramarginal gyrus, and lateral occipital cortex) in which removal of the genetic factor specific to one hemisphere resulted in a significantly worse fit (i.e., the same, or one, genetic factor did not explain the genetic variance for both left and right brain regions). For all subsequent analyses, unilateral left/right ROIs were retained for these brain regions, with bilateral (mean of left and right) measures used for all other ROIs. In total, for SA we derived 32 bilateral (mean of left and right) and 4 unilateral regional measures (total of 36) and for CT 30 bilateral and 8 unilateral measures (total of 38).
Next, bivariate analyses were used to estimate phenotypic correlations, and the genetic and environmental contributions to the phenotypic correlations, for all possible pairs of SA and CT ROI measures (74 in total; 36 SA and 38 CT). These 2 701 pairwise AE models populated a 74 by 74 correlation matrix (one each for phenotypic correlations, genetic and environmental contributions). The R package corrplot (Wei and Simko 2016) was used to illustrate the correlation matrices. We also applied hierarchical clustering using Euclidian distances to the genetic association matrix using the R package gplots (Warnes et al. 2016).
We used the Benjamini–Hochberg procedure to control the false discovery rate (FDR) for the multiple comparisons (Benjamini and Hochberg 1995). Here, P values obtained from model fit likelihood ratio tests are ordered and ranked from smallest to largest (e.g., the smallest P value is ranked i = 1, the next smallest is ranked i = 2, and so on). The adjusted P value (or q value) is calculated by multiplying the individual P value (P(i)) by the total number of multiple comparisons (m) divided by the rank number (i): P(i)*(m/i). A q value less than 0.05 was considered significant. The procedure was applied separately to SA and CT results for (1) covariate effects (m = 612; significance of 9 covariates tested for 68 ROIs), (2) heritability estimates (m = 204; significance of 3 model fits (AE, CE, E) tested for 68 ROIs), (3) bivariate analyses of corresponding left/right regions (m = 102; significance of 3 covariances (phenotypic, genetic, environmental) tested between 34 left/right ROIs), (4) bivariate analyses of specific left/right genetic influence (m = 34; significance of dropping specific genetic variance factor for 34 ROIs), and (5) bivariate analyses of the 74 ROIs across the cortex (m = 630 for pairwise SA, m = 703 for pairwise CT, m = 1368 for pairwise SA and CT). All bivariate analyses included simultaneous means regressions to adjust for covariate effects (same covariates as for heritability estimates).
Replication in an Independent Sample
Data from the Human Connectome Project (S1200 release) (Van Essen et al. 2012; Glasser et al. 2016) was used as a secondary analysis sample. The S1200 release contains imaging data for 1113 individuals from families; here, we included only participants classified as a twin (by genotype or self-report), as well as the nontwin siblings of these participants. The final sample of 698 adults (399 females, 299 males, mean age: 29.30 ± 3.60 years, age range: 22–36 years) consisted of 152 MZ pairs (93 females, 59 males), 85 DZ pairs (52 females, 33 males), 203 singleton siblings of twins (1–2 per family; 96 females, 107 males), 10 members of singleton families (2 per family; 7 females, 3 males), and 11 unpaired twins (6 females, 5 males). MZ and DZ twin zygosity was determined through genotyping, if available (215 of 237 pairs), otherwise by self-report (22 of 237 pairs). A subset of twins (n = 45 individuals) were scanned a second time (mean duration between first and second scan was 139.30 ± 68.99 days). Details relating to participant selection and MRI acquisition have been reported (Van Essen et al. 2012). Preprocessed FreeSurfer SA and CT measures (Glasser et al. 2013) were used. We used bilateral composite measures (mean of left and right hemispheres) for all ROIs, with the exception of parahippocampal SA, pericalcarine SA, superior temporal SA, transverse temporal SA, and rostral anterior cingulate CT, where the genetic influence for left and right regions could not be constrained to one factor. All univariate and bivariate genetic analyses completed on the QTIM sample were undertaken on the HCP data, with simultaneous means regressions to control for covariate effects (same covariates as QTIM data, apart from acquisition orientation, which did not vary in the HCP dataset). Univariate and bivariate models were extended to include nontwin siblings in order to increase statistical power (Posthuma and Boomsma 2000).
Results
Preliminary Analyses
The global effect of SA/CT (i.e., total SA and whole brain average CT) on each ROI was highly significant; standardized regression coefficients ranged from 0.26 (entorhinal cortex right) to 0.86 (superior frontal gyrus right) for SA and 0.16 (entorhinal cortex right) to 0.81 (inferior parietal cortex right) for CT (regression coefficients and associated q values of all covariates are presented in Supplementary Tables S2 and S3). Test–retest correlations varied across ROIs, ranging from 0.30 to 0.97, and were generally high for SA (test–retest correlation > 0.70 for 55/68 ROIs) and more moderate for CT (test–retest correlation > 0.70 for 39/68 ROIs), with the mean reliability estimate (weighted by ROI size) being higher for SA (0.84) than CT (0.72). Test–retest reliability estimates are shown in Figure 1a/b (also provided in Supplementary Table S4 and displayed in Supplementary Fig. S3).
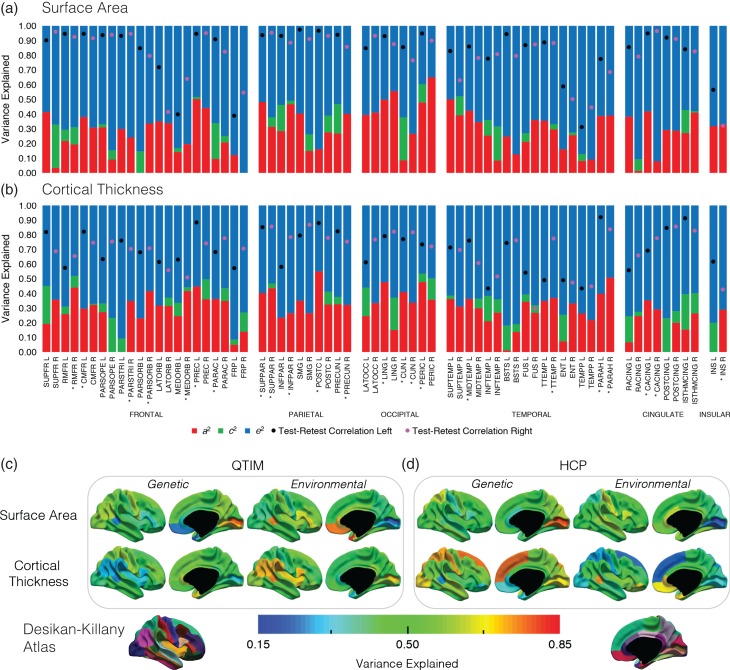
Figure 1.
Variance component estimates for SA (a) and CT (b) for 68 ROIs (34 in each of the left (L) and right (R) hemisphere). a2 = additive genetic (red), c2 = common environment (green), e2 = unique environment (blue). *ROIs with heritability estimates (a2) significantly different from zero. ROIs are grouped in lobar divisions (Frontal, Parietal, Occipital, Temporal, Cingulate, Insular cortex). Reliability of left and right ROIs are denoted by black and pink dots respectively. Heritability estimates for lobar divisions (mean of ROIs, weighted by size) were: frontal 0.27, parietal 0.33 occipital 0.43, temporal 0.31, cingulate 0.27, insular cortex 0.32 for SA and frontal 0.31, parietal 0.35, occipital 0.32, temporal 0.29, cingulate 0.18, insular cortex 0.15. The lower panel maps the genetic and environmental variance for SA (top row) and CT (bottom row) for up to 34 bilateral cortical regions in the QTIM (c) and HCP (d) samples. Heritability estimates range: 0.19–0.75 (QTIM SA), 0.27–0.68 (QTIM CT), 0.15–0.81 (HCP SA), 0.27–0.76 (HCP CT). For regions where genetic variance of left and right ROIs did not completely overlap (i.e., QTIM: SA of the superior temporal and parahippocampal gyrus, and CT of the superior frontal gyrus, pars opercularis, supramarginal gyri, and lateral occipital, HCP: SA of the superior temporal, parahippocampal, and transverse temporal gyri, pericalcarine cortex, and CT of the rostral anterior cingulate), the mean genetic (and environmental) variance of the left and right ROI is shown. N = 157/194 MZ/DZ pairs and 221 unpaired twins (QTIM), 152/85 MZ/DZ pairs and 224 siblings/unpaired twins (HCP). ROI abbreviations listed in Supplementary Figure S1.
Heritability of SA and CT
For almost all ROIs, for both SA (66/68) and CT (64/68) the MZ twin correlations were higher than the DZ correlations, suggesting individual variation in SA and CT is genetically influenced (Supplementary Table S4, Supplementary Fig. S4). ACE modeling indicated a range of heritability estimates across ROIs for both SA and CT (Supplementary Table S4; Fig. 1a/b), from not heritable up to 0.65 for SA and 0.55 for CT. Low heritability estimates were found for regions of poor reliability (e.g., insular cortex right [INS R] SA; heritability = 0.33, test–retest correlation = 0.32), but also for regions of high reliability (e.g., postcentral gyrus left [POSTC L] SA; heritability = 0.16, test–retest correlation = 0.97). As common environmental effects were small and nonsignificant, all remaining variance was due to environmental effects unique to the individual. Unique or nonshared environmental variance was greater than measurement error (i.e., e2 greater than unreliability (1 − r2 test–retest)) for the majority of SA (62/68) and CT (54/68) ROIs, suggesting that nongenetic variance was largely due to unique environmental factors rather than measurement error. Heritability estimates for total SA and whole brain average CT were 0.91 and 0.58, respectively (corrected for all covariates excluding total SA/mean CT).
In Figure 1c, using bilateral measures (mean of left and right ROIs, for regions in which this was suitable), we map both the genetic and environmental variance using an AE model. As with unilateral measures, heritability estimates varied across the cortex, ranging from 0.19 (frontal pole) to 0.75 (pericalcarine cortex) for SA and 0.28 (banks of the superior temporal sulcus) to 0.68 (pericalcarine cortex) for CT.
ROI Correlations Between Hemispheres
Phenotypic correlations between corresponding left/right ROIs, though varying widely in magnitude (rph −0.06 to 0.65; Fig. 2), were all significant. Phenotypic correlations greater than 0.40 were largely due to strong genetic covariation between left and right hemispheres. In contrast, phenotypic correlations less than 0.20 were due to weak genetic and environmental covariance, except for SA of the caudal anterior cingulate where the low phenotypic correlation between left and right hemispheres (rph −0.06) was due to genetic and environmental contributions of opposing directions (rph-a 0.17, rph-e −0.23).
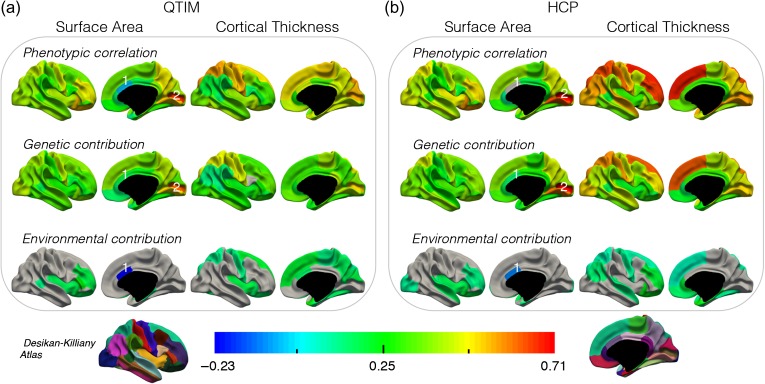
Figure 2.
Phenotypic correlations, with genetic and environmental contributions, between corresponding left/right ROIs for SA and CT in the QTIM (a) and HCP (b) samples. Phenotypic correlations between corresponding left/right ROIs ranged from −0.05 to 0.65 (all significant) for QTIM and from 0.01 to 0.71 (all significant except caudal anterior cingulate SA) for HCP. Genetic covariance accounted primarily for phenotypic correlations between hemispheres, but this was not always the case (e.g., genetic and environmental influence of a similar magnitude contributed to the phenotypic correlation left and right CT of the superior frontal gyrus in the QTIM sample). While low phenotypic correlations were typically due to very weak genetic and environmental contributions, they were also found to mask somewhat larger opposing genetic and environmental influences in the caudal anterior cingulate SA (see footnote 1 below). Regions with correlations not significantly different from zero are in grey. 1For one region there was significant opposing genetic and environmental influence (caudal anterior cingulate) SA: QTIM − rph −0.06, rph-a 0.17, rph-e −0.23, HCP − rph 0.01 (estimate not significant, q = 0.051), rph-a 0.13, rph-e −0.11. 2In both samples, the highest phenotypic correlation was for pericalcarine SA (rph 0.65 (QTIM) and 0.71 (HCP)), with associations due almost entirely to genetic contributions.
ROI Correlations Across the Cortex
There was a complex pattern of associations across cortical regions. For SA, strong correlations were found between occipital lobe ROIs (rph 0.23–0.63), with weaker and mainly negative associations across lobar divisions (Fig. 3a). Phenotypic associations (rph −0.30 to 0.63) were predominantly due to genetic contributions (rph-a −0.26 to 0.54; Fig. 3b, lower) with very little environmental covariance (rph-e −0.20 to 0.19; Fig. 3b, upper). A much stronger pattern of positive correlations between regions within a lobar division was found for CT (Fig. 3c). Moderate negative correlations were found between regions not sharing spatial proximity. Some regions (e.g., rostral middle frontal gyrus [RMFR], rostral anterior cingulate [RACING]) were associated with the majority of ROIs. The posterior (POSTCING) and isthmus (ISTHMCING) divisions of the cingulate were generally not associated with other regions across the cortex. Genetic contributions (rph-a −0.36 to 0.37; Fig. 3d, lower) largely accounted for phenotypic correlations (rph −0.42 to 0.59; Fig. 3c), though some sparse patterns of environmental covariance (rph-e −0.22 to 0.27; Fig. 3d, upper) were present between prefrontal and temporal ROIs. The application of hierarchical cluster analysis to the genetic association matrices generally resulted in patterns following lobar organization for CT, whereas for SA, outside of occipital ROIs, there was a greater clustering of regions from different lobes (Supplementary Fig. S5).
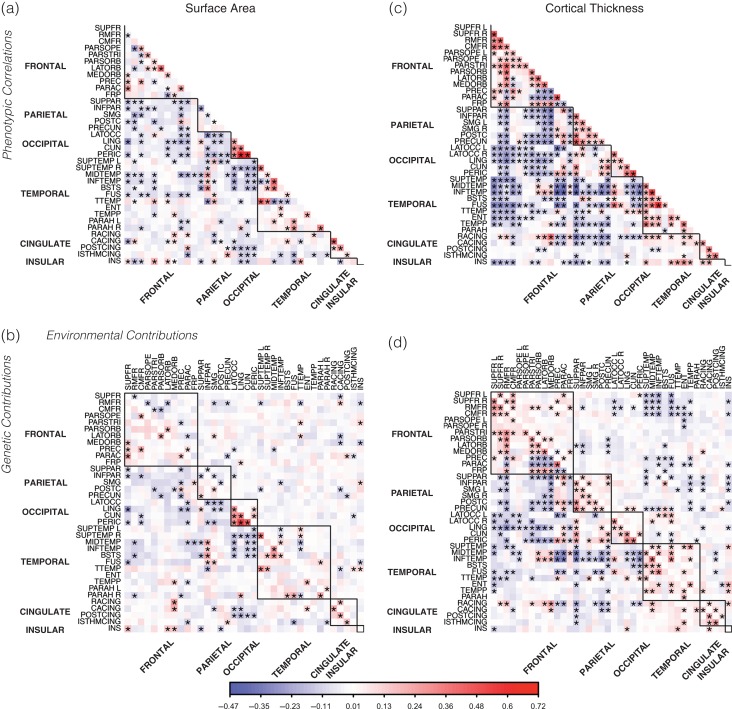
Figure 3.
Phenotypic correlations, with genetic and environmental contributions, for SA and CT across regions in the QTIM sample. SA (a) was mainly associated within rather than across lobar divisions, particularly for the occipital lobe. Phenotypic associations were predominantly due to genetic associations (c; lower triangle), with very few environmental associations (c; upper triangle). For CT (b) there is a much stronger pattern of associations. Correlations are positive within-lobe and negative between-lobes. These phenotypic associations are due largely to genetic covariance (d; lower triangle), however, some associations are due to environmental covariance (d; upper diagonal). *A significant correlation (q value < 0.05). ROI abbreviations listed in Supplementary Figure S1.
ROI Correlations Between SA and CT
While we found that whole brain total SA and mean CT were inversely associated (rph −0.26, rph-a = −0.21, rph-e = −0.05; measures corrected for all covariates excluding total SA/mean CT), at the regional level associations between SA and CT were sparse and while some were negative, others were positive (range: rph −0.34 to 0.25, rph-a −0.25 to 0.21, rph-e −0.31 to 0.15; Supplementary Fig. S6). These associations were weak, due to low genetic and environmental covariation, with the exception of pericalcarine SA and CT in which a positive genetic and negative environmental contribution resulted in a weak phenotypic correlation (rph 0.04, rph-a 0.13, rph-e −0.09).
Replication Using the HCP Sample
Patterns of genetic and environmental (co)variance were similar across the QTIM and HCP datasets (Figs 1d and 2b, Supplementary Tables S5–S8, Supplementary Figs S7–S11). Evidence of separate genetic influence on left and right regions for parahippocampal and superior temporal gyri SA was replicated. Further, the pattern of strong associations between SA measures for occipital lobe ROIs, as well as the same pattern of positive associations within, and negative associations between, CT ROIs, was found. These associations were predominantly due to genetic factors, with some thinly dispersed environmental influence contributing to several CT associations.
Discussion
Here we extend prior work, showing there is a strong, region-specific genetic influence on the area and thickness of the human cerebral cortex (SA up to 65%; CT up to 55%). A wide range of heritabilities, for both SA and CT, is evident across the cortex, largely independent of measurement error. We also show that there is substantial environmental variance, which is not due to measurement error, but find little evidence for any strong common (shared) environmental factors. In addition, we show that associations across regions are surprisingly complex. While for most regions genetic covariance accounts for phenotypic correlations between hemispheres, this was not always the case. For some ROIs the same genetic factor did not explain the genetic variance for both left and right hemisphere measures. Further, when we covaried for total SA, with the exception of the occipital lobe, regional SA was only weakly associated, suggesting that there are limited region-specific interrelationships for SA. In contrast, CT of neighboring regions was associated after controlling for mean CT; positively within lobar divisions and negatively across regions, mainly due to genetic covariance. Lastly, correlations between SA and CT at the regional level did not follow the inverse relationship observed at the global level, rather there was a complex pattern of negative and positive regional associations.
We report several novel results. Firstly, the striking finding that SA/CT heritability estimates vary widely, largely independent of test–retest reliability, that is, while high reliability was required to detect high heritability, a high reliability estimate did not guarantee a substantial genetic effect. Notably, across ROIs with high reliability (85 [QTIM] and 114 [HCP] out of 136 ROIs with test–retest correlation >0.75) heritability estimates ranged from zero to 0.65 (QTIM) and 0.79 (HCP). Also, while in general heritability estimates for QTIM and HCP were in line with those reported previously, particularly for ROIs with high test–retest reliability, there were some notable exceptions. For example, heritability for CT of the right parahippocampal gyrus (reliability ~ 0.85 in QTIM and HCP) ranged from 6% to 55% across 5 datasets: QTIM, HCP, National Institute of Mental Health (Schmitt et al. 2008), Genetics of Brain Structure and Function Study (Winkler et al. 2010), Vietnam Era Twin Study of Ageing (Eyler et al. 2012). In addition to differences across the samples, differences in imaging and/or genetic analyses may, in part, underlie these contrasting results.
The wide range of heritability estimates we report here may relate to cortical neuroplasticity; an intrinsic characteristic of the cortex (Pascual-Leone et al. 2005; Jancke 2009). Underlying differences in tissue organization and microstructure permit neural circuit plasticity (Zatorre et al. 2012), allowing neural circuity to be formed/altered due to sensory experience and learning (Lendvai et al. 2000; Pantev et al. 2001; Trachtenberg et al. 2002). Indeed, we found a substantial environmental influence (e2 > 0.60), which was not due to measurement error (test–retest correlation >0.75), on both SA and CT for several association areas in both the QTIM and HCP datasets (SA: rostral/caudal middle frontal gyrus left and right, pars opercularis left and right, CT: posterior cingulate left and right). Greater environmental variation may be indicative of functional cortical areas adapting to environmental stimuli for development/function. This notion is consistent with a comparative study of nonhuman primates, (Gomez-Robles et al. 2015), where human cortical morphology was shown to be substantially less heritable than in chimpanzees (lobe and sulcal dimension heritability estimates up to 0.65 in humans and 0.77 in chimpanzees), with the lower heritability of human cortical organization attributed to greater plasticity in humans. Though speculative, this capacity for greater plasticity in humans may also contribute to gene by environment interaction effects. Further, while plasticity is often discussed in terms of positive effects (e.g., learning), there are likely adverse effects associated with increased plasticity (e.g., a greater possibility of maladaptive brain circuits).
Interestingly, the broad range of cortical heritability estimates we report here contrasts with that found for subcortical brain structures in the same sample (Renteria et al. 2014). For subcortical structure volumes, environmental variance was smaller and genetic variance substantially larger, even though test–retest reliability estimates for subcortical and cortical measures are comparable. The supposition that cortical variation is more environmentally mediated than subcortical variation is reasonable, given the cortex’s role in social interaction and learning, characteristics essential for human cognitive function and development (Boyd et al. 2011). Conversely, the evolutionarily older subcortical system, responsible for behavior regulation, emotion and memory, may require and/or be less malleable to interaction with the environment. This is not to say that subcortical structures are incapable of plasticity; an increasing body of work provides evidence for hippocampal and amygdala plasticity (Leuner and Gould 2010; Sahay et al. 2011; Kuhn et al. 2014; Rabl et al. 2014; Jhaveri et al. 2017). Rather, we speculate that it is the uniquely human abilities afforded by the cerebral cortex (and their dependency upon environmental influence) that is responsible for the higher levels of environmental variance found in the cortex.
In addition to the wide range in heritability estimates, a second major finding was the complex patterning of genetic and environmental covariation, which had not been investigated previously. When we directly examined whether different genetic factors influence left/right cortical regions we found a specific (different) genetic influence between hemispheres for 2 regions—superior temporal gyrus SA, parahippocampal gyrus SA; findings replicated in the HCP sample. This specific left/right genetic influence has not been previously found (Eyler et al. 2014), likely due to the larger sample size of the present study. Further complexity was evident from the vastly different covariance patterns found for SA compared with CT. SA measures for neuroanatomical regions did not correlate highly (outside of the occipital lobe), suggesting that when covaried for total SA, there are limited, and generally weak, region-specific genetic and environmental contributions to covariance across the area of cortical regions. Intriguingly, the occipital lobe appears as an anomaly. Structural covariation between occipital regions, occurring not as a function of overall brain size, has previously been shown in ex-vivo measurements (Andrews et al. 1997). The authors related the importance of coordinated occipital variation to visual ability, though whether the degree of structural covariation relates to individual differences in visual ability is currently unknown. In the present study, structural interdependence between occipital regions appears mainly due to genetic factors, with some environmental influence, possibly in the form of experience-expectant plasticity (Greenough et al. 1987).
The finding of generally weak genetic associations across the cortex for SA appear to contrast with the work of Chen et al. (2012), in which 12, maximally genetically correlated divisions of the cortical surface were identified based on vertex-wise (i.e., continuous) SA measures (corrected for total SA). How could genetic covariance in SA be best explained by 12 divisions, when the present study found generally weak associations (outside of the occipital lobe) between regional SA? Firstly, vertex-based approaches to SA measurement are more heritable than ROI-based SA measures, possibly due to the degree of spatial averaging in vertex-based approaches (Eyler et al. 2012), which could result in higher genetic correlations. Secondly, it is important to reiterate that the findings of Chen et al. (2012) and those of the present study are based on different metrics. Chen et al. (2012) examined genetic covariance through genetic correlations (i.e., the extent to which 2 ROIs share genetic variance, regardless of their contribution to phenotypic covariance), whereas we examined genetic correlations weighted by heritability to standardize genetic covariance in terms of its contribution to phenotypic covariance. Thus, these 2 metrics answer different questions regarding the role of genetics in cortical organization.
In contrast to SA, we found CT covaried weakly to strongly across regions, and as effects of mean CT were removed, these associations were not due to a global factor. Highly correlated cortical regions share maturational trajectories (Mechelli et al. 2005; Alexander-Bloch, Raznahan, et al. 2013; Fjell et al. 2015) and form systems underlying perception, behavior and cognition (Alexander-Bloch, Giedd, et al. 2013; Evans 2013; Vertes and Bullmore 2015; Richmond et al. 2016). With genetic factors largely accounting for structural associations, these results suggest genes play an important role in the organizational principles of the cortex. Only Schmitt et al. (2008) has examined genetic covariance between CT measures of neuroanatomical regions. Using hierarchical cluster analysis, they identified 2 major blocks: a temporo-occipital cluster and a frontoparietal cluster. We did not find the same divisions, rather in QTIM we found a parietal/occipital cluster and a fronto-temporo-cingulate cluster, and in HCP we found a frontal cluster and temporo-occipital-cingulate-parietal cluster. These results suggest that patterns of genetic covariance in CT may not be stable from childhood to adulthood.
Our finding that SA and CT are only weakly associated (both negatively and positively) over a number of neuroanatomical regions, replicates and extends prior work showing regional SA and CT to be distinct characteristics of the cortex (Panizzon et al. 2009; Winkler et al. 2010). As noted previously (Panizzon et al. 2009), these differences likely correspond to the cellular architecture of the cortex; neurons within the cerebral cortex are organized into ontogenetic columns (Mountcastle 1997), with the size, number and density of cells within columns hypothesized to determine thickness, and the number of cortical columns responsible for SA (Rakic 1988). Thus, it is entirely rational that the genetic and environmental factors that influence variation in SA are separate from those responsible for CT variation. The unique contributions of SA and CT variation to cortical morphometry and function should not be undervalued.
In examining variation within the cerebral cortex, it is important not to disregard the effects of age and sex, as well as global factors. Very strong relationships between regional and global measures were found for SA and to a lesser extent, CT. Prior studies have reported a degree of genetic overlap between global and regional measures, stronger for SA than CT (Winkler et al. 2010; Eyler et al. 2012). Without adjustment for global effects, it is likely that genetic associations across cortical regions would be substantially higher, particularly for SA. Whether regions with a greater degree of region-specific genetic variance provide better phenotypes for genome association studies is an intriguing future research direction. Subtle but complex age and sex effects were found in the current sample. Age and sex effects are well documented for CT (Salat et al. 2004; Sowell et al. 2007; Tamnes et al. 2010; van Soelen et al. 2012), but effects for SA have been difficult to establish as studies have focused on cortical volume rather than area. Based on the present results, we posit that after controlling for total cortical area, small and sparse regional sex differences in cortical SA are present. Studies of sexual dimorphisms within the cortex may provide insights into disorders with sex specific risk factors.
A limitation of the present study is that SA and CT measures were estimated for cortical regions based on macroanatomical landmarks (gyri and sulci), with such regions not completely representing cytoarchitectural variation within the cortex. Measures of genetic covariation on a continuous basis throughout the cortical ribbon (Schmitt et al. 2009; Eyler et al. 2012; Chen et al. 2013; Cui et al. 2016) may provide an elegant means to circumvent this limitation. Also, cortical parcellations based on structural, functional, and connectional imaging data (Fan et al. 2016; Glasser et al. 2016) provide an enticing basis for ROI measures with increased neuroanatomical precision. Nevertheless, the Desikan–Killiany atlas has been, and will continue to be, one of the main cortical parcellations used in studying SA and CT. An understanding of genetically and environmentally mediated variance within these regions will aid interpretation of past and future studies using this cortical parcellation.
Conclusions
A complex pattern of genetic and environmental factors influence both the SA and CT of the cerebral cortex. When controlling for global effects, region-specific genetic factors account for much of the structural variation within anatomically distinct cortical regions, but environmental sources are clearly involved. Identifying cellular and molecular level changes within the brain which intercede between genetically and environmentally mediated variation is a challenging next step, but one that will likely go far in advancing our understanding of the origins of normal and abnormal brain circuitry.
Supplementary Material
Notes
We are forever grateful to the twins and siblings for their willingness to participate in our studies. We thank Marlene Grace and Ann Eldridge for participant recruitment; Kerrie McAloney for study co-ordination; Kori Johnson, Aaron Quiggle, Natalie Garden, Matthew Meredith, Peter Hobden, Kate Borg, Aiman Al Najjar and Anita Burns for data acquisition; David Butler and Daniel Park for IT support. Conflict of Interest: None declared.
Funding
The Queensland Twin IMaging (QTIM) study was supported by the National Institute of Child Health and Human Development (R01 HD050735), and the National Health and Medical Research Council (NHMRC 486682, 1009064), Australia. Lachlan Strike was supported by an Australian Government Research Training Program (RTP) scholarship and a Queensland Brain Institute (QBI) top-up scholarship. Data were provided [in part] by the Human Connectome Project, Washington University - University of Minnesota Consortium of the Human Connectome Project (WU-Minn HCP) (Principal Investigators: David Van Essen and Kamil Ugurbil; 1U54MH091657) funded by the 16 National Institutes of Health (NIH) Institutes and Centers that support the NIH Blueprint for Neuroscience Research; and by the McDonnell Center for Systems Neuroscience at Washington University.
References
- Alexander-Bloch A, Giedd JN, Bullmore E. 2013. Imaging structural co-variance between human brain regions. Nat Rev Neurosci. 14:322–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander-Bloch A, Raznahan A, Bullmore E, Giedd J. 2013. The convergence of maturational change and structural covariance in human cortical networks. J Neurosci. 33:2889–2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews TJ, Halpern SD, Purves D. 1997. Correlated size variations in human visual cortex, lateral geniculate nucleus, and optic tract. J Neurosci. 17:2859–2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakken TE, Roddey JC, Djurovic S, Akshoomoff N, Amaral DG, Bloss CS, Casey BJ, Chang L, Ernst TM, Gruen JR, et al. , Alzheimer’s Disease Neuroimaging Initiative; Pediatric Imaging, Neurocognition, and Genetics Study . 2012. Association of common genetic variants in GPCPD1 with scaling of visual cortical surface area in humans. Proc Natl Acad Sci USA. 109:3985–3990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. 1995. Controlling the false discovery rate—a practical and powerful approach to multiple testing. J R Stat Soc Series B Methodol. 57:289–300. [Google Scholar]
- Blokland GA, McMahon KL, Thompson PM, Hickie IB, Martin NG, de Zubicaray GI, Wright MJ. 2014. Genetic effects on the cerebellar role in working memory: same brain, different genes? Neuroimage. 86:392–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd R, Richerson PJ, Henrich J. 2011. The cultural niche: why social learning is essential for human adaptation. Proc Natl Acad Sci USA. 108(Suppl 2):10918–10925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CH, Fiecas M, Gutierrez ED, Panizzon MS, Eyler LT, Vuoksimaa E, Thompson WK, Fennema-Notestine C, Hagler DJ Jr, Jernigan TL, et al. 2013. Genetic topography of brain morphology. Proc Natl Acad Sci USA. 110:17089–17094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CH, Gutierrez ED, Thompson W, Panizzon MS, Jernigan TL, Eyler LT, Fennema-Notestine C, Jak AJ, Neale MC, Franz CE, et al. 2012. Hierarchical genetic organization of human cortical surface area. Science. 335:1634–1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang MC, Barysheva M, Shattuck DW, Lee AD, Madsen SK, Avedissian C, Klunder AD, Toga AW, McMahon KL, de Zubicaray GI, et al. 2009. Genetics of brain fiber architecture and intellectual performance. J Neurosci. 29:2212–2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Y, Liu B, Zhou Y, Fan L, Li J, Zhang Y, Wu H, Hou B, Wang C, Zheng F, et al. 2016. Genetic effects on fine-grained human cortical regionalization. Cereb Cortex. 26:3732–3743. [DOI] [PubMed] [Google Scholar]
- de Zubicaray GI, Chiang MC, McMahon KL, Shattuck DW, Toga AW, Martin NG, Wright MJ, Thompson PM. 2008. Meeting the challenges of neuroimaging genetics. Brain Imaging Behav. 2:258–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, et al. 2006. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 31:968–980. [DOI] [PubMed] [Google Scholar]
- Docherty AR, Sawyers CK, Panizzon MS, Neale MC, Eyler LT, Fennema-Notestine C, Franz CE, Chen CH, McEvoy LK, Verhulst B, et al. 2015. Genetic network properties of the human cortex based on regional thickness and surface area measures. Front Hum Neurosci. 9:440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson KI, Leckie RL, Weinstein AM. 2014. Physical activity, fitness, and gray matter volume. Neurobiol Aging. 35(Suppl 2):S20–S28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans AC. 2013. Networks of anatomical covariance. Neuroimage. 80:489–504. [DOI] [PubMed] [Google Scholar]
- Eyler LT, Chen CH, Panizzon MS, Fennema-Notestine C, Neale MC, Jak A, Jernigan TL, Fischl B, Franz CE, Lyons MJ, et al. 2012. A comparison of heritability maps of cortical surface area and thickness and the influence of adjustment for whole brain measures: a magnetic resonance imaging twin study. Twin Res Hum Genet. 15:304–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyler LT, Vuoksimaa E, Panizzon MS, Fennema-Notestine C, Neale MC, Chen CH, Jak A, Franz CE, Lyons MJ, Thompson WK, et al. 2014. Conceptual and data-based investigation of genetic influences and brain asymmetry: a twin study of multiple structural phenotypes. J Cogn Neurosci. 26:1100–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan L, Li H, Zhuo J, Zhang Y, Wang J, Chen L, Yang Z, Chu C, Xie S, Laird AR, et al. 2016. The Human Brainnetome Atlas: a new brain atlas based on connectional architecture. Cereb Cortex. 26:3508–3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Dale AM. 2000. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci USA. 97:11050–11055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fjell AM, Grydeland H, Krogsrud SK, Amlien I, Rohani DA, Ferschmann L, Storsve AB, Tamnes CK, Sala-Llonch R, Due-Tonnessen P, et al. 2015. Development and aging of cortical thickness correspond to genetic organization patterns. Proc Natl Acad Sci USA. 112:15462–15467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geschwind DH, Rakic P. 2013. Cortical evolution: judge the brain by its cover. Neuron. 80:633–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasser MF, Coalson TS, Robinson EC, Hacker CD, Harwell J, Yacoub E, Ugurbil K, Andersson J, Beckmann CF, Jenkinson M, et al. 2016. A multi-modal parcellation of human cerebral cortex. Nature. 536:171–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasser MF, Sotiropoulos SN, Wilson JA, Coalson TS, Fischl B, Andersson JL, Xu J, Jbabdi S, Webster M, Polimeni JR, et al. , WU-Minn HCP Consortium . 2013. The minimal preprocessing pipelines for the Human Connectome Project. Neuroimage. 80:105–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Robles A, Hopkins WD, Schapiro SJ, Sherwood CC. 2015. Relaxed genetic control of cortical organization in human brains compared with chimpanzees. Proc Natl Acad Sci USA. 112:14799–14804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenough WT, Black JE, Wallace CS. 1987. Experience and brain development. Child Dev. 58:539–559. [PubMed] [Google Scholar]
- Jackson DN. 1984. MAB, multidimensional aptitude battery: manual. Port Huron Michigan: Research Psychologists Press. [Google Scholar]
- Jancke L. 2009. The plastic human brain. Restor Neurol Neurosci. 27:521–538. [DOI] [PubMed] [Google Scholar]
- Jhaveri DJ, Tedoldi A, Hunt S, Sullivan R, Watts NR, Power JM, Bartlett PF, Sah P. 2017. Evidence for newly generated interneurons in the basolateral amygdala of adult mice. Mol Psychiatry. Advance accessed published August 15, 2017. doi:10.1038/mp.2017.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi AA, Lepore N, Joshi SH, Lee AD, Barysheva M, Stein JL, McMahon KL, Johnson K, de Zubicaray GI, Martin NG, et al. 2011. The contribution of genes to cortical thickness and volume. Neuroreport. 22:101–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS. 2013. What psychiatric genetics has taught us about the nature of psychiatric illness and what is left to learn. Mol Psychiatry. 18:1058–1066. [DOI] [PubMed] [Google Scholar]
- Kremen WS, Prom-Wormley E, Panizzon MS, Eyler LT, Fischl B, Neale MC, Franz CE, Lyons MJ, Pacheco J, Perry ME, et al. 2010. Genetic and environmental influences on the size of specific brain regions in midlife: the VETSA MRI study. Neuroimage. 49:1213–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn S, Gleich T, Lorenz RC, Lindenberger U, Gallinat J. 2014. Playing Super Mario induces structural brain plasticity: gray matter changes resulting from training with a commercial video game. Mol Psychiatry. 19:265–271. [DOI] [PubMed] [Google Scholar]
- Lendvai B, Stern EA, Chen B, Svoboda K. 2000. Experience-dependent plasticity of dendritic spines in the developing rat barrel cortex in vivo. Nature. 404:876–881. [DOI] [PubMed] [Google Scholar]
- Leuner B, Gould E. 2010. Structural plasticity and hippocampal function. Annu Rev Psychol. 61:111–140, C111–C113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luciano M, Wright MJ, Smith GA, Geffen GM, Geffen LB, Martin NG. 2001. Genetic covariance among measures of information processing speed, working memory, and IQ. Behav Genet. 31:581–592. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Morrison JH. 2013. The brain on stress: vulnerability and plasticity of the prefrontal cortex over the life course. Neuron. 79:16–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay DR, Knowles EE, Winkler AA, Sprooten E, Kochunov P, Olvera RL, Curran JE, Kent JW Jr, Carless MA, Goring HH, et al. 2014. Influence of age, sex and genetic factors on the human brain. Brain Imaging Behav. 8:143–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMillan J, Beavis A, Jones FL. 2009. The AUSEI06. J Soc. 45:123–149. [Google Scholar]
- Mechelli A, Friston KJ, Frackowiak RS, Price CJ. 2005. Structural covariance in the human cortex. J Neurosci. 25:8303–8310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mountcastle VB. 1997. The columnar organization of the neocortex. Brain. 120(Pt 4):701–722. [DOI] [PubMed] [Google Scholar]
- Neale MC, Cardon LR. 1992. Methodology for genetic studies of twins and families. Dordrecht, The Netherlands: Springer. [Google Scholar]
- Neale MC, Hunter MD, Pritikin JN, Zahery M, Brick TR, Kirkpatrick RM, Estabrook R, Bates TC, Maes HH, Boker SM. 2016. OpenMx 2.0: extended structural equation and statistical modeling. Psychometrika. 81:535–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panizzon MS, Fennema-Notestine C, Eyler LT, Jernigan TL, Prom-Wormley E, Neale MC, Jacobson K, Lyons MJ, Grant MD, Franz CE, et al. 2009. Distinct genetic influences on cortical surface area and cortical thickness. Cereb Cortex. 19:2728–2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantev C, Engelien A, Candia V, Elbert T. 2001. Representational cortex in musicians. Plastic alterations in response to musical practice. Ann NY Acad Sci. 930:300–314. [PubMed] [Google Scholar]
- Pascual-Leone A, Amedi A, Fregni F, Merabet LB. 2005. The plastic human brain cortex. Annu Rev Neurosci. 28:377–401. [DOI] [PubMed] [Google Scholar]
- Peng Q, Schork A, Bartsch H, Lo MT, Panizzon MS, Pediatric Imaging, Neurocognition and Genetics Study . Westlye LT, Kremen WS, Jernigan TL, Le Hellard S, Steen VM, et al. 2016. Conservation of distinct genetically-mediated human cortical pattern. PLoS Genet. 12:e1006143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posthuma D, Boomsma DI. 2000. A note on the statistical power in extended twin designs. Behav Genet. 30:147–158. [DOI] [PubMed] [Google Scholar]
- R Core Team 2015. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- Rabl U, Meyer BM, Diers K, Bartova L, Berger A, Mandorfer D, Popovic A, Scharinger C, Huemer J, Kalcher K, et al. 2014. Additive gene-environment effects on hippocampal structure in healthy humans. J Neurosci. 34:9917–9926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakic P. 1988. Specification of cerebral cortical areas. Science. 241:170–176. [DOI] [PubMed] [Google Scholar]
- Rakic P. 2009. Evolution of the neocortex: a perspective from developmental biology. Nat Rev Neurosci. 10:724–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renteria ME, Hansell NK, Strike LT, McMahon KL, de Zubicaray GI, Hickie IB, Thompson PM, Martin NG, Medland SE, Wright MJ. 2014. Genetic architecture of subcortical brain regions: common and region-specific genetic contributions. Genes Brain Behav. 13:821–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond S, Johnson KA, Seal ML, Allen NB, Whittle S. 2016. Development of brain networks and relevance of environmental and genetic factors: a systematic review. Neurosci Biobehav Rev. 71:215–239. [DOI] [PubMed] [Google Scholar]
- Sahay A, Scobie KN, Hill AS, O’Carroll CM, Kheirbek MA, Burghardt NS, Fenton AA, Dranovsky A, Hen R. 2011. Increasing adult hippocampal neurogenesis is sufficient to improve pattern separation. Nature. 472:466–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salat DH, Buckner RL, Snyder AZ, Greve DN, Desikan RS, Busa E, Morris JC, Dale AM, Fischl B. 2004. Thinning of the cerebral cortex in aging. Cereb Cortex. 14:721–730. [DOI] [PubMed] [Google Scholar]
- Schmitt JE, Lenroot RK, Ordaz SE, Wallace GL, Lerch JP, Evans AC, Prom EC, Kendler KS, Neale MC, Giedd JN. 2009. Variance decomposition of MRI-based covariance maps using genetically informative samples and structural equation modeling. Neuroimage. 47:56–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt JE, Lenroot RK, Wallace GL, Ordaz S, Taylor KN, Kabani N, Greenstein D, Lerch JP, Kendler KS, Neale MC, et al. 2008. Identification of genetically mediated cortical networks: a multivariate study of pediatric twins and siblings. Cereb Cortex. 18:1737–1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnack HG, van Haren NE, Brouwer RM, Evans A, Durston S, Boomsma DI, Kahn RS, Hulshoff Pol HE. 2015. Changes in thickness and surface area of the human cortex and their relationship with intelligence. Cereb Cortex. 25:1608–1617. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Peterson BS, Kan E, Woods RP, Yoshii J, Bansal R, Xu D, Zhu H, Thompson PM, Toga AW. 2007. Sex differences in cortical thickness mapped in 176 healthy individuals between 7 and 87 years of age. Cereb Cortex. 17:1550–1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamnes CK, Ostby Y, Fjell AM, Westlye LT, Due-Tonnessen P, Walhovd KB. 2010. Brain maturation in adolescence and young adulthood: regional age-related changes in cortical thickness and white matter volume and microstructure. Cereb Cortex. 20:534–548. [DOI] [PubMed] [Google Scholar]
- Trachtenberg JT, Chen BE, Knott GW, Feng G, Sanes JR, Welker E, Svoboda K. 2002. Long-term in vivo imaging of experience-dependent synaptic plasticity in adult cortex. Nature. 420:788–794. [DOI] [PubMed] [Google Scholar]
- Van Essen DC, Ugurbil K, Auerbach E, Barch D, Behrens TE, Bucholz R, Chang A, Chen L, Corbetta M, Curtiss SW, et al. , WU-Minn HCP Consortium . 2012. The Human Connectome Project: a data acquisition perspective. Neuroimage. 62:2222–2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Soelen IL, Brouwer RM, van Baal GC, Schnack HG, Peper JS, Collins DL, Evans AC, Kahn RS, Boomsma DI, Hulshoff Pol HE. 2012. Genetic influences on thinning of the cerebral cortex during development. Neuroimage. 59:3871–3880. [DOI] [PubMed] [Google Scholar]
- Vertes PE, Bullmore ET. 2015. Annual research review: growth connectomics—the organization and reorganization of brain networks during normal and abnormal development. J Child Psychol Psychiatry. 56:299–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warnes GR, Bolker B, Bonebakker L, Gentleman R, Huber W, Liaw A, Lumley T, Maechler M, Magnusson A, Moeller S, et al. 2016. gplots: Various R Programming Tools for Plotting Data https://cran.r-project.org/package=gplots.
- Wei T, Simko V. 2016. corrplot: Visualization of a correlation matrix.
- Whelan CD, Hibar DP, van Velzen LS, Zannas AS, Carrillo-Roa T, McMahon K, Prasad G, Kelly S, Faskowitz J, deZubiracay G, et al. 2016. Heritability and reliability of automatically segmented human hippocampal formation subregions. Neuroimage. 128:125–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler AM, Kochunov P, Blangero J, Almasy L, Zilles K, Fox PT, Duggirala R, Glahn DC. 2010. Cortical thickness or grey matter volume? The importance of selecting the phenotype for imaging genetics studies. Neuroimage. 53:1135–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zatorre RJ, Fields RD, Johansen-Berg H. 2012. Plasticity in gray and white: neuroimaging changes in brain structure during learning. Nat Neurosci. 15:528–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Castellanos FX. 2016. Annual Research Review: discovery science strategies in studies of the pathophysiology of child and adolescent psychiatric disorders—promises and limitations. J Child Psychol Psychiatry. 57:421–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.