Abstract
Treatment of mice with lipopolysaccharide (LPS) and the liver-specific transcriptional inhibitor D-(+)-galactosamine (GalN) induces fatal hepatitis, which is mediated by tumor necrosis factor α (TNF-α) and characterized by massive hepatic apoptosis. Previous studies suggest that GalN increases the sensitivity to LPS/TNF-α, probably by blocking the transcription of protective factors, but the identity of most of these factors is still unclear. Here, we report that Ifit1 protects against LPS/GalN-induced fatal hepatitis. Forced expression of Ifit1 in hepatocytes significantly diminished TNF-α–mediated apoptosis. Moreover, targeted expression of Ifit1 in the liver by recombinant adeno-associated virus serotype 8 protected mice from LPS/GalN-induced lethal hepatitis, which was associated with the inhibition of TNF-α–mediated activation of the c-Jun N-terminal kinase (JNK)–Bim cascade. Furthermore, Ifit1 bound to a scaffolding protein Axin and inhibited its function to mediate JNK activation. Together, our data demonstrate that Ifit1 is a novel protective factor that inhibits LPS/GalN-induced (TNF-α–mediated) fatal hepatitis, suggesting that Ifit1 is a potential therapeutic target for treatment of inflammatory liver diseases.
Keywords: Ifit1, LPS, fatal hepatitis, TNF-α, JNK
Lipopolysaccharide (LPS; also known as endotoxin) is a part of the pathology of gram-negative bacterial infections that elicits a variety of inflammatory responses, such as endotoxin shock, tissue injury, and death [1]. Simultaneous injection of low doses of LPS and the liver-specific transcriptional inhibitor D-(+)-galactosamine (GalN) is widely used to induce fatal hepatitis in animals, which results in typical hepatic apoptosis. In this model, tumor necrosis factor α (TNF-α) appears to be essential for hepatocyte killing and mouse mortality [2]. Although the mechanisms by which TNF-α kills hepatocytes are still unclear, recent studies have shown that TNF-α binds to TNF-α receptor 1 (TNFR1) and then triggers apoptosis through the adaptor proteins TRADD and FADD, which subsequently recruit and activate caspase-8 [3, 4]. In addition, c-Jun N-terminal kinase (JNK) signaling has also been reported to be required in the process of TNF-α–mediated apoptosis. Bid and Bim, 2 members of the proapoptotic BH3-only Bcl-2 family, seem to be the downstream death effectors of caspase-8 and JNK, respectively [4].
GalN inhibits RNA synthesis in hepatocytes primarily by depleting UTP [5], suggesting that it may sensitize hepatocytes to LPS-induced (TNF-α–mediated) apoptosis by blocking the transcription of protective factors. However, most of these protective factors have not yet been identified, which prompted us to carry out the current study.
Ifit1, one of the first identified IFN-stimulated genes, belongs to the IFN-stimulated protein with tetratricopeptide repeats family. It has been reported that Ifit1 interacts with the translation initiation factor eIF3, leading to the inhibition of protein translation [6]. Ifit1 has also been shown to be an antiviral protein that specifically binds to 5′-triphosphate RNA [7] or the human papillomavirus E1 protein [8] to inhibit viral replication. A recent study indicates that Ifit1 is associated with resistance to DNA damage induced by chemotherapy and/or radiation across multiple cancer cell lines [9], suggesting an antiapoptotic effect of Ifit1. Although Ifit1 is strongly induced by LPS, its function in LPS-induced hepatitis is poorly understood. Here, we report that forced expression of Ifit1 in hepatocytes protects against TNF-α–induced cell death in vitro. Similarly, gene transfer of Ifit1 to liver by using recombinant adeno-associated virus serotype 8 (rAAV8), a liver-specific delivery vehicle for gene therapy, protects mice from LPS/GalN-induced lethal hepatitis. Moreover, our data indicate that Ifit1 prevents TNF-α–induced hepatic apoptosis by binding to the scaffolding protein Axin and negatively regulating its activity, leading to inhibition of the JNK-Bim cascade. These findings identify Ifit1 as a novel hepatoprotective factor that mediates hepatocyte resistance to the toxicity of TNF-α.
MATERIALS AND METHODS
Animal Studies
Male C57BL/6 mice aged 9–10 weeks were purchased from the Academy of Military Medical Sciences, Laboratory Animal Center (Beijing, China), and received the human care under the guidelines on laboratory animals of Nankai University. For acute liver injury, mice were intraperitoneally injected with 0.01 µg of LPS (Sigma) plus 20 mg of GalN (Sigma). For gene transfer, mice were injected with 1 × 1011 rAAV8 through the caudal vein. Tissues were snap frozen in liquid nitrogen for protein extraction, stored in TRIzol reagent (Invitrogen) for RNA extraction, or fixed in OCT (Sakura) or 4% paraformaldehyde for histopathological analysis. Blood specimens were obtained through cardiac puncture.
Cell Culture
Mouse normal liver cell line BNL CL.2 was purchased from Shanghai Cell Bank (ATCC source, Shanghai, China) and cultured in Dulbecco's modified Eagle's medium containing 10% fetal bovine serum at 37°C in a humidified incubator containing 10% CO2.
RNA Analysis
Total RNA was transcribed to complementary DNA (cDNA) with the First-Strand cDNA Synthesis system (TransGen Biotech, China). SYBR Green–based real-time quantitative polymerase chain reaction (qPCR) analysis was performed using the Thermal Cycler system (Bio-Rad) in accordance with the manufacturer's instructions.
Vector Construction and Virus Production
Genes of interest (GOIs) were amplified by reverse-transcription PCR and then inserted into the pLV-cDNA cloning vector (Biosettia) with BamHI and MluI (a Flag-tag was inserted into the vector previously to form a fusion protein with each GOI). Single-stranded small hairpin RNA (shRNA) was annealed and then ligated to the pLV-RNAi vector (Biosettia). Lentiviral stocks were produced by transfecting a 4-plasmid system into 293T cells as indicated by the manufacturer (Biosettia). rAAV8 was provided by Beijing Five Plus Molecular Medicine Institute (Beijing, China).
Sodium Dodecyl Sulfate Polyacrylamide Gel Electrophoresis (SDS-PAGE) and Western Blotting
SDS-PAGE and immunoblotting were performed as described in the Supplementary Materials.
Apoptosis Analysis by Flow Cytometry
BNL CL.2 cells were cocultured with 0.01 µg/mL TNF-α (Sigma) and 1 µg/mL actinomycin D (ActD; Sigma) for 7 hours, incubated with fluorescein isothiocyanate–annexin V and propidium iodide (BD Biosciences) for 15 minutes, and then analyzed by flow cytometry.
Histological Analysis and In Situ Apoptosis Detection
Tissue sections were stained with hematoxylin and eosin and analyzed by microscopy. The in situ labeling of apoptotic cells was performed using a terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay with a commercial kit (Promega).
Immunofluorescence
Hepatocytes were grown on sterilized glass coverslips in 24-well plates for 2 days and then fixed and permeated. Staining was performed as described in the Supplementary Materials.
Biochemical Analysis
Serum levels of alanine aminotransferase (ALT) were determined using the ALT activity assay kit (Sigma). Serum TNF-α levels were determined by an enzyme-linked immunosorbent assay (R&D). Cell viability was measured by a commercial kit (Promega) with the multidetection system (Promega), based on quantitation of adenosine triphosphate.
Coimmunoprecipitation
Hepatocytes were seed in a 75-cm2 flask and cultured for 2 days, followed by treatment with ActD and TNF-α separately or simultaneously for 5 hours. Coimmunoprecipitation was then performed by using a commercial kit (CWBiotech, China) with indicated antibodies.
Statistical Analysis
Statistical significance was determined by the t test or Kaplan–Meier analysis, using GraphPad Prism 5.01. Data were expressed as means ± SD. P values of <.05 were considered statistically significant.
RESULTS
Gene Array Analysis Identifies Several Candidates of Genes That May Restrict LPS-Induced Liver Injury
Treatment of mice with GalN dramatically increased the LPS-induced lethality due to the development of fatal hepatitis (Supplementary Figure 1). Although TNF-α has been proven to play a central role in this process [2], our data revealed that GalN did not affect the level of TNF-α expression (Supplementary Figure 2), suggesting that GalN probably increased LPS-induced lethality by blocking the transcription of hepatoprotective factors.
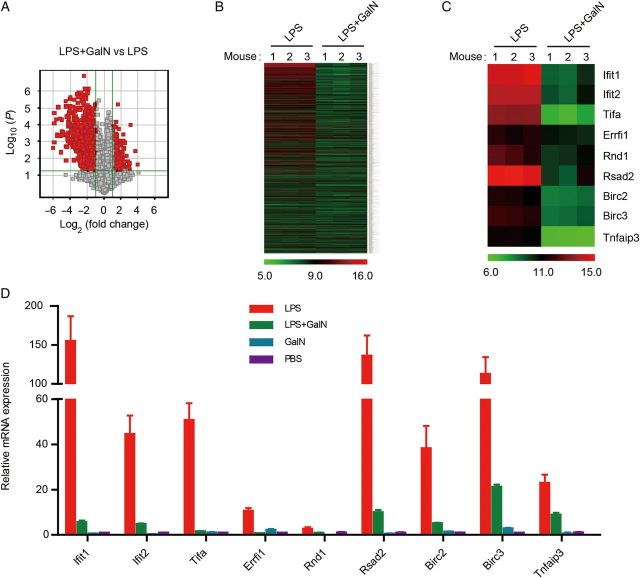
To search for these hepatoprotective factors, we performed gene array analysis of liver tissue specimens from mice treated with LPS or LPS/GalN. Compared with the LPS group, about 1400 genes were downregulated in the LPS/GalN group (Figure 1A and 1B). Interestingly, gene-set enrichment analysis indicated that 38% (Interferome v2.01 database) of downregulated genes, such as Ifit1 and Ifit2, were enriched in the type 1 interferon biological process (Supplementary Figure 3). We reasoned that these genes may play important roles in the process of bacterial infection and may have a hepatoprotective effect. Therefore, we chose 9 of these enriched genes to define their functions in LPS-induced hepatitis, which were strongly induced by LPS and seemed to have prosurvival or antiapoptosis effects. They were Ifit1, Ifit2, Tifa, Rsad2, Rnd1, Errfi1, Birc2, Birc3, and Tnfaip3 (Figure 1C). Tnfaip3 has been proven to have a hepatoprotective effect in the LPS/GalN model [10], so we used it as a positive control.
Figure 1.
Gene array analysis for protective factors that restrict lipopolysaccharide (LPS)–induced liver injury. Mice were treated with 0.01 µg of LPS alone or together with 20 mg of D-(+)-galactosamine (GalN) for 2.5 hours. Liver tissue specimens were isolated for gene array analysis. A, Volcano plots for visualizing differential gene expression between LPS and LPS/GalN treatments. B and C, Heat map depicting the messenger RNA (mRNA) levels of differentially expressed genes that were downregulated in the model. D, Real-time quantitative polymerase chain reaction analysis of the mRNA levels of genes of interest in mouse livers 2.5 hours after treatment (n = 3). Abbreviation: PBS, phosphate buffered saline.
To verify the microarray data, we performed real-time qPCR to measure the messenger RNA (mRNA) level of the 9 GOIs in liver tissue specimens treated with LPS or LPS/GalN. As shown in Figure 1D, all GOIs were strongly induced by LPS and dramatically inhibited in the presence of GalN.
Effect of GOIs on TNF-α–Induced Cell Death
Hepatocytes were sensitized to TNF-α by cotreatment with the transcriptional inhibitor ActD, which resembles the GalN-induced LPS/TNF-α sensitization of hepatocytes in mice [11] (Supplementary Figure 4). To investigate whether these GOIs have hepatoprotective effects, we used a lentivirus expression system to stably express them in BNL CL.2 cells and then cotreated the cells with TNF-α and ActD for 7 hours.
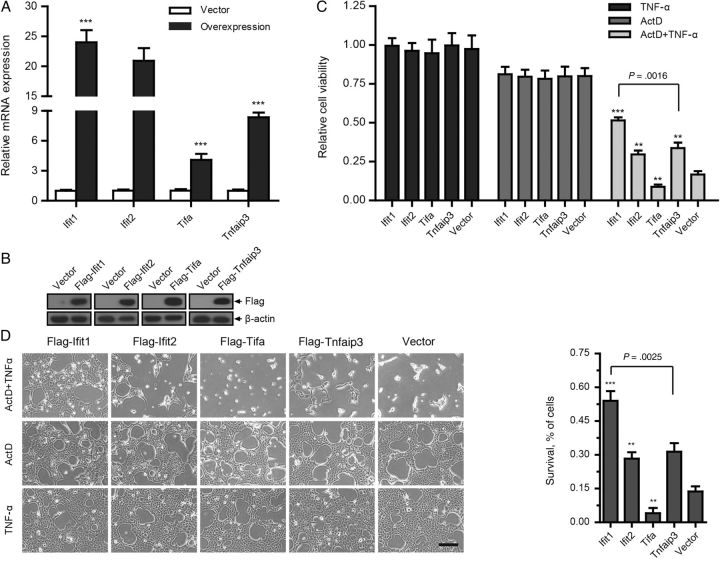
qPCR and immunoblotting analysis indicated that high transgene expression was achieved in all of the cells (Figure 2A and 2B and Supplementary Figure 5A and 5B). Notably, forced expression of Ifit1, Rsad2, and Tnfaip3 attenuated the cytotoxicity of TNF-α in hepatocytes (Figure 2C and 2D and Supplementary Figure 5C and 5D). We recorded cell viabilities of 52% and 34% among cells in which Ifit1 and Tnfaip3, respectively, were expressed, compared with only 17% in the empty vector group. We thus focused our attention on Ifit1, which displayed the highest cytoprotective effect.
Figure 2.
Expression of some genes of interest (GOIs) reduces tumor necrosis factor α (TNF-α)–induced cell death. BNL CL.2 cells expressing GOIs or empty vector were treated with 0.01 µg/mL TNF-α and 1 µg/mL actinomycin D (ActD) separately or simultaneously for 7 hours. A and B, Real-time quantitative polymerase chain reaction and immunoblotting analyses of the overexpression efficiency of GOIs. C, Cell viability assay, based on quantitation of adenosine triphosphate (n = 3). Cell viability is relative to the same type of cells treated with dimethyl sulfoxide. D, Cell counting analysis of survival among cells (n = 3). The survival percentage is relative to the group with that received TNF-α treatment. All data are presented as mean values ± SD. Scale bar represents 100 µm. **P < .01 and ***P < .001, by the 2-tailed t test, for comparison to groups that received empty vector. Abbreviation: mRNA, messenger RNA.
Ifit1 Expression Is Induced by LPS but Inhibited by GalN in Hepatocyte In Vivo
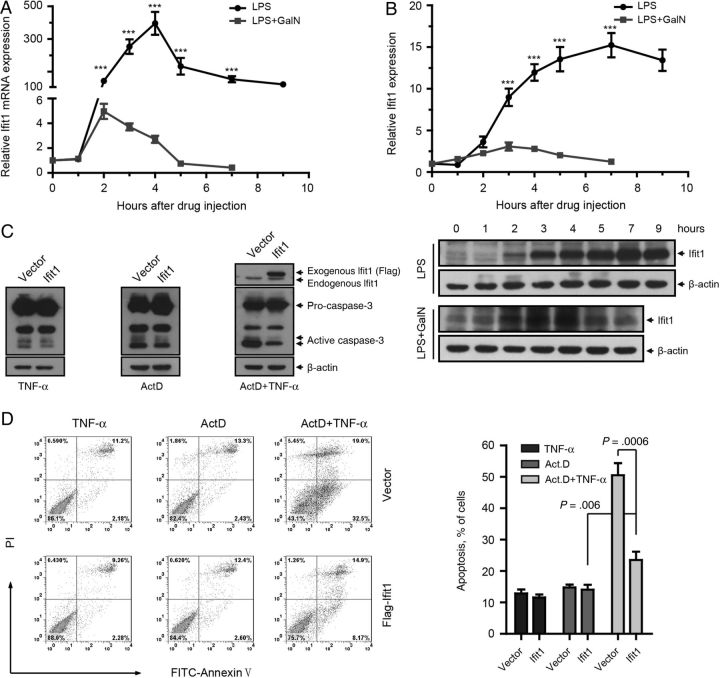
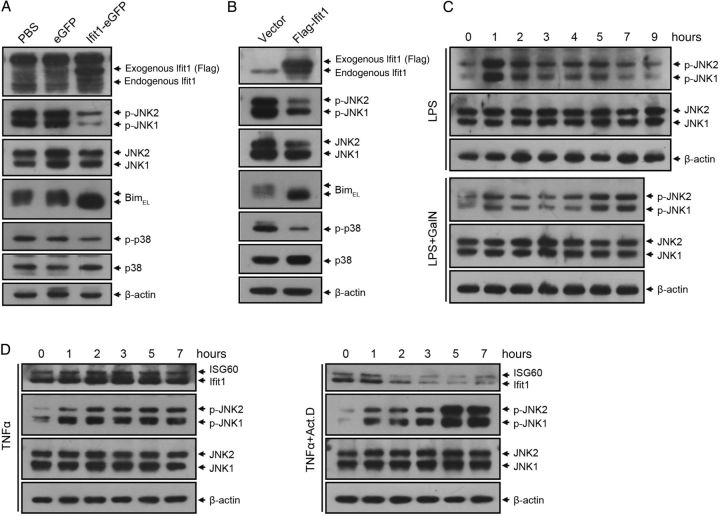
We next detected the pattern of Ifit1 expression in mouse liver during LPS stimulation. qPCR analysis showed that Ifit1 mRNA was induced rapidly by LPS and reached a peak 4 hours after treatment, whereas it was dramatically inhibited by cotreatment with GalN (Figure 3A). Similarly, levels of Ifit1 protein increased quickly and peaked 7 hours after LPS treatment and were dramatically inhibited in the presence of GalN (Figure 3B). These data indicated that the level of Ifit1 expression was conversely associated with liver injury.
Figure 3.
Expression of Ifit1 protects against tumor necrosis factor α (TNF-α)–mediated apoptosis in vitro. A and B, Mice were treated with 0.01 µg of lipopolysaccharide (LPS) alone or together with 20 mg D-(+)-galactosamine (GalN). Livers were isolated at indicated times after injection. A, Real-time quantitative polymerase chain reaction analysis of Ifit1 in mouse livers (n = 3). Expression levels are normalized to levels in untreated samples. B, Immunoblotting for Ifit1 in mice liver. Curves represent quantification of the immunoblotting results by ImageJ software, and expression levels are normalized to that in lane 1 (n = 3). Data are presented as mean values ± SD. ***P < .001, by the 2-tailed t test, for comparisons to LPS/GalN groups. C and D, BNL CL.2 cells expressing Ifit1 or empty vector were treated with 0.01 µg/mL TNF-α and 1 µg/mL actinomycin D (ActD) simultaneously or separately for 7 hours. C, Immunoblotting analysis of caspase-3 activation. D, Flow cytometry analysis of apoptosis. Comparisons were made using the 2-tailed t test. Abbreviation: mRNA, messenger RNA.
Ifit1 Inhibits TNF-α–Induced Apoptosis in Cultured Hepatocytes
To further investigate the antiapoptotic effect of Ifit1, we detected the proteolytic activation of caspase-3, which plays a critical role in apoptosis, in hepatocytes cotreated with TNF-α and ActD. Immunoblotting analysis indicated that forced expression of Ifit1 dramatically inhibited the cleavage of caspase-3 (Figure 3C), which was consistent with the fluorescence-activated cell-sorting (FACS) data showing that expression of Ifit1 diminished TNF-α–induced apoptosis in hepatocytes (Figure 3D). These results suggested that Ifit1 had a strong antiapoptotic effect in hepatocytes.
Targeted Expression of Ifit1 in Hepatocytes Protects Mice From LPS/GalN-Induced Lethal Hepatitis
rAAV8 has been widely used as therapeutic transgene cassette and has shown early promise in clinical trials [12, 13]. Intravenous injection of rAAV8 effectively and specifically targets transgene expression to the liver, mainly to hepatocytes [14] (Supplementary Figure 6). To investigate the protective effect of Ifit1 on LPS/GalN-induced lethal hepatitis in vivo, mice were injected with rAAV8 expressing Ifit1 (rAAV8-Ifit1-eGFP) or eGFP (rAAV8-eGFP) or with saline as a control and then challenged with a lethal dose of LPS and GalN 4 days later (Supplementary Figure 7A).
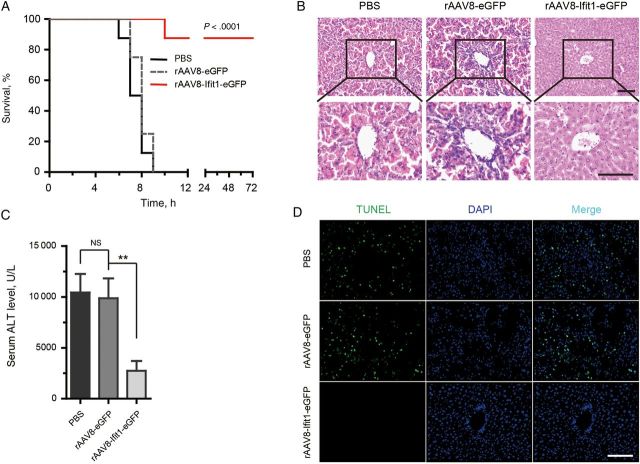
As shown in Supplementary Figure 7B and 7C, high expression of Ifit1 was achieved in the livers of mice with rAAV8-Ifit1-eGFP infection. Interestingly, whereas all control mice (both PBS recipients and eGFP recipients) died, almost all mice expressing Ifit1 survived and were healthy until euthanized 3 weeks later (Figure 4A and Supplementary Figure 7D). Notably, control mice had black livers due to massive hemorrhage, while the livers of mice infected with rAAV8-Ifit1 appeared normal (Supplementary Figure 7E). Histologically, the hepatic microarchitecture was destroyed and appeared to have massive hemorrhage at the site of the sinusoids in control mice, while mice expressing Ifit1 had an intact liver architecture with no signs of hemorrhage (Figure 4B).
Figure 4.
Gene transfer of Ifit1 protects mice against lipopolysaccharide (LPS)/D-(+)-galactosamine (GalN)–induced lethal hepatitis. Mice were injected intravenously with recombinant adeno-associated virus serotype 8 (rAAV8) encoding Ifit1-eGFP or eGFP or with saline and challenged 4 days later with 0.01 µg of LPS plus 20 mg of GalN. Livers and serum were harvested 7 hours after LPS/GalN challenge. A, Survival analysis of the mice injected with indicated rAAV8. B, Histological examination of hematoxylin-eosin–stained liver sections. C, Serum alanine aminotransferase (ALT) levels (n = 3). Data are presented as mean values ± SD. Comparisons were made using the 2-tailed t test. D, Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) staining of liver sections for apoptosis detection. All scale bars represent 100 µm. **P < .01. Abbreviations: NS, not significant; PBS, phosphate-buffered saline.
As a parameter for liver injury, serum ALT levels were measured using an ALT assay kit. Compared with control mice, the release of ALT was significantly diminished in mice expressing Ifit1 (Figure 4C). Similarly, the proteolytic cleavage of caspase-3 was dramatically reduced in rAAV8-Ifit1–infected mice (Supplementary Figure 7F). TUNEL staining is routine technique for detecting late apoptosis in cultured cells and animal models. Interestingly, no TUNEL stain was detectable in sections of liver tissue specimens from rAAV8-Ifit1–infected mice, while many hepatocyte nuclei in liver tissue specimens from control mice were TUNEL-stain positive (Figure 4D). Together, these results indicate that gene transfer of Ifit1 in the liver protects mice from LPS/GalN-induced lethal hepatitis by inhibiting hepatocyte apoptosis.
Ifit1 Prevents TNF-α–Mediated Activation of the JNK-Bim Cascade
We next investigated how Ifit1 inhibits TNF-α/ActD–induced apoptosis in vitro and in LPS/GalN-induced fatal hepatitis in vivo. Activation of casapase-8 in hepatocytes is required for TNF-α–mediated liver destruction [4]. Therefore, we tested the activation of caspase-8 and its downstream death effector, Bid, which triggers apoptosis by binding to the prosurvival Bcl-2 family members [15, 16]. Surprisingly, the proteolytic activation of caspase-8 and Bid showed no difference either in mice or in cell models (Supplementary Figure 8A and 8C).
Signaling through JNK has also been implicated in TNF-α–induced apoptosis [4]. In response to TNF-α stimuli, JNK-mediated phosphorylation causes the release of Bim from the dynein motor complex [17], characterized by a mobility shift in Western blot analysis. Released Bim is then localized to the membrane of mitochondria, where it triggers apoptosis by binding to the Bcl-2 family members [18]. We were excited to find that that JNK phosphorylation was dramatically diminished in mice expressing Ifit1, compared with control mice (Figure 5A and Supplementary Figure 8B). Notably, a mobility shift of BimEL, the most abundantly expressed isoform of Bim in liver, was observed in control mice but not in mice expressing Ifit1 (Figure 5A). Similarly, forced expression of Ifit1 in cultured hepatocytes dramatically inhibited the activation of JNK and BimEL when cotreated with TNF-α and ActD (Figure 5B).
Figure 5.
Ifit1 inhibits tumor necrosis factor α (TNF-α)–mediated activation of JNK and p38. A, Mice were injected with recombinant adeno-associated virus serotype 8 (rAAV8) or saline and challenged 4 days later with 0.01 µg of lipopolysaccharide (LPS) plus 20 mg of D-(+)-galactosamine (GalN). Liver extracts were harvested 7 hours after LPS/GalN challenge and probed by immunoblotting with antibodies against Ifit1, JNK, Bim, p38, and β-actin (loading control). B, BNL CL.2 cells expressing Ifit1 or empty vector were cotreated with 0.01 µg/mL TNF-α and 1 µg/mL actinomycin D (ActD) for 7 hours. Cell lysates were probed by immunoblotting for Ifit1, JNK, Bim, p38, and β-actin. C, Immunoblotting for JNK in mice treated with 0.01 µg of LPS alone or together with 20 mg of GalN. D, Immunoblotting for JNK in BNL CL.2 cells treated with 0.01 µg/mL TNF-α alone or together with 1 µg/mL ActD. Abbreviations: JNK, c-Jun N-terminal kinase; PBS, phosphate-buffered saline.
Previous studies showed that p38-MAPK signaling mediates TNF-α–induced apoptosis through downregulation of the antiapoptotic protein Bcl-xL [19, 20]. Notably, our data indicated that expression of Ifit1 significantly reduced the activation of p38 in both mice and cultured cells (Figure 5A and 5B and Supplementary Figure 8B).
We also detected the activation of ERK, another MAPK kinase that has been reported to play an important role in apoptosis [21, 22]. As shown in Supplementary Figure 8A, expression of Ifit1 had no effect on the activation of ERK in mice models.
Together, these results raised the possibility that Ifit1 attenuated LPS/GalN-induced lethal hepatitis by preventing TNF-α–mediated activation of the JNK and p38 signaling.
Level of Ifit1 Expression Is Conversely Associated With Activation of JNK
To further investigate the relationship between Ifit1 and JNK, we tested the pattern of endogenous Ifit1 expression and JNK activation in mouse livers. Notably, JNK was dramatically activated in liver tissue specimens 1 hour after LPS treatment and then decreased significantly with the induction of Ifit1. Moreover, in the presence of GalN, induction of Ifit1 was mild in the first 3 hours and then decreased continuously thereafter. Meanwhile, phosphorylation of JNK rapidly increased soon after injection, decreased slightly during the next 2 hours, and dramatically increased at the end of study interval (Figures 3B and 5C).
To confirm these results, we tested Ifit1 and JNK in cultured hepatocytes. Similarly, JNK was phosphorylated quickly after TNF-α stimulation and then remained at a steady level correlating with the constant expression of Ifit1. On the other hand, in the presence of ActD, phosphorylation of JNK dramatically increased in the last 2 hours, consistent with the transcriptional inhibition of Ifit1 by ActD (Figure 5D). These results indicated that the level of Ifit1 expression was conversely associated with the level of JNK activation.
As shown in Supplementary Figure 8D and 8E, the activation of p38 was also conversely related to the level of Ifit1 expression. Together, these results revealed that Ifit1 prevented TNF-α–mediated activation of JNK and p38.
Ifit1 Inhibits TNF-α–Mediated Activation of JNK by Binding to Axin
We next investigated how Ifit1 regulates the JNK and p38 pathways during TNF-α stimulation. It has been reported that JNK is activated by its upstream kinases, MKK4/7, in response to TNF-α stimuli [23, 24], which requires the formation of Axin homodimers and the direct physical interaction of Axin with MEKK1 [25, 26]. This complex also mediates the activation of p38 [25]. Thus, Axin acts as a scaffolding protein and is crucial for regulation of the JNK and p38 pathways.
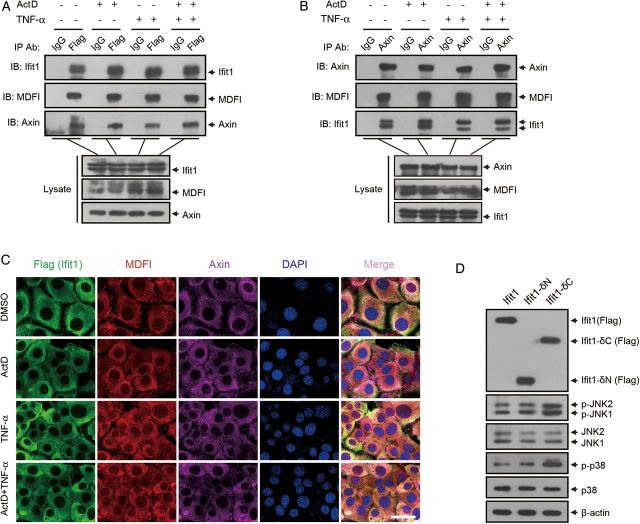
By referring to the 12D protein-protein interaction databases, we found that Ifit1 can interact with MDFI, an I-mfa domain protein that has been reported to directly interact with Axin and inhibit its function [27]. Therefore, we hypothesized that Ifit1 interacts with MDFI to inhibit the function of Axin. To certify our hypothesis, we performed a coimmunoprecipitation assay with Flag-Ifit1–infected hepatocytes, using an anti-Flag antibody. Data revealed that MDFI and Axin effectively coimmunoprecipitated with Ifit1 and that this interaction was independent of TNF-α or ActD stimulation (Figure 6A). Similarly, MDFI and Ifit1 also coimmunoprecipitated with Axin, and TNF-α and ActD were not necessary for this interaction (Figure 6B).
Figure 6.
Ifit1 inhibits tumor necrosis factor α (TNF-α)–induced apoptosis by binding to Axin. A–C, BNL CL.2 cells expressing Ifit1 were or were not treated with TNF-α and actinomycin D (ActD) for 5 hours. A, Coimmunoprecipitation with an anti-Flag antibody or control immunoglobulin G (IgG), followed by immunoblotting. B, Coimmunoprecipitation with an anti-Axin antibody or control IgG, followed by immunoblotting. C, Immunofluorescence staining for detecting the localization of Ifit1, MDFI, and Axin. D, BNL CL.2 cells expressing Ifit1-δN or Ifit1-δC were cotreated with TNF-α and ActD for 5 hours. Cell lysates were probed by immunoblotting for Ifit1, JNK, p38, and β-actin. Scale bar represents 30 µm. Abbreviations: JNK, c-Jun N-terminal kinase; DMSO, dimethyl sulfoxide.
To further investigate the interaction of these proteins, we detected the location of the 3 proteins in hepatocytes, using an immunofluorescence assay. As shown in Figure 6C, MDFI and Axin were tightly colocalized with Ifit1 in hepatocytes, with or without TNF-α and ActD treatment.
To confirm these interactions, we constructed a deletion mutation of Ifit1 at the N-terminus (Ifit1-δN) or C-terminus (Ifit1-δC) and stably expressed them in hepatocytes, using a lentivirus expression system (Supplementary Figure 9A). Then, we determined whether MDFI and Axin coimmunoprecipitated with Ifit1 in the hepatocytes. Interestingly, MDFI and Axin effectively coimmunoprecipitated with Ifit1-δN but not with Ifit1-δC (Supplementary Figure 9B). Similarly, MDFI and Ifit1-δN but not Ifit1-δC coimmunoprecipitated with Axin (Supplementary Figure 9B). These data suggested that the C-terminal domain of Ifit1 was required for the interaction with Axin and MDFI.
To investigate whether these interactions are necessary for the inhibition of Axin-mediated JNK and p38 activation, we determined whether JNK and p38 were activated in hepatocytes expressing Ifit1-δN or Ifit1-δC. As shown in Figure 6D, expression of Ifit1-δN inhibited the activation of JNK and p38, while Ifit1-δC had no effect, suggesting that the interaction of Ifit1 with Axin was necessary for the inhibition of Axin activity. Together, these results indicated that Ifit1 interacted with Axin and inhibited its ability to activate JNK and p38 in response to TNF-α.
Interaction of Ifit1 With MDFI Is Required for Inhibition of Axin
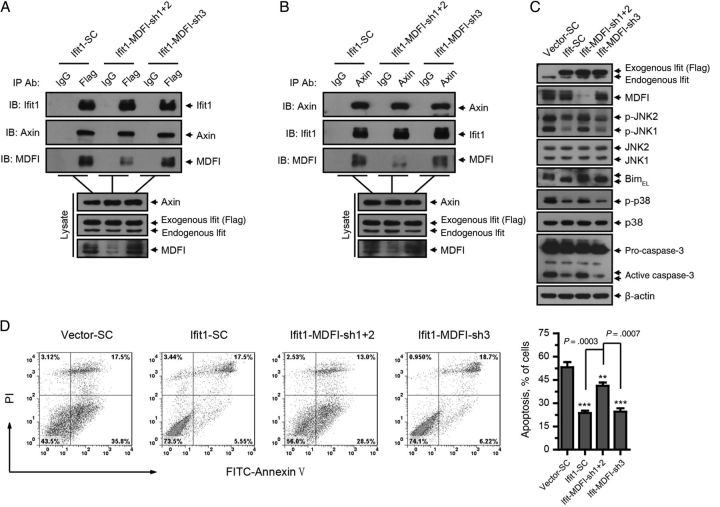
To investigate whether MDFI is required for the physical interaction of Ifit1 with Axin, we silenced MDFI in hepatocytes expressing Ifit1 by lentivirus-encapsulated shRNA and then performed a coimmunoprecipitation assay with Ifit1 and Axin. As shown in Figure 7A and 7B, silencing of MDFI (sh1 +2) did not interrupt the interaction between Ifit1 and Axin, which suggested that MDFI was not required for the physical interaction between Ifit1 and Axin.
Figure 7.
MDFI is not required for the physical interaction between Ifit1 and axin, but it is necessary for Ifit1-mediated inhibition of axin. BNL CL.2 cells expressing Ifit1 or empty vector were infected with lentivirus encoding small hairpin RNA for MDFI or β-galactosidase from Escherichia coli (SC) and then were cotreated with tumor necrosis factor α (TNF-α) and actinomycin D (ActD) for 5–7 hours. A, Coimmunoprecipitation with an anti-Flag antibody or control immunoglobulin G (IgG), followed by immunoblotting. B, Coimmunoprecipitation with an anti-Axin antibody or control IgG, followed by immunoblotting. C, Immunoblotting for Ifit1, MDFI, JNK, Bim, p38, casepase-3, and β-actin. D, Flow cytometry analysis of apoptosis. Data are presented as mean values ± SD. **P < .01 and ***P < .001, by the 2-tailed t test, for comparisons to SC groups. Abbreviation: JNK, c-Jun N-terminal kinase.
We next investigated whether MDFI is required for Ifit1-mediated inhibition of Axin. Notably, silence of MDFI eliminated the activity of Ifit1 in suppressing Axin function, resulting in increased activation of JNK, Bim, and p38, as well as the proteolytic cleavage of caspase-3, compared with the control vector (SC) group or the noneffective shRNA (sh3) group (Figure 7C).
We also performed FACS analysis to measure apoptosis in these cells. As shown in Figure 7D, silencing of MDFI disrupted the antiapoptotic effect of Ifit1. Collectively, these data demonstrated that, although MDFI was not required for the physical interaction between Ifit1 and Axin, it was necessary for Ifit1-mediated inhibition of Axin.
DISCUSSION
It has been suggested that GalN increases the sensitivity of hepatocytes to LPS or TNF-α by blocking the transcription of protective factors, which prompted us to seek hepatoprotective factors that may serve as targets for treatment of endotoxin-induced liver injury. In this study, we defined a protective role of Ifit1 in LPS/GalN-induced (TNF-α–mediated) hepatocyte killing. This protection was associated with the negative regulation of the JNK and p38 signaling pathways.
JNK signaling is crucial for TNF-α–induced apoptosis. In response to TNF-α stimuli, the adaptor proteins TRADD and ASK1 are rapidly recruited to TNFR1 and then activate MEKK1, which forms a complex with Axin and activates MKK4/7, the upstream kinase of JNK and p38 [28, 29]. Subsequently, JNK activates its downstream death effector, Bim, by releasing it from sequestration by the microtubular dynein motor complex [17, 30]. Then Bim interacts with the prosurvival Bcl-2 family member to activate Bax and Bak, triggering the initiation of a mitochondrial apoptosis pathway resulting in release of cytochrome c [16]. Interestingly, our data revealed that Ifit1 inhibited TNF-α–mediated activation of JNK and Bim, which was consistent with a previous report showing that injection of a JNK inhibitor reduced LPS/GalN-induced hepatocyte killing [31].
It has been reported that p38-MAPK mediates TNF-α–induced apoptosis in endothelial cells and kidney cells through phosphorylation and downregulation of the antiapoptotic protein Bcl-xL [19]. In our study, we found that expression of Ifit1 also reduced the activating phosphorylation of p38, suggesting that the antiapoptotic effect of Ifit1 was also associated with the inhibition of TNF-α–mediated p38 activation. However, the role of p38 in apoptosis is a matter of debate. Several articles have shown that activation of p38 attenuates TNF-α–induced apoptosis in neuronal PC12 cells and LNCaP prostatic cancer cells by an unknown mechanism [32, 33]. Thus, the explicit role of p38 in TNF-α–induced hepatocyte apoptosis needs to be further determined.
Axin is a scaffolding protein that has been proven to be involved in multiple signaling transduction, such as β-catenin regulation, and TNF-α–mediated JNK and p38 activation [25, 34, 35]. In response to TNF-α stimuli, Axin physically interacts with MEKK1 to activate MKK4/7, which can subsequently activate JNK and p38 [25, 26]. Our data showed that Ifit1 bound to Axin and negatively regulated its activity, resulting in inhibition of JNK and p38 activation. Moreover, the I-mfa domain protein MDFI was required for Ifit1-mediated inhibition of Axin through its direct binding to both Ifit1 and Axin [27]. The C-terminus of Ifit1 is required for its interaction with Axin and MDFI, but the structural basis for this requirement is still unclear. Moreover, because silencing MDFI did not eliminate the antiapoptotic activity of Ifit1, we reason that the antiapoptotic effect of Ifit1 is not only dependent on its function to inhibit Axin activity, but that other unknown mechanisms may also be involved and need to be further defined.
In conclusion, our findings identify Ifit1 as a hepatoprotective factor that mediates hepatocyte resistance to LPS/GalN-induced (TNF-α–mediated) cell death. Thus, any strategies that increase the expression or activity of Ifit1 might have potential for the prevention and treatment of endotoxin-induced liver injury and other inflammatory liver diseases.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. We thank Dr Ralph A. Reisfeld (Scripps Research Institute) and Dr Dwayne Stupack (UCSD) for their valuable suggestions and for proofreading this manuscript.
Financial support. This work was supported by National Key Scientific Research Projects (2013CB967200), the National Natural Science Foundation of China Overseas Cooperation Fund (31428013), the National Natural Science Foundation of China General Project (81272316 and 81273331), and the Program of International S&T Cooperation (2012DFA10650).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Ogikubo Y, Norimatsu M, Noda K et al. Evaluation of the bacterial endotoxin test for quantification of endotoxin contamination of porcine vaccines. Biologicals 2004; 32:88–93. [DOI] [PubMed] [Google Scholar]
- 2. Kuhla A, Eipel C, Siebert N, Abshagen K, Menger MD, Vollmar B. Hepatocellular apoptosis is mediated by TNFalpha-dependent Fas/FasLigand cytotoxicity in a murine model of acute liver failure. Apoptosis 2008; 13:1427–38. [DOI] [PubMed] [Google Scholar]
- 3. Ding WX, Yin XM. Dissection of the multiple mechanisms of TNF-alpha-induced apoptosis in liver injury. J Cell Mol Med 2004; 8:445–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kaufmann T, Jost PJ, Pellegrini M et al. Fatal hepatitis mediated by tumor necrosis factor TNFalpha requires caspase-8 and involves the BH3-only proteins Bid and Bim. Immunity 2009; 30:56–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Feng B, Wu S, Lv S et al. Metabolic profiling analysis of a D-galactosamine/lipopolysaccharide-induced mouse model of fulminant hepatic failure. J Proteome Res 2007; 6:2161–7. [DOI] [PubMed] [Google Scholar]
- 6. Terenzi F, Hui DJ, Merrick WC, Sen GC. Distinct induction patterns and functions of two closely related interferon-inducible human genes, ISG54 and ISG56. J Biol Chem 2006; 281:34064–71. [DOI] [PubMed] [Google Scholar]
- 7. Abbas YM, Pichlmair A, Gorna MW, Superti-Furga G, Nagar B. Structural basis for viral 5′-PPP-RNA recognition by human IFIT proteins. Nature 2013; 494:60–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Terenzi F, Saikia P, Sen GC. Interferon-inducible protein, P56, inhibits HPV DNA replication by binding to the viral protein E1. EMBO J 2008; 27:3311–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Weichselbaum RR, Ishwaran H, Yoon T et al. An interferon-related gene signature for DNA damage resistance is a predictive marker for chemotherapy and radiation for breast cancer. Proc Natl Acad Sci U S A 2008; 105:18490–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Arvelo MB, Cooper JT, Longo C et al. A20 protects mice from D-galactosamine/lipopolysaccharide acute toxic lethal hepatitis. Hepatology 2002; 35:535–43. [DOI] [PubMed] [Google Scholar]
- 11. Wullaert A, Wielockx B, Van Huffel S et al. Adenoviral gene transfer of ABIN-1 protects mice from TNF/galactosamine-induced acute liver failure and lethality. Hepatology 2005; 42:381–9. [DOI] [PubMed] [Google Scholar]
- 12. Paulk NK, Wursthorn K, Wang Z, Finegold MJ, Kay MA, Grompe M. Adeno-associated virus gene repair corrects a mouse model of hereditary tyrosinemia in vivo. Hepatology 2010; 51:1200–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nathwani AC, Tuddenham EG, Rangarajan S et al. Adenovirus-associated virus vector-mediated gene transfer in hemophilia B. N Engl J Med 2011; 365:2357–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wang L. Adeno-associated virus gene therapy prevents hepatocellular adenoma in murine model of glycogen storage disease type Ia. Hepatology 2012; 56:1593–5. [DOI] [PubMed] [Google Scholar]
- 15. Huang DC, Strasser A. BH3-Only proteins-essential initiators of apoptotic cell death. Cell 2000; 103:839–42. [DOI] [PubMed] [Google Scholar]
- 16. Kuwana T, Bouchier-Hayes L, Chipuk JE et al. BH3 domains of BH3-only proteins differentially regulate Bax-mediated mitochondrial membrane permeabilization both directly and indirectly. Mol Cell 2005; 17:525–35. [DOI] [PubMed] [Google Scholar]
- 17. Puthalakath H, Huang DC, O'Reilly LA, King SM, Strasser A. The proapoptotic activity of the Bcl-2 family member Bim is regulated by interaction with the dynein motor complex. Mol Cell 1999; 3:287–96. [DOI] [PubMed] [Google Scholar]
- 18. Lei K, Davis RJ. JNK phosphorylation of Bim-related members of the Bcl2 family induces Bax-dependent apoptosis. Proc Natl Acad Sci U S A 2003; 100:2432–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Grethe S, Ares MP, Andersson T, Porn-Ares MI. p38 MAPK mediates TNF-induced apoptosis in endothelial cells via phosphorylation and downregulation of Bcl-x(L). Exp Cell Res 2004; 298:632–42. [DOI] [PubMed] [Google Scholar]
- 20. Yeh CJ, Lin PY, Liao MH et al. TNF-alpha mediates pseudorabies virus-induced apoptosis via the activation of p38 MAPK and JNK/SAPK signaling. Virology 2008; 381:55–66. [DOI] [PubMed] [Google Scholar]
- 21. Cagnol S, Chambard JC. ERK and cell death: mechanisms of ERK-induced cell death--apoptosis, autophagy and senescence. FEBS J 2010; 277:2–21. [DOI] [PubMed] [Google Scholar]
- 22. Wang X, Martindale JL, Holbrook NJ. Requirement for ERK activation in cisplatin-induced apoptosis. J Biol Chem 2000; 275:39435–43. [DOI] [PubMed] [Google Scholar]
- 23. Zou H, Li Q, Lin SC, Wu Z, Han J, Ye Z. Differential requirement of MKK4 and MKK7 in JNK activation by distinct scaffold proteins. FEBS Lett 2007; 581:196–202. [DOI] [PubMed] [Google Scholar]
- 24. Abell AN, Granger DA, Johnson GL. MEKK4 stimulation of p38 and JNK activity is negatively regulated by GSK3beta. J Biol Chem 2007; 282:30476–84. [DOI] [PubMed] [Google Scholar]
- 25. Zhang Y, Neo SY, Wang X, Han J, Lin SC. Axin forms a complex with MEKK1 and activates c-Jun NH(2)-terminal kinase/stress-activated protein kinase through domains distinct from Wnt signaling. J Biol Chem 1999; 274:35247–54. [DOI] [PubMed] [Google Scholar]
- 26. Luo W, Ng WW, Jin LH, Ye Z, Han J, Lin SC. Axin utilizes distinct regions for competitive MEKK1 and MEKK4 binding and JNK activation. J Biol Chem 2003; 278:37451–8. [DOI] [PubMed] [Google Scholar]
- 27. Kusano S, Raab-Traub N. I-mfa domain proteins interact with Axin and affect its regulation of the Wnt and c-Jun N-terminal kinase signaling pathways. Mol Cell Biol 2002; 22:6393–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tobiume K, Matsuzawa A, Takahashi T et al. ASK1 is required for sustained activations of JNK/p38 MAP kinases and apoptosis. EMBO Rep 2001; 2:222–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ichijo H, Nishida E, Irie K et al. Induction of apoptosis by ASK1, a mammalian MAPKKK that activates SAPK/JNK and p38 signaling pathways. Science 1997; 275:90–4. [DOI] [PubMed] [Google Scholar]
- 30. Putcha GV, Le S, Frank S et al. JNK-mediated BIM phosphorylation potentiates BAX-dependent apoptosis. Neuron 2003; 38:899–914. [DOI] [PubMed] [Google Scholar]
- 31. Takamura M, Matsuda Y, Yamagiwa S et al. An inhibitor of c-Jun NH2-terminal kinase, SP600125, protects mice from D-galactosamine/lipopolysaccharide-induced hepatic failure by modulating BH3-only proteins. Life Sci 2007; 80:1335–44. [DOI] [PubMed] [Google Scholar]
- 32. Ricote M, Garcia-Tunon I, Fraile B et al. P38 MAPK protects against TNF-alpha-provoked apoptosis in LNCaP prostatic cancer cells. Apoptosis 2006; 11:1969–75. [DOI] [PubMed] [Google Scholar]
- 33. Park JG, Yuk Y, Rhim H, Yi SY, Yoo YS. Role of p38 MAPK in the regulation of apoptosis signaling induced by TNF-alpha in differentiated PC12 cells. J Biochem Mol Biol 2002; 35:267–72. [DOI] [PubMed] [Google Scholar]
- 34. Thompson MD, Monga SP. WNT/beta-catenin signaling in liver health and disease. Hepatology 2007; 45:1298–305. [DOI] [PubMed] [Google Scholar]
- 35. Neo SY, Zhang Y, Yaw LP, Li P, Lin SC. Axin-induced apoptosis depends on the extent of its JNK activation and its ability to down-regulate beta-catenin levels. Biochem Biophys Res Commun 2000; 272:144–50. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.