Abstract
The aim of this study was to evaluate the safety and clinical efficacy of a combined preoperative regimen consisting of volumetric modulated arc therapy–simultaneous integrated boost and capecitabine chemotherapy for distal rectal cancer. A total of 26 patients with locally advanced distal rectal cancer were enrolled from March 2015 to May 2016. The radiation dose fractionation was 58.75 Gy/25 fractions (2.35 Gy/fraction) for rectal tumor and pelvic lymph node metastasis and 50 Gy/25 fractions for pelvic lymph node stations, accompanied with simultaneous capecitabine chemotherapy. Completion of the simultaneous chemotherapy was ensued by 1 week of rest and then another cycle of induction chemotherapy with capecitabine. A radical rectal cancer surgery was performed 6 to 8 weeks after the simultaneous chemoradiotherapy. The primary end points were the complete pathological response rate and the postoperative sphincter preservation rate. All 26 patients completed the neoadjuvant chemoradiotherapy, among which 25 received surgical treatment. The postoperative complete pathological response rate was as high as 32% (8/25), while the sphincter preservation rate was 60% (15/25), the overall tumor/node (T/N) downstaging rate was 92% (23/25), and the R0 resection rate was 100%. During the chemoradiation, the most common adverse events were grade 1 and 2; grade 3 radiodermatitis occurred in 2 cases but no occurrence of acute adverse events occurred that were grade 4 and above. After the surgery, there was one case of ureteral injury and one case of intestinal obstruction, but no perioperative deaths occurred. In conclusion, the chemoradiation regimen of preoperative volumetric modulated arc therapy-simultaneous integrated boost (VMAT-SIB58.75Gy) and a single cycle of induction chemotherapy with capecitabine for patients with distal rectal cancer is safe and feasible with a satisfactory complete pathological response rate, sphincter preservation rate, and R0 resection rate.
Keywords: distal rectal cancer, volumetric modulated arc therapy, simultaneous integrated boost, neoadjuvant chemoradiation, sphincter-preserving surgery
Introduction
Mid and distal rectal cancer accounts for more than 70% of malignant rectal tumors.1 At the time of diagnosis, most cases are already in the intermediate to advanced stage. Clinical practices often rely on abdominoperineal resection (APR); however, the low sphincter preservation rate is detrimental to the patients’ quality of life and psychological and mental health. Currently, the multidisciplinary treatment of rectal cancer has been widely accepted. Compared with upfront surgical treatments, a combination of preoperative neoadjuvant chemoradiation and surgery can downstage rectal tumor and pelvic lymph node metastasis and reduce the risk of local tumor recurrence, while maximizing the surgical possibilities of sphincter preservation.1 Hence, its clinical application is increasingly widespread. At present, there is a relative lack of studies regarding preoperative neoadjuvant chemoradiation for treating distal rectal cancer. With the technology of image-guided radiotherapy and volumetric modulated arc therapy (VMAT), this study used VMAT-simultaneous integrated boost (SIB) to increase the local radiation dose for treating rectal tumors and incorporated a single cycle of induction chemotherapy with capecitabine in hopes of further downstaging the rectal tumors, enhancing the postoperative sphincter preservation rate, and, eventually, improving patient survival and quality of life without any significant increase in toxic side effects.
Patients and Methods
Eligibility Criteria
The eligibility criteria included resectable histologically confirmed distal rectal adenocarcinoma with an inferior border within 5 cm of the anal verge. The tumor had to have evidence of cT3-T4 with any N, or any T with N1 or N2 disease on pelvic magnetic resonance imaging (MRI), staged according to the 2009 classification of the American Joint Committee on Cancer (seventh edition). The additional eligibility criteria were a Karnofsky Performance Status score of at least 70, an age at the time of diagnosis between 18 years and 75 years, adequate blood counts, and adequate hepatic and renal function. This trial was approved by the institutional review board of PLA General Hospital (No. 2012019), in accordance with the Helsinki Declaration. All patients provided written informed consent before they were recruited for the study. This trial was registered with www.chictr.org before patient recruitment started.
Ineligibility Criteria
Patients with unresectable disease or distant metastases were excluded from the study. Patients with a history of other malignancy within the prior 5 years were also excluded. Other exclusion criteria included previous treatment of rectal cancer, prior pelvic radiotherapy, sensitivity to fluoropyrimidines, acute obstructive symptoms, uncontrolled concurrent infection, active inflammatory bowel disease, clinically significant cardiac disease, uncontrolled high-risk hypertension, pregnancy, or life expectancy ≤6 months.
Pretreatment Evaluation
All the patients underwent a complete history and physical examination, total colonoscopy, tumor biopsy, and computed tomography (CT) scan of the chest and abdomen. Pelvic MRI scans were used to determine the clinical tumor/node (T/N) classification. Positron emission tomography was not required but was performed when feasible. Complete laboratory tests included a complete blood cell count, blood electrolytes, carcinoembryonic antigen, creatinine, blood urea nitrogen, liver transaminases, γ-glutamyltransferase, alkaline phosphatase, and total bilirubin. Cardiac function was investigated using an electrocardiogram and an echocardiogram.
Study Design and Treatment
This study was a nonrandomized, open-labeled, single-arm, single-institution, phase II trial of capecitabine in combination with preoperative VMAT-SIB in patients with resectable locally advanced distal rectal cancer.
Radiotherapy
Each patient had undergone CT-based simulation with 5-mm slices in the supine position with a full bladder. Intravenous contrast was used in all of the patients. The target volume delineation took reference from ICRU reports 50 and 62; the gross target volume (GTV) encompassed the primary lesions of the tumors and the pelvic lymph nodes, while the planning GTV (PGTV) was obtained by expanding the GTV by a margin of 5 mm horizontally and a margin of 5 to 10 mm vertically. The clinical target volume (CTV) was defined as the GTV plus surrounding pelvic tissues that are at significant risk of harboring microscopic disease, including the mesorectal space, the presacral region, the mesorectal lymph nodes, and the perirectal and internal iliac lymph nodes. The perineum was included if the surgeon deemed an APR necessary. The external iliac lymph nodes were considered part of the CTV if there was tumor extension to the vagina, uterus, cervix, prostate, or bladder. The inguinal lymph nodes were included only when the tumor was invading the lower third of the vagina or anal canal. The planning target volume (PTV) was generated by expanding the CTV by a margin of 5 mm horizontally and a margin of 1 cm vertically. A representative case is shown in Figure 1. The organs at risk (OARs) included the small intestine, bladder, femur, and pelvis. Regimen design and prescribed dose: All the patients completed the regimen under the Eclipse 10.0 treatment planning system (Varian Medical Systems, Palo Alto, California). The prescribed dose (≥95% of the target dose) was PGTV, 58.75 Gy/25 fractions, 5 fractions/week and PTV, 50 Gy/2 fractions, 5 fractions/week. The dose limits for OARs including the small intestine were V30 <40%, V15 <120 cc, V45 <78 cc, V50 <17 cc; for the bladder the dose limit was V40 <45%; for the pelvis the dose limits were V40 <50%, V10 <95%, V20 <80%; and for the femur the limit was V40 <5%.
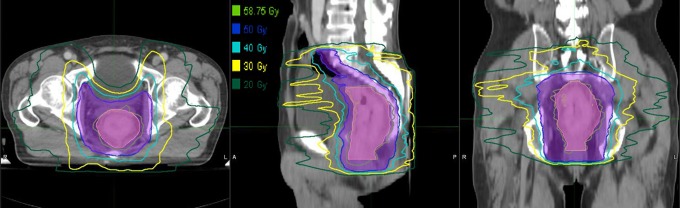
Figure 1.
Isodose distributions for patient 1 with cT3N0 lower rectal cancer. The pink and slate blue lines represent PGTV and PTV, respectively. The green, light blue, sky blue, yellow, and sea green lines represent 58.75, 50, 40, 30, and 20 Gy isodoses, respectively. PGTV indicates planning gross target volume; PTV, planning target volume.
Preoperative Chemotherapy
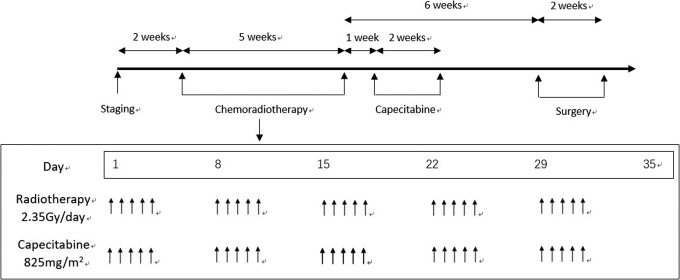
The preoperative chemotherapy included the following: Simultaneous chemotherapy, which consisted of 825 mg/m2 of oral capecitabine twice per day and 5 days per week for 5 weeks; and induction chemotherapy, which was provided after the chemotherapy. The patients rested for a week and proceeded to take oral capecitabine for a cycle (1250 mg/m2 twice per day for 14 consecutive days; Figure 2).
Figure 2.
The workflow of preoperative chemoradiotherapy in patients with locally advanced rectal cancer.
Surgery
Approximately 6 to 8 weeks after simultaneous chemotherapy, all the patients underwent total mesorectal excision surgery, which included the Miles, Dixon, and Hartman operations.
Assessment of Adverse Reactions and Adjustment of Chemoradiation Regimen
During the course of chemoradiation, all patients underwent weekly complete blood count and renal and liver function tests. The preoperative adverse reactions to chemoradiation were graded according to the Common Terminology Criteria for Adverse Events 4.0 standard. Adjustments to the chemoradiation regimens were as follows: If the patients experienced grade 3 or 4 hematologic adverse events during simultaneous chemoradiation, the treatment was halted and resumed only when the reactions recovered to grade 1 or 2. At that time, however, the dose of capecitabine was halved. Patients exhibiting grade 3 or 4 nonhematologic adverse reactions were administered half doses of capecitabine, while for those with severe symptoms, capecitabine was discontinued and only radiotherapy was administered. If grade 4 diarrhea developed, the simultaneous chemoradiation was stopped, and radiotherapy resumed only when the reaction recovered to grade 2 or 3.
Study End Points
The primary end points included the pathological complete response (ypCR) rate and the rate of sphincter-preserving surgery. The secondary end points included the rate of tumor downstaging, toxicity, and postoperative complications (within 30 days of surgery).
Statistical Analysis
The primary end point of this single-arm phase II trial was the ypCR rate. According to A’Hern’s 1-stage phase II design, the required number of patients was estimated to be 25 to accept the hypothesis that the ypCR rate was greater than 30% with 80% power and to reject the hypothesis that the ypCR rate was less than or equal to 10% with 5% significance.2
Results
Patients
A total of 26 patients with rectal cancer were enrolled between March 2015 and May 2016. For each patient included, the bottom edge of their tumor was 3 to 5 cm from the anal verge, with a median distance of 4 cm; their ages ranged from 18 to 75 (with the median of 55). Among them, 20 were male, and 6 were female. Clinical staging: There were 2 cases of T2 (7.7%), 22 cases of T3 (84.6%), and 2 cases of T4 (7.7%) as well as 7 cases of N1 (26.9%) and 12 cases of N2 (46.2%). There were 16 patients with moderately or poorly differentiated adenocarcinoma, 3 patients with well-differentiated adenocarcinoma, 3 patients with mucinous adenocarcinoma, and 4 patients with undifferentiated adenocarcinoma (Table 1). The median follow-up time was 16 months (range = 9-25 months).
Table 1.
Demographic and Clinical Features for All Patients.a
| Characteristics | Date |
|---|---|
| Age, median (range), years | 55 (18-75) |
| Gender, n (%) | |
| Male | 20 (76.9%) |
| Female | 6 (23.1%) |
| Clinical T stage, n (%) | |
| cT2 | 2 (7.7%) |
| cT3 | 22 (84.6%) |
| cT4 | 2 (7.7%) |
| Clinical N stage, n (%) | |
| cN0 | 7 (26.9%) |
| cN1 | 7 (26.9%) |
| cN2 | 12 (46.2%) |
| Distance from anal verge, median (range) (cm) | 4 (3-5) |
| Tumor differentiation, n (%) | |
| Well differentiated | 3 (11.5%) |
| Moderately or poorly differentiated | 16 (61.5%) |
| Mucinous adenocarcinoma | 3 (11.5%) |
| No differentiated | 4 (15.5%) |
a n = 26.
Completion of Chemoradiation
All 26 patients successfully completed preoperative simultaneous chemoradiation, with a radiotherapy duration of 34 to 41 days (the median duration was 35 days). During simultaneous chemotherapy, the capecitabine dose was 694 to 820 mg/m2 (with a median dose of 742 mg/m2 twice per day) and during induction chemotherapy, the capecitabine dose was 1087 to 1250 mg/m2 (with a median dose of 1165 mg/m2 twice per day).
Acute Adverse Reactions
During simultaneous chemoradiation, the patients mostly experienced grade 1 or 2 acute adverse reactions. The occurrence rate of grade 1 adverse reactions was 85% (22/26), including 10 hematologic toxicities, 11 cases of diarrhea, 12 cases of excretory response, 9 cases of radiodermatitis, and 5 cases of hand-foot syndrome; the occurrence rate of grade 2 acute adverse reactions was 53.8% (14/26), including 7 cases of hematologic responses, 8 cases of diarrhea, and 2 cases of radiodermatitis. There were 2 cases of grade 3 acute adverse reactions, both of which were radiodermatitis. None of the acute adverse reactions were more severe than grade 4. The detailed occurrences of adverse reactions are shown in Table 2.
Table 2.
Toxicity During the Course of Chemoradiation.
| Toxicity | Grade 1, n (%) | Grade 2, n (%) | Grade 3, n (%) | Grade 4, n (%) |
|---|---|---|---|---|
| Lower gastrointestinal toxicity | 11 (42.3) | 8 (30.8) | 0 | 0 |
| Hematologic toxicity | 10 (38.5) | 7 (27.0) | 0 | 0 |
| Excretory response | 12 (46.2) | 0 | 0 | 0 |
| Radiodermatitis | 9 (34.6) | 2 (7.7) | 2 (7.7) | 0 |
| Hand-foot syndrome | 5 (19.2) | 0 | 0 | 0 |
Surgery and Postoperative Complications
A total of 25 patients finished the preoperative assessment and surgical treatment, and 1 patient declined the surgery due to severe perianal edema. The median interval between the end of radiotherapy and the surgery was 53 days (34-88 days); 8 patients underwent the Miles operation, 15 had the Dixon operation, and 2 had the Hartmann operation. The sphincter preservation rate was 60% (15/25), whereas the R0 resection rate was 100%. The operation rate for preventive ostomies was 93.3% (14/15), with 12 cases of transverse colostomies and 2 cases of ileostomies. In terms of the postoperative complications, there was one case of intestinal obstruction and one case of ureteral injury. There were no perioperative deaths. Details about the operations are illustrated in Table 3.
Table 3.
Surgical Procedure.
| Operation | n | % |
|---|---|---|
| Miles operation | 8 | 32 |
| Dixon operation | 15 | 60 |
| Hartman operation | 2 | 8 |
| Sphincter-preserving operation rate | 15 | 60 |
| Ostomy operation rate | 24 | 96 |
| R0 resection rate | 25 | 100 |
Pathologic Responses
A total of 25 patients were subject to the postoperative assessment of pathologic responses. According to the 5-point tumor regression grading (TRG) system by Dworak et al,3 8 (32%) achieved ypCR, and 2 patients were close to achieving ypCR (TRG3); 23 (92%) cases achieved T/N downstaging, among which 18 (72%) of 25 achieved T downstaging, and 17 (94%) of 18 achieved N downstaging. Details can be found in Table 4.
Table 4.
Postoperative Pathological Tumor/Node Staging and TRG.
| Postoperative Pathology | No. (n) | % |
|---|---|---|
| ypT stage | ||
| T0 | 8 | 32 |
| T1 | 1 | 4 |
| T2 | 8 | 32 |
| T3 | 8 | 32 |
| ypN stage | ||
| N0 | 0 | 0 |
| N1 | 3 | 12 |
| N2 | 0 | 0 |
| Dworak TRG score | ||
| 0-1 | 7 | 28 |
| 2 | 8 | 8 |
| 3 | 2 | 12 |
| 4 (ypCR) | 8 | 32 |
Abbreviations: TRG, tumor regression grading; ypCR, complete pathological response.
Discussion
Currently, APR is the main treatment for distal rectal cancer; however, the highly invasive procedure fails to preserve the sphincter and, consequently, leads to poor postoperative quality of life among the patients. Preoperative neoadjuvant chemoradiation, on the other hand, is effective in shrinking the tumor, reducing the clinical stages, and improving the R0 resection rate and sphincter-preserving rate, while lowering the rate of local tumor recurrence. Therefore, this approach is of increasing significance to the integrated treatment of distal rectal cancer.4,5 The German CAO/ARO/AIO-94 trial incorporated 799 patients with locally advanced rectal cancer, who either received preoperative or postoperative chemoradiation. The results showed that preoperative chemoradiotherapy, as compared with postoperative chemoradiotherapy, improved local control (5-year: 6% vs 13%; P = .006) and was associated with reduced severe acute toxicities (27% vs 40%; P = .001). Among patients for whom surgeons assumed APR was necessary before random grouping, 19% (15/78) in the postoperative chemoradiation group received sphincter-preserving surgeries, while 39% (45/116) in the preoperative group underwent the same (P = .004).1 The trial R96-02 conducted in Lyon, France enrolled 88 patients with T2/3 rectal cancer at 6 cm from the anal verge and divided them randomly into 2 groups: the external-beam radiotherapy group (39 Gy/13 fractions) versus a boost group who received both external- and internal-beam radiotherapy (85Gy/3 fractions). At a median follow-up of 35 months, it was observed that the boost arm improved the clinical complete response rate (cCR) from 2% in the no boost group to 24% (P = .001) and the sphincter preservation from 44% of the no boost group to 76% (P = .004). There was no statistically significant difference in the sphincter functions between the groups.6
After the preoperative neoadjuvant treatment for distal rectal cancer, the sphincter preservation rate is generally around 40% to 50%.7 There have been academic debates on whether preoperative neoadjuvant chemoradiation can increase the chance of sphincter saving surgery.5,8 Factors such as the tumor length, clinical staging, the tumor distance from the anal verge, and pathological type are determinants of sphincter saving surgeries.7 Bujko et al summarized 10 randomized, controlled clinical studies and found that preoperative chemoradiation does not increase the patients’ sphincter preservation.9 Nevertheless, neoadjuvant chemoradiation leads to tumor shrinkage and increases the tumor distance from the anal verge. An adequate distal anastomotic length can avoid injuring the levator ani and sphincter muscles and reduce the occurrence of postoperative anastomotic edema, thereby increasing the chance of sphincter preservation.10 According to the NSASBP R-03 study, APR was intended for 69% of the preoperative arm and 67% of the postoperative arm. However, at surgery, two-thirds of the postoperative arm did undergo APR, whereas only one-half of the preoperative arm had the same operation. The remainder were able to undergo a sphincter-saving procedure. This represents a 60% increase in sphincter preservation using the combined preoperative regimen.11 The application of image-guided radiotherapy has provided a technological platform for VMAT-SIB. In addition, due to the proximity of the distal rectum to the anal canal, which facilitates the implementation of a local integrated boost. According to the systematic review by Burbach et al in the Netherlands, increasing the local dose for locally advanced rectal cancer to up to 60 Gy can improve the tumor ypCR rate while causing acceptable toxicities.12 This study adopted image-guided radiotherapy and increased the local dose through VMAT-SIB (EQD2 was 60.46 Gy), resulting in a 92% tumor downstaging rate and increasing the sphincter preservation rate to 60% using preventive ostomy. This approach also yielded the resection rate of 100%, the postoperative complication rate of 8%, and a median postoperative hospital stay of 10 days. This denoted the absence of any significant increase in the surgical difficulty and occurrence of postoperative complications. Indeed, the ypCR rate in this trial of 32% compares favorably with other ypCR rates reported in the literature (Table 5).
Table 5.
Trials Incorporating Preoperative Chemoradiation.
| First Author | No. Patients | Radiation | Chemotherapy | pCR (%) | >G3 Diarrhea (%) |
|---|---|---|---|---|---|
| Sauer et al1 | 415 | 50.4 Gy, 1.8 Gy/fx, daily | ci5-FU 1000 mg/m2/d, d1-5, 29-33 | 8 | 12 |
| Gerard et al13 | 375 | 45 Gy, 1.8 Gy/fx, daily | Bolus 5-FU 325 mg/m2/d, d1-5, 29-33 + leucovorin 20 mg/m2/d, d1, 29 | 11 | NR |
| Rodel et al14 | 104 | 50.4 Gy, 1.8 Gy/fx, daily | Capecitabine 825 mg/m2 BID d1-22, 22-35 + oxaliplatin 50 mg/m2/d, d1, 8, 22, 29 | 16 | 12 |
| Zhu et al15 | 78 | 55 Gy, 2.2 Gy/fx to gross disease and 2.0 Gy/fx to pelvis using IMRT-SIB | Concurrent chemotherapy of Xelox (Capecitabine 825 mg/m2 BID 5 d/wk + oxaliplatin 50 mg/m2 d1/wk); and 2 weeks after concurrent chemoradiation, induction chemotherapy of one cycle of Xelox (oxaliplatin 130 mg/m2 d1 + capecitabine 1000 mg/m2 BID 14 days) | 23.7 | 10.3 |
| This study | 25 | 58.75 Gy, 2.35 Gy/fx to gross disease and 2.0 Gy/fx to pelvis using VMAT-SIB | Concurrent chemotherapy of Capecitabine 825 mg/m2 BID 5 d/wk; and 1 week after concurrent chemoradiation, induction chemotherapy of Capecitabine, 1250 mg/m2 BID 14 days | 32 | 0 |
Abbreviations: BID, twice daily; ci, continuous infusion; fx, fraction; IMRT, intensity-modulated radiation therapy; NR, not reported; SIB, simultaneous integrated boost.
Preoperative chemotherapy has double effects of increasing the sensitivity to radiotherapy and killing tumor cells directly. It not only leads to a higher probability of tumor downstaging and a higher R0 resection rate but also reduces the risk of local recurrence and distant metastasis by eliminating or inhibiting micrometastatic lesions, in turn benefitting the patients’ survival and quality of life. In the literature review regarding preoperative chemotherapy for rectal cancer, Glynne-Jones et al proposed that a mix of preoperative simultaneous chemoradiation and preoperative chemotherapy can enhance the ypCR and R0 resection rates, while facilitating tumor downstaging.16 A Polish study randomized 312 patients with stage II and III resectable rectal cancer, among which 155 received preoperative short-course radiotherapy and 157 had preoperative long-course chemoradiotherapy.17 The results indicated that preoperative simultaneous chemoradiation had the same sphincter preservation rates as short-term radiotherapy (58% vs 61%; P = .57) but a significantly higher R0 resection rate and ypCR rate (4.4% vs 12.9% and 16.1% vs 0.7%, respectively). The Memorial Sloan Kettering Cancer Center conducted a retrospective study and enrolled 61 patients for trials of FOLFOX4 induction chemotherapy, ensued by simultaneous chemoradiation and surgery, which resulted in an ypCR rate of 27%.18 Multiple large-scale, randomized clinical studies revealed that using oxaliplatin to enhance the sensitivity to radiotherapy in addition to 5-FU/capecitabine increases the toxicity and has uncertain benefits.19-21 As a new fluorinated analog of uracil, capecitabine has similar short- and long-term effects as a 5-FU injection or drip.22 It is applied to an increasingly wide range of clinical uses due to the convenience, security, and reliability of oral administration. This study enrolled 26 patients for preoperative capecitabine monotherapy, most of which had grade 1 or 2 acute adverse reactions to the chemotherapy. No moderation of the dose was required, as there was no severe adverse reaction to radiotherapy.
It is worth noting that one patient experienced perianal edema after radiotherapy and postponed the surgery. After a follow-up of 10 months, there was no sign of tumor recurrence. When summarizing 4 retrospective studies, Roels et al found the recurrence rate of rectal cancer in the ischiorectal fossa to be approximately 4% (53/1188), with risk factors being the tumor distance of ≤6 cm from the anal verge and the APR, which account for the recurrence rates of 8% (18/234) and 11% (21/189), respectively.23 In this study, among patients with tumors that were 3 cm from the anal verge, the radiotherapy target included the ischiorectal fossa. On the other hand, Valentini et al believed that the levator ani can be considered an effective natural barrier against the metastasis of tumor cells to the ischiorectal fossa.24 Before the distal rectal tumor spreads to the levator ani or the internal or external sphincter, the preoperative radiotherapy target can leave out the ischiorectal fossa to minimize the perianal radiodermatitis and facilitate fast recovery of the perineal incision.
The extent of tumor shrinkage after preoperative neoadjuvant chemoradiation is positively correlated with the patients’ prognosis, with complete remission predictive of a high survival chance. Capirci et al performed a follow-up analysis of 566 patients with locally intermediate to advanced rectal cancer; patients achieving ypCR after preoperative chemotherapy had a low local recurrence rate of 1.6% and a staggering 5-year overall survival rate of 90%.25 Patients who cannot tolerate radical surgeries may consider high-dose local chemoradiation of the tumor, which can promote tumor shrinkage and downstaging until complete remission. Appelt enrolled 51 T2/3 patients with rectal cancer having tumors ≤6 cm from the anal verge; after high-dose preoperative chemoradiation, 40 (78%) of them achieved cCR without radical operations and, upon watchful waiting for 1 year and 2 years, had a local recurrence rate of 15.5% and 25.9%, respectively.26 Habr-Gama et al found that, among 71 patients achieving cCR after neoadjuvant chemoradiation, the 5-year disease-free survival before any surgical treatment was 92%.27 Another study added 0, 2, 4, and 6 cycles of mFOLFOX6 induction chemotherapy in the interval between preoperative chemoradiation and total mesorectal excision surgery; the findings revealed that, despite the absence of significant improvement in the sphincter preservation rate, the tumor shrinkage was increasingly evident with the postponement of the surgery and the increase in chemotherapy intensity.28 There was no apparent increase in the surgical difficulty or risks; the long-term survival rates were not reported. Whether patients with distal rectal cancer with satisfactory tumor shrinkage after radiotherapy can avoid radical surgeries by having local resection or increasing the chemotherapy intensity even if they have not achieved cCR is a research topic for future studies.
Limitations
There were limitations in our study, including the use of single-center, noncontrolled research, and small sample size. Additionally, the follow-up is relatively short, and longer follow-up would be required to assess whether the high rate of ypCR could translate into benefits in progression-free survival and overall survival.
Conclusions
The combined preoperative regimen of VMAT-SIB (VMAT-SIB58.75Gy) and induction chemotherapy for locally advanced distal rectal cancer yielded a satisfactory sphincter preservation rate and ypCR rate. The preliminary results showed good tolerance and a low occurrence rate of adverse events among the patients, but the long-term efficacy and their quality of life are worth further investigation.
Abbreviations
- APR
abdominoperineal resection
- cCR
clinical complete response
- CT
computed tomography
- CTV
clinical target volume
- GTV
gross target volume
- MRI
magnetic resonance imaging
- OAR
organ at risk
- PGTV
planning gross target volume
- PTV
planning target volume
- SIB
simultaneous integrated boost
- TRG
tumor regression grading
- VMAT
volumetric modulated arc therapy
- ypCR
complete pathological response
Footnotes
Authors’ Note: Y.Y. and Q.L. contributed equally to this work. Trial Number from the WHO-approved Chinese Clinical Trial Registry: ChiCTR-ONC-12002387.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding.: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was funded by the National Key Research and Development Program of Ministry of Science and Technology of China 2016YFC0105712, and Suzhou “Revitalizing Healthcare with Science and Education” Youth Science and Technology Project KJXW2016010.
ORCID iD: Yongqiang Yang, MD  https://orcid.org/0000-0002-6981-4833
https://orcid.org/0000-0002-6981-4833
References
- 1. Sauer R, Becker H, Hohenberger W, et al. ; German Rectal Cancer Study Group. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351(17):1731–1740. [DOI] [PubMed] [Google Scholar]
- 2. A’Hern RP. Sample size tables for exact single-stage phase II designs. Stat Med. 2001;20(6):859–866. [DOI] [PubMed] [Google Scholar]
- 3. Dworak O, Keilholz L, Hoffmann A. Pathological features of rectal cancer after preoperative radiochemotherapy. Int J Colorectal Dis. 1997;12(1):19–23. [DOI] [PubMed] [Google Scholar]
- 4. Crane CH, Skibber JM, Feig BW, et al. Response to preoperative chemoradiation increases the use of sphincter-preserving surgery in patients with locally advanced low rectal carcinoma. Cancer. 2003;97(2):517–524. [DOI] [PubMed] [Google Scholar]
- 5. Luna-Perez P, Rodriguez-Ramirez S, Rodriguez-Coria DF, et al. Preoperative chemoradiation therapy and anal sphincter preservation with locally advanced rectal adenocarcinoma. World J Surg. 2001;25(8):1006–1011. [DOI] [PubMed] [Google Scholar]
- 6. Gerard JP, Chapet O, Nemoz C, et al. Improved sphincter preservation in low rectal cancer with high-dose preoperative radiotherapy: the lyon R96-02 randomized trial. J Clin Oncol. 2004;22(12):2404–2409. [DOI] [PubMed] [Google Scholar]
- 7. Janjan NA, Khoo VS, Abbruzzese J, et al. Tumor downstaging and sphincter preservation with preoperative chemoradiation in locally advanced rectal cancer: the M. D. Anderson Cancer Center experience. Int J Radiat Oncol Biol Phys. 1999;44(5):1027–1038. [DOI] [PubMed] [Google Scholar]
- 8. Gerard JP, Rostom Y, Gal J, et al. Can we increase the chance of sphincter saving surgery in rectal cancer with neoadjuvant treatments: lessons from a systematic review of recent randomized trials. Crit Rev Oncol Hematol. 2012;81(1):21–28. [DOI] [PubMed] [Google Scholar]
- 9. Bujko K, Kepka L, Michalski W, Nowacki MP. Does rectal cancer shrinkage induced by preoperative radio(chemo)therapy increase the likelihood of anterior resection? A systematic review of randomised trials. Radiother Oncol. 2006;80(1):4–12. [DOI] [PubMed] [Google Scholar]
- 10. Mozafar M, Adhami F, Atqiaee K, et al. Neo-adjuvant chemoradiotherapy; an opportunity in sphincter preserving procedure for rectal cancer. Gastroenterol Hepatol Bed Bench. 2014;7(1):32–37. [PMC free article] [PubMed] [Google Scholar]
- 11. Hyams DM, Mamounas EP, Petrelli N, et al. A clinical trial to evaluate the worth of preoperative multimodality therapy in patients with operable carcinoma of the rectum: a progress report of National Surgical Breast and Bowel Project Protocol R-03. Dis Colon Rectum. 1997;40(2):131–139. [DOI] [PubMed] [Google Scholar]
- 12. Burbach JP, den Harder AM, Intven M, van Vulpen M, Verkooijen HM, Reerink O. Impact of radiotherapy boost on pathological complete response in patients with locally advanced rectal cancer: a systematic review and meta-analysis. Radiother Oncol. 2014;113(1):1–9. [DOI] [PubMed] [Google Scholar]
- 13. Gerard J, Conroy T, Bonnetain F, et al. Preoperative radiotherapy with or without fluorouracil and leucovorin in T3-4 rectal cancers: results of FFCD 9203. J Clin Oncol. 2006;24(28):4620–4625. [DOI] [PubMed] [Google Scholar]
- 14. Rodel C, Liersch T, Hermann RM, et al. Multicenter phase II trial of chemoradiation with oxaliplatin for rectal cancer. J Clin Oncol. 2007;25(1):110–117. [DOI] [PubMed] [Google Scholar]
- 15. Zhu J, Liu F, Gu W, et al. Concomitant boost IMRT-based neoadjuvant chemoradiotherapy for clinical stage II/III rectal adenocarcinoma: results of a phase II study. Radiat Oncol. 2014;9:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Glynne-Jones R, Anyamene N, Moran B, Harrison M. Neoadjuvant chemotherapy in MRI-staged high-risk rectal cancer in addition to or as an alternative to preoperative chemoradiation? Ann Oncol. 2012;23(10):2517–2526. [DOI] [PubMed] [Google Scholar]
- 17. Bujko K, Nowacki MP, Nasierowska-Guttmejer A, Michalski W, Bebenek M, Kryj M. Long-term results of a randomized trial comparing preoperative short-course radiotherapy with preoperative conventionally fractionated chemoradiation for rectal cancer. Br J Surg. 2006;93(10):1215–1223. [DOI] [PubMed] [Google Scholar]
- 18. Cercek A, Goodman KA, Hajj C, et al. Neoadjuvant chemotherapy first, followed by chemoradiation and then surgery, in the management of locally advanced rectal cancer. J Natl Compr Canc Netw. 2014;12(4):513–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Aschele C, Cionini L, Lonardi S, et al. Primary tumor response to preoperative chemoradiation with or without oxaliplatin in locally advanced rectal cancer: pathologic results of the STAR-01 randomized phase III trial. J Clin Oncol. 2011;29(20):2773–2780. [DOI] [PubMed] [Google Scholar]
- 20. O’Connell MJ, Colangelo LH, Beart RW, et al. Capecitabine and oxaliplatin in the preoperative multimodality treatment of rectal cancer: surgical end points from National Surgical Adjuvant Breast and Bowel Project trial R-04. J Clin Oncol. 2014;32(18):1927–1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gerard JP, Azria D, Gourgou-Bourgade S, et al. Comparison of two neoadjuvant chemoradiotherapy regimens for locally advanced rectal cancer: results of the phase III trial ACCORD 12/0405-Prodige 2. J Clin Oncol. 2010;28(10):1638–1644. [DOI] [PubMed] [Google Scholar]
- 22. Hofheinz RD, Wenz F, Post S, et al. Chemoradiotherapy with capecitabine versus fluorouracil for locally advanced rectal cancer: a randomised, multicentre, non-inferiority, phase 3 trial. Lancet Oncol. 2012;13(6):579–588. [DOI] [PubMed] [Google Scholar]
- 23. Roels S, Duthoy W, Haustermans K, et al. Definition and delineation of the clinical target volume for rectal cancer. Int J Radiat Oncol Biol Phys. 2006;65(4):1129–1142. [DOI] [PubMed] [Google Scholar]
- 24. Valentini V, Gambacorta MA, Barbaro B, et al. International consensus guidelines on clinical target volume delineation in rectal cancer. Radiother Oncol. 2016;120(2):195–201. [DOI] [PubMed] [Google Scholar]
- 25. Capirci C, Valentini V, Cionini L, et al. Prognostic value of pathologic complete response after neoadjuvant therapy in locally advanced rectal cancer: long-term analysis of 566 ypCR patients. Int J Radiat Oncol Biol Phys. 2008;72(1):99–107. [DOI] [PubMed] [Google Scholar]
- 26. Appelt AL, Pløen J, Harling H, et al. High-dose chemoradiotherapy and watchful waiting for distal rectal cancer: a prospective observational study. Lancet Oncol. 2015;16(8):919–927. [DOI] [PubMed] [Google Scholar]
- 27. Habr-Gama A, Perez RO, Nadalin W, et al. Operative versus nonoperative treatment for stage 0 distal rectal cancer following chemoradiation therapy: long-term results. Ann Surg. 2004;240(4):711–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Garcia-Aguilar J, Chow OS, Smith DD, et al. ; Timing of Rectal Cancer Response to Chemoradiation Consortium. Effect of adding mFOLFOX6 after neoadjuvant chemoradiation in locally advanced rectal cancer: a multicentre, phase 2 trial. Lancet Oncol. 2015;16(8):957–966. [DOI] [PMC free article] [PubMed] [Google Scholar]