Abstract
Backgrounds:
Recently Hong Kong Liver Cancer (HKLC) staging system has been proposed for staging of hepatocellular carcinoma (HCC), and has been shown to provide better prognostic ability than the Barcelona Clinic Liver Cancer (BCLC) system. However, the HKLC system lacks external validation, and its applicability remains uncertain. The present study was aimed to evaluate the prognostic performance of HKLC in HCC patients treated with curative intent.
Methods:
Medical records of HCC patients treated with either resection or radiofrequency ablation (RFA) from 2011 to 2016 were retrospectively reviewed. The overall survival and the prognostic ability of the HKLC and BCLC system were evaluated.
Results:
79 HCC patients were included, of which 64.56% had Child A cirrhosis. Chronic viral hepatitis B infection was the leading cause of HCC, followed by chronic viral hepatitis C infection, alcohol and alcohol with HBV or HCV infection. According to the BCLC system, 82.28% were in stage 0-A, and according to the HKLC system, 93.67% were in stage I-IIb. RFA and liver resection were the primary treatment in 56.96% and 43.04%, respectively. The 5-year survival rate of patients in HKLC stage I, IIa and IIb were 81.64%, 61.66%, and 54.42%, respectively (P<0.001). Whereas, the 5-year survival rate of patients in BCLC stage 0, A and B were 60.00%, 75.90%, and 26.65%, respectively (P=0.053). The AUROC curve of the HKLC and BCLC for the entire cohort was 0.77 and 0.64, respectively (P=0.15). Subgroup analysis showed the AUROC curve of the HKLC and BCLC for the patients with viral-associated HCC was 0.79 and 0.68, respectively (P=0.02).
Conclusions:
Applying the HKLC staging system provides a good discriminative ability for survival prediction in HCC patients treated with curative intent. Comparing with the BCLC system, the HKLC system tends to yield better prognostic accuracy, particularly in viral-associated HCC.
Keywords: Hepatocellular carcinoma, Hong Kong Liver Cancer Staging system, Radiofrequency ablation
Introduction
Hepatocellular carcinoma (HCC), a primary cancer of the liver, is one of the major global health issues due to its large population burden (Somboon et al., 2014; Intaraprasong et al., 2016; Wanich et al., 2016). Currently, it is ranked as the sixth most prevalent cancer, affecting around 6% of population worldwide. Despite an improvement in the diagnostic and treatment protocol, it is still ranked as the second leading cause of cancer death(Ferlay et al., 2015).
Since the launch of the renowned Barcelona Clinic Liver Cancer (BCLC) staging system in year 2002, it has been adopted worldwide by the major organizations such as AASLD and EASL as a standard system for staging and treatment decision for HCC (Bruix and Llovet, 2002; Pons et al., 2005). Over the last decade, several studies have successfully illustrated the efficacy of BCLC system for prognostic and therapeutic guidance in HCC (Pons et al., 2005; Bruix and Sherman, 2011; Gomez-Rodriguez et al., 2012; Santambrogio et al., 2013).
In 2014, a new staging system for HCC, namely Hong Kong Liver Cancer (HKLC), was firstly introduced. In the first report, HKLC system was shown to provide more precise discriminatory power and prognostic prediction than the traditional BCLC system(Yau et al., 2014). Hence, unlike the BCLC system, the HKLC staging system has not been widely acknowledged due to lacking of external validations. In this study, we aimed to validate the performance of HKLC system for staging and predicting prognosis in patients with HCC aiming for curative treatment in comparison to the traditional BCLC system.
Materials and Methods
Patients
A database of all newly diagnosed HCC patients treated with curative intent by either hepatic resection (HR) or radiofrequency ablation (RFA) during the period of January 2011 to September 2016 at Thammasat University Hospital, a referral university hospital in the central region of Thailand, was retrospectively reviewed. HCC was diagnosed either by delicate histology examination or by criteria endorsed by AASLD and EASL (Bruix and Sherman, 2011; 2012). Each patient was comprehensively inspected for age, sex, previous and current illnesses, etiology and staging of cirrhosis, presence of portal hypertension, preoperative MELD score, mass number, diameter of the largest mass, presence of intra-hepatic vascular involvement, treatment modality, disease free period, and overall survival.
In every subject, all the gathered information was used for HCC staging by both the BLCL and HKLC systems(Pons et al., 2005; Yau et al., 2014). Recurrent time was defined as the time from the beginning of curative treatment to the earliest evidence of HCC reappearance. Overall survival (OS) was defined as the time from HCC diagnosis to the last medical visit or death.
Statistical analysis
Continuous variables were compared by the Student’s t-test, and categorical variables were compared by Chi-square or Fisher’s exact test where appropriate. The time to recurrent and overall survival were evaluated by Kaplan-Meier method with log-rank test. The prognostic ability of BCLC and HKLC system was analyzed and compared by area under the receiver operator characteristic curve(AUROC). The p-value<0.05 was considered as statistically significant. All statistical analyses were performed by STATA version 13.0 (Stata Corp, College Station, TX, USA).
Results
Baseline characteristics
A total of 79 patients with HCC were included in this study. Sixty patients (75.9%) were male with a mean age of 62.55±11.55 years. Chronic hepatitis B virus (HBV) infection was the leading cause of HCC (n=37, 46.84%), followed by chronic hepatitis C (HCV) infection (n=19, 24.05%), alcohol (n=8, 10.13%), alcohol with HBV or HCV infection (n= 5, 6.33%) and hemochromatosis(n=1, 1.27%). The staging of liver cirrhosis was Child-Pugh A and B in 51 (64.56 %), and 23 (29.11%) patients, respectively. The pre-treatment MELD score was 10.49± 4.18. At the diagnosis of HCC, the mean size of the largest mass was 4.14±3.05 cm, and the number of HCC was 1, 2, 3 and >3 masses in 50 (63.29%), 15 (18.99%), 12 (15.19%) and 2 (2.53%), respectively. Forty-five patients (56.96%) received RFA as their primary treatment, whereas 34 patients (43.04%) were subjected to liver resection.
As shown in the Table 1, applying the BCLC system, 10 (12.66%), 55 (69.62%), 13 (16.45%), and 1 (1.27%) patients were classified in stage 0, A, B and C, respectively. Meanwhile, 41 (51.90%), 19 (24.05%), 14 (17.72%), 4(5.06%) and 1 (1.27%) patients were classified in stage 0, I, IIa, IIb, IIIa and IIIb, respectively, by the HKLC system.
Table 1.
Baseline Characteristics of Study Population
| Factors | Study Population |
|---|---|
| Baselines characteristics : | |
| Sex (% Male) | 60 (75.95%) |
| Age (years) | 62.55±11.55 |
| Presence of cirrhosis | 74 (93.67%) |
| - Child-Pugh A | 51 (64.56%) |
| - Child-Pugh B | 23 (29.11%) |
| Viral hepatitis infection | 55 (69.62%) |
| Etiology | |
| - HBV infection | 37 (46.84%) |
| - HCV infection | 19 (24.05%) |
| - Alcohol | 8 (10.13%) |
| - Alcohol + HBV/HCV infection | 5 (6.33%) |
| - Hemochromatosis | 1 (1.27%) |
| Preoperative MELD score | 10.49±4.18 |
| Largest mass diameter (cm.) | 4.14± 3.05 |
| Presence of portal hypertension* | 45 (56.96%) |
| Mass number | |
| - 1 | 50 (63.29%) |
| - 2 | 15 (18.99%) |
| - 3 | 12 (15.19%) |
| - > 3 | 2 (2.53%) |
| Intrahepatic vascular involvement | 9 (11.39%) |
| Staging : | |
| BCLC Staging | |
| - BCLC 0 | 10 (12.66%) |
| - BCLC A | 55 (69.62%) |
| - BCLC B | 13 (16.45%) |
| - BCLC C | 1 (1.27%) |
| HKLC Staging | |
| - HKLC I | 41 (51.90%) |
| - HKLC IIa | 19 (24.05%) |
| - HKLC IIb | 14 (17.72%) |
| - HKLC IIIa | 4 (5.06%) |
| - HKLC IIIb | 1 (1.27%) |
| Treatment : | |
| Radiofrequency ablation | 45 (56.96%) |
| Resection | 34 (43.04%) |
Portal hypertension is defined as presence of esophageal varices or splenomegaly with platelet count ≤ 100,000 cell
Patient outcomes
HCC recurrence
As shown in the table 2, the 1- and 5- year recurrent rates were 10% and 25% for those in BCLC stage 0, 26.55% and 80.50% for those in BCLC stage A and 22.22% and 67.59% for those in BCLC stage B (P= 0.46). Whereas, the 1- and 5- year recurrent rates were 18.46% and 52.75% for those in HKLC stage I, 22.57% and 100.00% for those in HKLC stage IIa and 28.57% and 100% for those in HKLC stage IIb, respectively (P= 0.18).
Table 2.
Recurrent Rate of Patients according to HKLC and BCLC Staging System
| All HCC patients (N=78) | ||||
|---|---|---|---|---|
| 1-year recurrence | 2-year recurrence | 5-year recurrence | P value | |
| HKLC | ||||
| - Stage I | 18.46% | 39.24% | 52.75% | 0.18 |
| - Stage IIa | 22.57% | 39.78% | 100.00% | |
| - Stage IIb | 28.57% | 64.29% | 100.00% | |
| BCLC | ||||
| - Stage 0 | 10.00% | 25.00% | 25.00% | 0.46 |
| - Stage A | 26.55% | 46.37% | 80.50% | |
| - Stage B | 22.22% | 67.59% | 67.59% | |
Overall survival
As shown in the table 3, the 1- and 5- year survival rates were 100.00% and 60.00% for those in BCLC stage 0, 86.79% and 75.90% for those in BCLC stage A, and 76.15% and 26.65% for those in BCLC stage B (P= 0.05). Whereas, the 1- and 5-year survival rates were 100% and 81.64% for those in HKLC stage I, 82.22% and 61.66% for those in HKLC stage IIa, and 76.29% and 54.42% for those in HKLC stage IIb (P <0.001).
Table 3.
Overall Survival Rate of Patients According to HKLC And BCLC Staging System
| All HCC patients (N=79) | Viral-associated HCC patients (N=55) | |||||||
|---|---|---|---|---|---|---|---|---|
| 1-year survival | 2-year survival | 5-year survival | P-value | 1-year survival | 2-year survival | 5-year survival | P-value | |
| HKLC | ||||||||
| - Stage I | 100.00% | 89.06% | 81.64% | <0.001 | 100.00% | 92.31% | 92.31% | 0.016 |
| - Stage IIa | 82.22% | 74.00% | 61.66% | 76.15% | 65.27% | 65.27% | ||
| - Stage IIb | 76.29% | 54.42% | 54.42% | 66.67% | 66.67% | 66.67% | ||
| BCLC | ||||||||
| - Stage 0 | 100.00% | 80.00% | 60.00% | 0.053 | 100.00% | 75.00% | 75.00% | 0.153 |
| - Stage A | 86.79% | 82.23% | 75.90% | 93.85% | 86.38% | 86.38% | ||
| - Stage B | 76.15% | 26.65% | 26.65% | 85.71% | 64.29% | 64.29% | ||
Viral-associated HCC (n=55)
Subgroup analysis was performed in 55 patients with chronic HBV or HCV infection. As demonstrated in the table 3, the 1- and 5- year survival rates were 100.00% and 75.00% for those in BCLC stage 0, 93.85% and 86.38% for those in BCLC stage A and 85.71% and 64.29% for those in BLCL B (P=0.15). Whereas, the 1- and 5- year survival rates were 100% and 92.31% for those in HKLC stage I, 76.15% and 65.27% for those in HKLC stage IIa and 66.67% and 66.67% for those in HKLC stage IIb (P= 0.016).
Comparing the prognostic performance of BCLC and HKLC systems
As shown in the Figure 1, the AUROC of the HKLC for the prediction of overall survival of the entire cohort was greater than that of BCLC system (0.77 and 0.64, respectively), however no statistical significant was reached (P=0.15). Whereas, the AUROC of the HKLC for the prediction of overall survival in patients with viral associated HCC was significantly greater than that of BCLC (0.79 and 0.68, respectively, P= 0.02).
Figure 1.
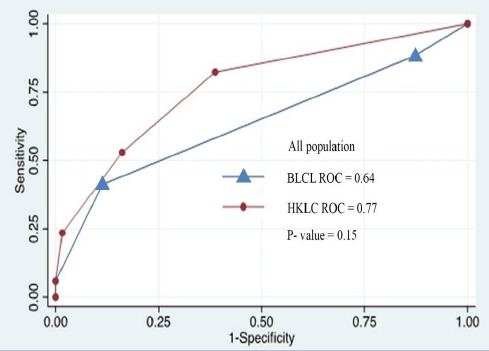
ROC Analysis for Overall Survival in HCC Patients Classified by HKLC and BCLC System
Figure 2.
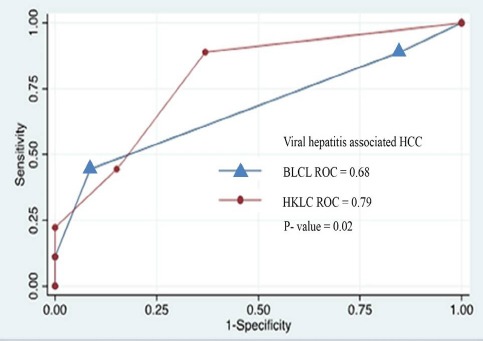
ROC Analysis for Viral Hepatitis Associated HCC Patients Classified by HKLC and BCLC System
Discussion
Over the last decade, the issue of hepatocellular carcinoma management has been heavily investigated (Pawlik et al., 2005; Gomez-Rodriguez et al., 2012; Lin et al., 2012; Hsu et al., 2013; Khan et al., 2014). Despite various algorithms proposed by many study groups, management decisions are still controversial in which protocol to be followed. The BCLC staging classification has been adopted as a standard staging system for HCC because this system takes into account clinical information affecting prognosis of patients and links with treatment application (Bruix and Sherman, 2011; 2012). However, over recent years, the prognostic ability and generalization of this system to all HCC patients has been questioned. For instance, several studies have demonstrated successful treatment of HCC by more aggressive treatment modalities as recommend by the BCLC system (Wu et al., 2000; Pawlik et al., 2005).
The HKLC staging classification was firstly introduced in 2014 by Yau and colleagues. In the original study, applying the HKLC system was able to provide better prognostic stratification than the conventional BCLC system, especially in patients with intermediate to advanced stages of HCC (Yau et al., 2014). Accordingly, the HKLC algorithm was recommended as a preferable guideline over the standard BCLC protocol. In addition, subsequent studies were able to demonstrate that HKLC protocol provided better prognostic ability than BCLC protocol (Liu et al., 2015; Wu et al., 2016). However, more data are needed to verify the performance of this system before it can be generally implemented.
The present study focuses on the prognostic ability of HKLC system in patients with early stage HCC aiming for curative treatment. Interestingly, our results show that both BCLC and HKLC staging systems were able to predict the overall survival in these patients. However, in the subgroup of HCC patients related with chronic HBV and HCV infection, HKLC system was able to provide better prognostic prediction than the traditional BCLC system. This finding encourages the use of HKLC system to allocate the patients with viral-associated HCC to receive suitable treatment options. This finding could possibly be explained by the fact that the HKLC system was originally developed from a cohort of patients with predominantly chronic HBV infection, whereas the BCLC system was developed in Caucasian population with high prevalence of chronic alcoholism and chronic HCV infection(Bruix and Llovet, 2002; Pons et al., 2005). Indeed, all of our patients were Asians and almost half were chronic HBV infection-associated HCC. These findings emphasize the diversity in natural history and prognosis of HCC patients across different continents and different etiologies.
Another possible explanation is that the role of hepatic resection in early and very early HCC has been expanded in the HKLC system comparing to standard BCLC system(Intaraprasong et al., 2016). This change is attributed to the advancement of novel surgical techniques and peri-operative care. Indeed, over the past few years, hepatic resection was shown to be effective in patients with HCC up to 3 masses or 5 cm in size or in HCC patients with mild portal hypertension or with intrahepatic vascular involvement (Wu et al., 2000; Pawlik et al., 2005).
Nevertheless, the present study has several limitations. First, data were collected retrospectively, therefore some relevant information could be missed. Secondly, we included only patients with early stage of HCC received curative treatment, therefore the results of this study could not be generally applied to all HCC patients.
In conclusion, the present study shows that HKLC staging system provides strong discriminative ability and therapy allocation in patients with HCC treated with curative intent. It clearly outperforms the traditional BCLC system in those with viral hepatitis associated HCC. Applying the HKLC system to prognostic stratification and treatment application in patients with HCC is a promising approach in a new era of HCC management.
List of abbreviations
AASLD, American Association for the Study of Liver Diseases; AUROC, Area under Reciever Operating Characteristic; BCLC, Barcelona Clinic Liver Cancer; EASL, European Association for the Study of the Liver; HBV, Hepatitis B Virus; HCC, hepatocellular carcinoma; HCV, Hepatitis C virus; HKLC, Hong Kong Liver Cancer; HR, Hepatic resection; MELD, Model for End-Stage Liver Disease; OS, Overall Survival; RFA, Radiofrequency Ablation
Statement conflicts of interest
The authors declare no conflict of interest.
References
- Bruix J, Llovet JM. Prognostic prediction and treatment strategy in hepatocellular carcinoma. Hepatology. 2002;35:519–24. doi: 10.1053/jhep.2002.32089. [DOI] [PubMed] [Google Scholar]
- Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020–2. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EASL-EORTC clinical practice guidelines. Management of hepatocellular carcinoma. J Hepatol. 2012;56:908–43. doi: 10.1016/j.jhep.2011.12.001. [DOI] [PubMed] [Google Scholar]
- Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:359–86. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- Gomez-Rodriguez R, Romero-Gutierrez M, Artaza-Varasa T, et al. The value of the Barcelona clinic liver cancer and alpha-fetoprotein in the prognosis of hepatocellular carcinoma. Rev Esp Enferm Dig. 2012;104:298–304. doi: 10.4321/s1130-01082012000600003. [DOI] [PubMed] [Google Scholar]
- Hsu CY, Lee YH, Hsia CY, et al. Performance status in patients with hepatocellular carcinoma: determinants, prognostic impact, and ability to improve the Barcelona Clinic Liver Cancer system. Hepatology. 2013;57:112–9. doi: 10.1002/hep.25950. [DOI] [PubMed] [Google Scholar]
- Intaraprasong P, Siramolpiwat S, Vilaichone RK. Advances in management of hepatocellular carcinoma. Asian Pac J Cancer Prev. 2016;17:3697–703. [PubMed] [Google Scholar]
- Khan AS, Fowler KJ, Chapman WC. Current surgical treatment strategies for hepatocellular carcinoma in North America. World J Gastroenterol. 2014;20:15007–17. doi: 10.3748/wjg.v20.i41.15007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S, Hoffmann K, Schemmer P. Treatment of hepatocellular carcinoma: a systematic review. Liver Cancer. 2012;1:144–58. doi: 10.1159/000343828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu PH, Hsu CY, Lee YH, et al. Hong Kong liver cancer staging system is associated with better performance for hepatocellular carcinoma: Special emphasis on viral etiology. Medicine (Baltimore) 2015;94:e1772. doi: 10.1097/MD.0000000000001772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawlik TM, Poon RT, Abdalla EK, et al. Hepatectomy for hepatocellular carcinoma with major portal or hepatic vein invasion: results of a multicenter study. Surgery. 2005;137:403–10. doi: 10.1016/j.surg.2004.12.012. [DOI] [PubMed] [Google Scholar]
- Pons F, Varela M, Llovet JM. Staging systems in hepatocellular carcinoma. HPB (Oxford) 2005;7:35–41. doi: 10.1080/13651820410024058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santambrogio R, Salceda J, Costa M, et al. External validation of a simplified BCLC staging system for early hepatocellular carcinoma. Eur J Surg Oncol. 2013;39:850–7. doi: 10.1016/j.ejso.2013.05.001. [DOI] [PubMed] [Google Scholar]
- Somboon K, Siramolpiwat S, Vilaichone RK. Epidemiology and survival of hepatocellular carcinoma in the central region of Thailand. Asian Pac J Cancer Prev. 2014;15:3567–70. doi: 10.7314/apjcp.2014.15.8.3567. [DOI] [PubMed] [Google Scholar]
- Wanich N, Vilaichone RK, Chotivitayatarakorn P, et al. High prevalence of hepatocellular carcinoma in patients with chronic hepatitis B infection in Thailand. Asian Pac J Cancer Prev. 2016;17:2857–60. [PubMed] [Google Scholar]
- Wu CC, Hsieh SR, Chen JT, et al. An appraisal of liver and portal vein resection for hepatocellular carcinoma with tumor thrombi extending to portal bifurcation. Arch Surg. 2000;135:1273–9. doi: 10.1001/archsurg.135.11.1273. [DOI] [PubMed] [Google Scholar]
- Wu L, Bartlett A, Plank L, et al. Validation of the Hong Kong liver cancer staging system in hepatocellular carcinoma patients treated with curative intent. J Hepatol. 2016;64:978–9. doi: 10.1016/j.jhep.2015.12.008. [DOI] [PubMed] [Google Scholar]
- Yau T, Tang VY, Yao TJ, et al. Development of Hong Kong Liver Cancer staging system with treatment stratification for patients with hepatocellular carcinoma. Gastroenterology. 2014;146:1691–700. doi: 10.1053/j.gastro.2014.02.032. [DOI] [PubMed] [Google Scholar]


