Abstract
Cortical thickness (CT) and surface area (SA) vary widely between individuals and are associated with intellectual ability and risk for various psychiatric and neurodevelopmental conditions. Factors influencing this variability remain poorly understood, but the radial unit hypothesis, as well as the more recent supragranular cortex expansion hypothesis, suggests that prenatal and perinatal influences may be particularly important. In this report, we examine the impact of 17 major demographic and obstetric history variables on interindividual variation in CT and SA in a unique sample of 805 neonates who received MRI scans of the brain around 2 weeks of age. Birth weight, postnatal age at MRI, gestational age at birth, and sex emerged as important predictors of SA. Postnatal age at MRI, paternal education, and maternal ethnicity emerged as important predictors of CT. These findings suggest that individual variation in infant CT and SA is explained by different sets of environmental factors with neonatal SA more strongly influenced by sex and obstetric history and CT more strongly influenced by socioeconomic and ethnic disparities. Findings raise the possibility that interventions aimed at reducing disparities and improving obstetric outcomes may alter prenatal/perinatal cortical development.
Keywords: brain development, neonate, neuroimaging, premature birth, socioeconomic status
Introduction
Cortical thickness (CT) and surface area (SA) are two independent components of cortical volume most commonly studied using structural magnetic resonance imaging (MRI). Although both measures change dynamically across the lifespan (Storsve et al. 2014; Lyall et al. 2015; Remer et al. 2017; Tamnes et al. 2017) recent research suggests that early-life events, especially those occurring in the pre- or perinatal period, have pervasive and long lasting effects (Raznahan et al. 2012; Walhovd et al. 2012; Walhovd et al. 2016b). Pre- and perinatal events may be especially important for atypical development as small differences early in life can have cascading effects on later outcomes (Karmiloff-Smith 1998; Masten and Cicchetti 2010). Notably, many neuropsychiatric disorders are characterized by altered global and/or regional CT and SA including schizophrenia and bipolar disorder (Rimol et al. 2012), autism (Ohta et al. 2016; Yang et al. 2016), and attention deficit and hyperactivity disorder (Silk et al. 2016).
Current theories of cortical development also point to the prenatal period as a foundational period in the emergence of individual differences in CT and SA. According to the radial unit hypothesis, differences in global and regional SA are driven by the number of cortical columns generated during the early embryonic period, while differences in CT are attributed to the number and size of cells within a column, packing density, and numbers of neuronal processes, glial processes, and synapses, features which arise primarily during the fetal and perinatal periods (Rakic 1995, 2009). More recently, a supragranular layer expansion hypothesis has been proposed which posits that outer radial glial (oRG) cells play a critical role in radial and tangential expansion of supragranular layers in primates with potential implications for individual differences in CT and SA (Nowakowski et al. 2016). Throughout the prenatal and early postnatal developmental window, these processes are influenced by tightly regulated patterns of gene expression and environmental signals (Kandel et al. 2013). How these factors influence individual variation in early CT and SA is not well understood.
In this paper, we report the first large scale neuroimaging study of environmental influences on CT and SA during infancy. Our aim was to understand how 17 major demographic and medical history variables affect neonatal CT and SA. Studying infants allows us to determine pre- and perinatal variables of interest with a high degree of accuracy and reduces potential confounding effects that can arise when studying children or adults, where pre- and perinatal variables of interest may be correlated with later environmental exposures. Previous work by our group (Knickmeyer et al. 2017) revealed that age, sex, gestational age at birth, and birth weight are highly significant predictors of neonatal brain volumes, but did not assess CT and SA. Based on the theories of cortical development reviewed above, we hypothesized that different sets of environmental factors would impact CT and SA. Additionally, based on previous findings relating birth weight, sex, and socioeconomic status (SES) to cortical structure in later childhood/adulthood (Raznahan et al. 2011, 2012; Walhovd et al. 2012; Wierenga et al. 2014; Noble et al. 2015), we hypothesized that these environmental influences would determine individual variation in SA to a greater extent than CT. Given that many complex psychiatric diseases are the end result of altered neurodevelopmental trajectories that commence in prenatal and early postnatal life (Wolff and Piven 2014; Birnbaum et al. 2015), our investigation of environmental influences on neonatal CT and SA represents a fundamental step in developing public health interventions to optimize early cortical development and reduce risk for later mental illness.
Materials and Methods
Subjects
Our study included 805 neonates (434 twins, 371 singletons; 429 males, 376 females) between the ages of 6 and 144 days post birth, drawn from two prospective longitudinal studies of early brain development being carried out at the University of North Carolina (UNC) at Chapel Hill (Gilmore et al. 2010a, 2010b, 2012). Pregnant mothers were recruited from outpatient obstetrics and gynecology clinics in central North Carolina. Women with major medical illnesses or abnormal fetal ultrasounds were excluded at enrollment. Maternal reports were used to determine parental demographic information such as maternal age, paternal age, paternal ethnicity, maternal ethnicity, maternal psychiatric history, and paternal psychiatric history and SES variables such as maternal education, paternal education, and household income. Psychiatric history variables were also determined using medical record review. Both maternal and paternal psychiatric history were categorized and binarized such that individuals were considered positive for psychiatric history if they reported a diagnosis in any of the following DSM-V categories, or if medical record review indicated such a diagnosis: schizophrenia spectrum and other psychotic disorders, bipolar and related disorders, depressive disorders, anxiety disorders, obsessive-compulsive and related disorders, attention-deficit hyperactivity disorders, Tourette’s syndrome, or autism-spectrum disorders. A breakdown of these categories in our sample is available in Supplementary Table 1. Maternal smoking during pregnancy was also collected using maternal reports. Labor, delivery, and pediatric medical records were used to collect obstetric variables such as birth weight, gestational age at birth, 5 min APGAR scores, stay in neonatal intensive care unit over 24 h, gestation number, and delivery method. Detailed demographic information can be viewed in Table 1. After complete description of the study to subjects’ parent(s), written informed consent was obtained. Study protocols were approved by the Institutional Review Board of the UNC School of Medicine.
Table 1.
Descriptive statistics for demographic and medical history variables
| Continuous variables | Average | SD | Min | Max | |
|---|---|---|---|---|---|
| Birth weight | 2843.511 | 706.544 | 790 | 4820 | |
| Gestational age at birth | 261.195 | 19.082 | 192 | 295 | |
| Postnatal age at MRI | 30.64 | 16.871 | 6 | 144 | |
| 5 min APGAR score | 8.72 | 0.693 | 3 | 10 | |
| Maternal education | 15.05 | 3.464 | 0 | 26 | |
| Paternal education | 14.86 | 3.488 | 0 | 26 | |
| Maternal age | 29.858 | 5.585 | 16 | 47 | |
| Paternal age | 32.379 | 6.553 | 17 | 64 | |
| Categorical variables | N | % | |||
| NICU stay > 24 h | No | 635 | 79 | ||
| Yes | 170 | 21 | |||
| Sex | Male | 429 | 53 | ||
| Female | 376 | 47 | |||
| Delivery method | Vaginal | 382 | 47 | ||
| C-section | 423 | 52 | |||
| Household income | High | 238 | 30 | ||
| Mid | 217 | 27 | |||
| Low | 299 | 37 | |||
| Missing | 51 | 6 | |||
| Maternal ethnicity | Caucasian | 612 | 76 | ||
| African American | 173 | 21 | |||
| Asian | 17 | 2 | |||
| Native American | 3 | <1 | |||
| Paternal ethnicity | Caucasian | 588 | 73 | ||
| African American | 184 | 23 | |||
| Asian | 26 | 3 | |||
| Native American | 7 | 1 | |||
| Gestational number | Singleton | 371 | 46 | ||
| Twin | 434 | 54 | |||
| Maternal psychiatric history | No | 508 | 63 | ||
| Yes | 297 | 37 | |||
| Paternal psychiatric history | No | 714 | 89 | ||
| Yes | 91 | 11 | |||
| Maternal smoking | No | 738 | 92 | ||
| Yes | 67 | 8 | |||
| T2 sequence type | Type1 | 287 | 36 | ||
| Type2 | 386 | 48 | |||
| Type3 | 12 | 1 | |||
| Type4 | 120 | 15 | |||
Image Acquisition
MRI images were obtained using either a Siemens Allegra head-only 3 T scanner (N = 673) or a Siemens TIM Trio 3 T scanner (N = 132) (Siemens Medical System, Inc., Erlangen, Germany) during unsedated natural sleep. Subjects were fitted with earplugs and secured into a vacuum-fixed immobilization device prior to the scan. Heart rate and oxygen saturation were monitored using a pulse oximeter. Proton density and T2 weighted structural images were acquired on the Allegra using a turbo-spin echo sequence (TSE, TR = 6200 ms, TE1 = 20 ms, TE2 = 119 ms, flip angle = 150°, spatial resolution = 1.25 mm × 1.25 mm × 1.95 mm, N = 287, sequence name = Type1). For neonates who were deemed unlikely to sleep through the scan session, a “fast” turbo-spin echo sequence was collected on the Allegra using a decreased TR, a smaller image matrix, and fewer slices (TSE, TR range = 5270 ms–5690 ms, TE1 range = 20 ms–21 ms, TE2 range = 119 ms–124 ms, flip angle = 150°, spatial resolution = 1.25 mm × 1.25 mm × 1.95 mm, N = 386, sequence name = Type2). For the Trio, subjects were initially scanned using a TSE protocol (TR = 6200 ms, TE1 = 17, TE2 = 116 ms, flip angle = 150°, spatial resolution = 1.25 mm × 1.25 mm × 1.95 mm, N = 12, sequence name = Type3) while the rest were scanned using a 3-D T2 SPACE protocol (TR = 3200 ms, TE = 406, flip angle = 120°, spatial resolution = 1 mm × 1 mm × 1 mm, N = 120, sequence name = Type4). We determined that sequence parameters had a significant influence on both CT and SA (see Supplementary Methods and Supplementary Tables 2 and 3) and therefore included T2 sequence name (Type1–Type4) as a covariate in all of the analyses described in this study.
Image Analysis
CT and SA measures were derived for all subjects using a pipeline previously described by Li et al. (2016). All MR images were preprocessed for tissue segmentation using a standard infant-specific pipeline (Li et al. 2013). Specific steps included skull stripping and manual editing of non-brain tissue, removal of the cerebellum and brain stem, and corrections for intensity inhomogeneity. Gray matter (GM), white matter (WM), and cerebrospinal fluid (CSF) were segmented by applying a standalone infant-specific patch-driven coupled level sets method (Wang et al. 2014). Non-cortical regions were masked and tissues were divided into the left and right hemispheres. A deformable surface method (Li et al. 2012, 2014) was applied to the tissue segmentations in order to reconstruct the inner, middle, and outer cortical surfaces. This method involved a topological correction of WM volume to ensure spherical topology, a tessellation of the corrected WM to generate a triangular mesh, and the deformation of the inner mesh towards the reconstruction of each cortical surface while preserving the initial topology. All inner, middle, and outer surfaces for the left and right hemispheres were visually examined for accurate mapping.
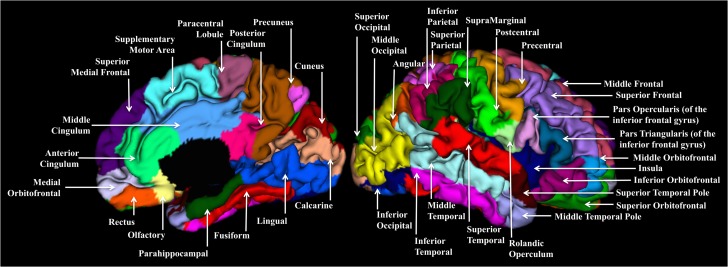
The inner surface was defined as the boundary between gray and white matter and the outer surface as the boundary between the gray matter and CSF. A third, middle cortical surface was defined as the layer lying in the geometric center of the inner and outer surfaces of the cortex. CT was computed for each vertex as the average value of the minimum distance from the inner to the outer surfaces and the minimum distance from the outer to the inner surfaces. SA was computed based on the central cortical surface. The cortical surface was parcellated into 78 regions of interest based on an infant-specific 90 region parcellation atlas (Tzourio-Mazoyer et al. 2002; Gilmore et al. 2012) as shown in Figure 1. Twelve regions represent subcortical structures and were therefore not examined. The average CT and total SA were calculated for each ROI based on corresponding values at each vertex.
Figure 1.
The 78 cortical regions of interest from the AAL atlas (Tzourio-Mazoyer et al., 2002) projected onto a representative neonatal brain. Due to its anatomical location, Heschl’s gyrus is not visible.
Statistical Analysis
Parental demographic and medical history variables included maternal age, paternal age, maternal education, paternal education, maternal ethnicity, paternal ethnicity, maternal psychiatric history, paternal psychiatric history, total household income, and maternal smoking during pregnancy. Infant demographic variables included sex, birth weight, gestational age at birth, postnatal age at MRI, 5 min APGAR scores, stay in neonatal intensive care unit over 24 h, gestation number, and delivery method. See Supplementary Table 4 for a correlation matrix of predictor variables (continuous and binary). See Supplementary Table 5 for a comparison of demographic variables between Caucasian and African American subjects. See Supplementary Table 6 for a comparison of demographic variables by income. To examine the effects of these variables on individual differences in neonatal CT and SA, we applied a moment-based method to select fixed effects in a linear mixed effects model (Ahn et al. 2012; Knickmeyer et al. 2017). For the selection of fixed effects, an adaptive lasso penalty was applied with all twin pairs treated as repeated measures. Variable selection was run separately for each outcome (total SA, average CT, 78 regional CT measures, 78 regional SA measures). Results were bootstrapped 1000 times to ensure stable results. Variables were considered to be important predictors for a specific outcome if they were selected more than 800 times for that outcome (an inclusion frequency of 80%). T2 sequence type was included as a fixed variable when model selection was run for all SA and CT measures. To account for overall brain size, total SA was also fixed for all regional SA model selections and the cubed root of intracranial volume (a sum of gray matter, white matter and cerebrospinal fluid) was fixed in the model selection for average and regional CT.
In the variable selection method, there are only two outcomes: a variable is included or not. Thus, after variable selection, linear mixed effects models were run using the selected variables for each region independently. These “selected models” were used to perform significance testing and to generate effect sizes and r2 values. Mixed effects models were also run including all variables for comparison, these are termed “full models”. To account for familial relatedness within monozygotic (MZ) and dizygotic (DZ) twins, we used a standard ACE model described in Xia et al. (2014), which includes additive genetic effects (A), common environmental effects (C) and random environmental effects (E) (see Supplementary Methods for more details). For all regional analyses, adjustments for multiple comparisons were made using Benjamini & Hochberg method. FDR < 0.05 was considered significant for each region of interest.
Sensitivity Analyses
The following sensitivity analyses were performed: (1) Model selections for CT and SA were run without adjusting for overall brain size. (2) In order to identify any effects of gestational number, selected models for CT and SA were performed separately for twins and singletons. (3) Selected models were run replacing paternal education with maternal education. (4) Selected models were also run replacing maternal ethnicity with paternal ethnicity.
Results
Results reported below in the average CT, regional CT, total SA, and regional SA sections are those generated using the adaptive lasso and selected models adjusting for global brain size.
Average CT
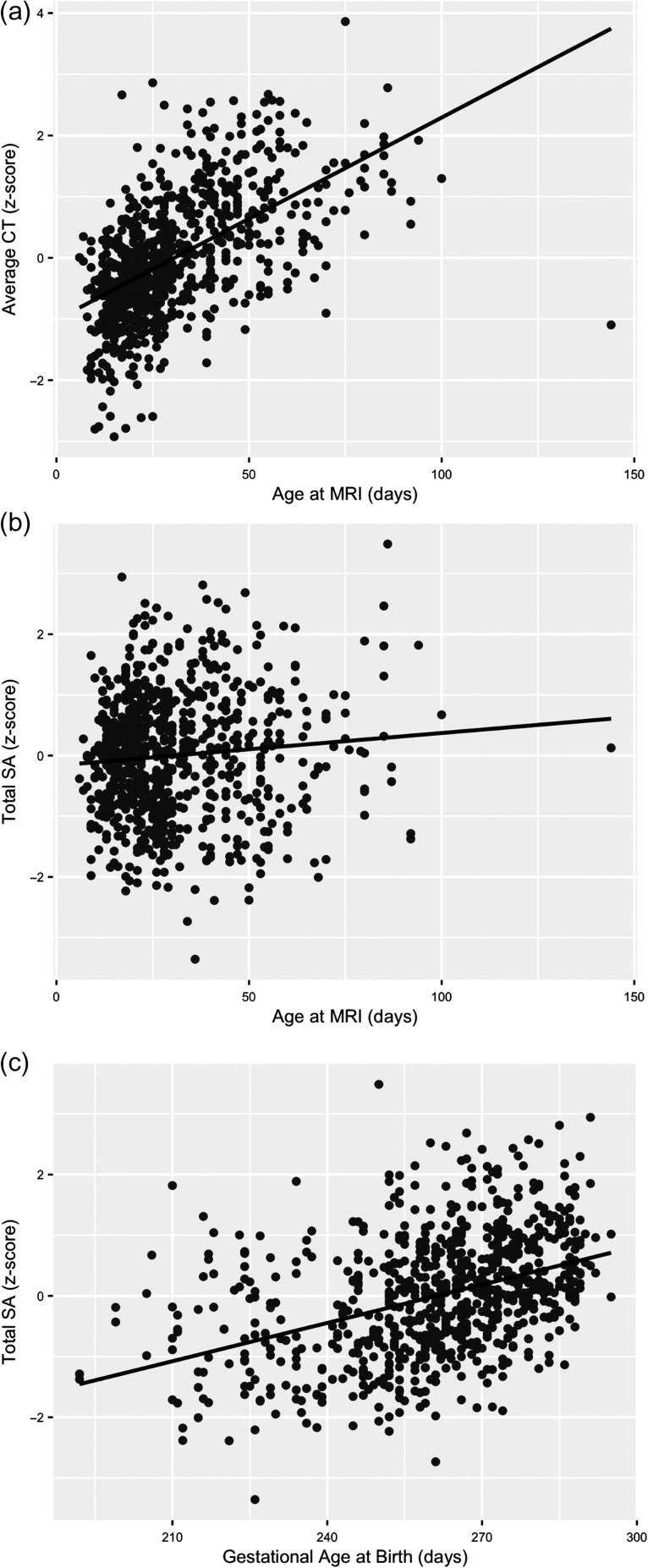
Postnatal age at MRI, paternal education, and maternal ethnicity emerged as important predictors of average neonatal CT (Table 2). Postnatal age at MRI showed a positive relationship, with average CT (Fig. 2a) increasing 0.09% every day. Paternal education was negatively associated with average CT. With every additional year of paternal education, there was a 0.13% decrease in average CT. Significant associations between CT and maternal ethnicity likely reflect differences between Caucasian and African American mothers as these make up the majority of the sample. Compared to offspring of Caucasian mothers, offspring of African American mothers showed 1.4% larger average CT, offspring of Asian mothers showed 0.45% larger average CT, and offspring of Native American mothers showed 0.29% smaller average CT.
Table 2.
Selected models for global cortical thickness and surface area
| Region of interest | R 2 | Predictors | Beta | r 2 | q-value | Relative difference |
|---|---|---|---|---|---|---|
| Average CT | 0.52 | Intercept | 1.14E+00 | |||
| Postnatal age at MRI | 1.64E–03 | 1.77E–01 | 6.67E–44 | 0.09% | ||
| Paternal education | −2.44E–03 | 1.67E–02 | 3.26E–06 | −0.13% | ||
| Maternal ethnicity – Asian | 8.66E–03 | 3.58E–04 | 3.39E–08 | 0.45% | ||
| Maternal ethnicity – African American | 2.74E–02 | 2.91E–02 | 1.40% | |||
| Maternal ethnicity – Native American | −5.44E–03 | 2.53E–05 | −0.29% | |||
| ICV 1/3 | 9.59E–03 | 1.87E–01 | 4.24E–49 | 0.50% | ||
| T2 Sequence Type (Type1 vs Type2) | 3.62E–03 | 7.53E–04 | 8.12E–01 | 0.19% | ||
| T2 Sequence Type (Type1 vs Type3) | 2.73E–03 | 2.52E–05 | 0.14% | |||
| T2 Sequence Type (Type1 vs Type4) | −1.86E–03 | 1.01E–04 | −0.10% | |||
| Total SA | 0.51 | Intercept | −2.11E+02 | |||
| Birth weight | 5.70E+00 | 1.89E–01 | 3.98E–24 | 3.6%a | ||
| Gestational age at birth | 2.78E+02 | 3.29E–01 | 1.78E–26 | 0.35% | ||
| Postnatal age at MRI | 4.08E+02 | 5.52E–01 | 1.16E–67 | 0.51% | ||
| Sex | −3.06E+03 | 2.71E–02 | 1.69E–10 | 3.90% | ||
| T2 sequence type (Type1 vs Type2) | 7.39E+02 | 1.59E–03 | 3.76E–01 | 0.93% | ||
| T2 sequence type (Type1 vs Type3) | −1.26E+03 | 2.72E–04 | −1.59% | |||
| T2 sequence type (Type1 vs Type4) | 4.33E+02 | 2.73E–04 | 0.55% |
aPer 500 g for birth weight.
Figure 2.
Age at MRI plotted against average CT (a), age at MRI plotted against total SA (b), and gestational age at birth plotted against total SA (c) for all individual subjects.
Regional CT
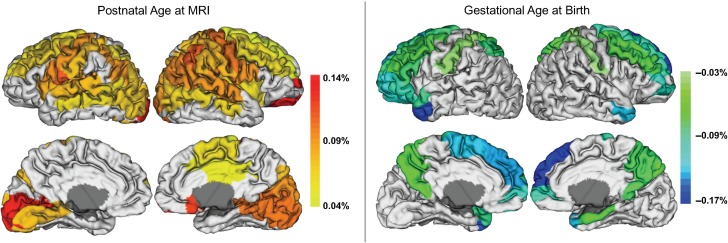
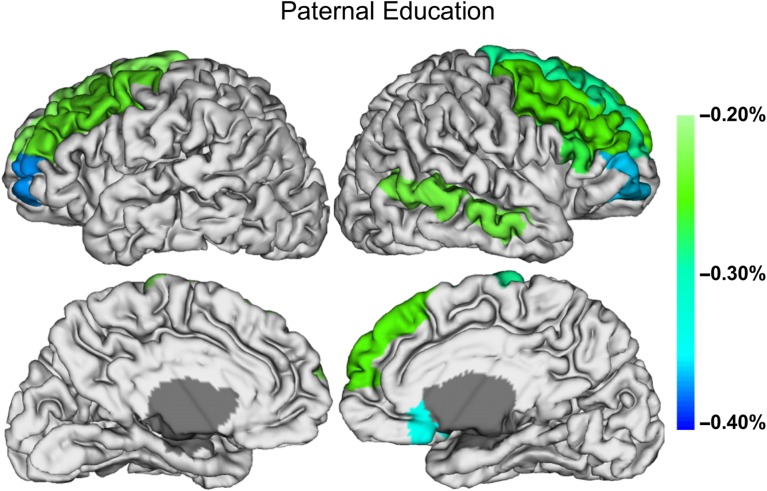
Postnatal age at MRI, gestational age at birth, maternal ethnicity, and paternal education emerged as important predictors of regional CT in at least 10% of regions examined (Supplementary Table 7). Postnatal age at MRI showed positive associations with regional CT (Fig. 3, Supplementary Table 8). Specifically, older babies had thicker cortices in the pre- and postcentral gyri, right supplementary motor area, right middle cingulate gyrus, insula, and portions of the lateral frontal, occipital, and parietal lobes. Gestational age at birth showed negative associations with regional CT (Fig. 3, Supplementary Table 9). Earlier born babies had thicker cortices in the medial and lateral frontal lobe, superior and middle temporal poles, right hippocampal gyrus, and postcentral gyrus. Paternal education also showed a negative association with regional CT (Fig. 4, Supplementary Table 10). Higher paternal education was associated with thinner cortices in superior frontal, middle frontal, and middle orbital frontal gyri as well as in the right inferior frontal pars triangularis, right medial superior frontal gyrus, right olfactory region, and right middle temporal gyrus.
Figure 3.
Significant associations between regional CT and postnatal age at MRI and gestational age at birth shown as percent change by day. Postnatal age at MRI ranges from 6 to 144 days and gestational age at birth ranges from 192 to 295 days. Regions where postnatal age at MRI and gestational age at birth did not emerge as important predictors in the variable selection are shown in white; these regions were not included in the selected models. Subcortical regions are in gray and were not analyzed.
Figure 4.
Regions having significant (negative) associations between CT measures and paternal education are presented onto the cortical surface. The percent difference in CT for every additional year of paternal education is indicated by the color bar. Regions where paternal education did not emerge as an important predictor in the variable selection are shown in white; these regions were not included in the selected models. Regions in gray were not analyzed.
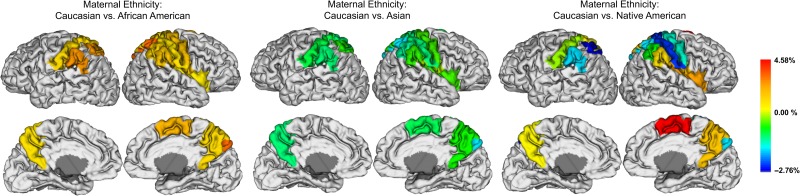
Associations between maternal ethnicity and regional CT likely reflect differences between infants of Caucasian and African American mothers as these make up the majority of the sample (Fig. 5, Supplementary Table 11). Compared to offspring of Caucasian mothers, offspring of African American mothers had thicker cortices in bilateral postcentral gyri, superior parietal lobules, precuneus, and the supramarginal gyri, as well as the right precentral gyrus, insula, inferior parietal lobule, supplementary motor area, and rolandic operculum. Compared to offspring of Caucasian mothers, offspring of Asian mothers had thicker cortices in the right precentral gyrus, rolandic operculum, supramarginal gyrus, insula, and precuneus as well as the left superior parietal lobule. Offspring of Asian mothers had thinner cortices in the postcentral gyrus, right superior parietal lobule, right inferior parietal lobule, right supplementary motor area, left supramarginal gyrus, and left precuneus. Compared to offspring of Caucasian mothers, offspring of Native American mothers had thicker cortices in the precuneus, left postcentral gyrus, right rolandic operculum, right supramarginal gyrus, right supplementary motor area, and right insula, and thinner cortices in the right precentral and postcentral gyri, superior parietal lobule, right inferior parietal lobule, and right supramarginal gyrus. Sex, birth weight and gestational number emerged as important predictors of average CT in a small number of cortical regions. These results can found in Supplementary Table 12.
Figure 5.
Significant associations between regional CT and maternal ethnicity are projected onto the cortical surface. Negative percent differences represent thinner cortices in infants of Caucasian mothers and positive percent differences show thicker cortices in infants of Caucasian mothers. Regions where maternal ethnicity did not emerge as an important predictor in the variable selection are shown in white; these regions were not included in the selected models. Regions in gray were not analyzed.
Total SA
Birth weight, gestational age at birth, postnatal age at MRI, and sex emerged as the most important predictors of total SA (Table 2 and Supplementary Table 13). Birth weight showed a strong positive association with total SA. For every 500 g increase in birth weight, there was a 3.6% increase in overall cortical SA. Gestational age at birth and postnatal age at MRI also showed strong positive associations with total SA (Fig. 2b,c). Total SA increased 0.35% for every additional day in the womb and 0.51% for every postnatal day. Additionally, sex was a significant predictor of total SA, with males having 3.9% larger cortical surfaces than females.
Regional SA
We found postnatal age at MRI, birth weight, paternal ethnicity, maternal ethnicity, sex, and gestational age at birth to be important predictors of regional SA in a small number of ROIs. These results can found in Supplementary Tables 14 and 15.
The following did not emerge as important predictors of neonatal CT and SA at either the global or regional level: APGAR scores at 5 min, delivery method, maternal education, total household income, maternal age, paternal age, maternal psychiatric history, paternal psychiatric history, and NICU stay over 24 h. Results of full mixed effects models containing all possible predictors (Supplementary Tables 16–17) were highly similar to results using the adaptive lasso. Exceptions included birth weight and maternal ethnicity, which did not emerge as significant predictors of regional CT in the full mixed models. Additionally, gestational age at birth, sex, and birth weight were significant predictors of regional SA in the full model but did not appear in the adaptive lasso.
Sensitivity Analyses
In an additional analysis, without adjustments for overall brain size, significant predictors for regional CT were similar to those in the primary analysis (Supplementary Table 18). For regional SA, we identified postnatal age at MRI, gestational age at birth, birth weight, gestation number, and sex as important predictors in widespread regions of the cortex (Supplementary Table 19). For selected models run separately in twin and singleton subgroups, beta values and directions of effect were similar to those observed in the primary analysis (Supplementary Tables 20 and 21 for singletons and Supplementary Tables 22 and 23 for twins). Overall patterns of results were also similar when paternal education was replaced with maternal education (Supplementary Table 24) and when maternal ethnicity was replaced with paternal ethnicity (Supplementary Table 25) in our selected models of CT.
Discussion
To our knowledge, this study is the first to examine environmental influences on CT and SA in a large normative sample of neonates. Our findings build on our previous work examining the influences of obstetric, demographic, and socioeconomic factors on neonatal brain volumes (Knickmeyer et al. 2017) and provide a more refined account of how these factors impact early cortical development.
We found that the cortical surface expanded 0.51% and CT increased 0.09% daily between the ages of 6 and 144 days post birth. These results capture extremely rapid expansion and growth of the cortex during early postnatal development, likely driven by dendritic development, synaptogenesis, and, gliogenesis, as well as complex patterns of cortical connectivity and cortical folding (Stiles 2008). By comparison, annual growth rates during middle and late childhood reach maximum values of only 0.005% and 0.015% for CT and SA, respectively (Raznahan et al. 2011). We also found that CT growth patterns were regionally heterogeneous, with primary visual, motor, and auditory regions representing some of the fastest growing cortices after birth. This is consistent with longitudinal studies of CT, SA (Li et al. 2015; Lyall et al. 2015), and cortical volume during early brain development (Gilmore et al. 2012) that also show heterogeneous patterns of growth across the cortex. Specifically, sensory and motor regions are shown to mature earlier during development compared to regions involved with higher order integrative functions. Similar hierarchical organization is observed in older children and adolescents, with sensory and motor regions reaching their peak thickness values earlier than association cortices (Sowell et al. 2004; Shaw et al. 2008). While our results were in line with these reports, faster growing cortices also included association regions within orbitofrontal and prestriate cortex. This suggests there are complex patterns of CT growth after birth in both primary sensory and association regions. This notion is supported by our previous assessments of global brain volumes which also revealed rapid postnatal brain development and complex regional growth patterns (Knickmeyer et al. 2017). Given minimal regional differences in gene expression during infancy (Pletikos et al. 2014), heterogeneous patterns of CT growth observed in our sample may reflect post-transcriptional processes and activity-dependent mechanisms sensitive to environmental input. Interestingly, we observed nominal regional heterogeneity in SA growth during this time period.
We found that gestational age at birth had opposing effects on SA and CT (positive and negative associations respectively). In keeping with published studies showing reduced cortical SA during infancy (Engelhardt et al. 2015) and childhood (Lax et al. 2013; Rogers et al. 2014; Zhang et al. 2015) in infants born preterm, total SA was larger in later born babies. During the late fetal stage, there is rapid growth in brain size driven by the accelerated development of cortical SA relative to cortical volume (Kapellou et al. 2006). This is likely influenced by the development of sulci, gyri, and corticocortical connectivity. Our results suggest that being born early disrupts these processes, even in children that are not technically premature (>37 weeks). In contrast to SA, later born babies had thinner cortices in widespread regions of the frontal lobe as well as the postcentral gyrus, precuneus, and the temporal poles. This finding suggests that exposure to the postnatal environment in earlier born babies may alter growth of the cortical mantle in these regions. Compared to the intrauterine environment, the extra-uterine environment is rich in sensory information and could promote synaptogenesis and complex dendritic morphology, leading to accelerated growth of the cortex. In fact, findings of smaller brain volumes in later born babies in our previous study (Knickmeyer et al. 2016) may be related to the rapid CT growth we observe in this current study. Alternatively, thicker cortices in earlier born babies may reflect cortical overgrowth resulting from disrupted apoptotic mechanisms which normally take place late in gestation. Thicker cortices in earlier born babies compared to later born babies may also reflect a lack of maturation of the underlying white matter (Keunen et al. 2016) which would influence tissue classification during automated MRI segmentation protocols. The intrauterine environment is critical for the organization of axonal pathways and the processes of premyelination and myelination that begin during the second half of pregnancy and are likely interrupted as a result of preterm birth (Qiu et al. 2015). Additional studies assessing white matter microstructure and myelination would help clarify the biological mechanisms underlying these findings.
Overall, the opposing effects of gestational age at birth on CT and SA reaffirm the conceptualization of CT and SA as relatively independent phenotypes. This conceptualization is further supported by our finding that individual variation in infant CT and SA is explained by different sets of environmental factors. Sex and obstetric history variables (especially birth weight) had a strong influence on neonatal SA whereas variables related to SES and ethnic disparities (paternal education and maternal ethnicity) had a strong influence on CT. Observed differences are in keeping with twin studies which consistently report CT and SA to be genetically independent (Jansen et al. 2015), and with current theories of prenatal cortical development. In particular, the radial unit hypothesis (Rakic 2009) suggests that the number of cortical minicolumns determines the size of the cortical surface and that the number of minicolumns depends on the rate of cell proliferation and/or programmed cell death within symmetrically-dividing radial glial cells of the ventricular zone (VZ). Differences in CT are ascribed to changes in proliferation kinetics of asymmetrically dividing neural progenitor cells, as well as to changes in the size of neurons and the amount of tissue situated between neuronal cell bodies, which is itself composed of neuronal and glial processes including dendrites, dendritic spines, axon terminals, and synapses (Rakic 1995, 2009). Additionally, the recently proposed supragranular layer expansion hypothesis suggests that at midneurogenesis, radial glial scaffolds become discontinuous (Nowakowski et al. 2016). During this discontinuous phase, self-renewing divisions of oRG cells increase the SA of supragranular layers, while neurogenic divisions of oRG cells increase the thickness of these layers.
Our findings regarding birth weight and sex are similar to studies in older children and adults, which reveal males and heavier born babies have larger SA but not CT (Raznahan et al. 2011, 2012; Walhovd et al. 2012, 2016a, Wierenga et al. 2014). Sex and birth weight were also significant predictors of global brain volumes in our previous study (Knickmeyer et al. 2016), further confirming the importance of these prenatal factors during early brain development. Keeping the above neurodevelopmental hypotheses in mind, the positive association of birth weight with SA may reflect the influence of genetic potential for growth, maternal nutrition and metabolism, endocrine factors, and placental perfusion and function on proliferation and apoptosis of radial glial cells, as well as on self-renewing divisions of oRG cells, and, in late pregnancy, the development of corticocortical connectivity. Larger total SA in males may reflect the influence of gonadal steroids on these same processes. It is notable that testosterone secretion in male fetuses is highest between weeks 14 and 18 (Prince 2001), encompassing the latter portion of the continuous scaffold stage and the early portions of the discontinuous scaffold stage.
The association between paternal education and CT may reflect the father’s ability to provide psychosocial resources during pregnancy and the early postpartum period, support healthy maternal behaviors, reduce stress, and provide greater cognitive stimulation in the home (Blumenshine et al. 2011; Shapiro et al. 2017). All of these factors may influence asymmetrically dividing neural progenitor cells, neurogenic divisions of oRG cells, synaptogenesis, and the formation/elaboration of neuronal and glial processes during development. Alternatively, associations between neonatal CT and paternal education could be driven by genetic influences. Given the rapid rates of CT growth observed in our study, it is somewhat surprising that this association is negative such that infants of more educated fathers have thinner cortices, especially in the frontal lobes. With that said, our findings are in keeping with previous work showing negative correlations between CT and intelligence during early childhood (Shaw et al. 2006). These findings have led to the hypothesis that children with higher IQs have more prolonged maturation of higher order regions. Thus, we hypothesize that infants born to more educated fathers may experience a slower, more extended developmental window of the frontal lobe that may be advantageous to later cognitive outcomes. It is also possible that thinner cortices in offspring of highly educated fathers reflect changes in image contrast caused by the myelination of underlying white matter (Sowell et al. 2004). Notably, environmental enrichment and social interactions promote oligodendrocyte lineage development and myelination (Tomlinson et al. 2016). Supporting this hypothesis, we previously found that higher paternal education is associated with larger overall white matter volume in neonates (Knickmeyer et al. 2016).
We observed that offspring of African American mothers had thicker cortices in parietal regions involved in somatosensory processes and sensory integration compared to offspring of white mothers. However, we note that these associations were not significant in the full mixed effects models. Furthermore, maternal ethnicity was not an important predictor of global brain volumes in this sample of neonates (Knickmeyer et al. 2016). Associations between maternal ethnicity and CT may reflect genetic differences and/or the influences of environmental factors associated with the sociocultural construct of race/ethnicity on the cellular processes described above. Additional studies are needed to determine whether these associations are robust and if they are temporary or represent persistent alterations with functional consequences. Furthermore, to effectively develop interventions aimed at optimizing infant brain development, future studies must delineate specific mechanisms underlying these associations. Specific variables that may be of importance include psychosocial stress, exposure to environmental pollutants, and reduced access to/utilization of prenatal care, which may be more common among racial and ethnic minorities (Grobman et al. 2016; Lorch and Enlow 2016). These variables were not assessed in the current study, but when comparing infants of Caucasian and African American (AA) mothers, we did observe significant differences in birth weight, maternal and paternal education, and maternal age (all lower in AA), in NICU stay greater than 24 h, maternal psychiatric history, and maternal smoking (all more common in AA), and in paternal psychiatric history (less common in AA). We note that Caucasian and African American women made up the majority of the sample examined. Comparisons between Asian and Native American women and other ethnic groups should be treated with caution until future studies with larger groups of Asian and Native American women are conducted.
We note that APGAR scores at 5 min, delivery method, maternal education, total household income, maternal age, paternal age, maternal psychiatric history, paternal psychiatric history, maternal smoking, and NICU stay over 24 h were not selected as important predictors of neonatal CT and SA. In some cases this may reflect high correlations between predictor variables (e.g., between paternal and maternal education). In such a situation, the moment-based method selects the best predictive variable. With specific regard to psychiatric history, the lack of associations may reflect the fact that our psychiatric history variables include multiple disorders with depression being the most common. Previous work by our group has shown that a maternal history of severe mental illness (specifically schizophrenia) does influence brain development (Gilmore et al. 2010a). Additionally, with only 8% of women reporting smoking during pregnancy, our study may be underpowered to detect associations between brain structure and maternal smoking during pregnancy.
In conclusion, CT and SA both exhibit rapid growth during the first postnatal month, but show distinct relationships with environmental factors. Gestational age at birth is positively associated with SA, but negatively associated with CT. Birth weight and sex influence SA, potentially through cellular processes active during early pregnancy and midgestation, while maternal ethnicity and paternal education influence CT, possibly through cellular processes active in the perinatal period. Strengths of this study include the use of detailed medical, obstetric, and demographic data, the collection of a large representative imaging dataset, and the application of cutting edge pediatric image analysis methods. Limitations reflect inherent difficulties in imaging infant subjects. Age-related changes in signal intensities and contrast may affect CT and SA measures (Walhovd et al. 2016a). In addition, compared to SA measurements at this age, CT measurements are much smaller, show less variation, and more prone to partial volume errors. We also note that paternal factors such as psychiatric history are generally obtained from mothers and may not always be provided with full accuracy. Despite these limitations, our results highlight the importance of obstetric, demographic, and socioeconomic factors in explaining individual variation in neonatal CT and SA. Ultimately, this line of research will allow the development and optimal application of interventions to support prenatal/perinatal cortical development, ensuring a strong foundation for a long, healthy, and productive life.
Supplementary Material
Notes
We thank our participating families and staff of the UNC MRI Research Center, the UNC Neuro Image Research and Analysis Laboratories, and the UNC Early Brain Development Program. Conflict of Interest: None declared.
Funding
This work was supported by the National Institutes of Health (MH064065, MH070890, and HD053000 to J.G., MH083045 to R.K., UL1 RR025747, and MH086633 to H.Z., MH091645, HD-003110 and HD079124 to M.S., MH108914 and MH107815 to G.L., MH100217 to D.S., MH109773 to L.W., and T32 NS007431 to S.J. and J.B.) and the National Science Foundation (SES-1357666 and DMS-1407655 to H.Z.).
References
- Ahn M, Zhang HH, Lu W. 2012. Moment-based method for random effects selection in linear mixed models. Stat Sin. 22:1539–1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnbaum R, Jaffe AE, Chen Q, Hyde TM, Kleinman JE, Weinberger DR. 2015. Investigation of the prenatal expression patterns of 108 schizophrenia-associated genetic loci. Biol Psychiatry. 77:e43–e51. [DOI] [PubMed] [Google Scholar]
- Blumenshine PM, Egerter SA, Libet ML, Braveman PA. 2011. Father’s education: an independent marker of risk for preterm birth. Matern Child Health J. 15:60–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelhardt E, Inder TE, Alexopoulos D, Dierker DL, Hill J, Van Essen D, Neil JJ. 2015. Regional impairments of cortical folding in premature infants. Ann Neurol. 77:154–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore JH, Kang C, Evans DD, Wolfe HM, Smith JK, Lieberman JA, Lin W, Hamer RM, Styner M, Gerig G. 2010. a. Prenatal and neonatal brain structure and white matter maturation in children at high risk for schizophrenia. Am J Psychiatry. 167:1083–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore JH, Schmitt JE, Knickmeyer RC, Smith JK, Lin W, Styner M, Gerig G, Neale MC. 2010. b. Genetic and environmental contributions to neonatal brain structure: a twin study. Hum Brain Mapp. 31:1174–1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore JH, Shi F, Woolson SL, Knickmeyer RC, Short SJ, Lin W, Zhu H, Hamer RM, Styner M, Shen D. 2012. Longitudinal development of cortical and subcortical gray matter from birth to 2 years. Cereb Cortex. 22:2478–2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grobman WA, Parker C, Wadhwa PD, Willinger M, Simhan H, Silver B, Wapner RJ, Parry S, Mercer B, Haas D, et al. Eunice Kennedy Shriver National Institute of Child Health Human Development nuMoM2b Network, Bethesda, MD . 2016. Racial/ethnic disparities in measures of self-reported psychosocial states and traits during pregnancy. Am J Perinatol. 33:1426–1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen AG, Mous SE, White T, Posthuma D, Polderman TJC. 2015. What twin studies tell us about the heritability of brain development, morphology, and function: a review. Neuropsychol Rev. 25:27–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandel E, Schwartz JH, Jessell TM, Siegelbaum SA, Hudspeth AJ. 2013. Principles of neural science. 5th ed.New York: McGraw Hill Professional. [Google Scholar]
- Kapellou O, Counsell SJ, Kennea N, Dyet L, Saeed N, Stark J, Maalouf E, Duggan P, Ajayi-Obe M, Hajnal J, et al. . 2006. Abnormal cortical development after premature birth shown by altered allometric scaling of brain growth. PLoS Med. 3:e265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karmiloff-Smith A. 1998. Development itself is the key to understanding developmental disorders. Trends Cogn Sci. 2:389–398. (Regul Ed) [DOI] [PubMed] [Google Scholar]
- Keunen K, Išgum I, van Kooij BJM, Anbeek P, van Haastert IC, Koopman-Esseboom C, Fieret-van Stam PC, Nievelstein RAJ, Viergever MA, de Vries LS, et al. . 2016. Brain volumes at term-equivalent age in preterm infants: imaging biomarkers for neurodevelopmental outcome through early school age. J Pediatr. 172:88–95. [DOI] [PubMed] [Google Scholar]
- Knickmeyer RC, Xia K, Lu Z, Ahn M, Jha SC, Zou F, Zhu H, Styner M, Gilmore JH. 2017. Impact of demographic and obstetric factors on infant brain volumes: a population neuroscience study. Cereb Cortex. 27:5616–5625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lax ID, Duerden EG, Lin SY, Mallar Chakravarty M, Donner EJ, Lerch JP, Taylor MJ. 2013. Neuroanatomical consequences of very preterm birth in middle childhood. Brain Struct Funct. 218:575–585. [DOI] [PubMed] [Google Scholar]
- Li G, Lin W, Gilmore JH, Shen D. 2015. Spatial patterns, longitudinal development, and hemispheric asymmetries of cortical thickness in infants from birth to 2 years of age. J Neurosci. 35:9150–9162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, Nie J, Wang L, Shi F, Gilmore JH, Lin W, Shen D. 2014. Measuring the dynamic longitudinal cortex development in infants by reconstruction of temporally consistent cortical surfaces. Neuroimage. 90:266–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, Nie J, Wang L, Shi F, Lin W, Gilmore JH, Shen D. 2013. Mapping region-specific longitudinal cortical surface expansion from birth to 2 years of age. Cereb Cortex. 23:2724–2733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, Nie J, Wu G, Wang Y, Shen D, Alzheimer’s Disease Neuroimaging Initiative . 2012. Consistent reconstruction of cortical surfaces from longitudinal brain MR images. Neuroimage. 59:3805–3820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, Wang L, Shi F, Lyall AE, Ahn M, Peng Z, Zhu H, Lin W, Gilmore JH, Shen D. 2016. Cortical thickness and surface area in neonates at high risk for schizophrenia. Brain Struct Funct. 221:447–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorch SA, Enlow E. 2016. The role of social determinants in explaining racial/ethnic disparities in perinatal outcomes. Pediatr Res. 79:141–147. [DOI] [PubMed] [Google Scholar]
- Lyall AE, Shi F, Geng X, Woolson S, Li G, Wang L, Hamer RM, Shen D, Gilmore JH. 2015. Dynamic development of regional cortical thickness and surface area in early childhood. Cereb Cortex. 25:2204–2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masten AS, Cicchetti D. 2010. Developmental cascades. Dev Psychopathol. 22:491–495. [DOI] [PubMed] [Google Scholar]
- Noble KG, Houston SM, Brito NH, Bartsch H, Kan E, Kuperman JM, Akshoomoff N, Amaral DG, Bloss CS, Libiger O, et al. . 2015. Family income, parental education and brain structure in children and adolescents. Nat Neurosci. 18:773–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowakowski TJ, Pollen AA, Sandoval-Espinosa C, Kriegstein AR. 2016. Transformation of the radial glia scaffold demarcates two stages of human cerebral cortex development. Neuron. 91:1219–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta H, Nordahl CW, Iosif A-M, Lee A, Rogers S, Amaral DG. 2016. Increased surface area, but not cortical thickness, in a subset of young boys with autism spectrum disorder. Autism Res. 9:232–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pletikos M, Sousa AMM, Sedmak G, Meyer KA, Zhu Y, Cheng F, Li M, Kawasawa YI, Sestan N. 2014. Temporal specification and bilaterality of human neocortical topographic gene expression. Neuron. 81:321–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince FP. 2001. The triphasic nature of Leydig cell development in humans, and comments on nomenclature. J Endocrinol. 168:213–216. [DOI] [PubMed] [Google Scholar]
- Qiu A, Mori S, Miller MI. 2015. Diffusion tensor imaging for understanding brain development in early life. Annu Rev Psychol. 66:853–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakic P. 1995. A small step for the cell, a giant leap for mankind: a hypothesis of neocortical expansion during evolution. Trends Neurosci. 18:383–388. [DOI] [PubMed] [Google Scholar]
- Rakic P. 2009. Evolution of the neocortex: a perspective from developmental biology. Nat Rev Neurosci. 10:724–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raznahan A, Greenstein D, Lee NR, Clasen LS, Giedd JN. 2012. Prenatal growth in humans and postnatal brain maturation into late adolescence. Proc Natl Acad Sci USA. 109:11366–11371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raznahan A, Shaw P, Lalonde F, Stockman M, Wallace GL, Greenstein D, Clasen L, Gogtay N, Giedd JN. 2011. How does your cortex grow? J Neurosci. 31:7174–7177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remer J, Croteau-Chonka E, Dean DC, D’Arpino S, Dirks H, Whiley D, Deoni SCL. 2017. Quantifying cortical development in typically developing toddlers and young children, 1–6 years of age. Neuroimage. 153:246–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimol LM, Nesvåg R, Hagler DJ, Bergmann O, Fennema-Notestine C, Hartberg CB, Haukvik UK, Lange E, Pung CJ, Server A, et al. . 2012. Cortical volume, surface area, and thickness in schizophrenia and bipolar disorder. Biol Psychiatry. 71:552–560. [DOI] [PubMed] [Google Scholar]
- Rogers CE, Barch DM, Sylvester CM, Pagliaccio D, Harms MP, Botteron KN, Luby JL. 2014. Altered gray matter volume and school age anxiety in children born late preterm. J Pediatr. 165:928–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro GD, Bushnik T, Sheppard AJ, Kramer MS, Kaufman JS, Yang S. 2017. Paternal education and adverse birth outcomes in Canada. J Epidemiol Community Health. 71:67–72. [DOI] [PubMed] [Google Scholar]
- Shaw P, Greenstein D, Lerch J, Clasen L, Lenroot R, Gogtay N, Evans A, Rapoport J, Giedd J. 2006. Intellectual ability and cortical development in children and adolescents. Nature. 440:676–679. [DOI] [PubMed] [Google Scholar]
- Shaw P, Kabani NJ, Lerch JP, Eckstrand K, Lenroot R, Gogtay N, Greenstein D, Clasen L, Evans A, Rapoport JL, et al. . 2008. Neurodevelopmental trajectories of the human cerebral cortex. J Neurosci. 28:3586–3594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silk TJ, Beare R, Malpas C, Adamson C, Vilgis V, Vance A, Bellgrove MA. 2016. Cortical morphometry in attention deficit/hyperactivity disorder: contribution of thickness and surface area to volume. Cortex. 82:1–10. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Leonard CM, Welcome SE, Kan E, Toga AW. 2004. Longitudinal mapping of cortical thickness and brain growth in normal children. J Neurosci. 24:8223–8231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiles J. 2008. The fundamentals of brain development. Cambridge, MA: Harvard University Press. [Google Scholar]
- Storsve AB, Fjell AM, Tamnes CK, Westlye LT, Overbye K, Aasland HW, Walhovd KB. 2014. Differential longitudinal changes in cortical thickness, surface area and volume across the adult life span: regions of accelerating and decelerating change. J Neurosci. 34:8488–8498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamnes CK, Herting MM, Goddings A-L, Meuwese R, Blakemore S-J, Dahl RE, Güroğlu B, Raznahan A, Sowell ER, Crone EA, et al. . 2017. Development of the cerebral cortex across adolescence: a multisample study of inter-related longitudinal changes in cortical volume, surface area, and thickness. J Neurosci. 37:3402–3412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomlinson L, Leiton CV, Colognato H. 2016. Behavioral experiences as drivers of oligodendrocyte lineage dynamics and myelin plasticity. Neuropharmacology. 110:548–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M. 2002. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 15:273–289. [DOI] [PubMed] [Google Scholar]
- Walhovd KB, Fjell AM, Brown TT, Kuperman JM, Chung Y, Hagler DJ, Roddey JC, Erhart M, McCabe C, Akshoomoff N, et al. , Pediatric Imaging, Neurocognition, and Genetics Study . 2012. Long-term influence of normal variation in neonatal characteristics on human brain development. Proc Natl Acad Sci USA. 109:20089–20094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walhovd KB, Fjell AM, Giedd J, Dale AM, Brown TT. 2016. Through thick and thin: a need to reconcile contradictory results on trajectories in human cortical development. Cereb Cortex. 27:1472–1481. bhv301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walhovd KB, Krogsrud SK, Amlien IK, Bartsch H, Bjørnerud A, Due-Tønnessen P, Grydeland H, Hagler DJ, Håberg AK, Kremen WS, et al. . 2016. Neurodevelopmental origins of lifespan changes in brain and cognition. Proc Natl Acad Sci USA. 113:9357–9362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Shi F, Li G, Gao Y, Lin W, Gilmore JH, Shen D. 2014. Segmentation of neonatal brain MR images using patch-driven level sets. Neuroimage. 84:141–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wierenga LM, Langen M, Oranje B, Durston S. 2014. Unique developmental trajectories of cortical thickness and surface area. Neuroimage. 87:120–126. [DOI] [PubMed] [Google Scholar]
- Wolff JJ, Piven J. 2014. Neurodevelopmental disorders: accelerating progress in autism through developmental research. Nat Rev Neurol. 10:431–432. [DOI] [PubMed] [Google Scholar]
- Xia K, Yu Y, Ahn M, Zhu H, Zou F, Gilmore JH, Knickmeyer RC. 2014. Environmental and genetic contributors to salivary testosterone levels in infants. Front Endocrinol (Lausanne). 5:187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang DY-J, Beam D, Pelphrey KA, Abdullahi S, Jou RJ. 2016. Cortical morphological markers in children with autism: a structural magnetic resonance imaging study of thickness, area, volume, and gyrification. Mol Autism. 7:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Inder TE, Neil JJ, Dierker DL, Alexopoulos D, Anderson PJ, Van Essen DC. 2015. Cortical structural abnormalities in very preterm children at 7 years of age. Neuroimage. 109:469–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.