Abstract
Cratoxylum formosum Dyer is the Thai vegetable which commonly consumed a fresh leaves. In this study, we extracted Cratoxylum formosum with water and tested the extract for genotoxicity and anti-genotoxicity effects. We carried out the experiment using micronucleus test and comet assay in TK6 cells. In micronucleus experiment, we used cytokinesis-block proliferation technique to stop cell division which produced a cell at binucleated (BNC) stage. The comet assay was carried out after pre-treatment the cell with C. formosum for 18 h. The results revealed not increased the micronucleus frequency of C. formosum at concentration ranging from 50-150 µg/ml. In contract, it showed that the combination between C. formosum at various concentrations (25, 50, 75, 100, 150 and 200 µg/ml) and mitomycin C could decrease significantly in frequency of micronuclei. The mean of micronucleus frequency in the sample were 23.17 ±3.33, 23.33 ±4.72, 21.00 ±3.61, 11.33 ±3.21, 16.67 ±2.08, and 23.33±1.53 MN/ 1,000 BNC, respectively whereas the MMC-treated group was 33.67 ± 8.96 MN/ 1,000 BNC. The comet assay result showed that pre-treatment with Cratoxylum formosum (25, 50, 100, 200 µg/ml) could inhibit the hydrogen peroxide induced DNA damage by 6.95, 12.99, 17.61, and 26.39 respectively.
Keywords: Cratoxylum formosum Dyer, comet assay, micronucleus test
Introduction
Cratoxylum formosum Dyer (CF) was a plant mostly found in many Southeast Asia countries including Thailand. Many people used this plant as food. They are commonly consumed the fresh young leaf or sometimes used a leaf as an ingredient in soup. There are studies found that its leaf have many biological activities such as anti-oxidant and anti-mutagenic properties. Moreover, the aqueous extracts from leaf possess strong free radical scavenging and protective effect to vascular also (Yingngam et al., 2014; Nakahara et al., 2002; Maisuthisakul et al., 2007). However, there are little data on DNA effect of its leaf.
According to OECD guideline, a well know genotoxicity assays which is the comet assay and micronucleus (MN) test have been approved for detect DNA damage (Pant et al., 2015; Araldi et al., 2015). The alkaline comet assay or single-cell gel electrophoresis (SCGE) is a sensitive and specific technique for measuring DNA damage. The damage expressed as comet after the electric current pulled the charged DNA from the nucleus. The comet cell composed of the head of normal DNA and the tail of DNA break. Thereby, the intensity of the DNA in tail is correlated to DNA damage (Collins, 2004; Tice et al., 2000). In the MN test is assay for detect the DNA damage result from clastogenic and aneugenic activities which lead to micronuclei formation in the cytoplasm during mitosis. Thus, an increase in the micronuclei frequency is an indication of chromosomal damage (OECD, 2010; Fenech, 2000). Therefore, the combination of two different assays has been mostly used to assess of DNA damage in many cells and tissue.
The aim of the present study is to evaluate the potential in vitro anti-genotoxic effect of C. formosum leaves using the comet assay and MN test. These tests using TK6 lymphoblastoid cell line were performed.
Materials and Methods
Extractionf of C. formosum leaves
Fresh leaves of CF were harvested and collected from Phayao Province, North of Thailand. The leaves were cleaned by tab water and air-dried. Two hundred-fifty grams of fresh leaves were blended with 2.5 L distilled water and heated for 1 h at 80°C in a water bath. Following, filtration through Whatman no.1 filter paper using a suction apparatus, the extract was lyophilized giving a reddish brown color. The dry extracted was weight and kept at -20°C. The yield of freeze-dried powder from fresh leaves was about 8%W/V.
Cell culture and maintaining
The TK6 human lymphoblastoid cell line (CRL-8015) were purchased from American Type Culture Collection (ATCC, USA) and were maintained as exponentially phase in tissue culture flasks in suspension in 37 °C in humid atmosphere and with 5% CO2. The culture medium consist of RPMI 1640 medium supplemented with 10%(v/v) heat inactivated Horse Serum (HS). Cells were sub-culture every 48 hours.
Cytotoxicity test
The cytotoxicity test was done in our laboratory. CF extract was investigated in TK6 cells by WST-1 assay (Ngamwongsatit et al., 2008). In the experiment, TK6 cells were cultured in the presence of CF leaf extracts at various concentrations for 24 h and the percentage of the cell viability was evaluated by WST-1 method. Then, cell viability values were calculated as inhibitory concentration (IC50). The leaf extract of CF showed the low cytotoxicity expressed as IC50 values of 381.48±36.13 µg/ml as reported previous.
Micronucleus test
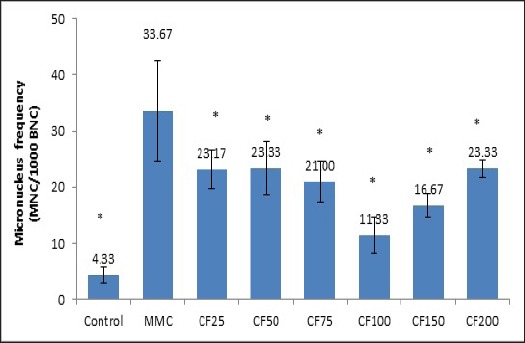
Prior to the anti-genotoxicity assay, the genotoxicity of CF leaves were evaluated at selected doses based on viability greater than 70%. In this study the extract at doses of 50, 100 and 150 µg/ml were incubated in TK6 cells for 24 h at 37°C. The anti-genotoxicity experiment was performed at doses (>70% cell viability) of 25, 50, 75, 100, 150 and 200 µg/ml RPMI in combination with a known mutagen, mitomycin C (MMC at 0.8 µg/ml) for 24 h (Elhajouji, 2010; Aardema et al., 2011; Fenech, 2007). After treatment, a cytochalasin B solution (Cyt B 3 µg/ml) was added during 6 h to collect the cells at a binucleated stage13. Following washing and harvesting steps, treated cells were prepared as monolayer on glass slides using cytospin equipment (Shadon, UK). Slides were left to dry at room temperature and then fixed in cold methanol for 30 mins. Cells on slides were stained using 10% Giemsa solution. Micronuclei (MN) formations were scored in 1000 binucleated (BNC) cells under light microscope (40x)(Figure 1).
Figure 1.
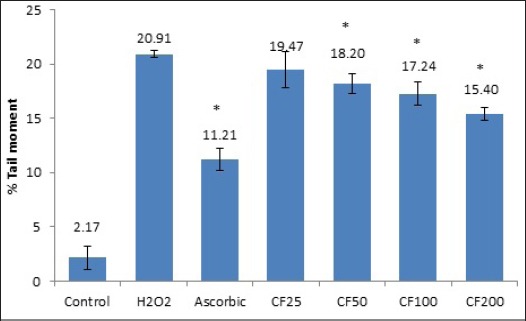
Bars Chart Showed the Frequencies of Micronucleus in TK6 Cells after Exposure to Different Doses of CF Leaf Extracts Ranging from 25- 200 µg/ml. The control group was untreated-cells, whereas MMC bar or positive control group was 0.8 µg/ml of mitomycin C treatment. The bar having asterisks are significant different from positive control at p≤ 0.05.
Comet assay
In the present study, alkaline condition (pH>13) was used in the lysing and electrophoresis steps which produce DNA denature. After seeding the TK6 cells (2×105 cells/ ml) into a 6-well plate, cells were treated for 18 h with CF leaf extract at doses of 25, 50, 100, 200 µg/ml RPMI. By the end of the treatment time, cells were harvested by to remove extract-containing medium. Cells were then treated with 50 µM H2O2 for 5 mins at 4 °C for induced DNA damage. Following treatment, cells were washed twice with cold HBSS and re-suspended in RPMI medium to be ready for comet assay.
The comet assay was performed following the method described by Tice, (2003). In brief, 20 µl of cell suspension was mixed with 75 µl of 0.5% low melting point (LMP) agarose at 37°C, layered onto a pre-coated slide with 0.75% normal melting point (NMP) agarose and covered with a coverslip allowing gel-solidification on a flat surface ice box. The coverslip was gently removed and 95 µl of LMP agarose was layered and covered with the coverslip. After coverslip removal, the slides immersed into a lysis solution (2.5M NaCl, 10 mM Na2EDTA·2H2O, 10 mM Trisma base, pH 10 with 1% triton X-100, 10% DMSO) for 2 h at 4°C. After lysis, slides were exposed to freshly make alkaline electrophoresis buffer (200 mM EDTA, 10 N NaOH, pH 13) for 20 min to allow DNA unwinding. The slides were then placed on an electrophoresis tank filled with sufficient electrophoresis solution and kept in an ice bath (4°C). Electrophoresis was carried out for 20 mins at constant 25V and a current of 300mA using a power pact supply. Then, the slides were neutralized in 0.4 M Trizma base buffer (pH 7.5) and stained with 100 µl of 20 µg/ml Ethidium bromide. At least 50 cells per slide and per treatment were randomly analyzed for comet images using the fluorescence microscope (at 40x magnification) connected to a computer equipped with an automated image analysis system (Comet assay III, Perceptive Instrument, UK)
Results of comet assay was used two parameters as indicator of DNA damage like tail length (TL= a distance of damage-DNA migration) and tail moment (TM; a DNA damage intensity) values.
Statistical analysis
The experiment was repeated in triplicate for MN test and duplicate for the comet assay. Data was expressed as mean ±SD of the medians were calculated for each group of treatments and then analyzed by SPSS software program. The statistical approach included the one-way ANOVA followed by LSD test which was to evaluate the significance of the differences in micronucleus frequency and comet assay data.
Results
Micronucleus test
In this study, we performed the micronucleus test to investigate the mutagenic and anti-mutagenic properties of the CF leaf extracts at various concentrations. Maximum concentrations were defined as those inducing around 40-50% toxicity which was 200 µg/ml of CF extract. Result of MN test showed that negative control (untreated cells) exhibited a baseline frequency of micronuclei of 1.33 MN/ 1,000 BNC. After exposure, CF leaf extracts did not induce the MN frequency in TK6 cells. The mean micronucleus frequencies in the CF treatment were 3.00, 2.50, and 2.67 MN/ 1,000 BNC for doses of 50, 100, and 150 µg/ml, whereas the induction of micronucleus found in MMC-treated cells were 5 MN/ 1,000 BNC.
For the anti-genotoxicity assay, the combination of CF leaf extract and MMC was performed in the MN test using TK6 cells. The mean frequency of micronucleus cells in negative control group was 2.33 ±3.21 MN/ 1,000 BNC. In contrast, MMC-treated group induce a significant increase in MN frequency from negative control group by 33.67 ± 8.96 MN/ 1,000 BNC. Interestingly, there were the significant decreases in mean frequency of micronucleus when cell treated with doses of 25, 50, 75, 100, 150 and 200 µg/ml of CF extracts. The micronucleus frequencies were 23.17 ±3.33, 23.33 ±4.72, 21.00 ±3.61, 11.33 ±3.21, 16.67 ±2.08, 23.33±1.53 MN/ 1000 BNC, respectively.
Comet assay
The result of effect of CF leaf on anti-DNA damage in TK6 cells induced by H2O2 was determined by the comet assay. As showed the results presented in Table 1 and Figure 2, demonstrated the untreated cells showed base line data on TL and TM values of 30.02± 3.81 and 2.17± 1.07 respectively. Whereas in H2O2-treated TK6 groups produced a marked increase in both TL and TM values (66.84± 2.77 and 20.91± 0.28). However, the ability of ascorbic acid to decrease DNA damage in cell culture treated with H2O2 by 57.13± 3.04 and 11.21± 1.07 for TL and TM values. Interestingly, pre-treatment of TK6 cells with 25, 50, 100 and 200 µg/ml of CF leaf significantly inhibition H2O2-induced DNA damage by 6.95%, 12.99%, 17.61%, and 26.39% (data based on TM values)
Table 1.
Mean and Standard Deviation of Comet assay Data (Tail Length and Tail Moment) of Cf Leaf Extract, Control, H2o2, and Ascorbic Acid. All Values are Expressed as Mean ±Sd.
| Concentration (µg/ml) | Tail length (µm)± SD | Tail moment (%)± SD | % Damage inhibition |
|---|---|---|---|
| Control | 30.02± 3.81 | 2.17± 1.07 | - |
| H2O2 µm | 66.84± 2.77 | 20.91± 0.28 | - |
| Ascorbic 100 µm | 57.13± 3.04 | 11.21± 1.07 | - |
| CF 25 | 62.42± 10.87 | 19.47± 1.66 | 6.95 |
| CF 50 | 64.31± 0.17 | 18.19± 0.92 | 12.99 |
| CF 100 | 58.52± 4.41 | 17.24± 1.07 | 17.61 |
| CF 200 | 57.10± 1.65 | 15.39± 0.63 | 26.39 |
Figure 2.
Bar Charts from Comet assay Results Represented by %Tail Moment (TM) Values. All values are expressed as mean ±SD. Pre-treatment with CF leaf extracts (50, 100, 200 µg/ml) exhibited significantly difference from H2O2-treated groups. Asterisk (*) show different from H2O2-treated groups. The mean difference is significant at the 0.05 level.
Discussion
The present study, we evaluated the anti-genotoxic effect of Cratoxylum formosum leaf extract by the micronucleus test and comet assay in TK6 cells. The advantages of these assays because the MN test and comet assay are two of the most widely used methods to characterize DNA damage induce by chemical compounds. Both assays are difference in endpoints of DNA damage. The MN test, indicates DNA damage as clastogenic or aneugenic activities, whereas the comet assay for detecting DNA strand, cross-links and alkaline-labile sites which utilized a wide variety of cells and tissues (Pilar et al., 2016; Zapata et al., 2016). However, in comet assay was relatively sensitivity than the MN test because the comet assays can detection of carcinogens which gave negative results in the MN test. Therefore, we suggested the used of both techniques for assessing whether the chemical can induced DNA damage (Pant et al., 2015; Araldi et al., 2015).
In consideration of the result of this study, the protective effect might be the phenolic compound found in C. formosum leaf due to anti-oxidant activity. These would lead to being oxidized the H2O2 to attack cellular component (Kaur and Kapoor, 2001). Similar results were reported that the extraction for phenolic compounds from C. formosum has protective ability against H2O2 –induced HEK293 cells death (Yingngam et al., 2014).
Our results reveal the absence of genotoxicity of C. formosum leaf extract in TK6 cells. For the anti-genotoxicity test, C. formosum leaf extract possess a moderate inhibition of DNA damage both in vitro comet assay and micronucleus test. Moreover, we suggest the in vivo data are required before its approval.
Acknowledgements
The author wish thank for University of Phayao, Thailand for the financial support of this study. I also would like to thank Dr. Prapaipat Klungsupya and colleagues, a scientist of Thailand Institute of Scientific and Technological Research (TISTR). They were performed the laboratory experiment of the micronucleus test and comet assay.
References
- Aardema MJ, Galloway S, Zeiger E, Cimino MC, Hayashi M. Guidance for understanding solubility as limiting factor for selecting the upper test concentration in the OECD In Vitro Micronucleus Assay Test Guideline No. 487. Mutat Res. 2011;722:89–90. doi: 10.1016/j.mrgentox.2011.03.007. [DOI] [PubMed] [Google Scholar]
- Araldi RP, Melo TC, Mendes TB, et al. Using the comet and micronucleus assays for genotoxicity studies: A review. Biomed Pharmacother. 2015;72:74–82. doi: 10.1016/j.biopha.2015.04.004. [DOI] [PubMed] [Google Scholar]
- Collins AR. The comet assay for DNA damage and repair: principles, applications, and limitations. Mol Biotechnol. 2004;26:249–61. doi: 10.1385/MB:26:3:249. [DOI] [PubMed] [Google Scholar]
- Elhajouji A. Mitonycin C, 5-fluoruracil, colchicine and etoposide tested in the in vitro mammalian cell micronucleus test (MNvit) in the human lymphoblastoid cell line TK6 at Novartis in support of OECD draft Test Guideline 487. Mutat Res. 2010;702:157–62. doi: 10.1016/j.mrgentox.2010.03.011. [DOI] [PubMed] [Google Scholar]
- Fenech M. The in vitro micronucleus technique. Mutat Res. 2000;20:81–95. doi: 10.1016/s0027-5107(00)00065-8. [DOI] [PubMed] [Google Scholar]
- Fenech M. Cytokinesis-block micronucleus cytome assay. Nat Protoc. 2007;2:1084–104. doi: 10.1038/nprot.2007.77. [DOI] [PubMed] [Google Scholar]
- Kaur C, Kapoor HC. Antioxidants in fruits and vegetables-the millenium’s health. Int J Food Sci Technol. 2001;36:703–25. [Google Scholar]
- Maisuthisakul P, Pongsawatmanit Gordon MH. Characterization of the phytochemicals and antioxidant properties of extracts from Teaw (Cratoxylum formosum Dyer) Food Chem. 2007;100:1620–29. [Google Scholar]
- Nakahara K, Trakoontivakorn G, Alzoreky N, et al. Antimutagenicity of some edible Thai plants, and a bioactive carbazole alkaloid, mahanine, isolated from Micromwlem minutum. J Agric Food Chem. 2002;50:4796–802. doi: 10.1021/jf025564w. [DOI] [PubMed] [Google Scholar]
- Ngamwongsatit P, Banada PP, Panbangred W, Bhunia AK. WST-1-based cell cytotoxicity assay as a substitute for MTT-based assay for rapid detection of toxigenic Bacillus species using CHO cell line. J Microbiol Methods. 2008;73:211–5. doi: 10.1016/j.mimet.2008.03.002. [DOI] [PubMed] [Google Scholar]
- OECD (Organisation for economic cooperation and development) Guideline for the testing of chemicals: In vitro mammalian cell micronucleus test. Guideline. 2010;487:1–23. [Google Scholar]
- Pant K, Krsmanovic L, Bruce SW, et al. Combination comet and micronucleus assay validation performed by BioReliance under the JaCVAM initiative. Mutat Res. 2015;788:87–97. doi: 10.1016/j.mrgentox.2015.03.010. [DOI] [PubMed] [Google Scholar]
- Pilar MC, Puerto M, Prieto AL, et al. Genotoxicity of a thiosulfonate compound derived from ALLium sp. Intented to be used in active food packaging: In vivo comet assay and micronucleus test. Mutat Res. 2016;800:1–11. doi: 10.1016/j.mrgentox.2016.03.001. [DOI] [PubMed] [Google Scholar]
- Tice E, Agurell D, Anderson B, et al. Single cell gel/Comet assay: guidelines for in vitro and in vivo genetic toxicology testing. Environ Mol Mutagen. 2000;35:206–21. doi: 10.1002/(sici)1098-2280(2000)35:3<206::aid-em8>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Tice RR, Agurell E, Anderson D, et al. Single cell gel/Comet assay: guidelines for in vitro and in vivo genetic toxicology testing. Environ Mol Mutagen. 2003;35:206–11. doi: 10.1002/(sici)1098-2280(2000)35:3<206::aid-em8>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Yingngam B, Monschein M, Brantner A. Ultrasound-assisted extraction of phenolic compounds from Cratoxylum formosum ssp. Formosum leaves using central composite design and evaluation of its protective ability against H2O2-induced cell death. Asian Pac J Trop Dis. 2014;7:497–505. doi: 10.1016/S1995-7645(14)60281-9. [DOI] [PubMed] [Google Scholar]
- Zapata ML, Bock BC, Orozco LY, Palacio JA. Application of the micronucleus test and comet assay in Trachemys callirostris erythrocytes as a model for in situ genotoxic monitoring. Ecotoxicol Environ Saf. 2016;127:108–16. doi: 10.1016/j.ecoenv.2016.01.016. [DOI] [PubMed] [Google Scholar]


