Abstract
Dengue fever (DEN) is the most common arboviral disease in the world and dengue virus (DENV) causes 390 million annual infections around the world, of which 240 million are inapparent and 96 million are symptomatic. During the past decade a changing epidemiological pattern has been observed in Africa, with DEN outbreaks reported in all regions. In Senegal, all DENV serotypes have been reported. These important changes in the epidemiological profile of DEN are occurring in a context where there is no qualified vaccine against DEN. Further there is significant gap of knowledge on the vector bionomics and transmission dynamics in the African region to effectively prevent and control epidemics. Except for DENV-2, few studies have been performed with serotypes 1, 3, and 4, so this study was undertaken to fill out this gap. We assessed the vector competence of Aedes (Diceromyia) furcifer, Ae. (Diceromyia) taylori, Ae. (Stegomyia) luteocephalus, sylvatic and urban Ae. (Stegomyia) aegypti populations from Senegal for DENV-1, DENV-3 and DENV-4 using experimental oral infection. Whole bodies and wings/legs were tested for DENV presence by cell culture assays and saliva samples were tested by real time RT-PCR to estimate infection, disseminated infection and transmission rates. Our results revealed a low capacity of sylvatic and urban Aedes mosquitoes from Senegal to transmit DENV-1, DENV-3 and DENV-4 and an impact of infection on their mortality. The highest potential transmission rate was 20% despite the high susceptibility and disseminated infection rates up to 93.7% for the 3 Ae. aegypti populations tested, and 84.6% for the sylvatic vectors Ae. furcifer, Ae. taylori and Ae. luteocephalus.
Author summary
Dengue fever remains a major public health problem in all tropical regions of the world and causes 390 million infections each year. In Africa, while dengue outbreaks were rare during the last century, recurrent urban epidemic have been reported in all regions the last decade. Serotype 3, never reported in West Africa, caused major outbreaks in 2009 in several capital cities while serotype 2, usually confined to the forest cycle, spilled over into urban areas in Senegal and Mauritania in 2014–2015. These changes are occurring in a context where vector control remains the only effective approach to prevent and control epidemics. However, the design and the implementation of efficient vector control require an accurate knowledge of the vector bionomics and competence while such data are lacking in the African region. To fill out this gap we experimentally infected domestic and wild mosquitoes from Senegal to assess their vector competence for dengue serotypes 1, 3 and 4. Finally both domestic and wild Senegalese mosquitoes showed a low ability to transmit dengue viruses.
Introduction
Dengue fever (DEN) is the most common arboviral disease in the world and is caused by four genetically distinct serotypes of virus (DENV-1, DENV-2, DENV-3, DENV-4) belonging to the genus Flavivirus of the family Flaviviridae. Among the 390 million annual infections estimated around the world, 240 million are inapparent and only 96 million are symptomatic [1]. Dengue fever causes a wide clinical spectrum similar for the four serotypes. The different clinical manifestations of DENV infection range from asymptomatic to several symptomatic forms ranging in severity from classical dengue fever, to Dengue Hemorrhagic Fever (DHF) and Dengue Shock Syndrome (DSS). Dengue viruses are transmitted to humans by mosquitoes of the genus Aedes, mainly by the peridomestic mosquito Aedes aegypti aegypti and secondarily by Ae. albopictus and other anthropophilic Aedes mosquitoes.
In Africa, the sylvatic circulation of DENV-2 appears to be predominant [2] in contrast to Asia and South America where endemic/epidemic DENV strains circulating in peridomestic cycles are most common, and a sylvatic, nonhuman primate-amplified enzootic cycle has not been identified except for in Malaysia. The first isolations of DENV-2 from naturally infected mosquitoes in Africa date to 1969 when two strains were isolated from Ibadan and Jos in Nigeria [3]. Thereafter, several epizootics of DENV-2 were reported through the periodic amplifications of the sylvatic cycle involving wild populations of mosquitoes and monkeys in several West African countries [4]. However, despite these frequent epizooties and the presence of the epidemic vector Ae. aegypti in all bioclimatic areas, only sporadic DEN cases were recorded in West Africa. This could be explained by the presence of Aedes aegypti formosus, the ancestral African sylvatic and zoophilic form that uses tree holes as its larval habitat. Indeed, both sub-species exist in Africa but the presence of Aedes aegypti aegypti (the domestic, highly anthropophilic and primarily endophilic subspecies) in West Africa remains debatable mainly because of the lack of reliable methods to distinguish the two subspecies. The first documented outbreak caused by DENV-2 in West Africa occurred in Burkina Faso in 1982 and was suspected to be triggered by an introduction from the east of an epidemic Seychelles strain [2].
Most African DEN outbreaks caused by DENV-2 have occurred in East Africa. The others DENV serotypes (1, 3 and 4) are only known from endemic-epidemic cycles in Africa with no evidence of enzootic circulation. Only DENV-1 has been found associated with Ae. aegypti. During the last century, DENV-1 epidemics were notified in South Africa in 1926–27, Sudan in 1984, and Nigeria in 1964 and 1975 while the unique DENV-3 outbreaks occurred in Mozambique in 1985 [5,6]. Serotype 4 was only reported in Senegal in contexts which still remains enigmatic [7]. Amarasinghe et al. 2011 [6] have presented an exhaustive review on dengue situation in Africa.
Over the last 2 decades a changing epidemiological pattern has been observed in Africa, with outbreaks of DEN reported in all regions and several cases exported to Europe [8].
DENV-2, responsible for several epidemics in East Africa (Somalia, Djibouti, Kenya and Tanzania) and usually circulating in a sylvatic cycle (between Aedes mosquitoes and non human primates) in West Africa, spilled over into urban areas in 2014–2015 in Senegal and Mauritania, Gabon in 2007, Angola in 2013 and Burkina Faso in 2016. Serotype 3 (DENV-3), never reported in Africa after its first emergence in 1985 in Mozambique, caused a major urban outbreak in 2009 in Cape Verde, Cote d’Ivoire, Gabon and Senegal. Since September 2017, Burkina Faso and Senegal face up to major urban outbreaks Ouagadougou and Louga respectively (S1 Table) [9,10,11,12,13,14,15,16,17,18,19,20,21,22,23]. In Senegal all DENV serotypes have been reported (S2 Table).
These important changes summarized above in the epidemiological African profile of DEN are occurring in a context where there is no vaccine against DENV recommended for all populations. Furthermore, there is a significant gap of knowledge on DENV vector bionomics and transmission dynamics in Africa to effectively prevent and control epidemics. The vector competence of mosquitoes associated with DENV in nature is poorly characterized. Except for DENV-2 [24,25] few studies have been performed with serotypes 1, 3, and 4 [26].
Following the 2009 Dakar DENV-3 epidemic, we initiated a vector competence study to evaluate the ability of Ae. aegypti populations from Dakar and Kedougou to transmit DENV-1 and -3, for which there is no evidence of enzootic, sylvatic circulation in Africa [27]; these two serotypes appear to circulate only in an endemic/epidemic cycle with peridomestic human amplification. Our prior results showed low susceptibility to DENV-3 but high infection and dissemination rates with DENV-1. However, the oral DENV doses used were low and transmission potential was not tested. Furthermore, only Ae. aegypti was tested and vector competence data for sylvatic vectors were generated for DENV-1, -3 and -4. Thereby the present study assessed the vector competence of Senegalese Ae. aegypti, Ae. furcifer, Ae. taylori and Ae. luteocephalus for DENV-1, -3 and -4.
Materials and methods
Ethics statement
The University of Texas Medical Branch (UTMB) Institutional Animal Care and Use Committee approved all experiments involving animal-derived cells/tissues/sera/samples under protocol 02-09-068. UTMB complies with all applicable regulatory provisions of the U.S. Department of Agriculture (USDA)-Animal Welfare Act; the National Institutes of Health (NIH), Office of Laboratory Animal Welfare-Public Health Service (PHS) Policy on Humane Care and Use of Laboratory Animals; the U.S Government Principles for the Utilization and Care of Vertebrate Animals Used in Research, Teaching, and Testing developed by the Interagency Research Animal Committee (IRAC), and other federal statutes and state regulations relating to animal research. The animal care and use program at UTMB conducts reviews involving animals in accordance with the Guide for the Care and Use of Laboratory Animals (2011) published by the National Research Council.
Mosquito species
Mosquito species used in this study were collected from three Senegalese localities: Dakar, Saint Louis and Kedougou (Fig 1). The Table 1 describes the characteristics and geographic origins of Ae. aegypti, Ae. furcifer, Ae. taylori, and Ae. luteocephalus populations tested. The sylvatic Ae. aegypti population from Kedougou breeding in tree holes represented Ae. aegypti formosus morphologically characterised by the lack of pales scales on the first abdominal tergite and the urban populations from Dakar and Saint Louis breeding in artificial containers were consistent with Ae. aegypti aegypti contrariwise characterised by the presence of pales scales. These species were chosen based on their abundance, anthrophophilic behavior and association with DENV in nature. For each population, several larval habitats were sampled and immature stages were collected and reared in the laboratory. For Ae. furcifer, Ae. taylori and Ae. luteocephalus adult females were caught in a gallery forest at Kedougou and reared in the laboratory. Progeny of these populations were considered as the F1 generation that we used for experimental infections. Adult mosquitoes were maintained with a 10% sucrose solution at 27 °C, 75–80% relative humidity (RH), 12:12 h (Light:Dark) photoperiod.
Fig 1. Map showing the three localities where mosquitoes were collected in Senegal for experimental infections with dengue viruses.
This map was built using a shapefile from the free domain of the Geographic Information System (http://www.diva-gis.org) with the R software version 3.3.1 and the package maptools.
Table 1. Mosquito species tested in this study.
| Species | Source | Geographic position | Year of collection | Habitat | Gene-ration |
|---|---|---|---|---|---|
| Ae. aegypti | Dakar | 17°28’24 W 14°43’29 N |
2014 | Domestic | F1 |
| Ae. aegypti | Saint Louis | 16°29’20 W 16°01’16 N |
2014 | Domestic | F1 |
| Ae. aegypti | Kedougou | 12°11’00 W 12°33’00 N |
2014 | Sylvatic | F1 |
| Ae. furcifer | Kedougou | 12°11’00 W 12°33’00 N |
2014 | Sylvatic | F1 |
| Ae. taylori | Kedougou | 12°11’00 W 12°33’00 N |
2014 | Sylvatic | F1 |
| Ae. luteocephalus | Kedougou | 12°11’00 W 12°33’00 N |
2014 | Sylvatic | F1 |
W: west, N: north
Virus strains and preparation of stocks
Hosts origin, year of collection and passage histories of the virus strains used in this study are presented in Table 2. DENV-1, DENV-3 and DENV-4 strains obtained from the World Reference Center for Emerging Viruses and Arboviruses at the University of Texas Medical Branch in Galveston, Texas. For the Ae. furcifer experiment we used the DENV-4 strain from Haiti (Haiti 73), and DENV-3 strain from Barbados in the Caribbean region of North America (Carec 01–11828). For other mosquito species we used the following African strains: DENV-1 (SH 29177); DENV-3 strain (S-162 TvP-3622), and DENV-4 strain SH 38549.
Table 2. Dengue virus strains used for this study.
| DENV strains | Reference | Host origin | Year of collection | Location | Passage history |
|---|---|---|---|---|---|
| DENV-1 | SH 29177 | Human | 1979 | Senegal (Bandia) | C6/36-3 |
| DENV-3 | Carec 01–11828 | Human | 2001 | Barbados | C6/36-3 |
| DENV-3 | S-162 TvP-3622 | Human | 1993 | Somalia | C6/36-2 |
| DENV-4 | SH 38549 | Human | 1983 | Senegal (Dakar) | C6/36-3 |
| DENV-4 | Haiti 73 | Human | 1994 | Haiti | C6/36-3 |
An additional passage on C6/36 cells was performed for each strain to obtain the viral stock used to infect mosquitoes. Cell lines were provided by the American Type Culture Collection (Manassas, Va.), and cultured in Gibco DMEM (Dulbecco’s Modified Eagle Medium), High glucose (Gibco Cat. No. 11965–092) supplemented with 10% fetal bovine serum (FBS; Atlanta Biologicals Cat. No. S11150) heat-inactivated in 56° C water bath for 60 min, penicillin-streptomycin (Gibco Cat. No. 15140–122) and 10% of Bacto Tryptose Phosphate Broth (Becton, USA). Virus in cell culture supernatants was concentrated using Millipore UFC910024 Amicon Ultra-15 Centrifugal Filter Concentrator with Ultracel 100 Regenerated Cellulose Membrane. Concentrated viruses were collected, aliquoted and frozen at -80°C, and used as viral stocks for mosquito infection. Virus stocks were titrated using the method focus forming assays and immunostaining described below.
Focus forming assays and immunostaining
Virus titers for stocks and infectious blood meals after 1 hour of exposure to mosquitoes were determined by focus forming assays and immunostaining as described previously [28]. Briefly, Ten-fold serial dilutions of virus in MEM supplemented with 2% FBS and antibiotics (Invitrogen, Carlsbad, CA), were added in duplicate to confluent C6/36 cell monolayers attached to 24-well Costar (Corning, NY) plates, and incubated for 1 h with periodic gentle rocking to facilitate virus adsorption at 28 °C. Wells were then overlaid with 1 ml of 0.8% methylcellulose (Sigma-Aldrich, St. Louis) diluted in warm Optimem (Invitrogen) supplemented with 2% FBS, antibiotics and 1% (w/v) L-glutamine and incubated undisturbed for 4 days at 28 °C. Methylcellulose overlay was aspirated and cell monolayer rinsed once with phosphate buffered saline (PBS), pH 7.4 (Invitrogen) followed by fixation with a mixture of ice-cold acetone and methanol (1:1) solution and allowed to incubate for 30 min at room temperature (RT). Fixation solution was aspirated and plates were allowed to air dry. Plates were washed thrice with PBS supplemented with 3% FBS, followed by hour-long incubation with a dengue-specific hyperimmune mouse ascitic fluid. Mouse hyperimmune sera (MIAF) to DENV were prepared in adult mice; using 10% crude homogenates of DENV- infected newborn mouse brain in phosphate-buffered saline as the immunogen. The immunization schedule consisted of four intraperitoneal injections of antigen mixed with Freund’s adjuvant, given at weekly intervals. After the final immunization, mice were inoculated with sarcoma 180 cells, and the resulting immune ascitic fluids were collected. All animal work was done at UTMB under an IACUC approved animal use protocol (number 9505045). Plates were washed thrice followed by hour-long incubation with a secondary antibody, goat anti-mouse conjugated to horseradish peroxidase (HRP) (KPL, Gaithersburg, MD). Detection proceeded with the addition of aminoethylcarbazole (AEC) substrate (ENZO Life sciences, Farmingdale, CT) prepared according to vendor instructions.
Oral infection of mosquitoes
Three- to 5-day-old F1 female mosquitoes were placed into 500 mL cardboard containers and sucrose-starved for 48 hours before being exposed to an infectious artificial blood meal (Hemotek Ltd, UK) using BALB/c mouse skins obtained from the University of Texas Medical Branch Animal Resource Center, as membranes. The blood meal contained a 33% volume of washed sheep erythrocytes and a 33% volume of a cell culture-derived virus stock supplemented with 21% FBS, and adenosine triphosphate (ATP) to a final concentration of 0.005 M as a phagostimulant, and sucrose at a final concentration of 10%. After feeding for up to 60 minutes, the remaining blood meal was kept at– 80 °C for virus titration using plaque assay then mosquitoes were cold-anaesthetized and fully engorged specimens were incubated with 10% sucrose at 27°±1°C, a relative humidity of 70–75% and 12:12 h (Light:Dark) photoperiod.
Virus detection in mosquitoes
At 7 or 15 days post bloodmeal (dpbm), mosquitoes were cold-anaesthetized and their legs and wings were removed. The proboscis of each mosquito was then inserted into a capillary tube containing 1–2 μL of FBS for salivation for up to 30 min then expectorated saliva was collected into a tube containing 100 μL of DMEM supplemented with 5% FBS. Detection of DENV in the mosquito body but not the wings/legs indicated a non-disseminated infection (limited to the midgut), whereas the presence of virus in both the body and wings/legs indicated dissemination into the hemocoel. Mosquito bodies as well as wings/legs of infected bodies were tested for DENV after homogenization in 400 μl of MEM containing 5% of FBS, and centrifugation for 2 min at 11,500 x g at 4 °C to separate virus supernatant and debris. For each sample, 100 μl of supernatant were cultured in 24-well plates containing Vero cell monolayers and DENV was detected by focus forming assays and immunostaining described above, but without the ten-fold serial dilutions. So detection was limited to presence/absence revelation. Saliva of infected wings/legs were tested to detect DENV presence by real-time RT-PCR using an internal control of 10 no-infected mosquito saliva pooled together; 100 μl of each sample was used for RNA extraction using the QIAamp Viral RNA Extraction Kit (QIAgen, Heiden, Germany), according to the manufacturer’s protocol. Dengue virus RNAs extracted from mosquito saliva were amplified using Bio-Rad iTaq universal probes one-step kit (Cat#172–5141) following Manufacturer’s protocol. For detecting DENV-1 and DENV-3, forward primer (5’ATTAGAGAGCAGATCTCTG 3’), reverse primer (5’TGACACGCGGTTTC 3’), and Probe 5’/56-FAM/TCAATATGCTGAAACGCG/3BHQ_1/-3’ were used; for DENV-4, forward primer 5’AAT AGA GAG CAG ATC TCTG 3’ was used. The RT‐PCR was performed by Quant Studio 6 Flex instrument made from applied BioSystems by life technologies. The cycling conditions were RT step at 50.0 °C for 10 min, at 95.0 °C for 3 min, and 43 cycles of 15 s at 94.0 °C and 1 min at 55 °C.
Impact of infection on mosquito longevity
During our experiment with Ae. furcifer, we observed 5 days after oral DENV exposure a high mortality rate. Based on this observation, we planned subsequent experiments to include a negative control cohort exposed to uninfected blood meals to assess the effect of DENV on mortality. The uninfected blood meals used as the negative control contained a 33% volume of washed sheep erythrocytes and 33% volume of cell culture media (Gibco DMEM, High glucose supplemented with 10% fetal bovine serum, penicillin-streptomycin and 10% of Bacto Tryptose Phosphate Broth) supplemented with 21% FBS, and adenosine triphosphate (ATP) to a final concentration of 0.005 M as a phagostimulant, and sucrose at a final concentration of 10%. The Table 3 showed the sample size for each virus strain and for each mosquito populations. These mosquitoes were monitored twice daily for mortality until 15 dpbm for Ae. taylori, Ae. aegypti from Kedougou and St. Louis and 20 dpbm for Ae. aegypti from Dakar, then surviving mosquitoes were tested for DENV infection as described above.
Table 3. Sample size for each virus strain and for each mosquito populations.
| Mosquito species | Sample sizes | ||
|---|---|---|---|
| DENV-1 | DENV-3 | DENV-4 | |
| Ae. aegypti aegypti Dakar | 158 | 156 | 165 |
| Ae. aegypti formosus Kedougou | 90 | 93 | 148 |
| Ae. aegypti aegypti St. Louis | 129 | 165 | 128 |
| Ae. taylori | 41 | 57 | 62 |
Data analysis
Infection (number of positive bodies/total number of engorged mosquitoes incubated and tested), disseminated infection (number of mosquitoes with positive wings-legs/ total number of engorged mosquitoes incubated and tested) and transmission (number of mosquitoes with infected saliva/ total number of engorged mosquitoes incubated and tested) rates were calculated for each species and each dpbm. The rates obtained were compared using Fisher’s exact test. For Ae. aegypti populations potential impact of the virus serotype, incubation and mosquito origin were estimated using beta regression model. A Wilcoxon test was performed to compare differences between survivals among groups. For all tests, differences were considered statistically significant at p < 0.05 using R v. 2.15.1 (R Foundation for Statistical Computing, Vienna, Austria) [29].
Results
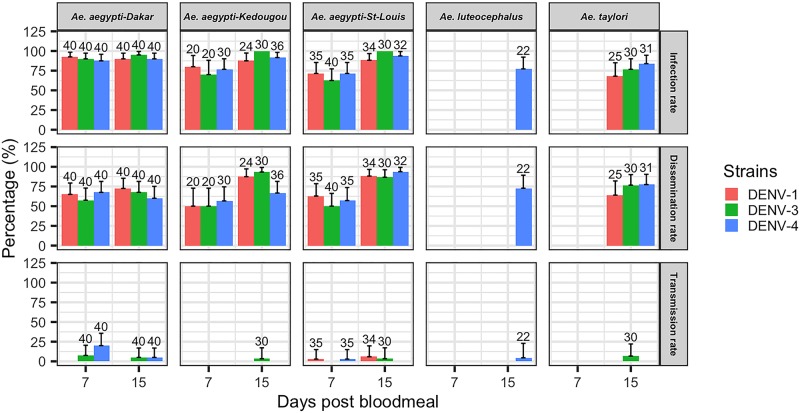
The titers of DENV stocks used ranged between 107 and 108 PFU/ml and the Table 4 presented the titers of the blood meals prepared from these stocks after 1-hour exposure at 37±1 °C for mosquitoes feeding in different days. These titers ranged between 1.2 x 106 and 4.7 x 107 PFU/ml. A total of 606 Ae. aegytpi (240 from Dakar, 206 from St. Louis and 160 from Kedougou), 86 Ae. taylori, 71 Ae. furcifer and 22 Ae. luteocephalus was tested after DENV exposure and incubation for 7 or 15 days. For Ae. aegypti, the minimum and maximum values of infection rates were 87.5–92.5% and 90–95% for the population from Dakar, 62.5–71.42% and 88.23–100% for the St. Louis population, and 70–80% and 87.5–100% for the Kedougou population, respectively, at 7 and 15 dpbm (Fig 2). Disseminated infection rates were 57.5–67.5% and 60–72.5% for the population from Dakar, 50–62.85% and 86.66–93.75% for the population from St. Louis and 50–56.66% and 66.66–93.33% for population from Kedougou respectively at 7 and 15 dpbm. While the infection and dissemination rates were high, the potential transmission (saliva infection) rates were globally low (0–20%), 0–5% for Dakar, 0–2.85% and 0–5.88% for St. Louis, and 0% and 0–3.33% for Kedougou, respectively at 7 and 15 dpbm.
Table 4. Titers of the infectious blood meal after 1 hour of exposure to the different mosquito species.
| Populations of Mosquito species | Blood meal titers (PFU/mL) | ||
|---|---|---|---|
| DENV-1 | DENV-3 | DENV-4 | |
| Ae. aegypti aegypti Dakar | 4.7 x 107 | 2.4 x 107 | 1.2 x 106 |
| Ae. aegypti formosus Kedougou | 4.9 x 106 | 3.5 x 106 | 2.6 x 107 |
| Ae. aegypti aegypti St. Louis | 4.9 x 106 | 3.5 x 106 | 2.6 x 107 |
| Ae. furcifer | NA | 3.1 x 106 | 1.6 x 106 |
| Ae. taylori | 4.9 x 106 | 3.5 x 106 | 2.6 x 107 |
| Ae. luteocephalus | NA | NA | 1.4 x 106 |
Fig 2. Infection, disseminated infection and transmission rates of four Senegalese Aedes mosquitoes orally exposed to different dengue serotypes at 7 and 15 days post bloodmeal.
Error bars represent the upper limits of the 95% confidence intervals of infection, dissemination and transmission rates.
Results showed that all species were susceptible to disseminated infection with DENV-1, -3 and -4 (Fig 2 and S1 Fig). Ae. aegytpi population from Dakar showed higher infection rates (IR) than populations from St. Louis and Kedougou for all 3 dengue serotypes at 7 dpbm. However, differences were significant only between the Dakar and St. Louis populations for DENV-1 (Fisher’s exact test: p = 0.01) and DENV-3 (Fisher’s exact test: p = 0.003). Infection rates of Ae. aegypti populations increased significantly between 7 and 15 dpbm for all 3 serotypes except for the population from Dakar. At 15 dpbm, IRs of the 3 populations did not differ significantly (Fisher’s exact test: p > 0.05). For all Ae. aegypti populations, IRs with DENV-3 were higher than those obtained with DENV-1 and DENV-4. However, the difference was statistically significant only for Ae. aegypti from Kedougou when we compare IR obtained with DENV-3 versus DENV-1 (Fisher’s exact test: p = 0.04). The minimum and maximum values of disseminated infection rates of the 3 populations of Ae. aegypti were 50–65% for DENV-1, 50–57.5% for DENV-3 and 56.66–67.5% for DENV-4 and were statistically comparable at 7 dpbm (Fisher’s exact test: p > 0.05), while at 15 dpbm Ae. aegypti from St. Louis showed significantly higher DIR than populations from Dakar (Fisher’s exact test: p = 0.001) and Kedougou (Fisher’s exact test: p = 0.005) for DENV-4. With Ae. aegypti populations from Kedougou and St. Louis the IRs and DIRs increased between 7 and 15 dpbm, however the population from Dakar were susceptible to infection and developed disseminated infection with same rates at 7 and 15 dpbm.
Among the sylvatic vectors, Ae. furcifer showed the highest IRs for DENV-3 and DENV-4 but differences were not significant (Fisher’s exact test: p > 0.05). No significant difference was observed for DENV-4 infection and dissemination among the three species despite the higher IR with Ae. furcifer and lower with Ae. luteocephalus.
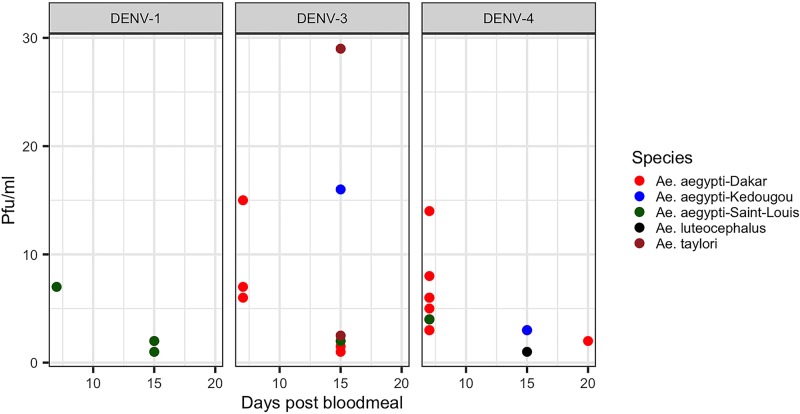
The Fig 3 shows titers of the infected saliva. Globally we observed mainly for Ae. aegypti a decreasing of titer between 7 dpbm and 15 dpbm. The highest titer (29 PFU/ml) was observed with Ae. taylori. For Ae. aegypti highest titers were 15 and 16 PFU/ml for Dakar and Kedougou mosquito strains at 7 and 15 dpbm respectively.
Fig 3. Virus titers of infected mosquito saliva at 7 and 15 dpbm (PFU/ml).
The regression model did not reveal an effect of the virus serotype on the infection rates of Ae. aegypti populations (Table 5). However, odds ratio of mosquito strain from Saint-Louis versus mosquito strains from Dakar, decreases significantly by a factor of 0.4 (p<0.001) while relative proportion of infected mosquitoes increases by a factor of 3.5 at 15 dpbm compared to 7 dpbm (p<0.001).
Table 5. Beta regression model estimating relationship between infection and virus serotype, incubation and mosquito origin.
| Infection | Dissemination | Transmission | |||||||
|---|---|---|---|---|---|---|---|---|---|
| OR | CI | P-value | OR | CI | P-value | OR | CI | P-value | |
| Geographic Origin | |||||||||
| Dakar | 1 | 1 | 1 | ||||||
| Kedougou | 0.55 | 0.29–1 | 0.06 | 1.14 | 0.7–1.8 | 0.5 | 0.65 | 0.03–4.47 | 0.7 |
| Saint-Louis | 0.41 | 0.23–0.7 | <0.001 | 1.44 | 0.96–2.2 | 0.08 | 0.27 | 0.04–1 | 0.09 |
| Days post Blood meal | |||||||||
| Day7 | 1 | 1 | 1 | ||||||
| Day15 | 3.5 | 2–6 | <0.001 | 2.59 | 1.8–3.7 | <0.001 | 0.55 | 0.19–1.4 | 0.2 |
| Virus serotypes | |||||||||
| DENV-1 | 1 | 1 | 1 | ||||||
| DENV-3 | 1.1 | 0.6–2 | 0.7 | 0.8 | 0.5–1.2 | 0.3 | 0.45 | 0.05–3.25 | 0.4 |
| DENV-4 | 1 | 0.56–1.78 | 0.9 | 0.79 | 0.5–1.2 | 0.2 | 0.80 | 0.1–5.12 | 0.8 |
OR: Odd Ratio; CI: Confidence Interval
For the dissemination rates, no effects of the virus serotype and mosquito origin were observed. The incubation period was the unique parameter affecting the dissemination with an odds ratio at 15 dpbm increasing by a factor of 2.59 compared to 7 dpbm.
No statistically significant relationship between transmission rate and mosquito origin, virus strains and dpbm.
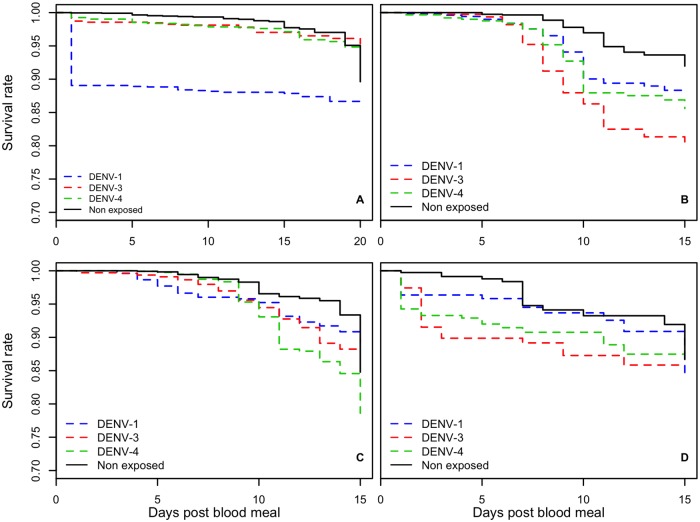
When we compared survival of Ae. aegypti mosquitoes from Dakar exposed to DENV-1, -3 and -4 with infection rates of 92.68, 93.02 and 91.66%, respectively, to that of the unexposed control group, globally the difference was statistically significant (Wilcoxon test: p <0.001) (Fig 4A). For Ae. aegypti mosquitoes from St. Louis exposed to DENV-1, -3 and -4 with infection rates of 88.23, 100 and 93.75% respectively compared to unexposed group (Fig 4B), significantly higher mortality was observed (Wilcoxon test: p = 2.57x10-12). For Ae. aegypti from Kedougou (Fig 4C) exposed to DENV-1, -3, -4 with infection rates of 87.5, 100, 91.66%, respectively, mortality was also significantly higher than that of the negative controls (Wilcoxon test: p = 0.0009). The difference was also overall significant for Ae. taylori (Wilcoxon test: p = 0.01) (Fig 4D), which showed infection rates of 68, 76.66, and 83.87% for DENV-1, DENV-3 and DENV-4 respectively.
Fig 4. Daily changes in survival after exposure of Ae. aegypti populations from Dakar (A), from St. Louis (B) and from Kedougou (C) and Aedes taylori (D) to infected blood meals with dengue viruses 1, 3 and 4 and uninfected blood used as control (Non exposed).
For Ae. aegypti from Kedougou and Ae. taylori, our analysis did not reveal any effect between DENV serotypes and the mosquito population survival rate (p = 0.344 and p = 0.378 respectively). But survivals were significantly different between DENV serotypes for Ae. aegypti Dakar (p <0.001) and Ae. aegypti Saint Louis (p = 0.002). For Ae. aegypti from Dakar the DENV-1 induced the highest mortality (Wilcoxon test: p<0.001).
Kaplan–Meier survival curves are shown that mortality was higher for Ae. aegypti from Dakar exposed to DENV versus unexposed. Also, survival of this population was more affected by DENV-1 than by DENV-4 and DENV-3.
Our results showed that DENV infection also affected the survival of Ae. aegypti from St. Louis. Survival of mosquitoes exposed to all three DENV was reduced compared to negative controls from the 6th dpbm.
Ae. aegypti from Kedougou and exposed to DENV-4 survived better until 9 dpbm, then mortality increased highly compared to controls from the 11th dpbm. Mosquitoes exposed to DENV-1 had reduced survival early compared to DENV-3 and DENV-4. From the 11th dpbm, survival of Ae. aegypti from Kedougou was significantly affected by all three DENV serotypes.
Survival of Ae. taylori exposed to DENV-4 or -3 was significantly lower than controls at all dpbm, but there was no significant difference between DENV serotypes. While mosquitoes exposed to DENV-1 showed reduced survival for the first 7 dpbm, no significant difference was observed later.
Discussion
Our study provides important information on the vector competence of both sylvatic and domestic populations of Ae. aegypti and three sylvatic species of Aedes while some aspect could be considered as minor limitations. First, due to limited number of specimens available we were not able to test Ae. furcifer with the African DENV strains after an early experiment performed with DENV-4 and DENV-3 strains respectively from Haiti and Barbados. For the same reason we also limited the experiment with Ae. furcifer, Ae. taylori and Ae. luteocephalus at 15 dpbm only. Furthermore, only the RT-PCR was used to detect DENV genomes whether infectious particles or not for 2 reasons: i) the purpose of this article is to show the competence of the vector and we have been focused on the detection of DENV in the different compartments of the mosquitoes and in the saliva. As we have shown that the virus reached the saliva, it implies that the vector is competent ii) In our experience with other viruses, (West Nile, Usutu), we have noticed that RT-PCR and infectious viral particles are generally very consistent and concordant in their conclusions and trends [30,31]. Such a trend has also been confirmed on C6/36 cells for other virus [32]. Despite the relevance of the capillary feeding method for virus transmission assessment there is no proof of salivation activity for each tested individual. However, as the use of animals has many limitations, it is currently the best alternative technique [33,34], successfully used over years with different media like defibrinated blood [35], mineral or immersion oils [36,37], foetal bovine serum [38] to measure virus transmission. One way to assess the presence of saliva could be the detection of saliva components like protein or carbohydrates. Such an approach will require, however, a larger volume of media for saliva collection to achieve the different analysis without compromising virus detection. During our experiments, no DENV-susceptible laboratory mosquito strain was used as control. In our knowledge, there is no unique mosquito strain that can serve as a single control for each of the DENV serotypes and genotypes. Beyond the current scope of the study, future experiments would take into account this aspect by integrating at least one laboratory susceptible mosquito strain for each DENV serotype.
Our results showed high infection and disseminated infection rates with DENV serotypes 1, 3 and 4, both for Ae. aegypti populations and for the sylvatic mosquito vectors Ae. furcifer, Ae. taylori and Ae. luteocephalus. IRs with Ae. aegypti population from Dakar reached their maximum values as early as 7 dpi, while for Ae. aegypti from Kedougou and St. Louis, IRs increased between 7 dpbm and 15 dpbm to reach their maximum values later. Ae. aegypti mosquitoes from Dakar develop DENV infection earlier than populations from Kedougou and St. Louis and this could be explained by highest blood meal titer for Ae. aegypti aegypti Dakar than others populations. However differences were not significant between populations from Dakar and Kedougou for DENV-1 and -3, as observed earlier [27], but IRs and DIRs were higher in the present study. This could be explained by difference of virus strains and especially by high oral virus doses (106–107 PFU/ml) compared to those used previously (103–104 MID50/ml (Mice Infectious Dose 50)) with different Ae. aegypti populations from Thailand [39]. Moreover Ae. aegypti populations from western (Burkina Faso: Koro, Bobo and Kari mosquito strains) and eastern (Kenya: Rabai and Shimba Hills mosquito strains) Africa showed lower rates despite the same viral titers (107.3–108.1 MID50/ml). Similarly in many DEN-endemic countries infection and dissemination rates obtained [40] were lower than those showed by this study.
Comparisons between serotypes show that Senegalese Ae. aegypti were more susceptibility to DENV-3 than to other serotypes. Also, DENV-3 was detected in the saliva of all three populations; this could explain recent DENV-3 outbreaks in many African countries (S1 Table). The St. Louis population showed transmission potential for all three DENVs even if TRs were low, suggesting a salivary gland barrier within some Ae. aegypti populations. However, the population from Dakar showed higher TRs than other populations and at 7 dpbm these rates for DENV-4 reached 20% of total engorged mosquitoes and 7.5% for DENV-3. These transmission rates observed just a week after mosquito infection may explain the Dakar DENV-3 epidemic like the Cape Verde Ae. aegypti population transmitting during the 2009 outbreak while it showed infection rates of 0% at 7 dpbm and until 10 dpbm the transmission rates did not exceed 20% of only mosquitoes which disseminated the infection [41]. The potential transmission rates obtained with DENV-4 show that even if a large outbreak has not been reported, the risk of a DENV-4 epidemic is present in Dakar and St. Louis because, as noted before, even with low transmission rates a vector can cause epidemics based on its abundance, density, survival and human feeding frequency [42].
Regarding enzootic, sylvatic vectors, infection and dissemination rates were relatively high for the different serotypes tested (Fig 2 and S1 Fig). The same population of Ae. furcifer, with different DENV-2 strains, showed similar IRs (26 to 97%) and DIRs (17 to 75%) to the rates we obtained [24]. But in our study we did not detect transmission potential by Ae. furcifer. The decreasing of viral titers in mosquito saliva observed at least in Aedes aegypti between 7 and 15 dpbm may explain the low transmission rates obtained.
Vectorial capacity is the efficiency of a vector in the transmission of a pathogen due to the combined effects of many factors, both intrinsic and extrinsic. The mortality rate is an important component [43,44,45]. Even if vectors become infected after taking an infectious blood meal, if they fail to survive to bite another host, the potential for transmission by this population is low. As a result, changes in the mosquito mortality rate would directly affect transmission of the pathogen. In our study we found that the exposure of Ae. aegypti from Dakar, Kedougou and St. Louis to the 3 DENV serotypes significantly increases mortality compared to negative control cohorts exposed to uninfected blood meals. Adverse effects on the fitness of Ae. aegypti due to DENV infection were also reported previously [46]. We also found increased mortality of infected Ae. taylori mosquitoes. Several other studies showed effects of other arboviruses on the survival of mosquito vectors [47,48].
Our results showed that for all 3 populations of Ae. aegypti, DENV-1 exposure affects mosquito survival. However, for the Ae. taylori population, after 7 dpbm we no longer detected an effect on survival of mosquitoes with DENV-1 infection. This absence of effect of DENV-1 infection on Ae. taylori is surprising because this species is not normally adapted to this DENV serotype, which is not known to circulate in the forest galleries frequented by Ae. taylori in Africa [49]. Indeed, only DENV-2 has been shown to circulate regularly in a sylvatic cycle in southeastern Senegal in the Kedougou region [7,50]. In summary, our results indicate that DENV-4 exposure did not affect survival of Ae. aegypti from Kedougou before 9 dpbm but it affected early the survival of the Ae. aegypti populations from Dakar and Saint Louis and Ae. taylori. DENV-3 caused high mortality in all mosquito populations tested, mainly in Ae. taylori and Ae. aegypti from Dakar. Survival was been most affected by DENV-1 which showed the highest potential transmission rates.
Supporting information
(DOC)
(DOC)
Error bars represent the upper limits of the 95% confidence intervals of infection and dissemination rates.
(TIF)
Acknowledgments
We thank Jing for her technical assistance during mosquito rearing.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was funded by National Institute of Health grants R24AI120942 and R01AI121452 and the Institut Pasteur de Dakar. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References:
- 1.Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, et al. (2013) The global distribution and burden of dengue. Nature 496: 504–507. 10.1038/nature12060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gonzalez J, Du Saussay C, Gautun J, McCormick J, Mouchet J (1984) [Dengue in Burkina Faso (ex-Upper Volta): seasonal epidemics in the urban area of Ouagadougou]. Bull Soc Path Exo Fil 78: 7–14. [PubMed] [Google Scholar]
- 3.Moore Dá, Causey O, Carey D, Reddy S, Cooke A, et al. (1975) Arthropod-borne viral infections of man in Nigeria, 1964–1970. Ann Trop Med Parasitol 69: 49–64. [DOI] [PubMed] [Google Scholar]
- 4.Diallo M, Ba Y, Sall AA, Diop OM, Ndione JA, et al. (2003) Amplification of the sylvatic cycle of dengue virus type 2, Senegal, 1999–2000: entomologic findings and epidemiologic considerations. Emerg Infect Dis 9: 362 10.3201/eid0903.020219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gubler D, Sather G, Kuno G, Cabral J (1986) Dengue 3 virus transmission in Africa. The Am J Trop Med Hyg 35: 1280–1284. [DOI] [PubMed] [Google Scholar]
- 6.Amarasinghe A, Kuritsk JN, Letson GW, Margolis HS (2011) Dengue virus infection in Africa. Emerg Infect Dis 17: 1349–1354. 10.3201/eid1708.101515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saluzzo J, Cornet M, Castagnet P, Rey C, Digoutte J (1986) Isolation of dengue 2 and dengue 4 viruses from patients in Senegal. Trans R Soc Trop Med Hyg 80: 5–5. [DOI] [PubMed] [Google Scholar]
- 8.Were F (2013) The dengue situation in Africa. Paediatrics and international child health. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Massangaie M, Pinto G, Padama F, Chambe G, da Silva M, et al. (2016) Clinical and Epidemiological Characterization of the First Recognized Outbreak of Dengue Virus-Type 2 in Mozambique, 2014. Am J Trop Med Hyg 94: 413–416. 10.4269/ajtmh.15-0543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chipwaza B, Mugasa JP, Selemani M, Amuri M, Mosha F, et al. (2014) Dengue and Chikungunya fever among viral diseases in outpatient febrile children in Kilosa district hospital, Tanzania. PLoS Negl Trop Dis 8: e3335 10.1371/journal.pntd.0003335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vairo F, Mboera LE, De Nardo P, Oriyo NM, Meschi S, et al. (2016) Clinical, virologic, and epidemiologic characteristics of dengue outbreak, Dar es Salaam, Tanzania, 2014. Emerg Infect Dis 22: 895 10.3201/eid2205.151462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ellis EM, Neatherlin JC, Delorey M, Ochieng M, Mohamed AH, et al. (2015) A household serosurvey to estimate the magnitude of a dengue outbreak in Mombasa, Kenya, 2013. PLoS Negl Trop Dis 9: e0003733 10.1371/journal.pntd.0003733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Woyessa AB, Mengesha M, Kassa W, Kifle E, Wondabeku M, et al. (2016) The first acute febrile illness investigation associated with dengue fever in Ethiopia, 2013: A descriptive analysis. Ethiop J Health Development (EJHD) 28. [Google Scholar]
- 14.Malik A, Earhart K, Mohareb E, Saad M, Saeed M, et al. (2011) Dengue hemorrhagic fever outbreak in children in Port Sudan. J Infect Pub Health 4: 1–6. [DOI] [PubMed] [Google Scholar]
- 15.Seidahmed O, Siam H, Soghaier M, Abubakr M, Osman H, et al. (2012) Dengue vector control and surveillance during a major outbreak in a coastal Red Sea area in Sudan. [PubMed] [Google Scholar]
- 16.Kyobe Bosa H, Montgomery JM, Kimuli I, Lutwama J (2014) Dengue fever outbreak in Mogadishu, Somalia 2011: Co-circulation of three dengue virus serotypes. 3 p. [Google Scholar]
- 17.Sessions OM, Khan K, Hou Ya, Meltzer E, Quam M, et al. (2013) Exploring the origin and potential for spread of the 2013 dengue outbreak in Luanda, Angola. Glob Health Act 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leroy EM, Nkoghe D, Ollomo B, Nze-Nkogue C, Becquart P, et al. (2009) Concurrent chikungunya and dengue virus infections during simultaneous outbreaks, Gabon, 2007. Emerg Infect Dis 15: 591 10.3201/eid1504.080664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Caron M, Grard G, Paupy C, Mombo IM, Nso BBB, et al. (2013) First evidence of simultaneous circulation of three different dengue virus serotypes in Africa. PLoS One 8: e78030 10.1371/journal.pone.0078030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ridde V, Agier I, Bonnet E, Carabali M, Dabiré KR, et al. (2016) Presence of three dengue serotypes in Ouagadougou (Burkina Faso): research and public health implications. Infect Dis Poverty 5: 23 10.1186/s40249-016-0120-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tarnagda Z, Cissé A, Bicaba BW, Diagbouga S, Sagna T, et al. (2018) Dengue Fever in Burkina Faso, 2016. Emerg Infect Dis 24: 170 10.3201/eid2401.170973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Faye O, Ba Y, Faye O, Talla C, Diallo D, et al. (2014) Urban epidemic of dengue virus serotype 3 infection, Senegal, 2009. Emerg Infect Dis 20: 456 10.3201/eid2003.121885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Organization WH (2009) Dengue in Africa: emergence of DENV-3, Côte d’Ivoire, 2008. Wkly Epidemiol Rec 84: 85–88. [PubMed] [Google Scholar]
- 24.Diallo M, Sall AA, Moncayo AC, Ba Y, Fernandez Z, et al. (2005) Potential role of sylvatic and domestic African mosquito species in dengue emergence. Am J Trop Med Hyg 73: 445–449. [PubMed] [Google Scholar]
- 25.Diallo M, Ba Y, Faye O, Soumare ML, Dia I (2008) Vector competence of Aedes aegypti populations from Senegal for sylvatic and epidemic dengue 2 virus isolated in West Africa. Trans R Soc Trop Med Hyg 102: 493–498. 10.1016/j.trstmh.2008.02.010 [DOI] [PubMed] [Google Scholar]
- 26.da Moura AJF, de Melo Santos MAV, Oliveira CMF, Guedes DRD, de Carvalho-Leandro D, et al. (2015) Vector competence of the Aedes aegypti population from Santiago Island, Cape Verde, to different serotypes of dengue virus. Parasit Vectors 8: 114 10.1186/s13071-015-0706-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gaye A, Faye O, Diagne CT, Faye O, Diallo D, et al. (2014) Oral susceptibility of Aedes aegypti (Diptera: Culicidae) from Senegal for dengue serotypes 1 and 3 viruses. Trop Med Int Health 19: 1355–1359. 10.1111/tmi.12373 [DOI] [PubMed] [Google Scholar]
- 28.Rossi SL, Nasar F, Cardosa J, Mayer SV, Tesh RB, et al. (2012) Genetic and phenotypic characterization of sylvatic dengue virus type 4 strains. Virol 423: 58–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Team RC(2013) R: A language and environment for statistical computing. [Google Scholar]
- 30.Fall G, Faye M, Weidmann M, Kaiser M, Dupressoir A, et al. (2016) Real-time RT-PCR assays for detection and genotyping of West Nile virus lineages circulating in Africa. Vect Born Zoo Dis 16: 781–789. [DOI] [PubMed] [Google Scholar]
- 31.Nikolay B, Diallo M, Faye O, Boye CS, Sall AA (2012) Vector competence of Culex neavei (Diptera: Culicidae) for Usutu virus. Am J Trop Med Hyg 86: 993–996. 10.4269/ajtmh.2012.11-0509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen KC, Kam Y-W, Lin RPT, Ng MM-L, Ng LF, et al. (2013) Comparative analysis of the genome sequences and replication profiles of chikungunya virus isolates within the East, Central and South African (ECSA) lineage. Virol J 10: 169 10.1186/1743-422X-10-169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Anderson SL, Richards SL, Smartt CT (2010) A simple method for determining arbovirus transmission in mosquitoes. J Am Mosquit Control Assoc 26: 108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Heitmann A, Jansen S, Lühken R, Leggewie M, Schmidt-Chanasit J, et al. (2018) Forced Salivation As a Method to Analyze Vector Competence of Mosquitoes. J Visual Exp: JoVE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aitken T (1977) An in vitro feeding technique for artificially demonstrating virus transmission by mosquitoes. Mosquit News 37: 130–133. [Google Scholar]
- 36.Hurlbut HS (1966) Mosquito salivation and virus transmission. Am J Trop Med Hyg 15: 989–993. [DOI] [PubMed] [Google Scholar]
- 37.Colton L, Biggerstaff BJ, Johnson A, Nasci RS (2005) Quantification of West Nile virus in vector mosquito saliva. J Am Mosquit Control Assoc 21: 49–53. [DOI] [PubMed] [Google Scholar]
- 38.Mores CN, Turell MJ, Dohm DJ, Blow JA, Carranza MT, et al. (2007) Experimental transmission of West Nile virus by Culex nigripalpus from Honduras. Vect Born Zoo Dis 7: 279–284. [DOI] [PubMed] [Google Scholar]
- 39.Thongrungkiat S, Jirakanjanakit N, Apiwathnasorn C, Prummongkol S, Samung Y (2003) Comparative susceptibility to oral infection with dengue viruses among local strains of Aedes aegypti (Diptera: Culicidae) collected at different seasons of the year. J Vect Ecol 28: 166–170. [PubMed] [Google Scholar]
- 40.Gubler DJ, Nalim S, Tan R, Saipan H, Sulianti Saroso J (1979) Variation in susceptibility to oral infection with dengue viruses among geographic strains of Aedes aegypti. Am J Trop Med Hyg 28: 1045–1052. [DOI] [PubMed] [Google Scholar]
- 41.Vazeille M, Yébakima A, Lourenço-de-Oliveira R, Andriamahefazafy B, Correira A, et al. (2013) Oral receptivity of Aedes aegypti from Cape Verde for yellow fever, dengue, and chikungunya viruses. Vect Born Zoo Dis 13: 37–40. [DOI] [PubMed] [Google Scholar]
- 42.Miller B, Monath T, Tabachnick W, Ezike V (1989) Epidemic yellow fever caused by an incompetent mosquito vector. Trop Med Parasitol: official organ of Deutsche Tropenmedizinische Gesellschaft and of Deutsche Gesellschaft fur Technische Zusammenarbeit (GTZ) 40: 396–399. [PubMed] [Google Scholar]
- 43.Garrett-Jones C (1964) The human blood index of malaria vectors in relation to epidemiological assessment. Bull World Health Organization 30: 241. [PMC free article] [PubMed] [Google Scholar]
- 44.Dye C (1992) The analysis of parasite transmission by bloodsucking insects. Ann Rev Entomol 37: 1–19. [DOI] [PubMed] [Google Scholar]
- 45.Luz PM, Codeço CT, Massad E, Struchiner CJ (2003) Uncertainties regarding dengue modeling in Rio de Janeiro, Brazil. Memórias do Instituto Oswaldo Cruz 98: 871–878. [PubMed] [Google Scholar]
- 46.Copeland R (1981) An electron microscopic study of the development of dengue virus in the salivary glands of Aedes aegypti (L.). Master's thesis, Louisiana State University and Agricultural and Mechanical College. [Google Scholar]
- 47.Moncayo AC, Edman JD, Turell MJ (2000) Effect of eastern equine encephalomyelitis virus on the survival of Aedes albopictus, Anopheles quadrimaculatus, and Coquillettidia perturbans (Diptera: Culicidae). J Med Entomol 37: 701–706. [DOI] [PubMed] [Google Scholar]
- 48.Mahmood F, Reisen WK, Chiles RE, Fang Y (2004) Western equine encephalomyelitis virus infection affects the life table characteristics of Culex tarsalis (Diptera: Culicidae). J Med Entomol 41: 982–986. [DOI] [PubMed] [Google Scholar]
- 49.Wang E, Ni H, Xu R, Barrett AD, Watowich SJ, et al. (2000) Evolutionary relationships of endemic/epidemic and sylvatic dengue viruses. J Virol 74: 3227–3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cornet M(1993) Dengue Virus in Africa Monograph on Dengue and Dengue Hemorrhagic Fever, SEARO 22. WHO, Geneva. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
(DOC)
Error bars represent the upper limits of the 95% confidence intervals of infection and dissemination rates.
(TIF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.