Abstract
Dibutyl phthalate (DBP) is present in consumer products and the coating of some oral medications. Acetyl tributyl citrate (ATBC) has been proposed as an alternative to DBP because DBP causes endocrine disruption in animal models. Following ingestion, DBP is converted to its main metabolite mono-butyl phthalate (MBP) which has been detected in >90% of human follicular fluid samples. Previous studies show that DBP reduces the number of antral follicles present in the ovaries of mice. Thus, this study was designed to evaluate the effects of DBP, MBP, and ATBC on in vitro growth and viability of mouse ovarian antral follicles. Antral follicles were isolated from CD-1 females (PND32-37) and treated with vehicle, DBP, MBP, or ATBC (starting at 0.001 and up to 1000 μg/ml for DBP; 24–72 h). Follicle diameter, ATP production, qPCR, and TUNEL were used to measure follicle growth, viability, cell cycle and apoptosis gene expression, and cell death-associated DNA fragmentation, respectively. While MBP did not cause toxicity, DBP exposure at ≥10 μg/ml resulted in growth inhibition followed by cytoxicity at ≥500 μg/ml. ATBC increased the number of nongrowing follicles at 0.01 μg/ml and did not affect ATP production, but increased TUNEL positive area in treated follicles. Gene expression results suggest that cytotoxicity in DBP-treated follicles occurs via activation of cell cycle arrest prior to follicular death. These findings suggest that concentrations of DBP ≥10 μg/ml are detrimental to antral follicles and that ATBC should be examined further as it may disrupt antral follicle function at low concentrations.
Keywords: dibutyl phthalate, mono-butyl phthalate, acetyl tributyl citrate, phthalate substitute, ovary, antral follicle, endocrine disruptor, toxicology, cell cycle, apoptosis
Summary Sentence
DBP and ATBC exposures resulted in dose-specific follicle growth inhibition with high concentrations of DBP causing cytoxicity via activation of cyclin-dependent kinase inhibitors and subsequent apoptosis.
Introduction
Endocrine-disrupting chemicals (EDCs) have been recognized as a significant threat to human fertility [1]. EDCs are exogenous agents that are able to interact with the endocrine system and influence the processes that regulate metabolic homeostasis, development, and reproduction. One example of EDCs are phthalate esters, a group of chemicals commonly used in products such as plastics, personal care products, medical equipment, and in the coating of some oral medications [2]. Risk assessment studies using animal models have demonstrated that phthalates can negatively impact the male and female reproductive systems (reviewed in [3]). In humans, phthalate exposures have been associated with adverse reproductive health outcomes including early menopause, endometriosis, early pregnancy loss, and decreased oocyte retrieval during in vitro fertilization cycles [4–8].
Dibutyl phthalate (DBP) is a low molecular weight phthalate commonly found in some food packaging materials, personal care products, and the coating of oral medications [9]. It is estimated that DBP exposure in the general population is 7–10 μg/kg/day, while individuals exposed in the workplace and through medications are exposed to 0.1–76 and 1–233 μg/kg/day, respectively [9–11]. High dose exposure risk assessment studies (for review, see [3]) have demonstrated that DBP increased mid-term abortions (500 mg/kg/day) and reproductive tract malformations (250 and 500 mg/kg/day) in treated rats [12]. In the female offspring of rats treated during pregnancy, DBP exposure delayed vaginal opening and onset of estrous cyclicity (12,000 and 50,000 mg/kg; [13]) and decreased lordosis quotient (50,000 mg/kg; [14]). DBP treatment decreased ovarian (1250 and 1500 mg/kg/day) and uterine (750–1500 mg/kg/day) weight in pseudopregnant rats [15] and in immature female rats (uterine; 100 mg/kg/day; [16]). Using dosages that resemble those estimated for humans (10 and 100 μg/kg/day), we have shown that mice exposed to DBP for 10 days have decreased 17β-estradiol and increased gonadotropin levels in their serum, as well as fewer antral follicles and disrupted apoptosis gene expression in their ovaries [17]. Taken together, these findings suggest that DBP may target the ovary and affect the survival of ovarian antral follicles.
Upon entering the body, DBP is quickly metabolized by esterases to form mono-butyl phthalate (MBP), which is commonly referred to as the toxic metabolite based on its ability to produce reproductive toxicity in rodents. In humans, MBP has been detected in follicular fluid obtained from women undergoing IVF with median concentrations ranging from 1.46 ng/ml in a small group of women in the USA [18] to 2.05 ng/ml in a large cohort of women in China [19]. Taken together, reports showing that DBP reduces the number of antral follicles in the ovaries of treated mice and that MBP is detected in >90% of human follicular fluid samples highlight the importance of assessing the effects of DBP and MBP on antral follicle function.
Acetyl tributyl citrate (ATBC) is an FDA-approved food contact chemical which has been proposed as an alternative to DBP [20]. Like DBP, ATBC has been shown to migrate from food packaging material and medical equipment into the products that they contain [20]. Interestingly, at least one study has reported ATBC to leach from medical equipment more rapidly than the potent EDC, di-2-ethylhexyl phthalate (DEHP) [20–22]. The systemic, reproductive, and developmental toxicity of ATBC have been evaluated in animal models by employing dosages ranging from 50 to 1000 mg/kg/day [20]. Collectively, these studies reported low systemic, reproductive, and developmental toxicity for ATBC (no observable adverse effect levels of 100 mg/kg/day for systemic and 250–1000 mg/kg/day for reproductive and developmental toxicity), thus leading to the conclusion that ATBC is a safe alternative to DBP. Biomonitoring data for ATBC is rare and limited to one study evaluating levels in children attending day care centers in Germany. In that study, ATBC levels in settled dust (24 mg/kg) were used to obtain a median estimated intake from dust in children of 0.1 μg/kg of body weight [23]. No studies have reported the levels of ATBC found in women of reproductive age, but these levels are assumed to be much lower than those tested in previous risk assessment studies. Therefore, determining whether lower levels of ATBC cause reproductive toxicity is critical to fully understand whether ATBC has endocrine disrupting ability like DBP.
The purpose of this study was to evaluate the ability of DBP, MBP, and ATBC to disrupt ovarian antral follicle function in vitro. We selected a wide range of concentrations including levels similar to those present in women and determined whether these chemicals interfere with follicle growth, decrease follicle viability, and, in the case of DBP, the mechanisms underlying its effects on follicle growth and viability.
Materials and methods
Animals
Cycling female CD-1 mice (32–37 days old) were obtained from Charles River Laboratories (Charles River, CA). Animals were housed four mice per cage at the University of Arizona Central Animal Facility with food and water provided ad libitum. Animals were housed in single-use BPA and phthalate-free Econo-Cage® Disposable Rodent Caging (Lab Products Inc., Seaford, DE) to reduce exposure to plasticizers commonly found and able to leach out of polycarbonate, polystyrene, and polysulfone plastic caging. Temperature was maintained at 22 ± 1°C and animals were subjected to 12L:12D cycles. Animals were euthanized at 32–37 days old by carbon dioxide (CO2) inhalation followed by cervical dislocation. The ovaries were removed and antral follicles isolated as described below. All experiments and methods involving animals were approved by the University of Arizona Institutional Animal Care and Use Committee and conformed to the Guide for the Care and Use of Experimental Animals [24].
Chemicals
Dimethylsulfoxide (DMSO), DBP, MBP, ATBC, and hydroxyurea (HU) were obtained from Sigma-Aldrich (St. Louis, MO). Alpha-minimal essential media (MEMα), ITS (10 ng/ml insulin, 5.5 ng/ml transferrin, 5.5 ng/ml selenium), penicillin/streptomycin, and charcoal-stripped fetal bovine serum were obtained from Life Technologies (Grand Island, NY). Human recombinant follicle-stimulating hormone (rFSH) was obtained from Dr. A.F. Parlow from the National Hormone and Peptide Program (Harbor-UCLA Medical Center, Torrance, CA). HistoGel was obtained from Thermo Fisher Scientific (Waltham, MA).
Antral follicle culture
Untreated mice were euthanized and their ovaries removed and trimmed of fat and fallopian tubes. Early antral follicles were mechanically isolated based on relative size (220–350 μm) and placed in culture as previously described [25]. Briefly, isolated follicles were plated one per well in standard or white clear-bottom 96-well plates (Corning, Corning, NY). All treatments were given in supplemented MEMα medium containing 5% FBS, 1% ITS, 1% penicillin/streptomycin (100 U/ml penicillin, 100 mg/ml streptomycin), and 5 IU/ml rFSH. DBP, MBP, and ATBC treatment solutions were prepared individually in supplemented MEMα using DMSO as vehicle (final vehicle concentration 0.075%). Final concentrations of each chemical in each well ranged from 0.001 up to 1000 μg/ml. The concentrations tested were selected with the objective of generating dose response data; however, it is important to note that the lowest concentrations of DBP and MBP applied (0.001 and 0.01 μg/ml) resemble levels of MBP previously reported in follicular fluid samples from women [18,19]. All experiments included an additional group of follicles treated with the DNA synthesis inhibitor HU (100 mM) as a positive control for the growth and viability assays. Follicle cultures were incubated for 24–72 h according to experiment at 37°C in 5% CO2. A total of two to four mice (20–60 follicles per mouse) were used to obtain enough follicles to ensure that each follicle culture experiment had 6–12 follicles per treatment group (treatment replicates). Each experiment was repeated at least four times (biological replicates). Isolated follicles were systematically distributed into the different treatment groups to ensure that each mouse was equally represented within each treatment group in the culture. At the end of each follicle culture experiment, follicles were processed for viability assays as described in the Viability Assays section, or pooled by treatment prior to snap freezing in liquid nitrogen and stored at –80°C for later RNA extraction and qPCR as described in the Real-time PCR section.
Analysis of follicular growth
Follicle growth was assessed by measuring the diameter of each follicle as described previously [25] starting on the day of plating and then every 24 h until the end of each timed culture experiment. Only follicles with diameters between 220 and 350 μm with a visible antrum were used for these experiments. Growth of antral follicles was assessed using two approaches. First, follicle diameter data values were converted to percent change by dividing the diameter of each follicle at each time point with their starting size and multiplying by 100 [25]. Second, follicles were stratified into three categories by adapting previously reported criteria used for primate follicles [26]. Briefly, we used the average growth of control-treated follicles at 72 h (18% growth from baseline) as a guide to divide follicles into “growing” (≥9% growth from baseline), “slow-growing” (between 1% and 8% growth) and “nongrowing” (<1% growth). The proportion of follicles within each strata was compared statistically between treatments as described in the Statistical Analysis section.
Viability assays
Antral follicle viability and cytotoxicity were assessed using commercially available kits following the manufacturer's instructions. All follicle cultures were done using white clear-bottomed 96-well plates (Costar). CellTiter-Glo Luminescent Cell Viability assay kits (Promega, Madison, WI) were used to determine the viability of antral follicles by lysing the cells and quantifying ATP concentration in each well as an indication of metabolically active follicles. On the last day of each culture, equilibration of the kit to room temperature was followed by the addition of CellTiter-Glo Reagent and induction of cell lysis by mixing on a microplate shaker according to the manufacturer's instructions. Following lysis, each plate was incubated at room temperature for 10 min to stabilize the luminescent signal prior to detection. Luminescent signals were detected using a Synergy H1m plate reader (BioTek, Winooski, VT). Specifically, luminescence was detected using top optics with a read height of 1 mm and integration time of one second. Each experiment consisted of 6–12 follicles per treatment group (treatment replicates) and was repeated at least four times (biological replicates) per time point. Luminescence data were compared statistically between treatments as described in the Statistical Analysis section.
Real-time PCR
At the end of the culture period, follicles were pooled by treatment and immediately snap-frozen and stored at –80°C for subsequent qPCR analysis as described previously [25]. Total RNA was extracted from pooled follicles using an RNeasy Micro Kit (Qiagen, Valencia, CA) including DNAse treatment to eliminate potential genomic DNA contamination. RNA concentration was determined at 260 nm using a Synergy H1m microplate reader equipped with a Take3 microvolume plate (Biotek). RNA samples (50 ng) were reverse transcribed using an iScript cDNA synthesis kit (Bio-Rad, Hercules, CA) according to the manufacturer's instructions. All qPCR reactions contained 1 μL of cDNA, 1 μL of gene-specific primers (500 nM), 3 μL of nuclease-free water, and 5 μL of SsoFast EvaGreen Supermix (Bio-Rad) for a final volume of 10 μL. All reactions were done in triplicate on a CFX Connect Real-time System (Bio-Rad) using the qPCR program previously published in Craig et al. [25].
All primers were obtained from Integrated DNA Technologies (IDT, Inc., Coralville, IA). Primers for cyclin-dependent kinase inhibitor 2A (Cdkn2a) were Forward: 5΄-GCT CTG GCT TTC GTG AAC AT-3΄ and Reverse: 5΄-CGA ATC TGC ACC GTA GTT GA-3΄ (Accession Number NM_177282.5). Primers for the housekeeping genes β-actin (Actb), glyceraldehyde 3-phosphate dehydrogenase (Gapdh), and TATA-box binding protein (Tbp) were purchased pre-made as PrimePCR® qPCR primer assays (Actb: Mm.PT.58.33540333, Gapdh: Mm.PT.39a.1, Tbp: Mm.PT.58.10867035; IDT, Inc.). All other primer sequences have been published previously [25]. Specificity of all primer sequences was validated by melt curve analysis and agarose gel electrophoresis.
Expression data were generated using CFX Manager Software, which uses the mathematical model for relative quantification of real-time PCR data developed by Pfaffl [27]. The mean expressions of the housekeeping genes Actb, Gapdh, and Tbp were used as reference to normalize gene expression. Reported data consist of mean relative mRNA expression ratios from four separate follicle culture experiments.
Terminal deoxynucleotidyl transferase dUTP nick end labeling analysis
At the end of culture, the spent media from each follicle was removed, replaced with methanol-free paraformaldehyde, and follicles fixed for 45 min at room temperature. After fixation, the paraformaldehyde was removed and 70% ethanol added until ready to process. To facilitate the processing of such small structures, follicles were embedded in HistoGel spheres prior to placing them in cassettes for subsequent paraffin embedding. Embedded follicles were sectioned (5 μm) and mounted four sections per slide. All sections from each follicle were examined under a light microscope and the sections with the widest diameters were selected for terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) staining.
TUNEL staining was performed using a DeadEnd Fluorometric TUNEL System (Promega) according to manufacturer's instructions. Prior to visualization, mounting media containing DAPI (Cell Signaling Technologies, Danvers, MA) was added to visualize cell nuclei. The stained sections were visualized and fluorescent images captured at ×200 magnification using a Leica CTR 5500 fluorescent microscope equipped with a Leica EC3 digital camera.
Fluorescent images were analyzed using SimplePCI version 6.5.1 (Hamamatsu Corporation, Sewickley, PA). Specifically, RGB images showing TUNEL (green) and DAPI (blue) staining were merged using Photoshop and the analysis software calibrated using validated stage micrometer images. A region of interest was manually drawn around each follicle section, each color was thresholded within that region, and all areas measured using the software. The total TUNEL-stained area was divided by the total DAPI-stained area to obtain a TUNEL/DAPI area percentage. A total of four to five follicles were analyzed per treatment. For each follicle, four sections were quantified and averaged to produce a TUNEL/DAPI percentage.
Statistical analysis
All data, except those generated by qPCR and TUNEL staining, were analyzed using SPSS 23.0 statistical software (IBM, Chicago, IL). For all comparisons, statistical significance was assigned at P ≤ 0.05. Since growth rate data were obtained on the same follicles over time, these data were compared between treatments and over time using Repeated Measures General Liner Model analysis. Viability data were end-point measurements obtained from different follicles over time,;thus, we compared these data using Univariate General Linear Model analysis. The proportion of follicles within each strata of growth was compared between treatments using Chi-square tests. Real-time PCR data were analyzed using the “Gene Expression Analysis” feature of the CFX Manager Software (Bio-Rad) that accompanies the CFX Connect Real-time PCR System. TUNEL/DAPI ratios were compared using Kruskal-Wallis nonparametric test followed by Mann-Whitney post hoc tests (SPSS 24.0, IBM). When necessary, outliers were identified using boxplots (SPSS 24.0) and tested using Grubb's Outlier Test (GraphPad QuickCalcs Website: http://graphpad.com/quickcalcs/Grubbs1.cfm, accessed January 2017) prior to removal. Instances when outliers were removed are indicated in the text along with disclosure of the statistical parameters obtained when outliers were still included.
Results
Effect of dibutyl phthalate, mono-butyl phthalate, and acetyl tributyl citrate on the growth of mouse antral follicles
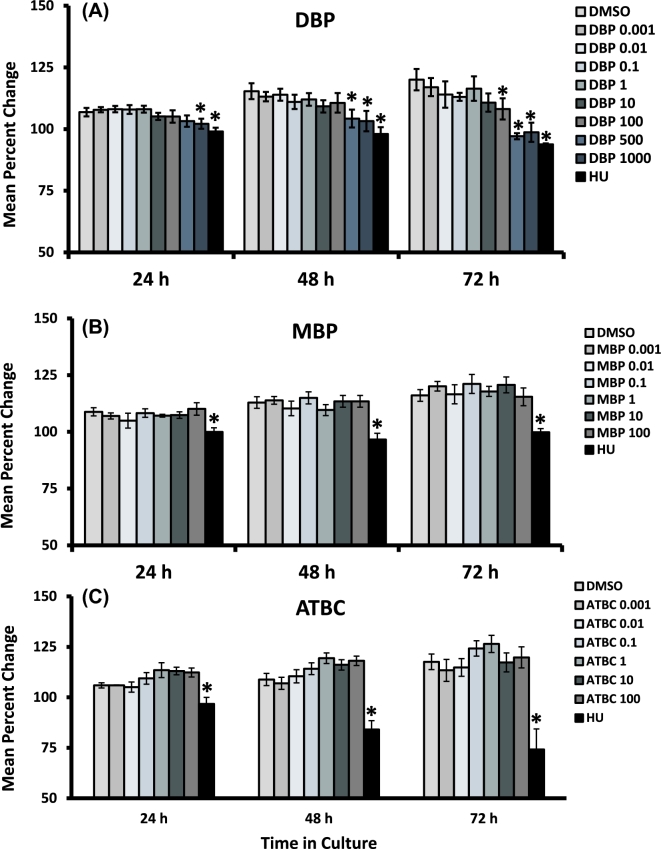
Our first objective was to establish the dose–response relationship between exposure to each chemical and in vitro follicular growth. To accomplish this objective, we exposed isolated mouse antral follicles with increasing concentrations of each phthalate or ATBC for 24–72 h and recorded their growth rate over time. At the end of 24 h, follicles treated with DBP at 1000 μg/ml and HU were significantly smaller than vehicle-treated controls. At the 48-h time point, growth inhibition was evident in follicles treated with 500 and 1000 μg/ml of DBP, as well as HU. Finally, at the end of 72 h, the growth of follicles treated with HU and DBP at all concentrations above 100 μg/ml was significantly reduced compared to follicles treated with vehicle control (P ≤ 0.05; n = 5 separate follicle culture experiments). In contrast, the growth of follicles treated with DBP at concentrations between 0.001 and 1 μg/ml did not differ from that observed in vehicle-treated follicles over the entire 72-h culture period.
We hypothesized that MBP is the main metabolite responsible for these effects as reported with other DBP-related toxicities. Surprisingly, antral follicles treated with MBP at concentrations ranging from 0.001 to 100 μg/ml were able to increase their diameter over time in a manner similar to that observed in vehicle-treated control follicles (Figure 1B). Finally, while exposure to HU inhibited follicle growth, exposure to the non-phthalate plasticizer ATBC did not affect the growth rate of antral follicles at any of the time points tested (Figure 1C).
Figure 1.
Effect of DBP, MBP, and ATBC exposure on the growth rate of antral follicles in vitro. Early antral follicles (n = 6–12 follicles per treatment) were obtained from the ovaries of untreated CD-1 mice and cultured as described in Materials and Methods section. Follicle diameter was measured every 24-h period and normalized to baseline diameter to obtain percent change. Data are presented as mean percent change ± SEM. Asterisks (*) indicate significantly different from control.
Effect of dibutyl phthalate, mono-butyl phthalate, and acetyl tributyl citrate on the number of growing antral follicles
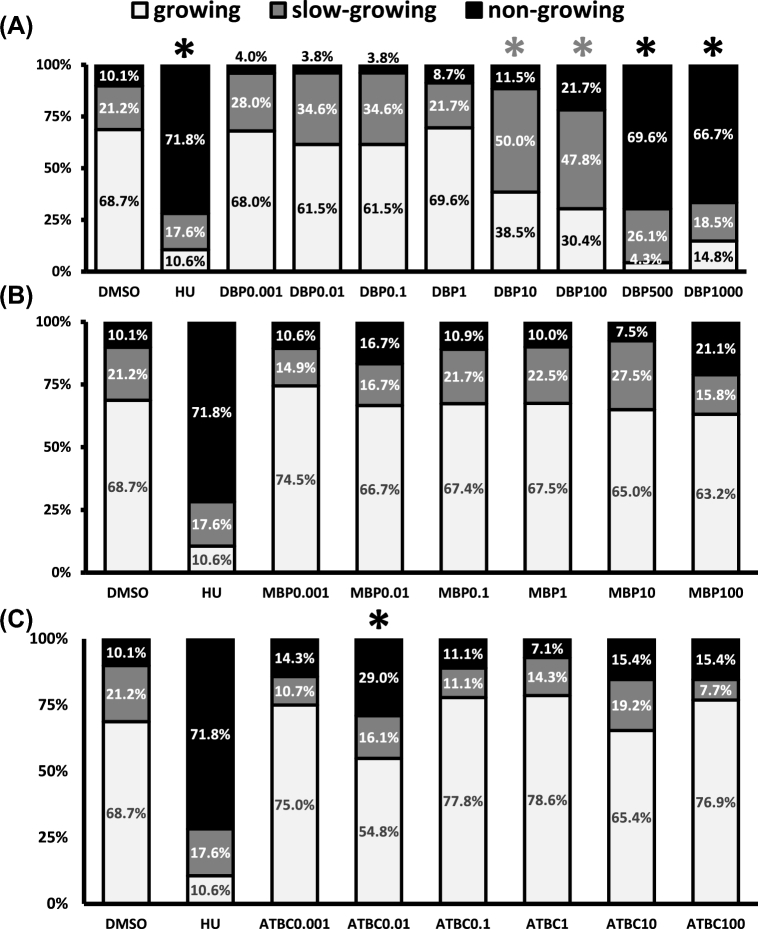
The transition from the early antral follicle to the preovulatory stage occurs quite rapidly and is mainly driven by the proliferation of granulosa cells [28]. This period of increased proliferation is reflected in our culture system as an increase in follicle diameter over the 72-h period. We used daily follicle diameter to determine the percentage of follicles growing in the entire experimental population. This follicle population-based approach is novel to ovarian toxicology, but has been adapted from work focused on characterizing hormonal influences on follicle growth and survival in primate follicle cultures [26]. Thus, we determined whether increasing concentrations of DBP, MBP, and ATBC altered the number of growing follicles in our experimental follicle population. Specifically, we determined whether each chemical caused the distribution of “growing,” “slow-growing,” and “nongrowing” follicles in our cultures to differ from that observed under vehicle control conditions. Under vehicle control conditions for 72 h, we found that 68.7% of cultured follicles were “growing,” 17.6% were “slow-growing,” and that 10.1% were “nongrowing” follicles. This was in contrast to the distribution observed for HU-treated follicles which was 10.6% “growing,” 17.6% “slow-growing,” and 71.8% “nongrowing” (Figure 2).
Figure 2.
Effect of DBP, MBP, and ATBC exposure on the growth pattern of antral follicles in vitro. Early antral follicles (n = 6–12 follicles per treatment) were obtained from the ovaries of untreated CD-1 mice and cultured as described in Materials and Methods section. Based on their growth rate, follicles were further classified into “growing,” “slow-growing,” and “nongrowing.” Data are presented as mean percent of follicles ± SEM with asterisks (*) indicating significantly different from control. Gray asterisks indicate statistically significant differences in the slow-growing follicles, while the black asterisks indicate so in nongrowing follicles.
Exposure to DBP for 72 h, caused dose–dependent deviations in the percentage of follicles falling under each of the selected strata (Figure 2A). Specifically, the percentage of follicles growing was significantly decreased by exposure to DBP at concentrations ≥10 μg/ml. The decrease in growing follicles observed with DBP treatment was attributed to a significant increase in slow-growing follicles in the 10 and 100 μg/ml groups and a significant increase in nongrowing follicles in the 500 and 1000 μg/ml groups. When the same analysis was performed for MBP and ATBC, we observed that MBP did not affect the distribution of follicles into the different growth patterns (Figure 2B); however, ATBC at 0.01 μg/ml caused a significant increase in nongrowing follicles (Figure 2C).
Effect of dibutyl phthalate, mono-butyl phthalate, and acetyl tributyl citrate exposure on antral follicle viability
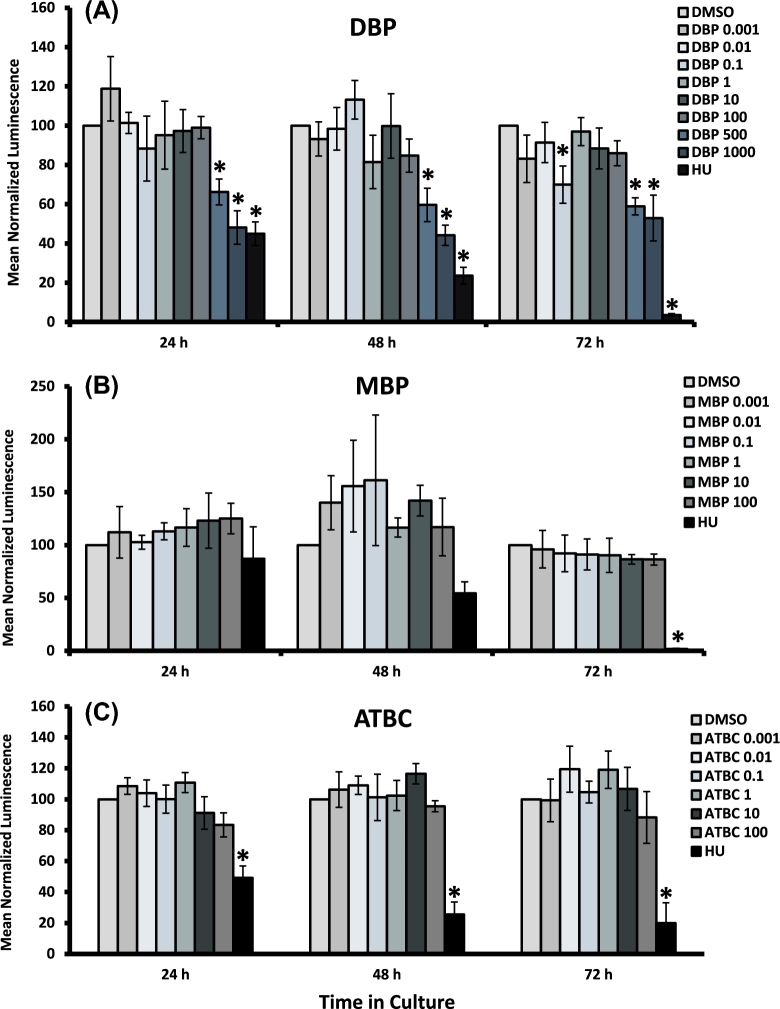
We also determined whether phthalates and ATBC compromise follicle viability by causing cytotoxicity. To accomplish this objective, we exposed isolated antral follicles from mice with increasing concentrations of DBP, MBP, and ATBC for 24–72 h and subjected the cultured follicles to commercially available viability assays as described in the Viability Assays section. Antral follicle viability was assessed by measuring ATP concentration as an indication of metabolically active cells (Figure 3). As expected for a known apoptosis inducer, follicles treated with HU showed decreased ATP levels compared to vehicle controls by 72 h. Similarly, treatment with DBP at 500 and 1000 μg/ml caused significant decreases in ATP levels versus vehicle control starting at 24 h and onwards. In contrast, ATP levels in follicles treated with lower concentrations of DBP (below 500 μg/ml with the exception of 0.1 μg/ml) were not different from those observed in vehicle-treated follicles, thus suggesting that DBP exposure at these concentrations does not decrease viability in cultured antral follicles. Interestingly, follicles exposed to DBP at 0.1 μg/ml showed a decrease in ATP levels when compared to vehicle controls (Figure 3A). No significant changes in ATP levels were observed in follicles treated with all concentrations of MBP and ATBC (Figure 3B and C).
Figure 3.
Effect of DBP, MBP, and ATBC exposure on the viability of antral follicle follicles in vitro. Early antral follicles (n = 6–12 follicles per treatment) were obtained from the ovaries of untreated CD-1 mice and cultured as described in Materials and Methods section. At the end of each culture, the ATP concentration within each follicle was determined using a luminescence assay. Data were normalized to the DMSO vehicle control and are presented as mean normalized luminescence ± SEM with asterisks (*) indicating significantly different from control.
Effect of dibutyl phthalate exposure on antral follicle cell cycle and apoptosis gene expression
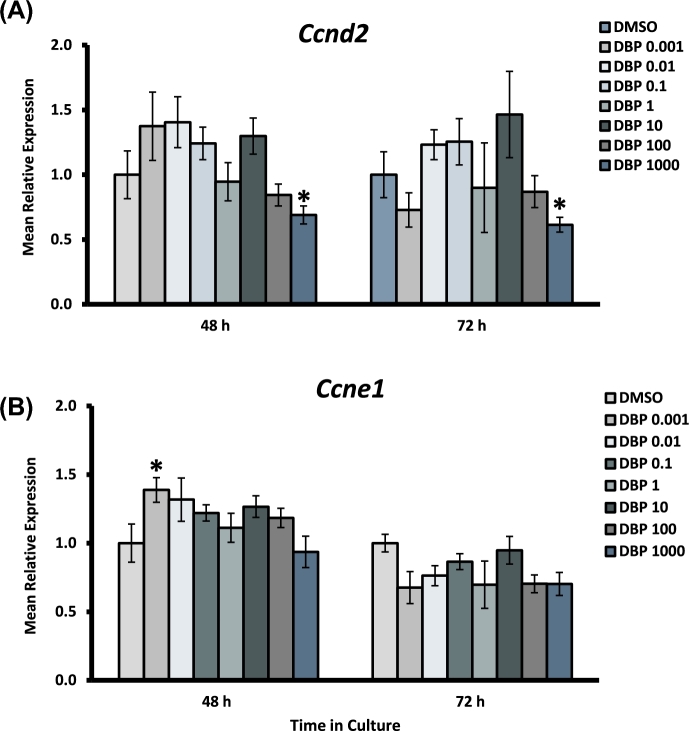
We evaluated whether DBP exposure led to dysregulated expression of cell cycle and apoptosis-related genes in mouse antral follicles. To accomplish this goal, follicles were exposed to increasing concentrations of DBP for 48 and 72 h and processed for qPCR analysis as described in the Materials and Methods section. To understand the effects of DBP on cell cycle regulation, we evaluated the expression of the regulators of cell cycle entry and progression cyclin D2 (Ccnd2) and cyclin E1 (Ccne1) (Figure 4), as well as the cell cycle arrest activators cyclin-dependent kinase inhibitors 1A and 2A (Cdkn1a and Cdkn2a) (Figure 5). To evaluate apoptosis regulation, we measured the expression of the pro-apoptotic factors BCL2-associated X protein (Bax) and BH3 interacting domain death agonist (Bid), and the anti-apoptosis factor, B cell leukemia/lymphoma 2 (Bcl2) (Figure 6).
Figure 4.
Effect of DBP exposure on cell cycle progression gene expression in cultured antral follicles. Early antral follicles (n = 6–12 follicles per treatment) were obtained from the ovaries of untreated CD-1 mice and cultured as described in Materials and Methods section. At the end of each culture, RNA was extracted and processed for qPCR analysis of Ccnd2 (A) and Ccne1 (B). Data were normalized to housekeeping genes and are presented as mean relative expression ± SEM with asterisks (*) indicating significantly different from control.
Figure 5.
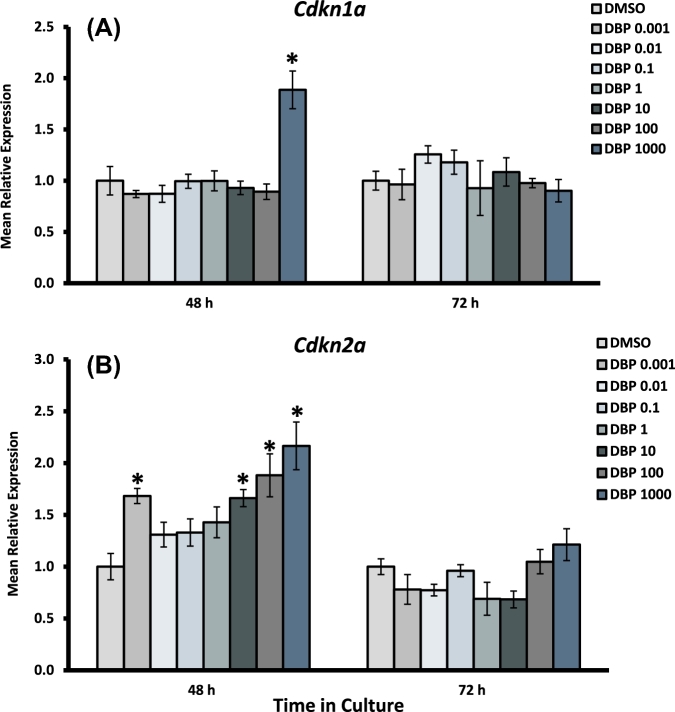
Effect of DBP exposure on cell cycle arrest gene expression in cultured antral follicles. Early antral follicles (n = 6–12 follicles per treatment) were obtained from the ovaries of untreated CD-1 mice and cultured as described in Materials and Methods section. At the end of each culture, RNA was extracted and processed for qPCR analysis of Cdkn1a (A) and Cdkn2a (B). Data were normalized to housekeeping genes and are presented as mean relative expression ± SEM with asterisks (*) indicating significantly different from control.
Figure 6.
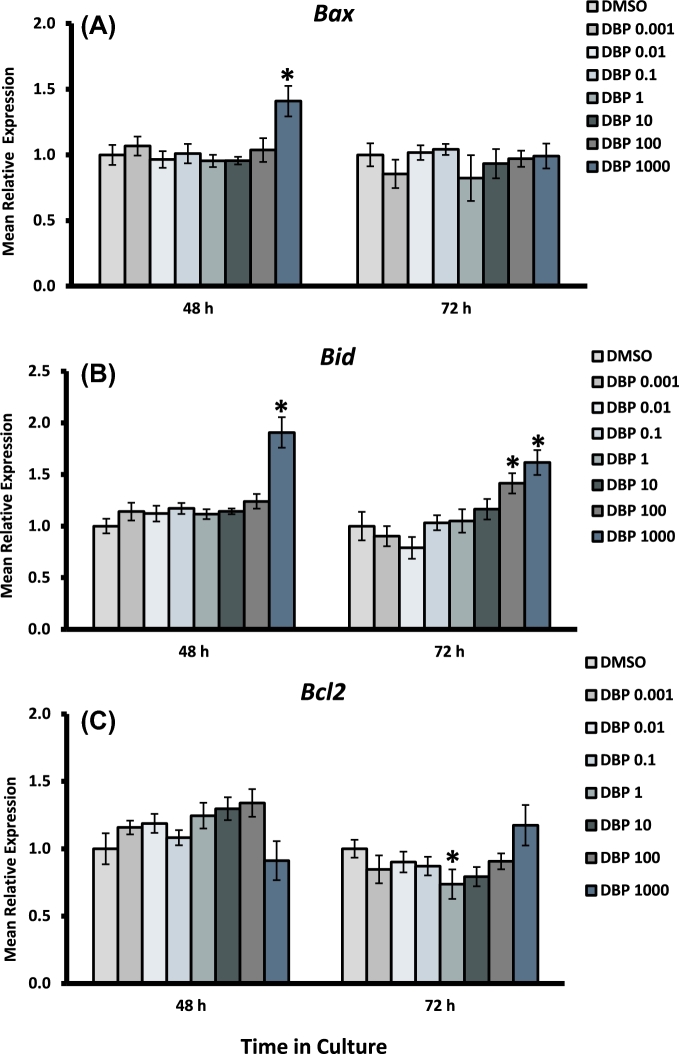
Effect of DBP exposure on apoptosis gene expression in cultured antral follicles. Early antral follicles (n = 6–12 follicles per treatment) were obtained from the ovaries of untreated CD-1 mice and cultured as described in Materials and Methods section. At the end of each culture, RNA was extracted and processed for qPCR analysis of Bax (A), Bid (B), and Bcl2 (C). Data were normalized to housekeeping genes and are presented as mean relative expression ± SEM with asterisks (*) indicating significantly different from control.
Expression of Ccnd2 was decreased in follicles treated with DBP 1000 μg/ml at both 48 and 72 h, but was unaffected at lower concentrations. Ccne1 was significantly increased in follicles treated with DBP at 0.001 μg/ml at 48 h, an effect that was not present at 72 h or in other treatment groups. The expression of the cell cycle arrest gene Cdkn1a was significantly increased in follicles treated with the highest concentration of DBP at 48 h. Interestingly, Cdkn2a expression was increased at 48 h in follicles treated with DBP at 0.001 and 10–1000 μg/ml, but returned to levels similar to those observed in controls by 72 h. Bax and Bid expression were both increased at 48 h in follicles treated with DBP at 1000 μg/ml in the absence of Bcl2 expression changes. Bax expression returned to control levels by 72 h, but Bid mRNA levels remained increased and included an increase in follicles treated with DBP at 100 μg/ml as well. Finally, there was a statistically significant decrease in the expression of Bcl2 mRNA in follicles treated with DBP at 1 μg/ml for 72 h.
Effect of dibutyl phthalate exposure on antral follicle death
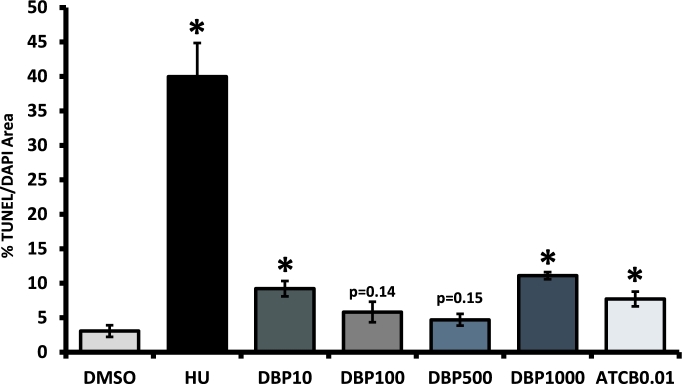
Based on our data showing that DBP treatment increased the number of slow-growing and nongrowing follicles at concentrations ≥10 μg/ml, and decreased ATP production and increased cell cycle arrest and apoptosis gene expression at concentrations ≥500 μg/ml, we hypothesized that these follicles had undergone follicular atresia. To test this hypothesis, we treated follicles with DBP at 10–1000 μg/ml for 48 h and determined the level of DNA fragmentation in each sample using TUNEL. DNA fragmentation in the treatment groups differed from that observed in vehicle control-treated antral follicles (Figure 7A–F). Interestingly, we observed a tendency for increased variability as TUNEL/DAPI ratio values increased. Thus, we tested for, identified, and removed significant outliers in the groups treated with DBP at 10, 500, and 1000 μg/ml. When the analysis was performed on the original data containing outliers, we observed statistical significance or strong trends for increased DNA fragmentation in follicles treated with DBP at 10 μg/ml (7.86 ± 1.61%; P = 0.05), 500 μg/ml (7.32 ± 2.7%; P = 0.09), and 1000 μg/ml (18.0 ± 9.2; P = 0.09). Figure 8 shows the results obtained after outlier removal. Using this approach, we observed that the differences in DNA fragmentation levels relative to vehicle control were statistically significant in follicles treated with DBP at 10 and 1000 μg/ml (Figure 8).
Figure 7.
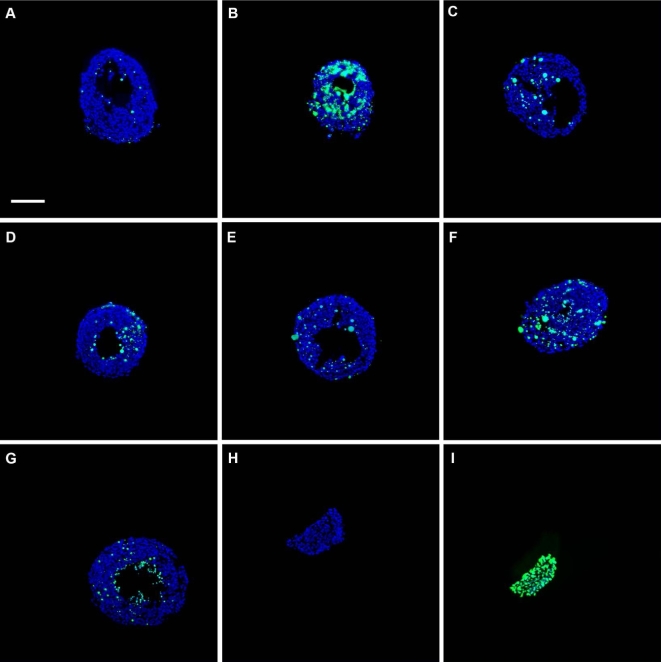
Representative images of antral follicles subjected to TUNEL. Early antral follicles (n = 6–12 follicles per treatment) were obtained from the ovaries of untreated CD-1 mice, cultured, and processed for TUNEL as described in Materials and Methods section. Representative merged images show DAPI (blue) and TUNEL (green) staining in follicles treated in vitro with vehicle control (DMSO, A), apoptosis positive control (HU, B), DBP 10 μg/ml (C), 100 μg/ml (D), 500 μg/ml (E), 1000 μg/ml (F), and ATBC 0.01 μg/ml (G) for 48 h. Panels H and I show representative images of the assay negative (no enzyme added) and positive (DNAse digestion of tissue) controls, respectively. Images were taken at ×200 magnification. Scale bar represents 100 μm.
Figure 8.
Effect of DBP and ATBC exposure on TUNEL. Early antral follicles (n = 6–12 follicles per treatment) were obtained from the ovaries of untreated CD-1 mice, cultured, subjected to TUNEL, and quantified as described in Materials and Methods section. Data are presented as the mean percentage of TUNEL/DAPI area ± SEM with asterisks (*) indicating significantly different from control at the P < 0.05 level. Actual P-values are provided for groups showing visual trends.
Effect of acetyl tributyl citrate exposure on antral follicle death
ATBC treatment at 0.01 μg/ml caused an increase in nongrowing antral follicles. To further understand this observation, we tested whether ATBC treatment increased follicle death under those conditions. As with DBP, we treated antral follicles with ATBC at 0.01 μg/ml for 48 h and determined the level of DNA fragmentation in each sample using TUNEL. DNA fragmentation in ATBC-treated follicles was significantly greater than that observed in the vehicle-treated group (Figures 7G and 8).
Discussion
We used an isolated ovarian follicle culture system to evaluate direct effects of DBP, MBP, and ATBC exposure on mouse antral follicles. Our main findings suggest that direct DBP exposure results in decreased growth of antral follicles and increased cytotoxicity associated with activation of cyclin-dependent kinase inhibitor-induced inhibition of cell cycle progression and initiation of apoptosis. Surprisingly, MBP, the proposed active metabolite of DBP, did not affect antral follicle growth and viability in this study. Finally, ATBC increased the number of nongrowing follicles and induced DNA fragmentation when follicles were exposed to 0.01 μg/ml without affecting ATP production. Our results showing increased number of slow-growing and nongrowing follicles and increased DNA fragmentation suggest that DBP exposures ≥10 μg/ml and ATBC exposures at 0.01 μg/ml are detrimental to ovarian antral follicles, respectively.
With the exception of our previous work showing that a 96-h exposure to high concentrations of DBP in vitro resulted in significantly smaller antral follicles that underwent atresia by 168 h [25], no other studies have addressed the effects of direct exposure to DBP using small antral follicles. Furthermore, no studies have focused on shorter exposure times and lower concentrations that more closely mimic the lifespan of early antral follicles and levels found in human follicular fluid, respectively. In this study, we show that as DBP concentration increases, the growth rate and the number of growing early antral follicles decreases causing slow growth initially and no growth once the higher concentrations are reached. Results from measurement of ATP levels as a surrogate for the presence of metabolically active cells and DNA fragmentation as a marker for follicular atresia suggest that increasing concentrations of DBP are cytotoxic to antral follicles. This is consistent with our previous work showing that follicles treated with 1000 μg/ml undergo atresia [25]. The idea that phthalates can target and disrupt ovarian follicle growth is further supported by work evaluating the effects of other phthalates on this process. Specifically, the effects of direct exposure to di-2-ethylhexyl phthalate (DEHP) and its metabolite mono-2-ethylhexyl phthalate (MEHP) in antral follicles and the underlying mechanisms for these effects have been well documented in various studies [29–32]. Based on these studies, DEHP and MEHP disrupt antral follicle growth, induce atresia, inhibit steroidogenesis, and increase oxidative stress by disrupting the proliferation, steroidogenic, and antioxidant machineries in the ovarian follicle. Of note, in this study we tested a range of concentrations that included low levels similar to those in follicular fluid as well as higher supraphysiological levels. This approach allowed us to differentiate between DBP levels that result in cytotoxicity from levels that potentially disrupt signaling pathways that control follicle growth with and without causing follicular death.
Proliferation of the granulosa cell layer is a major determinant of the size of an ovarian follicle. To divide, granulosa cells must progress through the cell cycle, a process favored by cyclins such as Ccnd2 and Ccne1 and opposed by cyclin-dependent kinase inhibitors such as Cdkn1a and Cdkn2a. At various stages during the cell cycle, cells must pass internal checkpoints to ensure that no errors exist and conditions are adequate to continue to cell division. If conditions are not adequate, cell cycle progression is halted via cell cycle arrest until conditions are favorable. If inadequate conditions are not reversed, cells remain arrested at the checkpoint and the build-up of stress signals eventually triggers apoptosis. In this study, some concentrations of DBP increased the number of nongrowing follicles without affecting ATP production. Therefore, it is very likely that exposure to these specific concentrations causes a transient cell cycle arrest that does not significantly impact metabolic activity. To further understand this mechanism, we characterized the effects of DBP on the expression of important regulators of cell cycle progression, cell cycle arrest, and apoptosis in treated antral follicles. Using this approach, we observed dose-specific effects of DBP on gene expression, which included strong concurrent activation of Cdkn2a and Cdkn1a and Bax and Bid along with downregulation of Ccnd2 within the first 48 h of exposure to the cytotoxic concentrations. Interestingly, follicles treated with concentrations below the cytotoxic levels of DBP only showed a transient increase in Ccne1 and Cdkn2a, but no activation of apoptosis-related genes. However, all DBP-treated follicles with defects in growth had greater levels of DNA fragmentation which is a strong indication that DBP-induced inhibition of growth is due to follicle atresia. Finally, we observed that the activation of the two cell cycle arrest- initiating pathways (i.e. Cdkn1a vs. Cdkn2a) seemed to be related to the magnitude of the DBP exposure received. Characterizing this pathway preference and its regulation at the molecular level will be the subject of future studies evaluating the role of cell cycle arrest in normal follicle growth, as well as EDC-mediated follicular disruption.
While previously proposed as the toxic metabolite of DBP [33, 34], MBP did not disrupt antral follicle growth and viability in this study. Pharmacokinetic studies have shown that, in the whole organism, DBP is metabolized by esterases in the stomach and intestines to form MBP and phthalic acid. MBP can then be excreted free, conjugated into a glucuronidated form, or oxidized into mono-3-oxo-n-butyl phthalate, mono-3-hydroxy-n-butyl phthalate, and mono-3-carboxypropryl phthalate [34–36]. Based on our observation that toxicity occurs when follicles are exposed to DBP but not when exposed to MBP, it is reasonable to speculate that a different metabolite (not generated when MBP is directly added to the culture vs. conversion of DBP to MBP) is required for toxicity. Based on what is known about the metabolism of DBP that metabolite could be phthalic acid. On the other hand, it is possible that MBP is either quickly inactivated by conjugation or oxidation when directly applied to the culture, thus preventing follicle toxicity. Finally, it is possible that MBP penetration into the follicle is limited by its increased polarity relative to DBP, an idea previously proposed in a study evaluating DBP and MBP exposures on cytotoxicity and differentiation in cultured rat embryonic limb bud cells [37]. In that study, authors speculated that differences in polarity between DBP and MBP are very likely to influence their toxicity mechanisms by decreasing cellular penetration and reducing their delivery to target molecules in the tissue under study. Additional studies will be undertaken to fully characterize metabolism and toxicity of DBP and MBP in ovarian follicles.
Prior to our study evaluating in vitro ATBC exposure on antral follicles, others had investigated its ability to inhibit cell growth in human KB and HeLa cells, monkey Vero cells, and dog MDCK cells. Specifically, ATBC inhibited cell growth (≥50% inhibition versus control) in all tested cell lines starting at concentrations as low as 40 μg/ml [38,39]. ATBC has also been shown to activate human and rat SXR-mediated expression of CYP3A4, an enzyme involved in metabolism of endogenous steroid hormones and prescription drugs, at concentrations as low as 4 μg/ml [40]. In our experiments, the growth of ATBC-treated follicles was not significantly inhibited and follicles did not have decreased ATP at most concentrations. Interestingly, ATBC treatment at 0.01 μg/ml resulted in a significant increase in nongrowing follicles which, according to TUNEL data, are undergoing follicular atresia. In the absence of biomonitoring data on the levels of ATBC found in serum and follicular fluid in women, we recently conducted a study where female CD-1 mice were exposed to doses 10 to 100-fold lower than reported risk assessment studies and determined whether reproductive function was disrupted by ATBC. ATBC did not affect fertility parameters, but daily exposure to ATBC (10 mg/kg/day for 15 days) resulted in a decrease in the number of primordial, primary, and secondary follicles in the ovaries of these mice [41]. Until data on ATBC levels in serum and follicular fluid become available, it is reasonable to suspect that low levels of ATBC could cause detrimental effects in women.
In conclusion, we characterized the dose–response relationship between direct DBP, MBP, and ATBC exposure and identified the exposure timing and DBP concentrations that cause growth inhibition and cytotoxicity in antral follicles. Although DBP was detrimental to follicle growth and viability and resulted in significant dysregulation of cell cycle and apoptosis gene expression in a dose-specific manner, our results suggest that MBP does not play a role in DBP toxicity in follicles exposed in vitro. The mechanisms underlying these differences in toxicity with related metabolites are intriguing and will be investigated in subsequent studies. Finally, these findings help inform risk assessment for phthalates and ATBC by highlighting the importance of including low environmentally relevant exposures in risk assessment, identifying the effects of toxic and nontoxic concentrations on key parameters related to follicle growth and function (i.e. follicle diameter, percent of follicles growing, and ATP production), and paving the way for future studies seeking to understand whether non-toxic exposures affect postfollicular processes such as fertilization and early embryo development.
Acknowledgments
The authors thank Nizigiyimana Ernest and Shade Rodriguez for technical help and Douglas Cromey (SWEHSC Cellular Imaging Facility Core) for performing the image analysis.
References
- 1. Jobling S, Bjerregaard P, Blumberg B, Brandt I, Brian J, Casey S, Frouin H, Guidice L, Heindel J, Iguchi T, Kidd K, Kortenkamp A et al. . Evidence for endocrine disruption in humans and wildlife. In: Bergman A, Heinder J, Jobling S, Kidd K, Zoeller R (eds.), State of the Science of Endocrine Disrupting Chemicals, Switzerland: United Nations Environment Programme and the World Health Organization; 2012: 23–188. [Google Scholar]
- 2. Centers for Disease Control and Prevention Third national report on human exposure to environmental chemicals. 2005; NCEH Pub. No. 05-0570.
- 3. Kay V, Chambers C, Foster W. Reproductive and developmental effects of phthalate diesters in females. Crit Rev Toxicol 2013; 43:200–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Grindler N, Allsworth J, Macones G, Kannan K, Roehl K, Cooper A. Persistent organic pollutants and early menopause in U.S. Women. PLoS One 2015; 10:e0116057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Meeker J, Ferguson K. Urinary phthalate metabolites are associated with decreased serum testosterone in men, women, and children from NHANES 2011–2012. J Clin Endocrinol Metab 2014; 99:4346–4352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Toft G, Jonsson B, Lindh C, Jense T, Hjollund N, Vested A, Bonde J. Association between pregancy loss and urinary phthalate levels around the time of conception. Environ Health Perspect 2012; 120:458–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jukic A, Calafat A, McConnaughey D, Longnecker M, Hoppin J, Weinberg C, Wilcox A, Baird D. Urinary concentrations of phthalate metabolites and bisphenol a and associations with follicular-phase length, luteal-phase length, fecundability, and early pregnancy loss. Environ Health Perspect 2015; 124:321–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hauser R, Gaskins A, Souter I, Smith K, Dodge L, Ehrlich S, Meeker J, Calafat A, Williams P. Urinary phthalate metabolite concentrations and reproductive outcomes among women undergoing in vitro fertilization: results from the EARTH study. Environ Health Perspect 2016; 124:831–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kavlock R, Boekelheide K, Chapin R, Cunningham M, Faustman E, Foster P, Golub M, Henderson R, Hinberg I, Little R, Seed J, Shea K et al. . NTP center for the evaluation of risks to human reproduction: phthalates expert panel report on the reproductive and developmental toxicity of di-n-butyl phthalate. Reprod Toxicol 2002; 16:489–527. [DOI] [PubMed] [Google Scholar]
- 10. International Programme on Chemical Safety (IPCS) Di-n-butyl phthalate. 1997, http://www.inchem.org/documents/ehc/ehc/ehc189.htm. Accessed July 2014.
- 11. Hines C, Hopf N, Deddens J, Silva M, Calafat A. Estimated daily intake of phthalates in occupationally exposed groups. J Expo Sci Environ Epidemiol 2011; 21:133–141. [DOI] [PubMed] [Google Scholar]
- 12. CPSC Chronic hazard advisory panel on phthalates and phthalate alternatives; 2016. http://www.Cpsc.Gov/PageFiles/169902/CHAP-REPORT-with-Appendices.Pdf. Accessed July 2014.
- 13. Salazar V, Castillo C, Ariznavarreta C, Campon R, Tresquerres J. Effect of oral intake of dibutyl phthalate on reproductive parameters of Long Evans rats and pre-pubertal development of their offspring. Toxicology 2004; 205:131–137. [DOI] [PubMed] [Google Scholar]
- 14. Lee H, Yamanouchi K, Nishihara M. Effects of perinatal exposure to phthalate/adipate esters on hypothalamic gene expression and sexual behavior in rats. J Reprod Dev 2006; 52:343–352. [DOI] [PubMed] [Google Scholar]
- 15. Ema M, Miyawaki E, Kawashima K. Effects of dibutyl phthalate on reproductive function in pregnant and pseudopregnant rats. Reprod Toxicol 2000; 14:13–19. [DOI] [PubMed] [Google Scholar]
- 16. Ahmad R, Verma Y, Gautam A, Kumar S. Assessment of estrogenic potential of di-n-butyl phthalate and butyl benzyl phthalate in vivo. Toxicol Ind Health 2015; 31:1296–1303. [DOI] [PubMed] [Google Scholar]
- 17. Sen N, Liu X, Craig Z. Short term exposure to di-n-butyl phthalate (DBP) disrupts ovarian function in young CD-1 mice. Reprod Toxicol 2015; 53:15–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Krotz S, Carson S, Tomey C, Buster J. Phthalates and bisphenol do not accumulate in human follicular fluid. J Assist Reprod Genet 2012; 29:773–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Du Y, Fang Y, Wang Y, Zeng Q, Guo N, Zhao H, Li Y. Follicular fluid and urinary concentrations of phthalate metabolites among infertile women and associations with in vitro fertilization parameters. Reprod Toxicol 2016; 61:142–150. [DOI] [PubMed] [Google Scholar]
- 20. CPSC Review of Exposure and Toxicity Data for Phthalate Substitutes. 2010, https://www.cpsc.gov/s3fs-public/phthalsub.pdf. Accessed July 2014.
- 21. Welle F, Wolz G, Franz R. Migration of plasticizers from PVC tubes into enteral feeding solutions. Pharma International 2005; 3:17–21. [Google Scholar]
- 22. SCENIHR Preliminary report on the safety of medical devices containing DEHP-plasticized PVC or other plasticizers on neonates and other groups possibly at risk. Scientific Committee on Emerging and Newly-Identified Health Risks 2008; 2016:http://ec.europa.eu/health/ph_risk/committees/04_scenihr/docs/scenihr_o_014.pdf.
- 23. Fromme H, Schütze A, Lahrz T, Kraft M, Fembacher L, Siewering S, Burkardt R, Dietrich S, Koch H, Volkel W. Non-phthalate plasticizers in German daycare centers and human biomonitoring of DINCH metabolites in children attending the centers (LUPE 3). Int J Hyg Environ Health 2016; 219:33–39. [DOI] [PubMed] [Google Scholar]
- 24. Institute of Laboratory Animal Resources Guide for the Care and Use of Laboratory Animals. Washington, DC: National Academies Press; 1996. [Google Scholar]
- 25. Craig Z, Hannon P, Wang W, Ziv-Gal A, Flaws J. Di-n-butyl phthalate disrupts the expression of genes involved in cell cycle and apoptotic pathways in mouse ovarian antral follicles. Biol Reprod 2013; 88:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ting A, Xu J, Stouffer R. Differential effects of estrogen and progesterone on development of primate secondary follicles in a steroid-depleted milieu in vitro. Hum Reprod 2015; 30:1907–1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pfaffl M. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acid Res 2001; 29:e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hirshfield A. Overview of ovarian follicular development: considerations for the toxicologist. Environ Mol Mutagen 1997; 29:10–15. [PubMed] [Google Scholar]
- 29. Craig Z, Singh J, Gupta R, Flaws J. Co-treatment of mouse antral follicles with 17β-estradiol interferes with mono-2-ethylhexyl phthalate (MEHP)-induced atresia and altered apoptosis gene expression. Reprod Toxicol 2014; 45:45–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wang W, Craig Z, Basavarajappa M, Gupta R, Flaws J. Di (2-ethylhexyl) phthalate inhibits growth of mouse antral follicles through an oxidative stress pathway. Toxicol Appl Pharmacol 2012; 258:288–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gupta RK, Singh JM, Leslie TC, Meachum S, Flaws JA, Yao HH. Di-(2-ethylhexyl) phthalate and mono-(2-ethylhexyl) phthalate inhibit growth and reduce estradiol levels of antral follicles in vitro. Toxicol Appl Pharmacol 2010; 242:224–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wang W, Craig Z, Basavarajappa M, Hafner K, Flaws J. Mono (2-ethylhexyl) phthalate induces oxidative stress and inhibits growth of mouse ovarian antral follicles. Biol Reprod 2012; 87:152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kremer J, Williams C, Parkinson H, Borghoff S. Pharmacokinetics of monobutylphthalate, the active metabolite of di-n-butylphthalate, in pregnant rats. Toxicol Lett 2005; 159:144–153. [DOI] [PubMed] [Google Scholar]
- 34. Fennell T, Krol W, Sumner S, Snyder R. Pharmacokinetics of dibutyl phthalate in pregnant rats. Toxicol Sci 2004; 82:407–418. [DOI] [PubMed] [Google Scholar]
- 35. Albro P, Moore B. Identification of the metabolites of simple phthalate diesters in rat urine. J Chromatogr 1974; 94:209–218. [DOI] [PubMed] [Google Scholar]
- 36. Tanaka A, Matsumoto A, Yamaha T. Biochemical studies on phthalic esters. III. Metabolism of dibutyl phthalate (DBP) in animals. Toxicology 1978; 9:109–123. [DOI] [PubMed] [Google Scholar]
- 37. Kim S, Kim S, Kwon Q, Sohn K, Kwack S, Choi Y, Han S, Lee M, Park K. Effects of dibutyl phthalate and monobutyl phthalate on cytotoxicity and differentiation in cultured rat embryonic limb bud cells; protection by antioxidants. J Toxicol Environ Health A 2002; 65:461–472. [DOI] [PubMed] [Google Scholar]
- 38. Mochida K, Gomyoda M, Fujita T. Acetyl tributyl citrate and dibutyl sebacate inhibit the growth of cultured mammalian cells. Bull Environ Contam Toxicol 1996; 56:635–637. [DOI] [PubMed] [Google Scholar]
- 39. Ekwall B, Nordensten C, Albanus L. Toxicity of 29 plasticizers to HeLa cells in the MIT-24 system. Toxicology 1982; 24:199–210. [DOI] [PubMed] [Google Scholar]
- 40. Takeshita A, Igarashi-Migitaka J, Nishiyama K, Takahashi H, Takeuchi Y, Koibuchi N. Acetyl tributyl citrate, the most widely used phthalate substitute plasticizer, induces cytochrome p450 3a through steroid and xenobiotic receptor. Toxicol Sci 2011; 123:460–470. [DOI] [PubMed] [Google Scholar]
- 41. Rasmussen LM, Sen N, Liu X, Craig ZR. Effects of oral exposure to the phthalate substitute acetyl tributyl citrate on female reproduction in mice. J Appl Toxicol 2016; 37:668–675. [DOI] [PMC free article] [PubMed] [Google Scholar]