Abstract
PURPOSE:
Atypical skeletal development is common in youth with myelomeningocele (MM), though the underlying reasons have not been fully elucidated. This study assessed skeletal maturity in children and adolescents with MM and examined the effects of sex, age, sexual development, ethnicity, anthropometrics and shunt status.
METHODS:
43 males and 35 females with MM, 6-16 years old, underwent hand radiographs for bone age determination. The difference between bone age and chronological age was evaluated using Wilcoxon sign rank tests. Relationships between age discrepancy (skeletal–chronological) and participant characteristics were assessed using multiple linear regression with forward selection.
RESULTS:
Overall, forty percent (31/78) of MM participants had an advanced bone age of 1 year or greater (median: 2.5 years), while 47% (37/78) were within 1 year above or below their chronological age (−0.001 years) and 13% (10/78) were delayed by more than 1 year (−1.4 years). Bone age was advanced compared to chronologic age in both males and females (p≤0.024). Advanced bone age was observed in early to late puberty and after maturation (p≤0.07), as well as in Hispanic participants (p=0.003) and in those with a shunt (p=0.0004). Advanced bone age was positively correlated with height, weight and body mass index (BMI) percentiles (p=0.004). In multiple linear regression analysis, advanced bone age was most strongly associated with higher Tanner stage of sexual development, and higher weight, height or BMI percentile.
CONCLUSIONS:
Advanced skeletal maturity is common in children/adolescents with MM over 8 years of age who have reached puberty (65%), particularly those who are overweight (80%). Hormonal effects associated with adiposity and sexual maturity likely influence skeletal maturation. Clinicians may use Tanner stage and weight or BMI to gain insight into skeletal maturity.
Keywords: skeletal maturity, myelomeningocele, bone age, spina bifida, pediatrics
Introduction:
Myelomeningocele (MM), the most common and severe form of spina bifida, results from failed neural tube formation in utero and affects approximately 3,000 pregnancies in the United States annually (1). In addition to muscle weakness and paralysis, skeletal development can be altered in those with MM, with decreased bone density and differences in skeletal maturity having been previously documented (2–6). The underlying causes of these pathologies have not been fully elucidated, but the effects of mechanical loading, nutrition, bone metabolism and the endocrine system have been investigated as possible contributors (2–6). Altered bone development can contribute to increased fracture risk in both the short and long term.
Reduced bone density and incongruent skeletal maturity are likely multifactorial. Previous studies have linked lower bone mineral density in children with MM to wheelchair use and overall limited ambulation (2, 3) while another study correlated increased calcium excretion to lower bone density (4). Incongruent skeletal maturity, both advanced and delayed, has been documented in the MM population. Previous work suggests that neither gender nor neurosegmental level plays an instrumental role in bone age; however, there are mixed reports on the effects of shunt status (5, 6). The presence or absence of a shunt has been used to evaluate the effect of hydrocephalus and dysfunctions of the hypothalamic-pituitary axis on skeletal maturation. Feeley et. al. found that presence of a shunt was associated with delayed bone age (5), whilst Kalen and Harding found no effect of hydrocephalus or number of shunt revisions on bone age in children with MM (6), possibly due to the relatively low number of participants with shunts in the Kalen study. The Kalen study did find advanced skeletal maturity in 32% of children with MM over 8 years of age. The inconclusive results of previous studies may be, at least in part, due to differences in the methodologies used to assess bone age (Gruelich and Pyle vs. Oxford method) as well as differences in the MM participants studied.
Skeletal maturity and sexual development are inter-related (7), but past work has not examined this relationship in children with MM who often have precocious puberty (8). Additionally, obesity has been correlated to advanced bone age in youth without disability, possibly due to hormonal effects associated with adipose tissue (9–12). However, even though 50–83% of patients with MM are overweight or obese (13, 14), the role of excess weight in incongruent skeletal maturity in MM has not been previously investigated. Finally, fracture rate and bone age have been shown to differ among ethnicities in populations without MM (15, 16). Given the increased incidence of MM amongst Hispanic populations and the high incidence of obesity in the MM population, ethnicity and body mass index (BMI) are important to consider when assessing differences in bone age.
An understanding of which patients with MM are likely to be advanced or delayed in skeletal development could help improve patient management and treatment decision making, particularly regarding the timing of orthopaedic interventions and treatment of precocious puberty. Fractures, spinal deformity and deformity of the lower limbs are common in children and adolescents with MM, and treatment decision making to address these problems necessitates an understanding of skeletal growth remaining, which depends on bone age (4, 17). The purpose of this study was to evaluate the relationship of multiple factors including age, sex, sexual maturity, anthropometric measures, ethnicity and shunt status on skeletal maturity in the MM population. It was hypothesized that chronological age, sexual maturity (Tanner Stage), anthropometric measures, ethnicity and shunt status would be related to advanced skeletal maturation in the MM population, while sex would not.
Methods and Materials:
Following approval by the institutional review board at Children’s Hospital Los Angeles, appropriate written assent and consent were obtained from all participants and their parent(s) or legal guardian(s). This prospective, cross-sectional study included participants who had a diagnosis of myelomeningocele and were between 6–16 years of age. Potential participants were excluded if current use of glucocorticoids or other medications affecting growth and/or development was reported, if they had metal implants in their tibias bilaterally, or if they had other diagnosis excluding hydrocephalus and asthma as determined from guardian and participant self-report. All participants were part of a larger study investigating the relationship between bone mass and ambulation (18).
Participant characteristics including height, weight, and BMI were measured by an experienced pediatric physical therapist. Height was measured in standing for participants who could stand upright and supine for those who could not. Laboratory-specific intra- and inter-rater reliability for height and weight measurements was within 0.4 cm and 0.9 kg, respectively. Height, weight, and BMI percentiles for age were determined using growth charts from the Centers for Disease Control and Prevention, and participants were classified into groups based on cutoffs for height (<20th percentile, >20th percentile) and BMI percentiles (healthy BMI: <85th percentile, overweight BMI: 85th – 95th percentile, obese BMI: >95% percentile) (19, 20). Manual muscle testing was also performed by a physical therapist, and neurosegmental level was classified based on criteria from the International Myelodysplasia Study Group (IMSG) (21) as sacral, low lumbar, mid lumbar or high lumbar and above. An experienced pediatric endocrinologist assessed the development of breasts or testes as well as pubic hair to determine the Tanner stage as a marker of sexual maturity (22, 23). Additionally, all participants underwent an x-ray of his/her left hand and wrist for determination of bone age using the Greulich and Pyle method (24). The imaging settings were 60 kVp, 2 mAs using high-resolution extremity radiographs; all appropriate radiologic protocols were followed. A research team member was trained by a pediatric radiologist to read bone age from hand x-rays using the Gruelich and Pyle method. Reliability between the evaluator and radiologist had a mean coefficient of variation (CoV) of 3.9% with a mean difference of 0.6 (standard deviation, SD 0.4) years. Intra-rater reliability for the evaluator had a mean CoV of 1.5% with a mean difference of 0.03 (SD 0.35) years. The evaluator, blinded to chronological age, completed all x-ray readings.
Statistical Analyses
Statistical analyses were performed using Stata14 (Stata Corp, Texas). Descriptive statistics are presented using median and interquartile range (IQR). Participant characteristics were compared between males and females using Mann Whitney rank sum tests for continuous variables (age, height, weight, BMI percentiles) and Fisher’s exact tests for categorical variables (sex, Hispanic ethnicity, Tanner stage, and shunt status). Differences between bone age and chronological age were assessed using Wilcoxon sign rank tests and are described by the median difference, IQR, and 95% confidence interval (CI) of the median difference. Age discrepancy was defined as age skeletal – age chronological such that positive values indicate advanced bone age while negative values indicate delayed bone age. The relationships between age discrepancy and chronologic age, sex, anthropometric measurements, shunt status and Tanner stage (independent variables) were assessed using simple and multiple linear regression with a forward selection process. Independent variables were added to the model when they increased the adjusted R2 of the model by at least 0.1. When related variables were considered (such as age and Tanner stage), the one producing the highest adjusted R2 was used.
Results:
Age, Sex, and Sexual Development
Seventy-eight participants with MM (35 female) were included in the analysis; 14 were classified as sacral level, 12 as low lumbar, 41 as mid lumbar and 11 as high lumbar and above (5 high lumbar, 6 thoracic). The overall median chronological age was 9.8 (IQR 7.7 to 12.2) years, and median bone age was 10.5 (IQR 7.5 to 14.0) years. The median difference between bone age and chronological age for all participants was 0.50 years (IQR −0.5 to 1.9; 95% CI 0.20 to 1.05; p=0.0008) with a positive value indicating advanced bone age (Table 1). Overall, 40% (31/78) of participants had advanced bone age greater than 1 year (median: 2.5, IQR: 1.6 to 3.3; range: 1.0 to 4.4), while 13% (10/78) were delayed more than 1 year (median: −1.4, IQR: −1.8 to −1.1; range: −1.0 to −2.9) and 47% (37/78) were within 1 year above or below their chronological age. Bone age was significantly advanced compared to chronologic age in both males (median difference 0.41 years, IQR −0.7 to 1.9, 95% CI −0.22 to 1.32, p= 0.024) and females (median difference 0.50 years, IQR −0.4 to 2.5, 95% CI 0.06 to 1.20, p=0.010) (Table 2). Forty-two percent (18/43) of males and 37% (13/35) of females had bone age that was advanced by more than 1 year. The difference between bone age and chronological age was similar between males and females (Table 1).
Table 1:
Demographic and anthropometric characteristics of the study participants
| Overall (n=78) | Males (n=43) | Females (n=35) | p-value Males vs. Females |
|
|---|---|---|---|---|
| Hispanic | 71/78 (91%) | 41/43 (95%) | 30/35 (86%) | 0.23 |
| Shunt | 62/78 (79%) | 36/43 (84%) | 26/35 (74%) | 0.40 |
| Chronological Age (years) | 9.8 (7.7, 12.2) [6.0, 15.8] |
9.9 (7.4, 12.5) [6.0, 15.8] |
9.3 (7.7, 11.9) [6.0, 14.0] |
0.78 |
| Bone Age (years) | 10.5 (7.5, 14.0) [4.0, 18.0] |
10.5 (6.5, 14.0) [4.5, 17.0] |
10.5 (8.0, 14.0) [4.0, 18.0] |
0.95 |
| Age difference (bone – chronological) | 0.50 (−0.52, 1.91) [−2.89, 4.40] |
0.41 (−0.73, 1.91) [−2.89, 3.93] |
0.50 (−0.37, 2.51) [−2.28, 4.40] |
0.67 |
| Height percentile | 11.5 (1.4, 46.0) | 8.1 (0.5, 38.2) | 15.9 (2.3, 54.0) | 0.49 |
| Weight percentile | 67.3 (24.2, 90.3) | 57.9 (24.2, 90.3) | 72.6 (24.2, 90.3) | 0.84 |
| BMI percentile | 88.5 (61.8, 97.1) | 88.5 (57.9, 98.2) | 88.5 (69.2, 96.4) | 0.81 |
| Tanner | 0.22 | |||
| 1 | 40 (51%) | 26 (60%) | 14 (40%) | |
| 2 | 7 (9%) | 5 (12%) | 2 (6%) | |
| 3 | 10 (13%) | 4 (9%) | 6 (17%) | |
| 4 | 7 (9%) | 3 (7%) | 4 (11%) | |
| 5 | 14 (18%) | 5 (12%) | 9 (26%) | |
Continuous variables (age, age difference, and anthropometric percentiles) are presented as median (interquartile range) [range]. Categorical variables (Hispanic, shunt, Tanner) are presented as n (%). Statistical significance was evaluated using Mann Whitney rank sum tests for continuous variables and Fisher’s exact tests for categorical variables.
Table 2:
Difference between skeletal and chronological age in participant subgroups.
| Chronological Age |
Bone Age | Age Diff | P-value | |
|---|---|---|---|---|
| Sex | 0.70 | |||
| Male (n=43) | 9.9 (7.4, 12.5) [6.0, 15.8] |
10.5 (6.5, 14.0) [4.5, 17.0] |
0.41 (−0.7, 1.9) [−2.9, 3.9] |
0.02 |
| Female (n=35) | 9.3 (7.7, 11.9) [6.0, 14.0] |
10.5 (8.0, 14.0) [4.0, 18.0] |
0.50 (−0.4, 2.5) [−2.3, 4.4] |
0.01 |
| Tanner | ||||
| 1 (n=40) | 8.0 (6.3, 9.2) [6.0, 12.0] |
7.5 (5.8, 10.0) [4.0, 13.0] |
−0.37 (−1.0, 0.5) [−2.9, 3.1] |
0.22 |
| 2 (n=7) | 12.6 (10.3, 12.2) [7.4, 13.4] |
12.5 (10.5, 14.0) [8.5, 15.0] |
1.1 (0.3, 1.9) [−0.08, 2.2] |
0.03 |
| 3 (n=10) | 11.2 (10.5, 13.2) [8.0, 15.8] |
12.0 (10.5, 14.5) [8.5, 17] |
0.39 (0.02, 1.2) [−1.4, 3.9] |
0.07 |
| 4 (n=7) | 11.1 (10.4, 12.5) [9.3, 13.5] |
13.0 (10.5, 14.5) [10.0, 15.5] |
1.4 (−0.4, 2.0) [−0.53, 4.4] |
0.06 |
| 5 (n=14) | 13.4 (13.1, 14.0) [10.5, 14.9] |
16.5 (16.0, 16.5) [13.5, 18.0] |
2.9 (2.5, 3.4) [0.86, 4.1] |
0.001 |
| BMI Percentile | 0.002 | |||
| Healthy Weight (n=33) | 8.8 (6.6, 11.9) [6.0, 14.0] |
10.0 (6.0, 10.5) [4.0, 16.5] |
−0.03 (−0.9, 1.0) [−2.3, 2.6] |
0.98 |
| Overweight (n=13) | 10.7 (8.2, 11.6) [6.4, 13.9] |
10.5 (8.0, 14.0) [6.0, 18.0] |
0.55 (0.2, 1.8) [−2.9, 4.4] |
0.09 |
| Obese (n=32) | 10.4 (8.1, 13.2) [6.0, 15.8] |
12.5 (8.0, 16.5) [5.0, 17.0] |
1.4 (0.1, 3.0) [−1.0, 3.9] |
0.0003 |
| Height Percentile | 0.004 | |||
| <20th Percentile (n=46) | 9.7 (7.1, 12.0) [6.0, 14.9) |
9.8 (6.5, 13.0) [4.0, 17.0] |
−0.05 (−1.0, 1.0) [−2.9, 3.7] |
0.82 |
| >20th Percentile (n=32) | 10.0 (8.1, 12.6) [6.0, 15.8] |
11.5 (8.8, 14.5) [6.0, 18.0] |
1.3 (0.5, 2.5) [−0.6, 4.4] |
<0.0001 |
| Ethnicity | 0.18 | |||
| Not Hispanic (n=7) | 11.1 (8.0, 13.4) [7.9, 13.5] |
14.0 (7.5, 16.0) [7.5, 17.0] |
0.86 (−0.4, 3.5) [−0.6, 4.4] |
0.09 |
| Hispanic (n=71) | 9.7 (7.4, 12.1) [6.0, 15.8] |
10.5 (7.5, 13.5) [4.0, 18.0] |
0.41 (−0.5, 1.8) [−1.5, 3.7] |
0.003 |
| Shunt Status | 0.28 | |||
| No Shunt (n=16) | 8.6 (7.9, 11.3) [6.2, 14.0] |
9.0 (7.3, 11.3) [5.0, 17.0] |
−0.07 (−1.0, 0.8) [−1.4, 4.4] |
0.96 |
| Shunt (n=62) | 10.2 (7.4, 12.6) [6.0, 15.8] |
10.5 (7.5, 14.0) [4.0, 18.0] |
0.62 (−0.4, 2.0) [−2.9, 4.1] |
0.0004 |
Values are presented as median (IQR) [range]. P-values are from Wilcoxon Sign Rank tests.
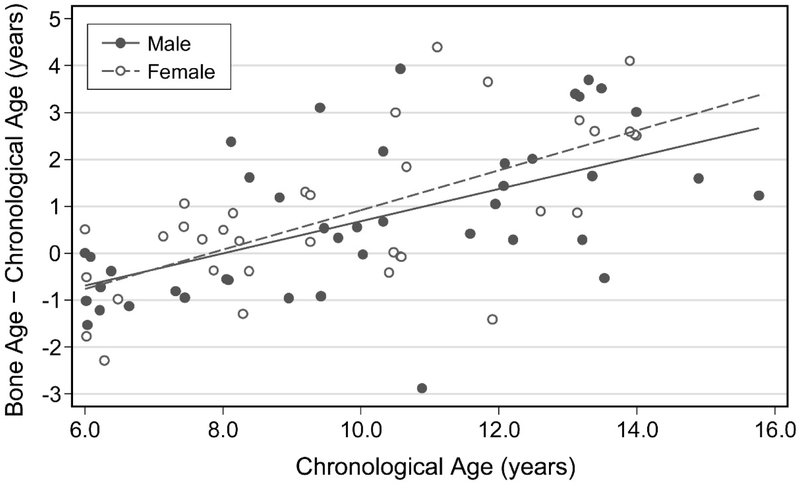
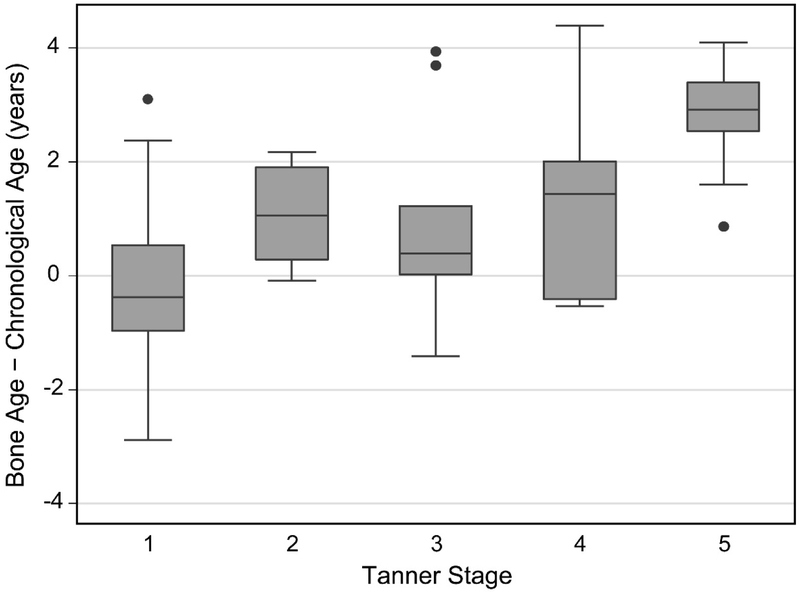
The difference between bone age and chronological age increased with age in both males and females, progressing from slightly delayed bone age in younger children to increasingly advanced bone age after a chronological age of approximately 8 years (β = 0.37, r2 = 0.38, p<0.001) (Figure 1). There was large variability in the difference between skeletal and chronological age in pre-pubertal children (Tanner 1, median difference −0.37 years, IQR −0.97 to 0.54, 95% CI −0.79 to 0.32, p=0.221) (Figure 2). Bone age tended to be advanced relative to chronological age in early (Tanner 2, median difference 1.06 years, IQR 0.29 to 1.91, 95% CI 0.03 to 2.09, p=0.028), mid (Tanner 3, median difference 0.39 years, IQR 0.02 to 1.23, 95% CI −0.04 to 2.89, p=0.075), and late (Tanner 4, median difference 1.43 years, IQR −0.41 to 2.01, 95% CI −0.49 to 3.64, p=0.063) puberty and was most advanced after sexual maturity (Tanner 5, median difference 2.91 years, IQR 2.53 to 3.39, 95% CI 2.53 to 3.41, p=0.001; Table 2). Bone age was advanced more than 1 year in 53% (30/57) of children over 8 years of age and in 65% (24/37) of children over age 8 years who had entered puberty (Tanner ≥2).
Figure 1:
Difference between skeletal age and chronological age as a function of chronological age in boys and girls.
Figure 2:
Difference between skeletal age and chronological age as a function of Tanner stage of sexual maturity.
Anthropometric Measures
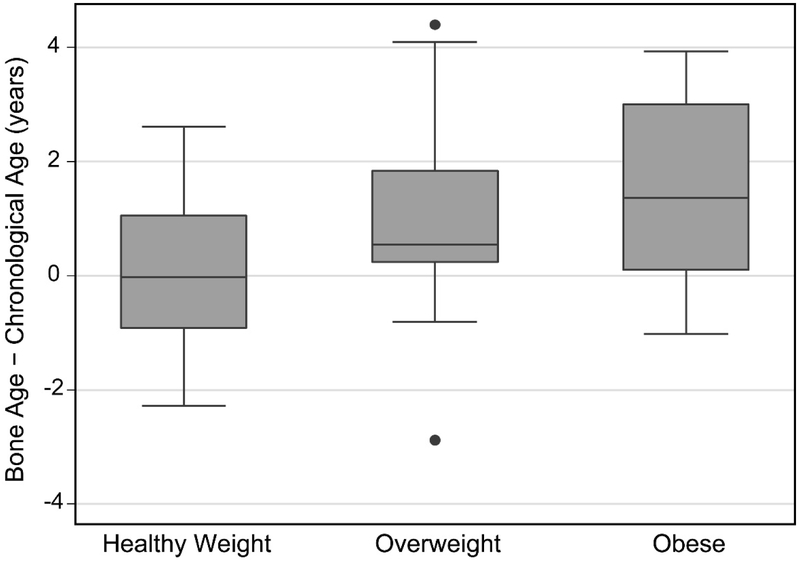
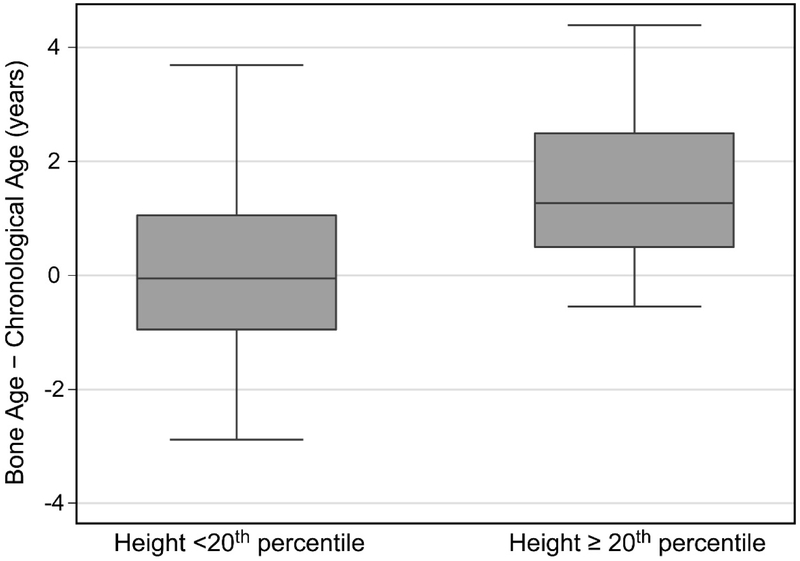
The difference between bone age and chronological age was positively correlated with height, weight, and BMI percentiles (Table 3). Most notably, advanced skeletal maturity manifested at the highest BMI percentiles. Bone age was advanced more than 1 year in 49% (22/45) of children who were overweight or obese and in 80% (20/25) of these who were also over 8 years of age had entered puberty (Tanner ≥2). Bone age did not differ significantly from chronological age for those of normal/healthy BMI, but tended to be advanced for children who were overweight or obese (Table 2, Figure 3). Bone age was also similar to chronological age for children with height under the 20th percentile, but was advanced for taller children (Table 2, Figure 4).
Table 3:
Results of simple linear regression predicting the difference between skeletal and chronological age.
| Coef. | SE | N | t | P | R2 | |
|---|---|---|---|---|---|---|
| Chronologic age (yr) | 0.37 | 0.05 | 78 | 6.85 | <0.001 | 0.37 |
| Tanner | 78 | 0.44 | ||||
| 2 | 1.23 | 0.50 | 2.44 | 0.02 | ||
| 3 | 1.10 | 0.44 | 2.54 | 0.01 | ||
| 4 | 1.60 | 0.50 | 3.16 | 0.002 | ||
| 5 | 3.00 | 0.38 | 7.84 | <0.001 | ||
| Sex (male) | −0.15 | 0.38 | 78 | −0.39 | 0.70 | 0.002 |
| Height Percentile | 0.018 | 0.006 | 78 | 3.01 | 0.004 | 0.09 |
| Weight Percentile | 0.025 | 0.005 | 78 | 5.41 | <0.001 | 0.27 |
| BMI Percentile | 0.023 | 0.007 | 78 | 3.22 | 0.002 | 0.11 |
| Hispanic | −0.88 | 0.65 | 78 | −1.36 | 0.18 | 0.01 |
| Shunt | 0.50 | 0.46 | 78 | 1.08 | 0.28 | 0.002 |
Coef. = unstandardized regression coefficient; SE = standard error of unstandardized coefficient; N = number of observations; t = t-statistic; P = P-value, R2 = adjusted coefficient of determination
Figure 3:
Difference between skeletal age and chronological age for normal/healthy weight (BMI ≤ 85th percentile), overweight (BMI between 85th and 95th percentile), and obese (BMI > 95th percentile) participants.
Figure 4:
Difference between skeletal age and chronological age for participants with height < 20th percentile and height ≥ 20th percentile.
Ethnicity and Shunt Status
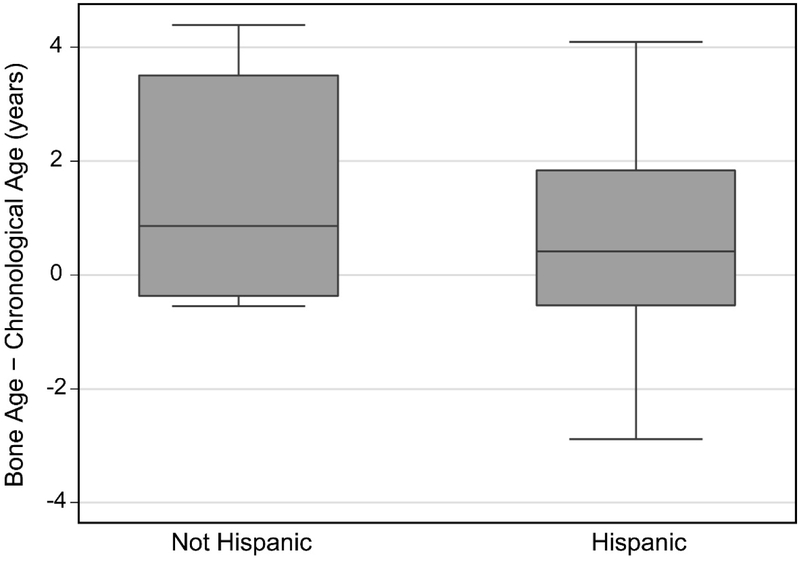
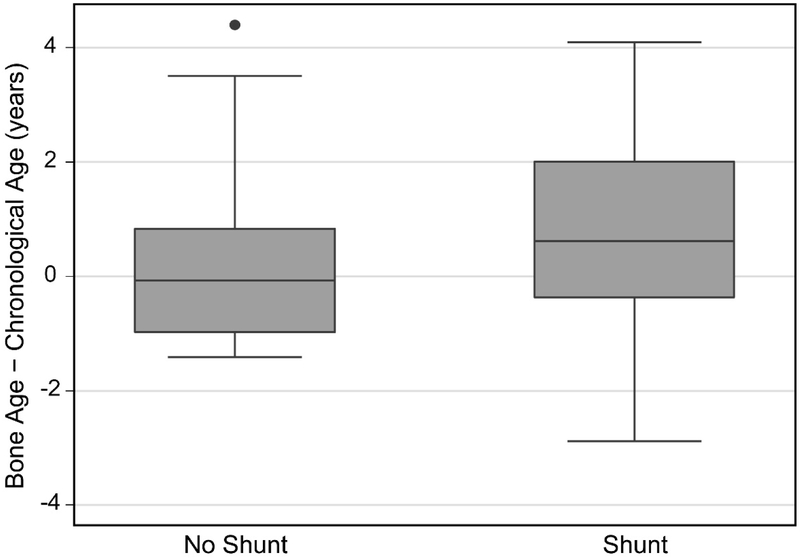
Bone age was advanced in Hispanic participants, but did not differ significantly from chronological age in non-Hispanic participants despite their having a larger median difference (Table 2, Figure 5). Bone age was significantly advanced in the participants with a shunt, but not in those without a shunt (Table 2, Figure 6).
Figure 5:
Difference between skeletal age and chronological age for non-Hispanic and Hispanic participants.
Figure 6:
Difference between skeletal age and chronological age for participants without and with a shunt.
Multiple Regression Analysis
In multiple regression analysis, the difference between skeletal and chronological age was most strongly associated with higher Tanner stage of sexual development and higher weight, height or BMI percentile. Chronological age had a significant effect only when Tanner stage was removed from the model. Of the anthropometric measures, weight percentile produced the strongest model although statistical significance was also obtained using height or BMI percentile instead (p≤0.05). Sex, Hispanic ethnicity and shunt status did not influence age incongruence in multiple regression analysis. Thus, the final multiple regression model included Tanner stage and weight percentile as the independent variables (adjusted R2 = 0.56, F(5, 72) = 20.55, p<0.0001; Table 4).
Table 4:
Results of multiple linear regression predicting the difference between skeletal and chronological age.
| Coef. | SE | N | t | p | R2 | |
|---|---|---|---|---|---|---|
| Bone age – Chronological age | 78 | 0.56 | ||||
| Tanner stage | ||||||
| 2 | 1.06 | 0.45 | 2.35 | 0.02 | ||
| 3 | 1.01 | 0.39 | 2.62 | 0.01 | ||
| 4 | 1.08 | 0.46 | 2.58 | 0.01 | ||
| 5 | 2.56 | 0.35 | 7.23 | <0.001 | ||
| Weight percentile | 0.017 | 0.004 | 4.53 | <0.001 | ||
| Constant | −1.01 | 0.25 | −3.99 | <0.001 | ||
Coef. = unstandardized regression coefficient; SE = standard error of unstandardized coefficient; N = number of observations; t = t-statistic; P = P-value, R2 = adjusted coefficient of multiple determination
Discussion
For the pediatric orthopedist, knowing a patient’s skeletal maturity and time to growth cessation is important in the timing of many orthopedic interventions such as epiphysiodesis, hemi-epiphysiodesis, limb lengthening, angular and rotational osteotomies and spinal fusion (25, 26). In fracture management, knowing the amount of skeletal growth remaining aides decision making as to an acceptable amount of angulation in fracture alignment that will remodel (27). Bone age is critical to decide on the timing of growth modulation in the axial and appendicular skeleton to correct angular deformity or determine how much scoliosis is likely to progress (28, 29). Additionally, information regarding skeletal maturity would help pediatricians in monitoring growth and development as short stature and precocious puberty are common in the MM population (30). Patients with delayed bone age and short stature but normal pubertal development might be referred to endocrinology for evaluation of possible growth hormone deficiency. Patients with advanced bone age and advanced pubertal development might be referred to endocrinology for evaluation of precocious puberty and possible GnRH analogue therapy.
Consistent with prior studies, the current study found a significant difference between chronologic age and bone age in children with MM. It was observed that bone age changed from being delayed to advanced at about 8 years of age for both girls and boys, consistent with prior work (6, 8). While the median discrepancy between skeletal age and chronological age was approximately 6 months, discrepancies as great as 4.4 years were observed. The underlying reason for the acceleration of skeletal maturation around 8 years of age cannot be fully ascertained from this study; however, the timing is synchronous with the typical onset of sexual development in the MM population and suggests a premature reactivation of the hypothalamic-pituitary-gonadal axis (31, 32). For clinicians caring for the children with MM, this would be an appropriate age to evaluate skeletal maturity and contemplate the timing of interventions such as growth modulation or scoliosis correction that may be reliant on time to growth cessation.
In univariate analysis, advanced bone age was found in the children with a shunt, but not in those without a shunt. This finding is contrary to past research reported by Kalen and Harding (6) and Feeley (5). Kalen and Harding found no relationship between shunt presence and bone age while Feeley et al. found that the presence of a shunt correlated with a younger bone age. In the present study, most participants (62 of 78) had shunts; additionally, the current study assessed the difference between bone age and chronological age within individuals, while the previous studies assessed group differences in bone age. Moreover, the relationship between shunt presence and age discrepancy in the current study was modulated by other variables, as it was no longer observed once Tanner stage and anthropometrics were considered. As prior studies have shown that participants with shunted hydrocephalus have lower levels of growth hormones (e.g., insulin-like growth factor-1, growth hormone, binding protein 3) (33), measuring hormone levels in this population might reveal if their hypothalamic-pituitary axis were dysfunctional and a possible source of abnormalities of skeletal maturation (32).
In the spina bifida population, short stature is common, and excess weight is more prevalent than in the typically developing population (9, 34). The current study found a relationship between higher weight or BMI and advanced bone age. This is consistent with prior studies in the typically developing population, which have shown advanced bone age with increased BMI (12). Knowing that increased BMI is associated with advanced skeletal maturity in MM is valuable because obesity is modifiable through physical activity (3). Decreased adiposity could affect maturation since production of hormones, including estradiol and leptin, by adipose tissue may stimulate early maturation in people with increased BMI (12). Incongruent bone age was not observed in participants below the 20th percentile for height, though it was advanced for those above the 20th percentile. The group below the 20th percentile for height had lower Tanner staging with lower weight/BMI percentiles. It is likely that the relationship between height and incongruent bone age is mediated by sexual development and weight or BMI.
Tanner stage of sexual development was positively related to advanced bone age consistent with prior studies in typically developing children that have shown a correlation between pubertal development and skeletal maturation (35, 36). In the cerebral palsy population, which also has discrepancies between chronologic age and skeletal maturation, Henderson et al. showed a relationship between Tanner stage and skeletal maturation (37). To our knowledge, the current study is the first to show a similar relationship in MM. The increase in discrepancy between chronologic age and skeletal age as the participants progressed through puberty may suggest an uninhibited hormonal positive feedback loop controlling skeletal maturation in the MM population (38). Studies in typically developing children have reported that increases in gonadotropin hormones and pubertal progression correlate with advances in bone age (36).
No effect of Hispanic ethnicity was observed in the multiple linear regression analysis. Previous studies in typically developing children have reported inconsistent results regarding the relationship between Hispanic ethnicity and bone age. Zhang et al. and Ontell et al. found advanced bone age in Hispanics around the time of adolescence and puberty, while Mansourvar et al. found normal in bone age in Hispanics (15, 39, 40). The relationship between skeletal maturity and Hispanic ethnicity may be of particular importance for those with MM as the Hispanic population has a higher prevalence of MM (41). Additional investigations are needed to fully understand if a relationship between ethnicity and bone age exists and to what extent.
Limitations
This study has several limitations. First, the study sample was skewed with the majority of participants having lesions at the mid-lumbar level and below, and is therefore not representative of the general MM population. Additionally, most participants in this study were ambulatory, with only nine being non-ambulatory. Qualitatively, similar results between ambulatory and non-ambulatory participants were found, but the study was not powered to examine statistical differences between ambulatory and non-ambulatory participants. However, it should be noted that previous studies have found no relationship between neurosegmental level and bone age in children with spina bifida (5, 6) although lack of ambulation is strongly related to low bone density (18, 42). Additionally, the standard height measure used and the resulting BMI may not be ideal for all children with MM. Standing or supine height may underestimate true height in the presence of joint contracture or scoliosis. Unfortunately, in this study alternative measures of height such as segmental measurement or arm span were not used. Due to this study’s high proportion of Hispanic participants the findings may not be generalizable to non-Hispanic youth with MM although there is a higher prevalence of spina bifida in the Hispanic population (41, 43), which is reflected in the current study’s sample. A larger multicenter study could also enable a larger sample size along with confirming generalizability of the study results.
Conclusions
In conclusion, in the MM population advanced skeletal maturation is common in children and adolescents over 8 years of age who have reached puberty, particularly those who are overweight or obese. Being cognizant that skeletal age can be advanced by as much as 4 years or more when compared to chronologic age may lead the orthopedic surgeon to investigate a patient’s bone age sooner when planning for growth modulation or spinal arthrodesis. The combination of physical examination, bone age and BMI data would greatly assist the pediatrician in clinical decision making, specifically regarding possible use of hormone therapies to treat precocious puberty and to obtain pediatric endocrinology consultation where most beneficial.
Acknowledgements:
Funding: Support provided by NIH-NICHD Grant # 5R01HD059826 from the National Institutes of Health – Eunice Kennedy Shriver National Institute of Child Health and Human Development.
Footnotes
Conflict of interest: The authors have no conflicts of interest to disclose.
References:
- 1.Bowman RM, Boshnjaku V, McLone DG. The changing incidence of myelomeningocele and its impact on pediatric neurosurgery: a review from the Children’s Memorial Hospital. Child’s nervous system : ChNS : official journal of the International Society for Pediatric Neurosurgery. 2009. July;25(7):801–6. PubMed PMID: 19326126. Epub 2009/03/28. eng. [DOI] [PubMed] [Google Scholar]
- 2.Apkon SD, Fenton L, Coll JR. Bone mineral density in children with myelomeningocele. Developmental medicine and child neurology. 2009. January;51(1):63–7. PubMed PMID: 18811711. Epub 2008/09/25. eng. [DOI] [PubMed] [Google Scholar]
- 3.Ausili E, Focarelli B, Tabacco F, Fortunelli G, Caradonna P, Massimi L, et al. Bone mineral density and body composition in a myelomeningocele children population: effects of walking ability and sport activity. European review for medical and pharmacological sciences. 2008. Nov-Dec;12(6):349–54. PubMed PMID: 19146196. Epub 2009/01/17. eng. [PubMed] [Google Scholar]
- 4.Quan A, Adams R, Ekmark E, Baum M. Bone mineral density in children with myelomeningocele. Pediatrics. 1998. September;102(3):E34. PubMed PMID: 9724682. Epub 1998/09/02. eng. [DOI] [PubMed] [Google Scholar]
- 5.Feeley BT, Ip TC, Otsuka NY. Skeletal maturity in myelomeningocele. Journal of pediatric orthopedics. 2003. Nov-Dec;23(6):718–21. PubMed PMID: 14581773. Epub 2003/10/29. eng. [DOI] [PubMed] [Google Scholar]
- 6.Kalen V, Harding CR. Skeletal maturity in myelodysplasia. Developmental medicine and child neurology. 1994. June;36(6):528–32. PubMed PMID: 8005364. Epub 1994/06/01. eng. [DOI] [PubMed] [Google Scholar]
- 7.Demirjian A, Buschang PH, Tanguay R, Patterson DK. Interrelationships among measures of somatic, skeletal, dental, and sexual maturity. American journal of orthodontics. 1985. November;88(5):433–8. PubMed PMID: 3864376. Epub 1985/11/01. eng. [DOI] [PubMed] [Google Scholar]
- 8.Greene SA, Frank M, Zachmann M, Prader A. Growth and sexual development in children with meningomyelocele. European journal of pediatrics. 1985. July;144(2):146–8. PubMed PMID: 2864250. Epub 1985/07/01. eng. [DOI] [PubMed] [Google Scholar]
- 9.Mueske NM, Ryan DD, Van Speybroeck AL, Chan LS, Wren TA. Fat distribution in children and adolescents with myelomeningocele. Developmental medicine and child neurology. 2015. March;57(3):273–8. PubMed PMID: 25251828. Pubmed Central PMCID: PMC4323886. Epub 2014/09/25. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Douyon L, Schteingart DE. Effect of obesity and starvation on thyroid hormone, growth hormone, and cortisol secretion. Endocrinology and metabolism clinics of North America. 2002. March;31(1):173–89. PubMed PMID: 12055988. Epub 2002/06/12. eng. [DOI] [PubMed] [Google Scholar]
- 11.Kokkoris P, Pi-Sunyer FX. Obesity and endocrine disease. Endocrinology and metabolism clinics of North America. 2003. December;32(4):895–914. PubMed PMID: 14711067. Epub 2004/01/09. eng. [DOI] [PubMed] [Google Scholar]
- 12.van Lenthe FJ, Kemper CG, van Mechelen W. Rapid maturation in adolescence results in greater obesity in adulthood: the Amsterdam Growth and Health Study. The American journal of clinical nutrition. 1996. July;64(1):18–24. PubMed PMID: 8669409. Epub 1996/07/01. eng. [DOI] [PubMed] [Google Scholar]
- 13.Hayes-Allen MC, Tring FC. Obesity: another hazard for spina bifida children. British journal of preventive & social medicine. 1973. August;27(3):192–6. PubMed PMID: 4585800. Pubmed Central PMCID: 478794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Finding Balance Obesity and Children with Special Needs. Ability Path 2011; 1–52. http://abilitypath.org/wp-content/uploads/2015/11/obesity-report.pdf (accessed 2017-09). [Google Scholar]
- 15.Ontell FK, Ivanovic M, Ablin DS, Barlow TW. Bone age in children of diverse ethnicity. AJR American journal of roentgenology. 1996. December;167(6):1395–8. PubMed PMID: 8956565. Epub 1996/12/01. eng. [DOI] [PubMed] [Google Scholar]
- 16.Wren TA, Shepherd JA, Kalkwarf HJ, Zemel BS, Lappe JM, Oberfield S, et al. Racial disparity in fracture risk between white and nonwhite children in the United States. J Pediatr. 2012. December;161(6):1035–40. PubMed PMID: 22974572. Pubmed Central PMCID: 3504618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morakis E, Wright J. Evidence-Based Treatment of Spina Bifida. Paediatric Orthopaedics: Springer; 2017. p. 255–61. [Google Scholar]
- 18.Horenstein RE, Shefelbine SJ, Mueske NM, Fisher CL, Wren TA. An approach for determining quantitative measures for bone volume and bone mass in the pediatric spina bifida population. Clinical biomechanics (Bristol, Avon). 2015. August;30(7):748–54. PubMed PMID: 26002057. Pubmed Central PMCID: PMC4523422. Epub 2015/05/24. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barlow SE. Expert committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: summary report. Pediatrics. 2007. December;120 Suppl 4:S164–92. PubMed PMID: 18055651. Epub 2007/12/18. eng. [DOI] [PubMed] [Google Scholar]
- 20.Mei Z, Ogden CL, Flegal KM, Grummer-Strawn LM. Comparison of the prevalence of shortness, underweight, and overweight among US children aged 0 to 59 months by using the CDC 2000 and the WHO 2006 growth charts. The Journal of pediatrics. 2008. November;153(5):622–8. PubMed PMID: 18619613. Epub 2008/07/16. eng. [DOI] [PubMed] [Google Scholar]
- 21.Wright J Caring for the child with spina bifida. 1 ed. Oak Brook, Illinois: American Academy of Orthopaedic Surgeons; 2001. [Google Scholar]
- 22.Marshall WA, Tanner JM. Variations in pattern of pubertal changes in girls. Archives of disease in childhood. 1969. June;44(235):291–303. PubMed PMID: 5785179. Pubmed Central PMCID: PMC2020314. Epub 1969/06/01. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marshall WA, Tanner JM. Variations in the pattern of pubertal changes in boys. Archives of disease in childhood. 1970. February;45(239):13–23. PubMed PMID: 5440182. Pubmed Central PMCID: PMC2020414. Epub 1970/02/01. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Greulich WW, Pyle SI. Radiographic atlas skeletal development of the hand and wrist. Am J Med Sci. 1959;238(3).13626967 [Google Scholar]
- 25.Swaroop VT, Dias L. Orthopedic management of spina bifida. Part I: hip, knee, and rotational deformities. Journal of children’s orthopaedics. 2009. December;3(6):441–9. PubMed PMID: 19856195. Pubmed Central PMCID: PMC2782071. Epub 2009/10/27. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thomson JD, Segal LS. Orthopedic management of spina bifida. Developmental disabilities research reviews. 2010;16(1):96–103. PubMed PMID: 20419777. Epub 2010/04/27. eng. [DOI] [PubMed] [Google Scholar]
- 27.Wilkins KE. Principles of fracture remodeling in children. Injury. 2005. February;36 Suppl 1:A3–11. PubMed PMID: 15652934. eng. [DOI] [PubMed] [Google Scholar]
- 28.Eastwood DM, Sanghrajka AP. Guided growth: recent advances in a deep-rooted concept. J Bone Joint Surg Br. 2011. January;93(1):12–8. PubMed PMID: 21196537. eng. [DOI] [PubMed] [Google Scholar]
- 29.Rupprecht M, Spiro AS, Breyer S, Vettorazzi E, Ridderbusch K, Stücker R. Growth modulation with a medial malleolar screw for ankle valgus deformity. 79 children with 125 affected ankles followed until correction or physeal closure. Acta Orthop. 2015;86(5):611–5. PubMed PMID: 25909385. Pubmed Central PMCID: PMC4564785. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rotenstein D, Bass AN. Treatment to near adult stature of patients with myelomeningocele with recombinant human growth hormone. J Pediatr Endocrinol Metab. 2004. September;17(9):1195–200. PubMed PMID: 15506678. eng. [DOI] [PubMed] [Google Scholar]
- 31.Léger J, Carel J-C. Precocious puberty. Puberty: Springer; 2016. p. 137–54. [Google Scholar]
- 32.Trollmann R, Strehl E, Dorr HG. Precocious puberty in children with myelomeningocele: treatment with gonadotropin-releasing hormone analogues. Developmental medicine and child neurology. 1998;40:38–43. [DOI] [PubMed] [Google Scholar]
- 33.Lopponen T, Saukkonen AL, Serlo W, Tapanainen P, Ruokonen A, Knip M. Reduced levels of growth hormone, insulin-like growth factor-I and binding protein-3 in patients with shunted hydrocephalus. Archives of disease in childhood. 1997. July;77(1):32–7. PubMed PMID: 9279148. Pubmed Central PMCID: PMC1717228. Epub 1997/07/01. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rotenstein D, Adams M, Reigel DH. Adult stature and anthropomorphic measurements of patients with myelomeningocele. European journal of pediatrics. 1995. May;154(5):398–402. PubMed PMID: 7641775. Epub 1995/05/01. eng. [DOI] [PubMed] [Google Scholar]
- 35.Marshall WA. Interrelationships of skeletal maturation, sexual development and somatic growth in man. Annals of human biology. 1974. January;1(1):29–40. PubMed PMID: 16431550. Epub 1974/01/01. eng. [DOI] [PubMed] [Google Scholar]
- 36.Sizonenko PC, Burr IM, Kaplan SL, Grumbach MM. Hormonal changes in puberty. II. Correlation of serum luteinizing hormone and follicle stimulating hormone with stages of puberty and bone age in normal girls. Pediatric research. 1970. January;4(1):36–45. PubMed PMID: 5416997. Epub 1970/01/01. eng. [DOI] [PubMed] [Google Scholar]
- 37.Henderson RC, Gilbert SR, Clement ME, Abbas A, Worley G, Stevenson RD. Altered skeletal maturation in moderate to severe cerebral palsy. Developmental medicine and child neurology. 2005. April;47(4):229–36. PubMed PMID: 15832545. Epub 2005/04/19. eng. [DOI] [PubMed] [Google Scholar]
- 38.Wojcicka A, Bassett JH, Williams GR. Mechanisms of action of thyroid hormones in the skeleton. Biochim Biophys Acta. 2013. July;1830(7):3979–86. PubMed PMID: 22634735. Epub 2012/05/25. eng. [DOI] [PubMed] [Google Scholar]
- 39.Zhang A, Sayre JW, Vachon L, Liu BJ, Huang HK. Racial differences in growth patterns of children assessed on the basis of bone age. Radiology. 2009. January;250(1):228–35. PubMed PMID: 18955510. Pubmed Central PMCID: PMC2817832. Epub 2008/10/29. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mansourvar M, Ismail MA, Raj RG, Kareem SA, Aik S, Gunalan R, et al. The applicability of Greulich and Pyle atlas to assess skeletal age for four ethnic groups. Journal of forensic and legal medicine. 2014. February;22:26–9. PubMed PMID: 24485416. Epub 2014/02/04. eng. [DOI] [PubMed] [Google Scholar]
- 41.Shin M, Besser LM, Siffel C, Kucik JE, Shaw GM, Lu C, et al. Prevalence of spina bifida among children and adolescents in 10 regions in the United States. Pediatrics. 2010. August;126(2):274–9. PubMed PMID: 20624803. Epub 2010/07/14. eng. [DOI] [PubMed] [Google Scholar]
- 42.Szalay EA, Cheema A. Children with spina bifida are at risk for low bone density. Clinical orthopaedics and related research. 2011. May;469(5):1253–7. PubMed PMID: 21042897. Pubmed Central PMCID: 3069300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Williams LJ, Rasmussen SA, Flores A, Kirby RS, Edmonds LD. Decline in the prevalence of spina bifida and anencephaly by race/ethnicity: 1995–2002. Pediatrics. 2005. September;116(3):580–6. PubMed PMID: 16140696. Epub 2005/09/06. eng. [DOI] [PubMed] [Google Scholar]