Abstract
Objective:
To characterize the pattern of neuron loss in hippocampal sclerosis of aging (HS-Aging) and age-related diseases and to evaluate its contribution to cognitive impairment in the elderly.
Methods:
Participants (n = 1,361) came from longitudinal observational studies of aging at the Knight Alzheimer Disease Research Center, Washington University, Saint Louis, Missouri, USA. Relative neuron loss in the hippocampus of HS-Aging was measured using unbiased stereological methods. Transactive response DNA-binding protein of 43 kDa (TDP-43) proteinopathy, a putative marker of HS-Aging, was assessed. Clinical and cognitive data were analyzed using parametric statistical methods.
Results:
Ninety-three cases had HS-Aging (6.8%), eight cases had ‘pure’ HS-Aging, and 37 cases had comorbid intermediate or high Alzheimer disease neuropathologic change (i/h ADNC). Relative neuron loss (ratio of neuron number in hippocampal subfield CA1 to the neuron number in parahippocampal gyrus) was 0.15 for HS-Aging; this was significantly lower than 0.64 for i/h ADNC and 0.66 for control cases (Kruskal-Wallis test, p <0.0001, p = 0.0003, respectively). TDP-43 proteinopathy was present in 92.4% of HS-Aging cases, higher than that in i/h ADNC (52%) and control (25%) cases. Pure HS-Aging cases were more likely to have cognitive impairment in the memory domain.
Interpretation:
Relative neuron loss in the hippocampus compared to the PHG may be useful in distinguishing HS-Aging in the context of comorbid ADNC. HS-Aging contributes to cognitive impairment which phenotypically resembles AD dementia. TDP proteinopathy is a frequent comorbidity in HS-Aging and may contribute to cognitive impairment to a modest degree.
Hippocampal sclerosis of aging (HS-Aging) is a pathological entity characterized by focal neuron loss and gliosis in the Cornu Ammonis field 1 (CA1) of the hippocampus and the subiculum. While HS was first described as a pathological lesion commonly found in autopsied brains of patients with temporal lobe epilepsy (TLE) in the early 19th century,1 HS has been used broadly to include cerebrovascular disease, Alzheimer disease neuropathologic change (ADNC) and frontotemporal lobar degeneration (FTLD). More recently, it has been identified as a neurodegenerative entity contributing to dementia in the elderly. To distinguish HS in the aging brain from HS in TLE, infarcts, FTLD and other neurodegenerative diseases, we use the appellation HS-Aging. HS-Aging is gaining increased attention because recent reports indicate that it is not an infrequent pathology in the elderly, especially in people aged over 80,2,3 and its prevalence is estimated to be even higher than that of ADNC after the age of 95.4 Intriguingly, HS-Aging often coexists with other pathologies which also cause dementia, such as ADNC, FTLD, Lewy body disease (LBD), vascular and other diseases.5,6 It remains to be determined whether aging alone contributes to these multiple pathologies or if one or more coexisting pathologies contribute to the pathogenesis of HS-Aging. A number of studies support an association between HS-Aging and FTLD with transactive response DNA-binding protein of 43 kDa (FTLD-TDP).6,7,8 In several neurodegenerative diseases there may be extensive neuron loss and gliosis in multiple fields of the hippocampus, but in HS-Aging the neuron loss is restricted mainly to the CA1 field and subiculum.9 The coexistence of multiple pathologies and the focal nature of HS-Aging raise two difficulties for the practicing neuropathologist. Firstly, in the context of moderate to severe comorbid disease, most frequently ADNC, the neuropathologic diagnosis of HS-Aging may be difficult. Although the most recent neuropathologic diagnostic criteria define HS-Aging by pyramidal neuron loss and gliosis in the CA1 hippocampal field and subiculum that is out of proportion to ADNC, which also contributes independently to hippocampal damage,9 it may be challenging to disentangle the neuronal loss attributed to ADNC against that generated by HS-Aging. Secondly, in the context of comorbid disease, it may be difficult to attribute clinical deficits to HS-Aging or ADNC, or both. As HS-Aging involves a relatively small but clinically eloquent part of the brain, more widespread neurodegenerative pathology, e.g., ADNC, may obscure any contribution to the clinical picture. However, some studies have demonstrated that HS-Aging may be associated with profound episodic memory deficits and preserved word fluency compared to Alzheimer disease dementia (AD dementia).4,10 Determining the contribution of HS-Aging in the presence of comorbidities to the clinical picture is likely to require pooling of data from multiple sites.
In this study, we reviewed the neuropathology of brains donated to the Knight Alzheimer Disease Research Center (Knight ADRC), Washington University, Saint Louis, Missouri, USA, as part of longitudinal, observational studies. Cases were identified as having HS-Aging according to the most recent neuropathologic criteria. To characterize more fully neuronal loss, stereologic methods were used with all cases of HS-Aging and cohorts of ADNC and normal aged control cases. To determine the presence of TDP-43 proteinopathy, all HS-Aging cases had additional TDP-43 immunohistochemistry performed. HS-Aging, ADNC and normal control cases were analyzed to determine the contribution of HS-Aging to the clinical phenotype.
Materials and Methods
Participants
Research participants were recruited from the Memory and Aging Project (MAP), a longitudinal, observational study directed by the Knight ADRC; all participants who made a brain donation were included. A few patients who undertook similar assessments to those in MAP were obtained from a separate outpatient clinic, the Memory Diagnostic Center (MDC), both at Washington University, St. Louis, Missouri. Additional brains were included from outside institutes where a referral for neuropathologic assessment of patients had been made and in whom cognitive impairment or dementia was reported, similar to the assessments performed by MAP.
Clinical and cognitive assessments
In MAP, each participant was annually assessed at a study visit by experienced clinicians using a semi-structured interview with a participant and his or her partner, spouse or child (the collateral source), as well as a neurological examination (Table 1). Clinicians were blinded to previously collected data and any previous cognitive assessment. Each participant underwent cognitive testing by an experienced psychometrician within two weeks of the clinical assessment (Table 2). The battery of psychometric tests included standard paper and pencil cognitive tests which assess global cognitive function, episodic memory, executive function, visuospatial ability and language ability as described previously.11 All the collected data were stored in the Knight ADRC research database. Longitudinal symptoms, signs, diagnostic impression and results of cognitive tests were obtained from the database. In a subset of patients assessed in MDC, only demographic data, clinical diagnosis and Clinical Dementia Rating (CDR) with age at assessment were available. During the thirty-year life of the MAP, the assessment protocol underwent modification over the years, but the CDR was used universally in MAP and MDC and there was no “drift” in the CDR over this time period.12 For this study, the clinical diagnosis of AD dementia included all participants who had ‘probable AD’ according to the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association (NINDS-ADRDA) criteria and probable AD dementia following the National Institute on Aging - Alzheimer’s Association (NIA-AA) criteria. Variables included: age at symptom onset, age at death, final clinical diagnosis and CDR at death. Study procedures and policies were approved by the Washington University - Institutional Review Board (WU-IRB).
TABLE 1.
Characteristics of Study Participants
| Variable | HS-Aging (pure) | Control | p value | HS-Aging + i/h ADNC | i/h ADNC | p value |
|---|---|---|---|---|---|---|
| Frequency | 7 | 8 | 33 | 32 | ||
| Age at onset, mean, y | 78.1 | 87.0 | 0.27 | 78.2 | 78.3 | 0.72 |
| Age at death, mean [range], y | 90 [72–96] | 91.1 [81–104] | 0.67 | 88.5 [78–101] | 88.1 [77–100] | 0.63 |
| Female, n (%) | 28.6 | 50 | 0.40 | 69.7 | 65.6 | 0.73 |
| Education, mean, y | 13.1 | 15.5 | 0.41 | 13.4 (n=32) | 13.6 (n=31) | 0.85 |
| Family history of dementia, n (%) | 50 (n=4) | 50 (n=0) | 0.99 | 83.3 (n=24) | 85.7 (n=21) | 0.82 |
| APOE ε4 allele (n) | 6 | 8 | 0.99 | 29 | 29 | 0.61 |
| 2 | 0 | 0 | 1 | 2 | ||
| 1 | 2 | 2 | 13 | 14 | ||
| 0 | 4 | 6 | 15 | 13 | ||
| Primary clinical diagnosis | ||||||
| AD dementia | 6 | 1 | 0.004 | 33 | 30 | 0.24 |
| Other | 0 | 2* | 0.16 | 0 | 2** | 0.14 |
| Normal cognition | 1 | 5 | 0.06 | 0 | 0 | ― |
| Symptoms | ||||||
| Seizure | 1 / 6 | 0 / 8 | 0.23 | 4 / 31 | 4 / 30 | 0.69 |
| Stroke or TIA | 0 / 6 | 3 / 8 | 0.16 | 5 / 31 | 7 / 30 | 0.50 |
| Head injury | 5 / 6 | 3 / 8 | 0.09 | 7 / 30 | 5 / 30 | 0.52 |
| Disease duration, mean, y | 11.9 | 2.5 | 0.01 | 10.5 | 9.8 | 0.55 |
| CDR at death | 0.004 | 0.94 | ||||
| ≥2 | 6 | 0 | 29 | 28 | ||
| 0.5 or 1 | 0 | 3 | 4 | 4 | ||
| 0 | 1 | 5 | 0 | 0 | ||
| CDR-SB | (N=6) | (N=31) | (N=30) | |||
| Last assessment | 11.8 | 1.4 | 0.01 | 11.7 | 10.4 | 0.45 |
| Annual rate of change | 0.44 | 0.08 | <0.0001 | 0.99 | 0.90 | 0.43 |
| MMSE | ||||||
| Last assessment | 17.2 (n=5) | 27.9 | 0.01 | 17.6 (n=23) | 18 (n=18) | 0.86 |
| Annual rate of change | −0.37 | −0.14 | 0.07 | −0.98 | −0.40 | 0.05 |
| Brain Weight, g | 1218 | 1213 | >0.99 | 1104 | 1126 | 0.47 |
| Pathology, ADNC | >0.99 | 0.01 | ||||
| Not ADNC | 2 | 3 | 0 | 0 | ||
| Low | 5 | 5 | 0 | 0 | ||
| Intermediate | 0 | 0 | 9 | 1 | ||
| High | 0 | 0 | 24 | 31 | ||
Numbers written with brackets are numbers of participants with available data. All values are given as mean or number of participants unless otherwise indicated. P values were determined by use of Mann-Whitney U test for age and CDR and MMSE at last assessment, Student's t-test for education and disease duration, chi-squared test for categorical variables, and linear regression analysis for annual change.
one dementia with Lewy bodies case and one with uncertain dementia
one frontotemporal dementia and one vascular dementia with ADNC case. Abbreviations: HS-Aging = hippocampal sclerosis of aging; ADNC = Alzheimer's disease neuropathologic change; i/h ADNC = intermediate or high ADNC; HS-Aging + i/h ADNC = HS-Aging with i/h ADNC; AD dementia = Alzheimer's disease dementia; TIA = transient ischemic attack; CDR = Clinical Dementia Rating; CDR-SB = CDR Sum of Boxes; MMSE = Mini-Mental State Examination.
TABLE 2.
Neuropsychology of Study Participants
| Variable | HS-Aging (pure) | Control | p value | HS-Aging + i/h ADNC | i/h ADNC | p value |
|---|---|---|---|---|---|---|
| Frequency | 7 | 8 | 33 | 32 | ||
| Digit span forward | (N=6) | (N=8) | (N=32) | (N=29) | ||
| last assessment | 5.8 | 6.6 | 0.46 | 5.3 | 5.1 | 0.95 |
| annual rate of change | −0.01 | 0.00 | 0.87 | −0.05 | −0.01 | 0.22 |
| Digit span backward | (N=6) | (N=32) | (N=28) | |||
| last assessment | 3.2 | 4.9 | 0.17 | 3.3 | 3.8 | 0.96 |
| annual rate of change | −0.06 | −0.03 | 0.25 | −0.08 | −0.10 | 0.64 |
| Digit symbol substitution | (N=6) | (N=28) | (N=26) | |||
| last assessment | 27.5 | 33.8 | 0.41 | 20.8 | 20.8 | >0.99 |
| annual rate of change | −0.20 | −0.58 | 0.16 | −2.13 | −1.49 | 0.27 |
| Trail Making Test-A | (N=6) | (N=31) | (N=26) | |||
| last assessment | 83.0 | 66.5 | 0.22 | 107.2 | 100.6 | 0.79 |
| annual rate of change | −0.83 | 1.7 | 0.0006 | 4.0 | 3.9 | 0.97 |
| Category Fluency | (N=4) | (N=22) | (N=18) | |||
| last assessment | 10.8 | 15.3 | 0.17 | 8.2 | 8.1 | 0.97 |
| annual rate of change | −0.98 | −0.43 | 0.04 | −0.54 | −0.33 | 0.54 |
| Logical Memory - immediate | (N=6) | (N=31) | (N=29) | |||
| last assessment | 4.2 | 6.5 | 0.04 | 1.7 | 1.6 | 0.52 |
| annual rate of change | −0.41 | 0.09 | <0.0001 | −0.84 | −0.28 | 0.008 |
| Logical Memory - delayed | (N=6) | (N=29) | (N=28) | |||
| last assessment | 1.7 | 5.8 | 0.01 | 0.5 | 0.6 | 0.20 |
| annual rate of change | −0.56 | 0.06 | <0.0001 | −0.72 | −0.12 | 0.001 |
| Boston Naming Test | (N=6) | (N=30) | (N=27) | |||
| last assessment | 45.7 | 52.9 | 0.39 | 29.0 | 32.4 | 0.47 |
| annual rate of change | −0.59 | −0.45 | 0.36 | −1.80 | −0.64 | 0.04 |
| Block Design | (N=6) | (N=30) | (N=26) | |||
| last assessment | 19.3 | 28.8 | 0.22 | 15.3 | 14.9 | 0.88 |
| annual rate of change | −0.25 | −0.47 | 0.32 | −1.06 | −0.50 | 0.21 |
Numbers written with brackets are numbers of participants with available data for each. All values are given as means. P values were determined by use of the Mann-Whitney U test for data at last assessment and use of linear regression analysis for annual change. Abbreviations: HS-Aging = hippocampal sclerosis of aging; i/h ADNC = intermediate or high Alzheimer's disease neuropathologic change; HS-Aging + i/h ADNC = having i/h ADNC in addition to HS-Aging.
APOE genotyping
DNA was extracted from antemortem blood samples or fresh-frozen brain tissue from a subset of participants according to the protocol of the WU-IRB. Apolipoprotein E (APOE) genotyping was performed within the Knight ADRC Genetics Core using established methods.13
Neuropathologic assessment
All brain donations were obtained with informed consent following WU-IRB protocols. At the time of the autopsy, usually limited to the removal of the brain, each brain was assessed macroscopically and digitally recorded. Routinely, the right hemibrain was coronally sliced, snap-frozen and preserved for biochemical studies and the left hemibrain was fixed in 10% buffered formalin using established protocols.14 Formalin-fixed tissue samples were embedded in paraffin and 6 μm sections were cut. Sections were stained with hematoxylin and eosin (H&E) and by a modified Bielschowsky silver impregnation. Immunohistochemistry (IHC) was performed with anti-phosphorylated tau (PHF1; a gift from Dr. P. Davies, Feinstein Institute for Medical Research, Manhasset, NY), anti-phosphorylation-dependent α-synuclein (pSyn #64; Wako Pure Chemical Industries, Osaka, Japan), anti-β-amyloid (10D5; Eli Lilly, Indianapolis, IN), and anti-phosphorylation-dependent TAR DNA-binding protein 43 (anti-TDP phospho-Ser409/410; Cosmo Bio, Tokyo, Japan) antibodies. Staining was performed on sections of the middle frontal gyrus, anterior cingulate gyrus, precentral gyrus, superior temporal gyrus, inferior parietal lobule, amygdala, hippocampus at the level of the lateral geniculate nucleus, striatum including the basal forebrain nuclei, pallidum, thalamus, midbrain, pons, medulla oblongata, and cerebellum.
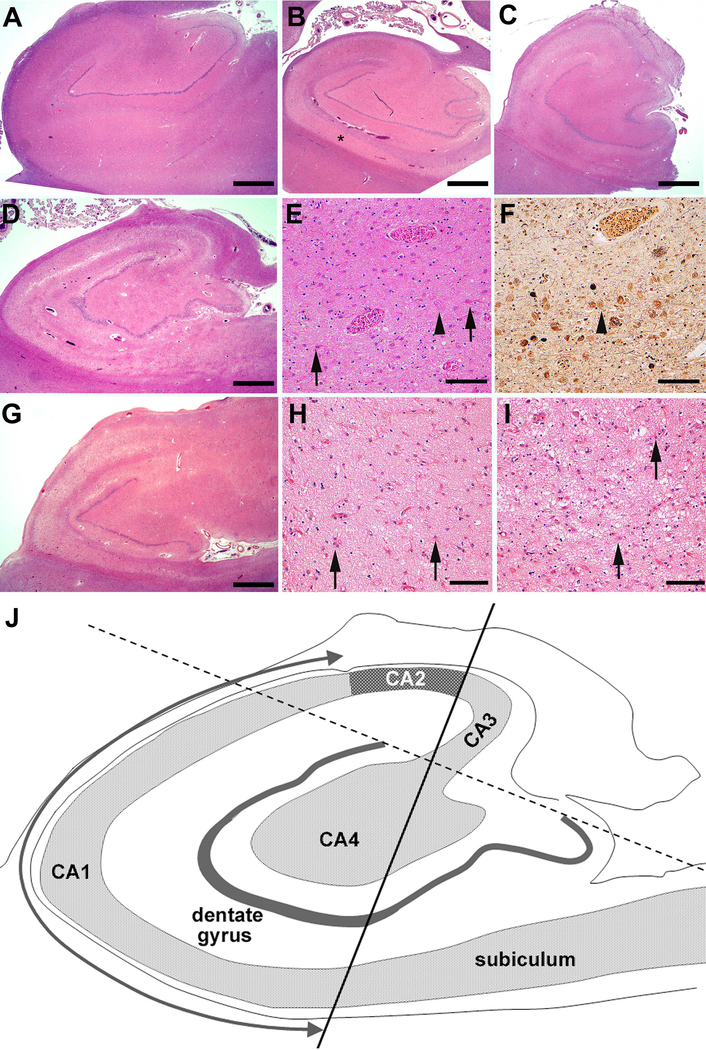
In total, 1,405 brains were assessed by the Neuropathology Core of the Knight ADRC using a standard protocol between January 1981 and December 2014. Some cases were excluded because of unavailability of hippocampal tissue or absence of clinical or demographic data. After excluding cases with missing tissue (hippocampus) and/or data 1,361 cases were available for neuropathologic assessment. Although HS was recorded in the research database, all 1,361 cases were reassessed to ensure uniformity in assessment across the three decades of brain collection. For example, in the 1980s, some descriptions included “HS due to Alzheimer disease.” All such cases were reassessed. To ensure a standard and uniform diagnosis of HS-Aging in all cases, the following criteria were applied: 1) there had to be severe neuronal loss and gliosis in CA1 field and subiculum of the hippocampus using a section stained with H&E; 2) the severity of neuronal loss in the CA1 field and the parahippocampal gyrus had to be out of proportion to neuronal loss in adjacent cortex using an H&E section; and 3) the case was excluded if coexisting ADNC, FTLD, or other neurodegenerative disease, or vascular disease was present and may have explained the neuronal loss in CA1 and determined by reviewing adjacent sections with a silver impregnation and/or IHC that detected Aβ amyloidosis, tauopathy, TDP-43 proteinopathy, or other neurodegenerative disease. Thus, cases exhibiting severe neuronal loss and gliosis in both CA1 and the subiculum and PHG in the context of ADNC, FTLD, or other neurodegenerative disease that mimic HS-Aging were excluded. Cases of typical HS-Aging, atypical HS-Aging and ADNC mimicking HS-Aging are shown in Fig 1A-I.
FIGURE 1:
Photomicrographs showing hippocampal sclerosis of Aging (HS-Aging) and frequent comorbidities that mimic hippocampal sclerosis. An 88-year-old male with a macroscopically normal hippocampus and Alzheimer disease neuropathologic change (ADNC) but without HS-Aging (A). A 68-year-old male with frontotemporal lobar degeneration and transactive response DNA-binding protein of 43 kDa (FTLD-TDP) + HS-Aging (B). There is marked atrophy of the CA1 (*). An 89-year-old female with intermediate or high ADNC + HS-Aging (C). There is atrophy, pallor and severe neuronal cell loss and gliosis in CA1. A 95-year-old female with high ADNC and severe neuronal loss mimicking HS-Aging (D-F). There is atrophy and pallor in both the CA1 and the subiculum (D); at higher magnification, several ‘ghost’ tangles are visible (E, F, arrows) and a reactive astrocyte is labeled (arrowhead). A 73-year-old female with FTLD-TDP mimicking HS-Aging (G to I). There is narrowing and pallor not only of CA1 and the subiculum but also of CA2 and CA3 (G). There is severe neuron loss and frequent reactive astrocytes (arrows) in CA1 (H) as well as in the PHG (I). (A - E and G - I) hematoxylin and eosin stain; (F), modified Bielschowsky silver impregnation. Scale bar = 500 μm (A-C, D and G), 100 μm (E, F, H and I). (J) Diagram of the hippocampus used to define hippocampal subfields for neuron number estimation. CA1 = Cornu Ammonis 1 field.
Neuropathologic staging of ADNC was determined using the NIA-AA guidelines. Where appropriate, established neuropathologic diagnostic criteria were used to determine the presence of cerebrovascular disease (CVD), FTLD, Lewy body disease (LBD), argyrophilic grain disease (AGD), corticobasal degeneration (CBD) and other tauopathies.9,15–19 These entities were found in combination with HS-Aging. Following the NIA-AA criteria, ‘low’ ADNC is not considered to contribute to clinical features. Thus, for this study, ‘intermediate’ or ‘high’ ADNC were grouped together as i/h ADNC. HS-Aging cases having i/h ADNC were defined as i/h ADNC + HS-Aging and HS-Aging cases having ‘low’ or ‘not’ ADNC were defined as HS-Aging (pure). Some cases with ‘not’ ADNC according to NIA-AA had primary age-related tauopathy (PART).20 In this study, cases lacking Aβ plaques but with evidence of PART were assigned a neurofibrillary tangle score of 1 or 2 according to the NIA-AA criteria, likely reflecting the older age of these cases.
Thirty-three of 37 i/h ADNC + HS-Aging cases and seven of eight pure HS-Aging cases from the MAP or MDC cohorts had clinical and cognitive data and were included in the study. Cases with i/h ADNC and no HS-Aging were matched by age at death, sex and CDR at death with i/h ADNC + HS-Aging cases. Normal control cases were matched with pure HS-Aging cases by age at death, gender, and other demographic variables. For the control groups, more recent cases were preferentially selected if multiple cases were available and complete demographic and other data were available. The selected cases underwent thorough neuropathologic review. Thirty-two i/h ADNC cases and eight normal control cases were available for neuropathologic, clinical and cognitive data analyses.
Stereology
A 25 μm-thick section was cut from the paraffin-embedded block of the middle part of the hippocampus by a rotary microtome and was stained with cresyl violet for neuron counting. Poor preservation of some tissue blocks more than 25 years old were unsuitable for cresyl violet staining and were excluded from the study. Suitable sections for analysis were obtained from 81 HS-Aging cases, 23 i/h ADNC cases and six normal control cases. Due to limitations of tissue availability, only one section at a single level was used for stereological analysis in each case. The section used for estimating neuron number, typically at the level of the lateral geniculate nucleus, was close to, but not always adjacent to, the section stained with H&E.
To estimate neuron number, cresyl violet-stained sections were examined using a Nikon Eclipse E800 research microscope (Nikon Instruments Inc., Melville, New York) and a computer-operated stage controller system (MAC 5000; Ludl Electronic Products Ltd., Hawthorne, New York). Estimates of neuron number in the CA1 field of the hippocampus and in the cortical ribbon of the PHG of the same section were performed using stereology software (Stereo Investigator Version 10; MicroBrightField Inc., Williston, Vermont). The ‘disector’ probe was used to estimate neuron numbers21,22. The boundary between CA1 and the subiculum was operationalized by using a line perpendicular to a line connecting both termini of the dentate gyrus and through the apex of the curve of CA2 and CA3. A boundary between CA1 and CA2 was identified by the size, shape and density of neurons in the hippocampus (Fig 1J). A line connecting both termini of the dentate gyrus was used as a boundary between CA1 and CA2. The parahippocampal gyrus was defined as the region of the cortex between a line perpendicular to the neocortex containing six layers and a line through the collateral fissure on the same section. Contours of CA1 and the parahippocampal gyrus were outlined at low magnification (X40). To determine the sampling site number, pilot experiments on ADNC cases were performed in order to generate a coefficient of error of < 0.1. The optical fractionator probe was used to generate more than 300 sampling grids in CA1 and > 200 sampling sites in the neocortex of the PHG. Each counting frame had an X-axis width of 50 μm and a Y-axis height of 50 μm (counting frame area = 2,500 μm2) and each disector probe had a z-axis depth of 10 μm. Counting frames were viewed with a 100X oil immersion objective lens. Neurons were defined as cells having a soma, stained cytoplasm, and a darkly stained single nucleolus following established morphological criteria.23 Nucleoli were counted with the disector probe using established counting rules for neurons.24 Approximately 100–150 neurons were counted in CA1 and 100–200 neurons were counted in the PHG; however, due to severe neuronal loss in HS-Aging, fewer than 25 neurons were counted in CA1 in many of the HS-Aging cases even after exhaustive sampling of more than 300 sites. Fewer than 100 neurons were counted in CA1 in several severely affected i/h ADNC cases; similar neuron numbers were observed in the PHG. As with HS-Aging cases, some i/h ADNC cases did not yield coefficients of error < 0.1.
TDP-43 proteinopathy
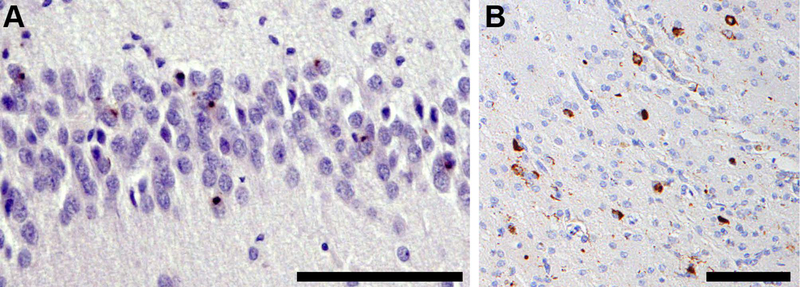
TDP-43-immunoreactive inclusions were counted visually in 6 μm sections from 92 HS-Aging cases, 33 i/h ADNC cases, and eight control cases. Sections underwent TDP IHC at the same time to ensure inter-batch consistency of staining. Sections were viewed using a Nikon Eclipse E800 research microscope and images were captured using CellSens (Olympus, Tokyo, Japan) software. Briefly, within an area of 25,000 μm2 (the width of a frame adjusted to the width of the dentate gyrus) in the most severely affected region of CA1 and within an area of 250,000 μm2 (500 × 500 μm frame) in that of the PHG, any type of TDP-immunoreactive inclusion body was counted (Fig 5A and B). 25 A six-point scale modified from Wilson et al. was used to grade cases: grade 0, no inclusion; grade 1, 1–5 inclusions; grade 2, 6–12 inclusions; grade 3, 13–20 inclusions; grade 4, 21–30 inclusions; grade 5, >30 inclusions per region of interest.26
FIGURE 5:
TDP-43 proteinopathy in a case of HS-Aging. (A) TDP-43-immunoreactive neuronal cytoplasmic inclusions (NCI) in the dentate gyrus of an 84-year-old female with FTLD-TDP + HS-Aging. (B) NCI and dystrophic neurites (DN) are present in the PHG of an 89-year-old female with ADNC + CVD + HS-Aging. Phospho-TDP-43 immunohistochemistry, scale bar = 100 μm (A, B).
Statistical Analysis
Statistical analyses were undertaken using GraphPad Prism 7.03 (GraphPad Software, La Jolla, California) or JMP Pro 13 (SAS Institute Inc., Cary, North Carolina) software. For continuous data, comparisons between two groups were performed using Student’s t-test, and the Mann-Whitney U test was used when an outlier existed. Group comparisons for ordinal data such as CDR and TDP-positive inclusion frequency were performed with the Mann-Whitney U test. Group comparisons for categorical measures, such as sex and presence of family history were performed with the chi-squared test. Group comparisons of neuronal cell counts were performed using the Kruskal-Wallis test followed by Dunn’s multiple comparison test. The correlation between two ordinal data sets was assessed using Spearman’s rank correlation coefficient. The annual rate of change of CDR and cognitive tests were compared by linear regression analysis with an interaction effect for the neuropathologic diagnosis. Analyses of cognitive performance were undertaken with time adjusted for years after symptom onset.
Results
Characteristics of Study Participants
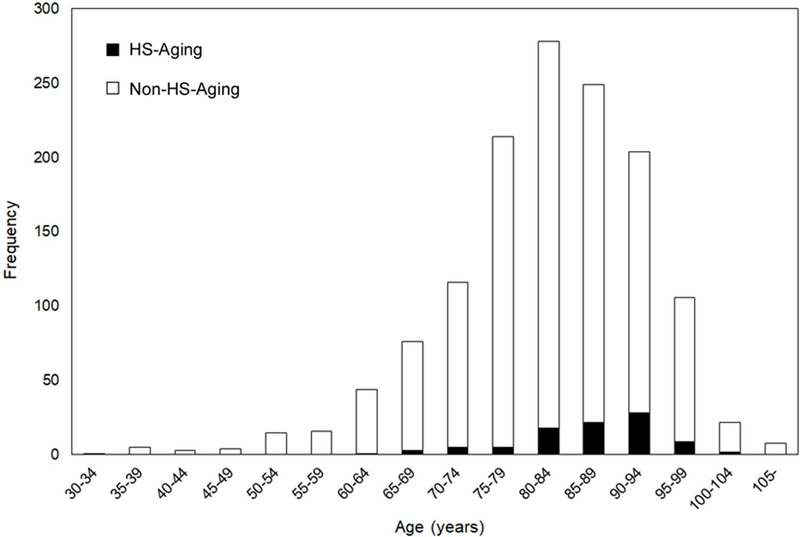
Hippocampal sclerosis of aging was present in 93 out of 1,361 participants of the Knight ADRC sample (6.8%). Mean and median age of the participants with HS-Aging (86.6 years, 88 years, range 64–101 years) were significantly higher than the entire cohort (81.9 years, 83 years, range 34–108 years, Mann-Whitney U test, p<0.0001, Fig 2). Individuals aged ≥ 90 years were significantly more likely to have HS-Aging compared to individuals aged < 90 (chi-squared test, p=0.0001). The prevalence of HS-Aging at age 90 or older was 11.5%. The frequency of HS-Aging in the Knight ADRC cohort was lower than that reported in some other studies, e.g., 13% of the total cohort and 18% of individuals aged > 90 years had HS-Aging in one study,6 and 17% of the participants aged ≥ 90 years had HS-Aging in another study.5 One study reported 6% of the autopsy cohort had HS-Aging, the same as our study.27 The lower frequency of HS-Aging in our study may be explained by sampling restricted to the hippocampus from only one level and from only one hemisphere. HS-Aging may be unilateral and affect only one segment of the hippocampus, thus, HS-Aging may be under represented in our sample. Gender did not account for any differences in our cohort (54.6% were female; chi-squared test, p=0.79).
FIGURE 2:
Frequencies of HS-Aging among 1,361 neuropathologically assessed cases. HS-Aging = hippocampal sclerosis of aging; non-HS-Aging = non-hippocampal sclerosis of Aging.
Comorbidity in individuals with HS-Aging
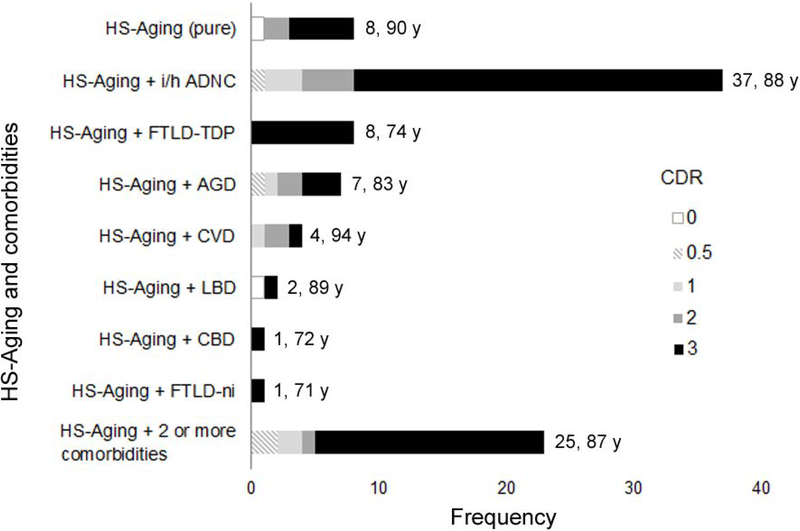
Comorbid pathology, most frequently i/h ADNC, was a common finding in this cohort (Fig 3). Sixty subjects had one or more comorbid diseases and 25 subjects had two or more pathologies in addition to HS-Aging. Eight subjects had HS-Aging alone (pure HS-Aging), which accounted for 8.6% of the HS-Aging cases and 0.59% of the entire cohort. Thirty-seven cases had i/h ADNC in addition to HS-Aging (i/h ADNC + HS-Aging) and eight cases had FTLD-TDP in addition to HS-Aging (FTLD-TDP + HS-Aging). Participants with FTLD-TDP + HS-Aging were more likely to be younger at the age at death (mean age at death: 74.1 years; Mann-Whitney U test, p<0.0001) and have more severe cognitive impairment (data not shown) than in i/h ADNC with HS-Aging or with HS-Aging alone.
FIGURE 3:
Frequencies of comorbid neurodegenerative diseases with HS-Aging. Numbers shown to the right of each bar indicate frequency and mean age at death. HS-Aging = hippocampal sclerosis of aging; i/h ADNC = intermediate or high Alzheimer disease neuropathologic change; FTLD-TDP = frontotemporal lobar degeneration with TDP-43 proteinopathy; AGD = argyrophilic grain disease; CVD = cerebrovascular disease; LBD = Lewy body disease; CBD = corticobasal degeneration; FTLD-ni = FTLD with no inclusions.
Neuron counts in HS-Aging
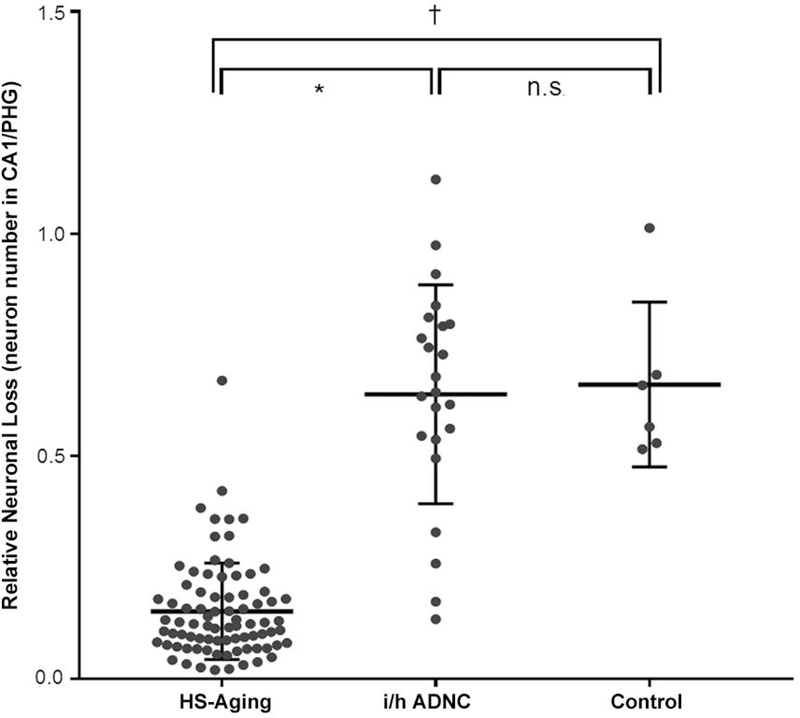
Summary data of the numbers of neurons in the CA1 subfield of the hippocampus and the parahippocampal gyrus are shown in Fig 4 and Supplementary Table. These data were comparable to those of previous studies using stereological methods to estimate neuron numbers.28–32 Although sections were cut at 25 μm, variations in processing over three decades combined with shrinkage due to dewaxing resulted in a measured mounted section thickness of 14.0 ± 2.1 μm in CA1 and 13.8 ± 1.9 μm in the PHG (mean ± SD, N=110). To eliminate this variability between and across a single section, the ratio of neuron number in CA1 to number in the PHG was calculated for each case. The ratio of neuronal numbers in CA1 to the PHG was significantly lower in HS-Aging cases (0.15 ± 0.11) than i/h ADNC cases (0.64 ± 0.25, Kruskal-Wallis test, p < 0.0001) and control cases (0.66 ± 0.19, Kruskal-Wallis test, p = 0.0003). Some i/h ADNC cases had severe neuron loss due to neurofibrillary tangle (NFT) pathology in the CA1 region mimicking HS-Aging (Fig 1D-F) and the variability of the ratio of neuron numbers in CA1 to PHG was larger in i/h ADNC cases (Fig 4). A comparison of the ratio of neuron numbers in pure HS-Aging, HS-Aging + i/h ADNC, HS-Aging + FTLD-TDP, HS-Aging + AGD and HS-Aging + CVD, showed no statistically significant differences between groups (Supplementary Table).
FIGURE 4:
Relative neuron loss in CA1 distinguishes HS-Aging from ADNC and normal aging. Dot plot shows relative neuron loss (CA1 neuron number/PHG neuron number) in HS-Aging, ADNC and normal control cases. Neuron numbers were estimated using the disector probe. Data are expressed as mean ± standard deviation; Kruskal-Wallis test, *p < 0.0001, †p = 0.0003, n.s. = not significant. HS = hippocampal sclerosis; i/h ADNC = intermediate or high Alzheimer disease neuropathologic change alone; CA1 = Cornu Ammonis 1 field; PHG = parahippocampal gyrus.
TDP-43-immunoreactive inclusion bodies
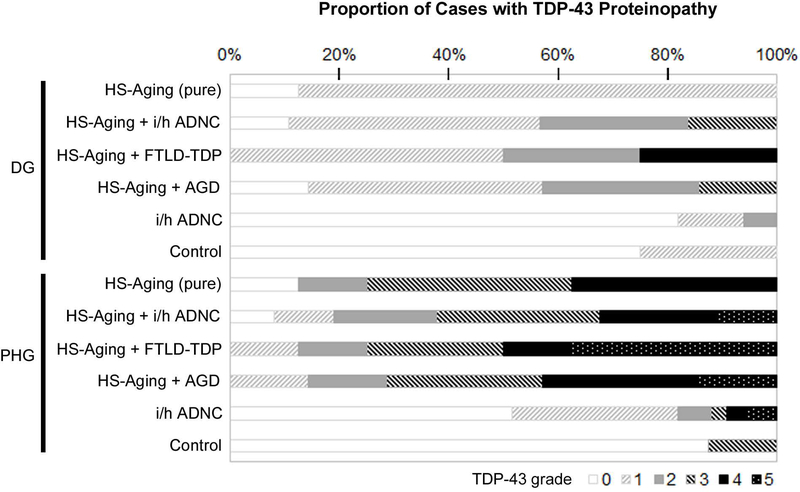
The frequency of TDP-43-immunoreactive inclusions graded with a 6-point scale within the most affected area were compared among HS-Aging cases, i/h ADNC cases and control cases. HS-Aging cases had a tendency to have more frequent TDP-43-positive inclusions both in the dentate gyrus (DG) and in the superficial laminae of the PHG compared to i/h ADNC and aged control cases (Kruskal-Wallis test, p < 0.0001 for the DG and p = 0.0012 vs i/h ADNC cases, and p = 0.0012 for the DG and p = 0.0002 for the PHG vs control cases). I/h ADNC cases also showed more frequent TDP-43-positive inclusions both in the DG and in the PHG than control cases, but these differences did not reach statistical significance. Each of the HS-Aging cohorts showed almost the same proportion of TDP-43-positive inclusions in both the DG and in the PHG, but TDP-43 proteinopathy was more severe in HS-Aging than i/h ADNC cases (Fig 6). Only in FTLD-TDP + HS-Aging cases was there a trend towards a greater number of TDP-43-positive inclusions in the DG. We found that pure HS-Aging cases had only sparse inclusions in the DG, but had frequent inclusions in the PHG, similar to the other HS-Aging cohorts.
FIGURE 6:
Frequency of TDP-43-immunoreactive inclusions in HS-Aging with or without comorbidities, intermediate or high (i/h) ADNC and normal aging. Data represent the frequencies of TDP-43-immunoreactive inclusions in the DG and PHG and assessed using a TDP-43 grading scale. DG = dentate gyrus; PHG = parahippocampal gyrus; HS-Aging = hippocampal sclerosis of aging; FTLD-TDP = frontotemporal lobar degeneration with TDP-43 proteinopathy; AGD = argyrophilic grain disease.
Seven of 92 HS-Aging cases (7.6%), 16 of 33 i/h ADNC cases (48%), and six of eight control cases (75%) had no TDP-43-positive inclusions in the DG or PHG. Interestingly, two cognitively normal HS-Aging cases (CDR 0 at death) had no TDP-43-positive inclusions. One had LBD in addition to HS-Aging and the other had pure HS-Aging. We did not find a significant correlation between the CDR score at death and the grade of TDP-43-positive inclusions in the DG (Spearman’s ρ = 0.20, p = 0.018), but there was a weak correlation between the CDR at death and the grade of TDP-43-positive inclusions in the PHG (Spearman’s ρ = 0.24, p = 0.005) in the HS-Aging cases overall.
Clinical and Cognitive Features of HS-Aging
To characterize the clinical features of HS-Aging, we compared clinical and cognitive data between groups matched by age and gender and cognitive status (CDR) (Tables 1 and 2). We found no significant associations between education, family history of dementia, or APOE ε4 allele frequency with HS-Aging. Six out of seven pure HS-Aging cases was diagnosed with AD dementia at death and all 33 i/h ADNC+HS-Aging cases and 30 out of 32 i/h ADNC cases had AD dementia. The clinical diagnosis of the remainder was frontotemporal dementia or vascular dementia. We examined the influence of seizure activity as this has been associated with HS in TLE in younger cohorts. We found no association between a history of cerebrovascular disease (stroke or transient ischemic attack) and head injury with HS-Aging. However, the severity of dementia was significantly greater in pure HS-Aging cases than control cases, and global cognitive function assessed by the Mini-Mental State Examination (MMSE) score at the last visit was also significantly lower in pure HS-Aging than control cases. ADNC was significantly milder in HS-Aging + i/h ADNC cases than i/h ADNC cases. Focal atrophy in the hippocampus (HS-Aging) was not correlated with global cerebral atrophy (brain weight).
To further characterize the cognitive deficits in HS-Aging, we analyzed: digit span forward, digit span backward, digit symbol substitution and block design test from the Wechsler Adult Intelligence Scale, Trail Making Test A, category fluency, logical memory immediate and delayed, from the Wechsler Memory Scale; and the Boston Naming Test. Each test was administered annually and at the last assessment (Table 2). Only scores from the logical memory tests resulted in significant differences between pure HS-Aging and control cases. Tests assessing executive function showed a trend towards slightly worse scores in pure HS-Aging cases but these did not reach significance. Comparison between HS-Aging + i/h ADNC cases and i/h ADNC cases did not show significant differences fat the last assessment; however, annual rates of change in logical memory and Boston Naming Test scores were significantly greater in i/h ADNC + HS-Aging cases.
Discussion
HS-Aging has been defined as severe neuronal loss and gliosis in CA1 and/or subiculum in aged individuals (>85 years) but the presence of severe comorbidity, typically ADNC or FTLD-TDP, may make determining the contribution of HS-Aging and other neurodegenerative diseases challenging for the neuropathologist. Using unbiased stereologic methods to assess neuron numbers, we show that a novel relative neuron loss ratio, the ratio of neuron number in CA1/PHG, helps to distinguish HS-Aging from confounding severe comorbid disease. A novel aspect of this study is the use of the most sensitive NIA-AA neuropathologic criteria for the diagnosis of ADNC. Using these approaches, we derived a relative neuron loss threshold ratio of 0.3, which identified HS-Aging with 90.1% sensitivity and 89.7% specificity. Even though the ratio of neuron counts in CA1 to PHG was used to exclude cases with i/h ADNC, three i/h ADNC cases were classified as HS-Aging false-positives and five HS-Aging cases were classified as false-negatives. One possible reason for this discrepancy is that neuronal loss in ADNC may correlate with NFTs which affect the entorhinal cortex more severely;29 however, some ADNC cases may not follow the regular spatial pattern of pathology. Although a single section containing both the hippocampus and the PHG is required to determine HS-Aging, the presence of other comorbidities requires a more extensive neuropathologic examination of molecular and other pathologies and their spatial distribution in order to more fully explain cognitive impairment.
Our data indicate that an individual with pure HS-Aging is likely to present with memory impairment and subsequently develop milder impairment in other domains; overall, the clinical phenotype of HS-Aging mimics AD dementia despite having no or low ADNC in the brain. When HS-Aging and i/h ADNC cases were directly compared, HS-Aging cases expressed a trend towards milder memory impairment when assessed with the Wechsler Memory Scale (WMS) - Immediate Memory Scale (t-test, p = 0.006) and milder impairment in language function assessed with the Boston Naming Test (t-test, p = 0.06) than i/h ADNC cases with the same severity of dementia. Some previous studies found that HS-Aging had an additional effect on cognitive impairment due to ADNC, and when HS-Aging was present it was associated with preserved verbal fluency and worse memory function compared to AD demetia.4,10 To identify the deficits in multiple cognitive domains in HS-Aging a study with greater power is required such as with a multicenter collaborative study. We are planning such a study using the neuropathologic and clinical data archived by the National Alzheimer Coordinating Center (NACC, Seattle, WA).
As HS-Aging typically presents with an amnestic phenotype, clinical features alone may not be adequate to distinguish HS-Aging from ADNC during life. However, if severe neuron loss in the CA1 and subiculum can be detected by neuroimaging techniques, it is likely to improve the accuracy of diagnosis of HS-Aging during life. For example, Botha et al. reported that cases in an autopsy cohort with neuropathologic evidence of HS-Aging had greater medial temporal lobe hypometabolism, as revealed by FDG-PET, than cases with ADNC.33 But as that study showed, measurement of AD biomarkers is necessary to exclude ADNC and other tauopathies.
HS-Aging has been defined historically as a pathology affecting only the CA1 subfield of the hippocampus and/or subiculum. This contrasts with PART, a recently described entity, which affects the medial temporal lobe and hippocampal subdivisions more globally; the latter is often clinically misdiagnosed as mild AD dementia.20 Our data show that pure HS-Aging presents with impairment in broader cognitive domains that are not readily predicted by the focal neuronal loss of HS-Aging. One possible explanation for the involvement of several cognitive domains is the presence of comorbid TDP-43 proteinopathy, at least in the majority of cases. In pure HS-Aging cases TDP-43-positive inclusions were rated grade 3 or 4 of in the PHG in all but one case; one case with no TDP-43 proteinopathy was cognitively normal until death (limited tissue precluded stereological assessment of neuron number). There was an additional HS-Aging case with normal cognition until death in our cohort and this case was also without TDP-43 proteinopathy, but it was excluded from the pure HS-Aging group due to comorbid LBD. Other HS-Aging cases without TDP-43-positive inclusions had comorbid pathologies (CVD not involving the medial temporal lobe) which likely accounted for the cognitive impairment. Amador-Ortiz et al. was the first to report that the majority of cases with HS-Aging have TDP-43 proteinopathy8 and Nag et al. reported that HS-Aging with TDP-43 proteinopathy was associated with lower function in multiple cognitive domains than HS-Aging without TDP-43-immunoreactive inclusion bodies.6 Our study supports these prior findings. We wanted to know if there is any association between the severity of TDP-43 proteinopathy and the degree of cognitive impairment or dementia. In our study, interestingly, pure HS-Aging cases with TDP-43-positive inclusions had only sparse inclusions in the DG indicating that TDP-43 pathology confined to the DG is only weakly associated with clinical symptoms. When all the HS-Aging cases were analyzed as a whole, we found a weak correlation between severity of dementia and the frequency of TDP-43-positive inclusions in the PHG. A prior study found TDP-43 proteinopathy in the medial temporal lobe and neocortex and it was proposed that this might reflect a more advanced stage of HS-Aging. In that study, more widespread TDP-43 proteinopathy was associated with lower function in multiple cognitive domains.34,35 One possible explanation for the weak correlation between the severity of dementia and frequency of TDP-43-positive inclusions in the PHG in our study may be that the correlation was influenced, in part, by comorbid ADNC, by differences in the sizes of cohorts available, and the ages of the respective cohorts. However, the spatial distribution of TDP-43 proteinopathy in the neocortex as reported by others may account for some of the impairment in multiple cognitive domains and may reflect the AD dementia seen in our pure HS-Aging cohort.
As with late-onset ADNC, our data show that HS-Aging can coexist with several comorbidities including other neurodegenerative diseases and vascular disease. These data indicate that HS-Aging and TDP-43 proteinopathy in the medial temporal lobe are likely independent of the pathogenesis of other comorbid pathologies. The severity of neuron loss in CA1 of HS-Aging appears to be independent of comorbid pathologies. Why the CA1 subfield is selectively vulnerable to neurodegeneration is the focus of intensive investigations. Although aging is considered a major risk factor for HS-Aging, FTLD-TDP + HS-Aging cases are significantly younger than the other subtypes of HS-Aging suggesting that FTLD-TDP may accelerate the pathogenesis of HS-Aging. Nag et al. propose that TDP-43 pathology may occur in HS-Aging independently of FTLD-TDP or ADNNC, and contribute to dementia in the aged population.35 Our data support this conclusion. Nelson et al. demonstrate that TDP-43 pathology may extend beyond the hippocampus in HS-Aging and this may contribute to some of the deficits in cognitive domains as we and others have demonstrated. To encompass cases solely with TDP-43 pathology, Nelson et al. propose a new term, ‘cerebral age-related TDP-43 and sclerosis’ (CARTS).36 Most of our cases would meet these criteria but a small, but significant proportion without TDP-43 proteinopathy, would be excluded. As the significance of TDP-43 pathology in the pathogenesis of HS-Aging or CARTS is still uncertain, caution is required in applying this term.
There are limitations to our approach. In this study, only a single section from one level and from one hemisphere was available for the estimation of neuron numbers in the hippocampus and the PHG. While HS-Aging may occur unilaterally or bilaterally, our data are likely underestimates of the true incidence of this pathology. Although we did not use the neuronal marker, NeuN, to identify neurons, cresyl violet stain and morphologic criteria are widely used to reliably estimate neuron numbers and our interrater reliability measures were acceptable (kappa = 0.8). Although we did not investigate FTLD-TDP cases without HS-Aging as a separate control group in this study, the hippocampus in FTLD-TDP, as with ADNC, may also be severely affected as demonstrated in Fig 1G-I. The sample size for analyses of clinical and cognitive data was limited by incomplete clinical data. Pooling of data from NIA-funded Alzheimer Disease Centers may overcome this difficulty.
In conclusion, our clinicopathologic analyses indicate that: 1) all HS-Aging cases have low numbers of neurons in CA1 and subiculum regardless of any comorbid pathology; 2) neuron loss in CA1 is more severe in HS-Aging than ADNC alone; 3) HS-Aging cases have more frequent TDP-43-immunoreactive inclusions both in the DG and in the PHG regardless of comorbid pathology; 4) an individual with pure HS-Aging is more likely to present with moderate dementia with deficits in memory being the most prominent deficit; and 5) HS-Aging cases are most likely to be identified clinically as AD dementia. Pure HS-Aging, uncomplicated by other pathologies, was uncommon in our cohort but representative of the frequency found in other studies. To determine the clinicopathologic correlates of pure HS-Aging much larger samples from a multicenter study will be required. From such a large cohort, the relative contribution to pathogenesis and clinical phenotype associated with comorbid TDP-43 proteinopathy may be more clearly elucidated.
Supplementary Material
Supplementary TABLE. Neuron counts and relative neuron loss in HS-Aging with comorbidities, HS-Aging (total), ADNC and normal controls.
Acknowledgement
We thank especially our participants, patients, donors and their families and M. Baxter and A. Burns of the Neuropathology Laboratory of the Knight ADRC. This project was supported by grants from the NIH National Institute on Aging (P50 AG05681, P01 AG03991, and P01 AG26276), Coins for Alzheimer’s Research Trust and the Alzheimer’s Drug Discovery Foundation. R.I. was supported by a Fellowship Grant from the Astellas Foundation of Research on Metabolic Disorders and a Fellowship Grant from the Mochida Memorial Foundation for Medical and Pharmaceutical Research.
Footnotes
Potential Conflicts of Interest
Nothing to report.
References
- 1.Bouchet C, Cazauvieilh J-B (1825): De l’épilepsie considérée dans ses rapports l’aliénation mentale. Recherches sur la nature et le siege de ces deux maladies: Mémoire qui remporté le prix au concours établi par M. Esquirol. Arch Gen Méd; 9: 510–542. [Google Scholar]
- 2.Dickson DW, Davies P, Bevona C, et al. Hippocampal sclerosis: a common pathological feature of dementia in very old (> or = 80 years of age) humans. Acta Neuropathol. 1994; 88: 212–21. [DOI] [PubMed] [Google Scholar]
- 3.Corey-Bloom J, Sabbagh MN, Bondi MW, et al. Hippocampal sclerosis contributes to dementia in the elderly. Neurology. 1997; 48: 154–60. [DOI] [PubMed] [Google Scholar]
- 4.Nelson PT, Schmitt FA, Lin Y, et al. Hippocampal sclerosis in advanced age: clinical and pathological features. Brain. 2011; 134: 1506–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kawas CH, Kim RC, Sonnen JA, et al. Multiple pathologies are common and related to dementia in the oldest-old: The 90+ Study. Neurology. 2015; 85: 535–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nag S, Yu L, Capuano AW, et al. Hippocampal sclerosis and TDP-43 pathology in aging and Alzheimer disease. Ann Neurol. 2015; 77: 942–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hatanpaa KJ, Blass DM, Pletnikova O, et al. Most cases of dementia with hippocampal sclerosis may represent frontotemporal dementia. Neurology. 2004; 63:538–542. [DOI] [PubMed] [Google Scholar]
- 8.Amador-Ortiz C, Ahmed Z, Zehr C, Dickson DW. Hippocampal sclerosis dementia differs from hippocampal sclerosis in frontal lobe degeneration. Acta Neuropathol. 2007; 113: 245–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Montine TJ, Phelps CH, Beach TG, et al. National Institute on Aging-Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease: a practical approach. Acta Neuropathol. 2012; 123: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dawe RJ, Bennett DA, Schneider JA, Arfanakis K. Neuropathologic correlates of hippocampal atrophy in the elderly: a clinical, pathologic, postmortem MRI study. PloS one. 2011; 6: e26286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Day GS, Lim TS, Hassenstab J, et al. Differentiating cognitive impairment due to corticobasal degeneration and Alzheimer disease. Neurology. 2017; 88: 1273–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Williams MM, Roe CM, Morris JC. Stability of the Clinical Dementia Rating, 1979–2007. Arch Neurol. 2009;66:773–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pastor P, Roe CM, Villegas A, et al. Apolipoprotein E epsilon 4 modifies Alzheimer’s disease onset in an E280A PS1 kindred. Ann Neurol. 2003; 54: 163–169. [DOI] [PubMed] [Google Scholar]
- 14.Cairns NJ, Perrin RJ, Franklin EE, et al. Neuropathologic assessment of participants in two multi-center longitudinal observational studies: the Alzheimer Disease Neuroimaging Initiative (ADNI) and the Dominantly Inherited Alzheimer Network (DIAN). Neuropathology. 2015; 35: 390–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cairns NJ, Neumann M, Bigio EH, et al. TDP-43 in familial and sporadic frontotemporal lobar degeneration with ubiquitin inclusions. Am J Pathol. 2007; 171: 227–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McKeith IG, Dickson DW, Lowe J, et al. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology. 2005; 65:1863–1872. [DOI] [PubMed] [Google Scholar]
- 17.Saito Y, Ruberu NN, Sawabe M, et al. Staging of argyrophilic grains: an age-associated tauopathy. J Neuropathol Exp Neurol. 2004; 63: 911–918. [DOI] [PubMed] [Google Scholar]
- 18.Kouri N, Murray ME, Hassan A, et al. Neuropathological features of corticobasal degeneration presenting as corticobasal syndrome or Richardson syndrome. Brain. 2011; 134: 3264–3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kovacs GG, Ferrer I, Grinberg LT, et al. Aging-related tau astrogliopathy (ARTAG): harmonized evaluation strategy. Acta Neuropathol. 2016; 131: 87–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Crary JF, Trojanowski JQ, Schneider JA, et al. Primary age-related tauopathy (PART): a common pathology associated with human aging. Acta Neuropathol. 2014; 128: 755–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.West MJ, Gundersen HJ. Unbiased stereological estimation of the number of neurons in the human hippocampus. J Comp Neurol. 1990; 296: 1–22. [DOI] [PubMed] [Google Scholar]
- 22.West MJ. New stereological methods for counting neurons. Neurobiol Aging. 1993; 14: 275–285. [DOI] [PubMed] [Google Scholar]
- 23.Rajkowska G, Miguel-Hidalgo JJ, Dubey P, et al. Prominent reduction in pyramidal neurons density in the orbitofrontal cortex of elderly depressed patients. Biol Psychiatry. 2005; 58: 297–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gundersen HJ. Notes on the estimation of the numerical density of arbitrary profiles: the edge effect. J Microsc. 1977; 111: 219–223. [Google Scholar]
- 25.Cairns NJ, Bigio EH, Mackenzie IR, et al. Neuropathologic diagnostic and nosologic criteria for frontotemporal lobar degeneration: consensus of the Consortium for Frontotemporal Lobar Degeneration. Acta Neuropathol. 2007; 114: 5–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wilson RS, Yu L, Trojanowski JQ, et al. TDP-43 pathology, cognitive decline, and dementia in old age. JAMA Neurol. 2013; 70: 1418–1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rauramaa T, Pikkarainen M, Englund E, et al. Consensus recommendations on pathologic changes in the hippocampus: a postmortem multicenter inter-rater study. J Neuropathol Exp Neurol. 2013; 72: 452–461. [DOI] [PubMed] [Google Scholar]
- 28.Gómez-Isla T, Price JL, McKeel DW Jr, et al. Profound loss of layer II entorhinal cortex neurons occurs in very mild Alzheimer’s disease. J Neurosci. 1996; 16: 4491–4500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gómez-Isla T, Hollister R, West H, et al. Neuronal loss correlates with but exceeds neurofibrillary tangles in Alzheimer’s disease. Ann Neurol. 1997; 41: 17–24. [DOI] [PubMed] [Google Scholar]
- 30.Price JL, Ko AI, Wade MJ, et al. Neuron number in the entorhinal cortex and CA1 in preclinical Alzheimer disease. Arch Neurol. 2001; 58: 1395–1402. [DOI] [PubMed] [Google Scholar]
- 31.von Gunten A, Kövari E, Bussière T, et al. Cognitive impact of neuronal pathology in the entorhinal cortex and CA1 field in Alzheimer’s disease. Neurobiol Aging. 2006; 27: 270–277. [DOI] [PubMed] [Google Scholar]
- 32.Gemmell E, Bosomworth H, Allan L, et al. Hippocampal neuronal atrophy and cognitive function in delayed poststroke and aging-related dementias. Stroke. 2012; 43: 808–814. [DOI] [PubMed] [Google Scholar]
- 33.Botha H, Mantyh WG, Murray ME, et al. FDG-PET in tau-negative amnestic dementia resembles that of autopsy-proven hippocampal sclerosis. Brain. 2018; 141: 1201–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Amador-Ortiz C, Lin WL, Ahmed Z, et al. TDP-43 immunoreactivity in hippocampal sclerosis and Alzheimer’s disease. Ann Neurol. 2007; 61: 435–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nag S, Yu L, Wilson RS, et al. TDP-43 pathology and memory impairment in elders without pathologic diagnoses of AD or FTLD. Neurology. 2017; 88: 653–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nelson PT, Trojanowski JQ, Abner EL, et al. “New Old Pathologies”: AD, PART, and Cerebral Age-Related TDP-43 With Sclerosis (CARTS). J Neuropathol Exp Neurol. 2016; 75: 482–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary TABLE. Neuron counts and relative neuron loss in HS-Aging with comorbidities, HS-Aging (total), ADNC and normal controls.