Abstract
Background:
Carboplatin is a drug that is used for treatment of many types of cancer. However, it may produce serious nephrotoxicity. Candesartan is angiotensin II receptor antagonist employed mainly for control of hypertension. Coenzyme Q10 (CoQ10) is a fat-soluble substance which was proven to have potent antioxidant and anti-inflammatory properties.
Aim:
Our aim was to study the effects of candesartan and/or CoQ10 on carboplatin-induced nephrotoxicity in mice.
Methods:
Sixty mice were divided into 6 equal groups: Control untreated; carboplatin; carboplatin + candesartan; carboplatin + CoQ10; carboplatin + carboxymethyl cellulose; and carboplatin + candesartan + CoQ10 group. Kidney weight/body weight ratio, blood urea, serum creatinine, creatinine clearance, urinary N-acetyl beta-D-glucosaminidase (NAG), gamma glutamyl transpeptidase (GGT) and the urinary albumin excretion rate (UAER) were determined. Renal tissue catalase (CAT), glutathione reductase (GR), nuclear factor (erythroid-derived 2)-like 2 (Nrf2), heme oxygenase-1 (HO-1), transforming growth factor beta-1 (TGF-β1), tumor necrosis factor alpha (TNF-α) and interleukin 6 (IL-6) were also determined, along with mitochondrial complex I activity. In addition, portions of the kidney were subjected to histopathological and immunohistochemical examination.
Results:
Candesartan and/or CoQ10 induced significant improvement of renal and mitochondrial functions with significant increase in tissue CAT, GR, Nrf2 and HO-1 content associated with significant decrease in the kidney weight/body weight ratio, tissue TGF-β1, TNF-α and IL-6 and alleviation of the histopathological and immunohistochemical changes as compared to carboplatin alone group. These effects were more significant in candesartan/CoQ10 combination group compared to either candesartan or CoQ10 alone.
Conclusion:
Candesartan/CoQ10 combination might represent a beneficial therapeutic modality for amelioration of carboplatin-induced nephrotoxicity.
Keywords: Candesartan, coenzyme Q10, carboplatin, nephrotoxicity, mice
Introduction
Carboplatin is an anticancer agent that is used for treatment of lung, ovarian and head malignancies (Chen et al., 2010). It undergoes activation inside the cells forming reactive complexes that cause intra- and inter-strand cross-linkage of DNA molecules which modifies DNA structure and inhibits DNA replication (Ho et al., 2016). The major limiting factor for carboplatin use is the possible development of nephrotoxicity. The byproducts of carboplatin may accumulate in the kidney and induce serious renal tubular damage (Moon et al., 2011). Yonezawa (2012) suggested that nephrotoxicity induced by platinum drugs, including carboplatin, is related to their effects on cell polarity and basolateral transport mechanisms in renal epithelial cells. Moreover, induction of oxidative stress, mitochondrial dysfunction and increased release of pro-inflammatory cytokines were suggested as potential contributing factors to carboplatin nephrotoxicity (Ozbek, 2012).
Candesartan is angiotensin II receptor antagonist used mainly for treatment of hypertension. Recent studies reported that it has potent antioxidant properties which may play a role in its nephroprotective effects (Callera et al., 2016). Moreover, it was proven that candesartan has anti-inflammatory and anti-apoptotic actions, possibly through inhibition of production of the pro-inflammatory cytokines such as tumor necrosis factor alpha (TNF-α) and suppression of the apoptotic markers such as caspase-3 in the renal tissues. Taken together, these properties may give candesartan the ability to serve as a potential nephroprotective agent against carboplatin nephrotoxicity (Sherif et al., 2015).
Coenzyme Q10 (CoQ10) is present in most eukaryotic cells, mainly in the mitochondria. It is a component of the electron transport chain and participates in energy production. It is widely used for management of a variety of diseases including heart disease, cancer and statin myopathy (Sarmiento et al., 2016). This wide range of uses was attributed to its potent antioxidant and anti-inflammatory properties. CoQ10 was reported to inhibit lipid peroxidation and suppress the expression of the proinflammatory cytokines (Mirmalek et al., 2016). These properties together with the ability of CoQ10 to restore mitochondrial functions may protect against carboplatin nephrotoxicity (Fatima et al., 2016). The aim of this work was to study the effect of candesartan and/or CoQ10 on carboplatin-induced nephrotoxicity in mice.
Materials and Methods
Drugs and chemicals
Carboplatin, candesartan and CoQ10 were purchased from Sigma-Aldrich Company (St. Louis, MO, USA). Carboxymethyl cellulose (CMC) was obtained from Sigma Pharmaceutical Company, Quesna, Egypt. Carboplatin was dissolved in distilled water. Both candesartan and CoQ10 were suspended in 1% CMC solution.
Animals and groups
In this study, we used sixty male Balb/c mice weighing about 18–25 grams. All the experiments were conducted according to the National Research Council’s guidelines. Animal handling was followed according to Helsinki declaration of animal ethics. Mice were randomized into six equal groups (Ten mice/group) as follows:
Group I: Control untreated group.
Group II: Received a single intraperitoneal injection of carboplatin in a dose of 100 mg/kg (Chen et al., 2010).
Group III: Received candesartan in a dose of 5 mg/kg once daily orally by gastric tube one week before and continued for one week after carboplatin injection (Callera et al., 2016).
Group IV: Received CoQ10 in a dose of 200 mg/kg once daily orally by gastric tube one week before and continued for one week after carboplatin injection (Cleren et al., 2008).
Group V: Vehicle control group, received CMC once daily orally by gastric tube one week before and continued for one week after carboplatin injection.
Group VI: Received candesartan in a dose of 5 mg/kg concomitantly with CoQ10 in a dose of 200 mg/kg once daily orally by gastric tube one week before and continued for one week after carboplatin injection.
Determination of renal function tests
One week after carboplatin injection, mice were kept in special metabolic cages for 24 hours urine collection. Urine samples were centrifuged at 1,400 rpm for 5 minutes after proper dilution and the supernatant was collected. Twenty-four hours urinary protein levels were measured using a colorimetric method described by Fujita et al., (1983). The urinary albumin excretion rate (UAER) was measured using the following formula:
UAER (mg/24 h) = 24 h total volume of urine (L) X Urinary protein levels (mg/L)
Urinary N-acetyl beta-D-glucosaminidase (NAG) activity was determined according to the method of Price (1979). Urinary gamma glutamyl transpeptidase (GGT) activity was measured according to the method of Szasz (1969).
After an overnight fasting, blood samples were taken from the retro-orbital sinus of mice. Then, serum was separated by centrifugation for 20 minutes at 3000 rpm. Serum was used to estimate blood urea according to Patton and Crouch (1977). Serum and urinary creatinine were measured according to Henry (1974). Creatinine clearance was calculated by applying the following formula (Cockroft and Gault, 1976):

Mice were killed by decerebration and both kidneys were removed and weighed for determination of the kidney weight/body weight ratio. The right kidney was homogenized and centrifuged at 2000 rpm, 4ºC for 15 min. The supernatant was used for determination of tissue biochemical parameters.
Determination of renal tissue antioxidant enzymes
The supernatant was used for determination of tissue catalase (CAT) according to Aebi method (1984) and tissue glutathione reductase (GR) using kits supplied by Sigma-Aldrich Company, St. Louis, MO, USA according to the instructions of the manufacturer.
Determination of renal tissue transforming growth factor beta 1 (TGF-β1), tumor necrosis factor alpha (TNF-α) and interleukin 6 (IL-6)
TGF-β1 was determined using kits supplied by Uscn Life Science Inc. Wuhan, according to the instructions of the manufacturer. TNF-α was determined by using mouse TNF-α ELISA kits of Ray Biotech, Inc. according to the instructions of the manufacturer. IL-6 was measured using ELISA kits supplied by Sigma Aldrich Co. according to the instructions of the manufacturer.
Assessment of renal tissue nuclear factor (erythroid-derived 2)-like 2 (Nrf2) and heme oxygenase-1 (HO-1) content
Tissue Nrf2 and HO-1 contents were measured using ELISA kits purchased from Cloud clone, Uscn Life Science, INC. USA, according to the instructions of the manufacturer.
Assessment of the mitochondrial complex I activity
A piece of the renal tissue was held in a special medium (10.94 g sucrose, 1.21 g Tris–HCl, 0.38 g EGTA, 100 ml distilled water, pH 7.8). Homogenization was done in 9 volumes of this cold medium with three or four strokes. Then, the homogenate was centrifuged at 700 × g for 10 min at 4 °C, the supernatant was centrifuged for 20 min at 1,000 × g to obtain mitochondria pellets that were washed with the previous collecting buffer to remove contamination. Then, mitochondria were resuspended in 9 volumes of the collecting buffer. Mitochondrial proteins were determined using Lowry method (1951). Mitochondrial complex I activity was measured according to Birch-Machin et al. (1994).
Determination of renal tissue caspase-3 activity
A piece of the renal tissue was homogenized and proteins were extracted and stored at -80 °C. The activity of caspase-3 in the renal tissues was determined according to Gurtu et al. (1997).
Histopathological examination
The left kidney was rinsed with ice-cold saline and fixed in 10% neutral-buffered formalin, embedded in paraffin wax, sectioned and stained with hematoxylin and eosin to evaluate the extent of renal injury. Apoptotic indices were determined according to the morphological criteria of Staunton and Gaffney (1995).
Immunohistochemical analysis of nuclear factor kappa-B (NF-kB) (p65) in the renal tissues
This was carried out using a sensitive Dako EnVision+ System (Dako Cytomation, Carpentaria, CA) according to the manufacturer’s instructions. These sections were examined by two pathologists independently using an inverted Olympus BH2 microscope (Olympus America Inc., Melville, NY) and images were acquired with Image Proplus software (Media Cybernetics, Carlsbad, CA), digitally scored on a computer. The intensity of staining was graded and assigned a score on a scale from 0 to 3, where 0 indicated negative immunostaining, 1 indicated weak immunostaining, 2 indicated moderate immunostaining, and 3 indicated strong immunostaining (Gupta et al., 2003). Calculation of the percentage of the positive staining was carried out using IHC profiler tool in image J software (1.49v) National Institute of Health, USA (Varghese et al., 2014).
Statistical analysis
The statistical analysis was performed using the statistical package for the social sciences (SPSS) version 18.0 (IBM Corp., Armonk, NY, USA). Multiple comparisons were performed using multiple measures analysis of variance (ANOVA) followed by Fisher LSD test. Data were presented as mean ± standard error of mean (S.E.M.). Differences between the means of the different groups were considered significant when p-value is less than 0.05.
Results
Effect of different treatments on the kidney weight/body weight ratio
Carboplatin administration resulted in significant increase in kidney weight/body weight ratio compared to the control group. Administration of CMC induced non-significant effect on kidney weight/body weight ratio compared to carboplatin group. Administration of candesartan or CoQ10 induced significant decrease in kidney weight/body weight ratio compared to carboplatin group. This decrease was significant with candesartan/CoQ10 combination group compared to the use of each of these drugs alone (Table 1).
Table 1.
Effect of Different Treatments on the Kidney Weight/Body Weight Ratio, Blood Urea, Serum Creatinine, Creatinine Clearance, UAER, Urinary NAG and Urinary GGT in the Studied Groups
| Control | Carboplatin | Carboplatin+ Candesartan | Carboplatin + CoQ10 | Carboplatin + CMC | Carboplatin + Candesartan+CoQ10 | F-value | df | |
|---|---|---|---|---|---|---|---|---|
| Kidney weight/body weight ratio | 6.54±0.2 | 20.6±0.58a | 13.01 ±0.54b | 17.39±0.47b | 19.37±0.45 | 9.18±0.15bcd | 174.34 | 51 |
| Blood urea (mg/dl) | 19.2 ± 0.54 | 74.32± 3.13a | 41.17±0.66b | 49.12±0.91b | 71.51±2.16 | 31.89 ±0.64bcd | 193.65 | 51 |
| Serum creatinine (mg/dl) | 0.52 ± 0.01 | 2.75 ± 0.06a | 1.82±0.04b | 1.98±0.04b | 2.83±0.07 | 1.32±0.03bcd | 107.21 | 51 |
| Creatinine clearance (ml/min) | 1.25 ± 0.04 | 0.43 ± 0.03a | 0.82±0.05b | 0.78±0.04b | 0.41±0.02 | 1.06±0.04bcd | 81.78 | 51 |
| Urinary GGT (U/L) | 821.34±22.8 | 2132 ±41.2a | 1435.2±31.2b | 1489.4±29.7b | 2214.2±46.1 | 1067.6±25.1bcd | 121.18 | 51 |
| Urinary NAG (U/L) | 0.22 ± 0.01 | 1.27 ± 0.04a | 0.68±0.03b | 0.73±0.03b | 1.31±0.05 | 0.41±0.02bcd | 273.80 | 51 |
| UAER (mg/day) | 3.08±0.18 | 13.17 ±0.53a | 6.81 ±0.43b | 7.34±0.48b | 12.95±0.5 | 4.23±0.21bcd | 94.01 | 51 |
Significant compared to the control group (p-value less than 0.05);
Significant compared to carboplatin group (p-value less than 0.05);
Significant compared to carboplatin + candesartan group (p-value less than 0.05);
Significant compared to carboplatin + CoQ10 group (p-value less than 0.05).
Effect of different treatments on the renal function tests
Carboplatin induced significant increase in blood urea, serum creatinine, urinary GGT, urinary NAG and UAER associated with significant decrease in creatinine clearance compared to the control group. Administration of CMC induced non-significant effect on the above-mentioned parameters compared to carboplatin group. Administration of candesartan or CoQ10 induced significant decrease in blood urea, serum creatinine, urinary GGT, urinary NAG and UAER associated with significant increase in creatinine clearance compared to carboplatin group. These changes were significant with candesartan/CoQ10 combination group compared to the use of each of these drugs alone (Table 1).
Effect of different treatments on renal tissue CAT and GR
Carboplatin induced significant decrease in tissue CAT and GR compared to the control group. Administration of CMC induced non-significant effect on the above-mentioned parameters compared to carboplatin group. Administration of candesartan or CoQ10 induced significant increase in tissue CAT and GR compared to carboplatin group. These changes were significant with candesartan/CoQ10 combination group compared to the use of each of these drugs alone (Table 2).
Table 2.
Effect of Different Treatments on Renal Tissue CAT, GR, Tissue HO-1, Nrf2 and Mitochondrial Complex I Activity in the Studied Groups
| Control | Carboplatin | Carboplatin+ Candesartan | Carboplatin + CoQ10 | Carboplatin + CMC | Carboplatin + Candesartan+CoQ10 | F-value | df | |
|---|---|---|---|---|---|---|---|---|
| Tissue CAT (U/mg tissue) | 29.2±0.4 | 13.54±0.19 a | 20.3±0.3 b | 18.9±0.28 b | 12.21±0.18 | 25.1±0.38 bcd | 71.93 | 51 |
| Tissue GR (U/g tissue) | 849.3±12.9 | 441.2±7.3 a | 652.4±8.74 b | 569.8±8.5 b | 445.5±7.72 | 743.55±10.3 bcd | 267.04 | 51 |
| Tissue HO-1 (ng/mg protein) | 0.32±0.02 | 0.14±0.01a | 0.21±0.01 b | 0.19±0.01 b | 0.13±0.01 | 0.26±0.01 bcd | 94.01 | 51 |
| Tissue Nrf2 (x10-1 ng/mg protein) | 0.27±0.02 | 0.11±0.01a | 0.19±0.01 b | 0.18±0.01 b | 0.12±0.01 | 0.23±0.02 bcd | 81.4 | 51 |
| Mitochondrial complex I activity (nmol NADH/min/mg protein) | 92.4±2.5 | 34.12±1.23 a | 48.6±1.7 b | 59.32±1.9 b | 37.4±1.42 | 72.35±2.2 bcd | 111.9 | 51 |
Significant compared to the control group (p-value less than 0.05);
Significant compared to carboplatin group (p-value less than 0.05);
Significant compared to carboplatin + candesartan group (p-value less than 0.05);
Significant compared to carboplatin + CoQ10 group (p-value less than 0.05),
Effect of different treatments on renal tissue Nrf2 and HO-1 content and mitochondrial complex I activity
Carboplatin administration resulted in significant decrease in renal tissue Nrf2 and HO-1 content and mitochondrial complex I activity compared to the control group. Administration of CMC induced non-significant effect on these parameters compared to carboplatin group. Administration of candesartan or CoQ10 induced significant increase in the renal tissue Nrf2 and HO-1 content and mitochondrial complex I activity compared to carboplatin group. These changes were significant with candesartan/CoQ10 combination group compared to the use of each of these drugs alone (Table 2).
Effect of different treatments on renal tissue TGF-β1, TNF-α and IL-6
Carboplatin resulted in significant increase in renal tissue TGF-β1, TNF-α and IL-6 compared to the control group. Administration of CMC induced non-significant effect on these parameters compared to carboplatin group. Candesartan or CoQ10 induced significant decrease in renal tissue TGF-β1, TNF-α and IL-6 compared to carboplatin group. This decrease was significant with candesartan/CoQ10 combination group compared to the use of each of these drugs alone (Table 3).
Table 3.
Effect of Different Treatments on Renal Tissue TNF-Α, IL-6, TGF-Β1, Caspases 3 Activity and Apoptotic Index in the Studied Groups
| Control | Carboplatin | Carboplatin + Candesartan | Carboplatin + CoQ10 | Carboplatin + CMC | Carboplatin + Candesartan+ CoQ10 | F-value | df | |
|---|---|---|---|---|---|---|---|---|
| Tissue TNF-α (pg/ mg protein) | 40.5± 6.4 | 654.3±11.2a | 432.8±9.3b | 468.2±9.8b | 668.1±10.7 | 251.6±7.4bcd | 233.32 | 51 |
| Tissue IL-6 (pg/ mg protein) | 234.1±4.32 | 892.13±14.4 a | 597.2±9.1 b | 656.4±9.3 b | 899.62±12.3 | 390.3±6.4bcd | 144.17 | 51 |
| Tissue TGF-β1 (pg/ mg protein) | 21.83±1.07 | 73.6±4.2 a | 55.12±2.8b | 60.01±2.9 b | 71.5±4.5 | 41.3±1.3bcd | 99.07 | 51 |
| Tissue caspases 3 activity (nmol/mg protein/min) | 4.32±0.06 | 11.34±0.21 a | 7.81±0.09 b | 8.14±0.11 b | 10.94±0.19 | 6.67±0.08 bcd | 45.06 | 51 |
| Apoptotic index (%) | 4.11±0.05 | 8.44±0.12 a | 6.23±0.08 b | 6.56±0.09 b | 8.25±0.11 | 5.37±0.07 bcd | 48.53 | 51 |
Values were represented as mean ± S.E.M;
Significant compared to the control group (p-value less than 0.05);
Significant compared to carboplatin group (p-value less than 0.05);
Significant compared to carboplatin + candesartan group (p-value less than 0.05);
Significant compared to carboplatin + CoQ10 group (p-value less than 0.05).
Histopathological and immunohistochemical results
Administration of carboplatin resulted in diffuse tubular degeneration, diffuse inflammatory cellular infiltration and severe interstitial hemorrhage (Figure 1b) associated with significant increase in the apoptotic index and caspase-3 expression (Table 3) and significant increase in positive immunostaining for NF-κB (p65) (Figure 2b, 3) compared to the control untreated group. CMC induced non-significant effect on the histopathological or immunohistochemical picture compared to carboplatin-treated group (Figure 1c, 2c, 3). Treatment with either candesartan or CoQ10 resulted in significant decrease in the number of infiltrated cells, hemorrhage, tubular degeneration and necrosis (Figure. 1d,e) with significant decrease in the apoptotic index and caspase-3 expression (Table 3) and significant decrease in NF-κB (p65) immunostaining (Figure 2d, e, Figure 3) compared to carboplatin group. Candesartan/CoQ10 combination resulted in minimal cellular infiltration, minimal haemorrhage and mild tubular necrosis (Figure. 1f) with significant decrease in the apoptotic index and caspase-3 expression (Table 3) and significant decrease in NF-κB (p65) immunostaining (Figure 2f, 3) compared to the use of each of these drugs alone.
Figure 1.
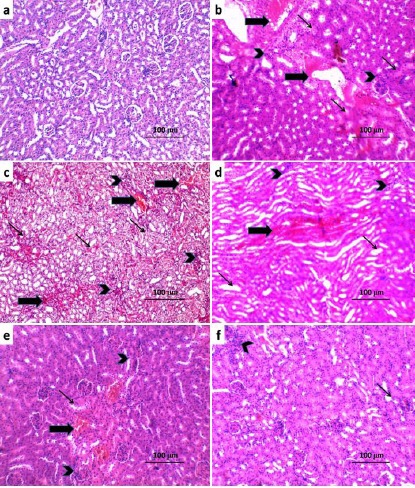
Sections from the Kidney (H and E x400) of a) Control Group with Normal Appearance of the Glomeruli, Tubules and Interstitium.; b) Carboplatin group showing diffuse tubular degeneration (↑), diffuse inflammatory cellular infiltration (Arrow head) and severe interstitial haemorrhage (↑); c) Carboplatin+CMC group showing non-significant effect on the histopathological picture compared to carboplatin group; d) Carboplatin + Candesartan group showing significant decrease in the number of infiltrated cells (Arrow head), haemorrhage (↑) and tubular degeneration (↑); e) Carboplatin + CoQ10 group showing mild inflammatory cellular infiltration (Arrow head), minimal haemorrhage (↑) and mild tubular degeneration (↑); f) Carboplatin + Candesartan + CoQ10 group showing minimal cellular infiltration (Arrow head), minimal haemorrhage (↑) and mild tubular degeneration (↑).
Figure 2.
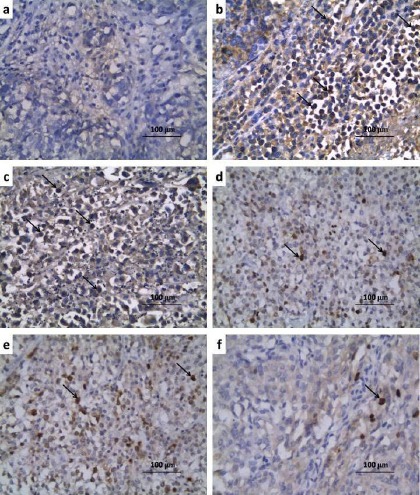
Renal Sections of Immunohistochemical Staining of NF-κB (p65) (x400) of a) Control Group Showing Minimal Positive Staining for NF-κB (p65); b) Carboplatin group showing strong positive staining for NF-κB (p65) (↑); c) Carboplatin + CMC group showing strong positive staining for NF-κB (p65) (↑);d) Carboplatin + Candesartan group showing moderate positive staining for NF-κB (p65) (↑);e) Carboplatin + CoQ10 group revealing moderate positive staining for NF-κB (p65) (↑); f) Carboplatin + Candesartan + CoQ10 group showing weak positive staining for NF-κB (p65) (↑).
Figure 3.
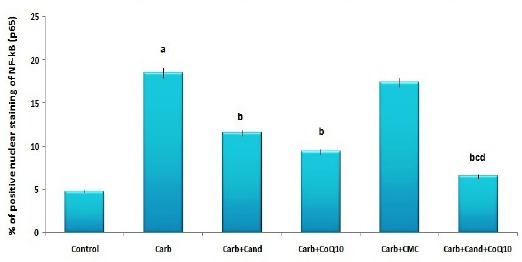
Percentage of Positive Nuclear Staining of NF-κB (p65) (Mean±S.E.M.).a, Significant compared to the control group; b, Significant compared to carboplatin group; c, Significant compared to carboplatin + candesartan group; d, Significant compared to carboplatin + CoQ10 group.
Discussion
Oxidative stress had been suggested to play a role in carboplatin nephrotoxicity (Desai et al., 2016; Hosohata, 2016). Experimental findings suggested that free radicals and reactive oxygen species (ROS) are involved in carboplatin nephrotoxicity (Kishore Reddy et al., 2010). They demonstrated that the activities of the antioxidant enzymes had been reduced in carboplatin treated animals compared to the control group which agreed with the results of the present study. These observations prove that the mechanism of carboplatin nephrotoxicity is strongly related to depletion of renal antioxidant defense mechanisms (Hosohata, 2016).
Recent studies reported that candesartan has potent antioxidant properties that may ameliorate chemotherapy-induced nephrotoxicity (Emam et al., 2013; Sherif et al., 2015). Candesartan was proven to decrease ROS production and enhance the activity of the antioxidant enzymes. Moreover, Nishida et al. (2011) reported that candesartan may improve renal functions, possibly through its effect on lipid peroxidation. Also, Luo et al., (2015) suggested that treatment with antioxidant agents that affect the rennin-angiotensin system (RAS) may resolve the imbalance of renal RAS created by oxidative stress. This was in agreement with the present study where candesartan induced significant increase in tissue CAT and GR compared to carboplatin group.
In the present study, CoQ10 induced significant increase in renal tissue antioxidant enzymes compared to the group that received carboplatin alone. This was in accordance with Sawicka et al., (2013) and Fatima et al., (2016) who suggested that CoQ10 may act as free radical scavenger and enhance the expression of the antioxidant enzymes. Moreover, Sawicka et al., (2013) suggested that CoQ10 can ameliorate platinum-induced nephrotoxicity through inhibition of lipid peroxidation and nitrosative stress.
Nrf2 is a redox sensing transcriptional factor that may play a crucial role in regulation of oxidant/anti-oxidant balance in various types of kidney diseases. Also, Nrf2 may have significant anti-inflammatory effects, possibly through suppression of TGF-β1, TNF-α and IL-6 expression (Choi et al., 2014). Zhu et al., (2017) attributed platinum-induced nephrotoxicity to suppression of Nrf2 expression which in turn suppresses the activity of the antioxidant enzymes and increases NF-κB expression which activate the inflammatory cascade and induce apoptosis.
Heme oxygenase (HO) is a rate limiting enzyme that regulates the oxidant and the inflammatory processes frequently encountered in platinum-induced nephrotoxicity (Kilic et al., 2013). Bolisetty et al., (2016) reported that deletion of HO-1 in the proximal tubules aggravates damage during platinum nephrotoxicity, possibly through increased ROS production and enhanced caspase-3 expression. Kilic et al., (2013) reported that platinum containing compounds may modulate Nrf2/HO-1 pathway leading to serious nephrotoxicity. In agreement with these studies, carboplatin in the present study significantly suppressed renal Nrf2/HO-1 expression which was associated with significant inhibition of the activity of the antioxidant enzymes and significant increase in the expression of the pro-inflammatory cytokines and the apoptotic markers resulting in significant nephrotoxicity.
In the present study, candesartan induced significant increase in Nrf2/HO-1 content compared to carboplatin group. This was in the same line with Ruiz et al., (2013) who reported that agents that affect angiotensin II receptors, such as candesartan, can modulate the Nrf2 expression which in turn may meliorate oxidative stress and inflammation in a wide range of kidney diseases. Also, Artham et al., (2015) found that candesartan can increase HO-1 production which may confer potent antioxidant and anti-inflammatory mechanisms.
In our study, CoQ10 was found to affect Nrf2/HO-1 pathway which may ameliorate carboplatin nephrotoxicity. This was in agreement with Khodir et al., (2017) who attributed the antioxidant, anti-inflammatory and anti-apoptotic properties of CoQ10 to its effect on Nrf2/HO-1 pathway. Pala et al., (2016) reported that the cytoprotective effect of CoQ10 is due to modulation of the expression of NF-κB, Nrf2 and HO-1, indicating the anti-inflammatory effect of CoQ10 and emphasizing its role in the antioxidant defenses.
There is increasing evidence that supports the role of inflammation in the pathophysiology of carboplatin nephrotoxicity. Chen et al., (2010) reported that carboplatin increases the expression of TGF-β1 which in turn enhances the production of the proinflammatory cytokines such as TNF-α and IL-6 and increases NF-κB expression which was in agreement with the results of the present study. Moreover, Freudlsperger et al., (2013) found that there is cross-talk between TGF-β1 and NF-κB signaling pathways which may play a crucial role in carboplatin nephrotoxicity.
Sherif et al., (2015) reported that candesartan decreases the concentration of TGF-β1, TNF-α and monocyte chemoattractant protein-1 in the renal tissues and suppresses NF-κB expression which is the cornerstone in the inflammatory process induced by platinum compounds. This was in context with the results of our study where candesartan induced significant decrease in tissue TGF-β1, TNF-α, IL-6 and NF-κB expression compared to carboplatin group.
Fatima et al., (2016) reported that CoQ10 may attenuate platinum-induced nephrotoxicity via suppression of the expression of the pro-inflammatory cytokines. Granata et al., (2015) found that CoQ10 can reduce the expression of NF-κB, TGF-β-1 and related transcription factors which may ameliorate inflammation in the renal tissues. These studies were in the same line with the results of our study where CoQ10 induced significant decrease in tissue TGF-β1, TNF-α, IL-6 and NF-κB expression compared to carboplatin group.
Recent studies found that platinum-induced nephrotoxicity is largely mediated through mitochondrial dysfunction (Inapurapu et al., 2017). This may be stimulated by DNA damage, pro-apoptotic protein attack and oxidative stress leading to inhibition of the activity of the mitochondrial complexes (Yang et al., 2014). This was in the same line with the results of our study where carboplatin induced significant decrease in mitochondrial complex I activity compared to the control group. This decrease was ameliorated by the use of candesartan and/or CoQ10. This may be attributed to the ability of candesartan to antagonize the harmful effects of angiotensin II on mitochondrial functions (Sherif et al., 2015). Also, Astolfi et al., (2016) reported that CoQ10 can improve platinum-induced cytotoxicity through restoration of the functions of the mitochondrial complexes.
Recent studies reported that oxidative stress leads to activation of caspase-3 which was proven to play an important role in tubular necrosis frequently encountered in carboplatin nephrotoxicity (Sue et al., 2014). Krüger et al., (2015) reported that carboplatin induces the expression of caspase 3 and caspase 7 which in turn causes DNA damage of renal tubular cells leading to apoptosis of renal tissues. This was in the same line with our study where carboplatin resulted in significant increase in the apoptotic index and caspase-3 expression compared to the control group.
Sherif et al., (2015) attributed the renoprotective effects of candesartan in platinum-induced nephrotoxicity to its potent anti-apoptotic properties. They reported that candesartan induces significant decrease in gene expression of caspase-3. Lv et al., (2009) documented that the antiapoptotic effects of candesartan were mediated through blocking the mesangial cell oxidative stress and apoptosis induced by angiotensin II. This was in agreement with the results of the present study where candesartan induced significant decrease in the apoptotic index and caspase-3 expression compared to carboplatin group.
In the present study, CoQ10 induced significant decrease in the apoptotic index and caspase-3 expression compared to carboplatin group. This was in accordance with El-Sheikh et al., (2012) who recorded significant antiapoptotic properties of CoQ10 manifested by significant decrease in caspase-3 expression. Papucci et al., (2003) reported that CoQ10 prevents apoptosis by inhibiting mitochondrial depolarization, attenuating ATP decrease, and preventing DNA fragmentation. Moreover, Li et al., (2014) found that CoQ10 inhibits nuclear translocation of apoptosis inducing factor and cell death caused by inhibition of mitochondrial complex I activity.
In the present study, candesartan/CoQ10 combination induced significant improvement in the renal functions with significant increase in Nrf2/HO-1 content associated with significant decrease in the inflammatory mediators compared to the use of each of these drugs alone. This may be attributed to their combined antioxidant and anti-inflammatory effects together with their ability to inhibit apoptosis of renal cells. Tsuneki et al., (2013) showed that CoQ10 prevented the actions of angiotensin II including decreased production of ROS. Moreover, CoQ10 prevented angiotensin II-induced upregulation of intercellular adhesion molecule 1 and vascular cell adhesion molecule 1 and inhibited their adhesion to the inflammatory cells. In addition, CoQ10 was proven to decrease renin and aldosterone concentrations which in turn will prevent renal vasoconstriction induced by activation of the RAS during renal injury (Carrasco et al., 2014). In conclusion, candesartan/CoQ10 combination might represent a beneficial therapeutic modality for amelioration of carboplatin-induced nephrotoxicity.
Conflict of interest
The authors declare that there is no conflict of interest.
Acknowledgements
Many thanks to Dr. Mohamed El Rashidy, Pathology Department, Faculty of Medicine, Tanta University, Egypt, for his kind help in the histopathological study.
References
- Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–6. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- Artham S, Fouda AY, El-Remessy AB, Fagan SC. Vascular protective effects of Angiotensin Receptor Blockers: Beyond Blood pressure. Receptors Clin Investig. 2015;2:e774. doi: 10.14800/rci.774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astolfi L, Simoni E, Valente F, et al. Coenzyme Q10 plus multivitamin treatment prevents cisplatin ototoxicity in rats. PLoS One. 2016;11:e0162106. doi: 10.1371/journal.pone.0162106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birch-Machin MA, Briggs HL, Saborido AA, Bindoff LA, Turnbull DM. An evaluation of the measurement of the activities of complexes I–IV in the respiratory chain of human skeletal muscle mitochondria. Biochem Med Metab Biol. 1994;51:35–42. doi: 10.1006/bmmb.1994.1004. [DOI] [PubMed] [Google Scholar]
- Bolisetty S, Traylor A, Joseph R, Zarjou A, Agarwal A. Proximal tubule-targeted heme oxygenase-1 in cisplatin-induced acute kidney injury. Am J Physiol Renal Physiol. 2016;310:385–94. doi: 10.1152/ajprenal.00335.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callera GE, Antunes TT, Correa JW, et al. Differential renal effects of candesartan at high and ultra-high doses in diabetic mice-potential role of the ACE2/AT2R/Mas axis. Biosci Rep. 2016;36:e00398. doi: 10.1042/BSR20160344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrasco J, Anglada FJ, Campos JP, et al. The protective role of coenzyme Q10 in renal injury associated with extracorporeal shockwave lithotripsy: a randomised, placebo-controlled clinical trial. BJU Int. 2014;113:942–50. doi: 10.1111/bju.12485. [DOI] [PubMed] [Google Scholar]
- Chen H-H, Chen T-W, Lin H. Pravastatin attenuates carboplatin-induced nephrotoxicity in rodents via peroxisome proliferator-activated receptor-regulated heme oxygenase-1. Mol Pharmacol. 2010;78:36–45. doi: 10.1124/mol.109.061101. [DOI] [PubMed] [Google Scholar]
- Choi BH, Kang KS, Kwak MK. Effect of redox modulating NRF2 activators on chronic kidney disease. Molecules. 2014;19:12727–59. doi: 10.3390/molecules190812727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleren C, Yang L, Lorenzo B, et al. Therapeutic effects of coenzyme Q10 (CoQ10) and reduced CoQ10 in the MPTP model of Parkinsonism. J Neurochem. 2008;104:1613–21. doi: 10.1111/j.1471-4159.2007.05097.x. [DOI] [PubMed] [Google Scholar]
- Cockroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephrol. 1976;16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- Desai AV, Heneghan MB, Li Y, et al. Toxicities of busulfan/melphalan versus carboplatin/etoposide/melphalan for high-dose chemotherapy with stem cell rescue for high-risk neuroblastoma. Bone Marrow Transplant. 2016;51:1204–10. doi: 10.1038/bmt.2016.84. [DOI] [PubMed] [Google Scholar]
- El-Sheikh AA, Morsy MA, Mahmoud MM, Rifaai RA, Abdelrahman AM. Effect of coenzyme-q10 on Doxorubicin-induced nephrotoxicity in rats. Adv Pharmacol Sci 2012. 2012:981461. doi: 10.1155/2012/981461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emam HT, Madboly AG, Shoman AA, Hussein NI. The possible protective role of candesartan on cyclosporine induced nephrotoxicity in rat. Med J Cairo Univ. 2013;81:271–8. [Google Scholar]
- Fatima S, Al-Mohaimeed N, Al-Shaikh Y, et al. Combined treatment of epigallocatechin gallate and Coenzyme Q10 attenuates cisplatin-induced nephrotoxicity via suppression of oxidative/nitrosative stress, inflammation and cellular damage. Food Chem Toxicol. 2016;94:213–20. doi: 10.1016/j.fct.2016.05.023. [DOI] [PubMed] [Google Scholar]
- Freudlsperger C, Bian Y, Contag Wise S, et al. TGF-βand NF-κB signal pathway cross-talk is mediated through TAK1 and SMAD7 in a subset of head and neck cancers. Oncogene. 2013;32:1549–59. doi: 10.1038/onc.2012.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita Y, Mori S, Kitano S. Color reaction between pyrogallol red-molybdenum (VI) complex and protein. Bunseki Kagaku. 1983;32:379–86. [Google Scholar]
- Granata S, Dalla Gassa A, Tomei P, Lupo A, Zaza G. Mitochondria: a new therapeutic target in chronic kidney disease. Nutr Metab. 2015;12:49. doi: 10.1186/s12986-015-0044-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S, Hussain T, MacLennan GT, et al. Differential expression of S100A2 and S100A4 during progression of human prostate adenocarcinoma. J Clin Oncol. 2003;21:106–12. doi: 10.1200/JCO.2003.03.024. [DOI] [PubMed] [Google Scholar]
- Gurtu V, Kain SR, Zhang G. Fluorometric and colorimetric detection of caspase activity associated with apoptosis. Anal Biochem. 1997;251:98–102. doi: 10.1006/abio.1997.2220. [DOI] [PubMed] [Google Scholar]
- Henry RJ. Clinical chemistry principles and techniques. 2nd ed. Hagerstown, MD, USA: Harper and Row; 1974. Determination of serum creatinine; p. 525. [Google Scholar]
- Ho GY, Woodward N, Coward JI. Cisplatin versus carboplatin: comparative review of therapeutic management in solid malignancies. Crit Rev Oncol Hematol. 2016;102:37–46. doi: 10.1016/j.critrevonc.2016.03.014. [DOI] [PubMed] [Google Scholar]
- Hosohata K. Masuda S, editor. Role of oxidative stress in drug-induced kidney injury. Int J Mol Sci. 2016;17:1826. doi: 10.3390/ijms17111826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inapurapu S, priya Kudle KR, Bodiga S, Bodiga VL. Cisplatin cytotoxicity is dependent on mitochondrial respiration in Saccharomyces cerevisiae. Iran J Basic Med Sci. 2017;20:83–9. doi: 10.22038/ijbms.2017.8099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khodir AE, Atef H, Said E, ElKashef HA, Salem HA. Implication of Nrf2/HO-1 pathway in the coloprotective effect of coenzyme Q10 against experimentally induced ulcerative colitis. Inflammopharmacol. 2017;25:119–35. doi: 10.1007/s10787-016-0305-0. [DOI] [PubMed] [Google Scholar]
- Kilic U, Kilic E, Tuzcu Z, et al. Melatonin suppresses cisplatin-induced nephrotoxicity via activation of Nrf-2/HO-1 pathway. Nutr Metabol. 2013;10:7. doi: 10.1186/1743-7075-10-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishore Reddy YV, Sreenivasula Reddy P, Shivalingam MR. Cisplatin or carboplatin caused suppression in anti–oxidant enzyme defense system in liver, kidney and testis of male albino rats. J Biomed Sci Res. 2010;2:23–8. [Google Scholar]
- Krüger K, Thomale J, Stojanović N, et al. Platinum-induced kidney damage: Unraveling the DNA damage response (DDR) of renal tubular epithelial and glomerular endothelial cells following platinum injury. Biochim Biophys Acta. 2015;1853:685–98. doi: 10.1016/j.bbamcr.2014.12.033. [DOI] [PubMed] [Google Scholar]
- Li H, Chen G, Ma W, Li P-AA. Water-soluble coenzyme Q10 inhibits nuclear translocation of apoptosis inducing factor and cell death caused by mitochondrial complex I inhibition. Int J Mol Sci. 2014;15:13388–400. doi: 10.3390/ijms150813388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with Folin phenol reagent. J Biol Chem. 1951;193:265–75. [PubMed] [Google Scholar]
- Luo H, Wang X, Chen C, et al. Oxidative stress causes imbalance of renal renin angiotensin system (RAS) components and hypertension in obese zucker rats. J Am Heart Assoc. 2015;4:e001559. doi: 10.1161/JAHA.114.001559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv J, Jia R, Yang D, Zhu J, Ding G. Candesartan attenuates Angiotensin II-induced mesangial cell apoptosis via TLR4/MyD88 pathway. Biochem Biophys Res Commun. 2009;380:81–6. doi: 10.1016/j.bbrc.2009.01.035. [DOI] [PubMed] [Google Scholar]
- Mirmalek SA, Gholamrezaei Boushehrinejad A, Yavari H, et al. Antioxidant and anti-inflammatory effects of coenzyme Q10 on L-arginine-induced acute pancreatitis in rat. Oxid Med Cell Longev. 2016;2016:5818479. doi: 10.1155/2016/5818479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon IJ, Kim KR, Chu H-S, et al. N-Acetylcysteine and N-nitroarginine methyl ester attenuate carboplatin-induced ototoxicity in dissociated spiral ganglion neuron cultures. Clin Exp Otorhinolaryngol. 2011;4:11–7. doi: 10.3342/ceo.2011.4.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishida Y, Takahashi Y, Nakayama T, Soma M, Asai S. Comparative effect of olmesartan and candesartan on lipid metabolism and renal function in patients with hypertension: a retrospective observational study. Cardiovasc Diabetol. 2011;10:74. doi: 10.1186/1475-2840-10-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozbek E. Induction of oxidative stress in Kidney. Int J Nephrol 2012. 2012:465897. doi: 10.1155/2012/465897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pala R, Orhan C, Tuzcu M, et al. Coenzyme Q10 supplementation modulates NFκB and Nrf2 pathways in exercise training. J Sports Sci Med. 2016;15:196–203. [PMC free article] [PubMed] [Google Scholar]
- Papucci L, Schiavone N, Witort E, et al. Coenzyme Q10 prevents apoptosis by inhibiting mitochondrial depolarization independently of its free radical scavenging property. J Biol Chem. 2003;278:28220–8. doi: 10.1074/jbc.M302297200. [DOI] [PubMed] [Google Scholar]
- Patton CJ, Crouch SR. Spectrophotometric and kinetic investigation of the Berthelot reaction for the determination of ammonia. Anal Chem. 1977;49:464–9. [Google Scholar]
- Price RJ. Urinary N-acetyl-â-D-glucosaminidase (NAG) as an indicator of renal disease. Curr Probl Clin Biochem. 1979;9:150–63. [PubMed] [Google Scholar]
- Ruiz S, Pergola PE, Zager RA, Vaziri ND. Targeting the transcription factor Nrf2 to ameliorate oxidative stress and inflammation in chronic kidney disease. Kidney Int. 2013;83:1029–41. doi: 10.1038/ki.2012.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarmiento A, Diaz-Castro J, Pulido-Moran M, et al. Coenzyme Q10 supplementation and exercise in healthy humans: A systematic review. Curr Drug Metab. 2016;17:345–58. doi: 10.2174/1389200216666151103115654. [DOI] [PubMed] [Google Scholar]
- Sawicka E, Długosz A, Rembacz KP, Guzik A. The effects of coenzyme Q10 and baicalin in cisplatin-induced lipid peroxidation and nitrosative stress. Acta Pol Pharm. 2013;70:977–85. [PubMed] [Google Scholar]
- Sherif IO, Al-Mutabagani LA, Alnakhli AM, Sobh MA, Mohammed HE. Renoprotective effects of angiotensin receptor blocker and stem cells in acute kidney injury: Involvement of inflammatory and apoptotic markers. Exp Biol Med (Maywood) 2015;240:1572–9. doi: 10.1177/1535370215577582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staunton MJ, Gaffney EF. Tumor type is a determinant of susceptibility to apoptosis. Am J Clin Pathol. 1995;103:300–7. doi: 10.1093/ajcp/103.3.300. [DOI] [PubMed] [Google Scholar]
- Sue Y-M, Chou H-C, Chang C-C, et al. Singh SR, editor. L-Carnitine Protects against carboplatin-mediated renal injury: AMPK- and PPARα-dependent inactivation of NFAT3. PLoS One. 2014;9:e104079. doi: 10.1371/journal.pone.0104079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szasz G. A kinetic photometric method for serum γ-glutamyl transpeptidase. Clin Chem. 1969;15:124–36. [PubMed] [Google Scholar]
- Tsuneki H, Tokai E, Suzuki T, et al. Protective effects of coenzyme Q10 against angiotensin II-induced oxidative stress in human umbilical vein endothelial cells. Eur J Pharmacol. 2013;701:218–27. doi: 10.1016/j.ejphar.2012.12.027. [DOI] [PubMed] [Google Scholar]
- Varghese F, Bukhari AB, Malhotra R, De A. IHC Profiler: an open source plugin for the quantitative evaluation and automated scoring of immunohistochemistry images of human tissue samples. PLoS One. 2014;9:e96801. doi: 10.1371/journal.pone.0096801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Liu H, Liu F, Dong Z. Mitochondrial dysregulation and protection in cisplatin nephrotoxicity. Arch Toxicol. 2014;88:1249–56. doi: 10.1007/s00204-014-1239-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonezawa A. Platinum agent-induced nephrotoxicity via organic cation transport system. Yakugaku Zasshi. 2012;132:1281–5. doi: 10.1248/yakushi.12-00211. [DOI] [PubMed] [Google Scholar]
- Zhu X, Jiang X, Li A, Zhao Z, Li S. S-Allylmercaptocysteine attenuates cisplatin-induced nephrotoxicity through suppression of apoptosis, oxidative stress, and inflammation. Nutrients. 2017;9:166. [Google Scholar]


