Abstract
Background:
Metal exposure alters neurodevelopmental outcomes; little is known about critical windows of susceptibility when exposure exerts the strongest effect.
Objective:
To examine associations between dentine biomarkers of manganese (Mn), zinc (Zn) and lead (Pb) and later childhood behaviors.
Methods:
Subjects enrolled in a longitudinal birth cohort study in Mexico City provided naturally shed deciduous teeth. We estimated weekly prenatal and postnatal dentine Mn, Zn and Pb concentrations in teeth using laser ablation-inductively coupled plasma-mass spectrometry (LA-ICP-MS) and measured behavior at ages 8–11 years of age using the Behavior Assessment System for Children, 2nd edition (BASC-2). We used distributed lag models and lagged weighted quantile sum regression to identify the role of individual and combined dentine biomarkers of Mn, Zn and Pb on behavioral outcomes controlling for maternal education and gestational age.
Results:
Among the 133 subjects included in this study, prenatal and early postnatal dentine Mn appeared protective against childhood behavioral problems, specifically hyperactivity and attention. Postnatal dentine Mn was associated with increased reporting of internalizing problems, specifically anxiety. At 6 months, a 1-unit increase (unit = 1 SD of log concentration) in Mn was associated with a 0.18-unit (unit =1 SD of BASC-2 score) increase in internalizing symptoms score and a 0.25-unit increase in anxiety. Postnatal Pb was associated with increasing anxiety symptoms; at 12 months, a 1-unit increase in Pb was associated with a 0.4 unit increase in anxiety symptoms. When examined as a metal mixture, we observed two potential windows of susceptibility to increased anxiety symptoms: the first window (0–8 months) appeared driven by Mn, the second window (8–12 months) was driven by the metal mixture and dominated by Pb. A 1-unit increase in the mixture index was associated with a 0.7-unit increase in SD of anxiety symtoms.
Conclusions:
Childhood behaviors may demonstrate postnatal windows of susceptibility to individual and mixed metal concentrations measured in deciduous teeth. Prenatal dentine Mn may be protective, while excessive early postnatal Mn may increase risk for adverse behaviors. In combination, higher concentrations of Mn, Zn and Pb may have an adverse impact on behavior.
Keywords: Dentine biomarkers, child behavior, metal mixtures, anxiety, manganese
Introduction
Up to one in five children worldwide experience mental health problems (Kieling et al. 2011), and the burden of childhood mental health problems may be increasing. Psychosocial problems identified by primary care practitioners in the Unites States increased from 7% in 1979 to 19% in 1996, with increases in both internalizing and externalizing problems (Kelleher et al. 2000). Internalizing disorders (i.e., negative behaviors that are focused inward, such as anxiety and depression), are now the most common psychiatric conditions afflicting children and adolescents (Anderson et al. 1987; Cohen et al. 1993a; Pine et al. 1998) representing the second and fifth, respectively, leading causes of disability in the United States (Murray et al. 2013). In Mexico, an estimated 40% of adolescents ages 12 to 17 have a mental health disorder, most commonly anxiety and depression (Benjet et al. 2009; Gallegos et al. 2012). Externalizing problems (i.e., negative behaviors directed outwards, such as hyperactivity and inattention) including attention-deficit/hyperactivity disorder (ADHD) and conduct disorders are also increasing (Cohen et al. 1993a; Loeber et al. 2000; Merikangas et al. 2009). Demonstration of internalizing and/or externalizing behaviors in childhood increases the risk for later-life mental health disorders (Campbell 1995), substance abuse, and suicidal behaviors (Beesdo et al. 2009; Clark et al. 2007). While behavioral disorders can present as early as 4 to 5 years of age, a growing body of literature indicates that the susceptibility to develop mood disorders may be programmed by events occurring during fetal and early postnatal life (Angold et al. 1998; Beesdo et al. 2009; Kim et al. 2015; Pine et al. 1998). The root causes of the increasing prevalence of neurodevelopmental disorders, including internalizing problems, are only partly understood. Although genetic factors play a role, strong evidence exists that early life exposure to environmental chemicals is an important contributor to this increasing prevalence of neurodevelopmental disorders (Grandjean and Landrigan 2006, 2014). While longstanding evidence suggests early-life metal exposures negatively impact cognitive outcomes such as IQ, more recent epidemiologic studies demonstrate associations between early life exposure to essential and nonessential metals and adverse behavioral outcomes such as internalizing and externalizing behaviors (Banks et al. 1997; Ericson et al. 2007; Gunier et al. 2015; Khan et al. 2012; Meyer-Baron et al. 2013; Mora et al. 2015; Needleman et al. 1990; Sanders et al. 2015; Yousef et al. 2011).
In this study, we focus on early-life exposure to three neuroactive metals; manganese (Mn), zinc (Zn) and lead (Pb), and their independent and combined associations with childhood internalizing and externalizing symptoms. Mn and Zn are both essential metals required by the body at low doses and neurotoxic at excessively high and/or low levels. Pb is a non-essential heavy metal, well-recognized as a neurotoxicant. We select these metals as 1) each is common in the environment due to anthropogenic activities such as mining, and smelting operations and industrial uses and they are often found together as a correlated mixture (Mehra and Thakur 2016), 2) experimental and epidemiological evidence links early life exposure to each metal individually with adverse neurobehavioral outcomes, specifically with internalizing symptoms including anxiety and depression. Much of the experimental and epidemiologic studies demonstrating associations between occupational exposure to these metals and higher rates of anxiety and depression focus on adults (Betharia and Maher 2012; Bouchard et al. 2009; Islam et al. 2013; Jung et al. 2016; Kim et al. 2015; Mlyniec et al. 2017; Shiue 2015). Less is understood of the role of early life metal uptake in the development of childhood anxiety and depression. Affective behaviors are regulated in the brain within the prefrontal (e.g., medial prefrontal cortex; mPFC) and limbic (e.g., amygdala) regions (Duval et al. 2015; Kim et al. 2011). These brain regions develop throughout early gestation and childhood, making them vulnerable to early-life neurotoxic insults, which can override a normal growth trajectory toward a maladaptive phenotype (i.e., internalizing or externalizing disorder). Indeed, emerging studies link early-life environmental insults with the pathophysiology of abnormal neuronal circuitry in neuropsychiatric disorders (Money and Stanwood 2013; Schlotz and Phillips 2009). However, the critical time windows of developmental susceptibility to neurotoxic exposures are illdefined (Adams et al. 2000; Bellinger 2013; Selevan et al. 2000). While we know many metals, including Mn, Zn and Pb, readily cross the placental barrier, increasing exposure during early-life windows of developmental susceptibility (Chen et al. 2014; Goyer 1990; Needham et al. 2011; Rudge et al. 2009; Yoon et al. 2009), limited data exist on the cumulative and life stage-specific effects of exposure on behavioral outcomes in childhood. Further, limited epidemiologic literature examine associations with co-exposure to correlated neurotoxicant metals. In one recent study examining metals in placenta, co-exposure to more than one toxicant metal increased the risk for adverse neurodevelopmental outcomes (Freire et al.).
In this paper, we use our dentine biomarker to evaluate associations between early-life metal uptake of individual and mixed metals and internalizing and externalizing behaviors in children, identifying potential critical windows of developmental susceptibility to adverse outcomes. We hypothesized that variable early life biomarkers of Mn, Zn and Pb during critical developmental windows may be associated with higher reporting of internalizing and externalizing behaviors (i.e., anxiety and depression, hyperactivity and attention problems). This study takes place among subjects enrolled in an ongoing longitudinal birth cohort study, Early Life Exposure in Mexico to Environmental Toxicants (ELEMENT). As recently summarized by Claus Henn et al (2018), this cohort is uniquely suited to a study of the effects of metal exposure because (1) air pollution, of which Mn and other metals are key components, is severe in Mexico City (Calderon-Garciduenas et al. 2013); (2) rich sources of Mn in the diet, such as beans, are commonly consumed in Mexican diets; (3) higher Pb and Mn exposure, measured in biological and environmental samples, has been reported in Mexico than in the U.S. and Canada (Claus Henn et al. 2018; Santos-Burgoa et al. 2001). We use deciduous “baby” teeth to reconstruct fine scale (1–2 weeks) exposure histories to Mn, Zn and Pb and identify the discrete developmental period(s) for each metal most associated with changes in children’s behavior. We then use a novel statistical method, lagged weighted quantile sum (WQS) regression, to capture the time-varying association between the 3-metal mixture and children’s behavioral outcomes.
Methods
Study population
Mother-child pairs in this study were drawn from four successively-enrolled longitudinal birth cohort studies in Mexico City that comprise the ELEMENT project. Detailed information on the study design and data collection procedures has been published previously detail elsewhere (Braun et al. 2012; Claus Henn et al. 2018; Ettinger et al. 2009; Hernandez-Avila et al. 2002). Between 1994 and 2001, ELEMENT enrolled healthy, low to moderate income mothers, between ages 18–39 from the National Institute of Perinatology (Instituto Nacional de Parinatologia) to investigate the long-term consequences of prenatal environmental factors on child development (Ettinger et al. 2009; Gonzalez-Cossio et al. 1997; Tellez-Rojo et al. 2004). Biomarkers, neurodevelopmental outcomes outcome, and covariate data from all cohorts were collected following standardized protocols by the same study staff, allowing us to pool data across cohorts. Exclusion criteria included an Apgar score of < or = 6 at 5 min, a condition requiring treatment in neonatal intensive care unit.
Of the 1,079 children born into ELEMENT and followed until 5 years of age, 826 (77%) participated in an additional follow-up study at 6–16 years of age. At this follow-up, participants were asked to bring or mail in deciduous teeth as they were naturally shed for analysis of dentine metals. At the time of analysis, we collected teeth from 141 participants, 3 of these teeth were excluded due to excessive decay or wear. The current study includes 133 subjects with complete data on dentine metals, BASC-2 behavioral outcomes from children between ages 8–11 years, and selected covariates.
All participating mothers signed a written consent form and received a detailed explanation of the study intent and research procedures. In addition, subjects older than 6 years of age signed a written consent of minor form and received a detailed explanation of the study. All participants were encouraged to ask questions about the study in order to ensure their understanding. The institutional review boards of the National Institute of Public Health of Mexico and all of the participating hospitals and institutions of the co-authors approved this research. Written informed consent and/or assent were obtained from all participants.
Exposure assessment: Dentine biomarkers of Mn, Zn and Pb
We analyzed deciduous teeth that were free of obvious defects such as caries and extensive tooth wear and measured dentine metals using laser ablation-inductively coupled plasma-mass spectrometry (LA-ICPMS). Analysis methods and validation procedures have been described in detail elsewhere (Andra et al. 2016; Arora et al. 2005; Arora et al. 2011; Arora et al. 2012; Arora and Austin 2013; Arora et al. 2014; Austin et al. 2013; Austin et al. 2017; Claus Henn et al. 2018; Hare et al. 2011). For validation of Mn, there was a significant positive association between dentine Mn formed in the second trimester with Mn loading in floor dust sampled during the second trimester of pregnancy (Arora et al. 2012). Tooth Pb measurements were validated using maternal pregnancy blood Pb levels and postpartum maternal bone Pb levels (Arora et al. 2014). Validation of additional metals (i.e. zinc) is discussed in Andra (2016).
Deciduous teeth accumulate metals and their mineralization proceeds in an incremental pattern (akin to growth rings) spanning the prenatal and early postnatal periods (commencing gestational week 13–16 for incisors and concluding postnatal age 10–11 months for molars) (Ash and Neslon 2002; Hillson 1996). Using the neonatal line (a histological feature formed in enamel and dentine at birth) and daily growth incremental markings to assign temporal information to sampling points, each tooth was sampled at approximately 50 timepoints between 3 months gestation through one year postnatal age, at a sampling frequency of approximately every 7–10 days. This reflects metal uptake at roughly weekly resolution. The detection limit was 0.05 μg/g for each metal. Because mineral density varies within teeth and between tooth type and individuals, metal intensities at each sampling point were normalized to calcium (Ca) by dividing the analyte concentration by Ca and expressing as a ratio (e.g., as 55Mn:43Ca, 66Zn:43Ca, or 208Pb:43Ca).
Behavioral assessments
At the additional follow-up visit when children were between 6–16 years of age, the Behavior Assessment System for Children, 2nd edition (BASC-2) (Reynolds and Kamphaus 1992) was administered to parents of subjects to assess their child’s behavior. The BASC-2 is a valid, reliable, 134-item parent-report assessment of 2–18 year old children’s behavior and self-perceptions. All behavioral assessments were administered in Spanish using the translated text provided by the publisher (Pearson Clinical). Spanish-speaking parents and children were included in the standardization of the assessment, however separate Spanish norms are not available. We analyzed the overall behavioral skills index (BSI) composite score, internalizing and externalizing composite scores and individual subscales including anxiety, depression, attention, and hyperactivity. Scores were analyzed as sex- and age-specific percentiles. Higher scores indicate an increase in reported behavioral problems.
Covariates and potential confounders
ELEMENT has detailed information on sociodemographic and other characteristics that may confound the relationship between metal concentrations and child neurodevelopment. Dating back through pregnancy, questionnaire data included maternal age at delivery, maternal education level, maternal smoking during pregnancy, and socioeconomic status (SES). An SES index was calculated based on the algorithm AMAI rule 13 × 6 from 1994 which determines six levels of SES based on questions about the characteristics of the household (i.e., type of floor, ownership of car, number of rooms) (Carrasco 2002). The six levels were then collapsed into low, medium and higher SES. Maternal IQ was estimated using selected subtests of the Wechsler Adult Intelligence Scale-Spanish (Information, Comprehension, Similarities, and Block Design) (Kaufman and Lichtenberger 2006). Characteristics of the birth and newborn period including gestational age, birth weight and birth length were extracted from the medical records.
Statistical analyses
We conducted standard univariate and bivariate explorations of the data and examined the distributional plots for all variables. Distributions of metal concentrations measured between 4-month gestational age (−4 mo.) to 12 months postnatally (12 mo.) and BASC-2 scores assessed at 8–11 years of age were examined, and appropriate transformations (e.g., natural log) performed, as necessary, to satisfy model assumptions.
We used a variation of a distributed lag model (DLM) (Coull BA 2015; Gasparrini et al. 2010) as our primary statistical approach to identify sensitive windows for neurodevelopmental outcomes associated with our biomarkers of early life metal exposure to individual metals. DLMs are regression models for time series data that yield an estimate of the effect of exposure incurred at specific time windows while adjusting for exposures at other times and for covariates, under the assumption that the effect of exposure varies smoothly over time. DLMs have been applied extensively to environmental health research (Baek et al. 2016a; Baek et al. 2016b; Gasparrini and Armstrong 2010; Heaton and Peng 2012; Lall et al. 2011; Wyzga 1978; Zhao et al. 2014). The typical form of the DLM requires that exposures are measured at identical time points so that a smoothed function of the lagged variables can be fit. Because of the natural curvature of teeth, the intervals between sampling times may vary within and across teeth. Following methods developed by Coull and colleagues, we interchange the role of the outcome and exposure and use a functional spline model with timevarying coefficients in single chemical analyses, i.e., a reversed DLM (rDLM) (Chen et al. 2015; Coull 2014). The time-varying association parameters are plotted with Bonferroni-adjusted confidence intervals overlaid with Holm-Bonferroni-adjusted confidence intervals at regular intervals. A sensitive window is identified when the estimated point wise 95% confidence intervals on the association parameter between the exposure and the outcome variable do not include zero (Chiu et al. 2016). Results are presented as the unit change in outcome (1 unit = 1 standard deviation (SD) of BASC-2 score) per unit change in exposure (1 unit = 1 SD of log-transformed concentration dentine metal).
We extend the rDLM to model time-dependent effects of co-exposure to the three metals as a mixture using lagged weighted quantile sum (WQS) regression (Bello et al. 2017). WQS regression utilizes a nonnegative, unit sum constraint that groups correlated variables (i.e., chemicals) into a uni-dimensional index. The index is regressed against the outcome variable to determine the association between the chemical mixture and the outcome and to make generalized inference concerning the relative or importance of each chemical, i.e., the proportional weight (Carrico et al. 2014; Czarnota et al. 2015). The lagged WQS is a penalized regression extension of the WQS to estimate mixture effects using a weighted index that allows us to identify time periods with the largest and most potentially biologically relevant mixture effects.
A priori, we tested for potential confounders that are known or suspected predictors of neurodevelopment or exposure including maternal education, maternal IQ, SES, birth weight, and gestational age (Claus Henn et al. 2010). Some participants were missing data on key potential confounders (e.g., maternal IQ, maternal education). In sensitivity analyses, we multiply imputed missing values using chained equations with the MRI procedure in SAS (SAS Institute, Inc., Cary, NC, USA) (van Buuren 2007; White et al. 2011). We assumed data were missing at random and generated 20 imputed datasets. We included all exposure outcome, and potential confounder data thought to be related to the process causing missing data. We combined results from models fit the multiply imputed datasets averaging the results for the 20 imputations to give the final effect estimates for sensitivity analyses. We calculated standard errors using methods that combine the within-and between imputation uncertainty (Rubin 2004). We also checked for confounding by study cohort. In the final models, we included variables the were associated with either the exposure or the outcome (e.g., maternal years of education and gestational age) and improved the model fit. Among our cohort, maternal education was significantly associated with SES (Spearman’s p < 0.05) and improved model fit over SES. To avoid collinearity, maternal education was included in the model rather than SES.
In sensitivity analyses to examine associations between cumulative dentine metal concentrations and BASC-2, we computed aggregated dentine metal concentrations by summing across all time 50 points for each individual metal and used linear regression model to examine associations between each single aggregated exposure measure and BASC-2 outcomes, adjusting for covariates. In addition, we summed across specific windows (birth ± 2 weeks, 3–5 months postnatal) to examine the associations between aggregated metal concentrations in these windows and BASC-2 outcomes.
Exposure outliers were identified using the generalized extreme studentized deviation procedure (Rosner 1983) and extreme observations were checked against original data. We identified one subject with consistently higher dentine Pb and Zn. Results with and without this extreme value were compared in sensitivity analyses and did not differ. Final models are presented with all 133 subjects. We conducted statistical analysis using SAS (version 9.4; SAS Institute, Inc., Cary, NC, USA), with alpha set at p < 0.05.
Results
Sociodemographic characteristics, neurodevelopmental scores, and dentine metal concentrations
Table 1 presents the sociodemographic characteristics for the ELEMENT subjects selected into this study based on availability of exposure, outcome and covariate data (n = 133). Subjects enrolled into ELEMENT were born between 1995–2004. There was a nearly equal distribution of females and males in the cohort (51.9% female). Very few of the subjects were born preterm (less than 37 weeks gestation, 5.3%) or had low birth weight (less than 2500 g, 3.0%). Subjects selected into this study differed from the parent ELEMENT cohort in birth weight (3.2 kg vs. 3.1 kg, p < 0.05) and maternal IQ (93.5 vs. 88.8, p < 0.05). The mean age at BASC-2 administration was 7.9 ± 1.3 years. The mean ± SD BASC-2 BSI Composite percentile score was 50.05 ± 0.09; internalizing problems, 52.23 ± 0.09; externalizing problems, 48.08 ± 0.85. Individual symptom scores were significantly and positively correlated with overall BASC-2 Behavioral Composite scores (r = 0.40.8, p < 0.001). Internalizing problems were significantly but less highly correlated with externalizing problems (r = 0.4, p < 0.001) (Table 1). BASC-2 outcomes were similar between males and females, only hyperactivity differed by sex. Mean percentile scores for hyperactivity were higher for females than males (mean ± SD = 57.7 ± 1.48 and 47.61 ± 1.01, respectively; p < 0.05
Table 1.
Sociodemographic characteristics of study participants included in the parent cohort ELEMENTa and those included in the current analysesb.
| Excluded from analysis (n = 693) Mean ± SD or % | Included in analysis (n = 133) Mean ± SD or % | |
|---|---|---|
| Child Sex (% female) | 48.8% | 51.9% |
| Estimated gestational age at birth (weeks) | 38.9 ± 1.5 | 38.9 ± 1.5 |
| Preterm birth (< 37 week gestation) | 6.2% | 5.3% |
| Birth weight (kg) | 3.10 ± 0.45 | 3.24 ± 0.44* |
| Low Birth weight (<2500 g) | 6.5% | 3.0% |
| Maternal age at delivery (years) | 28.9 ± 5.9 | 28.8 ± 5.6 |
| Maternal years of education at delivery | 9.0 ± 3.9 | 6.3 ± 4.6* |
| Family SES | ||
| Low | 54.2% | 47.4% |
| Medium | 43.2% | 36.9% |
| Higher | 2.6% | 3.4% |
| Maternal IQ (WAIS) | 88.6 ± 22.2 | 94.7 ± 20.1* |
| Maternal smoking during pregnancy (% yes) | 4.4% | 1.7% |
| Study cohort (years of subject birth) | ||
| 1 (1994–1995) | 29.1% | 1.5%* |
| 2 (1997–2002) | 35.3% | 13.5% |
| 3 (1998–2001) | 16.5% | 22.6% |
| 4 (2001–2006) | 19.2% | 62.4% |
| Age at BASC-2 administration (years) | 10.2 ± 2.7 | 8.4 ± 1.4* |
| BASC-2 percentiles | ||
| Behavioral Symptoms Index | 50.5 ± 9.5 | 50.1 ± 10.2 |
| Internalizing Problems | 52.1 ± 10.4 | 52.2 ± 10.0 |
| Anxiety | 53.3 ± 10.0 | 55.0 ± 10.2 |
| Depression | 51.7 ± 10.7 | 51.3 ± 11.2 |
| Externalizing Problems | 49.3 ± 9.5 | 48.1 ± 9.8 |
| Hyperactivity | 49.3 ± 9.8 | 49.6 ± 10.3 |
| Attention | 52.4 ± 10.8 | 52.6 ± 11.2 |
Indicates significant difference between groups, p < 0.05;
Not including those subjects included in current analyses.
Missing data for some covariates among subjects in current analyses; gestational age (n = 1), birth weight (n = 1), maternal age (n = 1), Family SES (n = 17), Maternal IQ (n = 19), maternal smoking (n = 18),
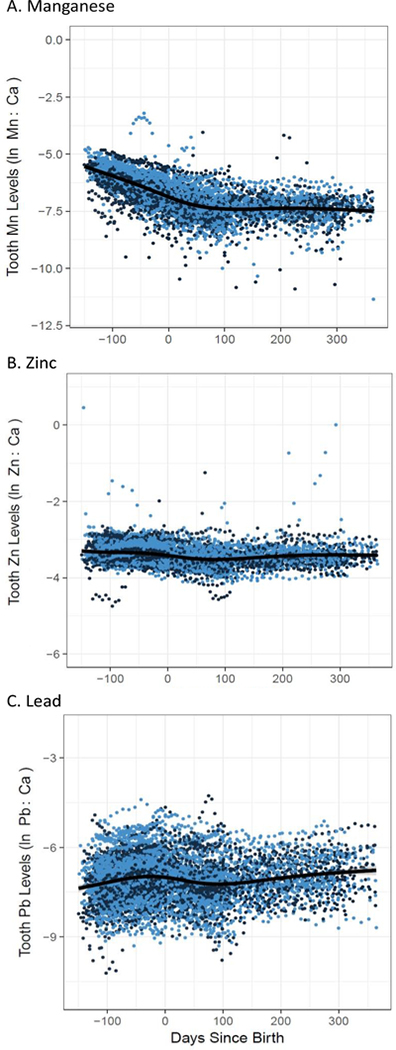
Figure 1 (A – C) depicts log concentrations of dentine Mn, Zn and Pb at approximately 50 timepoints between the second trimester of pregnancy (4 months gestation) and 12 months of age. Consistent with Claus Henn et al (2018), dentine Mn levels were highest in the second trimester and declined steeply over the prenatal period. Levels continued to decline after birth but at a slower rate (Figure 1A). Dentine Zn levels were mostly stable over the 16-month sampling period, declining slightly in the postpartum period (Figure 1B). Dentine Pb levels demonstrated a slight increase in the 3rd trimester of pregnancy, dipped slightly in the first several months after birth (average 0–100 days) and began increasing after 4 months. Dentine concentrations of all Mn, Zn and Pb were not associated with child sex, gestational age, birth weight and maternal anemia status. In sensitivity analyses, we used year of birth as a surrogate variable to determine differences in metal exposures across cohorts and did not observe differences in the associations between exposure and outcome.
Figure 1.
Tooth metal levels from early second trimester (−5 months before birth) to one year of age (12 months) for (A) Mn as ln 55Mn:42Ca ratio, (B) Zn as 66Zn:42Ca ratio and (C) Pb as 208Pb:42Ca ration. Dots (dark blue = male, light blue = female) represent individual tooth measurements for 133 participants with approximately 50 measurements per participant. Line represents Loess smoother.
Windows of neurodevelopmental susceptibility to individual metals
Manganese.
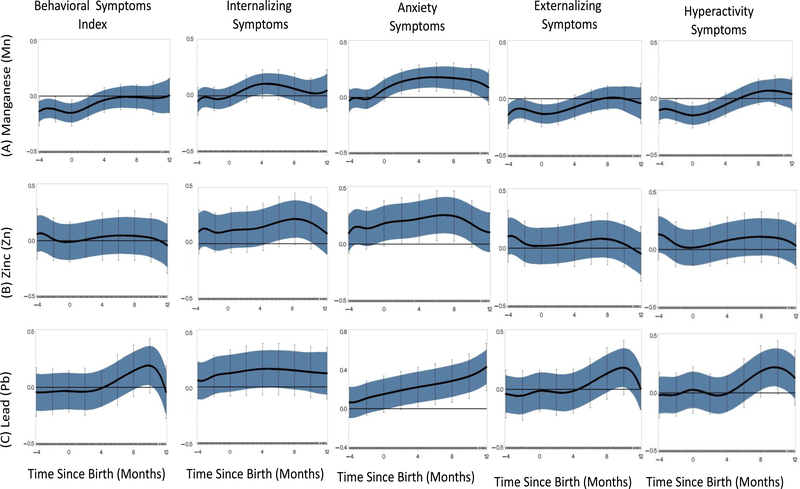
Using reverse distributed lag models (rDLMs), we examined associations between individual dentine metals with behavioral outcomes from the BASC2. Plots of the rDLMs showing the correlations between dentine Mn with the BSI suggest that dentine Mn uptake spanning the prenatal period through approximately 23 months postnatal age may be associated with reduced risk of overall behavioral problems (Figure 2). When internalizing and externalizing symptoms were examined separately, the association between dentine Mn and externalizing symptoms is similar to that demonstrated for overall BSI; dentine Mn spanning the prenatal period through approximately 4 months was associated with reduced externalizing symptoms. This association was significant (p < 0.05) for the window between 3 and 5 postnatal months. At birth, a one-unit increase in dentine Mn was associated with a −0.15 -unit (95% CI = −0.24, −0.07) reduction in BASC-2 BSI score, equivalent to approximately a 1.5 point decrease in BSI. This trend was consistent for associations between dentine Mn with hyperactivity and aggression, the two main components of the externalizing symptoms score. Conversely, early life dentine Mn, around 4 months, was associated with increased reporting of internalizing problems. The shape of the relationship with dentine Mn differed between two sub-scales of internalizing symptoms; anxiety and depression (anxiety association shown in Figure 2, depression not shown). For anxiety, a sensitive window appears shortly after birth and spans through postnatal 11 months. At approximately 6 months postnatal (the timing where we observe the strongest correlation between Mn and anxiety), a one-unit increase in dentine Mn was associated with a 0.18-unit (95% CI = 0.09,0.27) increase BASC-2 scores for anxiety symptoms. The rDLM plot of the association between dentine Mn and depression symptoms was largely nonsignificant, but similar to the shape of the relationship for the internalizing symptom score.
Figure 2.
Individual Reversed DLM plots demonstrating association between fine scale resolution dentine metal levels and BASC-2 Behavioral Symptoms; [(A) manganese (top), (B) zinc(middle), (C)lead (bottom)] adjusted for maternal education and gestational age (n = 133); Y axis = time varying correlation between exposure and outcome; X axis = time since birth representing timing of tooth sampling; central black line indicates = correlation; blue band represents the 95% confidence intervals and the vertical bars represent the 95% Holms-Bonferroni family-wise confidence intervals. A significant association indicating a critical window is defined as an area of the rDLM that does not include zero.
Zinc.
We observed an association between dentine Zn and anxiety symptoms similar to that observed for Mn; dentine Zn spanning from birth through approximately 10–11 months of age was associated with increased reporting of anxiety. At 6 months of age, a one-unit increase in dentine Zn was associated with a 0.25-unit increase in anxiety scores. We did not observe significant associations for dentine Zn and depression, dentine Zn and externalizing symptoms (including hyperactivity and aggression) or for dentine Zn and overall BSI.
Lead.
The rDLM examining correlations between dentine Pb with the BSI suggested dentine Pb between 9–10 months postnatal was significantly associated with increased reporting of overall behavioral problems. This relationship was consistent for that observed between dentine Pb and externalizing problems; dentine Pb during the window from 8–11 months postnatally was strongly associated with reported hyperactivity. At 10 months, a one-unit increase in dentine Pb is associated with a 0.22-unit (95% CI = 0.06, 0.38) and 0.19-unit (95% CI = 0.02, 0.37) increase in BASC-2 BSI and hyperactivity scores, respectively. No significant associations were found between dentine Pb uptake and overall internalizing symptoms. We observed a linear trend over time suggesting postnatal Pb uptake in teeth was associated with increased anxiety symptoms (Figure 2). At 12 months, a one-unit increase in dentine Pb was associated with a 0.4-unit increase in anxiety scores. The rDLM for dentine Pb and depression suggests postnatal dentine Pb may be associated with increased reports of depressive symptoms reported on the BASC-2 (data not shown).
Identification of time-varying windows of vulnerability to dentine metal mixtures
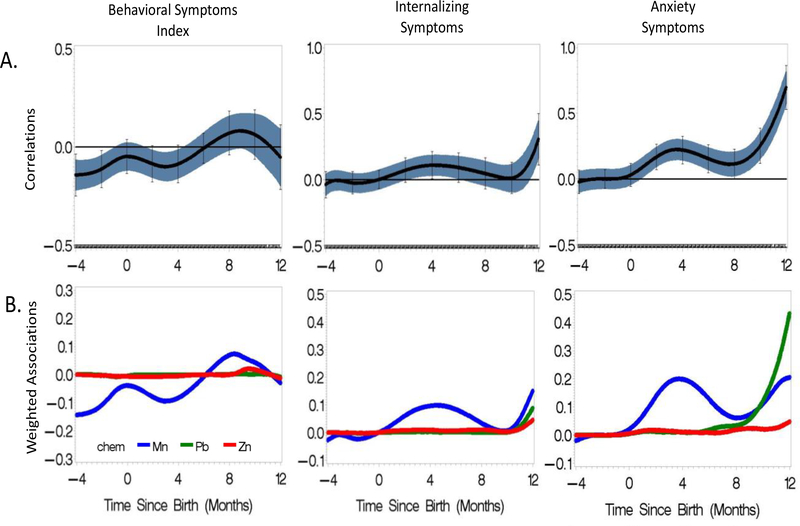
Figure 3 shows the results of the lagged WQS examining the time-varying associations between co-exposure to Mn, Zn, and Pb between 4 months gestational age through 12 months postnatal age with the BASC-2 BSI, Internalizing and Anxiety symptom scores. Throughout the prenatal period up to approximately 45 months, the metal mixture appears to be protective against adverse behavioral outcomes in the BSI. This association was statistically significant for −4 to −1 months and for 2.5 to 4 months. At −4 months prenatally, a one-unit increase in the mixture is associated with a 0.14 unit decrease in the BSI score. At 3 months postnatally, a one unit increase in the mixture index is associated with a 0.1 unit decrease in BSI score. The contributions of the individual metals to the observed sensitive windows are shown in Figure 3B. The early life protective associations are driven by dentine Mn (in blue), with dentine Zn and Pb having no contribution. In the postnatal period around 8 months, there is a nonsignificant suggestion of an association between the dentine metal mixture and increasing BSI scores. This association is driven largely by manganese (94%) with a small contribution by zinc (4%). The rDLM of the association between the 3-metal mixture and externalizing behaviors, hyperactivity and attention and the corresponding contributions of each metal is indistinguishable from the plot of the 3-metal mixture (data not shown).
Figure 3.
Lagged WQS representing time varying associations of co-exposure to dentine Mn, Zn and Pb with BASC-2 Behavioral Skills Index, Internalizing Symptoms, and Anxiety Symptoms assessed at 8–11 years of age among 133 ELEMENT subjects. (A) Lagged WQS plots with 95% piecewise confidence intervals (blue band) and 95% Holm-Bonferonni family-wise confidence intervals (vertical bars). The Yaxis represents time varying correlation between joint exposure and outcome. (B) Weighted associations of the individual metals to the observed mixture effects shown in plot A. In both figures, the X-axis represents the time since birth indicating the timing (in months) of tooth sampling.
The association between the 3-metal mixture and internalizing problems reveals two possible windows of developmental vulnerability. Around 4 months postnatal, the metal mixture is associated with increased internalizing problems and increased anxiety (Figure 3A). These relationships are driven largely by dentine Mn (90%), with little contribution from the other metals (Figure 3B). At 12 months, there is a significant positive association between possible mixture affect between the 3-metal mixture and higher internalizing behaviors around 12 months postnatally. At 12 months, a one-unit increase in the mixture is associated with a 0.3 unit increase in BASC-2 internalizing score. Figure 3B, showing the contribution of each individual metal to the association, suggests that the association between the 3-metal mixture and higher internalizing behavior is dominated by Mn, but also driven by dentine Pb and dentine Zn. The association between the 3-metal mixture and anxiety reflected this pattern. Dentine Mn between 0–8 months is significantly associated with higher anxiety. At 12 months, the significant positive association between the 3-metal mixture and anxiety is driven by Pb (71%), Mn (23%) and Zn (6%).
Sensitivity Analyses – Aggregated dentine metal concentrations
Using the cumulative measure of dentine metals aggregated across 50 timepoints, we observed significant associations between dentine Mn and overall BSI and externalizing problem (B = −0.47, SE = 0.16, P < 0.01; −0.470.16, p < 0.01, respecitively). We did not observe the association between Mn and internalizing problems or anxiety. Further, we did not observe associations between aggregated Pb or Zn and any BASC-2 outcomes. When we further restricted our cumulative Mn concentration to the period between 3–5 months, we did not observe an association between Mn and internalizing behaviors or anxiety.
Discussion
Using dentine biomarkers to reconstruct fine temporal resolution for Mn, Zn and Pb exposure throughout the prenatal and early postnatal periods, our results suggest: 1) individual metal exposure during specific developmental windows impacts the risk of reported behavioral problems in childhood and 2) early life Mn exposure appears protective against behavioral outcomes, yet postnatal Mn appears as a risk factor for behavioral outcomes, and 3) when examined as a mixture, manganese appears to drive the association with internalizing and externalizing problems. Specifically, dentine Mn and Zn levels in the early postnatal periods (from birth through 9 months of age) demonstrate significant associations with increased childhood anxiety symptoms. Beginning at birth, dentine Pb is associated with increasing anxiety symptoms, with the association increasing over time and appearing to extend beyond 12 months. When the three metals are examined as a mixture, the protective effect of prenatal and early postnatal Mn persists. Postnatally, we observe two distinct windows of susceptibility to internalizing and anxiety symptoms; the first window (around 4 months) appears to be driven largely by dentine Mn while the association at the second window (beginning after 8 months) is driven by the mixture of the three metals, and dominated by dentine Pb. The correlation between the metal mixture and anxiety at 12 months is higher than the correlations between any individual metal and anxiety symptoms, suggesting a mixtures effect. Taken together, these results suggest associations between dentine biomarkers of Mn and neurodevelopmental outcomes may depend upon timing. They also suggest that exposure to three metals may have a measurable association with behavior not observed in analyses of individual metals.
The timing of a neurotoxic exposure is an important determinant for the expression of a behavioral phenotype; not all neural structures and neuronal connections follow the same developmental trajectory, suggesting there may be unique windows of vulnerability for different developmental outcomes (Giedd et al. 1996; Huttenlocher and Dabholkar 1997). Traditional biomarkers collected at single time points may measure exposure in the wrong time window(s), leading to missed associations (Austin et al. 2017). Taking this developmental approach to understanding early life risk factors is particularly relevant to anxiety disorders as they may derive from early developmental events that affect the way an individual brain is “wired” (Kagan and Snidman 1999; Leonardo and Hen 2008; Van Ameringen et al. 1998). Anxiety disorders that manifest in childhood are fairly constant over a lifetime (Kagan and Snidman 1999). Increasing evidence from experimental rodent models suggests the period comprising the second and third weeks of life is a critical period for the development of the neural circuits and connections that mediate anxious behaviors (Leonardo and Hen 2008). This timeframe spanning postnatal days 7–21 in the rodent is comparable to 1–2 years of age in humans (Semple et al. 2013). Our observation of associations between higher metal exposures during specific developmental time windows and increasing anxiety symptoms support the concept of critical windows of development for behavioral outcomes.
Although it is well established that children are more susceptible than adults to the toxic effects of metal exposure (Aranda et al. 1997; Fraser 1992; Johansson et al. 2008; Kacew 1997; Landrigan 2004; Winder 2010), growing evidence suggests fetuses and neonates are even more vulnerable than children(Alcorn and McNamara 2002; Beath 2003; de Wildt et al. 1999; Ek et al. 2012; Erikson et al. 2007; Fechter 1999; Gow et al. 2001; Gregus et al. 1998; Hakkola et al. 1998; Hoffmann 1982; Juchau and Faustman-Watts 1983; Makri et al. 2004; Ring et al. 1999). Few epidemiologic studies examine time-resolved windows throughout development that may be more vulnerable to disrupted (excess or deficient) metal uptake. Using blood samples collected throughout childhood, previous work within our cohort examined associations between early-life blood Mn and Pb and children’s neurodevelopment. After adjusting for blood Pb, higher 12-month blood Mn was associated with poor infant neurodevelopment at 12 months(Claus Henn et al. 2010). This association was not observed with Mn levels at 24-months, suggesting a diminution of Mn neurotoxicity with increasing age and the possibility of a window of vulnerability to cognitive outcomes around one year of age. Notably, when Mn, Zn and Pb exposures occur jointly at 12 months, further decrements in IQ scores were observed, supporting the literature suggesting that the effect of a mixture of toxicant may differ from the effect of the individual metal components (Freire et al. 2018). Two recent studies have utilized dentine Mn as a biomarker of early life exposure, dividing the tooth into prenatal and postnatal time frames. Consistent with our results, prenatal dentine Mn levels were higher than postnatal, yet most adverse associations with neurodevelopmental outcomes were observed with postnatal values. Postnatal dentine Mn was associated with small decrements in cognition at 6 and 12 months of age (Gunier et al. 2013) and increases in internalizing and externalizing behaviors at 7 years of age (Mora et al. 2015). In a study of adolescent motor function, higher prenatal dentine Mn was largely protective from adverse motor outcomes in boys, but associated with increased tremor in girls (Chiu et al. 2017).
Results from these prior studies provide some insight into the timing of Mn exposure and the developmental windows of susceptibility, but fail to leverage the temporal exposure information available through dentine biomarkers and do not account for co-exposure to other neuroactive metals. Using lagged WQS, we examined time-resolved (i.e., weekly) associations between co-exposure to Mn, Pb and Zn and childhood behavioral outcomes. We observe a strong association between the 3-metal mixture at 4 and 12 months and childhood anxiety symptoms. Sensitivity analyses using metal concentrations aggregated at those specific time points failed to capture the associations with anxiety symptoms observed in the time series data. This suggests that approaches that our approach leveraging temporal data from deciduous teeth can identify realtionships that more traditional approaches focusing on spot samples or limited time windows may miss. Our results suggest that the impact of early life chemical exposures on behavioral outcomes may depend both on the timing as well as the dose of the exposure and highlight the need to study metal exposures as a mixture at different stages of development.
There are several limitations to our study. The fairly small sample size limited the statistical power to stratify the sample and perform the lagged WQS approach for both training and validation (Czarnota et al. 2015) and the exploratory approach limits our ability to generalize our findings on a population level. These findings should be confirmed in a larger sample. Further, while the lagged WQS method provides a comprehensive profile of the time-varying metal mixture, it does not allow for estimation of potentially multiplicative effects (i.e., metal interactions). While our use of deciduous teeth provides novel, retrospective data on early life exposures, deciduous teeth begin developing only in the early second trimester and we cannot examine the effects of very early metal exposure on neurodevelopmental outcomes occurring in the first trimester.
Despite these limitations, there are several unique strengths to our study. This study is among the first to present findings from an investigation linking prenatal and early postnatal dentine metal concentrations measured in deciduous teeth with children’s behavioral outcomes in later childhood (ages 8–11). This research explores new biomarkers of metal exposure, assessment of both individual and combined metal exposure, and employs a novel methodology (i.e., lagged WQS) to capture the time-varying association between exposure and outcome. Our results indicate that there are relatively small, statistically significant associations for each individual metal as well as for the combination of metals that vary by the time window when the metals are measured. Finally, we focus on relevant but understudied neurophenotypes. Adolescent anxiety disorders predict a broad range of subsequent psychiatric disorders (Bittner et al. 2004; Cohen et al. 1993b; Foley et al. 2004; Hayward et al. 2000; Orvaschel et al. 1995; Stein et al. 2001; Woodward and Fergusson 2001) yet early life environmental predictors of childhood anxiety disorders have received little attention.
Conclusion
Overall, we found that early life metal exposures, as measured in deciduous teeth, were associated with behavioral problems in children and the associations were dependent upon developmental timing. These findings add to a growing literature addressing the neurodevelopmental toxicity of neurotoxic metals, and suggest that the developing brain is uniquely vulnerable to the effect of metal exposure at different timepoints. In addition to addressing exposures over time, our findings suggest that studies addressing early life exposures should consider co-exposure to multiple neurotoxic chemicals, rather than single chemicals. Greater knowledge about the developmental timing of exposures and brain development would be valuable for informing interventions.
Highlights:
3 to 5 bullet points (maximum 85 characters, including spaces, per bullet point)
Tooth-matrix biomarkers were used to identify windows of susceptibility to Mn, Zn, and Pb
Prenatal and early postnatal Mn appears protective against externalizing behavior
Postnatal Mn is associated with higher internalizing behaviors, especially anxiety
Co-exposure to Mn, Zn and Pb at 12 months is associated with anxiety
Acknowledgements
We are grateful to all the participants and their families for taking part in this study. We thank the staff of the ELEMENT cohort studies and we acknowledge the American British Cowdray Hospital which provided facilities used for this research.
Fundingx
This study was supported and partially funded by the National Institute of Public Health/Ministry of Health of Mexico and the National Institute of Environmental Health Sciences (NIH/NIEHS) grants R00ES020364 (Horton), R01ES019597 (Arora), and DP2ES025453 (Arora). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Abbreviations
- Ca
Calcium
- Mn
Manganese
- Pb
Lead
- BASC-2
Behavioral Assessment System for Children, 2nd edition
- ELEMENT
Early Life Exposures in Mexico to Environmental Toxicants
- rDLM
Reversed Distributed Lag Model
- WQS
Weighted Quantile Sum Regression
Footnotes
Declarations
The authors declare they have no actual or potential competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams J, Barone S Jr., LaMantia A, Philen R, Rice DC, Spear L, et al. 2000. Workshop to identify critical windows of exposure for children’s health: Neurobehavioral work group summary. Environ Health Perspect 108 Suppl 3:535–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcorn J, McNamara PJ. 2002. Ontogeny of hepatic and renal systemic clearance pathways in infants: Part i. Clin Pharmacokinet 41:959–998. [DOI] [PubMed] [Google Scholar]
- Anderson JC, Williams S, McGee R, Silva PA. 1987. Dsm-iii disorders in preadolescent children. Prevalence in a large sample from the general population. Arch Gen Psychiatry 44:69–76. [DOI] [PubMed] [Google Scholar]
- Andra SS, Austin C, Arora M. 2016. The tooth exposome in children’s health research. Curr Opin Pediatr 28:221–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angold A, Costello EJ, Worthman CM. 1998. Puberty and depression: The roles of age, pubertal status and pubertal timing. Psychol Med 28:51–61. [DOI] [PubMed] [Google Scholar]
- Aranda JV, Hales BF, Rieder MF. 1997. Developmental pharmacology. In: Neonatal-perinatal medicine: Diseases of the fetus and infant, (Fanaroff AA, Martin RJ, eds). St. Louis, MO:Mosby. [Google Scholar]
- Arora M, Chan SW, Ryan CG, Kennedy BJ, Walker DM. 2005. Spatial distribution of lead in enamel and coronal dentine of wistar rats. Biological trace element research 105:159–170. [DOI] [PubMed] [Google Scholar]
- Arora M, Hare D, Austin C, Smith DR, Doble P. 2011. Spatial distribution of manganese in enamel and coronal dentine of human primary teeth. Sci Total Environ 409:1315–1319. [DOI] [PubMed] [Google Scholar]
- Arora M, Bradman A, Austin C, Vedar M, Holland N, Eskenazi B, et al. 2012. Determining fetal manganese exposure from mantle dentine of deciduous teeth. Environmental science & technology 46:5118–5125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arora M, Austin C. 2013. Teeth as a biomarker of past chemical exposure. Curr Opin Pediatr 25:261–267. [DOI] [PubMed] [Google Scholar]
- Arora M, Austin C, Sarrafpour B, Hernandez-Avila M, Hu H, Wright RO, et al. 2014. Determining prenatal, early childhood and cumulative long-term lead exposure using micro-spatial deciduous dentine levels. PLoS One 9:e97805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ash M, Neslon S. 2002. Wheeler’s dental anatomy, physiology, and occlusion 8th edition:Saunders. [Google Scholar]
- Austin C, Smith TM, Bradman A, Hinde K, Joannes-Boyau R, Bishop D, et al. 2013. Barium distributions in teeth reveal early-life dietary transitions in primates. Nature 498:216–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin C, Richardson C, Smith D, Arora M. 2017. Tooth manganese as a biomarker of exposure and body burden in rats. Environ Res 155:373–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baek J, Sanchez BN, Berrocal VJ, Sanchez-Vaznaugh EV. 2016a. Distributed lag models: Examining associations between the built environment and health. Epidemiology 27:116–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baek J, Sanchez-Vaznaugh EV, Sanchez BN. 2016b. Hierarchical distributed-lag models: Exploring varying geographic scale and magnitude in associations between the built environment and health. Am J Epidemiol 183:583–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks EC, Ferretti LE, Shucard DW. 1997. Effects of low level lead exposure on cognitive function in children: A review of behavioral, neuropsychological and biological evidence. Neurotoxicology 18:237–281. [PubMed] [Google Scholar]
- Beath SV. 2003. Hepatic function and physiology in the newborn. Semin Neonatol 8:337–346. [DOI] [PubMed] [Google Scholar]
- Beesdo K, Knappe S, Pine DS. 2009. Anxiety and anxiety disorders in children and adolescents: Developmental issues and implications for dsm-v. Psychiatr Clin North Am 32:483–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellinger DC. 2013. Prenatal exposures to environmental chemicals and children’s neurodevelopment: An update. Saf Health Work 4:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bello GA, Arora M, Austin C, Horton MK, Wright RO, Gennings C. 2017. Extending the distributed lag model framework to handle chemical mixtures. Environ Res 156:253–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjet C, Borges G, Medina-Mora ME, Zambrano J, Aguilar-Gaxiola S. 2009. Youth mental health in a populous city of the developing world: Results from the mexican adolescent mental health survey. J Child Psychol Psychiatry 50:386–395. [DOI] [PubMed] [Google Scholar]
- Betharia S, Maher TJ. 2012. Neurobehavioral effects of lead and manganese individually and in combination in developmentally exposed rats. Neurotoxicology 33:1117–1127. [DOI] [PubMed] [Google Scholar]
- Bittner A, Goodwin RD, Wittchen HU, Beesdo K, Hofler M, Lieb R. 2004. What characteristics of primary anxiety disorders predict subsequent major depressive disorder? J Clin Psychiatry 65:618626, quiz 730. [DOI] [PubMed] [Google Scholar]
- Bouchard MF, Bellinger DC, Weuve J, Matthews-Bellinger J, Gilman SE, Wright RO, et al. 2009. Blood lead levels and major depressive disorder, panic disorder, and generalized anxiety disorder in us young adults. Arch Gen Psychiatry 66:1313–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun JM, Hoffman E, Schwartz J, Sanchez B, Schnaas L, Mercado-Garcia A, et al. 2012. Assessing windows of susceptibility to lead-induced cognitive deficits in mexican children. Neurotoxicology 33:1040–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderon-Garciduenas L, Serrano-Sierra A, Torres-Jardon R, Zhu H, Yuan Y, Smith D, et al. 2013. The impact of environmental metals in young urbanites’ brains. Exp Toxicol Pathol 65:503–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell SB. 1995. Behavior problems in preschool children: A review of recent research. J Child Psychol Psychiatry 36:113–149. [DOI] [PubMed] [Google Scholar]
- Carrasco Av. 2002. The amai system of classifying households by socio-economic level.. <http://wwwEsomarorg>..
- Carrico C, Gennings C, Wheeler DC, Factor-Litvak P. 2014. Charaterization of weighted quantile sum regression for highly correlated data in a risk analyses setting. Journal of Agricultural, Biological and Environmental Statistics 20:100–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YH, Ferguson KK, Meeker JD, McElrath TF, Mukherjee B. 2015. Statistical methods for modeling repeated measures of maternal environmental exposure biomarkers during pregnancy in association with preterm birth. Environ Health 14:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Myers R, Wei T, Bind E, Kassim P, Wang G, et al. 2014. Placental transfer and concentrations of cadmium, mercury, lead, and selenium in mothers, newborns, and young children. J Expo Sci Environ Epidemiol 24:537–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu YH, Hsu HH, Coull BA, Bellinger DC, Kloog I, Schwartz J, et al. 2016. Prenatal particulate air pollution and neurodevelopment in urban children: Examining sensitive windows and sex-specific associations. Environ Int 87:56–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu YM, Claus Henn B, Hsu HL, Pendo MP, Coull BA, Austin C, et al. 2017. Sex differences in sensitivity to prenatal and early childhood manganese exposure on neuromotor function in adolescents. Environ Res 159:458–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark C, Rodgers B, Caldwell T, Power C, Stansfeld S. 2007. Childhood and adulthood psychological ill health as predictors of midlife affective and anxiety disorders: The 1958 british birth cohort. Arch Gen Psychiatry 64:668–678. [DOI] [PubMed] [Google Scholar]
- Claus Henn B, Ettinger AS, Schwartz J, Tellez-Rojo MM, Lamadrid-Figueroa H, Hernandez-Avila M, et al. 2010. Early postnatal blood manganese levels and children’s neurodevelopment. Epidemiology 21:433–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claus Henn B, Austin C, Coull BA, Schnaas L, Gennings C, Horton MK, et al. 2018. Uncovering neurodevelopmental windows of susceptibility to manganese exposure using dentine microspatial analyses. Environmental Research 161:588–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen P, Cohen J, Brook J. 1993a. An epidemiological study of disorders in late childhood and adolescence--ii. Persistence of disorders. J Child Psychol Psychiatry 34:869–877. [DOI] [PubMed] [Google Scholar]
- Cohen P, Cohen J, Kasen S, Velez CN, Hartmark C, Johnson J, et al. 1993b. An epidemiological study of disorders in late childhood and adolescence--i. Age- and gender-specific prevalence. J Child Psychol Psychiatry 34:851–867. [DOI] [PubMed] [Google Scholar]
- Coull BABJ, Wellenius GA, Kioumourtzoglou M, Mittleman MA, Koutrakis P, Godleski JJ.. 2015. Statistical learning methods for the effects of multiple air pollution constituents. Research report 183.Health Effects Institute; [PubMed] [Google Scholar]
- Coull BA, Claus HB, Wright RO, Arora M 2014. Statistical methods for analyzing critical windows of metal exposures using the tooth biomarker. In: ISES. [Google Scholar]
- Czarnota J, Gennings C, Wheeler DC. 2015. Assessment of weighted quantile sum regression for modeling chemical mixtures and cancer risk. Cancer Inform 14:159–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wildt SN, Kearns GL, Leeder JS, van den Anker JN. 1999. Glucuronidation in humans. Pharmacogenetic and developmental aspects. Clin Pharmacokinet 36:439–452. [DOI] [PubMed] [Google Scholar]
- Duval ER, Javanbakht A, Liberzon I. 2015. Neural circuits in anxiety and stress disorders: A focused review. Ther Clin Risk Manag 11:115–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ek CJ, Dziegielewska KM, Habgood MD, Saunders NR. 2012. Barriers in the developing brain and neurotoxicology. Neurotoxicology 33:586–604. [DOI] [PubMed] [Google Scholar]
- Ericson JE, Crinella FM, Clarke-Stewart KA, Allhusen VD, Chan T, Robertson RT. 2007. Prenatal manganese levels linked to childhood behavioral disinhibition. Neurotoxicology and teratology 29:181–187. [DOI] [PubMed] [Google Scholar]
- Erikson KM, Thompson K, Aschner J, Aschner M. 2007. Manganese neurotoxicity: A focus on the neonate. Pharmacol Ther 113:369–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ettinger AS, Lamadrid-Figueroa H, Tellez-Rojo MM, Mercado-Garcia A, Peterson KE, Schwartz J, et al. 2009. Effect of calcium supplementation on blood lead levels in pregnancy: A randomized placebo-controlled trial. Environ Health Perspect 117:26–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fechter LD. 1999. Distribution of manganese in development. Neurotoxicology 20:197–201. [PubMed] [Google Scholar]
- Foley DL, Pickles A, Maes HM, Silberg JL, Eaves LJ. 2004. Course and short-term outcomes of separation anxiety disorder in a community sample of twins. J Am Acad Child Adolesc Psychiatry 43:1107–1114. [DOI] [PubMed] [Google Scholar]
- Fraser FC. 1992. Teratology In: Pediatric pharmacology: Therapeutic principles in practice (Yaffe SJ, Aranda JV, eds). Philadelphia:Saunders, W.B; . [Google Scholar]
- Freire C, Amaya E, Gil F, Fernandez MF, Murcia M, Llop S, et al. 2018. Prenatal co-exposure to neurotoxic metals and neurodevelopment in preschool children: The environment and childhood (inma) project. The Science of the total environment 621:340–351. [DOI] [PubMed] [Google Scholar]
- Gallegos J, Langley A, Villegas D. 2012. Anxiety, depression, and coping skills among mexican school children: A comparison of students with and without learning disabilities. Learn Disabil Q 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasparrini A, Armstrong B. 2010. Time series analysis on the health effects of temperature: Advancements and limitations. Environ Res 110:633–638. [DOI] [PubMed] [Google Scholar]
- Gasparrini A, Armstrong B, Kenward MG. 2010. Distributed lag non-linear models. Stat Med 29:22242234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd JN, Snell JW, Lange N, Rajapakse JC, Casey BJ, Kozuch PL, et al. 1996. Quantitative magnetic resonance imaging of human brain development: Ages 4–18. Cereb Cortex 6:551–560. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Cossio T, Peterson KE, Sanin LH, Fishbein E, Palazuelos E, Aro A, et al. 1997. Decrease in birth weight in relation to maternal bone-lead burden. Pediatrics 100:856–862. [DOI] [PubMed] [Google Scholar]
- Gow PJ, Ghabrial H, Smallwood RA, Morgan DJ, Ching MS. 2001. Neonatal hepatic drug elimination. Pharmacol Toxicol 88:3–15. [DOI] [PubMed] [Google Scholar]
- Goyer RA. 1990. Transplacental transport of lead. Environ Health Perspect 89:101–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandjean P, Landrigan PJ. 2006. Developmental neurotoxicity of industrial chemicals. Lancet 368:2167–2178. [DOI] [PubMed] [Google Scholar]
- Grandjean P, Landrigan PJ. 2014. Neurobehavioural effects of developmental toxicity. Lancet Neurol 13:330–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregus Z, Fekete T, Halaszi E, Gyurasics A, Klaassen CD. 1998. Effects of fibrates on the glycine conjugation of benzoic acid in rats. Drug Metab Dispos 26:1082–1088. [PubMed] [Google Scholar]
- Gunier RB, Bradman A, Jerrett M, Smith DR, Harley KG, Austin C, et al. 2013. Determinants of manganese in prenatal dentin of shed teeth from chamacos children living in an agricultural community. Environmental science & technology 47:11249–11257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunier RB, Arora M, Jerrett M, Bradman A, Harley KG, Mora AM, et al. 2015. Manganese in teeth and neurodevelopment in young mexican-american children. Environ Res 142:688–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakkola J, Tanaka E, Pelkonen O. 1998. Developmental expression of cytochrome p450 enzymes in human liver. Pharmacol Toxicol 82:209–217. [DOI] [PubMed] [Google Scholar]
- Hare D, Austin C, Doble P, Arora M. 2011. Elemental bio-imaging of trace elements in teeth using laser ablation-inductively coupled plasma-mass spectrometry. Journal of dentistry 39:397–403. [DOI] [PubMed] [Google Scholar]
- Hayward C, Killen JD, Kraemer HC, Taylor CB. 2000. Predictors of panic attacks in adolescents. J Am Acad Child Adolesc Psychiatry 39:207–214. [DOI] [PubMed] [Google Scholar]
- Heaton MJ, Peng RD. 2012. Flexible distributed lag models using random functions with application to estimating mortality displacement from heat-related deaths. J Agric Biol Environ Stat 17:313–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Avila M, Peterson KE, Gonzalez-Cossio T, Sanin LH, Aro A, Schnaas L, et al. 2002. Effect of maternal bone lead on length and head circumference of newborns and 1-month-old infants. Arch Environ Health 57:482–488. [DOI] [PubMed] [Google Scholar]
- Hillson S 1996. Dental anthropology. New York:Cambridge University Press. [Google Scholar]
- Hoffmann H 1982. Absorption of drugs and other xenobiotics during development in experimental animals. Pharmacol Ther 16:247–260. [DOI] [PubMed] [Google Scholar]
- Huttenlocher PR, Dabholkar AS. 1997. Regional differences in synaptogenesis in human cerebral cortex. J Comp Neurol 387:167–178. [DOI] [PubMed] [Google Scholar]
- Islam MR, Ahmed MU, Mitu SA, Islam MS, Rahman GK, Qusar MM, et al. 2013. Comparative analysis of serum zinc, copper, manganese, iron, calcium, and magnesium level and complexity of interelement relations in generalized anxiety disorder patients. Biological trace element research 154:21–27. [DOI] [PubMed] [Google Scholar]
- Johansson PA, Dziegielewska KM, Liddelow SA, Saunders NR. 2008. The blood-csf barrier explained: When development is not immaturity. Bioessays 30:237–248. [DOI] [PubMed] [Google Scholar]
- Juchau MR, Faustman-Watts E. 1983. Pharmacokinetic considerations in the maternal-placental-fetal unit. Clin Obstet Gynecol 26:379–390. [DOI] [PubMed] [Google Scholar]
- Jung A, Spira D, Steinhagen-Thiessen E, Demuth I, Norman K. 2016. Zinc deficiency is associated with depressive symptoms-results from the berlin aging study ii. J Gerontol A Biol Sci Med Sci. [DOI] [PubMed] [Google Scholar]
- Kacew S 1997. General principles in pediatric pharmacology and toxicology In: Environmental toxicology and pharmacology of human development, (Kacew S, Lambert GH, eds). Washington, DC:Taylor & Francis. [Google Scholar]
- Kagan J, Snidman N. 1999. Early childhood predictors of adult anxiety disorders. Biol Psychiatry 46:1536–1541. [DOI] [PubMed] [Google Scholar]
- Kaufman A, Lichtenberger E. 2006. Assessing adolescent and adult intelligence, 3rd ed. Hoboken, NJ:Wiley. [Google Scholar]
- Kelleher KJ, McInerny TK, Gardner WP, Childs GE, Wasserman RC. 2000. Increasing identification of psychosocial problems: 1979–1996. Pediatrics 105:1313–1321. [DOI] [PubMed] [Google Scholar]
- Khan K, Wasserman GA, Liu X, Ahmed E, Parvez F, Slavkovich V, et al. 2012. Manganese exposure from drinking water and children’s academic achievement. Neurotoxicology 33:91–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieling C, Baker-Henningham H, Belfer M, Conti G, Ertem I, Omigbodun O, et al. 2011. Child and adolescent mental health worldwide: Evidence for action. Lancet 378:1515–1525. [DOI] [PubMed] [Google Scholar]
- Kim MG, Ryoo JH, Chang SJ, Kim CB, Park JK, Koh SB, et al. 2015. Blood lead levels and causespecific mortality of inorganic lead-exposed workers in south korea. PLoS One 10:e0140360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MJ, Loucks RA, Palmer AL, Brown AC, Solomon KM, Marchante AN, et al. 2011. The structural and functional connectivity of the amygdala: From normal emotion to pathological anxiety. Behav Brain Res 223:403–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lall R, Ito K, Thurston GD. 2011. Distributed lag analyses of daily hospital admissions and sourceapportioned fine particle air pollution. Environ Health Perspect 119:455–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landrigan PJ. 2004. Children as a vulnerable population. Int J Occup Med Environ Health 17:175–177. [PubMed] [Google Scholar]
- Leonardo ED, Hen R. 2008. Anxiety as a developmental disorder. Neuropsychopharmacology 33:134–140. [DOI] [PubMed] [Google Scholar]
- Loeber R, Green SM, Lahey BB, Frick PJ, McBurnett K. 2000. Findings on disruptive behavior disorders from the first decade of the developmental trends study. Clin Child Fam Psychol Rev 3:37–60. [DOI] [PubMed] [Google Scholar]
- Makri A, Goveia M, Balbus J, Parkin R. 2004. Children’s susceptibility to chemicals: A review by developmental stage. J Toxicol Environ Health B Crit Rev 7:417–435. [DOI] [PubMed] [Google Scholar]
- Mehra R, Thakur A. 2016. Relationship between lead, cadmium, zinc, manganese and iron in hair of environmentally exposed subjects. Arabian Journal of Chemistry 9:S1214–S1217 [Google Scholar]
- Merikangas KR, Nakamura EF, Kessler RC. 2009. Epidemiology of mental disorders in children and adolescents. Dialogues Clin Neurosci 11:7–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer-Baron M, Schaper M, Knapp G, Lucchini R, Zoni S, Bast-Pettersen R, et al. 2013. The neurobehavioral impact of manganese: Results and challenges obtained by a meta-analysis of individual participant data. Neurotoxicology 36:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mlyniec K, Gawel M, Doboszewska U, Starowicz G, Nowak G. 2017. The role of elements in anxiety. Vitam Horm 103:295–326. [DOI] [PubMed] [Google Scholar]
- Money KM, Stanwood GD. 2013. Developmental origins of brain disorders: Roles for dopamine. Front Cell Neurosci 7:260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mora AM, Arora M, Harley KG, Kogut K, Parra K, Hernandez-Bonilla D, et al. 2015. Prenatal and postnatal manganese teeth levels and neurodevelopment at 7, 9, and 10.5 years in the chamacos cohort. Environ Int 84:39–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray CJ, Atkinson C, Bhalla K, Birbeck G, Burstein R, Chou D, et al. 2013. The state of us health, 1990–2010: Burden of diseases, injuries, and risk factors. JAMA 310:591–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Needham LL, Grandjean P, Heinzow B, Jorgensen PJ, Nielsen F, Patterson DG, et al. 2011. Partition of environmental chemicals between maternal and fetal blood and tissues. Environmental science & technology 45:1121–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Needleman HL, Schell A, Bellinger D, Leviton A, Allred EN. 1990. The long-term effects of exposure to low doses of lead in childhood. An 11-year follow-up report. N Engl J Med 322:83–88. [DOI] [PubMed] [Google Scholar]
- Orvaschel H, Lewinsohn PM, Seeley JR. 1995. Continuity of psychopathology in a community sample of adolescents. J Am Acad Child Adolesc Psychiatry 34:1525–1535. [DOI] [PubMed] [Google Scholar]
- Pine DS, Cohen P, Gurley D, Brook J, Ma Y. 1998. The risk for early-adulthood anxiety and depressive disorders in adolescents with anxiety and depressive disorders. Arch Gen Psychiatry 55:56–64. [DOI] [PubMed] [Google Scholar]
- Reynolds C, Kamphaus R. 1992. Behavior assessment system for children, 2nd edition. American Guidance Service, INc. [Google Scholar]
- Ring JA, Ghabrial H, Ching MS, Smallwood RA, Morgan DJ. 1999. Fetal hepatic drug elimination. Pharmacol Ther 84:429–445. [DOI] [PubMed] [Google Scholar]
- Rosner B 1983. Percentage points for a generalized esd many-outlier procedure. Technometrics 25:165–172. [Google Scholar]
- Rubin DB. 2004. Multiple imputation for nonresponse in surveys. Hoboken, NJ:Wiley-Interscience. [Google Scholar]
- Rudge CV, Rollin HB, Nogueira CM, Thomassen Y, Rudge MC, Odland JO. 2009. The placenta as a barrier for toxic and essential elements in paired maternal and cord blood samples of south african delivering women. J Environ Monit 11:1322–1330. [DOI] [PubMed] [Google Scholar]
- Sanders AP, Claus Henn B, Wright RO. 2015. Perinatal and childhood exposure to cadmium, manganese, and metal mixtures and effects on cognition and behavior: A review of recent literature. Curr Environ Health Rep 2:284–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos-Burgoa C, Rios C, Mercado LA, Arechiga-Serrano R, Cano-Valle F, Eden-Wynter RA, et al. 2001. Exposure to manganese: Health effects on the general population, a pilot study in central mexico. Environ Res 85:90–104. [DOI] [PubMed] [Google Scholar]
- Schlotz W, Phillips DI. 2009. Fetal origins of mental health: Evidence and mechanisms. Brain Behav Immun 23:905–916. [DOI] [PubMed] [Google Scholar]
- Selevan SG, Kimmel CA, Mendola P. 2000. Identifying critical windows of exposure for children’s health. Environ Health Perspect 108 Suppl 3:451–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semple BD, Blomgren K, Gimlin K, Ferriero DM, Noble-Haeusslein LJ. 2013. Brain development in rodents and humans: Identifying benchmarks of maturation and vulnerability to injury across species. Prog Neurobiol 106–107:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiue I 2015. Urinary heavy metals, phthalates and polyaromatic hydrocarbons independent of health events are associated with adult depression: USA nhanes, 2011–2012. Environ Sci Pollut Res Int 22:17095–17103. [DOI] [PubMed] [Google Scholar]
- Stein MB, Chavira DA, Jang KL. 2001. Bringing up bashful baby. Developmental pathways to social phobia. Psychiatr Clin North Am 24:661–675. [DOI] [PubMed] [Google Scholar]
- Tellez-Rojo MM, Hernandez-Avila M, Lamadrid-Figueroa H, Smith D, Hernandez-Cadena L, Mercado A, et al. 2004. Impact of bone lead and bone resorption on plasma and whole blood lead levels during pregnancy. Am J Epidemiol 160:668–678. [DOI] [PubMed] [Google Scholar]
- Van Ameringen M, Mancini C, Oakman JM. 1998. The relationship of behavioral inhibition and shyness to anxiety disorder. J Nerv Ment Dis 186:425–431. [DOI] [PubMed] [Google Scholar]
- van Buuren S 2007. Multiple imputation of discrete and continuous data by fully conditional specification. Statistical Methods Research 16:219–242 [DOI] [PubMed] [Google Scholar]
- White IR, Royston P, Wood AM. 2011. Multiple imputation using chained equations: Issues and guidance for practice. Stat Med 30:377–399. [DOI] [PubMed] [Google Scholar]
- Winder BS. 2010. Manganese in the air: Are children at greater risk than adults? J Toxicol Environ Health A 73:156–158. [DOI] [PubMed] [Google Scholar]
- Woodward LJ, Fergusson DM. 2001. Life course outcomes of young people with anxiety disorders in adolescence. J Am Acad Child Adolesc Psychiatry 40:1086–1093. [DOI] [PubMed] [Google Scholar]
- Wyzga RE. 1978. The effect of air pollution upon mortality: A consideration of distributed lag models. Journal of the American Statistical Association 73:463–472. [DOI] [PubMed] [Google Scholar]
- Yoon M, Nong A, Clewell HJ 3rd, Taylor MD, Dorman DC, Andersen ME 2009. Evaluating placental transfer and tissue concentrations of manganese in the pregnant rat and fetuses after inhalation exposures with a pbpk model. Toxicol Sci 112:44–58. [DOI] [PubMed] [Google Scholar]
- Yousef S, Adem A, Zoubeidi T, Kosanovic M, Mabrouk AA, Eapen V. 2011. Attention deficit hyperactivity disorder and environmental toxic metal exposure in the united arab emirates. J Trop Pediatr 57:457–460. [DOI] [PubMed] [Google Scholar]
- Zhao X, Chen F, Feng Z, Li X, Zhou XH. 2014. The temporal lagged association between meteorological factors and malaria in 30 counties in south-west china: A multilevel distributed lag non-linear analysis. Malar J 13:57. [DOI] [PMC free article] [PubMed] [Google Scholar]