Abstract
Acute myeloid leukemia (AML), is a clonal disorder caused by acquired somatic mutations and chromosomal rearrangements. According to some evidence, progression of hematolymphoid malignancies depends on the induction of new blood vessel formation under the influence of acute leukemia. Various factors are produced by cancer cells under hypoxic conditions to increase vascular formation. Among these, vascular endothelial growth factor (VEGF) plays a crucial role. Cytotoxicity and anticancer effects of arsenic trioxide (ATO) have been reported in many cancers. Sorafenib, known as an angiogenic inhibitor, decreases leukemic cell survival. The aim of this study was to indicate combination effects of ATO and sorafenib in two AML cell lines, KG-1 and U937. Effective doses was determined by MTT assay for both single and combination treatments. Percentages of apoptotic cells were evaluated by Annexin V FITC staining and mRNA levels of VEGF isoforms and receptor expression were investigated by Real-Time PCR. Our data show that sorafenib (5μM and 7μM in KG-1 and U937 cell lines respectively), ATO (1.618μM and 1μM in KG-1 and U937 cell lines respectively), and also their combination significantly increased the percentage of apoptotic cells. In addition the mRNA level of VEGF isoforms was downregulated in the U937 cell line while upregulated in KG-1 cells. Taken together, our results suggest that the VEGF autocrine loop may have an influence on AML development and progression and could be consider as a therapeutic target. The combination of sorafenib as a VEGF inhibitor with ATO synergistically inhibits cell proliferation and promotes apoptosis.
Keywords: Anti-Vascular Endothelial Growth Factor, Sorafenib, Arsenic Trioxide, Acute Myeloid Leukemia, cell lines
Introduction
Acute myeloid leukemia (AML) is a clonal disorder as a result of acquired somatic mutations and chromosomal rearrangements (Rubnitz et al., 2010) which occurs in a hematopoietic progenitor (Mohammadi et al., 2016; Bohl et al., 2017). Progress in our comprehension as to pathophysiology of AML have not yet led to major advances in disease-free and overall survival of patients. While the particular reason for this biological abnormality in patients are usually unclear, advances in understanding of the genetic basis of leukemia could lead to a wide array of targeted therapies. The importance of angiogenesis in tumor growth has become evident in numerous studies (Lee et al., 2016; Mohammadi et al., 2017b). Different factors are being produced from malignant cells in hypoxic condition to promote vascular formation. Among those, vascular endothelial growth factor (VEGF) has crucial role in enhancing migration, proliferation, and differentiation of endothelial cells. VEGF have five isoforms; VEGF-A (key regulator of blood vessels growth, herein refer to VEGF), placenta growth factor (PGF), VEGF-B, VEGF-C and VEGF-D. There are 4 receptors for VEGF have been recognized: Flt-1 (VEGFR-1), Flk-1/KDR (VEGFR-2), Flt-4 (VEGFR-3), and neuropilin-1 (NRP-1) (Ferrara et al., 2003). Expression levels of VEGF and its receptors are accepted as an indicators of angiogenesis in solid tumors. Different types of leukemia like solid tumors were also shown to have high microvessel density (MVD) in bone marrow (Medinger et al., 2010; Lee et al., 2016). Based on various studies, acute leukemia cells secrete substantial amounts of VEGF in the serum and also malignant hematopoietic cells were detected to express VEGF and VEGFR (Zhu et al., 2003). Expression of VEGF and VEGF receptor (VEGFR) genes have been linked to reduced survival and lower remission rates in patients with different hematologic malignancies (Aguayo et al., 2002; Song et al., 2012b). VEGF signaling known as a key aspects of tumorgenesis (Goel and Mercurio, 2013). Co-expression of VEGF and VEGFR in lymphoma, multiple myeloma and leukemia, connected with its direct role in malignant cell survival, proliferation. These data indicate the pivotal function of autocrine VEGF loops in the pathogenesis of these sort of malignancies (Podar and Anderson, 2005; Mohammadi et al., 2017a). Variant direct and indirect VEGF and VEGFR inhibitor strategies are under clinical research for treatment of both solid tumors and hematologic malignancies. The production of anti-apoptotic and pro-apoptotic molecules changes within the course of conventional treatment for cancer. Specifically, previous researches indicate that chemotherapy, irradiation and surgery, could promote tumor angiogenesis through increasing production of VEGF and other endothelial cell survival and growth factors in tumor cells. High concentration of VEGF in bone marrow microenvironment in hematologic malignancies may repress the anti-proliferative effects of several chemotherapeutics which enhance multidrug resistance (Tran et al., 2002; Mohammadi et al., 2017a). Thus, combination of chemotherapies and irradiation with medicine that inhibit VEGF signaling may promote antitumor efficacy. (Teicher et al., 1992; Lee et al., 2000; Podar and Anderson, 2005). Sorafenib is known as a multi kinase inhibitor through activity against the Ser/ Thr kinase Raf that recognize to have important role in tumor cell signaling and proliferation (Liu et al., 2006) (Figure 1). In addition to targeting Raf serine/threonine kinases, also sorafenib potently inhibits the auto phosphorylation of various receptor tyrosine kinases including, VEGFR1, 2 and 3 and also PDGFRβ (Wilhelm et al., 2006) which play pivotal roles in tumor progression and angiogenesis. (Adnane et al., 2006; Wilhelm et al., 2008). Arsenic trioxide (ATO) as a single agent is able to target various cellular functions through multiple molecular factors (Emadi and Gore, 2010) (Figure 2). Likewise, ATO known as a current standard treatment for adult acute promyelocytic leukemia. The aim of present study was to evaluate the combination effect of ATO and sorafenib as a new strategy with unique anti-cancer properties on VEGF gene expression and apoptosis in KG-1 and U937 lines. Also, the effect of these compounds on mRNA expression level of different isoform of VEGF were evaluated in these cell lines.
Figure 1.
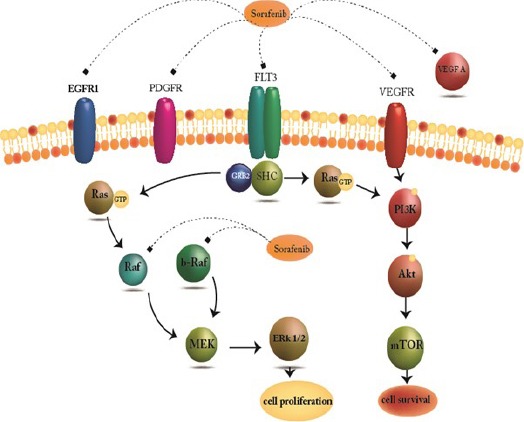
Molecular Target of Sorafenib. Sorafenib is Known as a Multi Kinase Inhibitor which acts via Activity Against the Ser/ Thr kinase Raf that recognize to Have Important Role in Tumor Cell Signaling and Proliferation
Figure 2.
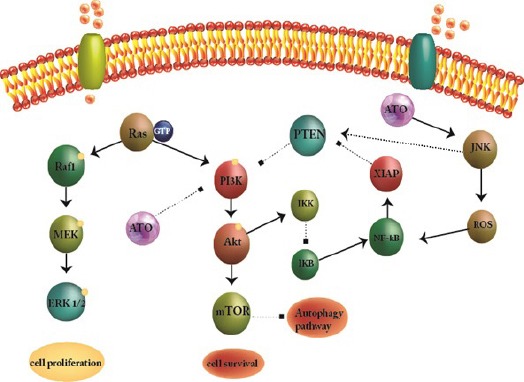
Molecular Target of Arsenic Trioxide (ATO). ATO acts as a single agent targets with various cellular functions through multiple molecular factors.
Material and Methods
Reagents
Annexin V-FITC aposptosis detection kit, dimethylsulfoxide (DMSO), DEPC treated water and ATO were obtained from the Sigma-Aldrich (Sigma-Aldrich, St. Louis, MO). Sorafenib was purchase from the Santa Cruz (Santa Cruz, Dallas, Texas). RPMI 1640 medium and fetal bovine serum (FBS) were purchased from (Gibco, Carlsbad, CA). The cDNA synthesis kit was purchased from Takara Bio Inc. (Takara Bio Inc., Otsu, Japan). TRI pure was obtained from Roche Applied Science (Roche Applied Science, Germany).
Cell line
The human leukemia cell lines U937 and KG-1 were obtained from the National Cell Bank of Iran (Pasteur Institute, Tehran, Iran). These cell lines were cultured in complete RPMI-1640 medium with 10% and 20% heat inactivated FBS for U937 and KG-1 cell line respectively, supplemented with 100 U/mL penicillin, 100 μg/mL streptomycin. All cells were cultured in a humidified atmosphere at 37°C with 5% CO2 incubator.
Proliferation assay
U937 and KG-1 cell line were seeded (at an initial density 5 × 103 cells per well) in 96-well plate. Cells were treated with ATO, Sorafenib and their combinations at 37ºC and 5% CO2 atmosphere for 24, 48 and 72 hours. The proliferation rate of cells was analyzed by MTT assay. Results were expressed as a proliferation rate, with 100% representing control cells treated with 0.1% DMSO alone.
Apoptosis assay
U937 and KG-1 cell lines were seeded at density of 3 × 105 cell/well and incubated for 48 hours in absence and presence of ATO, Sorafenib and their combinations. To assess the percentage of apoptosis induction by treated compounds, fluorescein-conjugated Annexin V (Annexin V-FITC) staining assay was accomplished based on manufacturer protocol. The percentage of apoptosis was determined as a percentage of the Annexin V+/ PI- cells through flow cytometry by BD flow cytometer instrument and analyzed with flowjo (Tree Star Inc., version 9.6.3, USA) program.
DNA cell cycle analysis
Cells were treated with indicated concentrations of ATO and Sorafenib for 48h, then fixed in 70% in ethanol and dye by PI. Cells were assessed by BD flow cytometer instrument analyzed with flowjo program. The apoptotic cell fraction could predict from hypodiploid sub-G0/G1 DNA fraction.
RNA isolation and Real Time PCR
RNA isolation
Total RNA was extracted by using the TRI pure (Roche Applied Science, Germany), according to the manufacturer instructions. The RNA pellets were reconstituted in DEPC treated water. The quality and quantity of total RNA was determined spectro-photometrically using Nanodrop ND-1000 (Nanodrop Technologies, Wilmington, DE) at 260 and 280 nm. Complementary DNAs (cDNAs) were reverse transcribed from 1-2µg of total RNA by use of cDNA synthesis kit (Takara Bio Inc., Otsu, Japan) according to the manufacturer instructions. The cDNA concentration was then normalized in series of PCR by using HPRT and GAPDH primers (Table 1). The normalized cDNAs were subjected to amplification, using Step One Plus™ ABI instrument (Apply Bio systems, USA). The levels of HPRT mRNA expression were used to estimate the relative expression levels. The comparative Ct method was used to compute relative expression values. The primers and their corresponding amplicon lengths were provided in Table 1.
Table 1.
Real-Time PCR Primers
| primer | sequence | Ref |
|---|---|---|
| GAPDH | TGAACGGGAAGCTCACTGG TCCACCACCCTGTTGCTGTA | (Kong et al., 2014b) |
| HPRT | GCTATAAATTCTTTGCTGACCTGCTG AATTACTTTTATGTCCCCTGTTGACTGG | (Gusenbauer et al., 2015) |
| VEGFA | AGGGCAGAATCATCACGAAGT AGGGTCTCGATTGGATGGCA | (Kong et al., 2014a) |
| VEGFB | GAGATGTCCCTGGAAGAACACA GAGTGGGATGGGTGATGTCAG | (Yang et al., 2009) |
| VEGFC | GAGGAGCAGTTACGGTCTGTG TCCTTTCCTTAGCTGACACTTGT | (Awad et al., 2015) |
| VEGF-R1 | CAGGCCCAGTTTCTGCCATT TTCCAGCTCAGCGTGGTCGTA | (Grellier et al., 2009) |
| VEGF-R2 | CCAGCAAAAGCAGGGAGTCTGT TGTCTGTGTCATCGGAGTGATATCC | (Giurdanella et al., 2015) |
Statistical Analysis
All in vitro experiments were performed in triplicate, and results have been expressed as the mean±standard deviation (SE). Student’s t test and one-way analysis of variance (one-way ANOVA) were used to determine statistical significances of difference. Statistical significance were defined at *P<0.05, **P<0.01, and ***P<0.001 compared to corresponding control.
Results
Evaluation of cell proliferation using MTT test
The cell proliferation of U937 and KG-1 which treated with indicated concentration of ATO (0.5μM - 5μM) and sorafenib (2μM - 12μM) were assessed by MTT assay for 24h, 48h and 72h. We perceived both time- and dose-dependent effect of compounds. As seen in Figure 3, we did not observed a significant difference between 48h and 72h. We evaluated synergic effect of selected dose of ATO and sorafenib (1.618μM and 2μM for KG-1 and 1μM for U937) and sorafenib (7μM for KG-1 and 5μM for U937) respectively. Our data indicate that ATO and sorafenib alone or their combination could significantly reduced the cell proliferation in both U937 and KG-1 cell lines in comparison to the control group (Figure 3).
Figure 3.
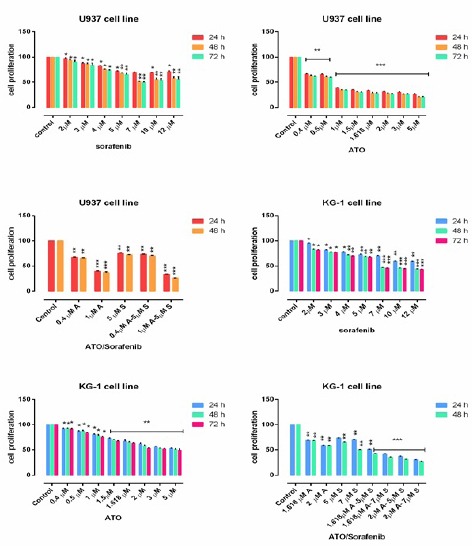
Cell Proliferation in U937 and KG-1. Effects of ATO and Sorafenib on cell proliferation of KG-1 and U937 cell lines. The anti-proliferative effects of ATO (0.4-5 μM), Sorafenib (2-12 μM) and their combinations on cited AML cell lines were assessed by MTT assay after 24 h-48h and 72 h treatment. Significant difference between 48h and 72h was not observed. After detection of suitable doses for ATO (1.618μM and 2μM for KG-1 and 1μM for U937) and sorafenib (7μM for KG-1 and 5μM for U937), we evaluated combination effect of ATO and sorafenib. Combination effect of ATO and sorafenib compared to the control or even single compound could significantly decrease cell proliferation in both U937 and KG-1 cell lines. Data are mean ± S.E of three independent experiments. Statistical significance were defined at *P<0.05, **P<0.01 and ***P<0.001compared to corresponding control.
Apoptosis assay
We performed flow cytometry assay by Annexin V FITC/PI staining to investigate apoptosis effects of indicated compounds. As seen in Figure 4 and Figure 5, we observed an increase in early apoptotic cells (Annexin +/PI-) and minimum percentage of necrosis in treated cells as compared with control in both U937 and KG-1 cell lines. In addition, significant increase (70 % in KG-1 and 80 % in U937) of apoptotic cells were seen in combination of ATO and sorafenib. The percentages of apoptotic cells in treated KG-1(ATO1.618μM+Sorafenib7μM and ATO 2μM+Sorafenib7μM) and U937 (ATO1μM+Sorafenib5μM) cell populations were statistically remarkable as compared to the control groups.
Figure 4.
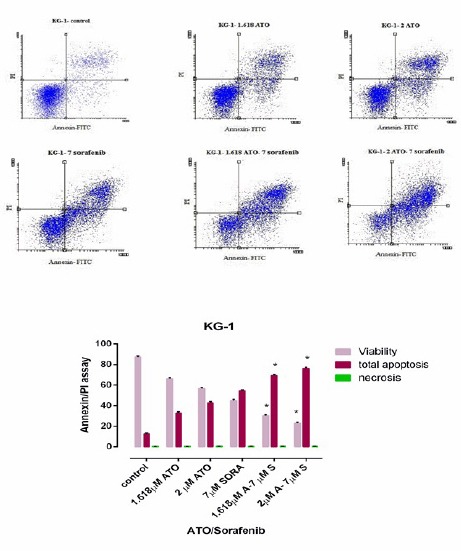
Apoptosis Assay in KG-1 Cell Line after 48h. Cells in the lower right quadrant represented apoptosis while in the upper right quadrant indicated post-apoptotic necrosis. Data are mean ± S.E of three independent experiments. Statistical significance were defined at *P<0.05, **P<0.01 and ***P<0.001compared to corresponding control.
Figure 5.
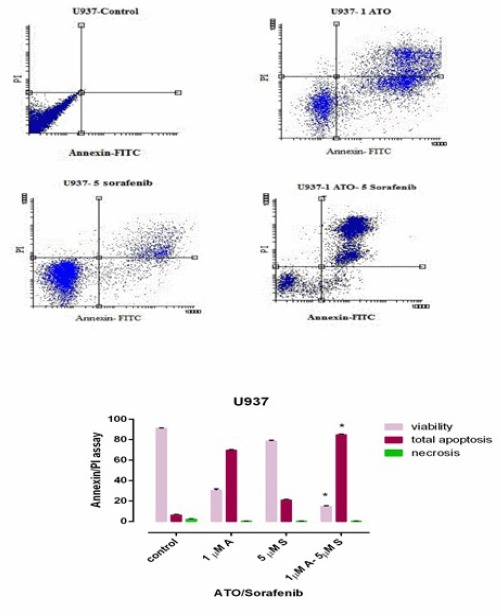
Apoptosis Assay in U937 Cell Line after 48h. Cells in the Lower Right Quadrant Represented Apoptosis while in the Upper Right Quadrant Indicated Post-Apoptotic Necrosis. Data are mean ± S.E of three independent experiments. Statistical significance were defined at *P<0.05, **P<0.01 and ***P<0.001compared to corresponding control.
Cell cycle assay
DNA content of KG-1 and U937 cells evaluated during cell cycle to get information about cell cycle progression. In present study after treatment of U937 the cell population in G0/G1 phase increased in all doses especially when cells treated with combination of Sorafenib and ATO for 48h. Moreover our data exhibited that combination of ATO and sorafenib increased the hypodiploid sub G0/G1 DNA fraction in dose depended manner (1.13% to 8.3% for KG-1 cell and 9.21% to 16.1% for U937 cell respectively) indicating apoptotic population (Figure 6 and Figure 7).
Figure 6.
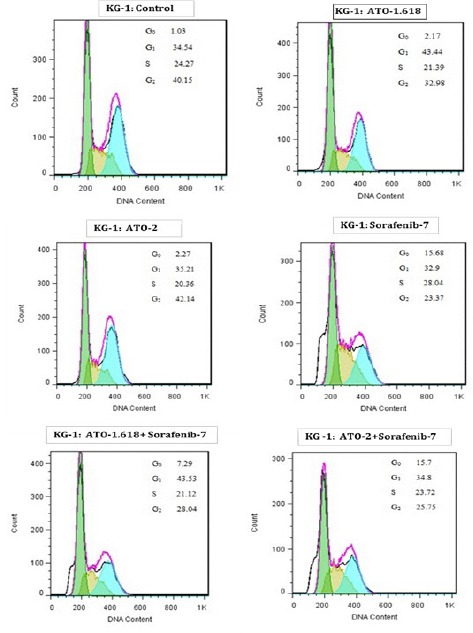
Cell Cycle Analysis for KG-1. Effect of ATO and Sorafenib on KG-1 induced sub-G0/G1 DNA population. Data are mean ± S.E of three independent experiments. Statistical significance were defined at *P<0.05, **P<0.01 and ***P<0.001compared to corresponding control.
Figure 7.
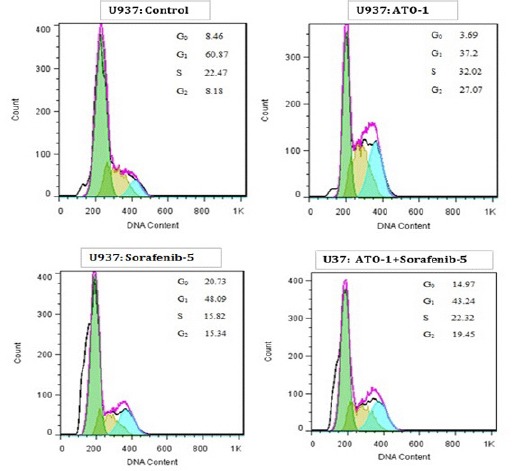
Cell Cycle Analysis for U937. Cell cycle analysis for U937. Effect of ATO and Sorafenib on KG-1 induced sub-G0/G1 DNA population. Data are mean ± S.E of three independent experiments. Statistical significance were defined at *P<0.05, **P<0.01 and ***P<0.001compared to corresponding control.
Real-Time PCR assay
The expression of VEGF isoforms and VEGFR1 and VEGFR2 in treated cells were evaluated by Real-time PCR. KG-1 cells were treated with selective concentrations of ATO (1.618μM and 2μM), sorafenib (7μM) and also their combination for 48h. We observed that the mRNA expression of VEGFR1, VEGFR2 and VEGFC downregulated when KG-1 cells were treated with 7μM sorafenib while the mRNA expression of VEGFA and VEGFB slightly upregulated. The significant downregulation VEGFB mRNA expression observed when cells treated with 1.618μM of ATO in KG-1 cells. (Figure 8).
Figure 8.
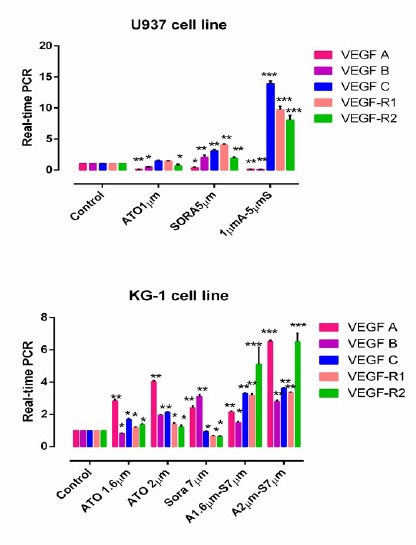
The Effects of ATO and Sorafenib on the mRNA Level of Indicated Genes in KG-1 and U937 Cells. The effect of ATO (1–2 μM) and Sorafenib (5-7 μM) on expression level of VEGF (A, B, C, R1 and R2) were determined by qRT-PCR analysis. Values were normalized using the expression of the housekeeping HPRT. Values are given as mean ± S.E. of three independent experiments. Statistical significance were defined at *P<0.05, **P<0.01 and ***P<0.001compared to corresponding control.
U937 cells were treated with selective concentrations of ATO (1μM), sorafenib (5μM) and also their combination for 48h. Our data indicate that ATO (1μm), sorafenib (5μm) and specially their combination significantly downregulate the mRNA expression of VEGFA in U937 cells. In addition our results showed that ATO and also combination of ATO and sorafenib lead to downregulation of mRNA expression of VEGFB gene. Likewise, slightly upregulation in mRNA expression of cited gene was observed in sorafenib treated cells. The expression of VEGFR2 downregulated by ATO (1μM) (Figure 8).
Discussion
Acute myeloid leukemia (AML) is known as one of the most common hematologic disorder in modern society based on the World Health Organization report. Beside of new treatment options which have improved overall survival in last decades, AML still remain as a life-threating disease (Panah et al., 2017). According to recent data, the proliferation of both solid tumors and hematological malignancies might be dependent on angiogenesis (Barbarroja et al., 2007; Rodriguez-Ariza et al., 2011). In present study we demonstrated the effect of ATO and sorafenib on expression pattern of VEGFA, VEGFB, VEGFC, VEGFR1 and VEGFR2 in leukemic cell lines. Previous studies demonstrated that AML blast increase autocrine VEGF pathway to promote proliferation which lead to cell survival (Mohammadi et al., 2017a). This might elucidate why AML patients with VEGF overexpression illustrate poor prognosis (Aguayo et al., 2000; Padro et al., 2002).
VEGF is known as a target in leukemia treatment and various strategies have been used to down-regulate or inhibit VEGF signaling pathway(Song et al., 2012b). This new strategy which inhibit VEGF pathway could block the autocrine VEGF pathway in AML cell lines as well as the atypical vessel development through the vascular endothelial cells (Song et al., 2012a). Perez-Atayde et al., 1997 have reported that blood vessel density increased in the bone marrow in children suffer from acute lymphoblastic leukemia.
ATO is known as a multi target agent which able to activate apoptosis through various molecular pathway in various cancer including solid tumor cells and hematological malignancies (Li and Broome, 1999; Rousselot et al., 1999; Zhu et al., 1999; Bonati et al., 2006). In this research we observed cytotoxicity of ATO and also induction of apoptosis in both U937 and KG-1 cell lines in a dose-dependent manner. In addition we observed that ATO could down-regulate the gene expression of VEGF. We tested various concentration of ATO in resistance cell line (KG-1). We observed that 1.618μM of ATO exhibit significant effects as compare with upper and lower doses of ATO in KG-1. Recent researches have confirmed that low concentrations of ATO (1-5 μM), induce partial apoptosis of T lymphocytes by promoting caspase activation and oxidative stress (Gupta et al., 2003). In good agreement with our result, Rojewski et al., showed that low concentration of ATO induce apoptosis in U937 cell population (Rojewski et al., 2002). Goel and Mercurio., (2013) noted that ATO disrupt a reciprocal stimulatory loop between endothelial cells and leukemic cells through promoting apoptosis in HUVECs cell line via inhibiting leukemic cell VEGF protein production (Roboz et al., 2000). Data from our laboratory also indicted that ATO down-regulate gene expression of VEGFA, VEGFB and VEGFR-2 in U937 cell population and down-regulate gene expression of VEGFB in KG-1 cell population.
The anti-angiogenic effect of sorafenib in breast, colorectal carcinomas renal cell and hepatocellular carcinoma has been reported (Wilhelm et al., 2008). The anti-angiogenic property of Sorafenib also is being evaluated to use for AML treatment (Mori et al., 2008). Considering the vital role of VEGF, we investigate the effect of sorafenib on VEGF isoforms and their receptors. However sorafenib evident to be more effective in leukemias with the FLT3-ITD mutation, antileukemic function has been elucidated in several patients with AML and wild-type form of FLT3(Crump et al., 2010). In line with our result, Shuiying et al, reported that sorafenib exhibited antileukemic activity in AML cell lines (Hu et al., 2011). Although previous studies confirmed the antiangiogenic effect of sorafenib in cell line with FLT3-ITD mutation, we exhibit antileukemic effect of sorafenib in wild-type FLT3 cell line including in U937 and KG-1 cell line.
In conclusion, The VEGF pathway may has potential impact on AML development and progression and could be consider as a therapeutic target. Sorafenib as a VEGF inhibitor in combination with ATO has a synergistic impact on inhibition of cell proliferation and promotion of apoptosis in AML cell line without FLT3 mutation. ATO enhances the antitumor activity of sorafenib in both U937 and KG-1 cell population when used simultaneously in combination. Our study indicated a potential mechanism underlying the interaction between ATO and sorafenib in U937 and KG-1 cell lines.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgments
This study was performed and funded by Hematology, Oncology and Stem Cell Transplantation Research Center, Tehran University of Medical Sciences.
References
- Adnane L, Trail PA, Taylor I, et al. Sorafenib (BAY 43-9006, Nexavar®), a dual-action inhibitor that targets RAF/MEK/ERK pathway in tumor cells and Tyrosine Kinases VEGFR/PDGFR in tumor vasculature. Methods Enzymol. 2006;407:597–612. doi: 10.1016/S0076-6879(05)07047-3. [DOI] [PubMed] [Google Scholar]
- Aguayo A, Kantarjian H, Manshouri T, et al. Angiogenesis in acute and chronic leukemias and myelodysplastic syndromes. Blood. 2000;96:2240–5. [PubMed] [Google Scholar]
- Aguayo A, Kantarjian HM, Estey EH, et al. Plasma vascular endothelial growth factor levels have prognostic significance in patients with acute myeloid leukemia but not in patients with myelodysplastic syndromes. Cancer. 2002;95:1923–30. doi: 10.1002/cncr.10900. [DOI] [PubMed] [Google Scholar]
- Barbarroja N, Aristides-Torres L, Hernandez V, et al. Coordinated deregulation of cellular receptors, proangiogenic factors and intracellular pathways in acute myeloid leukaemia. Leuk Lymphoma. 2007;48:1187–99. doi: 10.1080/10428190701340616. [DOI] [PubMed] [Google Scholar]
- Bohl SR, Dolnik A, Jensen T, et al. Gene expression analysis of decitabine treated AML: high impact of tumor suppressor gene expression changes. Leuk Lymphoma. 2017;45:1–4. doi: 10.1080/10428194.2017.1287360. [DOI] [PubMed] [Google Scholar]
- Bonati A, Rizzoli V, Lunghi P. Arsenic trioxide in hematological malignancies: the new discovery of an ancient drug. Curr Pharm Biotechnol. 2006;7:397–405. doi: 10.2174/138920106779116829. [DOI] [PubMed] [Google Scholar]
- Crump M, Hedley D, Kamel-Reid S, et al. A randomized phase I clinical and biologic study of two schedules of sorafenib in patients with myelodysplastic syndrome or acute myeloid leukemia: a NCIC (National Cancer Institute of Canada) clinical trials group study. Leuk Lymphoma. 2010;51:252–60. doi: 10.3109/10428190903585286. [DOI] [PubMed] [Google Scholar]
- Emadi A, Gore SD. Arsenic trioxide-an old drug rediscovered. Blood Rev. 2010;24:191–9. doi: 10.1016/j.blre.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrara N, Gerber H-P, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669–76. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- Goel HL, Mercurio AM. VEGF targets the tumour cell. Nat Rev Cancer. 2013;13:871–82. doi: 10.1038/nrc3627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S, Yel L, Kim D, et al. Arsenic trioxide induces apoptosis in peripheral blood T lymphocyte subsets by inducing oxidative stress: a role of Bcl-2. Mol Cancer Ther. 2003;2:711–9. [PubMed] [Google Scholar]
- Hu J, Liu Y-F, Wu C-F, et al. Long-term efficacy and safety of all-trans retinoic acid/arsenic trioxide-based therapy in newly diagnosed acute promyelocytic leukemia. Proc Natl Acad Sci U S A. 2009;106:3342–7. doi: 10.1073/pnas.0813280106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu S, Niu H, Inaba H, et al. Activity of the multikinase inhibitor sorafenib in combination with cytarabine in acute myeloid leukemia. J Natl Cancer Inst. 2011;103:893–905. doi: 10.1093/jnci/djr107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C-G, Heijn M, di Tomaso E, et al. Anti-vascular endothelial growth factor treatment augments tumor radiation response under normoxic or hypoxic conditions. Cancer Res. 2000;60:5565–70. [PubMed] [Google Scholar]
- Li YM, Broome JD. Arsenic targets tubulins to induce apoptosis in myeloid leukemia cells. Cancer Res. 1999;59:776–80. [PubMed] [Google Scholar]
- Liu L, Cao Y, Chen C, et al. Sorafenib blocks the RAF/MEK/ERK pathway, inhibits tumor angiogenesis, and induces tumor cell apoptosis in hepatocellular carcinoma model PLC/PRF/5. Cancer Res. 2006;66:11851–8. doi: 10.1158/0008-5472.CAN-06-1377. [DOI] [PubMed] [Google Scholar]
- Medinger M, Fischer N, Tzankov A. Vascular endothelial growth factor-related pathways in hemato-lymphoid malignancies. Int J Oncol 2010. 2010 doi: 10.1155/2010/729725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammadi S, Ghaffari SH, Shaiegan M, et al. Acquired expression of osteopontin selectively promotes enrichment of leukemia stem cells through AKT/mTOR/PTEN/beta-catenin pathways in AML cells. Life Sci. 2016;152:190–8. doi: 10.1016/j.lfs.2016.04.003. [DOI] [PubMed] [Google Scholar]
- Mohammadi S, Nikbakht M, Sajjadi SM, et al. Reciprocal interactions of leukemic cells with bone marrow stromal cells promote enrichment of leukemic stell cell compartments in response to curcumin and daunorubicin. Asian Pac J Cancer Prev. 2017a;18:831–40. doi: 10.22034/APJCP.2017.18.3.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammadi S, Zahedpanah M, Nikbakht M, et al. Parthenolide reduces gene transcription of prosurvival mediators in U937 cells. Exp Oncol. 2017b;39:30–5. [PubMed] [Google Scholar]
- Mori S, Cortes J, Kantarjian H, et al. Potential role of sorafenib in the treatment of acute myeloid leukemia. Leuk Lymphoma. 2008;49:2246–55. doi: 10.1080/10428190802510349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padro T, Bieker R, Ruiz S, et al. Overexpression of vascular endothelial growth factor (VEGF) and its cellular receptor KDR (VEGFR-2) in the bone marrow of patients with acute myeloid leukemia. Leukemia. 2002;16:1302. doi: 10.1038/sj.leu.2402534. [DOI] [PubMed] [Google Scholar]
- Panah MZ, Nikbakht M, Sajjadi SM, et al. Anti-apoptotic effects of osteopontin via the up-regulation of AKT/mTOR/β-catenin loop in acute myeloid leukemia cells. Int J Hematol Oncol Stem Cell Res. 2017;11:148–57. [PMC free article] [PubMed] [Google Scholar]
- Perez-Atayde AR, Sallan SE, Tedrow U, et al. Spectrum of tumor angiogenesis in the bone marrow of children with acute lymphoblastic leukemia. Am J Pathol. 1997;150:815. [PMC free article] [PubMed] [Google Scholar]
- Podar K, Anderson KC. The pathophysiologic role of VEGF in hematologic malignancies: therapeutic implications. Blood. 2005;105:1383–95. doi: 10.1182/blood-2004-07-2909. [DOI] [PubMed] [Google Scholar]
- Roboz GJ, Dias S, Lam G, et al. Arsenic trioxide induces dose-and time-dependent apoptosis of endothelium and may exert an antileukemic effect via inhibition of angiogenesis. Blood. 2000;96:1525–30. [PubMed] [Google Scholar]
- Rodriguez-Ariza A, Lopez-Pedrera C, Aranda E, et al. VEGF targeted therapy in acute myeloid leukemia. Crit Rev Oncol Hematol. 2011;80:241–56. doi: 10.1016/j.critrevonc.2010.09.009. [DOI] [PubMed] [Google Scholar]
- Rojewski M, Baldus C, Knauf W, et al. Dual effects of arsenic trioxide (As2O3) on non-acute promyelocytic leukaemia myeloid cell lines: induction of apoptosis and inhibition of proliferation. Br J Haematol. 2002;116:555–63. doi: 10.1046/j.0007-1048.2001.03298.x. [DOI] [PubMed] [Google Scholar]
- Rousselot P, Labaume S, Marolleau J-P, et al. Arsenic trioxide and melarsoprol induce apoptosis in plasma cell lines and in plasma cells from myeloma patients. Cancer Res. 1999;59:1041–8. [PubMed] [Google Scholar]
- Rubnitz JE, Gibson B, Smith FO. Acute myeloid leukemia. Hematol Oncol Clin North Am. 2010;24:35–63. doi: 10.1016/j.hoc.2009.11.008. [DOI] [PubMed] [Google Scholar]
- Song G, Li Y, Jiang G. Role of VEGF/VEGFR in the pathogenesis of leukemias and as treatment targets (Review) Oncol Rep. 2012a;28:1935–44. doi: 10.3892/or.2012.2045. [DOI] [PubMed] [Google Scholar]
- Song G, Li Y, Jiang G. Role of VEGF/VEGFR in the pathogenesis of leukemias and as treatment targets (Review) Oncol Rep. 2012b;28:1935–44. doi: 10.3892/or.2012.2045. [DOI] [PubMed] [Google Scholar]
- Teicher BA, Sotomayor EA, Huang ZD. Antiangiogenic agents potentiate cytotoxic cancer therapies against primary and metastatic disease. Cancer Res. 1992;52:6702–4. [PubMed] [Google Scholar]
- Tran J, Master Z, Joanne LY, et al. A role for survivin in chemoresistance of endothelial cells mediated by VEGF. roc Natl Acad Sci U S A. 2002;99:4349–54. doi: 10.1073/pnas.072586399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm S, Carter C, Lynch M, et al. Discovery and development of sorafenib: a multikinase inhibitor for treating cancer. Nat Rev Drug Discov. 2006;5:835–44. doi: 10.1038/nrd2130. [DOI] [PubMed] [Google Scholar]
- Wilhelm SM, Adnane L, Newell P, et al. Preclinical overview of sorafenib, a multikinase inhibitor that targets both Raf and VEGF and PDGF receptor tyrosine kinase signaling. Mol Cancer Ther. 2008;7:3129–40. doi: 10.1158/1535-7163.MCT-08-0013. [DOI] [PubMed] [Google Scholar]
- Zhu X-H, Shen Y-L, Jing Y-k, et al. Apoptosis and growth inhibition in malignant lymphocytes after treatment with arsenic trioxide at clinically achievable concentrations. J Natl Cancer Inst. 1999;91:772–8. doi: 10.1093/jnci/91.9.772. [DOI] [PubMed] [Google Scholar]
- Zhu Z, Hattori K, Zhang H, et al. Inhibition of human leukemia in an animal model with human antibodies directed against vascular endothelial growth factor receptor 2. Correlation between antibody affinity and biological activity. Leukemia. 2003;17:604–11. doi: 10.1038/sj.leu.2402831. [DOI] [PubMed] [Google Scholar]


