Abstract
Background:
Growing work points to the negative impact of early adverse experiences on the developing brain. An outstanding question concerns the extent to which early intervention can normalize trajectories of brain development in at-risk children. We tested this within the context of a randomized clinical trial (RCT) of an early parenting program, the Attachment and Biobehavioral Catch up (ABC), delivered to parents and infants monitored for maltreatment by Child Protective Services.
Methods:
Families participated in the RCT when children were 2.5 years of age and younger. Parenting and home adversity was measured at baseline. Children were followed longitudinally and resting brain activity was measured electrophysiologically (n=106) when children reached 8 years of age. Spectral power was quantified and compared across children assigned to the experimental intervention (ABC), a control intervention, and a low-risk comparison group (n=76) recruited at the follow up assessment.
Results:
Higher early home adversity was associated with electrophysiological profiles indicative of cortical delays/immaturity in middle childhood, based on relatively greater power in lower frequency bands (theta, 4–6Hz and low alpha, 6–9Hz) and lower power in a higher frequency band (high alpha, 9–12Hz). Children assigned to ABC showed relatively greater high frequency power (beta, 12–20Hz) than children assigned to the control intervention. Beta power in the ABC did not differ from those of the low risk comparison group.
Conclusions:
Maltreatment risk and home adversity can affect indicators of middle childhood brain maturation. Early parenting programs can support more normative patterns of neural function during middle childhood.
Keywords: Early adversity, resting EEG, brain maturation, childhood maltreatment, early intervention, parenting
Introduction.
Stable and responsive caregivers help provide the foundation for normative brain development. Early adverse rearing contexts are characterized by unresponsive, frightening, or unstable parental care, all of which increase the likelihood of cognitive and emotional problems. When experienced in the earliest years of life, a “window of vulnerability”(1), early adverse rearing can have a lasting negative impact on the developing brain, increasing risk for maladaptive outcomes(2–13). Importantly, the first years of life are also regarded as a “window of opportunity”(1), in that enriching or therapeutic input may have the greatest impact if experienced during the brain’s most malleable phases. Burgeoning evidence suggests that early interventions, especially delivered in the first years of life, can promote more normative neural outcomes in children exposed to unfavorable early rearing conditions.
Among the strongest experimental evidence supporting the impact of early intervention comes from the Bucharest Early Intervention Program (BEIP), a randomized clinical trial of foster care for institutionally reared, severely neglected children(14, 15). In this study, entry into highly responsive family settings was associated with more neurotypical trajectories of cortical function (measured with electroencephalography, EEG) in institutionally reared children than among children who continued in institutional care(16, 17). Specifically, children placed in foster care showed relatively greater magnitudes of spectral power in higher frequency alpha(17) and beta bands(16, 18) of resting EEG than children who remained in the institution. Notably, at eight years of age, only children placed in homes before the age of two showed significant intervention gains in alpha power estimates. Those placed after age two did not show evidence for normalization of cortical function in their EEG spectral power profiles(17), suggesting that intervention timing (age of intervention onset) is an important determinant of neural recovery.
To our knowledge, only one study has examined whether early intervention can normalize neural outcomes in children exposed to more common forms of maltreatment, such as abuse and neglect in family settings. Bruce and colleagues (2009) tested this question in the context of an intervention for maltreated preschool age children placed into foster care(19). Children and foster parents were randomly assigned to receive either multidimensional treatment foster care for preschoolers (MTFC-P), which promoted responsive parenting and enhanced preschool teacher support, or services as usual (SAU). A small subset of children (n=10 in MTFC-P and n=13 in SAU) participated in an EEG follow-up study where event-related potentials (ERPs) were recorded during an attentional and inhibitory control task. Children who received the MTFC-P showed a larger “feedback related negativity” (FRN) during the task than children who received SAU. Findings suggest that early intervention may promote more adaptive neural responses associated with self-monitoring and feedback sensitivity, which may be necessary for appropriate self regulation in social and academic contexts.
Current study.
Extant evidence suggests that early interventions can support more optimal neural functioning in children exposed to various forms of maltreatment, yet the body of literature is still in its very early stages. Studies to date have investigated extreme forms of psychosocial neglect (institutional rearing) and severe maltreatment warranting out-of-home placement(17, 19), but these represent only a subset of the contexts in which children may experience early adverse rearing. Emotional and physical neglect in family settings is the most common form of maltreatment experienced by children in the U.S., and children aged three and younger are at disproportionately high risk for being neglected(20). An important question is whether there are long-term effects on brain development in children reared in chronically neglecting and under-stimulating family settings. A second question is whether early interventions, designed to lessen neglect risk by improving parental responsiveness, can have a protective effect on the developing brain.
To address these questions, we examined the effectiveness of an early parenting intervention in a unique sample of families with infants/toddlers who were referred to Child Protective Services (CPS) for maltreatment-related concerns. The primary reason for referral was risk for family neglect, defined as the failure of the parent to support basic physical or emotional needs of the child(21), but children varied in the extent to which they experienced many other risk factors (see supplementary table S1). This was part of a larger randomized clinical trial of the Attachment and Biobehavioral Catch-up (ABC) intervention for neglecting families, shown to be effective in improving parental responsiveness(22) in previous studies. For example, children who received ABC have shown more optimal cognitive(23) and socio-emotional outcomes(24), and more normalized stress reactivity(25) during early childhood.
Following the completion of the intervention, children’s development was followed longitudinally throughout early and middle childhood. At eight years of age, EEG was recorded and examined for the current study. EEG is well-suited for examining neural outcomes in this sample for several reasons. Generally, EEG provides information about the excitability of neural networks; both macro-level cortical-cortical and cortico-subcortical dynamics are captured in the electrophysiological recording at the scalp(26). EEG power spectrum profiles are known to change as the brain develops. Low frequency activity, particularly in the theta band, decreases as the brain matures(27–31), whereas high frequency activity, particularly in the alpha band, increases from infancy throughout adolescence(32–34). These developmental changes have long been considered functional markers of cortical specialization, network organization, and enhanced neural efficiency. In terms of clinical significance, children with more immature EEG power spectral patterns (less higher frequency and more slower wave activity), including those exposed to severe early life neglect(35), are at increased risk for a range of neurocognitive and affective problems (27, 36–41).
Building on prior work, we used EEG as a means for detecting potential neurodevelopmental alterations in children exposed to chronically adverse early home environments including risk for neglect. We hypothesized that early home environment risk, specifically related to compromised parenting, would be associated with more immature middle childhood EEG spectral power profiles, defined as relatively greater proportions of slower frequency activity (in the theta band) and relatively lower proportions of high frequency activity (in the higher alpha and beta bands).
We also expected that children assigned to ABC would show more optimal patterns of neural function than children assigned to DEF. This stems from our prior work showing that, especially for maltreating families such as those in this sample, reducing maltreating behavior and increasing parental responsiveness is the critical agent for supporting optimal outcomes, cognitive, emotionally, and physiologically(23–25). We defined improved neural outcomes as patterns of relatively higher amounts of higher frequency activity, specifically in the higher alpha and beta bands as shown in prior work involving neglected children(16–18), and relatively lower amounts of slower frequency activity, specifically in the theta band. Given prior work in the BEIP(17), we also expected that children who received ABC at the earliest ages would be most likely to demonstrate more normative patterns of EEG spectral power at 8 years of age.
Methods and Materials
Procedure.
Data from this study came from an ongoing prospective longitudinal investigation testing the effectiveness of ABC for children reared in CPS-referred families at risk for maltreatment. This study began in 2006, when children were 2.5 years old or younger. Follow-up visits are ongoing, and children are now between 9 and 13 years of age. All procedures received ethics approval from the IRB at the University of Delaware where this study took place.
At study onset, children were randomized to ABC or the control intervention, DEF. Both intervention programs involved weekly one-hour sessions in the families’ homes for ten weeks. On average, families took 3.6 months to complete the program. Follow-up assessments took place one month subsequent to the intervention and when children reached 24 and 36 months of age (and at 48 months for a subgroup of children). Families were re-contacted when children reached 8 years of age and were invited to participate in additional follow-up assessments, including an EEG recording.
At the eight year assessment, a sample of non-maltreated children (n=83) from the community was recruited. See Tables 1–3 for demographic characteristics.
Table 1.
Child demographic characteristics
| Child Demographics | Low Risk Comparison | ABC | DEF | ||||
|---|---|---|---|---|---|---|---|
| % | n | % | n | % | n | ||
| Gender (Female) | 47.60 | 39 | 42.6 | 20 | 50.0 | 29 | |
| Race | African Am. | 46.98 | 39 | 59.6 | 28 | 69.0 | 40 |
| Caucasian | 21.68 | 18 | 12.8 | 6 | 22.4 | 13 | |
| Biracial/other | 26.50 | 22 | 25.5 | 12 | 8.6 | 5 | |
| Not reported | 4.81 | 4 | 2.1 | 1 | -- | -- | |
| Hispanic Ethnicity | 21.68 | 18 | 17.0 | 8 | 22.4 | 13 | |
| Min-Max | M(SD) | Min-Max | M(SD) | Min-Max | M(SD) | ||
|---|---|---|---|---|---|---|---|
| WJ-III Cog Score | 68–123 | 90.92(13.01) | 49–111 | 78.48 (13.13) | 56–106 | 83.45 (11.61) | |
| HOME total score | n/a | n/a | 22–43 | 32.86(6.12) | 20–44 | 34.03(5.29) | |
| Age at baseline (mos) | n/a | n/a | .49–19.52 | 8.49 (5.69) | .89–20.01 | 7.45 (5.63) | |
| Age at Intervention | n/a | n/a | .76–28.75 | 10.36 (6.53) | 1.68–21.95 | 8.98 (5.52) | |
| Age at EEG (yrs) | 6.9–9.08 | 8.51 (0.37) | 8.0–9.20 | 8.45(0.37) | 8.0–9.03 | 8.41 (0.33) | |
WJ-R = Woodcock Johnson III Assessment
Table 3.
Relative spectral power, early home adversity, early intervention status and timing
| Parameters | Model 1 | Model 2 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Fixed Effects | Estimates | Fixed Effects | Estimates | ||||||||
| Band | F | p val | B | SE | p val | F | p val | B | SE | p val | |
| Theta (4–6Hz) | Intercept | 33.243 | .000** | −1.641 | .282 | .000** | 32.633 | .000** | −1.69 | .294 | .000** |
| Region1 | 27.005 | .000** | - | - | - | 27.003 | .000** | - | - | - | |
| Malt. Risk | 0.032 | .858 | −.001 | .001 | .858 | 0.091 | .764 | −.001 | .001 | .764 | |
| Fam. Income Inf. | 1.476 | .227 | .008 | .006 | .227 | 1.659 | .201 | .008 | .006 | .201 | |
| Fam. Income 8 yr | 0.190 | .664 | .002 | .003 | .664 | 0.218 | .642 | .001 | .003 | .642 | |
| EEG age | 7.383 | .008** | −.009 | .003 | .008** | 7.695 | .007** | −.009 | .003 | .007** | |
| ABC2 | -- | -- | -- | -- | -- | 0.769 | .383 | .015 | .017 | .383 | |
| Intervention Timing | -- | -- | -- | -- | -- | 1.430 | .235 | .001 | .001 | .089 | |
| ABC*Timing | -- | -- | -- | -- | -- | 1.188 | .278 | −.001 | .001 | .278 | |
| Low Alpha (6–9Hz) | Intercept | 11.099 | .001** | −1.600 | .485 | .001** | 10.244 | .002** | −1.57 | .494 | .002** |
| Region1 | 25.531 | .000** | - | - | - | 25.494 | .000** | - | - | - | |
| Malt. Risk | 4.794 | .031* | .003 | .001 | .031* | 4.542 | .036* | ,003 | ,001 | .036* | |
| Fam. Income Inf. | 5.245 | .024* | .025 | .011 | .024* | 4.974 | .028* | ,025 | .011 | .028* | |
| Fam. Income 8 yr | 4.856 | .030* | −.013 | .005 | .030* | 5.413 | .022* | −.013 | .005 | .022* | |
| EEG age | 1.174 | .281 | −.006 | .006 | .281 | 0.802 | .373 | −.005 | .005 | .373 | |
| ABC2 | -- | -- | -- | -- | -- | 2.434 | .122 | .046 | .029 | .122 | |
| Intervention Timing | -- | -- | -- | -- | -- | 4.765 | .032* | −.001 | .001 | .783 | |
| ABC*Timing | -- | -- | -- | -- | -- | 3.329 | .071 | −.004 | .002 | .071 | |
| High Alpha (9–12Hz) | Intercept | 9.345 | .003** | −1.32 | .441 | .004** | 8.363 | .005** | −1.29 | .459 | .006** |
| Region1 | 75.515 | .000** | - | - | - | 75.405 | .000** | - | - | - | |
| Malt. Risk | 18.909 | .000** | −.005 | .001 | .000** | 18.559 | .000** | −.005 | .001 | .000** | |
| Fam. Income Inf. | 0.007 | .934 | .001 | .010 | .934 | 0.003 | .958 | .001 | .011 | .958 | |
| Fam. Income 8 yr | 3.534 | .063 | −.010 | .005 | .063 | 3.713 | .057 | −.010 | .005 | .057 | |
| EEG age | 6.091 | .015* | .013 | .005 | .015* | 6.963 | .010* | .014 | .005 | .010* | |
| ABC2 | -- | -- | -- | -- | -- | 0.001 | .980 | .001 | .027 | .098 | |
| Intervention Timing | -- | -- | -- | -- | -- | 2.740 | .101 | −.002 | .001 | .167 | |
| ABC*Timing | -- | -- | -- | -- | -- | 0.039 | .844 | .001 | .002 | .844 | |
| Beta (12–20Hz) | Intercept | 9.836 | .002** | −1.51 | .484 | .002** | 8.713 | .004** | −1.418 | .494 | .005** |
| Region1 | 13.139 | .000** | - | - | - | 13.096 | .000** | - | - | - | |
| Malt. Risk | 0.003 | .958 | −.001 | .001 | .958 | .001 | .980 | .001 | .001 | .980 | |
| Fam. Income Inf. | 1.472 | .228 | −.013 | .011 | .228 | 1.829 | .180 | −.015 | .011 | .180 | |
| Fam. Income 8 yr | 0.465 | .497 | .004 | .005 | .497 | 0.481 | .489 | .003 | .005 | .489 | |
| EEG age | 0.053 | .818 | .001 | .006 | .818 | 0.002 | .961 | −.001 | .005 | .961 | |
| ABC2 | -- | -- | -- | -- | -- | 4.270 | .042* | −.061 | .029 | .042* | |
| Intervention Timing | -- | -- | -- | -- | -- | 2.628 | .108 | −.001 | ,001 | .770 | |
| ABC*Timing | -- | -- | -- | -- | -- | 4.077 | .046* | .005 | .002 | .046* | |
Key:
= p<.05,
=p<.01.
B estimates for each of the 7 regions included in model not reported, Reference is parietal region;
Reference is ABC group. ABC=Attachment and Biobehavioral Catch up; DEF=Developmental Education for Children; Fam=Family.
Participants.
Of the original 183 families in the randomized clinical trial (RCT), 127 were successfully contacted to participate in the 8 year follow-up visit (ABC: n=58; DEF: n=69). Of those, 105 (ABC: n=47; DEF: n=58) completed the EEG portion of the study. See the consort diagram in the supplement for details (42). For this sub-study, child age at the start of the intervention ranged from 0.76 to 28.75 months. Of the total 83 children recruited in this community comparison sample, 76 participated in the EEG assessment.
Baseline Assessment:
Demographic data.
Socio-demographic risk was assessed at baseline middle childhood follow up assessments. All but four families provided demographic data. See Tables 1–2 for demographic characteristics and supplementary Table S2 for income distributions.
Table 2.
Caregiver demographic characteristics
| Caregiver Variable | Demographics | Comparison Group | ABC | DEF | |||
|---|---|---|---|---|---|---|---|
| % | n | % | n | % | n | ||
| Gender (Female) | 98.79 | 82 | 97.9 | 46 | 94.8 | 55 | |
| Marital | Married/living | -- | -- | 21.2 | 10 | 26.8 | 15 |
| together | -- | -- | |||||
| Status | Single | -- | -- | 68.1 | 32 | 63.8 | 37 |
| Divorced/separated | -- | -- | 6.4 | 3 | 5.2 | 3 | |
| Not reported | -- | -- | 4.3 | 2 | 5.2 | 3 | |
| Education | <High School | 7.2 | 6 | 68.1 | 32 | 50.0 | 29 |
| High School | 27.71 | 23 | 23.4 | 11 | 37.9 | 22 | |
| Some College | 30.12 | 25 | 2.1 | 1 | 8.6 | 5 | |
| College Graduate | 26.50 | 22 | 2.1 | 1 | -- | -- | |
| Not Reported | 10.84 | 9 | 4.3 | 2 | 3.4 | 2 | |
| Race | African American | 48.19 | 40 | 61.7 | 29 | 70.7 | 41 |
| Caucasian | 27.71 | 23 | 27.7 | 13 | 24.1 | 14 | |
| Biracial/other | 19.27 | 16 | 8.5 | 4 | 5.2 | 3 | |
| Not reported | 4.81 | 4 | 2.1 | 1 | -- | -- | |
| Hispanic Ethnicity | 21.68 | 18 | 17.0 | 8 | 20.7 | 12 | |
| Min-Max | M(SD) | Min-Max | M(SD) | Min-Max | M(SD) | ||
|---|---|---|---|---|---|---|---|
| Caregiver age at child’s birth | -- | -- | 15.21–56.27 | 27.53 (9.52) | 13.71–44.03 | 26.88 (9.58) | |
Note: ABC=Attachment and Biobehavioral Catch up; DEF=Developmental Education for Children. Data on marital status and caregiver age were not collected for the comparison group.
Early Home Adversity.
We used the Home Observation for Measurement of the Environment (HOME), Third Edition(43, 44), Infant Toddler version, to measure adversity in the early home environment at the baseline assessment. This instrument provides a total score of home environmental risk and six subscale scores that measure variability in parenting and environmental risk, including: 1) Parental emotional and verbal responsiveness, 2) Parental acceptance of suboptimal behavior and avoidance of restriction or punishment, 3) General home organization, 4) Presence of appropriate learning materials, 5) Parental Involvement, and 6) Variety in daily stimulation (see supplement for more details on the measure and these subscales). For ease of interpretation, scores were reverse coded so that higher scores indicated higher home risk.
Early intervention.
Attachment and Biobehavioral Catch-up.
ABC was designed to enhance parental sensitivity to children’s distress, lessen intrusive behaviors, and decrease frightening behavior. Throughout sessions, parent coaches gave in vivo and video-based feedback on the parents’ positive behavior (i.e., when the parent responded sensitively to her child’s distress, followed her child’s lead with delight rather than intrusively, or refrained from using frightening behavior). Each session was video recorded. For additional intervention details, see Lind et al., 2014(42).
Developmental Education for Families.
DEF was adapted from a home visitation program developed by Ramey and colleagues(45, 46). The implementation of this program focused on helping parents learn ways to enhance children’s cognitive and language development, and omitted aspects of the original intervention focused on parental sensitivity. Coaches helped parents practice these concepts using themed, developmentally appropriate activities. Parent coaches used in vivo and video feedback to point out positive parental behavior consistent with the intervention targets.
Intervention timing:
Children ranged from 0.76 to 28.75 months of age at baseline. As in prior work(17), we used child age to represent intervention timing.
8 year follow-up visit.
EEG recording:
EEG was recorded while participants sat quietly in front of a computer screen, alternating one minute epochs of keeping their eyes open and then closed, for a total of six minutes. EEG was recorded from an electrode cap consisting of 32 Ag/AgCl electrodes placed according to the International 10–20 system(47), and digitized at 1024 samples per second. See supplement for additional details.
EEG processing and analyses.
Preprocessing of EEG data was performed according to recommended guidelines(48) using the Boston EEG Automated Processing Pipeline/Harvard Automated Processing Pipeline(49, 50). See supplement for details. EEG data were collected from 105 children in the experimental group (ABC=47, DEF=58) and 76 children in the community comparison group. Of those, 8 participants’ data (ABC=3, DEF=3, Community comparison=2) were not included due to excessive artifact and/or too little data to compute spectral analyses. To limit the number of comparisons and increase the reliability of EEG estimates, data were averaged across eyes open/closed conditions, consistent with prior work(51). Data from 32 channels were reduced to 7 regions including Frontal pole, Frontal, Fronto-Central, Central, Central Parietal, Parietal, and Occipital sites.
Spectral power (μV2) was computed for the following frequency bands: theta (4–6Hz), low alpha (6–9Hz), high alpha (9–12Hz), and beta (12–20Hz) similar to prior studies with same-aged children(17). We quantified separate power estimates for low alpha (6–9Hz) and high alpha (9–12Hz) to account for established developmental increases in alpha power and frequency across development(28, 30).
We examined estimates of both absolute power and relative power in this study. Absolute power is the total amount of spectral power for a given frequency band measured at the specific site or region and can be influenced by non-functional properties such as anatomical features and skull thickness. Therefore, relative power is often computed as the proportion of power at a given frequency band and site, relative to the total amount of power at that site. As the proportion score is specific to each individual’s power spectrum, it minimizes contribution of inter-individual variability in neuroanatomical features(27, 52) and is useful for pediatric samples(28, 30). See supplement for more details on EEG quantification.
Results
We used separate marginal models for each frequency band; see supplement for more details. Region was entered as a within-subjects factor. Family income at both the baseline and 8 year assessment were included as predictors in each model. Maternal age, child gender, and child ethnicity were not significantly associated with variables of interest and were therefore not included in the models. A separate model was run for each frequency band. For each band, we used relative power as an outcome, and then repeated models with absolute power.
Early home adversity and EEG spectral power.
We tested associations between total scores on the HOME inventory and EEG spectral power, controlling for covariates. See Model 1 in Table 3 for relative power and in Table 4 for absolute power. When significant associations with total HOME scores emerged, post hoc analyses were performed to examine which HOME subscales contribute to the total effect.
Table 4.
Absolute spectral power, early home adversity, early intervention status and timing
| Parameters | Model 1 | Model 2 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Fixed Effects | Estimates | Fixed Effects | Estimates | ||||||||
| Band | F | p val | B | SE | p val | F | p val | B | SE | p val | |
| Theta (4–6Hz) | Intercept | 4.181 | .043* | −1.18 | .589 | .046* | 4.649 | .033* | −1,32 | ,611 | .032* |
| Region1 | 50.727 | .000** | - | - | - | 50.802 | .000** | - | - | - | |
| Malt. Risk | 4.990 | .027* | .004 | .001 | .027* | 4.533 | .035* | .003 | .001 | .035* | |
| Fam. Income Inf. | 3.749 | .055 | .026 | .013 | .055 | 4.164 | .043* | .028 | .013 | .043* | |
| Fam. Income 8 yr | 0.293 | .589 | −.004 | .007 | .589 | 0.275 | .601 | −.003 | .007 | .601 | |
| EEG age | 0.289 | .592 | .004 | .007 | .592 | 0.389 | .534 | .004 | .007 | .601 | |
| ABC2 | -- | -- | -- | -- | -- | 2.142 | .145 | .055 | .037 | .145 | |
| Intervention Timing | -- | -- | -- | -- | -- | 0.005 | .942 | .002 | .002 | .213 | |
| ABC*Timing | -- | -- | -- | -- | -- | 2.558 | .112 | −.005 | .003 | .112 | |
| Low Alpha (6–9Hz) | Intercept | 1.207 | .274 | −.885 | .857 | .304 | 1.170 | .282 | −.925 | .875 | .293 |
| Region1 | 58.586 | .000** | - | - | - | 58.650 | .000** | - | - | - | |
| Malt. Risk | 7.259 | .008** | .007 | .002 | .008** | 6.972 | .009** | −.006 | .002 | .009** | |
| Fam. Income Inf. | 3.697 | .057 | .038 | .019 | .057 | 3.773 | .054 | .038 | .019 | .054 | |
| Fam. Income 8 yr | 3.011 | .085 | −.018 | .010 | .085 | 3.259 | .074 | −.019 | .010 | .074 | |
| EEG age | 0.504 | .479 | .007 | .010 | .479 | 0.853 | .357 | .009 | .010 | .357 | |
| ABC2 | -- | -- | -- | -- | -- | 2.370 | .126 | .083 | .054 | .126 | |
| Intervention Timing | -- | -- | -- | -- | -- | 3.325 | .071 | −.001 | .003 | .968 | |
| ABC*Timing | -- | -- | -- | -- | -- | 3.135 | .079 | −.008 | .004 | .079 | |
| High Alpha (9–12Hz) | Intercept | 1.953 | .165 | −1.08 | .819 | .189 | 2.181 | .142 | −1.200 | .846 | .159 |
| Region1 | 87.337 | .000** | - | - | - | 87.318 | .000** | - | - | - | |
| Malt. Risk | 0.490 | .485 | −.001 | .002 | .485 | 0.410 | .523 | −.001 | .002 | .523 | |
| Fam. Income Inf. | 1.278 | .261 | .021 | .019 | .261 | 1.538 | .217 | .023 | .019 | .217 | |
| Fam. Income 8 yr | 1.686 | .197 | −.013 | .010 | .197 | 1.675 | .198 | −.013 | .010 | .198 | |
| EEG age | 1.953 | .165 | −1.08 | .819 | .189 | 10.124 | .002** | .033 | .011 | .002** | |
| ABC2 | -- | -- | -- | -- | -- | 0.674 | .413 | .042 | .052 | .413 | |
| Intervention Timing | -- | -- | -- | -- | -- | 1.647 | .202 | −.011 | .003 | .539 | |
| ABC*Timing | -- | -- | -- | -- | -- | 0.212 | .646 | −.002 | .004 | .646 | |
| Beta (12–20Hz) | Intercept | 1.599 | .209 | −1.049 | .854 | .222 | 1.331 | .251 | −.981 | .893 | .274 |
| Region1 | 74.117 | .000** | - | - | - | 74.120 | .000** | - | - | - | |
| Malt. Risk | 2.924 | .090 | .004 | .002 | .090 | 2.890 | .092 | .004 | .002 | .092 | |
| Fam. Income Inf. | 0.020 | .888 | .002 | .019 | .888 | 0.006 | .938 | .001 | .020 | .938 | |
| Fam. Income 8 yr | 0.001 | .971 | −.001 | .010 | .971 | 0.002 | .964 | −.001 | .010 | .964 | |
| EEG age | 2.533 | .114 | .017 | .010 | .114 | 2.251 | .136 | .016 | .011 | .136 | |
| ABC2 | -- | -- | -- | -- | -- | 0.190 | .664 | −.024 | .055 | .664 | |
| Intervention Timing | -- | -- | -- | -- | -- | 0.070 | .792 | −.001 | .003 | .948 | |
| ABC*Timing | -- | -- | -- | -- | -- | 0.124 | .725 | .001 | .004 | .725 | |
Key:
= p<.05,
=p<.01.
B estimates for each of the 7 regions included in model not reported, Reference is parietal region;
Reference is ABC group ABC=Attachment and Biobehavioral Catch up; DEF=Developmental Education for Children; Fam=Family.
Theta (4–6 Hz).
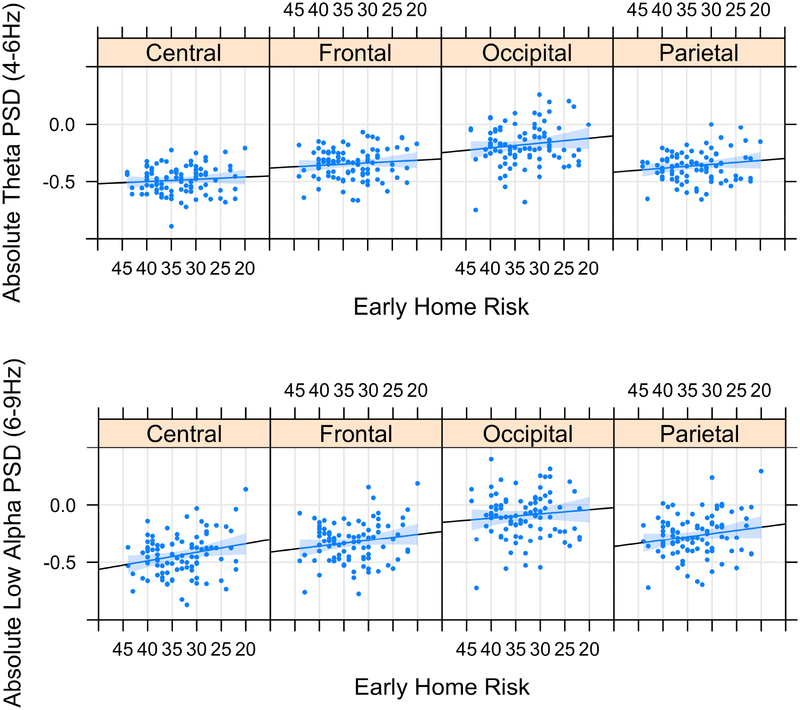
There was a significant main effect of early home adversity on absolute (p=.027) but not relative (p=.858) power. Consistent with hypotheses, higher early home adversity was associated with higher absolute power (see Figure 1A). Post hoc analyses revealed that associations were driven by the parental acceptance subscale (i.e., extent to which parents accept suboptimal behavior and avoid restriction or punishment). This emerged for relative (p=.014) and absolute (p=.010) estimates.
Figure 1.
The total Home Observation for Measurement of the Environment score was used as an assessment of early home adversity. Originally scaled (not reverse coded) HOME scores are presented in the figures, with lower scores indicating higher risk. For ease in interpretation, the scores on the x-axis are in descending order. Early home adversity is positively associated with spectral power in lower-frequency bands theta (4–6 Hz) and low alpha (6–9 Hz). Log-transformed values are displayed. PSD, power spectral density.
Low alpha (6–9 Hz).
There was a significant main effect of early home adversity on relative (p=.031) and absolute (p=.008) power in the low alpha band. High early home risk was associated with higher absolute power (see Figure 1B). Post hoc analyses of HOME subscales revealed that associations were driven by variability in parental acceptance for relative (p=.004) and absolute (p=.002) power, and with home organization/predictability for absolute power (p=.042).
High alpha (9–12 Hz).
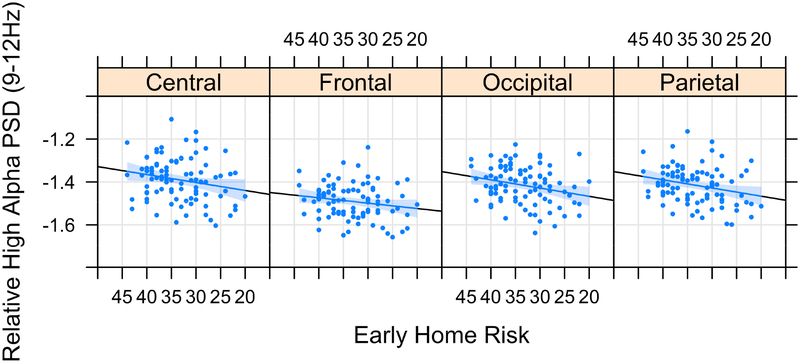
There was a significant main effect of early home adversity on relative power (p<.001) in the high alpha band. Consistent with expectations, higher early home adversity was associated with lower relative power (see Figure 2). Post-hoc examination of subscales revealed that relative power associations were driven by parental responsiveness (p=.005), caregiver involvement (p=.001), overall home organization/predictability (p=.001), and the provision of appropriate learning materials (p=.001). There were no associations between early home adversity and absolute power (p=.485).
Figure 2.
The total Home Observation for Measurement of the Environment score was used as an assessment of early home adversity. Originally scaled (not reverse coded) HOME scores are presented in the figures, with lower scores indicating higher risk. For ease in interpretation, the scores on the x-axis are in descending order. Early home adversity is negatively associated with spectral power in the high alpha band (9–12 Hz). Log-transformed values are displayed. PSD, power spectral density.
Beta (12–20 Hz).
Early home adversity was not significantly associated with relative (p=.958) or absolute (p=.090) power.
Intervention status, intervention timing, and EEG spectral power.
Next, we added intervention group, intervention timing, and their interaction to the previous model and tested their associations with spectral power for each frequency band. Intervention timing was a continuous variable based on age at the start of the intervention. See Model 2 in Table 3 for relative power and Table 4 for absolute power.
Theta (4–6 Hz).
The main effect of intervention group and timing was not significant for relative (group: p=.383, timing: p=.089) or absolute (group: p=.145, timing: p=.213) power. The interaction between intervention group and timing was also not significant for relative (p=.278) or absolute (p=.112) power.
Low alpha (6–9 Hz).
There was no main effect of intervention group or timing for relative (group: p=.122, timing: p=.783) or absolute (group: p=.126, timing: p=.968) power. The interaction between intervention group and timing was also not significant for relative (p=.071) or absolute (p=.079) power.
High alpha (9–12 Hz).
The main effect of intervention group and timing was not significant for relative (group: p=.098, timing: p=.167) and absolute (group: p=.413, timing: p=.539) power. The interaction between intervention group and timing was also not significant for relative (p=.844) or absolute (p=.646) power.
Beta (12–20 Hz).
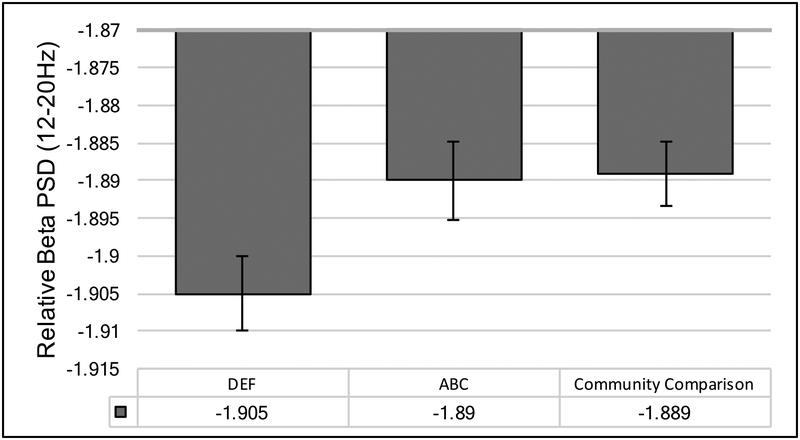
A significant main effect of intervention group emerged for relative (p=.042) but not absolute (p=.664) power. The main effect of intervention timing was not significant for relative (p=.770) or absolute (p=.948) power.
To better interpret the main effect of intervention group, we compared spectral power in the beta band among children assigned to ABC and DEF with the comparison group of children recruited at the time of the eight year assessment. Family income and child age at the 8 year assessment were included as covariates. Results of a linear mixed model revealed a main effect of group (B=−.001, p=.037). Children in DEF had significantly lower relative power than children in ABC (p = .038) and the community comparison group (p = .018). Children in ABC did not significantly differ in their spectral power estimates from the community comparison group (p = .816). These results suggest an intervention effect, and normalization in their spectral power in the beta band for children assigned to ABC (see Figure 3).
Figure 3.
Main effect of intervention group on spectral power in the beta band (12–20Hz). Log transformed values are displayed. ABC=Attachment and Biobehavioral Catch up program. DEF=Developmental Education for families program.
Results from the larger marginal model showed a significant interaction between intervention group and timing for relative (p=.046) but not absolute (p=.725) power. Post-hoc inspections revealed that timing (child age at the time of the intervention) was not significantly associated with relative power for children assigned to ABC (B=−.001, p=.684), but was associated with relative power for children assigned to DEF (B=.004, p=.023). Unexpectedly, children who received DEF at older ages showed significantly higher levels of relative power than children who received DEF at younger ages.
Discussion
This study examined EEG patterns of neural function in children reared in CPS-referred families at risk for maltreatment. Families with infants and toddlers participated in an RCT of an early parenting intervention. Children were followed longitudinally, and resting EEG was recorded when children were 8 years of age. Higher levels of at risk parenting and home adversity were associated with more neurodevelopmentally immature patterns of spectral power profiles (relatively greater spectral power in the lower frequency bands, theta (4–6Hz) and low alpha power (6–9Hz), and relatively lower spectral power in a higher frequency band, high alpha (9–12Hz)) during middle childhood. Consistent with prior work involving neglected children(17, 18), we show that an early intervention is associated with enhanced high frequency spectral power in the beta band (12–20Hz). These effects were observed when children were 8 years of age, 5–7 years after the completion of the intervention. This points to long lasting positive effects of the early parenting program on neural outcomes.
Data from this study contribute to the extant literature in several ways. First, we prospectively investigated an understudied and methodologically challenging population of children at risk for maltreatment who remained with their families of origin. Converging with prior work involving institutional rearing, we show that chronic family adversity and neglect risk exert a widespread effect on cortical development(2). Genearlly, observed variability in parenting (acceptance, involvement, and responsiveness) was more consistently associated with patterns of neural function, than non parenting domains (general organization of the home environment or provision of learning materials). This supports our hypotheses that problematic parenting was a key mechanism of influence on neural trajectories in this sample of children reared in families being monitored for maltreatment.
Our associations between higher early home risk and more immature patterns of cortical activity defined as relatively greater power in slower frequency bands have also been observed in children reared in contexts of psychosocial deprivation, social isolation, and in low resource homes(53, 54). In terms of the neurophysiological basis for these neural profiles, prior work using MRI suggests that early life adversity, particularly neglect, leads to patterns of over-pruning of cortical gray matter connections(2, 55) and reductions in white matter or myelination(5, 9, 56, 57). Future work examining alterations in structural and functional connectivity of the brain, particularly the cerebral cortex, may shed light on the specific neural changes that contribute to these EEG alterations in this sample.
Results also indicate that early parenting intervention for maltreating families supports more normative patterns of neural function in middle childhood. Relative to children in DEF, children who received ABC showed higher relative spectral power in the beta band (12–20Hz) at eight years of age. Spectral power estimates of children assigned to ABC did not significantly differ from those of children recruited from the nonmaltreating community sample, suggesting a normalization in neural function in this frequency band. A key component of ABC was to help parents engage with their child without being frightening, arousing, or excessively harsh when disciplining. This supports our hypothesis that, for maltreating families, interventions that specifically target parenting will be most effective for improving child outcomes. This may differ from other contexts adversity (i.e., low income families where parenting is not the central concern) in that additional aspects of the environment (learning environment, stimulation) may need to be targeted to achieve similar patterns of EEG normalization.
In terms of the functional implications of our findings, spectral power in the beta band has recently been associated with improved cognitive control and maintenance of current behavioral states(58), which may have been required for children to remain seated, control movements, and follow instructions during this EEG task. Although we assessed neural function in a task-free paradigm, beta spectral power has also been associated with better performance on tasks that involve working memory(59), language processing(60), emotional processing(61, 62) and motor performance(63). An important caveat is that it may be difficult to draw any direct connections between neural function measured in our task-free paradigm with performance in a specific cognitive or emotional domain. Examining the relevance of these neural profiles for specific improvements in cognitive or behavioral domains of risk in this sample is an important next step.
Findings from this study should be interpreted in the context of several limitations. As we describe, the adversity experienced by this sample is complex. In addition to risk for maltreatment, children were reared in impoverished early environments, and faced many additional risk factors that could contribute to neurodevelopmental profiles. We used an observational measure, the HOME, to assess early adversity. While the HOME assessed the domains that are most likely to be compromised in this population (parental responsiveness, acceptance, involvement), it is not a measure of maltreatment. Scores are largely based on naturalistic observations, which may overcome some biases associated with caregiver report(64). However, only one rater provided estimates for this study; future work should include multiple raters to ensure reliability of scores.
A second limitation is that we used child age at the time of intervention onset as our measure of intervention timing. Although this is consistent with prior work in this area(17), age of intervention is conflated with duration of early adverse exposures. In human work, adversity typically starts at birth, making it difficult to disassociate child age, intervention timing, and duration effects. Animal models that manipulate these factors can add to the understanding of how timing, duration, and age explain unique variance in neural outcomes. A third limitation is that there is only one measure of brain activity used in this study. While we theorize that group and intervention based differences in spectral power reflect variability in trajectories of brain development, future work will benefit from the inclusion of longitudinal assessments of neural activity.
Strengths of this study include the prospective design of a maltreatment-risk sample and use of a community comparison group, to assess the effects of early home and family adversity and intervention on children’s later neural function. Results extend findings from prior work involving children exposed to more severe forms of early deprivation and maltreatment risk. Our study results have public health implications in that they suggest that early home and family risk factors can predict later trajectories of brain function and that early intervention may mitigate these effects. Therefore, access to early parenting intervention for maltreating families should be prioritized.
Supplementary Material
ACKNOWLEDGEMENTS:
The project described was supported by funding to Mary Dozier from the National Institute of Mental Health (Grants: R01MH052135, R01MH074374, and R01MH084135) and funding from Edna Bennett Pierce.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Randomized Clinical Trial Name: Intervening Early with Neglected Children
Number: NCT02093052
Financial Disclosures
Johanna Bick, Erin Palmwood, Lindsay Zajac, Robert Simons, and Mary Dozier declare no biomedical financial interests or potential conflicts of interest.
References
- 1.Andersen SL (2003): Trajectories of brain development: point of vulnerability or window of opportunity? Neurosci Biobehav Rev. 27:3–18. [DOI] [PubMed] [Google Scholar]
- 2.McLaughlin KA, Sheridan MA, Winter W, Fox NA, Zeanah CH, Nelson CA (2013): Widespread Reductions in Cortical Thickness Following Severe Early-Life Deprivation: A Neurodevelopmental Pathway to Attention-Deficit/Hyperactivity Disorder. Biol Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chugani HT, Behen ME, Muzik O, Juhasz C, Nagy F, Chugani DC (2001): Local brain functional activity following early deprivation: a study of postinstitutionalized Romanian orphans. Neuroimage. 14:1290–1301. [DOI] [PubMed] [Google Scholar]
- 4.Maheu FS, Dozier M, Guyer AE, Mandell D, Peloso E, Poeth K, et al. (2010): A preliminary study of medial temporal lobe function in youths with a history of caregiver deprivation and emotional neglect. Cogn Affect Behav Neurosci. 10:34–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sheridan MA, Fox NA, Zeanah CH, McLaughlin KA, Nelson CA 3rd (2012): Variation in neural development as a result of exposure to institutionalization early in childhood. Proc Natl Acad Sci U S A. 109:12927–12932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tottenham N, Hare TA, Millner A, Gilhooly T, Zevin JD, Casey BJ (2011): Elevated amygdala response to faces following early deprivation. Dev Science. 14:190–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tottenham N, Hare TA, Quinn BT, McCarry TW, Nurse M, Gilhooly T, et al. (2010): Prolonged institutional rearing is associated with atypically large amygdala volume and difficulties in emotion regulation. Dev Science. 13:46–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gee DG, Gabard-Durnam LJ, Flannery J, Goff B, Humphreys KL, Telzer EH, et al. (2013): Early developmental emergence of human amygdala-prefrontal connectivity after maternal deprivation. Proc Natl Acad Sci U S A. 110:15638–15643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bick J, Zhu T, Stamoulis C, Fox NA, Zeanah C, Nelson CA (2015): Effect of early institutionalization and foster care on long-term white matter development: A randomized clinical trial. JAMA Pediatr. 169:211–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Govindan RM, Behen ME, Helder E, Makki MI, Chugani HT (2010): Altered water diffusivity in cortical association tracts in children with early deprivation identified with Tract-Based Spatial Statistics (TBSS). Cereb Cortex. 20:561–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eluvathingal TJ, Chugani HT, Behen ME, Juhasz C, Muzik O, Maqbool M, et al. (2006): Abnormal brain connectivity in children after early severe socioemotional deprivation: a diffusion tensor imaging study. Pediatrics. 117:2093–2100. [DOI] [PubMed] [Google Scholar]
- 12.Hart H, Rubia K (2012): Neuroimaging of child abuse: a critical review. Front Hum Neurosci. 6:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McCrory E, De Brito SA, Viding E (2011): The impact of childhood maltreatment: a review of neurobiological and genetic factors. Front Psychiatry. 2:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zeanah CH, Nelson CA, Fox NA, Smyke AT, Marshall P, Parker SW, et al. (2003): Designing research to study the effects of institutionalization on brain and behavioral development: The Bucharest Early Intervention Project. Dev Psychopathol. 15:885–907. [DOI] [PubMed] [Google Scholar]
- 15.Nelson CA, Fox NA, Zeanah CH (2014): Romania’s abandoned children: Deprivation, brain development and the struggle for recovery. Cambridge, MA: Harvard University Press. [Google Scholar]
- 16.Vanderwert RE, Zeanah CH, Fox NA, Nelson CA (2016): Normalization of EEG activity among previously institutionalized children placed into foster care: A 12-year follow-up of the Bucharest Early Intervention Project. Developmental Cognitive Neuroscience. 17:68–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vanderwert RE, Marshall PJ, Nelson CA 3rd, Zeanah CH, Fox NA (2010): Timing of intervention affects brain electrical activity in children exposed to severe psychosocial neglect. PLoS One. 5:e11415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stamoulis C, Vanderwert RE, Zeanah CH, Fox NA, Nelson CA (2015): Early Psychosocial Neglect Adversely Impacts Developmental Trajectories of Brain Oscillations and Their Interactions. J Cogn Neurosci. 27:2512–2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bruce J, McDermott JM, Fisher PA, Fox NA (2009): Using behavioral and electrophysiological measures to assess the effects of a preventive intervention: A preliminary study with preschool-aged foster children. Prevention Science. 10:129–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.DHHS (2017): Child Maltreatment, 2015. US Department of Health & Human Services, Administration for Children and Families, Administration on Children, Youth and Families, Children’s Bureau. Washington, DC: US Government Printing Office [Google Scholar]
- 21.CAPTA (2010): CAPTA Reauthorization Act of 2010 (P.L. 111–320), § 5101, Note (§ 3). [Google Scholar]
- 22.Bick J, Dozier M (2013): The effectiveness of an attachment‐based intervention in promoting foster mothers’ sensitivity toward foster infants. Infant Mental Health Journal. 34:95–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bernard K, Lee AH, Dozier M (2017): Effects of the ABC Intervention on Foster Children’s Receptive Vocabulary: Follow-Up Results From a Randomized Clinical Trial. Child Maltreatment. 22:174–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bernard K, Dozier M, Bick J, Lewis-Morrarty E, Lindhiem O, Carlson E (2012): Enhancing Attachment Organization Among Maltreated Children: Results of a Randomized Clinical Trial. Child Dev. 83:623–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bernard K, Dozier M, Bick J, Gordon MK (2015): Intervening to enhance cortisol regulation among children at risk for neglect: Results of a randomized clinical trial. Dev Psychopathol. 27:829–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nunez PL (2000): Toward a quantitative description of large-scale neocortical dynamic function and EEG. Behav Brain Sci. 23:371–398. [DOI] [PubMed] [Google Scholar]
- 27.Somsen RJ, van’t Klooster BJ, van der Molen MW, van Leeuwen HM, Licht R (1997): Growth spurts in brain maturation during middle childhood as indexed by EEG power spectra. Biol Psychol. 44:187–209. [DOI] [PubMed] [Google Scholar]
- 28.Marshall PJ, Bar-Haim Y, Fox NA (2002): Development of the EEG from 5 months to 4 years of age. Clin Neurophysiol. 113:1199–1208. [DOI] [PubMed] [Google Scholar]
- 29.Bell MA, Wolfe CD (2007): Changes in brain functioning from infancy to early childhood: Evidence from EEG power and coherence during working memory tasks. Developmental neuropsychology. 31:21–38. [DOI] [PubMed] [Google Scholar]
- 30.Gasser T, Verleger R, Bächer P, Sroka L (1988): Development of the EEG of school-age children and adolescents. I. Analysis of band power. Electroencephalogr Clin Neurophysiol. 69:91–99. [DOI] [PubMed] [Google Scholar]
- 31.Benninger C, Matthis P, Scheffner D (1984): EEG development of healthy boys and girls. Results of a longitudinal study. Clin Neurophysiol. 57:1–12. [DOI] [PubMed] [Google Scholar]
- 32.Britton JW, Frey LC, J H (2016): The Developmental EEG: Premature, Neonatal, Infant, and Children In: St. Louis E, Frey LC (Eds.). editor. Electroencephalography (EEG): An introductory text and atlas of normal and abnormal findings in adults, children and infants. Chicago, IL: American Epilepsy Society. [PubMed] [Google Scholar]
- 33.Blume W (1982): Atlas of Pediatric Encephalography. New York: Raven Press. [Google Scholar]
- 34.Petersen I, Olofsson O (1971): The development of the electroencephalogram in normal children from the age of 1 through 15 years. Neuropadiatrie. 2:247–304. [DOI] [PubMed] [Google Scholar]
- 35.McLaughlin KA, Fox NA, Zeanah CH, Sheridan MA, Marshall P, Nelson CA (2010): Delayed maturation in brain electrical activity partially explains the association between early environmental deprivation and symptoms of attention-deficit/hyperactivity disorder. Biol Psychiatry. 68:329–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Doppelmayr M, Klimesch W, Stadler W, Pöllhuber D, Heine C (2002): EEG alpha power and intelligence. Intelligence. 30:289–302. [Google Scholar]
- 37.Clarke AR, Barry RJ, McCarthy R, Selikowitz M (1998): EEG analysis in attention-deficit/hyperactivity disorder: a comparative study of two subtypes. Psychiatry Res. 81:19–29. [DOI] [PubMed] [Google Scholar]
- 38.Chabot RJ, di Michele F, Prichep L (2005): The role of quantitative electroencephalography in child and adolescent psychiatric disorders. Child Adolesc Psychiatr Clin N Am. 14:21–53, v–vi. [DOI] [PubMed] [Google Scholar]
- 39.Barry RJ, Clarke AR, Johnstone SJ (2003): A review of electrophysiology in attention-deficit/hyperactivity disorder: I. Qualitative and quantitative electroencephalography. Clin Neurophysiol. 114:171–183. [DOI] [PubMed] [Google Scholar]
- 40.Barry RJ, Clarke AR, Hajos M, Dupuy FE, McCarthy R, Selikowitz M (2011): EEG coherence and symptom profiles of children with Attention-Deficit/Hyperactivity Disorder. Clin Neurophysiol. 122:1327–1332. [DOI] [PubMed] [Google Scholar]
- 41.John ER, Ahn H, Prichep L, Trepetin M, Brown D, Kaye H (1980): Developmental equations for the electroencephalogram. Science. 210:1255. [DOI] [PubMed] [Google Scholar]
- 42.Lind T, Bernard K, Ross E, Dozier M (2014): Intervention effects on negative affect of CPS-referred children: Results of a randomized clinical trial. Child Abuse Negl. 38:1459–1467 %@ 0145–2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bradley RH, Caldwell BM, Rock SL, Ramey CT, Barnard KE, Gray C, et al. (1989): Home environment and cognitive development in the first 3 years of life: A collaborative study involving six sites and three ethnic groups in North America. Dev Psychol. 25:217. [Google Scholar]
- 44.Caldwell BM, Bradley RH (1984): Home observation for measurement of the environment. University of Arkansas at Little Rock Little Rock. [Google Scholar]
- 45.Ramey CT, McGinness G, Cross L, Collier A, Barrieblackley S, Borman K (1982): The social life of children in a changing society.
- 46.Ramey CT, Yeates KO, Short EJ (1984): The plasticity of intellectual development: Insights from preventive intervention. Child Dev.1913–1925. [PubMed] [Google Scholar]
- 47.Cooper R, Osselton J, Shaw J (1969): EEG technology. London: Butterworths. [Google Scholar]
- 48.Pivik RT, Broughton RJ, Coppola R, Davidson RJ, Fox N, Nuwer MR (1993): Guidelines for the recording and quantitative analysis of electroencephalographic activity in research contexts. Psychophysiology. 30:547–558. [DOI] [PubMed] [Google Scholar]
- 49.Levin A, Méndez Leal A, Gabard-Durnam L, O’Leary H (under review): BEAPP: The Batch Electroencephalography Automated Processing Platform. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gabard-Durnam LJ, Mendez Leal AS, Wilkinson CL, Levin AR (2018): The Harvard Automated Processing Pipeline for Electroencephalography (HAPPE): Standardized Processing Software for Developmental and High-Artifact Data. Frontiers in Neuroscience. 12:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hostinar CE, Davidson RJ, Graham EK, Mroczek DK, Lachman ME, Seeman TE, et al. (2017): Frontal brain asymmetry, childhood maltreatment, and low-grade inflammation at midlife. Psychoneuroendocrinology. 75:152–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Clarke AR, Barry RJ, McCarthy R, Selikowitz M (2001): Age and sex effects in the EEG: development of the normal child. Clin Neurophysiol. 112:806–814. [DOI] [PubMed] [Google Scholar]
- 53.Otero GA (1997): Poverty, cultural disadvantage and brain development: a study of pre-school children in Mexico. Clin Neurophysiol. 102:512–516. [DOI] [PubMed] [Google Scholar]
- 54.Otero GA, Pliego-Rivero FB, Fernandez T, Ricardo J (2003): EEG development in children with sociocultural disadvantages: a follow-up study. Clin Neurophysiol. 114:1918–1925. [DOI] [PubMed] [Google Scholar]
- 55.Kelly PA, Viding E, Wallace GL, Schaer M, De Brito SA, Robustelli B, et al. (2013): Cortical thickness, surface area, and gyrification abnormalities in children exposed to maltreatment: neural markers of vulnerability? Biol Psychiatry. 74:845–852. [DOI] [PubMed] [Google Scholar]
- 56.Tendolkar I, Mårtensson J, Kühn S, Klumpers F, Fernández G (2018): Physical neglect during childhood alters white matter connectivity in healthy young males. Hum Brain Mapp. 39:1283–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Greenough WT, Black JE, Wallace CS (1987): Experience and brain development. Child Dev. 58:539–559. [PubMed] [Google Scholar]
- 58.Engel AK, Fries P (2010): Beta-band oscillations—signalling the status quo? Curr Opin Neurobiol. 20:156–165. [DOI] [PubMed] [Google Scholar]
- 59.Fernandez T, Harmony T, Silva-Pereyra J, Fernandez-Bouzas A, Gersenowies J, Galan L, et al. (2000): Specific EEG frequencies at specific brain areas and performance. Neuroreport. 11:2663–2668. [DOI] [PubMed] [Google Scholar]
- 60.Papanicolaou AC, Loring DW, Deutsch G, Eisenberg HM (1986): Task-related EEG asymmetries: a comparison of alpha blocking and beta enhancement. Int J Neurosci. 30:81–85. [DOI] [PubMed] [Google Scholar]
- 61.Güntekin B, Başar E (2014): A review of brain oscillations in perception of faces and emotional pictures. Neuropsychologia. 58:33–51. [DOI] [PubMed] [Google Scholar]
- 62.Ray WJ, Cole HW (1985): EEG alpha activity reflects attentional demands, and beta activity reflects emotional and cognitive processes. Science. 228:750–752. [DOI] [PubMed] [Google Scholar]
- 63.Pfurtscheller G, Stancák A, Neuper C (1996): Post-movement beta synchronization. A correlate of an idling motor area? Electroencephalogr Clin Neurophysiol. 98:281–293. [DOI] [PubMed] [Google Scholar]
- 64.Jones PC, Pendergast LL, Schaefer BA, Rasheed M, Svensen E, Scharf R, et al. (2017): Measuring home environments across cultures: Invariance of the HOME scale across eight international sites from the MAL-ED study. Journal of School Psychology. 64:109–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.