Abstract
Stress and anxiety have been associated with changes in the microbiota of the gut and ultimately diminished resistance to pathogens. The objective of this study was to observe intestinal microbiota and susceptibility to Salmonella associated with stress hormones, cortisol (CORT), and norepinephrine (NE), in piglets. At weaning, 90 piglets (15 for a Salmonella challenge) were trained to take the carrier (apple juice) orally. At 2 wk after weaning, pens of piglets were assigned randomly to 1 of 3 treatments: control (CNT), NE, or CORT. Blood samples were collected prior to treatment, then piglets were dosed orally with treatments twice on day 0; at 0800 and 1600 h. Control piglets were administered 6.1 mL of the carrier only, NE pigs were administered 40 mg/mL of NE-bitartrate salt dissolved in the carrier, and CORT pigs were administered 12 mg/mL of hydrocortisone acetate dissolved in the carrier. Jugular blood samples were collected prior to necropsies (n = 5/treatment) at 0800 and 1600 h on day 1, and at 0800 h on days 2, 7, and 14 after treatments were started. A subset of pigs were subjected to a 24-h Salmonella challenge. Jejunal and ileal tissues and jejunal, ileal, cecal, and rectal contents were collected and colonies were counted. Microbial data and blood samples were analyzed using mixed models with fixed effects of treatment and day. Cortisol-treated piglets exhibited a spike in plasma CORT concentrations at 0800 h day 1 (P = 0.001) accompanied by greater concentrations of cecal Escherichia coli (P < 0.05) and a shift in intestinal environment to favor coliforms on day 2 (P < 0.05). Salmonella concentrations from rectal contents tended (P = 0.07) to be suppressed by CORT. Lactic acid–producing bacteria rectal concentrations were greater (P = 0.03) in CORT pigs on 0800 h on day 1 then NE pigs and tended to be greater than CNT (P = 0.09) and were greater on day 14 for both CNT (P = 0.003) and NE (P = 0.02). Norepinephrine spiked in NE piglets at 0800 h on day 1 (P = 0.001), 1600 h day 1 (P = 0.004), through day 2 (P = 0.04). Intestinal environment of NE pigs shifted to favor ileal anaerobes (P ≤ 0.05) and facultative anaerobes (E. coli; P = 0.01) compared to CNT. However, Salmonella concentrations in rectal contents were suppressed by NE compared to CNT (P = 0.05). Oral administration of NE and CORT had the desired effect of increasing concentrations of stress hormones and resulted in microbiome shifts throughout the intestines.
Keywords: cortisol, intestinal microbiota, norepinephrine, Salmonella
INTRODUCTION
The gut and the brain communicate via the gut–brain axis. The gut is innervated with contacts to the nervous system, through which bidirectional communication between the gut and the brain occur (Lyte, 2014). Anxiety and other negative affective states therefore influence the microorganisms living in the gut. An increase in stress and anxiety and impairments in learning and memory have been associated with infection and changes from the normal gut microbiome (Goehler et al., 2007; Li et al., 2009; Gareau et al., 2011). Therefore, stress with greater cortisol (CORT) and catecholamines as observed in piglets during weaning, may be changing the gut microbiome and susceptibility to disease.
One of the earliest sources of stress for production piglets is weaning. Both the HPA axis and SAM axis and their hormones have been studied as physiological causes of stress in weaned pigs (Stanton and Mueller, 1976). Neurotransmitters associated with that response have recently been implicated in gastrointestinal physiology and disease (Mittal et al., 2017); because the relationship of the gut system and enteric nervous system is bidirectional the relationship becomes complex. Neurotransmitters play a major role in gut homeostasis including the gut microbiome and overall gut motility and health. Additionally, catecholamines activate different receptors at different concentrations, further muddling our knowledge of this intricate model (Mittal et al., 2017). Catecholamines may come directly from the microbial population or transported by them to the site (Sudo, 2014). Additionally, exogenous manipulation of catecholamines has ameliorated symptoms of disease and disease (Liang et al., 2015) and catecholamines have been shown to effect immunity and pathogen growth and virulence (Freestone, 2013).
Reduced intestine Lactobacillus counts have been associated with environmental stress; such as no bedding, food, or water (Tannock and Savage (1974), maternal separation (Bailey and Cow, 1999), and even high-stress periods in humans (Knowles et al., 2008). In contrast, epinephrine and norepinephrine (NE) are known to increase virulence of some foodborne pathogens (Lustri et al., 2017). Still few studies have examined the impact on microbiota due to acute psychological stressors such as weaning in pigs. Based on evidence from other species, we hypothesize that we can induce intestine microbial shifts with oral administration of NE or CORT.
The objectives of this study were to administer CORT or NE to nursery pigs (after weaning stress has subsided) to determine changes in gut microbial communities and susceptibility to Salmonella.
MATERIALS AND METHODS
Animals and Treatments
Animal procedures were approved by the Purdue Animal Care and Use Committee using the 3rd edition of Guide for the care and use of agricultural animals in research and teaching (2010). At weaning (19 ± 2 d of age, mean ± SD), Yorkshire by Landrace cross barrows and gilts (n = 75 trial piglets, 5 per treatment per necropsy; and 15 for a Salmonella challenge, 5 per treatment) were blocked by weight into 15 pens and balanced by sex. The study was conducted in 2 replications for logistical reasons. Additional companion piglets were added to bring the total piglets per pen to 8. Pigs were fed a nursery diet (Table 1) ad libitum. Mean piglet BW ± SD was 12.8 kg ± 1.81 at 2 wk after weaning when the experiment began. Because pigs are known to like apple juice and it would have minimal effect on the diet, the piglets were trained to take the carrier (apple juice) orally by syringe (Ardes France Mod Depose, Wabash Valley Feeds, West Lafayette, IN). This route of administration has 2 distinct advantages to other methods. The pigs would be maintained in their social housing, so there was not the isolation stress of single housing. The second method of i.v. collection requires the stress of that process. Therefore, the oral delivery was chosen to circumvent unnecessary social and handling stress. Two wk post-weaning (experimental day 0), piglets were randomly assigned to control, NE, or CORT treatment. On day 0, piglets were administered treatments twice, at 0800 and 1600 h. Control piglets received 6.1 mL of carrier only, NE pigs received 244 mg of NE-bitartrate salt (Sigma-Aldrich; St. Louis, MO) dissolved in 6.1 mL of carrier, and CORT piglets received 73.2 mg of hydrocortisone acetate (Sigma-Aldrich) dissolved in 6.1 mL of carrier. These concentrations were based on Pullinger et al., (2010) for NE concentrations and on Kranendonk et al., (2005) for oral CORT delivery.
Table 1.
Phase 4 nursery basal diet
| Ingredient | %, as fed |
|---|---|
| PU Corn 2006 NRC | 62.540 |
| SBM | 31.340 |
| Soybean oil | 2.000 |
| Limestone | 1.180 |
| MonoCal | 1.020 |
| Vitamin | 0.250 |
| TM | 0.125 |
| Se 600 | 0.050 |
| Phytase | 0.100 |
| Salt | 0.350 |
| Lysine-HCL | 0.360 |
| DL-Methionine | 0.160 |
| l-Threonine | 0.155 |
| l-Tryptophan | 0.020 |
| Banmith, 48 | 0.100 |
| Corn treatment premix (diet 1–6) | 0.250 |
| Total | 100.000 |
Sample Collection and Processing
Prior to receiving treatments, at 0700 h and before euthanasia for each necropsy, jugular blood samples were collected into sodium heparin vacuum tubes (BD; Franklin Lakes, NJ) and the serum was harvested and frozen at −80°C until processed for CORT and NE concentrations. Trained individuals euthanized piglets with CO2 gas followed by exsanguination. Necropsies were performed at 0800 and 1600 h on day 1, and at 0800 h on days 2, 7, and 14. Jejunal tissue, ileal tissue, mesenteric lymph nodes (MLN), jejunal content, ileal content, cecal content, and rectal content were collected. Jejunal tissue (3 cm) was collected from the proximal jejunum. Ileal tissue (3 cm) was collected from the ileocecal junction. The tissues were flash frozen using liquid nitrogen, and stored on liquid nitrogen cooled ice for transportation to the lab where they were ground for collection of 1 g amounts. Content samples (up to 5 mL) were stored on ice for transportation to the lab where jejunal, ileal, cecal, and rectal contents were aliquoted into 1 g amounts. All samples were stored at −80°C until processing.
Salmonella Challenge
Because Salmonella is a common pathogens in neonatal pigs and has enhanced growth and virulence in response to catecholamines and alters genetic gene expression (Freestone, 2013; Bearson, 2016), we tested the ability of the stress hormones to reduce or exacerbate Salmonella attachment and translocation to MLN after weaning. At 0700 h on day 2 in replication 2, 5 pigs per treatment were moved from the nursery to the USDA BSL2 laboratory, where they were housed individually. Piglets were tested for fecal presumptive Salmonella (using plating on Rambach agar) on each of 2 separate days prior to delivery of Salmonella enterica serovar Typhimurium (ATCC strain χ4232, Walsh et al., 2012; Eicher et al., 2017). One milliliter containing 4 × 108 CFU/mL was delivered to each pig intranasally at immediately upon arrival. At 24-h post-infection (PI), necropsies were performed and ileal tissue, MLN, ileal content, cecal content, and rectal content were collected. Ileal tissue (1 g) was collected from the ileocecal junction. Ileal, cecal, and rectal contents were collected in 1 g amounts. Samples were transported and stored as above.
Microbial Community Profiles
Content samples (1 g) were serial diluted with buffered peptone water (BPW) (Fluka Analytical; St. Louis, MO). Tissue samples were diluted with 9 mL of BPW and homogenized using a mallet and stomacher (Seward; Bohemia, NY) machine. After homogenization, 3 mL of each sample were used for serial dilutions. Jejunal, ileal, and MLN tissue samples were diluted to 10−3. Jejunal and ileal content were diluted to 10−3 and cecum and rectal content were diluted to 10−6.
Tissue samples were tested for total coliforms and Escherichia coli. Content samples were tested for total coliforms, aerobes, anaerobes, E. coli, lactic acid bacteria (LAB), and Enterococcus. To determine total coliforms and aerobes, 1 mL of samples were plated on 3M Petrifilm plates. To enumerate anaerobes, 10 µL of samples were plated on brain heart infusion agar in 12 well plates; E. coli, 20 µL of samples were plated on EMB agar in 6 well plates; LAB, 10 µL of samples were plated on MRS agar in 12 well plates; Enterococcus, 20 µL of samples were plated on m-enterococcus agar in 6 well plates. Plates testing for anaerobes and LAB were incubated for 18 to 24 h anaerobically at 37°C. Plates testing for total coliforms, aerobes, E. coli, and enterococcus were incubated for 18 to 24 h aerobically at 37°C. After incubation, visible colonies were counted and recorded.
Stress Hormones and Body Temperature
Stress hormones.
Plasma samples were tested for CORT concentrations using radioimmunoassay (RIA) kits obtained from IBL International (Morrisville, NC). The RIA kit instructions were followed. Plasma samples were tested for NE concentration using ELISA kits from Rocky Mountain Diagnostics (Colorado Springs, CO). The ELISA kit instructions were followed.
Thermography recording and analysis.
Thermal images were used to record body temperature of all experimental piglets 5 d a week, at 1700 h, for the duration of the study. In accordance with Schmidt et al. (2013), the thermal images captured the back of the ear of the piglets (Fig. 1). Thermal images were captured using a FLIR (Wilsonville, OR) thermography camera and analyzed using the FLIR Tools software.
Figure 1.
Thermograph of the area behind the ear (+) that was used to quantify temperature of pigs given carrier only, cortisol, or norepinephrine at 0800 and 1600 h on day 0.
Statistical Methods
All measures were log-transformed and analyzed using mixed models (SAS 9.4) with fixed effects of treatment and day and their interactions and either compound symmetry or unstructured covariance structures as determined by Bayesian information criterion values. Generalized linear model was used when convergence could not be obtained.
Thermography body temperatures were analyzed using mixed models with fixed effects of treatment and day and compound symmetry. Microbial community profiles were log-transformed and analyzed using mixed models with fixed effects of treatment and day and either compound symmetry or unstructured covariance structures. Salmonella populations were log-transformed and analyzed using mixed models and compound symmetry covariance structures or generalized linear model. Plasma NE and CORT concentrations were log-transformed and analyzed using mixed models with fixed effects of treatment and day and compound symmetry. Results were considered significant at P value equal to or less than 0.05. Results were considered a trend when P value was less than or equal to 0.1 and greater than or equal to 0.06.
RESULTS
Stress Hormones
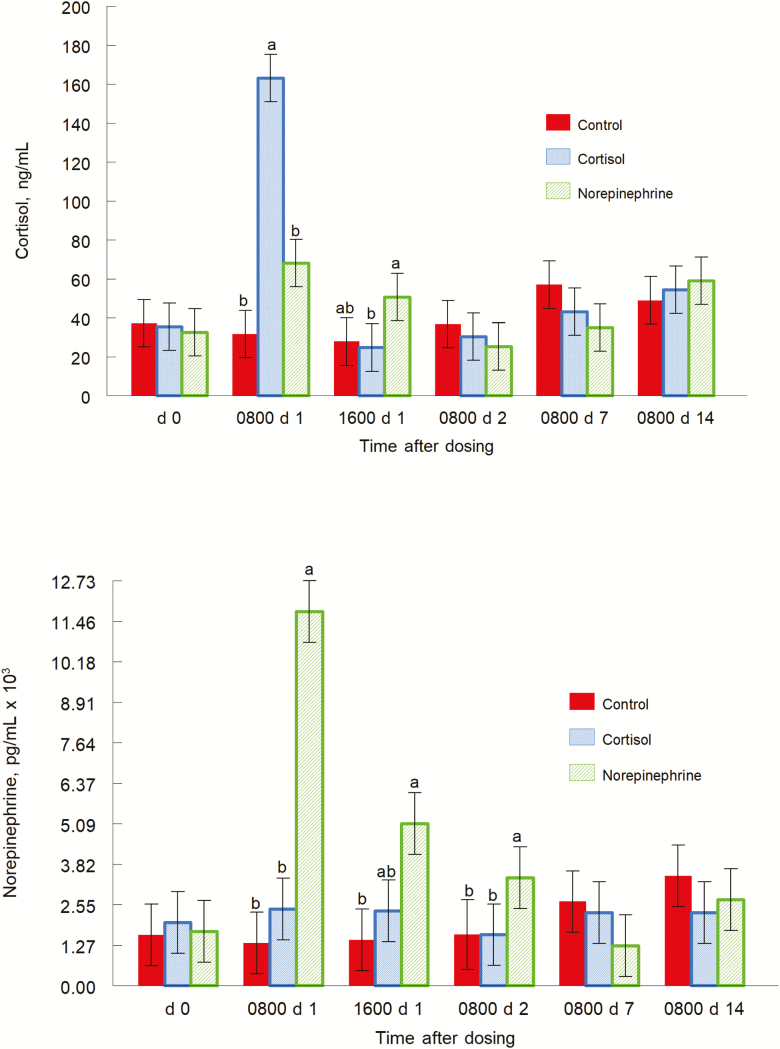
Plasma CORT concentrations had treatment by day interaction (P = 0.0001; Fig. 2 top panel). On day 0, there were no differences among treatments. Mean plasma CORT concentrations were 37.2, 35.4, and 32.6 ng/mL for control, CORT, and NE piglets, respectively. On day 1 at 0800 h, CORT-treated piglets had greater (P = 0.0001) plasma concentrations of CORT than control piglets, and greater (P = 0.0003) plasma concentrations of CORT than NE-treated piglets. Norepinephrine-treated piglets also tended to have greater (P = 0.1) plasma concentrations of CORT than control piglets. Mean plasma CORT concentrations were 31.7, 163.1, and 68.1 ng/mL for control, CORT, and NE piglets, respectively. On day 1 at 1600 h, NE-treated piglets had greater (P = 0.043) concentrations of CORT than CORT-treated piglets and tended to have greater (P = 0.1) concentrations than control piglets. Mean plasma CORT concentrations were 27.8, 24.7, and 50.6 ng/mL for control, CORT, and NE piglets, respectively. On day 2, control piglets tended to have greater concentrations of plasma CORT (P = 0.1) than NE-treated piglets. Mean plasma CORT concentrations were 36.8, 30.3, and 25.3 ng/mL for control, CORT, and NE piglets, respectively. On day 7, concentrations of plasma CORT tended to be greater for control piglets (P = 0.1) than NE-treated piglets. Mean plasma CORT concentrations were 57.0, 43.0, and 35.1 ng/mL for control, CORT, and NE piglets, respectively. On day 14, there were no differences among treatments. Mean plasma CORT concentrations were 49.7, 54.4, and 59.1 ng/mL for control, CORT, and NE piglets, respectively.
Figure 2.
Cortisol (top panel; treatment by day interaction, P = 0.0001) and norepinephrine (bottom panel, treatment by day interaction, P = 0.0001) concentrations of pigs given carrier only, cortisol, or norepinephrine at 0800 and 1600 h on day 0. Data are mean concentrations ± SE on day 0, at 0800 and 1600 h on day 1, and at 0800 h on days 2, 7, and 14. a,bP ≤ 0.05.
Plasma NE concentrations had a treatment by day interaction (P = 0.0001; Fig. 2, bottom panel). On day 0, there were no differences among treatments. Mean plasma NE concentrations were 1598.6, 1979.9, and 1718.7 pg/mL for control, CORT, and NE piglets, respectively. On day 1 at 0800 h, plasma NE concentrations were greater in NE-treated piglets than control (P = 0.0001) and CORT-treated piglets (P = 0.0001). Mean plasma NE concentrations were 1338.7, 2411.0, and 11,755.0 pg/mL for control, CORT, and NE piglets, respectively. On day 1 at 1600 h, NE-treated piglets had greater (P = 0.004) plasma NE concentrations than control piglets, and tended to have greater concentrations than CORT piglets (P = 0.1). Mean plasma NE concentrations were 1444.0, 2350.7, and 5129.5 pg/mL for control, CORT, and NE piglets, respectively. On day 2, plasma NE concentrations were greater for NE-treated piglets than for CORT-treated piglets (P = 0.05). Mean plasma NE concentrations were 1610.2, 1629.3, and 3393.1 pg/mL for control, CORT, and NE piglets, respectively. On day 7, control piglets tended to have greater plasma NE concentrations (P = 0.1) than NE-treated piglets. Mean plasma NE concentrations were 2637.8, 2280.3, and 1264.4 pg/mL for control, CORT, and NE piglets, respectively. On day 14, there were no differences in plasma NE concentrations among treatments. Mean plasma NE concentrations were 3449.0, 2293.0, and 2684.9 pg/mL for control, CORT, and NE piglets, respectively.
Body Temperature
Main effects for treatment (P = 0.006) and day (P = 0.001) were found for thermography (data not shown). No treatment by day interactions was found. Temperatures of CORT-treated piglets were greater (P = 0.002) than the temperatures of NE-treated piglets; 38.1°C and 37.5°C for CORT and NE piglets, respectively. Cortisol-treated piglets had greater temperatures (P = 0.03) than control piglets; 38.1°C and 37.7°C for CORT and control piglets, respectively. Temperatures of NE-treated piglets were not different (P = 0.24) than temperatures of control piglets; 37.5°C and 37.7°C for NE and control piglets, respectively.
Intestinal Bacterial Populations
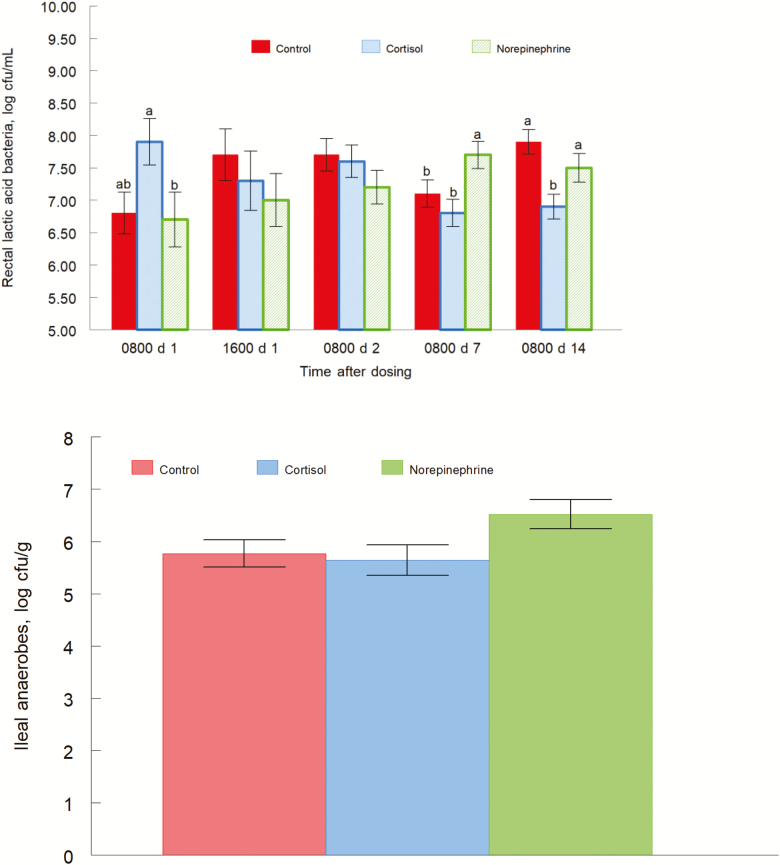
Lactic acid bacteria population from rectal content had a treatment by day interaction (P = 0.0001; Fig. 3, top panel). On day 1 at 0800 h, CORT-treated piglets had greater concentrations of LAB (P = 0.03) than NE-treated piglets, and tended to have greater concentrations of LAB (P = 0.09) than control piglets. Mean LAB concentrations were 7.7, 6.5, and 6.8 log CFU/g for CORT, NE, and control piglets, respectively. On day 1 at 1600 h and day 2, there were no differences among treatments. On day 7, NE-treated piglets exhibited greater LAB concentrations (P = 0.02) than CORT piglets, and greater concentrations (P = 0.02) than control piglets. Mean LAB concentrations were 6.9, 7.7, and 7.1 log CFU/g for CORT, NE, and control piglets, respectively. On day 14, the LAB concentrations of CORT piglets were lower (P = 0.003) than control piglets, and less (P = 0.02) than NE piglets. Mean LAB concentrations were 6.8, 7.5, and 7.8 log CFU/g for CORT, NE, and control piglets, respectively. LAB populations in jejunal content, ileal content, and cecal content were not different by treatment (P = 0.81, P = 0.49, and P = 0.99, respectively).
Figure 3.
Lactic acid bacteria concentration of rectal content (top panel, treatment by day interaction, P = 0.0001) and anaerobe concentration of ileal content (bottom panel, treatment effect P = 0.09) for pigs orally given the carrier only, cortisol, or norepinephrine at 0800 and 1600 h on day 0. Data are means ± SE at 0800 and 1600 h on day 1, and at 0800 h on days 2, 7, and 14. a,bP ≤ 0.05.
Anaerobe populations tended to have a treatment effect (P = 0.09; Fig. 3, bottom panel) for ileal content. Concentrations of anaerobes were greater (P = 0.05) in NE-treated piglets than CORT-treated piglets, and tended to be greater (P = 0.07) than control piglets. Mean anaerobe concentrations were 5.6, 6.5, and 5.8 log CFU/g for CORT, NE, and control piglets, respectively. Jejunal content was not different among treatments (P = 0.74). Similarly, cecum content was not different by treatment (P = 0.44). Additionally, rectal content was not different by treatment (P = 0.85).
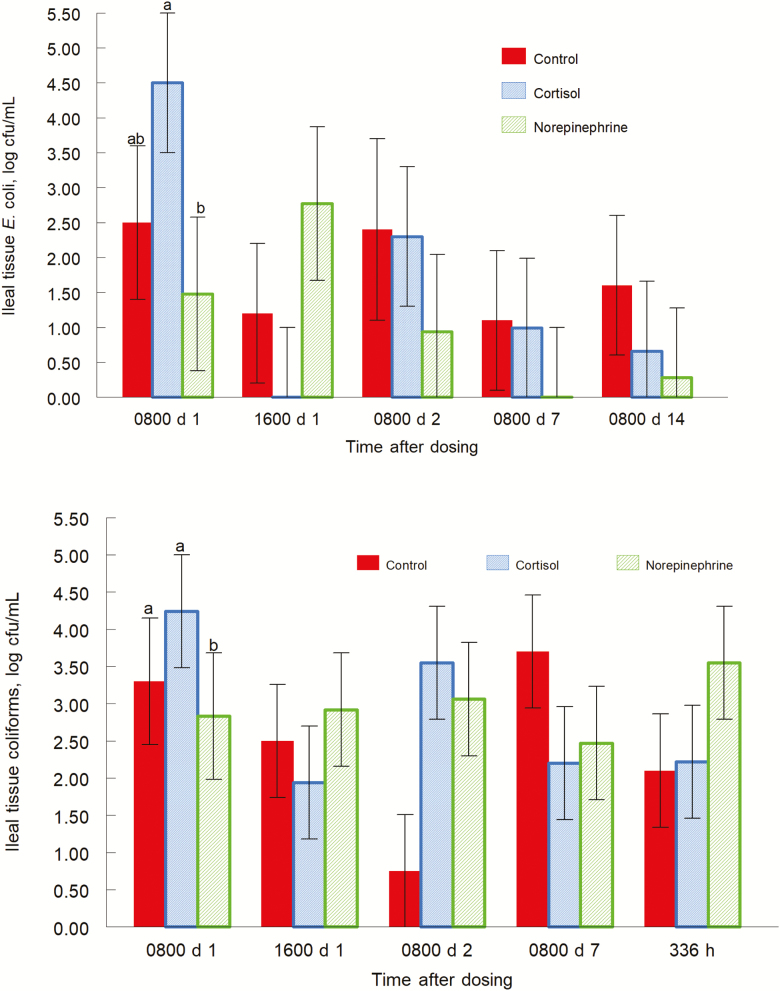
Escherichia coli populations had a treatment effect (P = 0.03; data not shown) for cecal content. Concentrations of E. coli were greater (P = 0.04) in CORT-treated piglets than control piglets. Norepinephrine-treated piglets also had higher concentrations of E. coli (P = 0.0126) than control piglets. Mean E. coli concentrations were 4.5, 4.8, and 3.3 log CFU/g for CORT, NE, and control piglets, respectively. Escherichia coli populations also tended (P = 0.1; Fig. 4) to have a treatment by day effect for ileal tissue. On day 1 at 0800 h, CORT-treated piglets had greater (P = 0.05) concentrations of E. coli than NE-treated piglets. Mean E. coli concentrations were 4.5, 1.5, and 2.5 log CFU/g for CORT, NE, and control piglets, respectively. On day 1 at 1600 h, NE-treated piglets tended (P = 0.08) to have greater concentrations of E. coli than CORT-treated piglets. Mean E. coli concentrations were 0, 2.8, and 1.2 log CFU/g for CORT, NE, and control piglets, respectively. There were no treatment by day interactions for days 2, 7, or 14. Escherichia coli populations in jejunal content, ileal content, rectal content, jejunal tissue, ileal tissue, and MLN were not different by treatment (P = 0.59, P = 0.50, P = 0.49, P = 0.63, P = 0.98, P = 0.77, respectively).
Figure 4.
Escherichia coli in ileal tissue (top panel, treatment by day interaction, P = 0.10) and coliforms in ileal tissue (bottom panel, treatment by day interaction, P = 0.07) for pigs orally give the carrier only, cortisol, or norepinephrine at 0800 and 1600 h on day 0. Data are means ± SE at 0800 and 1600 h on day 1, and at 0800 h on days 2, 7, and 14. a,bP ≤ 0.05.
Populations of coliforms tended to have a treatment by day interaction (P = 0.07; Fig. 4, bottom panel) for ileal tissues. On day 1 at 0800 and 1600 h, there were no treatment effects. On day 2, the control piglets had fewer coliforms than CORT-treated piglets (P = 0.01) and NE piglets (P = 0.04). Mean coliform concentrations were 3.5, 3.1, and 0.7 log CFU/g for CORT, NE, and control piglets, respectively. There were no treatment effects on days 7 and 14. There were no treatment effects for coliform populations in the jejunum content (P = 0.55), ileal content (P = 0.98), cecal content (P = 0.77), rectal content (P = 0.83), jejunal tissues (P = 0.98), MLN (P = 0.26).
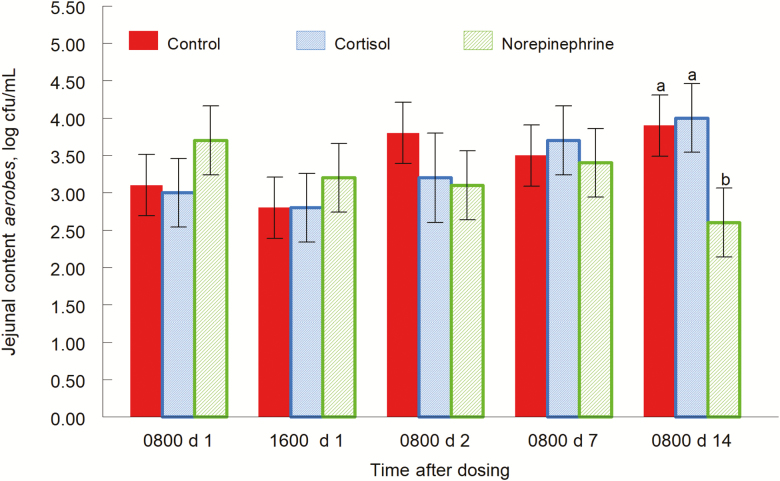
Populations of aerobes had a treatment by day interaction (P = 0.03; Fig. 5) for jejunal content. There were no significant treatment effects on day 1 at 0800 and 1600 h or day 1. On day 2, concentrations of aerobes tended to be greater in control piglets (P = 0.1) than NE-treated piglets. Mean aerobe concentrations were 3.2, 3.1, and 3.8 log CFU/g for CORT, NE, and control piglets, respectively. There were no treatment effects on day 7. On day 14, CORT-treated piglets had greater concentrations of aerobes (P = 0.006) than NE-treated piglets. Also, concentrations of aerobes in control piglets were greater (P = 0.001; data not shown) than concentrations in NE-treated piglets. Mean aerobe concentrations were 4.0, 2.6, and 3.9 log CFU/g for CORT, NE, and control piglets, respectively. Ileal content, cecum content, and rectal content were not different by treatment (P = 0.49, P = 0.20, and P = 0.16, respectively).
Figure 5.
Aerobe concentrations of jejunal content (treatment by day interaction, P = 0.03) for pigs orally give the carrier only, cortisol, or norepinephrine at 0800 and 1600 h on day 0. Data are means ± SE at 0800 and 1600 h on day 1, and at 0800 h on days 2, 7, and 14. a,bP ≤ 0.05.
There were no treatment effects for Enterococcus populations (data not shown) exhibited no significant treatment interactions in the jejunum (P = 0.58), ileum (P = 0.43), cecum (P = 0.77), or rectum (P = 0.59).
Salmonella Enumeration
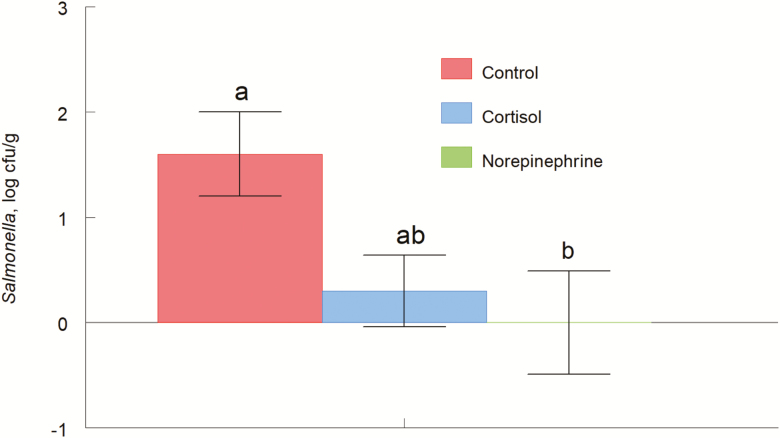
Salmonella enterica serovar Typhimurium tended to have main effects of treatment (P = 0.09; Fig. 6) for the rectal contents. Concentrations of Salmonella were greater (P = 0.05) for control piglets than NE-treated piglets, and tended to be greater (P = 0.07) than CORT-treated piglets. Mean Salmonella concentrations were 0.3, 0, and 1.6 log CFU/g for CORT, NE, and control piglets, respectively. Salmonella populations exhibited no treatment main effects in the cecal contents (P = 0.70), ileal tissue (P = 0.18), MLN (P = 0.27), or ileal contents (P = 0.24).
Figure 6.
Salmonella enterica serovar Typhimurium (ATCC; 4 × 108 CFU/mL) in the rectal content of pigs (treatment effect P = 0.09) orally given the carrier only, cortisol, or norepinephrine at 0800 and 1600 h on day 0 and given an internasal Salmonella enterica serovar Typhimurium challenge. Data are mean CFU ± SE at 24 h after inoculation. a,bP ≤ 0.05.
DISCUSSION
In this study, the administration of CORT resulted in a spike in blood CORT concentrations and a shift in intestinal environment to favor aerobes and pathogens. The administration of NE resulted in a lasting spike in NE concentrations and a shift in intestinal environment to favor anaerobes and facultative anaerobes. This suggests increased NE takes longer to return to homeostasis and decreased blood flow to the intestines.
The NE and CORT piglets each showed an increase in the blood CORT levels for their respective treatments, whereas the control piglets did not. The CORT-treated piglets had CORT concentrations that returned to baseline within 24 h, likely due to CORT’s feedback inhibition. When the adrenal cortex releases CORT, CORT interacts with the brain to inhibit the release of corticotropin-releasing factor (CRF) and adrenocorticotropic hormone (ACTH) (Sapolsky et al., 1986; Ljung et al., 1996; McEwen et al., 2015). Both CRF and ACTH are critical components in the stress response, and inhibition of these components by a spike in CORT concentrations will result in decreased CORT release. Norepinephrine piglets also exhibited a spike in CORT, which can be explained by the connection between the HPA axis and locus-ceruleus-norepinephrine (LCN) autonomic systems. The LCN is connected to the parvocellular corticotropin-releasing hormone neurons, which plays a critical role in the HPA axis. Through these connections, NE can stimulate release of CRF, which would increase CORT concentrations (Tsigos and Chrousos, 1994; Tsigos and Chrousos, 2002). Norepinephrine-treated piglets experienced a spike in NE that lasted for 24 h, longer than the spike in CORT concentrations. This may be due to the dosage of NE being greater than CORT, or because it might take more time for the body to regain homeostasis after a spike in NE. Deficits in CORT and NE, seen days after treatment, are most likely due to overcorrection as the body attempts to return to homeostasis (Saxbe, 2008).
The decrease in the generally beneficial LAB 2 wk after treatment and the increase in the opportunistic pathogenic species E. coli, may point to an opportunistic infection in CORT-treated piglets. Toruner et al. (2008) showed that IBD patients treated with corticosteroids were at a greater risk of developing opportunistic infections. Additionally, CORT and glucocorticoids have a history of suppressing the immune system (Kaattari and Tripp, 1987; de Bosscher et al., 2000). Thus, due to CORT’s structural similarities to prescribed corticosteroids and history of infection, it can be concluded that CORT acted as an immunosuppressant that left the piglets open to opportunistic infections, which in turn decreased beneficial bacteria and increased opportunistic pathogens. In contrast to that, it can be argued that a short and mild stressor may lead to increased immune responses (Dhabhar, 2009). There are many factors that determine the effects of glucocorticoids and catecholamines on immunity, including whether it is an acute or chronic stress, the microenvironment of the immune response, concentration and source (endogenous or synthetic), and timing of the stressor within an immune response. But, the effect of glucocorticoids or catecholamines on the microbiota may also modulate the immune response indirectly (Freestone, 2013).
Overall, it can be generalized that pathogenic bacteria are mostly aerobic and beneficial bacteria are mostly anaerobic. As well as an increase in E. coli populations, CORT-treated piglets exhibited increases in aerobes. Although the increased E. coli and aerobes occurred in different sections of the intestines, the overall intestinal environment of CORT piglets appears to favor aerobes. Again, this may be due to opportunistic infections brought on by the immunosuppressant function of CORT. Norepinephrine-treated piglets exhibited an increase in anaerobes, and LAB 1 wk post-treatment. The overall intestinal environment of NE piglets appears to favor anaerobes and facultative anaerobes. However, because the LAB populations nearly match the anaerobe populations, the differences in anaerobes may be due to changes in LAB populations. Due to a lack of literature on anaerobes and NE, the mechanism that would cause these observed changes is unknown. Although, NE is known to have direct effects on microbial populations (Lyte et al., 2011) and gene expression of the microbial populations (Bearson, 2016). Additionally, it increases internalization of Salmonella and E. coli O157:H7, but not commensal E. coli into jejunal Peyer’s Patch mucosa (Green et al., 2003), demonstrating some differential effects within bacterial species. In contrast, ex-vivo treatment with NE of jejunal tissue, did not alter Salmonella recovery (Brown and Price, 2008).
In our initial work with this Salmonella (Rostagno et al., 2011), we saw differences beginning at 24 h after infection in rectal contents and less than 1 log increase after that. During a challenge with S. Typhimurium, NE treatment resulted in decreased Salmonella populations in piglet rectal contents. However, we saw treatment main effects of treatments in the proximal intestinal regions (data not shown). This may be explained by NE’s role in vasoconstriction. During stress, NE causes vasoconstriction to the intestines, slowing digestion, and thus slowing the movement of intestinal contents. The differences in S. Typhimurium in NE-treated piglets were detected only in the rectal contents. Thus, it might be concluded that the slow-moving contents of the NE-treated piglets resulted in S. Typhimurium populations delayed arrival to the rectum. However, when NE was delivered orally to pigs already infected with Salmonella Typhimurium, an increase in shedding occurred, but preculture of the bacteria with NE did not affect the infection (Pullinger et al., 2010). Thus, it appears that the sequence of exposure to NE and the targeted cells (bacterial or tissue) alters the dynamics of a Salmonella infection.
CONCLUSION
The oral administration of NE and CORT had the desired effect of increasing concentrations of the stress hormones in the body. Administration of treatments also resulted in microbiome shifts throughout the intestines. High E. coli populations and low LAB populations indicate that the oral administration of CORT may have resulted in opportunistic infections. Piglets treated with NE exhibited an increase in anaerobes and LAB. In this, work stress hormones altered microbial communities of the intestine.
Footnotes
Mention of trade names or commercial products in this publication is solely for providing specific information and does not imply recommendation or endorsement by the U.S. Department of Agriculture. USDA is an equal opportunity provider and employer.
LITERATURE CITED
- Bailey M. T. and Coe C. L.. 1999. Maternal separation disrupts the integrity of the intestinal microflora in infant rhesus monkeys. Dev. Psychobiol. 35:146–155. doi: 10.1002/(SICI)1098-2302(199909)35:2 [DOI] [PubMed] [Google Scholar]
- Bearson B. L, 2016. In Microbial endocrinology: interkingdom signaling in Infectious Disease and Health. M. Lyte (ed.). Adv. Exp. Med. Biol. vol. 874; p. 167–182. doi: 10.1007/978-3-319-20215-0_7 [DOI] [Google Scholar]
- Brown D. R. and Price L. D.. 2008. Catecholamines and sympathomimetic drugs decrease early Salmonella Typhimurium uptake into porcine Peyer’s patches. FEMS Immunol. Med. Microbiol. 52:29–35. doi: 10.1111/j.1574-695X.2007.00348.x [DOI] [PubMed] [Google Scholar]
- De Bosscher K., Vanden Berghe W., and Haegeman G.. 2000. Mechanisms of anti-inflammatory action and of immunosuppression by glucocorticoids: negative interference of activated glucocorticoid receptor with transcription factors. J. Neuroimmunol. 109:16–22. [DOI] [PubMed] [Google Scholar]
- Dhabhar F. S. 2009. Enhancing versus suppressive effects of stress on immune function: implications for immunoprotection and immunopathology. Neuroimmunomodulation. 16:300–317. doi: 10.1159/000216188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eicher S. D., Rostagno M. H., and Lay D. C.. 2017. Feed withdrawal and transportation effects on salmonella enterica levels in market-weight pigs. J. Anim. Sci. 95:2848–2858. doi: 10.2527/jas.2017.1454 [DOI] [PubMed] [Google Scholar]
- Freestone P. 2013. Communication between bacteria and their hosts. Scientifica (Cairo). 2013:361073. doi: 10.1155/2013/361073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gareau M. G., Wine E., Rodrigues D. M., Cho J. H., Whary M. T., Philpott D. J., Macqueen G., and Sherman P. M.. 2011. Bacterial infection causes stress-induced memory dysfunction in mice. Gut. 60:307–317. doi: 10.1136/gut.2009.202515 [DOI] [PubMed] [Google Scholar]
- Goehler L. E., Lyte M., and Gaykema R. P.. 2007. Infection-induced viscerosensory signals from the gut enhance anxiety: implications for psychoneuroimmunology. Brain. Behav. Immun. 21:721–726. doi: 10.1016/j.bbi.2007.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green B. T., Lyte M., Kulkarni-Narla A., and Brown D. R.. 2003. Neuromodulation of enteropathogen internalization in Peyer’s patches from porcine jejunum. J. Neuroimmunol. 141:74–82. doi: 10.1016/S0165-5728(03)00225-X [DOI] [PubMed] [Google Scholar]
- Kaattari S. L., and Tripp R. A.. 1987. Cellular mechanisms of glucocorticoid mmunosuppression in salmon. J. Fish Biol. 31:129–132. doi: 10.1111/j.1095-8649.1987.tb05304.x [DOI] [Google Scholar]
- Knowles S. R., Nelson E. A., and Palombo E. A.. 2008. Investigating the role of perceived stress on bacterial flora activity and salivary cortisol secretion” a possible mechanism underlying susceptibility to illness. Biol Psychol. 77:132–137. doi: 10.1016/j.biopsycho.2007.09.010 [DOI] [PubMed] [Google Scholar]
- Kranendonk G., Hopster H., van Eerdenburg F., van Reenen K., Fillerup M., de Groot J., Korte M., and Taverne M.. 2005. Evaluation of oral administration of cortisol as a model for prenatal stress in pregnant sows. Am. J. Vet. Res. 66:780–790. [DOI] [PubMed] [Google Scholar]
- Li W., Dowd D., Scurlock B., Acosta-Martinez V., and Lyte M.. 2009. Memory and learning behavior in mice is temporally associated with diet-induced alterations in gut bacteria. Physiol. Behav. 96:557–567. doi: 10.1016/j.physbeh.2008.12.004 [DOI] [PubMed] [Google Scholar]
- Liang S., Wang T., Hu X., Luo J., Li W., Wu X., Duan Y., and Jin F.. 2015. Administration of Lactobacillus helveticus NS8 improves behavioral, cognitive, and biochemical aberrations caused by chronic restraint stress. Neuroscience. 310:561–577. doi: 10.1016/j.neuroscience.2015.09.033 [DOI] [PubMed] [Google Scholar]
- Ljung T., Andersson B., Bengtsson B. A., Björntorp P., and Mårin P.. 1996. Inhibition of cortisol secretion by dexamethasone in relation to body fat distribution: a dose-response study. Obes. Res. 4:277–282. doi: 10.1002/j.1550-8528.1996.tb00546.x [DOI] [PubMed] [Google Scholar]
- Lustri B. C., Sperandio V., and Moreira C. G.. 2017. Bacterial chat: intestinal metabolites and signals in host-microbiota-pathogen interactions. Infect. Immun. 12:1–14. doi: 10.1128/IAI.00476-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyte M. 2014. Microbial endocrinology and the microbiota-gut-brain axis. Adv. Exp. Med. Biol. 817:3–24. doi: 10.1007/978-1-4939-0897-4_1 [DOI] [PubMed] [Google Scholar]
- Lyte M., Vulchanova L., and Brown D. R.. 2011. Stress at the intestinal surface: catecholamines and mucosa-bacteria interactions. Cell Tissue Res. 343:23–32. doi: 10.1007/s00441-010-1050-0 [DOI] [PubMed] [Google Scholar]
- McEwen B. S., Bowles N. P., Gray J. D., Hill M. N., Hunter R. G., Karatsoreos I. N., and Nasca C.. 2015. Mechanisms of stress in the brain. Nat. Neurosci. 18:1353–1363. doi: 10.1038/nn.4086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittal R., Debs L. H., Patel A. P., Nguyen D., Patel K., O’Connor G., Grati M., Mittal J., Yan D., Eshraghi A., et al. . 2017. Neutrotrnasmitters: the critical modulators regulating gut-brain axis. J. Cell Physiol. 22:2359–2372. doi: 10.1002/jcp.25518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pullinger G. D., van Diemen P. M., Carnell S. C., Davies H., Lyte M., and Stevens M. P.. 2010. 6-hydroxydopamine-mediated release of norepinephrine increases faecal excretion of Salmonella enterica serovar Typhimurium in pigs. Vet. Res. 41:68. doi: 10.1051/vetres/2010040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rostagno M. H., Eicher S. D., and Lay D. C. Jr. 2011. Immunological, physiological, and behavioral effects of salmonella enterica carriage and shedding in experimentally infected finishing pigs. Foodborne Pathog. Dis. 8:623–630. doi: 10.1089/fpd.2010.0735 [DOI] [PubMed] [Google Scholar]
- Sapolsky R. M., Krey L. C., and McEwen B. S.. 1986. The neuroendocrinology of stress and aging: the glucocorticoid cascade hypothesis. Endocr. Rev. 7:284–301. doi: 10.1210/edrv-7-3-284 [DOI] [PubMed] [Google Scholar]
- Saxbe D. E. 2008. A field (researcher’s) guide to cortisol: tracking HPA axis functioning in everyday life. Health Psychol. Rev. 2:163–190. doi: 10.1080/17437190802530812 [DOI] [Google Scholar]
- Schmidt M., Lahrmann K. H., Ammon C., Berg W., Schön P., and Hoffmann G.. 2013. Assessment of body temperature in sows by two infrared thermography methods at various body surface locations. J. Swine Health Prod. 21:203–209. [Google Scholar]
- Stanton H. C. and Mueller R. L.. 1976. Sympathoadrenal neurochemistry and early weaning of swine. Am. J. Vet. Res. 37:779–783. [PubMed] [Google Scholar]
- Sudo N. 2014. Microbiome, HPA axis and production of endocrine hormones in the gut. In: Lyte N. and Cryan J. F., editors, Microbial endocrinology: The microbiota-gut-brain axis in health and disease. Adv. Exp. Med. Biol., Springer, New York, NY: P. 177–194. doi: 10.1007/978-1-4939-0897-4_8 [DOI] [PubMed] [Google Scholar]
- Tannock G. W. and Savage D. C.. 1974. Influences of dietary and environmental stress on microbial populations in the murine gastrointestinal tract. Infect. Immun. 9:591–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toruner M., Loftus E. V. Jr, Harmsen W. S., Zinsmeister A. R., Orenstein R., Sandborn W. J., Colombel J. F., and Egan L. J.. 2008. Risk factors for opportunistic infections in patients with inflammatory bowel disease. Gastroenterology. 134:929–936. doi: 10.1053/j.gastro.2008.01.012 [DOI] [PubMed] [Google Scholar]
- Tsigos C. and Chrousos G. P.. 1994. Physiology of the hypothalamic-pituitary-adrenal axis in health and dysregulation in psychiatric and autoimmune disorders. Endocrinol. Metab. Clin. North Am. 23:451–466. [PubMed] [Google Scholar]
- Tsigos C. and Chrousos G. P.. 2002. Hypothalamic-pituitary-adrenal axis, neuroendocrine factors and stress. J. Psychosom. Res. 53:865–871. [DOI] [PubMed] [Google Scholar]
- Walsh M. C., Rostagno M. H., Gardiner G. E., Sutton A. L., Richert B. T., and Radcliffe J. S.. 2012. Controlling Salmonella infection in weanling pigs through water delivery of direct-fed microbials or organic acids. Part I: effects on growth performance, microbial populations, and immune status. J. Anim. Sci. 90:261–271. doi: 10.2527/jas.2010-3598 [DOI] [PubMed] [Google Scholar]