Abstract
Background:
Effective treatments for cholangiocarcinoma (CCA) are still lacking. There are promising results of checkpoint inhibitor programmed cell death ligand-1 (PD-L1) activities in early phase trials. This study aimed to investigate the expression of PD-L1 and its relation to possible treatments for CCA.
Methods:
Formalin-fixed paraffin-embedded tumor samples from 46 patients with cholangiocarcinoma were retrieved. PD-L1 expression was evaluated by immunohistochemistry using anti-PD-L1 antibody, clone 5H1. A PD-L1 positive response on tumor cells was defined as >1% of tumor cell membranes stained. The association between PD-L1, clinico-pathological characteristics was analyzed using Fisher’s exact test, and survival analysis was done with the Cox regression model.
Results:
Out of 46 samples, 32 (70%) had positive PD-L1 expression in tumor cell membranes. The median level of PD-L1 expression was 1.75% (0-34.7). PD-L1 expression was significantly associated with stage IV disease (OR 3.98, p=0.046) and a high neutrophil/lymphocyte ratio (OR 5.36, p=0.018). PD-L1 positivity was associated with worse overall survival compared with those with a PD-L1 negative tumor but did not reach a level of significance (7.2 vs. 7.9 months, p=0.32).
Conclusion:
PD-L1 is widely expressed in CCA but was not predictive for overall survival. PD-L1 positivity was (7.2 and 7.9 months, p=0.32). Significantly associated with stage IV disease and a high neutrophil/lymphocyte ratio.
Keywords: Biliary tract cancer, cholangiocarcinoma, immune studies, neutrophil-lymphocyte ratio, PD-L1
Introduction
Cholangiocarcinoma (CCA) is a rare and aggressive solid tumor. Currently, the only potentially curative treatment is radical surgery but it is only suitable for localized disease which accounts for fewer than 20% of the patients and the recurrence rate is high(Hasegawa et al., 2007). Most of the patients present with unresectable disease or metastasis to distant organs. The current systemic treatment is not impressive with a median survival of about 12 months despite the combination chemotherapy of gemcitabine and cisplatin (Valle et al., 2010).
The molecular pathogenesis of CCA involves the long-term inflammatory responses with the production of many cytokines associated with or without liver flukes (Chan-On et al., 2009). Recent studies also suggested that neutrophil/lymphocyte ratio, an inflammatory marker, is a poor prognostic marker for CCA(McNamara et al., 2014; Lin et al., 2016). The T-lymphocyte is the most prevalent cell type for inflammation in biliary tract cancer and patients with intra-tumoural T and B lymphocytes are correlated with longer survival (Goeppert et al., 2013).
The Programmed Death-1 (PD-1) T cell co-receptor and its ligands, programmed death-ligands 1 and 2 (PD-L1 and PD-L2) are pivotal in evading immune surveillance for tumor microenvironment by serving as negative regulators (Topalian et al., 2012). With growing evidence, it is becoming clear that blocking the PD-1/ PD-L1 pathway could benefit in a durable response and prolong overall survival in many cancers, including melanoma, lung, and kidney cancer (Motzer et al., 2015; Robert et al., 2015; Reck et al., 2016). Therefore, this study looks forward in searching for a cure for CCA from this novel immunotherapy.
PD-L1 expression has been reported in many tumors and has been linked to worse prognoses (Wu et al., 2015; Zhou et al., 2015). Since present knowledge does not reveal data in CCA, however, the aim in this study was to characterize PD-L1 expression and its correlation with clinico-pathological data as well as overall survival in CCA patients.
Materials and Methods
Patients and samples
A total of 46 CCA patients treated surgically at Srinagarind Hospital, Khon Kaen University were identified. Formalin-fixed paraffin-embedded blocks were retrieved and re-reviewed. Baseline clinico-pathological features such as age, gender, tumor characteristics, TNM staging, and clinical follow-up data were retrospectively collected. Institutional Review Board approval was obtained before data acquisition and tumor staining.
Immunohistochemistry
PD-L1 expression was evaluated in a tissue microarray by immunohistochemistry using a mouse IgG antibody against PD-L1 (Figure 1). Three-micron-thick tumor sections were stained with an anti-PD-L1 antibody (1:1000, Roche Diagnostic GmbH, USA) in an autostainer system (Ventana Medical System, Tucson, AZ). The UltraView Universal DAB Detection kit (Ventana) was used according to the manufacturer’s instructions. Counterstaining using hematoxylin was carried out as part of the automated staining protocol (Ventana). After staining, slides were then washed, dehydrated, mounted and cover slipped.
Figure 1.

PD-L1 Expression in FFPE Samples Stained with Anti-PD-L1 Antibody (Clone 5H1) in Cholangiocarcinoma. Positive brown fine granular staining is present in tumor cells with moderate signal intensity in the cytoplasm (a). In panel (b), tumor cells are negative for PD-L1.
Scoring of PD-L1 expression
Membranous expression in tumor cells was quantified by two independent pathologists (SS and PU) blinded to clinical outcome. PD-L1 tumor positivity was defined as >1% tumor cell membrane staining.
Pretreatment systemic inflammatory, nutritional, and tumor markers analysis
Pretreatment blood samples were taken for routine analysis of complete blood counts and albumin. The white blood cell, platelet, neutrophil, and lymphocyte count were analyzed with an automated hematological blood analyzer. Serum concentrations of albumin, carcinoembryogenic antigen (CEA), and cancer antigen 19-9 (CA 19-9) were measured in an automated analyzer.
Systemic inflammation was determined by the neutrophil-lymphocyte ratio (NLR) which was defined as the absolute neutrophil count divided by the absolute lymphocyte count. The range was 0.8-13.7. No patient had a clinical record of infection or sepsis at the time of blood sampling.
Statistical analysis
The primary objective of this study was to characterize the PD-L1 expression in patients with CCA and to correlate the positivity of expression with clinic-pathological features and disease outcome. Overall survival (OS) was defined as the time from diagnosis to death from any cause. In the absence of death or other event, the end point was considered as the last follow-up time. Fisher’s exact tests were used to assess the associations of clinical characteristics and PD-L1 positivity. The Cox regression model was used to assess the association of PD-L1 positivity with OS. All statistical computations were performed using SPSS v.17.0 (SPSS, Inc. Chicago, IL) and a p-value of <0.05 was considered statistically significant.
Results
Patients and tumor characteristics are described in Table 1. The study included a total of 46 patients with CCA. The median age was 57.5 years (range; 45-76 years) and most of the patients were male (72%). The subtypes included intrahepatic CCA (n=32) and extrahepatic CCA (n=13). Moreover, 39 patients had a moderate to well differentiated grade and 7 patients had poorly differentiated tumors. TNM clinical stages I, II, III and IV at diagnosis were identified in 4, 10, 12, and 20 patients, respectively. Seven patients (15.2%) were found to have distant metastasis at diagnosis and 18 patients had lymph node involvement (39.1%). All patients received fluorouracil-based chemotherapy after the surgery.
Table 1.
Baseline Characteristics of the CCA Patients (N=46)
| Variables | Number (%) |
|---|---|
| Age (median; range) | (57.5; 45-76) |
| < 60 years | 25 (54.3) |
| ≥ 60 years | 21 (45.7) |
| Gender | |
| Male | 33 (71.7) |
| Female | 13 (28.3) |
| Tumor location | |
| Intrahepatic | 33 (71.7) |
| Extrahepatic | 13 (28.3) |
| Tumor grading | |
| Well differentiated | 8 (17.4) |
| Moderately differentiated | 31 (67.4) |
| Poorly differentiated | 7 (15.2) |
| TNM staging | |
| I | 4 (8.7) |
| II | 10 (21.7) |
| III | 12 (26.1) |
| IV | 20 (43.5) |
| Neutrophil/lymphocyte ratio | |
| < 3 | 24 (52.2) |
| ≥ 3 | 22 (47.8) |
| Nodal status | |
| Negative | 28 (60.9) |
| Positive | 18 (39.1) |
| Metastatic status | |
| Negative | 39 (84.8) |
| Positive | 7 (15.2) |
PD-L1 expression in tumor cells and clinico-pathological features
The PD-L1 expression in cholangiocarcinoma tissue was positive in 32 cases (69.6%). A variable PD-L1 expression ranging from 1.2 to 34.7% was observed with most of the cases expressing a low level of positivity; median 1.75%.
The associations between clinical variables stratified by PD-L1 status of 46 patients are summarized in Table 2. There was a significant association of high NLR (>3) and PD-L1 expression (OR=5.36, p=0.018) and stage IV disease (OR=3.98, p=0.046).
Table 2.
Correlation between Clinical Variables and PDL1 Status
| Variables | PDL1 status | Odds ratio | p-value | |
|---|---|---|---|---|
| Negative | Positive | |||
| N (%) | N (%) | |||
| Lymph node status | ||||
| Negative | 10 (71.4) | 18 (56.2) | 2.2 | 0.246 |
| Positive | 4 (28.6) | 14 (43.8) | ||
| Tumor grade | ||||
| Well/moderately differentiated | 12 (85.7) | 27 (84.4) | 0.014 | 0.642 |
| Poorly differentiated | 2 (14.3) | 5 (15.6) | ||
| Staging | ||||
| I-III | 11 (78.6) | 15 (46.9) | 3.98 | 0.046* |
| IV | 3 (21.4) | 17 (53.1) | ||
| Serum albumin | ||||
| < 3 g/dL | 1 (7.1) | 7 (22.6) | 0.26 | 0.402 |
| ≥ 3 g/dL | 13 (92.9) | 24 (77.4) | ||
| Serum CA 19-9 | ||||
| < 100 U/ml | 6 (46.2) | 14 (51.9) | 0.8 | 0.736 |
| ≥ 100 U/ml | 7 (53.8) | 13 (48.1) | ||
| Serum CEA | ||||
| < 2.5 ng/ml | 3 (23.1) | 4 (13.8) | 1.87 | 0.657 |
| ≥ 2.5 ng/ml | 10 (76.9) | 25 (86.2) | ||
| NLR | ||||
| <3 | 11 (78.6) | 13 (40.6) | 5.36 | 0.018* |
| >3 | 3 (21.4) | 19 (59.4) | ||
CA 19-9, Cancer antigen 19-9; CEA, Carcinoembryonic antigen; NLR, Neutrophil/lymphocyte ratio
PD-L1 positivity was not significantly associated with patients’ age, gender, pathological lymph node status, tumor grade, serum albumin, or serum tumor markers that were found as shown in Table 2. Moreover, PD-L1 positivity was also not related with tumor markers, CEA and CA 19-9.
PD-L1 positivity and overall survival
At the time of final data analysis, 44 patients (95.6%) had died. The median overall survival was 7.77 months (95%CI 5.3-10.20). The median survival for those with PD-L1 negative was 7.97 months (95%CI 1.43-14.51) compared with 7.23 months (95%CI 4.27-10.19) in PD-L1 positive patients. There was a non-significant trend toward increased risk of death in patients with PD-L1+ tumor (HR=1.39, 95% CI 0.72-2.68, p=0.32) (Figure 2).
Figure 2.
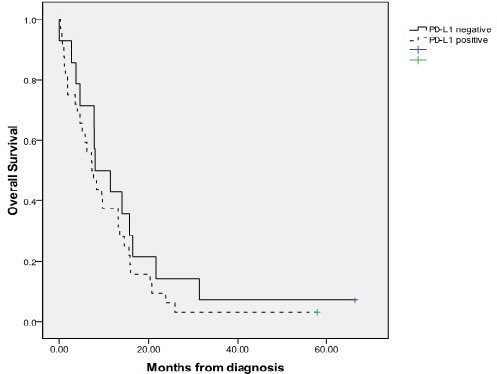
Kaplan Meier Analysis of Survival. According to PD-L1 (<1% vs > 1%), the median survival were 7.97 vs 7.23 months, respectively. The hazard ratio was 1.39 (95% CI 0.72-2.68).
Discussion
In the present study the PD-L1 expression in cholangiocarcinoma patients was obtained and two major findings were analyzed. It was found that PD-L1 expression is up regulated in 70% of cholangiocarcinoma tissues and PD-L1 expression was increased from baseline NLR and significantly associated with systemic inflammation.
In many cancers, PD-L1 has been found to be expressed and served as a therapeutic target for immune-checkpoint inhibitors. There were only four studies which demonstrated PD-L1 expression in CCA (Ye et al., 2009; Suleiman et al., 2015 (suppl 3; abstr 294); Gani et al., 2016; Mody et al., 2016 (suppl 4S; abstr 289)). In this study it was found that PD-L1 was up-regulated in 70% of samples, similar to three other series (72-100%) (Ye et al., 2009; Suleiman et al., 2015 (suppl 3; abstr 294); Gani et al., 2016). In one series which included all hepatobiliary patients, however, PD-L1 positivity was demonstrated in only 4% of intrahepatic CCA and 18% in extrahepatic CCA, likely due to different techniques and scoring systems ((Mody et al., 2016 (suppl 4S; abstr 289)).
Correlations between PD-L1 expression and clinical features among patients with CCA are conflicting among studies. The current study found that PD-L1 was associated with a higher stage of disease similar to two other studies. In the current study no association between PD-L1 expression and patients’ age, sex, lymph node status, or tumor grade was found. Ye et al., (2009) found that increased PD-L1 expression was associated with higher grades and higher stages of tumors and Gani et al., (2016) found a significant correlation between nodal metastasis and PD-L1 expression. An unfavorable prognostic value of PD-L1 expression was demonstrated herein, which is similar to an earlier study in CCA.
Interestingly, in this study, a significant association between an increased neutrophil/lymphocyte ratio (NLR), a marker of systemic inflammation, and high PD-L1 expression was demonstrated. NLR was studied in many cancers, including CCA, and was found to be an adverse prognostic factor (McNamara et al., 2014; Lin et al., 2016). In the recent meta-analysis suggested that elevated preoperative NLR is associated with shorter survival in cholangiocarcinoma patients. Most included studies assessing the NLR used the cut-off of 3.0 (Tan et al., 2016). In the present authors’ best knowledge, this is the first report of an association between NLR and PD-L1 in cholangiocarcinoma. The findings are consistent with earlier data, given the strong relationship between CCA and chronic inflammation especially in Thailand where liver fluke is the major cause of CCA (Chan-On et al., 2009).
Limitations of this study were the small sample size and the lack of some of the clinical data. Furthermore, since the pathogenesis of CCA in Thailand may be different from other populations given the strong association with liver fluke (Sithithaworn et al., 2014), PD-L1 expression might also differ.
In summary, PD-L1 expression in tumors was observed in 70% of cholangiocarcinomas and PD-L1 positivity was associated with high neutrophil/lymphocyte ratio and a higher tumor stage. The PD-1/PD-L1 pathway is a potential therapeutic target for advanced CCA and further studies are warranted.
Conflict of interest
The authors declare that they have no conflict of interest.
Acknowledgements
This project was supported by the Faculty of Medicine, Khon Kaen University. We would like to acknowledge Prof. James A. Will for editing the manuscript via Publication Clinic KKU, Thailand.
References
- Chan-On W, Kuwahara K, Kobayashi N, et al. Cholangiocarcinomas associated with long-term inflammation express the activation-induced cytidine deaminase and germinal center-associated nuclear protein involved in immunoglobulin V-region diversification. Int J Oncol. 2009;35:287–95. [PubMed] [Google Scholar]
- Gani F, Nagarajan N, Kim Y, et al. Program death 1 immune checkpoint and tumor microenvironment: Implications for patients with intrahepatic cholangiocarcinoma. Ann Surg Oncol. 2016;23:2610–7. doi: 10.1245/s10434-016-5101-y. [DOI] [PubMed] [Google Scholar]
- Goeppert B, Frauenschuh L, Zucknick M, et al. Prognostic impact of tumour-infiltrating immune cells on biliary tract cancer. Br J Cancer. 2013;109:2665–74. doi: 10.1038/bjc.2013.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa S, Ikai I, Fujii H, et al. Surgical resection of hilar cholangiocarcinoma: analysis of survival and postoperative complications. World J Surg. 2007;31:1256–63. doi: 10.1007/s00268-007-9001-y. [DOI] [PubMed] [Google Scholar]
- Lin G, Liu Y, Li S, et al. Elevated neutrophil-to-lymphocyte ratio is an independent poor prognostic factor in patients with intrahepatic cholangiocarcinoma. Oncotarget. 2016;7:50963–71. doi: 10.18632/oncotarget.7680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara MG, Templeton AJ, Maganti M, et al. Neutrophil/lymphocyte ratio as a prognostic factor in biliary tract cancer. Eur J Cancer. 2014;50:1581–9. doi: 10.1016/j.ejca.2014.02.015. [DOI] [PubMed] [Google Scholar]
- Mody K, Feldman R, Reddy S, et al. (suppl 4S;abstr 289)). PD1/PDL1 expression and molecular associations in HPB malignancies. J Clin Oncol. 2016:34. [Google Scholar]
- Motzer RJ, Escudier B, McDermott DF, et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med. 2015;373:1803–13. doi: 10.1056/NEJMoa1510665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reck M, Rodriguez-Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016;375:1823–33. doi: 10.1056/NEJMoa1606774. [DOI] [PubMed] [Google Scholar]
- Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372:320–30. doi: 10.1056/NEJMoa1412082. [DOI] [PubMed] [Google Scholar]
- Sithithaworn P, Yongvanit P, Duenngai K, et al. Roles of liver fluke infection as risk factor for cholangiocarcinoma. J Hepatobiliary Pancreat Sci. 2014;21:301–8. doi: 10.1002/jhbp.62. [DOI] [PubMed] [Google Scholar]
- Suleiman Y, Coppola D, Zibadi S, et al. (suppl 3;abstr 294). Prognostic value of tumorinfiltrating lymphocytes (TILs) and expression of PDL1 in cholangiocarcinoma. J Clin Oncol. 2015:33. [Google Scholar]
- Tan DW, Fu Y, Su Q, et al. Prognostic significance of neutrophil to lymphocyte ratio in oncologic outcomes of cholangiocarcinoma: A meta-analysis. Sci Rep. 2016;6:33789. doi: 10.1038/srep33789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topalian SL, Drake CG, Pardoll DM. Targeting the PD-1/B7-H1(PD-L1) pathway to activate anti-tumor immunity. Curr Opin Immunol. 2012;24:207–12. doi: 10.1016/j.coi.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valle J, Wasan H, Palmer DH, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010;362:1273–81. doi: 10.1056/NEJMoa0908721. [DOI] [PubMed] [Google Scholar]
- Wu P, Wu D, Li L, et al. PD-L1 and survival in solid tumors: A meta-analysis. PLoS One. 2015;10:e0131403. doi: 10.1371/journal.pone.0131403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Y, Zhou L, Xie X, et al. Interaction of B7-H1 on intrahepatic cholangiocarcinoma cells with PD-1 on tumor-infiltrating T cells as a mechanism of immune evasion. J Surg Oncol. 2009;100:500–4. doi: 10.1002/jso.21376. [DOI] [PubMed] [Google Scholar]
- Zhou ZJ, Zhan P, Song Y. PD-L1 over-expression and survival in patients with non-small cell lung cancer: a meta-analysis. Transl Lung Cancer Res. 2015;4:203–8. doi: 10.3978/j.issn.2218-6751.2015.03.02. [DOI] [PMC free article] [PubMed] [Google Scholar]


