Abstract
Background:
The aim of the present study was to investigate any prognostic value of pre-treatment anemia, leukocytosis and thrombocytosis in patients with advanced pretreated NSCLC.
Methods:
A randomized, multicenter phase II study comparing the IGF-1R modulator AXL with standard docetaxel in the treatment of previously treated stage IIIB or IV NSCLC patients was conducted in 2011-2013. Clinical and laboratory data were collected, including serum values for hemoglobin (Hgb), white blood cells (WBC) and platelets (Plt) at baseline. These hematological parameters were studied in relation to overall survival using Kaplan–Meier product-limit estimates and multivariate Cox proportional hazards regression models.
Results:
The median overall survival for all patients was 8.9 months. Patients with leukocytosis (WBC > 9 x 109/L) had a significantly shorter median overall survival (4.2 months) as compared with those with a WBC ≤ 9 x 109/L at baseline (12.3 months) with a corresponding of HR 2.10 (95% CI: 1.29-3.43). Patients with anemia (Hgb < 110 g/L) had a non-significant (p = 0.097) shorter median overall survival (6.1 months) as compared with their counterparts with Hgb ≥ 110 g/L at baseline (9.4 months). As for thrombocytosis (Plt > 350 x 109/L), there was no statistically significant impact on overall survival. Leukocytosis retained its prognostic significance in a multivariate model where other clinical factors such as age, sex and WHO performance status were taken into consideration (HR: 1.83, 95% CI: 1.06-3.13, p = 0.029).
Conclusion:
Pre-treatment leukocytosis is a strong and independent prognostic marker for shorter overall survival in previously treated stage IIIB or IV NSCLC patients receiving docetaxel or AXL1717. Combined use of pre-treatment leukocytosis assessments together with established prognostic factors such as performance status could be of help when making treatment decisions in this clinical setting.
Keywords: NSCLC, second-line, anemia, leukocytosis, thrombocytosis
Introduction
Lung cancer is the leading cause of cancer-related death in the western world (DeSantis et al., 2014). Patients who relapse after initial therapy of non-small cell lung cancer (NSCLC) have, prior to the recent introduction of the PD-1 inhibitors in second-line therapy, often been given docetaxel in a palliative second-line setting (Noble et al., 2006). The prognosis for this group of patients has been poor with a 5-year survival rate of less than 5% (Bonomi, 2004).
The Insulin-like Growth Factor type 1 Receptor (IGF-1R) signaling pathway has been reviewed as a promising target for anti-cancer pharmaceutical development. (Camidge et al., 2009; Rosenzweig and Atreya, 2010; Scagliotti and Novello, 2012; Tabernero et al., 2014; Tran et al., 2014; Zhang et al., 2014). AXL1717 (AXL) is a novel modulator of IGF-1R signaling with a second anti-tumoral effect deriving from indirect effects on microtubule dynamics. It was first studied in the phase I setting on patients with various pre-treated tumors and showed promising results particularly in patients with squamous cell NSCLC (Ekman et al., 2011). Subsequently, a randomized phase II study including a total of 99 patients with previously treated, locally advanced or metastatic squamous cell cancer (SCC) or adenocarcinoma (AC) subtypes of NSCLC was conducted, in which the patients were randomized to either docetaxel (DCT) as monotherapy or AXL as monotherapy(Bergqvist et al., 2016). When comparing the rate of progression-free survival (PFS) at 12 weeks it was shown that neither of the treatments was superior to the other.
Considering the poor prognosis in these patients, regardless of given treatment, finding prognostic factors that can reveal beforehand which patients will benefit/not benefit from treatment is important. The prognostic factors should preferably be reliable as well as readily available in the clinical setting. The role of standard hematopoietic serum samples as prognostic markers has emerged as potential prognostic factors in several malignancies (Paik et al., 2014; Aldemir et al., 2015; Koh et al., 2015; Langsenlehner et al., 2015; Zhang et al., 2015a; Zhang et al., 2015b). These tests are inexpensive and samples are routinely taken from virtually every patient that is projected to receive oncologic treatment. In NSCLC, there are indications that standard hematopoietic laboratory tests may have a prognostic value. However previous studies have generally been small and/or included heterogeneous study populations (Engan and Hannisdal, 1990; Pedersen and Milman, 1996; Kasuga et al., 2001; MacRae et al., 2002; Ferrigno and Buccheri, 2003; Aoe et al., 2004; Chamogeorgakis et al., 2008; Tibaldi et al., 2008; Tomita et al., 2008a; Tomita et al., 2008b; Kim et al., 2014). In addition, there has previously been little focus on patients in the second-line setting, in which prognostic factors are of importance in order to decide whether or not to treat the patient. In the present study, we used data from the aforementioned randomized phase II trial in order to investigate the prognostic value of anemia, leukocytosis and thrombocytosis at baseline in the second-line setting of advanced NSCLC patients treated with either docetaxel or AXL.
Material and Methods
A randomized, multicenter phase II study was performed with the primary aim of comparing the PFS after 12 weeks of treatment with the IGF-1R modulator AXL1717 to treatment with standard docetaxel11. In total, 99 previously treated stage IIIB or IV NSCLC patients were included and randomized to treatment with either AXL or docetaxel and the study was conducted 2011 to 2013 in 5 countries in Eastern Europe.
Patients were treated continuously in the primary study treatment period for a maximum of 4 treatment cycles of 21 days (in total 12 weeks). Patients treated with AXL, who were treatment responders or had stable disease at the end of 4 cycles, could be offered an extension of treatment with AXL. Extension treatment cycles were 42 days (28 days of AXL treatment followed by a 14-day treatment-free interval) with the same dose as in the primary study period, and the extension period could continue for up to 4 extension cycles.
AXL was administered as 400 mg given twice daily (BID) as an oral suspension. After a protocol amendment following safety concerns; AXL was administered as 300 mg BID for the first 28 days. Then, depending on absolute neutrophil count (ANC) levels measured during the first 28 days, subsequent doses could be increased to 400 mg BID, remain at 300 mg BID, or be temporarily interrupted and, when ANC levels returned to an acceptable level, be resumed at the same dose or one dose level lower.
Docetaxel (DCT) was administered as a standard 75 mg/m2 on Day 1 of each 21-day treatment cycle for up to 4 cycles. Dose delays and adjustments were made for toxicities.
Baseline data, including gender, age at diagnosis, histology (squamous cell carcinoma or adenocarcinoma) and the serum values of hemoglobin (Hgb), white blood cells (WBC) and platelets (Plt) were prospectively collected for all patients during the screening period and the blood cell values were again collected at baseline directly before first dose of study treatments (AXL or DCT). The reference limits for thrombocytosis (Plt > 350 x 109/L) and leukocytosis (WBC > 9 x 109/L) are the limits presently used at the Uppsala University Hospital, Uppsala, Sweden and anemia was defined as Hgb < 110 g/L in both genders as previously published (Holgersson et al., 2012).
Statistics
Patients’ characteristics at baseline were presented with standard descriptive statistics. Overall survival was analyzed with Kaplan–Meier product-limit estimates and log-rank test was used to compare the survival curves of the different categories. Survival time was calculated from the date of randomization to the date of death or last follow-up date. Patients lost to follow up were censored at last date known alive. Overall survival was also analyzed using multivariate Cox proportional hazards regression models. The multivariate model was adjusted by gender, age at diagnosis, histology, WHO performance status, Hgb, WBC and Plt at baseline and treatment arm. Results were presented as hazard ratios with 95 % confidence intervals (95 % CI). P-values were given where p < 0.05 was considered statistically significant.
Results
Of the 99 patients available for analysis, there were 28 (28%) women and 71 (72%) men. The median age was 57 years (range: 42 – 81 years). Concerning histology, 49 patients (49%) had adenocarcinoma (AC) and 50 (51%) had squamous cell carcinoma (SCC). Most patients (72%) were considered to be in WHO Performance Status (PS) 1, whereas 24% were in PS 0 and only four patients (4%) were in PS 2. The median value of Hgb at baseline was 124 g/L (range 92-167 g/L) and 17 (17%) patients were defined as being anemic. For WBC, the median baseline value was 7.3 x 109/L (range 3.8-19.9 x 109/L) and 33 (33%) of the patients fulfilled the definition of leukocytosis. For Plt, the median value at baseline was 284 x 109/L (range 155-668 x 109/L) and 26 (27%) of patients fulfilled the definition of thrombocytosis. The patient characteristics are summarized in Table 1.
Table 1.
Baseline Characteristics
| Docetaxel (N=41) | AXL1717 (N=58) | Total (N=99) | ||
|---|---|---|---|---|
| Age | ||||
| Mean (SD) | 58.6 (7.6) | 57.5 (7.0) | 57.9 (7.2) | |
| Median | 58 | 57 | 57 | |
| Range | 44 to 73 | 42 to 81 | 42 to 81 | |
| n | 41 | 58 | 99 | |
| Gender | n (%) | |||
| Female | 10 (24) | 18 (31) | 28 (28) | |
| Male | 31 (76) | 40 (69) | 71 (72) | |
| Histology | n (%) | |||
| Adenocarcinoma | 20 (49) | 29 (50) | 49 (49) | |
| Squamous cell carcinoma | 21 (51) | 29 (50) | 50 (51) | |
| WHO Performance status | n (%) | |||
| 0 | 12 (29) | 12 (21) | 24 (24) | |
| 1 | 28 (68) | 43 (74) | 71 (72) | |
| 2 | 1 (2) | 3 (5) | 4 (4) | |
| Hgb | ||||
| Mean (SD) | 126 (17) | 125 (16) | 125 (16) | |
| Median | 125 | 122 | 124 | |
| Range | 92 to 167 | 93 to 164 | 92 to 167 | |
| n | 40 | 57 | 97 | |
| Hgb | n (%) | |||
| <110 g/L | 6 (15) | 11 (19) | 17 (18) | |
| ≥110 g/L | 34 (85) | 46 (81) | 80 (82) | |
| WBC | ||||
| Mean (SD) | 8.3 (3.7) | 8.1 (2.8) | 8.2 (3.1) | |
| Median | 7.3 | 7.3 | 7.3 | |
| Range | 3.8 to 19.9 | 3.8 to 15.5 | 3.8 to 19.9 | |
| n | 41 | 58 | 99 | |
| WBC | n (%) | |||
| ≤9 x 109/L | 29 (71) | 37 (64) | 66 (67) | |
| >9 x 109/L | 12 (29) | 21 (36) | 33 (33) | |
| Plt | ||||
| Mean (SD) | 313 (102) | 307 (98) | 309 (99) | |
| Median | 301 | 276 | 284 | |
| Range | 155 to 668 | 196 to 588 | 155 to 668 | |
| n | 40 | 57 | 97 | |
| Plt | n (%) | |||
| ≤350 x 109/L | 27 (68) | 44 (77) | 71 (73) | |
| >350 x 109/L | 13 (33) | 13 (23) | 26 (27) | |
Hgb, Hemoglobin; WBC, White blood cells; Plt, Platelets; SD, Standard deviation
The median overall survival for all patients was 8.9 months and there was no statistically significant survival difference between the patients receiving docetaxel (8.6 months) and those receiving AXL (8.9 months). There was no statistically significant difference in overall survival between patients aged <65 years as compared to those ≥65 years. Male patients had a shorter median overall survival than female patients (7.0 vs. 13.6 months), however the difference did not reach statistical significance (p = 0.16, log rank test). Patients with SCC histology had shorter median overall survival than those with AC histology (8.1 and 12.6 months, respectively) but the difference was not statistically significant (p = 0.12, log rank test). Patients with PS 0 had better median overall survival (16.5 months) than patients in PS 1 and PS 2 (7.1 and 10.1 months, respectively) but the difference was not statistically significant (p = 0.13, log rank test).
When comparing patients with and without anemia (Figure 1) using Kaplan-Meier methodology, the patients with anemia had a shorter median survival (6.1 months) as compared with the patients without anemia (9.4 months), a non-significant difference with a HR of 1.63 (95% CI: 0.91-2.93, p = 0.097, log rank test). For patients with and without leukocytosis (Figure 2), the patients with leukocytosis had a shorter median survival (4.2 months) as compared with the patients with a WBC ≤ 9 x 109/L at baseline (12.3 months) with Kaplan-Meier curves showing a HR of 2.10 (95% CI: 1.29-3.43). This HR was also statistically significant (p = 0.002, log rank test). When comparing patients with and without thrombocytosis (Figure 3), the patients with thrombocytosis had a shorter median survival (4.2 months) as compared with the patients with Plt ≤ 350 x 109/L at baseline (12.6 months) however, the corresponding Kaplan-Meier curves did not show a statistically significant difference (HR 1.54, 95% CI: 0.93-2.65, p = 0.094, log rank test). For a summary of median overall survival in different subgroups see Table 2. In a multivariate Cox analysis including gender, age, histology, WHO performance status, treatment arm in addition to all three pathological lab parameters (Table 3) the prognostic significance was retained for leukocytosis (HR: 1.83, 95% CI: 1.06-3.13, p = 0.029).
Figure 1.
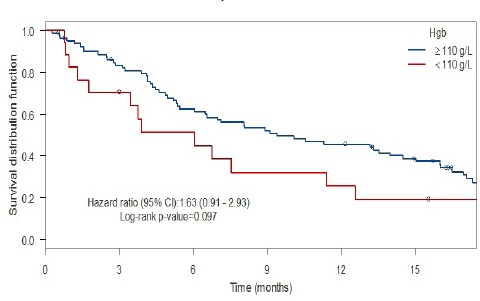
Anemia and Survival
Figure 2.
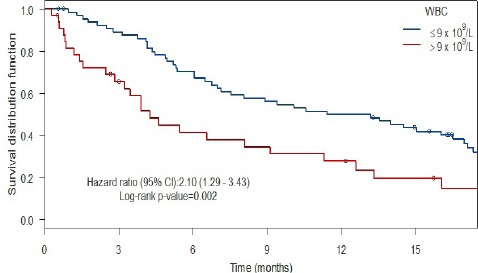
Leukocytosis and Survival
Figure 3.
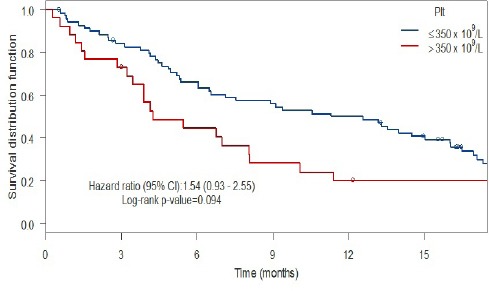
Thrombocytosis and Survival
Table 2.
Median Overall Survival Times (Months) by Subgroup
| Median OS (95 % CI) | Log-rank p-value | |
|---|---|---|
| Total | 8.91 (6.05-13.55) | |
| Treatment | ||
| DCT | 8.60 (5.36-16.1) | |
| AXL | 8.91 (5.46-16.5) | 0.66 |
| Age | ||
| <65 years | 8.91 (5.36-14.5) | |
| ≥65 years | 7.14 (6.05-NA) | 0.4 |
| Sex | ||
| Female | 13.6 (7.53-22.4) | |
| Male | 7.00 (5.36-12.6) | 0.16 |
| Subtype | ||
| Adenocarcinoma | 12.6 (4.87-18.3) | |
| Squamous cell carcinoma | 8.09 (6.55-13.32) | 0.12 |
| WHO performance status | ||
| 0 | 16.5 (5.36-NA) | |
| 1 | 7.14 (5.46-13.3) | |
| 2 | 10.1 (1.32-NA) | 0.13 |
| Hgb | ||
| <110 g/L | 6.05 (3.45-NA) | |
| ≥110 g/L | 9.41 (6.48-15.0) | 0.097 |
| WBC | ||
| ≤9 x 109/L | 12.3 (7.53-17.1) | |
| >9 x 109/L | 4.24 (3.22-11.3) | 0.002 |
| Plt | ||
| ≤350 x 109/L | 12.6 (6.55-16.1) | |
| >350 x 109/L | 4.24 (3.45-23.6) | 0.094 |
DCT, Docetaxel; AXL, AXL1717; Hgb, Hemoglobin; WBC, White blood cells; Plt, Platelets; SD, Standard deviation; OS, Overall survival
Table 3.
Multivariate Cox Analysis for Overall Survival
| Hazard ratio | 95% CI | P-value | |
|---|---|---|---|
| Male gender | 1.38 | (0.80-2.39) | 0.24 |
| Age (per year) | 1.00 | (0.97-1.04) | 0.89 |
| Squamous cell carcinoma vs Adenocarcinoma | 1.26 | (0.76-2.10) | 0.37 |
| WHO Performance status 1 vs 0 | 1.48 | (0.81-2.71) | 0.21 |
| WHO Performance status 2 vs 0 | 1.23 | (0.34-4.48) | 0.76 |
| Hgb <110 g/L vs ≥110 g/L | 1.48 | (0.76-2.86) | 0.25 |
| WBC >9 x 109/L vs ≤9 x 109/L | 1.83 | (1.06-3.13) | 0.029 |
| Plt >350 x 109/L vs ≤350 x 109/L | 1.25 | (0.71-2.22) | 0.44 |
| AXL vs DCT | 0.86 | (0.54-1.38) | 0.54 |
Hgb, Hemoglobin; WBC, White blood cells; Plt, Platelets; DCT, Docetaxel; AXL, AXL1717; SD, Standard deviation; CI, Confidence interval
Discussion
In the present study, we show that for patients with advanced, pretreated NSCLC treated with either docetaxel or the IGF-1R modulator AXL1717, baseline leukocytosis (WBC > 9 x 109/L) was shown to be a strong and independent prognostic marker for overall survival. For anemia (Hgb < 110 g/L) and thrombocytosis (Plt > 350 x 109/L), there was a trend towards worse survival but no statistically significant relationship was found. Also, female patients and patients with adenocarcinoma histology had a better overall survival than male patients and patients with squamous histology, although the association was not statistically significant in the present study of limited size.
The strengths of the present study consist of its homogenous and well-defined patient population and reliable data collected prospectively according to a specified protocol for the performance of a phase II trial. Limitations include a retrospective post hoc review of data, a limited patient population and the experimental setting associated with clinical trials, which does not always reflect clinical reality.
Several clinical prognostic factors influencing survival have been previously studied in NSCLC patients and some of these, including tumor stage, age and performance status, are being used presently for treatment decision making (Brundage et al., 2002). Much research has been conducted concerning novel immunological and histological prognostic biomarkers such as epidermal growth factor receptor (EGFR), however these markers are often expensive and time-consuming to measure and their prognostic value in NSCLC patients remains to be confirmed (Donnem et al., 2012). Therefore, identifying additional reliable prognostic markers that are inexpensive and easy to use are of importance to tailor the treatment for each individual NSCLC patient.
Leukocytosis in patients with cancer can be caused by infection or bone marrow metastases. However, leukocytosis can also be detected in patients without other signs of infectious disease, which is thought to be caused by tumoral production of hematopoietic cytokines; a paraneoplastic phenomenon known as tumor-related leukocytosis (TRL) (Maione et al., 2009). This has been associated with significantly shorter survival in lung cancer as compared with patients without leukocytosis and patients with leukocytosis caused by apparent infection or bone marrow metastasis (Kasuga et al., 2001). A large number of studies, including a pooled analysis of North Central Cancer Treatment Group (NCCTG) trials with data from over 1,000 patients, have reported leukocytosis to be associated with poorer outcome in NSCLC in the first line setting (Ferrigno and Buccheri, 2003; Mandrekar et al., 2006; Tibaldi et al., 2008). In the NCCTG analyses, elevated WBC (>10.2 x 109/L for males and >10.6 x 109/L for females) was associated with worse overall survival with a HR of 1.43 compared to patients with normal (non-elevated) WBC. The relationship between WBC and survival in NSCLC in the second-line setting is less investigated and the reason for the strong prognostic significance of leukocytosis with an impressive HR of 1.83 in the present study is unclear. It is, however, well-known that cancer-related inflammation is a hallmark of cancer progression and the tumor micro-environment plays an important role in tumor progression (Hanahan and Weinberg, 2011). It could be hypothesized that high levels of circulating WBC in a patient that has already been exposed to first-line systemic treatment correlates with a higher level of pro-inflammatory mediators in the tumor microenvironment, which has previously been found to be associated with chemoresistance in NSCLC (Wang et al., 2007). In recent trials, immune checkpoint inhibitors such as PD-1 antibodies, which activates lymphocytes in the tumor microenvironment, have proven to be superior to chemotherapy in the second-line setting (Borghaei et al., 2015; Brahmer et al., 2015). Thus, evaluating whether leukocytosis also has a predictive role for response to treatment with PD-1 inhibitors would be of interest.
Anemia leads to tumor hypoxia, which is proposed to increase the resistance of the tumor cells to chemotherapy through modulation of gene expression and cell-cycle progression which makes the tumor cells less susceptible to treatment (Teicher, 1995; Vaupel et al., 2002; Harrison and Blackwell, 2004). In the present study anemia, was associated with worse survival but the difference was not statistically significant. Thrombocytosis has been suggested to facilitate tissue invasion and formation of metastases by affecting the blood vessel endothelium (Karpatkin and Pearlstein, 1981; Gastpar et al., 1982; Mehta, 1984) and, similarly to leukocytosis, it has been shown to be a negative prognostic marker in NSCLC in several studies (Gislason and Nou, 1985; Engan and Hannisdal, 1990; Pedersen and Milman, 1996; Aoe et al., 2004; Tomita et al., 2008b; Kim et al., 2014). However, like anemia, thrombocytosis was in the present study associated with worse survival but the difference did not reach statistical significance.
To summarize, the results from the present study shows a strong and independent negative prognostic significance of pre-treatment leukocytosis in patients with NSCLC given second-line treatment with either docetaxel or the IGF-1R modulator AXL1717 with a HR of 1.83. A WBC > 9 x 109/L, which is widely used in clinical practice as a denotation of leukocytosis, seems to be a good cut-off level. Creating a prognostic index by combining pre-treatment leukocytosis with established prognostic factors such as performance status and disease stage could improve treatment decision making when selecting patients for palliative second-line chemotherapy. The results need to be validated prospectively with larger cohorts of patients stratified according to pre-treatment WBC levels. These studies should preferably also include patients treated with PD-1 inhibitors, which are increasingly used in the second-line setting, in order to investigate whether pre-treatment WBC levels also may also have a role as a predictive marker for PD-1 inhibitor response in addition to being a prognostic marker for survival.
Conflicts of interest
Marcus Thuresson is presently employed by Statisticon, a company supplying statistical services. This company has received compensation from Axelar AB for the present work.
Johan Harmenberg was previously employed by Axelar AB but is not presently affiliated with the company. He owns stocks in Axelar AB. He has not received any compensation for the present work.
Acknowledgements
The authors would like to thank Axelar AB for making this study possible.
References
- Aldemir MN, Turkeli M, Simsek M, et al. Prognostic Value of baseline neutrophil-lymphocyte and platelet-lymphocyte ratios in local and advanced gastric cancer patients. Asian Pac J Cancer Prev. 2015;16:5933–7. doi: 10.7314/apjcp.2015.16.14.5933. [DOI] [PubMed] [Google Scholar]
- Aoe K, Hiraki A, Ueoka H, et al. Thrombocytosis as a useful prognostic indicator in patients with lung cancer. Respiration. 2004;71:170–3. doi: 10.1159/000076679. [DOI] [PubMed] [Google Scholar]
- Bergqvist M, Holgersson G, Bondarenko I, et al. Phase II randomized study of the IGF-1R pathway modulator AXL1717 compared to docetaxel in patients with previously treated, locally advanced or metastatic non-small cell lung cancer. Acta Oncol. 2016;20:1–7. doi: 10.1080/0284186X.2016.1253866. [DOI] [PubMed] [Google Scholar]
- Bonomi PD. Therapeutic advances in second-line treatment of advanced non-small-cell lung cancer. Clin Lung Cancer. 2004;6:154–61. doi: 10.3816/CLC.2004.n.028. [DOI] [PubMed] [Google Scholar]
- Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung Cancer. N Engl J Med. 2015;373:1627–39. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373:123–35. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brundage MD, Davies D, Mackillop WJ. Prognostic factors in non-small cell lung cancer: a decade of progress. Chest. 2002;122:1037–57. doi: 10.1378/chest.122.3.1037. [DOI] [PubMed] [Google Scholar]
- Camidge DR, Dziadziuszko R, Hirsch FR. The rationale and development of therapeutic insulin-like growth factor axis inhibition for lung and other cancers. Clin Lung Cancer. 2009;10:262–72. doi: 10.3816/CLC.2009.n.037. [DOI] [PubMed] [Google Scholar]
- Chamogeorgakis T, Anagnostopoulos C, Kostopanagiotou G, et al. Does anemia affect outcome after lobectomy or pneumonectomy in early stage lung cancer patients who have not received neo-adjuvant treatment? Thorac Cardiovasc Surg. 2008;56:148–53. doi: 10.1055/s-2007-989455. [DOI] [PubMed] [Google Scholar]
- DeSantis CE, Lin CC, Mariotto AB, et al. Cancer treatment and survivorship statistics 2014. CA Cancer J Clin. 2014;64:252–71. doi: 10.3322/caac.21235. [DOI] [PubMed] [Google Scholar]
- Donnem T, Bremnes RM, Busund LT, et al. Gene expression assays as prognostic and predictive markers in early stage non-small cell lung cancer. J Thorac Dis. 2012;4:212–3. doi: 10.3978/j.issn.2072-1439.2012.03.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekman S, Frodin JE, Harmenberg J, et al. Clinical phase I study with an insulin-like growth factor-1 receptor inhibitor: experiences in patients with squamous non-small cell lung carcinoma. Acta Oncol. 2011;50:441–7. doi: 10.3109/0284186X.2010.499370. [DOI] [PubMed] [Google Scholar]
- Engan T, Hannisdal E. Blood analyses as prognostic factors in primary lung cancer. Acta Oncol. 1990;29:151–4. doi: 10.3109/02841869009126536. [DOI] [PubMed] [Google Scholar]
- Ferrigno D, Buccheri G. Hematologic counts and clinical correlates in 1201 newly diagnosed lung cancer patients. Monaldi Arch Chest Dis. 2003;59:193–8. [PubMed] [Google Scholar]
- Gastpar H, Ambrus JL, Ambrus CM. Platelet cancer cell interaction in metastasis formation. Platelet aggregation inhibitors: a possible approach to metastasis prevention. Prog Clin Biol Res. 1982;89:63–82. [PubMed] [Google Scholar]
- Gislason T, Nou E. Sedimentation rate, leucocytes, platelet count and haemoglobin in bronchial carcinoma: an epidemiological study. Eur J Respir Dis. 1985;66:141–6. [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Harrison L, Blackwell K. Hypoxia and anemia: factors in decreased sensitivity to radiation therapy and chemotherapy? Oncologist. 2004;9:31–40. doi: 10.1634/theoncologist.9-90005-31. [DOI] [PubMed] [Google Scholar]
- Holgersson G, Sandelin M, Hoye E, et al. Swedish lung cancer radiation study group: the prognostic value of anaemia, thrombocytosis and leukocytosis at time of diagnosis in patients with non-small cell lung cancer. Med Oncol. 2012;29:3176–82. doi: 10.1007/s12032-012-0247-3. [DOI] [PubMed] [Google Scholar]
- Karpatkin S, Pearlstein E. Role of platelets in tumor cell metastases. Ann Intern Med. 1981;95:636–41. doi: 10.7326/0003-4819-95-5-636. [DOI] [PubMed] [Google Scholar]
- Kasuga I, Makino S, Kiyokawa H, et al. Tumor-related leukocytosis is linked with poor prognosis in patients with lung carcinoma. Cancer. 2001;92:2399–405. doi: 10.1002/1097-0142(20011101)92:9<2399::aid-cncr1588>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Kim KH, Park TY, Lee JY, et al. Prognostic significance of initial platelet counts and fibrinogen level in advanced non-small cell lung cancer. J Korean Med Sci. 2014;29:507–11. doi: 10.3346/jkms.2014.29.4.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh CH, Bhoo-Pathy N, Ng KL, et al. Utility of pre-treatment neutrophil-lymphocyte ratio and platelet-lymphocyte ratio as prognostic factors in breast cancer. Br J Cancer. 2015;113:150–8. doi: 10.1038/bjc.2015.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langsenlehner T, Pichler M, Thurner EM, et al. Evaluation of the platelet-to-lymphocyte ratio as a prognostic indicator in a European cohort of patients with prostate cancer treated with radiotherapy. Urol Oncol. 2015;33:9–16. doi: 10.1016/j.urolonc.2015.02.002. [DOI] [PubMed] [Google Scholar]
- MacRae R, Shyr Y, Johnson D, et al. Declining hemoglobin during chemoradiotherapy for locally advanced non-small cell lung cancer is significant. Radiother Oncol. 2002;64:37–40. doi: 10.1016/s0167-8140(02)00151-2. [DOI] [PubMed] [Google Scholar]
- Maione P, Rossi A, Di Maio M, et al. Tumor-related leucocytosis and chemotherapy-induced neutropenia: linked or independent prognostic factors for advanced non-small cell lung cancer? Lung Cancer. 2009;66:8–14. doi: 10.1016/j.lungcan.2009.02.022. [DOI] [PubMed] [Google Scholar]
- Mandrekar SJ, Schild SE, Hillman SL, et al. A prognostic model for advanced stage nonsmall cell lung cancer. Pooled analysis of North central cancer treatment group trials. Cancer. 2006;107:781–92. doi: 10.1002/cncr.22049. [DOI] [PubMed] [Google Scholar]
- Mehta P. Potential role of platelets in the pathogenesis of tumor metastasis. Blood. 1984;63:55–63. [PubMed] [Google Scholar]
- Noble J, Ellis PM, Mackay JA, et al. Second-line or subsequent systemic therapy for recurrent or progressive non-small cell lung cancer: a systematic review and practice guideline. J Thorac Oncol. 2006;1:1042–58. [PubMed] [Google Scholar]
- Paik KY, Lee IK, Lee YS, et al. Clinical implications of systemic inflammatory response markers as independent prognostic factors in colorectal cancer patients. Cancer Res Treat. 2014;46:65–73. doi: 10.4143/crt.2014.46.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen LM, Milman N. Prognostic significance of thrombocytosis in patients with primary lung cancer. Eur Respir J. 1996;9:1826–30. doi: 10.1183/09031936.96.09091826. [DOI] [PubMed] [Google Scholar]
- Rosenzweig SA, Atreya HS. Defining the pathway to insulin-like growth factor system targeting in cancer. Biochem Pharmacol. 2010;80:1115–24. doi: 10.1016/j.bcp.2010.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scagliotti GV, Novello S. The role of the insulin-like growth factor signaling pathway in non-small cell lung cancer and other solid tumors. Cancer Treat Rev. 2012;38:292–302. doi: 10.1016/j.ctrv.2011.07.008. [DOI] [PubMed] [Google Scholar]
- Tabernero J, Chawla SP, Kindler H, et al. Anticancer activity of the type I insulin-like growth factor receptor antagonist, ganitumab, in combination with the death receptor 5 agonist, conatumumab. Target Oncol. 2014;10:65–76. doi: 10.1007/s11523-014-0315-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teicher BA. Physiologic mechanisms of therapeutic resistance. Blood flow and hypoxia. Hematol Oncol Clin North Am. 1995;9:475–506. [PubMed] [Google Scholar]
- Tibaldi C, Vasile E, Bernardini I, et al. Baseline elevated leukocyte count in peripheral blood is associated with poor survival in patients with advanced non-small cell lung cancer: a prognostic model. J Cancer Res Clin Oncol. 2008;134:1143–9. doi: 10.1007/s00432-008-0378-2. [DOI] [PubMed] [Google Scholar]
- Tomita M, Shimizu T, Hara M, et al. Impact of preoperative hemoglobin level on survival of non-small cell lung cancer patients. Anticancer Res. 2008a;28:1947–50. [PubMed] [Google Scholar]
- Tomita M, Shimizu T, Hara M, et al. Prognostic impact of thrombocytosis in resectable non-small cell lung cancer. Interact Cardiovasc Thorac Surg. 2008b;7:613–5. doi: 10.1510/icvts.2007.174391. [DOI] [PubMed] [Google Scholar]
- Tran TN, Selinger CI, Yu B, et al. Alterations of insulin-like growth factor-1 receptor gene copy number and protein expression are common in non-small cell lung cancer. J Clin Pathol. 2014;67:985–91. doi: 10.1136/jclinpath-2014-202347. [DOI] [PubMed] [Google Scholar]
- Wang HW, Lin CP, Chiu JH, et al. Reversal of inflammation-associated dihydrodiol dehydrogenases (AKR1C1 and AKR1C2) overexpression and drug resistance in nonsmall cell lung cancer cells by wogonin and chrysin. Int J Cancer. 2007;120:2019–27. doi: 10.1002/ijc.22402. [DOI] [PubMed] [Google Scholar]
- Vaupel P, Briest S, Hockel M. Hypoxia in breast cancer: pathogenesis, characterization and biological/therapeutic implications. Wien Med Wochenschr. 2002;152:334–42. doi: 10.1046/j.1563-258x.2002.02032.x. [DOI] [PubMed] [Google Scholar]
- Zhang GM, Zhu Y, Luo L, et al. Preoperative lymphocyte-monocyte and platelet-lymphocyte ratios as predictors of overall survival in patients with bladder cancer undergoing radical cystectomy. Tumour Biol. 2015a;36:8537–43. doi: 10.1007/s13277-015-3613-x. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Pan J, Lubet RA, et al. Targeting the insulin-like growth factor-1 receptor by picropodophyllin for lung cancer chemoprevention. Mol Carcinog. 2014;54:129–37. doi: 10.1002/mc.22206. [DOI] [PubMed] [Google Scholar]
- Zhang WW, Liu KJ, Hu GL, et al. Preoperative platelet/lymphocyte ratio is a superior prognostic factor compared to other systemic inflammatory response markers in ovarian cancer patients. Tumour Biol. 2015b;36:8831–7. doi: 10.1007/s13277-015-3533-9. [DOI] [PubMed] [Google Scholar]


