Key Points
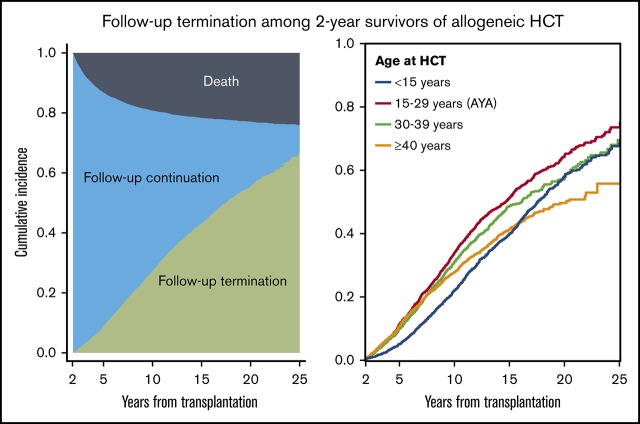
The cumulative incidence of follow-up termination is 28% at 10 years, increasing to 67% at 25 years after allogeneic HCT.
Follow-up termination at HCT centers is most often made by physicians based on the patient’s good physical condition.
Abstract
The need for long-term follow-up (LTFU) after allogeneic hematopoietic cell transplantation (HCT) has been increasingly recognized for managing late effects such as subsequent cancers and cardiovascular events. A substantial population, however, has already terminated LTFU at HCT centers. To better characterize follow-up termination, we analyzed the Japanese transplant registry database. The study cohort included 17 980 survivors beyond 2 years who underwent their first allogeneic HCT between 1974 and 2013. The median patient age at HCT was 34 years (range, 0-76 years). Follow-up at their HCT center was terminated in 4987 patients. The cumulative incidence of follow-up termination was 28% (95% confidence interval [CI], 27%-29%) at 10 years, increasing to 67% (95% CI, 65%-69%) at 25 years after HCT. Pediatric patients showed the lowest probability of follow-up termination for up to 16 years after HCT, whereas adolescent and young adult (AYA) patients showed the highest probability of follow-up termination throughout the period. Follow-up termination was most often made by physicians based on the patient’s good physical condition. Multivariate analysis identified 6 factors associated with follow-up termination: AYA patients, female patients, standard-risk malignancy or nonmalignant disease, unrelated bone marrow transplantation, HCT between 2000 and 2005, and absence of chronic graft-versus-host disease. These results suggest the need for education of both physicians and patients about the importance of LTFU, even in survivors with good physical condition. The decreased risk for follow-up termination after 2005 may suggest the increasing focus on LTFU in recent years.
Visual Abstract
Introduction
Expanding indications for allogeneic hematopoietic cell transplantation (HCT), advances in treatment of complications after HCT, and increased availability of alternative graft sources have resulted in a growing number of HCT survivors. By 2012, 1 million HCTs had been performed worldwide.1 Approximately 100 000 patients underwent allogeneic HCT before 2009 in the United States, with an estimated number of survivors of 42 000.2 Approximately 49 000 patients underwent allogeneic HCT until 2013 in Japan.3 Although patients who have survived for at least 5 years after HCT without recurrence of the primary disease have a high probability of surviving for an additional 15 years, mortality rates remained four- to ninefold higher than the expected general population rate for at least 30 years after HCT.4 Even though they overcome early complications, they have a higher risk for many late effects, such as subsequent cancers and cardiovascular events, than the general population.5-11 Recently, the need for long-term follow-up (LTFU) has been increasingly recognized for managing late effects and psychosocial problems, and several guidelines have been proposed.12,13 Until a decade ago, many HCT recipients who had no complications and no medications often terminated their follow-up at HCT centers. These patients may encounter several challenges when general physicians do not have sufficient knowledge about late effects or complicated comorbidities in HCT survivors.14
To keep all HCT survivors in the LTFU network, several strategies could be planned, including education, routine LTFU outpatient appointments, and the reunion of HCT survivors. It is very difficult to bring back patients who already terminated follow-up at HCT centers. Information is lacking regarding the actual number of patients who have terminated follow-up at HCT centers in Japan, as well as in other countries. To characterize these patients, we analyzed information regarding follow-up status at HCT centers, using the Japanese national transplant registry database.
Methods
Patients and data collection
The study cohort included consecutive patients who underwent their first allogeneic HCT between 1974 and 2013 and were alive at 2 years after HCT. The Japanese Data Center for Hematopoietic Cell Transplantation collects information on recipient, donor, and outcomes of HCT in Japan.15,16 For more than 20 years, these data have been collected from more than 300 transplant centers throughout the country, in collaboration with the Japan Society for Hematopoietic Cell Transplantation, the Japan Society for Pediatric Hematology and Oncology, the Japan Marrow Donor Program, Cord Blood Banks, and the Japanese Red Cross Society. More than 99% of all transplant centers in Japan reported and updated outcomes every year.15 Participation to the HCT registry was approved by the institutional review board of each center. Observational studies conducted by the Japan Society for Hematopoietic Cell Transplantation/Japanese Data Center for Hematopoietic Cell Transplantation were carried out with a waiver of informed consent during the study period, as clinical information was anonymized according to the Ethical Guideline for Epidemiological Research in Japan. The details of follow-up termination were retrospectively reviewed at 1 representative center (Japanese Red Cross Nagoya First Hospital). This study was approved by the institutional review board of the Japanese Red Cross Nagoya First Hospital, and was conducted in accordance with the Declaration of Helsinki.
Definition
Follow-up termination was defined when the centers reported that clinic visits were terminated. Patients who did not have follow-up information for more than 3 years were also considered as having terminated follow-up. The age range of the adolescent and young adult (AYA) generation was defined as from 15 to 29 years.17-19 Disease risk for hematological malignancies was defined according to the 2006 American Society for Blood and Marrow Transplantation schema.20 Acute leukemia in first or second complete remission, chronic myeloid leukemia in first chronic phase, Hodgkin or non-Hodgkin lymphoma in complete or partial chemotherapy-sensitive remission, chronic lymphocytic leukemia in first remission, myelodysplastic syndrome, or myeloproliferative disorder without excess blasts were considered standard risk. All others were defined as high-risk diseases. HLA matching for sibling and cord blood transplantation was assessed by serological data for HLA-A, HLA-B, and HLA-DR loci. HLA matching for unrelated transplantation was assessed using allele data for the HLA-A, HLA-B, HLA-C, and HLA-DRB1 loci.16 HLA mismatch was defined in the graft-versus-host disease (GVHD) vector when recipient antigens were not shared by the donor. The intensity of conditioning regimens was defined as described previously.21 Diagnosis and clinical grading of acute and chronic GVHD were performed according to the established criteria.22,23
Statistical analysis
The actual number of patients belonging to the following 4 groups were calculated in each year, using the HCT registry database: deceased, alive less than 2 years after HCT, alive at least 2 years after HCT, and follow-up termination.
The primary endpoint of this study was termination of follow-up at HCT centers. The cumulative incidence of follow-up termination was estimated, treating death before follow-up termination as a competing event. The Fine-Gray proportional hazards model was used to examine factors associated with follow-up termination.24 A backward stepwise procedure was used to develop a final model, based on a P-value threshold of .05. Covariates included patient age at HCT, patient sex, disease risk, conditioning intensity, HLA and donor type, graft source, year of HCT, maximum grade of acute GVHD, and severity of chronic GVHD before 2 years. The patient age at HCT violated the proportional hazards assumption, and an optimal cut point of 16 years was determined based on the maximum likelihood method and separate hazard ratios before and after 16 years were estimated.
Results
Estimation of yearly HCT survivors
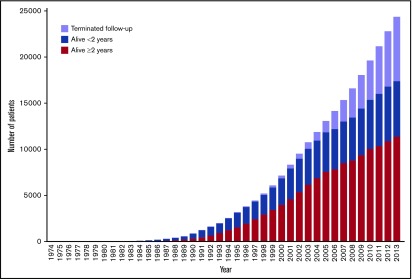
A total of 47 299 patients had their first allogeneic HCT between 1974 and 2013. The number of HCT survivors in each year is shown in Figure 1. There were at least 17 379 survivors in Japan in 2013. Among them, 11 364 patients were survivors of at least 2 years, and 6015 were survivors of less than 2 years. A total of 6922 patients terminated follow-up at their HCT centers in 2013.
Figure 1.
The actual number of survivors after allogeneic hematopoietic cell transplantation in each year from 1974 to 2013.
Characteristics of 2-year survivors
The main study cohort included 17 980 consecutive patients who had their first allogeneic HCT between 1974 and 2013 and were alive at 2 years after HCT. Characteristics of patients are summarized in Table 1. The median age of patients at HCT was 34 years (range, 0-76 years). A total of 3918 patients (22%) underwent HCT at younger than 15 years, 3922 (22%) at age 15 to 29 years, 2911 (16%) at age 30 to 39 years, and 7229 (40%) at age 40 years or older. The primary diagnosis was acute leukemia in 9866 patients (55%), other hematological malignancies in 6128 patients (34%), and nonmalignant disorders in 1986 patients (11%). Most patients underwent bone marrow transplantation (BMT) from an HLA-matched related or unrelated donor. More than half of patients underwent HCT after 2006. Grade II-IV acute GVHD developed in 6077 patients (34%), and extensive chronic GVHD developed in 4333 patients (24%) by 2 years after HCT.
Table 1.
Patient characteristics
| Characteristic | No. (%) |
|---|---|
| Total number of patients | 17 980 |
| Patient age at transplantation (range), y | 34 (0-76) |
| <15 | 3918 (22) |
| 15-29 | 3922 (22) |
| 30-39 | 2911 (16) |
| 40-49 | 3098 (17) |
| ≥50 | 4131 (23) |
| Patient sex | |
| Male | 10 248 (57) |
| Female | 7732 (43) |
| Diagnosis | |
| AML | 5789 (32) |
| ALL | 4077 (23) |
| Lymphoma/CLL | 1782 (10) |
| CML | 1678 (9) |
| MDS | 1663 (9) |
| ATL | 440 (2) |
| MPN | 322 (2) |
| Plasma cell neoplasms | 243 (1) |
| Aplastic anemia | 1322 (7) |
| Immunodeficiency | 306 (2) |
| Bone marrow failure | 182 (1) |
| Inborn metabolic disorders | 176 (1) |
| Disease risk | |
| Standard | 10 547 (59) |
| High | 5447 (30) |
| Nonmalignant | 1986 (11) |
| Conditioning | |
| Myeloablative | 11 198 (62) |
| Reduced intensity | 5184 (29) |
| Unknown intensity | 1598 (9) |
| Donor type | |
| Related bone marrow | 5061 (28) |
| Related mobilized blood cells | 2509 (14) |
| Unrelated bone marrow | 7241 (40) |
| Unrelated mobilized blood cells | 18 (<1) |
| Cord blood | 2845 (16) |
| Haploidentical related | 306 (2) |
| HLA matching* | |
| Match | 10 909 (74) |
| Mismatch | 3920 (26) |
| Year of transplantation | |
| 1974-1999 | 3613 (20) |
| 2000-2005 | 5260 (29) |
| 2006-2013 | 9107 (51) |
| Maximum grade of acute GVHD | |
| Grade 0-I | 11 903 (66) |
| Grade II | 4528 (25) |
| Grade III-IV | 1549 (9) |
| Chronic GVHD before 2 y | |
| None | 10 228 (57) |
| Limited | 3419 (19) |
| Extensive | 4333 (24) |
AML, acute myeloid leukemia; ALL, acute lymphoblastic leukemia; ATL, adult T-cell leukemia/lymphoma; CLL, chronic lymphocytic leukemia; CML, chronic myeloid leukemia; MDS, myelodysplastic syndrome; MPN, myeloproliferative neoplasm.
Cord blood and haploidentical transplantation are excluded.
Follow-up termination at HCT centers
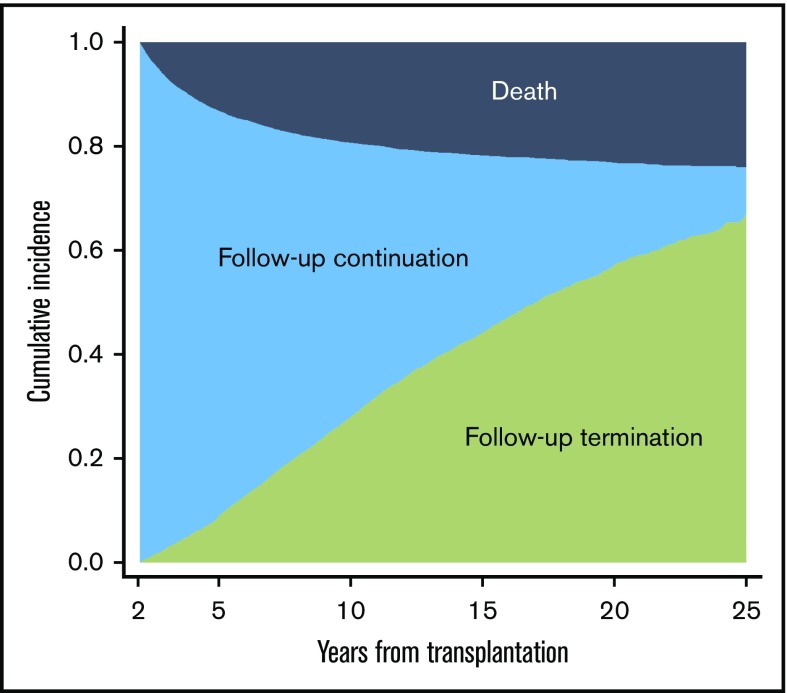
Among 17 980 patients who survived beyond 2 years, the HCT centers reported follow-up termination in 3905 patients. An additional 1082 patients did not have follow-up information at their HCT centers for more than 3 years and were considered as having terminated follow-up. The cumulative incidence of follow-up termination was 28% (95% CI, 27% to 29%) at 10 years, increasing to 67% (95% CI, 65% to 69%) at 25 years after HCT (Figure 2). The median duration from HCT to follow-up termination was 7.3 years (range, 2.0-26 years). The probability of patients continuing follow-up at their HCT center was 53% at 10 years after HCT, and the probability decreased to only 9% at 25 years.
Figure 2.
Cumulative incidence of follow-up termination among 2-year survivors. The gray area represents death without follow-up termination, the blue area represents follow-up continuation, and the green area represents follow-up termination as mutually exclusive competing events.
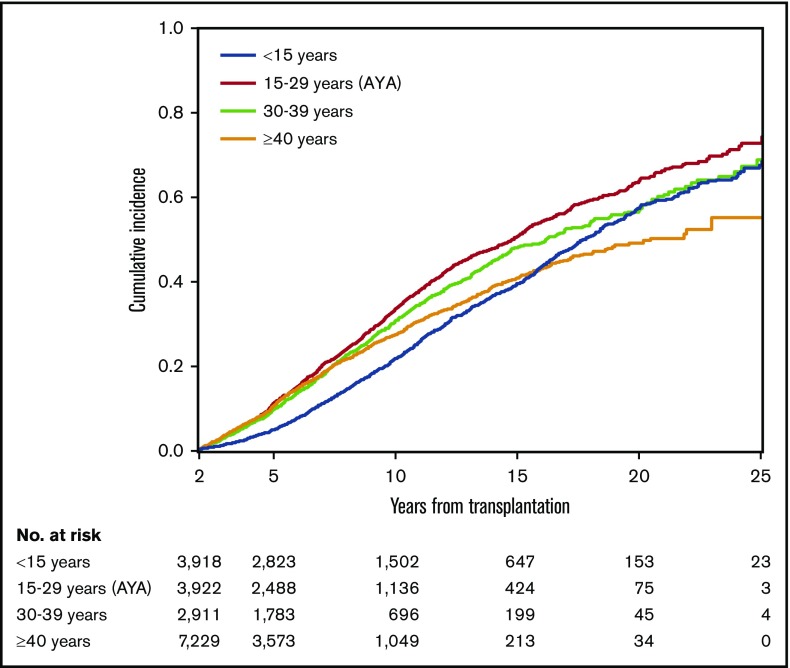
The cumulative incidences of follow-up termination according to patient age group is shown in Figure 3. AYA patients showed the highest probability of follow-up termination throughout the period, followed by those aged 30 to 39 years and those aged at least 40 years. Pediatric patients showed the lowest probability of follow-up termination up to 16 years after HCT, and the curve crossed at 16 years with those aged at least 40 years. The probability of follow-up termination in pediatric patients approached similar values to those aged 30 to 39 years beyond 16 years after HCT. The median patient age at the last follow-up among 1169 pediatric survivors who terminated follow-up was 17 years (range, 2-36 years), and 573 (49%) of them were at least 18 years old at their last follow-up.
Figure 3.
Cumulative incidence of follow-up termination according to patient age at transplantation.
Factors associated with follow-up termination
Multivariate analysis identified 6 factors associated with the risk for follow-up termination (Table 2). Compared with AYA patients, patients in other age groups had a lower risk for follow-up termination. Female patients had a higher risk for follow-up termination. Compared with patients with standard-risk malignancy, those with high-risk malignancy had a lower risk for follow-up termination, whereas those with nonmalignant disease had a higher risk for follow-up termination. Compared with patients who had BMT from a related donor, those who had BMT from an unrelated donor had a higher risk for follow-up termination. Compared with HCT between 2000 and 2005, HCT before 2000 and HCT after 2005 were associated with a lower risk for follow-up termination. Development of chronic GVHD before 2 years after HCT, particularly extensive chronic GVHD, was associated with a lower risk for follow-up termination.
Table 2.
Factors associated with follow-up termination
| Factor | Univariate | Multivariate | ||
|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |
| Patient age (up to 16 y after HCT), y* | ||||
| <15 | 0.65 (0.60-0.70) | <.001 | 0.60 (0.56-0.66) | <.001 |
| 15-29 | 1.00 (reference) | 1.00 (reference) | ||
| 30-39 | 0.86 (0.79-0.94) | .001 | 0.89 (0.82-0.97) | .011 |
| ≥40 | 0.74 (0.68-0.79) | <.001 | 0.79 (0.73-0.86) | <.001 |
| Patient age (beyond 16 y after HCT), y* | ||||
| <15 | 0.79 (0.62-1.01) | .062 | 0.77 (0.60-0.99) | .04 |
| 15-29 | 1.00 (reference) | 1.00 (reference) | ||
| 30-39 | 0.89 (0.64-1.24) | .49 | 0.87 (0.62-1.22) | .43 |
| ≥40 | 0.66 (0.44-0.99) | .05 | 0.66 (0.44-1.00) | .05 |
| Patient sex | ||||
| Male | 1.00 (reference) | 1.00 (reference) | ||
| Female | 1.11 (1.05-1.18) | <.001 | 1.12 (1.06-1.19) | <.001 |
| Disease | ||||
| Standard-risk malignancy | 1.00 (reference) | 1.00 (reference) | ||
| High-risk malignancy | 0.84 (0.79-0.90) | <.001 | 0.86 (0.81-0.92) | <.001 |
| Nonmalignant disease | 1.21 (1.12-1.30) | <.001 | 1.14 (1.06-1.24) | .001 |
| Conditioning | ||||
| Myeloablative | 1.00 (reference) | |||
| Reduced intensity | 0.97 (0.91-1.04) | .40 | ||
| Unknown intensity | 1.11 (1.03-1.20) | .005 | ||
| Donor type | ||||
| Related BM | 1.00 (reference) | 1.00 (reference) | ||
| Related PBSC | 0.92 (0.84-1.01) | .081 | 1.05 (0.95-1.16) | .37 |
| Unrelated BM | 1.13 (1.06-1.20) | <.001 | 1.19 (1.11-1.27) | <.001 |
| Unrelated PBSC | 0.52 (0.08-3.47) | .50 | 0.56 (0.09-3.33) | .53 |
| Cord blood | 0.82 (0.64-1.05) | .11 | 0.88 (0.68-1.13) | .31 |
| Haploidentical related | 0.94 (0.85-1.03) | .19 | 0.98 (0.88-1.09) | .73 |
| HLA matching† | ||||
| Match | 1.00 (reference) | |||
| Mismatch | 1.00 (0.94-1.07) | .89 | ||
| Year of transplantation | ||||
| 1974-1999 | 0.96 (0.90-1.02) | .16 | 0.92 (0.86-0.99) | .017 |
| 2000-2005 | 1.00 (reference) | 1.00 (reference) | ||
| 2006-2013 | 0.86 (0.80-0.93) | <.001 | 0.86 (0.80-0.93) | <.001 |
| Maximum grade of acute GVHD | ||||
| Grade 0-I | 1.00 (reference) | |||
| Grade II | 0.91 (0.85-0.97) | .005 | ||
| Grade III-IV | 0.84 (0.76-0.93) | .001 | ||
| Chronic GVHD before 2 y | ||||
| None | 1.00 (reference) | 1.00 (reference) | ||
| Limited | 0.82 (0.77-0.88) | <.001 | 0.82 (0.77-0.89) | <.001 |
| Extensive | 0.67 (0.63-0.72) | <.001 | 0.67 (0.62-0.72) | <.001 |
BM, bone marrow; PBSC, mobilized blood cells.
Separate analyses were performed before and after 16 years because of the violation of proportional assumption.
Cord blood and haploidentical transplantation were excluded.
Details of follow-up termination
Although the HCT registry did not collect reasons of follow-up termination, the registry collected ECOG performance status (PS) at the patient’s last follow-up. Among 4987 patients who terminated follow-up, the last PS was 0 in 3502 patients (70%), at least 1 in 868 patients (17%), and unknown in 617 patients (12%; Table 3).
Table 3.
The last performance status in patients who terminated follow-up (N = 4987)
| Patient age at transplantation, y | ECOG performance status at the last follow-up | ||
|---|---|---|---|
| 0 | ≥1 | Unknown | |
| All patients | 3502 (70) | 868 (17) | 617 (12) |
| <15 | 676 (58) | 112 (10) | 379 (32) |
| 15-29 | 1180 (79) | 182 (12) | 123 (8) |
| 30-39 | 678 (79) | 152 (18) | 31 (4) |
| ≥40 | 968 (66) | 422 (29) | 84 (6) |
To characterize the details of follow-up termination according to the patient’s last PS, we retrospectively examined 355 consecutive 2-year survivors of allogeneic HCT between 1974 and 2013 who terminated follow-up at 1 representative center (Japanese Red Cross Nagoya First Hospital; Table 4). The decision of follow-up termination was made by physicians in 241 cases (83%) with the last PS of 0, in 13 cases (39%) with the last PS of at least 1, and in 21 cases (66%) with unknown last PS. Among the 80 cases where patients decided to terminate their follow-up, 33 (41%) lived distant from the hospital, 13 (16%) had socioeconomic reasons, and 34 (42%) had other reasons. These proportions were similar across all the age groups.
Table 4.
Details of follow-up termination at 1 representative center (N = 355)
| ECOG performance status at the last follow-up | Person who made the decision, no. (%) | |
|---|---|---|
| Physician | Patient | |
| All patients | ||
| 0 | 241 (83) | 49 (17) |
| ≥1 | 13 (39) | 20 (61) |
| Unknown | 21 (66) | 11 (34) |
| Age <15 y at HCT | ||
| 0 | 129 (83) | 26 (17) |
| ≥1 | 7 (58) | 5 (42) |
| Unknown | 12 (63) | 7 (37) |
| Age 15-29 y at HCT | ||
| 0 | 50 (81) | 12 (19) |
| ≥1 | 0 (0) | 1 (100) |
| Unknown | 3 (60) | 2 (40) |
| Age 30-39 y at HCT | ||
| 0 | 27 (87) | 4 (13) |
| ≥1 | 1 (13) | 7 (88) |
| Unknown | 3 (75) | 1 (25) |
| Age ≥40 y at HCT | ||
| 0 | 35 (83) | 7 (17) |
| ≥1 | 5 (42) | 7 (58) |
| Unknown | 3 (75) | 1 (25) |
Discussion
Using the national HCT registry database in Japan, we showed that the cumulative incidence of follow-up termination at HCT centers was 28% at 10 years, increasing to 67% at 25 years, after allogeneic HCT. Multivariate analysis identified 6 factors associated with follow-up termination: AYA patients, female patients, standard-risk or nonmalignant disease, unrelated BMT, HCT between 2000 and 2005, and absence of chronic GVHD.
Khera et al reported on the adherence to preventive care practices using patient questionnaires. The probability of adherence to recommended preventive care was 75%, with a median follow-up of 11 years after HCT. The lower adherence was associated with concerns regarding medical costs, male sex, lower physical functioning, the absence of chronic GVHD, a longer time after HCT, and poor knowledge of recommended tests.25 Although different analytic methods were used in both studies, the probability of continued LTFU at 11 years after HCT was 48% in the current study.
Follow-up termination was most often made by physicians based on the patient’s good physical condition. Indeed, younger adult patients, those with standard-risk malignancy or nonmalignant disease, and those without chronic GVHD showed a higher risk for follow-up termination in the current study. The reason why the use of unrelated bone marrow donors was associated with a higher risk for follow-up termination than the use of related bone marrow donors remains to be elucidated. Several studies showed that the use of HLA-matched related bone marrow donors resulted in the best GVHD-free, relapse-free survival.26-28 The lower risk for follow-up termination after 2005 compared with 2000 to 2005 may suggest that the importance of LTFU has been increasingly recognized after 2005.
Female sex was found to be a risk factor for follow-up termination, an unexpected result because female patients usually have better adherence in several studies.29-31 A registry study of Japanese patients showed higher GVHD-free, relapse-free survival in female patients than in male patients.27 Thus, better physical condition might explain the higher risk for follow-up termination in female patients. The greater concern regarding medical costs among female patients may also contribute to this effect.25 The discrepancy between current studies and other studies may be a result of different study methods. Follow-up termination in this study could represent better physical condition or poor adherence, although it represented only poor adherence in other studies.29-31
Pediatric patients aged 0 to 15 years showed the lowest probability of follow-up termination up to 16 years after HCT. This observation could represent parental contributions until the patients became adults and independent. Pediatric patients usually receive comprehensive care from a medical team including social workers, psychologists, and pediatricians.32 Thus, patients and their families may find it difficult to leave this very supportive environment, and many childhood survivors continue follow-up by pediatricians. Based on our data, approximately half the pediatric patients who terminated follow-up were followed by pediatricians after they became adults. To avoid the loss in transition from a pediatrician to a physician, cooperation between the pediatric and adult LTFU system is needed.14,33,34
Recent studies have shown that even healthy HCT survivors have greater risks for subsequent cancers, cardiovascular events, and other late effects such as metabolic syndrome, avascular necrosis, and iron overload than the general population.5-7,27,35-38 Some survivors may have very late cardiac or pulmonary complications.39-42 The onset of bronchiolitis obliterans varies from 3 months to more than 10 years after HCT.43 If these complications occurred after patients had terminated follow-up at their HCT center, it might be difficult for a general physician to provide adequate medical care including diagnosis and treatment. Thus, we should maintain the communication with all long-term survivors after HCT and establish the efficient network and collaboration between transplant centers and community healthcare providers.
This study has several limitations. First, the registry database did not collect reasons for follow-up termination. Although our results suggested that follow-up was most often terminated by physicians based on the patient’s good physical condition, other reasons such as employment remain to be characterized.44 It is possible that HCT survivors have huge difficulty in finding employment, as the national statistics shows that even healthy people have a high turnover rate.45 If HCT survivors succeed in getting a job, they are likely to prioritize the job over medical treatment. Second, LTFU termination at HCT centers does not represent termination of all medical follow-up, as patients may have a health check-up in their workplaces or communities. The HCT centers should maintain contact with these patients and should update their data for better characterization of their lifelong late effects. Last, the Japanese HCT registry data do not incorporate death data from the national vital statistics registry, and death information is derived from only HCT centers.
In conclusion, follow-up termination at HCT centers is most often made by physicians, based on the patient’s good physical condition. Thus, it is very important to educate both physicians and patients about the importance of LTFU, even in survivors with good physical condition. Some patients have socioeconomic challenges, and improvement of social infrastructures may be needed in those patients.
Acknowledgments
The authors appreciate the contributions of all the physicians and data managers at the centers, who provided important data regarding transplantation to the Japan Society for Hematopoietic Cell Transplantation, the Japan Marrow Donor Program, and the Japan Cord Blood Bank Network. They also thank all of the members of the TRUMP committees of the Japan Society for Hematopoietic Cell Transplantation, Japan Marrow Donor Program, and the Japan Cord Blood Bank Network for their dedication and contribution to data management.
This study is funded by the Japanese Red Cross Nagoya First Hospital Research Grant.
Authorship
Contribution: K.M., T.Y., Y.A., T.I., K.K., S.O., and Y.I. designed the study and collected data; K.M and Y.I performed statistical analysis, interpreted data, and wrote the manuscript; N.U., T.F., K.O., H.O., T.E., M.I., S.T., T.M., H.K., H.Y., and A.H. provided data; and all authors interpreted data, critically revised the manuscript for important intellectual content, and approved the final manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Yoshihiro Inamoto, Department of Hematopoietic Stem Cell Transplantation, National Cancer Center Hospital, 5-1-1 Tsukiji, Chuo-ku, Tokyo 104-0045, Japan; e-mail: yinamoto@ncc.go.jp.
References
- 1.Gratwohl A, Pasquini MC, Aljurf M, et al. ; Worldwide Network for Blood and Marrow Transplantation (WBMT). One million haemopoietic stem-cell transplants: a retrospective observational study. Lancet Haematol. 2015;2(3):e91-e100. [DOI] [PubMed] [Google Scholar]
- 2.Majhail NS, Tao L, Bredeson C, et al. Prevalence of hematopoietic cell transplant survivors in the United States. Biol Blood Marrow Transplant. 2013;19(10):1498-1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Activities and Outcomes of Hematopoietic Cell Transplantation in Japan (2016). http://www.jdchct.or.jp/en/data/slide/2016. Accessed 29 October 2018.
- 4.Martin PJ, Counts GW Jr, Appelbaum FR, et al. Life expectancy in patients surviving more than 5 years after hematopoietic cell transplantation. J Clin Oncol. 2010;28(6):1011-1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhatia S, Louie AD, Bhatia R, et al. Solid cancers after bone marrow transplantation. J Clin Oncol. 2001;19(2):464-471. [DOI] [PubMed] [Google Scholar]
- 6.Rizzo JD, Curtis RE, Socié G, et al. Solid cancers after allogeneic hematopoietic cell transplantation. Blood. 2009;113(5):1175-1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chow EJ, Mueller BA, Baker KS, et al. Cardiovascular hospitalizations and mortality among recipients of hematopoietic stem cell transplantation. Ann Intern Med. 2011;155(1):21-32. [DOI] [PubMed] [Google Scholar]
- 8.Atsuta Y, Suzuki R, Yamashita T, et al. ; Japan Society for Hematopoietic Cell Transplantation. Continuing increased risk of oral/esophageal cancer after allogeneic hematopoietic stem cell transplantation in adults in association with chronic graft-versus-host disease. Ann Oncol. 2014;25(2):435-441. [DOI] [PubMed] [Google Scholar]
- 9.Inamoto Y, Shah NN, Savani BN, et al. Secondary solid cancer screening following hematopoietic cell transplantation. Bone Marrow Transplant. 2015;50(8):1013-1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shimoni A, Labopin M, Savani B, et al. Long-term survival and late events after allogeneic stem cell transplantation from HLA-matched siblings for acute myeloid leukemia with myeloablative compared to reduced-intensity conditioning: a report on behalf of the acute leukemia working party of European group for blood and marrow transplantation. J Hematol Oncol. 2016;9(1):118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Majhail NS. Long-term complications after hematopoietic cell transplantation. Hematol Oncol Stem Cell Ther. 2017;10(4):220-227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rizzo JD, Wingard JR, Tichelli A, et al. Recommended screening and preventive practices for long-term survivors after hematopoietic cell transplantation: joint recommendations of the European Group for Blood and Marrow Transplantation, the Center for International Blood and Marrow Transplant Research, and the American Society of Blood and Marrow Transplantation. Biol Blood Marrow Transplant. 2006;12(2):138-151. [DOI] [PubMed] [Google Scholar]
- 13.Majhail NS, Rizzo JD, Lee SJ, et al. ; Sociedade Brasileira de Transplante de Medula Ossea (SBTMO). Recommended screening and preventive practices for long-term survivors after hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2012;18(3):348-371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hashmi S, Carpenter P, Khera N, Tichelli A, Savani BN. Lost in transition: the essential need for long-term follow-up clinic for blood and marrow transplantation survivors. Biol Blood Marrow Transplant. 2015;21(2):225-232. [DOI] [PubMed] [Google Scholar]
- 15.Atsuta Y. Introduction of Transplant Registry Unified Management Program 2 (TRUMP2): scripts for TRUMP data analyses, part I (variables other than HLA-related data). Int J Hematol. 2016;103(1):3-10. [DOI] [PubMed] [Google Scholar]
- 16.Kanda J. Scripts for TRUMP data analyses. Part II (HLA-related data): statistical analyses specific for hematopoietic stem cell transplantation. Int J Hematol. 2016;103(1):11-19. [DOI] [PubMed] [Google Scholar]
- 17.Bleyer A, O’Leary M, Barr R, Ries LAG, eds. Cancer epidemiology in older adolescents and young adults 15 to 29 years of age, including SEER incidence and survival: 1975-2000. National Cancer Institute, NIH Pub. No. 06-5767. https://seer.cancer.gov/archive/publications/aya/aya_mono_complete.pdf. Accessed 29 October 2018.
- 18.Ledford H. Who exactly counts as an adolescent? Nature. 2018;554:429-431. [DOI] [PubMed] [Google Scholar]
- 19.Sawyer SM, Azzopardi PS, Wickremarathne D, Patton GC. The age of adolescence. Lancet Child Adolesc Health. 2018;2(3):223-228. [DOI] [PubMed] [Google Scholar]
- 20.RFI disease classifications and corresponding CIBMTR classifications. https://www.asbmt.org/practice-resources/rfi-forms. Accessed 29 October 2018.
- 21.Giralt S, Ballen K, Rizzo D, et al. Reduced-intensity conditioning regimen workshop: defining the dose spectrum. Report of a workshop convened by the center for international blood and marrow transplant research. Biol Blood Marrow Transplant. 2009;15(3):367-369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Przepiorka D, Ippoliti C, Koberda J, et al. Interleukin-2 for prevention of graft-versus-host disease after haploidentical marrow transplantation. Transplantation. 1994;58(7):858-860. [PubMed] [Google Scholar]
- 23.Shulman HM, Sullivan KM, Weiden PL, et al. Chronic graft-versus-host syndrome in man. A long-term clinicopathologic study of 20 Seattle patients. Am J Med. 1980;69(2):204-217. [DOI] [PubMed] [Google Scholar]
- 24.Fine J, Gray R. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94(446):496-509. [Google Scholar]
- 25.Khera N, Chow EJ, Leisenring WM, et al. Factors associated with adherence to preventive care practices among hematopoietic cell transplantation survivors. Biol Blood Marrow Transplant. 2011;17(7):995-1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Holtan SG, DeFor TE, Lazaryan A, et al. Composite end point of graft-versus-host disease-free, relapse-free survival after allogeneic hematopoietic cell transplantation. Blood. 2015;125(8):1333-1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Inamoto Y, Kimura F, Kanda J, et al. ; JSHCT GVHD Working Group. Comparison of graft-versus-host disease-free, relapse-free survival according to a variety of graft sources: antithymocyte globulin and single cord blood provide favorable outcomes in some subgroups. Haematologica. 2016;101(12):1592-1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mehta RS, Peffault de Latour R, DeFor TE, et al. Improved graft-versus-host disease-free, relapse-free survival associated with bone marrow as the stem cell source in adults. Haematologica. 2016;101(6):764-772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bishop MM, Lee SJ, Beaumont JL, et al. The preventive health behaviors of long-term survivors of cancer and hematopoietic stem cell transplantation compared with matched controls. Biol Blood Marrow Transplant. 2010;16(2):207-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Prasad PK, Sun CL, Baker KS, et al. Health care utilization by adult Hispanic long-term survivors of hematopoietic stem cell transplantation: report from the Bone Marrow Transplant Survivor Study. Cancer. 2008;113(10):2724-2733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shankar SM, Carter A, Sun CL, et al. Health care utilization by adult long-term survivors of hematopoietic cell transplant: report from the Bone Marrow Transplant Survivor Study. Cancer Epidemiol Biomarkers Prev. 2007;16(4):834-839. [DOI] [PubMed] [Google Scholar]
- 32.Margolis R, Wiener L, Pao M, Malech HL, Holland SM, Driscoll P. Transition from pediatric to adult care by young adults with chronic granulomatous disease: the patient’s viewpoint. J Adolesc Health. 2017;61(6):716-721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wojciechowski EA, Hurtig A, Dorn L. A natural history study of adolescents and young adults with sickle cell disease as they transfer to adult care: a need for case management services. J Pediatr Nurs. 2002;17(1):18-27. [DOI] [PubMed] [Google Scholar]
- 34.Tuchman LK, Slap GB, Britto MT. Transition to adult care: experiences and expectations of adolescents with a chronic illness. Child Care Health Dev. 2008;34(5):557-563. [DOI] [PubMed] [Google Scholar]
- 35.Majhail NS, DeFor T, Lazarus HM, Burns LJ. High prevalence of iron overload in adult allogeneic hematopoietic cell transplant survivors. Biol Blood Marrow Transplant. 2008;14(7):790-794. [DOI] [PubMed] [Google Scholar]
- 36.Majhail NS, Flowers ME, Ness KK, et al. High prevalence of metabolic syndrome after allogeneic hematopoietic cell transplantation. Bone Marrow Transplant. 2009;43(1):49-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goto T, Ikuta K, Inamoto Y, et al. Hyperferritinemia after adult allogeneic hematopoietic cell transplantation: quantification of iron burden by determining non-transferrin-bound iron. Int J Hematol. 2013;97(1):125-134 [DOI] [PubMed] [Google Scholar]
- 38.Inamoto Y, Matsuda T, Tabuchi K, et al. ; Japan Society for Hematopoietic Cell Transplantation Late Effects and Quality of Life Working Group. Outcomes of patients who developed subsequent solid cancer after hematopoietic cell transplantation. Blood Adv. 2018;2(15):1901-1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kawano H, Tanaka H, Yamashita T, et al. Very late-onset reversible cardiomyopathy in patients with chronic GvHD. Bone Marrow Transplant. 2015;50(6):870-872. [DOI] [PubMed] [Google Scholar]
- 40.Bergeron A. Late-onset noninfectious pulmonary complications after allogeneic hematopoietic stem cell transplantation. Clin Chest Med. 2017;38(2):249-262. [DOI] [PubMed] [Google Scholar]
- 41.Hamada S, Yoshioka T, Higuchi K. Cellular bronchiolitis: a late-onset non-infectious pulmonary complication after allogenic bone marrow transplantation. Arch Bronconeumol. 2017;53(4):220-221. [DOI] [PubMed] [Google Scholar]
- 42.Nagasawa M, Mitsuiki N, Aoki Y, et al. Effect of reduced-intensity conditioning and the risk of late-onset non-infectious pulmonary complications in pediatric patients. Eur J Haematol. 2017;99(6):525-531. [DOI] [PubMed] [Google Scholar]
- 43.Yoshihara S, Yanik G, Cooke KR, Mineishi S. Bronchiolitis obliterans syndrome (BOS), bronchiolitis obliterans organizing pneumonia (BOOP), and other late-onset noninfectious pulmonary complications following allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2007;13(7):749-759. [DOI] [PubMed] [Google Scholar]
- 44.Tichelli A, Gerull S, Holbro A, et al. Inability to work and need for disability pension among long-term survivors of hematopoietic stem cell transplantation. Bone Marrow Transplant. 2017;52(10):1436-1442. [DOI] [PubMed] [Google Scholar]
- 45.Outline of results of survey on employment trends in the year of 2016. [Japanese]. http://www.mhlw.go.jp/toukei/itiran/roudou/koyou/doukou/17-2/dl/gaikyou.pdf. Accessed 29 October 2018.