Abstract
Backgrounds:
Cisplatin (CDDP) is a choice of anti-cancer drug for cancer chemotherapy with serious side effects such as nephrotoxicity. It seems that age is an important factor influencing the side effects of CDDP. This study was designed to determine the role of age and gender simultaneously in CDDP induced renal toxicity.
Methods:
40 Wistar male and female rats were assigned as 6 groups in 3 different age categories (10, 16, and 20 weeks old). The single dose of CDDP (7.5 mg/kg, ip) was administrated, and a week later measurements were performed.
Results:
Body weight changes in male (not in female) animals aged 16 and 20 weeks were more than 10 weeks old animals (P<0.05). In male rats, the serum levels of creatinine (Cr) and blood urea nitrogen (BUN), and Cr-clearance in aged 10 weeks, normalized kidney weight (KW) in aged 20 weeks, and serum nitrite, aspartate aminotransferase (AST) and malondialdehyde (MDA) levels and kidney tissue damage score (KTDS) in rats aged 16 weeks were significantly altered (P<0.05). Gender difference in serum level of Cr, BUN and nitrite, and Cr-clearance were observed in animals aged10 weeks (P<0.05).
Conclusion:
The side effects of CDDP are gender depended, and may be different at various ages.
Keywords: Cisplatin, cancer, age, gender, Rat
Introduction
Cisplatin (cis-diamminedichloroplatinum II, CDDP) is one of the most common anti-cancer drug in clinic which is accompanied with side effects of toxicity including nephrotoxicity, hepatotoxicity and ototoxicity. The induced nephrotoxicity is considered as an early drug side effect which limits the CDDP therapy. CDDP induced nephrotoxicity and the protective supplements against this side effect are gender related (Zamani et al., 2016; Ghayyoomi et al., 2015; El-Arabey AA., 2015; Naseem et al., 2015; Aydin et al., 2014; Nematbakhsh et al., 2013a; Nasri H., 2013; Eshraghi-Jazi et al., 2011; Haghigh et al., 2012; Pinches et al., 2012; Stakisaitis et al., 2010). The exact mechanism is not yet well understood, but it may not be related to female sex hormones (Pezeshki et al., 2013; Ghasemi et al., 2016). CDDP reduces glomerular filtration rate (GFR) (Daniel et al., 1997) while, it has been reported that male rats which were subjected to CDDP therapy excreted more sodium than female (Stakisaitis et al., 2010). On the other hand, Lu et al. reported that CDDP-induced toxicity in renal tubules was not sex- and age-related (Lu et al., 2005). Although, there are many studies related to sex- difference of CDDP, but the age related difference was less reflected. In this study, the role of age in CDDP induced nephrotoxicity was considered in rat model in connection with each gender.
Materials and Methods
40 Wistar male and female rats (purchased from Animal Center, Isfahan University of Medical Sciences, Isfahan, Iran, 231 ± 9.9 g) were assigned as 6 groups in 3 different categories of ages. The animals were kept under standard conditions and were fed with rat chow and water. All experiments were approved by the Isfahan University of Medical Sciences Ethics Committee. The experimental groups were as following:
Groups 1 (male, 219.7 ± 4.5 g, n=8) and 4 (female,169.0 ± 3.6 g, n=8): 10 weeks old rats.
Groups 2 (male, 292.0 ± 6.7 g, n=6) and 5 (female,176.2 ± 4.8 g, n=6): 16 weeks old rats.
Groups 3 (male, 348.2 ± 10.3 g, n=6) and 6 (female,193.8 ± 5.5 g, n=6): 20 weeks old rats.
The rats from all the groups received CDDP (7.5 mg/kg, ip, Mylan Pharmaceuticals, Inc) as a single dose, and one week later they were anesthetized with urethane (1.5 g/kg) and after abdominal incision a catheter was inserted in the bladder to collect the urine for 3 hours. Then blood sample from each rat was obtained, after sacrificing, kidneys were removed and weighted rapidly. The serum levels of creatinine (Cr) blood urea nitrogen (BUN), aspartate aminotransferase (AST), Alkaline phosphatase (ALP) and urine Cr level were determined using the quantitative diagnostic kits (Pars Azmoon, Iran). The serum levels of malondialdehyde (MDA) and nitrite (Griess Method) were quantified according to the manual methodology. The left kidney was fixed in 10% neutral formalin solution and was embedded in paraffin for the histopathological staining. The hematoxylin and eosin, staining was applied to examine the tubular damages. The tubular lesions were scored from 1 to 4 (in the form of a parameter named as kidney tissue damage score, KTDS) while the score of zero was assigned to normal tubules without damage. The Cr clearance was calculated based on renal clearance formula.
Data are presented as mean ± SEM. Analysis of variance (ANOVA) followed by LSD as the post hoc was applied to compare quantitative data between the groups, and the Student t-test was used to compare the data between genders. The Kruskal-Wallis and Mann-Whitney U tests were employed to compare the KTDS between the groups. P values <0.05 were considered statistically significant.
Results
The weight loss/gain induced by CDDP were 4.9±3.2, -15.1±4.9 and -13.5±5.1g in male, and -3.3±3.7, -7.8±4.8 and -8.8±4.3 g in female, respectively in animal aged 10, 16 and 20 weeks. The weight change in 16 and 20 weeks old male (not female) rats were significantly different from younger one (P<0.05). The serum levels of Cr and BUN, and Cr-clearance in male rats aged 10 weeks were significantly different from the others older rats, while such observations were not seen in female rats. The net kidney weight (KW) after one week of CDDP therapy were 1.65±0.03, 2.02±0.09 and 2.1±0.15g in male, and 1.36±0.05,1.52±0.06 and 1.49±0.05 in female aged 10, 16 and 20 weeks rats respectively. The KW in 16 and 20 weeks old male (not female) rats were significantly different from younger one (P<0.05) and KW was different between sexes in each age category (P<0.05), however different result was obtained when it normalized to body weight (Figure.1). The normalized KW in 20 weeks old male (not female) rats was significantly different from younger one (P<0.05). In addition, the normalized KW in male rats treated with CDDP was significantly different from the other gender (P<0.05). The serum nitrite, MDA and AST levels and KTDS in 16 weeks old male rats were statistically different from the rats in two other categories of ages (P<0.05). The results are presented in Figure 1. Actually both age and gender influenced in CDDP induced side effects.
Figure 1.
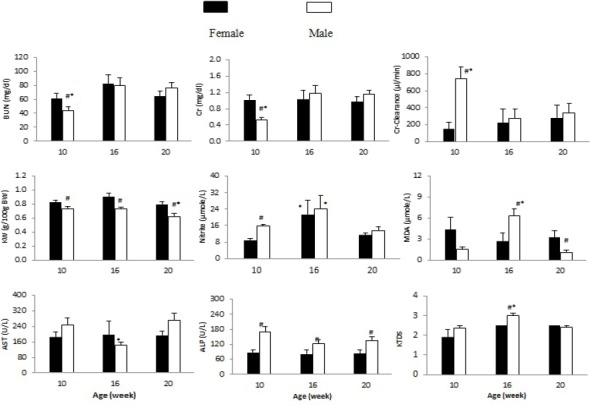
The Serum Levels of BUN, Cr, Nitrite, MDA, AST and ALT, and KW and KTDS in the Experimental Groups. * represent for significant difference from other age groups in similar gender (P<0.05) and # represent significant difference from male in similar group (comparison between males and females in each age range) (P<0.05).
Discussion
The major findings of this short study indicated that the serum levels of BUN and Cr, and Cr-clearance were age and gender related, and young male animals (10 weeks old) had the lower BUN and Cr and the higher Cr-clearance responses to CDDP administration. Opposite to this finding, it is reported that increased BUN and Cr and KTDS induced by CDDP in male rats were more than in female (Nematbakhsh et al., 2013a), and possibly the difference was related to age, because in animals aged 16 and 20 weeks, almost the mean values for BUN and Cr and KTDS in male were greater than female rats (Figure 1). The higher Cr-clearance in young male rats may reveal the existence of more suitable renal blood flow when compared with the other groups. This result also is in contrast with Winston and Safirstein study that reported the reduction of renal blood flow by CDDP (Winston and Safirstein, 1985). In laboratory, usually adult rats (> 11 weeks old) use for animal research, and therefore the impact of age may be ignored. Nitrite and MDA alterations responses to CDDP administration were different, and they were increased in 16 weeks old rats. Nitrite increase in adult rats treated with CDDP was reported previously (Nematbakhsh et al., 2013b), and also it was considered also gender related (Taskiran et al., 1997; Watanabe et al., 2000; Ahmed et al., 2007). The liver enzymes also act differently in different ages and gender. However, there is no exact interpretation for it.
As a conclusion, cancer is a major problem in today’s society, especially at an early age in both male and female, and CDDP as anti-cancer is one of the choice by clinician for chemotherapy. Using CDDP to treat cancer in different genders and ages may be associated with different side effects (Nematbakhsh et al., 2017). Therefore, the side effects of this medication need to be assessed in different categories of ages for both sexes, and clinical trials studies for this subject are suggested.
Conflict of interest
The authors have declared that no conflict of interest exists
Acknowledgments
This research was supported by Isfahan University of Medical Sciences (Grant # 249250).
References
- Ahmed SB, Fisher NDL, Hollenberg NK. Gender and the renal nitric oxide synthase system in healthy humans. Clin J Am Soc Nephrol. 2007;2:916–31. doi: 10.2215/CJN.00110107. [DOI] [PubMed] [Google Scholar]
- Aydin I, Agilli M, Aydin FN. Gender differences influence renal injury in cisplatin- treated rats: biochemical evaluation. Biol Trace Elem Res. 2014;158:275. doi: 10.1007/s12011-014-9945-3. [DOI] [PubMed] [Google Scholar]
- Daniel G, Hahn K, Bravo L, et al. The effect of a single therapeutic dose of cisplatin on GFR in dogs. Oncol Rep. 1997;4:153–6. [PubMed] [Google Scholar]
- El-Arabey AA. Gender difference in Cisplatin-induced nephrotoxicity in a rat model. Nephrourol Mon. 2015;7:e23595. doi: 10.5812/numonthly.23595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eshraghi-Jazi F, Nematbakhsh M, Nasri H, et al. The protective role of endogenous nitric oxide donor (L-arginine) in cisplatin-induced nephrotoxicity: Gender related differences in rat model. J Res Med Sci. 2011;16:1389–96. [PMC free article] [PubMed] [Google Scholar]
- Ghasemi M, Nematbakhsh M, Pezeshki Z, et al. Nephroprotective effect of estrogen and progesterone combination on cisplatin- induced nephrotoxicity in ovariectomized female rats. Indian J Nephrol. 2016;26:167–75. doi: 10.4103/0971-4065.160337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghayyoomi M, Soltani N, Nematbakhsh M, et al. The effect of an specific inducible NO synthase inhibitor, S-methylisothiourea hemisulfate on cisplatin-induced nephrotoxicity;gender-related differences. Adv Biomed Res. 2015;4:130. doi: 10.4103/2277-9175.161223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haghighi M, Nematbakhsh M, Talebi A, et al. The role of angiotensin II receptor 1 (AT1) blockade in cisplatin-induced nephrotoxicity in rats: gender-related differences. Ren Fail. 2012;34:1046–51. doi: 10.3109/0886022X.2012.700886. [DOI] [PubMed] [Google Scholar]
- Lu Y, Kawashima A, Horii I, et al. Cisplatin-induced cytotoxicity in BSO-exposed renal proximal tubular epithelial cells: sex, age, and species. Ren Fail. 2005;27:629–33. doi: 10.1080/08860220500200668. [DOI] [PubMed] [Google Scholar]
- Naseem I, Hassan I, Alhazza IM, et al. Protective effect of riboflavin on cisplatin induced toxicities: a gender-dependent study. J Trace Elem Med Biol. 2015;29:303–14. doi: 10.1016/j.jtemb.2014.08.003. [DOI] [PubMed] [Google Scholar]
- Nasri H. Cisplatin therapy and the problem of gender-related nephrotoxicity. J Nephropharmacol. 2013;2:13–4. [PMC free article] [PubMed] [Google Scholar]
- Nematbakhsh M, Ebrahimian S, Tooyserkani M, et al. Gender difference in Cisplatin-induced nephrotoxicity in a rat model: greater intensity of damage in male than female. Nephrourol Mon. 2013a;5:818–21. doi: 10.5812/numonthly.10128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nematbakhsh M, Pezeshki Z. Sex-related difference in nitric oxide metabolites levels after nephroprotectant supplementation administration against cisplatin-induced nephrotoxicity in Wistar rat model: The role of vitamin E, erythropoietin, or n-acetylcysteine. ISRN Nephrol 2013b. 2013:e612675. doi: 10.5402/2013/612675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nematbakhsh M, Pezeshki Z, Eshraghi Jazi F, et al. Cisplatin-induced ephrotoxicity;protective supplements and gender differences. Asian Pac J Cancer Prev. 2017;18:295–314. doi: 10.22034/APJCP.2017.18.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezeshki Z, Nematbakhsh M, Nasri H, et al. Evidence against protective role of sex hormone estrogen in Cisplatin-induced nephrotoxicity in ovarectomized rat model. Toxicol Int. 2013;20:43–7. doi: 10.4103/0971-6580.111568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinches M, Betts C, Bickerton S, et al. Evaluation of novel renal biomarkers with a cisplatin model of kidney injury: gender and dosage differences. Toxicol Pathol. 2012;40:522–33. doi: 10.1177/0192623311432438. [DOI] [PubMed] [Google Scholar]
- Stakisaitis D, Dudeniene G, Jankūnas RJ, et al. Cisplatin increases urinary sodium excretion in rats: gender-related differences. Medicina (Kaunas) 2010;46:45–50. [PubMed] [Google Scholar]
- Taskiran D, Kutay FZ, Sozmen E, et al. Sex differences in nitrite/nitrate levels and antioxidant defense in rat brain. Neuro Report. 1997;8:881–4. doi: 10.1097/00001756-199703030-00013. [DOI] [PubMed] [Google Scholar]
- Watanabe T, Akishita M, Toba K, et al. Influence of sex and age on serum nitrite/nitrate concentration in healthy subjects. Clin Chim Acta. 2000;301:169–79. doi: 10.1016/s0009-8981(00)00340-5. [DOI] [PubMed] [Google Scholar]
- Winston JA, Safirstein R. Reduced renal blood flow in early cisplatin-induced acute renal failure in the rat. Am J Physiol. 1985;249:490–6. doi: 10.1152/ajprenal.1985.249.4.F490. [DOI] [PubMed] [Google Scholar]
- Zamani Z, Nematbakhsh M, Eshraghi-Jazi F. Effect of enalapril in cisplatin-induced nephrotoxicity in rats;gender-related difference. Adv Biomed Res. 2016;5:14. doi: 10.4103/2277-9175.175253. [DOI] [PMC free article] [PubMed] [Google Scholar]


