Abstract
Current regulatory requirements impede clinical translation and market introduction of many new antimicrobial combination implants and devices, causing unnecessary patient suffering, doctor frustration, and costs to healthcare payers. Regulatory requirements of antimicrobial combination implants and devices should be thoroughly revisited and their approval allowed based on enrichment of benefit demonstrations from high-risk patient groups and populations or device components to facilitate their clinical translation. Biomaterial implant and devices equipped with antimicrobial strategies and approved based on enrichment claims should be mandatorily enrolled in global registry studies supervised by regulatory agencies for a minimum five-year period or until statistically validated evidence for noninferiority or superiority of claims is demonstrated. With these recommendations, this trans-Atlantic consortium of academicians and clinicians takes its responsibility to actively seek to relieve the factors that stagnate downward clinical translation and availability of antimicrobial combination implants and devices. Improved dialogue between the various key players involved in the current translational blockade, which include patients, academicians and doctors, policymakers, regulatory agencies, manufacturers, and healthcare payers, is urgently needed.
Keywords: biomaterial-associated infection, downward clinical translation, prosthesis, enrichment, benefit assessment, risk assessment, biomaterials, surface modification
Clinical Need for Antimicrobial Combination Devices
Biomaterial implants and devices such as prosthetic joints and other implants are used routinely to alleviate pain, ameliorate function, and improve appearance to improve quality of life and extend it. A major complication of these biomaterial implants and devices is infection risk, occurring across nearly all applications of biomaterials, whether internally implanted, like total hip and knee arthroplasties,1,2 cardiovascular electronic devices such as permanent pacemakers and cardioverter defibrillators3,4 and intraocular lenses,5 or externally carried like contact lenses.6 On average, infection rates may reach 5% across different biomaterials applications. Percutaneous devices, like bone fixation pins and central venous and urinary catheters, have infection rates much higher than those of totally implantable devices, ranging up to several tens of percentages,7 with the exception of dental implants that resist infection better than most percutaneous implants.8 Regardless of the particular biomaterials implant or device in question, biomaterials-related infections have caused dramatic increases in patient morbidity and mortality9 and high financial burden to health care payers, including patients.10 A sobering finding, for example, is that prosthetic joint infection places patients at a mortality risk exceeding that of many cancers.11
Regulatory Landscape: A Blockade to Downward Clinical Translation
Numerous antimicrobial combination implants and devices are reported annually in scientific publications. Most are based on biomaterial (surface) modifications or (on-demand) drug-releasing coatings to reduce microbial colonization.12 Many of them have been primarily academically designed and are too complex to be scaled and manufactured in a commercially feasible scenario. In addition, their complexity makes it difficult to comply with the requirements defined by national policymakers and regulatory agencies. Although the United States Food and Drug Administration (US FDA) and the European Union (EU) have a similar focus to expedite approval and clearance of safe and effective medical biomaterial implants and devices to the market, combination devices still present a challenge13 with no united approach to adoption. Combinational device approach is becoming even more important since the recent issue of the new Europe’s Medical Device Regulation (MDR) 2017/745,14 in which notified body consultation with medicines authority is required for devices whose substance or metabolites are systemically absorbed to achieve intended action. A combination device that incorporates medicinal substances always fell under the old European Communities authorization rule, regardless of the concentration of the substance, but combination devices that previously fell below a minimum medicinal threshold under MDR 2001/83/EC will now also be classified as combination products. This elevates the challenges to obtain regulatory approval and clearance for combination implants and devices. Although the overall impact of this new MDR remains to be seen, it may well yield higher risks and higher costs, reducing innovation incentives for antimicrobial technologies and their market introduction.
US FDA requires demonstration of both human benefit and assessment of risk prior to introducing new antimicrobial combination implants and devices to the market, while the European Union’s focus is on risk assessment. Regulatory agencies struggle to define requirements for human benefit and risk assessment. Both industry and academia realize that designing and conducting expensive clinical trials adequately addressing regulatory requirements often have ambiguous performance end points that are impossible to reach with statistical confidence with only 1–5 implant recipients in 100 developing serious infection that requires additional surgery or treatment.15 This clearly hampers commercial feasibility.16,17 Moreover, end points of in vitro or animal studies (to the extend that they are predictable for human clinical outcome) are frequently expressed in terms of limiting microbial colonization or infectious biofilm formation, biofilm removal and degradation, inhibition of microbial colonization effects on biofilm growth rate, and other fairly imprecise terminology that does not address potential human benefits in terms of reduced infection rates. This, in the current regulatory landscape, causes regulatory agencies to struggle with the risk/benefit profile18−20 and leaves potential industrial partners interested in bringing novel antimicrobial combination implants and devices into clinical use in the dark with respect to financial returns in the marketplace.21
The few commercially available implants and devices equipped with antimicrobial strategies, such as the CE-approved gentamicin-releasing coating on intramedullary nails, have been brought to the market at extreme costs, which have been spent primarily to meet regulatory requirements.22,23 In contrast and admittedly in a slightly different context, drug eluting stents, for example, benefiting hundreds of thousands of recipients globally, have been much more easily analyzed to provide the necessary statistical numbers24,25 with good prospects for return of investment to companies.
Collectively, these factors have caused a formidable hurdle that has largely precluded translation of antimicrobial combination implants and devices from bench to bedside.
Preventive or Therapeutic Goals of a Combination Device
In academic research, the distinction between preventive and therapeutic goals of perceived innovations with respect to the control of implant or device infections is not always made clear. In a therapeutic approach, a matured biofilm community with all its well-known recalcitrance features to treatment needs to be eradicated, which is much more difficult than the killing of peri-operatively introduced bacteria adhering as individuals on an implant or device surface, required to prevent development of infection.16 Herewith, distinction between preventive and therapeutic goals forms the key to success in the development of many antimicrobial implant and device strategies. Generally, the preventive approach is for device companies a more interesting one. However, preventive claims would, under the current regulatory requirements, necessitate either an immense clinical trial enrollment to provide sufficient power to delineate the small fraction of patients actually benefiting from antimicrobial implants, or patient stratification and triage to streamline trial cohorts for preventive treatment based on identifiable infection risk factors. Yet, also from a patients perspective, preventive goals are clearly preferable above therapeutic ones.
Recommendations to Facilitate Downward Clinical Translation
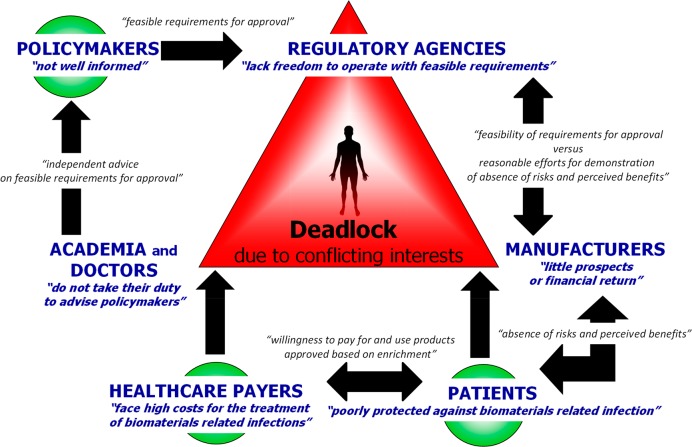
Seeking to relieve the factors that stagnate downward clinical translation and availability of antimicrobial combination implants and devices that prevent or reduce the likelihood of biomaterial-implant-associated infections, a change in dialogue between the various key players involved in the current translational blockade is required (see Figure 1). For policymakers and regulatory agencies, this trans-Atlantic consortium recommends that
-
(1)
Clinical benefit and efficacy assessments performed in high-risk patient groups for antimicrobial combination implants and devices should be considered for extrapolation to general patient populations without necessarily requiring clinical demonstration of benefit (“enrichment-from-patient”).
Rationale: Clinical improvements can be demonstrated with statistical significance with a far lower number of implant or device recipients enrolled in clinical trials when carried out in high-risk patients (i.e., trauma patients or patients suffering from diabetes) than when carried out in “healthy” patients, often requiring enrollment of tens or hundreds of thousands of patients. However, regulatory agencies demand a control group with infection rates similar to patients without comorbidity, but the need for such a demand remains unclear. We therefore recommend that clinical improvements demonstrated in high-risk patient groups be enriched to claims for the general patient population requiring a specific implant or device.
-
(2)
Clinical trials in less-developed countries with infection rates higher than global averages for a certain device should be accepted for benefit demonstration of antimicrobial combination implants and devices of the same type (“enrichment-from-population”).
Rationale: Biomaterial implant and device manufacturers and academicians often seek sites in less-developed countries to host clinical trials for antimicrobial benefit demonstration, as they usually have higher infection rates. In such trials, clinical improvements can be demonstrated with statistical significance without reaching out to tens or hundreds of thousands of patients required for a similar study within the United States or Europe. However, regulatory agencies demand a control group with infection rates similar to domestic ones, but the need for such a requirement remains unclear. We therefore recommend that clinical improvements demonstrated in less-developed countries be enriched to approved domestic claims in the absence of other confounding factors affecting outcomes (i.e., physiological, genomic, or anatomical differences, clinical treatment or standard of care differences).
-
(3)
In cases of an already-approved combination implant or device with antimicrobial coating, replacement of that coating with another coating or application to an implant or device with different designs or geometries should not require the same type and extent of regulatory assessment of risk/benefit as for the already-approved device (“enrichment-from-component”).
Rationale: An approved combination implant or device equipped, for instance, with an antibiotic-releasing coating is often modified in small steps to meet new requirements stimulated, for instance, by the development of new, antibiotic-resistant strains or changes in geometry. In such cases, a change in geometry or replacement of an already established and implemented antibiotic by another one should be approved by enrichment of an approved product without the same type and extent of regulatory assessment.
Figure 1.
Key players involved in the current translational blockade that impedes clinical availability of new antimicrobial combination devices to prevent biomaterial-associated infections, placing the patient against the will of each individual key player, trapped in a deadlock situation. Current positions assumed by key players (blue italic text) and new dialogue content needed between different key players (black italic text) to free the patient from this position, as based on our recommendations, are both indicated.
Regulatory bodies are often blamed for the current deadlocked translational situation, but regulatory bodies only enforce rules defined by policymakers (see also Figure 1). This leads to our next recommendation on relevant policies, addressing academicians and clinicians alike:
-
4.
Academicians and clinicians should be more active in advising policymakers about more realistic regulations concerning new approvals of antimicrobial combination implants and devices.
Rationale: Academicians and clinicians are the only experts in the field who can independently advise policymakers on changes necessary in regulatory requirements. Advising policymakers on more realistic regulations should be done equally with professional academic and clinical duties to modify current regulations as a first step to produce feasible regulatory requirements to obtain approval for antimicrobial combination implants and devices. The current trend in academia that performance evaluation should go beyond bibliometric analysis and include an outreach portfolio (“what did I do, why does it matter”)26 may constitute the stimulus for academic researchers and clinicians to pick up this indispensable role to change regulatory requirements.
These recommendations do not relieve medical device manufacturers from their duties to demonstrate benefit, for which we propose the following:
-
5.
Infection-resistant combination devices conditionally approved on an enrichment basis should be mandatorily enrolled in global registry studies supervised by regulatory agencies for a minimum five-year period or until statistically validated evidence for noninferiority or superiority of claims is demonstrated.
Rationale: Clinical practice should be evidence-based. In the absence of possibilities for prospective clinical trials for the diverse reasons mentioned above, retrospective clinical trials should be allowed to provide the necessary evidence for noninferiority or superiority of antimicrobial combination implants or devices approved on an enrichment basis.
Finally, both healthcare payers and patients must realize that the costs of bringing new antimicrobial combination implants and devices to the market not only comprise substantive research and development costs but also expenditures for clinical trials to demonstrate human benefit and patient safety. Our consortium believes that implementation of the above-cited recommendations will help to facilitate downward clinical translation of new antimicrobial combination devices to the market and therewith clinical use and patient benefits. Our recommendations, however, leave manufacturers uncertain about the ultimate approval prospects for a new antimicrobial device approved on an enrichment basis. This creates uncertainty about financial returns. Therefore, healthcare payers and patients should express equal willingness to pay for procedures using antimicrobial combination implants and devices approved on an enrichment basis as when performed with otherwise approved ones, even when possibly more expensive.
These recommendations (summarized in Box 1) are proposed with the intent of stimulating the needed dialogue between academicians, clinicians, manufacturers, policymakers and regulatory agencies, healthcare payers, and patients, as these key-players all must act in unison to further advance this field on which much of the future of healthcare is based.
Box 1. Key Concepts Box.
Biomaterial-implant-associated infections are causing dramatic increases in patient morbidity and mortality and financial burden to health care payers, including patients.
Although numerous antimicrobial combination implants and devices are reported annually in scientific publications, the current regulatory landscape makes clinical benefit demonstration impossible, presenting a blockade to their downward clinical translation.
Healthcare payers, academicians and doctors, policymakers, regulatory agencies, manufacturers, and patients should take their respective responsibilities to facilitate more efficient dialogue to create more realistic regulations allowing clinical translation of antimicrobial combination implants and devices.
Regulatory approval of antimicrobial combination implants and devices should be allowed based on enrichment of claims from (1) high- to low-risk patient groups, (2) from high- to low-risk population groups, and (3) one to another component of the same device.
The authors declare the following competing financial interest(s): H.J.B. is also director of a consulting company, SASA BV (GN Schutterlaan 4, 9797 PC Thesinge, The Netherlands). J.P. received royalties from Elsevier, Wolters Kluwer, and Jaypee Publishers. M.J.R. is consultant of DePuy Synthes and holds a patent for antimicrobial coatings. R.B. receives speaker fees from DePuy-Synthes and has patents on antimicrobial materials; all benefits go to the University of Nottingham. R.O.D. has patents, all benefits going to the Baylor College of Medicine, and has acted as a consultant on device-related infections.
References
- Kurtz S. M.; Ong K. L.; Lau E.; Mowat E. F.; Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005–2030. J. Bone Joint Surg. Am. 2007, 89, 780–785. 10.2106/00004623-200704000-00012. [DOI] [PubMed] [Google Scholar]
- Kurtz S. M.; Lau E.; Schmier J.; Ong K. L.; Zhao K.; Parvizi J. Infection burden for hip and knee arthroplasty in the United States. J. Arthroplasty 2008, 23, 984–991. 10.1016/j.arth.2007.10.017. [DOI] [PubMed] [Google Scholar]
- Greenspon A. J.; Patel J. D.; Lau E.; Ochoa J. A.; Frisch D. R.; Ho R. T.; Pavri B. B.; Kurtz S. M. 16-Year trends in the infection burden for pacemakers and implantable cardioverter-defibrillators in the United States 1993 to 2008. J. Am. Coll. Cardiol. 2011, 58, 1001–1006. 10.1016/j.jacc.2011.04.033. [DOI] [PubMed] [Google Scholar]
- Nagpal A.; Baddour L. M.; Sohail M. R. Microbiology and pathogenesis of cardiovascular implantable electronic device infections. Circ.: Arrhythmia Electrophysiol. 2012, 5, 433–441. 10.1161/CIRCEP.111.962753. [DOI] [PubMed] [Google Scholar]
- Sadaka A.; Durand M. L.; Gilmore M. S. Bacterial endophthalmitis in the age of outpatient intravitreal therapies and cataract surgeries: Host–microbe interactions in intraocular infection. Prog. Retinal Eye Res. 2012, 31, 316–331. 10.1016/j.preteyeres.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng K. H.; Leung S. L.; Hoekman H. W.; Beekhuis W. H.; Mulder P. G. H.; Geerards A. J. M.; Kijlstra A. Incidence of contact-lens-associated microbial keratitis and its related morbidity. Lancet 1999, 354, 181–185. 10.1016/S0140-6736(98)09385-4. [DOI] [PubMed] [Google Scholar]
- Lai N. M.; Chaiyakunapruk N.; Lai N. A.; O’Riordan E.; Pau W. S. C.; Saint S. Catheter impregnation, coating or bonding for reducing central venous catheter-related infections in adults. Coch. Data Syst. Rev. 2013, 6, CD007878. 10.1002/14651858.CD007878.pub2. [DOI] [PubMed] [Google Scholar]
- Yue C.; Zhao B.; Ren Y.; Kuijer R.; Van der Mei H. C.; Busscher H. J.; Rochford E. T. J. The implant infection paradox: why do some succeed when others fail? Opinion and discussion paper. Eur. Cells Mater. 2015, 29, 303–313. 10.22203/eCM.v029a23. [DOI] [PubMed] [Google Scholar]
- Helwig P.; Morlock J.; Oberst M.; Hauschild O.; Hubner J.; Borde J.; Sudkamp N. P.; Konstantinidis L. Periprosthetic joint infection-effect on quality of life. Int. Orthop. 2014, 38, 1077–1081. 10.1007/s00264-013-2265-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanner J.; Padley W.; Kiernan M.; Leaper D.; Norrie P.; Baggott R. A benchmark too far: findings from a national survey of surgical site infection surveillance. J. Hosp. Infect. 2013, 83, 87–91. 10.1016/j.jhin.2012.11.010. [DOI] [PubMed] [Google Scholar]
- Zmistowski B.; Karam J. A.; Durinka J. B.; Casper D. S.; Parvizi J. Periprosthetic joint infection increases the risk of one-year mortality. J. Bone Joint Surg. 2013, 95, 2177–2184. 10.2106/JBJS.L.00789. [DOI] [PubMed] [Google Scholar]
- Wu P.; Grainger D. W. Drug/device combinations for local drug therapies and infection prophylaxis. Biomaterials 2006, 27, 2450–2467. 10.1016/j.biomaterials.2005.11.031. [DOI] [PubMed] [Google Scholar]
- Krueger A. C.CDRH/ODE Perspectives on adding anti-biofilm technologies/agents to devices: Biofilms, medical devices and anti-biofilm technology. Challenges and Opportunities Workshop: Center for Biofilm Engineering at the Montana State University. February 21, 2014. (http://www.fda.gov/downloads/MedicalDevices/NewsEvents/WorkshopsConferences/UCM387641.pdf).
- Europe’s Medical Device Regulation. https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:32017R0745 (accessed November 2018).
- Darouiche R. O. Antimicrobial approaches for preventing infections associated with surgical implants. Clin. Infect. Dis. 2003, 36, 1284–1289. 10.1086/374842. [DOI] [PubMed] [Google Scholar]
- Busscher H. J.; Van der Mei H. C.; Subbiahdoss G.; Jutte P. C.; Van den Dungen J. J. A. M.; Zaat S. A. J.; Schultz M. J.; Grainger D. W. Biomaterial-associated infection: Locating the finish line in the race for the surface. Sci. Transl. Med. 2012, 4, 153rv10. 10.1126/scitranslmed.3004528. [DOI] [PubMed] [Google Scholar]
- Grainger D. W.; Van der Mei H. C.; Jutte P. C.; Van den Dungen J. J. A. M.; Schultz M. J.; Van der Laan B. F. A. M.; Zaat S. A. J.; Busscher H. J. Critical factors in the translation of improved antimicrobial strategies for medical implants and devices. Biomaterials 2013, 34, 9237–9243. 10.1016/j.biomaterials.2013.08.043. [DOI] [PubMed] [Google Scholar]
- French-Mowat E.; Burnett J. How are medical devices regulated in the European Union?. J. R. Soc. Med. 2012, 105, S22–S28. 10.1258/jrsm.2012.120036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covance A. F.Clinical trial requirements: Similarities and differences US vs. EU. RAPS Annual Conference & Exhibition: Philadelphia, Pennsylvania, September 17, 2009. http://www.slideshare.net/RETIRE/clinical-trial-requirements-us-vs-eu-similarities-and-differences.
- Kashyap U. N.; Gupta V.; Raghunandan H. V. Comparison of drug approval process in United States & Europe. J. Pharm. Sci. Res. 2013, 5, 131. [Google Scholar]
- Abou-El-Enein M.; Duda G. N.; Gruskin E.; Grainger D. W. Strategies for de-risking translational processes in biomedical science and technology. Trends Biotechnol. 2017, 35, 100–108. 10.1016/j.tibtech.2016.07.007. [DOI] [PubMed] [Google Scholar]
- Schmidmaier G.; Lucke M.; Wildemann B.; Haas N. P.; Raschke M. Prophylaxis and treatment of implant-related infections by antibiotic-coated implants: a review. Injury 2006, 37 (Suppl 2), S105–112. 10.1016/j.injury.2006.04.016. [DOI] [PubMed] [Google Scholar]
- Fuchs T.; Stange R.; Schmidmaier G.; Raschke M. J. The use of gentamicin-coated nails in the tibia: preliminary results of a prospective study. Arch. Orthop. Trauma Surg. 2011, 131, 1419–1425. 10.1007/s00402-011-1321-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg M. J.; Konnyu K. J. Review of randomized clinical trials of drug-eluting stents for the prevention of in-stent restenosis. Am. J. Cardiol. 2006, 98, 375–382. 10.1016/j.amjcard.2006.02.042. [DOI] [PubMed] [Google Scholar]
- Kandzari D. E.; Leon M. B.; Meredith I.; Fajadet J.; Wijns W.; Mauri L. Final 5-year outcomes from the endeavor Zotarolimus-eluting stent clinical trial program: Comparison of safety and efficacy with first-generation drug-eluting and bare-metal stents. JACC Cardiovasc. Interv. 2013, 6, 504–512. 10.1016/j.jcin.2012.12.125. [DOI] [PubMed] [Google Scholar]
- Benedictus R.; Miedema F. Fewer numbers, better science. Nature 2016, 538, 453–455. 10.1038/538453a. [DOI] [PubMed] [Google Scholar]